Open Access Article
Open Access ArticleUnveiling the potential of bismuth-based catalysts for electrochemical CO2 reduction
Negar
Sabouhanian
,
Jacek
Lipkowski
* and
Aicheng
Chen
 *
*
Electrochemical Technology Centre, Department of Chemistry, University of Guelph, 50 Stone Road East, Guelph, Ontario N1G 2W1, Canada. E-mail: jlipkows@uoguelph.ca; aicheng@uoguelph.ca
First published on 4th December 2024
Abstract
Electrochemical CO2 reduction has favorable industrial relevance due to its integrability with renewable energies and controllable product generation. Bismuth-based catalysts have emerged as promising candidates in this regard due to their intriguing electrochemical properties and cost-effectiveness. This review focuses on recent advances in bismuth-based catalysts for the electrochemical reduction of CO2, including synthesis methods and approaches for performance improvements. Insights into product formations using Bi-based catalysts are also presented, where in situ FTIR and Raman spectroscopic studies are highlighted to understand the structural evolution of the catalysts and to decipher the mechanisms of CO2 reduction. Further, recent progress of electrochemical CO2 reduction from an industrial perspective and strategies for further development of the bismuth-based catalysts with high activity, selectivity and stability towards practical applications are discussed.
Keywords: Electrochemical CO2 reduction; Bismuth; Nanomaterials; Electrocatalysts; In situ spectroscopy.
1. Introduction
The incessant increase in atmospheric carbon dioxide (CO2) levels is widely recognized as the primary driver of climate change/global warming.1,2 Potential strategies for carbon capture and utilization (CCU) to mitigate CO2 emissions have attracted considerable interest.3–5 The electrochemical CO2 reduction reaction (CO2RR) has significant potential for industrial applications as it can integrate renewable energies as power sources.6–8 In this context, reports in the literature have revealed the intensive investigation of diverse electrocatalysts and CO2RR mechanisms.8–10 Despite extensive research, implementing CO2 electroreduction remains constrained due to the excessive costs involved.11Formate is one of the products of CO2RR having substantial market potential as a valuable industrial feedstock.12 The formic acid/formate market is anticipated to reach one megaton per year by 2030 with various applications in medical, agriculture, and textile industries.13 They are regarded as a vital intermediate for synthesizing valuable oxygen-containing compounds such as alcohols, esters, and acids in syngas catalysis. Additionally, the high density of formic acid (1.22 kg L−1) gives it a significant volumetric hydrogen capacity of 53 g H2 per liter, making it a promising candidate as a liquid hydrogen carrier.14 It may be utilized in energy production and storage systems such as hydrogen fuel cells.13 Formate/formic acid formation by electrochemical reduction of CO2 is a promising approach due to its integrability with renewable energies, relatively low cost, practical operation and being environmentally friendly.15 Among various metal-based catalysts, bismuth-based materials have exhibited significant potential for the electrochemical reduction of CO2 to formate with high selectivity.16 Bismuth is mainly found on the ores bismuthinite (bismuth sulfide) and bismite (bismuth oxide) as well as Bi crystals with an oxide layer. Bismuth is not very reactive and can sometimes be found as a native metal.17 Bi-based catalysts exhibited a lower overpotential and higher faradaic efficiency for formate formation compared with other metals. The high formate selectivity can be attributed to the low energy barrier of *COO− intermediate formation. In addition, hydrogen evolution reaction (HER) as a competitive reaction exhibits a relatively high overpotential at Bi-based catalysts.18 These factors along with the cost-effectiveness, low toxicity and high abundance in nature in contrast to other metals make Bi-based catalysts promising for CO2 reduction to formate in large-scale applications.19–24 Different nanostructured monometallic Bi catalysts (e.g., nanoparticles, nanorods, nanodendrites, and nanosheets) have been synthesized and investigated for CO2 reduction.25 However, the weaker *OCHO binding energy makes it difficult to boost formate generation.26 It has been shown that the addition of secondary elements to form bimetallic Bi-based catalysts might effectively improve their catalytic performance.27 The binding energy of oxygen in *OCHO may be adjusted by introduction of another metal (e.g., CuBi,28 BiSn,29 InBi,30 and ZnBi (ref. 31)), which may enhance the *OCHO adsorption through compressive strain and changes of surface electronic bands structure.26 Further, it is revealed that bimetallic Bi-based catalysts not only electrochemically reduce CO2 to formate, but also lead to the creation of other products such as propane and ethylene.32,33In situ characterization techniques have been developed to study the reaction mechanisms and identify the key intermediates formed during CO2 reduction. Great efforts have been made to attain industrial-compatible current densities and high stability for CO2 reduction to formate on Bi-based catalysts using flow cells or membrane electrode assemblies (MEAs). However, achieving ampere level current densities and high electrode stability remains challenging.
In this review, we discuss the recent advances in the development of bismuth-based catalysts with a focus on synthesis methods and strategies for performance enhancement. The possibility of additional product generation using Bi-based catalysts reported in the recent literature is featured. The detection of key intermediates and monitoring of product formation via in situ FTIR and Raman spectroscopy are highlighted. Finally, recent achievements from an industrial perspective are described, and future research directions are proposed.
2. Monometallic Bi-based catalysts
2.1. Synthesis methods
Several strategies have been employed to fabricate monometallic Bi-based catalysts, including top-down exfoliation techniques, chemical reduction reactions, and the electroreduction of pre-synthesized materials (e.g., BiOX where X is Cl, Br, or I,34 (BiO)2CO3,35 Bi2O3,21 and Bi-metal organic frameworks (Bi-MOFs)).36 The morphologies21 and coordination environments37 of Bi-containing precursors significantly affect the final structure of the Bi-based catalyst. Various surface engineering techniques have been applied to improve the faradaic efficiency (FE) of formate production, including defect engineering, heteroatom doping, and the active site reconstitution in Bi nanosheets (Bi NSs).38,39 In this section, recent advances in strategies for monometallic Bi-based catalysts synthesis, and proposed strategies for enhancing their catalytic performance are reviewed.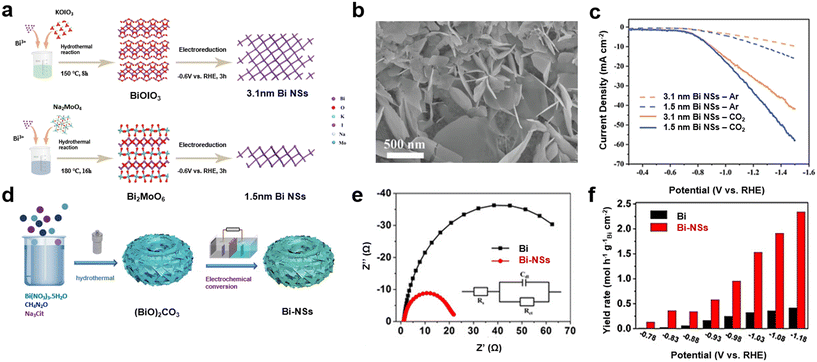 | ||
| Fig. 1 (a) Schematic of the preparation of ultrathin Bi NSs; (b) SEM image of 1.5 nm Bi NSs; (c) LSV curves for 3.1 nm Bi NSs and 1.5 nm Bi NSs in a CO2 and Ar saturated 0.5 M KHCO3 solution36 (copyright 2023 Elsevier); (d) schematic of the preparation of Bi NSs; (e) Nyquist plots and (f) formate yielded rates for CO2RR over Bi NSs and Bi nanopowders35 (copyright 2021 John Wiley and Sons). | ||
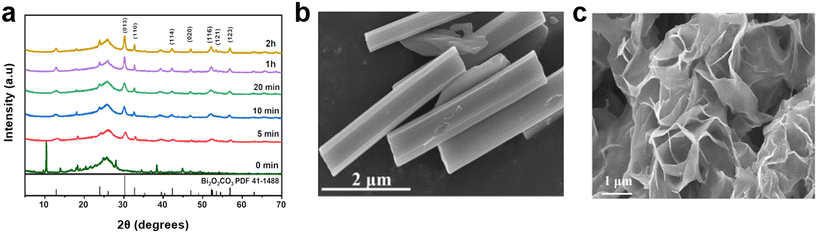 | ||
| Fig. 2 (a) XRD patterns of Bi-BTC after different electrolysis times (0 min, 5 min, 10 min, 20 min, 1 h, and 2 h); SEM image of Bi-BTC (b) before and (c) following electrolysis57 (copyright 2024 American Chemical Society). | ||
2.2. Strategies for improving performance
Defect engineering has been employed as an effective strategy to enhance CO2RR performance by improving selectivity, activity and stability of the catalyst. The grain boundaries in polycrystalline materials may create sites with improved activity for CO2 reduction in comparison to the competing HER.60 The selectivity of Bi-based catalysts is enhanced by improving the affinity to the *OCHO intermediate. It has been shown that the adequate exposure of edge sites and defects is a successful approach for improving the selectivity of the Bi-based catalysts in CO2RR.16,34,36 The defects of reconstructed Bi strongly depend on the initial morphology and coordination environment of Bi-based pre-catalysts.37 For example, bismuth oxide nanosheets could be converted into porous bismuth nanosheets (Bi PNSs) with abundant kink sites on the pore walls,61 or converted to Bi nanoribbons by an in-plane confined hydrogen-reduction strategy with abundant Bi–O edge sites.62 Bismuth sulfide (Bi2S3) nanorods were electroreduced to defect-rich metallic Bi.63 The major impacts of the preferential exposure sites and defect engineering were studied by Xu and coworkers.34 The authors synthesized BiOBr nanosheets as Bi-containing precursors using a hydrothermal method, which was then converted to Bi NSs by topotactic transformation. As distinct Bi sources, cetyltrimethylammonium bromide (CTAB) and KBr were used during the hydrothermal process to control crystallization and form BiOBr NS with rich edge and terrace sites, respectively. Following topotactic transformation, preferential exposure sites were maintained, and a certain quantity of defect sites was also produced. DFT calculations were performed to evaluate the effects of edge sites, terrace sites, and defects on *OCHO intermediate formation as the most energetically favored reaction pathway toward formate creation. It was revealed that the formation energy of *OCHO on the Bi-edge sites was lower than that of the terrace sites, and defects on the edge sites could further decrease its Gibbs-free energy. It was determined that the edge/defect-rich Bi NS with a dramatically enlarged surface area exhibited high performance for CO2RR with a current density of up to 870 mA cm−2 at −1.08 V vs. RHE and FE higher than 90% for formate generation. In another study conducted by Wang et al.,64 metal Bi with abundant defects (Bi-D) was synthesized via a solvothermal method and showed a 93.9% FE of formate at −0.9 V vs. RHE. The presence of amorphous phases introduced abundant defects and unsaturated active sites that could enhance the FE compared with commercial Bi powder. The introduction of oxygen vacancies as defects was shown by Ren et al.65 to increase the activity and selectivity in a wide potential window, where the 2D Bi/Bi2O3 catalyst that possessed abundant oxygen vacancies (Bi/Bi2O3–Ov) exhibited high selectivity for the generation of formate with an FE of >90% in a wide potential range of −0.7 to −1.35 V (RHE). It was shown that the modified adsorbing-desorbing property was due to the abundant oxygen vacancies provided adsorption sites for CO2 molecules on the catalyst surface. CO2 molecules were thus ready to be reduced even at high negative potentials (up to −1.35 V (RHE)). The FE of formate remained at around 90% with no obvious decrease after 200 h at −0.8 and −0.85 V (RHE), showing the high stability of Bi/Bi2O3–Ov catalyst. In addition to oxygen vacancies, amorphous regions as indicated in Fig. 3a (red dotted circles) were observed. The Bi/Bi2O3–Ov catalyst showed a higher formate FE than the same catalysts without oxygen vacancies (Fig. 3b). DFT calculations revealed that the Bi/Bi2O3–Ov catalyst exhibited a lower energy barrier for *OCHO formation than the Bi/Bi2O3 catalyst (Fig. 3c), confirming the higher activity and selectivity of Bi/Bi2O3–Ov for CO2RR to formate in a wide range of potential. The numerous O vacancies were suggested to create frustrated Lewis pairs (FLPs) on the surface to promote CO2RR.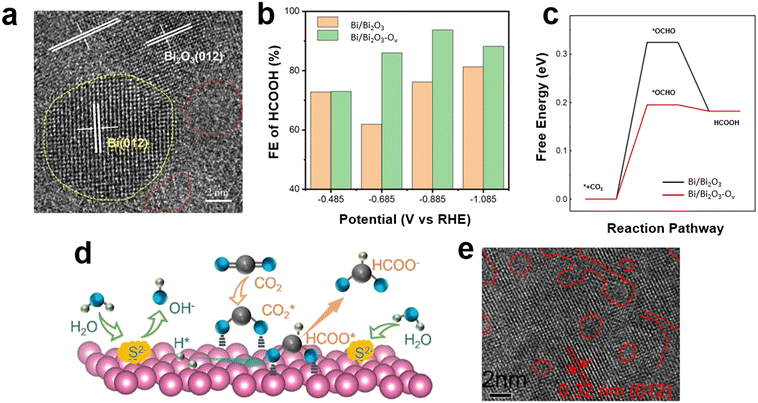 | ||
| Fig. 3 (a) HRTEM image of Bi/Bi2O3–Ov; (b) FE of formate for Bi/Bi2O3 and Bi/Bi2O3–Ov catalysts measured at different potentials in flow cells using 1 M KOH electrolyte; (c) free energy diagrams of CO2RR to formate using Bi/Bi2O3 and Bi/Bi2O3–Ov catalysts65 (copyright 2022 John Wiley and Sons); (d) schematic of CO2 reduction mechanism using Bi-S2 catalyst; (e) HR-TEM image for Bi-S2 catalysts66 (copyright 2023 Elsevier). | ||
The creation of disordered metal sites has been proposed by Wang et al.19 as an efficient defect engineering strategy to activate CO2 and enhance activity for formate formation. It has been suggested that CO2 molecules tended to be adsorbed to and activated on the disorder-engineered Bi sites. It was observed that the distorted metal sites could enhance localized electron transfer to the antibonding π* orbital of adsorbed CO2 to bend the linear CO2 molecules. The richly lattice distorted Bi NSs exhibited a high value of formate FE, reaching ∼100% at a current density of 200 mA cm−2.
It was confirmed that the introduction of p-block atoms into bismuth could modify its electronic structure and alter the energy required for the generation of intermediates.67 The introduction of appropriate heteroatoms may modify the electronic density of Bi p-orbitals, thus enhancing the adsorption of the *OCHO intermediates and improving the intrinsic activity for CO2 reduction.68 For instance, the edge defects of Bi NSs may be coordinated by heteroatom dopants such as sulfur,37,66 titanium,69 or copper39 to enhance CO2RR selectivity, while suppressing the competing HER. Chen et al.67 showed that boron doping could induce the formation of electron-rich Bi, thus facilitating the reduction of *OCHO. A similar electron enrichment effect was also observed by Ti doping, which enhanced interactions between the active sites and *OCHO intermediates. For pure Bi, *OCHO intermediates are strongly adsorbed on the surface, making them difficult to desorb as formate.67 It was revealed that the electron enrichment of Bi could weaken the binding strengths between the active metal centers and oxygen atoms, thereby lowering the barrier for generating *OCHO intermediate.69 Furthermore, the formation of H* as a key intermediate for HER may be strongly suppressed by doping.67 A recent study conducted by Wang et al.66 demonstrated that sulfur doping not only induced charge redistribution around Bi atoms but also activated water molecules to provide sufficient 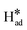 for CO2RR rather than HER (Fig. 3d) to optimize the reaction pathway toward formate formation. The HR-STEM image of S-doped Bi in Fig. 3e revealed a profusion of defects due to the breaking of Bi–O bonds in the initial Bi-containing precursor following topotactic transformation. The effects of sulfur doping on the catalytic activity of Bi NSs were investigated using DFT calculations as reported by Lv et al.,37 indicating that sulfur dopants existed primarily at the edge sites of Bi NSs. This translated to the strong adsorption capacities of *OCHO intermediates while inhibiting the production of CO and H2.
for CO2RR rather than HER (Fig. 3d) to optimize the reaction pathway toward formate formation. The HR-STEM image of S-doped Bi in Fig. 3e revealed a profusion of defects due to the breaking of Bi–O bonds in the initial Bi-containing precursor following topotactic transformation. The effects of sulfur doping on the catalytic activity of Bi NSs were investigated using DFT calculations as reported by Lv et al.,37 indicating that sulfur dopants existed primarily at the edge sites of Bi NSs. This translated to the strong adsorption capacities of *OCHO intermediates while inhibiting the production of CO and H2.
Recent studies have claimed that the bismuth subcarbonate (Bi2O2CO3) is a stable Bi phase under CO2RR that may serve as the active phase for the generation of formate.70 Bi2O2CO3 can provide high carrier mobility due to its thin thickness and limits nanoparticle growth, preventing disordered aggregation and preserving the number of active sites.26 The establishment of the Bi–Bi2O2CO3 interface has been suggested as an efficient strategy to promote CO2 activation and the formation of key intermediates.63 Liu et al.25 synthesized flower-like Bi NSs and confirmed the formation of stable Bi–Bi2O2CO3 interfaces after exposure to air. It was shown that the electrode containing Bi–Bi2O2CO3 interfaces exhibited improved CO2 reduction activity in contrast to the bulk Bi electrode, and the FE of formate could be enhanced up to 89% at −1.07 V vs. RHE. Two-dimensional nanoflake Bi2O2CO3 was utilized as a substrate to load InOx nanodots for efficient CO2 reduction to formate. A good performance of the catalysts with a high FE of 90.83% at a current density of 200 mA cm−2 was attributed to the exposure of the active sites.26
3. Bimetallic Bi-based catalysts
Bimetallic electrocatalysts typically exhibit higher activity and selectivity for CO2 reduction in contrast to monometallic catalysts since it is an effective approach for adjusting the composition, stabilizing key intermediates, optimizing the electronic structure, and suppressing competing reactions.20,71,72 The careful and rational design of the alloy composition and structure can enhance the selective adsorption of intermediates at active sites, lowering activation barriers and favoring desired reaction pathways. In this section, recently reported Bi-based bimetallic catalysts for the generation of formate and other products are reviewed.3.1. Formate generation
Cu and Bi have been widely investigated in bimetallic systems to augment the generation of formate.73 The introduction of Cu into Bi enables the reduction of the energy barriers for intermediate formation through effective electronic structure modifications, facilitating the creation of formate.74,75 Yang et al.76 studied the effect of electronic structure modifications on the CO2 adsorption at Bi, Bi/Cu9S5, and Bi/Cu9S8 catalysts. The projected density of states (PDOS) results showed that the interaction of *OCHO with Bi was mainly due to the s-p orbital hybridization. However, the heterojunctions Bi/Cu9S5 and Bi/Cu9S8 provided more Bi orbital hybridization mainly due to the p-d orbital hybridization, enhancing the adsorption of *OCHO intermediate. Recently, differently structured CuBi catalysts were synthesized utilizing various techniques (e.g., electrodeposition,72 hydrothermal,77 derivations from MOFs,78 and galvanic exchange reactions11). Liu et al.79 synthesized BiCu on a Cu foam by coupling a hydrothermal reaction followed by electrochemical transformation. Cu2+ and Bi3+ ions were co-deposited on a Cu foam to create a BiCu pre-catalyst (BiCu/CF-p), which had close interactions between Bi and Cu. After further electrochemical transformation, the obtained bimetallic BiCu catalyst on Cu foam (BiCu/CF) exhibited an unexpectedly high formate current density. Fig. 4a illustrates their synthesis procedures, which show that the delocalization of Bi p-orbitals induced by nearby metal Cu atoms enhanced the CO2RR pathway. This interaction facilitated the hybridization of orbitals of Bi atoms and *OCHO intermediates, which created additional anti-bonding orbitals. Consequently, the *OCHO intermediates were stabilized and the thermodynamic barrier of CO2RR was reduced. Similarly, Lou et al.73 successfully co-electrodeposited CuBi bimetallic catalysts on a derived copper foam using complexing agents like trisodium citrate dehydrate in the electrolyte solution. In this work, the researchers confirmed that the applied potential for co-electrodeposition had a significant impact on the growth mode of the catalyst, which altered its performance to selectively convert CO2 to formate. It was observed that a needle-like bimetallic CuBi structure (Fig. 4b) was formed at −0.6 V (Ag/AgCl), which had irregular coverage and showed the highest formate FE (94.4%) at −0.97 V (RHE). The tip of the needles could enhance the concentration of the adsorbed CO2 on the catalyst surface due to the field-induced reagent concentration (FIRC) effect. In another study conducted by Xue et al.78 a novel Cu/Bi bimetallic catalyst with a cylindrical morphology containing bimetallic nanoparticles was derived from MOFs (Fig. 4c). The FE of formate attained 93% at −0.94 V (RHE), which was attributed to the stronger adsorption of CO2− intermediates. The electron transfer of Cu to Bi could tune the binding strength of CO2− intermediates, leading to improving electrocatalytic selectivity toward formate. A new strategy for the synthesis of 3D Cu–Bi nanofoam electrodes was reported by Yang et al.,80 who employed a rapid thermal shock synthesis technique followed by porosity engineering via acid etching and electroreduction.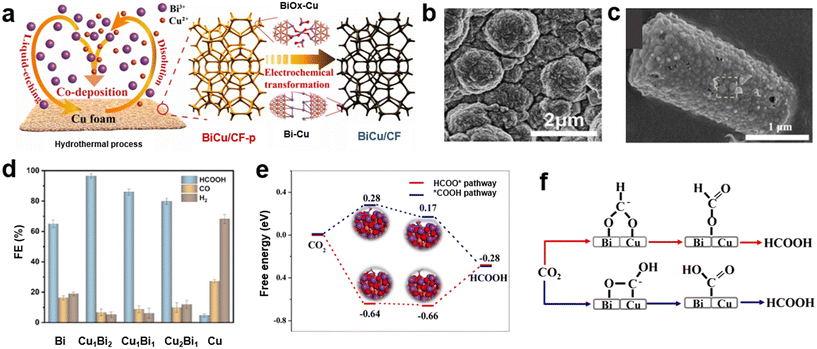 | ||
| Fig. 4 (a) Schematic of fabrication pathway for a BiCu/CF electrode79 (copyright 2022 Elsevier); (b) SEM image of CuBi bimetallic catalyst electrode synthesized at −0.6 V73 (copyright 2022 Elsevier); (c) SEM image of the optimized Cu1–Bi/Bi2O3@C catalyst78 (copyright 2022 John Wiley and Sons); (d) FE of CO2RR over Cu–Bi electrocatalysts at −0.9 V (vs. RHE)81 (copyright 2022 John Wiley and Sons); (e) free energy diagrams of various pathways of CO2RR to formate on a CuBi75 (211) plane and (f) proposed mechanism of CO2 reduction on the CuBi75 catalyst82 (copyright 2021 Elsevier). | ||
In addition to the morphologies of the bimetallic CuBi catalysts, their compositions played key roles in determining their activities77 and selectivities.32 Li et al.81 prepared self-supporting Cu–Bi aerogel catalysts at different molar ratios (Fig. 4d) and showed that the selectivity could be modified by altering the molar ratio of Cu/Bi. A high formate FE (96.57%) was achieved using a Cu1Bi2 catalyst. They suggested that the 3D self-supporting structure and high surface area of Cu–Bi aerogels could facilitate electron transfer through more transport channels. As a result, it enhanced the reaction of the intermediates with protons/electrons, leading to higher CO2RR catalytic efficiency. In another study, a composite Cu1Bi1 bimetallic catalyst with a ginger root-like structure (CuO/CuBi2O4) was synthesized by Ren et al.,83 which exhibited a high FE (98.07%) for formate at −0.98 V vs. RHE. It was demonstrated that the Cu–Bi interface could provide abundant active sites for CO2RR, and the presence of bismuth–oxygen bonds stabilized the adsorbed CO2*− intermediate. According to the literature, it was demonstrated that the conversion of CO2 to formate on CuBi catalysts was more favorable through the HCOO* pathway since it was considerably downhill in energy (−0.66 eV). The corresponding free energy diagram of different pathways and the proposed mechanism of CO2 reduction are shown in Fig. 4e and f.82 CO2 was preferably converted to formate with the HCOO* intermediate.
Recently, bimetallic bismuth-based catalysts integrated with other metals such as Sn, In, and Zn have been explored. For instance, bimetallic Bi/Sn catalysts were prepared by a two-step electrodeposition method,29 and it was found that the morphology and catalytic activity could be greatly affected by the deposition time. The needle-like structure in Fig. 5a was created through the deposition of metallic Bi for 5 min followed by the deposition of metallic Sn for 60 min (Bi5Sn60) on a copper mesh substrate. It was demonstrated that Bi5Sn60 had a high formate production rate (634.3 μmol cm−2 h−1) at −1.0 V (vs. RHE). This remarkable formate generation rate was related to the modification of the electronic structure, which enhanced the interactions between the active sites and *OCHO intermediates. A facile co-electrodeposition method was suggested by Yang et al.84 for the synthesis of bimetallic SnBi catalysts. They utilized the specifically formulated electrolyte solution with an adjusted pH of 8–10 at the temperature of 60 °C to obtain a desirable alloy with the best performance for CO2 reduction. This group reported a high formate FE of 96.1% at −1.06 V vs. RHE for a Sn0.5Bi catalyst (0.5 Sn2+/Bi3+ molar ratio), which was attributed to a large surface area with an abundance of active sites and defects. It was reported that the tin metal oxide/bismuth metal oxide interface stabilized the CO2− intermediate and suppressed HER. Also, the electronic coupling at the interfaces of Sn and Bi led to the *OCHO formation pathway, thus promoting the formate generation. Xu et al.85 prepared a Sn-doped Bi nanowire bundle (NB) through the in situ reconstruction of Sn-doped Bi2S3 precursors. By optimizing the doping concentration, a remarkable performance for CO2RR to formate could be achieved. The Sn1/24-Bi NBs showed a high FE of formate >90% over a wide potential window of −0.5 to −1.9 V vs. RHE. The excellent activity of the Sn1/24-Bi NBs catalysts originated from the electron-rich surface, as well as a lower reaction kinetic barrier. The calculation of the Fermi level of both Bi and Sn1/24-Bi catalysts exhibited the availability of more free electrons on the surface of Sn1/24-Bi catalyst, improving the CO2− adsorption.
 | ||
| Fig. 5 (a) SEM image of Bi5Sn60 catalyst29 (copyright 2022 Elsevier); (b) the measured FE of formate for InBi catalysts with different compositions at −0.94 V (solid bar) and the DFT-calculated WF for In, In16Bi84, and Bi (orange line)30 (copyright 2022 American Chemical Society); (c) SEM image of the ZnBi-3 electrode at 3000× magnification. (d) LSV curves of Zn and ZnBi electrodes recorded at a 20 mV s−1 scan rate for CO2RR (solid lines) and HER (dashed lines)86 (copyright 2024 American Chemical Society). | ||
Zhou et al.87 synthesized a defective BiIn catalyst and studied the influences of defective surfaces on the promotion of HCOOH formation, showing that the introduction of indium to bismuth is an effective approach to enhance the FE of formate via the optimization of the binding energy of *OCHO. Phosphorus-doped BiIn was used as a pre-catalyst to create defective surfaces during the self-reconstruction. It was demonstrated that the defective sites increased *OH adsorption, promoted water dissociation, and enhanced CO2RR kinetics. The Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) In ratio in BiIn catalysts can be optimized to attain high activity and selectivity. For this purpose, Wang et al.88 prepared Bi-In2O3 nanoflower catalysts with different Bi/In ratios and investigated the relationship between the catalyst composition and CO2RR performance. It was found that the catalyst with a Bi/In ratio of 6
In ratio in BiIn catalysts can be optimized to attain high activity and selectivity. For this purpose, Wang et al.88 prepared Bi-In2O3 nanoflower catalysts with different Bi/In ratios and investigated the relationship between the catalyst composition and CO2RR performance. It was found that the catalyst with a Bi/In ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 94 had a high FE of formate (88.1%) at −0.7 V vs. RHE. Likewise, indium–bismuth nanosphere catalysts with different compositions were synthesized by Tan et al.30 and tested for CO2RR to formate. The In16Bi84 had the highest FE (∼100%) of formate at −0.94 V vs. RHE as seen in Fig. 5b. It was concluded that the presence of Bi enabled electrons to flow from Bi to In and provided additional active sites for CO2RR. Improvements in the performance of bimetallic BiIn catalysts via defect engineering were proposed by Yang et al.89 The researchers showed that the oxygen vacancies originating from the lattice mismatches of Bi2O3 and In2O3 could reduce the CO2 activation energy. DFT calculations confirmed that CO2 molecules were mainly adsorbed by the oxygen vacancies, and the dominant pathway was through oxygen vacancies. MOF-derived Bi/In bimetallic oxide nanoparticles embedded in carbon networks showed excellent selectivity for formate due to the high surface area, desirable pore size distribution, and high electrical conductivity of the carbon network as well as the synergistic effect of Bi and In bimetallic components.90
94 had a high FE of formate (88.1%) at −0.7 V vs. RHE. Likewise, indium–bismuth nanosphere catalysts with different compositions were synthesized by Tan et al.30 and tested for CO2RR to formate. The In16Bi84 had the highest FE (∼100%) of formate at −0.94 V vs. RHE as seen in Fig. 5b. It was concluded that the presence of Bi enabled electrons to flow from Bi to In and provided additional active sites for CO2RR. Improvements in the performance of bimetallic BiIn catalysts via defect engineering were proposed by Yang et al.89 The researchers showed that the oxygen vacancies originating from the lattice mismatches of Bi2O3 and In2O3 could reduce the CO2 activation energy. DFT calculations confirmed that CO2 molecules were mainly adsorbed by the oxygen vacancies, and the dominant pathway was through oxygen vacancies. MOF-derived Bi/In bimetallic oxide nanoparticles embedded in carbon networks showed excellent selectivity for formate due to the high surface area, desirable pore size distribution, and high electrical conductivity of the carbon network as well as the synergistic effect of Bi and In bimetallic components.90
As a nonprecious earth-abundant metal, Zn is a promising electrocatalyst for CO2RR. Recently, the synergistic effects of Zn and Bi have been recognized, and bimetallic ZnBi catalysts have been studied for the CO2RR to formate.91 Wang et al.31 synthesized a Zn–Bi bimetallic catalyst (Zn-Bi2O3/CN) and revealed that the surface Bi–O structure and synergistic Zn–Bi effect might enhance the CO2RR. DFT calculations indicated that the presence of Zn reduced the energy barrier of HCOO* formation, thus facilitating the production of formate. A hydrothermal process was employed by Zhang et al.91 to prepare bimetallic ZnBi catalysts with a formate FE of 94% at −0.8 V vs. RHE. A feasible and cost-effective strategy was proposed by Sabouhanian et al.86 to grow bismuth nanodendrites on the Zn surface. The ZnBi catalysts were synthesized by immersing the electrodeposited Zn in a bismuth nitrate solution. Due to differences in the reduction potentials of Zn2+ and Bi3+, galvanic replacement took place and Bi nanodendrites grew uniformly on the surface (Fig. 5c). Fig. 5d depicts the activities of Zn and ZnBi electrodes for CO2RR (solid lines) and HER (dashed lines). It was observed that the CO2RR activity increased significantly after 60 s of immersion (ZnBi-1) and was boosted further at 90 s (ZnBi-2). After 90 s, the CO2RR was only slightly improved for 120 s of the galvanic replacement time (ZnBi-3). Furthermore, the additional incorporation of Bi decreased the onset potential for CO2RR. In contrast, the HER activity remained almost the same by introducing Bi as a secondary element. In situ IR spectroscopy determined that CO2 reduction at the ZnBi catalysts proceeded through the generation of the adsorbed *COO− intermediate.
3.2. Generation of other products
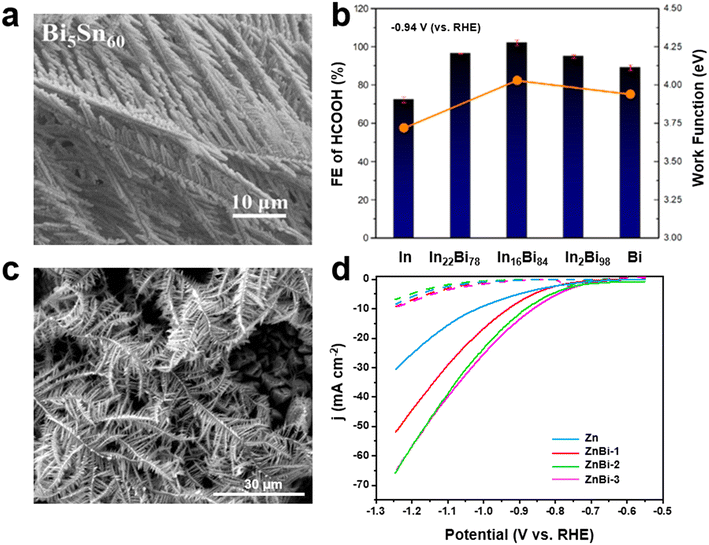
Although most of the reported bimetallic CuBi catalysts exhibited high selectivity for the production of formate, recent research has suggested the feasibility of generating other products.32 Azenha et al.92 reported the deposition of Bi on CuO NWs under various charge levels passed through the substrate; the formed catalyst demonstrated an exceptionally high selectivity of CO2RR for the generation of propane in a 0.1 M KHCO3 electrolyte, which achieved an FE of 85.4% (Fig. 6a). This remarkable catalytic performance was attributed to enhanced CO2 and CO adsorption capacities due to abundant oxygen defects. In another study, Wang et al.32 developed bimetallic CuxBi aerogel catalysts by simultaneously reducing CuCl2·2H2O and BiCl3 with NaBH4, and controlled the composition of CuxBi aerogels where X was 5, 10, 50, and 100. Interestingly, the product selectivity varied from CO to CH4, C2H4, or formate. A schematic for the synthesis of CuxBi aerogels with different selectivity is presented in Fig. 6b. It was demonstrated that the introduction of varying amounts of Bi resulted in changes to the Cu(II)/Cu(I) ratios on the catalyst surface; thus, regulating the hydrogenation capacities of intermediates. Similarly, Cu–Bi NPs were prepared with different stoichiometric ratios via chemical reduction, which revealed that the FE of CH4 and C2H4 was sensitive to the quantity of Bi.93 Cu7Bi1 had a high FE (70.6%) for CH4 at −1.2 V (vs. RHE). Further, lowering the C–C coupling energy barrier to enhance the FE of C2H4 was verified through the integration of single Bi atoms and oxygen vacancies with CuO (Bi–CuO (VO)).33 Interestingly, Bi–CuO (VO) with a FE that exceeded 48% of C2H4 at −1.05 V (vs. RHE) significantly outperformed the other Cu-based electrocatalysts. Cao et al.94 further showed the C–C coupling ability of a single Bi atom-decorated Cu alloy (BiCu-SAA) by employing operando FTIR based on a synchrotron radiation (SR-FTIR) technique. The appearance of an absorption peak at 1563 cm−1 corresponded to the *COCOH species, which verified the C–C coupling for C2 products.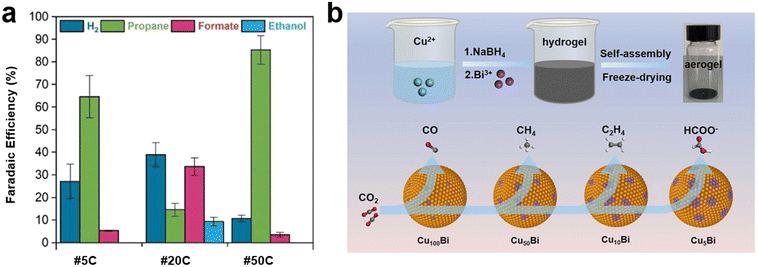 | ||
| Fig. 6 (a) Faradaic efficiencies of CO2RR on CuBi catalysts prepared with different charge values passed through the substrate using 0.1 M KHCO3 catholyte92 (copyright 2022 Elsevier); (b) schematic of synthesis procedure for CuxBi aerogels and different product selectivity32 (copyright 2022 Elsevier). | ||
4. In situ spectroscopic studies
The Bi-based catalysts were often synthesized through the in situ electrochemical transformation of the initial Bi-containing precursors. Thus, it is vital to monitor and understand the in situ structural reconstruction during the CO2RR. In situ Raman spectroscopy has been employed to monitor the dynamic reconstruction and evolution of Bi-based catalysts under CO2RR conditions.95 The transformation of the initial Bi-containing precursors to Bi NSs can be explored via in situ Raman spectroscopy through changes in the Raman peaks assigned to the vibration of the Bi–M or Bi–O bonds.36 Shen et al.39 showed the structural reconstruction of the CuS–Bi2S3 precursor to metallic Bi. In situ Raman spectra revealed that the band assigned to Bi2S3 and CuS quickly disappeared and two broad bands at 72 and 96 cm−1 appeared, which were ascribed to the Eg and A1g stretching modes of Bi–Bi bonds. Although most studies have shown the complete reduction of Bi2O3 to metallic Bi during the CO2 reduction, Deng et al.96 demonstrated the partial reduction of Bi2O3 by in situ Raman spectroscopy. They reported that the presence of Bi–O structure at the near surface was the main incentive for the selective conversion of CO2 to formate. The Bi–O structure could enhance CO2 adsorption and improve the stabilization of CO2− intermediate.The formation of bismuth subcarbonate (Bi2O2CO3) was reported and detected by in situ Raman spectroscopy during the CO2RR, contingent on the electrolyte and initial composition of the Bi precursor.95 An et al.97 reported that the formation of Bi2O2CO3 originated from the formation of a surface oxide layer (Bi3+) or oxidized metal Bi exposed to air (BiO+). In the case of Bi3+ formation, bismuth hydroxide (Bi(OH)3) was generated by the reaction of the Bi3+ and OH− from local alkalinity. However, the Bi(OH)3 was not stable and reacted with CO2 to form Bi2O2CO3 as shown in reaction (1) and (2). In the case of BiO+ formation, it further reacted with carbonate that was present in the highly alkaline CO2-purged electrolyte to form Bi2O2CO3 species as shown in reaction (3) and (4).
| Bi3+ + OH− ↔ Bi(OH)3 | (1) |
| 2Bi(OH)3 + CO2 ↔ Bi2O2CO3 + 3H2O | (2) |
| 4Bi + 3O2 + 2H2O ↔ 4BiO+ + 4OH− | (3) |
| 2BiO+ + CO32− ↔ Bi2O2CO3 | (4) |
The existence of Bi2O2CO3 species during the CO2RR process was detected using the in situ shell-isolated nanoparticle enhanced Raman spectroscopy (SHINERS) method. The appearance of the Bi–O stretching vibration of Bi2O2CO3 located at 182 cm−1 confirmed the formation of Bi2O2CO3 on the electrode surface.95 Wu et al.38 demonstrated that a thin layer of Bi2O2CO3 initially formed on the surface; however, it could be completely diminished and converted to metallic Bi prior to the occurrence of CO2RR. As shown in Fig. 7a, the peak at 162 cm−1 assigned to the Bi![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration mode of the Bi2O2CO3 appeared at the open circuit potential (OCP), which verified the formation of the Bi2O2CO3. With the application of more negative potentials from OCP to −0.9 V, the peak intensity decreased and finally disappeared. Similarly, the transformation of Bi2O3 microcrystals to Bi2O2CO3 under electrochemical conditions was reported by Zeng et al.,98 it was further reduced to metallic Bi at potentials higher than −0.6 V vs. RHE. Bi2O2CO3 has also been directly employed as a catalyst for CO2RR.57,70 The abundant oxygen vacancies in Bi2O2CO3 nanosheets (VO-BOC-NS) served as durable electrocatalysts for CO2 reduction to formate with an FE of >95% at −0.62 V vs. RHE. The stability of the VO-BOC-NS catalyst under CO2 reduction conditions was characterized using in situ Raman spectroscopy in a 0.5 M KCl electrolyte. No notable change of the intensity of peak at 1066 cm−1 derived from CO32− in VO-BOC-NS was observed even at more negative potentials, showing excellent stability.99
O vibration mode of the Bi2O2CO3 appeared at the open circuit potential (OCP), which verified the formation of the Bi2O2CO3. With the application of more negative potentials from OCP to −0.9 V, the peak intensity decreased and finally disappeared. Similarly, the transformation of Bi2O3 microcrystals to Bi2O2CO3 under electrochemical conditions was reported by Zeng et al.,98 it was further reduced to metallic Bi at potentials higher than −0.6 V vs. RHE. Bi2O2CO3 has also been directly employed as a catalyst for CO2RR.57,70 The abundant oxygen vacancies in Bi2O2CO3 nanosheets (VO-BOC-NS) served as durable electrocatalysts for CO2 reduction to formate with an FE of >95% at −0.62 V vs. RHE. The stability of the VO-BOC-NS catalyst under CO2 reduction conditions was characterized using in situ Raman spectroscopy in a 0.5 M KCl electrolyte. No notable change of the intensity of peak at 1066 cm−1 derived from CO32− in VO-BOC-NS was observed even at more negative potentials, showing excellent stability.99
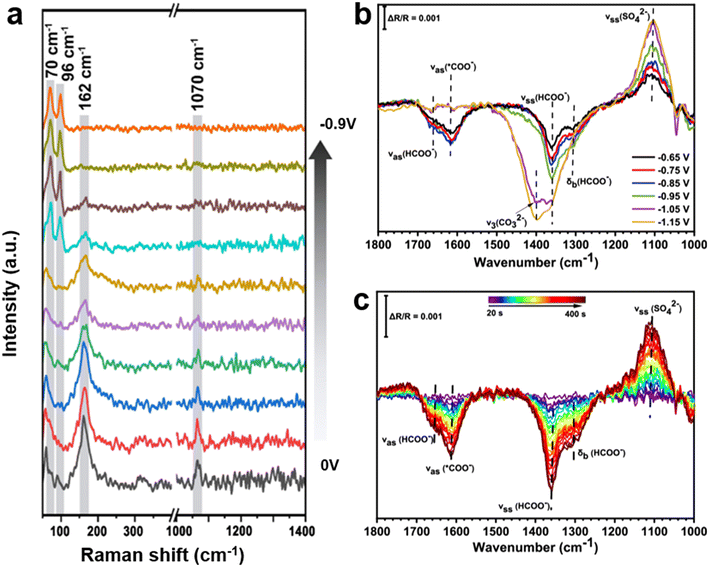 | ||
| Fig. 7 (a) In situ Raman spectra for the transformation of the Bi2O3 precursor from Bi2O2CO3 to Bi during the CO2RR38 (copyright 2022 American Chemical Society); potential-dependent (b) and time-dependent (c) IR spectra measured in a CO2-saturated 0.1 M K2SO4 solution for the ZnBi-3 electrode86 (copyright 2024 American Chemical Society). | ||
In addition to studying the structural evolution of materials, the detection of key intermediates and the elucidation of reaction pathways are vital for gaining better insights into reaction mechanisms, and further improving performance by modifying the binding energy of intermediates.100 It was reported that the generation of formate often occurs through the oxygen-bridged *OCHO intermediate on Bi-based catalysts.57,76 The stability of the adsorbates with Bi–O bonds is systematically higher than those with C–Bi bonds. Thus, the *OCHO pathway is dominant compared with *COOH pathway, leading to higher selectivity of formate generation. Some studies have shown that the formation of the *OCHO intermediate is accompanied by the adsorption of HCO3− groups.101 Sabouhanian et al.86 studied the CO2 reduction mechanism at ZnBi catalysts using an in situ electrochemical ATR-FTIR technique. As shown in Fig. 7b, the peaks assigned to formate at 1305 cm−1 (C–H bending mode), 1360 cm−1 (C–O symmetric stretch), and 1660 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O asymmetric stretch) were observed. The signal that appeared at 1614 cm−1 was attributed to the asymmetric stretch of the adsorbed *COO− intermediate, revealing that the CO2 molecule was adsorbed by the carbon atom in a monodentate orientation. The time-dependent FTIR spectra of the ZnBi catalyst are presented in Fig. 7c to monitor the formation and consumption of species over time. It was observed that the peaks assigned to the symmetric and asymmetric stretches of formate followed the same trend as the peak allocated to *COO−, and they became stronger as the reaction progressed. This proves that more *COO− species resulted in the production of more formate.
O asymmetric stretch) were observed. The signal that appeared at 1614 cm−1 was attributed to the asymmetric stretch of the adsorbed *COO− intermediate, revealing that the CO2 molecule was adsorbed by the carbon atom in a monodentate orientation. The time-dependent FTIR spectra of the ZnBi catalyst are presented in Fig. 7c to monitor the formation and consumption of species over time. It was observed that the peaks assigned to the symmetric and asymmetric stretches of formate followed the same trend as the peak allocated to *COO−, and they became stronger as the reaction progressed. This proves that more *COO− species resulted in the production of more formate.
5. Industrial perspective
Although Bi-based catalysts exhibit a high FE and selectivity for formate, the required high activity, high stability, and low overpotential hinder them from being employed on an industrial scale. Consequently, multiple challenges need to be resolved in terms of their stabilities and activities for industrial applications.34,39 To meet the required criteria for commercialization, it is necessary to achieve current densities of >200 mA cm−2 and long-term (>100 h) stability.16 Encouraging progress has been made recently toward upgrading the electrochemical stability of Bi-based catalysts, while maintaining high activity. Flow cells and membrane electrode electrolyzers were developed for scalable CO2RR systems as they can address mass transport issues.77,102,103 The gas diffusion electrode (GDE) configuration allowed CO2 to access the electrode surface as a gas and facilitated its mass transportation. There are some pending patents using Bi-based catalysts for CO2 reduction to formate.104,105 A superior high current density of 2.0 A cm−2 with 93% FE of formate at −0.95 V vs. RHE (Fig. 8a) was achieved by Lin et al.63 in a flow cell. This group synthesized a Bi2S3 precursor that underwent structural evolution and created a nanocomposite catalyst containing Bi0 clusters and Bi2O2CO3 nanosheets. They showed that the FE of formate increased from 66% at −0.38 V vs. RHE to 96% at −0.52 V vs. RHE. This catalyst maintained a stable and industrial-level current density for 100 h. Interestingly, Shen et al.39 prepared a Bi2S3-containing precursor through the integration of CuS. The CuS–Bi2S3 nano-heterojunction precursor was observed to be reconstructed to Cu-doped Bi (CDB) nanosheet electrocatalysts. An industrial-compatible current density of −1132 mA cm−2 at −0.86 V vs. RHE was recorded in a flow cell. Moreover, the long-term stability of over 100 h at −400 mA cm−2 was attained in a membrane electrode assembly.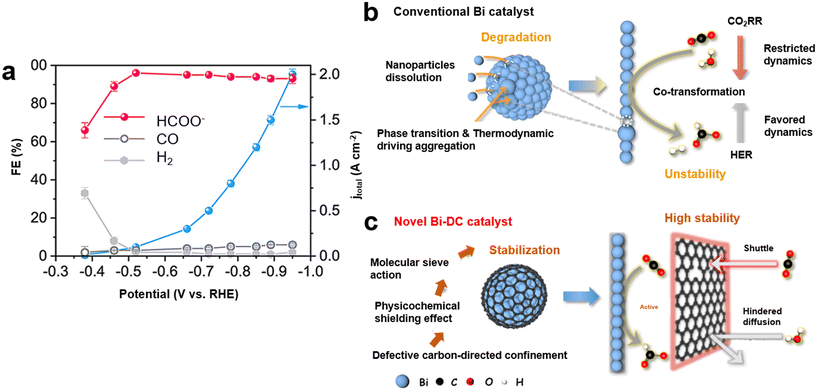 | ||
| Fig. 8 (a) The measured FE of each product and total current density for CO2 reduction over a Bi2S3-derived catalyst using 1 M KOH electrolyte in a flow cell63 (copyright 2023 John Wiley and Sons); (b) schematic of the degradation of a conventional Bi catalyst and limited CO2RR dynamics; (c) schematic of structural stabilization using a novel Bi-DC catalyst with a special sp2/sp3 carbon hybridization structure for efficient and stable formate formation106 (copyright 2024 American Chemical Society). | ||
In addition to the activity, stability is the most important issue for Bi-based catalysts due to thermodynamically driven aggregation of nanoparticles, the dissolution of reactive species, and structural reconstruction during CO2RR.39,45,106 Only a few research papers have reported a stability time of ∼100 h. As catalytic reactions take place on the catalyst's surface, surface modifications can greatly influence the catalytic reactions. The microenvironmental modulation using the molecularly modified surface layers can impact the adsorption behavior of ions and molecules on the catalyst, improving the stability during CO2 reduction.107 For example, HER on Cu nanoneedles was suppressed by a hydrophobic polytetrafluoroethylene (PTFE) coating, leading to the high C2 selectivity with 47% FE. Moreover, the Cu nanoneedle structures were well maintained during the CO2 reduction due to the PTFE coating, resulting in high stability.108 Li et al.106 recorded the most prolonged stability (120 h at 0.4 A) in a membrane electrode assembly cell. A CO2-philic defective carbon (DC) was formed over a Bi catalyst (Bi-DC), where the presence of sp3-hybrid defects in the carbon exhibited a unique sieving effect on CO2 molecules. Thus, it could effectively suppress the degradation and structural evolution of active Bi species during CO2RR. The DC species facilitated the formation of an interconnected carbon network, which enhanced the dispersion of the active Bi component. This resulted in a larger electrochemical surface area (ECSA) and, consequently, a higher current density. Schematics of the degradation of a conventional Bi catalyst and structural stabilization using a novel Bi-DC catalyst are presented in Fig. 8b and c, respectively. It has been reported that the stability of the Bi-based catalysts might be affected by the oxygenated species from the electrolyte leading to poisoning of the active sites. Zhu et al.107 introduced a molecular passivation layer of oxyphilic ascorbic acid to inhibit the poisoning of the hydroxyl. So, the free OH− preferred to bind to the outer ascorbic acid layer, and the possibility of binding to defective Bi sites was reduced. As a result, the high stability of over 120 hours was achieved at 50 mA cm−2. It has been reported that liquid metals as catalysts might have higher stability compared with solid ones due to dynamic surface properties and surface renewal ability. Liquid Bi alloy catalysts were generated by Guo et al.,109 showing 98% FE of formate over 80 h. They overcame the problem of deterioration of the traditional solid-state Bi-based catalysts during CO2 reduction. Compared with the liquid Bi alloy, the solid one exhibited a lower formate FE and less stability. To date, alkaline electrolytes have exhibited the highest activity for CO2RR to formate using Bi-based catalysts.110 For example, Peng et al.111 demonstrated that KOH solutions had improved activities over KHCO3 due to their lower solution resistance, which was confirmed by EIS. However, CO2 molecules can react with OH− ions in alkaline electrolytes and be converted to carbonate, which leads to carbonate deposition and the consumption of CO2 feedstocks.112 More importantly, the formation of carbonates can significantly threaten the stability of CO2RR, as they may obstruct the porous channels required for CO2 transport in the gas diffusion electrode, accelerate electrolyte leakage and raise cell resistance. These challenges greatly constrain the industrial potential of an alkaline or neutral CO2RR system.113 Recently, Bi-based catalysts have been utilized for CO2RR in acidic electrolytes to overcome the aforementioned issues. Formic acid is generated in acidic electrolytes rather than formate, which can reduce the industrial expenses associated with subsequent separation and purification from the electrolyte.20 However, HER typically dominates under acidic conditions. For example, in a strong acid with a pH of 1 or lower, the FE for CO2RR products is nearly zero.113 Recently, a strategy for the engineering of the local microenvironment has been proposed to enhance the CO2RR under acidic conditions. It was found that the HER in acidic electrolytes could be suppressed by creating a hydrophobic interfacial environment,112 or reducing proton coverage113 on the catalyst surface. The FE of formate up to 92.2% at a current density of −237.1 mA cm−2 was reported for CO2 reduction over Bi NSs in acidic electrolytes.113 The recently reported Bi-based catalysts with an industrially compatible current density of formate are summarized in Table 1, showing that over 90% FE was achieved.
| Catalyst | Electrolyte | Potential (V vs. RHE) | Current density (mA cm−2) | Stability (h) | FE (%) | Ref. |
|---|---|---|---|---|---|---|
| Ti–Bi NS | 1 M KOH | −1.01 | 224.1 | 12 | 96.3 | 69 |
| Heterophase Bi | 1 M KOH | −0.68 | 140 | 10 | 95.2 | 114 |
| Cu@Bi NW/Cu | 0.5 M KHCO3 | −1.07 | 129 | 6 | 98.7 | 42 |
| BOC@GDY | 1 M KOH | −1.1 | 200 | 10 | 93.5 | 115 |
| Bi NS | 1 M KOH | −0.63 | 400 | 26 | >90 | 19 |
| Bi-ene-NW | 1 M KOH | −0.7 | 200 | 110 | >90 | 16 |
| In/Bi-750 | 1 M KOH | −1.2 | 200 | 13 | 90.8 | 26 |
| Bi-GDE | 1 M KOH | −1.2 | 355 | 60 | 94 | 98 |
| BiIn@P | 1 M KOH | −0.92 | 500 | 17 | 97.3 | 87 |
| BOC-NS | 1 M KOH | −1.55 | 1000 | 24 | 93 | 116 |
| Copper-doped bismuth | 5 M KOH | −0.86 | 1132 | 100 | >90 | 39 |
| Sn1/24-Bi | 1 M KOH | −0.5–1.9 | >200 | 84 | >90 | 85 |
| Bi2S3-derived | 1 M KOH | −0.95 | 2000 | 100 | 93 | 63 |
6. Conclusions and perspectives
The recent advances in Bi-based catalysts for CO2 reduction are compared in Table 2 and Fig. 9, which highlight various morphologies and structures obtained for Bi-based catalysts along with their FE of formate. Bi-based catalysts have shown a high FE and selectivity for the generation of formate and may serve in the future as a potential electrocatalyst for CO2RR to formate for industrial applications. Through innovative synthesis techniques and the careful engineering of catalyst structures, researchers have achieved remarkable advancements to improve the activity and selectivity. Further, the introduction of secondary elements to synthesize bimetallic Bi-based catalysts has been extensively studied to decrease the energy barrier for intermediate formation. Not only formate but also other products such as propane, methane, and ethylene could be produced using CuBi catalysts by regulating the hydrogenation capacities of intermediates, enhancing CO2 and CO adsorption capacities, and lowering the C–C coupling energy barrier. Significant advances have been made using flow cell and membrane electrode assembly electrolyzers utilizing gas-diffusion electrodes to achieve high current densities owing to rapid mass transfer. Selectivity of up to 100% and current densities higher than −200 mA cm−2 have been reached in most recent studies. However, achieving ampere-level current densities and long-term stabilities of >100 h remains challenging. Consequently, despite several notable achievements, there is still a need to direct future research as follows to narrow the gap between laboratory and industrial scales.| Catalyst | Synthesis method | Electrolyte | Potential (RHE) | Stability (h) | FE (%) | Ref. | |
|---|---|---|---|---|---|---|---|
| Mono-metallic Bi-based | Bi NS | Electroreduction | 0.5 M KHCO3 | −0.8–−1.2 | 22 | 90 | 38 |
| OD-BiNS | Electrochemical transformation | 0.5 M KHCO3 | −0.95 | 10 | 93 | 21 | |
| Bi-PNS | Solvothermal | 0.5 M KHCO3 | −1.0 | 9 | 95 | 59 | |
| Bi NS | Electroreduction | 0.5 M KHCO3 | −0.98 | 12 | 94.5 | 36 | |
| Bi–S | Electrochemical transformation | 0.5 M KHCO3 | −0.9 | 35 | 96.7 | 66 | |
| Bi PNS | Electroreduction | 0.1 M KHCO3 | −1.2 | 10 | 95.31 | 61 | |
| Bi2S3/CNTs | Sonochemical | 0.5 M KHCO3 | −0.91 | 78 | 99.3 | 117 | |
| Bi/BiOx NS | Electrochemical conversion | 0.5 M KHCO3 | −0.78–−1.18 | 10 | 94 | 48 | |
| Bi-metallic Bi-based | CuBi | Co-deposition | 0.5 M KHCO3 | −0.97 | 20 | 94.4 | 73 |
Bi–Cu (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Ion-assisted codeposition | 0.1 M KHCO3 | −1.0 | 20 | 94.1 | 74 | |
| Cu–Bi aerogel | One-step reduction | 0.5 M KHCO3 | −0.9 | 36 | 96.57 | 81 | |
| Cu0.8Bi0.2 | Thermal evaporation | 1 M KOH | ∼−0.72 | 24 | >95 | 118 | |
| Bi3Cu1 | Hydrothermal | 0.5 M KOH | −0.75 | 20 | 95.1 | 77 | |
| P–Cu–BiNF | Fast reduction | 0.5 M KHCO3 | −0.78–−1.08 | 12 | 90 | 119 | |
| Bi–Cu | Electrochemical deposition | 0.5 M KHCO3 | −0.91 | 50 | 94.37 | 111 | |
| CuBi | CuBi-MOF | 0.5 M KHCO3 | −0.77 | 24 | 100 | 82 | |
| Bi on Cu foil | Electrodeposition | 0.1 M KHCO3 | −0.65 | 24 | 100 | 120 | |
| Cu@Bi nanocone | Electrodeposition | 0.5 M KHCO3 | −0.95 | 10 | 96.9 | 98 | |
| Bi/Cu foam | Electrodeposition | 0.1 M KHCO3 | −1.0 | 20 | 92 | 97 | |
| Cu1Bi1 | Co-precipitation | 0.5 M KHCO3 | −0.98 | 60 | 98.07 | 83 | |
| Bi5Sn60 | Electrodeposition | 0.1 M KHCO3 | −1.0 | 20 | 94.8 | 29 | |
| Bi–Sn aerogel | Chemical reduction | 0.1 M KHCO3 | −1.0 | 10 | 93.9 | 99 | |
| Sn-doped Bi2O3 NSs | Solvothermal | 0.5 M KHCO3 | −0.97 | 8 | 93.4 | 121 | |
| SnBi | Electrodeposition | 0.5 M KHCO3 | −1.06 | 100 | 96.1 | 84 | |
| Sn–Bi | Hydrothermal | 0.5 M KHCO3 | −0.74–−1.14 | 160 | >90 | 122 | |
| Sn1−xBix | Co-reduction | 0.5 M KHCO3 | −0.67–−0.92 | 50 | >90 | 123 | |
| In/Bi-750 | Electrochemical transformation | 0.5 M KHCO3 | −1.0 | 48 | 97.17 | 26 | |
| BiIn5-500@C | MOF | 0.5 M KHCO3 | −0.86 | 15 | 97.5 | 124 | |
| Bi-In2O3 | Wet chemical | 0.5 M KHCO3 | −0.7 | 10 | 88.1 | 88 | |
| In16Bi84 NS | Liquid-polyol | 0.5 M KHCO3 | −0.94 | 10 | ∼100 | 30 | |
| In2O3/Bi2O3 | MOF | 0.5 M KHCO3 | −0.4–−1.6 | 30 | 99.9 | 89 | |
| Zn–Bi | Hydrothermal | 0.5 M NaHCO3 | −0.8 | 7 | 94 | 91 |
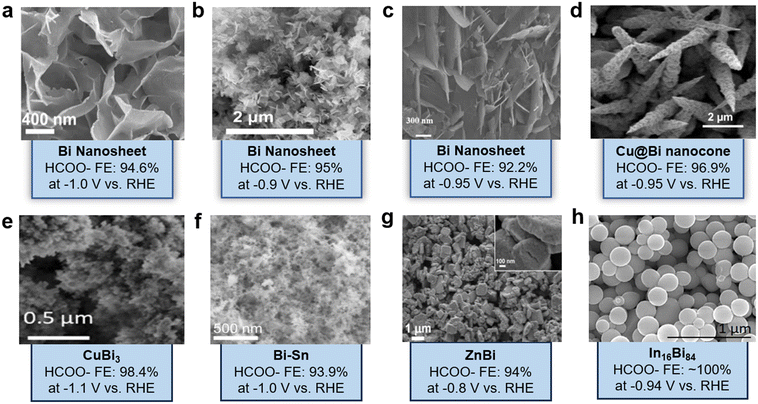 | ||
| Fig. 9 SEM images of (a) Bi NSs38 (copyright 2022 American Chemical Society); (b) P-nanoplates-Bi catalysts40 (copyright 2021 Elsevier); (c) nitrogen-doped Bi NSs125 (copyright 2022 MDPI); (d) Cu@Bi nanocone126 (copyright 2020 Elsevier); (e) CuBi3 (ref. 71) (copyright 2023 Elsevier); (f) Bi–Sn aerogel127 (copyright 2021 John Wiley and Sons); (g) ZnBi (ref. 91) (copyright 2024 American Chemical Society); (h) In16Bi84 (ref. 30) (copyright 2022 American Chemical Society). | ||
Continued efforts should be made to enhance the stability of Bi-based catalysts for industrial applications. It is also necessary to conduct further research into scalable synthesis techniques and cost-effective catalyst design for large-scale applications. It is vital to gain insights into the kinetics of CO2RR and identify intermediates in real-world applications. Thus, in situ characterization techniques should be developed to be compatible with high current densities. Further research should be conducted to optimize the conditions and designs of cells to generate concentrated formic acid to reduce the expenses of product purification and separation. Integrating Bi-based catalysts with renewable energy sources such as solar and wind power should be considered. The coupling of electrochemical CO2 reduction with renewable energy will reduce the reliance on fossil fuels and contribute to a greener energy landscape. Finally, CO2RR should be assessed regarding the economic perspective and environmental impact. Building a pilot-scale system that allows for real-world testing is crucial to evaluating the stability of the catalysts, the costs and the efficiency of the CO2 reduction process. It would provide valuable data on operational expenses, overall system performance, and long-term durability, offering key insights into the economic viability and potential scalability.
Data availability
This is a review article. All the data are extracted from the published papers, which are specially described in the related figures and tables.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by Discovery Grants from the Natural Sciences and Engineering Research Council of Canada (J.L.: RGPIN-2022-03958; A.C.: RGPIN-2022-04238). A.C. acknowledges the NSERC and Canada Foundation for Innovation (CFI) for the Canada Research Chair Award in Electrochemistry and Nanoscience.References
- S. Abner and A. Chen, Design and mechanistic study of advanced cobalt-based nanostructured catalysts for electrochemical carbon dioxide reduction, Appl. Catal., B, 2022, 301, 120761 CrossRef CAS.
- A. Salverda, S. Abner, E. Mena-Morcillo, A. Zimmer, A. Elsayed and A. Chen, Electrochemical, scanning electrochemical microscopic, and in situ electrochemical fourier transform infrared studies of CO2 reduction at porous copper surfaces, J. Phys. Chem. C, 2023, 127, 7151–7161 CrossRef CAS.
- Y.-X. Duan, R.-C. Cui and Q. Jiang, Recent progress on the electroreduction of carbon dioxide to C1 liquid products, Curr. Opin. Electrochem., 2023, 38, 101219 CrossRef CAS.
- P. Roy, A. K. Mohanty and M. Misra, Prospects of carbon capture, utilization and storage for mitigating climate change, Environ. Sci.:Adv., 2023, 2, 409–423 CAS.
- Q. Zhu, Y. Zeng and Y. Zheng, Overview of CO2 capture and electrolysis technology in molten salts: operational parameters and their effects, Ind. Chem. Mater., 2023, 1, 595–617 RSC.
- X. Li, X. Wu, X. Lv, J. Wang and H. B. Wu, Recent advances in metal-based electrocatalysts with hetero-interfaces for CO2 reduction reaction, Chem Catal., 2022, 2, 262–291 CrossRef CAS.
- Z. Yang, R. Chen, L. Zhang, Y. Li and C. Li, Recent progress in nickel single-atom catalysts for the electroreduction of CO2 to CO, Ind. Chem. Mater., 2024, 2, 533–555 RSC.
- W. Chen, Y. Wang, Y. Li and C. Li, Electrocatalytic CO2 reduction over bimetallic Bi-based catalysts: A review, CCS Chem., 2023, 5, 544–567 CrossRef CAS.
- F. Rahmati, N. Sabouhanian, J. Lipkowski and A. Chen, Synthesis of 3D porous Cu nanostructures on Ag thin film using dynamic hydrogen bubble template for electrochemical conversion of CO2 to ethanol, Nanomaterials, 2023, 13, 778 CrossRef CAS PubMed.
- X. Li, Z. Li, Z. Zhang, Y. Zhao, Q. Fang, J. Tang and J. He, Design and synthesis of magnesium-modified copper oxide nanosheets as efficient electrocatalysts for CO2 reduction, Nanoscale, 2024, 16, 17527–17536 RSC.
- I. Husein, J. M. Hadi, A. Surendar, N. N. Gryzunova, R. G. Khairullina, D. O. Bokov and H. T. Hoi, Bismuth oxide nanostructure supported on Cu foam as efficient electrocatalyst toward carbon dioxide electroreduction, Ionics, 2023, 29, 3213–3223 CrossRef CAS.
- S. Qiao, G. Zhang, D. Tian, W. Xu, W. Jiang, Y. Cao, J. Qian, J. Zhang, Q. He and L. Song, Stepped copper sites coupling voltage-induced surfactant assembly to achieve efficient CO2 electroreduction to formate, Energy Environ. Sci., 2024, 17, 6779–6786 RSC.
- B. Ávila-Bolívar, R. Cepitis, M. Alam, J.-M. Assafrei, K. Ping, J. Aruväli, A. Kikas, V. Kisand, S. Vlassov, M. Käärik, J. Leis, V. Ivaništštev, P. Starkov, V. Montiel, J. Solla-Gullón and N. Kongi, CO2 reduction to formate on an affordable bismuth metal-organic framework based catalyst, J. CO2 Util., 2022, 59, 101937 CrossRef.
- R. Sun, Y. Liao, S.-T. Bai, M. Zheng, C. Zhou, T. Zhang and B. F. Sels, Heterogeneous catalysts for CO2 hydrogenation to formic acid/formate: from nanoscale to single atom, Energy Environ. Sci., 2021, 14, 1247–1285 RSC.
- W. Mao, Q. Yuan, H. Qi, Z. Wang, H. Ma and T. Chen, Recent progress in metabolic engineering of microbial formate assimilation, Appl. Microbiol. Biotechnol., 2020, 104, 6905–6917 CrossRef CAS PubMed.
- M. Zhang, W. Wei, S. Zhou, D.-D. Ma, A. Cao, X.-T. Wu and Q.-L. Zhu, Engineering a conductive network of atomically thin bismuthene with rich defects enables CO2 reduction to formate with industry-compatible current densities and stability, Energy Environ. Sci., 2021, 14, 4998–5008 RSC.
- R. Mohan, Green bismuth, Nat. Chem., 2010, 2, 336 CrossRef CAS PubMed.
- Y. Guan, M. Liu, X. Rao, Y. Liu and J. Zhang, Electrochemical reduction of carbon dioxide (CO2): bismuth-based electrocatalysts, J. Mater. Chem. A, 2021, 9, 13770–13803 RSC.
- Z. Wang, X. Zu, X. Li, L. Li, Y. Wu, S. Wang, P. Ling, Y. Zhao, Y. Sun and Y. Xie, Industrial-current-density CO2-to-formate conversion with low overpotentials enabled by disorder-engineered metal sites, Nano Res., 2022, 15, 6999–7007 CrossRef CAS.
- X.-D. Liang, N. Tian, S.-N. Hu, Z.-Y. Zhou and S.-G. Sun, Recent advances of bismuth-based electrocatalysts for CO2 reduction: Strategies, mechanism and applications, Mater. Rep.: Energy, 2023, 3, 100191 CAS.
- J. Lee, H. Liu, Y. Chen and W. Li, Bismuth nanosheets derived by in situ morphology transformation of bismuth oxides for selective electrochemical CO2 reduction to formate, ACS Appl. Mater. Interfaces, 2022, 14, 14210–14217 CrossRef CAS PubMed.
- W. Lai, Y. Liu, M. Zeng, D. Han, M. Xiao, S. Wang, S. Ren and Y. Meng, One-step electrochemical dealloying of 3D Bi-continuous micro-nanoporous bismuth electrodes and CO2RR performance, Nanomaterials, 2023, 13, 1767 CrossRef CAS PubMed.
- D. Xia, H. Yu, H. Xie, P. Huang, R. Menzel, M. M. Titirici and G. Chai, Recent progress of Bi-based electrocatalysts for electrocatalytic CO2 reduction, Nanoscale, 2022, 14, 7957–7973 RSC.
- M. Wu, B. Xu, Y. Zhang, S. Qi, W. Ni, J. Hu and J. Ma, Perspectives in emerging bismuth electrochemistry, Chem. Eng. J., 2020, 381, 122558 CrossRef CAS.
- S. Liu, B. Hu, J. Zhao, W. Jiang, D. Feng, C. Zhang and W. Yao, Enhanced electrocatalytic CO2 reduction of bismuth nanosheets with introducing surface bismuth subcarbonate, Coatings, 2022, 12, 233 CrossRef CAS.
- Q. Wang, X. Yang, H. Zang, C. Liu, J. Wang, N. Yu, L. Kuai, Q. Qin and B. Geng, InBi bimetallic sites for efficient electrochemical reduction of CO2 to HCOOH, Small, 2023, 19, 2303172 CrossRef CAS PubMed.
- Z. Liu, M. N. Hossain, J. Wen and A. Chen, Copper decorated with nanoporous gold by galvanic displacement acts as an efficient electrocatalyst for the electrochemical reduction of CO2, Nanoscale, 2021, 13, 1155–1163 RSC.
- Z. Zhang, W. Liu, W. Zhang, M. Liu and S. Huo, Interface interaction in CuBi catalysts with tunable product selectivity for electrochemical CO2 reduction reaction, Colloids Surf., A, 2021, 631, 127637 CrossRef CAS.
- Z. Li, Y. Feng, Y. Li, X. Chen, N. Li, W. He and J. Liu, Fabrication of Bi/Sn bimetallic electrode for high-performance electrochemical reduction of carbon dioxide to formate, Chem. Eng. J., 2022, 428, 130901 CrossRef CAS.
- D. Tan, W. Lee, Y. E. Kim, Y. N. Ko, M. H. Youn, Y. E. Jeon, J. Hong, J. E. Park, J. Seo, S. K. Jeong, Y. Choi, H. Choi, H. Y. Kim and K. T. Park, In–Bi electrocatalyst for the reduction of CO2 to formate in a wide potential window, ACS Appl. Mater. Interfaces, 2022, 14, 28890–28899 CrossRef CAS PubMed.
- C. Wang, A. He, N. Zhang, H. Sui, Z. Wen, C. Xu, G. Yan and R. Xue, Zn-Bi bimetallic catalyst for electrochemical synthesis of C1 products by highly selective CO2 reduction, J. Catal., 2023, 428, 115128 CrossRef CAS.
- Y. Wang, L. Cheng, Y. Zhu, J. Liu, C. Xiao, R. Chen, L. Zhang, Y. Li and C. Li, Tunable selectivity on copper–bismuth bimetallic aerogels for electrochemical CO2 reduction, Appl. Catal., B, 2022, 317, 121650 CrossRef CAS.
- W. Li, L. Li, Q. Xia, S. Hong, L. Wang, Z. Yao, T.-S. Wu, Y.-L. Soo, H. Zhang, T. W. B. Lo, A. W. Robertson, Q. Liu, L. Hao and Z. Sun, Lowering C−C coupling barriers for efficient electrochemical CO2 reduction to C2H4 by jointly engineering single Bi atoms and oxygen vacancies on CuO, Appl. Catal., B, 2022, 318, 121823 CrossRef CAS.
- J. Xu, S. Yang, L. Ji, J. Mao, W. Zhang, X. Zheng, H. Fu, M. Yuan, C. Yang, H. Chen and R. Li, High current CO2 reduction realized by edge/defect-rich bismuth nanosheets, Nano Res., 2023, 16, 53–61 CrossRef CAS.
- C.-J. Peng, X.-T. Wu, G. Zeng and Q.-L. Zhu, In situ bismuth nanosheet assembly for highly selective electrocatalytic CO2 reduction to formate, Chem. – Asian J., 2021, 16, 1539–1544 CrossRef CAS PubMed.
- A. Xu, D. Wei, X. Chen, T. Yang, Y. Huang, H. He and J. Xu, In situ transformation of bismuth-containing precursors into ultrathin bismuth nanosheets for enhanced electrochemical CO2 reduction, Chem. Eng. J., 2023, 452, 139227 CrossRef CAS.
- L. Lv, R. Lu, J. Zhu, R. Yu, W. Zhang, E. Cui, X. Chen, Y. Dai, L. Cui, J. Li, L. Zhou, W. Chen, Z. Wang and L. Mai, Coordinating the edge defects of bismuth with sulfur for enhanced CO2 electroreduction to formate, Angew. Chem., Int. Ed., 2023, 62, e202303117 CrossRef CAS PubMed.
- J. Wu, X. Yu, H. He, C. Yang, D. Xia, L. Wang, J. Huang, N. Zhao, F. Tang, L. Deng and Y.-N. Liu, Bismuth-nanosheet-based catalysts with a reconstituted Bi0 atom for promoting the electrocatalytic reduction of CO2 to formate, Ind. Eng. Chem. Res., 2022, 61, 12383–12391 CrossRef CAS.
- H. Shen, Y. Zhao, L. Zhang, Y. He, S. Yang, T. Wang, Y. Cao, Y. Guo, Q. Zhang and H. Zhang, In-situ constructuring of copper-doped bismuth catalyst for highly efficient CO2 electrolysis to formate in ampere-level, Adv. Energy Mater., 2023, 13, 2202818 CrossRef CAS.
- P. Liu, H. Liu, S. Zhang, J. Wang and C. Wang, A general strategy for obtaining BiOX nanoplates derived Bi nanosheets as efficient CO2 reduction catalysts by enhancing CO2•− adsorption and electron transfer, J. Colloid Interface Sci., 2021, 602, 740–747 CrossRef CAS PubMed.
- T. Wissink, A. J. W. Man, W. Chen, J. M. J. J. Heinrichs, R. C. J. van de Poll, M. C. Figueiredo and E. J. M. Hensen, Evolution of bismuth oxide catalysts during electrochemical CO2 reduction, J. CO2 Util., 2023, 77, 102604 CrossRef CAS.
- Y. Hu, D. Lu, W. Zhou, X. Wang and Y. Li, In situ construction of 3D low-coordinated bismuth nanosheets@Cu nanowire core–shell nanoarchitectures for superior CO2 electroreduction activity, J. Mater. Chem. A, 2023, 11, 1937–1943 RSC.
- H. Zheng, G. Wu, G. Gao and X. Wang, The bismuth architecture assembled by nanotubes used as highly efficient electrocatalyst for CO2 reduction to formate, Chem. Eng. J., 2021, 421, 129606 CrossRef CAS.
- D. Yao, C. Tang, A. Vasileff, X. Zhi, Y. Jiao and S.-Z. Qiao, The controllable reconstruction of Bi-MOFs for electrochemical CO2 reduction through electrolyte and potential mediation, Angew. Chem., Int. Ed., 2021, 60, 18178–18184 CrossRef CAS PubMed.
- B. Ávila-Bolívar, M. Lopez Luna, F. Yang, A. Yoon, V. Montiel, J. Solla-Gullón, S. W. Chee and B. Roldan Cuenya, Revealing the intrinsic restructuring of Bi2O3 nanoparticles into Bi nanosheets during electrochemical CO2 reduction, ACS Appl. Mater. Interfaces, 2024, 16, 11552–11560 CrossRef PubMed.
- Y. Wang, L. Cheng, J. Liu, C. Xiao, B. Zhang, Q. Xiong, T. Zhang, Z. Jiang, H. Jiang, Y. Zhu, Y. Li and C. Li, Rich bismuth-oxygen bonds in bismuth derivatives from Bi2S3 pre-catalysts promote the electrochemical reduction of CO2, ChemElectroChem, 2020, 7, 2864–2868 CrossRef CAS.
- D. Wang, K. Chang, Y. Zhang, Y. Wang, Q. Liu, Z. Wang, D. Ding, Y. Cui, C. Pan, Y. Lou, Y. Zhu and Y. Zhang, Unravelling the electrocatalytic activity of bismuth nanosheets towards carbon dioxide reduction: Edge plane versus basal plane, Appl. Catal., B, 2021, 299, 120693 CrossRef.
- Y. Jiang, Q. Chen, D. Wang, X. Li, Y. Xu, Z. Xu and G. Guo, In situ structural evolution of BiOCOOH nanowires and their performance towards electrocatalytic CO2 reduction, Nano Res., 2023, 16, 6661–6669 CrossRef CAS.
- X. Zeng, C. Xiao, L. Liao, Z. Tu, Z. Lai, K. Xiong and Y. Wen, Two-dimensional (2D) TM-tetrahydroxyquinone metal–organic framework for selective CO2 electrocatalysis: A DFT investigation, Nanomaterials, 2022, 12, 4049 CrossRef CAS PubMed.
- Q. Liu, X. Bai, H. Pham, J. Hu and C. Z. Dinu, Active nanointerfaces based on enzyme carbonic anhydrase and metal–organic framework for carbon dioxide reduction, Nanomaterials, 2021, 11, 1008 CrossRef CAS PubMed.
- F. Li, G. H. Gu, C. Choi, P. Kolla, S. Hong, T.-S. Wu, Y.-L. Soo, J. Masa, S. Mukerjee, Y. Jung, J. Qiu and Z. Sun, Highly stable two-dimensional bismuth metal-organic frameworks for efficient electrochemical reduction of CO2, Appl. Catal., B, 2020, 277, 119241 CrossRef CAS.
- J. Wang, G. Chao, W. Zong, K. Chu, J. Zhu, R. Chen, Y. Zheng, L. Zhang and T. Liu, Efficient nitrate electroreduction to ammonia over copper catalysts supported on electron-delocalized covalent organic frameworks, Chem. Eng. J., 2024, 499, 156343 CrossRef CAS.
- P. Lamagni, M. Miola, J. Catalano, M. S. Hvid, M. A. H. Mamakhel, M. Christensen, M. R. Madsen, H. S. Jeppesen, X.-M. Hu, K. Daasbjerg, T. Skrydstrup and N. Lock, Restructuring metal–organic frameworks to nanoscale bismuth electrocatalysts for highly active and selective CO2 reduction to formate, Adv. Funct. Mater., 2020, 30, 1910408 CrossRef CAS.
- L. Liu, K. Yao, J. Fu, Y. Huang, N. Li and H. Liang, Bismuth metal-organic framework for electroreduction of carbon dioxide, Colloids Surf., A, 2022, 633, 127840 CrossRef CAS.
- W.-J. Xie, O. M. Mulina, A. O. Terent'ev and L.-N. He, Metal–organic frameworks for electrocatalytic CO2 reduction into formic acid, Catalysts, 2023, 13, 1109 CrossRef CAS.
- L. Cao, J. Huang, X. Wu, B. Ma, Q. Xu, Y. Zhong, Y. Wu, M. Sun and L. Yu, Active-site stabilized Bi metal–organic framework-based catalyst for highly active and selective electroreduction of CO2 to formate over a wide potential window, Nanoscale, 2023, 15, 19522–19532 RSC.
- Q. Huang, X. Sha, R. Yang, H. Li and J. Peng, Electrochemical conversion of CO2 into formate boosted by in situ reconstruction of Bi-MOF to Bi2O2CO3 ultrathin nanosheets, ACS Appl. Mater. Interfaces, 2024, 16, 13882–13892 CrossRef CAS PubMed.
- F. Yang, A. O. Elnabawy, R. Schimmenti, P. Song, J. Wang, Z. Peng, S. Yao, R. Deng, S. Song, Y. Lin, M. Mavrikakis and W. Xu, Bismuthene for highly efficient carbon dioxide electroreduction reaction, Nat. Commun., 2020, 11, 1088 CrossRef CAS PubMed.
- Z.-L. Yu, S.-Q. Wu, L.-W. Chen, Y.-C. Hao, X. Su, Z. Zhu, W.-Y. Gao, B. Wang and A.-X. Yin, Promoting the electrocatalytic reduction of CO2 on ultrathin porous bismuth nanosheets with tunable surface-active sites and local pH environments, ACS Appl. Mater. Interfaces, 2022, 14, 10648–10655 CrossRef CAS PubMed.
- R. G. Mariano, K. McKelvey, H. S. White and M. W. Kanan, Selective increase in CO2 electroreduction activity at grain-boundary surface terminations, Science, 2017, 358, 1187–1192 CrossRef CAS PubMed.
- Y.-M. Bai, C.-Q. Cheng, Z.-Z. Shi, X.-Z. Hu, S.-W. Yan, Z.-Y. Zhang, J. Yang, G.-R. Shen, P.-F. Yin, C.-K. Dong, H. Liu and X.-W. Du, Clean synthesis of bismuth porous nanosheets for efficient CO2 electroreduction, ACS Appl. Energy Mater., 2022, 5, 11561–11567 CrossRef CAS.
- Y. Li, J. Chen, S. Chen, X. Liao, T. Zhao, F. Cheng and H. Wang, In situ confined growth of bismuth nanoribbons with active and robust edge sites for boosted CO2 electroreduction, ACS Energy Lett., 2022, 7, 1454–1461 CrossRef CAS.
- L. Lin, X. He, X.-G. Zhang, W. Ma, B. Zhang, D. Wei, S. Xie, Q. Zhang, X. Yi and Y. Wang, A nanocomposite of bismuth clusters and Bi2O2CO3 sheets for highly efficient electrocatalytic reduction of CO2 to formate, Angew. Chem., Int. Ed., 2023, 62, e202214959 CrossRef CAS PubMed.
- Y. Wang, Z. Huang, Y. Lei, J. Wu, Y. Bai, X. Zhao, M. Liu, L. Zhan, S. Tang, X. Zhang, F. Luo and X. Xiong, Bismuth with abundant defects for electrocatalytic CO2 reduction and Zn–CO2 batteries, Chem. Commun., 2022, 58, 3621–3624 RSC.
- J. Ren, X. Long, X. Wang, Z. Lin, R. Cai, M. Ju, Y. Qiu and S. Yang, Defect-rich heterostructured Bi-Based catalysts for efficient CO2 reduction reaction to formate in wide operable windows, Energy Technol., 2022, 10, 2200561 CrossRef CAS.
- M. Wang, S. Liu, B. Chen, M. Huang and C. Peng, Co-regulation of intermediate binding and water activation in sulfur-doped bismuth nanosheets for electrocatalytic CO2 reduction to formate, Chem. Eng. J., 2023, 451, 139056 CrossRef CAS.
- X. Chen, H. Chen, W. Zhou, Q. Zhang, Z. Yang, Z. Li, F. Yang, D. Wang, J. Ye and L. Liu, Boron dopant induced electron-rich bismuth for electrochemical CO2 reduction with high solar energy conversion efficiency, Small, 2021, 17, 2101128 CrossRef CAS PubMed.
- Y. Zhang, S. Liu, N. Ji, L. Wei, Q. Liang, J. Li, Z. Tian, J. Su and Q. Chen, Modulation of the electronic structure of metallic bismuth catalysts by cerium doping to facilitate electrocatalytic CO2 reduction to formate, J. Mater. Chem. A, 2024, 12, 7528–7535 RSC.
- A. Xu, X. Chen, D. Wei, B. Chu, M. Yu, X. Yin and J. Xu, Regulating the electronic structure of bismuth nanosheets by titanium doping to boost CO2 electroreduction and Zn–CO2 batteries, Small, 2023, 19, 2302253 CrossRef CAS PubMed.
- Y. Wang, B. Wang, W. Jiang, Z. Liu, J. Zhang, L. Gao and W. Yao, Sub-2 nm ultra-thin Bi2O2CO3 nanosheets with abundant Bi-O structures toward formic acid electrosynthesis over a wide potential window, Nano Res., 2022, 15, 2919–2927 CrossRef CAS.
- Y. Fu, K. Leng, H. Zhuo, W. Liu, L. Liu, G. Zhou and J. Tang, Nanoconfinement effects on CuBi3 alloy catalyst for efficient CO2 electroreduction to formic acid, J. CO2 Util., 2023, 70, 102456 CrossRef CAS.
- Y. Xiong, B. Wei, M. Wu, B. Hu, F. Zhu, J. Hao and W. Shi, Rapid synthesis of amorphous bimetallic copper-bismuth electrocatalysts for efficient electrochemical CO2 reduction to formate in a wide potential window, J. CO2 Util., 2021, 51, 101621 CrossRef CAS.
- W. Lou, L. Peng, R. He, Y. Liu and J. Qiao, CuBi electrocatalysts modulated to grow on derived copper foam for efficient CO2-to-formate conversion, J. Colloid Interface Sci., 2022, 606, 994–1003 CrossRef CAS PubMed.
- M. Wang, S. Liu, B. Chen, F. Tian and C. Peng, Synergistic geometric and electronic effects in Bi–Cu bimetallic catalysts for CO2 electroreduction to formate over a wide potential window, ACS Sustainable Chem. Eng., 2022, 10, 5693–5701 CrossRef CAS.
- M. Tian, S. Wu, Y. Hu, Z. Mu, Z. Li, Y. Hou, P. Xi and C.-H. Yan, Doping and pretreatment optimized the adsorption of *OCHO on bismuth for the electrocatalytic reduction of CO2 to formate, Nanoscale, 2023, 15, 4477–4487 RSC.
- X. Yang, Q. Wang, F. Chen, H. Zang, C. Liu, N. Yu and B. Geng, In-situ electrochemical restructuring of Cu2BiSx solid solution into Bi/CuxSy heterointerfaces enabling stabilization intermediates for high-performance CO2 electroreduction to formate, Nano Res., 2023, 16, 7974–7981 CrossRef CAS.
- X. Zhang, L. Peng, B. Xu, P. Liu, X. Jiao, H. Kang, Z. Song, X. Yan, Y. Mao and J. Qiao, Bi-Cu bimetallic electrocatalysts prepared using electrochemical deposition effluent for highly converting CO2 to formate, Process Saf. Environ. Prot., 2022, 158, 560–566 CrossRef CAS.
- Y. Xue, C. Li, X. Zhou, Z. Kuang, W. Zhao, Q. Zhang and H. Chen, MOF-derived Cu/Bi Bi-metallic catalyst to enhance selectivity toward formate for CO2 electroreduction, ChemElectroChem, 2022, 9, e202101648 CrossRef CAS.
- B. Liu, Y. Xie, X. Wang, C. Gao, Z. Chen, J. Wu, H. Meng, Z. Song, S. Du and Z. Ren, Copper-triggered delocalization of bismuth p-orbital favours high-throughput CO2 electroreduction, Appl. Catal., B, 2022, 301, 120781 CrossRef CAS.
- S. Yang, H. Wang, Y. Xiong, M. Zhu, J. Sun, M. Jiang, P. Zhang, J. Wei, Y. Xing, Z. Tie and Z. Jin, Ultrafast Thermal shock synthesis and porosity engineering of 3D hierarchical Cu–Bi nanofoam electrodes for highly selective electrochemical CO2 reduction, Nano Lett., 2023, 23, 10140–10147 CrossRef CAS PubMed.
- H. Li, X. Yue, J. Che, Z. Xiao, X. Yu, F. Sun, C. Xue and J. Xiang, High performance 3D self-supporting Cu−Bi aerogels for electrocatalytic reduction of CO2 to formate, ChemSusChem, 2022, 15, e202200226 CrossRef CAS PubMed.
- Z. Yang, H. Wang, X. Fei, W. Wang, Y. Zhao, X. Wang, X. Tan, Q. Zhao, H. Wang, J. Zhu, L. Zhou, H. Ning and M. Wu, MOF derived bimetallic CuBi catalysts with ultra-wide potential window for high-efficient electrochemical reduction of CO2 to formate, Appl. Catal., B, 2021, 298, 120571 CrossRef CAS.
- H. Ren, X. Wang, X. Zhou, T. Wang, Y. Liu, C. Wang, Q. Guan and W. Li, In-situ constructing Cu1Bi1 bimetallic catalyst to promote the electroreduction of CO2 to formate by synergistic electronic and geometric effects, J. Energy Chem., 2023, 79, 263–271 CrossRef CAS.
- S. Yang, Y. Sun, C. Wang, L. Lv, M. Hu, J. Jin and H. Xie, One-step co-electrodeposition of SnBi for efficient electrochemical reduction of carbon dioxide to formic acid, Catal. Sci. Technol., 2023, 13, 758–766 RSC.
- X. Xu, Y. Wei, L. Mi, G. Pan, Y. He, S. Cai, C. Zheng, Y. Jiang, B. Chen, L. Li, S. Zhong, J. Huang, W. Hu and Y. Yu, Interstitial Sn-doping promotes electrocatalytic CO2-to-formate conversion on bismuth, Sci. China Mater., 2023, 66, 3539–3546 CrossRef CAS.
- N. Sabouhanian, J. Lipkowski and A. Chen, Growth and electrochemical study of bismuth nanodendrites as an efficient catalyst for CO2 reduction, ACS Appl. Mater. Interfaces, 2024, 16, 21895–21904 CrossRef CAS PubMed.
- J. Zhou, L. Li, H. Ren, H. Wang, Y. Li, K. Liu, L. Huang, X. Yang, Z. Hao, Y. Zhang, Z. Wang, X. Wang, J. Ding, Y. Ji, L. Wang and H. Liang, A defective bismuth–indium catalyst promotes water dissociation for selective carbon dioxide electroreduction to HCOOH, Inorg. Chem. Front., 2024, 11, 1703–1709 RSC.
- R. Wang, S. Deng, Y. Pang and G. Chai, Designing Bi-In2O3 nanoflower catalysts for enhanced performance of electrochemical CO2 reduction to formate, ChemNanoMat, 2024, 10, e202400008 CrossRef CAS.
- Z. Yang, H. Wang, X. Bi, X. Tan, Y. Zhao, W. Wang, Y. Zou, H. Wang, H. Ning and M. Wu, Bimetallic In2O3/Bi2O3 catalysts enable highly selective CO2 electroreduction to formate within ultra-broad potential windows, Energy Environ. Mater., 2024, 7, e12508 CrossRef CAS.
- Q. Wang, X. Yang, H. Zang, F. Chen, C. Wang, N. Yu and B. Geng, Metal–organic framework-derived BiIn bimetallic oxide nanoparticles embedded in carbon networks for efficient electrochemical reduction of CO2 to formate, Inorg. Chem., 2022, 61, 12003–12011 CrossRef CAS PubMed.
- T. Zhang, Y. Qiu, P. Yao, X. Li and H. Zhang, Bi-modified Zn catalyst for efficient CO2 electrochemical reduction to formate, ACS Sustainable Chem. Eng., 2019, 7, 15190–15196 CrossRef CAS.
- C. Azenha, C. Mateos-Pedrero, M. Alvarez-Guerra, A. Irabien and A. Mendes, Binary copper-bismuth catalysts for the electrochemical reduction of CO2: Study on surface properties and catalytic activity, Chem. Eng. J., 2022, 445, 136575 CrossRef CAS.
- Z. Wang, Q. Yuan, J. Shan, Z. Jiang, P. Xu, Y. Hu, J. Zhou, L. Wu, Z. Niu, J. Sun, T. Cheng and W. A. I. Goddard, Highly selective electrocatalytic reduction of CO2 into methane on Cu–Bi nanoalloys, J. Phys. Chem. Lett., 2020, 11, 7261–7266 CrossRef CAS PubMed.
- Y. Cao, S. Chen, S. Bo, W. Fan, J. Li, C. Jia, Z. Zhou, Q. Liu, L. Zheng and F. Zhang, Single atom Bi decorated copper alloy enables C−C coupling for electrocatalytic reduction of CO2 into C2+ Products, Angew. Chem., Int. Ed., 2023, 62, e202303048 CrossRef CAS PubMed.
- X.-D. Liang, Q.-Z. Zheng, N. Wei, Y.-Y. Lou, S.-N. Hu, K.-M. Zhao, H.-G. Liao, N. Tian, Z.-Y. Zhou and S.-G. Sun, In-situ constructing Bi@Bi2O2CO3 nanosheet catalyst for ampere-level CO2 electroreduction to formate, Nano Energy, 2023, 114, 108638 CrossRef CAS.
- P. Deng, H. Wang, R. Qi, J. Zhu, S. Chen, F. Yang, L. Zhou, K. Qi, H. Liu and B. Y. Xia, Bismuth oxides with enhanced bismuth–oxygen structure for efficient electrochemical reduction of carbon dioxide to formate, ACS Catal., 2020, 10, 743–750 CrossRef CAS.
- X. An, S. Li, X. Hao, X. Du, T. Yu, Z. Wang, X. Hao, A. Abudula and G. Guan, The in situ morphology transformation of bismuth-based catalysts for the effective electroreduction of carbon dioxide, Sustainable Energy Fuels, 2020, 4, 2831–2840 RSC.
- J. Zeng, N. B. D. Monti, T. Chen, M. Castellino, W. Ju, M. A. O. Lourenço, P. Jagdale and C. F. Pirri, Evolution of bismuth electrodes activating electrosynthesis of formate from carbon dioxide reduction, Catal. Today, 2024, 437, 114743 CrossRef CAS.
- Y. Zhang, Y. Chen, R. Liu, X. Wang, H. Liu, Y. Zhu, Q. Qian, Y. Feng, M. Cheng and G. Zhang, Oxygen vacancy stabilized Bi2O2CO3 nanosheet for CO2 electroreduction at low overpotential enables energy efficient CO-production of formate, InfoMat, 2023, 5, e12375 CrossRef CAS.
- S. Abner and A. Chen, Nanostructured cobalt/copper catalysts for efficient electrochemical carbon dioxide reduction, Nanoscale, 2024, 16, 12967–12981 RSC.
- C. Cao, D.-D. Ma, J.-F. Gu, X. Xie, G. Zeng, X. Li, S.-G. Han, Q.-L. Zhu, X.-T. Wu and Q. Xu, Metal–organic layers leading to atomically thin bismuthene for efficient carbon dioxide electroreduction to liquid fuel, Angew. Chem., Int. Ed., 2020, 59, 15014–15020 CrossRef CAS PubMed.
- M. Serafini, F. Mariani, F. Basile, E. Scavetta and D. Tonelli, From traditional to new benchmark catalysts for CO2 electroreduction, Nanomaterials, 2023, 13, 1723 CrossRef CAS PubMed.
- K. Wang, T. Qu, Q. Li, S. Tan and X. Chen, Techno-economic analysis of electrocatalytic CO2 reduction into methanol: A comparative study between alkaline flow cell and neutral membrane electrode assembly, Catalysts, 2023, 13, 1005 CrossRef CAS.
- Z. Mi, A. Arbor and W. Jae Dong, US Pat., 20240301573A1, 2024 Search PubMed.
- R. Parajuli, D. Ansovini, M. Philips and K. Schouten, World Intellectual Property Organization Pat., 2019141827A1, 2019 Search PubMed.
- W. Li, C. Yu, X. Tan, Y. Ren, Y. Zhang, S. Cui, Y. Yang and J. Qiu, Beyond leverage in activity and stability toward CO2 electroreduction to formate over a bismuth catalyst, ACS Catal., 2024, 14, 8050–8061 CrossRef CAS.
- J. Zhu, J. Li, R. Lu, R. Yu, S. Zhao, C. Li, L. Lv, L. Xia, X. Chen, W. Cai, J. Meng, W. Zhang, X. Pan, X. Hong, Y. Dai, Y. Mao, J. Li, L. Zhou, G. He, Q. Pang, Y. Zhao, C. Xia, Z. Wang, L. Dai and L. Mai, Surface passivation for highly active, selective, stable, and scalable CO2 electroreduction, Nat. Commun., 2023, 14, 4670 CrossRef CAS PubMed.
- P. An, L. Wei, H. Li, B. Yang, K. Liu, J. Fu, H. Li, H. Liu, J. Hu, Y.-R. Lu, H. Pan, T.-S. Chan, N. Zhang and M. Liu, Enhancing CO2 reduction by suppressing hydrogen evolution with polytetrafluoroethylene protected copper nanoneedles, J. Mater. Chem. A, 2020, 8, 15936–15941 RSC.
- J. Guo, X. Zhi, D. Wang, L. Qu, A. Zavabeti, Q. Fan, Y. Zhang, J. D. Butson, J. Yang, C. Wu, J. Z. Liu, G. Hu, X. Fan and G. K. Li, Surface-enriched room-temperature liquid bismuth for catalytic CO2 reduction, Small, 2024, 20, 2401777 CrossRef CAS PubMed.
- C. Lin, Y. Liu, X. Kong, Z. Geng and J. Zeng, Electrodeposited highly-oriented bismuth microparticles for efficient CO2 electroreduction into formate, Nano Res., 2022, 15, 10078–10083 CrossRef CAS.
- L. Peng, Y. Wang, Y. Wang, N. Xu, W. Lou, P. Liu, D. Cai, H. Huang and J. Qiao, Separated growth of Bi-Cu bimetallic electrocatalysts on defective copper foam for highly converting CO2 to formate with alkaline anion-exchange membrane beyond KHCO3 electrolyte, Appl. Catal., B, 2021, 288, 120003 CrossRef CAS.
- T. Dong, H. Li, Z. Wang, Y. Geng, R. Chang, X. Tian, J. Lai, S. Feng and L. Wang, Acidic electroreduction CO2 to formic acid via interfacial modification of Bi nanoparticles at industrial-level current, Nano Res., 2023, 17, 5817–5825 CrossRef.
- Y. Qiao, W. Lai, K. Huang, T. Yu, Q. Wang, L. Gao, Z. Yang, Z. Ma, T. Sun, M. Liu, C. Lian and H. Huang, Engineering the local microenvironment over Bi nanosheets for highly selective electrocatalytic conversion of CO2 to HCOOH in strong acid, ACS Catal., 2022, 12, 2357–2364 CrossRef CAS.
- C. Qin, L. Xu, J. Zhang, J. Wang, J. He, D. Liu, J. Yang, J.-D. Xiao, X. Chen, H.-B. Li, Z. Yang and J. Wang, Phase interface regulating on amorphous/crystalline bismuth catalyst for boosted electrocatalytic CO2 reduction to formate, ACS Appl. Mater. Interfaces, 2023, 15, 47016–47024 CrossRef CAS PubMed.
- S.-F. Tang, X.-L. Lu, C. Zhang, Z.-W. Wei, R. Si and T.-B. Lu, Decorating graphdiyne on ultrathin bismuth subcarbonate nanosheets to promote CO2 electroreduction to formate, Sci. Bull., 2021, 66, 1533–1541 CrossRef CAS PubMed.
- T. Fan, W. Ma, M. Xie, H. Liu, J. Zhang, S. Yang, P. Huang, Y. Dong, Z. Chen and X. Yi, Achieving high current density for electrocatalytic reduction of CO2 to formate on bismuth-based catalysts, Cell Rep. Phys. Sci., 2021, 2, 100353 CrossRef CAS.
- F. Yang, Z. Xie, X. Huang, X. Yin, W. Zhang, Y. Huang and D. Zhang, Bi2S3 nanorods grown on multiwalled carbon nanotubes as highly active catalysts for CO2 electroreduction to formate, Phys. Chem. Chem. Phys., 2023, 25, 9198–9207 RSC.
- L. Li, X. Jin, X. Yu and M. Zhong, Bimetallic Cu-Bi catalysts for efficient electroreduction of CO2 to formate, Front. Chem., 2022, 10, 983778 CrossRef CAS PubMed.
- Y. Zhang, C. Cao, X.-T. Wu and Q.-L. Zhu, Three-dimensional porous copper-decorated bismuth-based nanofoam for boosting the electrochemical reduction of CO2 to formate, Inorg. Chem. Front., 2021, 8, 2461–2467 RSC.
- H. Jiang, L. Wang, Y. Li, B. Gao, Y. Guo, C. Yan, M. Zhuo, H. Wang and S. Zhao, High-selectivity electrochemical CO2 reduction to formate at low overpotential over Bi catalyst with hexagonal sheet structure, Appl. Surf. Sci., 2021, 541, 148577 CrossRef CAS.
- X. Li, X. Wu, J. Li, J. Huang, L. Ji, Z. Leng, N. Qian, D. Yang and H. Zhang, Sn-Doped Bi2O3 nanosheets for highly efficient electrochemical CO2 reduction toward formate production, Nanoscale, 2021, 13, 19610–19616 RSC.
- B. Ren, G. Wen, R. Gao, D. Luo, Z. Zhang, W. Qiu, Q. Ma, X. Wang, Y. Cui, L. Ricardez-Sandoval, A. Yu and Z. Chen, Nano-crumples induced Sn-Bi bimetallic interface pattern with moderate electron bank for highly efficient CO2 electroreduction, Nat. Commun., 2022, 13, 2486 CrossRef CAS PubMed.
- Q. Yang, Q. Wu, Y. Liu, S. Luo, X. Wu, X. Zhao, H. Zou, B. Long, W. Chen, Y. Liao, L. Li, P. K. Shen, L. Duan and Z. Quan, Novel Bi-doped amorphous SnOx nanoshells for efficient electrochemical CO2 reduction into formate at low overpotentials, Adv. Mater., 2020, 32, 2002822 CrossRef CAS PubMed.
- Y. Guan, X. Zhang, Y. Zhang, T. N. V. Karsili, M. Fan, Y. Liu, B. Marchetti and X.-D. Zhou, Achieving high selectivity towards electro-conversion of CO2 using In-doped Bi derived from metal-organic frameworks, J. Colloid Interface Sci., 2022, 612, 235–245 CrossRef CAS PubMed.
- S. Li, Y. Kang, C. Mo, Y. Peng, H. Ma and J. Peng, Nitrogen-doped bismuth nanosheet as an efficient electrocatalyst to CO2 reduction for production of formate, Int. J. Mol. Sci., 2022, 23, 14485 CrossRef CAS PubMed.
- F. Zhang, C. Chen, S. Yan, J. Zhong, B. Zhang and Z. Cheng, Cu@Bi nanocone induced efficient reduction of CO2 to formate with high current density, Appl. Catal., A, 2020, 598, 117545 CrossRef CAS.
- Z. Wu, H. Wu, W. Cai, Z. Wen, B. Jia, L. Wang, W. Jin and T. Ma, Engineering bismuth–tin interface in bimetallic aerogel with a 3D porous structure for highly selective electrocatalytic CO2 reduction to HCOOH, Angew. Chem., Int. Ed., 2021, 60, 12554–12559 CrossRef CAS PubMed.
| This journal is © Institute of Process Engineering of CAS 2025 |