 Open Access Article
Open Access ArticleEnhanced lithium extraction from brine using surface-modified LiMn2O4 electrode with nanoparticle islands†
Guiling
Luo
a,
Muyao
He
b,
Li
Zhang
b,
Jianquan
Deng
a,
Linlin
Chen
c,
Yanhong
Chao
*b,
Haiyan
Liu
 a,
Wenshuai
Zhu
a,
Wenshuai
Zhu
 *a and
Zhichang
Liu
a
*a and
Zhichang
Liu
a
aCollege of Chemical Engineering and Environment, State Key Laboratory of Heavy Oil Processing, China University of Petroleum-Beijing, Beijing 102249, China. E-mail: zhuws@cup.edu.cn
bCollege of Science, State Key Laboratory of Heavy Oil Processing, China University of Petroleum-Beijing, Beijing 102249, China. E-mail: chaoyh@cup.edu.cn
cSchool of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, Jiangsu, China
First published on 31st January 2025
Abstract
Lithium is an important raw material for new energy-powered vehicles, and ensuring its supply is of great significance for global green and sustainable development. Salt lake brine is the main lithium resource, but the separation of Li+ from coexisting metals poses a major challenge. In this work, a lithium-storing metal oxide SnO2 nanoparticle island-modified LiMn2O4 electrode material is designed to endow LiMn2O4 with higher lithium extraction capacity and cycling stability. The SnO2 nanoparticle islands effectively mitigate stress during the charge–discharge process of LiMn2O4, thereby enhancing cycling stability and promoting the diffusion of Li+. The lithium adsorption capacity of the LiMn2O4 electrode material modified with SnO2 nanoparticles reaches 19.76 mg g−1 within 1 hour, which is 1.7 times higher than that of LiMn2O4 (11.45 mg g−1). The LiMn2O4 electrode material modified with SnO2 nanoparticles shows good selectivity and cycling stability for the separation of lithium ions.
Keywords: Electrochemical adsorption; Extraction lithium; Surface modified; LiMn2O4.
1 Introduction
The increasing demand for lithium, primarily driven by the proliferation of lithium-ion batteries, is expected to result in a significant supply shortage by 2030.1,2 Lithium resources include continental brines, geothermal brines, seawater, lithium spodumene, lithium montmorillonite, lithium feldspar, and lithium mica, with China's salt lake brine reserves accounting for 80% of the global reserves.3–5 To address this impending imbalance, extracting lithium from salt lake brines has emerged as a highly promising strategy.6,7 Salt lake lithium extraction can partially replace lithium extraction from solid-state ores, reducing reliance on imported ores and reducing energy consumption and carbon emissions.8–10 Electrochemical methods for lithium extraction from salt lake brines are particularly advantageous due to their simplicity, high recovery efficiency, and cost-effectiveness.11,12 By leveraging these methods, it is possible to enhance the sustainability of lithium production, ensuring a more stable supply chain and contributing to the reduction of energy consumption and carbon emissions in the process.Electrochemical methods involve applying a certain strength of electric field to assist the selective capture/release of Li+ on electrode materials, enabling the extraction and enrichment of Li+ from the mixed solution phase.13,14 LiMn2O4 (LMO), is widely used as electrode materials for selective Li+ capture, attributed to the spinel-type LMO having specific lithium vacancies and appropriate potential windows, resulting in excellent Li+ selectivity.15,16 The selectivity of LMO for Li+ is primarily influenced by the size difference between Li+ and other cations present in the solution.17,18 The ionic radii of common cations found in salt lakes are as follows: Mg2+ (0.072 nm), Li+ (0.076 nm), Na+ (0.102 nm), Ca2+ (0.106 nm), and K+ (0.138 nm).19,20 While LMO can selectively insert and extract Li+, the similar size of Mg2+ poses a challenge for exclusive Li+ recovery based solely on ionic radius. Studies have shown that solvation radius is related to the transport in aqueous solutions, the difference in hydration energy between these ions significantly aids in selective extraction.21,22 Mg2+ has a much higher hydration energy (1922 kJ mol−1) compared to Li+ (515 kJ mol−1), which, combined with the synergy between the hydrated ion radius and the ion radius, enhances the selective extraction mechanism for Li+.23 This combination of factors makes LMO-based electrochemical methods effective for lithium recovery.
However, the inherent properties of LMO determine its poor cycling stability, and there is a gap between the actual lithium extraction capacity and the theoretical capacity, limiting its application in electrochemistry. Surface coating, doping, and core–shell structures are common techniques for modifying LMO electrodes, each with distinct advantages and limitations. Surface coatings improve cycling stability by suppressing side reactions and Mn dissolution but can hinder lithium-ion transport if not uniformly applied.24,25 Doping stabilizes the crystal structure and enhances conductivity; however, excessive doping can disrupt the spinel structure and reduce performance.26 Core–shell structures offer structural stability and surface protection but face challenges with synthesis complexity, scalability, and lithium-ion transport across interfaces.27 Surface modification of metal oxides is an effective method to improve surface chemical stability, resist stresses during charge and discharge processes, and inhibit the Jahn–Teller effect of LMO.28–30 In recent years, lithium storage anodes prepared using metal oxide materials such as TiO2, SnO2, and Fe2O3 have attracted considerable attention due to their high chemical stability and reversible capacity.31–33 SnO2 nanoparticles exhibit excellent Li+ storage capability, with a theoretical Li+ storage capacity of up to 740 mAh g−1.34 The SnO2 nanoparticle island modification approach stands out by offering a uniform and scalable method to enhance the cycling stability of LMO electrodes while suppressing Mn dissolution during charge–discharge cycles.35 The nanoparticle islands effectively mitigate structural stress, ensuring better long-term stability compared to conventional surface coatings or core–shell structures.36 Additionally, SnO2 possesses high thermal stability and oxidation resistance, is insoluble in water, and is also difficult to dissolve in acidic or alkaline solutions, making it suitable for various aqueous environments.
Herein, this work designed and synthesized LMO electrode materials modified with SnO2 nanoparticles with high lithium capacity and chemical surface stability, and constructed an electrochemical lithium extraction system from brine. The physical and chemical properties of samples were systematically characterized using SEM, TEM, HRTEM, Raman, XRD, XPS, and other techniques. The electrochemical quasi in situ spectroscopy characterization elucidated the deintercalation mechanism of Li+ in the SnO2 nanoparticle-modified LMO (SnLMO) structure, and the XRD spectra of the material after cycling clarified the influence of SnLMO on the Jahn–Teller effect. In addition, the effects of interfering ions on the electrode polarization of SnLMO, extraction capacity and cycling stability of lithium were also investigated.
2 Results and discussion
2.1 Structure and morphology
The surface of LMO is smooth and presents a truncated octahedral morphology (Fig. 1a). The particle size follows a normal distribution with an average of 570 ± 20 nm (Fig. S1†), and there are a few large particles exceeding 2 μm. SnLMO-1 exhibits a truncated octahedral morphology with uniformly attached agglomerated nanoscale particles on the surface, with very few nanowires present (Fig. 1b). TEM images of SnLMO-1 indicate that SnO2 nanoparticles are uniformly loaded onto the surface of LMO, with a small amount of nanowires present (Fig. 1c and d). From the HRTEM image (Fig. 1e), it is evident that the SnO2 nanoparticles on SnLMO-1 possess a clear lattice structure with a lattice size of 0.26 nm, belonging to the (101) plane.33 EDX elemental analysis and mapping results demonstrate the uniform distribution of Sn elements on the surface of lithium manganese oxide (Fig. 1f and S2†). Furthermore, with increasing loading amounts, SnLMO-2 and SnLMO-3 exhibit a higher density of nanowires without altering the basic morphology of the truncated octahedron (Fig. S3 and S4†). In LMO materials loaded with SnO2 nanowires, clear lattice fringes of the SnO2 (110) plane are observed, with a lattice size of 0.31 nm (Fig. S5†). | ||
| Fig. 1 SEM images of LMO (a) and SnLMO-1 (b); TEM (c and d), HRTEM (e), mapping (f) images of SnLMO-1. | ||
The characteristic peaks in the XPS spectrum of Mn3+ and Mn4+ (Mn 2p3/2 and Mn 2p1/2) in LMO are located at 640.1, 651.6 eV and 641.5, 653.0 eV, respectively; with proportions of 42.28% and 57.72%, respectively (Fig. 2a). After being modified with 0.5 wt% SnO2, the binding energies of Mn3+ and Mn4+ are shifted, with characteristic peak positions at 640.4, 651.8 eV and 641.9, 653.1 eV, respectively; with proportions of 53.06% and 46.94%, respectively. The O 1s peaks of LMO are located at 528.5, 529.8, and 531.4 eV, corresponding to lattice oxygen, Mn–O, and surface physically adsorbed water molecules O–H,30,37 respectively; with proportions of 16.60%, 31.93%, and 51.47%, respectively. The O 1s peaks of SnLMO-1 are located at 528.3, 530.4, and 532.2 eV, corresponding to lattice oxygen, Mn–O, and surface physically adsorbed water molecules O–H, respectively; with proportions of 23.52%, 29.22%, and 47.26%, respectively (Fig. 2b). The characteristic peaks at 493.5 eV and 485.1 eV are attributed to Sn 3d3/2 and Sn 3d5/2, respectively (Fig. 2c), indicating the successful modification of Sn on LMO.38 The presence of SnO2 enhances the binding energy of LMO, improving surface stability.39 XRD spectra show consistent characteristic peaks for LMO and SnLMO, with no shift in characteristic peaks with increasing modification, indicating that the internal crystal structure of LMO remains unchanged after being modified with SnO2 (Fig. 2d). There are also no characteristic peaks of SnO2 appearing after modification, possibly due to the low modified amount or uniform distribution of the modified.
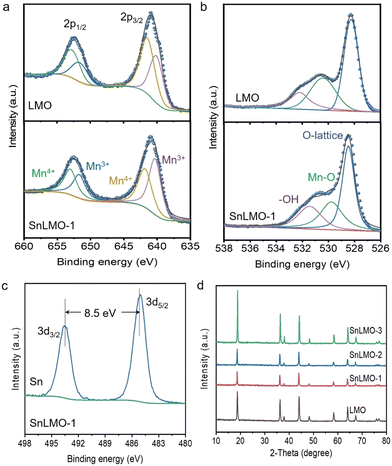 | ||
| Fig. 2 High-resolution XPS spectra of Mn 2p (a), O 1s (b), and Sn 3d (c) of LMO and SnLMO-1; XRD patterns (d) of LMO and SnLMO. | ||
2.2 Electrochemical selectivity and dynamics
However, compared to the LiCl solution, the presence of cations such as Na+, K+, Mg2+, and Ca2+ in a solution affects the electrochemical behavior of Li+. The redox voltage in the CV curve is mainly influenced by the Li+ migration channel in LMO.42 The current in the CV curve is determined by the amount of Li+ inserted into the structure, which is governed by the transport properties of the lithium-ion channels.43 The shifts in peak voltages, therefore, can be attributed to changes in the ion transport dynamics, particularly due to the interference of additional cations in the electrolyte. A small amount of Na+, K+, Mg2+, and Ca2+ ions can adsorb onto the lithium-ion migration channels of LMO, hindering the insertion of Li+.44 This adsorption effect can increase the reduction potential (or discharge voltage), as seen in Fig. 3a, S6 and S7.† These ions occupy part of the space in the migration channels, making it more difficult for Li+ to diffuse and intercalate into the material, thus leading to an increase in the reduction voltage.45 Additionally, the presence of cations in the electrolyte also affects the electric field distribution at the electrode/electrolyte interface, which can lead to electrochemical polarization. This polarization affects the extraction of Li+, resulting in an increase in the oxidation potential.46 In summary, the observed shifts in the CV curves are primarily due to the combined effects of ionic competition in the migration channels and the electrochemical polarization caused by the cations.
| D Li + | O1 | O2 | R1 | R2 | Average |
|---|---|---|---|---|---|
| LMO | 5.02 × 10−12 | 6.85 × 10−12 | 2.93 × 10−12 | 4.81 × 10−12 | 4.90 × 10−12 |
| SnLMO-1 | 8.92 × 10−12 | 1.36 × 10−11 | 8.14 × 10−12 | 1.27 × 10−11 | 1.08 × 10−11 |
| SnLMO-2 | 5.98 × 10−12 | 1.10 × 10−11 | 6.02 × 10−12 | 1.13 × 10−11 | 8.58 × 10−12 |
| SnLMO-3 | 4.81 × 10−12 | 8.13 × 10−12 | 4.15 × 10−12 | 8.02 × 10−12 | 6.28 × 10−12 |
2.3 The mechanism of the lithium deintercalation process
To study the mechanism of Li+ extraction selectivity of SnLMO-1, the structural characteristics of electrode materials at different potentials during charging and discharging processes were analyzed. Previous literature has indicated that under the influence of an electric field, LMO may undergo reactions represented by reactions (1) and (2) with Li+.52,53 Additionally, in a LiCl solution, charging and discharging were conducted within the range of 0–1.0 V at a current density of 50 mA g−1, and the corresponding XRD profiles at the respective voltages are shown in Fig. 4. During charging, with increasing potential, the (111) crystal plane of SnLMO-1 shifted towards higher angles (from 18.84° to 19.06°), indicating a decrease in lattice spacing, transforming the electrode material from SnLMO-1 to SnO2–λ-MnO2.54 During discharging, with decreasing potential, the (111) crystal plane of SnLMO-1 shifted towards lower angles (from 19.06° to 18.64°). After completing one cycle, the characteristic peaks of the (111) crystal plane return to their initial positions, indicating the reversible deintercalation of Li+. This reversible change demonstrates the structural stability and efficiency of SnLMO-1 as an electrode material for lithium extraction, highlighting its potential application in lithium recovery.2LiMn2O4 ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) 2Li0.5Mn2O4 + Li+ + e− 2Li0.5Mn2O4 + Li+ + e− | (1) |
2Li0.5Mn2O4 ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) 4λ-MnO2 + Li+ + e− 4λ-MnO2 + Li+ + e− | (2) |
2.4 Performance
We studied the cycling performance of SnLMO with different modified amounts in a 30 mL of 1.0 mol L−1 LiCl (three-electrode system, current density of 50 mA g−1), as shown in Fig. 5a. After 30 cycles, the capacity retention rates of LMO, SnLMO-1, SnLMO-2, and SnLMO-3 were 36.52%, 61.03%, 55.83%, and 30.45%, respectively. The specific surface area of the material has a significant impact on the lithium extraction capacity. The N2 isothermal adsorption curve results show that the specific surface areas of LMO, SnLMO-1, SnLMO-2, and SnLMO-3 are 1.9, 4.3, 9.1, and 13.3 cm2 g−1, respectively (Fig. S10†). Due to the increased specific surface area of SnO2 nanowires loaded, SnLMO-3 exhibited better discharge capacity than other materials in the first 15 cycles. However, it experienced significant decay after 15 cycles, possibly due to volume expansion of SnO2 nanowires upon lithiation, leading to poor reversible cycling performance.52 Modification of SnO2 nanoparticles into island-like structures is beneficial for reducing the stress of LMO (SnLMO-1) during redox reaction processes and enhancing the surface area, thereby enhancing the material's cycling stability. Additionally, the dissolution of Mn in the electrolyte after cycling was examined, with results shown in Fig. 5b. In LiCl electrolyte, during the SnLMO and LMO redox reaction processes, partial Mn3+ underwent disproportionation reactions (Jahn–Teller effect), generating soluble Mn2+ that dissolved and diffused into the electrolyte.55 The RMn in SnLMO-1 was 0.14%, lower than that of LMO (0.16%), SnLMO-2 (0.15%), and SnLMO-3 (0.16%). This indicates that modified SnO2 nanoparticles can reduce stress on the electrode surface during Mn3+/4+ redox reaction processes and suppress the occurrence of the Jahn–Teller effect. Furthermore, through XRD spectra of electrode materials before and after cycling, it was observed that SnLMO-1 exhibited a low-angle shift after the first cycle (attributed to electrode activation during the first charge–discharge cycle), and the characteristic peaks at the 5th, 10th, 15th, and 30th cycles remained consistent with the initial state, indicating its excellent cycling stability (Fig. 5c), demonstrating good chemical stability of the composite material.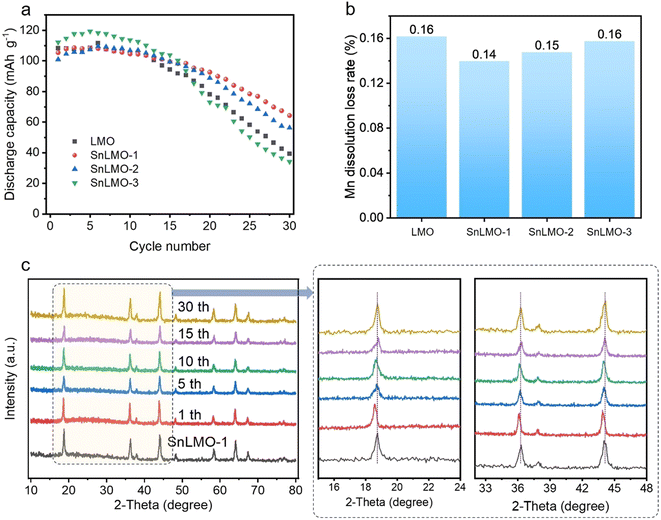 | ||
| Fig. 5 Cycling performance (a) and Mn dissolution loss rate RMn (b) of LMO and SnLMO; XRD patterns of SnLMO-1 cycling process (c). | ||
The study evaluates the lithium recovery performance of LMO and SnLMO in a simulated brine solution containing 30 mmol L−1 concentrations of various chloride salts (LiCl, NaCl, KCl, MgCl2, and CaCl2) using electrochemical methods (Fig. 6a and b). The performance was measured over five capture and release cycles. The average extraction capacities of LMO, SnLMO-1, SnLMO-2, and SnLMO-3 were 11.45, 19.76, 17.97, and 18.76 mg g−1, respectively; and the average release capacities were 7.61, 18.45, 10.77, and 14.36 mg g−1, respectively. The results indicate that SnO2 modified LMO can increase Li+ adsorption capacity, attributed to larger specific surface area and smaller electron transfer resistance. Further, when utilizing the SnLMO-1//Ag electrochemical system for lithium extraction from the mixed chloride solution, the average extraction and release capacities were 19.76 and 18.45 mg g−1, respectively, after five cycles (Fig. 6c). The system also demonstrated minimal changes in other cations within the solution and achieved a lithium purity of 96.90% in the recovery solution (Fig. 6d). Compared with previous studies (Table 2), SnLMO-1 exhibits good selectivity and extraction capacity, attributed to SnO2 as an excellent lithium storage material effectively increasing lithium ion extraction capacity, and Ag demonstrating good reversibility to the electrode.
| Electrochemical system | Purity (%) | Capacity (mg g−1) | Ref. |
|---|---|---|---|
| PPy: polypyrrole; NCM: Li1−xNi0.33Co1/3Mn1/3O2; LMNC: Li1.16Mn0.6Ni0.12Co0.12O2; LNMM: LiNi0.038Mo0.012Mn1.95O4; rGO/NCM: graphene-coated LiNi0.6Co0.2Mn0.2O2; HTO/rGO-TA: H2TiO3/reduced graphene oxide–tannic acid. | |||
| LMO//PPy | — | 4.43 | 56 |
| NCM//Ag | 96.4 | 10.83 | 57 |
| LMNC//Bi | 92.5 | 13.05 | 58 |
| LMO//AEM//MnO2 | — | 22 | 44 |
| LNMM//AC | 97.2 | 14.4 | 59 |
| rGO/NCM//AC | 93.4 | 13.84 | 60 |
| α-LiAlO2//AC | — | 8.25 | 61 |
| LMO//Li1−xMn2O4 | — | 17.20 | 48 |
| PPy/Al2O3/LMO//AC | 97.4 | 12.84 | 41 |
| HTO/rGO-TA//AC | — | 25.2 | 62 |
| SnLMO-1//Ag | 96.9 | 19.76 | This work |
3 Conclusion
This work designs lithium storage type metal oxide SnO2 island-modified LMO electrode materials, endowing them with high Li+ diffusion capability and electro-adsorption capacity. The electro-adsorption capacity in simulated brine (210 mg L−1 Li+) is 19.76 mg g−1, the Li+ diffusion coefficient is 1.08 × 10−11 cm2 s−1, and the capacity retention rate after 30 cycles is 61.03%, which is higher than that of unmodified LMO. However, SnO2 nanowire-modified LMO exhibits a higher initial discharge capacity but poor cycling stability (30-cycle capacity retention rate of 30.45%). Therefore, SnO2 nanoparticle modification has better stability than nanowires, attributed to the island modification of nanoparticles reducing the stress generated during the charge–discharge process, and suppressing the distortion of electrode material structure.Data availability
Data will be made available on request.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2022YFE0208300), the National Natural Science Foundation of China (No. 22278426 and 21908082), the Science Foundation of China University of Petroleum, Beijing (No. 2462024XKBH001, 2462022YJRC003, 2462022YJRC002).References
- Y. Song, S. Fang, N. Xu, M. Wang, S. Chen, J. Chen, B. Mi and J. Zhu, Solar transpiration–powered lithium extraction and storage, Science, 2024, 385, 1444–1449 CrossRef CAS PubMed.
- Z. Li, I.-C. Chen, L. Cao, X. Liu, K.-W. Huang and Z. Lai, Lithium extraction from brine through a decoupled and membrane-free electrochemical cell design, Science, 2024, 385, 1438–1444 CrossRef CAS PubMed.
- S. Zavahir, T. Elmakki, M. Gulied, Z. Ahmad, L. Al-Sulaiti, H. K. Shon, Y. Chen, H. Park, B. Batchelor and D. S. Han, A review on lithium recovery using electrochemical capturing systems, Desalination, 2021, 500, 114883 CrossRef CAS.
- P. K. Choubey, M.-S. Kim, R. R. Srivastava, J.-C. Lee and J.-Y. Lee, Advance review on the exploitation of the prominent energy-storage element: Lithium. Part I: From mineral and brine resources, Miner. Eng., 2016, 89, 119–137 CrossRef CAS.
- Y. Zhang, W. Sun, R. Xu, L. Wang and H. Tang, Lithium extraction from water lithium resources through green electrochemical-battery approaches: A comprehensive review, J. Cleaner Prod., 2021, 285, 124905 CrossRef CAS.
- G. Zhou, X. Li, L. Chen, G. Luo, J. Gu, J. Zhu, J. Yu, J. Yin, Y. Chao and W. Zhu, Construction of porous disc-like lithium manganate for rapid and selective electrochemical lithium extraction from brine, Chin. J. Chem. Eng., 2023, 54, 316–322 CrossRef CAS.
- G. Zhou, L. Chen, X. Li, G. Luo, Z. Yu, J. Yin, L. Fan, Y. Chao, L. Jiang and W. Zhu, Construction of truncated-octahedral LiMn2O4 for battery-like electrochemical lithium recovery from brine, Green Energy Environ., 2023, 8, 1081–1090 CrossRef CAS.
- J. Yu, J. Zhu, G. Luo, L. Chen, X. Li, P. Cui, P. Wu, Y. Chao, W. Zhu and Z. Liu, 3D-printed titanium-based ionic sieve monolithic adsorbent for selective lithium recovery from salt lakes, Desalination, 2023, 560, 116651 CrossRef CAS.
- T. Ding, M. Zheng, S. Peng, Y. Lin, X. Zhang and M. Li, Lithium extraction from salt lakes with different hydrochemical types in the Tibet Plateau, Geosci. Front., 2023, 14, 101485 CrossRef CAS.
- P. Xu, J. Hong, X. Qian, Z. Xu, H. Xia, X. Tao, Z. Xu and Q. Ni, Materials for lithium recovery from salt lake brine, J. Mater. Sci., 2020, 56, 16–63 CrossRef.
- R. Bagheri, M. Ghaedi, A. Asfaram, E. Alipanahpour Dil and H. Javadian, RSM-CCD design of malachite green adsorption onto activated carbon with multimodal pore size distribution prepared from Amygdalus scoparia: Kinetic and isotherm studies, Polyhedron, 2019, 171, 464–472 CrossRef CAS.
- X. Li, Y. Chao, L. Chen, W. Chen, J. Luo, C. Wang, P. Wu, H. Li and W. Zhu, Taming wettability of lithium ion sieve via different TiO2 precursors for effective Li recovery from aqueous lithium resources, Chem. Eng. J., 2020, 392, 123731 CrossRef CAS.
- A. Battistel, M. S. Palagonia, D. Brogioli, F. La Mantia and R. Trocoli, Electrochemical methods for lithium recovery: A comprehensive and critical review, Adv. Mater., 2020, 32, e1905440 CrossRef PubMed.
- W. Yu, Y. Wang, A. Wu, A. Li, Z. Qiu, X. Dong, C. Dong and H. Huang, Suppress oxygen evolution of lithium-rich manganese-based cathode materials via an integrated strategy, Green Energy Environ., 2024, 9, 138–151 CrossRef CAS.
- H. Joo, J. Lee and J. Yoon, Short Review: Timeline of the electrochemical lithium recovery system using the spinel LiMn2O4 as a positive electrode, Energies, 2020, 13, 6235 CrossRef CAS.
- J. Wang, X. Yue, P. Wang, T. Yu, X. Du, X. Hao, A. Abudula and G. Guan, Electrochemical technologies for lithium recovery from liquid resources: A review, Renewable Sustainable Energy Rev., 2022, 154, 111813 CrossRef CAS.
- B. Hu, X. Shang, P. Nie, B. Zhang, J. Yang and J. Liu, Lithium ion sieve modified three-dimensional graphene electrode for selective extraction of lithium by capacitive deionization, J. Colloid Interface Sci., 2022, 612, 392–400 CrossRef CAS PubMed.
- K. T. Kubra, M. S. Salman, M. N. Hasan, A. Islam, S. H. Teo, M. M. Hasan, M. C. Sheikh and M. R. Awual, Sustainable detection and capturing of cerium(III) using ligand embedded solid-state conjugate adsorbent, J. Mol. Liq., 2021, 338, 116667 CrossRef CAS.
- R. Trócoli, A. Battistel and F. L. Mantia, Selectivity of a lithium-recovery process based on LiFePO4, Chemistry, 2014, 20, 9888–9891 CrossRef PubMed.
- M. N. Hasan, M. A. Shenashen, M. M. Hasan, H. Znad and M. R. Awual, Assessing of cesium removal from wastewater using functionalized wood cellulosic adsorbent, Chemosphere, 2021, 270, 128668 CrossRef CAS PubMed.
- X. Jia, M. Hu, K. Soundarapandian, X. Yu, Z. Liu, Z. Chen, A. Narita, K. Mullen, F. H. L. Koppens, J. Jiang, K. J. Tielrooij, M. Bonn and H. I. Wang, Kinetic ionic permeation and interfacial doping of supported graphene, Nano Lett., 2019, 19, 9029–9036 CrossRef CAS PubMed.
- K. Hayamizu, Y. Chiba and T. Haishi, Dynamic ionic radius of alkali metal ions in aqueous solution: A pulsed-field gradient NMR study, RSC Adv., 2021, 11, 20252–20257 RSC.
- B. Tansel, Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: Hydrated radius, hydration free energy and viscous effects, Sep. Purif. Technol., 2012, 86, 119–126 CrossRef CAS.
- Y. Gu, K. Yang, H. Yao, W. Li, H. Zhan, X. Ming, G. Huang, G. Li and F. Zhan, Surface modification of Li3InCl6 provides superior electrochemical performance for LiMn2O4 cathode materials, Chin. Chem. Lett., 2023, 34, 108047 CrossRef CAS.
- G. Luo, M. Zhou, Y. Chao, P. Cui, X. Li, L. Chen, G. Jiang, W. Zhu, Z. Liu and C. Xu, Augmented electrochemical extraction lithium performance via interface alloying modification, Sep. Purif. Technol., 2025, 354, 128683 CrossRef CAS.
- C. P. Lawagon, G. M. Nisola, R. A. I. Cuevas, R. E. C. Torrejos, H. Kim, S. P. Lee and W. J. Chung, Li Ni0.5Mn1.5O4/Ag for electrochemical lithium recovery from brine and its optimized performance via response surface methodology, Sep. Purif. Technol., 2019, 212, 416–426 CrossRef CAS.
- Y. Sun, Y. Wang, Y. Liu and X. Xiang, Highly efficient lithium extraction from brine with a high sodium content by adsorption-Coupled electrochemical technology, ACS Sustainable Chem. Eng., 2021, 9, 11022–11031 CrossRef CAS.
- Z. Zhang, X. Du, Q. Wang, F. Gao, T. Jin, X. Hao, P. Ma, J. Li and G. Guan, A scalable three-dimensional porous λ-MnO2/rGO/Ca-alginate composite electroactive film with potential-responsive ion-pumping effect for selective recovery of lithium ions, Sep. Purif. Technol., 2021, 259, 118111 CrossRef CAS.
- G. Luo, L. Zhu, X. Li, G. Zhou, J. Sun, L. Chen, Y. Chao, L. Jiang and W. Zhu, Electrochemical lithium ions pump for lithium recovery from brine by using a surface stability Al2O3–ZrO2 coated LiMn2O4 electrode, J. Energy Chem., 2022, 69, 244–252 CrossRef CAS.
- Y. Wang, J. Zhang, Z. Cheng and X. Xiang, Hydrophilic modification using polydopamine on core–shell Li1.6Mn1.6O4@Carbon electrodes for lithium extraction from lake brine, ACS Sustainable Chem. Eng., 2022, 10, 8970–8979 CrossRef CAS.
- T. Song and U. Paik, TiO2 as an active or supplemental material for lithium batteries, J. Mater. Chem. A, 2016, 4, 14–31 RSC.
- T. L. Nguyen, J. Hur and I. T. Kim, Facile Synthesis of quantum dots SnO2/Fe3O4 hybrid composites for superior reversible lithium-ion storage, J. Ind. Eng. Chem., 2019, 72, 504–511 CrossRef CAS.
- T. P. Nguyen and I. T. Kim, Self-assembled few-layered MoS2 on SnO2 anode for enhancing lithium-ion storage, Nanomaterials, 2020, 10, 2558 CrossRef CAS PubMed.
- J. Li, Y. Zhao, N. Wang and L. Guan, A high performance carrier for SnO2 nanoparticles used in lithium ion battery, Chem. Commun., 2011, 47, 5238 RSC.
- Y. Lee, T. Y. Kim, D.-W. Kim, J. K. Lee and W. Choi, Coating of spinel LiNi0.5Mn1.5O4 cathodes with SnO2 by an electron cyclotron resonance metal–organic chemical vapor deposition method for high-voltage applications in lithium ion batteries, J. Electroanal. Chem., 2015, 736, 16–21 CrossRef CAS.
- Y. Chen, W. Qin, J. An, J. Zhang and X. Wen, Island-like MoO3-x film grown on Mo foil as high-performance lithium-ion battery anode: a new strategy for preparing flexible electrode, J. Mater. Sci., 2022, 57, 5552–5565 CrossRef CAS.
- F. Lv, H. Cheng, W. Nie, Q. Sun, Y. Liu, T. Duan, Q. Xu and X. Lu, Enhancing rate capacity and cycle stability of LiNi1/3Co1/3Mn1/3O2 cathode material by laminar V2O5 coating for lithium-ion batteries, ChemistrySelect, 2021, 6, 6339–6347 CrossRef CAS.
- Z. Wang, T. Han, T. Fei, S. Liu and T. Zhang, Investigation of microstructure effect on NO2 sensors based on SnO2 nanoparticles/reduced graphene oxide hybrids, ACS Appl. Mater. Interfaces, 2018, 10, 41773–41783 CrossRef CAS PubMed.
- S. Wang, C. Luo, Y. Feng, G. Fan, L. Feng, M. Ren and B. Liu, Electrochemical properties and microstructures of LiMn2O4 cathodes coated with aluminum zirconium coupling agents, Ceram. Int., 2020, 46, 13003–13013 CrossRef CAS.
- N. Xie, Y. Li, Y. Lu, J. Gong and X. Hu, Electrochemically controlled reversible lithium capture and release enabled by LiMn2O4 nanorods, ChemElectroChem, 2019, 7, 105–111 CrossRef.
- X. Zhao, Y. Jiao, P. Xue, M. Feng, Y. Wang and Z. Sha, Efficient lithium extraction from brine using a three-dimensional nanostructured hybrid inorganic-gel framework electrode, ACS Sustainable Chem. Eng., 2020, 8, 4827–4837 CrossRef CAS.
- H. Fu, W. Xu, Z. Yu, L. He and Z. Zhao, Identifying electrochemical performance of LiFePO4 with different exposed crystal facets for voltammetric determination of Li+, J. Electroanal. Chem., 2024, 973, 118672 CrossRef CAS.
- W. Xu, L. He and Z. Zhao, Lithium extraction from high Mg/Li brine via electrochemical intercalation/de-intercalation system using LiMn2O4 materials, Desalination, 2021, 503, 114935 CrossRef CAS.
- M. Zhao, Z. Ji, Y. Zhang, Z. Guo, Y. Zhao, J. Liu and J. Yuan, Study on lithium extraction from brines based on LiMn2O4/Li1-xMn2O4 by electrochemical method, Electrochim. Acta, 2017, 252, 350–361 CrossRef CAS.
- D. F. Liu, S. Y. Sun and J. G. Yu, Electrochemical and adsorption behaviour of Li+, Na+, K+, Ca2+, and Mg2+ in LiMn2O4/λ-MnO2 structures, Can. J. Chem. Eng., 2018, 97, 1589–1595 CrossRef.
- M. Kunitski, N. Eicke, P. Huber, J. Kohler, S. Zeller, J. Voigtsberger, N. Schlott, K. Henrichs, H. Sann, F. Trinter, L. P. H. Schmidt, A. Kalinin, M. S. Schoffler, T. Jahnke, M. Lein and R. Dorner, Double-slit photoelectron interference in strong-field ionization of the neon dimer, Nat. Commun., 2019, 10, 1 CrossRef CAS PubMed.
- B. Liu, B. Xiao, L. Cui and M. Wang, Molecularly imprinted electrochemical sensor for the highly selective and sensitive determination of melamine, Mater. Sci. Eng., C, 2015, 55, 457–461 CrossRef CAS PubMed.
- D. Liu, W. Xu, J. Xiong, L. He and Z. Zhao, Electrochemical system with LiMn2O4 porous electrode for lithium recovery and its kinetics, Sep. Purif. Technol., 2021, 270, 118809 CrossRef CAS.
- Y. Mu, C. Zhang, W. Zhang and Y. Wang, Electrochemical lithium recovery from brine with high Mg2+/Li+ ratio using mesoporous λ-MnO2/LiMn2O4 modified 3D graphite felt electrodes, Desalination, 2021, 511, 115112 CrossRef CAS.
- C. Kim, M. Noh, M. Choi, J. Cho and B. Park, Critical size of a nano SnO2 electrode for Li-secondary battery, Chem. Mater., 2005, 17, 3297–3301 CrossRef CAS.
- H. Wang, X. Shan, H. Yu, Y. Wang, W. Schmickler, H. Y. Chen and N. Tao, Determining electrochemical surface stress of single nanowires, Angew. Chem., Int. Ed., 2017, 56, 2132–2135 CrossRef CAS PubMed.
- S. Zhao, C. D. Sewell, R. Liu, S. Jia, Z. Wang, Y. He, K. Yuan, H. Jin, S. Wang, X. Liu and Z. Lin, SnO2 as advanced anode of alkali-ion batteries: Inhibiting Sn coarsening by crafting robust physical barriers, void boundaries, and heterophase interfaces for superior electrochemical reaction reversibility, Adv. Energy Mater., 2019, 10, 1902657 CrossRef.
- X. Xu, Y. Zhou, Z. Feng, N. U. Kahn, Z. U. Haq Khan, Y. Tang, Y. Sun, P. Wan, Y. Chen and M. Fan, A self-supported lambda-MnO2 film electrode used for electrochemical lithium recovery from brines, ChemPlusChem, 2018, 83, 521–528 CrossRef CAS PubMed.
- G. Luo, X. Li, L. Chen, Y. Zhang, J. Gu, Y. Chao, W. Zhu, Z. Liu and C. Xu, Island-like CeO2 decorated LiMn2O4: Surface modification enhancing electrochemical lithium extraction and cycle performance, Chem. Eng. J., 2023, 455, 140928 CrossRef CAS.
- C. Zuo, Z. Hu, R. Qi, J. Liu, Z. Li, J. Lu, C. Dong, K. Yang, W. Huang, C. Chen, Z. Song, S. Song, Y. Yu, J. Zheng and F. Pan, Double the capacity of manganese spinel for lithium-ion storage by suppression of cooperative Jahn–Teller distortion, Adv. Energy Mater., 2020, 10, 2000363 CrossRef CAS.
- L. L. Missoni, F. Marchini, M. í. D. Pozo and E. J. Calvo, A LiMn2O4-polypyrrole system for the extraction of LiCl from natural brine, J. Electrochem. Soc., 2016, 163, A1898–A1902 CrossRef CAS.
- C. P. Lawagon, G. M. Nisola, R. A. I. Cuevas, H. Kim, S. P. Lee and W. J. Chung, Li1-xNi0.33Co1/3Mn1/3O2/Ag for electrochemical lithium recovery from brine, Chem. Eng. J., 2018, 348, 1000–1011 CrossRef CAS.
- X. Zhao, H. Yang, Y. Wang, L. Yang and L. Zhu, Lithium extraction from brine by an asymmetric hybrid capacitor composed of heterostructured lithium-rich cathode and nano-bismuth anode, Sep. Purif. Technol., 2021, 274, 119078 CrossRef CAS.
- X. Zhao, G. Li, M. Feng and Y. Wang, Semi-continuous electrochemical extraction of lithium from brine using CF-NMMO/AC asymmetric hybrid capacitors, Electrochim. Acta, 2020, 331, 135285 CrossRef CAS.
- X. Zhao, M. Feng, Y. Jiao, Y. Zhang, Y. Wang and Z. Sha, Lithium extraction from brine in an ionic selective desalination battery, Desalination, 2020, 481, 114360 CrossRef CAS.
- T. Elmakki, S. Zavahir, U. Hafsa, L. Al-Sulaiti, Z. Ahmad, Y. Chen, H. Park, H. K. Shon, Y. C. Ho and D. S. Han, Novel LiAlO2 material for scalable and facile lithium recovery using electrochemical ion pumping, Nanomaterials, 2023, 13, 895 CrossRef CAS PubMed.
- J. Zhang, Z. Cheng, X. Qin, X. Gao, R. Yun and X. Xiang, Bifunctional modification enhances lithium extraction from brine using a titanium-based ion sieve membrane electrode, ACS Appl. Mater. Interfaces, 2023, 15, 29586–29596 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4im00159a |
| This journal is © Institute of Process Engineering of CAS 2025 |



