Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Amorphous nanostructured Ni–Fe oxide as a notably active and low-cost oxygen evolution reaction electrocatalyst for anion exchange membrane water electrolysis†
Lorenzo Mirizzia,
Mohsin Muhyuddina,
Carmelo Lo Vecchio b,
Erminia Moscab,
Vincenzo Bagliob,
Irene Gattob,
Enrico Berretti
b,
Erminia Moscab,
Vincenzo Bagliob,
Irene Gattob,
Enrico Berretti c,
Alessandro Lavacchic,
Valerio C. A. Ficca
c,
Alessandro Lavacchic,
Valerio C. A. Ficca d,
Rosanna Viscardi
d,
Rosanna Viscardi d,
Roberto Nisticò
d,
Roberto Nisticò *a and
Carlo Santoro
*a and
Carlo Santoro *a
*a
aDepartment of Materials Science, University of Milano-Bicocca, U5, Via R. Cozzi 55, 20125, Milano, Italy. E-mail: roberto.nistico@unimib.it; carlo.santoro@unimib.it
bInstitute for Advanced Energy Technologies “Nicola Giordano” CNR-ITAE, Via Salita S. Lucia sopra Contesse 5, 98126 Messina, Italy
cIstituto di Chimica Dei Composti OrganoMetallici (ICCOM), Consiglio Nazionale Delle Ricerche (CNR), Via Madonna Del Piano 10, 50019 Sesto Fiorentino, Firenze, Italy
dENEA Casaccia Research Center, Via Anguillarese 301, Rome 00123, Italy
First published on 26th March 2025
Abstract
The oxygen evolution reaction (OER) is a critical bottleneck in the commercial evolution of anion exchange membrane water electrolyzers (AEMWEs). As a potential substitute for the scarce and expensive noble metal-based electrocatalysts typically used to improve the OER activity, here amorphous NiFe oxides with varying Ni/Fe ratios were synthesized using a simplistic and cost-effective sol–gel method. After carefully investigating the structural and morphological attributes of the derived electrocatalysts, their OER activities were analyzed by acquiring the half-cell measurements. First, the influence of the electrochemical ink formulation and additives on the activity of the electrocatalyst was studied, followed by elucidating the electrocatalyst loading to configure the working electrode on the rotating disk electrode (RDE). By comparing the activities of different synthesized NiFe oxides, it was observed that Ni0.75Fe0.25O delivers the peak performance with a minimum overpotential of ca. 290 mV. Therefore, the aforementioned sample was utilized to configure the anode electrode for a lab-scale AEMWE, achieving 3.7 A cm−2 at 2 V and 80 °C while demonstrating promising stability trends.
Keywords: NiFe oxide; AEM-WE; Alkaline media; Inorganic oxides; OER; PGM-free electrocatalysts.
1 Introduction
The prime dependence on dwindling fossil fuels is not only engendering severe energy crises but also leading to critical environmental issues that could have adverse repercussions for the next generations. Above all, the release of greenhouse gases and linked climate change are the major concerns of today's world. To alleviate such a pressing scenario, the transition toward cleaner and renewable power sources will ensure global sustainability.1 Green hydrogen, owing to its high energy density (more than two-fold higher than traditional fuels), chemical simplicity, and eco-friendliness, is emerging as a reliable and novel energy vector without any contribution to planetary carbon footprints.2–4 Moreover, due to the huge consumption in a variety of sectors, hydrogen has become a high-demand commodity, exceeding the global market value of 117 billion US dollars.4 So far, the current hydrogen demands, up to 96% of total hydrogen production, still rely on non-renewable and fossil fuel-based routes, which contradicts the ambitions of environmental sustainability.5,6 Nevertheless, renewable (solar, wind, and hydro) energy-driven electrochemical water splitting demonstrates potential to produce extra-pure hydrogen with net zero CO2 emissions;3 yet, the mass-scale deployment of this process is limited by the weaker economic competitiveness and system inefficiencies.5 Among the different water electrolysis technologies, proton exchange membrane water electrolyzers (PEMWEs), with the merits of lower operational temperature, compact design, and pressurized and efficient hydrogen production, can be regarded as reliable development.7,8 The system involves two half-cell reactions: the hydrogen evolution reaction (HER) at the cathode and the oxygen evolution reaction (OER) at the anode. As compared to the HER, the multielectron transfer OER is a thermodynamically complex and kinetically sluggish reaction, and thus it represents the major hindrance to the overall water splitting.9,10 To improve the kinetics of the OER, scarce and overpriced noble metal oxides such as IrO2 and RuO2 are typically used as anode side electrocatalysts in PEMWEs, while an acidic environment requires the utilization of Pt-based electrocatalysts for the HER, making it unfeasible from the economic point of view.2,8 Also, in acidic media, the lower stability of noble metal oxides is known.2 Shifting to the analogous technology of an anion-exchange membrane water electrolyzer (AEMWE) opens the opportunity to use low-cost transition metal-based electrocatalysts for the OER and HER. This medium shift may also rationalize the usage of abundant seawater since chlorine evolution reactions are hindered when the OER in alkaline media has an overpotential below 480 mV.11 Nevertheless, compared to the HER, the OER remains a limiting reaction in the basic media, contributing to greater overpotentials at 10 mA cm−2, a typical performance descriptor. While the development of Pt-free HER electrocatalysts is of great interest,12–14 a sustainable solution for the anodic bottleneck is urgently required. Therefore, devising innovative strategies to fabricate robust and cost-effective OER electrocatalysts is of critical priority in the given research domain to realize water electrolysis.15–20Being a reliable substitute for noble metal-based OER electrocatalysts, NiFe materials, especially oxides, are drawing scientific attention, which is attributed to their higher abundance, low cost, favorable electronic structure, adjustable stoichiometries, and intrinsic electrocatalytic activities.21,22 Nevertheless, there remains a considerable allowance for optimizing their electrocatalytic activity by improving the structural characteristics.23–25 Recently, Shi et al. disclosed an interesting relationship between the crystallographic structure of NiFe oxide and electrocatalytic activities.25 They found that crystalline NiFe oxide facilitates the HER activity, whereas amorphous oxide significantly uplifts the kinetics of the OER. Later, Li and coworkers observed that the amorphousness improves the OER performance in NiFe oxides as the tiny grain boundaries provide additional active sites.26 Recently, Poudel et al. reported NiFe alloy nanoparticles encased in pyridinic-N enriched carbon nanotubes that realized outstanding OER performance.27 Furthermore, Zuber et al. showed that the choice of precursor materials for the synthesis of NiFe oxide also influences the OER activity by giving rise to the evolution of multiple phases. Hence, the experimental route is also an important parameter to control the structure and then the corresponding electrocatalysis.28 In every experimental route, the metallic stoichiometries are one of the main factors controlling the OER performance, and therefore, the Ni to Fe ratio needs to be optimized. Yu et al. experienced peak current densities when the Ni/Fe ratio approached 32/1;29 in contrast, Kumar et al. reported the best performance with a Ni/Fe molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in the cubic NiFe oxides.24 Meanwhile Fominykh et al. claimed the highest OER performance for Fe0.1Ni0.9O.30 Therefore, such discrepancies have to be resolved. Another way to increase the electrocatalytic activity of Ni–Fe materials is to increase defective sites where active species are generated. For example, Kaiser et al. found that the intrinsic Ni defect of a NiOx thin film can improve its electrocatalytic activity.31 Next, Koper's group identified that deprotonation of NiOOH gives rise to proton-deficient surface species, which are responsible for the enhanced OER activity of NiOOH at highly alkaline pH.32 Recently, there has been considerable research interest in improving the characteristics, durability, and performance of the AEM in AEMWEs. While this area remains outside the scope of the present work, the relevant literature may still be of interest to readers.33–36
2 in the cubic NiFe oxides.24 Meanwhile Fominykh et al. claimed the highest OER performance for Fe0.1Ni0.9O.30 Therefore, such discrepancies have to be resolved. Another way to increase the electrocatalytic activity of Ni–Fe materials is to increase defective sites where active species are generated. For example, Kaiser et al. found that the intrinsic Ni defect of a NiOx thin film can improve its electrocatalytic activity.31 Next, Koper's group identified that deprotonation of NiOOH gives rise to proton-deficient surface species, which are responsible for the enhanced OER activity of NiOOH at highly alkaline pH.32 Recently, there has been considerable research interest in improving the characteristics, durability, and performance of the AEM in AEMWEs. While this area remains outside the scope of the present work, the relevant literature may still be of interest to readers.33–36
In any case, a porous architecture is a prerequisite to improve mass transport and ensure the exposure of active moieties. Regarding high surface area systems, aerogels are highly porous, covalent frameworks that are emerging as interesting materials for the production of self-standing electrodes for the OER.37,38 Due to their high porosity, the interactions between the electrocatalyst and electrolyte are enhanced, and thus, the activity of the aerogel-electrocatalysts is improved. Other typical methods to fabricate NiFe oxide may involve evaporation-induced self-assembly, hard templating, electrodeposition, solvothermal methods, and dip coating, each having various advantages and disadvantages.29,30,39,40 For instance, though the hard-templating method might produce performing electrocatalysts, removing silica templates always demands energy-intensive processes and harsh acids.29 However, it should be noted that even if their synthesis is relatively simple, it is generally time-consuming and requires the use of high-pressure and specialized equipment,37 which ultimately challenges the deployment of electrocatalysts for the targeted application. In addition to the synthesis route, the experimental conditions, particularly electrocatalyst loading and electrode configuration, can influence the OER activity.41–43 Nonetheless, at the state of the art, non-uniformity and inconsistency prevail in benchmark measurements and data communications.
As already discussed, the synthesis design, stoichiometries, and physicochemical characteristics of the evolved electrocatalysts dictate the overall OER activity. A comprehensive elucidation of these factors on the progress of NiFe amorphous oxide via a simplistic and controllable fabrication pathway is an important task. In light of the aforementioned considerations, herein, nanostructured amorphous Ni–Fe oxide materials with different Ni/Fe ratios have been synthesized to study the synergic effect of intrinsic Ni and Fe-induced structural defects. An easy and cost-effective sol–gel method was used to obtain oxo/hydroxo Ni–Fe materials that were morphologically, structurally, and physicochemically characterized. Afterward, electrochemical characterization was commenced to optimize the electrocatalyst ink formulation and electrocatalyst loading to configure the electrode. Different formulations involving electrode deposition inks were produced, and the effect of the additives on the electrocatalytic activity was studied by linear sweep voltammetry (LSV) on a rotating disk electrode (RDE). Eventually, with the optimum electrocatalyst loading and ink formulation, the produced electrocatalysts with varying Ni/Fe ratios were compared, and it was found that Ni0.75Fe0.25O is the best-performing electrocatalyst in alkaline media with an overpotential of ca. 290 mV. In addition, laboratory-scale full-cell AEMWE performance and stability tests over 100 hours were conducted.
2 Results and discussion
Based on the simplified sol–gel method, reported in the Materials and methods section, different NiFe oxides were synthesized with varying Ni and Fe ratios. Fig. 1 schematically illustrates the synthesis route, while Table 1 reports the proportion of Ni and Fe, along with the nomenclature of the derived samples.| Sample name | %mol Ni | %mol Fe |
|---|---|---|
| Ni1Fe0O | 100 | 0 |
| Ni0.9975Fe0.0025O | 99.75 | 0.25 |
| Ni0.995Fe0.005O | 99.50 | 0.50 |
| Ni0.98Fe0.02O | 98 | 2 |
| Ni0.95Fe0.05O | 95 | 5 |
| Ni0.90Fe0.1O | 90 | 10 |
| Ni0.75Fe0.25O | 75 | 25 |
| Ni0.5Fe0.5O | 50 | 50 |
| Ni0Fe1O | 0 | 100 |
After the fabrication of the samples, their physicochemical and electrochemical characteristics were thoroughly investigated, which will be discussed in the following sections.
2.1 Physicochemical characterization
Characterization of the as-developed materials was commenced with semi-quantitative compositional analysis via X-ray fluorescence spectroscopy (XRF). The spectra reported in Fig. S1a† show peaks at 6.4 and 7.5 keV that are respectively related to the K12 X-ray emission of Fe and Ni elements. The ratio between the amount of Fe and Ni was estimated considering the ratio between the areas of the peaks that were obtained from the computation of the XRF spectra performed using the instrument software. As reported in Fig. S1b,† the ratio between the two areas increases as the Fe nominal content in the material increases, thus confirming the good outcomes of the synthesis. Subsequently, using X-ray diffraction (XRD) the crystallographic structures of the derived samples were investigated, and the results are reported in Fig. 2. In the absence of Ni, the sample Ni0Fe1O appeared to be typically crystalline, despite being treated at a relatively low temperature, and the observed diffraction pattern was consistent with thermodynamically stable iron oxide phase hematite α-Fe2O3 (card number 01-089-2810, ICDD).44 Quite interestingly, the gradual substitution of Ni led to the amorphization of the system. In the sample containing 50%mol of both iron and nickel (Ni0.50Fe0.50O), the crystallinity tended to be lost, and instead, three broad peaks at ca. 36°, 42° and 62° 2θ emerged which could be attributed to either the formation of a short-range ordered NiO phase (2θ = 6.97°, 43.26° and 62.8°)31 or the formation of nickel ferrite with a spinel-like structure (2θ = 35.7°, 43.4°, and 63.0°, card number 00-054-0964, ICDD).45 However, above 75%mol Ni, just two amorphous humps were visible which were further reduced to just one between ca. 30 and 45° in the sample Ni0.995Fe0.005O and no extra ferritic phases were observed, suggesting the complete dissolution of iron into the amorphous NiO matrix. Shi et al. also witnessed a loss in the crystallinity when the Ni proportion was slightly increased, and the arisen amorphousness contributed to the OER.46 Similarly, Cai et al. reported an amorphous NiFe alloy that showed higher OER activity than the crystalline counterparts, and they concluded that the exposure of the active sites in the amorphous electrocatalyst can be enhanced due to the short-range order, which in turn uplifts the electrocatalytic performance.47Next, the surface chemistry of the derived electrocatalysts was examined using XPS. More detailed surface chemistry characterization of the synthesized Ni/Fe electrocatalysts was conducted using XPS on some selected samples, namely Ni1Fe0O, Ni0.95Fe0.05O, Ni0.75Fe0.25O, Ni0.50Fe0.50O, and Ni0Fe1O. In particular, the analysis of the Ni 2p emission spectra allowed for the characterization of the metallic Ni (not present), Ni2+, and Ni3+ oxidation states, as shown in Fig. 3 Ni2+ is associated with the fitting peaks at 855.1 eV and 872.7 eV, whereas Ni3+ is accountable for the peaks at 856.6 eV and 874.4 eV.48,49 Furthermore, two strong shake-up-type peaks were also detected at 861.2 and 879.7 eV.49,50 The Ni3+/Ni2+ ratio rises, passing from Ni1Fe0O to the Ni0.75Fe0.25O samples, highlighting a more significant contribution of Ni(III) compounds. For Ni0.5Fe0.5O, there is a strong reversal trend, suggesting that the equimolar content of Fe stabilizes a predominant Ni(II) phase. The Fe 2p peaks of Ni0Fe1O and the other Ni/Fe ECs are reported in Fig. S2,† where the Fe 2p3/2 and Fe 2p1/2 XPS peaks are displayed. The Fe 2p3/2 significantly overlaps with the Ni L3M23M45 Auger lines, hindering the quantitative evaluation at higher Ni content. For Ni0.95Fe0.05O, the peaks are not visible due to the low Fe abundance in this compound. Ni0.75Fe0.25O is characterized by an increment of the Fe signals that remained broad, whereas both Ni0.5Fe0.5O and Ni0Fe1O have defined peaks as expected.51,52 The satellite peak of Fe 2p3/2 for Fe2O3 (Ni0Fe1O) is about 8 eV higher than the primary Fe 2p3/2 peak. The current investigation yielded binding energies of Fe 2p3/2 and Fe 2p1/2 of 710.6 and 724.0 eV, respectively, and a satellite peak at 718.6 eV, clearly visible only for Ni0Fe1O. The XPS spectra of O 1s for the Ni0.95Fe0.05O, Ni0.75Fe0.25O, and Ni0.5Fe0.5O samples are reported in Fig. S3,† showing three peaks located at 529.4 eV, 530.9 eV, and 532.7 eV related to the lattice oxygen, the bond seen in metal oxides, hydroxides or defective oxide (M–OH), and the surface-adsorbed oxygen, respectively.53,54 Upon fitting the O 1s spectra, the contribution of the lattice oxygen peak intensifies with increasing Fe content in the NiFeOx species, confirming the transition from an amorphous phase to a reticular species, as revealed by XRD analysis.
 | ||
| Fig. 3 XPS spectra of Ni 2p for (a) Ni1Fe0O, (b) Ni0.95Fe0.05O, (c) Ni0.75Fe0.25O, and (d) Ni0.5Fe0.5O ECs. | ||
The morphology and the composition of the as-produced Ni/Fe electrocatalysts were determined by high-resolution transmission electron microscopy (HR-TEM) coupled with energy-dispersive X-ray detection (EDX). In Fig. 4, the most indicative acquired images are shown. The structure of the Ni-containing materials can all be associated with the base structure of Ni1Fe0O and only small changes can be addressed at high Fe loadings. The morphology of these systems is composed of big and thick particles. Moreover, NiO is peculiarly sensitive to the electron beam, being able to reduce to Ni if irradiated with a sufficiently low dose. It is thus difficult to determine the smaller structure. As expected, diffraction fringes are not detected, confirming their amorphous nature. Analogous results were also obtained for Ni0.5Fe0.5O. Ni0Fe1O results in being composed of nanoparticles with a size ranging from 20 to 150 nm, and the crystal plane reflexes suggest the occurrence of a more crystalline structure, in agreement with the XRD analysis.
 | ||
| Fig. 4 Low and high magnification TEM images of Ni1Fe0O (left), Ni0.5Fe0.5O (middle) and Ni0Fe1O (right). | ||
Scanning TEM images and the relative EDX mapping (Fig. 5) give the presence of iron on/in the NiO particles in a ratio that strictly resembles the theoretical one (Fig. S4†). Moreover, the ratio between Fe and O in Ni0Fe1O is equal to 2.
2.2 Electrochemical characterization
| Compound | Ink 1 | Ink 2 | Ink 3 | Ink 4 |
|---|---|---|---|---|
| ECs | 4 mg | 4 mg | 5 mg | 5 mg |
| Isopropyl alcohol | 1000 μL | 500 μL | 985 μL | 800 μL |
| Water | — | 475 μL | — | 185 μL |
| TritonX-100, 5% solution in water | — | 25 μL | — | — |
| Nafion® solution, 5% in water/ethanol | — | — | 15 μL | — |
| PiperION-A TP-85, 5% in ethanol | — | — | — | 15 μL |
Two electrocatalyst loadings were studied, 0.2 and 0.6 mg cm−2, respectively. In Fig. S5,† the LSVs of Ni1Fe0O and Ni0.95Fe0.05O are reported, whereas in Fig. 6a and b, the overpotentials measured at a current density of 10 mA cm−2 are reported for Ni1Fe0O and Ni0.95Fe0.05O, respectively. In both cases, the ink composition was varied, as reported in Table 2. The rationale behind the selection of different ingredients to fabricate the ink consists of: i) the use of a binder-free ink (ink 1), ii) the use of a surfactant with the intention of better dispersing the electrocatalyst (ink 2), iii) the use of Nafion as a binder (ink 3), and iv) the use of an anion exchange ionomer (AEI) that operates as a binder and an ionomer at the same time (ink 4).
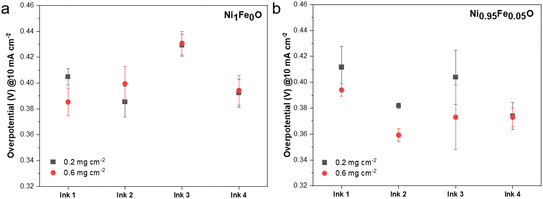 | ||
| Fig. 6 OER overpotentials of (a) Ni1Fe0O and (b) Ni0.95Fe0.05O measured at 10 mA cm−2. The effect of different ink formulations on the OER electrocatalytic activity is reported. | ||
When Ni1Fe0O, simple nickel oxide, was tested, no notable differences were visible in the electrocatalytic activity between the different ink formulations used, except for the Nafion®-containing ink (ink 3), resulting in higher overpotentials (0.43 ± 0.01 V) (Fig. 6a). This behavior could be explained by the fact that Nafion® self-assembles on the electrocatalyst surface, inhibiting the OER and hindering the mass transport of OH− and O2.60,61 Moreover, Nafion operates as a binder, but it does not contribute to the OH− transport. The difference in ink formulation for ink 3 is more evident for the loading comparison of Ni0.95Fe0.05O due to higher electrocatalytic activity, showing an overpotential of 0.40 ± 0.03 V (Fig. 6b). Usually, the ink composed of Nafion is the one that is commonly used in the community.
TritonX-100 is a non-ionic surfactant commonly used as a dispersant for different applications.62 Compared to ink 1, which is only a dispersion of the electrocatalyst in IPA, the addition of TritonX-100 (ink 2) permits enhancing the deposition, resulting in lower overpotential values and lower variability, especially for Ni0.95Fe0.05O at a higher loading of 0.6 mg cm−2 (Fig. 6b).
Interestingly, the substitution of Nafion® (ink 3) with PiperION-A TP-85 (ink 4), an anion exchange ionomer (AEI) acting as both an OH− exchange ionomer and a binder, showed improved performance for both electrocatalysts and both loadings as reported in Fig. 6a and b.
Interestingly, the use of ink 4 containing PiperION-A TP-85 did not show variation in the overpotentials despite the increase of electrocatalyst loading deposited on the surface of the electrode and the two different electrocatalysts investigated (Fig. 6a and b).
 | ||
| Fig. 7 Overpotentials of (a) Ni1Fe0O and (b) Ni0.95Fe0.05O measured at 10 mA cm−2. The effect of different electrocatalyst loadings on the OER electrocatalytic activity is reported. | ||
The results showed that Ni1Fe0O performed differently from Ni0.95Fe0.05O; in fact, when no Fe is present in the electrocatalyst, the increase of the loading seems not to affect the overpotentials (Fig. 7a). This might be due to the organization of the EC on the electrode surface. For Ni0.95Fe0.05O, instead, the overpotential decreases while increasing the EC loading on the electrode surface (Fig. 7b). This was visible while increasing the loading from 0.1 to 0.6 mg cm−2, and then a plateau value was reached. The lowest overpotential value is obtained starting from 0.6 mg cm−2 with a value of ca. 0.359 V.
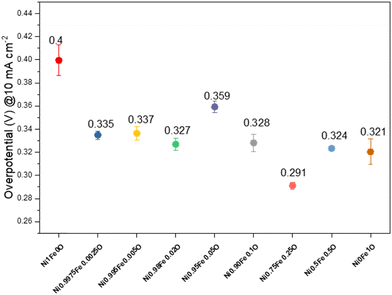
The optimized conditions in terms of both ink and EC loading were used to perform LSV for all the produced ECs. LSVs are reported in Fig. S8,† whereas the overpotentials at 10 mA cm−2 are reported in Fig. 8. The overpotentials during the OER measured at 50 mA cm−2 and 100 mA cm−2 are reported in Fig. S9.† The electrocatalytic activities toward the OER of the synthesized ECs are strongly dependent on the amount of Fe. The Fe introduction in the NiO structure, even at a low amount (0.25%mol), led to the reduction of the OER overpotential compared to pure NiO. The lowest overpotential of 0.291 V was obtained when 25%mol of iron was substituted in the material (sample Ni0.75Fe0.25O), whereas further increments in the Fe proportion in the material led to a slight increase of the overpotential reaching the Ni0Fe1O overpotential, which contained only α-Fe2O3 (Fig. 8).
2.3 Anion exchange membrane water electrolyzer integration and results
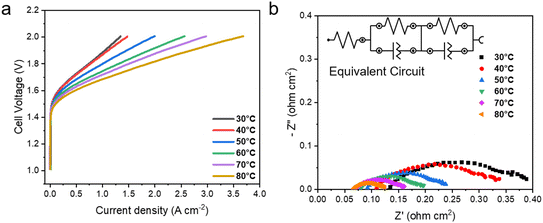
Considering the simplified synthesis methodology that led to the promising OER activity demonstrated in the half-cell tests, Ni0.75Fe0.25O was tested at the anode side of a 5 cm2 AEMWE full electrolysis cell, using a PiperION membrane and an ionomer solution on both anode and cathode inks. To the best of our knowledge, this is the first time that an amorphous structure of the NiFeOx anode electrocatalyst, prepared using a simple sol–gel method, has been integrated and studied in an AEMWE. Regarding the scalability of the system, the lower temperature required to form the amorphous phase, as opposed to the crystalline structure, could be advantageous for energy savings throughout the entire process. Polarization curves in the 30–80 °C temperature range were acquired to assess the impact of temperature on the cell performance. The results in Fig. 9a demonstrate how the raising of the temperature causes an increase in the performance. The higher performance was achieved at 80 °C where, at 2.0 V, a current density of 3.7 A cm−2 was reached, while at a lower voltage of 1.8 V, more than 2 A cm−2 was delivered. Moreover, it is feasible to observe that a 10 °C rise in cell temperature results in about 0.5 A cm−2 improvement in current density. This effect could be attributed to the enhanced ionic conductivity of the anionic membrane, as well as the improved kinetics of the processes taking place at the electrodes, as demonstrated by the Nyquist plots recorded at 1.8 V, which show a decrease both in the series (Rs) and charge transfer resistance (Rct) (Fig. 9b and Table 3). A direct comparison of these results with the current literature is not straightforward, as different researchers often use varying AEMs, supporting electrolyte concentrations, electrocatalyst compositions, and loadings. However, the results, displayed in Table 4, appear consistent when compared with recent studies in AEMWEs based on non-precious anode electrocatalysts.| Temperature | Rs (ohm cm2) | Rct (ohm cm2) |
|---|---|---|
| 30 °C | 0.131 | 0.284 |
| 40 °C | 0.105 | 0.260 |
| 50 °C | 0.087 | 0.167 |
| 60 °C | 0.074 | 0.128 |
| 70 °C | 0.066 | 0.094 |
| 80 °C | 0.060 | 0.064 |
| MEA | Anode loading (mg cm−2) | Current density (A cm−2) @1.8 V | Current density (A cm−2) @2.0 V | Ref. |
|---|---|---|---|---|
| Ni0.75Fe0.25O/Ni-felt//PiperION//Pt/C | 2.0 | 1.4 | 2.6 | This work |
| Ni-foam//PiperION//Pt/C | — | 0.30 | 0.62 | 63 |
| Ni-foam//PBP//Pt/C | — | 0.56 | 1.25 | 63 |
| NiFe2O4//FAA3-50//Pt/C | 3.0 | 1.5 | 2.5 | 64 |
| g-CN-CNF-800//FAA3-50//Pt/C | 4.0 | 0.48 | 0.98 | 65 |
| NiFe2O4//Sustainion//NiFeCo | 2.0 | 0.16 | 0.94 | 66 |
| NiFe2O4//FAS-50//NiFeCo | 2.0 | 0.25 | 0.90 | 66 |
| NiFe2O4//Sustainion X37-50//Ni RANEY® | 1.8 | 0.74 | — | 67 |
| NiFe2O4//Sustainion//NiFeCo | — | 0.25 | 0.90 | 68 |
| NiFe//PFTP-13//NiFe | 20.0 | 0.60 | 1.2 | 69 |
| Ni-felt//Fumion Recast//Pt/C | — | 0.74 | 1.3 | 70 |
A short-term (ca. 100 h) durability test under galvanostatic operating conditions (1 A cm−2) was performed, and the obtained chronopotentiometric curve is reported in Fig. 10a. The curve, after a slight increase in initial potential, shows a constant behavior over time. After replacing the KOH solution with a fresh one, a decrease in voltage was observed, but subsequently, the potential increased to reach the same value recorded before the replacement. Thus, the synthesized electrocatalyst demonstrated not only high performance but also exceptional stability. J–V plots (Fig. 10b) after this short-term durability test (end of test, EOT) showed even higher performance compared with the beginning of the test (BOT), clearly attributable to the electrocatalyst activation during the time.
3 Conclusions
Amorphous NiFe oxides were synthesized using a simple and cost-effective sol–gel method for oxygen evolution reaction (OER) applications in alkaline media. Various oxide variants were prepared by altering the Ni/Fe ratios. The material properties were characterized, and their electrocatalytic activities were evaluated as a function of ink formulations and electrocatalyst loading. Under optimized conditions, the OER performances of the developed NiFe oxides were analyzed using the rotating disk electrode (RDE) methodology. Among the variants, Ni0.75Fe0.25O exhibited the best performance, achieving a low overpotential of 0.291 V. This superior performance is attributed to a higher concentration of Ni3+ (NiOOH), a highly active species for the OER. Furthermore, the same amorphous sample was integrated for the first time, to our knowledge, in an anion exchange membrane water electrolyzer (AEMWE), demonstrating promising performance and good operational durability on a lab scale.4 Materials and methods
4.1 Materials
All chemicals were used without further purification. Nickel(II) nitrate hexahydrate (Ni(NO3)2·6H2O, >98.5%, 13478-00-7), potassium hydroxide (KOH, 85%, 1310-58-3), citric acid (C6H8O7, >99.5%, 77-92-9) and Triton X-100 were purchased from Sigma Aldrich. Iron(II) chloride tetrahydrate (FeCl3·4H2O, 98%, 13478-10-9) and Nafion® 5%wt dispersion in water/ethanol were purchased from Alfa Aesar. PiperION-A TP-85 5%wt dispersion in ethanol was purchased from Versogen. Milli-Q water with a resistivity of 18.2 MΩ cm was used. 40 wt% platinum on carbon (Pt/C, Alfa Aesar) was also used for comparison.4.2 Synthesis of Ni/Fe mixed oxides
The synthesis of Ni/Fe mixed oxide nanoparticles (NPs) was prepared following a modified procedure taken from Danial et al.71 In a typical synthesis, 0.01 mol of salt precursors (i.e., nickel(II) nitrate hexahydrate and iron(III) chloride hexahydrate, in different ratios) were dissolved in 50 mL of deionized water. The Ni/Fe molar ratios are 100/0, 99.75/0.25, 99.50/0.50, 98/2, 95/5, 90/10, 75/25, 50/50, and 0/100. This solution was then dripped in a previously prepared solution containing 2.1 g of citric acid and 50 mL of deionized water under magnetic stirring. The mixed solution was then heated from RT to 70 °C and maintained under isothermal conditions for 12 h. Subsequently, the solution was transferred to a Petri dish, and the solvent was allowed to completely evaporate at RT until a gel was formed. The gel was then dried and aged under an air atmosphere and isothermal conditions at 110 °C for 24 h. The obtained powder was then ground, placed in an alumina boat, and heated in a muffle furnace from RT to 250 °C (heating rate speed of 10 °C min−1), and then maintained under isothermal conditions for 4 h.4.3 Material characterization
4.4 Electrochemical measurements using a rotating disk electrode (RDE)
Electrochemical measurements were carried out in a standard three-electrode glass cell (Pine) with a glassy carbon rotating disk electrode (Ø 5 mm, area of 0.1963 cm2) as the working electrode, a titanium spring as the counter electrode, and an Ag/AgCl/Cl−(sat) electrode as the reference electrode. The potentials were then converted to a reversible hydrogen electrode (RHE) using the formula:where
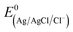 = 0.1976 V.
= 0.1976 V.
Linear sweep voltammograms (LSV) were performed in 1 M KOH solution from 1.2 to 1.9 V vs. RHE at 5 mV s−1, and the rotation of the electrode was set to 1600 rpm. Before starting the measurements, the solution was deaerated by fluxing nitrogen; a smaller flux was also present during the measurement to help the produced gas escape the solution.
4.5 Anion exchange membrane water electrolysis test (AEMWE)
The membrane-electrode assembly (MEA), with a geometrical active area of 5 cm2, was realized by a cold assembling procedure. The anode ink, based on the Ni0.75Fe0.25O EC and 20 wt% of a PiperION® ionomer, was applied by the spray coating technique directly onto the commercial PiperION® membrane (thickness 40 μm) surface to realize a one-side electrocatalyst coated membrane (CCM). The electrocatalyst loading was maintained at 2.0 ± 0.1 mg cm−2. Afterward, Ni felt (Bekaert), acting as a backing layer and current collector, was coupled to the anodic compartment. The cathode electrode was made by mixing commercial 40 wt% platinum on carbon (Pt/C, Alfa Aesar) and 20 wt% of a PiperION® ionomer. The ink was sprayed onto a Sigracet 25-BC (SGL group) gas diffusion layer (GDL) to obtain a catalyst-coated electrode (CCE), with a Pt loading of 0.5 ± 0.05 mg cm−2, as reported elsewhere.72 Before the MEA realization, the CCE and CCM were exchanged in a 1 M KOH aqueous solution for 1 h to convert the bicarbonate counter ions present in the pristine membrane and ionomer in the active hydroxyl species. The electrochemical characterization, in a 5 cm2 single-cell configuration, was carried out in a temperature range of 30–80 °C, at atmospheric pressure. As depicted in Fig. S10,† the hardware setup for AEMWE tests comprises, from the external to internal part, a gold plate, a Ni plate with a gasket and flow field (anode), the Ni felt, the MEA containing the CCM and CCE, another gasket on a graphite plate (cathode) and another gold plate for the closure frame. An alkaline aqueous solution (1 M KOH) was supplied by a peristaltic pump to the anode side of the single cell, with a flow rate of 5 mL min−1. The electrochemical measurements were carried out by using a potentiostat–galvanostat device PGSTAT302 equipped with an FRA module (Autolab). The electrochemical impedance spectroscopy (EIS) measurements were performed under potentiostatic control (at a cell voltage of 1.8 V) in a frequency range between 10 kHz and 100 mHz by frequency sweeping in the single sine mode. The amplitude of the sinusoidal excitation signal was 0.01 V r.m.s.A short stability test was carried out by chronopotentiometric analysis, maintaining the current at 1 A cm−2 for 100 h.
Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
C. S. and M. M. would like to acknowledge the NextGeneration EU from the Italian Ministry of Environment and Energy Security POR H2 AdP MMES/ENEA with involvement of CNR and RSE, PNRR – Mission 2, Component 2, Investment 3.5 “Ricerca e sviluppo sull'idrogeno” under the ENEA – UNIMIB agreement (Procedure 1.1.3 PNRR POR H2). C. S. and M. M. would like to acknowledge the National Recovery and Resilience Plan (PNRR), Mission 2 “Green Revolution and Ecological Transition”, Component 2 “Renewable Energy, Hydrogen, Network and Sustainable Mobility”, Investment 3.5 “Hydrogen Research and Development”, European Union – Next Generation EU – Italian Ministry of Environment and Energy Security (MASE), project AMBITION. E. B. wants to acknowledge the Circular and Sustainable Made in Italy Extended Partnership (MICS) funded by the European Union Next-Generation EU (Piano Nazionale di Ripresa e Resilienza (PNRR) – Mission 4, Component 2, Investment 1.3 – D.D. 1551.11-10-2022, PE00000004) for financial support.References
- Q. Hassan, S. Algburi, A. Z. Sameen, T. J. Al-Musawi, A. K. Al-Jiboory, H. M. Salman, B. M. Ali and M. Jaszczur, A comprehensive review of international renewable energy growth, Energy Built Environ., 2024 DOI:10.1016/j.enbenv.2023.12.002.
- A. Munir, J. Abdul Nasir, T. U. Haq, J. Iqbal, I. Hussain and A. Qurashi, Benchmarking stable electrocatalysts for green hydrogen production: A chemist perspective, Coord. Chem. Rev., 2024, 521, 216112 CrossRef.
- J. Chi and H. Yu, Water electrolysis based on renewable energy for hydrogen production, Chin. J. Catal., 2018, 39, 390–394 CrossRef.
- N. Du, C. Roy, R. Peach, M. Turnbull, S. Thiele and C. Bock, Anion-exchange membrane water electrolyzers, Chem. Rev., 2022, 122, 11830–11895 CrossRef.
- S. Kumar and V. Himabindu, Hydrogen production by PEM water electrolysis – A review, Mater. Sci. Energy Technol., 2019, 2, 442–454 Search PubMed.
- P. Hota, A. Das and D. K. Maiti, A short review on generation of green fuel hydrogen through water splitting, Int. J. Hydrogen Energy, 2023, 48, 523–541 CrossRef.
- T. Wang, X. Cao and L. Jiao, PEM water electrolysis for hydrogen production: Fundamentals, advances, and prospects, Carbon Neutrality, 2022, 1, 21 CrossRef.
- M. Carmo, D. L. Fritz, J. Mergel and D. Stolten, A comprehensive review on PEM water electrolysis, Int. J. Hydrogen Energy, 2013, 38, 4901–4934 CrossRef.
- L. Gao, X. Cui, C. D. Sewell, J. Li and Z. Lin, Recent advances in activating surface reconstruction for the high-efficiency oxygen evolution reaction, Chem. Soc. Rev., 2021, 50, 8428–8469 RSC.
- J. Song, C. Wei, Z. F. Huang, C. Liu, L. Zeng, X. Wang and Z. J. Xu, A review on fundamentals for designing oxygen evolution electrocatalysts, Chem. Soc. Rev., 2020, 49, 2196–2214 RSC.
- W. Tong, M. Forster, F. Dionigi, S. Dresp, R. Sadeghi Erami, P. Strasser, A. J. Cowan and P. Farràs, Electrolysis of low-grade and saline surface water, Nat. Energy, 2020, 5, 367–377 CrossRef CAS.
- F. Wang, L. Xiao, Y. Jiang, X. Liu, X. Zhao, Q. Kong, A. Abdukayum and G. Hu, Recent achievements in noble metal-based oxide electrocatalysts for water splitting, Mater. Horiz., 2025, 12, 1757–1795 RSC.
- S. A. Mirshokraee, M. Muhyuddin, J. Orsilli, E. Berretti, L. Capozzoli, A. Lavacchi, C. L. Vecchio, V. Baglio, A. Galli, A. Zaffora, F. D. Franco, M. Santamaria, L. Olivi, S. Pollastri and C. Santoro, Mono-, bi- and tri-metallic platinum group metal-free electrocatalysts for hydrogen evolution reaction following a facile synthetic route, Ind. Chem. Mater., 2023, 1, 343–359 RSC.
- S. A. Mirshokraee, M. Muhyuddin, N. Pianta, E. Berretti, L. Capozzoli, J. Orsilli, F. D'Acapito, R. Viscardi, A. Cosenza, P. Atanassov, C. Santoro and A. Lavacchi, Ni-phthalocyanine derived electrocatalysts for oxygen reduction reaction and hydrogen evolution reaction: Active sites formation and electrocatalytic activity, ACS Catal., 2024, 14, 14524–14538 CrossRef.
- Z.-P. Wu, X. F. Lu, S.-Q. Zang and X. W. Lou, Non-noble-metal-based electrocatalysts toward the oxygen evolution reaction, Adv. Funct. Mater., 2020, 30, 1910274 CrossRef.
- J. Zhao, J.-J. Zhang, Z.-Y. Li and X.-H. Bu, Recent progress on NiFe-based electrocatalysts for the oxygen evolution reaction, Small, 2020, 16, 2003916 CrossRef.
- L. Gong, H. Yang, A. I. Douka, Y. Yan and B. Y. Xia, Recent progress on NiFe-based electrocatalysts for alkaline oxygen evolution, Adv. Sustainable Syst., 2021, 5, 2000136 CrossRef.
- N. Yuan, Q. Jiang, J. Li and J. Tang, A review on non-noble metal based electrocatalysis for the oxygen evolution reaction, Arabian J. Chem., 2020, 13, 4294–4309 CrossRef.
- M. B. Poudel, M. P. Balanay, P. C. Lohani, K. Sekar and D. J. Yoo, Atomic engineering of 3D self-supported bifunctional oxygen electrodes for rechargeable zinc-air batteries and fuel cell applications, Adv. Energy Mater., 2024, 14, 2400347 CrossRef CAS.
- S. Ramakrishnan, J. Balamurugan, M. Vinothkannan, A. R. Kim, S. Sengodan and D. J. Yoo, Nitrogen-doped graphene encapsulated FeCoMoS nanoparticles as advanced trifunctional catalyst for water splitting devices and zinc–air batteries, Appl. Catal., B, 2020, 279, 119381 CrossRef CAS.
- Y. Han, J. Wang, Y. Liu, T. Li, T. Wang, X. Li, X. Ye, G. Li, J. Li, W. Hu and Y. Deng, Stability challenges and opportunities of NiFe-based electrocatalysts for oxygen evolution reaction in alkaline media, Carbon Neutralization, 2024, 3, 172–198 CrossRef CAS.
- Q. Kang, D. Lai, W. Tang, Q. Lu and F. Gao, Intrinsic activity modulation and structural design of NiFe alloy catalysts for an efficient oxygen evolution reaction, Chem. Sci., 2021, 12, 3818–3835 RSC.
- A. Kleiman-Shwarsctein, Y. S. Hu, G. D. Stucky and E. W. McFarland, NiFe-oxide electrocatalysts for the oxygen evolution reaction on Ti doped hematite photoelectrodes, Electrochem. Commun., 2009, 11, 1150–1153 CrossRef CAS.
- A. Kumar and S. Bhattacharyya, Porous NiFe-oxide nanocubes as bifunctional electrocatalysts for efficient water-splitting, ACS Appl. Mater. Interfaces, 2017, 9, 41906–41915 CrossRef CAS.
- G. Shi, C. Arata, D. A. Tryk, T. Tano, M. Yamaguchi, A. Iiyama, M. Uchida, K. Iida, S. Watanabe and K. Kakinuma, NiFe alloy integrated with amorphous/crystalline NiFe oxide as an electrocatalyst for alkaline hydrogen and oxygen evolution reactions, ACS Omega, 2023, 8, 13068–13077 CrossRef CAS.
- X. Li, H. Zhang, Q. Hu, W. Zhou, J. Shao, X. Jiang, C. Feng, H. Yang and C. He, Amorphous NiFe oxide-based nanoreactors for efficient electrocatalytic water oxidation, Angew. Chem., Int. Ed., 2023, 62, e202300478 CrossRef CAS.
- M. B. Poudel, S. Vijayapradeep, K. Sekar, J. S. Kim and D. J. Yoo, Pyridinic-N exclusively enriched CNT-encapsulated NiFe interfacial alloy nanoparticles on knitted carbon fiber cloth as bifunctional oxygen catalysts for biaxially flexible zinc–air batteries, J. Mater. Chem. A, 2024, 12, 10185–10195 RSC.
- A. Zuber, I. M. Oikonomou, L. Gannon, I. Chunin, L. Reith, B. Can, M. Lounasvuori, T. Schultz, N. Koch, C. McGuinness, P. W. Menezes, V. Nicolosi and M. P. Browne, Effect of the precursor metal salt on the oxygen evolution reaction for NiFe oxide materials, ChemElectroChem, 2024, 11, e202400151 CrossRef CAS.
- M. Yu, G. Moon, E. Bill and H. Tüysüz, Optimizing Ni–Fe oxide electrocatalysts for oxygen evolution reaction by using hard templating as a toolbox, ACS Appl. Energy Mater., 2019, 2, 1199–1209 CrossRef CAS.
- K. Fominykh, P. Chernev, I. Zaharieva, J. Sicklinger, G. Stefanic, M. Döblinger, A. Müller, A. Pokharel, S. Böcklein, C. Scheu, T. Bein and D. Fattakhova-Rohlfing, Iron-doped nickel oxide nanocrystals as highly efficient electrocatalysts for alkaline water splitting, ACS Nano, 2015, 9, 5180–5188 CrossRef CAS PubMed.
- H. Radinger, P. Connor, S. Tengeler, R. W. Stark, W. Jaegermann and B. Kaiser, Importance of nickel oxide lattice defects for efficient oxygen evolution reaction, Chem. Mater., 2021, 33, 8259–8266 CrossRef CAS.
- O. Diaz-Morales, D. Ferrus-Suspedra and M. T. M. Koper, The importance of nickel oxyhydroxide deprotonation on its activity towards electrochemical water oxidation, Chem. Sci., 2016, 7, 2639–2645 RSC.
- B. H. Oh, A. R. Kim and D. J. Yoo, Profile of extended chemical stability and mechanical integrity and high hydroxide ion conductivity of poly(ether imide) based membranes for anion exchange membrane fuel cells, Int. J. Hydrogen Energy, 2019, 44, 4281–4292 CrossRef CAS.
- A. Sahul Hameed, S. Munusamy, R. Gokulapriyan and D. J. Yoo, Comprehensive review of anion exchange membrane with ether and ether-free backbone, ACS Appl. Polym. Mater., 2024, 6, 12341–12361 CrossRef CAS.
- G. H. A. Wijaya, K. S. Im and S. Y. Nam, Advancements in commercial anion exchange membranes: A review of membrane properties in water electrolysis applications, Desalin. Water Treat., 2024, 320, 100605 CrossRef CAS.
- D. Hua, J. Huang, E. Fabbri, M. Rafique and B. Song, Development of anion exchange membrane water electrolysis and the associated challenges: A review, ChemElectroChem, 2023, 10, e202200999 CrossRef CAS.
- L. Osmieri, H. Yu, R. P. Hermann, M. E. Kreider, H. M. Meyer, A. J. Kropf, J. H. Park, S. M. Alia, D. A. Cullen, D. J. Myers and P. Zelenay, Aerogel-derived nickel-iron oxide catalysts for oxygen evolution reaction in alkaline media, Appl. Catal., B, 2024, 348, 123843 CrossRef CAS.
- W. Moschkowitsch, N. Zion, H. C. Honig, N. Levy, D. A. Cullen and L. Elbaz, Mixed-metal nickel–iron oxide aerogels for oxygen evolution reaction, ACS Catal., 2022, 12, 12162–12169 CrossRef CAS.
- M. W. Louie and A. T. Bell, An investigation of thin-film Ni–Fe oxide catalysts for the electrochemical evolution of oxygen, J. Am. Chem. Soc., 2013, 135, 12329–12337 CrossRef CAS PubMed.
- J. Landon, E. Demeter, N. İnoğlu, C. Keturakis, I. E. Wachs, R. Vasić, A. I. Frenkel and J. R. Kitchin, Spectroscopic characterization of mixed Fe–Ni oxide electrocatalysts for the oxygen evolution reaction in alkaline electrolytes, ACS Catal., 2012, 2, 1793–1801 CrossRef CAS.
- C. Dong, T. Kou, H. Gao, Z. Peng and Z. Zhang, Eutectic-derived mesoporous Ni-Fe-O nanowire network catalyzing oxygen evolution and overall water splitting, Adv. Energy Mater., 2018, 8, 1701347 CrossRef CAS.
- W. Jiang, W. Lehnert and M. Shviro, The influence of loadings and substrates on the performance of nickel-based catalysts for the oxygen evolution reaction, ChemElectroChem, 2023, 10, e202200991 CrossRef CAS.
- F. Malaj, A. Tampucci, D. Lentini, L. Brogi, E. Berretti, C. Coletti, S. Forti, A. Rossi and C. Santoro, One-pot synthesis of FeNi3/FeNiOx nanoparticles for PGM-free anion exchange membrane water electrolysis, Electrochim. Acta, 2024, 507, 145109 CrossRef CAS.
- G. Bona, G. Bragaggia, M. Cantoni, B. Di Credico, S. Mostoni, G. Capitani, R. Scotti, S. Gross and R. Nisticò, Polyethylene glycol-assisted hydro-solvothermal growth of anisotropic magnetic iron oxides: The role of mixed environment conditions, Colloids Surf., A, 2024, 702, 135117 CrossRef CAS.
- R. Nisticò, R. Mantovan, M. Cantoni, C. Rinaldi, M. Malandrino, S. Mostoni, M. D'Arienzo, B. Di Credico and R. Scotti, Hydrothermal synthesis of Cu-substituted Ni ferrites: Structural, morphological, and magnetic properties, J. Alloys Compd., 2024, 981, 173628 CrossRef.
- G. Shi, C. Arata, D. A. Tryk, T. Tano, M. Yamaguchi, A. Iiyama, M. Uchida, K. Iida, S. Watanabe and K. Kakinuma, NiFe alloy integrated with amorphous/crystalline NiFe oxide as an electrocatalyst for alkaline hydrogen and oxygen evolution reactions, ACS Omega, 2023, 8, 13068–13077 CrossRef CAS.
- W. Cai, R. Chen, H. Yang, H. B. Tao, H.-Y. Wang, J. Gao, W. Liu, S. Liu, S.-F. Hung and B. Liu, Amorphous versus crystalline in water oxidation catalysis: A case study of NiFe alloy, Nano Lett., 2020, 20, 4278–4285 CrossRef CAS.
- D. Xiong, W. Li and L. Liu, Vertically aligned porous nickel(II) hydroxide nanosheets supported on carbon paper with long-term oxygen evolution performance, Chem. – Asian J., 2017, 12, 543–551 CrossRef CAS PubMed.
- M. Cheng, H. Fan, Y. Song, Y. Cui and R. Wang, Interconnected hierarchical NiCo2O4 microspheres as high-performance electrode materials for supercapacitors, Dalton Trans., 2017, 46, 9201–9209 RSC.
- L. Liu, H. Zhang, L. Fang, Y. Mu and Y. Wang, Facile preparation of novel dandelion-like Fe-doped NiCo2O4 microspheres@nanomeshes for excellent capacitive property in asymmetric supercapacitors, J. Power Sources, 2016, 327, 135–144 CrossRef CAS.
- M. C. Biesinger, B. P. Payne, A. P. Grosvenor, L. W. M. Lau, A. R. Gerson, R. St and C. Smart, Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni, Appl. Surf. Sci., 2011, 257, 2717–2730 CrossRef CAS.
- T. Yamashita and P. Hayes, Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials, Appl. Surf. Sci., 2008, 254, 2441–2449 CrossRef CAS.
- A. Caprì, I. Gatto, C. L. Vecchio and V. Baglio, Effect of the calcination temperature on the characteristics of Ni/Fe-oxide electrocatalysts for application in anion exchange membrane electrolysers, Ind. Chem. Mater., 2023, 1, 553–562 RSC.
- A. Caprì, I. Gatto, C. Lo Vecchio, S. Trocino, A. Carbone and V. Baglio, Anion exchange membrane water electrolysis based on nickel ferrite catalysts, ChemElectroChem, 2023, 10, e202201056 CrossRef.
- W. Moschkowitsch, N. Zion, H. C. Honig, N. Levy, D. A. Cullen and L. Elbaz, Mixed-metal nickel–iron oxide aerogels for oxygen evolution reaction, ACS Catal., 2022, 12, 12162–12169 CrossRef.
- S. S. Kocha, J. W. Zack, S. M. Alia, K. C. Neyerlin and B. S. Pivovar, Influence of ink composition on the electrochemical properties of Pt/C electrocatalysts, ECS Trans., 2013, 50, 1475 CrossRef.
- L. Chen, Q. Xu, S. Z. Oener, K. Fabrizio and S. W. Boettcher, Design principles for water dissociation catalysts in high-performance bipolar membranes, Nat. Commun., 2022, 13, 3846 CrossRef CAS PubMed.
- M. Muhyuddin, S. Mostoni, H. C. Honig, L. Mirizzi, L. Elbaz, R. Scotti, M. D'Arienzo and C. Santoro, Enhancing electrocatalysis: Engineering the Fe–Nx–C electrocatalyst for oxygen reduction reaction using Fe-functionalized silica hard templates, ACS Appl. Energy Mater., 2024, 7, 11691–11702 CrossRef CAS.
- N. Giulini, M. Muhyuddin, S. Mattiello, M. Sassi, C. Lo Vecchio, V. Baglio, E. Berretti, A. Lavacchi, E. Fazio, L. Beverina and C. Santoro, Repurposing discarded porphyrin waste as electrocatalysts for the oxygen reduction reaction, Electrochim. Acta, 2024, 507, 145113 CrossRef CAS.
- J. Chlistunoff and J.-M. Sansiñena, On the use of Nafion® in electrochemical studies of carbon supported oxygen reduction catalysts in aqueous media, J. Electroanal. Chem., 2016, 780, 134–146 CrossRef CAS.
- G.-F. Li, D. Yang and P.-Y. Abel Chuang, Defining Nafion ionomer roles for enhancing alkaline oxygen evolution electrocatalysis, ACS Catal., 2018, 8, 11688–11698 CrossRef CAS.
- T. Li, T. Chen, X. Shen, H. H. Shi, E. Jabari and H. E. Naguib, A binder jet 3D printed MXene composite for strain sensing and energy storage application, Nanoscale Adv., 2022, 4, 916–925 RSC.
- T. Caielli, A. R. Ferrari, S. Bonizzoni, E. Sediva, A. Caprì, M. Santoro, I. Gatto, V. Baglio and P. Mustarelli, Synthesis, characterization and water electrolyzer cell tests of poly(biphenyl piperidinium) anion exchange membranes, J. Power Sources, 2023, 557, 232532 CrossRef CAS.
- A. Caprì, I. Gatto, C. Lo Vecchio, S. Trocino, A. Carbone and V. Baglio, Anion exchange membrane water electrolysis based on nickel ferrite catalysts, ChemElectroChem, 2023, 10, e202201056 CrossRef.
- J. E. Park, M.-J. Kim, M. S. Lim, S. Y. Kang, J. K. Kim, S.-H. Oh, M. Her, Y.-H. Cho and Y.-E. Sung, Graphitic carbon nitride-carbon nanofiber as oxygen catalyst in anion-exchange membrane water electrolyzer and rechargeable metal–air cells, Appl. Catal., B, 2018, 237, 140–148 CrossRef CAS.
- Z. Liu, S. D. Sajjad, Y. Gao, H. Yang, J. J. Kaczur and R. I. Masel, The effect of membrane on an alkaline water electrolyzer, Int. J. Hydrogen Energy, 2017, 42, 29661–29665 CrossRef CAS.
- B. Motealleh, Z. Liu, R. I. Masel, J. P. Sculley, Z. Richard Ni and L. Meroueh, Next-generation anion exchange membrane water electrolyzers operating for commercially relevant lifetimes, Int. J. Hydrogen Energy, 2021, 46, 3379–3386 CrossRef.
- I. V. Pushkareva, A. S. Pushkarev, S. A. Grigoriev, P. Modisha and D. G. Bessarabov, Comparative study of anion exchange membranes for low-cost water electrolysis, Int. J. Hydrogen Energy, 2020, 45, 26070–26079 CrossRef.
- N. Chen, S. Y. Paek, J. Y. Lee, J. H. Park, S. Y. Lee and Y. M. Lee, High-performance anion exchange membrane water electrolyzers with a current density of 7.68 A cm−2 and a durability of 1000 hours, Energy Environ. Sci., 2021, 14, 6338–6348 RSC.
- N. Carboni, L. Mazzapioda, A. Caprì, I. Gatto, A. Carbone, V. Baglio and M. A. Navarra, Composite anion exchange membranes based on graphene oxide for water electrolyzer applications, Electrochim. Acta, 2024, 486, 144090 CrossRef.
- A. Danial, M. Saleh, S. A. Salih and M. I. Awad, On the synthesis of nickel oxide nanoparticles by sol–gel technique and its electrocatalytic oxidation of glucose, J. Power Sources, 2015, 293, 101–108 CrossRef.
- A. Caprì, A. Martínez-Lázaro, J. Béjar, I. Gatto, L. Álvarez-Contreras, M. P. Gurrola, J. Ledesma-García, V. Baglio and L. G. Arriaga, Three-dimensionally ordered macroporous trimetallic spinel for anion exchange membrane water electrolysis, Electrochim. Acta, 2023, 463, 142851 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5im00008d |
| This journal is © Institute of Process Engineering of CAS 2025 |