Open Access Article
Open Access ArticleRecent advances in chemical composition imaging operation based on laser-induced breakdown spectroscopy
Shangyong Zhao a,
Yuchen Zhaob,
Yujia Daia,
Ziyuan Liu
a,
Yuchen Zhaob,
Yujia Daia,
Ziyuan Liu a,
Zongyu Hou
a,
Zongyu Hou c,
Xun Gao
c,
Xun Gao d and
Zhe Wang
d and
Zhe Wang *c
*c
aZhejiang A & F University, College of Opto-Electro-Mechanical Engineering, Hangzhou, 311300, P. R. China
bNottingham University Business School China, University of Nottingham Ningbo China, Ningbo, 315100, P. R. China
cState Key Laboratory of Power System Operation and Control, Tsinghua-Rio Tinto Joint Research Centre for Resources, Energy and Sustainable Development, International Joint Laboratory on Low Carbon Clean Energy Innovation, Institute for Carbon Neutrality, Department of Energy and Power Engineering, Tsinghua University, Beijing 100084, P. R. China. E-mail: zhewang@tsinghua.edu.cn
dSchool of Science, Ministry of Education Key Laboratory for Cross-Scale Micro and Nano Manufacturing, Changchun University of Science and Technology, Jilin, 130022, P. R. China
First published on 22nd January 2025
Abstract
With the rapid development of laser-induced breakdown spectroscopy (LIBS) imaging technology, it has attracted widespread attention. A LIBS imaging system based on laser ablation generates two- and three-dimensional (2D/3D) images through the point scanning method. This paper reviews the recent developments based on LIBS chemical composition imaging technology, including its background, fundamental principles, operation types, applications, and technical upgrade schemes. Here, the types of LIBS imaging operations and proposals for technological upgrades are the focus of this review. More importantly, we point out the existing problems in LIBS imaging and provide our own insights into its future development directions, including LIBS signal optimization, LIBS imaging resolution improvement and multiple technology integration. As a newly recent development direction, the technology fusion method is also a very promising research field in chemical composition imaging based on LIBS. This review is of great significance for the research and application of LIBS chemical composition imaging operations, which will provide basic knowledge guidance for LIBS researchers.
1. Introduction
Characterization technology has been playing a big role in research applications such as materials science, biomedicine, industry, agriculture, and environmental science, enabling a better understanding of the morphology, structure, composition, performance, and other characteristics of materials.1–3 In general, researchers will match the corresponding detection technologies for characterization analysis according to the detection demands for one or more characteristics of the analysis object. For example, the scanning electron microscopy (SEM), laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) and other technologies commonly used for comprehensive testing of lithium batteries include experimental techniques such as composition, morphology, crystal structure, functional group, material ion transport observation, material micromechanical properties, and material surface work function,4–6 but they are time-consuming and costly. Hence, the diversity of material properties, simplification of operations, and cost savings put forward higher requirements for the traditional detection technology and means.The proposed chemical composition imaging operation method provides a new research idea and technical direction to solve the above problems, which can provide the distribution of chemical composition on the surface of materials.7 This operation can simultaneously detect the surface morphology, composition, and other characteristics of the analysis object.8 Common methods of chemical composition analysis include spectral technology, mass spectrometry, energy spectroscopy, and chromatographic and chemical analyses. In particular, spectral analysis technology has shown a strong development momentum in recent years, and is constantly evolving towards miniaturization, high performance, multi-functionality, intelligence, and green environmental protection, showing great application potential. Typical spectral analysis technologies include ultraviolet radiation (UV),9 optical emission spectroscopy (OES),10 Raman spectroscopy (RS),11 infrared and near-infrared spectroscopy (IR/NIR),12 X-ray fluorescence spectrometry (XRF),13 laser-induced breakdown spectroscopy (LIBS),14 etc. Among them, LIBS technology is known as the “future superstar for chemical analysis” due to its advantages such as rapid, in situ, micro-invasive, simultaneous multi-element detection, simple operation, and safety.15 Over recent years, with the continuous improvement of LIBS basic theory and the increasingly mature application, chemical composition imaging based on LIBS has become one of the key research highlights.
Compared to other imaging techniques based on chemical element analysis, such as XRF's strict requirements for radiation protection, low sensitivity to light elements, and specific needs for sample preparation, as well as the problems faced by LA-ICP-MS, including high instrument complexity, high cost, complex operation, and high dependence on sample preparation, LIBS imaging technology demonstrates higher cost-effectiveness and wide adaptability. Besides, in contrast to conventional imaging techniques such as SEM, scanning tunneling microscopy (STM), and atomic force microscopy (AFM), LIBS imaging operation starts with point-to-point spectral lines obtained from different locations on the analysis object's surface and goes through the process of elements analysis to create a photograph that contains plenty of information, such as the chemical components and concentration at a certain location, the distribution of edge features, shape features, or texture features of the material surface chemical composition.16,17 However, this point-to-point approach causes a contradiction between spatial resolution and time consumption. The more point data collected per unit area, the better the spatial resolution, but the collection time will also increase.18 Furthermore, the instability of LIBS spectra, quantification issues, and the processing of large amounts of data also need to be solved.
Previous work has summarized the principles and applications of LIBS imaging technology.19,20 This paper aims to provide a comparatively comprehensive and systematic introduction to recent developments in chemical composition imaging based on LIBS, with a focus on the principle, operation, and development of LIBS imaging. We first briefly introduced LIBS technology, LIBS imaging operation, and spectral data processing. Then, the types and applications of LIBS imaging operations were summarized and discussed. Finally, we have put forward some constructive conclusions and suggestions regarding the current situation and existing problems of LIBS imaging operation.
2. Fundamental principle
2.1 LIBS technology
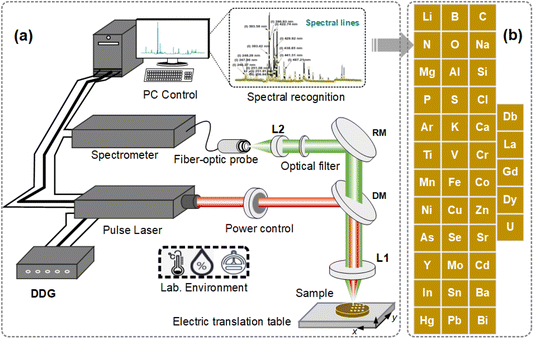
The schematic diagram of LIBS technology is shown in Fig. 1. A typical LIBS system is composed of a pulse laser, an optical spectrometer, a focusing lens, samples, a two- or three-dimensional (2D/3D) automatic objective table, a coupling lens, optical fibers, and other optical devices.11,21 When a high-energy pulse laser radiation focuses and is irradiated onto the target surface, after a series of physical processes such as melting, evaporation, expression, cooling, and disappearing, the laser plasma is generated and spectral radiation occurs.22 Qualitative and quantitative analysis of the elements corresponding to the spectrum is then performed to measure the types and contents of elements in the substance,23,24 as shown in Fig. 1(a). LIBS technology can detect all elements in the periodic table, and in practical applications, light elements to radioactive elements have been reported,17 as shown in Fig. 1(b).2.2 LIBS imaging operation
The crater of laser ablation is considered the point or pixel of LIBS imaging, and there are two parameters, the ablative crater diameter and ablative crater depth, as shown in Fig. 2(a). The size and shape of the ablative crater are related to the beam polarization state, focusing diameter, laser energy, sample physical parameters, etc.25–29 By systematically collecting point-to-point spectral information on the sample surface, a LIBS imaging cube (x, y, λ) with both positional information (x, y) and spectral information (λ) can be obtained,30 as shown in Fig. 2(b). The principle of LIBS imaging operation is based on the clustering-oriented image segmentation approach, and each minimum subset is called a region of interest (ROI). The greater the ROI per unit area, the higher the spatial resolution of the image, as shown in Fig. 2(c and d). However, the higher resolution of LIBS imaging operation is clearly associated with the longer acquisition time of the source data.31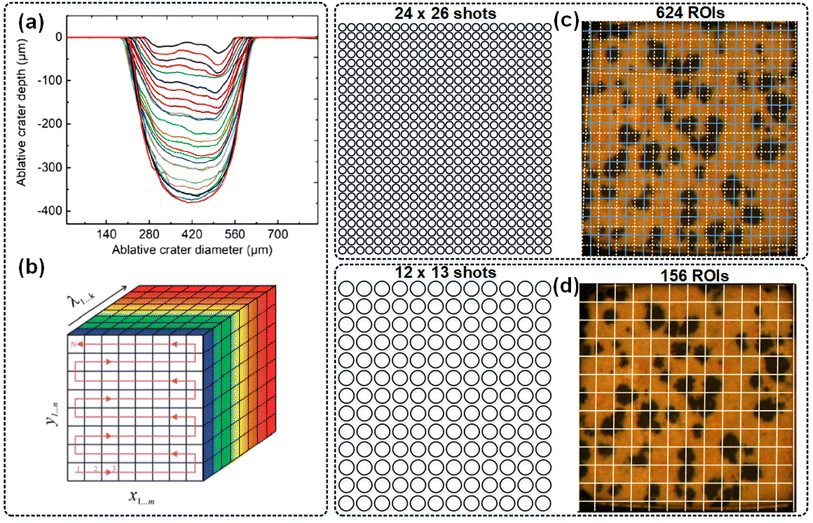 | ||
| Fig. 2 LIBS imaging operation.25,30,31 | ||
The analytical resolution, spot size, and detection limit of commonly used characterization techniques are shown in Fig. 3. The overall system performance of LIBS is effective, with the minimum size of the detection point reaching several micrometers and the optimal performance detecting the level of hundreds of ppb.32 In terms of single performance, such as sensitivity and analytical spot size, the advantages of LIBS technology are not obvious, but it is acceptable considering the cost-effectiveness of the technology, including sample processing, instrument complexity and portability, system construction cost, detection ability, and other factors. There is no doubt that LIBS technology still has huge space for further enhancement.33,34 Besides, compared with traditional imaging analysis techniques like SEM,35 LIBS also has the capability of chemical composition identification and concentration detection, as well as scanning imaging. As shown in Fig. 3, it can be seen that LIBS technology is in the middle of performance comparison, both of the sensitivity and analytical spot size are better, which means it becomes an attractive alternative to other technologies. In summary, the superiority of chemical composition imaging operations based on LIBS has been well confirmed.
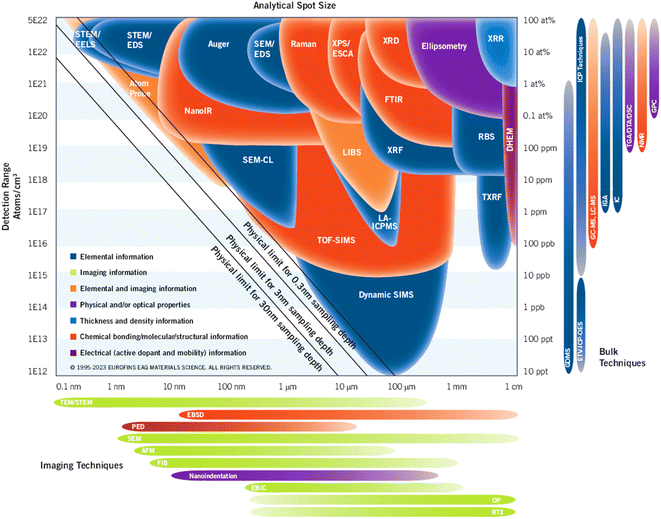 | ||
| Fig. 3 Analytical resolution, spot size, and detection limit of common characterization techniques (the data source comes from the EAG Laboratories website). | ||
2.3 Methodology
The schematic diagram of the original LIBS spectral processing workflow is shown in Fig. 4, which mainly consists of two parts: data preprocessing and LIBS imaging operation. Data preprocessing can significantly improve data quality and make the data more suitable for subsequent analysis and modeling, thereby obtaining more accurate and reliable results.36 Meanwhile, data preprocessing also helps to improve the execution efficiency of algorithms and the performance of models. And the LIBS imaging operation is to sequentially stitch various ROI segments, which can obtain the distribution of chemical components within the LIBS scanning area, achieving the effect of information visualization.The spectral preprocessing process and some important modeling tools are as follows: (1) data dimensionality reduction: common models include principal component analysis (PCA), linear discriminant analysis (LDA), and t-distributed stochastic neighbor embedding (t-SNE).37–39 (2) Baseline correction: the baseline correction process can eliminate baseline drift in spectral data and improve data stability and accuracy. Commonly used models include the polynomial fitting method and the least squares method.40 (3) Noise removal: the de-noising process can eliminate random noise in spectral data and improve the signal-to-noise ratio, and common models include smooth filtering and wavelet transform.41 (4) Standardization: the standardization process can standardize spectral data to zero mean and unit variance, eliminate the dimensional and magnitude differences in data, and improve its analyzability. Common models include minimum–maximum normalization and standard deviation normalization.42 (5) Interpolation: the interpolation process can be used to fill missing values in spectral data or smooth out missing values in outlier data to improve data integrity and reliability. Commonly used models include linear interpolation and polynomial interpolation.43 (6) Principal component selection and identification based on the NIST atomic database.31 The information obtained through these methods forms the basis for clearly identifying, documenting, and evaluating the importance of each chemical element in matter.
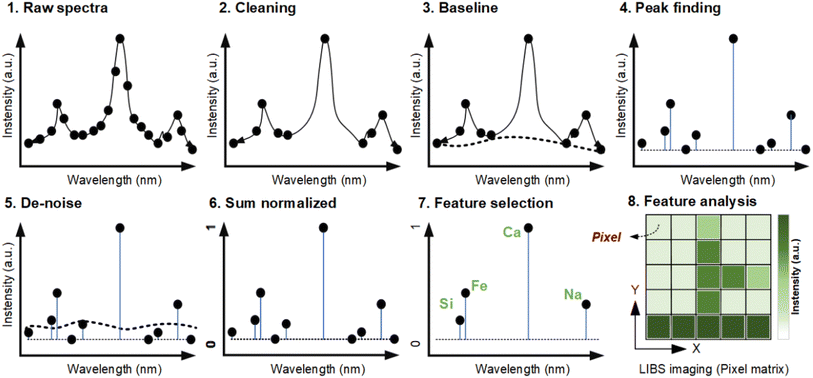
The basic principle of LIBS imaging is image region segmentation and stitching, which involves the orderly arrangement of multiple segments (or pixels) of the ROI.31 Each pixel corresponds to a spectrum, which contains many variables (i.e., spectral lines of different elements in the material after excitation, such as Si, Fe, Ga, and Na, as shown in Fig. 1(b) and 4). In general, most of the reported research work is based on semi-quantitative analysis of characteristic variables, that is, the element distribution and corresponding spectral intensity of LIBS imaging. However, it still remains a challenge to predict the concentration of each pixel in a substance more accurately and precisely. The existing solution is assisted by other technologies, such as ICP-MS, which will be discussed in Section 5. In addition, the number of pixels can reach 2 million over a surface of 488 mm2 with a step resolution reaching 15 μm,30 and how to deal with such a large scale of data is still the major problem that computer hardware devices and software tools will face.19,20
3. Types of LIBS imaging operation
3.1 Laser device selection
The morphology and formation of surface and internal craters also depend on the laser pulse width, as shown in Fig. 5(a). There are three main pulse-width types of laser sources commonly used in LIBS, including nanosecond (ns), picosecond (ps), and femtosecond (fs) lasers, among which ns lasers are the most widely used.44–47 The mechanisms that lead to energy absorption and target ablation are entirely different in both cases. During the laser pulse duration, fs-LIBS greatly reduces thermal damage and the heat-affected zone of the target due to negligible heat conduction and hydrodynamic motion. In addition, the spatial resolution obtained by fs pulses is superior to that of ns pulses. Other advantages of fs-LIBS compared to ns-LIBS include reduced mixing of continuum and atmospheric plasma, as well as the generation of atomic plumes. However, the main disadvantages of fs lasers compared with ns lasers include high equipment cost, high technical requirements, strict environmental requirements, and unknown long-term effects. But with the development of fs laser technology, we believe that its cost performance will continue to improve and its applicability will be increasingly wide. At present, it is regrettable that we have not found the “right way” to use it and can only say that the fs laser has great potential for future development in the field of LIBS imaging.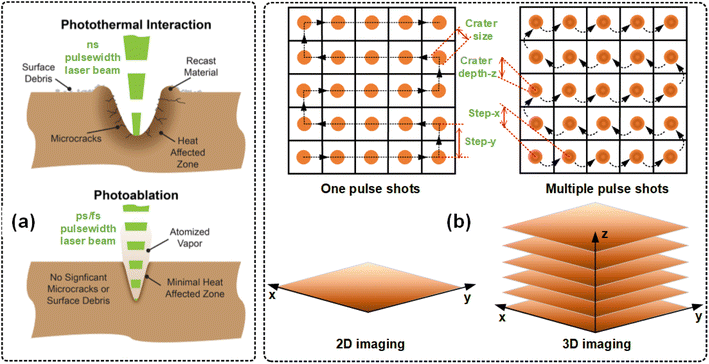 | ||
| Fig. 5 Schematic diagram of LIBS 2D/3D imaging operation: the (a) method of laser bombardments (the data source comes from the Coherent website) and (b) number of laser bombardments. | ||
3.2 LIBS 2D/3D imaging
As shown in Fig. 5(b), the LIBS 2D/3D imaging operation is based on the number of pulse shots, with single-pulse shooting and multi-pulse shooting corresponding to two types of 2D imaging and 3D imaging, respectively. In the case of multiple laser pulses striking the same position on the target material surface, the depth of the crater increases with the number of strikes (see Fig. 2(a)),48 and a 3D LIBS imaging data cube (x, y, z, λ) with both positional information (x, y, z) and spectral information (λ) can be obtained (see Fig. 5(b)). Therefore, the 3D imaging of LIBS technology is realized by adding depth information on the basis of 2D imaging. This usually requires changing the focusing position or scanning path of the laser beam to obtain the distribution information of elements at different depths in the sample. Then, these 2D images with different depths are superimposed by computer image processing technology to form a 3D element distribution image of the sample. It can be said that the 2D imaging of LIBS technology can quickly obtain the element distribution information on the sample surface, while the 3D imaging can further reveal the internal structure and element distribution characteristics of the sample.3.3 Micro-LIBS imaging
Micro-LIBS, or μ-LIBS, is a new element analysis technique based on the combination of LIBS technology and microscopic optics.49,50 Compared with the traditional LIBS imaging method, the μ-LIBS imaging method has smaller laser energy and focusing spots. The μ-LIBS system employs μJ energy laser pulses for laser ablation, which can achieve minimally invasive craters, high-spectral resolution composition detection, and high-spatial resolution (laser spot size <10 μm). Besides, μ-LIBS can also complete point-to-point scanning detection and analysis of the target object by docking with the precision load displacement platform. The lower μJ laser power allows the sample to be very small in volume (about 10 to 103 μm3) and in mass (10 pg to ng). Meanwhile, μ-LIBS also has good compatibility with Raman technology, and multifunctional detection of elements and molecular groups can be achieved by using the combined LIBS-Raman operation.51 Compared with the μ-LIBS system, traditional LIBS technology uses laser energy and spot size ranging from dozens to hundreds μm and mm levels, respectively.52,53 Based on the analysis of target size and dimensions, LIBS and micro-LIBS imaging should consider the actual situation of chemical composition imaging operations.4. LIBS imaging application
4.1 Biomedicine
Minor changes in elements within living organisms can directly affect their metabolism and physiological processes. The correlation between the composition and content of elements in organisms and the development of biological metabolism and diseases has been widely studied and has become important supporting information for medical diagnosis and treatment. Studies have found that some essential biological elements participate in oxidation reverse reactions, regulate biological metabolic balance, control biological processes, and have adaptive immune regulation functions.54–58 In recent years, LIBS imaging has been continuously enhanced as an influential biomedicine technique, showing intriguing potential in the field of clinical and medical analysis, such as for occupational lung disease,59 tablet coating,60 human breast cancer,61 lung tumor,62 lung cancer,63 skin tumor,64,65 cerebral distribution,66 human tissue,67,68 clinical specimens,69 and melanoma tissue.70 Some of the application results and diagrams are shown in Fig. 6. On the basis of 2D LIBS imaging operations, 3D LIBS imaging is investigated. There are two methods to collect longitudinal data: multiple pulse shots71 and slice-by-slice cutting,72 and then a 3D database is constructed. Generally, the former is used for the analysis of solid samples (with a small longitudinal depth and hundred-micron scale), while the latter is used for three-dimensional imaging analysis of biological tissues (with a large longitudinal depth and complex operation).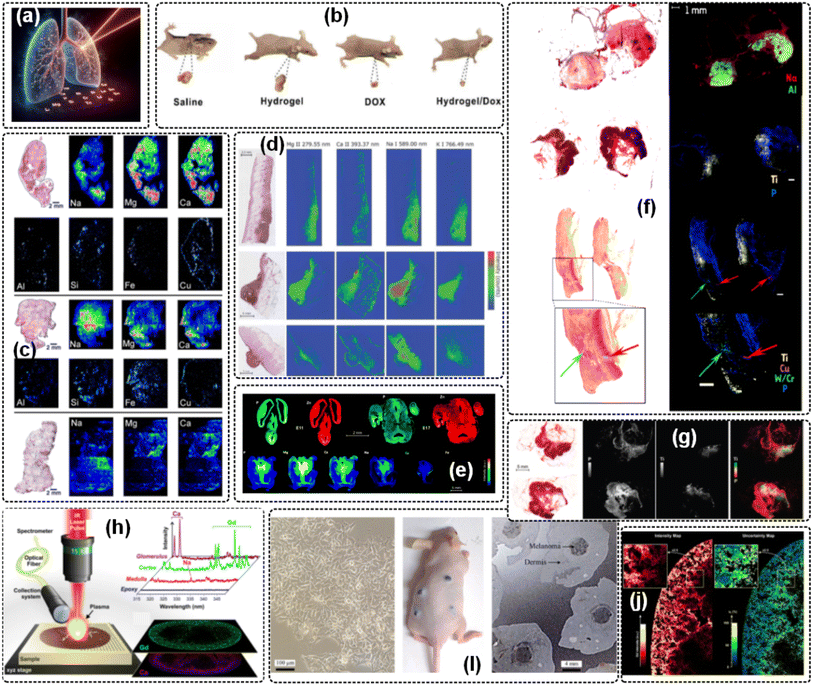 | ||
| Fig. 6 Biomedicine analysis by the LIBS imaging approach: (a) enhancing diagnostic capabilities for occupational lung diseases using LIBS imaging on biopsy tissue,59 (b) the representative breast cancer xenograft tumor mice after tail vein injection of 0.9% saline, 100 μg mL−1 DOX, hydrogel/DOX (125 μm DNA nanohydrogels, 100 μg mL−1 DOX), and 125 μm DNA nanohydrogels, respectively,61 (c) multi-elemental imaging of different lung tissue types,63 (d) histological H & E images complemented with LIBS elemental images of Mg, Ca, Na, and K for selected tumor samples,64 (e) spatio-temporal comparison for P and Zn elements, and an example of various elemental images in a selected section of the brain of a mouse,66 (f) high-resolution HES histological images of a healthy skin sample before LIBS analysis,65 (g) titanium accumulation in a lymph node,68 (h) general protocol for LIBS imaging,67 (i) microscope image of 70% cell confluence, clinical images of mice after 10 days of subcutaneous melanoma cell implantation, and optical image of cryosectioned tissues for LIBS mapping, respectively,69 and (j) elemental images of Gd distribution in a murine kidney.70 | ||
4.2 Minerals
The mineral composition and ore structure are key factors affecting the sustained economic extraction of base and precious metals and require in-depth understanding from upstream ore characterization, metallurgical testing, and process design, to mineral processing. Therefore, mineral analysis is an important basis and premise for geological work and the mining industry, which helps researchers obtain mineral information in time and quickly understand the physical and chemical properties of a certain region or a certain type of mineral.73 At present, LIBS imaging has been applied to mineral phases,30,74,75 luminescence phenomena,76 trace compounds,77 rock classification,78 mineral core surfaces,79 irregularly shaped minerals,80 elemental identification,81–83 heterogeneous inorganic archaeological samples,84 uranium mineralization in uranium ores,85 and other aspects. Part of the application results and schematic diagrams are shown in Fig. 7. Besides, multiple combined techniques have been applied for mineral analysis, improving the diversity of mineral detection information. Existing methods include LIBS-RS,86 LIBS-HSI,87–89 Micro-LIBS/Micro-XRF,90 and LIBS/LA-ICP-MS.91 Finally, the research on data processing algorithms (such as principal component analysis, feature lines selection),92,93 double pulse Micro-LIBS,94,95 miniaturized devices,96 etc. Finally, the matching technology and data analysis methods for large-scale and small-scale scanning objects have further optimized the application of imaging technology in the field of mineral analysis.97,98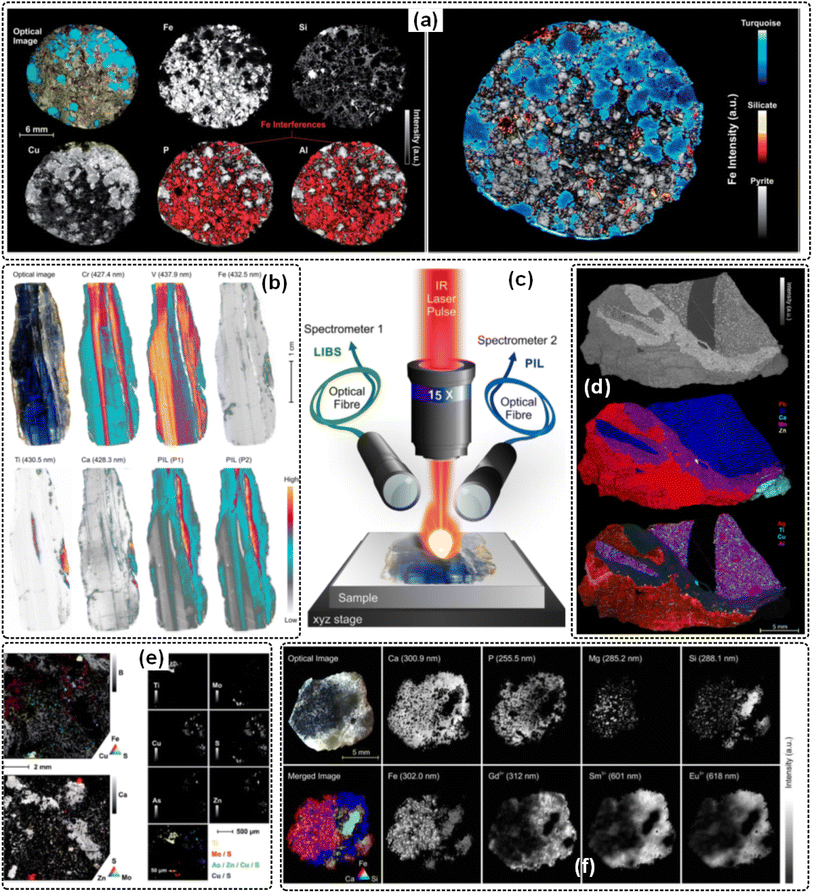 | ||
| Fig. 7 Mineral analysis by the LIBS imaging approach: (a) optical images of the gemstone and five LIBS elemental images of Fe, Si, Cu, P, and Al in a gray color scale,30 (b) distribution images of elements generated from the classical signal integration method from LIBS and PIL spectra,76 (c) principle of elemental LIBS, molecular LIBS, and PIL experiments,82 (d) the mean spectrum of the LIBS data set, the global intensity image, and elemental images generated with the conventional approach,77 (e) elemental images (B, Ca, S, Mo, Fe, and Zn) of the mine core surface in the region represented by the rectangle,79 (f) elemental and luminescence imaging of Kovdor fluorapatite.82 | ||
4.3 Industry and materials
Due to the simple sample preparation, fast analysis speed, remote online telemetry, 2D/3D surface distribution, and multi-element depth analysis, LIBS imaging operation has attracted the attention of researchers in the industrial and materials fields.25,31 LIBS imaging has the characteristics of real-time monitoring of the production process, traceability of detection data, representative analysis data, industrial transmission of data, product quality alarm function, simultaneous detection of multi-component parameters, etc., so it has great advantages in the quality control of industrial production processes. In recent years, the application results and diagrams of LIBS imaging in metal scrap identification,99 fluoropolymer materials,100 building materials,101 Li-ion solid-state electrolytes,102 trace metal impurity diffusion,103 fresh impregnated catalysts,104 mineral fertilizers,105 printed circuit board compositions,106 lithium phosphorus oxide thin films,107 superconductor thin films,108 and others,109–111 and the typical work (ref. 97, 102, 104, 106 and 107) are shown in Fig. 8. In addition, three-dimensional elemental imaging of material surfaces112 and nanoscale subcellular resolution imaging113 based on LIBS technology have also been studied. Moreover, related research work on quantitative LIBS imaging based on the multiple-calibration approach114 and LIBS-ICP-MS115 has been carried out.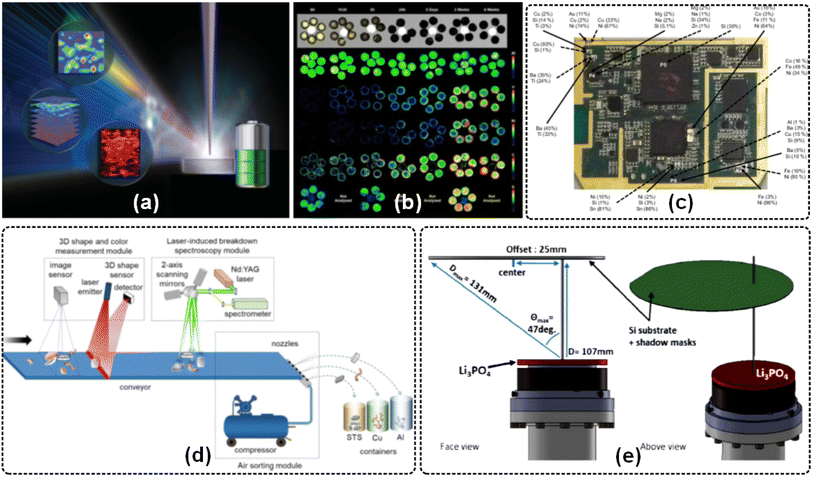 | ||
| Fig. 8 Industry and materials analysis by the LIBS imaging approach: (a) three-dimensional elemental imaging of Li-ion solid-state electrolytes using fs-LIBS,102 (b) optical images of supports impregnated with asphaltenes and the corresponding LIBS images of the asphaltenes,104(c) semi-quantitative information obtained using SEM-EDS for the surface (solid arrows) and crater (dotted arrows),106(d) laboratory-scale automatic sorting system based on laser-induced breakdown spectroscopy,97 and (e) relative positions of the target (Li3PO4; red) and substrate (Si wafer; green) during magnetron sputtering.107 | ||
4.4 Other applications
In order to investigate changes in ancient climate, such as precipitation, major climate changes on Earth, and changes in biological growth processes, there have been reports of extracting reference ancient climate data from fossil samples based on element distribution imaging technology.116,117 Also, LIBS imaging technology can monitor the environment and growth status of seeds and plant samples, including detecting the absorption, transportation, and accumulation of relevant elements (including nutrients and toxic elements), which is of great significance for understanding the growth status of plant samples, environmental quality, and the interaction between plants and the environment.118–121 Besides, LIBS imaging technology has shown great application potential in food safety,122 public security investigation,123 archaeological mortar,124 extraterrestrial exploration,125 and other fields.5. LIBS imaging development
5.1 Basic requirements in LIBS imaging
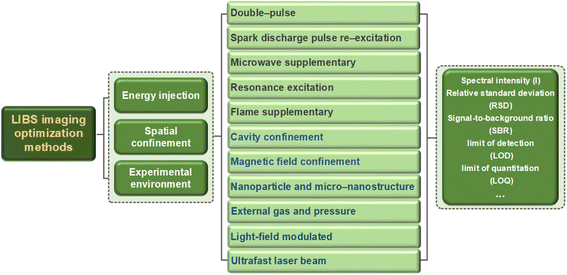
Currently, LIBS imaging technology is still in the stage of qualitative analysis, and how to achieve quantification is a problem that needs to be solved in the future.126,127 The traditional LIBS quantitative method is based on standard samples. Because the signal intensity of atomic spectral lines can reflect the quantitative relationship corresponding to element content, for samples with high purity and a simple structure, the quantitative method based on standard samples can well reflect the composition of the material.128 Another approach is called calibration-free LIBS (CF-LIBS), which is a physical process that studies laser-induced plasma. According to the corresponding theories and practical assumptions, quantitative analysis can be conducted using experimental data measured by experiments and physical theoretical formulae.129,130 But it is a consensus that developing a universal LIBS quantitative method is almost impossible. A more feasible approach is to develop specialized LIBS quantitative methods in specific fields to meet their application needs. Besides, how to improve the accuracy, precision, and sensitivity of LIBS quantitative imaging is also a key issue to be addressed in the future.5.2 Technology upgrade proposal
5.2.2.1 Spectral resolution. On the basis of LIBS signal optimization, from the perspective of software and algorithms, image super-resolution reconstruction (ISRR) technology may become an effective way to solve the problem of lower spectral resolution.151,152 ISRR technology refers to the restoration of a given low-resolution image to a corresponding high-resolution image by using a specific algorithm. The traditional super-resolution reconstruction algorithm mainly relies on basic digital image processing technology for reconstruction, which generally includes (1) interpolation-based super-resolution reconstruction, (2) degenerate model-based super-resolution reconstruction, and (3) learning-based super-resolution reconstruction. Besides, deep learning has begun to be widely applied in various fields, especially in the fields of image and vision, and convolutional neural networks have made great achievements, which has led more and more researchers to attempt to introduce deep learning into the field of super-resolution reconstruction.153,154
5.2.2.2 Spatial resolution. High-resolution LIBS images typically have greater pixel density, richer texture details, and higher reliability than low-resolution images. The most direct way to improve spatial resolution is to increase the point-to-point density of laser ablation, which puts forward higher requirements for beam quality and spot diameter. However, the LIBS system faces great challenges in providing the minimum spot size while maintaining overall beam parameter stability. Several solutions have been proposed at present: changing the laser wavelength (the limit value of the focused spot is proportional to the wavelength), changing the focal length of the objective lens (the focused spot is proportional to the focal length), and changing the diameter of the entrance pupil spot (the entrance pupil spot diameter is inversely proportional to the focal length); that is, using a short wavelength laser, a small focal length objective lens, a beam expander, or a spatial filter might be able to resolve the existing spatial resolution problem.
5.2.2.3 Spectral sensitivity. Existing LIBS imaging operations have high requirements for the flatness of samples. For some samples with uneven surfaces, the commonly used methods currently have low spectral sensitivity. A promising solution is to elongate the spatial distance of the focused beam, using optical field modulation, self-focusing lens, femtosecond laser filamentation, and so on. This can further improve the spectral sensitivity and accuracy of LIBS imaging.
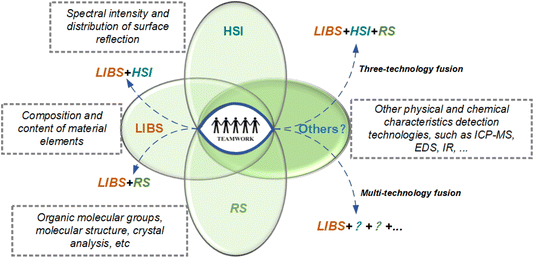
Modern spectral technology allows rapid, micro/non-destructive analysis of targets directly. Different types of spectrum measuring methods have different analysis focuses. In order to achieve a more comprehensive and accurate analysis, it is necessary to combine various analysis methods. The combination method optimizes and integrates different types of spectra to realize the complementary advantages of a single spectrum so as to obtain more comprehensive, reliable, and abundant characteristic data, achieving the purpose of improving the accuracy and stability of model prediction.31 The schematic diagram of multiple technology integration is shown in Fig. 10. There are currently mature technology integration cases, such as LIBS + HSI,156 LIBS + RS,157 LIBS + ICP-MS,158 and so on. The joint usage of two or more technologies is the trend of future development and has important application value.
There are two ways to achieve the multi-technology integration. One depends on the degree of matching between hardware devices, such as HSI, RS, IR, etc., and these technologies have the characteristics of being fast, in situ, and simple. Therefore, the combination of these technologies can improve the performance of LIBS spectral imaging technology without losing their respective advantages. Another way is low hardware compatibility, such as ICP-MS and EDS. First, image segmentation, ROI recognition, and feature classification are performed based on LIBS data. A more accurate component analysis of small sample ROIs is then performed using mass spectrometry or other techniques. Besides, LIBS-ICP-MS can provide full spectral and elemental information. Finally, a component content distribution map is drawn on the basis of feature correlation analysis.
6. Conclusion
LIBS has received more and more attention in the field of elemental imaging, mainly due to its advantages of rapid, in situ, small-invasive, and simultaneous detection of multi-elements, simple operation, and safety compared to other techniques. This review introduced the fundamental principle, operation, and data-processing methods of LIBS imaging and compared and discussed the types of LIBS 2D/3D imaging and micro-LIBS imaging. On this basis, the relevant applications in the fields of biomedicine, minerals, industry, materials, etc., were introduced. Finally, four technological upgrade proposals, including LIBS signal optimization, spatial resolution improvement, multiple technology integration, and quantitative analysis, were proposed and discussed. All these will be further studied and overcome in the future, and LIBS imaging technology will be applied in more fields.Data availability
The data that support the findings of this study are available from the corresponding author or some open source website, upon reasonable request.Author contributions
Shangyong Zhao: investigation, conceptualization, writing – original draft & review, supervision, funding acquisition. Yuchen Zhao: writing – original draft & review & editing, validation. Yujia Dai: investigation. Ziyuan Liu: investigation. Zongyu Hou: investigation. Xun Gao: investigation, writing – original draft. Zhe Wang: writing – original draft, supervision, project administration, funding acquisition.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the National Key Research and Development Program of China (2023YFB4102900), the National Natural Science Foundation of China (61575030), the Scientific Research Foundation of Zhejiang A&F University (2024LFR047), and the Department of Education of Zhejiang Province (Y202455661).References
- D. Wu, Y. Hu, H. Cheng and X. Ye, Molecules, 2023, 28, 3601 CrossRef CAS PubMed.
- X. Jia, S. Li, D. Han, R. Chen, Z. Yao, B. Ning, Z. Gao and Z. Fan, Crit. Rev. Food Sci. Nutr., 2021, 62, 4706–4725 CrossRef PubMed.
- Y. Qiu, X. Zhou, X. Tang, Q. Hao and M. Chen, Materials, 2023, 16, 2253 CrossRef CAS PubMed.
- M. Yang, R. Bi, J. Wang, R. Yu and D. Wang, Int. J. Miner., Metall. Mater., 2022, 29, 965–989 CrossRef.
- N. Balke, S. Kalnaus, N. J. Dudney, C. Daniel, S. Jesse and S. V. Kalinin, Nano Lett., 2012, 12, 3399–3403 CrossRef CAS PubMed.
- Z. Deng, X. Lin, Z. Huang, J. Meng, Y. Zhang, G. Ma, Y. Zhou, Y. Shen, H. Ding and Y. Huang, Adv. Energy Mater., 2021, 11, 2000806 CrossRef CAS.
- A. Limbeck, L. Brunnbauer, H. Lohninger, P. Porízka, P. Modlitbov, J. Kaiser, P. Janovszky, A. Keri and G. Galbacs, Anal. Chim. Acta, 2021, 1147, 72e98 CrossRef PubMed.
- B. Busser, S. Moncayo, J. Coll, L. Sancey and V. Motto-Ros, Coord. Chem. Rev., 2018, 358, 70–79 CrossRef CAS.
- G. L. P. A. Ramos, L. M. R. Esper and A. G. M. Gonzalez, Int. J. Food Sci. Technol., 2024, 59, 1187–1196 CrossRef CAS.
- I. Kim, C. Park, D. Shin and I. Yun, IEEE Sens. J., 2021, 21, 2256–2262 Search PubMed.
- Q. Wu, D. Xiao, N. Wang, F. Masis, W. Langbin and B. Li, Talanta, 2024, 266, 125067 CrossRef CAS PubMed.
- R. Fritzsch, S. Hume, L. Minnes, M. J. J. Baker, G. A. A. Burley and N. T. T. Hunt, Analyst, 2020, 145, 2014–2024 RSC.
- J. Orsilli and S. Caglio, Minerals, 2024, 14, 192 CrossRef CAS.
- S. S. Harilal, A. K. Shaik, E. J. Kautz, A. Devaraj, A. M. Casella and D. J. Senor, J. Anal. At. Spectrom., 2024, 39, 699–703 RSC.
- J. D. Winefordner, I. B. Gornushkin, T. Correll, E. Gibb, B. W. Smith and N. Omenetto, J. Anal. At. Spectrom., 2004, 19, 1061e1083 RSC.
- D. S. Francischini and M. A. Z. Arruda, Microchem. J., 2021, 169, 106526 CrossRef CAS.
- J. Caceres, F. Pelascini, V. Motto-Ros, S. Moncayo, F. Trichard, G. Panczer, A. Marín-Roldán, J. Cruz, I. Coronado and J. Martín-Chivelet, Sci. Rep., 2017, 7, 5080 CrossRef CAS PubMed.
- C. J. M. Lawley, A. M. Somers and B. A. Kjarsgaard, J. Geochem. Explor., 2021, 222, 106694 CrossRef CAS.
- V. Gardette, V. Motto-Ros, C. Alvarez-Llamas, L. Sancey, L. Duponchel and B. Busser, Anal. Chem., 2023, 95, 49–69 CrossRef CAS PubMed.
- L. Jolivet, M. Leprince, S. Moncayo, L. Sorbier, C.-P. Lienemann and V. Motto-Ros, Spectrochim. Acta, Part B, 2019, 151, 41–53 CrossRef CAS.
- S. Zhao, Y. Zhao, Y. Dai, Z. Liu, H. Zha and X. Gao, Front. Phys., 2024, 19, 62500 CrossRef.
- S. Zhao, C. Song, X. Gao, K. Guo, Z. Hao and J. Lin, Results Phys., 2020, 19, 103601 CrossRef.
- S. Zhao, W. Song, Z. Houa and Z. Wang, J. Anal. At. Spectrom., 2021, 36, 1704–1711 RSC.
- S. Zhao, W. Song, Y. Zhao, Z. Hou and Z. Wang, Microchem. J., 2022, 183, 107986 CrossRef CAS.
- D. Zhang, Y. Chu, S. Ma, Z. Sheng, F. Chen, Z. Hu, S. Zhang, B. Wang and L. Guo, Appl. Surf. Sci., 2020, 534, 147601 CrossRef CAS.
- E. M. Garcell and C. Guo, J. Appl. Phys., 2018, 123, 213103 CrossRef.
- S. M. Pimenov, E. V. Zavedeev, B. Jaeggi and B. Neuenschwander, Materials, 2023, 16, 795 CrossRef CAS PubMed.
- M. E. Shaheen, J. E. Gagnon and B. J. Fryer, Opt. Lasers Eng., 2019, 119, 18–25 CrossRef.
- M. E. Shaheen and J. E. Gagnon, Laser Phys., 2016, 26, 116102 CrossRef.
- S. Moncayo, L. Duponchel, N. Mousavipak, G. Panczer, F. Trichard, B. Bousquet, F. Pelascini and V. Motto-Ros, J. Anal. At. Spectrom., 2018, 33, 210–220 RSC.
- S. Zhao, Y. Zhao, Z. Hou and Z. Wang, Appl. Surf. Sci., 2023, 629, 157415 CrossRef CAS.
- Z. Wang, M. S. Afgan, W. L. Gu, Y. Z. Song, Y. Wang, Z. Y. Hou, W. R. Song and Z. Li, TrAC, Trends Anal. Chem., 2021, 143, 116385 CrossRef CAS.
- P. Yin, E. Yang, Y. Chen, Z. Peng, D. Li, Y. Duan and Q. Lin, Chem. Commun., 2021, 57, 7312–7315 RSC.
- M. Markiewicz-Keszycka, X. Cama-Moncunill, M. P. Casado-Gavalda, Y. Dixit, R. Cama-Moncunill, P. J. Cullen and C. Sullivan, Trends Food Sci. Technol., 2017, 65, 80–93 CrossRef CAS.
- J. Schreiner, F. Goetz-Neunhoeffer, J. Neubauer, S. Volkmann, S. Bergold, R. Webler and D. Jansen, Powder Diffr., 2019, 34, 143–150 CrossRef CAS.
- P. Mishra, A. Biancolillo, J. M. Roger, F. Marini and D. N. Rutledge, TrAC, Trends Anal. Chem., 2020, 132, 116045 CrossRef CAS.
- S. Marukatat, Artif. Intell. Rev., 2023, 56, 5445–5477 CrossRef.
- J. Oh and N. Kwak, Signal Process., 2024, 219, 109421 CrossRef.
- M. Kimura, IEEE Access, 2021, 9, 129619–129625 Search PubMed.
- Z. Liu, R. Zheng, Y. Tian, B. Wang, J. Guo and Y. Lu, J. Anal. At. Spectrom., 2022, 37, 1134–1140 RSC.
- S. Zhao, M. S. Afgan, H. Zhu and X. Gao, Optik, 2022, 25, 16844 Search PubMed.
- W. Song, Z. Hou, W. Gu, H. Wang, J. Cui, Z. Zhou, G. Yan, Q. Ye, Z. Li and Z. Wang, Fuel, 2021, 306, 121667 CrossRef CAS.
- K. R. Kim, G. Kim, J. Y. Kim, K. Park and K. W. Kim, J. Anal. At. Spectrom., 2014, 29, 76–84 RSC.
- M. Gragston, P. Hsu, N. Jiang, S. Roy and Z. Zhang, Appl. Opt., 2021, 60, C114–C120 CrossRef PubMed.
- A. Marín Roldán, M. Pisarcík, M. Veis, M. Drzík and P. Veis, Spectrochim. Acta, Part B, 2021, 177, 10655 CrossRef.
- Z. Feng, J. Zhang, X. Li, Q. Gao and B. Li, J. Phys. D: Appl. Phys., 2022, 55, 505206 CrossRef CAS.
- S. A. Kalam, N. L. Murthy, P. Mathi, N. Kommu, A. K. Singh and S. V. Rao, J. Anal. At. Spectrom., 2017, 32, 1535–1546 RSC.
- M. Shi, J. Wu, D. Wu, X. Guo, Y. Qiu, Y. Zhou, J. Li, H. Sun, X. Li and A. Qiu, Spectrochim. Acta, Part B, 2023, 209, 106797 CrossRef CAS.
- C. Fabre, K. Trebus, A. Tarantola, J. Cauzid, V. Motto-Ros and P. Voudouris, Spectrochim. Acta, Part B, 2022, 194, 106470 CrossRef CAS.
- C. Alvarez-Llamas, A. Tercier, C. Ballouard, C. Fabre, S. Hermelin, J. Margueritat, L. Duponchel, C. Dujardin and V. Motto-Ros, J. Anal. At. Spectrom., 2024, 39, 1077–1086 RSC.
- R. Glaus and D. W. Hahn, Spectrochim. Acta, Part B, 2014, 100, 116–122 CrossRef CAS.
- M. Martin, D. Hamm, S. Martin, S. Allman, G. Bell and R. Martin, Minerals, 2019, 9, 103 CrossRef CAS.
- A. Botto, B. Campanella, S. Legnaioli, M. Lezzerini, G. Lorenzetti, S. Pagnotta, F. Poggialini and V. Palleschi, J. Anal. At. Spectrom., 2019, 34, 81–103 RSC.
- K. Zabłocka-Słowińska, S. Płaczkowska, A. Prescha, K. Pawelczyk, I. Porebska, M. Kosacka, L. Pawlik-Sobecka and H. Grajeta, J. Trace Elem. Med. Biol., 2018, 45, 78–84 CrossRef PubMed.
- S. Liao, S. O. Omage, L. Börmel L, S. Kluge, M. Schubert, M. Wallert and S. Lorkowski, Antioxidants, 2022, 11, 1785 CrossRef CAS PubMed.
- S. LI, X. Jiang, Y. Luo, B. Zhou, M. Shi, F. Liu and A. Sha, Chemosphere, 2019, 234, 579–588 CrossRef CAS PubMed.
- R. B. N. Renata, G. R. A. Arely, L. M. A. Gabriela and M. L. M. Esther, Biol. Trace Elem. Res., 2023, 201, 1596–1614 CrossRef CAS PubMed.
- V. Gardette, L. Sancey, M. Leprince, L. Gate, F. Cosnier, C. Seidel, S. Valentino, F. Pelascini, J. -L. Coll, M. Peoc'h, V. Scolan, F. Paysant, V. Bonneterre, C. Dujardin, B. Busser and V. Motto-Ros, Small Sci., 2024, 4, 2300307 CrossRef CAS.
- V. H. C. Ferreira, V. Gardette, B. Busser, L. Sancey, S. Ronsmans, V. Bonneterre, V. Motto-Ros and L. Duponchel, Anal. Chem., 2024, 96, 7038–7046 CrossRef CAS PubMed.
- L. Zou, B. Kassim, J. P. Smith, J. D. Ormes, Y. Liu, Q. Tu and X. Bu, Analyst, 2018, 143, 5000–5007 RSC.
- H. Wei, Z. Zhao, Q. Lin and Y. Duan, Biol. Trace Elem. Res., 2021, 199, 1686–1692 CrossRef CAS PubMed.
- Q. Lian, X. Li, B. Lu, C. Zhu, J. Li and J. Chen, IEEE Access, 2023, 11, 141915–141925 Search PubMed.
- P. Yin, B. Hu, Q. Li, Y. Duan and Q. Lin, IEEE Trans. Instrum. Meas., 2021, 70, 4006207 CAS.
- K. Kiss, A. Sindelaova, L. Krbal, V. Stejskal, K. Mrazova, J. Vrabel, M. Kaska, P. Modlitbová, P. Porízka and J. Kaiser, J. Anal. At. Spectrom., 2021, 36, 909–916 RSC.
- S. Moncayo, F. Trichard, B. Busser, M. Sabatier-Vincent, F. Pelascini, N. Pinel, I. Templier, J. Charles, L. Sancey and V. Motto-Ros, Spectrochim. Acta, Part B, 2017, 133, 40–44 CrossRef CAS.
- B. Busser, A. Bulin, V. Gardette, H. Elleaume, F. Pelascini, A. Bouron, V. Motto-Ros and L. Sancey, J. Neurosci. Methods, 2022, 379, 109676 CrossRef CAS PubMed.
- M. Leprince, L. Sancey, J. L. Coll, V. Motto-Ros and B. Busser, Med. Sci., 2019, 35, 682–688 Search PubMed.
- Y. Gimenez, B. Busser, F. Trichard, A. Kulesza, J. M. Laurent, V. Zaun, F. Lux, J. M. Benoit, G. Panczer, P. Dugourd, O. Tillement, F. Pelascini, L. Sancey and V. Motto-Ros, Sci. Rep., 2016, 6, 29936 CrossRef CAS PubMed.
- B. Busser, S. Moncayo, F. Trichard, V. Bonneterre, N. Pinel, F. Pelascini, P. Dugourd, J. L. Coll, M. D'Incan, J. Charles, V. Motto-Ros and L. Sancey, Mod. Pathol., 2018, 31, 378–384 CrossRef CAS PubMed.
- J. Choi, S. Shin, Y. Moon, J. Han, E. Hwang and S. Jeong, Spectrochim. Acta, Part B, 2021, 179, 106090 CrossRef CAS.
- J. P. Smith, L. Zou, Y. Liu and X. Bu, Spectrochim. Acta, Part B, 2020, 165, 105769 CrossRef CAS.
- Q. Lin, S. Wang, Y. Duan and V. V. Tuchin, J. Biophotonics, 2021, 14, e202000479 CrossRef CAS PubMed.
- A. Buzatu, G. Damian, N. Buzgar, P. Andras, A. L. Apopeia, A. E. Maftei and S. Milovska, Vib. Spectrosc., 2017, 89, 49–56 CrossRef CAS.
- J. A. Meima and D. Rammlmair, Chem. Geol., 2020, 532, 119376 CrossRef CAS.
- C. Fabre, D. Devismes, S. Moncayo, F. Pelascini, F. Trichard, A. Lecomte, B. Bousquet, J. Cauzid and V. Motto-Ros, J. Anal. At. Spectrom., 2018, 33, 1345–1353 RSC.
- A. Nardecchia, A. de Juan, V. Motto-Ros, M. Gaft and L. Duponchel, Anal. Chim. Acta, 2022, 1192, 339368 CrossRef CAS PubMed.
- A. Nardecchia, C. Fabre, J. Cauzid, F. Pelascini, V. Motto-Ros and L. C. Duponchel, Anal. Chim. Acta, 2020, 1114, 66–73 CrossRef CAS PubMed.
- T. Chen, L. Sun, H. Yu, W. Wang, L. Qi, P. Zhang and P. Zeng, Appl. Geochem., 2022, 136, 105135 CrossRef CAS.
- F. Trichard, S. Moncayo, D. Devismes, F. Pelascini, J. Maurelli, A. Feugier, C. Sasseville, F. Surmad and V. Motto-Ros, J. Anal. At. Spectrom., 2017, 32, 1527–1534 RSC.
- W. Huang, C. He, Y. Wang, W. Zhao and L. Qiu, J. Anal. At. Spectrom., 2020, 35, 2530–2535 RSC.
- K. Rifai, L. Ç. Özcan, F. R. Doucet, K. Rhoderick and F. Vidal, Minerals, 2020, 10, 207 CrossRef CAS.
- M. Gaft, Y. Raichlin, F. Pelascini, G. Panzer and V. Motto Ros, Spectrochim. Acta, Part B, 2019, 151, 12–19 CrossRef CAS.
- J. Klus, P. Mikysek, D. Prochazka, P. Pořízka, P. Prochazkova, J. Novotny, T. Trojek, K. Novotny, M. Slobodník and J. Kaiser, Spectrochim. Acta, Part B, 2016, 123, 143–149 CrossRef CAS.
- O. Syta, B. Wagner, E. Bulska, D. Zielińska, G. Z. Zukowska, J. Gonzalez and R. Russo, Talanta, 2108, 179, 784–791 CrossRef PubMed.
- I. Krempl, K. Novotný, V. Wertich, R. Skoda, V. Kanický and J. Leichmann, Spectrochim. Acta, Part B, 2023, 206, 106734 CrossRef CAS.
- J. Aramendia, L. Gómez-Nubla, S. F. O. de Vallejuelo, K. Castro, G. Arana and J. M. Madariaga, J. Raman Spectrosc., 2019, 50, 193–201 CrossRef CAS.
- M. A. Speranc, F. W. B. de Aquino, M. A. Fernandes, A. Lopez-Castillo, R. L. Carneiro and E. R. Pereira-Filho, Geostand. Geoanal. Res., 2017, 41, 273–282 CrossRef.
- C. Sandoval-Munoz, G. Velasquez, J. Alvarez, F. Perez, M. Velasquez, S. Torres, D. Sbarbaro-Hofer, V. Motto-Ros and J. Yanez, J. Anal. At. Spectrom., 2022, 37, 1981–1993 RSC.
- W. Nikonow, D. Rammlmair, J. A. Meima and M. C. Schodlok, Mineral. Petrol., 2019, 113, 417–431 CrossRef CAS.
- C. Fabre, K. Trebus, A. Tarantola, J. Cauzid, V. Motto-Ros and P. Voudouris, Spectrochim. Acta, Part B, 2022, 194, 106470 CrossRef CAS.
- J. R. Chirinos, D. D. Oropeza, J. J. Gonzalez, H. Hou, M. Morey, V. Zorba and R. E. Russo, J. Anal. At. Spectrom., 2014, 29, 1292–1298 RSC.
- T. Lopes, D. Capela, M. F. S. Ferreira, D. Guimarães, P. A. S. Jorge and N. A. Silva, Appl. Spectrosc., 2024, 78, 753–759 CrossRef CAS PubMed.
- J. A. Meima and D. Rammlmair, Chem. Geol., 2020, 532, 119376 CrossRef CAS.
- C. Schiavo, L. Menichetti, E. Grifoni, S. Legnaioli, G. Lorenzetti and F. Poggialini, J. Instrum., 2016, 11, C080002 Search PubMed.
- G. S. Senesi, B. Campanella, E. Grifoni, S. Legnaioli, G. Lorenzetti, S. Pagnotta, F. Poggialini, V. Palleschi and O. D. Pascale, Spectrochim. Acta, Part B, 2018, 143, 91–97 CrossRef CAS.
- X. Wang, G. Zhang, A. Li, Y. Wang, H. Cui, W. Zhao and L. Qiu, Spectrochim. Acta, Part B, 2023, 207, 106759 CrossRef CAS.
- A. Cugerone, B. Cenki-Tok, E. Oliot, M. Muñoz, F. Barou, V. Motto-Ros and E. Le Goff, Geology, 2020, 48, 236–241 CrossRef CAS.
- D. Fougerouse, A. Cugerone, S. M. Reddy, K. Luo and V. Motto-Ros, Geochim. Cosmochim. Acta, 2023, 346, 223–230 CrossRef CAS.
- S. Park, J. Lee, E. Kwon, D. Kim, S. Shin, S. Jeong and K. Park, Int. J. Precis. Eng. Manuf. – Green Technol., 2022, 9, 695–707 CrossRef.
- M. Weiss, Z. Gajarska, H. Lohninger, M. Marchetti-Deschmann, G. Ramer, B. Lendl and A. Limbeck, Anal. Chim. Acta, 2022, 1195, 339422 CrossRef CAS PubMed.
- W. Song, Y. Fu, S. Zhao, Y. Zhao, H. Wang and Z. Wang, Constr. Build. Mater., 2023, 392, 131834 CrossRef.
- H. Hou, L. Cheng, T. Richardson, G. Chen, M. Doeff, R. Zheng, R. Russo and V. Zorba, J. Anal. At. Spectrom., 2015, 30, 2295–2302 RSC.
- F. Trichard, F. Gaulier, J. Barbier, D. Espinat, B. Guichard, C. P. Lienemann, L. Sorbier, P. Levitz and V. Motto-Ros, J. Catal., 2018, 363, 183–190 CrossRef CAS.
- L. Jolivet, L. Catita, O. Delpoux, C. P. Lienemann, L. Sorbier and V. Motto-Ros, J. Catal., 2021, 401, 183–187 CrossRef CAS.
- D. V. Babos, J. F. K. Ramos, G. C. Francisco, V. D. M. Benites and D. M. B. P. Milori, J. Opt. Soc. Am. B, 2023, 40, 654–660 CrossRef CAS.
- R. R. V. Carvalho, J. A. O. Coelho, J. M. Santos, F. W. B. Aquino, R. L. Carneiro and E. R. Pereira-Filho, Talanta, 2015, 134, 278–283 CrossRef CAS PubMed.
- W. Berthou, M. Legallais, B. Bousquet, V. Motto-Ros and F. L. Cras, Spectrochim. Acta, Part B, 2024, 215, 106906 CrossRef CAS.
- C. M. Ahamer, K. M. Riepl, N. Huber and J. D. Pedarnig, Spectrochim. Acta, Part B, 2017, 136, 56–65 CrossRef CAS.
- J. Fernandes, L. Sorbier, S. Hermelin, J.-M. Benoit, C. Dujardin, C.-P. Lienemann, J. Bernard and V. Motto-Ros, Spectrochim. Acta, Part B, 2024, 221, 107047 CrossRef CAS.
- W. Berthou, M. Legallais, S. Sorieul, G. Yildirim, B. Bousquet, V. Motto-Ros and F. Le Cras, Adv. Energy Mater., 2024, 14, 2400656 CrossRef CAS.
- B. le Bellego, V. Motto-Ros, B. Luais, C. Fabre, C. Dalou, P. Condamine and L. Tissandier, Spectrochim. Acta, Part B, 2024, 222, 107059 CrossRef CAS.
- D. Zhang, Y. Chu, S. Ma, Z. Sheng, F. Chen, Z. Hu, S. Zhang, B. Wang and L. Guo, Appl. Surf. Sci., 2020, 534, 147601 CrossRef CAS.
- Y. Meng, C. Gao, Z. Lin, W. Hang and B. Huang, Nanoscale Adv., 2020, 2, 3983–3990 RSC.
- F. Trichard, L. Sorbier, S. Moncayo, Y. Blouët, C. P. Lienemann and V. Motto-Ros, Spectrochim. Acta, Part B, 2017, 133, 45–51 CrossRef CAS.
- L. Brunnbauer, M. Jirku, C. D. Quarles Jr and A. Limbeck, Talanta, 2024, 269, 125500 CrossRef CAS PubMed.
- J. O. Caceres, F. Pelascini, V. Motto-Ros, S. Moncayo, F. Trichard, G. Panczer, A. Marín-Roldán, J. A. Cruz, I. Coronado and J. Martín-Chivelet, Sci. Rep., 2017, 7, 5080 CrossRef CAS PubMed.
- N. Hausmann, P. Siozos, A. Lemonis, A. C. Colonese, H. K. Robson and D. Anglos, J. Anal. At. Spectrom., 2017, 32, 1467–1472 RSC.
- L. Krajcarova, K. Novotny, M. Kummerova, J. Dubova, V. Gloser and J. Kaiser, Talanta, 2017, 173, 28–35 CrossRef CAS PubMed.
- C. Zhao, D. Dong, X. Du and W. Zheng, Sensors, 2016, 16, 1764 CrossRef PubMed.
- R. R. Gamela, M. A. Speranca, D. F. Andrade and E. R. Pereira-Filho, Anal. Methods, 2019, 11, 5543–5552 RSC.
- Q. Tang, M. Zhong, P. Yin, Z. Zhang, Z. Chen, G. Wu and Q. Lin, Spectrosc. Spectr. Anal., 2023, 43, 1485–1488 CAS.
- Y. Dixit, M. P. Casado-Gavalda, R. Cama-Moncunill, X. Cama-Moncunill, M. Markiewicz-Keszycka, F. Jacoby, P. J. Cullen and C. Sullivan, J. Food Eng., 2018, 216, 120–124 CrossRef CAS.
- M. Lopez-Lopez, C. Alvarez-Llamas, J. Pisonero, C. Garcıa-Ruiz and N. Bordel, Forensic Sci. Int., 2017, 273, 124–131 CrossRef CAS PubMed.
- N. Herreyre, A. Cormier, S. Hermelin, C. Oberlin, A. Schmitt, V. Thirion-Merle, A. Borlenghi, D. Prigent, C. Coquide, A. Valois, C. Dujardin, P. Dugourd, L. Duponchel, C. Comby-Zerbino and V. Motto-Ros, J. Anal. At. Spectrom., 2023, 38, 730–741 RSC.
- Y. Zhang, X. Ren, Z. Chen, W. Chen, Z. Zhang, X. Liu, W. Xu, J. Liu and C. Li, Remote Sens., 2023, 15, 1494 CrossRef.
- L. Guo, Z. Hao, M. Shen, W. Xiong, X. He, Z. Xie, M. Gao, X. Li, X. Zeng and F. Lu, Opt. Express, 2013, 21, 18188–18195 CrossRef CAS PubMed.
- J. Yu, Z. Hou, Y. Ma, T. Li, Y. Fu, Y. Wang, Z. Li and Z. Wang, Spectrochim. Acta Part B At. Spectrosc., 2020, 174, 105992 CrossRef CAS.
- W. Song, Z. Song, J. Vincent, H. Wang and Z. Wang, Talanta, 2020, 216, 120920 CrossRef CAS PubMed.
- T. A. Alrebdi, A. Fayyaz, H. Asghar, A. Zaman, M. Asghar, F. H. Alkallas, A. Hussain, J. Iqbal and W. Khan, Molecules, 2022, 27, 3754 CrossRef CAS PubMed.
- Z. Lu, D. Zhang, W. Wang, F. Chen, Y. Xu, J. Nie, Y. Chu and L. Guo, TrAC, Trends Anal. Chem., 2022, 152, 116618 CrossRef.
- Y. Fu, Z. Hou, T. Li, Z. Li and Z. Wang, Spectrochim. Acta, Part B, 2019, 155, 67–78 CrossRef CAS.
- Y. Fu, W. Gu, Z. Hou, S. A. Muhammed, T. Li, Y. Wang and Z. Wang, Front. Phys., 2021, 16, 22502 CrossRef.
- S. Zhao, C. Song, X. Gao and J. Lin, Results Phys., 2019, 15, 102736 CrossRef.
- J. Shao, Y. Zhang and A. Chen, Photonics, 2023, 10, 783 CrossRef CAS.
- C. P. D. Morais, D. V. Babos, V. C. Costa, J. B. Neris, G. Nicolodelli, M. C. Mitsuyuki, F. F. Mauad, S. Mounier and D. M. B. P. Milori, Sci. Total Environ., 2022, 837, 155699 CrossRef PubMed.
- Q. Wang, A. Chen and X. Gao, J. Anal. At. Spectrom., 2024, 39, 261–268 RSC.
- Y. Wang, X. He and C. Wang, J. Anal. At. Spectrom., 2023, 38, 1643–1651 RSC.
- S. J. Chen, A. Iqbal, M. Wall, C. Fumeauxa and Z. T. Alwahabi, J. Anal. At. Spectrom., 2017, 32, 1508–1518 RSC.
- Y. Ikeda, J. Ampadu Ofosu and I. Wakaida, Spectrochim. Acta, Part B, 2020, 171, 105933 CrossRef CAS.
- S. Ma, Y. Liu, H. Tian, L. Guo and D. Dong, J. Anal. At. Spectrom., 2023, 38, 342–346 RSC.
- Y. Song, W. Song, L. Li, W. Gu, K. Kou, M. S. Afgan, Z. Hou, Z. Li and Z. Wang, Opt. Lasers Eng., 2023, 162, 107433 CrossRef.
- S. Zhao, X. Gao, A. Chen and J. Lin, Appl. Phys., 2020, 126, 7 CrossRef CAS.
- Z. Wang, Z. Hou, S. Lui, D. Jiang, J. Liu and Z. Li, Opt. Express, 2012, 20, A1011–A1018 CrossRef PubMed.
- M. A. Khan, S. Bashir, N. A. Chishti, E. Bonyah, A. Dawood and Z. Ahmad, AIP Adv., 2023, 13, 015017 CrossRef CAS.
- S. Mushtaq, K. Siraj, M. S. A. Rahim, S. U. Q. Younas, W. Asad, N. Shahzad and A. Latif, Appl. Spectrosc., 2023, 77, 393–404 CrossRef CAS PubMed.
- Y. Wang, Y. Bu, Y. Cai and X. Wang, J. Anal. At. Spectrom., 2023, 38, 121–130 RSC.
- J. Yu, Z. Hou, Y. Ma, T. Li, Y. Fu, Y. Wang, Z. Li and Z. Wang, Spectrochim. Acta, Part B, 2020, 174, 105992 CrossRef CAS.
- S. Zhao, Y. Zhao, Z. Hou and Z. Wang, Spectrochim. Acta, Part B, 2023, 203, 106666 CrossRef CAS.
- Z. Hou, M. S. Afgan, S. Sheta, J. Liu and Z. Wang, J. Anal. At. Spectrom., 2020, 35, 1671–1677 RSC.
- S. Zhao, Y. Zhao, Y. Dai, Z. Liu and X. Gao, Spectrochim. Acta, Part B, 2024, 218, 106982 CrossRef CAS.
- M. Yamanaka, N. I. Smith and K. Fujita, Microscopy, 2014, 63, 177–192 CrossRef PubMed.
- C. Zhou and A. Xiong, Sensors, 2023, 23, 1923 CrossRef PubMed.
- Y. Huang, W. Bian, B. Jie, Z. Zhu and W. Li, Signal Image Video P, 2024, 18, 1619–2641 Search PubMed.
- X. Chen, Y. Wu, J. Chen, J. Wang and K. Zeng, IET Signal Process., 2023, 17, e12205 CrossRef.
- Z. Wang, T. Yuan, Z. Hou, W. Zhou, J. Lu, H. Ding and X. Zeng, Front. Phys., 2014, 9, 419–438 CrossRef.
- S. Zhao, Z. Hou and Z. Wang, J. Anal. At. Spectrom., 2024, 39, 306–309 RSC.
- A. Nardecchia, A. D. Juan, V. Motto-Ros, C. Fabre and L. Duponchel, Spectrochim. Acta Part B At. Spectrosc., 2022, 198, 106571 CrossRef CAS.
- M. Hola, K. Novotny, J. Dobes, I. Krempl, V. Wertich, J. Mozola, M. Kubes, V. Faltusova, J. Leichmann and V. Kanicky, Spectrochim. Acta, Part B, 2021, 186, 106312 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |