In situ Re–Os geochronology of Re-rich Palaeogene molybdenite by LA-ICP-MS/MS†
Stijn
Glorie
 *a,
Jay M.
Thompson
*a,
Jay M.
Thompson
 b,
Sarah E.
Gilbert
b,
Sarah E.
Gilbert
 c and
A. Kate
Souders
c and
A. Kate
Souders
 b
b
aDept. of Earth Sciences, University of Adelaide, Adelaide, SA 5005, Australia. E-mail: stijn.glorie@adelaide.edu.au
bU.S. Geological Survey, Denver, CO 80225, USA
cAdelaide Microscopy, University of Adelaide, Adelaide, SA 5005, Australia
First published on 23rd April 2025
Abstract
In situ Re–Os geochronology by LA-ICP-MS/MS was previously demonstrated by reacting Os with CH4 or N2O reaction gasses. However, for both reactions, a minor proportion of the Re parent isotope also reacts, potentially leading to significant isobaric interferences of 187Re on 187Os, especially for young samples with little radiogenic in-growth. Here we present an interlaboratory comparison and compare three reaction gas mixtures (CH4 + H2 + He, N2O and N2O + He) with the aim to robustly date Palaeogene (66–23 Ma) molybdenite from the Bingham Canyon and Henderson deposits. CH4 mixed with H2 gas gives the highest sensitivity, while N2O and He gas buffer Re reaction. On balance, the analytical method involving N2O + He reaction gas is most suitable for dating Palaeogene molybdenite, resulting in age precision of 2.6% for Bingham and 5.8% for Henderson. For older, >1 Ga molybdenite, CH4 + H2 + He may give comparatively better age precision.
Introduction
Molybdenite Re–Os geochronology is widely used in ore and hydrocarbon exploration (e.g. ref. 1 and 2). The conventional analytical approach involves isotope dilution followed by isotope ratio measurements with a Thermal Ionization Mass Spectrometer (TIMS), which is a laborious and time-consuming method that is conducted at highly specialized laboratories.3,4 Recent developments in reaction gas mass-spectrometry now allow Re and Os isotopes to be rapidly measured in situ using laser ablation inductively coupled plasma tandem mass-spectrometry (LA-ICP-MS/MS) at high spatial resolution.5–7 Hogmalm et al.5 and Tamblyn et al.6 demonstrated that Os efficiently reacts with CH4 to form OsCH2+, inducing a +14 amu mass-shift. This reaction occurs at a much higher rate (ca. 120×) compared to isobaric ReCH2+ production. However, the ca. 1–2% Re reaction accounts for potentially significant interference on mass 201 amu (187Os12C1H2, referred here as 187+14Os), especially for young samples with relatively low 187Os ingrowth. More recently, Simpson et al.7 showed that Os reacts with N2O to form OsO4+, inducing a +64 amu mass-shift for 187Os16O4 (referred here as 187+64Os). The equivalent reaction of Re can be reduced to ca. 0.15%, which is about an order of magnitude lower than for the CH4 method.6 However, while the interference correction is larger, generally, higher sensitivity (count rates) can be achieved with the CH4 method. The obtainable precision on the resulting Re–Os date is a balance between (1) increasing sensitivity and better counting statistics, and (2) reducing the interference correction, which also reduces count rates. Here we explore the limitations of the LA-ICP-MS/MS method on Cenozoic (<66 Ma) samples, with both the N2O and CH4 reaction gas methods. In addition to published reaction gas methodologies, we also explore the effects of mixing reaction gasses by adding H2 and/or He to CH4 or N2O in the reaction cell. We further present the first interlaboratory comparison for the in situ Re–Os molybdenite dating method.Sample descriptions
Bingham Canyon molybdenite
Molybdenite was sampled from the high-grade ore zone of the Bingham Canyon porphyry deposit in northern Utah, United States (US). This sample is a porphyritic intrusive that contains the following minerals: quartz (45%, 2 to 10 mm), altered feldspar (40%, 2 to 5 mm), biotite (∼2%, 0.2 to 0.5 mm), chalcopyrite (∼1%, <0.2 mm) and molybdenite (∼12%, 0.5 to >5 mm). Molybdenite occurs as aggregates and veins up to 10 mm in size of several millimetre-sized individual molybdenite crystals. The selected molybdenite grains were 0.5 to 2 mm in size and separated from the whole rock sample by gentle crushing and picking of grains onto double-sided tape prior to mounting in epoxy resin. The sample was then polished using fine SiC sandpaper (1000 and 2000 grit), finished using 1 μm suspended diamond paste and cleaned with ethanol. The age of the molybdenite from this deposit is dated by conventional N-TIMS Re–Os at 37.0 ± 0.27 Ma.8 The reported uncertainty is 2 SEM (=2 standard error of the mean).Henderson mine/RM 8599
Molybdenite was sampled from the high-grade ore concentrate in the Henderson mine, Colorado, US. This sample was measured as individual molybdenite grains from the original porphyritic rock sample as well as the mechanically homogenized RM 8599 powder purchased from the National Institute of Standards and Technology (NIST). The molybdenite grains from the whole rock sample were 0.5 to 2 mm in size and separated from the rock matrix by gentle crushing and picking of grains onto double-sided tape prior to mounting in epoxy resin. The sample was then polished using fine SiC sandpaper (1000 and 2000 grit), finished using 1 μm suspended diamond paste and cleaned with ethanol. The RM 8599 powder was prepared by pressing ∼2 grams into a 11 mm pellet at 10 tons of pressure (30 second holding time). The conventional N-TIMS Re–Os reference age of the RM 8599 sample is 27.656 ± 0.022 Ma.9 The individual molybdenite grains are assumed to be the same age as this molybdenite powder.Analytical methods
The molybdenite samples were analysed at the United States Geological Survey (USGS) Denver Federal Centre (Geology, Geophysics, and Geochemistry Science Center), Colorado, US and Adelaide Microscopy, University of Adelaide, Australia, for laboratory comparison purposes.US geological survey
At the USGS the analyses were performed in the USGS-LTRACE laboratory. A total of 6 analytical sessions are reported. Analyses from 2021 and 2022 were conducted using a Photon Machines Analyte G2 laser system with an ATL ArF excimer source operating at 193 nm wavelength and ∼5 n pulse width. Analyses from 2023 onward were conducted using a RESOlution-SE 193 nm laser ablation system also with an ATL excimer laser source. Both laser systems were coupled to an Agilent 8900x ICP-MS/MS. The laser fluence was varied between 3.5 and 6 J cm−2, depending on the session, spot size was between 80 and 120 microns, and laser repetition rate was between 10 and 20 Hz. All analyses were performed in a helium atmosphere and signal smoothing of laser pulses was achieved using the ‘squid’ signal smoother. Nitrogen (N2) was added to the Ar carrier gas before the ICP-MS to increase sensitivity.The ICP-MS tuning was first performed in single-quad mode for maximum heavy mass sensitivity while achieving a ThO/Th rate of <0.2% and U/Th <1.1 for the S-155 ablation cell and ∼1.2 for the HelEx cell (Analyte G2). Tuning was performed using the NIST612 glass with a ∼40 micron square beam (38 micron beam for the RESOlution-SE system), 10 Hz, 3.5 J cm−2 and 3 microns per s line scan speed. Under these conditions, the count rate for 238U was ∼1 Mcps. Once optimized in single-quad mode, the instrument was set to MS/MS mode with reaction gases CH4 (6% or 0.07 ml min−1), He (4.8 to 6.3 ml min−1) and H2 (5.0 to 5.4 ml min−1). See ESI 1†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 for further ICP-MS/MS setting details. The 185Re12CH2/185Re ratio was monitored during tuning and reaction gas flow rates and octupole settings were adjusted to minimize this ratio (∼0.3 to ∼0.4) while still maintaining sensitivity for the 185Re signal. The MASS-3 FeS pressed powder from the USGS was used for monitoring Os signal, but tuning specifically for Os was not feasible due to heterogeneities in the Os content of this material (5 to 10% variation). The isotopes measured during analysis vary between sessions (ESI 1†).10 Isotopes measured in each session (with dwell times in milliseconds in parenthesis) are: 57Fe (2), 185Re (20), 185+14Re (50–80), 187Os (20), 187+14Os (200), 188+14Os (10), 189+14Os (200).
10 for further ICP-MS/MS setting details. The 185Re12CH2/185Re ratio was monitored during tuning and reaction gas flow rates and octupole settings were adjusted to minimize this ratio (∼0.3 to ∼0.4) while still maintaining sensitivity for the 185Re signal. The MASS-3 FeS pressed powder from the USGS was used for monitoring Os signal, but tuning specifically for Os was not feasible due to heterogeneities in the Os content of this material (5 to 10% variation). The isotopes measured during analysis vary between sessions (ESI 1†).10 Isotopes measured in each session (with dwell times in milliseconds in parenthesis) are: 57Fe (2), 185Re (20), 185+14Re (50–80), 187Os (20), 187+14Os (200), 188+14Os (10), 189+14Os (200).
The correction for reacted Re with the CH4 gas was calculated using Os-free NIST612 glass using the mass shifted Re at masses 199 (185Re12CH2) and 201 (187Re12CH2) and the methodology presented in ref. 5 and 6 assuming natural Re abundances (185Re/187Re = 0.59738 ± 0.00039 (ref. 11)). Subsequently, an in-house Moly Hill molybdenite was used to calibrate the Re/Os ratio of the Henderson and Bingham molybdenites assuming an age of 2680 ± 90 Ma (187Os/187Re = 0.04566 ± 0.00153).12 Note that this is a different piece of Moly Hill molybdenite to the reference material characterised in ref. 6188Os/187Os ratios are not reported for the USGS data as all 189Os data (used as a proxy for 188Os) were effectively below detection limit. Data reduction, involving background subtraction, interference, drift corrections, and ratio normalisation, were conducted using the LADR software v. 1.1.7.13 Given interference subtracted count rates on 187+14Os in the time-resolved signals fall occasionally below zero, LADR fails to accurately calculate the signal precision uncertainty on the corrected 185Re/187+14Os ratios. Hence, signal precision uncertainties were calculated manually using spreadsheets by setting negative values to zero prior to calculating the standard deviation on the 187+14Os signal. All other sources of uncertainty (Table 1) are subsequently propagated to the calculated signal precision uncertainties. Reported fully propagated uncertainties on the isotope ratios are 2 SEM. No correction for down-hole Re–Os fractionation was made.6 Age calculations were conducted as weighted means in IsoplotR from the corrected 187Os/187Re ratios14 and age uncertainties are reported as 95% confidence uncertainties.
| Propagated uncertainties | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adelaide | USGS | ||||||||
| Systematic uncertainties | S1: 7/12/2023 | S2a: 14/12/2023 | S2b: 14/12/2023 | S1: 17/11/2021 | S2: 22/11/2021 | S3: 23/11/2021 | S4: 4/07/2022 | S5: 29/9/2023 | S6: 26/6/2024 |
| Calibration curve missfit Re/Os ratio | 0.92% | 0.86% | 1.69% | 1.39% | 1.71% | 0.61% | 1.02% | 0.74% | 0.56% |
| Calibration curve missfit Os/Os ratio | 0.55% | 0.62% | 0.62% | 0.34% | 0.38% | 0.51% | 0.72% | 0.04% | 0.24% |
| Uncertainty in measured Re/Os ratio for RM (Qmoly Hill) | 0.11% | 0.14% | 0.24% | 0.36% | 0.25% | 0.24% | 0.50% | 0.26% | 0.24% |
| Uncertainty in measured Os/Os ratio for RM (NiS3) | 0.09% | 0.14% | 0.14% | 0.23% | 0.21% | 0.20% | 0.32% | 0.16% | 0.11% |
| Uncertainty in mass bias | 0.04% | 0.12% | 0.11% | 0.34% | 0.37% | 0.35% | 0.24% | 0.34% | 0.14% |
| Long-term reproducibility of reference materials | Not propagated uncertainties (insufficient data) | ||||||||
| Random uncertainties |
|---|
| Signal precision of interference corrected 187+XOs |
| Signal precision of 185Re |
| Signal precision of 189+XOs |
| Uncertainty in blank subtraction |
| Uncertainty in interference correction factor (∼signal precision of 185+XRe) |
| Added age uncertainty for overdispersion if present (IsoplotR) |
| Constants | |
|---|---|
| Uncertainty in reference IDTIMS Re/Os ratio for RM (Qmoly Hill) | 0.38% |
| Theoretical uncertainty in reference Os/Os ratio for RM (NiS3) | 0.10% |
| Uncertainty in decay constant (IsoplotR default) | 0.51% |
| Uncertainty in initial Os/Os ratio anchor (IsoplotR default) | 0.06% |
Adelaide microscopy
At Adelaide microscopy, Re–Os isotope analysis was conducted on a RESOlution-SE 193 nm laser ablation system coupled to an Agilent 8900× ICP-MS/MS over two analytical sessions. The molybdenites were sampled by static spot ablation at 3 J cm−2 and the aerosol was transported to the plasma in a gas atmosphere of 1 l min−1 Ar, 0.38 l min−1 He and 4 ml min−1 N2. Given the absence of Re–Os down-hole fractionation,6 laser beam diameters and repetition rates were variable (30–100 μm, 7–10 Hz) between reference materials and samples, with the aim to maximize count rates while keeping Re count rates under the pulse/analog threshold for the detector (see ESI 1† for details).For each session, the mass-spectrometer was first tuned in absence of reaction gas to demonstrate a robust plasma (e.g. ThO/Th rate of <0.2% and U/Th <1.1). Subsequently, for session 1, a mixture of CH4 (0.22 ml min−1) + He (5 ml min−1) + H2 (6 ml min−1) was used in the reaction cell, tuned to maximise count rates. H2 was used to enhance sensitivity, while He was used to buffer 187Re12CH2 interference production. In the second session, N2O (0.32 ml min−1) was used as the reaction gas, first (session 2a) without added He (maximum sensitivity) and secondly (session 2b) with added He (5 ml min−1) to reduce the interference. Lense parameters and reaction cell settings were similar between both methods, detailed in ESI 1.† The isotopes measured during analysis vary between sessions (ESI 1†). Isotopes measured in each session (with dwell times in milliseconds in parenthesis) are: 95Mo (2), 185Re (20), 185+XRe (50–100), 187Os (50), 187+XOs (100), 189Os (50), 189+XOs (100–200). 189+XOs was measured as a proxy for ‘common’ 188Os.
The measured 185Re/187+xOs ratios (with x = 14 amu for CH4 method, x = 64 amu for N2O method) were corrected for 187+xRe interference on 187+xOs, taking into account the mass-bias on the 187Re/185Re ratio, measured in Os-free NIST610 glass (see details in ESI 1†), and subsequently calibrated to the QMolyHill reference molybdenite (N-TIMS 187Os/187Re ratio = 0.044699 ± 0.000166, age = 2624 ± 5 Ma, 2SEM uncertainties6). The 188Os/187Os ratios were calibrated using NiS-3,15 using measured 189+XOs as a proxy for 188Os and assuming a present-day 188Os/187Os ratio of 6.740 ± 0.004.16 Data reduction, involving background subtraction, interference, drift corrections, and ratio normalisation, were conducted using the LADR software v. 1.1.7.13 As above, signal precision uncertainties were calculated manually using a script by setting negative values to zero prior to calculating the standard deviation on the 187+14Os signal. All other sources of uncertainty (Table 1) are subsequently propagated to the calculated signal precision uncertainties. Reported fully propagated uncertainties on the isotope ratios are 2 SEM. Age calculations were conducted as weighted means in IsoplotR from the corrected 187Os/187Re ratios14 and age uncertainties are reported as 95% confidence uncertainties.
Reference molybdenite M252 from the Merlin deposit (Queensland, Australia) was used as secondary reference material to verify accuracy in isotope ratio determinations (N-TIMS 187Os/187Re ratio = 0.025649 ± 0.000105, age = 1520 ± 4 Ma (ref. 6)). The obtained Re–Os dates are 1505 ± 16 Ma (session 1), 1500 ± 20 Ma (session 2a) and 1514 ± 28 Ma (session 2b), in agreement with the reference age. Isotopic ratio uncertainties and age uncertainties are quoted as 2 standard error of the mean.
Results
Sensitivity and interferences
For the USGS sessions (all with CH4 + H2 + He reaction gas, abbreviated as U-sessions), the average sensitivity measured for a 40 μm/10 Hz laser beam on 185Re (measured on NIST-612) varied between ca. 5.7 and 10.3 kcps ppm−1. For the Adelaide sessions (with variable reaction gas mixtures, abbreviated as A-sessions), the average sensitivity for a 50 μm/10 Hz spot ablation on 185Re (measured on NIST-610) was 9.1 kcps ppm−1 for A-session 1 (CH4 + H2 + He), 7.3 kcps ppm−1 for A-session 2a (N2O), and 6.3 kcps ppm−1 for A-session 2b (N2O + He). While the CH4-method (U-sessions and A-session 1) produced the highest sensitivity, it also induced the highest Re interference with ca. 0.5% (average USGS) and ca. 0.6% (average Adelaide) Re reacting to form ReCH2+ (Table 2). This is ca. 45–65% lower compared to previously reported Re reaction rates in absence of H2 in the reaction cell.6,7 For the N2O method, ca. 0.17% Re reacts to the equivalent ReO4+ reaction product (A-session 2a), which is further reduced to 0.02% with added He (5 ml min-1; A-session 2b). Hence, although count rates are compromised, the N2O + He method requires a much smaller 187+xRe interference correction on 187+xOs. For example, on the secondary reference molybdenite (M252), the interference correction requires removal of 28% Re from Os on mass 187 + 14 amu in A-session 1, 10% on mass 187 + 64 amu for A-session 2a and 3% on mass 187 + 64 amu for A-session 2b (Table 2). Applied to the Cenozoic molybdenite samples, which are much younger and thus have considerably less radiogenic 187Os ingrowth compared to the Mesoproterozoic M252 molybdenite, the interference correction accounts for ca. 87–97% in the U-Sessions, 93–95% in A-session 1, 83–87% in A-session 2a and 49–58% in A-session 2b.| Session | Reaction gas | n | 185Re (cps) | ±2SEM | 185+xRe (cps) | ±2SEM | 187+xOsb (cps) | ±2SEM | 187+xOsb (cps) | ±2SEM | Interf.e (%) | Re RR (%) | Age (Ma)g | ±CI (Ma) | ±CIh (%) | MSWDi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adelaide | Measured | Correctedd | ||||||||||||||
| a All cps (=counts per second) values are back-ground substracted. b x = 14 amu for CH4 reaction to OsCH2+, = 64 amu for N2O reaction to OsO4+. c n = Number of analyses per sample. d Corrected refers to the interference correction of 187+xRe on 187+xOs by cps subtraction. e Interf. is the percentage inteference of 187+xRe on 187+xOs. f Re RR is the Re reaction rate calculated as the ratio of 185+xRe on 185Re. g Age is the calculated weighted mean Re–Os age in IsoplotR. h ±CI is the 95% confidence interval uncertainty on the age, calculated using added uncertainty for over dispersion where required. The second number also includes the uncertainty on the decay constant. % is only reported for the maximum propagated uncertainty. i MSWD = Mean squared weighted deviation. | ||||||||||||||||
| QMolyHill primary RM (IDTIMS: 2624 ± 5 Ma) | ||||||||||||||||
| 1 | CH4 + He + H2 | 30 | 545![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 008 008 |
58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 015 015 |
3120 | 334 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 097 097 |
3184 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 746 746 |
2611 | 18% | 0.57% | 2625 | 9|28 | 1.1% | 1.0 |
| 2a | N2O | 16 | 462![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 690 690 |
97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 879 879 |
863 | 198 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 508 508 |
5605 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 023 023 |
5265 | 6% | 0.19% | 2625 | 12|29 | 1.1% | 1.0 |
| 2b | N2O + He | 6 | 518![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 434 434 |
95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 257 257 |
118 | 19 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 167 167 |
2783 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 968 968 |
2750 | 2% | 0.02% | 2624 | 38|46 | 1.8% | 1.0 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||||||
| M252 secondary RM (IDTIMS: 1520 ± 4 Ma) | ||||||||||||||||
| 1 | CH4 + He + H2 | 30 | 790![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 180 |
149![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 834 834 |
4463 | 838 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 946 946 |
5283 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 307 307 |
3848 | 28% | 0.56% | 1505 | 6.5|16 | 1.1% | 0.83 |
| 2a | N2O | 16 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 574 574![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 831 831 |
243![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 405 405 |
2766 | 418 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 691 691 |
7116 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 929 929 |
6397 | 10% | 0.18% | 1500 | 11|20 | 1.3% | 1.9 |
| 2b | N2O + He | 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 953 953![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 411 411 |
761![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 730 730 |
474 | 196 | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 925 925 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 571 571 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 107 107 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 235 235 |
3% | 0.02% | 1514 | 23|28 | 1.8% | 0.5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||||||
| Bingham (IDTIMS: 37.0 ± 0.27 Ma) | ||||||||||||||||
| 1 | CH4 + He + H2 | 28 | 879![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 007 007 |
64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 792 792 |
5150 | 379 | 9499 | 701 | 683 | 168 | 93% | 0.59% | 45.4 | 1.9|2.0 | 4.4% | 0.62 |
| 2a | N2O | 18 | 724![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 950 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 786 786 |
1193 | 58 | 2495 | 121 | 443 | 68 | 83% | 0.16% | 36.5 | 1.3|1.3 | 3.6% | 0.52 |
| 2b | N2O + He | 18 | 711![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 728 728 |
38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 923 923 |
157 | 9 | 542 | 29 | 272 | 30 | 49% | 0.02% | 37.9 | 0.9|1.0 | 2.6% | 0.35 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||||||
| Henderson (IDTIMS: 27.656 ± 0.022 Ma) | ||||||||||||||||
| 1 | CH4 + He + H2 | 26 | 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280 280 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 584 584 |
1185 | 162 | 2151 | 296 | 121 | 38 | 95% | 0.59% | 30.9 | 3.9|3.9 | 13% | 3.7 |
| 2a | N2O | 18 | 117![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 332 332 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 678 678 |
188 | 19 | 375 | 39 | 50 | 14 | 87% | 0.16% | 23.3 | 3.1|3.1 | 13% | 3.3 |
| 2b | N2O + He | 18 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 570 570 |
8575 | 23 | 2 | 66 | 6 | 26 | 7 | 58% | 0.02% | 27.5 | 1.6|1.6 | 5.8% | 1.6 |
| Session | Reaction gas | 185Re (cps) | ±2SEM | 185+xRe (cps) | ±2SEM | 187+xOsb (cps) | ±2SEM | 187+xOsb (cps) | ±2SEM | Interf.d (%) | Re RRe (%) | Agef (Ma) | ±CIg (Ma) | ±CIg (%) | MSWDh | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| USGS | Measured | Correctedc | ||||||||||||||
| Moly Hill primary RM (IDTIMS: 2680 ± 90 Ma) | ||||||||||||||||
| 1 | CH4 + He + H2 | 9 | 866![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 292 292 |
213![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 714 714 |
2719 | 669 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 328 328 |
9056 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 705 705 |
7916 | 13% | 0.31% | 2687 | 28|36 | 1.3% | 11 |
| 2 | CH4 + He + H2 | 11 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 046 046![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 608 608 |
357![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 149 149 |
6674 | 1141 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 533 533 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 383 383 |
82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 195 195 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 448 448 |
12% | 0.33% | 2689 | 28|38 | 1.4% | 0.7 |
| 3 | CH4 + He + H2 | 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 332 332![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 768 768 |
251![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 245 245 |
4248 | 803 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 408 408 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 493 493 |
53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 172 172 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 124 124 |
16% | 0.32% | 2687 | 12|29 | 1.1% | 1.6 |
| 4 | CH4 + He + H2 | 12 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 167 167![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 149 149 |
191![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 059 059 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850 |
2580 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 312 312 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 677 677 |
43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 514 514 |
8495 | 33% | 1.10% | 2689 | 24|36 | 1.3% | 0.7 |
| 5 | CH4 + He + H2 | 14 | 644![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 773 773 |
127![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330 330 |
2605 | 521 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 428 428 |
8206 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 015 015 |
7334 | 10% | 0.40% | 2690 | 12|29 | 1.1% | 0.7 |
| 6 | CH4 + He + H2 | 19 | 466![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 349 349 |
62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 345 345 |
1927 | 255 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 604 604 |
3972 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 306 306 |
3539 | 10% | 0.41% | 2690 | 2.7|27 | 1.0% | 0.8 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||||||
| Bingham (IDTIMS: 37.0 ± 0.27 Ma) | ||||||||||||||||
| 1 | CH4 + He + H2 | 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 063 063![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 763 763 |
241![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 044 044 |
3379 | 778 | 6256 | 1425 | 500 | 152 | 92% | 0.32% | 36.1 | 4.8|4.8 | 13% | 0.6 |
| 2 | CH4 + He + H2 | 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 359 359![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 430 430 |
106![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 220 220 |
4678 | 359 | 8729 | 658 | 772 | 201 | 91% | 0.34% | 39.5 | 4.1|4.1 | 10% | 0.9 |
| 3 | CH4 + He + H2 | 5 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 449 449![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 963 963 |
461![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 924 924 |
7805 | 1456 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820 820 |
2771 | 1545 | 333 | 90% | 0.32% | 42.9 | 4.1| 4.1 | 9.6% | 0.04 |
| 4 | CH4 + He + H2 | 8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 756 756![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 781 781 |
283![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 965 965 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 551 551 |
3035 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 826 826 |
5334 | 1352 | 423 | 96% | 1.06% | 36.9 | 4.0|4.0 | 11% | 0.9 |
| 5 | CH4 + He + H2 | 10 | 880![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 153 153 |
88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 079 079 |
3518 | 358 | 7010 | 629 | 777 | 265 | 86% | 0.40% | 40.0 | 3.5|3.5 | 8.8% | 0.5 |
| 6 | CH4 + He + H2 | 16 | 450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 317 317 |
48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 575 575 |
1809 | 198 | 3459 | 384 | 372 | 175 | 88% | 0.40% | 33.2 | 5.2|5.2 | 16% | 2.6 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||||||
| Henderson (IDTIMS: 27.656 ± 0.022 Ma) | ||||||||||||||||
| 1 | CH4 + He + H2 | 8 | 292![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 726 726 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 817 817 |
930 | 44 | 1701 | 95 | 116 | 37 | 93% | 0.32% | 25.5 | 5.9| 5.9 | 23% | 2.8 |
| 2 | CH4 + He + H2 | 6 | 126![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 604 604 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 237 237 |
434 | 91 | 794 | 164 | 59 | 20 | 93% | 0.34% | 29.4 | 9.1|9.1 | 31% | 4.6 |
| 3 | CH4 + He + H2 | 8 | 463![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 603 603 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 291 291 |
1497 | 34 | 2759 | 58 | 216 | 56 | 93% | 0.32% | 29.3 | 4.5| 4.5 | 15% | 2.8 |
| 4 | CH4 + He + H2 | 14 | 453![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 338 338 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 020 020 |
4537 | 115 | 8002 | 196 | 288 | 109 | 96% | 1.00% | 22.1 | 6.7 |6.7 | 30% | 6.9 |
| 5 | CH4 + He + H2 | 11 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 184 184 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 293 293 |
380 | 62 | 714 | 120 | 60 | 22 | 91% | 0.41% | 20.1 | 5.6| 5.6 | 28% | 5.4 |
| 6 | CH4 + He + H2 | 17 | 161![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 548 548 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 540 540 |
644 | 135 | 1205 | 253 | 93 | 40 | 90% | 0.40% | 26.1 | 3.3|3.3 | 13% | 1.7 |
Cenozoic molybdenite Re–Os dates
The extensive interference subtraction significantly affects the accuracy and precision (as a function of count rate statistics and age dispersion) of the in situ Re–Os dates (Table 2 and Fig. 1). When CH4 + H2 + He is used in the reaction cell (A-session 1, all U-sessions), the Re–Os dates for the Henderson molybdenites are consistently over-dispersed (MSWD between 1.7 and 6.9) and at least for one analytical session (U5), the resulting weighted mean Re–Os date is too young (20.1 ± 5.6 Ma) compared to the IDTIMS reference age (27.66 ± 0.02 Ma; Fig. 1, 2 and Table 2). For the Bingham molybdenites, the CH4 + H2 + He method in Adelaide (A-session 1) produced an inaccurate date of 45.4 ± 2.0 Ma, compared to the IDTIMS reference age of 37.0 ± 0.3 Ma (Fig. 1 and Table 2). Furthermore, precision is compromised with the CH4 + H2 + He method, producing fully propagated age uncertainties up to 13% in Adelaide and as high as 31% at the USGS. Age precision and accuracy is improved with the N2O reaction gas (A-session 2a), producing dates of 36.5 ± 1.3 Ma for Bingham (in agreement with reference age) and 23.3 ± 3.1 Ma for Henderson (younger than reference age). Age dispersion remains large for Henderson with an MSWD of 3.3. For A-session 2b, where He is added to N2O in the reaction cell, both molybdenite dates are accurate and at the highest precision: 37.9 ± 1.0 Ma (2.6% uncertainty) for Bingham and 27.5 ± 1.6 Ma (5.8% uncertainty) for Henderson. For both samples, the dataset statistically constitutes a single age population (MSWD = 0.35 for Bingham and 1.6 for Henderson). For the N2O ± He sessions, the background and interference subtracted count rates on 187+64Os are ≤ 50 cps for the Henderson molybdenite, approaching the limits of the analytical method, while still producing accurate and precise dates.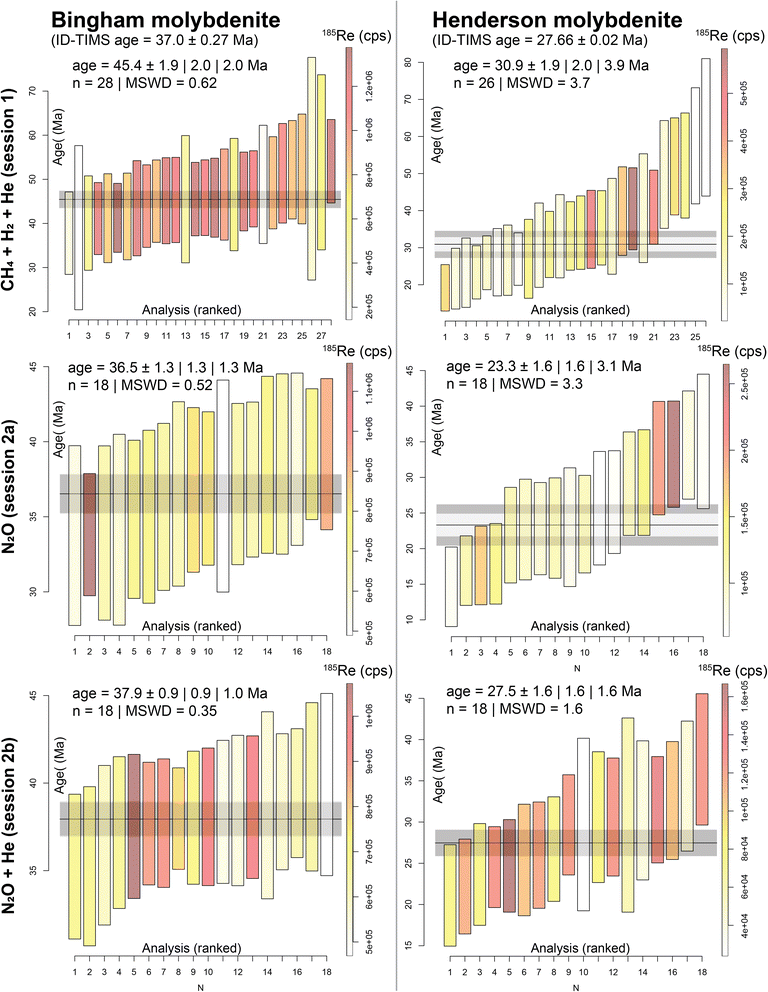 | ||
| Fig. 1 In situ Re–Os dates for the Bingham and Henderson molybdenite, analysed at Adelaide Microscopy, calculated as weighted means in IsoplotR.14 Analyses are ranked by age, plotted with 2 SEM uncertainties, and colour coded to 185Re count rate (cps). Reported weighted mean age uncertainties are 95% confidence intervals, without overdispersion, with overdispersion and with added uncertainty on the decay constant. MSWD = mean squared weighted deviation on the weighted mean Re–Os date. | ||
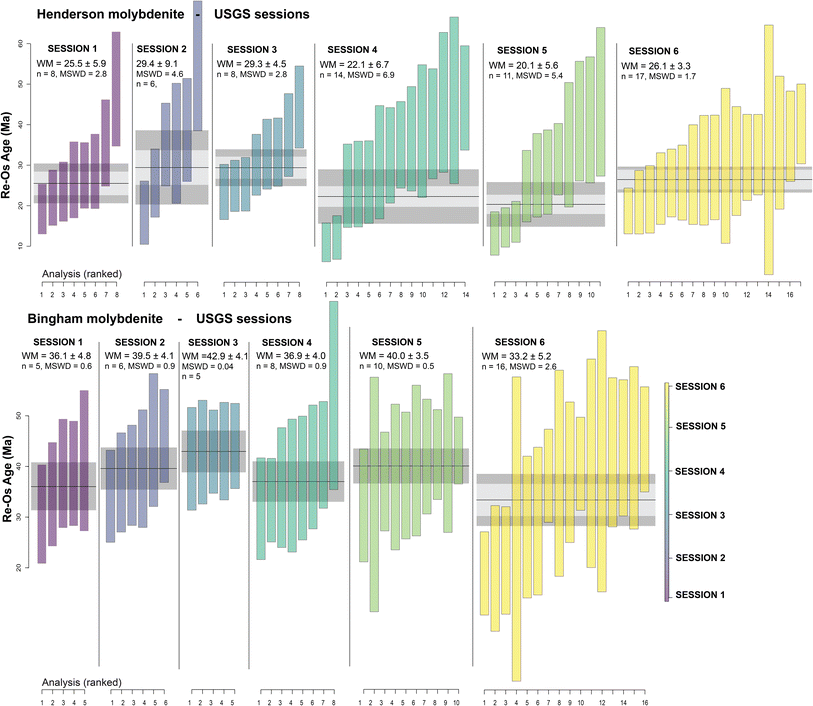 | ||
| Fig. 2 In situ Re–Os dates for the Bingham and Henderson molybdenite, analysed at the U.S. Geological Survey (USGS), calculated as weighted means in IsoplotR.14 Analyses are ranked by age and plotted with 2 SEM uncertainties. The resulting Re–Os age uncertainties are 95% confidence intervals including overdispersion (other uncertainties are shown in Tables 1 and 2). MSWD = mean squared weighted deviation on the weighted mean Re–Os date. | ||
Discussion
Interference correction in function of reaction rate and age
While it's important to maximize sensitivity (total count rates), the magnitude of the interference correction of 187+xRe on 187+xOs exerts a dominant control on the accuracy of in situ Re–Os age results, especially for young samples. Hence, an ability to predict the percentage interference would be an important screening tool prior to Re–Os analysis, increasing the likelihood of useful age calculations. Simpson et al.7 determined the interference as a function of Re reaction rate and age: 187+xRe (%) = RR × [F × (eλt − 1) + RR]−1. Given RR (= Re reaction rate) and F (=187Os transmission factor) are reaction-gas specific constants that should be largely invariable once determined for given mass-spectrometer tuning conditions, the interference correction can be predicted in function of age (Fig. 3). For Palaeogene (ca. 66–23 Ma) molybdenite, the interference is predicted to vary between ca. 87% and 95% for the CH4 + H2 + He reaction gas and between ca. 72% and 88% for the N2O reaction gas. Unless very high count rates can be measured (Re-rich molybdenite), such large correction will lead to over-dispersed and likely inaccurate dates, assuming that the samples are internally homogenous in terms of Re–Os ratios. For the N2O + He method, the interference correction remains significant (ca. 35–60%) but we demonstrate accurate and robust dates can be obtained with this approach. | ||
| Fig. 3 Percentage 187+xRe interference on 187+xOs plotted as a function of age for the three Adelaide analytical sessions with different reaction gas mixtures. Open symbols represent measured interference percentages (M.), while filled symbols were theorized (T.) based on a theorical formula from ref. 7. The curves are second-order interpolation polynomials (I.) for the theorized values. RR refers to the Re reaction rate (ratio of 185+xRe/185Re), λ is the decay constant, t is age in Ma and F is a method-specific 187Os transmission factor. For the CH4 and N2O methods, F was adapted from ref. 7 For the new N2O + He method, F was calculated as the ratio between measured and predicted interference curves. This plot can be used to predict the interference percentage based on age and method-specific constants (RR and F). | ||
Limitations and advantages of in situ Re–Os geochronology
Compared to the conventional ID-TIMS approach, higher sensitivity is required to enable accurate age determination by LA-ICP-MS/MS for young molybdenites. Therefore, Re concentrations need to be sufficiently high (185Re >100k cps) before attempting in situ Re–Os analysis. As demonstrated, an optimized gas mixture is crucial to minimize interference from 187+xRe on 187+xOs, with the N2O + He reaction gas being most promising. However, for older (Precambrian) molybdenites, Simpson et al.7 demonstrated fewer differences in obtainable age precision comparing reaction gasses, with the CH4 method potentially giving better precision for >1 Ga molybdenites. Thus, different reaction gas mixtures should be evaluated as some cater better for old versus young molybdenite samples.In contrast to ID-TIMS, which relies on bulk sample dissolution methods, the in situ method is a micro-sampling technique that has the ability to evaluate potential age zonation and/or isotopic disturbance (heterogeneity) across crystals. While age heterogeneity was not observed in the samples for this study (within the obtainable precision of a single analysis), the in situ technique is suitable for homogeneity assessments. Isotopic decoupling has been described previously3,17 but was not observed within the resolution of our analyses.
However, the most important advantage of the in situ method is the speed of analysis, where up to 1000 single spot dates can be obtained within a single (ca. 24 hours) analytical session. This opens a new window of opportunities for mineral exploration (e.g. ref. 18) that can now be extended to young (Palaeogene) molybdenite systems when Re concentrations are sufficiently high.
Conclusions
We evaluated three reaction gas mixtures for in situ (LA-ICP-MS/MS) Re–Os geochronology of young (Cenozoic) molybdenites and demonstrate that N2O (0.3 ml min−1) + He (5 ml min−1) is the optimal reaction gas mixture to sufficiently reduce the isobaric interference of Re onto Os (ca. 0.02% Re reaction rate). Robust Re–Os dates were obtained for the Re-rich Palaeogene Bingham Canyon and Henderson molybdenite, validating the approach.Data availability
All isotope ratio data and meta-data (reference materials, instrument conditions) are provided in the ESI† and ref. 10.Author contributions
S. Gl. J. T., S. E. G.: method development, analysis, data interpretation, writing, review and editing. A. K. S.: data interpretation, writing, review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. Gl. was supported by an Australian Research Council Future Fellowship (FT210100906). Jarred Lloyd is thanked for assistance with script writing for uncertainty calculations. JMT and AKS are supported by the U.S. Geological Survey Mineral Resources Program ‘From Outcrop to Ions’ project (RK00V44). William (Bill) Benzel is thanked for the sample of the Henderson Mine molybdenite. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Two anonymous reviewers are thanked for their reviews.References
- N. J. Saintilan, D. Selby, R. A. Creaser and S. Dewaele, Sci. Rep., 2018, 8, 14946 CrossRef CAS PubMed.
- A. J. Finlay, D. Selby and M. J. Osborne, Geology, 2011, 39, 475–478 CrossRef CAS.
- D. Selby and R. A. Creaser, Geochim. Cosmochim. Acta, 2004, 68, 3897–3908 CrossRef CAS.
- H. J. Stein, R. J. Markey, J. W. Morgan, J. L. Hannah and A. Scherstén, Terra Nova, 2001, 13, 479–486 CrossRef CAS.
- K. J. Hogmalm, I. Dahlgren, I. Fridolfsson and T. Zack, Miner. Deposita, 2019, 54, 821–828 CrossRef CAS.
- R. Tamblyn, S. Gilbert, S. Glorie, C. Spandler, A. Simpson, M. Hand, D. Hasterok, B. Ware and S. Tessalina, Geostand. Geoanal. Res., 2024, 48, 393–410 CrossRef CAS.
- A. Simpson, S. Glorie, S. Gilbert, R. Tamblyn and M. G. Gadd, Chem. Geol., 2024, 670, 122384 CrossRef CAS.
- J. T. Chesley and J. Ruiz, in Geology and Ore Deposits of the Oquirrh and Wasatch Mountains, Society of Economic Geologists, Utah, 1998, vol. 29 Search PubMed.
- R. Markey, H. J. Stein, J. L. Hannah, A. Zimmerman, D. Selby and R. A. Creaser, Chem. Geol., 2007, 244, 74–87 CrossRef CAS.
- J. M. Thompson and A. K. Souders, Re-Os Geochronology of Molybdenite and Metalliferous Black Shales, U.S. Geological Survey Data Release, 2025, DOI:10.5066/P13ZTY3I.
- J. W. Gramlich, T. J. Murphy, E. L. Garner and W. R. Shields, J. Res. Natl. Bur. Stand., Sect. A, 1973, 77, 691–698 CrossRef CAS PubMed.
- K. Suzuki, L. Qi, H. Shimizu and A. Masuda, Geochim. Cosmochim. Acta, 1993, 57, 1625–1628 CrossRef CAS.
- A. Norris and L. Danyushevsky, Towards estimating the complete uncertainty budget of quantified results measured by LA-ICP-MS, Goldschmidt, Boston, 2018 Search PubMed.
- P. Vermeesch, Geosci. Front., 2018, 9, 1479–1493 CrossRef CAS.
- S. Gilbert, L. Danyushevsky, P. Robinson, C. Wohlgemuth-Ueberwasser, N. Pearson, D. Savard, M. Norman and J. Hanley, Geostand. Geoanal. Res., 2013, 37, 51–64 CrossRef CAS.
- J. Völkening, T. Walczyk and K. G. Heumann, Int. J. Mass Spectrom. Ion Processes, 1991, 105, 147–159 CrossRef.
- H. Stein, A. Scherstén, J. Hannah and R. Markey, Geochim. Cosmochim. Acta, 2003, 67, 3673–3686 CrossRef CAS.
- A. Simpson, S. Glorie, M. Hand, S. E. Gilbert, C. Spandler, M. Dmitrijeva, G. Swain, A. Nixon, J. Mulder and C. Münker, Geosci. Front., 2024, 15, 101867 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ja00030k |
| This journal is © The Royal Society of Chemistry 2025 |
