Droplets in open microfluidics: generation, manipulation, and application in cell analysis
Jiaxu
Lin
,
Ying
Hou
,
Qiang
Zhang
and
Jin-Ming
Lin
 *
*
Department of Chemistry, Beijing Key Laboratory of Microanalytical Methods and Instrumentation, Key Laboratory of Bioorganic Phosphorus Chemistry and Chemical Biology (Ministry of Education), Tsinghua University, Beijing 100084, P. R. China. E-mail: jmlin@mail.tsinghua.edu.cn
First published on 8th January 2025
Abstract
Open droplet microfluidics is an emerging technology that generates, manipulates, and analyzes droplets in open configuration systems. Droplets function as miniaturized reactors for high-throughput analysis due to their compartmentalization and parallelization, while openness enables addressing and accessing the targeted contents. The convergence of two technologies facilitates the localization and intricate manipulation of droplets using external tools, showing great potential in large-scale chemical and biological applications, particularly in cell analysis. In this review, we first introduce various methods of droplet generation and manipulation in open environments. Next, we summarize the typical applications of open droplet systems in cell culture. Then, a comprehensive overview of cell analysis is provided, including nucleic acids, proteins, metabolites, and behaviors. Finally, we present a discussion of current challenges and perspectives in this field.
1. Introduction
As an emerging technology, open droplet microfluidics is becoming a versatile and powerful platform in chemical and biological applications. The combination of open configuration and droplet microfluidics allows for generation, manipulation, and analysis of discrete droplets in open space.1 Each droplet functions as an independent reaction chamber for parallel analysis without cross-contamination,2 which retains the advantages of miniaturization, compartmentalization, and parallelization from closed droplet microfluidics.3–5 The volume of droplets is extremely small and even reaches the picoliter level.6 Efficient production of large amounts of droplets with uniform size and shape offers a high-throughput tool for large-scale applications.7 Due to its remarkable advantages, droplet-based microfluidics has been widely employed in cell analysis, including nucleic acids,8,9 proteins,10,11 and metabolites12,13 in the recent two decades.The introduction of open configuration further enhances the flexibility and maneuverability of closed droplet microfluidics. Open microfluidic systems are characterized by at least one side of the device being exposed to the outside (air or oil phase) and not limited by the walls.14,15 The absence of barriers allows for in situ fluid manipulation16 and integration of external tools for easy access to the contents.17 Meanwhile, the openness feature brings additional benefits such as simplicity in device fabrication and flexibility in liquid handling.18 In particular, droplets in most open systems usually attach to the substrates and remain stationary. The static feature facilitates droplet addressing, which is the prerequisite for accurate addition and extraction operations.
Combining the advantages of both technologies, open droplet microfluidics has demonstrated its potential in various domains, such as biomolecular detection,19 chemical synthesis,20 drug discovery,21 and artificial cells.22 Notably, it has emerged as a valuable tool to address the challenges in cell research, providing a platform for in-depth investigation and tracking of cellular biology.23 The typical formats of open droplet microfluidics include open droplet arrays, hanging droplets, droplet networks and others. Although parts of digital microfluidic systems are also presented in open format, most situations are configured with closed tops. Therefore, digital microfluidics is not covered in this review; interested readers are referred to other reviews.24
In this review, we first introduce a series of typical methods for droplet generation together with droplet manipulation in open microfluidics. Next, we summarize the common scenarios of cell culture in open droplets. Then, the application of cell analysis was covered, including nucleic acids, proteins, metabolites, and behaviors. Finally, we give a brief discussion of the challenges and prospects in this field (Fig. 1).
 | ||
| Fig. 1 Schematic of generation, manipulation, and applications in cell analysis of open droplet microfluidics. | ||
2. Droplet generation
Droplet generation is the essential step in open droplet systems. In conventional closed droplet microfluidics, the methods are usually divided into passive and active generation depending on the acting forces.25 In open microfluidics, some droplet generation methods inherit the concepts of closed systems and extend upon them, while others exploit the advantages of open space and adopt completely innovative modes. Herein, several methods for generating droplets in open microfluidics are described in detail below (Fig. 2 and Table 1). It is worth noting that these methods are not exclusive, as some studies may employ two or multiple strategies simultaneously to enhance the efficiency of droplet generation. | ||
| Fig. 2 Droplet generation methods in open microfluidics. (A) Droplets are generated in a T-junction closed microfluidic system and transferred into the substrate in an online manner. Reproduced with permission from ref. 26. Copyright 2019, American Chemical Society. (B) Droplets generated in the closed microfluidics are collected in emulsions and dispersed into the microchip with an offline mode. Reproduced with permission from ref. 27. Copyright 2019, American Chemical Society. (C) Direct printing of droplets using inkjet printing technique. Reproduced with permission from ref. 28. Copyright 2018, American Chemical Society. (D) Direct printing of droplets using capillary-based liquid handling technique. Reproduced with permission from ref. 29. Copyright 2014, Springer Nature. (E) Through patterned surface modification, a large volume of liquid is rolled on the surface to form droplet arrays by a discontinuous dewetting effect. Reproduced with permission from ref. 30. Copyright 2017, Wiley-VCH. (F) Droplets are generated on a bionic structure chip based on a lotus leaf through physical effect. Reproduced with permission from ref. 31. Copyright 2020, Springer Nature. | ||
| Generation method | Droplet size (volume/diameter) | Throughput | Scale | Advantages (+)/limitations (−) |
|---|---|---|---|---|
| Closed systems-based transfer | 500 pL–6.2 nL (ref. 32) | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 drops in 36 min 000 drops in 36 min |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
+ Derived from closed droplet microfluidics and easy to get started |
| 270–500 pL (ref. 33) | ∼1–3 Hz | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
+ Relatively higher throughput and scale | |
| 10–15 pL (ref. 34) | — | 900 | − Requires device modification for droplet positioning (on-line method) | |
| 250 pL (ref. 35) | — | >3000 | − Requires prior preparation of droplets and microstructured chips for storage (off-line method) | |
| 33 pL (∼40 μm)27 | — | ∼1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
||
| Direct printing | 4–8 nL (ref. 36) | 81 drops in 9 min | 100 | + Droplet-on-demand printing |
| 50–110 μm (ref. 28) | 0.33 Hz | — | + Adjustable and independent control of droplet property | |
| 500 nL (ref. 37) | 342 drops in 2 h | 342 | − Relatively lower throughput and scale | |
| 4 nL (ref. 29) | 24 drops in 70 s | 3025 | − Requires highly automated instruments and sophisticated printing tips | |
| Patterned surface modification | 333.6 ± 14.2 pL (ref. 38) | — | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
+ One-step fabrication of large-scale arrays |
| 200–400 μm (ref. 39) | — | >100 | + Easy to operate | |
| 335 μm (ref. 40) | — | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
+ Controllable multiparameter geometry (i.e., shape, size, and arrangement) | |
| 0.66 nL (ref. 41) | 5100 drops in 2.5 min | 5100 | − Requires prior preparation of patterned substrates | |
| − Minimum droplet size is limited | ||||
| Interfacial tension | 100 nl (ref. 42) | 2 Hz | — | + Autonomous generation without direct actuation |
| − Lower throughput and weaker monodispersity | ||||
| Microstructure chip | 0.9–29.5 pL (ref. 31) | — | 736 | + One-step fabrication of large-scale arrays |
| + Produces droplets below the picoliter range | ||||
| − Requires complex microchip design |
2.1 Closed system-based transfer
This method originates from a straightforward strategy: generating droplets through established technology in closed microfluidic systems, then transferring them into open space. The method is an extension of existing developed techniques and retains the advantages of closed systems such as stability and high throughput. Commonly, the initial part of this method involves passive generation to produce monodisperse droplets in co-flow or flow-focusing designed microchips under the interaction between hydrodynamic forces and interfacial tension. Subsequently, these droplets are loaded onto substrates in open space, such as plates or microchips with microstructures.The method can be categorized into two modes based on the transfer process. The first mode is an on-line method, where droplets are produced within closed devices and then directly loaded onto the substrate through transfer tools.26,32,33 Haidas et al. developed a facile microfluidic workflow to form sessile droplet arrays.26 The device comprised a droplet generator, a capillary for delivery and a motorized plate as shown in (Fig. 2A). Monodisperse droplets were generated by the T-junction flow-focus configuration from the sample reagent and carrier oil. Then, droplets directly flowed into the connected capillary and were deposited on the open plate. Once the successful generation of droplets was detected by the embedded optical sensor, the motorized plate simultaneously moved to load the droplet into the next position and form a droplet array efficiently. Cole et al. fabricated a similar device, in which a poly(dimethylsiloxane) (PDMS) print head was used as the transfer tool instead of the capillary.33 In addition, a fluorescence activated cell sorting module was embedded into the closed microfluidic chip, which can filter negative droplets before entering the open space.
Another mode is an off-line method: droplets generated in closed systems are collected in emulsions, then loaded into microchips with capture structures.34,35,43 Um et al. developed a mesh-grid integrated microwell array to trap the droplets.34 The metal mesh was placed above the PDMS microchip as a microchannel structure and guided droplets into microwells. After loading, the residual droplets were flushed out by the oil flow. Xu et al. designed a novel microcage structure to improve the microwells, for the oil phase filled in the one-side open microwells first, increasing the pressure and hindering droplet sedimentation.27 The microcage structure with more open outlets can exclude oil and support the droplets to sink down. They also slid a coverslip along the chip to accelerate the droplet movement and remove redundant droplets (Fig. 2B).
The droplet generation method based on a closed-system transfer effectively maximizes the retention of the original advantages, including high throughput and controllability. This approach is user-friendly for experimenters who are already familiar with droplet fabrication in conventional microfluidics. The on-line generation mode requires only the addition of a connection device to the droplet outlet, while the off-line mode necessitates the preparation of a microstructured chip for droplet storage, which allows users to select the method that best meets their experimental needs and preferences.
2.2 Direct printing
The direct printing method takes a droplet-on-demand strategy, where droplets are generated above the target spots in open space, avoiding potential contamination during transfer steps. Droplet arrays can be created in a one-by-one way, with independent adjustable droplet sizes and printing rates. Typically, inkjet printing or capillary-based liquid handling techniques are used, relying on high-precision printing devices and automated programs.36,44 Lin's group developed a series of studies on droplet generation via inkjet printing method.28,44 A piezoelectric-driven print head and laboratory-made control software were utilized. By adjusting the driving conditions of the piezoelectric actuator (pulse voltage, width, and frequency), the size and generation rate of monodisperse droplets were regulated (Fig. 2C). Droplets can be generated in air as well as in the oil phase, showing their versatility within different system configurations.A capillary-based liquid handling technique is also utilized for direct droplet generation (Fig. 2D).36 Fang's group developed a sequential operation droplet array (SODA) technique over the past decade.29,37 The SODA system was mainly composed of a capillary probe, a microdroplet-holding chip, and a movement-controlling module. To achieve the creation of nanoliter or picoliter droplets, the end of the capillary tip was pretreated into a tapered shape by a heating and pulling method. Hydrophobic modification was applied on the tip to reduce the reagent remaining and adsorption, avoiding cross-contamination among samples. The top end of the capillary was connected to a high-precision syringe pump for fluid control.
In addition, electrohydrodynamic (EHD)45 or gas shear46 techniques were also utilized to detach droplets from a continuous flow. Overall, direct printing is a highly flexible and convenient method, as the properties of each droplet can be regulated independently through the automated procedure. However, compared to the passive generation method in closed microchips, its throughput is reduced because the formation of large-scale droplet arrays requires the repetition of “position–printing–reposition” procedures. The properties of droplet generation are limited by the sophistication of the device and the print tip. Nevertheless, commercially available devices present a recommended option for laboratories new to this field, as they can readily get started without complicated preparation or design expertise.
2.3 Patterned surface modification
Surface modification is a rapid method to create a large number of droplets based on a discontinuous dewetting effect. The surface is commonly modified with hydrophilic spots dispensed in a hydrophobic background. When a large volume of aqueous solution is swept over the substrate, portions are adsorbed on the hydrophilic spots and separate from the bulk liquid to form isolated droplets. Droplet arrays in open space are quickly generated with adjustable formats, which rely on the features of patterned spots such as surface properties, size, quantity, and spatial distribution. Designed surface patterns can be obtained by several methods, for example, mask-based photolithography,47 inkjet printing,48 and microcontact printing.38,39,49 Levkin's group developed a series of studies based on surface micropatterning.30,40,50 First, a thin superhydrophilic nanoporous film was coated on a glass plate. Then, a superhydrophobic grid pattern was created by a UV-initiated photografting reaction. The geometry and size of superhydrophilic spots and superhydrophobic background can be precisely controlled through the designed photomask. By spreading the cell suspension on the patterned chip and slightly tilting, homogenous droplets were generated through the effect of discontinuous dewetting (Fig. 2E). Despite the requirement for complex chemical pretreatment to obtain modified surfaces, the simple one-step dragging operation is characterized by high efficiency and throughput, enabling rapid generation of large-scale droplet arrays. This strategy minimizes the lag in droplet printing or transfer methods, ensuring optimal parallelism between independent droplets for cellular applications that require high immediacy. It is worth noting that superhydrophilic dots are usually biocompatible, allowing cells to attach and proliferate in the superhydrophilic region.2.4 Other methods
Recently, several emerging methods have also attracted interest in this field. Khor et al. demonstrated an open droplet generation approach driven by interfacial tension.42 They designed a narrow structure in an open channel, where the liquid plug inside can overcome the difference between hydrostatic and capillary pressure, thereby droplets were spontaneously extruded. Although the current method still suffers from slow speed and weak monodispersity, this power-free design provides a new idea to simplify the experimental setup without the need for direct actuations.The generation of water droplet in nature has inspired researchers to utilize microstructures for droplet production. Zhou's group designed a PDMS chip that mimics the surface of a lotus leaf with a series of micropores with angled walls (Fig. 2F).31,51 The special design provided surface energy in an advancing direction when a large volume of liquid is smeared, thereby liquid preferred to enter the micropores under the surface tension. Compared to the surface modification method, the physical structure-based method retains the convenience of one-step preparation and is suitable for generation of droplets below the picoliter level.
3. Droplet manipulation
Various droplet manipulation is critical for diverse droplet analysis, including moving, merging, splitting, extraction, and sorting. This section will mainly focus on the representative manipulations in open droplet systems (Fig. 3 and Table 2).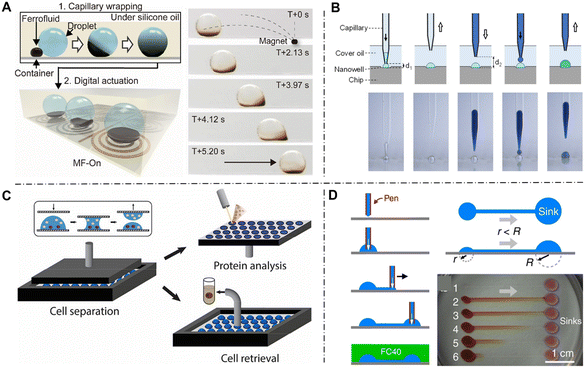 | ||
| Fig. 3 Droplet manipulation in open microfluidics. (A) Schematic of droplet moving driven by magnetic force. Reproduced with permission from ref. 52. Copyright 2024, Royal Society of Chemistry. (B) Using the capillary-based liquid handling method to position the existing droplet and add new reagents. Reproduced with permission from ref. 53. Copyright 2019, American Chemical Society. (C) Using the sandwich method to split and sample droplets in a high-throughput manner. Reproduced with permission from ref. 54. Copyright 2020, American Chemical Society. (D) Using a writing pen to create fluid circuits in open space, enables the configuration of droplet network. Reproduced with permission from ref. 55. Copyright 2017, Springer. | ||
| Manipulation type | Method | Advantages (+) | Limitations (−) |
|---|---|---|---|
| Droplet moving | Electricity56–59 | + High spatiotemporal precision maneuverability | − Potential cell damage due to high voltage |
| + Rapid response and real-time control | − Requires electrode arrays and sophisticated circuit control | ||
| Magnets52,60 | + High precision operation | − Invasive magnetic material may lead to contamination and biocompatibility issues | |
| + Rapid and long-distance control | |||
| Light61 | + Non-invasive interaction | − Complicated substrate modification | |
| + Ready controllability | − Potential photothermal damage to cells | ||
| Acoustics62 | + Contactless operation | − Complex principal design and device setup | |
| + Label-free selectivity | |||
| Droplet addition and retrieval | One-by-one mode53 | + Flexible, high-precision control | − Lower throughput and efficacy |
| Droplet array sandwiching63 | + High throughput for parallel large-scale analysis | − Limited operation precision | |
| Droplet network configuration | Writing pen,55,64,65 microjet,66 laser,67 pipettes68 | + Reconfigurable customized droplet network functionality | − Special surface treatment required |
| + Stationary droplets for in situ adherent cell analysis | − Lower control precision, suitable only for larger droplets |
3.1 Droplet moving
Droplet moving is a common operation in dynamic open droplet systems. Unlike closed microfluidic systems, open droplet systems without pumps require active forces to drive droplet transportation. For instance, electricity-based approaches have been widely used to move droplets, especially electrowetting on dielectric (EWOD).56 In EWOD-based systems, droplets are placed on a plate with an array of electrodes. When the electrode is electrically energized, the surface density of charges alters the wetting properties and contact angle. Therefore, droplets are activated and move to the adjacent electrode, and continuous transportation can be achieved by the sequential actuation of electrodes. EWOD-based methods process the advantages of rapid response and stability but require sophisticated electrode patterns and multilayer chip fabrication. Other charge-based methods have been investigated to eliminate the need for electrodes and explore more flexible operations, such as triboelectric wetting,57 electrostatic tweezer,58 and charge shielding knife.59 In general, electricity-based approaches offer advantages of automated control, high spatiotemporal precision and maneuverability, making them suitable for applications requiring rapid response and real-time control. Nonetheless, the interference of electricity with cellular viability and activity is a notable concern.Magnetic-based methods control droplet movement by applying a homogeneous or inhomogeneous magnetic field, which process the advantages such as biosafety, long-distance and rapid response. Li et al. proposed a magnetic-actuated robot to achieve programmable droplet manipulation, which consisted of a couple of steel beads and a magnetic control system.60 By adjusting the programmable magnetic field, the structure of the robots was controlled and determined the forces on droplets, thereby driving droplet transport. However, the requirement of inserting magnetic particles inside the droplets raises concerns about biocompatibility due to potential contamination. Wang et al. proposed a non-invasive droplet moving strategy through an outside ferrofluid transporter as shown in Fig. 3A.52 The ferrofluid transporter can spontaneously wrap the droplet by spreading on the droplet interface, forming an adaptive three-phase system. When applying an external magnetic field, the ferrofluid responded to deform and carry the wrapped droplets for transportation. The authors performed bioanalysis of cell spheroid culture and drug screen using this non-contact method, proving outstanding compatibility in biomedical applications.
In addition to those mentioned above, methods based on other active forces such as light61 and acoustics62 have been developed for droplet moving. As a fundamental operation, droplet moving significantly increases the maneuverability of open droplet systems, enabling workflows that consist of diverse process such as droplet merging, reagent addition, and medium replenishment. Despite the advanced moving operations, there are some issues that need to be addressed in cellular analysis, such as large volume limitations, incompatibility with adherent cells, and surface residues of biological samples. We look forward to the emergence of more transportation methods that are compatible with cellular analysis and believe that they can become more versatile and convenient tools in both scientific and industrial fields in the future.
3.2 Droplet addition and retrieval
Compared with closed microfluidics, a remarkable advantage of open microfluidics is that reagents can be easily added or retrieved from the open side of devices. A common approach for adding reagents into existing droplets is capillary-based liquid handling technique. Similar to droplet generation, a capillary can be positioned at the location of a targeted droplet and produce a new droplet for merging (Fig. 3B).53 Cross-contamination from sample adsorption on the capillary tip is a notable problem when performing sequential operations among multiple droplets. A hydrophobic coat modified on the capillary helps reduce adsorption.69 Non-contact capillary operation mode or inkjet printing technique70 are introduced to fundamentally avoid contamination. Moreover, capillary-based operations can also be employed to extract droplets. Through a suction strategy with precise volume control, the targeted droplets were aspirated and maintained in the tip of the capillary for the following analysis.Although the capillary-based method is sufficiently flexible to realize multiple droplet manipulations, its one-by-one mode restricts its throughput in large-scale droplet arrays. A droplet array sandwiching technology has been introduced to satisfy the demand for high-throughput applications.50,63,71 The device comprises two opposing substrates, where original droplets are seeded in the lower substrate while additional droplets are distributed in the upper substrate. When the two substrates are aligned and close together, droplet fusion occurs and enables simultaneous addition of droplet arrays. Konishi et al. used this method to investigate cellular calcium oscillations with different chemical concentrations.72 HeLa cells were cultured in lower droplets, while the stimulating reagent histamine was distributed in upper droplets. With the contact between two substrates, the droplets merged and histamine gradually diffused from the upper to the lower droplets. Moreover, EWOD technology was introduced to control the droplet height, thus adjusting the fusion time and histamine concentration.73 Likewise, the sandwich method was also employed for droplet extraction. Haidas et al. developed a parallel sampling method to collect a cell culture supernatant for mass spectrometry (MS) analysis (Fig. 3C).54 A parallel plate with a hydrophilic coating was gradually placed over the droplet array containing cells until the droplets wetted the upper substrate and formed a hemispherical shape. By slowly moving up the top plate, droplets were split and portions attached to the upper plate. Afterward, they obtained a plate with sample droplets and performed MS analysis in situ. The method significantly increased the throughput of droplet extraction; however, the separation mode can only retrieve portions of droplets instead of recovering all of the droplets, limiting its applications in the detection of low-abundance substances. Overall, droplet array sandwiching technology greatly expands system throughput and efficiency, allowing for parallel screening, cell seeding, reagent addition, and partial adoption in thousands of droplets or even larger-scale applications. The capillary-based single-point mode of operation, on the other hand, offers the advantage of greater flexibility and is suitable for small-scale screening of cells of interest as well as for accomplishing more precise manipulations, such as whole-droplet or controlled-volume recovery.
3.3 Droplet network configuration
In addition to methods based on droplet fusion, material exchange between droplets can also be achieved through droplet networks. In these specialized open systems, droplets are kept in situ and arranged with spatial structures, which is favorable for maintaining the stability of droplets. Communication between neighboring droplets is realized via intricate fluid channels or circuits. Walsh's lab created fluid circuits to implement the formation and configuration of droplet networks in open space, which they called “freestyle fluidics”.55,64,65 They used a writing pen made from a needle or plastic tube connected to a syringe pump. As the liquid was pumped and the pen moved continuously, patterned fluid circuits were deposited on the substrate and pinned by interfacial tension (Fig. 3D). Under the effect of Laplace and hydrostatic pressures between connected droplets, passive pumping was achieved as demonstrated by the dye transfer through channels. Additionally, the droplet networks can be broken and reconfigured by using a hydrophobic stylus to split droplets. When the stylus brushed the surface, the oil wetted the substrate and replaced the aqueous phase, separating the bigger chamber into two smaller droplets. Apart from the writing pens, other tools are employed to construct and configure the fluid geometry, such as microjet,66 laser,67 and ordinary pipettes.68 Compared to other methods of droplet movement and addition, the configuration of the droplet network enables material interaction and circulation without affecting the original droplets, making it suitable for constructing microenvironments for adherent cell culture. With these open and flexible operations, users can create complex networks of droplet functionality and dynamically reconfigure them during the detection process. However, current reconfiguration methods can only manipulate larger droplet, and finer control techniques need to be developed to construct more microscopic droplet networks.4. Cell culture in open droplets
The compartmentalized property of droplet microfluidics provides isolated microenvironments for containing cells, avoiding interference from other cells. However, there are some issues regarding the compatibility of droplets with cell culture that need to be addressed, such as adhesion support for adherent cells, nutrient limitation in long-term cultivation, and the observation of cells.74 In conventional droplet microfluidic systems, droplets are completely suspended in the oil phase. Adherent cells are unable to adhere to the water–oil interface, which may affect their normal physiological functions. The amount of nutrient material is limited by the miniaturized size of droplets and will deplete over time. In addition, droplets in the flowing phase may change and lose their original positions, causing disturbances during continuous observations. Fortunately, we find that droplets in open microfluidics may overcome the challenges. In most open droplet systems, a stable substrate is usually introduced, facilitating the anchorage of droplets and supporting the normal behavior of adherent cells through appropriate surface modification. Meanwhile, the openness enables the direct supplementation of fresh culture medium into droplets, promising a balanced biochemical environment for long-time experiments. Additionally, open droplet systems offer higher gas exchange efficiency compared to closed systems, which depends on the gas permeability of the microchip materials.1 Despite these advantages, there are also issues that need to be addressed when culturing cells in open droplet systems. The substrates are required to be transparent for applications that involve optical imaging observation, which may limit the usage of special materials. Another notable problem is the evaporation management of droplet volume. Exposure to open space accelerates droplet evaporation, especially in single-cell analysis. Various strategies have been adopted to prevent the process, such as oil cover,75 water moats,76 and reversible lid seal.77The feasibility of cell culture in open droplets was promised by previous work, which discovered that cells cultured in open droplets were comparable in transcriptomic expression with cells grown in traditional culture systems.78 Therefore, open droplet systems can serve as alternative platforms for high-throughput cell culture and related analysis. In this section, we presented some typical examples including single-cell culture, cell spheroid culture and cell co-culture in open droplet systems (Fig. 4 and Table 3).
 | ||
| Fig. 4 Cell culture in open droplet microfluidics. (A) Open microdroplets serve as single-cell culture dishes to investigate single-cell behavior. Reproduced with permission from ref. 77. Copyright 2022, Wiley-VCH. (B) Hanging droplets are used for cell spheroid formation and culture. Reproduced with permission from ref. 79. Copyright 2014, Springer Nature. (C) Cell co-culture of single-cell pairs through a sequential inkjet printing method. Reproduced with permission from ref. 80. Copyright 2024, Wiley-VCH. (D) By merging the adjacent droplets, spheroids can fuse into a multi-spheroid complex. Reproduced with permission from ref. 81. Copyright 2021, Wiley-VCH. | ||
| Culture form | Droplet carrier | Droplet size (volume or diameter) | Cell type | Culture duration |
|---|---|---|---|---|
| Single-cell culture | Droplet arrays | ∼100 pL | U-87 MG77 | 6 h |
| 31 pL | Escherichia coli 82 | 3 d | ||
| Cell spheroid culture | Hanging droplets | 3.5 mm | HCT-116 eGFP79 | 4 d |
| 0.36–7.6 nL | LN229 (ref. 83) | 3 d | ||
| Sidewall hanging droplets | 500 nL | HepG2 (ref. 84) | 4 d | |
| Cell co-culture | Merged sessile droplet arrays | ∼80–106 μm | U87 MG, THP-1 (ref. 80) | 24 h |
| 500 nL | HUVEC, C6 (ref. 85) | 2 d | ||
| Merged hanging droplets | 2.5 mm | ES-D3, MDA-MB-231, MCF-7 (ref. 86) | 10 d | |
| 200–1100 nL | HepG2, HEK 293T81 | 5 d |
4.1 Single-cell encapsulation and culture
Single-cell study can reveal cell heterogeneity and information obscured by population averages. Droplet microfluidics is widely used in single-cell research because the miniaturized droplets are of a comparable size to single cells. A challenging issue for single-cell culture in droplets is obtaining the maximized single-cell encapsulation rate. In most systems, cells are encapsulated randomly and follow the Poisson distribution. When the average number of cells in each droplet is 1, the Poisson distribution leads to many empty or multi-cell droplets. A versatile approach is regulating the density of the cell suspension and the size of droplets to reach the optimal encapsulation rate through preliminary experiments.44,87 This method is applicable to most experiments but improvement is still limited, and non-single-cell droplets still enter the system. Another approach is to integrate droplet sorting units into the generation device, thereby removing unwanted droplets directly from waste channels. Common droplet sorting technologies include fluorescence-activated cell sorting (FACS),33,88 impedance detection,89 and machine learning-based optical image processing.90Single-cell culture models were constructed in open droplet systems and focused on continuous monitoring of single-cell behaviors to understand the cell heterogeneity.82,91 Xie et al. proposed the concept of “single-cell culture dish” and realized it in open droplet microfluidics (Fig. 4A).77 The inkjet printing method was utilized to create an array of hydrophilic polylysine blots, followed by manually generating droplets containing single cells on the spots. Cells with negative charges could adhere to the positively charged matrix via the electrostatic attraction effect, retaining normal adhesive functions. Finally, the adhesion strength was determined by an in situ open microfluidic extractor. The authors were surprised to find that single cells showed an entirely distinct polarization pattern compared to those cultured in conventional Petri dishes, revealing a potential impact of intercellular communication which was only exhibited in single-cell studies.
4.2 Cell spheroid culture
Spheroids are formed by three-dimensional (3D) cell aggregates, which are believed to mimic tumor behavior more effectively than regular two-dimensional (2D) models. Hanging droplets, as a typical form of open droplet microfluidics, are specifically developed for 3D cell spheroid culture.79,83,92,93 Similar to other systems, cells are initially distributed in the droplets. To prevent adhesion on the substrate, the device is inverted to suspend the droplets in mid-air (Fig. 4B). Consequently, cells sink to the bottom of the droplets under gravity and spontaneously aggregate into spheroids in the liquid–air interface. However, the inverted structure is not conducive to subsequent droplet manipulations like reagent addition. Zhao et al. addressed the problem by setting the droplets on the sidewall instead of the bottom.84 This sidewall hanging mode reserved the function of spheroid formation in the bottom mode and enabled reagent addition from the open top direction.4.3 Cell co-culture
Droplet fusion operations enable the co-encapsulation of different types of cells, establishing microenvironments for cell co-culture. Lin et al. proposed a sessile microdroplet system method for single heterogeneous cell co-culture (Fig. 4C).80 Through a sequential inkjet printing technique, individual macrophage cells and tumor cells were encapsulated in microdroplets respectively and printed in the same position to fuse together. The heterotypic cell pairs were formed in an isolated droplet environment to enable the interaction study at single-cell resolution.This fusion strategy was also utilized for fusion of spheroids.81,85,86 Cui et al. developed a multi-spheroid architecture assembly method based on programmable droplet merging (Fig. 4D).81 The hanging droplet method was initially used to generate cell spheroids and the chip is oriented upright before droplet fusion. By dispensing an additional volume of culture medium, adjacent droplets spontaneously merged into larger droplets. Then, the chip was inverted again, two separate spheroids were allowed to come in contact at the bottom of the droplet, leading to their adhesion and fusion into a multi-spheroid complex. Through various merging combinations, they constructed cell spheroids with diverse numbers and configurations. The immunostaining of cell surface adhesion protein E-cadherin showed the cell–cell junctions formed between two merged spheroids. The function of the co-culture model was verified by Wnt signaling propagation within hetero-spheroids.
5. Cell analysis in open droplets
Droplets in open microfluidics can serve as multifunctional microreactors in cellular analysis and enable the efficient readout of detection signals. In this section, we summarize the applications of cell analysis in open droplets, including nucleic acids, proteins, metabolites, and behaviors (Table 4).| Analysis object | Droplet generation/manipulation | Detection object | Cell type | Detection method |
|---|---|---|---|---|
| Nucleic acid | Direct printing; droplet addition | GAPDH and ACTB genes | HeLa-CCL2 (ref. 94) | qPCR |
| Direct printing; droplet addition | Mir-122 | Huh-7 (ref. 95) | qPCR | |
| Closed system transfer; droplet addition and retrieval | Whole genome | Yeast cell96 | MDA | |
| Protein | Direct printing; droplet addition and retrieval | Single cell proteome | HeLa97–99 | LC-MS/MS |
| Patterned surface modification; droplet retrieval | MMP9 | A549 (ref. 41) | Fluorescence | |
| Direct printing | P53 proteins | BE100 | Antibody sandwich assay | |
| Direct printing | IgG, IL-2 | Clonal mouse hybridoma cells101 | Surface plasmon resonance based optical image | |
| Patterned surface modification; droplet network configuration | VEGF | U87 (ref. 68) | Rolling circle amplification (RCA) | |
| Metabolite | Direct printing; droplet retrieval | Glucose-phosphate | K562 (ref. 102) | ESI-MS |
| Direct printing; droplet retrieval | Phosphatidylcholines | A172, HA103 | ESI-MS | |
| Direct printing | Malonyl-CoA | A549 (ref. 104) | MALDI-MS | |
| Closed system transfer | Metabolite profile | Yeast cell105 | MALDI-MS | |
| Direct printing | Lactate | A549 (ref. 106) | Fluorescence | |
| Direct printing | Oxygen | MCF-7 (ref. 107) | Electrochemistry | |
| Droplet network configuration | Glucose | HCT116 (ref. 108) | Electrochemistry | |
| Behavior | Patterned surface modification | Drug screen | Lung cancer organoids109 | Optical image; qPCR |
| Direct printing; droplet network configuration | Cell migration | MDA-MB-231 (ref. 110) | Optical image | |
| Droplet network configuration | Wound healing | C2C12 myoblasts111 | Optical image |
5.1 Nucleic acid analysis
Nucleic acids are the fundamental substances in living things, functioning as carriers of genetic information. However, the total amount of nucleic acid in cells is extremely low, making it hard to detect directly. Therefore, it is necessary to amplify the nucleic acids for precise analysis. Polymerase chain reaction (PCR) is a general tool for nucleic acid amplification, which can produce millions of copies from the original templates. Droplet microfluidics has been combined with PCR assays for they reduce the reagent consumption and improve efficiency due to the limited volume. However, as a multistep analysis, multiple reagents need to be added sequentially at specific times, which demands complex chip design and procedures in closed microfluidic systems. Open droplet microfluidics enables the multistep addition of different reagents at the same target positions, supporting the in situ PCR assay.94,112,113 Zhu et al. proposed a nanoliter droplet array-based single-cell reverse transcription quantitative PCR (RT-qPCR) method (Fig. 5A).95 A capillary-based microfluidic robot was introduced to realize all the liquid addition steps automatically, consisting of single-cell encapsulation, cell lysis, and addition of reverse transcription mix and PCR mix. They integrated a fluorescence microscopy unit to record the real-time fluorescence changes and address the spatial information of droplet array. The fluorescence changes were accurately recorded during the thermocycling process due to the immobilization of droplets. The feasibility of the method was validated by measuring the mir-122 expression in single Huh-7 cells. Instead of PCR, which requires temperature-changing cycles, some isothermal amplification strategies carried out at a constant temperature have also been combined with open droplet systems, such as multiple displacement amplification (MDA),114 loop-mediated isothermal amplification (LAMP)115 and strand invasion-based amplification (SIBA).116 | ||
| Fig. 5 Cell nucleic acid analysis in open droplet microfluidics. (A) Single-cell RT-PCR analysis is performed in open droplet arrays, and a capillary-based microfluidic robot is used to dispense single cells and add the RT mix and PCR mix for multistep reaction. Reproduced with permission from ref. 95. Copyright 2015, Springer. (B) Single-cell whole genome sequencing is performed in an addressable dynamic droplet array, where microfluidic chips are employed for single-cell isolation, lysis, and amplification. The upper cover can be peeled off for droplet retrieval. Reproduced with permission from ref. 96. Copyright 2021, Wiley-VCH. | ||
The preparation of nucleic acid sequencing was also performed in open droplet systems. Li et al. presented an addressable dynamic droplet array (aDDA) for high-coverage genome sequencing of single yeast cells (Fig. 5B).96 They combined the continuous-flow droplet operation with a static-droplet-array strategy, in which the microchip was reversibly bonded by two layers: the lower layer contained microwells for cell droplet storage, while the upper layer consisted of T-shaped channels and trapping structures for reagent droplet addition. By aligning the units between two layers, the multi-stage reactants can be added into each droplet precisely. The droplets containing single yeast cells were dispensed in the lower layer and lysed. Then, droplets that carried amplification reagents were injected into the fluid channels and coalesced with cell droplets, triggering on-chip multiple displacement amplification. Lastly, the upper layer was peeled off to expose the droplets to the open space, and addressable droplets were picked up for whole genome sequencing.
5.2 Protein analysis
Proteins are macromolecules that carry out cellular functions and are involved in virtually every cellular process. Cell protein analysis is aiming to obtain the protein expression profiles, which is critical in understanding the underlying cellular mechanisms.Single-cell proteomics is an emerging field aiming to explore the diversity of proteins in individual cells. Currently, one of the common strategies for single-cell proteomic analysis involves a “bottom-up” workflow based on mass spectrometry (MS), which requires multiple preprocessing steps including cell lysis, protein reduction, alkylation, and enzymatic digestion.117 However, due to the extremely low abundance of proteins within single cells, these preparation steps may lead to undesired adsorption losses, thereby reducing the sensitivity and coverage of the results. Open droplet systems are compatible with the pretreatment workflow in single-cell proteomic studies in two aspects: sample loss is minimized benefiting from the tiny volume of droplets, and the openness facilitates the addition of reaction reagents and recovery of samples. Zhu's group developed an open droplet-based platform named nanoPOTS (nanodroplet processing in one-pot for trace samples) for single-cell proteomic analysis.97,98 As shown in Fig. 6A, an open microchip with photolithographically patterned nanowells was used to contain all reagents in single droplets, including cell suspension, MS-compatible surfactant, reducing agent, alkylating agent, and multiple proteases. The pretreatment workflow was implemented through a capillary with a liquid handling robot, including dispensing single cells, adding reagents sequentially, recovering, and injecting samples to the LC-MS interface. The final volume of droplets is controlled at about 200 nL to minimize the surface adsorption, thereby enhancing sample recovery and significantly improving the sensitivity. Using the nanoPOTS platform, they successfully achieved quantitative proteomic analysis in a small cell population97 and even in single mammalian cells containing only 0.1–0.2 ng of total protein.98 Moreover, they further worked on improving the sensitivity and throughput of the system by introducing a nested microchip118 or automated sampling robots.119 Fang's lab also worked on single-cell proteomic analysis through capillary-based robots and open droplet systems.99,120 Recent research reported a pick-up single-cell proteomic analysis (PiSPA) workflow,99 which successfully identified and quantified up to 3000 proteins in mammalian cells, nearly half the level of multi-cell samples. In the PiSPA system, they used the conical bottom tip of a commercial tube as a microreactor instead of a microchip, further simplifying the workflow compared to nanoPOTs. In the future, we believe that more advanced droplet manipulation techniques will be developed to further increase the sensitivity of the workflow, moving toward the whole coverage of single-cell proteomics.
 | ||
| Fig. 6 Cell protein analysis in open droplet microfluidics. (A) A nanoliter droplet-based workflow is used to improve the performance of single-cell proteomics. Reproduced with permission from ref. 97. Copyright 2018, Springer Nature. (B) Cell enzymatic activity is continuously monitored by the fluorescence images in droplet arrays. Reproduced with permission from ref. 41. Copyright 2021, Wiley-VCH. (C) Cell spatiotemporal secretion profiles are obtained through the surface plasmon resonance principle. Reproduced with permission from ref. 101. Copyright 2023, Springer Nature. (D) Reconfigurable open droplet systems for cell culture and secreted VEGF online detection. Reproduced with permission from ref. 68. Copyright 2019, Royal Society of Chemistry. | ||
Apart from the intracellular proteins, the proteins secreted into extracellular space can be confined in droplets for sensitive detection. The immobilized property of open droplet systems also offers stable environments for the real-time monitoring of secretion dynamics. Several studies have been performed on enzyme kinetics assays based on fluorescence imaging.26,41,121 As shown in Fig. 6B, Xiao et al. performed a single-cell enzymatic screening assay by monitoring the activity of matrix metalloproteinase 9 (MMP9) in circulating tumor cells (CTCs).41 A peptide sequence conjugated with fluorophore/quencher groups was added into droplets as the probe, which can be specifically cleaved by secreted MMP9 and release fluorescence. Therefore, the enzymatic activity was continuously monitored and acted as the biomarker to distinguish CTCs. Moreover, the open droplet system enabled single-cell addressing and recovery for downstream analysis, including gene mutation sequencing, immunostaining, and transcriptome analysis. The associated analysis further revealed new insights into the mechanisms of MMP9 secretion in the CTCs.
Antibody-based immunoassay was also utilized in open droplets for cytokine secretion analysis.100,122 Salehi-Reyhani et al. reported a droplet array to analyze the tumor-suppressor p53 protein by an antibody sandwich assay.100 The capture antibody was printed on the substrate to form a patterned spot array previously, and droplets containing single cells and fluorescently labelled anti-p53 antibody were dispensed at spot locations. The free p53 protein was first bound with detection antibody, then captured by surface antibody spots to indicate the expression level by fluorescence intensity. Considering the potential cytotoxicity of fluorescent labels and procedural complexity, a label-free optical detection method based on the surface plasmon resonance (SPR) principle was presented (Fig. 6C).101 Plasmonic gold nanohole array substrates were prepared and functionalized with receptors for specific detection. Then, picoliter droplets containing single cells were distributed into microwells made of PDMS micromesh. The binding of secretion–receptor caused spectral shifts in surface plasmon resonance and were recorded by a camera as variations in the intensity of the transmitted light. This method enabled the acquisition of protein secretion profiles of hybridoma cells with a high spatiotemporal resolution.
In addition to continuous spatiotemporal monitoring of cell secretion, open droplet systems also enable end-point measurement. Feng et al. presented a simple droplet network in open space, integrating cell culture and on-line semiquantitative detection of vascular endothelial growth factor (VEGF).68 The system consisted of two droplet chambers and a reconfigurable liquid channel between them, where one droplet was used for cell culture and another was modified with DNA aptamers for protein capture (Fig. 6D). The channel was disconnected to provide an isolated environment for cell culture at first. After 12 h, the channel was linked by moving the pipette in open space, therefore secreted VEGF can diffuse towards the detection chamber via the connected channel, where they were captured and quantified via rolling circle amplification (RCA) reaction.
5.3 Metabolite analysis
As the end-point of cell genome–environment interaction, cell metabolism is composed of various chemical reactions in living cells. Analysis of cellular metabolites is critical for revealing the status of cells and understanding the biological and biochemical processes.Similar to proteins, cellular metabolites are maintained at low abundance and cannot be amplified. MS is regarded as a promising technique for metabolite detection due to its advantages of being label-free, multiplex, and highly sensitive. Open droplet systems serve as a flexible pretreatment tool for MS analysis. Zhang's group used droplet microextraction combined with electrospray ionization mass spectrometry (ESI-MS) to detect metabolites in single cells (Fig. 7A).102,103 A capillary pre-filled with extraction reagent was positioned at single cells and extracted cellular metabolites through a small droplet. Then, the extraction reagent was aspirated back into the capillary tip and moved to the ESI-MS interface for detection. The droplets avoided the direct contact between the capillary and the metabolites, allowing retrieval of components using specific extraction solvents. Through this workflow, they successfully detected glucose-phosphate metabolites to study the glycolysis process102 and identified more than 300 phospholipids from single cells.103 The method was compatible with the dry surface as well as droplets for liquid–liquid microextraction.123 In addition, to eliminate the potential loss during capillary transition, Jin et al. developed a U-shaped capillary probe to perform in situ sampling, delivery and on-line MS detection.124,125 The bottom of the U-shape was located on the droplets and allowed to come in contact with the droplets, and two top openings were connected to a syringe pump and the ESI-MS inlet, respectively.
 | ||
| Fig. 7 Cell metabolite analysis in open droplet microfluidics. (A) Open droplets are located on the single cells and extracted metabolites for ESI-MS detection. Reproduced with permission from ref. 103. Copyright 2019, American Chemical Society. (B) Droplets containing cells are dispensed in the open substrate and MALDI-MS analysis is directly performed. Reproduced with permission from ref. 105. Copyright 2021, Springer. (C) The secretion of cell lactate in droplets is monitored using a fluorescence method to distinguish the tumor and normal cells. Reproduced with permission from ref. 106. Copyright 2021, American Chemical Society. (D) Electrochemical sensors are integrated on the substrate, which can detect the oxygen metabolism of cell spheroids continuously. Reproduced with permission from ref. 107. Copyright 2022, Royal Society of Chemistry. | ||
Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) is another developed technology, where samples can be bombarded by a laser beam and vaporized into a vacuum and ionized for detection. Open droplet microarrays are compatible with MALDI-MS, for the ionized analytes would not be blocked by the closed cover, and the substrate can be directly substituted for the dedicated MALDI plate into the instrument without transfer.126,127 It is notable that indium tin oxide coating is commonly used to enable the substrate to be conductive in the MALDI instrument.26,104,106 Xu et al. performed enzyme catalytic screening by studying the metabolism changes of host cells through this approach (Fig. 7B).105 Glass slides modified with chromium electrode patterns were used to hold droplets, which was conductive to high ionization efficiency and strong signal readout. After drying and coating with the matrix, MALDI-MS ion imaging was conducted to obtain intensity distributions with diverse mass-to-charge ratios (m/z) for subsequent data analysis. In addition, the soft ionization properties of MALDI preserved the DNA, allowing the recovery of droplets of interest for further PCR analysis.
MS-based methods provide the metabolic profile of cells at a specific moment, while optical-based methods can track the metabolic dynamics at specific time points. Zhang et al. developed a single-cell metabolic monitoring device to distinguish the normal cells and tumor cells by the Warburg effect, which indicates that more lactate was secreted in tumor microenvironments (Fig. 7C).106 They mixed cell suspensions with an acid-sensitive fluorescent dye, SNARF-5F, and printed a single-cell containing droplet array in open space. The extracellular pH in cell-occupied droplets was monitored by optical imaging via the fluorescent reporter, indicating lactate accumulation in droplet environments. The validation results from blood samples indicated that the method can distinguish tumor cells efficiently through metabolic monitoring within the droplets. In subsequent work, their group also used a similar system to monitor the metabolic activity of cells generating nitric oxide.80 Fluctuations in fluorescence intensity revealed the regulatory role of the tumor microenvironment in macrophage phenotypes. Fluorescent methods offer a low-cost alternative; however, the usage of fluorescent labels may interfere with the extracellular environment. Additionally, they are not suitable for continuous signal readout at high temporal resolution because prolonged fluorescent irradiation leads to quenching and thus affects experimental results.
Electrochemical detection is a less invasive and high temporal resolution technology for studying cell metabolites. Through the fabrication of electrodes on the substrate with attached droplets, metabolites in droplets can be detected with high sensitivity.128,129 Dornhof et al. developed an electrochemical method to measure cellular respiration rates (Fig. 7D).107 Platinum-based electrochemical sensors were manufactured on glass substrate. Using a bioprinting-based automated deposition technique, droplets encapsulated with tumor spheroids were printed above the electrodes, and drugs were added at specific time points. Changes in oxygen concentration following drug exposure were recorded over several hours using chronoamperometric and active potentiometric measurement, demonstrating the ability of the method to be reproducible and accurately determined. Misun's group designed an electrochemical chip to monitor the metabolism of tumor cell spheroids in hanging droplets.108,130 Pt-based electrochemical sensors were functionalized with oxidase enzymes and integrated into the ceiling substrate. Glucose consumption and lactate secretion of human colon cancer cells were monitored in parallel.
5.4 Behavior analysis
Cell behavior is the external manifestation of cellular physiological processes, including adhesion, migration, proliferation, and others. Featured by the addressability and biocompatibility of most open droplet systems, they are well-suited for continuous tracking and evaluation of cell behaviors in long-time scenarios.Determination and analysis based on cell activity have been used for large-scale screening applications, which are typically accomplished in traditional multi-well plates. Open droplet systems, especially droplet microarrays, provide a better alternative as they reduce reagent consumption and costs. Drug screening is a widespread application based on cell viability evaluation.83,93,109,131 Cui et al. used droplet microarray chips to conduct a highly miniaturized screening of 2208 drugs for recurrent glioma treatment.131 Each drug was printed on the chips as a single agent in specific order, then 3D cell spheroids were seeded on the sites and cultured in hanging droplets. The effect of the drug was evaluated by the cell viability image based on live/dead fluorescence staining. Primary screening identified over 20 potential drug candidates that significantly affected tumor spheroid formation and viability. In addition to pre-printing drugs on the substrate, Hu et al. used a droplet sandwich technique to add drugs and viability-test reagents into cells (Fig. 8A).109 They cultured lung cancer organoids in droplet arrays, and following analysis confirmed that one-week cell viability tests can effectively predict the drug response of patients.
 | ||
| Fig. 8 Cell behavior analysis in open droplet microfluidics. (A) High-throughput drug screen assays are performed in open droplet arrays based on the images of cell viability. Reproduced with permission from ref. 109. Copyright 2021, Springer Nature. (B) Droplet chain arrays are constructed to create specific cell microenvironments for cell migration assays. Reproduced with permission from ref. 110. Copyright 2016, Royal of Society Chemistry. (C) The micro-jets technique is used to create patterned wounds in cell layers, tracing the wound-healing behaviors in droplets. Reproduced with permission from ref. 111. Copyright 2021, AIP Publishing. | ||
Cell migration is a fundamental behavior in biological processes. The flexible configuration of the open droplet systems allows the construction of microenvironments with specific chemical concentration gradients for cell migration studies. Droplets containing cells and stimulating reagents are initially separated and connected via fluidic channels132 or porous membranes110 to allow cell migration. Ma et al. developed a droplet chain array for multiple modes of cell migration assays.110 The system consisted of a support layer, droplets above and below, and a porous membrane. Cells are seeded into the upper droplets and can migrate across the porous membrane under the induced concentration gradient from lower droplets. Fig. 8B illustrates a flower-like chain for a multi-tissue migration assay. Cells from different tissues were symmetrically seeded in the lower droplets, while the upper cancer cells showed different tendencies to migrate towards these directions.
Wound-healing studies have been conducted by scratched patterns in open space. Soitu et al. used a Teflon stylus as the cell knife and dragged across the cell monolayer to create patterns of wound conveniently.65 Afterwards, they also introduced the micro-jet technique to produce the wound (Fig. 8C).111 Immiscible fluorinated oil was jetted and detached the cells. The wound dimensions were regulated by the nozzle diameter, volumetric flow rate, and traverse speed.
Conclusions and perspectives
Open droplet microfluidics is becoming a powerful tool for understanding broader and deeper insights into cell research. In this review, we overviewed open droplet microfluidics by introducing the typical generation and manipulation method, several formats of cell culture, and applications of cell analysis including nucleic acids, proteins, metabolites, and behavior analysis in open droplet systems.The integration of droplets and open microfluidics exhibits a synergistic effect. Droplet microfluidics serves as a powerful tool in high-throughput cellular analysis due to the properties of compartmentalization and parallelization. The introduction of open configuration further simplifies the system setup and enables the droplets to be addressable and accessible. Therefore, interaction between the internal and external droplet environments is facilitated: reagents can be added at specific times to meet the requirements of multiple-step reactions, while droplet contents can be recovered for downstream analysis coupled with other methods. The relatively stable environment allows for long-term culture and continuous tracing of cell status. Open droplet systems integrate several in situ operations including cell isolation, culture, observation, manipulation, and recovery, serving as a flexible and robust workflow for cellular assays.
Nevertheless, several challenges remain to be addressed in the future, as follows: (1) limited throughput. Although most studies describe system performance as “high throughput”, this is often relative to non-droplet systems. In fact, most existing liquid handling technologies operate in a one-by-one manner, resulting in lower throughput than traditional closed droplet systems which use high-flow-rate syringe pumps. In certain time-sensitive, large-scale analytical scenarios, this serialized workflow may introduce significant delays between samples and potentially compromise the accuracy of results. One promising solution is to employ parallelized multi-channel tools, such as using sandwich arrays or side-by-side capillary tools. By implementing more sophisticated operational procedures, the number of droplets handled simultaneously can be significantly increased. Another approach is to develop a robust droplet moving method that enables droplets to autonomously navigate to specific working points to perform operations such as reagent addition and sorting. With algorithmic path planning and network scheduling, the operational throughput of large-scale systems can be significantly enhanced. (2) Evaporation management. Since droplets are directly exposed to open environments, increased evaporation rate becomes a critical concern. The accelerated reduction in volume can disrupt the nutrient supply and even lead to cell death. One solution is to improve external conditions, such as temperature and humidity, allowing for evaporation compensation. Alternatively, reconfigurable system designs such as disassembled top covers can be employed. Droplets are exposed to the open space only during generation and manipulation, while at other times they remain enclosed to minimize interference. Notably, these reversible devices also offer protection from external perturbations. (3) Lower standardization and commercialization place high demands on non-specialist experimenters. Compared to traditional droplet microfluidic devices, open systems are still in an early development stage with various operational modes. Precision-controlled droplet handling techniques need to be integrated into advanced devices that can be controlled by user-defined programs. Additionally, disposable kits for droplet generation via surface patterning, including prepared patterned substrates, could be developed as convenient and low-cost options for experimenters. These efforts will help promote the widespread use of open droplet microfluidics as a more pervasive and generalized platform in various laboratories.
As an emerging microfluidic technology, the integration of open droplets with other advanced technologies holds great promise. For example, the rapid development of artificial intelligence (AI) is driving a technological revolution, and its combination with open droplets is envisioned to yield more innovative functionalities. Data acquisition and processing of large-scale droplet arrays can be efficiently realized through machine learning or deep learning, which significantly reduces the complexity of manual data processing and provide support for larger-scale droplet experiments. Moreover, highly automated robotic processing can execute more intelligent droplet workflows, optimizing the system efficiency and performance. Envision a scenario of autonomously controlled systems, where the AI technology unifies the real-time sensing, data processing and immediate operation of the droplets, enabling intelligent adjustment of the subsequent operation based on contextual detection results. For example, flow rates could be adjusted according to the uniformity of the existing droplet during the generation process, or subsequent droplet size could be modified based on the encapsulation rate of single cells. This AI-assisted open droplet system can establish a closed-loop feedback control mechanism, enhancing the intelligence and efficiency of droplet analysis.
The combination of open droplets and single-cell multi-omics holds the potential to catalyze groundbreaking discoveries. The inherent accessibility and addressability of the open droplet system make it exceptionally well suited for in situ multistep and multiplexed analyses of the same sample, while effectively handling large-scale data. This capability is particularly advantageous for conducting single-cell multi-omics studies. By encoding and decoding the droplet of individual cells, functional indexing methods can be developed to facilitate multimodal analysis and connect measurements to genotypic, metabolic, and phenotypic features of single cells, thus revealing cellular heterogeneity and underlying biological pathways. Furthermore, the stabilizing characteristics of the open droplet system support the tracking of dynamic information, allowing for the observation of temporal changes during cellular development. Combined with sensitive real-time sensing and data analysis techniques, the trajectory of cells over time can be reconstructed. We envision utilizing open droplets as culture carriers and detection interfaces for single cells, enabling more comprehensive analyses to create a detailed blueprint of single-cell behavior.
Overall, despite the remaining challenges, droplets in open microfluidics will evolve into a powerful tool in various scientific and industrial fields and is expected to decipher more challenging chemical and biological mysteries in the future.
Data availability
Data for this article including figures are available and can be obtained from the published publication which have been cited at the Notes and references of this work.Author contributions
Jiaxu Lin: investigation and writing – original draft. Ying Hou: reviewing and editing. Qiang Zhang: reviewing and editing. Jin-Ming Lin: reviewing and editing, supervision and funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (No. 2022YFC3400700) and the National Natural Science Foundation of China (No. 22034005).Notes and references
- Y. Zeng, J. W. Khor, T. L. van Neel, W. Tu, J. Berthier, S. Thongpang, E. Berthier and A. B. Theberge, Nat. Rev. Chem., 2023, 1–17 Search PubMed.
- M. T. Guo, A. Rotem, J. A. Heyman and D. A. Weitz, Lab Chip, 2012, 12, 2146–2155 RSC.
- L. Shang, Y. Cheng and Y. Zhao, Chem. Rev., 2017, 117, 7964–8040 CrossRef CAS PubMed.
- T. Moragues, D. Arguijo, T. Beneyton, C. Modavi, K. Simutis, A. R. Abate, J.-C. Baret, A. J. deMello, D. Densmore and A. D. Griffiths, Nat. Rev. Methods Primers, 2023, 3, 1–22 CrossRef.
- Y. Ding, P. D. Howes and A. J. deMello, Anal. Chem., 2020, 92, 132–149 CrossRef CAS PubMed.
- A. J. deMello, Nature, 2006, 442, 394–402 CrossRef CAS PubMed.
- L. Nan, H. Zhang, D. A. Weitz and H. C. Shum, Lab Chip, 2024, 24, 1135–1153 RSC.
- E. Z. Macosko, A. Basu, R. Satija, J. Nemesh, K. Shekhar, M. Goldman, I. Tirosh, A. R. Bialas, N. Kamitaki, E. M. Martersteck, J. J. Trombetta, D. A. Weitz, J. R. Sanes, A. K. Shalek, A. Regev and S. A. McCarroll, Cell, 2015, 161, 1202–1214 CrossRef CAS.
- A. M. Klein, L. Mazutis, I. Akartuna, N. Tallapragada, A. Veres, V. Li, L. Peshkin, D. A. Weitz and M. W. Kirschner, Cell, 2015, 161, 1187–1201 CrossRef PubMed.
- K. Eyer, R. C. L. Doineau, C. E. Castrillon, L. Briseño-Roa, V. Menrath, G. Mottet, P. England, A. Godina, E. Brient-Litzler, C. Nizak, A. Jensen, A. D. Griffiths, J. Bibette, P. Bruhns and J. Baudry, Nat. Biotechnol., 2017, 35, 977–982 CrossRef CAS PubMed.
- Y. Bounab, K. Eyer, S. Dixneuf, M. Rybczynska, C. Chauvel, M. Mistretta, T. Tran, N. Aymerich, G. Chenon, J.-F. Llitjos, F. Venet, G. Monneret, I. A. Gillespie, P. Cortez, V. Moucadel, A. Pachot, A. Troesch, P. Leissner, J. Textoris, J. Bibette, C. Guyard, J. Baudry, A. D. Griffiths and C. Védrine, Nat. Protoc., 2020, 15, 2920–2955 CrossRef CAS PubMed.
- F. Del Ben, M. Turetta, G. Celetti, A. Piruska, M. Bulfoni, D. Cesselli, W. T. S. Huck and G. Scoles, Angew. Chem., Int. Ed., 2016, 55, 8581–8584 CrossRef CAS PubMed.
- K. Wink, M. van der Loh, N. Hartner, M. Polack, C. Dusny, A. Schmid and D. Belder, Angew. Chem., Int. Ed., 2022, 61, e202204098 CrossRef CAS PubMed.
- G. V. Kaigala, R. D. Lovchik and E. Delamarche, Angew. Chem., Int. Ed., 2012, 51, 11224–11240 CrossRef CAS PubMed.
- Q. Zhang, S. Feng, L. Lin, S. Mao and J.-M. Lin, Chem. Soc. Rev., 2021, 50, 5333–5348 RSC.
- Q. Zhang, T. Xie, X. Yi, G. Xing, S. Feng, S. Chen, Y. Li and J.-M. Lin, ACS Appl. Mater. Interfaces, 2023, 15(39), 45640–45650 CrossRef CAS PubMed.
- Q. Zhang, L. Lin, X. Yi, T. Xie, G. Xing, Y. Li, X. Wang and J.-M. Lin, Anal. Chem., 2023, 95(49), 18082–18090 CrossRef CAS PubMed.
- N. M. Oliveira, S. Vilabril, M. B. Oliveira, R. L. Reis and J. F. Mano, Mater. Sci. Eng., C, 2019, 97, 851–863 CrossRef CAS PubMed.
- H. Han, J. S. Lee, H. Kim, S. Shin, J. Lee, J. Kim, X. Hou, S.-W. Cho, J. Seo and T. Lee, ACS Nano, 2018, 12, 932–941 CrossRef CAS PubMed.
- J. Höpfner, M. Brehm and P. A. Levkin, Small, 2024, 20, 2304325 CrossRef PubMed.
- M. Tang, X. Duan, A. Yang, S. He, Y. Zhou, Y. Liu, L. Zhang, X. Luo, P. Shi, H. Li and X. Lin, Adv. Sci., 2022, 9, 2104449 CrossRef CAS PubMed.
- A. D. Pizarro, C. L. A. Berli, G. J. A. A. Soler-Illia and M. G. Bellino, Nat. Commun., 2022, 13, 3047 CrossRef CAS PubMed.
- Y. Song, L. Wang, T. Xu, G. Zhang and X. Zhang, Natl. Sci. Rev., 2023, 10, nwad106 CrossRef CAS PubMed.
- X. Xu, L. Cai, S. Liang, Q. Zhang, S. Lin, M. Li, Q. Yang, C. Li, Z. Han and C. Yang, Lab Chip, 2023, 23, 1169–1191 RSC.
- D. Xu, W. Zhang, H. Li, N. Li and J.-M. Lin, Lab Chip, 2023, 23, 1258–1278 RSC.
- D. Haidas, S. Bachler, M. Köhler, L. M. Blank, R. Zenobi and P. S. Dittrich, Anal. Chem., 2019, 91, 2066–2073 CrossRef CAS PubMed.
- J.-G. Xu, M.-S. Huang, H.-F. Wang and Q. Fang, Anal. Chem., 2019, 91, 10757–10763 CrossRef CAS.
- W. Zhang, N. Li, D. Koga, Y. Zhang, H. Zeng, H. Nakajima, J.-M. Lin and K. Uchiyama, Anal. Chem., 2018, 90, 5329–5334 CrossRef CAS PubMed.
- Y. Wei, Y. Zhu and Q. Fang, Anal. Chem., 2019, 91, 4995–5003 CrossRef CAS.
- T. Tronser, A. A. Popova, M. Jaggy, M. Bastmeyer and P. A. Levkin, Adv. Healthcare Mater., 2017, 6, 1700622 CrossRef.
- L. Du, H. Liu and J. Zhou, Microsyst. Nanoeng., 2020, 6, 1–9 CrossRef.
- C.-Y. Jiang, L. Dong, J.-K. Zhao, X. Hu, C. Shen, Y. Qiao, X. Zhang, Y. Wang, R. F. Ismagilov, S.-J. Liu and W. Du, Appl. Environ. Microbiol., 2016, 82, 2210–2218 CrossRef CAS.
- R. H. Cole, S.-Y. Tang, C. A. Siltanen, P. Shahi, J. Q. Zhang, S. Poust, Z. J. Gartner and A. R. Abate, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 8728–8733 CrossRef CAS PubMed.
- E. Um, E. Rha, S.-L. Choi, S.-G. Lee and J.-K. Park, Lab Chip, 2012, 12, 1594–1597 RSC.
- K. Hattori, Y. Goda, M. Yamashita, Y. Yoshioka, R. Kojima and S. Ota, Anal. Chem., 2022, 94, 11209–11215 CrossRef CAS PubMed.
- Y. Zhu, L.-N. Zhu, R. Guo, H.-J. Cui, S. Ye and Q. Fang, Sci. Rep., 2014, 4, 5046 CrossRef CAS PubMed.
- G.-S. Du, J.-Z. Pan, S.-P. Zhao, Y. Zhu, J. M. J. den Toonder and Q. Fang, Anal. Chem., 2013, 85, 6740–6747 CrossRef CAS PubMed.
- Y. Choi, Y. Song, Y. T. Kim, S. J. Lee, K. G. Lee and S. G. Im, ACS Appl. Mater. Interfaces, 2021, 13, 3098–3108 CrossRef CAS PubMed.
- M. Sun, J. Zhang, T. Xuanyuan, X. Liu and W. Liu, ACS Appl. Mater. Interfaces, 2024, 16, 20132–20142 CAS.
- F. L. Geyer, E. Ueda, U. Liebel, N. Grau and P. A. Levkin, Angew. Chem., Int. Ed., 2011, 50, 8424–8427 CrossRef CAS.
- X. Xiao, X. Miao, S. Duan, S. Liu, Q. Cao, R. Wu, C. Tao, J. Zhao, Q. Qu, A. Markiewicz, R. Peng, Y. Chen, A. Żaczek and J. Liu, Small Methods, 2023, 7, 2300096 CrossRef CAS PubMed.
- J. W. Khor, U. N. Lee, J. Berthier, E. Berthier and A. B. Theberge, Adv. Mater. Interfaces, 2023, 10, 2202234 CrossRef.
- D. Cai, Y. Wang, J. Zou, Z. Li, E. Huang, X. Ouyang, Z. Que, Y. Luo, Z. Chen, Y. Jiang, G. Zhang, H. Wu and D. Liu, Adv. Sci., 2023, 10, 2205863 CrossRef CAS.
- W. Zhang, N. Li, L. Lin, Q. Huang, K. Uchiyama and J.-M. Lin, Small, 2020, 16, 1903402 CrossRef CAS PubMed.
- Z. Wu, Y. Zheng, L. Lin, Y. Lin, T. Xie, J. Lin, G. Xing and J.-M. Lin, Small, 2024, 20, 2306814 CrossRef CAS PubMed.
- Z. Wu, Y. Zheng, J.-M. Lin, Y. Li, Y. Lin, X. Wang and L. Lin, Chem. Eng. J., 2024, 481, 148403 CrossRef CAS.
- H. Li, W. Fang, Z. Zhao, A. Li, Z. Li, M. Li, Q. Li, X. Feng and Y. Song, Angew. Chem., Int. Ed., 2020, 59, 10535–10539 CrossRef CAS PubMed.
- Y. Sun, W. Song, X. Sun and S. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 31054–31060 CrossRef CAS PubMed.
- D. Decrop, G. Pardon, L. Brancato, D. Kil, R. Zandi Shafagh, T. Kokalj, T. Haraldsson, R. Puers, W. van der Wijngaart and J. Lammertyn, ACS Appl. Mater. Interfaces, 2017, 9, 10418–10426 CrossRef CAS PubMed.
- A. A. Popova, S. M. Schillo, K. Demir, E. Ueda, A. Nesterov-Mueller and P. A. Levkin, Adv. Mater., 2015, 27, 5217–5222 CrossRef CAS PubMed.
- L. Du, Y. Li, X. Zhang, Z. Zhou, Y. Wang, D. Jing and J. Zhou, ACS Appl. Mater. Interfaces, 2023, 15, 17413–17420 CrossRef CAS PubMed.
- X. Wang, X. Li, A. Pu, H. B. Shun, C. Chen, L. Ai, Z. Tan, J. Zhang, K. Liu, J. Gao, K. Ban and X. Yao, Lab Chip, 2024, 24, 1782–1793 RSC.
- J.-W. Wang, J. Gao, H.-F. Wang, Q.-H. Jin, B. Rao, W. Deng, Y. Cao, M. Lei, S. Ye and Q. Fang, Anal. Chem., 2019, 91, 10132–10140 CrossRef CAS PubMed.
- D. Haidas, M. Napiorkowska, S. Schmitt and P. S. Dittrich, Anal. Chem., 2020, 92, 3810–3818 CrossRef CAS PubMed.
- E. J. Walsh, A. Feuerborn, J. H. R. Wheeler, A. N. Tan, W. M. Durham, K. R. Foster and P. R. Cook, Nat. Commun., 2017, 8, 816 CrossRef PubMed.
- X. Liu, D. Ma, H. Ye, Y. Hou, X. Bai, Y. Xing, X. Cheng, B. Lin and Y. Lu, TrAC, Trends Anal. Chem., 2023, 166, 117153 CrossRef CAS.
- W. Xu, Y. Jin, W. Li, Y. Song, S. Gao, B. Zhang, L. Wang, M. Cui, X. Yan and Z. Wang, Sci. Adv., 2022, 8, eade2085 CrossRef CAS PubMed.
- Y. Jin, W. Xu, H. Zhang, R. Li, J. Sun, S. Yang, M. Liu, H. Mao and Z. Wang, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2105459119 CrossRef CAS.
- X. Han, S. Tan, R. Jin, L. Jiang and L. Heng, J. Am. Chem. Soc., 2023, 145, 6420–6427 CrossRef CAS PubMed.
- A. Li, H. Li, Z. Li, Z. Zhao, K. Li, M. Li and Y. Song, Sci. Adv., 2020, 6, eaay5808 CrossRef CAS PubMed.
- F. Wang, M. Liu, C. Liu, Q. Zhao, T. Wang, Z. Wang and X. Du, Sci. Adv., 2022, 8, eabp9369 CrossRef CAS PubMed.
- Z. Yuan, C. Lu, C. Liu, X. Bai, L. Zhao, S. Feng and Y. Liu, Sci. Adv., 2023, 9, eadg2352 CrossRef PubMed.
- A. A. Popova, K. Demir, T. G. Hartanto, E. Schmitt and P. A. Levkin, RSC Adv., 2016, 6, 38263–38276 RSC.
- C. Soitu, A. Feuerborn, A. N. Tan, H. Walker, P. A. Walsh, A. A. Castrejón-Pita, P. R. Cook and E. J. Walsh, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, E5926–E5933 CrossRef PubMed.
- C. Soitu, A. Feuerborn, C. Deroy, A. A. Castrejón-Pita, P. R. Cook and E. J. Walsh, Sci. Adv., 2019, 5, eaav8002 CrossRef PubMed.
- C. Soitu, N. Stovall-Kurtz, C. Deroy, A. A. Castrejón-Pita, P. R. Cook and E. J. Walsh, Adv. Sci., 2020, 7, 2001854 CrossRef CAS PubMed.
- J. Niu, W. Liu, J. X. Li, X. Pang, Y. Liu, C. Zhang, K. Yue, Y. Zhou, F. Xu, X. Li and F. Li, Nat. Commun., 2023, 14, 3853 CrossRef CAS PubMed.
- S. Feng, S. Mao, J. Dou, W. Li, H. Li and J.-M. Lin, Chem. Sci., 2019, 10, 8571–8576 RSC.
- Y. Zhu, Y.-X. Zhang, L.-F. Cai and Q. Fang, Anal. Chem., 2013, 85, 6723–6731 CrossRef CAS PubMed.
- Y. Sun, X. Chen, X. Zhou, J. Zhu and Y. Yu, Lab Chip, 2015, 15, 2429–2436 RSC.
- Y. Zhang and T.-H. Wang, RSC Adv., 2019, 9, 21741–21747 RSC.
- S. Konishi, Y. Higuchi and A. Tamayori, Sens. Actuators, B, 2022, 370, 132435 CrossRef CAS.
- S. Konishi, C. Ohya and T. Yamada, Sci. Rep., 2021, 11, 12355 CrossRef PubMed.
- S. Sart, G. Ronteix, S. Jain, G. Amselem and C. N. Baroud, Chem. Rev., 2022, 122, 7061–7096 CrossRef CAS PubMed.
- D. S. Juang, T. D. Juang, D. M. Dudley, C. M. Newman, M. A. Accola, W. M. Rehrauer, T. C. Friedrich, D. H. O'Connor and D. J. Beebe, Nat. Commun., 2021, 12, 4317 CrossRef CAS PubMed.
- X.-L. Guo, Y. Wei, Q. Lou, Y. Zhu and Q. Fang, Anal. Chem., 2018, 90, 5810–5817 CrossRef CAS PubMed.
- T. Xie, Q. Zhang, W. Zhang, S. Feng and J. Lin, Small, 2022, 18, 2107992 CrossRef CAS PubMed.
- S. Chakraborty, V. Gourain, M. Benz, J. M. Scheiger, P. A. Levkin and A. A. Popova, Mater. Today Bio, 2021, 11, 100112 CrossRef CAS PubMed.
- O. Frey, P. M. Misun, D. A. Fluri, J. G. Hengstler and A. Hierlemann, Nat. Commun., 2014, 5, 4250 CrossRef CAS PubMed.
- J. Lin, Q. Zhang, T. Xie, Z. Wu, Y. Hou, Y. Song, Y. Lin and J.-M. Lin, Small Methods, 2024, 8, 2301659 CrossRef CAS PubMed.
- H. Cui, X. Wang, J. Wesslowski, T. Tronser, J. Rosenbauer, A. Schug, G. Davidson, A. A. Popova and P. A. Levkin, Adv. Mater., 2021, 33, 2006434 CrossRef CAS PubMed.
- H. Wu, X. Chen, X. Gao, M. Zhang, J. Wu and W. Wen, Anal. Chem., 2018, 90, 4303–4309 CrossRef CAS PubMed.
- A. Ganguli, A. Mostafa, C. Saavedra, Y. Kim, P. Le, V. Faramarzi, R. W. Feathers, J. Berger, K. P. Ramos-Cruz, O. Adeniba, G. J. P. Diaz, J. Drnevich, C. L. Wright, A. G. Hernandez, W. Lin, A. M. Smith, F. Kosari, G. Vasmatzis, P. Z. Anastasiadis and R. Bashir, Sci. Adv., 2021, 7, eabc1323 CrossRef CAS PubMed.
- S.-P. Zhao, Y. Ma, Q. Lou, H. Zhu, B. Yang and Q. Fang, Anal. Chem., 2017, 89, 10153–10157 CrossRef CAS PubMed.
- X. Du, W. Li, G. Du, H. Cho, M. Yu, Q. Fang, L. P. Lee and J. Fang, Anal. Chem., 2018, 90, 3253–3261 CrossRef CAS PubMed.
- D. Rodoplu, J. S. Matahum and C.-H. Hsu, Lab Chip, 2022, 22, 1275–1285 RSC.
- F. Chen, L. Lin, J. Zhang, Z. He, K. Uchiyama and J.-M. Lin, Anal. Chem., 2016, 88, 4354–4360 CrossRef CAS PubMed.
- P. Zhang and A. R. Abate, Adv. Mater., 2020, 32, 2005346 CrossRef PubMed.
- J. Schoendube, D. Wright, R. Zengerle and P. Koltay, Biomicrofluidics, 2015, 9, 014117 CrossRef CAS PubMed.
- Y. Wang, X. Wang, T. Pan, B. Li and J. Chu, Lab Chip, 2021, 21, 3695–3706 RSC.
- W. H. Yoon, H.-R. Lee, S. Kim, E. Kim, J. H. Ku, K. Shin and S. Jung, Biofabrication, 2020, 12, 035030 CrossRef CAS PubMed.
- M. B. Oliveira, A. I. Neto, C. R. Correia, M. I. Rial-Hermida, C. Alvarez-Lorenzo and J. F. Mano, ACS Appl. Mater. Interfaces, 2014, 6, 9488–9495 CrossRef CAS PubMed.
- A. A. Popova, T. Tronser, K. Demir, P. Haitz, K. Kuodyte, V. Starkuviene, P. Wajda and P. A. Levkin, Small, 2019, 15, 1901299 CrossRef PubMed.
- S. Chakraborty, C. Luchena, J. J. Elton, M. P. Schilling, M. Reischl, M. Roux, P. A. Levkin and A. A. Popova, Adv. Healthcare Mater., 2022, 11, 2102493 CrossRef CAS PubMed.
- Y. Zhu, Y.-X. Zhang, W.-W. Liu, Y. Ma, Q. Fang and B. Yao, Sci. Rep., 2015, 5, 9551 CrossRef PubMed.
- C. Li, Y. Gong, X. Wang, J. Xu and B. Ma, Small, 2021, 17, 2100325 CrossRef CAS PubMed.
- Y. Zhu, P. D. Piehowski, R. Zhao, J. Chen, Y. Shen, R. J. Moore, A. K. Shukla, V. A. Petyuk, M. Campbell-Thompson, C. E. Mathews, R. D. Smith, W.-J. Qian and R. T. Kelly, Nat. Commun., 2018, 9, 882 CrossRef PubMed.
- Y. Zhu, G. Clair, W. B. Chrisler, Y. Shen, R. Zhao, A. K. Shukla, R. J. Moore, R. S. Misra, G. S. Pryhuber, R. D. Smith, C. Ansong and R. T. Kelly, Angew. Chem., Int. Ed., 2018, 57, 12370–12374 CrossRef CAS PubMed.
- Y. Wang, Z.-Y. Guan, S.-W. Shi, Y.-R. Jiang, J. Zhang, Y. Yang, Q. Wu, J. Wu, J.-B. Chen, W.-X. Ying, Q.-Q. Xu, Q.-X. Fan, H.-F. Wang, L. Zhou, L. Wang, J. Fang, J.-Z. Pan and Q. Fang, Nat. Commun., 2024, 15, 1–13 Search PubMed.
- A. Salehi-Reyhani, E. Burgin, O. Ces, K. R. Willison and D. R. Klug, Analyst, 2014, 139, 5367–5374 RSC.
- S. Ansaryan, Y.-C. Liu, X. Li, A. M. Economou, C. S. Eberhardt, C. Jandus and H. Altug, Nat. Biomed. Eng., 2023, 7, 943–958 CrossRef CAS PubMed.
- J. Feng, X. Zhang, L. Huang, H. Yao, C. Yang, X. Ma, S. Zhang and X. Zhang, Anal. Chem., 2019, 91, 5613–5620 CrossRef CAS PubMed.
- X.-C. Zhang, Q. Zang, H. Zhao, X. Ma, X. Pan, J. Feng, S. Zhang, R. Zhang, Z. Abliz and X. Zhang, Anal. Chem., 2018, 90, 9897–9903 CrossRef CAS.
- C. RamalloGuevara, D. Paulssen, A. A. Popova, C. Hopf and P. A. Levkin, Adv. Biol., 2021, 5, 2000279 CrossRef CAS.
- L. Xu, K.-C. Chang, E. M. Payne, C. Modavi, L. Liu, C. M. Palmer, N. Tao, H. S. Alper, R. T. Kennedy, D. S. Cornett and A. R. Abate, Nat. Commun., 2021, 12, 6803 CrossRef CAS PubMed.
- W. Zhang, N. Li, L. Lin, H. Li and J.-M. Lin, Anal. Chem., 2021, 93, 6955–6960 CrossRef CAS PubMed.
- J. Dornhof, V. Zieger, J. Kieninger, D. Frejek, R. Zengerle, G. A. Urban, S. Kartmann and A. Weltin, Lab Chip, 2022, 22, 4369–4381 RSC.
- N. Rousset, R. L. Sandoval, M. M. Modena, A. Hierlemann and P. M. Misun, Microsyst. Nanoeng., 2022, 8, 1–18 CrossRef PubMed.
- Y. Hu, X. Sui, F. Song, Y. Li, K. Li, Z. Chen, F. Yang, X. Chen, Y. Zhang, X. Wang, Q. Liu, C. Li, B. Zou, X. Chen, J. Wang and P. Liu, Nat. Commun., 2021, 12, 2581 CrossRef CAS PubMed.
- Y. Ma, J.-Z. Pan, S.-P. Zhao, Q. Lou, Y. Zhu and Q. Fang, Lab Chip, 2016, 16, 4658–4665 RSC.
- C. Soitu, M. Panea, A. A. Castrejón-Pita, P. R. Cook and E. J. Walsh, Biomicrofluidics, 2021, 15, 014108 CrossRef CAS PubMed.
- W.-W. Liu, Y. Zhu, Y.-M. Feng, J. Fang and Q. Fang, Anal. Chem., 2017, 89, 822–829 CrossRef CAS PubMed.
- M. Rezaei, P. Radfar, M. Winter, L. McClements, B. Thierry and M. E. Warkiani, Anal. Chem., 2021, 93, 4584–4592 CrossRef CAS PubMed.
- K. Leung, A. Klaus, B. K. Lin, E. Laks, J. Biele, D. Lai, A. Bashashati, Y.-F. Huang, R. Aniba, M. Moksa, A. Steif, A.-M. Mes-Masson, M. Hirst, S. P. Shah, S. Aparicio and C. L. Hansen, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 8484–8489 CrossRef CAS PubMed.
- P. Mao, L. Cao, Z. Li, M. You, B. Gao, X. Xie, Z. Xue, P. Peng, C. Yao and F. Xu, Analyst, 2021, 146, 6960–6969 RSC.
- M. Awashra, P. Elomaa, T. Ojalehto, P. Saavalainen and V. Jokinen, Adv. Mater. Interfaces, 2024, 11, 2300596 CrossRef CAS.
- M. Labib and S. O. Kelley, Nat. Rev. Chem., 2020, 4, 143–158 CrossRef PubMed.
- J. Woo, S. M. Williams, L. M. Markillie, S. Feng, C.-F. Tsai, V. Aguilera-Vazquez, R. L. Sontag, R. J. Moore, D. Hu, H. S. Mehta, J. Cantlon-Bruce, T. Liu, J. N. Adkins, R. D. Smith, G. C. Clair, L. Pasa-Tolic and Y. Zhu, Nat. Commun., 2021, 12, 6246 CrossRef CAS PubMed.
- S. M. Williams, A. V. Liyu, C.-F. Tsai, R. J. Moore, D. J. Orton, W. B. Chrisler, M. J. Gaffrey, T. Liu, R. D. Smith, R. T. Kelly, L. Pasa-Tolic and Y. Zhu, Anal. Chem., 2020, 92, 10588–10596 CrossRef CAS PubMed.
- Z.-Y. Li, M. Huang, X.-K. Wang, Y. Zhu, J.-S. Li, C. C. L. Wong and Q. Fang, Anal. Chem., 2018, 90, 5430–5438 CrossRef CAS PubMed.
- X.-L. Guo, Y. Wei, Q. Lou, Y. Zhu and Q. Fang, Anal. Chem., 2018, 90, 5810–5817 CrossRef CAS PubMed.
- Q. Han, N. Bagheri, E. M. Bradshaw, D. A. Hafler, D. A. Lauffenburger and J. C. Love, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 1607–1612 CrossRef CAS PubMed.
- W.-H. Sun, Y. Wei, X.-L. Guo, Q. Wu, X. Di and Q. Fang, Anal. Chem., 2020, 92, 8759–8767 CrossRef CAS PubMed.
- D.-Q. Jin, Y. Zhu and Q. Fang, Anal. Chem., 2014, 86, 10796–10803 CrossRef CAS PubMed.
- D.-Q. Jin, S.-W. Shi, Y. Ma and Q. Fang, Anal. Chem., 2020, 92, 9214–9222 CrossRef CAS PubMed.
- S. K. Küster, M. Pabst, K. Jefimovs, R. Zenobi and P. S. Dittrich, Anal. Chem., 2014, 86, 4848–4855 CrossRef PubMed.
- S. K. Küster, S. R. Fagerer, P. E. Verboket, K. Eyer, K. Jefimovs, R. Zenobi and P. S. Dittrich, Anal. Chem., 2013, 85, 1285–1289 CrossRef PubMed.
- H. Zhang, T. Oellers, W. Feng, T. Abdulazim, E. N. Saw, A. Ludwig, P. A. Levkin and N. Plumeré, Anal. Chem., 2017, 89, 5832–5839 CrossRef CAS PubMed.
- Y. Song, T. Xu, J. Xiu and X. Zhang, Biosens. Bioelectron., 2020, 149, 111845 CrossRef CAS PubMed.
- P. M. Misun, J. Rothe, Y. R. F. Schmid, A. Hierlemann and O. Frey, Microsyst. Nanoeng., 2016, 2, 1–9 Search PubMed.
- H. Cui, X. Sun, M. Schilling, C. Herold-Mende, M. Reischl, P. A. Levkin, A. A. Popova and Ş. Turcan, Adv. Healthcare Mater., 2023, 12, 2300591 CrossRef CAS PubMed.
- C. Deroy, A. N. Rumianek, J. H. R. Wheeler, F. Nebuloni, P. R. Cook, D. R. Greaves and E. J. Walsh, Adv. Mater. Technol., 2022, 7, 2200279 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |




