 Open Access Article
Open Access ArticleCancer-on-a-chip for precision cancer medicine
Lunan Liu
a,
Huishu Wanga,
Ruiqi Chenb,
Yujing Songa,
William Weic,
David Baekb,
Mahan Gillinc,
Katsuo Kurabayashi
ac and
Weiqiang Chen
 *abd
*abd
aDepartment of Mechanical and Aerospace Engineering, New York University Tandon School of Engineering, Brooklyn, NY 11201, USA. E-mail: wchen@nyu.edu
bDepartment of Biomedical Engineering, New York University Tandon School of Engineering, Brooklyn, NY 11201, USA
cDepartment of Chemical and Biomolecular Engineering, New York University Tandon School of Engineering, Brooklyn, NY 11201, USA
dPerlmutter Cancer Center, NYU Grossman School of Medicine, New York, NY 10016, USA
First published on 15th April 2025
Abstract
Many cancer therapies fail in clinical trials despite showing potent efficacy in preclinical studies. One of the key reasons is the adopted preclinical models cannot recapitulate the complex tumor microenvironment (TME) and reflect the heterogeneity and patient specificity in human cancer. Cancer-on-a-chip (CoC) microphysiological systems can closely mimic the complex anatomical features and microenvironment interactions in an actual tumor, enabling more accurate disease modeling and therapy testing. This review article concisely summarizes and highlights the state-of-the-art progresses in CoC development for modeling critical TME compartments including the tumor vasculature, stromal and immune niche, as well as its applications in therapying screening. Current dilemma in cancer therapy development demonstrates that future preclinical models should reflect patient specific pathophysiology and heterogeneity with high accuracy and enable high-throughput screening for anticancer drug discovery and development. Therefore, CoC should be evolved as well. We explore future directions and discuss the pathway to develop the next generation of CoC models for precision cancer medicine, such as patient-derived chip, organoids-on-a-chip, and multi-organs-on-a-chip with high fidelity. We also discuss how the integration of sensors and microenvironmental control modules can provide a more comprehensive investigation of disease mechanisms and therapies. Next, we outline the roadmap of future standardization and translation of CoC technology toward real-world applications in pharmaceutical development and clinical settings for precision cancer medicine and the practical challenges and ethical concerns. Finally, we overview how applying advanced artificial intelligence tools and computational models could exploit CoC-derived data and augment the analytical ability of CoC.
Introduction
Cancer is a leading cause of death and a growing burden in the United States (US) and worldwide,1–3 urgently requiring novel and effective therapeutics. However, cancer drugs were reported to have the lowest rate of approval from the US Food and Drug Administration (FDA) after entering phase I clinical trials,4 and it can take a median time of 7.3 years and median cost of $648 million to develop an approved cancer drug.5 One key reason for this challenge is the lack of reliably preclinical models to mimic in vivo scenarios with sufficient predictive power, thus most anti-cancer drugs failed in clinical trials despite initial promising results in preclinical studies.6–8 Cancer therapeutic drugs or cells undergo several complicated pathological processes such as transportation through blood vessels and interactions with the tumor microenvironment (TME) before taking effect.9,10 More importantly, as cancer is a highly heterogeneous disease, the intra- and inter-tumor heterogeneity is a leading reason for the distinct patient responses to therapies.11,12 The efficacy of therapy is therefore highly dependent on patient-specific characteristics, which are difficult to be assessed in clinical trials and current model systems. Thus the “one-size-fits-all” approach inherent in conventional preclinical studies is no longer suitable for future advancements.13 Hence, development of more accurate preclinical and clinical screening systems is crucial yet remains a major challenge for new cancer therapy development.Various types of preclinical models have been developed and applied for cancer study, but most of them are still not ideal for accurate disease modeling and therapy testing (Fig. 1). Animal models have been the gold standard in preclinical cancer studies for decades, but they differ inherently from humans in both physiological and anatomical aspects.14 For example, genetically engineered mouse models (GEMMs) might fail to preserve the intra-tumor heterogeneity of human cancer, while patient-derived xenograft (PDX) tumor models are constrained by a limited number of sources, low engraftment success rate, and a high time and labor cost.15–17 Additionally, the widely used immunocompromised mice cannot fully recapitulate the human immune responses, which make them ill-suited to serve as predictive models for cancer immunotherapies. Observing and measuring cellular and molecular interactions in the TME in real time is also challenging in animal models, leading to a loss of valuable spatiotemporal information on disease progression and drug response.18 Furthermore, the use of animal models faces growing ethical concerns.19,20 Instead, different kinds of in vitro models have been developed as complements or alternatives to animal models. Traditional two-dimensional (2D) culture in well-plate is low cost and high throughput, but lacks physiologically relevant three-dimensional (3D) structures and function.21 Tumor spheroids22,23 and patient-derived organoids (PDOs), on the other hand, have recently emerged as novel tools for cancer modeling because they can retain the characteristics of original tumor with self-organizing biomimic 3D structures.24,25 Yet, even though these models can mimic the 3D structure of tumors to some extent, they do not well reproduce the in vivo TME features such as tumor vasculature, the spatial distribution of various type of stromal and immune cells as well as biophysical cues like hypoxia, blood flow and interstitial flow,26,27 let alone that many primary cancer cells simply cannot form spheroids or PDOs.28 Therefore, there is a critical unmet need of a humanized organotypic oncology model to fill the gap between the preclinical studies and clinical trials to better assess anticancer therapies, and improve the mechanistic understanding of therapy failures within a pathophysiologically relevant context.
Originated from the recent microfluidic organ-on-a-chip technology, bioengineered cancer-on-a-chip (CoC) microphysiological systems have emerged as novel transformative tools for precision cancer medicine, as they can closely mimic the complex features and microenvironment interactions in an actual tumor.29–32 Combined with patient samples or patient-derived organoids, CoC models have potentials to retain the original patient characteristics and tumor heterogeneity, making them a valuable precision medicine tool to predict potential therapeutic efficacy and screen for personalized and optimized anti-cancer therapies for individual patients.33–35 Moreover, novel multi-organ-on-a-chip cancer models can simulate the interactions across different organs within a single system, making it possible to study complex pathological processes such as tumor cell intravasation, circulation, and extravasation through the vascular system and TME during cancer metastasis.36–38 Multi-organ-on-a-chip cancer models can also be used to test the efficacy of potential cancer drugs on multiple organs simultaneously, helping to identify potential side effects and therapeutic targets. CoC can also integrate microfluidics-based microenvironmental control functions to precisely tune key biophysical and biochemical cues such as matrix stiffness, fluid flow, and gradients of nutrients, cytokines, chemokines and oxygen, mimicking the complex and dynamic environment in tumor under highly controlled, physiologically relevant conditions.39–41 In addition, the microfluidic CoC system present unique advantages for a high-throughput screening with arrays of testing units, ensuring uniformity and reproducibility across all samples while with good controllability, accelerating screening efficiency and accuracy. Moreover, CoC is compatible with live cell imaging and many existing bioassays for a multiparametric and spatiotemporal characterization, and further integration of novel in situ sensors, advanced analytical methodologies and artificial intelligence (AI) tools could provide rich and high-resolution biological information for cancer physiological study and therapeutic predictions.42–44
The recent FDA Modernization Act 2.0's approval in 2022 is a major step forward for the development and adoption of organ-on-chip technology as alternatives to traditional animal testing in the pharmaceutical industry.45,46 The cutting-edge CoC technology provide a new paradigm for a “clinical trials on a chip” study, thus holds a great translational potential for pharmaceutical development and precision medicine, ultimately leading to the development of more effective and safer treatments for cancer patients. A series of reviews have summarized types of CoC models and related tools for profiling cancer cascade and characterizing TME.47–51 Specifically, in this review, we will focus on the state-of-the-art progress in CoC development for modeling different key niches in TME including vascular, stromal and immune microenvironments, and CoC applications in screening cancer treatments like chemo and immunotherapy. Moreover, we will look into the future and discuss the path to build the next generation of CoC models for precision cancer medicine with high accuracy, translational potential, and analytical ability.
State-of-the-art progress in CoC development
Despite various cancer treatment methods like surgery, chemotherapy, radiotherapy and even novel immunotherapy options being developed over the last several decades, cancer remains among the current leading causes of death worldwide and is considered a major public health concern.1–3 Critical challenges including tumor heterogeneity, inherent histologic properties and the immunosuppressive nature of the TME significantly impede effective treatment. The TME is a “milieu” of distinct elements including aberrant ECM, stroma, infiltrating immunosuppressive cells [e.g., tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs) and regulatory T (Treg) cells], as well as accumulating inflammatory or immunosuppressive cytokines and chemokines.9,10 Cancer is a heterogeneous disease with high variability of patient clinical pathology. The patient-specific TME characteristics lead to distinct response to therapies. To address these critical challenges, CoC has been applied in modeling different types of cancers including solid tumors like lung,52 breast,53 liver,54 pancreatic,55 brain56 and colorectal cancer,57 and blood cancers like leukemia58 and lymphoma.59 Compared to animal models and traditional in vitro models, CoC allows for a more accurate representation of the human TME, as they can directly incorporate human cancer cells and niche cells, mimic the complex 3D anatomical structure and features like tumor vasculatures, stromal, immune, and extracellular matrix (ECM) components.60,61 Various therapies have been tested on CoC including chemotherapy,62 radiation therapy,63 immunotherapy64 and cellular therapy.65 In this section, we will discuss the current progress in CoC development for TME modeling and therapy screening (Fig. 2) and the major examples are summarized in Table 1. | ||
| Fig. 2 Cancer-on-a-chip microphysiological systems for tumor microenvironment modeling and precision cancer medicine screening. | ||
| Type | Model | Setup | Application | Ref. |
|---|---|---|---|---|
| Vasculature | In vitro capillary network | Self-assembly vasculogenesis | Building perfusable and interconnected vasculature | 66 |
| 3D endothelial-lined microvessels | 3D lumen-based vasculature structure with sprouting angiogenesis | Studying the role of angiogenesis in tumor growth and metastasis | 67 | |
| Vascularized tumor spheroids | Anastomosis between tumor and vascular bed | Creating perfusion in tumor for biomimic drug administration | 68 | |
| 3D perfusable microvascular networks | Microvessel bed with tumor cell perfusion | Single-cell level spatial–temporal characterization of tumor cell extravasation | 69 | |
| Pancreatic cancer chip with 3D perfusable endothelial lumens | Juxtaposed cancer and vessel lumens mimicking the cancer cell invasion process | Investigating the mechanism of how tumor reshapes blood vessels | 70 | |
| Stromal microenvironment | Breast cancer chip replicating ECM activation | Epithelial cells invaded into stromal chambers causing ECM activation | Studying ECM activation process by on-line monitoring of ECM evolution | 53 |
| Microfluidic model integrating 3D tumor spheroids and CAFs | Tumor spheroids and CAFs cultured in proximity in a hydrogel on chip | Validating CAFs promoting tumor growth while tumor cells inducing CAF activation and migration | 71 | |
| Omentum-on-a-chip studying stroma-mediated metastasis | Layer-by-layer loading at different days creating biomimic tissue-like structures | Investigating how stromal cells affect tumor cell attachment and growth leading to metastasis | 72 | |
| Tumor-on-a-chip incorporating human platelet lysate hydrogels for tumor metastasis study | Bone marrow mesenchymal stem cells and tumor cells embedded in human based platelet lysate hydrogels on chip | Reproducing the early cancer metastasis process and studying tumor–stromal cell–ECM interactions in a fully human derived TME | 73 | |
| Immune microenvironment | Glioblastoma-on-a-chip dissecting immunosuppression | Incorporating tumor, macrophages, 3D vessels and engineered ECM in a chip | Modeling the macrophage-associated immunosuppression and angiogenesis | 74 |
| Multi-channel tumor-macrophage co-culture model investigating EMT | Tumor aggregates cultured in contact or separately with macrophages in a multi-channel device | Investigating the role of different subtypes of macrophages in causing tumor aggregate dispersion as an indication of EMT | 75 | |
| 3D microfluidic chip modeling tumor-induced DC migration | DCs and tumor cells cultured in interconnected chambers | Tracking DC migration towards tumor cells and investigating potential chemokine axis | 76 | |
| 3D microfluidic tumor model with cytokine gradients | Center channel loaded with tumor cells and two side channels loaded with chemokine flow to build linear gradient in the center channel | Studying role of cytokine gradient in regulating tumor cell migration | 77 | |
| Therapy modeling and screening | Arrayed vascularized micro tumors for drug screening | Multi-unit array contained perfused and vascularized tumor in each unit, and drugs were delivered through hydrostatic pressure gradient | Large-scale chemo drug screening | 78 |
| Organotypic tumor spheroids for PD-1 blockades profiling | Patient-derived tumor spheroids integrated with microfluidic culture | Immune checkpoint blockade testing | 79 | |
| Immunocompetent leukemia chip for CAR T cell therapy screening | Reproducing bone marrow niche structures on chip and infused CAR T cells through vessels | CAR T cell therapy modeling and screening | 80 | |
| Breast cancer chip for CAR T cell efficacy and safety testing | Two-chamber structure with top chamber CAR T cell extravasating through endothelial monolayer to interact with bottom chamber tumor aggregates | Assessing the kinetics of cytokine secretion during CAR T cell therapy for safety evaluation and testing the patient-specific efficacy related to antigen expression | 65 | |
| Cancer chip model to evaluate NK cell therapy | Tumor cells were co-cultured with NK cells and a vessel lumen were applied to perfuse cells, medium or drugs | Investigating the mechanism of NK cell exhaustion in TME and testing potential therapies to alleviate the exhaustion | 81 | |
| Automatic microfluidic platform for drug testing | A high-throughput 3D cell culture chamber integrated with a multiplex fluid control system | Automatic testing of patient responses with different therapies | 82 | |
| Microfluidic CoC model with tumor slices for valuating drug response | Tumor slices were cultured in microfluidic chip with a pumping system providing perfusion | Maintaining the original characteristics of tumors and investigating their chemosensitivity | 83 |
Modeling tumor vasculature
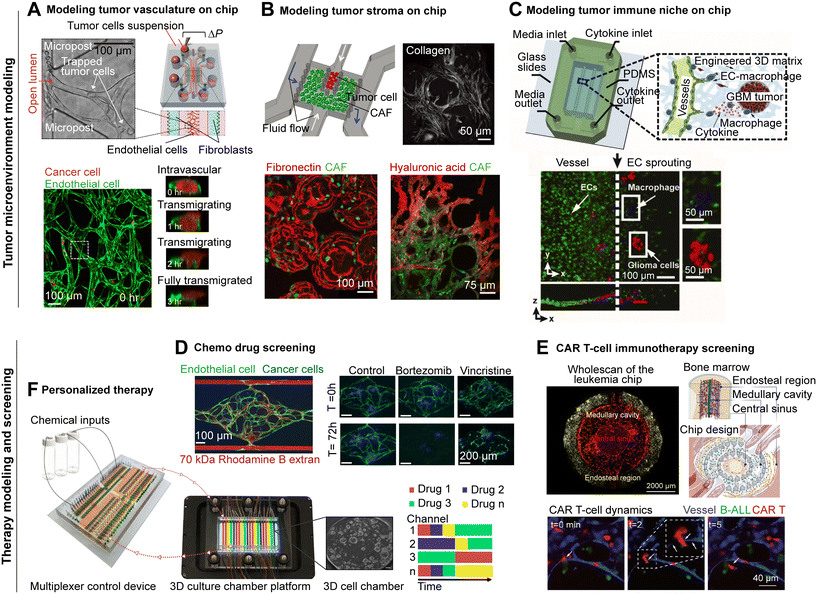
Tumor vasculature is a key component of the TME that significantly influence tumor behavior, including its growth, invasion, metastasis, and response to therapies.84,85 It often contributes to therapeutic resistance due to its abnormal structure and function which can hinder drug delivery and immune cell infiltration in the TME. In vitro preclinical models, particularly those 2D cell co-culture, 3D spheroids and PDOs lacking perfusable vasculature, are not ideal to study the tumor–vascular interactions in TME.86 CoC which excels in mimicking the tumor vasculature formation, has emerged as a promising solution.87–90 Biomimicry tumor blood vessels can be formed on chip through vasculogenesis, sprouting angiogenesis, or anastomosis. The vasculogenesis self-assembly method simply mixes primary vascular endothelial cells with hydrogels (e.g., fibrin gels) to spontaneously form an interconnected 3D vascular network.66,91,92 As tumor grows in size, tumor cells release pro-angiogenic factors such as vascular endothelial growth factor (VEGF) to facilitate tumor angiogenesis to form new blood vessels.93 The sprouting angiogenesis method forms new blood vessels sprouts by growing endothelial cells from existing blood vessels toward an angiogenic stimulus such as cancer cells, VEGF, or hypoxia in parallel microfluidic channels.67,94 Alternatively, the anastomosis method creates capillary networks through the sprouting and anastomosing of endothelial cells to form perfusable interconnections from established vasculature beds in two side-channels.68,95–98The formation of tumor blood vessels on chip relies highly on various factors including the source of endothelial cells, proper co-culturing cells, ECMs, pro-angiogenic factors and biophysical cues like interstitial flow and hypoxic conditions. Primary endothelial cells of healthy donors from commercial supplies (e.g., human umbilical vein endothelial cells, HUVECs) are often used for blood vessel construction in current CoC models, due to their readily available nature, well-studied characteristics and angiogenesis potential.36,38 However, tumor blood vessels could exhibit an aberrant, immature structure, which are more permeable than normal vasculatures.99,100 The vascular properties may vary depending on the functional state of the endothelium or organ-specific characteristics.37,101 Therefore, selecting appropriate endothelial cells especially tumor-derived endothelial cells are critical for better reproducing tumor vasculatures on chip for different modeling scenarios. For example, in a glioblastoma (GBM)-on-a-chip model, brain-specific endothelial cells formed tighter and more biomimic microvessels compared to umbilical cord or the lung-derived endothelial cells.102 A micro-tumor model has also demonstrated that the source and passage of endothelial cells affects the perfusability and robustness of vessel network.103 In addition to the cell source, the ECMs used in the model also significantly affect the formation of tumor blood vessels, thus requiring an optimization to better support tumor vasculature growth. Fibrin or a fibrin-based mixture has been the most commonly used scaffold structure in CoC models to support tumor and vasculature.104–106 Moreover, pro-angiogenic supporting cells such as human lung fibroblasts are often used to support the vasculature formation in vascularized CoC systems.107,108 Other cells like platelets have also been found to promote angiogenesis under the influence of tumor cells.109 In addition, pro-angiogenic soluble growth factors like VEGF, HB-EGF (heparin-binding epidermal growth factor-like growth factor) and PIGF (placental growth factor),90,110 and biophysical cues such as interstitial flow and hypoxia in TME can promote vasculature growth on chip.111,112
Vascularized CoC platforms provide an effective tool for studying tumor–vascular interactions in the TME.36 For example, CoC models have been used to study how tumor actively participate in driving angiogenesis and shaping blood vessels.113 For instance, ovarian and lung tumors were found to promote the formation of stable vascular network and increase the permeability and necrosis of surrounding vasculature.114 A pancreatic cancer model emulated vascular invasion and tumor–blood vessel interactions and determined the mediator of endothelial ablation from cancer.70 Immune–vascular–tumor interactions in glioblastoma were also studied in a 3D microfluidic angiogenesis model, and validated that tumor-induced polarization of immunosuppressive macrophages fostered a proangiogenic niche.74 Perfusable, vascularized CoC also made it possible to study metastasis.54,115–117 CoC models have been used to study cancer cell intravasation through mosaic vessels,118 cancer cell migration along the vasculature119 and cancer cell extravasation from blood vessels in metastasis.120,121 A CoC model established microvessels and allowed for visualization and characterization of tumor cell extravasation dynamics69 (Fig. 3A). The engineered perfusable vasculatures also allowed for the study of drug delivery86,122–124 as well as immune cell recruitment and extravasation in cancer immunotherapy.80,81,125–128 For example, poor blood–brain barrier (BBB) penetration, a major obstacle for targeted drug delivery in brain tumors,129 can be modeled with a BBB-on-a-chip model to recapitulate BBB function and mimic drug delivery and efficacy.62,130–132
 | ||
| Fig. 3 Representative cancer-on-a-chip models for tumor microenvironment modeling and therapy screening. (A) A tumor vasculature CoC model mimics the extravasation of tumor cells from vasculature. Reproduced from ref. 69 with permission from Springer Nature, copyright 2017. (B) A tumor stromal niche model studies CAFs activated by tumor cells and induced the over deposition of ECM components collagen, fibronectin and hyaluronic acid. Reproduced from ref. 53 with permission from Wiley, copyright 2016. (C) An GBM immune niche model studies TAM associated immunosuppression and promoted angiogenesis. Reproduced from ref. 74 with permission from Elsevier, copyright 2018. (D) A CoC with vascularized micro tumors screened effective chemo drugs with high reproducibility and biomimicry. Reproduced from ref. 78 with permission from Royal Society of Chemistry, copyright 2021. (E) A leukemia chip modeled the in vivo leukemic bone marrow niche and CAR T cell therapy on chip. Reproduced from ref. 80, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). (F) An automatic microfluidic CoC platform enabled personalized drug screening of for different patients. Reproduced from ref. 82, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). | ||
Modeling tumor stromal microenvironments
The tumor stromal niche, involving various types of cells such as tumor-associated fibroblasts (CAFs), mesenchymal stem cells (MSCs), endothelial cells, tumor-associated adipocytes, pericytes, osteoblasts and ECM, plays a pivotal role in forming a TME that promotes tumor progression, metastasis, and drug resistance.133–135 Advanced CoC in vitro models that faithfully recapitulate the complexity of stromal microenvironment, hold significant potential as platforms for elucidating the mechanisms underlying tumor–stromal crosstalk and for the development and screening of novel therapies. CAFs are the most abundant stromal cell type and are the primary source of ECM deposition in the TME, contributing to the physical and biochemical structure of the TME.136,137 CAFs can be recruited from nearby fibroblasts by tumor cells or be transdifferentiated from normal fibroblasts138,139 or from tumor-associated stromal cells such as MSCs.140,141 Cancer–stroma chip models have been leveraged to study the interactions between cancer cells and CAFs, recapitulating how cancer cells induce specific CAF phenotypes, activation, and migration.142 A breast CoC model replicated how cancer cells induced the activation of CAFs and the subsequent excessive deposition of ECM in stromal niche during cancer invasion53 (Fig. 3B). Compared to normal fibroblasts, CAFs exhibit different phenotypes, signaling pathways and protein expression, and promote tumor growth, angiogenesis and metastasis.143,144 In addition, CAFs inhibit anti-tumor immune cells,145,146 provide metabolites to tumor cells147,148 and participate in resistance to anti-tumor treatment.149,150 Using cancer–stroma chip models, one can investigate the potential mechanisms of how CAFs promote cancer cell invasion and therapy resistance.71,151–153 The migration ability of tumor cells influenced by CAFs was studied through an invasion assay on chip and was combined with transcriptome analysis to determine the gene of interest related to invasion.154 The crosstalk between CAFs and lymphatic vessels, was investigated on chip and found that CAF-secreted cytokines can impair vessel barrier function and mediate patient-specific cancer cell migration with clinical relevance.117,155 A multi-compartmentalized CoC model verified that CAFs can lower the killing effects of anti-tumor drugs, and that such drug resistance can be rescued by targeted therapy avoiding CAF-induced ECM remodeling.156 Beside CAFs, other tumor-associated stromal cells such as MSCs, osteoblasts, adipocytes and pericytes were also found to be critically involved in TME.157–160 For instance, a peritoneal omentum-on-a-chip model was used to study the distinct effects of stromal cells including mesothelial cells and adipocytes on tumor cell attachment and growth, as well as the microvascular network formation.72ECM is a major non-cellular component in tumor stromal niche. Its composition and biophysical characteristics (e.g., stiffness) can be indicators of tumor progression, metastasis161,162 and therapy resistance.163,164 CoC models utilize natural hydrogels (like fibrin gel, collagen or Matrigel)165–167 or mimicking hybrid hydrogels,56 allowing for 3D cell culture and mimicking the tissue-like conditions of the TME. More biomimicking ECM with anisotropic architectures168 or various natural or synthetic components like polyethylene glycol (PEG)169 and polylactide-co-glycolide acid (PLGA),170 can be tailored to mimic the TMEs of specific cancer types. ECM can be also obtained from tissues through decellularization instead of reconstituted hydrogels to better mimic the mechanical and physiological properties of the original tumor.52 CoC models enable the investigation of the evolution of cell-assembled ECM over time such as the high deposition of hyaluronic acid (HA) during tumor progression.53,165 Integrated with engineered ECM of various structures or densities, CoC can create a physiologically relevant stromal niche and allow for the behaviors of cancer cells under different biophysical cues to be studied.38,171–173 In vitro CoC models can recapitulate stromal cells, ECM characteristics, and physical properties at different metastatic sites, providing a platform for studying the mechanisms of metastasis and predicting metastatic potential.174 On-chip metastasis models have been applied to study how stromal cells and cancer cells facilitate tumor invasiveness via remodeling matrix stiffness, adjusting collagen expression and inducing certain gene expression.73,175,176 Likewise, an ovarian CoC has demonstrated how ECM components and biophysical cues like shear pressure and can affect cancer cell migratory behavior.177
Modeling tumor immune microenvironments
Immune cells (e.g., myeloid cells and lymphocytes) and acellular components (e.g., cytokines) can interact with tumor cells and other niche components and lead to immunosuppression in TME, critically regulating tumor progression, immune escape and drug resistance.178,179 Immunocompetent CoC incorporated with critical immune components can serve as an ideal tool for tumor immune microenvironment modeling to systematically investigate immune response, immune cell infiltration, antitumor cytotoxicity or protumor immunosuppression.180 TAM is abundant in the tumor immune niche and play a central role in supporting tumor development and immunosuppression in TME.181,182 CoC models have shown cancer cells can induce the recruitment183 and activation processes of macrophages into tumor cites,184 and in turn, TAM can enhance the speed and migration directedness of cancer cells through a matrix metalloproteinases (MMP)-dependent manner.185 Macrophages can polarized into different subtypes as a spectrum from anti-tumor M1 subtype to pro-tumor M2 subtype in the TME, exerting distinct functions in mediating immunosuppression, tumor progression and metastasis.186 CoC study demonstrated that M1 macrophages can inhibit tumor invasion, growth and angiogenesis while M2 macrophages promote tumor migration.187 A GBM-on-a-chip model demonstrated that TAM in the GBM TME were more polarized towards M2 phenotype and promoted tumor angiogenesis and immunosuppression through immune–vascular and cell–matrix interactions74 (Fig. 3C). By measuring the dispersion of carcinoma aggregate as a representation of epithelial-mesenchymal transition (EMT), a CoC platform that introduced different subtypes of macrophages found that macrophages of M2a subtype might promote cancer metastasis through a contact-mediated mechanism.75Dendritic cells (DCs) are the major cells participating in tumor antigen presentation.188 A CoC model which tracked the motion of DCs towards tumor cells as well as the subsequent phagocytosis events, was used to determine that the CXCR4/CXCL12 axis as the key signaling pathway guiding DC movement.76 Natural killer (NK) cells have strong cytotoxic activity and can directly kill cancer cells.189 However, immunosuppression can lead to the lack of presence of NK cells in TME.190 CoC models allow for a study of NK cell migration towards tumor cells under DC-induced chemical gradient,191 indicating the recruitment and anti-tumor potency of NK cells might be influenced by the crosstalk with DCs. In the future, more types of important immune cells such as Treg cells and MDSCs can be incorporated in the CoC models to better recapitulate the immunosuppressive TME. A 3D organotypic and immunocompetent leukemia chip constructed with healthy donor or patients' bone marrow mononuclear cells included all key bone marrow immune cells on chip, well mirrored the in vivo leukemic bone marrow immune microenvironment with biomimic cell compositions and functions.80 Another bone marrow on-a-chip model that incorporated four major niches and utilized recirculating perfusion system investigated the distinct patterns of homing and retention between malignant and healthy hematopoietic stem and progenitor cells (HSPC).192 Moreover, immune cell secreted cytokines in TME are critically involved in regulating cancer initiation, EMT, invasion and metastasis.193 The stable chemical gradient established on chip enables investigation of how specific cytokines facilitate the invasion of cancer cells in TME77 and formation of immunosuppressive TME.74
Despite the recent significant advances in CoC development, many challenges remain to be addressed to enhance the fidelity of the platform in modeling the TME. It should be noted that due to the limitation of available cell samples and the complexity in TME, it is usually not realistic to include all types of TME components on chip. A system with too high complexity would compromise the robustness, while a system that is too simple cannot accurately reflect the true niche. Therefore, the minimum system that satisfactorily recapitulates the TME should be determined. With various types of niche cells present on chip, a careful balancing of culture conditions, such as the nutrient requirements for each cell type, is required. Moreover, human leukocyte antigen (HLA)-mismatch in allogeneic cells is a particularly considerable problem in modeling patient-specific TME on chip as it will cause artifacts in immune responses. To attenuate this issue, one possible solution is to knockout of immune-related genes like β2 microglobulin (B2M) gene in allogeneic niche cells.194,195 Alternatively, adopting autologous patient-derived immune cells or patient induced pluripotent stem cell (iPSC)-derived cells from one patient may be a promising choice in the future.196–199 Lastly, mechanosensation of immune cells is an important factor in modulating their phenotypes and immune responses,200 and the mechanism of how immune cells respond to mechanical stimuli is promising to be investigated by CoC in the future.
Therapy modeling and screening
Despite significant progress in the field of oncology and continuing improvement in cancer treatment have been made, the complex nature of tumors, including their heterogeneity and ability to develop resistance to drug, often results in unreliable predictions of treatment efficacy and safety in preclinical models and clinical trials.201–203 Current simple in vitro models and animal models fail to reflect the tumor heterogeneity and TME characteristics, thus cause significant discrepancies between the preclinical and clinical results. CoC models with physiologically relevant TME, enable accurate assessments of new cancer therapies and patient responses, thus facilitating drug development and precision cancer medicine.204–206Chemotherapy remains one of the most prevalent cancer treatments, though challenges including cancer drug resistance, toxicity and patient-specific response make it difficult to achieve consistent success.207 CoC model can investigate chemo drug resistance by dissecting the effects of TME niche factors,208–210 establishing wide ranges of drug gradients for dose testing,211 and applying potential combinational therapies to overcome chemoresistance.212 A leukemia-on-a-chip study systematically explored how the bone marrow stromal niche cells such as vascular cells, MSCs and endosteal osteoblasts are able to maintain the survival of leukemia cells and support chemoresistance though cytokine and adhesive signaling.58 A following leukemia chip study further verified that leukemia cells could promote the non-classical monocyte differentiation, which is related to the leukemia patient's survival and chemotherapy response.213 A multi-compartmentalized CoC model verified that CAFs can lower the killing effects of anti-tumor drugs, and that such drug resistance can be rescued by targeted therapy avoiding CAF-induced ECM remodeling.156 Aligned stromal topography mimicking the in vivo tumor migration front was recreated on a hybrid nanopatterned model, and validated that such topography can mediate the chemoresistance of cancer cell clusters to different treatments.214 The efficacy of chemotherapy also depends on the structure and function of vessels to transport drugs.215 Vascularized and perfused CoC models enable mimicking chemo drug transport with high biomimicry.216 A CoC model established vascularized micro tumors on chip and identified effective chemo drugs with high reproducibility and physiological relevance78 (Fig. 3D). Such kinds of platforms have been used to investigate the mechanisms of tumor therapy resistance due to the obstruction of drug delivery and the effects of perfusion on drug transport.68,217,218
Immunotherapy is becoming a promising treatment for cancer, but human immune responses to immunotherapies cannot be well recapitulated in animal models.219 Novel CoC immune-oncology models could recapitulate the complexity and heterogeneity of immune niche, thus suitable for assessing cancer immunotherapies.220–222 Immune checkpoint inhibitors (ICIs) are most widely used immunotherapies clinically223 and have been modeled with CoC.224–226 For example, a GBM-on-a-chip model built with patient-derived GBM cells as well as TAMs and 3D vasculature has been used to model PD-1 checkpoint based immunotherapy and combination therapy.56 The GBM chip study validated that different GBM subtypes had different levels of TAM M2 polarization, immunosuppression, and cytotoxic T cell infiltration, thus resulting in distinct responses to the PD-1 ICI. In another study, patient and murine derived organotypic tumor spheroids retaining autologous key TME lymphoid and myeloid cell populations were cultured in 3D microfluidic chips, and were applied to determine the key TME immune cell and cytokine features associated with response and resistance to PD-1 ICI treatment.79 Such chip was further combined with dynamic single-cell RNA sequencing to determine a subpopulation of anti-PD-1 therapy persister cells.227 In addition to T cell based ICI studies, immunotherapies targeting other immune cells in TME like TAM were studied on chip as well.228 The microfluidic CoC system has been employed to investigate a promising immunotherapeutic approach that combines anti-epidermal growth factor receptor (EGFR) IgA with an anti-CD47 innate ICI to activate M2-like macrophage phagocytic function to eliminate cancer cells. In addition to the immune niche factors, incorporating stromal cells like CAFs and MSCs on chip has revealed a potential for more precise prediction of immunotherapy efficacy than conventional models.229 CoC models have demonstrated that CAFs can suppress the functions of immunotherapies like trastuzumab230 and PD-1 ICI.64 It was validated on chip that when reducing the expression level of immune checkpoints like PD-L1 on CAFs with pirfenidone, CAFs and cancer cells showed lower invasion and migration capacity.231 As salient features of solid tumors, dense stroma, abundant immunosuppressive cells and cytokines can inhibit the infiltration of cytotoxic T cells and cause an immune cold TME, significantly lowering immunotherapy efficacy.232–234 With real-time monitoring functions and 3D organotypic TME features, T cell infiltration and their interactions with the TME under immunotherapy can be investigated through time-lapse live cell imaging on chip.224–226 As targeting stroma or immune niche cells can overcome the immune cold TME and enhance immune cells infiltration, CoC models are suitable for testing and screening niche-targeted therapies in the future due to its high spatiotemporal resolution and accessible readouts.56
Cellular therapy is an emerging immunotherapy strategy by engineering immune cells to fight cancer.235 CoC models have been applied to study engineered chimeric antigen receptor (CAR) T cell therapy. A leukemia chip has enabled real-time spatiotemporal monitoring of CAR T cell dynamics and functions, including infiltration, activation, tumor killing and cytokine secretion, modeling distinct clinically observed responses including remission, resistance and relapse on chip80 (Fig. 3E). Besides, different types of CAR T cell products were examined on the chip, indicating the potential of the CoC model for CAR T cell development and personalized therapy screening. This leukemia CoC model has been applied to investigate potential factors leading to therapy failure, e.g., exploring how leukemia intrinsic drivers regulate CD19 antigen presentation on leukemia cells and impact patient response to CAR T cell therapy.236 CoC models have been applied to study CAR T cell therapy for solid tumors, and investigate the hurdles encountered during CAR T cell infiltration in ECM and killing to cancer cells.237 Another CoC model monitored the cytokine release kinetics during CAR T cell therapy and studied how to attenuate cytokine release syndrome (CRS), a main adverse event in CAR T cell therapy, with drug intervention to achieve on/off functional control of CAR T cells.65 The chip also accessed antigen-dependent CAR T cell killing efficacy by including different PDOs in the system. Beside CAR T cells, T cell receptor (TCR)-based T cell therapy was modeled on chip as well to study how immune cells like monocytes,238 environmental cues like inflammation and oxygen level239 and novel base editing technology like CRIPSR can affect therapy efficacy.240 In another study, CoC model assessed the on-target off-tumor effect of T cell bispecific antibodies (TCBs) immunotherapy by monitoring epithelial cell death, immune cell activation, attachment and pro-inflammatory cytokine secretion, demonstrating its potential in immunotherapy safety evaluation.241 Besides, NK cell therapy was evaluated on chip to investigate the penetration of NK cells into tumor spheroids127 and how tumor-induced immunosuppression leading to NK cells exhaustion, which can be alleviated by combinational ICIs and immunomodulatory agents.81 Oncolytic vaccinia virus (OVV), another novel immunotherapy, was modeled on chip. An automatic imaging processing algorithm was developed to analyze the dynamics of tumor and immune cells and revealed that OOV and immune cells together mediate the cytotoxicity on chip.242
The evolving landscape of cancer treatment is increasingly focusing on more personalized and precise interventions, accentuating the need for reliable and innovative screening technologies like CoC. For example, autologous tumor cells, CAFs and cytotoxic T cells obtained from patient samples have been utilized to establish a personalized lung CoC model.64 Various on-chip responses to anti-PD-1 therapy were observed on chip and showed similar trends with clinical results. CoC is suitable for high-throughput preclinical antitumor drug screening. For instance, a 3D-bioprinted cholangiocarcinoma-on-a-chip model resembled the anatomical microstructure of the hepato–vascular–biliary system, permitted a high-content antitumor drug screening.243 Another way to enable large-scale therapy screening is to develop 3D microtumors like tumor spheroids or organoids and incorporate them into array patterns on chip.244–249 An automatic microfluidic platform enabled the growth of PDOs and in parallel drug testing of 20 different regimens and 10 different patient samples under individual, combinational or sequencing therapy (Fig. 3F).82 Multicellular tumor spheroids derived from different patients were integrated into a 3D-printed microfluid chip and treated with different chemotherapies, demonstrating correlations with clinical results.250 Besides, patient tumor tissues can be directly dissected into submillimeter size and integrated on the chip through trapping them with microfluidic circuits for therapy screening.251–253 Sliced tumor tissues were laid on the porous membrane on the chip and drugs were perfused through the adjacent delivery channel for large-scale chemosensitivity testing254 and drug response prediction.83 Liquid biopsy derived from cancer patients' blood is another choice to be tested on chip for drug resistance or tolerance screening and evaluation.255
The path to build the next generation CoC model for precision cancer medicine
CoC has become a promising tool in disease modeling and therapy screening.256–258 However, there are still gaps to translate this new technique into real-world applications. To reflect patient-specific features for accurate and personalized drug testing, patient-derived cell samples should be integrated on chip. Novel 3D in vitro models like organoids have great potential to be combined with CoC with synergistic engineering to build a more biomimic model. Moreover, through linking multiple organs into one system and integrating sensors and microenvironmental control modules, the complexity and fidelity of the CoC system can be further improved. Advanced analytical methods like computational models and AI-based tools can utilized to process the CoC readouts, generating new insights and aiding therapy response prediction in a more precise and quantitative manner. Lastly, to translate the CoC into the market, standardized and scalable CoC with economical manufacturing and high-throughput function is essential. In this section, we will discuss the approaches to evolve CoC with high accuracy, analytical ability and translational applications. In this section, we will discuss strategies to build next generation CoC model for precision cancer medicine (Fig. 4) and the major examples are summarized in Table 2.| Type | Model | Setup | Application | Ref. |
|---|---|---|---|---|
| Patient-derived chip | Colon cancer chip with autologous patient tumor cells, stromal cells and lymphocytes | Patient-derived cells were accommodated in a micropatterned hydrogel chamber with mini colon structure on chip | Accessing drug efficacy and toxicity and investigating the interplays among different TME components | 57 |
| Patient-derived tumor vessel on chip | Tumor or normal adjacent tissue-isolated endothelial cells were loaded on chip forming vessel lumen | Reproducing patient tumor vessel features and tailoring treatments for specific patients | 259 | |
| Microfluidic model maintaining tumor biopsy with perfusion | Tumor biopsy was loaded in the microfluidic chamber perfused with a syringe pump | Maintaining tumor biopsy viability and architecture in vitro and testing drug responses | 260 | |
| Cancer organoids-on-a-chip | Vascularized colon organoids-on-a-chip | Colon organoids were co-cultured with self-assembled vascular network under oscillated perfusion | Modeling the recruitment of immune cells from vessels and their infiltration into organoids | 261 |
| Mini-colon modeling colorectal oncogenesis | Spatiotemporal control of tumorigenic transformation in organoids-on-chip with mini-colon topology | Recreating key pathophysiological features of colorectal cancer and screening tumorigenic factors | 262 | |
| Pancreatic cancer organoids-on-a-chip | Organoids were co-cultured with fibroblasts and macrophages to recapitulate the TME | Testing TME-modulating drugs on augmenting chemo therapy efficacy | 29 | |
| Multi-organs-on-chip system | Liver cancer and heart on-a-chip system integrated with sensors | A breadboard enabling microfluidic routing via pneumatic valves and individual modules connected with Teflon tubes | Automated drug screening and acute toxicity study | 263 |
| Cancer-liver–heart multi-organs system for drug evaluation | Drugs initially passed over liver then move on cancer and heart parts | Mimicking the first pass metabolism of liver and evaluating drug efficacy and off-tumor toxicity | 264 | |
| Multi-organ-on-chip system linked by vascular flow | Heart, liver, bone and skin tissue niches were connected by vascular flow through endothelial barrier | Maintaining viability and phenotype of multiple organs and study the PK/PD of cancer drugs | 265 | |
| Microenvironmental control integration | Chip model creating chemokine gradients | Two channels forming a V-shaped structure and parallel connecting channels in between | Establishing gradients and investigating effects on cancer stem cell migration | 266 |
| A hypoxic CoC model for evaluating CAR T cell therapy | A polycarbonate-made cap was inserted on chip as an oxygen diffusion barrier to create hypoxic landscape | Investigating the function and infiltration of CAR T cells in hypoxia | 267 | |
| A chip system integrating programmable flow control | Micropumps transferred fluids between different wells and created fluid pressure and flow | Achieving physiologically relevant flow and studying their effects on tissue function | 268 | |
| A lung cancer model with mechanical cues | Breathing motion in lung was recreated on chip by vacuum with cyclic strain | Mimicking in vivo physical cues and dissecting their effects on tumor growth and drug responses | 269 | |
| Sensor integration | A multi-sensor brain cancer-on-a-chip | Electrode-based sensors were integrated on chip to monitor biophysical and biochemical cues | Real-time and in situ monitoring oxygen level, pH values, lactate and glucose | 270 |
| Bead-based electrochemical immunosensor integrated with liver cancer chip | Electrochemical immunosensor was linked with the bioreactor and achieved programmable and automatic operations with microvalves | In situ and continual monitoring of biomarkers secreted from the bioreactor | 271 | |
| Cytokine secretion measurement for a brain tissue chip | Microfluidic ELISA-based digital immunosensor were integrated below the tissue barrier | Achieving multiplexed, ultrasensitive and longitudinal profiling of secreted cytokines on chip | 272 | |
| Chip translation | High-throughput plate for drug response measurement | 40 units parallel culture on the plate with automatic imaging to evaluate barrier integrity | Investigating the exposure time and concentration responses of drugs | 273 |
| Robotic system enabling automatic chip operation | Liquid handling robots integrated with mobile microscope and custom software | Achieving automatic chip culture, sample transferring and collecting and in situ imaging | 274 | |
| Microphysiological system with inbuilt environmental control | Environmental chamber with temperature and inflow control modules | Keeping the system sterile and maintaining temperature and CO2 level | 275 | |
| AI enabled precision cancer medicine | Deep learning approach classifying cell trajectory patterns | Cancer cell trajectories were derived from chip model and input in pre-trained convolutional neural network | In vitro evaluation of cancer drug treatments | 276 |
| Chip model aided by deep learning for immunotherapy screening | The infiltration images of T cells on the spheroids-on-a-chip platform were applied in a clinical data-trained deep learning model | Identifying immunotherapies enhancing T cell infiltration and treatment efficacy | 277 | |
| Machine learning-based tool for analysis of immune cell and cancer organoids interactions | Engineered T cells were co-cultured with organoids and their behaviors were analyzed by a machine learning-based tool by means of imaging and transcriptomics | Characterizing of T cell behavioral-phenotypic heterogeneity of cellular immunotherapies | 278 | |
| In silico model combination | Integrating the data from glioblastoma-on-a-chip model for in silico simulation | An ODE model was established to depict tumor and immune cell behaviors and interactions and calibrated with CoC data | Dissecting the mechanism of immunotherapy resistance and testing potential combinational therapy | 279 |
| Computational model of a spheroids-on-a-chip | Simulating flow and drug transport and sweeping parameters of the chip with CFD | Accelerating the optimization of chip design | 280 | |
| Quantitative PK/PD model coupling with vascularized chip | On-chip data were scaled with in vitro–in vivo transition | Predicting in vivo PK/PD parameters and aiding the design of phase-I clinical trial | 281 |
Building patient-derived chips
As cancer has high intra-tumoral and inter-tumoral heterogeneity,282 patient samples will better recapitulate the original characteristics of specific patients and enable tailored therapy with CoC model. Conventional CoC were mostly primed with cancer cell lines and commercial primary cells from different donors. Despite the easy access and the simple preparation of such samples, the human physiological relevance and the ability to recapitulate the patient-specific TME are largely impaired. To enable precision medicine with CoC, incorporation of patient cell samples on chip to reflect patient-specific characteristics is indispensable.To build patient-derived CoC models, one common approach is to dissociate and isolate different types of cells from patient biopsy samples, then reconstitute these dissociated cells into CoC to form patient-specific TMEs on chip. Various types of patient samples can be utilized to develop patient-derived CoC, including but not limited to tumor resections and biopsies,283 ascites,284 bone marrow aspirates285 and PDXs.286 For example, primary tumor biopsies can be mechanically disrupted and chemically dissociated into single cells, then be loaded into a microfluidic CoC.287 The platform enabled live and fixed-cell imaging and phenotypic biomarker quantification and combined machine-learning for risk stratification of cancer patients. Another microfluidic platform also adopted dissociated single cells from tumor biopsies which can generate more than 1200 data points with 56 different drug testing conditions.288 A patient-derived mini-colons CoC model reproduced the complexity in TME with colorectal cancer PDOs and their autologous CAFs and tumor-infiltrating lymphocytes (TILs) from colorectal cancer biopsies, and enabled discovery of CAF-triggered mechanism that drives cancer invasion and a comprehensive evaluation of drug effectivity, toxicity and resistance in anticancer therapies57 (Fig. 5A). Drug testing results showed the platform can recapitulate the heterogeneous patient responses and screen potential combinational therapy regimen for different patients. Tumor vessel is an important yet often omitted component during patient-derived model establishment. A study dissociated kidney cancer tissue samples and isolated CD31+ endothelial cells to generate tumor-associated vessels on chip.259 Comparing with normal vessels, tumor-associated vessels demonstrated higher permeability and angiogenesis ability. Also, tumor associated vessels showed patient-specific gene expression profiles on chip. Another method to establish patient-derived model is to mince or slice the resected tumors or biopsies into small-sized fragments and directly culture them in microfluidic CoC. The on-chip culture of these tumor fragments remains challenging. Perfusion system to continuously provide media and remove waste is key in maintaining the viability of tissue samples on chip. For example, microfluidic chambers were designed to maintain minced milliliter sized tissue biopsies, with continuous media perfusion to recapitulate the in vivo flow and diffusion conditions on chip.260 Another microfluidic platform cultured sliced tumor tissue in tissue chambers with integrated perfusion system and monitoring of the oxygen transport on chip.289 It was demonstrated that this chip system can offer satisfying oxygenation and maintain higher viability of tumor samples than conventional well-plate culture.
 | ||
| Fig. 5 Strategies to evolve CoC with higher accuracy, analytical ability and translational applications. (A) A patient-derived colorectal cancer (CRC) chip reproduced in vivo pathophysiology and anatomical structure built with autologous patient cancer cells, CAFs, TILs, and colon mucosa components including colonocytes (CCs), transit-amplifying cells (TAs) and intestinal stem cells (ISCs). Reproduced from ref. 57 with permission from Springer Nature, copyright 2024. (B) A vascularization of colon organoids-on-a-chip showed enhanced growth under perfusable culture on chip comparing with conventional static condition. Reproduced from ref. 261 with permission from Wiley, copyright 2020. (C) A heart and liver cancer multi-organs-on-chip model built with human iPSC-derived cardiomyocytes and hepatocellular carcinoma cells for investigating the acute toxicity induced by anti-tumor drugs. Reproduced from ref. 263 with permission from National Academy of Sciences, copyright 2017. (D) Programmable flow control on CoC chip. Reproduced from ref. 268, CC BY-NC 3.0 (https://creativecommons.org/licenses/by-nc/3.0/). (E) A multi-sensor integrated chip system with electrode-based O2, pH sensors and lactate and glucose biosensors. Reproduced from ref. 270 with permission from Royal Society of Chemistry, copyright 2014. (F) A standardized high-throughput microfluidic platform in 384-well plate format containing 40 units of colorectal cancer tubes for studying drug-induced toxicity on epithelial barriers. Reproduced from ref. 273, CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/). (G) A deep-learning model trained with clinical data and integrated with on-chip readouts to predict patient survival and identify drug candidates based on T cell infiltration in tumor sites. Reproduced from ref. 277 with permission from the authors, copyright 2022. (H) An ODE-based computational model calibrated with GBM CoC-derived data depicted the interactions between T cells, TAMs and GBM cells in TMEs of different GBM subtypes (proneural, classical, mesenchymal) and tested combinational immunotherapies to enhance treatment efficacy. Reproduced from ref. 279 with permission from Wiley, copyright 2021. | ||
Nonetheless, there are hurdles to be solved in developing patient-derived CoC models. First, the access of patient samples is still limited. Biobanks of cancer patient samples like National Cancer Institute Patient-Derived Models Repository (PDMR) are growing and increase the accessibility of precious patient samples. Besides, since patient samples are heterogeneous containing various types of cells, the isolation and in vitro co-culture of different types of autologous stromal and immune cells on chip with high viability and in vivo cell function are challenging, let alone many patients' primary cells do not survive, grow and loss their original characteristics in vitro, requiring well optimizations of on chip culture conditions.50 The ultimate goal of the patient-derived model is to utilize fully autologous samples without allogenic concerns. One possible solution is to use patient iPSCs to derive different autologous niche cells to build the CoC model. In a CoC model studying CAR T cell therapy, human iPSC-derived endothelial cells from the same donor were applied on chip.65 It was demonstrated that comparing with allogenic endothelial cells, patient iPSC-derived endothelial cells induced lower level of cytokine secretion, mitigating the alloreactive responses caused by CAR T cells. In another chip model, four types of cells representing different organs have been differentiated from the iPSCs of the same healthy donor and were integrated in one system.198 Such concept can be applied to develop fully patient-derived CoC model as well. Moreover, a study obtained iPSCs from the skin fibroblasts of different breast cancer patients and induced them into cardiomyocytes.290 The induced cardiomyocytes can reproduce the heterogeneous cardiotoxicity caused by chemo drugs on individual patient level, and such protocol is promising to be applied on CoC models.
Cancer organoids-on-a-chip
Cancer PDOs and organ-on-a-chip represent two distinct yet complementary 3D in vitro models. PDOs can maintain the original characteristics of the primary tumor with high fidelity,291,292 however, such models usually lack tumor vasculature and microenvironmental cues.293 Another obstacle is that PDOs usually grow with substantial variability in size, structural organization, or functional capacity due to uncontrolled culture and niche factors, raising a major concern on their robustness. Organ-on-a-chip, on the other hand, has strengths in these aspects and could be integrated with organoids model.294 Through synergistic engineering, cancer organoids-on-a-chip has showed the potential to become the avatar of patients and excel in precision cancer medicine.295,296Vascularized cancer organoids-on-a-chip platform can build organoids with biomimic vascular network, which is largely absent in conventional organoids yet essential in cancer genesis, progression and metastasis.297 CoC model is moving towards the goal of providing biophysical cues like flow and perfusion control to organoids with vasculature.55 A microfluidic platform named IFlowPlate enabled perfusion and vascularization of colon organoids with a programmable rocker, and the perfusable vascular network resulted in a different drug efficacy comparing from static conditions261 (Fig. 5B). Cancer organoids-on-a-chip also enables controlling the spatial distribution of vasculatures to mimic the mass transport between PDOs and the arterial end of capillary in TME.216 Another chip model enhanced the vascularization of organoids through flow,298 which was further leveraged to enable immune cells infiltration in the vascularized organoids.299
Cancer PDOs model, generated from patient tumor samples and was solely with cancer cells when created decades ago, requires the inclusion of patient stromal and immune cells to build a more complete TME.300 Efforts have been made to establish simplified spheroids-like co-culture models in well-plates. For example, patient-specific cancer assembloid model was constituted with cancer organoids and tumor-isolated TME cells.301 PDOs, stromal cells and peripheral blood lymphocytes co-culture platforms were used for enriching tumor-reactive T cells killing302 and studying tumor–stroma and tumor–immune interactions.303 Moreover, CoC can aid the integration of TME components with PDOs for real-time, high-resolution evaluation of cellular dynamics. Human colorectal cancer organoids,262 colon organoids304 or intestinal stem cell-revived tube-shaped epithelia organoids305 have been integrated with in vivo-like cellular components on chip and applied to study the spatiotemporally resolved colorectal oncogenesis and the human gut physiology and pathology for drug safety assessment. By co-culturing PDOs with stromal cells and immunes cells on a microfluidic chip with medium flow, a pancreatic cancer organoids-on-a-chip was proposed to recapitulate the desmoplastic stromal niche and immune niche.29 The model was applied to test anti-stroma agents and its feasibility in drug testing was proved.
Besides including TME cellar components, the innovation in biomaterials is another a promising solution for improving the establishment and integration of organoids on chip. For instance, one current obstacle in culturing immune organoids in vitro is to maintain their phenotype and viability, especially for non-epithelial cancers like lymphoma, an immune cell-related cancer.306 Synthetic hydrogels have been developed to mimic the lymphoid tissue microenvironment to enhance the survival of lymphoma organoids307 and applied in a CoC model.308 Such lymphoma chip studied immune responses of patients to chemotherapy and indicated a weakened post-chemotherapy immunity. In addition, as organoids-on-a-chip models excel in capturing the morphology and functions of human organs, they can be leveraged to extrinsically guide the self-organization of organoids with more physiologically relevant sizes, shapes and functions. Microfluidic devices have been applied to increase the dimensional uniformity of organoids by culturing them in microarrays,309 radial patterns310 and permeable membranes.311 Similar designs can be utilized by CoC model to reduce the viability of PDOs as well.
Modeling cancer in a multi-organs-on-chip system
Cancer is considered a “systemic” disease interacting with multiple organ systems beyond the initial tumor site. By integrating two or more organs with sophisticated fluid systems, multi-organs-on-chip systems are suitable to investigate the interactions across different organs in cancer and study complex disease mechanisms. For instance, multi-organs-on-chip can be applied for studying the interactions between distant organs like liver and brain to assess the hepatic metabolism-dependent drug cytotoxicity.312 Also, multi-organs-on-chip could be engineered with integrated control and sensing modules to capture and control the biological cues. For example, a fully integrated liver cancer and heart chip integrated with modular physical, biochemical, and optical sensing components was developed to operate the chip units in a continual dynamic and automatic manner, and was used for automated drug screening263 (Fig. 5C). A heart-breast CoC platform integrated with immune-aptasensor was developed to monitor cell-secreted biomarkers from organ interactions.313 Multi-organs-on-chip CoC platform can offer a comprehensive evaluation of drug effects on multiple organs and compound bioactivation and efficacy for pharmacokinetic (PK) and pharmacodynamic (PD) profiles in concurrent organs, which leads to the determination of efficacy and off-target toxicity anti-cancer therapeutics in a one-stop system.264,281,314 A comprehensive human-on-a-chip system contained heart, liver, bone, and skin compartments connected by a biomimic vascular system, and can accurately represent the overall physiological interactions in human body when treated with various cancer therapies.265 This allowed independent organ function, and each tissue was cultured in its environment from the common vascular flow by a permeable endothelial barrier. Such multi-organ system can maintain organ-specific molecular, structural, and functional phenotypes and showed PK/PD, and cardiotoxicity of anticancer drugs.Multi-organs-on-chip can investigate important adverse events of chemotherapy including hepatotoxicity and cardiotoxicity.315–317 By culturing tumor and liver microtissues in different chambers of a multi-organs-on-chip CoC platform, the drug-induced hepatoxicity and anti-tumor bioactivity can be determined simultaneously by measuring the cell viabilities.318 More importantly, the metabolism of anti-tumor prodrug can be simulated through liver components.319–322 On-chip models enable characterizing chemotherapy-caused cardiotoxicity by measuring heart cell functions like beat frequency323 or heart cell damages.324 Immune organs like bone marrow, lymph node and spleen can be integrated on multi-organs-on-chip system to recapitulate the complex immune responses and functions in cancer pathophysiology. As immune responses are systematic, incorporating different immune organs, recapitulating key functions and anatomical structure of primary and secondary immune organs on chip will provide a more biomimic immune microenvironment for modeling the interactions among different compartments.325–328 A tumor and lymph node on-a-chip model built with slices of tumor and lymph node tissue samples recapitulated the two-way communications between them under continuous recirculating flow.329 The on-chip results showed tumor-educated lymph node exhibited higher immunosuppression than in healthy tissue-cocultured lymph node. Moreover, spleen-derived cells with immune deficiency were applied on CoC models to investigate spleen–tumor crosstalk.330 It was demonstrated that immunodeficient spleen cells cannot exert strong immunosurveillance and that cancer cells showed aggressive invasion and poor interaction with spleen cells.
Modeling different organs in a single system is a long-cherished wish, yet facing several challenges. Multi-organs-on-chip systems are more complex than their single-organ chip counterparts, it is challenging to maintain the viability and function of different organs in one chip considering that they require different culture conditions. These systems also require intricate designs, physiological connections between organs, and sophisticated fluid control mechanisms that control the distribution of fluidics across multiple organ compartments. Additionally, creation of biomimic circulation system and blood substitutes remain a major hurdle for developing multi-organ on-chips human microphysiological systems. To mitigate this issue, researchers should try to balance the complexity, physiological accuracy, and reliability of these multi-organ on-chip models.329,331 Instead of creating overly complex models, a practical approach is to design systems that focus on organ functions and inter-organ crosstalk essential to a specific problem of interest in cancer. Despite these challenges, the ability of multi-organs-on-chip systems to mimic systemic interactions between organs presents an opportunity for cancer research and drug testing that alternative in vitro models are unable to offer.
Microenvironmental control integration
Microenvironmental cues such as chemical gradients, pH values, oxygen concentration, mechanical cues including fluidic flow and force, can contributing to tumor angiogenesis, invasion, progression, metastasis and resistance to therapies and are important factors to be considered in cancer modeling.332–340 Upgrading CoC platforms with microfluidics-based microenvironmental control functions could enable establishing a biomimic TME with more physiological relevance to in vivo conditions. CoC can provide precise spatiotemporal control of gradient-induced chemotaxis and aberrant pH values with customized design. For example, a V-shaped microfluidic chip with channels connecting the two sides can create natural gradient and study effect of chemical gradients on cancer stem cell migration.266 Bifurcated microfluidic device mimicked culture conditions of different pH values for direct comparison of cancer cell proliferation and aggressiveness.341 To tune oxygen level in CoC, one straightforward way is to culture chips in a hypoxia chamber.239,342 Another approach is to provide mixed gas into the device and control the oxygen concentration dissolved in culture media.343,344 In another approach, chemicals can be infused in side channels to enable reactions generating or scavenging oxygens.345,346 Such model has validated the hypoxia-induced cytotoxicity of cancer drugs.347 Moreover, by embedding a membrane with low oxygen permeability in the chip to block oxygen diffusion, oxygen gradient can also be established.348,349 A polycarbonate-made hypoxia cap was inserted in a CoC model to investigate how hypoxia spatially and temporally modulate CAR T cell functions.267 Besides, customized hydrogels capable of generating oxygen gradients and modeling hypoxia have been developed and are promising to be integrated with CoC model in the future.350,351CoC can control flow to reproduce the in vivo fluidic dynamics like blood flow and aberrant interstitial fluid flow. Peristaltic and syringe pumps were applied to control the perfusion on CoC.275,352 Pneumatic micropumps were integrated with high-throughput microfluidic system enabling individually managing fluid levels of each unit and generating different flow regimes: perfusion flow or high shear stress flow to model different in vivo conditions (Fig. 5D).268 Pumpless and gravity-driven rocker platform is another approach to control on-chip perfusion by creating reciprocating flow between the pairs of reservoirs.353 Comparing with pump-based systems, it requires much less space and avoids complex connections and air bubble formation problems, thus it is especially compatible with culturing in CO2 incubators or testing in high-throughput.273,354 Mechanical cues like cyclic stretching force can be applied through vacuum pump system355 in CoC, mimicking breathing motions in lung cancer269 and peristalsis in colorectal cancer.356 Similarly, another CoC model investigated the behavior of normal fibroblasts under mechanical stretching and found their enhanced ability to mediate cancer cell migration and similar phenotypes with CAFs.357
Despite CoC has successfully integrated different types of control modules, the scope of current models is often limited to controlling single microenvironmental cue on a single cancer chip unit. A multi-functional system integrated with interchangeable microenvironmental control modules in one chip is highly demanded, enabling a more accurate modeling of the in vivo pathophysiological processes in the TME for precision medicine applications. For example, a platform was demonstrated to integrate fluid flow control, oxygen sensing and tissue resistance measurement in one system.268 In addition, future development of a scale-up system with automatic control functions over an array of cancer chip units will allow a high-throughput of personalized therapy screening.
Sensor integration for in situ and real-time measurement
The integration of in situ and real-time sensors in CoC platforms is crucial for comprehensive study of TME and therapy screening. Traditional static assays, relying on endpoint measurements, often fail to capture the nuanced temporal and spatial variations within the TME, leading to missed insights into transient cellular interactions, signaling cascades, and metabolic shifts. In situ and real-time sensors address these challenges by providing continuous, localized measurements that reveal rapid changes within the TME. For example, in situ cytokine sensing could uncover spatially-resolved data, such as localized inflammation levels or regions of immunosuppression, which cannot be obtained through conventional endpoint or supernatant-based enzyme-linked immunosorbent assay (ELISA) assays. This capability is also beneficial for the application of CoC in precision cancer medicine, where integrated sensors allow for continuous monitoring of cellular and molecular responses to various drugs, providing immediate feedback.Label-free biosensing methods provide real-time, in situ monitoring of biomolecular interactions in CoC. For example, fluorescent ruthenium or platinum octaethylporphyrin (PtOEP) dye, which can be quenched by oxygen, were coated on film and inserted in the media flow on chip as real-time oxygen sensor.358–360 Cytochrome, derived from hepatoma cells, can be monitored on chip through the enzymatic conversion mediated by cytochrome to convert substrate ethoxyresorufin into fluorescent product resorufin.361 Label-free electrophysiological biosensors detect biomolecular interactions by monitoring electrical property changes such as current, voltage, or impedance arising from interactions at the sensor surface. Electrodes were patterned on the top and bottom of a lung cancer chip to achieve transepithelial electrical resistance (TEER) measurement. The TEER sensor accessed the integrity of cancer cell barrier indicating the toxicity of chemo drugs.362 Electrochemical sensors can be integrated into the CoC to continuously track important biomarkers, including cytokines, metabolites, and immune checkpoint molecules.42,363,364 Electrodes were functionalized with oxidase enzymes and integrated on the chip to real-time monitoring lactate and glucose by measuring current readouts.365 Thin-film platinum or iridium oxide electrodes were applied to monitor oxygen level and pH values, and together with electrode-based lactate and glucose biosensors, were integrated on brain cancer chip to achieve a multi-sensor microsystem270 (Fig. 5E). Moreover, microelectrode arrays of cantilever shape can be applied to monitor the contractility of cells.366 A significant recent breakthrough is the development of modular, regeneratable electrochemical sensors that support repeated use, continuous biomarker tracking, essential for applications requiring durable and high temporal resolution.367 Pendulum-type sensors represent a novel approach for real-time, drift-resistant electrochemical sensing.368,369 These sensors utilize a DNA-based or aptamer-based “pendulum” that moves in response to an electric field, providing real-time data on binding interactions via rapid current decay changes. Optical biosensors, particularly surface plasmon resonance (SPR) and localized surface plasmon resonance (LSPR) technologies, are among the most sensitive label-free tools for real-time molecular detection in CoC. These methods detect refractive index changes near the sensor surface, which occur due to biomolecular interactions. SPR and LSPR technologies are particularly advantageous for studying interactions like PD-1/PD-L1 binding in the TME, monitoring tumor-derived exosomes370 and cytokine release,371 and mapping single-cell secretion profiles.372,373 A recent advancement involves LSPR-based microwell arrays with nanohole substrates that allow the spatiotemporal mapping of cytokine secretion from individual cells.374 Multiplexed LSPR microarrays, plasmon rulers using patterned nanostructures offer simultaneous detection of multiple cytokines in complex samples.375–378 One example employs Fe3O4/Au core-shell nanoparticles patterned in an array for real-time, in situ monitoring of key signaling molecules, which are vital for studying immune responses and drug efficacy in CoC models.379 Recent research also integrated a LSPR-based digital nanoplasmonic microarray immunosensor to monitor in situ cytokine profiles on-chip in a biomimetic leukemia-on-a-chip TME model during CAR T cell therapy.380
Unlike label-free technologies, microfluidic ELISA and protein microarrays do not provide continuous or real-time monitoring; however, they offer a cost-effective, highly sensitive, and high-throughput solution for detecting cytokines, growth factors, and other soluble proteins in TME at a specific end-point. These methods are ideal for applications where high spatial resolution, high sensitivity and specificity, and multiplexed analysis are prioritized over real-time monitoring. High temporal resolution is also possible by running multiple integrated sensing chips in parallel at different time point. By leveraging microfluidics, these platforms achieve significant reductions in reagent volume and analysis time, making them particularly valuable for in situ protein detection within CoC. A reusable immunosensor has been linked with a chip system and used disposable magnetic microbeads to capture and measure the secreted biomarkers from hepatocytes on chip.271 Recent advancements in single-molecule detection techniques,381 integrated with microfluidic systems,382 have pushed the sensitivity and multiplexing capabilities of microfluidic ELISA and protein microarrays even further. As an example, the DigiTACK platform integrates digital immunosensors into a tissue chip for in situ, multiplexed cytokine detection, achieving fg mL−1 sensitivity and supporting longitudinal cytokine profiling for inflammatory studies.272,383 For high spatial resolution imaging, a plasmon-enhanced multiplexed FluoroDOT assay was developed to enable the visualization of single-cell protein secretions with spatially resolved digital resolution.384 Techniques such as tyramide signal amplification (TSA)385,386 and rolling circle amplification (RCA)387 have demonstrated superior sensitivity by amplifying detection signals at the molecular level, allowing for precise quantification of low-abundance biomarkers. These amplification approaches, coupled with microfluidic integration, enable highly multiplexed, ultra-sensitive in situ detection, making them invaluable for complex TME studies where both high sensitivity and spatial specificity are critical.
In situ multi-omics technologies are advancing our ability to study cellular interactions and molecular networks within the TME by enabling simultaneous analysis of multiple omics layers—such as transcriptomics, proteomics, and epigenomics—at single-cell resolution.388 By preserving spatial context and capturing multi-layered data from the same cells, these approaches provide a detailed understanding of the functional heterogeneity, regulatory mechanisms, and microenvironmental influences that drive cancer progression and immune responses in TME-mimicking CoC. For transcriptomics, in situ hybridization and sequencing techniques, particularly MERFISH (multiplexed error-robust fluorescence in situ hybridization), offer high-resolution spatial transcriptomic data by labeling individual mRNA molecules across thousands of genes in single cells.389 MERFISH is highly suitable for cancer chip applications, as it allows spatially resolved, multiplexed detection of gene expression within 3D thick tissues.390 For 2D multi-omics, techniques like spatial-CITE-seq391 and DBiT-seq392,393 integrate transcriptomics and proteomics in situ, allowing researchers to spatially map mRNA and protein expression patterns within TME regions. In CoC, these methods can reveal how gene and protein expression vary with location, shedding light on cell–cell interactions and how environmental factors contribute to immune suppression or tumor growth dynamics. Integrating transcriptomics with epigenetic assays like spatial-ATAC-seq394 provides insights into chromatin accessibility and epigenetic regulation within specific TME regions. This is especially valuable in CoC that seek to study tumor cell plasticity, immune evasion, and how chromatin states respond to drug treatments within a microfluidic setup. While multi-omic approaches provide comprehensive snapshots of the TME, they are costly and impractical for regular use, serving more as supplementary tools than primary monitoring solutions.
In situ and real-time sensing technologies within CoC offer significant potential for advancing our understanding of the TME. For effective translation into precision medicine, CoC should prioritize sensors that deliver high-quality, meaningful data relevant to clinical decision-making, such as immune response, therapeutic efficacy, and key biomarker levels. While researchers frequently seek higher spatiotemporal resolution, a fundamental challenge lies in balancing this with the need for comprehensive profiling, such as multi-omic studies, versus the requirement for real-time, continuous monitoring, while maintaining a clinically actionable perspective. For instance, label-free biosensing excels at continuous, real-time monitoring but is often limited in sensitivity and specificity, especially when multiplexed. The future direction for label-free technologies, therefore, could prioritize the development of self-regenerative biosensors to enable long-term, continuous monitoring. Such sensors could revolutionize CoC applications by providing uninterrupted data on dynamic TME processes over extended periods, essential for understanding tumor progression and treatment responses. Meanwhile, improving sensitivity, specificity, and resistance to environmental interferences and bio-fouling are crucial for advancing label-free sensors. Innovations such as nanostructured surfaces and anti-fouling coatings could enhance sensor longevity and data accuracy, particularly for electrochemical biosensors, where fouling reduces signal reliability. On the other hand, for sensors aimed at capturing comprehensive high-content data (e.g., single-molecule counting), seamless integration within CoC for in situ localized measurements remains challenging compared to conventional supernatant-based assays, due to the need for multi-step labeling. Another major opportunity lies in developing multi-modal platforms that integrate mechanical, chemical, and environmental sensors into a single CoC platform. A high-throughput organ-on-a-chip platform supported the culture of different tissues like liver, vasculature, gut and kidney, integrated pneumatic micropumps for programmable and physiologically relevant fluid control, TEER sensor for barrier function monitoring, and the optical luminescence-based oxygen sensor for drug development workflows, increasing the predictability of drug screening.268 The chip was constructed with an industry standard plate-based platform included 96 independent units and was compatible with high-throughput data collection tools like high content imaging system and enables the extraction of samples for RNA-seq. Such platforms could detect shifts in TME properties across multiple parameters, offering a holistic view that surpasses single-sensor setups.
Translation of CoC technology toward real-world applications in pharmaceutical development and precision cancer medicine in clinical settings
Despite the great potential of CoC platforms for improving the drug development pipeline, the current bottlenecks are the low throughput and reproducibility in chip fabrication and biological analyses. Future translation of CoC for real preclinical and clinical studies requires standardizations in chip design, materials, fabrication methods and operation that are compatible with standard biological experiments and workflows in industry.395 Polydimethylsiloxane (PDMS) based microfluidic chip design and soft-lithography fabrication are most commonly used in current CoC models.152 The major flaw of the PDMS-based soft lithography fabrication method is of low throughput and reproducibility due to extensive manual labor and batch variance, limiting its large-scale production of chips for clinical use. PDMS is often chosen for its optical quality, permeability, and low cost, but it has been found to absorb small molecules and leach uncured oligomers that can have effects on the cell culture.396,397 Therefore, materials such as polyurethane, styrene–ethylene–butylene–styrene (SEBS) elastomers, and other thermoplastics have been used as alternatives to PDMS, alongside manufacturing methods like micromilling and 3D bioprinting instead of soft lithography.398–402 Poly(methyl methacrylate) (PMMA), processed with laser cutting or milling machine, was utilized to create high-throughput drug screening and microtissue culture platforms compatible with microscopy275,403,404 and demonstrated superior drug cytotoxicity testing results comparing with PDMS due to their low absorbance to small molecules.405 Poly(ethylene) glycol diacrylate (PEGDA) hydrogel was used to build high-throughput brain cancer chip for drug screening,406 which demonstrates less nonspecific adsorption than PDMS.407 Thus, proper materials and a scalable chip fabrication method should be standardized to enable practical applications of CoC platforms. Another promising fabrication method can be adopted on CoC is 3D bioprinting, an additive manufacturing technology to establish complex TME in anatomic size with programmable and precise spatial control, faithfully recapitulating the original tumor structure and biophysiological properties.408 3D bioprinting of tumor tissue can be directly written in a microfluidic device, or the tumor tissue can be firstly bioprinted off-chip then assembled with the device.409 For example, a CoC model first printed silicon ink to form the chamber wall, then bioprinted the vasculature ring and the center cancer area, establishing a concentric-ring structure.410 The 3D bioprinted chip maintained the radial oxygen gradient and the tumor structures and characteristics like tumor invasion and hyperplasia of vessels. In another CoC model, hepatoma cell clusters were 3D bioprinted with controlled size and perfused into the microfluidic channel. 3D printing is also a compelling approach to directly fabricate microfluidic devices by providing rapid design iteration and prototyping capabilities. Ideally, both the microfluidic device and tumor tissue can be 3D printed through a single-step biofabrication approach combining the tissue bioprinting and 3D-printed.411Moreover, a complementary high-throughput analysis platform becomes essential, allowing compatibility with standard experiments and seamless integration with laboratory automation to efficiently process and analyze the larger volume of data produced, which further cascades into higher reproducibility and standardization. To optimize benchtop research into elegant, commercially-ready and cost-effective commercial products, the lab developed chips need to be redesigned into multi-well plate based high-throughput platforms and established with industrial grade high-volume manufacturing process. To enable high-throughput function, 384-well plate based261 and 96-well plate based78 platform could aim the compatibility with standard equipment, scale-up fabrication method, and integration with multi-plex microfluidic microenvironmental controls and automations. For example, the commercial organ-on-a-chip product OrganoPlate® developed by MIMETAS has achieved high-throughput fabrication and significant improvements in experimental capacity.412 The microfluidic structures on the plate were made of glass and polymers with biocompatibility and low-compound-absorbance. The bottom of the platform was made of optical quality think glass makes the platform compatible with high-resolution confocal microscope. Meniscus and step-wise shaped barriers were designed to create a stable liquid–air interface separating different chambers on the platform and largely lower the fabrication difficulty and cost.413–415 Such platform has been applied to evaluate tumor invasion and intravasation,416 study drug-induced epithelial barrier dysfunction,273 and test chemo and targeted drugs.417–419 40 colorectal cancer models were established on the platform by forming tubes with by cancerous epithelial cells and perfusing controllable flows273 (Fig. 5F). The model was applied to investigate drug-induced epithelial barrier dysfunction. Integrating the OrganoPlate®, MIMETAS proposed a OrganoCore® Discovery Platform to provide oncology service including cell sourcing and establishing automatic and high-throughput model of different cancer types.420 The service platform also enables robustly characterizing immune cells migration (e.g., CAR T cells), inflammation, cell viability, gene expression, etc. and predicting therapy responses. Another commercial high-throughput microfluidic organ-on-a-chip plate, idenTx, uses cyclic olefin co-polymers and polymethylpentene fabricated through injection molding.421 The thermoplastic idenTx plate makes it easy to create 3D microphysiological systems for various biology and drug discovery researches, especially for 3D culture of tumor, angiogenesis, and immuno-oncology studies.
To enable automatic and high-throughput chip operation, the development and integration of auxiliary equipment and modules are essential, including but not limited to high-content imaging system, liquid handler, automated CO2 incubator, automated microfluidic cartridge storage and movement and control and analysis software. For example, a robotic platform deployed liquid handler to transfer blood substitute culture medium through different types of vascularized organ chips to achieve fluidic linking mimicking the coupling through different organs.274 Besides, external environmental chamber can accommodate the chip system for keeping a sterile environment and maintaining appropriate temperature and CO2 concentration,275 while storage rack and robot arm can be installed in the chamber enabling automatic plate storage and transfer.422 Emulate Inc. has developed a comprehensive solution with multiple modules for chip experiments automation.423 The system includes the customized cartridge to house chips, an automatic culture module with flow and cyclic stretching force control, and a supporting module providing gas, vacuum and power. In addition to hardware, customized software enables the users to monitor and change their experiment settings. The future of the auxiliary equipment and modules integrated on chip might be focused on increasing the reliability and compatibility. Also, current schemes mainly concentrate on the pathophysiological processes on the chip, however, other important processes in the whole workflow, including gel loading and downstream analysis like RNA and DNA extracting, should be improved with automation and high-throughput function as well.
Although the translation of CoC into clinical settings is promising and can be hugely rewarded, it is still in early-stage and facing practical challenges and ethical concerns. Previous efforts have been made on translation of different types of in vitro models for clinical utilities like predicting patient-specific treatment respones291,424,425 and some have been incorporated in clinical trials to optimize decision-making.426 For example, organoids were established from cancer patients recruited in phase 1/2 clinical trials and their responses to anticancer treatments were compared with clinical responses, validating that in vitro model can reproduce tumor heterogeneity and recapitulate clinical scenarios with high sensitivity and specificity.427 Micro-organospheres can be generated with microfluidics from low-volume patient tissue retaining stromal and immune cells and rapidly access tumor drug responses in 2 weeks, timely aiding guiding clinical decisions with prospective clinical study.428 To make the CoC more adaptable in real-world clinical settings, firstly, the throughput and reproducibility of CoC should be increased with high standardization. Besides, most of current in vitro models require several weeks even months from preparing patient sample to getting experimental results, largely compromised their timeliness and lower the enthusiasm in clinics. Therefore, a rapid and standard process to acquire patient sample and establish patient niches on CoC is required. Moreover, the criteria on how to evaluate on-chip testing results and compare them with clinical responses still need rigorous validations. A clear scientific standard agreed by clinicians would fill the gap between CoC testing and clinical applications. Finally, unlike animal models or human subjects, the regulatory standard for in vitro models like CoC is immature.19 Since CoC can be a tool for conducting parallel clinical trials, ethical issues like informed consent, risk/benefit ratio evaluation should be considered and discussed by experts of different fields.
Artificial intelligence (AI) enabled precision cancer medicine on chip
With recent advances in AI, machine learning, and the computing power of graphics processing units (GPUs), the integration of AI into CoC systems hold enormous potential for high-throughput systems to accelerate anticancer drug development and to enhance precision cancer medicine.429 In particular, deep learning, a subfield of machine learning, has been revolutionary for the medical field. Several different types of deep learning models such as convolutional neural networks (CNNs) and recurrent neural networks (RNNs) have been used in medical imaging and diagnosis due to their ability to quickly and accurately identify features of cells and make accurate predictions using images and videos.430–433 Given the widespread use of deep learning algorithms in current medical research, CoC systems will likely include deep learning models to greatly improve diagnostic accuracy and increase the total throughput of the system.Deep learning has been previously integrated into CoC systems to evaluate the effectiveness of immunotherapy treatments by providing real-time cell morphology434 and trajectory data276 and by quantifying the interactions between tumors and immune cells.435 More recently though, deep learning has also been applied to high-throughput drug screening pipelines. A deep learning model has been trained on recognizing infiltration patterns of TILs in pathology images of solid tumors and correlated them to patient survival data.277 The deep learning model was then able to assign a TIL score based on infiltration patterns which described the efficacy of a specific anticancer treatment. Applying the deep learning model to the automated microfluidic system to screen a drug library of compounds, drugs that enhance T cell infiltration can be quickly identified, demonstrating the potential of deep learning in drug screening for precision medicine (Fig. 5G). In addition to drug screening, methods to model and understand T cell-tumor dynamics in patient-derived tumors on a mass scale are crucial for tailoring treatment to specific patients. Hence, a machine learning-based tool named BEHAV3D was developed enabling rapid analysis of T cell responses by classification of distinct T cell behaviors.278 Combining such pipeline with other omics techniques could provide a more complete characterization of the specific T cell-tumor response and optimize personalized immunotherapy treatments. Moreover, deep learning integrated into CoC systems is not only limited to analysis of the T cell immune response or immunotherapy treatments, it may also classify tumors into distinct subtypes, allowing for high-throughput, precise diagnoses and targeted treatments to certain subtypes of tumors. A deep learning model named ATMQcD was developed implementing multiple object tracking, cell segmentation, automated training set generation.436 It was combined with the microfluidic system to measure cellular deformability and classify tumor cells based on predicted invasiveness. The deep learning model was able to predict tumor invasiveness with remarkable accuracy and demonstrated the immense potential of deep learning on CoC systems to enable low-cost, rapid methods of tumor classification and subsequent tailed treatment to those subtypes.
Along with recent adoption of AI into CoC devices by some pharmaceutical companies294 and 2024 Nobel prizes being awarded to machine learning-based approaches to research in physics and chemistry, it is clear that the future of research in CoC applications will include deep learning and AI. For instance, MIMETAS is running a large scale on-chip screening of over 1000 drug compounds, integrated machine learning to process the imaging data collected from the drug screening.437 Similarly, Quris which recently acquired Nortis, an organ-on-a-chip company, is researching applications of AI in organ-on-a-chip systems to automatically generate a massive dataset from patient-on-a-chip data and then train a machine learning model that accurately predicts drug safety and efficacy before clinical trials.438 Future research integrating deep learning into CoC systems will likely be in a variety of topics from improved cell tracking to improved tumor modeling and even to AI-based drug screening and precision cancer medicine. With improved cell segmentation and cell tracking capability, future deep learning models will likely be able to identify more features relevant to tumor invasiveness and metastasis. While current deep learning models for image processing have been applied to whole cell tracking, organelle tracking may be a worthwhile endeavor for CoC devices. For example, lipid droplets have been identified to play an important role in cancer progression.439 Deep learning-based tracking and analysis of lipid droplets proposed on CoC devices show the potential of organelle tracking in high-throughput systems for cancer screening.440 Furthermore, deep learning models have been used to predict cancer drug responses and is currently a rapidly advancing field which when integrated into microfluidic systems may offer an even faster high-throughput method to select and only test the drugs that are relevant to specific patients.441 Such systems could be further enhanced by integrating other forms of data that may be relevant such as multiple omics data.442 In a similar manner, while current CoC systems are able to replicate tissues from a singular organ, tumor treatments must also take into account that a cancer treatment for one organ may be detrimental to the health of another. Future body-on-a-chip systems may integrate deep learning to evaluate and model the entire, fully-connected system in real-time, enabling personalized, whole-body analysis of immunotherapy drugs.443 Overall, although current CoC have yet to be fully adopted for diagnostic and prediction purposes, further research into applications of deep learning and AI into CoC as well as improvements to efficiency and accuracy will inevitably propel the field closer to full application in precision medicine.
Combining in silico and in vitro on-chip modeling
CoC models can provide a large amount of valuable data for cancer research. However, the pathophysiological processes in TME are complex, involving various cellular and molecular interactions during cancer progression and treatment. It is a challenge to interpret these multi-dimensional experimental readouts measured by different experimental approaches from clinical studies and in vitro models for better understanding the cancer biology. By harnessing data both from clinical studies and cancer chip models, in silico modeling or patient-specific ‘digital twins’ of TME can serve as powerful precision medicine tools to enable high-throughput yet low-cost virtual experimentation or even virtual clinical trials, aiding for cancer mechanism study, drug screening and treatment response prediction.444–446Computational models can provide quantitative tools to aid mechanistic study and therapy testing on chips by simulate the complex physiological processes in TME. Many biological processes in cancer can be modeled mathematically by using differential equations. Ordinary differential equation (ODE)-based computational models build mathematical frameworks to simulate and analyze key pathophysiological processes of tumor and microenvironmental cells, such as cell growth, death, migration, differentiation, and interaction kinetics, as well as signaling pathway network, metabolic functions, molecular, genetic and epigenetic changes in the TME in response to different factors like treatment interventions. For instance, an ODE-based in silico model was established for modeling glioma TME involving multiple types of cells, cytokines and signaling pathways.447 A microglia depletion therapy was applied in silico and early treated patients demonstrated significant benefits while it was not the case for late treated patients. Another ODE model quantified the dynamic interconversion between chemo drug sensitive and resistance cell populations, and validated that transition of cell phenotypes instead of selection of resistant cells might play a more important role in chemotherapy resistance.448 ODE models are especially suitable for model cellular therapies like CAR T cell therapy as they can simulate the dynamics of effector and target cells.449 An ODE model parametrized anti-CD19 CAR T cell functions like activation and proliferation and correlate them with the various clinical responses of leukemia patients.450 After calibrating with clinical data, the model enabled predicting potential patient responses including remission, resistance and relapse, based on the early stagy CAR T cell dynamics during treatment. Another model proposed the maximum naïve CAR T cell concentrations/baseline tumor burden ratio as a predictor for patient survival and determine the cut-off value enabling optimal predictive capability based on patient data.451 Besides, cytokine release syndrome (CRS), a lethal adverse event during CAR T cell therapy, can be modeled with ODE framework. An ODE model depicted the secretion of a series of cytokines by CAR T cells and monocytes and established an interactive network of cytokines including self-feedback and induced-responses from other kinds of cytokines.452 This CRS model also validated that periodic switching and conditionally triggered CAR T cell products can mitigate CRS and maintain anti-tumor efficacy. When applying ODE models for cancer mechanism study and therapy response prediction, one of the largest hurdles is the lack of access to sufficient and standard-formatted clinical data. Data obtained from patient-derived chips could be ideal complements, as CoC models built with autologous patient samples have the potential to conduct “clinical trial-on-a-chip” study to reproduce patient responses in vitro with high fidelity, which is especially necessary and valuable for rare diseases.453–457 In turn, computational ODE models could provide more arsenals to analyze and validate on-chip results and inspire the development of treatment regimen tested on chip.80
Computational ODE models can make use of CoC-derived readouts to investigate cancer biological mechanisms, in particular, to study the dynamics of treatment response and the emergence of resistance, and to suggest more effective and personalized anticancer treatment. A recent ODE model were calibrated with a GBM CoC measured data like tumor cell growth and apoptosis, T cell activation rate and cytotoxic efficiency, TAM abundance and immunosuppression level, and were applied for ICI immunotherapy screening279 (Fig. 5H). Another ODE model depicted the interactions between cancer cells and oxygen concentration and the cell migrations driven by hypoxia in a GBM CoC model.458 The model reproduced on-chip experiments and analyzed how parameters like cell initial density can affect the formation of the necrotic core in tumor. Computational fluid dynamics (CFD) modeling based on the governing equations of fluid mechanics, can simulate the physiologically relevant tumor fluid flows,459 oxygen diffusion and transport of anti-tumor drugs in microfluidic cancer chips.460 CFD models enable large parameter space sweeping to simulate vast flow conditions to optimize chip parameters,280 improve on-chip cell culture and trapping,461,462 help identifying determinant factors affecting therapeutic outcomes463 and optimizing combination therapy with CoC systems.464
Usually established with ODEs, PK model depicted the change of drug concentration with time and PD model depicted the relationship between drug efficacy and concentration, which are high useful in drug development and applications.465 CoC is a good fit for PK/PD modeling and can be coupled with mechanism-based computational model to provide quantitative PK/PD parameters, which cannot be easily obtained through conventional models.466–470 For example, multi-organs-on-a-chip system including liver part for metabolism and cancer part for drug targeting was applied to estimate unknown parameters in PK/PD model.471 To understand the PK of drug–drug interactions (DDI), on-chip and computational PK/PD model simulated results were compared to validate the combination of organ-on-a-chip and in silico model is an effective way for DDI prediction and study. Computational model is also required in the in vitro–in vivo translation (IVIVT) of the on-chip results to in vivo physiology, which is key for the translation of CoC into real-world applications. To achieve this, CoC model with accessible and reliable readouts of drug concentration from on-chip modules such as arteriovenous reservoir was established.274 With the data obtained from the CoC system, physiologically based PK model can be created to scale data and conduct quantitative IVIVT, and the model should be further validated to predict the PK/PD parameters matching the previously reported patient data.281 Despite the power of integrating mechanistic model with CoC for precision medicine, there are challenges to be solved. First, for PK/PD application, to correlate the on-chip results with in vivo measurements, scaling strategy of the computational model is essential.468,472 As PK/PD models are mostly combined with multi-organ chip model for applications, a multi-organ system with reliable design and high biomimicry is required.
Concluding remarks
A great need hasn't been met on developing reliable cancer models for cancer preclinical studies and precision medicine. The 3R principles (i.e., replacement, reduction, and refinement) also call for in vitro model alternatives reducing ethical concerns related to animal use.473 The cutting-edge CoC technology represents a significant paradigm shift in cancer research, because it allows researchers to study complex tumor biology and drug responses in a highly controlled, 3D environment that closely mimics the TME, providing a more accurate and relevant alternative to traditional animal models for disease modeling and drug screening. Despite remarkable achievements have been made on utilizing CoC for TME modeling and therapy testing, most of the models are still proof-of-concepts, demonstrating that there are gaps between scientific research and real-world applications. Applying patient-derived samples on CoC would make the “avatar” of specific patient possible, a premise to achieve a “clinical trials on a chip” study, thus holds a great translational potential for personalized medicine and improved pharmaceutical development strategies for cancer. Integrated CoC systems incorporated with cancer organoids, multiple organs, microenvironmental control and in situ sensing modules will enable a more comprehensive and multidimensional testing for different clinical scenarios and pharmaceutical industry applications. By increasing the standardization level, throughput and timeliness of the model as well as setting up standards and reaching a consensus with clinicians, the bottleneck on the translation of CoC is expected to be solved. Harnessing advanced analytical methodologies, including AI tools and computational models, will further unleash the potential in the rich readouts from the CoC to generate a smart system. We envision that as the CoC technology continuously evolves, it holds a great potential to revolutionize the precision cancer medicine, ultimately leading to the development of more effective and safer treatments for cancer patients.Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the US National Institutes of Health (R35GM133646, R21AR083247, R21GM151528), the US National Science Foundation (CBET-2103219), the International Foundation for Ethical Research and the American Heart Association (24IAUST1187087). Fig. 1, 2 and 4 were created with https://BioRender.com.References
- F. B. Ahmad, J. A. Cisewski and R. N. Anderson, MMWR Morb. Mortal. Wkly. Rep., 2024, 73, 677–681 CrossRef PubMed.
- R. L. Siegel, A. N. Giaquinto and A. Jemal, Ca-Cancer J. Clin., 2024, 74, 12–49 CrossRef.
- F. Bray, M. Laversanne, H. Sung, J. Ferlay, R. L. Siegel, I. Soerjomataram and A. Jemal, Ca-Cancer J. Clin., 2024, 74, 229–263 CrossRef PubMed.
- S. J. Hachey and C. C. W. Hughes, Lab Chip, 2018, 18, 2893–2912 RSC.
- V. Prasad and S. Mailankody, JAMA Intern. Med., 2017, 177, 1569–1575 CrossRef PubMed.
- C. H. Wong, K. W. Siah and A. W. Lo, Biostatistics, 2019, 20, 273–286 CrossRef PubMed.
- D. Bhattacharjee, J. Bakar, S. P. Chitnis, E. L. Sausville, K. D. Ashtekar, B. E. Mendelson, K. Long, J. C. Smith, D. E. Heppner and J. M. Sheltzer, Cell Chem. Biol., 2023, 30, 1211–1222.e1215 CrossRef CAS.
- J. Hu, Anesthesiology, 2024, 140, 349–351 CrossRef PubMed.
- Q. Wang, X. Shao, Y. Zhang, M. Zhu, F. X. C. Wang, J. Mu, J. Li, H. Yao and K. Chen, Cancer Med., 2023, 12, 11149–11165 CrossRef PubMed.
- K. E. de Visser and J. A. Joyce, Cancer Cell, 2023, 41, 374–403 CrossRef CAS.
- I. Dagogo-Jack and A. T. Shaw, Nat. Rev. Clin. Oncol., 2018, 15, 81–94 CrossRef CAS PubMed.
- M. Proietto, M. Crippa, C. Damiani, V. Pasquale, E. Sacco, M. Vanoni and M. Gilardi, Front. Oncol., 2023, 13, 1164535 CrossRef CAS PubMed.
- J. M. Ayuso, M. Virumbrales-Muñoz, J. M. Lang and D. J. Beebe, Nat. Commun., 2022, 13, 3086 CrossRef CAS PubMed.
- C. Horejs, Nat. Rev. Mater., 2021, 6, 372–373 CrossRef.
- C. R. Ireson, M. S. Alavijeh, A. M. Palmer, E. R. Fowler and H. J. Jones, Br. J. Cancer, 2019, 121, 101–108 CrossRef PubMed.
- J. Jin, K. Yoshimura, M. Sewastjanow-Silva, S. Song and J. A. Ajani, Cancers, 2023, 15, 4352 CrossRef CAS.
- S. Bose, H. Clevers and X. Shen, Med, 2021, 2, 1011–1026 CrossRef PubMed.
- Z. Li, W. Zheng, H. Wang, Y. Cheng, Y. Fang, F. Wu, G. Sun, G. Sun, C. Lv and B. Hui, Cancer Manage. Res., 2021, 13, 2455–2475 CrossRef CAS PubMed.
- R. G. Thakar and K. N. Fenton, Artif. Organs, 2023, 47, 1553–1558 CrossRef PubMed.
- S. K. Srivastava, G. W. Foo, N. Aggarwal and M. W. Chang, Biotechnol. Notes, 2024, 5, 8–12 CrossRef CAS PubMed.
- M. Kapałczyńska, T. Kolenda, W. Przybyła, M. Zajączkowska, A. Teresiak, V. Filas, M. Ibbs, R. Bliźniak, Ł. Łuczewski and K. Lamperska, Arch. Med. Sci., 2018, 14, 910–919 Search PubMed.
- S. J. Han, S. Kwon and K. S. Kim, Cancer Cell Int., 2021, 21, 152 CrossRef PubMed.
- G. Mehta, A. Y. Hsiao, M. Ingram, G. D. Luker and S. Takayama, J. Controlled Release, 2012, 164, 192–204 CrossRef CAS PubMed.
- Z. Fang, P. Li, F. Du, L. Shang and L. Li, Exp. Hematol. Oncol., 2023, 12, 69 CrossRef PubMed.
- R. J. Porter, G. I. Murray and M. H. McLean, Br. J. Cancer, 2020, 123, 1209–1218 CrossRef PubMed.
- Y. Huang, Z. Huang, Z. Tang, Y. Chen, M. Huang, H. Liu, W. Huang, Q. Ye and B. Jia, Front. Cell Dev. Biol., 2021, 9, 740574 CrossRef PubMed.
- J. M. Munson and A. C. Shieh, Cancer Manage. Res., 2014, 6, 317–328 CrossRef PubMed.
- A. G. Mitrakas, A. Tsolou, S. Didaskalou, L. Karkaletsou, C. Efstathiou, E. Eftalitsidis, K. Marmanis and M. Koffa, Int. J. Mol. Sci., 2023, 24, 6949 CrossRef CAS PubMed.
- M. R. Haque, C. R. Wessel, D. D. Leary, C. Wang, A. Bhushan and F. Bishehsari, Microsyst. Nanoeng., 2022, 8, 36 CrossRef PubMed.
- D. Caballero, R. L. Reis and S. C. Kundu, Adv. Exp. Med. Biol., 2020, 1230, 43–64 CrossRef CAS PubMed.
- M. A. Polidoro, E. Ferrari, C. Soldani, B. Franceschini, G. Saladino, A. Rosina, A. Mainardi, F. D'Autilia, N. Pugliese, G. Costa, M. Donadon, G. Torzilli, S. Marzorati, M. Rasponi and A. Lleo, JHEP Rep., 2024, 6, 100910 CrossRef PubMed.
- M. R. Haque, T. H. Rempert, T. A. Al-Hilal, C. Wang, A. Bhushan and F. Bishehsari, Cancers, 2021, 13, 4487 CrossRef CAS PubMed.
- A. van den Berg, C. L. Mummery, R. Passier and A. D. van der Meer, Lab Chip, 2019, 19, 198–205 RSC.
- C. Y. Lu, V. Terry and D. M. Thomas, npj Precis. Oncol., 2023, 7, 3 CrossRef PubMed.
- L. Marques, B. Costa, M. Pereira, A. Silva, J. Santos, L. Saldanha, I. Silva, P. Magalhães, S. Schmidt and N. Vale, Pharmaceutics, 2024, 16, 332 CrossRef CAS PubMed.
- R. Wang, C. Zhang, D. Li and Y. Yao, Front. Bioeng. Biotechnol., 2022, 10, 1057913 CrossRef PubMed.
- N. Del Piccolo, V. S. Shirure, Y. Bi, S. P. Goedegebuure, S. Gholami, C. C. W. Hughes, R. C. Fields and S. C. George, Adv. Drug Delivery Rev., 2021, 175, 113798 CrossRef CAS PubMed.
- M. Jouybar, C. M. de Winde, K. Wolf, P. Friedl, R. E. Mebius and J. M. J. den Toonder, Trends Biotechnol., 2024, 42, 431–448 CrossRef CAS PubMed.
- J. J. F. Sleeboom, J. Toonder and C. M. Sahlgren, Int. J. Mol. Sci., 2018, 19, 3047 CrossRef PubMed.
- V. S. Shirure, S. F. Lam, B. Shergill, Y. E. Chu, N. R. Ng and S. C. George, Lab Chip, 2020, 20, 3036–3050 RSC.
- D. W. Lee, N. Choi and J. H. Sung, Biotechnol. Prog., 2019, 35, e2701 CrossRef PubMed.
- L. Zhou, L. Liu, M. A. Chang, C. Ma, W. Chen and P. Chen, Biosens. Bioelectron., 2023, 225, 115064 CrossRef CAS PubMed.
- G. Imparato, F. Urciuolo, C. Mazio and P. A. Netti, Lab Chip, 2022, 23, 25–43 RSC.
- K. Seaman, Y. Sun and L. You, Med-X, 2023, 1, 11 CrossRef CAS.
- J. J. Han, Artif. Organs, 2023, 47, 449–450 CrossRef PubMed.
- P.-J. H. Zushin, S. Mukherjee and J. C. Wu, J. Clin. Invest., 2023, 133, e175824 CrossRef CAS PubMed.
- H. N. Kim, N. L. Habbit, C.-Y. Su, N. Choi, E. H. Ahn, E. A. Lipke and D.-H. Kim, Adv. Funct. Mater., 2019, 29, 1807553 CrossRef.
- E. Cauli, M. A. Polidoro, S. Marzorati, C. Bernardi, M. Rasponi and A. Lleo, J. Biol. Eng., 2023, 17, 53 CrossRef PubMed.
- R. Y. Lee, Y. Wu, D. Goh, V. Tan, C. W. Ng, J. C. T. Lim, M. C. Lau and J. P. S. Yeong, Adv. Healthcare Mater., 2023, 12, 2202457 CrossRef CAS PubMed.
- A. Sontheimer-Phelps, B. A. Hassell and D. E. Ingber, Nat. Rev. Cancer, 2019, 19, 65–81 CrossRef CAS PubMed.
- X. Liu, J. Fang, S. Huang, X. Wu, X. Xie, J. Wang, F. Liu, M. Zhang, Z. Peng and N. Hu, Microsyst. Nanoeng., 2021, 7, 50 CrossRef CAS PubMed.
- S. Park, T. H. Kim, S. H. Kim, S. You and Y. Jung, Cancers, 2021, 13, 3930 CrossRef CAS PubMed.
- F. Gioiella, F. Urciuolo, G. Imparato, V. Brancato and P. A. Netti, Adv. Healthcare Mater., 2016, 5, 3074–3084 CrossRef CAS PubMed.
- S. Nagaraju, D. Truong, G. Mouneimne and M. Nikkhah, Adv. Healthcare Mater., 2018, 7, 1701257 CrossRef PubMed.
- B. F. L. Lai, R. X. Z. Lu, Y. Hu, L. Davenport Huyer, W. Dou, E. Y. Wang, N. Radulovich, M. S. Tsao, Y. Sun and M. Radisic, Adv. Funct. Mater., 2020, 30, 2000545 CrossRef CAS PubMed.
- X. Cui, C. Ma, V. Vasudevaraja, J. Serrano, J. Tong, Y. Peng, M. Delorenzo, G. Shen, J. Frenster, R.-T. T. Morales, W. Qian, A. Tsirigos, A. S. Chi, R. Jain, S. C. Kurz, E. P. Sulman, D. G. Placantonakis, M. Snuderl and W. Chen, eLife, 2020, 9, e52253 CrossRef CAS PubMed.
- L. F. Lorenzo-Martín, N. Broguiere and J. Langer, et al., Nat. Biotechnol., 2024 DOI:10.1038/s41587-024-02301-4.
- C. Ma, M. T. Witkowski, J. Harris, I. Dolgalev, S. Sreeram, W. Qian, J. Tong, X. Chen, I. Aifantis and W. Chen, Sci. Adv., 2020, 6, eaba5536 CrossRef CAS PubMed.
- R. G. Mannino, A. N. Santiago-Miranda, P. Pradhan, Y. Qiu, J. C. Mejias, S. S. Neelapu, K. Roy and W. A. Lam, Lab Chip, 2017, 17, 407–414 RSC.
- J. Nolan, O. M. T. Pearce, H. R. C. Screen, M. M. Knight and S. W. Verbruggen, Cancers, 2023, 15, 635 CrossRef CAS PubMed.
- D. E. Ingber, Adv. Sci., 2020, 7, 2002030 CrossRef CAS PubMed.
- S. Seo, S.-Y. Nah, K. Lee, N. Choi and H. N. Kim, Adv. Funct. Mater., 2022, 32, 2106860 CrossRef CAS.
- R. Kennedy, D. Kuvshinov, A. Sdrolia, E. Kuvshinova, K. Hilton, S. Crank, A. W. Beavis, V. Green and J. Greenman, Sci. Rep., 2019, 9, 6327 CrossRef CAS PubMed.
- I. Veith, M. Nurmik, A. Mencattini, I. Damei, C. Lansche, S. Brosseau, G. Gropplero, S. Corgnac, J. Filippi, N. Poté, E. Guenzi, A. Chassac, P. Mordant, J. Tosello, C. Sedlik, E. Piaggio, N. Girard, J. Camonis, H. Shirvani, F. Mami-Chouaib, F. Mechta-Grigoriou, S. Descroix, E. Martinelli, G. Zalcman and M. C. Parrini, Cell Rep. Med., 2024, 5, 101549 CrossRef CAS PubMed.
- T. I. Maulana, C. Teufel, M. Cipriano, J. Roosz, L. Lazarevski, F. E. van den Hil, L. Scheller, V. Orlova, A. Koch, M. Hudecek, M. Alb and P. Loskill, Cell Stem Cell, 2024, 31, 989–1002.e1009 CrossRef CAS PubMed.
- Y.-H. Hsu, M. L. Moya, C. C. W. Hughes, S. C. George and A. P. Lee, Lab Chip, 2013, 13, 2990–2998 RSC.
- L. L. Bischel, E. W. Young, B. R. Mader and D. J. Beebe, Biomaterials, 2013, 34, 1471–1477 CrossRef CAS PubMed.
- Y. Nashimoto, R. Okada, S. Hanada, Y. Arima, K. Nishiyama, T. Miura and R. Yokokawa, Biomaterials, 2020, 229, 119547 CrossRef CAS PubMed.
- M. B. Chen, J. A. Whisler, J. Fröse, C. Yu, Y. Shin and R. D. Kamm, Nat. Protoc., 2017, 12, 865–880 CrossRef CAS PubMed.
- D.-H. T. Nguyen, E. Lee, S. Alimperti, R. J. Norgard, A. Wong, J. J.-K. Lee, J. Eyckmans, B. Z. Stanger and C. S. Chen, Sci. Adv., 2019, 5, eaav6789 CrossRef CAS PubMed.
- S.-Y. Jeong, J.-H. Lee, Y. Shin, S. Chung and H.-J. Kuh, PLoS One, 2016, 11, e0159013 CrossRef PubMed.
- L. I. Ibrahim, C. Hajal, G. S. Offeddu, M. R. Gillrie and R. D. Kamm, Biomaterials, 2022, 288, 121728 CrossRef CAS PubMed.
- C. F. Monteiro, I. A. Deus, I. B. Silva, I. F. Duarte, C. A. Custódio and J. F. Mano, Adv. Funct. Mater., 2024, 34, 2315940 CrossRef CAS.
- X. Cui, R. T. Morales, W. Qian, H. Wang, J. P. Gagner, I. Dolgalev, D. Placantonakis, D. Zagzag, L. Cimmino, M. Snuderl, R. H. W. Lam and W. Chen, Biomaterials, 2018, 161, 164–178 CrossRef CAS PubMed.
- J. Bai, G. Adriani, T.-M. Dang, T.-Y. Tu, H.-X. L. Penny, S.-C. Wong, R. D. Kamm and J.-P. Thiery, Onco Targets Ther, 2015, 6(28), 25295 Search PubMed.
- S. Parlato, A. De Ninno, R. Molfetta, E. Toschi, D. Salerno, A. Mencattini, G. Romagnoli, A. Fragale, L. Roccazzello, M. Buoncervello, I. Canini, E. Bentivegna, M. Falchi, F. R. Bertani, A. Gerardino, E. Martinelli, C. Natale, R. Paolini, L. Businaro and L. Gabriele, Sci. Rep., 2017, 7, 1093 CrossRef PubMed.
- B. J. Kim, P. Hannanta-anan, M. Chau, Y. S. Kim, M. A. Swartz and M. Wu, PLoS One, 2013, 8, e68422 CrossRef CAS PubMed.
- D. T. T. Phan, X. Wang, B. M. Craver, A. Sobrino, D. Zhao, J. C. Chen, L. Y. N. Lee, S. C. George, A. P. Lee and C. C. W. Hughes, Lab Chip, 2017, 17, 511–520 RSC.
- R. W. Jenkins, A. R. Aref, P. H. Lizotte, E. Ivanova, S. Stinson, C. W. Zhou, M. Bowden, J. Deng, H. Liu, D. Miao, M. X. He, W. Walker, G. Zhang, T. Tian, C. Cheng, Z. Wei, S. Palakurthi, M. Bittinger, H. Vitzthum, J. W. Kim, A. Merlino, M. Quinn, C. Venkataramani, J. A. Kaplan, A. Portell, P. C. Gokhale, B. Phillips, A. Smart, A. Rotem, R. E. Jones, L. Keogh, M. Anguiano, L. Stapleton, Z. Jia, M. Barzily-Rokni, I. Cañadas, T. C. Thai, M. R. Hammond, R. Vlahos, E. S. Wang, H. Zhang, S. Li, G. J. Hanna, W. Huang, M. P. Hoang, A. Piris, J. P. Eliane, A. O. Stemmer-Rachamimov, L. Cameron, M. J. Su, P. Shah, B. Izar, M. Thakuria, N. R. LeBoeuf, G. Rabinowits, V. Gunda, S. Parangi, J. M. Cleary, B. C. Miller, S. Kitajima, R. Thummalapalli, B. Miao, T. U. Barbie, V. Sivathanu, J. Wong, W. G. Richards, R. Bueno, C. H. Yoon, J. Miret, M. Herlyn, L. A. Garraway, E. M. Van Allen, G. J. Freeman, P. T. Kirschmeier, J. H. Lorch, P. A. Ott, F. S. Hodi, K. T. Flaherty, R. D. Kamm, G. M. Boland, K. K. Wong, D. Dornan, C. P. Paweletz and D. A. Barbie, Cancer Discovery, 2018, 8, 196–215 CrossRef CAS PubMed.
- C. Ma, H. Wang, L. Liu, J. Tong, M. T. Witkowski, I. Aifantis, S. Ghassemi and W. Chen, Res. Sq., 2023, DOI:10.21203/rs.3.rs-2762929/v1.
- J. M. Ayuso, S. Rehman, M. Virumbrales-Munoz, P. H. McMinn, P. Geiger, C. Fitzgerald, T. Heaster, M. C. Skala and D. J. Beebe, Sci. Adv., 2021, 7, eabc2331 CrossRef CAS PubMed.
- B. Schuster, M. Junkin, S. S. Kashaf, I. Romero-Calvo, K. Kirby, J. Matthews, C. R. Weber, A. Rzhetsky, K. P. White and S. Tay, Nat. Commun., 2020, 11, 5271 CrossRef CAS PubMed.
- S. Chakrabarty, W. F. Quiros-Solano, M. M. P. Kuijten, B. Haspels, S. Mallya, C. S. Y. Lo, A. Othman, C. Silvestri, A. van de Stolpe, N. Gaio, H. Odijk, M. van de Ven, C. M. A. de Ridder, W. M. van Weerden, J. Jonkers, R. Dekker, N. Taneja, R. Kanaar and D. C. van Gent, Cancer Res., 2022, 82, 510–520 CrossRef CAS PubMed.
- C. Viallard and B. Larrivée, Angiogenesis, 2017, 20, 409–426 CrossRef CAS PubMed.
- A. Singh, V. Veeriah, P. Xi, R. Labella, J. Chen, S. G. Romeo, S. K. Ramasamy and A. P. Kusumbe, JCI Insight, 2019, 4, e125679 CrossRef PubMed.
- Y. Zhou, Y. Wu, R. Paul, X. Qin and Y. Liu, ACS Appl. Mater. Interfaces, 2023, 15, 6431–6441 CrossRef CAS PubMed.
- A. R. Aref, R. Y.-J. Huang, W. Yu, K.-N. Chua, W. Sun, T.-Y. Tu, J. Bai, W.-J. Sim, I. K. Zervantonakis, J. P. Thiery and R. D. Kamm, Integr. Biol., 2013, 5, 381–389 CrossRef CAS PubMed.
- J. Paek, S. E. Park, Q. Lu, K.-T. Park, M. Cho, J. M. Oh, K. W. Kwon, Y.-s. Yi, J. W. Song, H. I. Edelstein, J. Ishibashi, W. Yang, J. W. Myerson, R. Y. Kiseleva, P. Aprelev, E. D. Hood, D. Stambolian, P. Seale, V. R. Muzykantov and D. Huh, ACS Nano, 2019, 13, 7627–7643 CrossRef CAS PubMed.
- M. B. Chen, J. A. Whisler, J. S. Jeon and R. D. Kamm, Integr. Biol., 2013, 5, 1262–1271 CrossRef CAS PubMed.
- T. J. Kwak and E. Lee, Sci. Rep., 2020, 10, 20142 CrossRef CAS PubMed.
- M. L. Moya, Y.-H. Hsu, A. P. Lee, C. C. W. Hughes and S. C. George, Tissue Eng., Part C, 2013, 19, 730–737 CrossRef CAS PubMed.
- J. A. Whisler, M. B. Chen and R. D. Kamm, Tissue Eng., Part C, 2012, 20, 543–552 CrossRef PubMed.
- J. Majidpoor and K. Mortezaee, Cell. Oncol., 2021, 44, 715–737 CrossRef CAS PubMed.
- B. M. Baker, B. Trappmann, S. C. Stapleton, E. Toro and C. S. Chen, Lab Chip, 2013, 13, 3246–3252 RSC.
- X. Wang, D. T. T. Phan, A. Sobrino, S. C. George, C. C. W. Hughes and A. P. Lee, Lab Chip, 2016, 16, 282–290 RSC.
- Z. Hu, Y. Cao, E. A. Galan, L. Hao, H. Zhao, J. Tang, G. Sang, H. Wang, B. Xu and S. Ma, ACS Biomater. Sci. Eng., 2022, 8, 1215–1225 CrossRef CAS PubMed.
- Y. Nashimoto, T. Hayashi, I. Kunita, A. Nakamasu, Y.-s. Torisawa, M. Nakayama, H. Takigawa-Imamura, H. Kotera, K. Nishiyama, T. Miura and R. Yokokawa, Integr. Biol., 2017, 9, 506–518 CrossRef PubMed.
- E. Sano, C. Mori, Y. Nashimoto, R. Yokokawa, H. Kotera and Y.-s. Torisawa, Biomicrofluidics, 2018, 12, 042204 CrossRef PubMed.
- J. A. Nagy, S. H. Chang, A. M. Dvorak and H. F. Dvorak, Br. J. Cancer, 2009, 100, 865–869 CrossRef CAS PubMed.
- L. L. Munn, Drug Discovery Today, 2003, 8, 396–403 CrossRef PubMed.
- C. B. X. Huang and T.-Y. Tu, Front. Oncol., 2023, 13, 1150332 CrossRef CAS PubMed.
- M. Gerigk, H. Bulstrode, H. H. Shi, F. Tönisen, C. Cerutti, G. Morrison, D. Rowitch and Y. Y. S. Huang, Lab Chip, 2021, 21, 2343–2358 RSC.
- Y. Liu, C. Sakolish, Z. Chen, D. T. T. Phan, R. H. F. Bender, C. C. W. Hughes and I. Rusyn, Toxicology, 2020, 445, 152601 CrossRef CAS PubMed.
- A. Aazmi, H. Zhou, Y. Li, M. Yu, X. Xu, Y. Wu, L. Ma, B. Zhang and H. Yang, Engineering, 2022, 9, 131–147 CrossRef.
- D. E. Glaser, M. B. Curtis, P. A. Sariano, Z. A. Rollins, B. S. Shergill, A. Anand, A. M. Deely, V. S. Shirure, L. Anderson, J. M. Lowen, N. R. Ng, K. Weilbaecher, D. C. Link and S. C. George, Biomaterials, 2022, 280, 121245 CrossRef CAS PubMed.
- C. Quintard, E. Tubbs, G. Jonsson, J. Jiao, J. Wang, N. Werschler, C. Laporte, A. Pitaval, T.-S. Bah, G. Pomeranz, C. Bissardon, J. Kaal, A. Leopoldi, D. A. Long, P. Blandin, J.-L. Achard, C. Battail, A. Hagelkruys, F. Navarro, Y. Fouillet, J. M. Penninger and X. Gidrol, Nat. Commun., 2024, 15, 1452 CrossRef CAS PubMed.
- J. Song, A. Miermont, C. T. Lim and R. D. Kamm, Sci. Rep., 2018, 8, 17949 CrossRef CAS PubMed.
- M. D. Bourn, S. Z. Mohajerani, G. Mavria, N. Ingram, P. L. Coletta, S. D. Evans and S. A. Peyman, Lab Chip, 2023, 23, 1674–1693 RSC.
- L. D. Ghosh, T. Mathur, J. J. Tronolone, A. Chuong, K. Rangel, S. Corvigno, A. K. Sood and A. Jain, Adv. Healthcare Mater., 2024, 13, 2304263 CrossRef CAS PubMed.
- Y. Liu, J. Li, J. Zhou, X. Liu, H. Li, Y. Lu, B. Lin, X. Li and T. Liu, Micromachines, 2022, 13, 225 CrossRef PubMed.
- Y. H. Hsu, M. L. Moya, P. Abiri, C. C. Hughes, S. C. George and A. P. Lee, Lab Chip, 2013, 13, 81–89 RSC.
- H.-H. Hsu, P.-L. Ko, C.-C. Peng, Y.-J. Cheng, H.-M. Wu and Y.-C. Tung, Mater. Today Bio, 2023, 21, 100703 CrossRef CAS PubMed.
- C. P. Miller, C. Tsuchida, Y. Zheng, J. Himmelfarb and S. Akilesh, Neoplasia, 2018, 20, 610–620 CrossRef CAS PubMed.
- K. Haase, G. S. Offeddu, M. R. Gillrie and R. D. Kamm, Adv. Funct. Mater., 2020, 30, 2002444 CrossRef CAS PubMed.
- M. B. Chen, C. Hajal, D. C. Benjamin, C. Yu, H. Azizgolshani, R. O. Hynes and R. D. Kamm, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 7022–7027 CrossRef CAS PubMed.
- A. Boussommier-Calleja, Y. Atiyas, K. Haase, M. Headley, C. Lewis and R. D. Kamm, Biomaterials, 2019, 198, 180–193 CrossRef CAS PubMed.
- R. C. Yada, D. E. Desa, A. A. Gillette, E. Bartels, P. M. Harari, M. C. Skala, D. J. Beebe and S. C. Kerr, Biomaterials, 2023, 298, 122136 CrossRef CAS PubMed.
- V. L. Silvestri, E. Henriet, R. M. Linville, A. D. Wong, P. C. Searson and A. J. Ewald, Cancer Res., 2020, 80, 4288–4301 CrossRef CAS PubMed.
- Y. Xiao, D. Kim, B. Dura, K. Zhang, R. Yan, H. Li, E. Han, J. Ip, P. Zou, J. Liu, A. T. Chen, A. O. Vortmeyer, J. Zhou and R. Fan, Adv. Sci., 2019, 6, 1801531 CrossRef PubMed.
- J. S. Jeon, S. Bersini, M. Gilardi, G. Dubini, J. L. Charest, M. Moretti and R. D. Kamm, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 214–219 CrossRef CAS PubMed.
- S. Kim, H. Lee, M. Chung and N. L. Jeon, Lab Chip, 2013, 13, 1489–1500 RSC.
- E. R. Shamir and A. J. Ewald, Nat. Rev. Mol. Cell Biol., 2014, 15, 647–664 CrossRef CAS PubMed.
- P. M. Glassman, J. W. Myerson, L. T. Ferguson, R. Y. Kiseleva, V. V. Shuvaev, J. S. Brenner and V. R. Muzykantov, Adv. Drug Delivery Rev., 2020, 157, 96–117 CrossRef CAS PubMed.
- S. Chuaychob, R. Lyu, M. Tanaka, A. Haginiwa, A. Kitada, T. Nakamura and R. Yokokawa, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2312472121 CrossRef CAS PubMed.
- X. Wu, M. A. Newbold and C. L. Haynes, Analyst, 2015, 140, 5055–5064 RSC.
- X. Wu, M. A. Newbold, Z. Gao and C. L. Haynes, Biochim. Biophys. Acta, Gen. Subj., 2017, 1861, 1122–1130 CrossRef CAS PubMed.
- J. M. Ayuso, R. Truttschel, M. M. Gong, M. Humayun, M. Virumbrales-Munoz, R. Vitek, M. Felder, S. D. Gillies, P. Sondel, K. B. Wisinski, M. Patankar, D. J. Beebe and M. C. Skala, Onco Targets Ther, 2019, 8, 1553477 Search PubMed.
- J. M. Ayuso, M. Farooqui, M. Virumbrales-Muñoz, K. Denecke, S. Rehman, R. Schmitz, J. F. Guerrero, C. Sanchez-de-Diego, S. A. Campo, E. M. Maly, M. H. Forsberg, S. C. Kerr, R. Striker, N. M. Sherer, P. M. Harari, C. M. Capitini, M. C. Skala and D. J. Beebe, Nat. Commun., 2023, 14, 6681 CrossRef CAS PubMed.
- D. Nathanson and P. S. Mischel, J. Clin. Invest., 2011, 121, 31–33 CrossRef CAS PubMed.
- J. P. Straehla, C. Hajal, H. C. Safford, G. S. Offeddu, N. Boehnke, T. G. Dacoba, J. Wyckoff, R. D. Kamm and P. T. Hammond, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2118697119 CrossRef CAS PubMed.
- H. Xu, Z. Li, Y. Yu, S. Sizdahkhani, W. S. Ho, F. Yin, L. Wang, G. Zhu, M. Zhang, L. Jiang, Z. Zhuang and J. Qin, Sci. Rep., 2016, 6, 36670 CrossRef CAS PubMed.
- T.-E. Park, N. Mustafaoglu, A. Herland, R. Hasselkus, R. Mannix, E. A. FitzGerald, R. Prantil-Baun, A. Watters, O. Henry, M. Benz, H. Sanchez, H. J. McCrea, L. C. Goumnerova, H. W. Song, S. P. Palecek, E. Shusta and D. E. Ingber, Nat. Commun., 2019, 10, 2621 CrossRef PubMed.
- S. Davidson, M. Efremova, A. Riedel, B. Mahata, J. Pramanik, J. Huuhtanen, G. Kar, R. Vento-Tormo, T. Hagai, X. Chen, M. A. Haniffa, J. D. Shields and S. A. Teichmann, Cell Rep., 2020, 31, 107628 CrossRef CAS PubMed.
- A. Orimo, P. B. Gupta, D. C. Sgroi, F. Arenzana-Seisdedos, T. Delaunay, R. Naeem, V. J. Carey, A. L. Richardson and R. A. Weinberg, Cell, 2005, 121, 335–348 CrossRef CAS PubMed.
- Z. Xiao, L. Todd, L. Huang, E. Noguera-Ortega, Z. Lu, L. Huang, M. Kopp, Y. Li, N. Pattada, W. Zhong, W. Guo, J. Scholler, M. Liousia, C.-A. Assenmacher, C. H. June, S. M. Albelda and E. Puré, Nat. Commun., 2023, 14, 5110 CrossRef CAS PubMed.
- L. Cords, S. Engler, M. Haberecker, J. H. Rüschoff, H. Moch, N. de Souza and B. Bodenmiller, Cancer Cell, 2024, 42, 396–412.e395 CrossRef CAS PubMed.
- H. Zhang, X. Yue, Z. Chen, C. Liu, W. Wu, N. Zhang, Z. Liu, L. Yang, Q. Jiang, Q. Cheng, P. Luo and G. Liu, Mol. Cancer, 2023, 22, 159 CrossRef PubMed.
- D. Yang, J. Liu, H. Qian and Q. Zhuang, Exp. Mol. Med., 2023, 55, 1322–1332 CrossRef CAS PubMed.
- E. Sahai, I. Astsaturov, E. Cukierman, D. G. DeNardo, M. Egeblad, R. M. Evans, D. Fearon, F. R. Greten, S. R. Hingorani, T. Hunter, R. O. Hynes, R. K. Jain, T. Janowitz, C. Jorgensen, A. C. Kimmelman, M. G. Kolonin, R. G. Maki, R. S. Powers, E. Puré, D. C. Ramirez, R. Scherz-Shouval, M. H. Sherman, S. Stewart, T. D. Tlsty, D. A. Tuveson, F. M. Watt, V. Weaver, A. T. Weeraratna and Z. Werb, Nat. Rev. Cancer, 2020, 20, 174–186 CrossRef CAS PubMed.
- K. Aoto, K. Ito and S. Aoki, Onco Targets Ther, 2018, 9, 34090–34102 Search PubMed.
- C. Pan, Q. Fang, P. Liu, D. Ma, S. Cao, L. Zhang, Q. Chen, T. Hu and J. Wang, Front. Cell Dev. Biol., 2021, 9, 708513 CrossRef PubMed.
- L. Jiang, H. Khawaja, S. Tahsin, T. A. Clarkson, C. K. Miranti and Y. Zohar, Front. Bioeng. Biotechnol., 2024, 12, 1302223 CrossRef PubMed.
- Z. Shen, X. Qin, M. Yan, R. Li, G. Chen, J. Zhang and W. Chen, Onco Targets Ther, 2017, 8, 1290–1303 Search PubMed.
- H. Zhang, T. Deng, R. Liu, T. Ning, H. Yang, D. Liu, Q. Zhang, D. Lin, S. Ge, M. Bai, X. Wang, L. Zhang, H. Li, Y. Yang, Z. Ji, H. Wang, G. Ying and Y. Ba, Mol. Cancer, 2020, 19, 43 CrossRef CAS PubMed.
- L. Jenkins, U. Jungwirth, A. Avgustinova, M. Iravani, A. Mills, S. Haider, J. Harper and C. M. Isacke, Cancer Res., 2022, 82, 2904–2917 CrossRef CAS PubMed.
- H. Huang, Z. Wang, Y. Zhang, R. N. Pradhan, D. Ganguly, R. Chandra, G. Murimwa, S. Wright, X. Gu, R. Maddipati, S. Müller, S. J. Turley and R. A. Brekken, Cancer Cell, 2022, 40, 656–673.e657 CrossRef CAS PubMed.
- M. Yuan, B. Tu, H. Li, H. Pang, N. Zhang, M. Fan, J. Bai, W. Wang, Z. Shu, C. C. DuFort, S. Huo, J. Zhai, K. Yao, L. Wang, H. Ying, W.-G. Zhu, D. Fu, Z. Hu and Y. Zhao, Nat. Cancer, 2022, 3, 945–960 CrossRef CAS PubMed.
- T. Shan, S. Chen, X. Chen, W. R. Lin, W. Li, J. Ma, T. Wu, X. Cui, H. Ji, Y. Li and Y. Kang, Oncol. Rep., 2017, 37, 1971–1979 CrossRef CAS PubMed.
- S. Kunou, K. Shimada, M. Takai, A. Sakamoto, T. Aoki, T. Hikita, Y. Kagaya, E. Iwamoto, M. Sanada, S. Shimada, F. Hayakawa, C. Oneyama and H. Kiyoi, Oncogene, 2021, 40, 3989–4003 CrossRef CAS PubMed.
- Q. Hu, L. L. R. Rix, X. Li, E. A. Welsh, B. Fang, S. Yun, J. Kroeger, H. R. Lawrence, A. Marusyk, J. M. Koomen, E. B. Haura and U. Rix, Eur. J. Cancer, 2020, 138, S48 CrossRef.
- Z. Li, T. Guo, L. Fang, N. Li, X. Wang, P. Wang, S. Zhao, F. Li, Y. Cui, X. Shu, L. Zhao, J. Li and C. Gu, Int. J. Oncol., 2019, 54, 1367–1375 CAS.
- C. W. Chi, Y. H. Lao, A. H. R. Ahmed, E. C. Benoy, C. Li, Z. Dereli-Korkut, B. M. Fu, K. W. Leong and S. Wang, Adv. Healthcare Mater., 2020, 9, e2000880 CrossRef PubMed.
- M. S. Poddar, Y.-D. Chu, G. Pendharkar, C.-H. Liu and C.-T. Yeh, Lab Chip, 2024, 24, 5043–5054 RSC.
- D. D. Truong, A. Kratz, J. G. Park, E. S. Barrientos, H. Saini, T. Nguyen, B. Pockaj, G. Mouneimne, J. LaBaer and M. Nikkhah, Cancer Res., 2019, 79, 3139–3151 CrossRef CAS PubMed.
- M. M. Gong, K. M. Lugo-Cintron, B. R. White, S. C. Kerr, P. M. Harari and D. J. Beebe, Biomaterials, 2019, 214, 119225 CrossRef CAS PubMed.
- S. Plesselova, K. Calar, H. Axemaker, E. Sahly, A. Bhagia, J. L. Faragher, D. M. Fink and P. de la Puente, Cell. Mol. Bioeng., 2024, 17, 345–367 CrossRef CAS PubMed.
- Y. C. Chen, M. E. Gonzalez, B. Burman, X. Zhao, T. Anwar, M. Tran, N. Medhora, A. B. Hiziroglu, W. Lee, Y. H. Cheng, Y. Choi, E. Yoon and C. G. Kleer, Cell Rep., 2019, 27, 3916–3926.e3915 CrossRef CAS PubMed.
- X. Zhang, F. Hu, G. Li, G. Li, X. Yang, L. Liu, R. Zhang, B. Zhang and Y. Feng, Cell Death Dis., 2018, 9, 25 CrossRef PubMed.
- B. Dirat, L. Bochet, M. Dabek, D. Daviaud, S. Dauvillier, B. Majed, Y. Y. Wang, A. Meulle, B. Salles, S. Le Gonidec, I. Garrido, G. Escourrou, P. Valet and C. Muller, Cancer Res., 2011, 71, 2455–2465 CrossRef CAS PubMed.
- Y. Wang, Q. Sun, Y. Ye, X. Sun, S. Xie, Y. Zhan, J. Song, X. Fan, B. Zhang, M. Yang, L. Lv, K. Hosaka, Y. Yang and G. Nie, JCI Insight, 2022, 7, e157874 CrossRef PubMed.
- J. Prakash and Y. Shaked, Cancer Discovery, 2024, 14, 1375–1388 CrossRef CAS PubMed.
- J. Winkler, A. Abisoye-Ogunniyan, K. J. Metcalf and Z. Werb, Nat. Commun., 2020, 11, 5120 CrossRef CAS PubMed.
- C. J. Whatcott, C. H. Diep, P. Jiang, A. Watanabe, J. LoBello, C. Sima, G. Hostetter, H. M. Shepard, D. D. Von Hoff and H. Han, Clin. Cancer Res., 2015, 21, 3561–3568 CrossRef CAS PubMed.
- M. Egeblad, M. G. Rasch and V. M. Weaver, Curr. Opin. Cell Biol., 2010, 22, 697–706 CrossRef CAS PubMed.
- G. S. Offeddu, E. Cambria, S. E. Shelton, K. Haase, Z. Wan, L. Possenti, H. T. Nguyen, M. R. Gillrie, D. Hickman, C. G. Knutson and R. D. Kamm, Adv. Sci., 2024, 11, 2402757 CrossRef PubMed.
- M. R. Carvalho, D. Barata, L. M. Teixeira, S. Giselbrecht, R. L. Reis, J. M. Oliveira, R. Truckenmüller and P. Habibovic, Sci. Adv., 2019, 5, eaaw1317 CrossRef CAS PubMed.
- N. Dhiman, N. Shagaghi, M. Bhave, H. Sumer, P. Kingshott and S. N. Rath, Cytotherapy, 2021, 23, 25–36 CrossRef CAS PubMed.
- B. A. Nerger, P. T. Brun and C. M. Nelson, Soft Matter, 2019, 15, 5728–5738 RSC.
- M. S. Weiss, B. P. Bernabé, A. Shikanov, D. A. Bluver, M. D. Mui, S. Shin, L. J. Broadbelt and L. D. Shea, Biomaterials, 2012, 33, 3548–3559 CrossRef CAS PubMed.
- S.-W. Kang and Y. H. Bae, Biomaterials, 2009, 30, 4227–4232 CrossRef CAS PubMed.
- X. Gong, J. Kulwatno and K. L. Mills, Acta Biomater., 2020, 108, 128–141 CrossRef CAS PubMed.
- E. Dogan, C. A. Galifi, B. Cecen, R. Shukla, T. L. Wood and A. K. Miri, Acta Biomater., 2024, 186, 156–166 CrossRef CAS PubMed.
- S. I. Montanez-Sauri, K. E. Sung, E. Berthier and D. J. Beebe, Integr. Biol., 2013, 5, 631–640 CrossRef CAS PubMed.
- A. Brooks, Y. Zhang, J. Chen and C.-X. Zhao, Adv. Healthcare Mater., 2024, 13, 2302436 CrossRef CAS PubMed.
- B. Firatligil-Yildirir, G. Bati-Ayaz, Nonappa, D. Pesen-Okvur and O. Yalcin-Ozuysal, PLoS One, 2024, 19, e0309285 CrossRef CAS PubMed.
- T. Yu, Z. Guo, H. Fan, J. Song, Y. Liu, Z. Gao and Q. Wang, Onco Targets Ther, 2016, 7, 25593–25603 Search PubMed.
- C. Chen, A. Boché, Z. Wang, E. Lopez, J. Peng, F. Carreiras, M. C. Schanne-Klein, Y. Chen, A. Lambert and C. Aimé, Adv. Healthcare Mater., 2024, 13, e2400938 CrossRef PubMed.
- G. P. Dunn, A. T. Bruce, H. Ikeda, L. J. Old and R. D. Schreiber, Nat. Immunol., 2002, 3, 991–998 CrossRef CAS PubMed.
- W. Zou, Nat. Rev. Cancer, 2005, 5, 263–274 CrossRef CAS PubMed.
- C. P. Miller, W. Shin, E. H. Ahn, H. J. Kim and D.-H. Kim, Trends Biotechnol., 2020, 38, 857–872 CrossRef CAS PubMed.
- M. Bied, W. W. Ho, F. Ginhoux and C. Blériot, Cell. Mol. Immunol., 2023, 20, 983–992 CrossRef CAS PubMed.
- J. Zhou, Z. Tang, S. Gao, C. Li, Y. Feng and X. Zhou, Front. Oncol., 2020, 10, 188 CrossRef PubMed.
- C. P. Huang, J. Lu, H. Seon, A. P. Lee, L. A. Flanagan, H.-Y. Kim, A. J. Putnam and N. L. Jeon, Lab Chip, 2009, 9, 1740–1748 RSC.
- P.-f. Liu, Y.-w. Cao, S.-d. Zhang, Y. Zhao, X.-g. Liu, H.-q. Shi, K.-y. Hu, G.-q. Zhu, B. Ma and H.-t. Niu, Onco Targets Ther, 2015, 6(35), 37695 Search PubMed.
- R. Li, J. D. Hebert, T. A. Lee, H. Xing, A. Boussommier-Calleja, R. O. Hynes, D. A. Lauffenburger and R. D. Kamm, Cancer Res., 2017, 77, 279–290 CrossRef CAS PubMed.
- A. J. Boutilier and S. F. Elsawa, Int. J. Mol. Sci., 2021, 22, 6995 CrossRef CAS PubMed.
- Y. Bi, V. S. Shirure, R. Liu, C. Cunningham, L. Ding, J. M. Meacham, S. P. Goedegebuure, S. C. George and R. C. Fields, Integr. Biol., 2020, 12, 221–232 CrossRef PubMed.
- A. Del Prete, V. Salvi, A. Soriani, M. Laffranchi, F. Sozio, D. Bosisio and S. Sozzani, Cell. Mol. Immunol., 2023, 20, 432–447 CrossRef CAS PubMed.
- M. Vitale, C. Cantoni, G. Pietra, M. C. Mingari and L. Moretta, Eur. J. Immunol., 2014, 44, 1582–1592 CrossRef CAS PubMed.
- Y. Zhou, L. Cheng, L. Liu and X. Li, Mol. Cancer, 2023, 22, 34 CrossRef PubMed.
- X. Ren, A. Alamri, J. Hipolito, F. Lin and S. K. P. Kung, in Methods Enzymol., ed. L. Galluzzi and N.-P. Rudqvist, Academic Press, 2020, vol. 631, pp. 357–370 Search PubMed.
- J. Aleman, S. K. George, S. Herberg, M. Devarasetty, C. D. Porada, A. Skardal and G. Almeida-Porada, Small, 2019, 15, 1902971 CrossRef CAS PubMed.
- R. Pradhan, A. Kundu and C. N. Kundu, Crit. Rev. Oncol. Hematol., 2024, 196, 104311 CrossRef PubMed.
- Y. Zhang, Y. Wang, L. Shao, X. Pan, C. Liang, B. Liu, Y. Zhang, W. Xie, B. Yan, F. Liu, X. Y. Yu and Y. Li, J. Cell. Mol. Med., 2020, 24, 695–710 CrossRef CAS PubMed.
- K. Hoerster, M. Uhrberg, C. Wiek, P. A. Horn, H. Hanenberg and S. Heinrichs, Front. Immunol., 2021, 11, 586168 CrossRef PubMed.
- Q. Ramadan, R. Hazaymeh and M. Zourob, Adv. Biol., 2023, 7, e2200312 CrossRef PubMed.
- V. E. J. M. Palasantzas, I. Tamargo-Rubio, K. Le, J. Slager, C. Wijmenga, I. H. Jonkers, V. Kumar, J. Fu and S. Withoff, Trends Genet., 2023, 39, 268–284 CrossRef CAS PubMed.
- A. P. Ramme, L. Koenig, T. Hasenberg, C. Schwenk, C. Magauer, D. Faust, A. K. Lorenz, A. C. Krebs, C. Drewell, K. Schirrmann, A. Vladetic, G. C. Lin, S. Pabinger, W. Neuhaus, F. Bois, R. Lauster, U. Marx and E. M. Dehne, Future Sci. OA, 2019, 5, Fso413 CrossRef CAS PubMed.
- J. Rogal, J. Roosz, C. Teufel, M. Cipriano, R. Xu, W. Eisler, M. Weiss, K. Schenke-Layland and P. Loskill, Adv. Sci., 2022, 9, 2104451 CrossRef CAS PubMed.
- J.-K. Kim, Y. J. Shin, L. J. Ha, D.-H. Kim and D.-H. Kim, Adv. Healthcare Mater., 2019, 8, 1801332 CrossRef PubMed.
- W. Zhao, J. Li, M.-J. M. Chen, Y. Luo, Z. Ju, N. K. Nesser, K. Johnson-Camacho, C. T. Boniface, Y. Lawrence, N. T. Pande, M. A. Davies, M. Herlyn, T. Muranen, I. K. Zervantonakis, E. von Euw, A. Schultz, S. V. Kumar, A. Korkut, P. T. Spellman, R. Akbani, D. J. Slamon, J. W. Gray, J. S. Brugge, Y. Lu, G. B. Mills and H. Liang, Cancer Cell, 2020, 38, 829–843.e824 CrossRef CAS PubMed.
- S. Sinha, R. Vegesna, S. Mukherjee, A. V. Kammula, S. R. Dhruba, W. Wu, D. L. Kerr, N. U. Nair, M. G. Jones, N. Yosef, O. V. Stroganov, I. Grishagin, K. D. Aldape, C. M. Blakely, P. Jiang, C. J. Thomas, C. H. Benes, T. G. Bivona, A. A. Schäffer and E. Ruppin, Nat. Cancer, 2024, 5, 938–952 CrossRef PubMed.
- N. Vasan, J. Baselga and D. M. Hyman, Nature, 2019, 575, 299–309 CrossRef CAS PubMed.
- N. S. Bhise, J. Ribas, V. Manoharan, Y. S. Zhang, A. Polini, S. Massa, M. R. Dokmeci and A. Khademhosseini, J. Controlled Release, 2014, 190, 82–93 CrossRef CAS PubMed.
- J. Sun, A. R. Warden and X. Ding, Biomicrofluidics, 2019, 13, 061503 CrossRef PubMed.
- M. Monjezi, M. Rismanian, H. Jamaati and N. Kashaninejad, Appl. Sci., 2021, 11, 9418 CrossRef.
- N. Dhiman, P. Kingshott, H. Sumer, C. S. Sharma and S. N. Rath, Biosens. Bioelectron., 2019, 137, 236–254 CrossRef CAS PubMed.
- L. Ying, Z. Zhu, Z. Xu, T. He, E. Li, Z. Guo, F. Liu, C. Jiang and Q. Wang, PLoS One, 2015, 10, e0129593 CrossRef PubMed.
- J. Bai, T.-Y. Tu, C. Kim, J. P. Thiery and R. D. Kamm, Onco Targets Ther, 2015, 6(34), 36603 Search PubMed.
- A. Sobrino, D. T. Phan, R. Datta, X. Wang, S. J. Hachey, M. Romero-López, E. Gratton, A. P. Lee, S. C. George and C. C. Hughes, Sci. Rep., 2016, 6, 31589 CrossRef CAS PubMed.
- J. Han, Y. Jun, S. H. Kim, H.-H. Hoang, Y. Jung, S. Kim, J. Kim, R. H. Austin, S. Lee and S. Park, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 14283–14288 CrossRef CAS PubMed.
- D. A. Tatosian and M. L. Shuler, Biotechnol. Bioeng., 2009, 103, 187–198 CrossRef CAS PubMed.
- M. T. Witkowski, I. Dolgalev, N. A. Evensen, C. Ma, T. Chambers, K. G. Roberts, S. Sreeram, Y. Dai, A. N. Tikhonova, A. Lasry, C. Qu, D. Pei, C. Cheng, G. A. Robbins, J. Pierro, S. Selvaraj, V. Mezzano, M. Daves, P. J. Lupo, M. E. Scheurer, C. A. Loomis, C. G. Mullighan, W. Chen, K. R. Rabin, A. Tsirigos, W. L. Carroll and I. Aifantis, Cancer Cell, 2020, 37, 867–882.e812 CrossRef CAS PubMed.
- C.-Y. Su, A. Wu, Z. Dong, C. P. Miller, A. Suarez, A. J. Ewald, E. H. Ahn and D.-H. Kim, Biomaterials, 2023, 298, 122128 CrossRef CAS PubMed.
- M. W. Dewhirst and T. W. Secomb, Nat. Rev. Cancer, 2017, 17, 738–750 CrossRef CAS PubMed.
- V. S. Shirure, Y. Bi, M. B. Curtis, A. Lezia, M. M. Goedegebuure, S. P. Goedegebuure, R. Aft, R. C. Fields and S. C. George, Lab Chip, 2018, 18, 3687–3702 RSC.
- D. Kim, K. S. Hwang, E. U. Seo, S. Seo, B. C. Lee, N. Choi, J. Choi and H. N. Kim, Adv. Healthcare Mater., 2022, 11, e2102581 CrossRef PubMed.
- P. Agarwal, H. Wang, M. Sun, J. Xu, S. Zhao, Z. Liu, K. J. Gooch, Y. Zhao, X. Lu and X. He, ACS Nano, 2017, 11, 6691–6702 CrossRef CAS PubMed.
- B. Olson, Y. Li, Y. Lin, E. T. Liu and A. Patnaik, Cancer Discovery, 2018, 8, 1358–1365 CrossRef CAS PubMed.
- Y. Li, H. Fan, J. Ding, J. Xu, C. Liu and H. Wang, Front. Genet., 2022, 13, 969723 CrossRef CAS PubMed.
- H. Xie, J. W. Appelt and R. W. Jenkins, Cancers, 2021, 13, 6052 CrossRef CAS PubMed.
- K. Paterson, S. Zanivan, R. Glasspool, S. B. Coffelt and M. Zagnoni, Lab Chip, 2021, 21, 2306–2329 RSC.
- Y. Shiravand, F. Khodadadi, S. M. A. Kashani, S. R. Hosseini-Fard, S. Hosseini, H. Sadeghirad, R. Ladwa, K. O'Byrne and A. Kulasinghe, Curr. Oncol., 2022, 29, 3044–3060 CrossRef PubMed.
- X. Jiang, L. Ren, P. Tebon, C. Wang, X. Zhou, M. Qu, J. Zhu, H. Ling, S. Zhang, Y. Xue, Q. Wu, P. Bandaru, J. Lee, H.-J. Kim, S. Ahadian, N. Ashammakhi, M. R. Dokmeci, J. Wu, Z. Gu, W. Sun and A. Khademhosseini, Small, 2021, 17, 2004282 CrossRef CAS PubMed.
- Y.-C. Chen, K.-Y. Lee, H.-J. Liao, W.-L. Sun, W.-C. Huang, Y.-S. Wang, W.-C. Chang and C.-H. Liu, Sens. Actuators, B, 2024, 406, 135409 CrossRef CAS.
- Z. Ao, H. Cai, Z. Wu, L. Hu, X. Li, C. Kaurich, M. Gu, L. Cheng, X. Lu and F. Guo, Theranostics, 2022, 12, 3628–3636 CrossRef CAS PubMed.
- K. Sehgal, A. Portell, E. V. Ivanova, P. H. Lizotte, N. R. Mahadevan, J. R. Greene, A. Vajdi, C. Gurjao, T. Teceno, L. J. Taus, T. C. Thai, S. Kitajima, D. Liu, T. Tani, M. Noureddine, C. J. Lau, P. T. Kirschmeier, D. Liu, M. Giannakis, R. W. Jenkins, P. C. Gokhale, S. Goldoni, M. Pinzon-Ortiz, W. D. Hastings, P. S. Hammerman, J. J. Miret, C. P. Paweletz and D. A. Barbie, J. Clin. Invest., 2021, 131, e135038 CrossRef CAS PubMed.
- M. Chernyavska, C. K. J. C. Hermans, C. Chan, N. Baumann, T. Rösner, J. H. W. Leusen, T. Valerius and W. P. R. Verdurmen, Organs-on-a-Chip, 2022, 4, 100019 CrossRef CAS.
- Z. Zou, Z. Lin, C. Wu, J. Tan, J. Zhang, Y. Peng, K. Zhang, J. Li, M. Wu and Y. Zhang, Adv. Sci., 2023, 10, 2302640 CrossRef CAS PubMed.
- M. Nguyen, A. De Ninno, A. Mencattini, F. Mermet-Meillon, G. Fornabaio, S. S. Evans, M. Cossutta, Y. Khira, W. Han, P. Sirven, F. Pelon, D. Di Giuseppe, F. R. Bertani, A. Gerardino, A. Yamada, S. Descroix, V. Soumelis, F. Mechta-Grigoriou, G. Zalcman, J. Camonis, E. Martinelli, L. Businaro and M. C. Parrini, Cell Rep., 2018, 25, 3884–3893.e3883 CrossRef CAS PubMed.
- H. Aboulkheyr Es, S. Zhand, J. P. Thiery and M. E. Warkiani, Integr. Biol., 2020, 12, 188–197 CrossRef PubMed.
- M. Kraman, P. J. Bambrough, J. N. Arnold, E. W. Roberts, L. Magiera, J. O. Jones, A. Gopinathan, D. A. Tuveson and D. T. Fearon, Science, 2010, 330, 827–830 CrossRef CAS PubMed.
- S. Singh, S. R. Ross, M. Acena, D. A. Rowley and H. Schreiber, J. Exp. Med., 1992, 175, 139–146 CrossRef CAS PubMed.
- K. C. Valkenburg, A. E. de Groot and K. J. Pienta, Nat. Rev. Clin. Oncol., 2018, 15, 366–381 CrossRef PubMed.
- S. Guedan, M. Ruella and C. H. June, Annu. Rev. Immunol., 2019, 37, 145–171 CrossRef CAS PubMed.
- M. T. Witkowski, S. Lee, E. Wang, A. K. Lee, A. Talbot, C. Ma, N. Tsopoulidis, J. Brumbaugh, Y. Zhao, K. G. Roberts, S. J. Hogg, S. Nomikou, Y. E. Ghebrechristos, P. Thandapani, C. G. Mullighan, K. Hochedlinger, W. Chen, O. Abdel-Wahab, J. Eyquem and I. Aifantis, Nat. Immunol., 2022, 23, 1424–1432, DOI:10.1038/s41590-022-01314-y.
- C. P. Miller, M. Fung, C. A. Jaeger-Ruckstuhl, Y. Xu, E. H. Warren, S. Akilesh and S. S. Tykodi, Neoplasia, 2023, 46, 100948 CrossRef CAS PubMed.
- S. W. L. Lee, G. Adriani, E. Ceccarello, A. Pavesi, A. T. Tan, A. Bertoletti, R. D. Kamm and S. C. Wong, Front. Immunol., 2018, 9, 416 CrossRef PubMed.
- A. Pavesi, A. T. Tan, S. Koh, A. Chia, M. Colombo, E. Antonecchia, C. Miccolis, E. Ceccarello, G. Adriani, M. T. Raimondi, R. D. Kamm and A. Bertoletti, JCI Insight, 2017, 2, e89762 CrossRef PubMed.
- R. Preece, A. Pavesi, S. A. Gkazi, K. A. Stegmann, C. Georgiadis, Z. M. Tan, J. Y. J. Aw, M. K. Maini, A. Bertoletti and W. Qasim, Mol. Ther.--Methods Clin. Dev., 2020, 19, 149–161 CrossRef CAS PubMed.
- S. J. Kerns, C. Belgur, D. Petropolis, M. Kanellias, R. Barrile, J. Sam, T. Weinzierl, T. Fauti, A. Freimoser-Grundschober, J. Eckmann, C. Hage, M. Geiger, P. R. Ng, W. Tien-Street, D. V. Manatakis, V. Micallef, R. Gerard, M. Bscheider, E. Breous-Nystrom, A. Schneider, A. M. Giusti, C. Bertinetti-Lapatki, H. S. Grant, A. B. Roth, G. A. Hamilton, T. Singer, K. Karalis, A. Moisan, P. Bruenker, C. Klein, M. Bacac, N. Gjorevski and L. Cabon, eLife, 2021, 10, e67106 CrossRef CAS PubMed.
- A. Mencattini, C. Lansche, I. Veith, P. Erbs, J.-M. Balloul, E. Quemeneur, S. Descroix, F. Mechta-Grigoriou, G. Zalcman, C. Zaupa, M. C. Parrini and E. Martinelli, Biosens. Bioelectron., 2022, 215, 114571 CrossRef CAS PubMed.
- Q. Liu, L. S. Mille, C. Villalobos, I. Anaya, M. Vostatek, S. Yi, W. Li, J. Liao, H. Wu, Y. Song, L. Xiong and Y. S. Zhang, Bio-Des. Manuf., 2023, 6, 373–389 CrossRef CAS.
- J. Ruppen, F. D. Wildhaber, C. Strub, S. R. Hall, R. A. Schmid, T. Geiser and O. T. Guenat, Lab Chip, 2015, 15, 3076–3085 RSC.
- Z. Zhang, Y.-C. Chen, S. Urs, L. Chen, D. M. Simeone and E. Yoon, Small, 2018, 14, 1703617 CrossRef PubMed.
- E. Prince, S. Kheiri, Y. Wang, F. Xu, J. Cruickshank, V. Topolskaia, H. Tao, E. W. K. Young, A. P. McGuigan, D. W. Cescon and E. Kumacheva, Adv. Healthcare Mater., 2022, 11, 2101085 CrossRef CAS PubMed.
- W. Liu, J. Xu, T. Li, L. Zhao, C. Ma, S. Shen and J. Wang, Anal. Chem., 2015, 87, 9752–9760 CrossRef CAS PubMed.
- T. Mulholland, M. McAllister, S. Patek, D. Flint, M. Underwood, A. Sim, J. Edwards and M. Zagnoni, Sci. Rep., 2018, 8, 14672 CrossRef PubMed.
- R. R. Xiao, B. Jing, L. Yan, J. Li, P. Tu and X. Ai, Lab Chip, 2022, 22, 4481–4492 RSC.
- E. Steinberg, R. Friedman, Y. Goldstein, N. Friedman, O. Beharier, J. A. Demma, G. Zamir, A. Hubert and O. Benny, Commun. Biol., 2023, 6, 1157 CrossRef PubMed.
- N. Moore, D. Doty, M. Zielstorff, I. Kariv, L. Y. Moy, A. Gimbel, J. R. Chevillet, N. Lowry, J. Santos, V. Mott, L. Kratchman, T. Lau, G. Addona, H. Chen and J. T. Borenstein, Lab Chip, 2018, 18, 1844–1858 RSC.
- M. Astolfi, B. Péant, M. A. Lateef, N. Rousset, J. Kendall-Dupont, E. Carmona, F. Monet, F. Saad, D. Provencher, A. M. Mes-Masson and T. Gervais, Lab Chip, 2016, 16, 312–325 RSC.
- L. F. Horowitz, A. D. Rodriguez, A. Au-Yeung, K. W. Bishop, L. A. Barner, G. Mishra, A. Raman, P. Delgado, J. T. C. Liu, T. S. Gujral, M. Mehrabi, M. Yang, R. H. Pierce and A. Folch, Lab Chip, 2021, 21, 122–142 RSC.
- T. C. Chang, A. M. Mikheev, W. Huynh, R. J. Monnat, R. C. Rostomily and A. Folch, Lab Chip, 2014, 14, 4540–4551 RSC.
- B. L. Khoo, G. Grenci, T. Jing, Y. B. Lim, S. C. Lee, J. P. Thiery, J. Han and C. T. Lim, Sci. Adv., 2016, 2, e1600274 CrossRef PubMed.
- M. Chernyavska, M. Masoudnia, T. Valerius and W. P. R. Verdurmen, Cancer Immunol., Immunother., 2023, 72, 3971–3983 CrossRef CAS PubMed.
- S. Parlato, G. Grisanti, G. Sinibaldi, G. Peruzzi, C. M. Casciola and L. Gabriele, Lab Chip, 2021, 21, 234–253 RSC.
- C. Bouquerel, A. Dubrova, I. Hofer, D. T. T. Phan, M. Bernheim, S. Ladaigue, C. Cavaniol, D. Maddalo, L. Cabel, F. Mechta-Grigoriou, C. Wilhelm, G. Zalcman, M. C. Parrini and S. Descroix, Lab Chip, 2023, 23, 3906–3935 RSC.
- M. Virumbrales-Muñoz, J. Chen, J. Ayuso, M. Lee, E. J. Abel and D. J. Beebe, Lab Chip, 2020, 20, 4420–4432 RSC.
- S. M. Hattersley, D. C. Sylvester, C. E. Dyer, N. D. Stafford, S. J. Haswell and J. Greenman, Ann. Biomed. Eng., 2012, 40, 1277–1288 CrossRef PubMed.
- S. Rajasekar, D. S. Y. Lin, L. Abdul, A. Liu, A. Sotra, F. Zhang and B. Zhang, Adv. Mater., 2020, 32, 2002974 CrossRef CAS PubMed.
- L. F. Lorenzo-Martín, T. Hübscher, A. D. Bowler, N. Broguiere, J. Langer, L. Tillard, M. Nikolaev, F. Radtke and M. P. Lutolf, Nature, 2024, 629, 450–457 CrossRef PubMed.
- Y. S. Zhang, J. Aleman, S. R. Shin, T. Kilic, D. Kim, S. A. Mousavi Shaegh, S. Massa, R. Riahi, S. Chae, N. Hu, H. Avci, W. Zhang, A. Silvestri, A. Sanati Nezhad, A. Manbohi, F. De Ferrari, A. Polini, G. Calzone, N. Shaikh, P. Alerasool, E. Budina, J. Kang, N. Bhise, J. Ribas, A. Pourmand, A. Skardal, T. Shupe, C. E. Bishop, M. R. Dokmeci, A. Atala and A. Khademhosseini, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E2293–E2302 CAS.
- C. W. McAleer, C. J. Long, D. Elbrecht, T. Sasserath, L. R. Bridges, J. W. Rumsey, C. Martin, M. Schnepper, Y. Wang, F. Schuler, A. B. Roth, C. Funk, M. L. Shuler and J. J. Hickman, Sci. Transl. Med., 2019, 11, eaav1386 CrossRef CAS PubMed.
- K. Ronaldson-Bouchard, D. Teles, K. Yeager, D. N. Tavakol, Y. Zhao, A. Chramiec, S. Tagore, M. Summers, S. Stylianos, M. Tamargo, B. M. Lee, S. P. Halligan, E. H. Abaci, Z. Guo, J. Jacków, A. Pappalardo, J. Shih, R. K. Soni, S. Sonar, C. German, A. M. Christiano, A. Califano, K. K. Hirschi, C. S. Chen, A. Przekwas and G. Vunjak-Novakovic, Nat. Biomed. Eng., 2022, 6, 351–371 CrossRef CAS PubMed.
- H. Zou, W. Yue, W.-K. Yu, D. Liu, C.-C. Fong, J. Zhao and M. Yang, Anal. Chem., 2015, 87, 7098–7108 CrossRef CAS PubMed.
- Y. Ando, E. L. Siegler, H. P. Ta, G. E. Cinay, H. Zhou, K. A. Gorrell, H. Au, B. M. Jarvis, P. Wang and K. Shen, Adv. Healthcare Mater., 2019, 8, 1900001 CrossRef PubMed.
- H. Azizgolshani, J. R. Coppeta, E. M. Vedula, E. E. Marr, B. P. Cain, R. J. Luu, M. P. Lech, S. H. Kann, T. J. Mulhern, V. Tandon, K. Tan, N. J. Haroutunian, P. Keegan, M. Rogers, A. L. Gard, K. B. Baldwin, J. C. de Souza, B. C. Hoefler, S. S. Bale, L. B. Kratchman, A. Zorn, A. Patterson, E. S. Kim, T. A. Petrie, E. L. Wiellette, C. Williams, B. C. Isenberg and J. L. Charest, Lab Chip, 2021, 21, 1454–1474 RSC.
- B. A. Hassell, G. Goyal, E. Lee, A. Sontheimer-Phelps, O. Levy, C. S. Chen and D. E. Ingber, Cell Rep., 2017, 21, 508–516 CrossRef CAS PubMed.
- A. Weltin, K. Slotwinski, J. Kieninger, I. Moser, G. Jobst, M. Wego, R. Ehret and G. A. Urban, Lab Chip, 2014, 14, 138–146 RSC.
- R. Riahi, S. A. M. Shaegh, M. Ghaderi, Y. S. Zhang, S. R. Shin, J. Aleman, S. Massa, D. Kim, M. R. Dokmeci and A. Khademhosseini, Sci. Rep., 2016, 6, 24598 CrossRef CAS PubMed.
- S.-H. Su, Y. Song, A. Stephens, M. Situ, M. C. McCloskey, J. L. McGrath, A. V. Andjelkovic, B. H. Singer and K. Kurabayashi, Biosens. Bioelectron., 2023, 224, 115030 CrossRef CAS PubMed.
- S. J. Trietsch, E. Naumovska, D. Kurek, M. C. Setyawati, M. K. Vormann, K. J. Wilschut, H. L. Lanz, A. Nicolas, C. P. Ng, J. Joore, S. Kustermann, A. Roth, T. Hankemeier, A. Moisan and P. Vulto, Nat. Commun., 2017, 8, 262 CrossRef PubMed.
- R. Novak, M. Ingram, S. Marquez, D. Das, A. Delahanty, A. Herland, B. M. Maoz, S. S. F. Jeanty, M. R. Somayaji, M. Burt, E. Calamari, A. Chalkiadaki, A. Cho, Y. Choe, D. B. Chou, M. Cronce, S. Dauth, T. Divic, J. Fernandez-Alcon, T. Ferrante, J. Ferrier, E. A. FitzGerald, R. Fleming, S. Jalili-Firoozinezhad, T. Grevesse, J. A. Goss, T. Hamkins-Indik, O. Henry, C. Hinojosa, T. Huffstater, K.-J. Jang, V. Kujala, L. Leng, R. Mannix, Y. Milton, J. Nawroth, B. A. Nestor, C. F. Ng, B. O'Connor, T.-E. Park, H. Sanchez, J. Sliz, A. Sontheimer-Phelps, B. Swenor, G. Thompson, G. J. Touloumes, Z. Tranchemontagne, N. Wen, M. Yadid, A. Bahinski, G. A. Hamilton, D. Levner, O. Levy, A. Przekwas, R. Prantil-Baun, K. K. Parker and D. E. Ingber, Nat. Biomed. Eng., 2020, 4, 407–420 CrossRef PubMed.
- F. E. Cliffe, C. Madden, P. Costello, S. Devitt, S. R. Mukkunda, B. B. Keshava, H. O. Fearnhead, A. Vitkauskaite, M. H. Dehkordi, W. Chingwaru, M. Przyjalgowski, N. Rebrova and M. Lyons, SLAS Technol., 2023, 28, 230–242 CrossRef CAS PubMed.
- A. Mencattini, D. Di Giuseppe, M. C. Comes, P. Casti, F. Corsi, F. R. Bertani, L. Ghibelli, L. Businaro, C. Di Natale, M. C. Parrini and E. Martinelli, Sci. Rep., 2020, 10, 7653 CrossRef CAS PubMed.
- Z. Ao, H. Cai, Z. Wu, L. Hu, A. Nunez, Z. Zhou, H. Liu, M. Bondesson, X. Lu, X. Lu, M. Dao and F. Guo, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2214569119 CrossRef CAS PubMed.
- J. F. Dekkers, M. Alieva, A. Cleven, F. Keramati, A. K. L. Wezenaar, E. J. van Vliet, J. Puschhof, P. Brazda, I. Johanna, A. D. Meringa, H. G. Rebel, M. B. Buchholz, M. Barrera Román, A. L. Zeeman, S. de Blank, D. Fasci, M. H. Geurts, A. M. Cornel, E. Driehuis, R. Millen, T. Straetemans, M. J. T. Nicolasen, T. Aarts-Riemens, H. C. R. Ariese, H. R. Johnson, R. L. van Ineveld, F. Karaiskaki, O. Kopper, Y. E. Bar-Ephraim, K. Kretzschmar, A. M. M. Eggermont, S. Nierkens, E. J. Wehrens, H. G. Stunnenberg, H. Clevers, J. Kuball, Z. Sebestyen and A. C. Rios, Nat. Biotechnol., 2023, 41, 60–69 CrossRef CAS PubMed.
- Z. Zhang, L. Liu, C. Ma, X. Cui, R. H. W. Lam and W. Chen, Small Methods, 2021, 5, 2100197 CrossRef CAS PubMed.
- S. Kheiri, E. Kumacheva and E. W. K. Young, Front. Bioeng. Biotechnol., 2021, 9, 781566 CrossRef PubMed.
- A. Herland, B. M. Maoz, D. Das, M. R. Somayaji, R. Prantil-Baun, R. Novak, M. Cronce, T. Huffstater, S. S. F. Jeanty, M. Ingram, A. Chalkiadaki, D. Benson Chou, S. Marquez, A. Delahanty, S. Jalili-Firoozinezhad, Y. Milton, A. Sontheimer-Phelps, B. Swenor, O. Levy, K. K. Parker, A. Przekwas and D. E. Ingber, Nat. Biomed. Eng., 2020, 4, 421–436 CrossRef CAS PubMed.
- C. E. Meacham and S. J. Morrison, Nature, 2013, 501, 328–337 CrossRef CAS PubMed.
- A. R. Mazzocchi, S. A. P. Rajan, K. I. Votanopoulos, A. R. Hall and A. Skardal, Sci. Rep., 2018, 8, 2886 CrossRef PubMed.
- T. J. Gerton, A. Green, M. Campisi, M. Chen, I. Gjeci, N. Mahadevan, C. A. A. Lee, R. Mishra, H. V. Vo, K. Haratani, Z.-H. Li, K. T. Hasselblatt, B. Testino, T. Connor, C. G. Lian, K. M. Elias, P. Lizotte, E. V. Ivanova, D. A. Barbie and D. M. Dinulescu, Cancers, 2023, 15, 4128 CrossRef CAS PubMed.
- Z. P. Khin, M. L. C. Ribeiro, T. Jacobson, L. Hazlehurst, L. Perez, R. Baz, K. Shain and A. S. Silva, Cancer Res., 2014, 74, 56–67 CrossRef CAS PubMed.
- L. J. Y. Ong, S. Chia, S. Q. R. Wong, X. Zhang, H. Chua, J. M. Loo, W. Y. Chua, C. Chua, E. Tan, H. Hentze, I. B. Tan, R. DasGupta and Y.-C. Toh, Front. Bioeng. Biotechnol., 2022, 10, 952726 CrossRef PubMed.
- M. S. Manak, J. S. Varsanik, B. J. Hogan, M. J. Whitfield, W. R. Su, N. Joshi, N. Steinke, A. Min, D. Berger, R. J. Saphirstein, G. Dixit, T. Meyyappan, H.-M. Chu, K. B. Knopf, D. M. Albala, G. R. Sant and A. C. Chander, Nat. Biomed. Eng., 2018, 2, 761–772 CrossRef CAS PubMed.
- F. Eduati, R. Utharala, D. Madhavan, U. P. Neumann, T. Longerich, T. Cramer, J. Saez-Rodriguez and C. A. Merten, Nat. Commun., 2018, 9, 2434 CrossRef PubMed.
- M. Parsian, P. Mutlu, E. Yildirim, C. Ildiz, C. Ozen and U. Gunduz, Biomicrofluidics, 2022, 16, 034103 CrossRef CAS PubMed.
- P. W. Burridge, Y. F. Li, E. Matsa, H. Wu, S.-G. Ong, A. Sharma, A. Holmström, A. C. Chang, M. J. Coronado, A. D. Ebert, J. W. Knowles, M. L. Telli, R. M. Witteles, H. M. Blau, D. Bernstein, R. B. Altman and J. C. Wu, Nat. Med., 2016, 22, 547–556 CrossRef CAS PubMed.
- H. Tiriac, P. Belleau, D. D. Engle, D. Plenker, A. Deschênes, T. D. D. Somerville, F. E. M. Froeling, R. A. Burkhart, R. E. Denroche, G. H. Jang, K. Miyabayashi, C. M. Young, H. Patel, M. Ma, J. F. LaComb, R. L. D. Palmaira, A. A. Javed, J. C. Huynh, M. Johnson, K. Arora, N. Robine, M. Shah, R. Sanghvi, A. B. Goetz, C. Y. Lowder, L. Martello, E. Driehuis, N. LeComte, G. Askan, C. A. Iacobuzio-Donahue, H. Clevers, L. D. Wood, R. H. Hruban, E. Thompson, A. J. Aguirre, B. M. Wolpin, A. Sasson, J. Kim, M. Wu, J. C. Bucobo, P. Allen, D. V. Sejpal, W. Nealon, J. D. Sullivan, J. M. Winter, P. A. Gimotty, J. L. Grem, D. J. DiMaio, J. M. Buscaglia, P. M. Grandgenett, J. R. Brody, M. A. Hollingsworth, G. M. O'Kane, F. Notta, E. Kim, J. M. Crawford, C. Devoe, A. Ocean, C. L. Wolfgang, K. H. Yu, E. Li, C. R. Vakoc, B. Hubert, S. E. Fischer, J. M. Wilson, R. Moffitt, J. Knox, A. Krasnitz, S. Gallinger and D. A. Tuveson, Cancer Discovery, 2018, 8, 1112–1129 CrossRef CAS PubMed.
- H. Yang, L. Sun, M. Liu and Y. Mao, Gastroenterol. Rep., 2018, 6, 243–245 CrossRef PubMed.
- S. E. Park, A. Georgescu and D. Huh, Science, 2019, 364, 960–965 CrossRef CAS PubMed.
- Y. Zhao, S. Landau, S. Okhovatian, C. Liu, R. X. Z. Lu, B. F. L. Lai, Q. Wu, J. Kieda, K. Cheung, S. Rajasekar, K. Jozani, B. Zhang and M. Radisic, Nat. Rev. Bioeng., 2024, 2, 588–608 CrossRef CAS.
- T. Takebe, B. Zhang and M. Radisic, Cell Stem Cell, 2017, 21, 297–300 CrossRef CAS PubMed.
- J. Zhu, L. Ji, Y. Chen, H. Li, M. Huang, Z. Dai, J. Wang, D. Xiang, G. Fu, Z. Lei and X. Chu, Cell Death Discovery, 2023, 9, 72 CrossRef PubMed.
- V. S. Shirure, C. C. W. Hughes and S. C. George, Annu. Rev. Biomed. Eng., 2021, 23, 141–167 CrossRef CAS PubMed.
- K. A. Homan, N. Gupta, K. T. Kroll, D. B. Kolesky, M. Skylar-Scott, T. Miyoshi, D. Mau, M. T. Valerius, T. Ferrante, J. V. Bonventre, J. A. Lewis and R. Morizane, Nat. Methods, 2019, 16, 255–262 CrossRef CAS PubMed.
- K. T. Kroll, M. M. Mata, K. A. Homan, V. Micallef, A. Carpy, K. Hiratsuka, R. Morizane, A. Moisan, M. Gubler, A.-C. Walz, E. Marrer-Berger and J. A. Lewis, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2305322120 CrossRef CAS PubMed.
- R. Polak, E. T. Zhang and C. J. Kuo, Nat. Rev. Cancer, 2024, 24, 523–539 CrossRef CAS PubMed.
- Y. Zhang, Q. Hu, Y. Pei, H. Luo, Z. Wang, X. Xu, Q. Zhang, J. Dai, Q. Wang, Z. Fan, Y. Fang, M. Ye, B. Li, M. Chen, Q. Xue, Q. Zheng, S. Zhang, M. Huang, T. Zhang, J. Gu and Z. Xiong, Nat. Commun., 2024, 15, 3382 CrossRef CAS PubMed.
- K. K. Dijkstra, C. M. Cattaneo, F. Weeber, M. Chalabi, J. van de Haar, L. F. Fanchi, M. Slagter, D. L. van der Velden, S. Kaing, S. Kelderman, N. van Rooij, M. E. van Leerdam, A. Depla, E. F. Smit, K. J. Hartemink, R. de Groot, M. C. Wolkers, N. Sachs, P. Snaebjornsson, K. Monkhorst, J. Haanen, H. Clevers, T. N. Schumacher and E. E. Voest, Cell, 2018, 174, 1586–1598.e1512 CrossRef CAS PubMed.
- S. Tsai, L. McOlash, K. Palen, B. Johnson, C. Duris, Q. Yang, M. B. Dwinell, B. Hunt, D. B. Evans, J. Gershan and M. A. James, BMC Cancer, 2018, 18, 335 CrossRef PubMed.
- O. Mitrofanova, M. Nikolaev, Q. Xu, N. Broguiere, I. Cubela, J. G. Camp, M. Bscheider and M. P. Lutolf, Cell Stem Cell, 2024, 31, 1175–1186.e1177 CrossRef CAS PubMed.
- M. Nikolaev, O. Mitrofanova, N. Broguiere, S. Geraldo, D. Dutta, Y. Tabata, B. Elci, N. Brandenberg, I. Kolotuev, N. Gjorevski, H. Clevers and M. P. Lutolf, Nature, 2020, 585, 574–578 CrossRef PubMed.
- L. E. Wagar, A. Salahudeen, C. M. Constantz, B. S. Wendel, M. M. Lyons, V. Mallajosyula, L. P. Jatt, J. Z. Adamska, L. K. Blum, N. Gupta, K. J. L. Jackson, F. Yang, K. Röltgen, K. M. Roskin, K. M. Blaine, K. D. Meister, I. N. Ahmad, M. Cortese, E. G. Dora, S. N. Tucker, A. I. Sperling, A. Jain, D. H. Davies, P. L. Felgner, G. B. Hammer, P. S. Kim, W. H. Robinson, S. D. Boyd, C. J. Kuo and M. M. Davis, Nat. Med., 2021, 27, 125–135 CrossRef CAS PubMed.
- S. B. Shah, C. R. Carlson, K. Lai, Z. Zhong, G. Marsico, K. M. Lee, N. E. Félix Vélez, E. B. Abeles, M. Allam, T. Hu, L. D. Walter, K. E. Martin, K. Gandhi, S. D. Butler, R. Puri, A. L. McCleary-Wheeler, W. Tam, O. Elemento, K. Takata, C. Steidl, D. W. Scott, L. Fontan, H. Ueno, B. D. Cosgrove, G. Inghirami, A. J. García, A. F. Coskun, J. L. Koff, A. Melnick and A. Singh, Nat. Mater., 2023, 22, 511–523 CrossRef CAS PubMed.
- Z. Zhong, M. Quiñones-Pérez, Z. Dai, V. M. Juarez, E. Bhatia, C. R. Carlson, S. B. Shah, A. Patel, Z. Fang, T. Hu, M. Allam, S. L. Hicks, M. Gupta, S. L. Gupta, E. Weeks, S. D. Vagelos, A. Molina, A. Mulero-Russe, A. Mora-Boza, D. J. Joshi, R. P. Sekaly, T. Sulchek, S. L. Goudy, J. Wrammert, K. Roy, J. M. Boss, A. F. Coskun, C. D. Scharer, A. J. García, J. L. Koff and A. Singh, Nat. Mater., 2025, 24, 297–311, DOI:10.1038/s41563-024-02037-1.
- D. Choi, A. M. Gonzalez-Suarez, M. G. Dumbrava, M. Medlyn, J. M. de Hoyos-Vega, F. Cichocki, J. S. Miller, L. Ding, M. Zhu, G. Stybayeva, A. Gaspar-Maia, D. D. Billadeau, W. W. Ma and A. Revzin, Adv. Sci., 2024, 11, 2303088 CrossRef CAS PubMed.
- S. E. Park, S. Kang, J. Paek, A. Georgescu, J. Chang, A. Y. Yi, B. J. Wilkins, T. A. Karakasheva, K. E. Hamilton and D. D. Huh, Nat. Methods, 2022, 19, 1449–1460 CrossRef CAS PubMed.
- D. Kim, H. Lim, J. Youn, T.-E. Park and D. S. Kim, Nat. Commun., 2024, 15, 9420 CrossRef CAS PubMed.
- Z. Li, D. Li, Y. Guo, Y. Wang and W. Su, Biotechnol. Lett., 2021, 43, 383–392 CrossRef CAS PubMed.
- J. Lee, S. Mehrotra, E. Zare-Eelanjegh, R. O. Rodrigues, A. Akbarinejad, D. Ge, L. Amato, K. Kiaee, Y. Fang, A. Rosenkranz, W. Keung, B. B. Mandal, R. A. Li, T. Zhang, H. Lee, M. R. Dokmeci, Y. S. Zhang, A. Khademhosseini and S. R. Shin, Small, 2021, 17, e2004258 CrossRef PubMed.
- E. Ferrari, R. Visone, E. Monti, E. Torretta, M. Moretti, P. Occhetta and M. Rasponi, Adv. Mater. Technol., 2023, 8, 2201435 CrossRef CAS.
- A. Grigorian and C. B. O'Brien, J. Clin. Transl. Hepatol., 2014, 2, 95–102 Search PubMed.
- M. Florescu, M. Cinteza and D. Vinereanu, Maedica, 2013, 8, 59–67 Search PubMed.
- Y. Wang, Y. Gao, Y. Pan, D. Zhou, Y. Liu, Y. Yin, J. Yang, Y. Wang and Y. Song, Acta Pharm. Sin. B, 2023, 13, 2483–2509 CrossRef PubMed.
- L. Ma, J. Barker, C. Zhou, W. Li, J. Zhang, B. Lin, G. Foltz, J. Küblbeck and P. Honkakoski, Biomaterials, 2012, 33, 4353–4361 CrossRef CAS PubMed.
- Y. Imura, K. Sato and E. Yoshimura, Anal. Chem., 2010, 82, 9983–9988 CrossRef CAS PubMed.
- T. Satoh, S. Sugiura, K. Shin, R. Onuki-Nagasaki, S. Ishida, K. Kikuchi, M. Kakiki and T. Kanamori, Lab Chip, 2017, 18, 115–125 RSC.
- Y. Hou, X. Ai, L. Zhao, Z. Gao, Y. Wang, Y. Lu, P. Tu and Y. Jiang, Lab Chip, 2020, 20, 2482–2494 RSC.
- C. Lohasz, F. Bonanini, L. Hoelting, K. Renggli, O. Frey and A. Hierlemann, Adv. Biosyst., 2020, 4, 2000079 CrossRef CAS PubMed.
- K.-C. Weng, Y. K. Kurokawa, B. S. Hajek, J. A. Paladin, V. S. Shirure and S. C. George, Tissue Eng., Part C, 2019, 26, 44–55 CrossRef PubMed.
- K.-i. Kamei, Y. Kato, Y. Hirai, S. Ito, J. Satoh, A. Oka, T. Tsuchiya, Y. Chen and O. Tabata, RSC Adv., 2017, 7, 36777–36786 RSC.
- M. A. J. Morsink, N. G. A. Willemen, J. Leijten, R. Bansal and S. R. Shin, Micromachines, 2020, 11, 849 CrossRef PubMed.
- P. Moura Rosa, N. Gopalakrishnan, H. Ibrahim, M. Haug and Ø. Halaas, Lab Chip, 2016, 16, 3728–3740 RSC.
- Y.-s. Torisawa, C. S. Spina, T. Mammoto, A. Mammoto, J. C. Weaver, T. Tat, J. J. Collins and D. E. Ingber, Nat. Methods, 2014, 11, 663–669 CrossRef CAS PubMed.
- L. G. Rigat-Brugarolas, A. Elizalde-Torrent, M. Bernabeu, M. De Niz, L. Martin-Jaular, C. Fernandez-Becerra, A. Homs-Corbera, J. Samitier and H. A. del Portillo, Lab Chip, 2014, 14, 1715–1724 RSC.
- S. Shim, M. C. Belanger, A. R. Harris, J. M. Munson and R. R. Pompano, Lab Chip, 2019, 19, 1013–1026 RSC.
- L. Businaro, A. De Ninno, G. Schiavoni, V. Lucarini, G. Ciasca, A. Gerardino, F. Belardelli, L. Gabriele and F. Mattei, Lab Chip, 2013, 13, 229–239 RSC.
- J. Hübner, M. Raschke, I. Rütschle, S. Gräßle, T. Hasenberg, K. Schirrmann, A. Lorenz, S. Schnurre, R. Lauster, I. Maschmeyer, T. Steger-Hartmann and U. Marx, Sci. Rep., 2018, 8, 15010 CrossRef PubMed.
- C. S. Teo, W. Hor Keong Tan, T. Lee and C.-H. Wang, Chem. Eng. Sci., 2005, 60, 4803–4821 CrossRef CAS.
- A. Müller, B. Homey, H. Soto, N. Ge, D. Catron, M. E. Buchanan, T. McClanahan, E. Murphy, W. Yuan, S. N. Wagner, J. L. Barrera, A. Mohar, E. Verástegui and A. Zlotnik, Nature, 2001, 410, 50–56 CrossRef PubMed.
- V. Estrella, T. Chen, M. Lloyd, J. Wojtkowiak, H. H. Cornnell, A. Ibrahim-Hashim, K. Bailey, Y. Balagurunathan, J. M. Rothberg, B. F. Sloane, J. Johnson, R. A. Gatenby and R. J. Gillies, Cancer Res., 2013, 73, 1524–1535 CrossRef CAS PubMed.
- H. Zhong, K. Chiles, D. Feldser, E. Laughner, C. Hanrahan, M. M. Georgescu, J. W. Simons and G. L. Semenza, Cancer Res., 2000, 60, 1541–1545 CAS.
- M. L. Pinzón-Daza, Y. Cuellar-Saenz, F. Nualart, A. Ondo-Mendez, L. Del Riesgo, F. Castillo-Rivera and R. Garzón, J. Cell. Biochem., 2017, 118, 1868–1878 CrossRef PubMed.
- J. D. Shields, M. E. Fleury, C. Yong, A. A. Tomei, G. J. Randolph and M. A. Swartz, Cancer Cell, 2007, 11, 526–538 CrossRef CAS PubMed.
- J. M. Munson, R. V. Bellamkonda and M. A. Swartz, Cancer Res., 2013, 73, 1536–1546 CrossRef CAS PubMed.
- F. Font-Clos, S. Zapperi and C. A. M. La Porta, iScience, 2020, 23, 101073 CrossRef PubMed.
- C. L. Avvisato, X. Yang, S. Shah, B. Hoxter, W. Li, R. Gaynor, R. Pestell, A. Tozeren and S. W. Byers, J. Cell Sci., 2007, 120, 2672–2682 CrossRef CAS PubMed.
- S. F. Lam, K. W. Bishop, R. Mintz, L. Fang and S. Achilefu, Sci. Rep., 2021, 11, 9246 CrossRef CAS PubMed.
- G. Khanal, S. Hiemstra and D. Pappas, Analyst, 2014, 139, 3274–3280 RSC.
- M. A. Acosta, X. Jiang, P.-K. Huang, K. B. Cutler, C. S. Grant, G. M. Walker and M. P. Gamcsik, Biomicrofluidics, 2014, 8, 054117 CrossRef PubMed.
- S. M. Grist, S. S. Nasseri, L. Laplatine, J. C. Schmok, D. Yao, J. Hua, L. Chrostowski and K. C. Cheung, Sci. Rep., 2019, 9, 17782 CrossRef PubMed.
- W. Wang, L. Li, M. Ding, G. Luo and Q. Liang, BioChip J., 2018, 12, 93–101 CrossRef CAS.
- W. Sun, Y. Chen, Y. Wang, P. Luo, M. Zhang, H. Zhang and P. Hu, Analyst, 2018, 143, 5431–5437 RSC.
- Y.-A. Chen, A. D. King, H.-C. Shih, C.-C. Peng, C.-Y. Wu, W.-H. Liao and Y.-C. Tung, Lab Chip, 2011, 11, 3626–3633 RSC.
- K. Funamoto, I. K. Zervantonakis, Y. Liu, C. J. Ochs, C. Kim and R. D. Kamm, Lab Chip, 2012, 12, 4855–4863 RSC.
- V. Palacio-Castañeda, L. Kooijman, B. Venzac, W. P. R. Verdurmen and S. Le Gac, Micromachines, 2020, 11, 382 CrossRef PubMed.
- D. M. Lewis, M. R. Blatchley, K. M. Park and S. Gerecht, Nat. Protoc., 2017, 12, 1620–1638 CrossRef CAS PubMed.
- D. M. Lewis, V. Tang, N. Jain, A. Isser, Z. Xia and S. Gerecht, ACS Biomater. Sci. Eng., 2018, 4, 400–409 CrossRef CAS PubMed.
- J. Kong, Y. Luo, D. Jin, F. An, W. Zhang, L. Liu, J. Li, S. Fang, X. Li, X. Yang, B. Lin and T. Liu, Onco Targets Ther, 2016, 7, 78421–78432 Search PubMed.
- S. Kheiri, Z. Chen, I. Yakavets, F. Rakhshani, E. W. K. Young and E. Kumacheva, Biotechnol. J., 2023, 18, 2200621 CrossRef CAS PubMed.
- Y. I. Wang and M. L. Shuler, Lab Chip, 2018, 18, 2563–2574 RSC.
- D. Huh, H. J. Kim, J. P. Fraser, D. E. Shea, M. Khan, A. Bahinski, G. A. Hamilton and D. E. Ingber, Nat. Protoc., 2013, 8, 2135–2157 CrossRef CAS PubMed.
- C. Strelez, S. Chilakala, K. Ghaffarian, R. Lau, E. Spiller, N. Ung, D. Hixon, A. Y. Yoon, R. X. Sun, H.-J. Lenz, J. E. Katz and S. M. Mumenthaler, iScience, 2021, 24, 102509 CrossRef CAS PubMed.
- M. Ao, B. M. Brewer, L. Yang, O. E. Franco Coronel, S. W. Hayward, D. J. Webb and D. Li, Sci. Rep., 2015, 5, 8334 CrossRef CAS PubMed.
- A. Sin, K. C. Chin, M. F. Jamil, Y. Kostov, G. Rao and M. L. Shuler, Biotechnol. Prog., 2004, 20, 338–345 CrossRef CAS PubMed.
- S. Matsumoto, A. R. Safitri, M. Danoy, T. Maekawa, H. Kinoshita, M. Shinohara, Y. Sakai, T. Fujii and E. Leclerc, Biotechnol. Prog., 2019, 35, e2854 CrossRef CAS PubMed.
- R. E. H. Karsten, K. Gier, J.-P. S. H. Mulder, M. Grajewski, P. Olinga and E. Verpoorte, Anal. Chem., 2024, 96, 15871–15879 CrossRef CAS PubMed.
- J. H. Sung, J.-r. Choi, D. Kim and M. L. Shuler, Biotechnol. Bioeng., 2009, 104, 516–525 CrossRef CAS PubMed.
- M. A. U. Khalid, Y. S. Kim, M. Ali, B. G. Lee, Y.-J. Cho and K. H. Choi, Biochem. Eng. J., 2020, 155, 107469 CrossRef CAS.
- S. Fuchs, S. Johansson, A. Tjell, G. Werr, T. Mayr and M. Tenje, ACS Biomater. Sci. Eng., 2021, 7, 2926–2948 CrossRef CAS PubMed.
- C. Lou, H. Yang, Y. Hou, H. Huang, J. Qiu, C. Wang, Y. Sang, H. Liu and L. Han, Adv. Mater., 2024, 36, e2307051 CrossRef PubMed.
- P. M. Misun, J. Rothe, Y. R. F. Schmid, A. Hierlemann and O. Frey, Microsyst. Nanoeng., 2016, 2, 16022 CrossRef CAS PubMed.
- C. Oleaga, A. Lavado, A. Riu, S. Rothemund, C. A. Carmona-Moran, K. Persaud, A. Yurko, J. Lear, N. S. Narasimhan, C. J. Long, F. Sommerhage, L. R. Bridges, Y. Cai, C. Martin, M. T. Schnepper, A. Goswami, R. Note, J. Langer, S. Teissier, J. Cotovio and J. J. Hickman, Adv. Funct. Mater., 2019, 29, 1805792 CrossRef PubMed.
- J. Aleman, T. Kilic, L. S. Mille, S. R. Shin and Y. S. Zhang, Nat. Protoc., 2021, 16, 2564–2593 CrossRef CAS PubMed.
- J. Das, S. Gomis, J. B. Chen, H. Yousefi, S. Ahmed, A. Mahmud, W. Zhou, E. H. Sargent and S. O. Kelley, Nat. Chem., 2021, 13, 428–434 CrossRef CAS PubMed.
- Y. Wang, H. Duan, Y. Yalikun, S. Cheng and M. Li, Talanta, 2024, 266, 125026 CrossRef CAS PubMed.
- C. Wang, C.-H. Huang, Z. Gao, J. Shen, J. He, A. MacLachlan, C. Ma, Y. Chang, W. Yang, Y. Cai, Y. Lou, S. Dai, W. Chen, F. Li and P. Chen, ACS Sens., 2021, 6, 3308–3319 CrossRef CAS PubMed.
- J. Zhu, J. He, M. Verano, A. T. Brimmo, A. Glia, M. A. Qasaimeh, P. Chen, J. O. Aleman and W. Chen, Lab Chip, 2018, 18, 3550–3560 RSC.
- S. Lee, Y. Sun, Y. Cao and S. H. Kang, TrAC, Trends Anal. Chem., 2019, 117, 58–68 CrossRef CAS.
- X.-W. Liao, Q.-Y. Xu, Z. Tan, Y. Liu and C. Wang, Electroanalysis, 2022, 34, 923–936 CrossRef CAS.
- S. Ansaryan, Y.-C. Liu, X. Li, A. M. Economou, C. S. Eberhardt, C. Jandus and H. Altug, Nat. Biomed. Eng., 2023, 7, 943–958 CrossRef CAS PubMed.
- S. E. Lee, Q. Chen, R. Bhat, S. Petkiewicz, J. M. Smith, V. E. Ferry, A. L. Correia, A. P. Alivisatos and M. J. Bissell, Nano Lett., 2015, 15, 4564–4570 CrossRef CAS PubMed.
- Y. Park, B. Ryu, B. R. Oh, Y. Song, X. Liang and K. Kurabayashi, ACS Nano, 2017, 11, 5697–5705 CrossRef CAS PubMed.
- P. Chen, M. T. Chung, W. McHugh, R. Nidetz, Y. Li, J. Fu, T. T. Cornell, T. P. Shanley and K. Kurabayashi, ACS Nano, 2015, 9, 4173–4181 CrossRef CAS PubMed.
- Y. Song, P. Chen, M. T. Chung, R. Nidetz, Y. Park, Z. Liu, W. McHugh, T. T. Cornell, J. Fu and K. Kurabayashi, Nano Lett., 2017, 17, 2374–2380 CrossRef CAS PubMed.
- Y. Cai, J. Zhu, J. He, W. Yang, C. Ma, F. Xiong, F. Li, W. Chen and P. Chen, Adv. Healthcare Mater., 2019, 8, 1801478 CrossRef PubMed.
- B. Ma, X. Liu, Z. Zhang, C. Ma, R. Chand, S. Patwardhan, C. Wang, S. D. Thamphiwatana, P. Chen and W. Chen, Biosens. Bioelectron., 2023, 230, 115247 CrossRef CAS PubMed.
- D. C. Duffy, Lab Chip, 2023, 23, 818–847 RSC.
- Z. Gao, Y. Song, T. Y. Hsiao, J. He, C. Wang, J. Shen, A. MacLachlan, S. Dai, B. H. Singer, K. Kurabayashi and P. Chen, ACS Nano, 2021, 15, 18023–18036 CrossRef CAS PubMed.
- S.-H. Su, Y. Song, M. W. Newstead, T. Cai, M. Wu, A. Stephens, B. H. Singer and K. Kurabayashi, Small, 2021, 17, 2101743 CrossRef CAS PubMed.
- A. Seth, E. Mittal, J. Luan, S. Kolla, M. B. Mazer, H. Joshi, R. Gupta, P. Rathi, Z. Wang, J. J. Morrissey, J. D. Ernst, C. Portal-Celhay, S. C. Morley, J. A. Philips and S. Singamaneni, Cells Rep. Methods, 2022, 2, 100267 CrossRef CAS PubMed.
- D. E. Reynolds, M. Pan, J. Yang, G. Galanis, Y. H. Roh, R.-T. T. Morales, S. S. Kumar, S.-J. Heo, X. Xu, W. Guo and J. Ko, Adv. Sci., 2023, 10, 2303619 CrossRef CAS PubMed.
- A. M. Maley, P. M. Garden and D. R. Walt, ACS Sens., 2020, 5, 3037–3042 CrossRef CAS PubMed.
- C. Wu, T. J. Dougan and D. R. Walt, ACS Nano, 2022, 16, 1025–1035 CrossRef CAS PubMed.
- Y. Deng, Z. Bai and R. Fan, Nat. Rev. Bioeng., 2023, 1, 769–784 CrossRef CAS.
- K. H. Chen, A. N. Boettiger, J. R. Moffitt, S. Wang and X. Zhuang, Science, 2015, 348, aaa6090 CrossRef PubMed.
- R. Fang, A. Halpern, M. M. Rahman, Z. Huang, Z. Lei, S. J. Hell, C. Dulac and X. Zhuang, eLife, 2024, 12, RP90029 CrossRef PubMed.
- Y. Liu, M. DiStasio, G. Su, H. Asashima, A. Enninful, X. Qin, Y. Deng, J. Nam, F. Gao, P. Bordignon, M. Cassano, M. Tomayko, M. Xu, S. Halene, J. E. Craft, D. Hafler and R. Fan, Nat. Biotechnol., 2023, 41, 1405–1409 CrossRef CAS PubMed.
- Y. Liu, M. Yang, Y. Deng, G. Su, A. Enninful, C. C. Guo, T. Tebaldi, D. Zhang, D. Kim, Z. Bai, E. Norris, A. Pan, J. Li, Y. Xiao, S. Halene and R. Fan, Cell, 2020, 183, 1665–1681.e1618 CrossRef CAS PubMed.
- J. Wirth, N. Huber, K. Yin, S. Brood, S. Chang, C. P. Martinez-Jimenez and M. Meier, Nat. Commun., 2023, 14, 1523 CrossRef CAS PubMed.
- Y. Deng, M. Bartosovic, S. Ma, D. Zhang, P. Kukanja, Y. Xiao, G. Su, Y. Liu, X. Qin, G. B. Rosoklija, A. J. Dwork, J. J. Mann, M. L. Xu, S. Halene, J. E. Craft, K. W. Leong, M. Boldrini, G. Castelo-Branco and R. Fan, Nature, 2022, 609, 375–383 CrossRef CAS PubMed.
- C. Ma, Y. Peng, H. Li and W. Chen, Trends Pharmacol. Sci., 2021, 42, 119–133 CrossRef CAS PubMed.
- R. Prantil-Baun, R. Novak, D. Das, M. R. Somayaji, A. Przekwas and D. E. Ingber, Annu. Rev. Pharmacol. Toxicol., 2018, 58, 37–64 CrossRef CAS PubMed.
- K. J. Regehr, M. Domenech, J. T. Koepsel, K. C. Carver, S. J. Ellison-Zelski, W. L. Murphy, L. A. Schuler, E. T. Alarid and D. J. Beebe, Lab Chip, 2009, 9, 2132–2139 RSC.
- K. Domansky, D. C. Leslie, J. McKinney, J. P. Fraser, J. D. Sliz, T. Hamkins-Indik, G. A. Hamilton, A. Bahinski and D. E. Ingber, Lab Chip, 2013, 13, 3956–3964 RSC.
- K. Domansky, J. D. Sliz, N. Wen, C. Hinojosa, G. Thompson, J. P. Fraser, T. Hamkins-Indik, G. A. Hamilton, D. Levner and D. E. Ingber, Microfluid. Nanofluid., 2017, 21, 107 CrossRef.
- P. M. van Midwoud, A. Janse, M. T. Merema, G. M. Groothuis and E. Verpoorte, Anal. Chem., 2012, 84, 3938–3944 CrossRef CAS PubMed.
- K. A. Homan, D. B. Kolesky, M. A. Skylar-Scott, J. Herrmann, H. Obuobi, A. Moisan and J. A. Lewis, Sci. Rep., 2016, 6, 34845 CrossRef CAS PubMed.
- D. J. Guckenberger, T. E. de Groot, A. M. D. Wan, D. J. Beebe and E. W. K. Young, Lab Chip, 2015, 15, 2364–2378 RSC.
- T. D. Do, U. T. Pham, L. P. Nguyen, T. M. Nguyen, C. N. Bui, S. Oliver, P. Pham, T. Q. Tran, B. T. Hoang, M. T. H. Pham, D. T. N. Pham and D. T. Nguyen, Diagnostics, 2023, 13, 1394 CrossRef CAS PubMed.
- L. Li, Y. Chen, H. Wang, G. An, H. Wu and W. Huang, Lab Chip, 2021, 21, 3924–3932 RSC.
- T. Nguyen, S. H. Jung, M. S. Lee, T.-E. Park, S.-k. Ahn and J. H. Kang, Lab Chip, 2019, 19, 3706–3713 RSC.
- Y. Fan, D. T. Nguyen, Y. Akay, F. Xu and M. Akay, Sci. Rep., 2016, 6, 25062 CrossRef CAS PubMed.
- C. I. Rogers, J. V. Pagaduan, G. P. Nordin and A. T. Woolley, Anal. Chem., 2011, 83, 6418–6425 CrossRef CAS PubMed.
- M. V. Monteiro, Y. S. Zhang, V. M. Gaspar and J. F. Mano, Trends Biotechnol., 2022, 40, 432–447 CrossRef CAS PubMed.
- N. S. Bhise, V. Manoharan, S. Massa, A. Tamayol, M. Ghaderi, M. Miscuglio, Q. Lang, Y. Shrike Zhang, S. R. Shin, G. Calzone, N. Annabi, T. D. Shupe, C. E. Bishop, A. Atala, M. R. Dokmeci and A. Khademhosseini, Biofabrication, 2016, 8, 014101 CrossRef PubMed.
- H.-G. Yi, Y. H. Jeong, Y. Kim, Y.-J. Choi, H. E. Moon, S. H. Park, K. S. Kang, M. Bae, J. Jang, H. Youn, S. H. Paek and D.-W. Cho, Nat. Biomed. Eng., 2019, 3, 509–519 CrossRef CAS PubMed.
- S. Knowlton, B. Yenilmez and S. Tasoglu, Trends Biotechnol., 2016, 34, 685–688 CrossRef CAS PubMed.
- N. R. Wevers, R. van Vught, K. J. Wilschut, A. Nicolas, C. Chiang, H. L. Lanz, S. J. Trietsch, J. Joore and P. Vulto, Sci. Rep., 2016, 6, 38856 CrossRef CAS PubMed.
- P. Vulto, S. Podszun, P. Meyer, C. Hermann, A. Manz and G. A. Urban, Lab Chip, 2011, 11, 1596–1602 RSC.
- S. J. Trietsch, G. D. Israëls, J. Joore, T. Hankemeier and P. Vulto, Lab Chip, 2013, 13, 3548–3554 RSC.
- M. Jang, P. Neuzil, T. Volk, A. Manz and A. Kleber, Biomicrofluidics, 2015, 9, 034113 CrossRef PubMed.
- L. Y. Ozer, H. S. Fayed, J. Ericsson and A. Al Haj Zen, Front. Oncol., 2023, 13, 1269376 CrossRef CAS PubMed.
- M. Geyer, D. Schreyer, L. M. Gaul, S. Pfeffer, C. Pilarsky and K. Queiroz, Cell Death Discovery, 2023, 9, 20 CrossRef CAS PubMed.
- H. L. Lanz, A. Saleh, B. Kramer, J. Cairns, C. P. Ng, J. Yu, S. J. Trietsch, T. Hankemeier, J. Joore, P. Vulto, R. Weinshilboum and L. Wang, BMC Cancer, 2017, 17, 709 CrossRef PubMed.
- E. Cavarzerani, I. Caligiuri, M. Bartoletti, V. Canzonieri and F. Rizzolio, Front. Bioeng. Biotechnol., 2023, 11, 1135374 CrossRef PubMed.
- MIMETAS, Cancer Drug Discovery, https://www.mimetas.com/en/cancer-drug-discovery/ Search PubMed.
- C. J. Ochs, J. Kasuya, A. Pavesi and R. D. Kamm, Lab Chip, 2014, 14, 459–462 RSC.
- H. Kimura, H. Nakamura, T. Goto, W. Uchida, T. Uozumi, D. Nishizawa, K. Shinha, J. Sakagami and K. Doi, Lab Chip, 2024, 24, 408–421 RSC.
- A. Apostolou, R. A. Panchakshari, A. Banerjee, D. V. Manatakis, M. D. Paraskevopoulou, R. Luc, G. Abu-Ali, A. Dimitriou, C. Lucchesi, G. Kulkarni, T. I. Maulana, M. Kasendra, J. S. Kerns, B. Bleck, L. Ewart, E. S. Manolakos, G. A. Hamilton, C. Giallourakis and K. Karalis, Cell. Mol. Gastroenterol. Hepatol., 2021, 12, 1719–1741 CrossRef CAS PubMed.
- X. Shi, Y. Li, Q. Yuan, S. Tang, S. Guo, Y. Zhang, J. He, X. Zhang, M. Han, Z. Liu, Y. Zhu, S. Gao, H. Wang, X. Xu, K. Zheng, W. Jing, L. Chen, Y. Wang, G. Jin and D. Gao, Nat. Commun., 2022, 13, 2169 CrossRef CAS PubMed.
- S. Yin, Y. Yu, N. Wu, M. Zhuo, Y. Wang, Y. Niu, Y. Ni, F. Hu, C. Ding, H. Liu, X. Cheng, J. Peng, J. Li, Y. He, J. Li, J. Wang, H. Zhang, X. Zhai, B. Liu, Y. Wang, S. Yan, M. Chen, W. Li, J. Peng, F. Peng, R. Xi, B. Ye, L. Jiang and J. J. Xi, Cell Stem Cell, 2024, 31, 717–733.e718 CrossRef CAS PubMed.
- G. Weng, J. Tao, Y. Liu, J. Qiu, D. Su, R. Wang, W. Luo and T. Zhang, Cancer Lett., 2023, 572, 216353 CrossRef CAS PubMed.
- G. Vlachogiannis, S. Hedayat, A. Vatsiou, Y. Jamin, J. Fernández-Mateos, K. Khan, A. Lampis, K. Eason, I. Huntingford, R. Burke, M. Rata, D.-M. Koh, N. Tunariu, D. Collins, S. Hulkki-Wilson, C. Ragulan, I. Spiteri, S. Y. Moorcraft, I. Chau, S. Rao, D. Watkins, N. Fotiadis, M. Bali, M. Darvish-Damavandi, H. Lote, Z. Eltahir, E. C. Smyth, R. Begum, P. A. Clarke, J. C. Hahne, M. Dowsett, J. de Bono, P. Workman, A. Sadanandam, M. Fassan, O. J. Sansom, S. Eccles, N. Starling, C. Braconi, A. Sottoriva, S. P. Robinson, D. Cunningham and N. Valeri, Science, 2018, 359, 920–926 CrossRef CAS PubMed.
- S. Ding, C. Hsu, Z. Wang, N. R. Natesh, R. Millen, M. Negrete, N. Giroux, G. O. Rivera, A. Dohlman, S. Bose, T. Rotstein, K. Spiller, A. Yeung, Z. Sun, C. Jiang, R. Xi, B. Wilkin, P. M. Randon, I. Williamson, D. A. Nelson, D. Delubac, S. Oh, G. Rupprecht, J. Isaacs, J. Jia, C. Chen, J. P. Shen, S. Kopetz, S. McCall, A. Smith, N. Gjorevski, A. C. Walz, S. Antonia, E. Marrer-Berger, H. Clevers, D. Hsu and X. Shen, Cell Stem Cell, 2022, 29, 905–917.e906 CrossRef CAS PubMed.
- J. Li, J. Chen, H. Bai, H. Wang, S. Hao, Y. Ding, B. Peng, J. Zhang, L. Li and W. Huang, Research, 2022, 2022, 9869518 Search PubMed.
- C. C. Chiang, R. Anne, P. Chawla, R. M. Shaw, S. He, E. C. Rock, M. Zhou, J. Cheng, Y. N. Gong and Y. C. Chen, Lab Chip, 2024, 24, 3169–3182 RSC.
- N. Vora, P. Shekar, T. Hanulia, M. Esmail, A. Patra and I. Georgakoudi, Lab Chip, 2024, 24, 2237–2252 RSC.
- H. Hashemzadeh, S. Shojaeilangari, A. Allahverdi, M. Rothbauer, P. Ertl and H. Naderi-Manesh, Sci. Rep., 2021, 11, 9804 CrossRef CAS PubMed.
- A. Esteva, A. Robicquet, B. Ramsundar, V. Kuleshov, M. DePristo, K. Chou, C. Cui, G. Corrado, S. Thrun and J. Dean, Nat. Med., 2019, 25, 24–29 CrossRef CAS PubMed.
- Y. J. Heo, D. Lee, J. Kang, K. Lee and W. K. Chung, Sci. Rep., 2017, 7, 11651 CrossRef PubMed.
- C. P. Tostado, L. X. Da Ong, J. J. W. Heng, C. Miccolis, S. Chia, J. J. W. Seow, Y.-C. Toh and R. DasGupta, Bioeng. Transl. Med., 2024, 9, e10628 CrossRef CAS PubMed.
- H. Hua, S. Zou, Z. Ma, W. Guo, C. Y. Fong and B. L. Khoo, Microsyst. Nanoeng., 2023, 9, 120 CrossRef CAS PubMed.
- BiotechNewswire, MIMETAS Opens Phenotypic Screening Center, https://www.biotechnewswire.ai/202105272228/mimetas-opens-phenotypic-screening-center.html Search PubMed.
- GlobeNewswire, Quris-AI Acquires Nortis, https://www.globenewswire.com/news-release/2024/10/29/2970665/0/en/Quris-AI-Acquires-Nortis.html Search PubMed.
- A. L. S. Cruz, E. d. A. Barreto, N. P. B. Fazolini, J. P. B. Viola and P. T. Bozza, Cell Death Dis., 2020, 11, 105 CrossRef PubMed.
- S. J. Hong, J. U. Hou, M. J. Chung, S. H. Kang, B. S. Shim, S. L. Lee, D. H. Park, A. Choi, J. Y. Oh, K. J. Lee, E. Shin, E. Cho and S. W. Park, Comput. Methods Programs Biomed., 2024, 246, 108041 CrossRef PubMed.
- A. Partin, T. S. Brettin, Y. Zhu, O. Narykov, A. Clyde, J. Overbeek and R. L. Stevens, Front. Med., 2023, 10, 1086097 CrossRef PubMed.
- R. Li, L. Li, Y. Xu and J. Yang, Briefings Bioinf., 2022, 23, bbab460 CrossRef PubMed.
- J. H. Sung, Y. I. Wang, N. Narasimhan Sriram, M. Jackson, C. Long, J. J. Hickman and M. L. Shuler, Anal. Chem., 2019, 91, 330–351 CrossRef CAS PubMed.
- A. Korkut, W. Wang, E. Demir, B. A. Aksoy, X. Jing, E. J. Molinelli, Ö. Babur, D. L. Bemis, S. Onur Sumer, D. B. Solit, C. A. Pratilas and C. Sander, eLife, 2015, 4, e04640 CrossRef PubMed.
- F. Eduati, V. Doldàn-Martelli, B. Klinger, T. Cokelaer, A. Sieber, F. Kogera, M. Dorel, M. J. Garnett, N. Blüthgen and J. Saez-Rodriguez, Cancer Res., 2017, 77, 3364–3375 CrossRef CAS PubMed.
- D. Alemani, F. Pappalardo, M. Pennisi, S. Motta and V. Brusic, J. Immunol. Methods, 2012, 376, 55–68 CrossRef CAS PubMed.
- Y. Wu, Y. Lu, W. Chen, J. Fu and R. Fan, PLoS Comput. Biol., 2012, 8, e1002355 CrossRef CAS PubMed.
- A. O. Pisco, A. Brock, J. Zhou, A. Moor, M. Mojtahedi, D. Jackson and S. Huang, Nat. Commun., 2013, 4, 2467 CrossRef PubMed.
- P. Sahoo, X. Yang, D. Abler, D. Maestrini, V. Adhikarla, D. Frankhouser, H. Cho, V. Machuca, D. Wang, M. Barish, M. Gutova, S. Branciamore, C. E. Brown and R. C. Rockne, J. R. Soc. Interface, 2020, 17, 20190734 CrossRef CAS PubMed.
- L. Liu, C. Ma, Z. Zhang, M. T. Witkowski, I. Aifantis, S. Ghassemi and W. Chen, J. Immunother. Cancer, 2022, 10, e005360 CrossRef PubMed.
- A. Mueller-Schoell, N. Puebla-Osorio, R. Michelet, M. R. Green, A. Künkele, W. Huisinga, P. Strati, B. Chasen, S. S. Neelapu, C. Yee and C. Kloft, Cancers, 2021, 13, 2782 CrossRef CAS PubMed.
- Z. Zhang, L. Liu, C. Ma and W. Chen, Adv. Ther., 2022, 5, 2200130 CrossRef CAS PubMed.
- S. H. Blumenrath, B. Y. Lee, L. Low, R. Prithviraj and D. Tagle, Exp. Biol. Med., 2020, 245, 1155–1162 CrossRef CAS PubMed.
- A. K. Capulli, K. Tian, N. Mehandru, A. Bukhta, S. F. Choudhury, M. Suchyta and K. K. Parker, Lab Chip, 2014, 14, 3181–3186 RSC.
- G. Wang, M. L. McCain, L. Yang, A. He, F. S. Pasqualini, A. Agarwal, H. Yuan, D. Jiang, D. Zhang, L. Zangi, J. Geva, A. E. Roberts, Q. Ma, J. Ding, J. Chen, D. Z. Wang, K. Li, J. Wang, R. J. Wanders, W. Kulik, F. M. Vaz, M. A. Laflamme, C. E. Murry, K. R. Chien, R. I. Kelley, G. M. Church, K. K. Parker and W. T. Pu, Nat. Med., 2014, 20, 616–623 CrossRef CAS PubMed.
- J. Ribas, Y. S. Zhang, P. R. Pitrez, J. Leijten, M. Miscuglio, J. Rouwkema, M. R. Dokmeci, X. Nissan, L. Ferreira and A. Khademhosseini, Small, 2017, 13, 1603737 CrossRef PubMed.
- A. Virlogeux, E. Moutaux, W. Christaller, A. Genoux, J. Bruyère, E. Fino, B. Charlot, M. Cazorla and F. Saudou, Cell Rep., 2018, 22, 110–122 CrossRef CAS PubMed.
- J. Ayensa-Jiménez, M. Pérez-Aliacar, T. Randelovic, S. Oliván, L. Fernández, J. A. Sanz-Herrera, I. Ochoa, M. H. Doweidar and M. Doblaré, Sci. Rep., 2020, 10, 21193 CrossRef PubMed.
- S. Lee, J. H. Kim, S. J. Kang, I. H. Chang and J. Y. Park, BioChip J., 2022, 16, 67–81 CrossRef CAS.
- J. Grenier, B. David, C. Journé, I. Cicha, D. Letourneur and H. Duval, Bioengineering, 2023, 10, 849 CrossRef CAS PubMed.
- M.-C. Kim, Z. Wang, R. H. W. Lam and T. Thorsen, J. Appl. Phys., 2008, 103, 044701 CrossRef.
- N. Rousset, F. Monet and T. Gervais, Sci. Rep., 2017, 7, 245 CrossRef PubMed.
- H. Kazempour, F. Teymouri, M. Khatami and S. N. Hosseini, J. Photochem. Photobiol., B, 2024, 258, 112960 CrossRef CAS PubMed.
- M. A. Hajari, S. Baheri Islami and X. Chen, Biomech. Model. Mechanobiol., 2021, 20, 983–1002 CrossRef PubMed.
- B. Meibohm and H. Derendorf, Int. J. Clin. Pharmacol. Ther., 1997, 35, 401–413 CAS.
- Y. Yang, Y. Chen, L. Wang, S. Xu, G. Fang, X. Guo, Z. Chen and Z. Gu, Front. Bioeng. Biotechnol., 2022, 10, 900481 CrossRef PubMed.
- M. Keuper-Navis, M. Walles, B. Poller, A. Myszczyszyn, T. K. van der Made, J. Donkers, H. Eslami Amirabadi, M. J. Wilmer, S. Aan, B. Spee, R. Masereeuw and E. van de Steeg, Pharmacol. Res., 2023, 195, 106853 CrossRef CAS PubMed.
- A. Przekwas and M. R. Somayaji, in Organ-on-a-chip, ed. J. Hoeng, D. Bovard and M. C. Peitsch, Academic Press, 2020, pp. 311–361, DOI:10.1016/B978-0-12-817202-5.00011-5.
- N. Tsamandouras, W. L. K. Chen, C. D. Edington, C. L. Stokes, L. G. Griffith and M. Cirit, AAPS J., 2017, 19, 1499–1512 CrossRef CAS PubMed.
- C. D. Edington, W. L. K. Chen, E. Geishecker, T. Kassis, L. R. Soenksen, B. M. Bhushan, D. Freake, J. Kirschner, C. Maass, N. Tsamandouras, J. Valdez, C. D. Cook, T. Parent, S. Snyder, J. Yu, E. Suter, M. Shockley, J. Velazquez, J. J. Velazquez, L. Stockdale, J. P. Papps, I. Lee, N. Vann, M. Gamboa, M. E. LaBarge, Z. Zhong, X. Wang, L. A. Boyer, D. A. Lauffenburger, R. L. Carrier, C. Communal, S. R. Tannenbaum, C. L. Stokes, D. J. Hughes, G. Rohatgi, D. L. Trumper, M. Cirit and L. G. Griffith, Sci. Rep., 2018, 8, 4530 CrossRef PubMed.
- K. Shinha, W. Nihei, T. Ono, R. Nakazato and H. Kimura, Biomicrofluidics, 2020, 14, 044108 CrossRef CAS PubMed.
- C. L. Stokes, M. Cirit and D. A. Lauffenburger, CPT: Pharmacometrics Syst. Pharmacol., 2015, 4, 559–562 CAS.
- S. B. Gorzalczany and A. G. Rodriguez Basso, Pharmacol. Res. Perspect., 2021, 9, e00863 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |





