Halochromism of rosolic acid: a pH-sensitive colorimetric dye combined with a smartphone technique for quantification of DNA in molecular diagnostics†
Rajamanickam
Sivakumar
 ,
Seo Yeon
Park
,
Seung Kyun
Park
and
Nae Yoon
Lee
,
Seo Yeon
Park
,
Seung Kyun
Park
and
Nae Yoon
Lee
 *
*
Department of BioNano Technology, Gachon University, 1342 Seongnam-daero, Sujeong-gu, Seongnam-si, Gyeonggi-do 13120, Korea. E-mail: nylee@gachon.ac.kr
First published on 22nd May 2025
Abstract
Infectious foodborne pathogens are responsible for serious illnesses and socioeconomic losses worldwide, making early detection crucial to control excessive damage. Colorimetric loop-mediated isothermal amplification (LAMP) assays are a promising molecular detection technique that produces visually discernible color changes. However, factors such as color blindness, age, and sex can cause individuals to perceive colors differently, limiting the effectiveness of colorimetric assays. To address this, quantitative chromatic analysis was employed to reliably distinguish colors and analyze their digital images. We developed a red-green-blue (RGB) channel-based method using a smartphone application (color picker) to quantify colorimetric LAMP amplicons. Two genes, hlyA of Listeria monocytogenes (L. monocytogenes) and esp of Enterococcus faecium (E. faecium), were investigated using LAMP, the most widely used isothermal amplification technique. In this study, the pH indicator “rosolic acid (RA)” was introduced to identify weakly buffered LAMP amplicons based on the colorimetric shift from red to yellow, and the color was quantified using the R/(R + G + B) computation. The limit of quantification was as low as 10 fg μL−1 for L. monocytogenes and 1 fg μL−1 for E. faecium. Furthermore, the sensitivity of the proposed method was comparable to that of the traditional LAMP assay, which uses phenol red as a pH indicator. The practical application of the RA-based colorimetric LAMP assay was demonstrated by detecting the blaOXA-23-like carbapenemase gene of A. baumannii in saliva. Thus, the RA-mediated colorimetric LAMP assay, combined with the RGB-based quantitative approach using a smartphone APP, holds significant potential in molecular diagnostics for detecting foodborne pathogens.
Introduction
Viral and bacterial pathogens transmitted through food pose major challenges to public health and the food industry. The World Health Organization estimates that over 600 million foodborne diseases and 0.42 million fatalities occur annually due to contaminated foods.1 Moreover, biological contamination of foods accounts for 75% of diarrheal illnesses, leading to around 3 million fatalities.2 According to the World Bank analysis, developing nations spend approximately 95 billion USD annually on treating foodborne diseases.3Listeria monocytogenes (L. monocytogenes), a Gram-positive bacterium commonly present in foods and the environment,4,5 is the cause of invasive listeriosis, a serious infection. The foodborne disease burden epidemiology reference group has confirmed 127 listeriosis outbreaks globally, with over 94% of cases requiring hospitalization and a fatality rate of 17%.6 Among harmful pathogens, L. monocytogenes stands out for its high fatality rate, underscoring the need for practical and cost-effective detection systems for controlling and preventing foodborne pathogens.7,8Pathogenic nucleic acids are crucial biomarkers that can be rapidly identified using molecular diagnostic methods compared to conventional culture-based techniques.9 Loop-mediated isothermal amplification (LAMP), a nucleic acid amplification technique, offers high sensitivity, specificity, simplicity, speed, and low cost.10 LAMP operates at isothermal temperatures (60–65 °C) and is less affected by inhibitors usually present in biological substances.11 While turbidimetry and fluorescence-based techniques are commonly used for real-time quantitative monitoring of the LAMP process,12,13 they are less ideal for point-of-care (POC) testing due to the limitations of turbidimeters and the cost of real-time fluorescence equipment. In addition, fluorescent LAMP often encounters significant background interference; similarly, polymerase chain reaction amplification is frequently impeded by an increase in fluorescent dye concentration. Thus, endpoint colorimetric LAMP has been examined using various chromogenic dyes, as naked-eye observation offers a unique and potentially efficient alternative to conventional detection methods.14–16
Indeed, significant quantities of hydrogen ions (H+) and pyrophosphate moieties were produced as byproducts of the weakly buffered LAMP reaction. The produced H+ lowers the initial alkaline pH (8.2–8.5) of the LAMP assay to an acidic range (6.5–7.0). This substantial pH shift produces distinct colors changes when pH-sensitive dyes are introduced, allowing efficient identification of the LAMP amplicon.17,18 Notably, only a limited number of pH-responsive dyes have been successfully involved in LAMP amplicon detection to date.16 Thus, the development of a new synthetic pH indicator capable of producing distinct color differences in the pH transition between 6.5 and 8.5 is in high demand. Rosolic acid (RA) an affordable, easy-to-handle, and highly stable pH-responsive dye, demonstrates different colors in response to pH variations and is widely used in food safety applications.19–21 For instance, RA-based testing techniques can detect excessive alkalinity in milk, a common form of adulteration.22 Therefore, RA was chosen in this study to examine its ability for colorimetric detection of foodborne pathogens based on pH differences. However, identifying color variations with the naked eye is inherently subjective, and readings can vary depending on individual color perception. Furthermore, variations in background color and ambient illumination further exacerbate visual subjectivity. Moreover, colorimetric LAMP assays offer only qualitative (yes/no) results, necessitating further calibration to quantify the exact concentrations of target samples.
Smartphones can be an efficient tool for fieldwork and testing in situations where conventional laboratory instruments are not feasible due to their portability, convenience, and widespread use.23,24 With high-megapixel cameras, smartphones can capture high-quality images that can be converted into numerical form using several customized smartphone applications (APPs) for quantitative analysis.17,25 Moreover, the internet connectivity of smartphones allows easy integration with other devices for remote monitoring and data sharing. Recently, there has been significant interest in employing smartphones to identify bacterial pathogens associated with foodborne diseases.26,27 For example, Nguyen et al. employed erichrome black T-dye for quantifying foodborne pathogens in real-time using a smartphone APP.28 Similarly, pH-sensitive polydiacetylene vesicles have been introduced for identifying antibiotic-resistant Staphylococcus aureus with a limit of detection (LOD) of 1.344 × 10−7 ng μL−1.29 Despite the potential of smartphone-based techniques to quantify low concentrations of infectious pathogens, only a few research groups have adopted this approach, and the number of published articles remains limited.
In this study, we identified foodborne pathogens using pH-responsive colorimetric LAMP in POC settings. In contrast to traditional real-time fluorescence-based detection techniques,30,31 this platform achieves highly sensitive, cost-effective, and quantitative molecular detection in real-time by combining smartphone-based analysis with pH-responsive colorimetric LAMP (Scheme 1). The distinctive color signal developed in this system was captured by a smartphone camera and analyzed using a customized smartphone APP “color picker.” Using a simple microtube for simultaneous amplification and detection of pathogens eliminates the possibility of contamination.
To date, the use of RA as a pH indicator in colorimetric LAMP amplicon detection combined with smartphone analysis has not been reported. As a proof-of-concept experiment, the proposed method was effectively utilized to identify the infectious pathogen A. baumannii in saliva samples.
Materials and methods
Designing of primers for weakly buffered LAMP
The PrimerExplorer V5 program was used to generate LAMP primers for hlyA and esp in L. monocytogenes and E. faecium. Primers were selected to target specific regions of the hlyA gene and consisted of one loop primer (LB, to speed up the reaction), a set of two inner primers (FIP (F1c and F2) and BIP (B1c and B2)), and outer primers (F3 and B3). The primer sequences are listed in Table S1.† The reaction mixture of 25 μL was prepared using 2× PHD LAMP premix (12.5 μL), primer mix (2.5 μL) [consisting of 10 μM of F3 (0.5 μL) and B3 (0.5 μL), 80 μM of FIP (0.5 μL) and BIP (0.5 μL), and 40 μM LB (0.5 μL) mixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio], target DNA (1 μL), and water (9 μL) to carry out the LAMP process in weakly buffered conditions. For the negative control, 1 μL of water was used in place of the target DNA. Amplification was performed at 65 °C for 45 min, and the amplified products were visualized via gel electrophoresis using 1.5% agarose. The experiments were repeated three times to verify the repeatability of the process. Weakly buffered LAMP assays for E. faecium and A. baumannii are shown in the Supplementary material (Table S2†). The real-time LAMP assay for L. monocytogenes is shown in the ESI† (Table S3).
1 ratio], target DNA (1 μL), and water (9 μL) to carry out the LAMP process in weakly buffered conditions. For the negative control, 1 μL of water was used in place of the target DNA. Amplification was performed at 65 °C for 45 min, and the amplified products were visualized via gel electrophoresis using 1.5% agarose. The experiments were repeated three times to verify the repeatability of the process. Weakly buffered LAMP assays for E. faecium and A. baumannii are shown in the Supplementary material (Table S2†). The real-time LAMP assay for L. monocytogenes is shown in the ESI† (Table S3).
Colorimetric detection of LAMP amplicons using a pH-responsive dye
Under acidic or alkaline conditions, the color of RA changes due to the halochromic properties (Fig. 1a). In acidic conditions, the lone pair electron on the hydroxyl group delocalizes (yellow color), whereas in alkaline conditions, RA undergoes deprotonation (red color). Based on this phenomenon, we introduced RA into a weakly buffered LAMP, where pH changes occur during the amplification process. This study introduced two colorimetric processes for detecting foodborne pathogens, in which RA served as a pH indicator (Fig. 1b). For end-point LAMP, 7.5 mM RA (1 μL) was mixed well with LAMP amplicon (9 μL) and kept at room temperature (RT) for a few seconds. For real-time LAMP, 7.5 mM RA (1 μL) was directly mixed with the LAMP assay (24 μL), which was heated at 65 °C for 45 min. A smartphone camera was used to capture the color variations, and a “color picker” APP analyzed the digital images. | ||
| Fig. 1 (a) Structure of RA under acidic and alkaline conditions. (b) Colorimetric detection processes performed in end-point LAMP and real-time LAMP using RA as a pH indicator. | ||
Performance of RA-based colorimetric LAMP
To determine the performance of the introduced method, serial dilutions were performed using ddH2O on the hlyA and esp genes in the range of 1 ng μL−1–0.1 fg μL−1. The lowest concentration of DNA that produced a significant color in the presence of RA, differing from the negative sample, was known as LOD. All the experiments were carried out three times to confirm the reproducibility of the process.Smartphone-based quantification of LAMP amplicons
A smartphone (iPhone 14 Pro) camera was used to capture color images of target DNA at various concentrations. An appropriate distance (~15 cm) was maintained between the visual object and the camera. After importing visual images of the target DNA into the “color picker” APP for image processing, the region of interest from the original image was cropped to obtain the average value of the red (R), green (G), and blue (B) channel intensities. The sensitivity of the introduced method was examined using the correlation between R/(R + G + B) and the diluted targets. The standard deviation (SD) of the blank samples and slope (b) of the calibration curve were used to measure the LOD, which was determined to be LOD = 3 SD/b. A model for data analysis was developed to quantitatively identify LAMP amplicons based on the relationship between R/RGB values and target concentrations. Relative standard deviation (RSD) of the experimental results were measured to assess the reproducibility of the process.Results and discussion
Optimization of ethanol and rosolic acid concentrations
Phenol red, neutral red, m-cresol purple, and phenolphthalein are commonly used synthetic dyes in pH monitoring applications.16,32 However, despite their unique color changes over a narrow pH range, RA is less known. RA, a triphenylmethane dye, is readily soluble in ethanol but not in aqueous medium. Thus, ethanol was used to dissolve RA (7.5 mM) at different concentrations (20–100%) to optimize LAMP amplicon detection. Due to insufficient dissolution, RA in 20% ethanol failed to produce color changes even though there were adequate pH variations between the positive and negative samples (Fig. S1†). In contrast, concentrations of 40–100% ethanol successfully identified the LAMP amplicons by the naked eye (Fig. 2a). Based on these results, 100% ethanol was chosen for dissolving RA in subsequent experiments since it showed the most color variation (Fig. 2b). Given that the technique for identifying LAMP amplicons is pH-dependent, determining the concentration of RA is crucial. To address this, 100% ethanol was used to prepare various concentrations of RA (1.25–10 mM), which produced noticeable color changes in the presence or absence of LAMP amplicons (Fig. 2c). Although visual differences were observed by the naked eye, ImageJ analysis revealed varying color intensities between the concentrations (Fig. 2d). Notably, 7.5 mM RA produced the highest contrast between the positive and negative samples, making it the optimal concentration for further experiments. Moreover, the optimization of the ethanol and RA concentrations for the colorimetric detection of the LAMP amplicon is shown in the flowchart (Fig. S2†). | ||
| Fig. 2 Optimization of ethanol and RA concentrations confirmed by (a and c) colorimetry and (b and d) grayscale analyses for the quantification of color intensities. | ||
Sensitivity test
To demonstrate this unique colorimetric readout approach using RA, a pH indicator never before used for the detection of LAMP amplicons, we evaluated its sensitivity. During amplification, dNTPs incorporated into the target DNA release pyrophosphate and H+ as byproducts. Indeed, the production of H+ can potentially lower the initial pH of the LAMP assay. To determine the lowest detectable sensitivity and investigate the effect of RA in monitoring LAMP amplicons, we performed LAMP reactions using 10-fold serial dilutions of L. monocytogenes as a template (Fig. 3).In the RA-based technique, samples containing more than 10 fg μL−1 of L. monocytogenes displayed a yellow color, indicating successful amplification (Fig. 3a). Similar results were confirmed by agarose gel electrophoresis (Fig. 3b). However, with respect to detection time, RA-based colorimetric detection allows easier visual distinction between positive and negative samples compared to agarose gel electrophoresis. Moreover, the RA-based method is a better choice than the agarose gel electrophoresis method in terms of simplicity and applicability because it does not require a UV illuminator for detection. Grayscale analysis was performed to measure the color intensities, and three successive trials were conducted to verify the reliability of the RA-based LAMP method. The color intensities of the 1 fg μL−1L. monocytogenes and negative samples were almost identical (Fig. 3c). The LOD for L. monocytogenes was determined to be 10 fg μL−1 using the pH-responsive RA-based visual detection technique.
Recently, functionalized gold nanoparticle probes, nanozyme strips, horseradish peroxidase-mimicking molecular beacons, and G-quadruplex DNAzyme-based methods combined with LAMP have been extensively utilized for the colorimetric detection of L. monocytogenes.33–36 Although these techniques are essential for monitoring L. monocytogenes, they have many limitations, such as low sensitivity, high cost, and labor-intensive processes. In contrast, the RA-based pH-dependent approach allowed for the rapid identification of positive and negative samples based on distinct color variations. Furthermore, the colorimetric chemical utilized in the detection process is highly stable and affordable, making it a promising tool for applications in medical diagnostics and food safety, for the detection of L. monocytogenes. A comparison between pH-dependent RA-based colorimetry and other relevant techniques for identifying L. monocytogenes is shown in Table S4.† Moreover, the introduced method efficiently detected another foodborne pathogen, E. faecium. Initially, high concentrations of E. faecium (1 ng μL−1) were serially diluted down to 0.1 fg μL−1. Notably, both agarose gel electrophoresis and the RA-based techniques detected E. faecium at concentrations as low as 1 fg μL−1 (Fig. S3a–c†).
Quantification of DNA concentration
Colorimetric indicators are preferred over turbidity measurements for interpreting nucleic acid amplification results. Although the human eye can detect a wide range of color combinations with perfect sensitivity, color variations in individual perception, background settings, lighting, and low volume of samples can complicate color interpretation. Thus, we introduced a smartphone APP for quantifying the color variations developed in the LAMP process when RA was used as a pH indicator.To verify the proposed approach experimentally, LAMP reactions were performed for L. monocytogenes followed by the addition of the colorimetric dye RA, and the results were imaged using an iPhone 14 Pro camera. Moreover, the colors produced in the microtubes were captured on a white light background to prevent the influence of external circumstances. The captured images were imported and subjected to quantitative analysis using the “color picker” APP. Recently, the RGB-based method has been widely used among various color models for the quantitative analysis of visual readouts.37 The intensity of the color was gradually altered between high and low concentrations of L. monocytogenes (Fig. 3a), and their RGB values were measured using a “color picker” APP (Table S5†). After collecting the RGB values of each concentration, many calculations were performed to achieve good linearity (R2 value ≥ 0.95). Fig. 4a–c shows plots of the three measured ratios (R/RGB, G/RGB, and B/RGB) against the concentrations of L. monocytogenes. Based on the results, R/RGB against the concentration of target DNA fit best, producing a good linear correlation between 1 ng μL−1 and 10 fg μL−1 (y = ax + b; “a” represents slope; “x” represents the concentration of DNA; “b” represents intercept, adjusted R2 = 0.97335). Thus, the limit of quantification (LOQ) and LOD (3SD/b) were found to be 10 fg μL−1 and 0.4 fg μL−1, respectively, using the color picker APP. Notably, the degree of color development in the DNA-containing solution was directly proportional to its concentration. In contrast, there was poor linearity in the concentration range of 1 ng μL−1 to 10 fg μL−1 for both the G/RGB (R2 = 0.53825) and B/RGB (R2 = 0.50168) ratios. These observations confirmed that the R/RGB ratio is more appropriate for quantifying DNA employing this method. Furthermore, the reproducibility of the RA-based colorimetry was evaluated (Table S6†). According to the results, the RSD of the five different concentrations ranged from 1.9 to 4.25%, which is an acceptable threshold; thus, the introduced method could be reproducible. Significantly, the color images were captured by different smartphones (Samsung S25 and OPPO F15) and various megapixel (MP) cameras. The results showed no obvious difference in their R2 value and produced good linearity (Fig. S4 and S5†). Therefore, the introduced approach provided reliable and comparable results for DNA quantification based on the R/RGB ratio using various smartphones with megapixel cameras.
 | ||
| Fig. 4 Quantification of L. monocytogenes and E. faecium based on (a and d) R/RGB, (b and e) G/RGB, and (c and f) B/RGB ratios using the “color picker” APP. | ||
Using the same approach, the concentration of E. faecium was also quantified (Fig. 4d–f). Due to the digital interpretation of the visual change, the smartphone APP-based image processing technique allowed for the accurate quantification of LAMP amplicon concentration.
Real-time LAMP
Real-time LAMP was performed to assess the possibility of cross-contamination and on-site detection. The main advantage of RA is that it does not interfere with amplification at certain concentrations, allowing it to be added to microtubes together with LAMP reagents prior to amplification. Since RA is a pre-added colorimetric dye, the microtube remains unopened post-amplification, causing a minimal risk of contamination in the entire process. Significantly, 7.5 mM RA was prepared using various percentages of ethanol (20 to 100%), and real-time LAMP was performed (Fig. 5a). The results showed clear differentiation between positive and negative samples in all cases, except for RA in 20% ethanol. However, the positive sample appeared colorless for RA when 40% ethanol was used, making it the optimal concentration for the real-time LAMP process.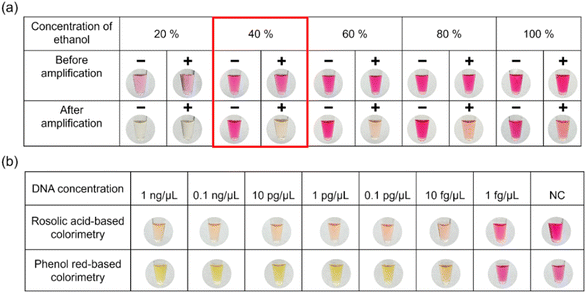 | ||
| Fig. 5 (a) Optimization of real-time LAMP using RA as a pH-responsive dye, and (b) comparison of the introduced method with the existing commercial method that employs phenol red as a pH indicator. | ||
Compared to SYBR Green and other dyes, RA is affordable and easier to use in outdoor settings. Traditional PCR can amplify and detect a single copy of DNA in 2 h,38 while real-time LAMP drastically minimizes the overall detection process to 45 min as compared to traditional PCR, leading to a 62.5% improvement in efficiency. The introduced real-time LAMP assay is more effective than traditional techniques because it provides results rapidly without needing a thermocycler or gel electrophoresis for amplification and result interpretation. The sensitivity of the RA-based real-time LAMP assay demonstrated comparable results to those of the commercial method that uses phenol red as a pH indicator. The color variation for L. monocytogenes was detectable by the naked eye at concentrations ranging from 1 ng μL−1 to 10 fg μL−1 (Fig. 5b). This highlights the suitability of the weakly buffered LAMP assay for field detection of food pathogens using RA as a pH indicator.
Assessment of specificity and interference study
The key function of primer design in the proposed LAMP assay ensures high selectivity in amplifying the hlyA and esp genes, preventing false positives and negative results. Agarose gel electrophoresis and colorimetry experiments revealed that the RA-based LAMP assay is species-specific for foodborne pathogens (Fig. 6a–c). Moreover, hlyA and esp were effectively amplified even in the presence of other pathogens and no cross-reactivity was observed (Fig. 6d). These results demonstrated that nonspecific amplification was avoided using the designed primers, preventing the formation of typical “dumbbell” structures in non-target DNA sequences (Fig. 6d). Thus, this LAMP assay showed 100% selectivity towards L. monocytogenes and E. faecium.Applicability of the RA-based colorimetric LAMP assay
A. baumannii is an antibiotic-resistant bacterium that causes hospital-acquired infections. To evaluate the versatile behavior and application viewpoint of the introduced method, we carried out a real sample analysis to detect the Gram-negative bacterium A. baumannii in saliva. Initially, A. baumannii (bla OXA-23-like carbapenemase gene) stored on an FTA card was used for LAMP at 65 °C for 45 min. The LAMP amplicon was detected within a few seconds using the RA-based colorimetric strategy, demonstrating the effectiveness of the proposed method (Fig. 7a and b).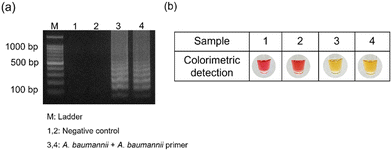 | ||
| Fig. 7 Detection of A. baumannii in saliva based on (a) agarose gel electrophoresis and (b) RA-based colorimetry method. | ||
Conclusions
In this study, we developed a smartphone-assisted methodology that incorporates a colorimetric LAMP assay with a smartphone APP for the quantification of infectious pathogens on a POC testing platform. RA was introduced for the first time to distinguish between positive and negative samples based on its distinct halochromic properties. The RA concentration and volume were optimized through various experiments, and an unmodified smartphone camera was used to capture images of the visual readouts. A “color picker” APP converted the color information into digital form for RGB analysis, which was used for quantification. Significantly, the R/(R + G + B) value successfully detected L. monocytogenes and E. faecium with a LOQ of 10 fg μL−1 and 1 fg μL−1, respectively. The introduced RA-based colorimetric LAMP test demonstrated 100% selectivity for identifying L. monocytogenes and E. faecium, respectively, and no cross-reactivity was observed with other infectious pathogens. Importantly, colorimetric real-time monitoring of infectious microorganisms was achieved with satisfactory results using the RA-based LAMP assay. In the real-time LAMP, RA was added prior to the reaction, eliminating the need to open the lid post-amplification for the colorimetry process, thereby reducing the risk of contamination. Significantly, the presented approach could eventually be commercialized since the real-time LAMP employing RA as a pH indicator achieved comparable sensitivity with a readily available pH-sensing LAMP kit (NanoHelix Co., Ltd, South Korea).In summary, with the advent of smartphones, which avoid the need for expensive instruments, high-throughput analyses can be performed using this method. LAMP combined with a halochromic dye offers significant advantages, including high sensitivity, ease of use, and rapidity. This approach holds promise for broad applications in food safety, medicine, and environmental monitoring, particularly for detecting infectious pathogens.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Rajamanickam Sivakumar: writing – review & editing, writing – original draft, visualization, methodology, formal analysis, data curation, conceptualization. Seo Yeon Park: writing – review & editing, visualization, formal analysis, data curation. Seung Kyun Park: formal analysis, data curation. Nae Yoon Lee: writing – review & editing, supervision, project administration, funding acquisition, conceptualization.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2023-00208684) and also by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (No. RS-2021-NR060117). A multidrug-resistant bacterium, Acinetobacter baumannii, was generously provided by Professor Richard A. Stabler from the Department of Infection Biology, London School of Hygiene & Tropical Medicine, London, United Kingdom.References
- E. Todd, Int. J. Environ. Res. Public Health, 2020, 17, 5129 CrossRef PubMed.
- Y. Lu, J. Zhang, X. Lu and Q. Liu, Trends Food Sci. Technol., 2024, 148, 104482 CrossRef.
- D. Grace, Food Secur., 2023, 15, 1475–1488 CrossRef.
- I. Shakuntala, S. Das, S. Ghatak, A. A. P. Milton, R. Sanjukta, K. U. Puro, R. K. Pegu, A. Duarah, S. B. Barbuddhe and A. Sen, Food Biotechnol., 2019, 33, 237–250 Search PubMed.
- L. Zhang, R. Huang, W. Liu, H. Liu, X. Zhou and D. Xing, Biosens. Bioelectron., 2016, 86, 1–7 CrossRef PubMed.
- M. C. B. Prasad, A. A. P. Milton, V. K. Menon, K. Srinivas, D. Bhargavi, S. Das, S. Ghatak, S. L. Vineesha, B. Sunil, C. Latha and P. M. Priya, Food Control, 2024, 155, 110081 Search PubMed.
- J. Du, H. Singh, W. J. Dong, Y. H. Bai and T. H. Yi, Sens. Actuators, B, 2018, 265, 285–292 Search PubMed.
- X. Wu, Q. Chen, C. Yang, Q. Ning and Z. Liu, Food Control, 2022, 134, 108721 CrossRef.
- R. Sivakumar, V. P. Dinh and N. Y. Lee, ACS Sustainable Chem. Eng., 2023, 11, 2079–2088 CrossRef.
- A. S. Sharma and N. Y. Lee, Coord. Chem. Rev., 2024, 509, 215769 Search PubMed.
- G. Papadakis, A. K. Pantazis, N. Fikas, S. Chatziioannidou, V. Tsiakalou, K. Michaelidou, V. Pogka, M. Megariti, M. Vardaki, K. Giarentis and J. Heaney, Sci. Rep., 2022, 12, 3775 CrossRef PubMed.
- S. Wachiralurpan, T. Sriyapai, S. Areekit, P. Sriyapai, D. Thongphueak, S. Santiwatanakul and K. Chansiri, Anal. Methods, 2017, 9, 6403–6410 RSC.
- A. T. Scott, T. R. Layne, K. C. O'Connell, N. A. Tanner and J. P. Landers, Anal. Chem., 2020, 92, 13343–13353 CrossRef PubMed.
- S. J. Oh, B. H. Park, J. H. Jung, G. Choi, D. C. Lee and T. S. Seo, Biosens. Bioelectron., 2016, 75, 293–300 CrossRef CAS PubMed.
- W. Jaroenram, P. Cecere and P. P. Pompa, J. Microbiol. Methods, 2019, 156, 9–14 CrossRef CAS.
- N. A. Tanner, Y. Zhang and T. C. Evans Jr, BioTechniques, 2015, 58, 59–68 CrossRef CAS.
- J. Park, S. Woo, J. Kim, H. Lee, Y. E. Yoo and S. Hong, Theranostics, 2021, 11, 6735 CrossRef CAS.
- F. Zhang, C. Gao, L. Bai, Y. Chen, S. Liang, X. Lv, J. Sun and S. Wang, Food Chem.:X, 2022, 13, 100201 CAS.
- J. Chalitangkoon and P. Monvisade, Carbohydr. Polym., 2021, 260, 117836 CrossRef CAS.
- M. K. Morsy, K. Zór, N. Kostesha, T. S. Alstrøm, A. Heiskanen, H. El-Tanahi, A. Sharoba, D. Papkovsky, J. Larsen, H. Khalaf and M. H. Jakobsen, Food Control, 2016, 60, 346–352 CrossRef CAS.
- H. Xiao-wei, Z. Xiao-bo, S. Ji-yong, L. Zhi-hua and Z. Jie-wen, Trends Food Sci. Technol., 2018, 81, 90–107 CrossRef CAS.
- C. D. S. Gondim, P. P. B. D. Santos, R. C. D. Sousa, R. G. Junqueira and S. V. C. D. Souza, Quim. Nova, 2021, 44, 625–635 Search PubMed.
- R. Rajamanikandan, M. Ilanchelian and H. Ju, Chemosphere, 2023, 340, 139838 CrossRef CAS PubMed.
- K. Yin, V. Pandian, K. Kadimisetty, X. Zhang, C. Ruiz, K. Cooper and C. Liu, Sci. Rep., 2020, 10, 9009 CrossRef CAS PubMed.
- K. Fan, J. Zeng, C. Yang, G. Wang, K. Lian, X. Zhou, Y. Deng and G. Liu, ACS Sens., 2022, 7, 2049–2057 CrossRef PubMed.
- H. V. Nguyen, V. D. Nguyen, F. Liu and T. S. Seo, ACS Omega, 2020, 5, 22208–22214 CrossRef.
- X. Ding, M. G. Mauk, K. Yin, K. Kadimisetty and C. Liu, Anal. Chem., 2018, 91, 655–672 CrossRef.
- H. Q. Nguyen, V. D. Nguyen, H. Van Nguyen and T. S. Seo, Sci. Rep., 2020, 10, 15123 CrossRef PubMed.
- Q. Li, Z. An, T. Sun, S. Ji, W. Wang, Y. Peng, Z. Wang, G. I. Salentijn, Z. Gao and D. Han, Biosens. Bioelectron., 2023, 219, 114824 CrossRef PubMed.
- J. E. Kong, Q. Wei, D. Tseng, J. Zhang, E. Pan, M. Lewinski, O. B. Garner, A. Ozcan and D. Di Carlo, ACS Nano, 2017, 11, 2934–2943 CrossRef.
- W. Chen, H. Yu, F. Sun, A. Ornob, R. Brisbin, A. Ganguli, V. Vemuri, P. Strzebonski, G. Cui, K. J. Allen and S. A. Desai, Anal. Chem., 2017, 89, 11219–11226 CrossRef.
- S. Y. Park, D. S. Chae, J. S. Lee, B. K. Cho and N. Y. Lee, Biosensors, 2023, 13, 535 CrossRef.
- S. Wachiralurpan, T. Sriyapai, S. Areekit, P. Sriyapai, S. Augkarawaritsawong, S. Santiwatanakul and K. Chansiri, Front. Chem., 2018, 6, 90 CrossRef PubMed.
- J. E. Lee, S. A. Kim, H. Mun, S. R. Kim, K. S. Ha and W. B. A. Shim, Food Control, 2022, 133, 108569 CrossRef.
- D. Shi and H. Shi, LWT--Food Sci. Technol., 2022, 154, 112641 CrossRef.
- Z. Liu, C. Yao, Y. Wang and C. Yang, Anal. Methods, 2018, 10, 848–854 RSC.
- K. Kaarj, P. Akarapipad and J. Y. Yoon, Sci. Rep., 2018, 8, 12438 CrossRef PubMed.
- J. Q. Chen, S. Healey, P. Regan, P. Laksanalamai and Z. Hu, Food Sci. Hum. Wellness, 2017, 6, 39–59 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5lc00036j |
| This journal is © The Royal Society of Chemistry 2025 |



