 Open Access Article
Open Access ArticleActive implantable drug delivery systems: engineering factors, challenges, opportunities
Fabiana Del Bono
 *a,
Nicola Di Trani
*a,
Nicola Di Trani
 b,
Danilo Demarchi
b,
Danilo Demarchi
 a,
Alessandro Grattoni
a,
Alessandro Grattoni
 b and
Paolo Motto Ros
b and
Paolo Motto Ros
 a
a
aDepartment of Electronics and Telecommunications, Politecnico di Torino, Turin, TO, Italy. E-mail: fabiana.delbono@polito.it
bDepartment of Nanomedicine, Houston Methodist Research Institute, Houston, TX, USA
First published on 10th July 2025
Abstract
Implantable drug delivery systems represent a transformative approach in modern pharmacology, offering precise and controlled drug administration tailored to individual patient needs. By circumventing physiological barriers such as the gastrointestinal tract and the blood–brain barrier, these systems enhance bioavailability and therapeutic efficacy while reducing systemic side effects. Key features include sustained or on-demand drug release, remote activation, and programmable dosing, which collectively improve patient compliance and minimize the frequency of interventions. Innovations in actuation mechanisms, powering technologies, and biocompatible materials have advanced the field, enabling the development of miniaturized, energy-efficient, and scalable devices. Applications range from chronic disease management to localized therapies for neurological and cardiovascular conditions. Despite significant progress, challenges remain in integrating power systems, communication protocols, and regulatory compliance for clinical translation. This review synthesizes the current state of active implantable drug delivery systems, discussing engineering trade-offs, system requirements, and future research directions toward achieving reliable, patient-centered solutions to guide system designers toward developing reliable, scalable, and patient-centered solutions that bridge the gap between cutting-edge research and clinical application.
1 Introduction
In the realm of modern healthcare, the quest for advanced drug delivery systems (DDS) that can precisely administer therapeutic agents to targeted sites while ensuring optimal patient compliance – adherence to prescribed treatments and health-related advice1 – remains a crucial endeavor. Among these innovative approaches, implantable drug delivery systems (IDDS) have emerged as a promising solution. To grasp the landscape of drug delivery, it is essential to distill the main challenges faced by the field. Pharmacological treatments are an essential part of managing many diseases. The most prevalent chronic conditions necessitating pharmacological therapy encompass arthritis, hypertension, and diabetes.2 Additionally, concurrent chronic obstructive pulmonary disease and heart failure mandate pharmacological intervention.3 Chronic pain disorders requiring pharmacological therapy include neuropathic pain, low back pain, fibromyalgia, and osteoarthritis.4According to US government statistics, retail spending on prescription drugs in the United States constituted nearly 11% of total personal healthcare expenses in 2021.6 Over the years, US expenditure on prescription medications has experienced significant growth, rising from $30 billion in 1980 to $335 billion in 2018.7 Additionally, in 2019, the pharmaceutical sector in the United States saw a 5.4% increase in overall expenditures compared to the previous year, reaching a total of $507.9 billion.8 Prior to the COVID-19 pandemic, controlled drug delivery systems were a substantial market, generating annual revenues exceeding $100 billion,9 with the implantable drug delivery systems market identified as significant.10 When speaking of pharmacotreatments, medication adherence – the degree to which patients follow their prescribed medication regimens11 – significantly influences therapeutic outcomes, patient health, and healthcare costs, particularly in the management of chronic conditions. Patients with chronic conditions such as stroke, coronary heart disease, asthma, diabetes, hypercholesterolemia and rheumatoid arthritis frequently experience challenges in adhering to new medications and encounter difficulties in meeting their medication and information needs.12 Polypharmacy, inappropriate prescribing, medication non-adherence, and adverse drug reactions are prevalent issues in managing chronic conditions.13 Therapy adherence hovers around 50% in developed countries. Improving adherence with existing drugs could potentially have as significant an impact on outcomes as the introduction of new therapeutic agents,14 being fundamental for therapeutic outcomes15,16 and reducing risk of complications and hospitalization.17,18 While increased medication adherence may lead to higher drug spending,19 this is often counterbalanced by reductions in other healthcare costs such as hospitalizations and emergency department visits.18,20,21 Traditional routes of drug administration encompass a range of methods, offering distinct advantages and limitations. Enteral and parenteral routes are conventional and widely utilized, while topical and transdermal routes provide localized treatment options.22–24 However, these modalities may transiently induce toxicity states due to the necessity of administering quantified drug doses, leading to an initial surge in plasma concentration beyond the toxicity threshold, followed by transitions to the efficacy and ultimately inefficacy windows.25 Traditional drug administration routes face challenges in controlling drug release and sustaining constant plasma therapeutic concentrations over extended periods.26 Furthermore, they are hampered by biological barriers and off-target side effects.27 Factors such as slow and incomplete dissolution, formation of insoluble complexes, poor net permeability, and first-pass metabolism limit oral drug absorption,28 resulting in low bioavailability.29 Challenges posed by biological barriers like the blood–retinal and blood–brain barriers drive innovations aimed at augmenting drug efficacy while mitigating side effects and enhancing patient compliance. These advancements hold significant promise for enhancing treatment outcomes across a multitude of diseases.30–40
Several diseases can benefit from timing drug administration to align with biological rhythms.41 Chronotherapy is a therapeutic approach that aligns the timing of treatment with the body's natural biological rhythms, particularly the circadian rhythms, to enhance the efficacy and minimize the side effects of medications.42 Coupled with the attainment of specific release profiles, it presents a promising avenue for optimizing drug delivery strategies. Chronopharmaceutical research is identified as a critical need, with the goal of developing customizable drug delivery systems tailored for chronotherapy. Such systems have the potential to enhance safety, efficacy, and patient compliance.43 Pulsatile drug delivery systems are highlighted as particularly promising for chronotherapy, offering programmable drug release characterized by well-defined lag phases.41
Due to their significant social and economic impact, drug delivery systems that meet the aforementioned requirements are the subject of extensive research, involving diverse approaches and paradigms, widely investigated and reported in the literature.44 Examining the history and taxonomy of drug delivery devices reveals that these systems have undergone substantial evolution over time, following different approaches and paradigms.
The development of implantable systems represents a specific segment within this broader landscape. Drug delivery implants represent a response to the imperative of enhancing patient compliance while maintaining drug concentrations within the therapeutic window.9 Various authors have reported recent advances in the field, focusing on different strategies such as transdermal delivery,45 fully implantable non-biodegradable devices,46 desired profiles,25,47 drug storage,48,49 and application.50–52 The emergence of digital health,53 the widespread adoption of mHealth platforms with application in closed-loop treatments,54 and even the prospects of space medicine55 open up new horizons for the development and potential of these technologies. The rich scientific output evidences the diverse taxonomy of drug delivery implants, which can be categorized and analyzed from various perspectives, and ultimately reflects a shared effort in the literature and scientific endeavors to address specific therapeutic needs and challenges.
This review aims to offer a biomedical system engineering perspective in the specific field of fully implantable, active and externally controlled drug delivery devices, which to the best of our knowledge remains uncovered. These devices, hereby referred to as active implantable drug delivery systems (AIDDSs), represent a specialized subset of active implantable medical devices (AIMDs). An AIDDS is characterized by the presence of internal actuation or control mechanisms that actively regulate the release of a therapeutic agent. The term active refers to the fact that the device includes one or more energy-dependent subsystems that enable drug release to be modulated in a time-dependent, programmable, or stimulus-responsive manner. Unlike passive systems, which rely solely on natural diffusion, degradation, or osmotic gradients, active systems require energy input to trigger, sustain, or stop delivery functions. The designation system underscores the integration of multiple functional modules, including drug reservoirs, actuation units, control electronics, power sources, and often communication interfaces. As such, AIDDSs embody the convergence of pharmacological efficacy with engineered control, positioning them uniquely within the land-scape of implantable therapeutic technologies. As outlined in Fig. 1, AIDDSs exist at the intersection of active medical devices, implantable medical devices, and drug delivery systems, with the distinguishing feature of incorporating a therapeutic agent and thereby qualifying as combination products. The first AIDDS emerged in the 1970s with the peristaltic infusion pump for continuous subcutaneous drug delivery. It enabled programmable, sustained release under external control, offering benefits like precise dosing and improved patient compliance. However, due to its bulk and limited battery life, it was mainly used in end-of-life palliative care, where portability was less critical. Despite its limitations, it laid the groundwork for future fully implantable and remotely controlled systems. In recent decades, research on actuators for implantable drug delivery technology has led to the development of numerous innovations in AIDDSs. This field offers significant therapeutic and commercial opportunities, as evidenced by the widespread regulatory approvals from FDA for partially-implanted commercial devices like Vyalev, iLet, and Omnipod.56–58 The key milestones in the evolution of commercial AIDDSs are summarized in Fig. 2. However, many devices developed in basic research never reach the market. Additionally, a recent shift in focus has been observed, with increasing attention towards new transport mechanisms, formulations, and carriers for therapeutic agents.47,59 For different reasons, AIDDSs still represent a research target for achieving the aforementioned challenges. First of all, while emerging biotechnologies and biomaterials show significant promise, their clinical translation readiness is yet to be fully established.46 Active implantable drug delivery systems represent an alternative paradigm with respect to innovative formulations and carriers,60 with the advantage of a faster pathway to clinical translation, supported by a more favorable regulatory framework. Second, from a systems engineering perspective, the advantage of AIMDs for controlled drug delivery lies in the availability of many actuation technologies. The scalability of dimensions and process transferability enabled by the use of MEMS in recent decades61 has unlocked opportunities in this field with immense potential. While noteworthy and highly valid attempts have been made,62 they have garnered little momentum and remain far from reaching the market. Progress in powering technology and remote communication adds up to this, making it possible to have complete devices and eventually seamless integration into the mHealth technological paradigm at the cost of taking up the challenges on integration and porting to mass production.63 The objective of this article is to focus on the landscape of remotely controlled AIDDSs and to highlight the engineering milestones and challenges, to better clarify the opportunities offered by this drug delivery paradigm. This aims to provide system designers with guidance in navigating the landscape of AIDDSs, awareness of engineering trade-offs in designing such systems, and an understanding of what is needed to bring these devices to market. The article is thus structured as follows: section 2 discusses the essential engineering principles and design considerations, including system requirements, biocompatibility, and regulatory constraints. Section 3 reviews state-of-the-art technologies, categorizing solutions based on actuation mechanisms, powering methods, and communication strategies. Section 4 highlights fully developed systems from the literature, showcasing their applications, limitations, and potential for clinical translation. Section 5 synthesizes insights from the review, highlighting key opportunities for advancing implantable drug delivery systems. Section 5 discusses future trends and draws conclusions.
 | ||
| Fig. 1 Overview of medical and pharmaceutical systems. Active implantable drug delivery systems (AIDDSs) are a subset of active implantable medical devices (AIMDs), at the intersection of active medical devices, implantable medical devices and drug delivery systems. Created in BioRender.5 | ||
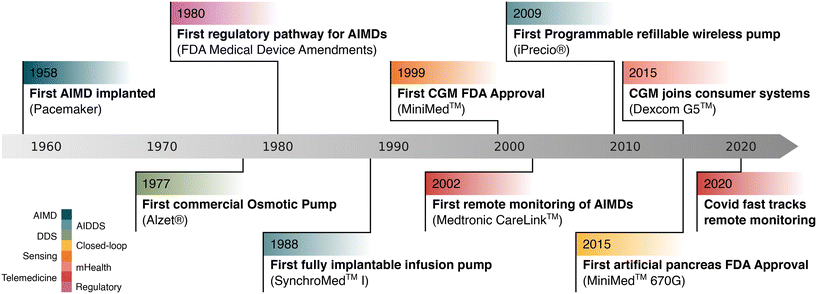 | ||
| Fig. 2 Key milestones in the development of AIDDSs, including foundational technologies and related systems that have contributed to their evolution. | ||
2 Engineering factors
Research in medical device technology, particularly drug delivery systems, typically begins with proof-of-concept studies and progresses toward clinical translation and market entry. The development of an AIMD for drug delivery must be addressed with a system engineering mindset, integrating comprehensive and systematic device design early in development to accelerate scaling to viable implantable technologies while minimizing design conflicts during later stages.Engineering a drug delivery device within a research setting encompasses a spectrum of activities spanning from basic research to translational research. In the realm of basic research, scientists delve into fundamental principles underlying drug delivery, exploring novel materials, mechanisms, and technologies. This foundational work lays the groundwork for innovation. However, as research progresses towards translational efforts, the focus must shift towards practical application, where collaboration across disciplines is paramount and a consistent design process must be followed.
Clinical translation refers to the process of advancing a medical technology from experimental validation to human application. For AIDDSs, this means demonstrating that the system can consistently deliver therapeutic agents in vivo under real-world constraints, while being safe, user-friendly, and compliant with medical device regulations. Adopting a translational mindset includes considerations of marketability, scalability, manufacturability, and regulatory approval, all while focusing on patient benefits.64 Specifically, the engineering of AIDDSs integrates the multifaceted tasks required for designing any AIMD with the specific requirements and constraints inherent in the sector of pharmacological treatments. Applying effective design methods streamlines development, facilitating faster clinical translation while optimizing time and resources beyond the exploratory research phase. It is advantageous to offer a comprehensive overview of the ideal device development process.
2.1 Design process steps
An overview of the medical device design process, from ideation to market, is the following:1. Requirement analysis: identify clinical needs, technical requirements, regulatory requirements, and safety considerations for the device.
2. Conceptual design: develop initial concepts of the device, including the drug release mechanism, power system, and control architecture.
3. Feasibility study: conduct preliminary studies to assess the feasibility of the design concepts, including initial prototyping and simulations.
4. Detailed design: refine the design details, selecting specific components, materials, software, and layout. Develop detailed schematics and models.
5. Prototyping: create functional prototypes to test and evaluate the device's performance, functionality, and safety.
6. Preclinical testing: perform in vitro and in vivo tests to assess the safety, efficacy, and biocompatibility of the device in animal models.
7. Design optimization: iterate on the design based on preclinical test results, optimizing for performance, safety, and manufacturability.
8. Design verification and validation: verify that the device meets design specifications and validate that it fulfills its intended purpose and complies with regulatory standards.
9. Regulatory submission: prepare and submit regulatory documentation, including preclinical and clinical data, to obtain approval from relevant authorities (e.g., FDA, EMA).
10. Clinical trials: conduct clinical studies to evaluate the device's safety and effectiveness in human subjects, collecting data for regulatory submission.
11. Manufacturing scale-up: develop and optimize the manufacturing process for large-scale production, ensuring compliance with quality standards.
12. Market launch: release the device to the market following regulatory approval, with ongoing monitoring and support.
13. Post-market surveillance: continuously monitor the device's performance, safety, and efficacy in the market, gathering feedback and data to inform future improvements.
Steps 7 through 10 represent the core of the clinical translation process, where the device transitions from validated prototypes toward human application under regulatory and clinical constraints.
2.2 Design framework and development for AIMDs
Product design process models serve as a conceptual framework for designers, enabling them to adeptly navigate the intricacies of medical device development.72 Utilizing iterative design and prototyping, addressing technical and clinical questions early on, and aligning with intellectual property and regulatory considerations is paramount to clinical success.73,74 Relevant research has shown that the ideal medical device development stage-gate model progresses through phases including initiation, opportunity and risk analysis, formulation, concept and feasibility, design and development, verification and validation, and finally product launch and post-launch assessments.75 Each phase can be iteratively performed, ensuring that each design issue is promptly addressed, minimizing time and resource loss. This process translates into moving from design inputs, consisting of clinical needs and regulations, to design outputs, which must be verified and validated before being transferred into a clinically translated product.76 Following these steps, a device can move from discovery and basic research, through pre-clinical development, and towards clinical development and eventual market approval.772.3 The role of medical device regulations
A key challenge in the field of drug delivery is the regulatory landscape, which significantly impacts the market entry of many devices, even those in late development stages. Implantable drug delivery systems face regulatory challenges due to their variety and novelty. Regulatory directives, such as the medical device regulation (MDR) in the European Union and the stringent guidelines set forth by the Food and Drug Administration (FDA) in the United States, outline a series of steps and requisites for obtaining approval for products deemed safe and effective.78 The regulatory classification determines their regulatory pathway and quality control requirements.77The significance of these regulatory principles cannot be underestimated, even in basic research with translational aspirations, as they can accelerate development and reduce the risk of device failure.74,75,79 Failure to consider these regulatory aspects early in the research may lead to developments that fail to meet regulatory standards for clinical translation, resulting in the loss of crucial safety and efficacy data necessary for evaluating novel therapeutic approaches.76
For drug delivery systems, acknowledging the combination nature of the product, and the overlap between medical device and pharmacological therapy regulations, facilitates better decision-making in the design of devices. Preferring implantable drug delivery systems compatible with existing drug therapies, which do not alter the drug formulation and only allow for better tailored dosing, timing, and release, is advantageous for faster clinical translation compared to investigating new formulations.80
2.4 From medical needs to system requirements: integration challenges
Central to the design process is the identification of system specifications derived from the medical needs of the intended use case. These integrate with consequent considerations such as size constraints, energy autonomy, and biocompatibility, which are fundamental to all active implantable devices, along with mandatory safety and efficacy requirements defined by regulations.Biocompatibility is crucial for both short and long-term use, considering sterilization, thermal considerations, shape, and degradation products.81,82 General safety, therapeutic effect, small scale, tunable release, and energetic autonomy are key factors,83,84 to be joined with reduction of patient's burden, unobtrusiveness, and consideration of human factors.85 Communication and security-privacy aspects, if applicable, must also be addressed.86 The actuation, energetic autonomy, and communication are the most technologically constrained, and will be explored in section 3. These requirements should be integrated with the definition of the following, summarized in Fig. 3.
2.5 Design conflicts and trade-offs
During the translation of requirements into specifications, potential design conflicts may arise. These conflicts can include size constraints, the complexity of actuation mechanisms (such as peristaltic, pressurized gated, or simple diffusion systems), and energy transfer limitations. For example, opting for a refillable reservoir may limit implant depth due to refill access needs, while implanting a rechargeable device deeply may restrict available recharge methods. Generally, the more constraints involved, the more challenging the implementation becomes.Ultimately, all these inputs converge into a design output that integrates possible solutions, balancing trade-offs to create a device with specified residency time, the presence or absence of a reservoir and its refill procedures, defined volume and volume efficiency, an appropriate release mechanism and technology, energy transfer or storage methodology, and potentially communication or trigger systems.
3 Current solutions
3.1 Release sites
3.2 Drug release strategy and pattern
Drug delivery devices can actuate drug release with different strategies and patterns. The drug release strategy can involve active processes, where energy is converted to drive the release, or passive methods, where diffusion or pressure gradients naturally enable drug transport, often following the rupture of a barrier. In some cases, the release can be modulated by integrating components that regulate the flow, such as valves or membranes that respond to specific triggers, allowing for greater control over the timing and dosage.The drug release pattern reflects how the drug is administered over time. This can manifest as a continuous flow, ensuring a steady release over a prolonged period, or as discrete events where the drug is delivered in a single bolus or at defined intervals in a pulsed manner. In general, release change in pattern is designed to occur on-demand, allowing for external or internal cues to initiate or modify delivery, providing a dynamic and responsive approach tailored to patient needs or physiological conditions.
3.3 Release actuation technology
The release technology often represents the central focus of research on these systems and serves as the core around which the requirements are defined. This section highlights various implementation strategies found in the literature for controlled and triggerable drug release.Drug delivery systems primarily rely on transport mechanisms to transfer drugs from the device to the physiological environment. The main mechanisms for controlled release can be categorized into two broad approaches: passive diffusion with molecular passage modulation and pressure gradient-driven release. These approaches are implemented in various practical ways. Passive diffusion can be controlled through the opening and closing of channels to achieve specific release profiles. Alternatively, the dissolution or state change of a carrier material allows the passage of drug molecules previously contained within. Pressure gradient-based release is implemented through peristaltic pumps or pressurized reservoirs. Pressurization may involve maintaining a constant internal pressure while modifying the fluid's resistance through valves or dynamically regulating the reservoir pressure to generate the required gradient in real time. These transport strategies are explored further in the subsequent sections. Some examples are represented in Fig. 4.
 | ||
| Fig. 4 Examples of actuation technologies relying on different approaches: (a) heating-mediated delivery based on a shape-memory polymer, reproduced from ref. 122 with permission from MDPI, copyright 2016; (b) electrolysis delivery based on pressure, reproduced from ref. 89 with permission from AAAS, copyright 2021; (c) magnetically actuated device based on tube compression, reproduced from ref. 120 with permission from AAAS, copyright 2021; (d) electrically controlled delivery through nanochannels in a silicon membrane, reproduced from ref. 123 with permission from RSC, copyright 2020. | ||
3.4 Use of reservoirs
Many drug delivery devices in the literature rely on reservoir systems for drug storage and release, particularly when alternative strategies, such as material property changes enabling molecular release, are not employed. Reservoir-based systems allow for controlled drug release over time, reducing the frequency of administration and improving patient adherence to treatments. The introduction of transcutaneous refill capabilities further extends the duration of drug release without requiring repeated surgical interventions, a feature primarily applicable to devices with superficial implantation sites.88Reservoir-based devices can be designed for various release profiles. Some are optimized for sustained release, while others enable on-demand, pulsed releases. In certain cases, they deliver single-dose drug “shots” after which the device terminates its function. When designing active reservoir-based systems, the volume of the reservoir relative to the total device volume defines the volume efficiency (ηvol), which measures the usable volume of the device. Maximizing volume efficiency is crucial for enhancing biocompatibility, reducing invasiveness, and minimizing the obtrusiveness of the device.
Key factors contributing to increased ηvol include the miniaturization of the actuation system, as discussed earlier, and the powering methods, which are analyzed in the following section.152 The possibility for transcutaneous refill further extends the IDDS lifespan, avoiding surgical replacement and extending the possibility for continuous treatment.153 A summary of the reservoir-based devices found in the literature is reported in Table 1.
| Ref. | Year | Release strategy | Release pattern | Actuation technology | Powering (communication) technology | Volume (mm3) | ηvol% |
|---|---|---|---|---|---|---|---|
| a Not complete devices.b Refillable reservoirs. | |||||||
| 96 | 2008 | Active | Sustained/pulsed | Piezoelectric | Rech. battery + inductive (IR interface) | b | |
| 134a | 2009 | Active | One-shot | Electrolytic degradation and pumping | — | 222.6 | 6.74 |
| 105 | 2010 | Active | One-shot | Thruster | Battery (RF) | 786.5 | 89.00 |
| 141 | 2010 | Modulated | Pulsed | Osmotic + piezoelectric valve | Battery (N/A) | 137![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160.0 160.0 |
b30.00 |
| 62 | 2012 | Passive | Multiple-shot | Electrothermal ablation | Battery | ||
| 126a | 2012 | Active | Sustained/pulsed | Electrolytic pumping | — | b | |
| 106 | 2013 | Active | One-shot | Mechanical | Battery (RF) | ||
| 127a | 2014 | Active | Pulsed | Electrolytic pumping | Inductive (ASK on WPT) | ||
| 47a | 2014 | Modulated | Pulsed | Thermoresponsive membrane + NIR laser | — | ||
| 97 | 2014 | Active | Sustained | Electrolytic pumping | Rech. battery (N/A) | b | |
| 124 | 2015 | Active | Pulsed | Shape-memory alloy | RF | 220.5 | b34.47 |
| 107 | 2015 | Active | One-shot | Electromagnetic coil + permanent magnets | Rech. battery (RF) | 786.5 | 38.14 |
| 130a | 2015 | Active | Pulsed | Electrolytic pumping | — | ||
| 143 | 2015 | Modulated | Sustained/pulsed | Osmotic + thermoresponsive membrane | External alternating magnetic field | ||
| 154a | 2016 | Active | Sustained/pulsed | Electroresponsive polymer | Ultrasonic | ||
| 118 | 2016 | Active | Sustained/pulsed | Electrolytic pumping | Inductive (telemetry on WPT) | 2430.0 | b7.61 |
| 109 | 2016 | Active | One-shot | Magnetic | External solenoid | 1188.0 | 65.66 |
| 131 | 2016 | Active | Pulsed | Electrolytic pumping | US | 770.0 | 1.69 |
| 114a | 2017 | Active | Sustained/pulsed | Electrolytic pumping | Triboelectric nanogenerator | b | |
| 132a | 2017 | Active | Sustained/pulsed | Electrolytic pumping | Near-field resonant inductive coupling | b | |
| 125 | 2017 | Active | Pulsed | Shape-memory polymer | RF | 125.0 | 4.00 |
| 102 | 2017 | Active | One-shot | Thermal expansion of composite | RF | 125.0 | 58.48 |
| 119 | 2018 | Active | Pulsed | Magnetic | — | ||
| 115 | 2018 | Active | Pulsed | Magnetic | — | 105.0 | 11.90 |
| 111 | 2018 | Passive | Pulsed | Magnetic | — | ||
| 93 | 2019 | Active | Sustained | Peristaltic based on phase-change | Rech. battery (BLE) | ||
| 155 | 2019 | Active | One-shot/pulsed | Electrolytic pumping | Inductive | 16.4 | 23.20 |
| 87 | 2020 | Passive | Multiple-shot | Electrolytic degradation | Inductive | ||
| 89 | 2021 | Passive | One-shot | Electrolytic degradation | RF | 250.0 | 48.00 |
| 120 | 2021 | Active | Pulsed | Mechanical + external solenoid | Ext. battery (N/A) | 184.0 | b81.52 |
| 133a | 2021 | Active | Pulsed | Electrolytic pumping | DC power supply | b | |
| 99 | 2023 | Passive | Multiple-shot | Electrolytic degradation | Biodegradable battery (light trigger) | ||
3.5 Powering technology
Powering technologies for AIDDSs are crucial for ensuring the long-term functionality and reliability of therapeutic and diagnostic systems.156 These devices, which include drug delivery systems, neurostimulators, and biosensors, often require energy sources that are compact, biocompatible, and capable of sustaining operations over extended periods. Traditional battery-powered implants have been widely used, but they pose challenges in terms of size, longevity, and the need for surgical replacement or recharging. As a result, there has been significant progress in the development of alternative powering methods that offer greater efficiency and longevity. Several authors have provided comprehensive reviews on the field of biomedical engineering.157–161 Wireless power transfer (WPT) techniques, such as capacitive and inductive coupling162 and radiofrequency (RF) energy harvesting, have emerged as promising solutions, enabling devices to be powered or recharged without the need for invasive procedures. Ultrasound and optical energy transfer methods are also being explored for their ability to reach deep implants with minimal energy loss.161 Additionally, advances in energy harvesting technologies allow devices to harness energy from the body's own physiological processes, such as heat, movement, or bioelectric signals, to power microelectronic components. Piezoelectric and triboelectric materials have been leveraged to convert mechanical energy from body movement into electrical power, offering a self-sustaining solution for long-term implantation.163Recent efforts have explored the use of magnetoelectric (ME) materials to further miniaturize receiver modules in implantable systems. ME structures can operate efficiently at smaller scales compared to traditional inductive coils, making them attractive for compact device integration164 light-based powering has also emerged as a promising approach, using visible to near-infrared (NIR) wavelengths captured by photodiodes, photovoltaic elements,165 or through photothermal conversion.166 These methods may help reduce tissue heating and improve biocompatibility, but remain in an early development phase, with ongoing efforts aimed at overcoming power transfer and penetration limitations. These innovations in powering technologies are essential for expanding the capabilities of implantable devices, reducing the need for invasive maintenance, and improving patient outcomes. However, challenges such as power efficiency, safety, miniaturization, and biocompatibility continue to drive ongoing research in this field.167 This section analyzes the powering methods applied to IDDDs. Fig. 5 graphically illustrates the size and power consumption requirements of devices available in the literature, some of which are already equipped with powering solutions, and relates them to a representation of the powering methods generally available in the field of AIMDs.
 | ||
| Fig. 5 Size and power consumptions of devices in the state of the art: (a) ref. 95, (b) ref. 118, (c) ref. 92, (d) ref. 96, (e) ref. 108 (f) ref. 105 and (g) ref. 103 report both size and power consumption. Colored areas in the background indicate qualitatively available powering technologies in the literature. | ||
3.6 Remote communication methods
The communication methods employed in implantable drug delivery systems can be categorized as either relying on the same technology as the energy transfer system, or not. Among the methods relying on energy transfer, modulations on energy transfer via NFC are notable,94,103 together with amplitude-shift keying (ASK) modulation on inductive power transfer.127 Communication methods that operate independently include generic RF communication,105 communication in the MICS band,62 and Bluetooth low energy communication.88,1134 Complete systems from the state of the art
In this section, we highlight exemplary systems from the literature that showcase clever and thoughtful integration across various engineering domains. These designs are strategically developed to stay within close reach of regulatory requirements, offering realistic pathways to clinical translation and paving the way for scalable and clinically impactful drug delivery solutions. These examples serve as benchmarks for understanding current progress and as inspiration for further innovation in the field. Table 2 summarizes the main trade-offs and challenges associated with the devices discussed in the remainder of this section, highlighting their advantages and disadvantages, as well as the challenges on technical implementation and regulatory considerations that have been addressed or remain open, respectively.| Device | Trade-offs | Challenges |
|---|---|---|
| Legend: (+) = advantages; (−) = disadvantages; (T) = technical addressed challenges; (R) = regulatory challenge considerations. | ||
| 4.1 (ref. 89) | (+) highly responsive; integration with sensing (−) not refillable; wearable instrumentation always on | (T) Alignment of WPT; system development/integration; (R) management after use to be addressed; high complexity complicates certification |
| 4.2 (ref. 87) | (+) Bioresorbable; on-demand; multi-reservoir; (−) limited programmability; single-use; limited residency time | (T) Optimisation of material corrosion; RF tuning; low-cost process; (R) byproduct safety and toxicity studies; lack of standardization complicates certification |
| 4.2 (ref. 99) | (+) Bioresorbable; self-powered; selectable multi reservoir; (−) single use; limited programmability | (T) Creation of biodegradable battery; calibration of photoselectivity; (R) byproduct safety and toxicity studies; lack of standardization complicates certification |
| 4.3 (ref. 94) | (+) dual use – optogenetics and pharmacology; multiple drugs; (−) limited by receiver depth and use of wearable | (T) Encapsulation; addressable reservoir activation; RF coupling; miniaturization; refill missing; (R) dual use complicates certification; longer term tests needed |
| 4.4 (ref. 110) | (+) targeted release; endoscopy-compatible; expellable; (−) single-use; mechanical assembly complexity | (T) Safe thermal activation; miniaturized rotating parts; flow optimization (R) missing biocompatibility studies; complex fabrication; evaluation of cost-effectiveness |
| 4.5 (ref. 133) | (+) integrated dose sensor; simple; low-cost (−) limited delivered volume; limited programmability | (T) Fabrication with carbon ink within PDMS; simple wireless activation; (R) certification of included materials; in vivo validation needed |
| 4.6 (ref. 113) | (+) wireless, refillable, programmable; consistent manufacturing; (−) limited battery; no feedback | (T) Fine flux control; miniaturization; optimization of fabrication; recharge missing; (R) implant > 6 mo. reached; extension for chronic use needed |
| 4.7 (ref. 91) | (+) deep implant, localized release; on-demand (−) anionic drugs only; not refillable; limited programmability | (T) Drug retention optimization; system design for consistent release; (R) definition of explant procedures; sterilization and certification of used materials |
| 4.8 (ref. 88) | (+) sustained release; remote control; low-power; refillable; (−) limited battery; assembly complexity | (T) System miniaturization; power consumption optimization; innovative actuator design; (R) need for chronic testing; addressing standardization of assembly/sterilization |
4.1 Soft implantable device for treatment of seizures
Joo et al.89 designed a soft implantable device (SID) (Fig. 6a) for seizure treatment, continuously monitoring EEG signals and using wireless power transmission to trigger drug release during emergencies. The minimally invasive subcutaneous implantation allows rapid pharmacological intervention while bypassing skin barriers. Although not a replacement for conventional methods, the SID addresses critical situations requiring swift and targeted responses. Currently, the device must be explanted after use, but future developments may include bioabsorbable or degradable materials to simplify its management after drug depletion. | ||
| Fig. 6 Examples of successful integration of complete active drug delivery systems: (a) soft implantable device for the treatment of seizures, reproduced from ref. 89 with permission from AAAS, copyright 2021; (b) bioresorbable and i. self-powered system, reproduced from ref. 87 with permission from AAAS, copyright 2020; ii. inductively activated system, reproduced from ref. 99 with permission from PNAS, copyright 2023; (c) battery-free multireservoir system for neural research, reproduced from ref. 94 with permission from PNAS, copyright 2019; (d) wireless endoscopic capsule for drug delivery, reproduced from ref. 108 with permission from Wiley, copyright 2015; (e) 3D printed peristaltic drug delivery system, reproduced from ref. 113 with permission from MDPI, copyright 2021; (f) ultrasound-activated drug delivery system, reproduced from ref. 91 with permission from RSC, copyright 2022; (g) electrically controlled nanochannel delivery system, reproduced from ref. 88 with permission from RSC, copyright 2019. | ||
4.2 Bioresorbable, on-demand devices
Koo et al.87 (Fig. 6b-i) introduced a bioresorbable, self-powered device. Drug release is triggered by specific light frequencies that activate photodiodes, creating a short circuit in the biodegradable electrochemical cell, and causing the degradation of anodes covering the reservoirs. Similarly, Zhang et al.99 (Fig. 6b-ii) presented a bioresorbable device for wirelessly controlled drug delivery, utilizing active valves based on electrochemically triggered crevice corrosion for precise drug release, powered with inductive transfer. The bioresorbable nature of these devices eliminates the need for surgical removal post-treatment, reducing associated risks. These systems are suitable for applications requiring shallow implantation and localized, on-demand, and time-sensitive drug delivery, particularly in acute scenarios, after which the device naturally degrades.4.3 Battery-free optofluidic microsystem for neural research
Zhang et al.94 (Fig. 6c) presented a compact, wireless, battery-free optofluidic microsystem designed for studying neural circuits in freely moving animals. Combining pharmacology and optogenetics, the device integrates an electrochemical micropump for drug delivery, refillable reservoirs for multiple administrations, and a micro-LED for programmable optogenetic stimulation with precise control of light frequency and intensity. Powered via near-field communication (NFC), the system eliminates the need for batteries. Its soft, flexible design minimizes tissue damage, making it suitable for long-term experiments without repeated trauma. The design is scalable and supports integration into closed-loop systems for real-time monitoring and manipulation of neuronal activity.4.4 Next-generation wireless endoscopic capsule
Woods et al.110 (Fig. 6d) introduced a next-generation wireless endoscopic capsule designed for targeted drug delivery in the intestinal tract. The capsule features a conical compression spring made of stainless steel, which, when fully compressed, generates enough force to release the stored drug through a needle operated by a mechanical system. The device integrates an RF controller, a camera for imaging, and is powered by a battery, enabling precise and controlled drug administration. This system represents an example of a microrobotic platform operating within the gastrointestinal tract.4.5 Electrolytic drug delivery device with integrated monitoring
Yi et al.133 introduced an electrolytic drug delivery device that uses electrolysis-induced bubble expansion to deform a polydimethylsiloxane (PDMS) membrane, driving precise drug release. A flexible piezoresistive sensor integrated into the membrane enables real-time monitoring of drug volume and flow rate with high accuracy. The device ensures controlled flow rates, reducing risks of overdose or underdelivery, and is optimized for small-scale drug volumes, making it ideal for micro-dosing applications. It is particularly suited for localized treatments in ophthalmology, neurology, and cancer therapies. The system envisions telemetry and wireless power transfer for remote monitoring and control, while currently focusing on in situ dosage sensing.4.6 Miniaturized 3D-printed drug delivery system
Forouzandeh et al.113 (Fig. 6e) presented a miniaturized drug delivery microsystem fabricated through 3D printing and wirelessly controllable. The device features a refillable microreservoir and a peristaltic micropump based on phase-change materials, suitable for subcutaneous implants and transdermal applications. The system's structure is fabricated using stereolithography with biocompatible resins, while the micropump is directly integrated onto the back of a printed circuit board using inkjet printing technology. It is powered by a rechargeable battery, although no charging system is included, and offers Bluetooth connectivity for remote control.4.7 Ultrasound-activated drug delivery system
Wang et al.91 (Fig. 6f) introduced a wireless, miniaturized drug delivery mechanism (DDM) for localized release, powered by an external ultrasound transmitter. Ultrasound waves are converted into electrical energy by a piezoelectric receiver, triggering electrochemical reduction in a PPy nanoparticulate film to release negatively charged drugs. The system is designed for deep tissue implantation and is particularly suited for rapid or localized drug delivery in severe hypoglycemia, localized cancer therapy, and epilepsy. While it eliminates bulky batteries and invasive procedures, its effectiveness depends on precise transmitter alignment, and its drug storage capacity is limited and non-refillable. Wang et al.170 build upon this design with a closed-loop control mechanism that measures redox currents during drug release and transmits this feedback to an external controller. This allows to dynamically adjust the potentiostat's voltage in real-time and improves precision to meet therapeutic needs.4.8 Electrically controlled drug delivery system
Di Trani et al.88 (Fig. 6g) presented a subcutaneous drug delivery device designed for chronic disease management. The system uses a silicon-based nanofluidic membrane to mediate drug release via concentration-driven diffusion from a refillable reservoir, modulated by a low-intensity electric field applied through platinum electrodes. The device has a battery powered electronic control system, and modulation of drug release is achieved thanks to remote control with Bluetooth low energy. The device supports continuous drug administration, avoiding the fluctuations typical of pulsatile systems, which improves treatment efficacy. Subsequent work was done to address the biocompatibility of the actuation mechanism123,171 and to recharge the device to extend its useful life to years.172,1735 Discussion on active DDS research progress
Reservoir-based drug delivery systems provide a versatile and promising approach for treating diseases, particularly chronic conditions. As outlined in Table 1, these systems support a wide range of release profiles, including continuous and on-demand drug delivery. Devices with refill capabilities are particularly advantageous as they allow for indefinite usage, making them suitable for chronic diseases requiring sustained treatment and reducing the need for invasive procedures. This flexibility often necessitates superficial implantation to facilitate refilling, aligning well with the needs of chronic disease management. Their compatibility with various actuation technologies further broadens clinical applications, offering customized solutions for different therapeutic needs.Batteries currently represent the most reliable and widely used power source, offering consistent and possibly long-term (but still limited) energy delivery. However, as the size of devices is reduced, batteries often occupy a disproportionate share of the device's volume, creating a barrier to further miniaturization. The use of secondary batteries allows for battery miniaturization, without reducing, instead increasing (virtually to infinity) the overall device residency time within the body thanks to their rechargeability. The necessity of both recharging batteries and power batteryless devices has led to growing interest in alternative powering methods, such as wireless power transfer and energy harvesting, which can enable smaller and less invasive designs without sacrificing functionality. Inductive and radiofrequency systems are gaining popularity for their ability to facilitate miniaturization and simultaneous communication, though they necessitate external instrumentation for power supply. Capacitive coupling, despite its promise in terms of power delivery and size compatibility, is underreported in the context of drug delivery systems. Ultrasound-based systems are rarely implemented, and their reliance on external transducers and gel mediums for efficient energy transfer presents significant challenges to large-scale diffusion. Though rarely addressed in the literature, addressing power-related challenges early in the design phase is essential for the development of scalable and clinically viable devices. An important but often overlooked aspect is the security of these devices. While enabling technologies have been the focus, once a certain level of development has been reached, it will be essential to incorporate security considerations into the iterative design process to support safe remote access and data integrity.
Successful examples of system integration in drug delivery devices combine the harmonization of actuation mechanisms and power supply with applicability and product and lifecycle considerations for in vivo considerations, providing valuable guidance for future developments. Examples from the literature show that achieving this integration enhances clinical applicability and supports scalability to mass production. Additionally, system fabrication and scalability to mass production remain critical considerations. Simplifying device architecture while carefully managing trade-offs between performance and complexity is critical for advancing these technologies. Other important considerations include guaranteeing interoperability and minimizing the burden on patients, alongside ensuring a straightforward setup, as patients cannot be expected to manage complex external sensors.174,175
Most existing drug delivery devices lack true remote control capabilities. While remote activation is often implemented, it usually coincides with the energy transfer and limited to triggering the actuator activation, as a matter of fact requiring the user's intervention, with all the possible consequent issues (see section 1). Only a limited number of devices, as highlighted in section 3.6, can be classified as systems with communication capabilities. Even in these cases, communication is typically linked to the energy transfer system. Independent communication, implemented through RF or Bluetooth technologies, remains rare. Despite demanding greater energy autonomy from the device, such communication facilitates integration with IoT systems, enabling remote patient management, increasing automation, and reducing the tasks required of patients or healthcare providers during drug administration.174–177 Through IoT integration, implantable drug delivery systems can become nodes in a broader digital health framework, continuously exchanging data with mobile health (mHealth) platforms, clinician dashboards, or cloud-based analytics engines. This enables autonomous and continuous remote control, entirely decoupled from energy transfer sessions. It can support real-time physiological monitoring by interfacing with wearable or implantable biosensors, allowing dynamic therapy adjustment based on contextual, patient-specific data. Such infrastructure can empower clinicians to proactively manage treatment regimens, automatically trigger drug administration in response to critical biomarker changes, and receive alerts for potential anomalies or emergencies. In particular, this approach can potentially enhance patient safety by providing responses to emergencies requiring pharmacological intervention, or, in less critical scenarios, therapy adjustments tailored to the patient's physiological needs.167,178,179
Integration with physiological monitoring remains an open and essential topic, aligning with the development of autonomous systems for disease management. Achieving this integration necessitates either energy and communication autonomy within the system or accessibility through an external activation system. However, not all externally activated devices provide details about the size of the external control system or the potential burden on the patient. Moving the combined control and energy source externally while maintaining the internal release system may allow for miniaturization but obligates the patient to more critically rely on the correct and timely use of external instrumentation, as a matter of fact increasing the patient's burden. Nevertheless, integration with physiological monitoring aligns with the trend toward patient-specific medicine.
While these functionalities increase system complexity, they can significantly improve cost-effectiveness of care by reducing hospital access, improving adherence, and enabling early intervention. Ensuring affordability, however, requires careful balance between performance and economic sustainability of the devices. In this regard, MEMS technologies offer a key advantage: they enable miniaturization and integration of actuators, sensors, and valves within compact devices, while remaining compatible with scalable, high-precision manufacturing. Their use supports both functional sophistication and production efficiency, making them instrumental in developing accessible and clinically viable AIDDSs. The integration of sensing capabilities and connectivity with mHealth platforms opens the door to implementing artificial intelligence (AI) for autonomous and adaptive drug delivery. AI algorithms could analyze real-time data from implantable or wearable sensors – such as vital signs, metabolic markers, or behavioral patterns – to support predictive models and closed-loop control strategies. This would enable dynamic adjustment of therapy based on individual physiological needs, enhancing treatment precision while reducing patient and clinician workload. Combined with remote monitoring infrastructure, AI can facilitate early detection of deviations from therapeutic targets, optimize dosing schedules, and support the development of fully autonomous drug delivery systems, representing a key opportunity for next-generation AIDDSs within digital healthcare ecosystems. Future advancements in integrating with the biological environment and creating unobtrusive devices hinge on material selection. Flexible materials that integrate well with surrounding soft tissue while offering durability represent a promising trend compatible with electronic systems.180 Bioresorbable electronics present significant opportunities for temporary and deeply implanted drug delivery systems, eliminating the need for explantation. However, further advancements in material science are required to develop materials whose by-products are soluble, non-toxic, and easily metabolized. Despite this, successful preliminary approaches are documented in the literature,181–183 and these concepts have been applied to drug delivery devices.87,99 The current bioresorbable paradigm conflicts with the integration of standard communication protocols due to the complexity required. Nonetheless, it represents a complementary and alternative paradigm.
Although these are research devices, the designs described in section 4 already are promising from a development perspective, addressing proactively emerging challenges in integration and scalability. As summarized in Table 2, these systems exemplify different engineering trade-offs and highlight both technical and regulatory barriers that must be overcome to transition from research prototypes to viable medical products. While unresolved issues remain, these designs provide a clear perspective on the challenges, allowing the implementation of iterative design processes that enabling iterative design processes that maintain the integrity of the device concept and are compatible with previous developments. By anchoring to well-defined requirements and challenges, these technologies offer greater realism, fostering innovations that could significantly impact pharmacology. The widespread adoption of systems with these characteristics would enable remote patient treatment, eliminating the need for healthcare facility visits for drug administration. This would reduce treatment costs, especially as these technologies are scaled, and improve public health outcomes. In this context, technologies advancing toward infrastructure simplification and interoperability with mHealth platforms176,177 are particularly impactful. Systems enabling the release of pre-existing and approved drug formulations, rather than requiring reformulated drugs, present the potential advantage of a streamlined regulatory pathway. Reservoir-based systems, which do not alter the drug formulation, could expedite market access for controlled release systems by following the regulatory framework for medical devices. To date, this pathway has primarily been applied to large devices for end-stage diseases. However, given the advancements in miniaturization highlighted in the literature, this represents a significant opportunity for prevalent diseases. The key technological challenges and future directions to realize this potential are summarized in Table 3, offering a framework to guide further development and clinical translation.
| Key challenges | Future directions |
|---|---|
| Energy autonomy and miniaturization | Wireless power transfer, energy harvesting, and ultra-low-power design to reduce size and extend operational lifetime in vivo |
| Reliable and autonomous communication | Independent RF/Bluetooth communication enabling real-time, bidirectional data flow and integration with IoT/mHealth platforms for remote therapy control and monitoring |
| Security and privacy risks | Incorporation of secure, encrypted protocols and authentication mechanisms from early design stages to support safe remote access and data integrity |
| Digital health and AI integration | Leveraging AI and mHealth platforms for real-time data analysis, predictive modeling, and closed-loop control for adaptive, patient-specific drug delivery |
| Patient burden and usability | Development of interoperable systems with intuitive interfaces and minimal external components to reduce complexity and support user-independent operation |
| Manufacturability and scalability | Adoption of MEMS and microfabrication techniques for reproducible, cost-effective production at scale, while preserving performance |
| Validation and clinical translation | Iterative prototyping with in vivo testing and alignment with clinical workflows to streamline preclinical-to-clinical transition |
| Biocompatibility and material constraints | Use of soft, durable, and bioresorbable materials compatible with long-term tissue integration and safe biodegradation |
| Regulatory complexity | Streamlined approval pathways via use of approved drug formulations and early-stage regulatory strategy alignment |
| Affordability and access | Modular and scalable architectures enabling economically sustainable, intelligent systems accessible for widespread chronic disease management |
Conclusions
The evolution of AIDDSs highlights their transformative potential to meet critical medical needs while advancing precision medicine. From the analysis conducted, several resolved issues, open challenges, opportunities, and potentialities emerge. Resolved challenges include miniaturization of actuation technologies and enhanced biocompatibility, enabling less invasive implantation and better patient comfort. Open challenges revolve around enhancing scalability, achieving true energy autonomy, and developing robust communication capabilities. Significant opportunities lie in integrating these systems within IoT frameworks, which can enable dynamic and adaptive responses not just at the timepoint of injection or implantation, but continuously throughout the therapy. These advancements will play a pivotal role in improving patient safety, particularly through real-time monitoring, emergency responsiveness, and therapy customization. To achieve this, it is foreseeable that new challenges related to security and privacy, not yet fully addressed, will emerge as remote control and communication become standard.However, the convergence of enabling technologies – such as flexible electronics, energy-efficient powering methods, and IoT frameworks – offers a clear pathway for advancing these systems. By tackling the remaining challenges through collaborative innovation and systematic design processes, researchers and engineers can position AIDDSs as scalable, safe, and impactful tools in modern healthcare.
The AIDDS of the future must encompass a dynamic response capable of ensuring efficacy, efficiency and, most importantly, safety for the patient throughout its operation. Furthermore, it should provide adaptive and continuous care, extending beyond the moment of injection or implantation to deliver a constantly tailored response at every stage of therapy. Finally, the system should operate in alignment with the principles of precision medicine, offering real-time, patient-specific adjustments to therapy and ensuring maximum therapeutic benefit while minimizing side effects. These features will not only elevate the functionality of AIDDSs but also position them as a cornerstone of future healthcare, enabling a seamless blend of advanced technology and personalized therapeutic approaches. To fully unlock their potential, a sustained focus on real-world validation, regulatory alignment, and patient-centered design is essential. This will ensure that future systems not only achieve clinical readiness but also drive a meaningful shift toward autonomous and accessible therapeutic solutions.
Data availability
No primary research results, software, or code have been included, and no new data were generated or analyzed as part of this review.Author contributions
Fabiana Del Bono: conceptualization, data curation, formal analysis, investigation, visualization, writing – original draft, writing – review & editing (lead). Nicola Di Trani: writing – review & editing (equal); visualization (supporting). Danilo Demarchi: conceptualization, project administration, supervision (equal); funding acquisition, resources, writing – review & editing (supporting). Alessandro Grattoni: supervision (equal); funding acquisition, project administration, resources, writing – review & editing (supporting). Paolo Motto Ros: supervision (lead); conceptualization, funding acquisition, project administration, writing – review & editing (equal); resources (supporting).Conflicts of interest
A. Grattoni is an inventor of intellectual property licensed by Continuity Biosciences. All other authors declare that they have no competing interests or consulting engagements.References
- J. Murphy and G. Coster, Drugs, 1997, 54, 797–800 CrossRef CAS PubMed.
- B. W. Ward and J. S. Schiller, Prev. Chronic Dis., 2013, 10, E65 Search PubMed.
- N. M. Hawkins, S. Virani and C. Ceconi, Eur. Heart J., 2013, 34, 2795–2807 CrossRef PubMed.
- K. Kroenke, E. E. Krebs and M. J. Bair, Gen. Hosp. Psychiatry, 2009, 31, 206–219 CrossRef PubMed.
- Biorender, BioRender: Scientific Image and Illustration Software, https://BioRender.com/l58n428, Accessed: 2025-02-05.
- U.S. Government Accountability Office, Prescription Drug Spending, 2023, Accessed: 2024-11-21 Search PubMed.
- Congressional Budget Office, Prescription Drugs: Spending, Use, and Prices, Congressional budget office technical report, 2022 Search PubMed.
- E. M. Tichy, G. T. Schumock, J. M. Hoffman, K. J. Suda, M. H. Rim, M. Tadrous, M. D. Wiest, L. M. Matusiak, J. S. Clark, S. Cuellar, J. A. Stubbings, L. C. Vermeulen and R. J. Hunkler, Am. J. Health-Syst. Pharm., 2020, 77, 1213–1230 CrossRef PubMed.
- A. C. Anselmo and S. Mitragotri, J. Controlled Release, 2014, 190, 15–28 CrossRef CAS PubMed.
- Grand View Research, Implantable Drug Delivery Devices Market Size, Share & Trends Analysis Report By Product, By Type (Biodegradable, Nonbiodegradable), By Technology, By Application, By Region, And Segment Forecasts, 2024–2030, 2024 Search PubMed.
- L. Osterberg and T. Blaschke, N. Engl. J. Med., 2005, 353(5), 487–497 CrossRef CAS PubMed.
- N. Barber, Qual. Saf. Health Care, 2004, 13, 172–175 CrossRef CAS PubMed.
- G. DeSevo and J. Klootwyk, Prim. Care - Clin. Off. Pract., 2012, 39, 345–362 CrossRef.
- World Health Organization, Adherence to Long-Term Therapies: Evidence for Action, World Health Organization, Geneva, Switzerland, 2003 Search PubMed.
- L. Epstein, Health Psychol., 1984, 3(4), 385–393 CAS.
- J. Urquhart, Eur. Heart J., 1996, 17 Suppl A, 8–15 CrossRef CAS.
- A. Golay, J. Med. Econ., 2011, 14, 594–608 CrossRef PubMed.
- M. C. Sokol, K. A. McGuigan, R. R. Verbrugge and R. S. Epstein, Med. Care, 2005, 43, 521–530 CrossRef PubMed.
- M. C. Roebuck, J. N. Liberman, M. Gemmill-Toyama and T. A. Brennan, Health Aff., 2011, 30, 91–99 CrossRef PubMed.
- T. Simon-Tuval, A. Shmueli and I. Harman-Boehm, Value Health, 2016, 19, 844–851 CrossRef PubMed.
- L. Lizàn, M. Comellas, S. Paz, J. L. Poveda, D. Meletiche and C. Polanco, Patient Prefer. Adherence, 2014, 8, 1653–1664 Search PubMed.
- S. Mignani, S. E. Kazzouli, M. Bousmina and J. P. Majoral, Adv. Drug Delivery Rev., 2013, 65, 1316–1330 CrossRef CAS PubMed.
- C. T. Dollery, D. S. Davies and M. E. Conolly, Ann. N. Y. Acad. Sci., 1971, 179, 108–112 CrossRef CAS PubMed.
- M. S. Alqahtani, M. Kazi, A. Ma and A. Mz, Front. Pharmacol., 2021, 12, 618411 CrossRef CAS PubMed.
- M.-L. Laracuente, M. H. Yu and K. J. McHugh, J. Controlled Release, 2020, 327, 834–856 CrossRef CAS PubMed.
- S. Amreen, S. M. Shahidulla, A. Sultana and N. Fatima, J. Drug Delivery Ther., 2023, 13, 98–105 CrossRef CAS.
- H. C. Zierden, A. Josyula, R. L. Shapiro, H. T. Hsueh, J. Hanes and L. M. Ensign, Trends Mol. Med., 2021, 27, 436–450 CrossRef CAS PubMed.
- J. B. Dressman, K. Thelen and E. Jantratid, Clin. Pharmacokinet., 2008, 47, 655–667 CrossRef CAS.
- U. Bhutani, T. Basu and S. Majumdar, Eur. J. Pharm. Biopharm., 2021, 162, 23–42 CrossRef CAS.
- A. D. Stefano, A. Iannitelli, S. Laserra and P. Sozio, Expert Opin. Drug Delivery, 2011, 8, 581–603 CrossRef.
- S. S. Lee and M. Robinson, Ophthalmic Res., 2009, 41, 124–135 CrossRef CAS.
- T. H. Johnston, S. Fox and J. Brotchie, Expert Opin. Drug Delivery, 2005, 2, 1059–1073 CrossRef CAS.
- M. Wen, N. S. El-Salamouni, W. M. El-Refaie, H. A. Hazzah, M. M. Ali, G. Tosi, R. Farid, M. Blanco-Prieto, N. Billa and A. Hanafy, J. Controlled Release, 2017, 245, 95–107 CrossRef CAS PubMed.
- G. Martino, R. Furlan, G. Comi and L. Adorini, Trends Immunol., 2001, 22(9), 483–490 CrossRef CAS PubMed.
- S. Shi, N. Kong, C. Feng, A. Shajii, C. Bejgrowicz, W. Tao and O. Farokhzad, Adv. Healthcare Mater., 2019, 8, 1801655 CrossRef PubMed.
- F. Yasmin, H. Najeeb, S. Shaikh, M. Hasanain, U. Naeem, A. Moeed, T. Koritala, S. Hasan and S. Surani, World J. Gastroenterol., 2022, 28, 1922–1933 CrossRef CAS PubMed.
- L. C. Fonseca, J. A. Lopes, J. Vieira, C. Viegas, C. S. Oliveira, R. P. Hartmann and P. Fonte, Drug Delivery Transl. Res., 2021, 11, 411–425 CrossRef PubMed.
- R. Zhao, Z. Lu, J. Yang, L. Zhang, Y. Li and X. Zhang, Front. Bioeng. Biotechnol., 2020, 8, 880 CrossRef.
- J. Lam, C. C. Cheung, M. Y. T. Chow, E. Harrop, S. Lapwood, S. Barclay and I. K. Wong, Adv. Drug Delivery Rev., 2020, 160, 234–243 CrossRef CAS.
- J. J. Chen and D. Swope, Pharmacotherapy, 2007, 27, 161S–173S CrossRef CAS.
- S. S. Patil and A. Shahiwala, Expert Opin. Ther. Pat., 2014, 24, 845–856 CrossRef CAS PubMed.
- A. Ballesta, P. Innominato, R. Dallmann, D. Rand and F. Lévi, Pharmacol. Rev., 2017, 69, 161–199 CrossRef CAS PubMed.
- B. B. C. Youan, Adv. Drug Delivery Rev., 2010, 62, 898–903 CrossRef CAS PubMed.
- S. Adepu and S. Ramakrishna, Molecules, 2021, 26, 5905 CrossRef CAS PubMed.
- M. R. Prausnitz and R. Langer, Nat. Biotechnol., 2008, 26, 1261–1268 CrossRef CAS PubMed.
- F. P. Pons-Faudoa, A. Ballerini, J. Sakamoto and A. Grattoni, Biomed. Microdevices, 2019, 21, 47 CrossRef PubMed.
- B. P. Timko, M. Arruebo, S. A. Shankarappa, J. B. McAlvin, O. S. Okonkwo, B. Mizrahi, C. F. Stefanescu, L. Gomez, J. Zhu, A. Zhu, J. Santamaria, R. Langer and D. S. Kohane, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 1349–1354 CrossRef CAS PubMed.
- C. L. Stevenson, J. T. Santini and R. Langer, Adv. Drug Delivery Rev., 2012, 64, 1590–1602 CrossRef CAS.
- J. C. Quarterman, S. M. Geary and A. K. Salem, Eur. J. Pharm. Biopharm., 2021, 159, 21–35 CrossRef CAS.
- F. Tan, Y. Zhu, Z. Ma and M. Al-Rubeai, Acta Biomater., 2020, 108, 46–55 CrossRef CAS.
- F. Munoz, G. Alici and W. Li, Adv. Drug Delivery Rev., 2014, 71, 77–85 CrossRef CAS.
- J. Zhang, J. Xu, J. Lim, J. K. Nolan, H. Lee and C. H. Lee, Adv. Healthcare Mater., 2021, 10, 2100194 CrossRef CAS PubMed.
- D. K. Raijada, K. Wac, E. Greisen, J. Rantanen and N. Genina, Adv. Drug Delivery Rev., 2021, 113857 CrossRef CAS PubMed.
- R. A. Lal, L. Ekhlaspour, K. Hood and B. Buckingham, Endocr. Rev., 2019, 40, 1521–1546 CrossRef PubMed.
- C. Y. X. Chua, M. Jimenez, M. Mozneb, G. Traverso, R. Lugo, A. Sharma, C. N. Svendsen, W. R. Wagner, R. Langer and A. Grattoni, Nat. Rev. Mater., 2024, 9, 808–821 CrossRef.
- AbbVie Inc., Vyalev – Advanced Drug Delivery Systems, https://www.vyalev.com/, Accessed: 2025-01-13.
- Insulet Corporation, Omnipod – Insulin Management Simplified, https://www.omnipod.com/, Accessed: 2025-01-13.
- Beta Bionics, iLet Bionic Pancreas – Automated Insulin and Glucagon Delivery System, https://www.betabionics.com/ilet-bionic-pancreas/, Accessed: 2025-01-13.
- M. J. Mitchell, M. M. Billingsley, R. M. Haley, M. E. Wechsler, N. A. Peppas and R. Langer, Nat. Rev. Drug Discovery, 2021, 20, 101–124 CrossRef CAS PubMed.
- C. M. Dawidczyk, C. Kim, J. H. Park, L. M. Russell, K. H. Lee, M. G. Pomper and P. C. Searson, J. Controlled Release, 2014, 187, 133–144 CrossRef CAS PubMed.
- E. Meng and T. Hoang, Adv. Drug Delivery Rev., 2012, 64, 1628–1638 CrossRef CAS PubMed.
- R. Farra, N. F. Sheppard, L. McCabe, R. M. Neer, J. M. Anderson, J. T. Santini, M. J. Cima and R. Langer, Sci. Transl. Med., 2012, 4, 122ra21 Search PubMed.
- S. M. Mirvakili and R. Langer, Nat. Electron., 2021, 4, 464–477 CrossRef.
- N. Tandon and S. Bhumiratana, ACS Biomater. Sci. Eng., 2022, 8, 4629–4633 CrossRef CAS PubMed.
- J. T. Santini, M. J. Cima and R. Langer, Nature, 1999, 397, 335–338 CrossRef CAS.
- R. Matheson, Major step for implantable drug-delivery device, https://news.mit.edu/2015/implantable-drug-delivery-microchip-device-0629, 2015, Accessed: 2025-05-12.
- Daré Bioscience, DARE-LARC1 – User-Controlled, Long-Acting Reversible Contraception, https://darebioscience.com/pipeline/dare-larc1/, 2025, Accessed: 2025-05-12.
- R. R. Henry, J. Rosenstock, D. Logan, T. Alessi, K. Luskey and M. A. Baron, J. Diabetes Its Complications, 2014, 28, 393–398 CrossRef PubMed.
- U.S. Food and Drug Administration, Final Decision on the Proposal To Refuse To Approve a New Drug Application for ITCA 650, https://www.federalregister.gov/documents/2024/08/23/2024-18898/final-decision-on-the-proposal-to-refuse-to-approve-a-new-drug-application-for-itca-650, 2024, Federal Register, 89 FR 68168.
- Medtronic Diabetes, MiniMed™ 780G Insulin Pump System, https://www.medtronicdiabetes.com/products/minimed-780g-insulin-pump-system, 2023, Accessed: 2025-05-12.
- U.S. Food and Drug Administration, MiniMed™ 780G System – P160017/S091, https://www.fda.gov/medical-devices/recently-approved-devices/minimed-780g-system-p160017s091, 2023, Accessed: 2025-05-12.
- L. A. Medina, G. E. Kremer and R. A. Wysk, J. Eng. Des., 2013, 24, 83–119 CrossRef.
- D. M. Beswick, A. Kaushik, D. Beinart, S. Mcgarry, M. K. Yew, B. F. Kennedy, P. Luke and S. Maria, J. Biomed. Opt., 2017, 23, 021102, DOI:10.1117/1.JBO.23.2.021102.
- N. A. White, T. J. C. O. Vrielink, K. E. A. van der Bogt, A. F. Cohen, J. I. Rotmans and T. Horeman, Br. J. Clin. Pharmacol., 2023, 89, 2144–2159 CrossRef PubMed.
- J. B. Pietzsch, L. A. Shluzas, M. E. Paté-Cornell, P. G. Yock and J. H. Linehan, J. Med. Devices Trans. ASME, 2009, 3, 021004 CrossRef.
- R. J. Morrison, K. N. Kashlan, C. L. Flanangan, J. K. Wright, G. E. Green, S. J. Hollister and K. J. Weatherwax, Clin. Transl. Sci., 2015, 8, 594–600 CrossRef.
- S. Al-Jawadi, P. Capasso and M. Sharma, Pharm. Dev. Technol., 2018, 23, 953–963 CrossRef CAS PubMed.
- N. A. White, T. J. O. Vrielink, K. E. van der Bogt, A. F. Cohen, J. I. Rotmans and T. Horeman, Br. J. Clin. Pharmacol., 2023, 89, 2144–2159 CrossRef PubMed.
- N. Jiang, J. E. Mück and A. K. Yetisen, Trends Biotechnol., 2020, 38, 129–133 CrossRef CAS PubMed.
- H. H. Bauer and M. Fischer, Int. Bus. Rev., 2000, 9, 703–725 CrossRef.
- G. D. Cha, D. Kang, J. Lee and D.-H. Kim, Adv. Healthcare Mater., 2019, 8, 1801660 CrossRef.
- D. S. Kohane and R. Langer, Chem. Sci., 2010, 1, 441–446 RSC.
- D. A. LaVan, T. McGuire and R. Langer, Nat. Biotechnol., 2003, 21, 1184–1191 CrossRef CAS PubMed.
- M. A. P. Mahmud, N. Huda, S. H. Farjana, M. Asadnia and C. Lang, Adv. Energy Mater., 2018, 8, 1701210 CrossRef.
- M. Privitera, M. Evans and D. Southee, Appl. Ergon., 2017, 59 Pt A, 251–263 CrossRef PubMed.
- D. Halperin, T. Kohno, T. S. Heydt-Benjamin, K. Fu and W. H. Maisel, IEEE Pervasive Comput., 2008, 7, 30–39 Search PubMed.
- J. Koo, S. B. Kim, Y. S. Choi, Z. Xie, A. J. Bandodkar, J. Khalifeh, Y. Yan, H. Kim, M. K. Pezhouh, K. Doty, G. Lee, Y.-Y. Chen, S. M. Lee, D. D'Andrea, K. Jung, K. Lee, K. Li, S. Jo, H. Wang, J.-H. Kim, J. Kim, S.-G. Choi, W. J. Jang, Y. S. Oh, I. Park, S. S. Kwak, J.-H. Park, D. Hong, X. Feng, C.-H. Lee, A. Banks, C. Leal, H. M. Lee, Y. Huang, C. K. Franz, W. Z. Ray, M. MacEwan, S.-K. Kang and J. A. Rogers, Sci. Adv., 2020, 6, eabb1093 CrossRef CAS.
- N. Di Trani, A. Silvestri, G. Bruno, T. Geninatti, C. Y. X. Chua, A. Gilbert, G. Rizzo, C. S. Filgueira, D. Demarchi and A. Grattoni, Lab Chip, 2019, 19, 2192–2204 RSC.
- H. Joo, Y. Lee, J. Kim, J.-S. Yoo, S. Yoo, S. Kim, A. K. Arya, S. Kim, S. H. Choi, N. Lu, H. S. Lee, S. Kim, S.-T. Lee and D.-H. Kim, Sci. Adv., 2021, 7, eabd4639 CrossRef CAS PubMed.
- M. L. Wang, P. Yeon, C. F. Chamberlayne, M. Mofidfar, H. Xu, J. P. Annes, R. N. Zare and A. Arbabian, 2021 IEEE Biomedical Circuits and Systems Conference (BioCAS), 2021, pp. 1–4 Search PubMed.
- M. L. Wang, C. F. Chamberlayne, H. Xu, M. Mofidfar, S. Baltsavias, J. P. Annes, R. N. Zare and A. Arbabian, RSC Adv., 2022, 12, 23337–23345 RSC.
- A. T. Evans, S. Chiravuri and Y. B. Gianchandani, J. Microelectromech. Syst., 2011, 20, 231–238 CAS.
- F. Forouzandeh, X. Zhu, A. Alfadhel, B. Ding, J. P. Walton, D. Cormier, R. D. Frisina and D. A. Borkholder, J. Controlled Release, 2019, 298, 27–37 CrossRef CAS PubMed.
- Y. Zhang, D. C. Castro, Y. Han, Y. Wu, H. Guo, Z. Weng, Y. Xue, J. Ausra, X. Wang, R. Li, G. Wu, A. Vázquez-Guardado, Y. Xie, Z. Xie, D. Ostojich, D. Peng, R. Sun, B. Wang, Y. Yu, J. P. Leshock, S. Qu, C.-J. Su, W. Shen, T. Hang, A. Banks, Y. Huang, J. Radulovic, P. Gutruf, M. R. Bruchas and J. A. Rogers, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 21427–21437 CrossRef CAS PubMed.
- C.-W. Dong, L.-G. Tran and W.-T. Park, J. Mech. Sci. Technol., 2021, 35, 697–706 CrossRef.
- A. Geipel, F. Goldschmidtboeing, P. Jantscheff, N. Esser, U. Massing and P. Woias, Biomed. Microdevices, 2008, 10, 469–478 CrossRef CAS PubMed.
- M. Humayun, A. Santos, J. C. Altamirano, R. Ribeiro, R. Gonzalez, A. de la Rosa, J. Shih, C. Pang, F. Jiang, P. Calvillo, J. Huculak, J. Zimmerman and S. Caffey, Transl. Vis. Sci. Technol., 2014, 3, 5 CrossRef PubMed.
- D. Samanta, R. Mehrotra, K. Margulis and R. N. Zare, Nanoscale, 2017, 9, 16429–16436 RSC.
- Y. Zhang, F. Liu, Y. Zhang, J. Wang, D. D'Andrea, J. B. Walters, S. Li, H.-J. Yoon, M. Wu, S. Li, Z. Hu, T. Wang, J. Choi, K. Bailey, E. Dempsey, K. Zhao, A. Lantsova, Y. Bouricha, I. Huang, H. Guo, X. Ni, Y. Wu, G. Lee, F. Jiang, Y. Huang, C. K. Franz and J. A. Rogers, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2217734120 CrossRef CAS PubMed.
- P.-Y. Li, J. Shih, R. Lo, S. Saati, R. Agrawal, M. S. Humayun, Y.-C. Tai and E. Meng, Sens. Actuators, A, 2008, 143, 41–48 CrossRef CAS.
- V. B. Bednar and K. Takahata, Biomed. Microdevices, 2021, 23, 18 CrossRef CAS PubMed.
- K. N. Noh, S. I. Park, R. Qazi, Z. Zou, A. D. Mickle, J. G. Grajales-Reyes, K.-I. Jang, R. W. Gereau IV, J. Xiao, J. A. Rogers and J.-W. Jeong, Small, 2017, 14, 1702479 CrossRef PubMed.
- Y. Zhang, A. D. Mickle, P. Gutruf, L. A. McIlvried, H. Guo, Y. Wu, J. P. Golden, Y. Xue, J. G. Grajales-Reyes, X. Wang, S. Krishnan, Y. Xie, D. Peng, C.-J. Su, F. Zhang, J. T. Reeder, S. K. Vogt, Y. Huang, J. A. Rogers and R. W. Gereau, Sci. Adv., 2019, 5, eaaw5296 CrossRef CAS PubMed.
- B. A. Darmawan, S. B. Lee, V. D. Nguyen, G. Go, K. T. Nguyen, H.-S. Lee, M. Nan, A. Hong, C.-S. Kim, H. Li, D. Bang, J.-O. Park and E. Choi, Sens. Actuators, B, 2020, 324, 128752 CrossRef CAS.
- X. Pi, Y. Lin, K. Wei, H. Liu, G. Wang, X. Zheng, Z. Wen and D. Li, Sens. Actuators, A, 2010, 159, 227–232 CrossRef CAS.
- S. P. Woods and T. G. Constandinou, IEEE Trans. Biomed. Eng., 2013, 60, 945–953 Search PubMed.
- M. Beccani, C. D. Natali, G. Aiello, C. Benjamin, E. Susilo and P. Valdastri, Procedia Eng., 2015, 120, 53–56 CrossRef.
- S. Woods and T. Constandinou, BioMed Res. Int., 2015, 2015, 741867 Search PubMed.
- V. H. Le, H. L. Rodriguez, C. Lee, G. Go, J. Zhen, V. D. Nguyen, H. Choi, S. Y. Ko, J.-O. Park and S. Park, Sens. Actuators, A, 2016, 243, 81–89 CrossRef CAS.
- S. P. Woods and T. G. Constandinou, J. Micro-Bio Robot., 2016, 11, 19–34 CrossRef PubMed.
- F. Munoz, G. Alici, H. Zhou, W. Li and M. Sitti, IEEE/ASME Trans. Mechatron., 2018, 23, 298–310 Search PubMed.
- J. Min, Y. Yang, Z. Wu and W. Gao, Adv. Ther., 2020, 3, 1900125 CrossRef.
- F. Forouzandeh, N. N. Ahamed, X. Zhu, P. Bazard, K. Goyal, J. P. Walton, R. D. Frisina and D. A. Borkholder, Pharmaceuticals, 2021, 14, 27–37 CrossRef PubMed.
- P. Song, S. Kuang, N. Panwar, G. Yang, D. J. H. Tng, S. C. Tjin, W. J. Ng, M. B. A. Majid, G. Zhu, K.-T. Yong and Z. L. Wang, Adv. Mater., 2017, 29, 1605668 CrossRef PubMed.
- C. Wang, S.-J. Seo, J.-S. Kim, S.-H. Lee, J.-K. Jeon, J.-W. Kim, K.-H. Kim, J.-K. Kim and J. Park, J. Controlled Release, 2018, 283, 105–112 CrossRef CAS PubMed.
- P.-Y. Li, T. K. Givrad, R. Sheybani, D. P. Holschneider, J.-M. I. Maarek and E. Meng, Lab Chip, 2010, 10, 101–110 RSC.
- G. Bruno, G. Canavese, X. Liu, C. S. Filgueira, A. Sacco, D. Demarchi, M. Ferrari and A. Grattoni, Nanoscale, 2016, 8, 18718–18725 RSC.
- A. Cobo, R. Sheybani, H. Tu and E. Meng, Sens. Actuators, A, 2016, 239, 18–25 CrossRef CAS PubMed.
- S. H. Lee, B. H. Kim, C. G. Park, C. Lee, B. Y. Lim and Y. B. Choy, J. Controlled Release, 2018, 286, 224–230 CrossRef CAS PubMed.
- S. H. Lee, Q. Wan, A. Wentworth, I. Ballinger, K. Ishida, J. E. Collins, S. Tamang, H.-W. Huang, C. Li, K. Hess, A. Lopes, A. R. Kirtane, J. S. Lee, S. Lee, W. Chen, K. Wong, G. Selsing, H. Kim, S. T. Buckley, A. Hayward, R. Langer and G. Traverso, Sci. Adv., 2021, 7, eabj4624 CrossRef CAS PubMed.
- A. T. Evans, S. Chiravuri and Y. B. Gianchandani, 2009 IEEE 22nd International Conference on Micro Electro Mechanical Systems, 2009, pp. 252–255 Search PubMed.
- D. G. Johnson and D. A. Borkholder, Micromachines, 2016, 7, 99 CrossRef PubMed.
- N. Di Trani, A. Silvestri, A. Sizovs, Y. Wang, D. R. Erm, D. Demarchi, X. Liu and A. Grattoni, Lab Chip, 2020, 20, 1562–1576 RSC.
- J. Fong, Z. Xiao and K. Takahata, Lab Chip, 2015, 15, 1050–1058 RSC.
- M. A. Zainal, A. Ahmad and M. S. Mohamed Ali, Biomed. Microdevices, 2017, 19, 8 CrossRef CAS PubMed.
- H. Gensler, R. Sheybani, P. Y. Li and E. Meng, Biomed. Microdevices, 2012, 14, 483–496 CrossRef CAS PubMed.
- R. Sheybani and E. Meng, 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2014, pp. 882–885 Search PubMed.
- R. Sheybani, A. Cobo and E. Meng, Biomed. Microdevices, 2015, 17, 74 CrossRef PubMed.
- Y. Yi, U. Buttner and I. G. Foulds, Lab Chip, 2015, 15, 3540–3548 RSC.
- Y. Yi, U. Buttner, A. A. A. Carreno, D. Conchouso and I. G. Foulds, J. Micromech. Microeng., 2015, 25, 105011 CrossRef.
- J. Zhou, A. Kim, M. Ochoa, H. Jiang and B. Ziaie, 2016 IEEE 29th International Conference on Micro Electro Mechanical Systems (MEMS), 2016, pp. 349–352 Search PubMed.
- Y. Yi and J. Kosel, Sens. Actuators, A, 2017, 261, 177–183 CrossRef CAS.
- Y. Yi, M. Chiao and B. Wang, Smart Mater. Struct., 2021, 30, 055003 CrossRef CAS.
- A. J. Chung, Y. S. Huh and D. Erickson, Biomed. Microdevices, 2009, 11, 861–867 CrossRef CAS PubMed.
- G. Jeon, S. Y. Yang, J. Byun and J. K. Kim, Nano Lett., 2011, 11, 1284–1288 CrossRef CAS PubMed.
- G. Bruno, T. Geninatti, R. L. Hood, D. Fine, G. Scorrano, J. Schmulen, S. Hosali, M. Ferrari and A. Grattoni, Nanoscale, 2015, 7, 5240–5248 RSC.
- G. Bruno, N. D. Trani, R. L. Hood, E. Zabre, C. S. Filgueira, G. Canavese, P. Jain, Z. Smith, D. Demarchi, S. Hosali, A. Pimpinelli, M. Ferrari and A. Grattoni, Nat. Commun., 2018, 9, 1682 CrossRef PubMed.
- T. T. Nguyen, M. Pham and N. S. Goo, J. Bionic Eng., 2008, 5, 135–141 CrossRef.
- P.-H. Cazorla, O. Fuchs, M. Cochet, S. Maubert, G. Le Rhun, Y. Fouillet and E. Defay, 2014 IEEE International Ultrasonics Symposium, 2014, pp. 491–494 Search PubMed.
- P.-H. Cazorla, O. Fuchs, M. Cochet, S. Maubert, G. Le Rhun, Y. Fouillet and E. Defay, Sens. Actuators, A, 2016, 250, 35–39 CrossRef CAS.
- A. T. Evans, J. M. Park, S. Chiravuri and Y. B. Gianchandani, Biomed. Microdevices, 2010, 12, 159–168 CrossRef CAS PubMed.
- N. Kumar, D. George, P. Sajeesh, P. V. Manivannan and A. K. Sen, J. Micromech. Microeng., 2016, 26, 075013 CrossRef.
- A. Zaher, S. Li, K. T. Wolf, F. Pirmoradi, O. Yassine, L. Lin, N. M. Khashab and J. Kosel, Biomicrofluidics, 2015, 9(5), 054113 CrossRef CAS PubMed.
- L. Zhang, Z. Lin, L. Zeng, F. Zhang, L. Sun, S. Sun, P. Wang, M. Xu, J. Zhang, X. Liang and H. Ge, Sci. China: Life Sci., 2021, 65, 896–908 CrossRef PubMed.
- S. Sirsi and M. Borden, Adv. Drug Delivery Rev., 2014, 72, 3–14 CrossRef CAS PubMed.
- B. Geers, H. Dewitte, S. D. D. Smedt and I. Lentacker, J. Controlled Release, 2012, 164(3), 248–255 CrossRef CAS PubMed.
- I. Lentacker, S. Smedt and N. Sanders, Soft Matter, 2009, 5, 2161–2170 RSC.
- M. Peng, Q. Zhao, A. Chai, Y. Wang, M. Wang and X. Du, Matter, 2025, 8, 101901 CrossRef CAS.
- J. Rivnay, R. Raman, J. T. Robinson, C. Schreib, T. Cohen-Karni, K. E. Galloway and O. Veiseh, Nat. Rev. Bioeng., 2025, 317–332 CrossRef CAS.
- Y. Zhang, H.-H. Hsu and X. Jiang, Nano Res., 2014, 1205–1213 CAS.
- J. T. Atkinson, M. S. Chavez, C. M. Niman and M. Y. El-Naggar, Microb. Biotechnol., 2023, 16, 507–533 CrossRef CAS PubMed.
- D. J. H. Tng, R. Hu, P. Song, I. Roy and K.-T. Yong, Micromachines, 2012, 3, 615–631 CrossRef.
- N. Di Trani, F. P. Pons-Faudoa, A. Sizovs, K. A. Shelton, M. A. Marzinke, P. N. Nehete and A. Grattoni, Adv. Ther., 2022, 5, 2100214 CrossRef CAS PubMed.
- J. Charthad, S. Baltsavias, D. Samanta, T. C. Chang, M. J. Weber, N. Hosseini-Nassab, R. N. Zare and A. Arbabian, 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2016, pp. 541–544 Search PubMed.
- K. Moussi, M. AlDajani and J. Kosel, 2019 IEEE 14th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), 2019, pp. 97–100 Search PubMed.
- A. B. Amar, A. Kouki and H. Cao, Sensors, 2015, 15, 28889–28914 CrossRef PubMed.
- K. Agarwal, R. Jegadeesan, Y. X. Guo and N. Thakor, IEEE Rev. Biomed. Eng., 2017, 10, 136–161 Search PubMed.
- S. R. Khan, S. Pavuluri, G. Cummins and M. Desmulliez, Sensors, 2020, 20, 3487 CrossRef CAS PubMed.
- G. L. Barbruni, P. M. Ros, D. Demarchi, S. Carrara and D. Ghezzi, IEEE Trans. Biomed. Circuits Syst., 2020, 14, 1160–1178 Search PubMed.
- M. Haerinia and R. Shadid, Signals, 2020, 1, 209–229 CrossRef.
- V. Nair, A. N. Dalrymple, Z. Yu, G. Balakrishnan, C. J. Bettinger, D. J. Weber, K. Yang and J. T. Robinson, Science, 2023, 382, eabn4732 CrossRef CAS PubMed.
- M. Karimi, A. Schmid and C. Dehollain, IEEE Sens. J., 2021, 21, 7145–7161 Search PubMed.
- Z. Gao, Y. Zhou, J. Zhang, J. Foroughi, S. Peng, R. H. Baughman, Z. L. Wang and C. H. Wang, Adv. Mater., 2024, 36, 2404492 CrossRef CAS PubMed.
- O. Saha, B. D. Truong and S. Roundy, Smart Mater. Struct., 2022, 31, 113001 CrossRef.
- J. Kim, J. Seo, D. Jung, T. Lee, H. Ju, J. Han, N. Kim, J. Jeong, S. Cho, J. H. Seol and J. Lee, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 16856–16863 CrossRef CAS PubMed.
- S. Lyu, Y. He, X. Tao, Y. Yao, X. Huang, Y. Ma, Z. Peng, Y. Ding and Y. Wang, Nat. Commun., 2022, 13, 6596 CrossRef CAS PubMed.
- H. Kim, B. Rigo and G. Wong, et al., Nano-Micro Lett., 2024, 16, 52 CrossRef PubMed.
- D. Bock, A. Marschilok, K. Takeuchi and E. Takeuchi, Electrochim. Acta, 2012, 84, 155–164 CrossRef CAS PubMed.
- R. V. Taalla, M. S. Arefin, A. Kaynak and A. Kouzani, IEEE Access, 2019, 7, 2092–2106 Search PubMed.
- M. L. Wang, P. Yeon, M. Mofidfar, C. Chamberlayne, H. Xu, J. P. Annes, R. N. Zare and A. Arbabian, IEEE Trans. Biomed. Circuits Syst., 2024, 1–14 Search PubMed.
- A. Silvestri, N. Di Trani, G. Canavese, P. Motto Ros, L. Iannucci, S. Grassini, Y. Wang, X. Liu, D. Demarchi and A. Grattoni, Membranes, 2021, 11, 535 CrossRef CAS PubMed.
- F. Del Bono, A. Bontempi, A. Dentis, N. D. Trani, D. Demarchi, A. Grattoni and P. M. Ros, IEEE Sens. J., 2024, 24, 7345–7354 CAS.
- F. Del Bono, N. Di Trani, D. Demarchi, A. Grattoni and P. M. Ros, 2024 IEEE Sensors, 2024, pp. 1–4 Search PubMed.
- M. Osama, A. A. Ateya, M. S. Sayed, M. Hammad, P. Pławiak, A. A. Abd El-Latif and R. A. Elsayed, Sensors, 2023, 23, 7435 CrossRef PubMed.
- G. R. Pradyumna, R. B. Hegde, K. B. Bommegowda, T. Jan and G. R. Naik, IEEE Access, 2024, 12, 20603–20623 Search PubMed.
- Y. Yang, H. Wang, R. Jiang, X. Guo, J. Q. Cheng and Y. Chen, IEEE Internet Things J., 2022, 9, 9478–9502 Search PubMed.
- M. Akkaş, R. Sokullu and H. E. Çetin, IEEE Internet Things J., 2020, 11, 100173 CrossRef.
- G. Traverso, V. Finomore, J. Mahoney, J. Kupec, R. Stansbury, D. Bacher, B. Pless, S. Schuetz, A. Hayward, R. Langer and A. Rezai, Device, 2023, 1, 100125 CrossRef.
- P. Ahmmed, J. Reynolds and A. Bozkurt, IEEE Sens. J., 2024, 24, 11205–11216 CAS.
- M. Mariello, I. Eş and C. M. Proctor, Adv. Healthcare Mater., 2024, 13, 2302969 CAS.
- S.-K. Kang, J. Koo, Y. K. Lee and J. A. Rogers, Acc. Chem. Res., 2018, 51, 988–998 CrossRef CAS PubMed.
- X. Huang, D. Wang, Z. Yuan, W. Xie, Y. Wu, R. Li, Y. Zhao, D. Luo, L. Cen, B. Chen, H. Wu, H. Xu, X. Sheng, M. Zhang, L. Zhao and L. Yin, Small, 2018, 14, 1800994 CrossRef PubMed.
- C. Fernandes and I. Taurino, Sensors, 2022, 22, 3062 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |






