Open Access Article
Open Access ArticlePhotocatalytically active metal–organic derived ensembles for organic pollutant degradation†
Clara
López-García
,
Celia
Castillo-Blas‡
 ,
Marta
Iglesias
,
Marta
Iglesias
 ,
M. Angeles
Monge
,
M. Angeles
Monge
 ,
Enrique
Gutiérrez-Puebla
,
Enrique
Gutiérrez-Puebla
 * and
Felipe
Gándara
* and
Felipe
Gándara
 *
*
Materials Science Institute of Madrid – Spanish National Research Council (ICMM-CSIC), C/Sor Juana Inés de la Cruz 3, 28049, Madrid, Spain. E-mail: egutierrez@icmm.csic.es; gandara@icmm.csic.es
First published on 15th August 2024
Abstract
The thermal treatment of metal–organic frameworks (MOFs) incorporating multiple metal elements produces solids with specific features that strongly depend on the parent MOF composition. Thus, metal–organic derived ensembles (MODEs) can be obtained from the pyrolysis process of multi-metal MOFs and can be composed of differently distributed chemical components. In particular, here we show how a series of isoreticular multi-metal MOFs with different combinations of up to four metal elements (Zn, Co, Mn, Ca) subjected to the same thermal process produce complex materials with similar yet distinct composition and distribution of their components. Their characterization using a combination of techniques shows that thermal treatment under a nitrogen atmosphere results in the formation of solids with the presence of metal nanoparticles supported on metal fluoride, which are embedded in a carbonaceous matrix. We prove that they are effective in the photocatalytic degradation of organic pollutants, such as organic dyes or drugs.
Introduction
Metal–organic frameworks (MOFs) are a class of materials built from the combination of metal elements and organic linkers to create periodic structures with potential porosity.1 Multi-variate (MTV), multi-metal (MM) MOFs incorporate different metal elements in topologically equivalent positions, and they provide a suitable platform for creating materials of increasing complexity by combining different arrangements of metal cations.2,3 In addition, the thermal treatment of MM-MTV-MOFs is an attractive strategy to obtain other types of complex solids with adjustable activity, for example, to be used as catalysts in reactions that require conditions that are not compatible with the direct use of the MOFs.4–7 Thus, calcination of multi-metal MOFs results in the formation of multi-metal oxides with various compositions,8–13 although exerting control over the metal ratios is still challenging. We have already shown that these metal–organic derived oxides might then be used as electrocatalysts in the oxygen reduction reaction,14 or as pre-catalysts for the reverse water gas shift reaction.15 Alternatively, pyrolysis of MM-MTV-MOFs typically leads to the formation of hybrid materials, such as carbonaceous species containing metal or metal oxide nanoparticles. Materials obtained through this route have already been employed for several applications,16–18 including the catalytic degradation of organic pollutants from water.19–28 Nowadays, the presence of organic pollutants in water represents a relevant societal problem for a number of reasons. Many organic contaminants have been linked to health risks, and exposure to them through water consumption or contact might represent a health issue. Besides, their presence might have a significant impact on the environment and damage aquatic ecosystems, negatively affecting the availability of resources. Moreover, water contamination by organic pollutants results in increased economic costs associated with their removal, or in decreased productivity from water-dependent industries, which negatively affects communities. Therefore, removing pollutants from water has become a major concern and great efforts are being made to develop efficient methods for removing them.Two classes of the most commonly found organic pollutants are dyes and pharmaceuticals. Removal of organic pollutants might be achieved by different methods, including adsorption, coagulation, filtration, biological treatment, or chemical oxidation.29–33 Photocatalytic degradation is a very attractive approach, particularly if it can be accomplished with the use of visible light.34 Different types of materials are being investigated for this purpose.35 Among the most studied materials are inorganic semiconductors such as titanium and zinc oxides (TiO2 and ZnO), which, due to their powerful oxidation capability, have been successfully used in organic,36 inorganic37 and pathogenic38,39 pollutant degradation processes. Other semiconductors with band gaps of less than 3 eV have also been used as photocatalysts for pollutant degradation.40,41 Among photocatalysts whose activity has been studied and shown favourable results are nanomaterials doped with different metals, successfully used in water remediation processes,42–44 and composites formed from different semiconductors, which show an improvement of photocatalytic activity for pollutant degradation compared to isolated semiconductors.45,46 To improve photocatalyst recyclability and recovery in water decontamination processes, immobilised photocatalysts have been extensively studied in the last few years.47,48 Carbon-based nanomaterials,49,50 glass,51,52 clays,53,54 polymers55,56 and zeolites57 have been studied as substrates or support materials for these processes.
Following, we have investigated MM-MTV-MOFs comprising combinations of up to four different metal elements as precursors to generate complex solids with photocatalytic activity in the degradation of pollutants. Particularly, we have employed MOFs with various combinations of zinc, cobalt, calcium and manganese to generate new metal–organic derived ensembles (MODEs) through a pyrolysis process. The resulting materials have been characterized using a combination of techniques showing that each one of the different metal elements initially incorporated in the MOF atomic sequence affords a different chemical component in the resulting MODE (Scheme 1). We show that they are effective photocatalysts for the degradation of organic dyes under visible light irradiation, and one of the studied materials is also active in the photodegradation of paracetamol.
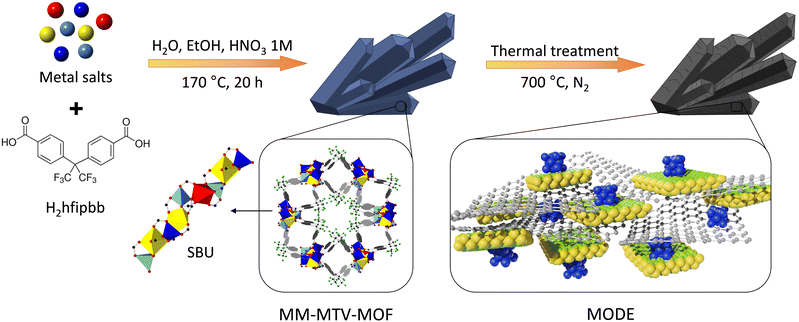 | ||
| Scheme 1 Different metal elements are arranged in the secondary building unit of MOFs and subsequently pyrolyzed to produce metal–organic derived ensembles. | ||
Results and discussion
To synthesize new metal–organic derived ensembles, various isoreticular ZnMnCoCa MOFs were synthesized by previously reported solvothermal methods.58 In a typical synthesis, the organic linker 4,4′-(hexafluoroisopropylidene)bis(benzoic acid) (H2hfipbb) and the selected metal elements were mixed and dissolved in a mixture of 10 mL of water, 10 mL of ethanol, and 0.6 mL of 1 M HNO3, keeping a linker to metal ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.65. The mixtures were heated at 170 °C for an overnight period, and the resulting solids were filtered and washed with water and ethanol. For the MOF synthesis, we chose initial Zn
1.65. The mixtures were heated at 170 °C for an overnight period, and the resulting solids were filtered and washed with water and ethanol. For the MOF synthesis, we chose initial Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn
Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co
Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca metal ratios of 1
Ca metal ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 1
7, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, which afforded compounds with compositions Zn0.37Mn0.28Co0.13Ca0.22(hfipbb), Zn0.16Mn0.47Co0.33Ca0.03(hfipbb), and Zn0.46Mn0.21Co0.17Ca0.15(hfipbb), respectively, as determined by ICP-MS analysis (see S1† for details). We chose these initial ratios, as they were previously proved to generate highly active multimetal oxides.
2, which afforded compounds with compositions Zn0.37Mn0.28Co0.13Ca0.22(hfipbb), Zn0.16Mn0.47Co0.33Ca0.03(hfipbb), and Zn0.46Mn0.21Co0.17Ca0.15(hfipbb), respectively, as determined by ICP-MS analysis (see S1† for details). We chose these initial ratios, as they were previously proved to generate highly active multimetal oxides.
Their corresponding MODEs (Scheme 1, right) were obtained from a pyrolysis process by heating the selected MOF precursor in a furnace under a N2 flow in two steps, first from room temperature to 80 °C with a heating rate of 0.3 °C min−1, and then from 80 °C to 700 °C with a heating rate of 5 °C min−1. Once this temperature was reached, the samples were kept at 700 °C for 4 h, and then cooled down to room temperature. The resulting solids, which are herein labelled with the number corresponding to the molar ratio employed for the synthesis of their MOF precursors, were characterized by means of powder X-ray diffraction (PXRD), scanning electron microscopy (SEM) and transmission electron microscopy (TEM).
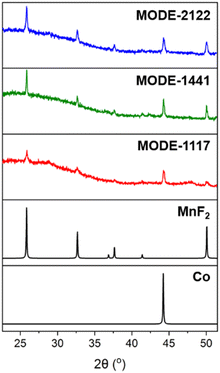
The experimental PXRD patterns for the samples are consistent with the presence of metallic cobalt and MnF2 as the only detectable crystalline phases (Fig. 1). Previously, we observed that under a reducing environment, such as a hydrogen atmosphere and high temperature (700 °C), cobalt atoms present in metal–organic derived oxides (MODOs) are reduced and form metal nanoparticles. In the present case, metallic cobalt is formed directly from the heating of the MOF under a nitrogen atmosphere, with a reducing environment that is likely provided by the simultaneous decomposition of the organic linker, as previously observed with other MOFs,59 which in addition provides the fluorine atoms for the formation of manganese fluoride.
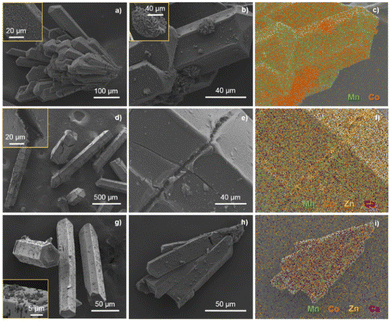
Scanning electron microscopy (SEM) images show that the morphology of the MOF precursor particles (Fig. S1†) is generally preserved after the pyrolysis process, as shown in Fig. 2 where the SEM images of the prepared MODEs are displayed. In the case of MODE-1441, the particles are formed by aggregates, which are clearly visible on the surface. For the remaining MODE combinations, the metal distribution is more uniform along the surface, with only minor aggregates observed for Co in MODE-2122 (Fig. 2, right). The energy dispersive X-ray spectroscopy (EDX) study (Fig. 2c) revealed that in all cases, carbon is clearly present on the particle surfaces. As for the presence of the different metal elements, these appear with varying distributions for each material, illustrating the influence of the initial distribution in the MOF on the resulting MODE. Thus, in the case of MODE-1441, elemental mapping shows areas with higher concentrations of cobalt, while manganese is more evenly distributed. Signals for zinc and calcium only appear as background noise, which is consistent with the point EDX measurements carried out for this sample that did not show the presence of these elements (Fig. S2†). The absence of zinc is consistent with the low melting point of this element (419.5 °C), indicating that it is mostly carried away during the pyrolysis process.60 Nevertheless, the use of zinc is required as a structure directing element for the preparation of the MOFs.58 The use of calcium is motivated by its previously demonstrated beneficial role in activity;15 however, the small initial amount of this element results in the impossibility to detect it within the surfaces (Fig. S3†).
In contrast, the elemental mapping of MODE-1117, which is obtained from a MOF with a much higher amount of calcium, shows the clear presence of this element (Fig. S5†). Moreover, calcium is found to be distributed similarly to manganese and fluoride and no particular area of high calcium concentration is visible in the EDX maps, suggesting that it is incorporated now in the same manganese fluoride structure that was identified with PXRD. Cobalt is found to be evenly distributed along the entire MODE. Interestingly, zinc is now also detected on the particle surfaces, particularly concentrated in areas where the MOF crystals are cracked, and small (sub-micron) needles are observed (Fig. 2e, f and S4†).
Finally, elemental mapping of MODE-2122 shows the absence of zinc on the surfaces, but the presence of calcium, although in a significantly smaller amount, but distributed along the entire MODE. Cobalt is specifically found on visible small surface particles, whereas Mn distribution follows Ca and F (Fig. S6 and S7†).
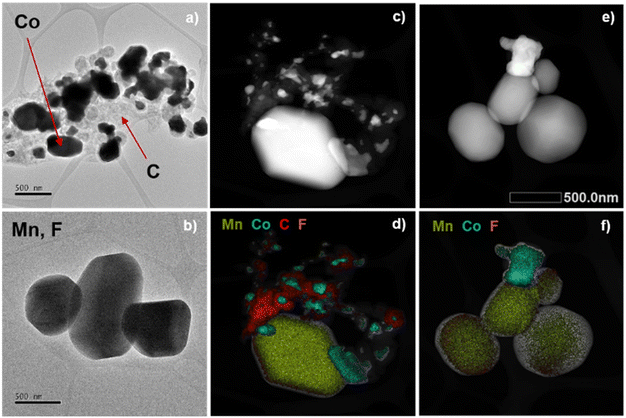
To further investigate the different components in one of the MODE materials, a transmission electron microscopy (TEM) analysis was carried out. For this, the particles of MODE-1441 were first dispersed with the help of ultrasound. The TEM images show the presence of two types of particles with different sizes, supported on a visible carbonaceous matrix (Fig. 3a and b and S8–S10†). The EDX mapping of a sample region clearly shows that cobalt and manganese are segregated in different particles, with some of them being mostly composed of manganese and fluoride, while others are made of just cobalt (Fig. 3c–f, S11 and S12†). The combined results of the PXRD, SEM, TEM and EDX analyses confirm that the MODEs resulting from the pyrolysis process are formed by cobalt nanoparticles supported on manganese fluoride and embedded in a carbonaceous matrix. For samples obtained from MOFs with a larger amount of calcium, this element is incorporated into the MnF2 particles, whereas zinc is typically lost as a volatile species during the pyrolysis process, and thus it is not observed in the MODEs.
Photocatalytic activity studies
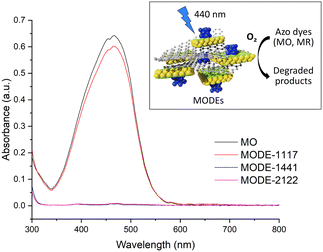
The ability of MODEs as photocatalysts was evaluated by UV-visible spectroscopy (290–800 nm) in the degradation of two organic dyes namely methyl orange (MO) and methyl red (MR). Experiments were carried out in a closed reactor, into which a radiation source (blue light, λ = 440 nm) was introduced together with the vials arranged in an adapter holder (see S3.1† for details). The MODE samples (5 mg) were stirred in 5 mL of a 10 ppm dye solution under an O2 atmosphere at room temperature, whereupon they were separated by centrifugation and the UV-vis absorption spectrum of the resulting solution was measured. Fig. 4 shows the absorption spectra for the MO solutions after 18 h reaction in the presence of the three MODEs. Blank experiments indicate that no photodegradation occurs under an air or O2 atmosphere in the absence of any catalyst. The spectra show significant differences in the activity of MODE-1117, which only achieves 9% degradation, while MODE-1441 and MODE-2122 reach 100% degradation. In the case of MODE-2122, 58% and 85% degradation rates are achieved after only 1 and 5 h, respectively (Fig. S14†).Considering that the main observed difference among the three MODEs during the SEM study was that the presence of Co particles aggregated with MnF2 particles was visible for MODE-1441 and MODE-2122, but not for MODE-1117, it is reasonable to think that this combination has a major influence on the photodegradation activity for MO.
The synergetic effect offered by the multiple components present in the MODEs is further demonstrated by the fact that the MOF precursor (1441) did not show any degradation ability (Fig. S15†). Conversely, other MOF derived oxides with various compositions were also tested, equally showing nearly null photodegradation activity (Fig. S17†), further demonstrating that the supported particles conforming the MODEs are the active species for this process.
To investigate the capability to degrade other contaminants, we also tested the activity of MODE-1441 and MODE-1117 in the photodegradation of another dye (methyl red) as well as in the photodegradation of the drug paracetamol. In the case of MR, using the same light source and O2 atmosphere, 100% and 72% photodegradation was achieved after 18 h for MODE-1441 and MODE-1117, respectively (Fig. S16†).
Paracetamol (PMA) photodegradation studies were performed following a similar procedure, but under a violet light ring as a radiation source (λ = 405 nm), under an air atmosphere and with a reaction time of 18 hours. For all tests, 4 mL of a 1000 ppm paracetamol solution was used. Paracetamol quantification after the photocatalytic reaction was monitored by means of chromatographic techniques, specifically HPLC combined with UV-vis detection. A solution of naphthol (Nph) (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm) was used as a reference. The HPLC conditions used for all measurements were as follows: flow rate 0.7 mL min−1, mobile phase distilled water
000 ppm) was used as a reference. The HPLC conditions used for all measurements were as follows: flow rate 0.7 mL min−1, mobile phase distilled water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile (80
acetonitrile (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) and wavelength 297 nm. For reference conditions, paracetamol and naphthol signals appear at 2.992 min and 2.835 min, respectively. A PMA
20) and wavelength 297 nm. For reference conditions, paracetamol and naphthol signals appear at 2.992 min and 2.835 min, respectively. A PMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Nph mixture was used to obtain the calibration curve for the paracetamol concentration, using PMA standards in concentrations between 0 and 1000 ppm and a fixed Nph 10
Nph mixture was used to obtain the calibration curve for the paracetamol concentration, using PMA standards in concentrations between 0 and 1000 ppm and a fixed Nph 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm volume. To determine the PMA concentration, the area ratio between Nph and NPh + PMA was measured. The obtained data fit the equation:
000 ppm volume. To determine the PMA concentration, the area ratio between Nph and NPh + PMA was measured. The obtained data fit the equation:
| ANph/APMA = 0.34 + 6.46 × e(−CPMA/132.5) (Fig. S18†) |
In previous studies of paracetamol degradation, sulphuric acid was used to adjust the pH of the medium,61 so the studies were carried out in the presence and absence of the acid. It was observed that under the above-mentioned reaction conditions and in the absence of a catalyst, a 1000 ppm paracetamol solution was degraded by 7.7%, and by 16.3% with the addition of 100 μl H2SO4. In the presence of MODE-1441 and under the same conditions, the paracetamol degradation rate amounted to 26.9% in the absence of the acid, and up to 45.9% by adding 100 μl of H2SO4 (pH = 3), indicating a three-fold increase in the degradation rate thanks to the activity of the catalyst and demonstrating the potential use of the material under harsh conditions (see S3.3† for details).
Conclusions
In summary, we have reported here the preparation and characterization of three new materials obtained through the pyrolysis process of multi-metal MOFs. The resulting metal–organic derived ensembles, MODEs, are demonstrated to be formed by a carbonaceous matrix, with embedded manganese fluoride particles that contain cobalt nanoparticles on their surface. These materials are demonstrated to be highly active in the photodegradation processes of water contaminants such as organic dyes or paracetamol, thanks to the synergetic activity of the different chemical components.In light of these results, the present study provides additional evidence of the potential applications of complex systems derived from multi-metal MOFs, thereby encouraging further research on the correlation between the composition of the initial MOF and the final composition in its complex derived systems. This may facilitate the development of MOF-derived ensembles with optimized ratios for a range of applications.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Conceptualization: MAM, EGP, and FG; formal analysis: CLG, MI, MAM, EGP, and FG; funding acquisition: MI, EGP, MAM, and FG; investigation: CLG and CCB; writing – original draft: CLG and FG; writing – review & editing: all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has received financial support through projects PID2021-123287OB-I00, PID2020-112590GB-C22, and PID2019-107675RB-100, funded by MCIN/AEI/10.13039/501100011033 and ERDF A way to make Europe, and PLEC2021-007906 (SOLFuture) funded by MCIN/AEI/10.13039/501100011033 and European Union NextGenerationEU/PRTR.Notes and references
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- L. Chen, H.-F. Wang, C. Li and Q. Xu, Chem. Sci., 2020, 11, 5369–5403 RSC.
- J. Castells-Gil, N. Almora-Barrios, B. Lerma-Berlanga, N. M. Padial and C. Martí-Gastaldo, Chem. Sci., 2023, 14, 6826–6840 RSC.
- S. Wang, W. Xu and W. Ma, Mater. Lett., 2024, 362, 136205 CrossRef CAS.
- P. Duan, H. Wang, H. Zhou, S. Zhang, X. Meng, Q. Duan, K. Jin and J. Sun, J. Colloid Interface Sci., 2024, 660, 974–988 CrossRef CAS PubMed.
- G. Asghar, M. Fiaz, M. A. Farid, M. N. Ashiq and M. Athar, Int. J. Hydrogen Energy, 2024, 51, 1435–1447 CrossRef CAS.
- T. Wu, F. Ren, Z. Guo, J. Zhang, X. Hou, Z. Chen, Y. Jin and P. Ren, J. Alloys Compd., 2024, 976, 172984 CrossRef CAS.
- D. Huang, G. Wang, M. Cheng, G. Zhang, S. Chen, Y. Liu, Z. Li, W. Xue, L. Lei and R. Xiao, Chem. Eng. J., 2021, 421, 127817 CrossRef CAS.
- S.-R. Zhu, M.-K. Wu, W.-N. Zhao, F.-Y. Yi, K. Tao and L. Han, J. Solid State Chem., 2017, 255, 17–26 CrossRef CAS.
- J.-L. Niu, H.-J. Peng, C.-H. Zeng, X.-M. Lin, P. Sathishkumar, Y.-P. Cai and A.-W. Xu, Chem. Eng. J., 2018, 336, 510–517 CrossRef CAS.
- X. Zhang, S. Xiang, Q. Du, F. Bi, K. Xie and L. Wang, Mol. Catal., 2022, 522, 112226 CrossRef CAS.
- Y. Hao, G. Du, Y. Fan, L. Jia, D. Han, W. Zhao, Q. Su and B. Xu, Appl. Surf. Sci., 2023, 614, 156237 CrossRef CAS.
- N. Sun, L. Wang, Y. Zhang, Z. Cao and J. Sun, ACS Appl. Nano Mater., 2023, 6, 18823–18836 CrossRef CAS.
- C. Castillo-Blas, N. López-Salas, M. C. Gutiérrez, I. Puente-Orench, E. Gutiérrez-Puebla, M. L. Ferrer, M. Á. Monge and F. Gándara, J. Am. Chem. Soc., 2019, 141, 1766–1774 CrossRef CAS PubMed.
- C. Castillo-Blas, C. Álvarez-Galván, I. Puente-Orench, A. García-Sánchez, F. E. Oropeza, E. Gutiérrez-Puebla, Á. Monge, V. A. de la Peña-O'Shea and F. Gándara, Nano Res., 2021, 14, 493–500 CrossRef CAS.
- Z. Lu, L. Guo, Q. Shen, F. Bi, C. Li and X. Zhang, Sep. Purif. Technol., 2024, 340, 126772 CrossRef CAS.
- S. Ren, P. Mo, A. Shui, J. Qian and B. Du, J. Mater. Sci.: Mater. Electron., 2024, 35, 530 CrossRef CAS.
- X. Chen, M. X. Li, J. L. Yan and L. L. Zhang, Xinxing Tan Cailiao, 2024, 39, 78–99 CAS.
- F. Wang, Y. Gao, S.-S. Liu, X.-H. Yi, C.-C. Wang and H. Fu, Chem. Eng. J., 2023, 463, 142466 CrossRef CAS.
- J. Xu, J. Gao, Y. Liu, Q. Li and L. Wang, Mater. Res. Bull., 2017, 91, 1–8 CrossRef CAS.
- S.-W. Lv, J.-M. Liu, N. Zhao, C.-Y. Li, F.-E. Yang, Z.-H. Wang and S. Wang, Sep. Purif. Technol., 2020, 253, 117413 CrossRef CAS.
- Y. Wang, X. Liu, L. Guo, L. Shang, S. Ge, G. Song, N. Naik, Q. Shao, J. Lin and Z. Guo, J. Colloid Interface Sci., 2021, 599, 566–576 CrossRef CAS PubMed.
- L. Yu, Y. Zhao, S. Guo and J. Xue, Catal. Lett., 2024 DOI:10.1007/s10562-024-04654-6.
- T. Jiang, X. Wang, J. Zhang, Y. Mai and J. Chen, Microporous Mesoporous Mater., 2024, 368, 113035 CrossRef CAS.
- W. L. Ma, Y. Q. Zhang, W. Z. Li, J. Li and J. Luan, Dalton Trans., 2024, 53, 4314–4324 RSC.
- W. Ji, W. Li, Y. Wang, T. C. Zhang and S. Yuan, Sep. Purif. Technol., 2024, 334, 126003 CrossRef CAS.
- Y. Li, D. Ren, S. Guo, M. Wang, J. Zhai, S. Zhang, X. Gong and X. Zhang, J. Mol. Struct., 2024, 1299, 137058 CrossRef CAS.
- L. Zhang, J. Chen, L. B. Song, J. J. Pan and Q. Luo, Prog. Nat. Sci.: Mater. Int., 2023, 33, 544–550 CrossRef CAS.
- F. Yu, X. Bai, M. Liang and J. Ma, Chem. Eng. J., 2021, 405, 126960 CrossRef CAS.
- G. Zhu, Y. Bian, A. S. Hursthouse, S. Xu, N. Xiong and P. Wan, Chemosphere, 2020, 247, 125921 CrossRef CAS PubMed.
- X. Yin, S. Tang, Q. Yong, X. Zhang and J. M. Catchmark, Sep. Purif. Technol., 2021, 276, 119366 CrossRef CAS.
- Z. A. Stoll, C. Forrestal, Z. J. Ren and P. Xu, J. Hazard. Mater., 2015, 283, 847–855 CrossRef CAS PubMed.
- J. Liu, L. Zhao, H. Geng, B. Wang, X. Tong, Y. Li, D. Chen, P. Sun and Y. Yang, J. Environ. Chem. Eng., 2023, 11, 110649 CrossRef CAS.
- A. J. Chacón-García, S. Rojas, E. S. Grape, F. Salles, T. Willhammar, A. K. Inge, Y. Pérez and P. Horcajada, Sci. Rep., 2024, 14, 7882 CrossRef PubMed.
- V. K. Parida, S. K. Srivastava, A. K. Gupta and A. Rawat, Mater. Express, 2023, 13, 1–38 CrossRef CAS.
- X. Chen, Z. Wu, D. Liu and Z. Gao, Nanoscale Res. Lett., 2017, 12, 143 CrossRef PubMed.
- Z. Wei, Y. Fang, Z. Wang, Y. Liu, Y. Wu, K. Liang, J. Yan, Z. Pan and G. Hu, Chemosphere, 2019, 225, 434–442 CrossRef CAS PubMed.
- C.-Y. Chen, L.-C. Wu, H.-Y. Chen and Y.-C. Chung, Water, Air, Soil Pollut., 2010, 212, 231–238 CrossRef CAS.
- J. Lee, K. Zoh and G. Ko, Appl. Environ. Microbiol., 2008, 74, 2111–2117 CrossRef CAS PubMed.
- T. O. Ajiboye, O. A. Oyewo and D. C. Onwudiwe, Surf. Interfaces, 2021, 23, 100927 CrossRef CAS.
- Q. Zheng, D. P. Durkin, J. E. Elenewski, Y. Sun, N. A. Banek, L. Hua, H. Chen, M. J. Wagner, W. Zhang and D. Shuai, Environ. Sci. Technol., 2016, 50, 12938–12948 CrossRef CAS PubMed.
- G. Palani, R. Apsari, M. M. Hanafiah, K. Venkateswarlu, S. K. Lakkaboyana, K. Kannan, A. T. Shivanna, A. M. Idris and C. H. Yadav, Nanomaterials, 2022, 12, 1754 CrossRef CAS PubMed.
- C. Adán, A. Bahamonde, I. Oller, S. Malato and A. Martínez-Arias, Appl. Catal., B, 2014, 144, 269–276 CrossRef.
- Y. An, D. J. de Ridder, C. Zhao, K. Schoutteten, J. Vanden Bussche, H. Zheng, G. Chen and L. Vanhaecke, Water Sci. Technol., 2016, 73, 2868–2881 CrossRef CAS PubMed.
- N. Li, J. Zhang, Y. Tian, J. Zhao, J. Zhang and W. Zuo, Chem. Eng. J., 2017, 308, 377–385 CrossRef CAS.
- C. Belver, J. Bedia, M. Peñas-Garzón, V. Muelas-Ramos, A. Gómez-Avilés and J. J. Rodriguez, in Visible Light Active Structured Photocatalysts for the Removal of Emerging Contaminants, Elsevier, 2020, pp. 41–98 Search PubMed.
- H. Chawla, S. Garg, P. P. Ingole and A. Chandra, in Green Photocatalytic Semiconductors, Springer US, 2022, pp. 445–473 Search PubMed.
- V. Parvulescu, M. Ciobanu and G. Petcu, in Handbook of Smart Photocatalytic Materials, Elsevier, 2020, pp. 103–140 Search PubMed.
- T. V. H. Luu, M. D. Luu, N. N. Dao, V. T. Le, H. T. Nguyen and V. D. Doan, J. Dispersion Sci. Technol., 2021, 42, 1311–1322 CrossRef CAS.
- W. Liu, Y. Li, F. Liu, W. Jiang, D. Zhang and J. Liang, Water Res., 2019, 151, 8–19 CrossRef CAS PubMed.
- A. Akbari Shorgoli and M. Shokri, Chem. Eng. Commun., 2017, 204, 1061–1069 CrossRef CAS.
- V. Vaiano and G. Iervolino, J. Colloid Interface Sci., 2018, 518, 192–199 CrossRef CAS PubMed.
- E. Hass Caetano Lacerda, F. C. Monteiro, J. R. Kloss and S. T. Fujiwara, J. Photochem. Photobiol., A, 2020, 388, 112084 CrossRef CAS.
- E. Magnone, M. K. Kim, H. J. Lee and J. H. Park, Ceram. Int., 2021, 47, 7764–7775 CrossRef CAS.
- H. S. Zakria, M. H. D. Othman, R. Kamaludin, S. H. Sheikh Abdul Kadir, T. A. Kurniawan and A. Jilani, RSC Adv., 2021, 11, 6985–7014 RSC.
- F. Gao, X. Hou, A. Wang, G. Chu, W. Wu, J. Chen and H. Zou, Particuology, 2016, 26, 73–78 CrossRef CAS.
- J. Liu, H. Lin, Y. Dong, Y. He and C. Liu, Chemosphere, 2022, 287, 132211 CrossRef CAS PubMed.
- C. Castillo-Blas, V. A. De La Peña-O'Shea, I. Puente-Orench, J. R. De Paz, R. Sáez-Puche, E. Gutiérrez-Puebla, F. Gándara and Á. Monge, Sci. Adv., 2017, 3, 1–11 Search PubMed.
- Y. Wu, Z. Huang, H. Jiang, C. Wang, Y. Zhou, W. Shen, H. Xu and H. Deng, ACS Appl. Mater. Interfaces, 2019, 11, 44573–44581 CrossRef CAS PubMed.
- P. Yin, T. Yao, Y. Wu, L. Zheng, Y. Lin, W. Liu, H. Ju, J. Zhu, X. Hong, Z. Deng, G. Zhou, S. Wei and Y. Li, Angew. Chem., Int. Ed., 2016, 55, 10800–10805 CrossRef CAS PubMed.
- G. E. do Nascimento, M. A. Soares Oliveira, R. M. da Rocha Santana, B. G. Ribeiro, D. C. Silva Sales, J. M. Rodríguez-Díaz, D. C. Napoleão, M. A. da Motta Sobrinho and M. M. M. B. Duarte, Water Sci. Technol., 2020, 81, 2545–2558 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: https://doi.org/10.1039/d4lf00233d |
| ‡ Present address: Department of Materials Science and Metallurgy, University of Cambridge, 27 Charles Babbage Road, CB30FS, Cambridge, United Kingdom. |
| This journal is © The Royal Society of Chemistry 2025 |