Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A naphthalimide-derived chemosensor for ratiometric detection of sulphide ions: insights into the S2−-driven reduction cascade, real-time applications and live cell imaging of bacterial cells†
Saurabh
Gupta
ab,
Gulshan
Kumar
 c,
Vijay
Luxami
c,
Vijay
Luxami
 a and
Kamaldeep
Paul
a and
Kamaldeep
Paul
 *ab
*ab
aDepartment of Chemistry and Biochemistry, Thapar Institute of Engineering and Technology, Patiala, 147004, India. E-mail: kpaul@thapar.edu
bTIET-VT, Centre of Excellence in Emerging Materials, Thapar Institute of Engineering and Technology, Patiala, Punjab, India 147004
cDepartment of Chemistry, Banasthali University, Banasthali Newai, 304022, Rajasthan, India
First published on 16th May 2025
Abstract
Hydrogen sulphide (H2S) is an unpleasant, harmful gas commonly found in the environment, released from geothermal vents, and produced as a byproduct in industries such as oil refining and wastewater treatment. Because of its extreme toxicity, there is growing concern about its presence, necessitating timely detection to ensure human welfare. However, detecting H2S in various environments, including air and water, remains a significant challenge. To develop a probe for sulphide ion detection, herein, we report the synthesis of a highly selective, sensitive, and colorimetric chemosensor (NATRP) for the detection of sulphide ions (S2−) in a 50% aqueous medium. NATRP demonstrates exceptional sensitivity and selectivity for S2− ions relative to other ions, with a limit of quantification of 26 nM and a detection limit of 7.9 nM. It shows aggregation-induced emission quenching, which upon the addition of S2− ions, disaggregates with enhancement in fluorescence intensity. This enables NATRP to detect S2− ions within 15 seconds and it demonstrates good pH stability, suggesting that NATRP can detect sulphide ions across a broad pH range. The mechanism underlying the detection involves the reduction of azide groups to amine groups in the presence of S2− ions, confirmed by NMR titrations and HRMS analysis. Furthermore, NATRP successfully detects S2− ions in water, serum and solid samples, as well as in live cell imaging in bacterial cells. Moreover, UV-visible and fluorescence data have been employed to construct 1-to-2 decoders.
1. Introduction
Sulphide ions (S2−) are highly toxic and cause various health issues, including respiratory system failure. They are mostly derived from the growth of the petrochemical and leather manufacturing industries, as well as human metabolism.1,2 Both protonated (HS− and H2S) and non-protonated (S2− ions) species are hazardous, which makes the situation worse, due to their presence in the environment and wastewater in certain quantities.3 Exposure to water contaminated with S2− ions, which dissociate into HS− and H2S, has been linked to diseases such as Alzheimer's, liver cirrhosis, and Down's syndrome.4,5 Hydrogen sulphide (H2S) is involved in various critical physiological processes, including inflammation, angiogenesis, neuromodulation, etc., and it has drawn interest as an essential endogenous gasotransmitter.6,7 In addition, it plays a vital role in the biological system and is extensively distributed throughout various organs, including the brain, spleen, liver, and heart.8 This underscores the importance of regulating sulfide discharge and adhering to environmental protection guidelines. However, accurate and efficient detection of S2− ions in aqueous environments and living systems has become an urgent necessity.9,10 A number of techniques for detecting S2− ions have been ascribed, including ion chromatography, colorimetric, electrochemical methods, and inductively coupled plasma atomic emission spectroscopy.11 These conventional techniques have a number of inherent shortcomings, such as operation complexity, limited sensitivity, and poor precision, which limit their use for quick and precise detection of S2− ions, especially in biological systems.12,13 Addressing these challenges requires innovative, user-friendly, and efficient detection methods capable of tracking S2− ions in complex and dynamic physiological environments.14 In recent years, the fluorogenic probes have gained significant attention because of their non-invasive nature, ease of use, high selectivity, amazing fluorescence changes, and real-time imaging capabilities that can also be applied to living cells, and organisms.15,16 While several fluorescent probes for S2− detection have been reported, their design strategies typically rely on the reactive characteristics of S2− ions, such as amide-iminol tautomerism, nitro and azide reduction, H2S-mediated hydroxyl amide reactions, copper-sulfide precipitation, spiro-lactam ring opening, and dual nucleophilic addition.17–19 The development of innovative S2− ion probes is still important because of the intricacy of the molecular activities involved in signaling transduction and other issues connected to the probes.20 Recently, fluorescent probes and transition metal complexes have been specifically produced for the detection of H2S in aqueous media using a coordination approach. Organic azide-based chemosensors have also been explored,21 but challenges such as synthetic complexity, reliance on organic solvents, interference from other biothiols, and limited biological imaging capabilities remain to be addressed.22 Naphthalimide-based sensor systems have emerged as promising tools for H2S detection, offering several advantages, including a simple single-step reaction, good yield, high sensitivity and selectivity, low cost, rapid response, and non-destructive nature.23 These attributes make naphthalimide-based sensors an appealing solution for detecting sulfide ions in diverse applications.1.1 Design of the NATRP
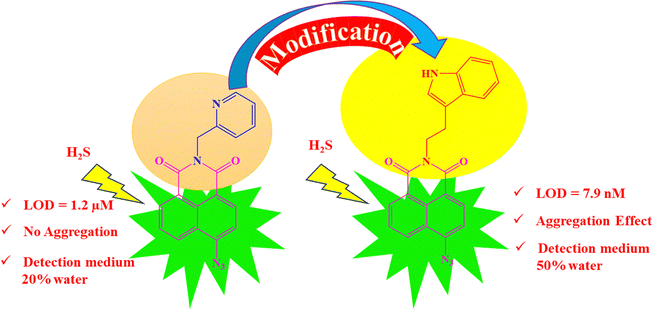
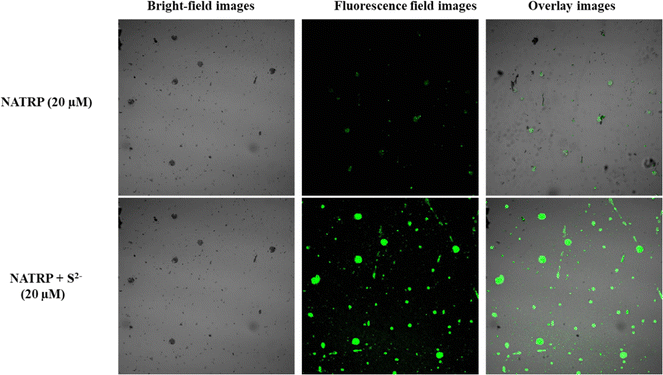
Over the past few years, naphthalimide-based fluorescent probes have been extensively developed for the detection of H2S, utilizing the azide group as the primary site for sensing (Table S1, ESI†). However, these sensors often exhibit limitations, including detection limits in the micromolar (μM) range and slow response times, which hinder their practical applicability for rapid and efficient H2S detection across diverse sample types. In 2022, Jothi et al. developed a naphthalimide-based fluorescent matrix for detecting H2S with the pyridyl group at an anhydride position.6 This probe detected H2S in a 20% water medium with a detection limit of 1.2 μM. To address the above issues, we modified the probe, replacing the pyridyl group with tryptamine. Tryptamine is known to regulate gastrointestinal motility by activating 5-HT4 receptors in the human gut, which may aid in the specific detection of sulphide ions. The synthesized probe demonstrated remarkable selectivity and sensitivity, detecting S2− ions in a 50% aqueous medium with a detection limit in the nanomolar (nM) range. Additionally, the synthesized compound exhibited aggregation-induced emission quenching, which was reversed upon interaction with S2− ions, resulting in “turn-on” fluorescence. This feature not only enhances sensitivity but also provides a reliable fluorescence-based tool for selective recognition of S2− ions. The probe proved effective in detecting sulfide ions in water, serum, and solid samples, as well as in live cell imaging in bacterial cells, establishing it as a versatile and efficient solution for real-world applications (Fig. 1).2. Experimental section
2.1 Synthesis of 2-(2-(1H-indol-3-yl)ethyl)-6-azido-1H-benzo[de]isoquinoline-1,3(2H)-dione (NATRP)
To a stirred solution of compound 4 (0.5 g, 2.09 mmol) in ethanol (30 mL), tryptamine (0.33 g, 2.09 mmol) was added in the presence of zinc acetate (0.038 g, 0.16 mmol), and the reaction mixture was refluxed for 8 h. The reaction was monitored with the help of TLC, and on completion of the reaction, 100 mL cold water was added resulting in the formation of dark yellow precipitates, which were filtered and vacuum-dried to obtain the crude product. The product was purified by column chromatography using chloroform and ethyl acetate (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as eluents to get the pure brownish-yellow product with 83% yield. The formation of compound NATRP was confirmed by the NMR spectroscopy technique (Fig. S1 and S2, ESI†). 1H NMR (400 MHz, CDCl3 + DMSO-d6): δ 8.50 (dd, 2J = 11.9, 3J = 7.5 Hz, 2H, ArH), 8.38 (d, J = 7.7 Hz, 1H, ArH), 7.79–7.73 (m, 1H, ArH), 7.70 (d, J = 7.7 Hz, 1H, ArH), 7.60 (d, J = 8.0 Hz, 1H, ArH), 7.27 (d, J = 7.9 Hz, 1H, ArH), 7.09 (d, J = 2.0 Hz, 1H, ArH), 7.00 (t, J = 7.1 Hz, 1H, ArH), 6.94 (t, J = 7.2 Hz, 1H, ArH), 4.31–4.23 (m, 2H, CH2), 3.04–2.95 (m, 2H, CH2); 13C NMR (DMSO-d6, 100 MHz): δ (ppm) 162.3, 161.8, 142.0, 135.3, 130.6, 130.4, 127.5, 126.2, 125.8, 122.9, 121.5, 120.0, 117.4, 114.1, 110.3, 28.2, 22.8; HRMS (ESI) calcd for C27H22N8O3 [M + H]+ 382.1297 found; 382.1299 (Scheme 1).
2) as eluents to get the pure brownish-yellow product with 83% yield. The formation of compound NATRP was confirmed by the NMR spectroscopy technique (Fig. S1 and S2, ESI†). 1H NMR (400 MHz, CDCl3 + DMSO-d6): δ 8.50 (dd, 2J = 11.9, 3J = 7.5 Hz, 2H, ArH), 8.38 (d, J = 7.7 Hz, 1H, ArH), 7.79–7.73 (m, 1H, ArH), 7.70 (d, J = 7.7 Hz, 1H, ArH), 7.60 (d, J = 8.0 Hz, 1H, ArH), 7.27 (d, J = 7.9 Hz, 1H, ArH), 7.09 (d, J = 2.0 Hz, 1H, ArH), 7.00 (t, J = 7.1 Hz, 1H, ArH), 6.94 (t, J = 7.2 Hz, 1H, ArH), 4.31–4.23 (m, 2H, CH2), 3.04–2.95 (m, 2H, CH2); 13C NMR (DMSO-d6, 100 MHz): δ (ppm) 162.3, 161.8, 142.0, 135.3, 130.6, 130.4, 127.5, 126.2, 125.8, 122.9, 121.5, 120.0, 117.4, 114.1, 110.3, 28.2, 22.8; HRMS (ESI) calcd for C27H22N8O3 [M + H]+ 382.1297 found; 382.1299 (Scheme 1).
2.2 Detection of Na2S in real samples
To detect S2− ions for real time applications, the water samples were collected from different sources like Ganga river water (Haridwar), Ghaggar river water (Ambala), and tap water (from the laboratory). The quantitative applications of S2− ions were estimated through a calibration curve. All these samples were further spiked with different concentrations of S2− ions (10, 20, 30, 40 μM). NATRP (20 μM) was added to these solutions (3 mL) having different S2− ion concentrations. The spiked samples were estimated over the calibration curve.3. Results and discussion
3.1 Photophysical behaviour of NATRP
Additional measurements of steady-state absorption and emission spectra were observed in a variety of solvents. It was found that the absorption maxima lie between 344 and 378 nm and the emission maxima exist between 435 and 530 nm, in different solvent systems (Fig. S3, ESI†). These redshifts in the absorption and emission spectra suggested the occurrence of intramolecular charge transfer (ICT). These findings were also corroborated with subsequent computational calculations, which showed shifting of electron density from tryptamine to the naphthalimide unit.3.2 Aggregation studies
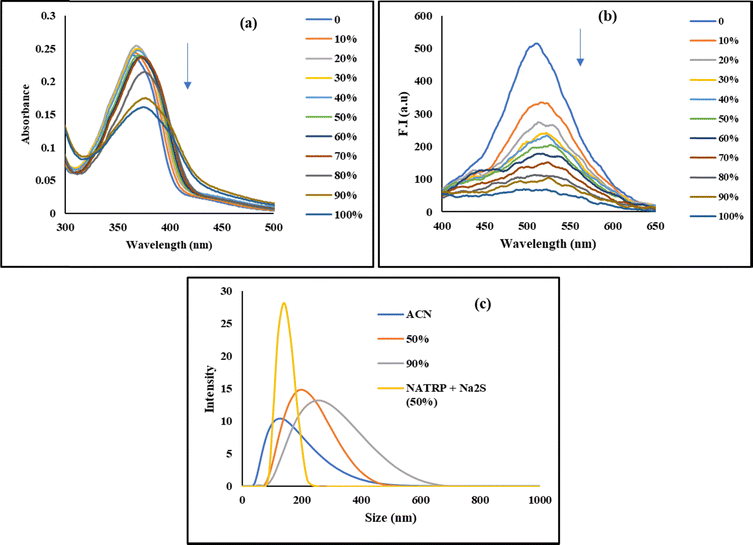
Aggregation studies were conducted by recording absorption and emission spectra in CH3CN with progressive addition of H2O in order to check the aggregation behaviour of the synthesized compound. The solution of NATRP (20 μM, CH3CN) exhibited an absorption band at 370 nm. With gradual addition of water from 0 to 40%, a slight increase and a shift in absorption maxima were observed. With a further increase in water ratio from 50 to 100%, a decrease in absorption maximum was recorded with a levelling off at 750 nm, indicative of the formation of aggregates in the medium (Fig. 2a). Emission studies were also performed to confirm aggregation in the solution of NATRP, which exhibited an intense emission band at 530 nm in CH3CN with a quantum yield (Φf) of 0.45. With progressive addition of water in solution, a constant decrease in emission intensity of NATRP was observed with a decrease in quantum yield to 0.27 in CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); and in pure water, the emission intensity of the compound was quenched entirely with a quantum yield (Φf) of 0.10, indicating the existence of aggregation-induced emission quenching (Fig. 2b).
1); and in pure water, the emission intensity of the compound was quenched entirely with a quantum yield (Φf) of 0.10, indicating the existence of aggregation-induced emission quenching (Fig. 2b).
A dynamic light scattering (DLS) experiment was performed to examine the effect of increasing water concentration on the particle size of NATRP, which also confirmed the aggregation behaviour of the compound. In pure CH3CN, NATRP exhibited an average particle size of 133 nm with a PDI value of 0.262. On increasing the water ratio in CH3CN, the average hydrodynamic size of NATRP was increased. In 50% H2O/CH3CN, the average particle size was found to be 226 nm having a PDI value of 0.347, and in 90% H2O/CH3CN, the particle size was increased to 269 nm with a PDI value of 0.363, indicating the formation of aggregates, and the increase in PDI value assisted the homogeneity of the particles with an increase in H2O. The maximum change in particle size of approximately 100 nm was recorded from pure CH3CN to 50% H2O/CH3CN ratio; afterwards, no such significant change in the size of the particles was observed and only a change of 40 nm was recorded from 50% to 90% H2O/CH3CN ratio. Furthermore, upon addition of S2− to the solution of NATRP in 50% H2O/CH3CN, the average particle size of a molecule was recorded to be 142 nm with a PDI value of 0.753, implying the disaggregation of the formed aggregates and resulting in homogeneity of the solution (Fig. 2c).
Furthermore, FE-SEM images of NATRP were recorded to check the morphological changes in the structure upon increasing the water concentration. NATRP exhibited a circular shape morphology in pure CH3CN. In contrast, upon increase in the water concentration to 50%, the compound exhibited needle-like structures. With a further increase to 80% water, a long needle-like structure was observed. In contrast, in 90% water, the particle size increased, showing more random structures whereas in pure H2O, the compound exhibited needles as well as rod-like morphologies (Fig. 3a–e). This change in the morphology of the structure confirmed the formation of aggregates. Furthermore, with addition of S2− ions to the solution of NATRP in 50% H2O/CH3CN, the compound displayed a cubical shape morphology (Fig. 3f). This might be due to the reduction of the azide group into an amine upon the addition of sulfide ions. Furthermore, the size of a particle of NATRP in pure CH3CN was found to be 163.2 nm and in 50% H2O:CH3CN, aggregates were formed with a particle size of 230 nm, while upon addition of S2− ions, the particle size was reduced to 150 nm. Thus, both the FE-SEM and DLS results were in coherence.
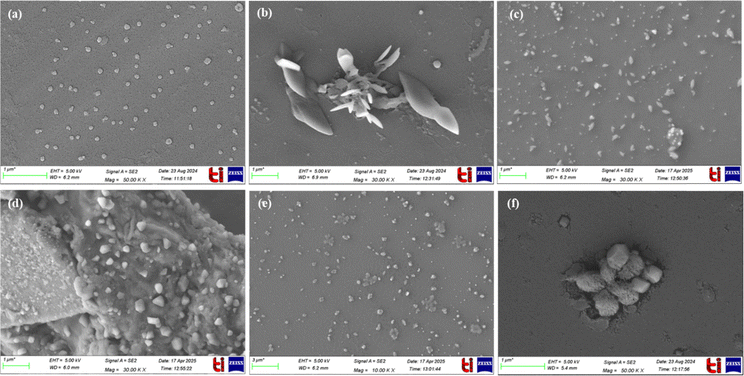 | ||
| Fig. 3 FE-SEM images of NATRP in (a) CH3CN; (b) 50% (H2O:CH3CN); (c) 80% (H2O:CH3CN); (d) 90% (H2O:CH3CN); (e) H2O; (f) the presence of S2− ions in 50% H2O:CH3CN. | ||
In addition to this, we further explored the aggregation-induced emission quenching behaviour of NATRP upon increasing the water concentration by performing time-correlated single photon counting studies. We recorded the average lifetime of NATRP in CH3CN and with increasing water concentration. In CH3CN, NATRP exhibited an average lifetime of 4.52 ns with two decay components having lifetime values of 1.86 ns and 7.52 ns, with populations of 53% and 47% (Table 1). On increasing the water concentration, i.e., CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (8
H2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), a drop in lifetime of NATRP from 4.52 ns to 3.63 ns was observed, having two decay components with lifetime values of 1.77 ns and 6.32 ns and populations of 59% and 41%. Further increasing the water content to 50%, i.e., CH3CN
2), a drop in lifetime of NATRP from 4.52 ns to 3.63 ns was observed, having two decay components with lifetime values of 1.77 ns and 6.32 ns and populations of 59% and 41%. Further increasing the water content to 50%, i.e., CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), a significant decrease in average lifetime of NATRP was recorded, and the lifetime was reduced to 2.98 ns with decay components having lifetime values and populations of 1.66 ns, 6.46 ns and 72%, 26%, respectively. On further increasing the water content, i.e. CH3CN
1), a significant decrease in average lifetime of NATRP was recorded, and the lifetime was reduced to 2.98 ns with decay components having lifetime values and populations of 1.66 ns, 6.46 ns and 72%, 26%, respectively. On further increasing the water content, i.e. CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2
H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8), no significant change in the average lifetime of NATRP was observed compared to the average lifetime of NATRP in CH3CN
8), no significant change in the average lifetime of NATRP was observed compared to the average lifetime of NATRP in CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The average lifetime of NATRP in CH3CN
1). The average lifetime of NATRP in CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2
H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) was found to be 2.67 ns with two decay components having lifetime values of 1.54 ns and 5.80 ns, with populations of 74% and 26%. Meanwhile, in water, the average lifetime of NATRP was found to be 2.63 ns, with two decay components having lifetime values of 1.60 ns and 6.77 ns, with populations of 80% and 20%. The decrease in average lifetime of NATRP with increasing water concentration may be due to the formation of aggregates, which causes quenching; therefore, the lifetime decreases. The results obtained from time-resolved studies were in accordance with the results obtained from steady-state fluorescence, DLS, and SEM studies.
8) was found to be 2.67 ns with two decay components having lifetime values of 1.54 ns and 5.80 ns, with populations of 74% and 26%. Meanwhile, in water, the average lifetime of NATRP was found to be 2.63 ns, with two decay components having lifetime values of 1.60 ns and 6.77 ns, with populations of 80% and 20%. The decrease in average lifetime of NATRP with increasing water concentration may be due to the formation of aggregates, which causes quenching; therefore, the lifetime decreases. The results obtained from time-resolved studies were in accordance with the results obtained from steady-state fluorescence, DLS, and SEM studies.
| τ 1 (ns) | τ 2 (ns) | α 1 | α 2 | τ av (ns) | χ 2 | |
|---|---|---|---|---|---|---|
| CH3CN | 1.86 | 7.52 | 0.53 | 0.47 | 4.52 | 1.10 |
CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (8 H2O (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
1.77 | 6.32 | 0.59 | 0.41 | 3.63 | 0.97 |
CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1.66 | 6.46 | 0.72 | 0.28 | 2.98 | 1.10 |
CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (2 H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) 8) |
1.54 | 5.80 | 0.74 | 0.26 | 2.67 | 1.12 |
| H2O | 1.60 | 6.77 | 0.80 | 0.2 | 2.63 | 1.10 |
Summing up the above studies conducted to understand the aggregation behaviour of NATRP with increasing concentration of water, it is observed that maximum aggregates of NATRP are formed up to 50% CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with a significant decrease in lifetime of NATRP. Further increasing the water concentration, little change in the particle size or lifetime of NATRP was observed, indicating that maximum changes are achieved until 50% CH3CN
1) with a significant decrease in lifetime of NATRP. Further increasing the water concentration, little change in the particle size or lifetime of NATRP was observed, indicating that maximum changes are achieved until 50% CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); after that, less changes are seen due to quenching in the emission intensity of NATRP.
1); after that, less changes are seen due to quenching in the emission intensity of NATRP.
3.3 Sensing properties of NATRP
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), where the maximum sensitivity and selectivity have been achieved. Therefore, preliminary studies were performed in CH3CN
1), where the maximum sensitivity and selectivity have been achieved. Therefore, preliminary studies were performed in CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
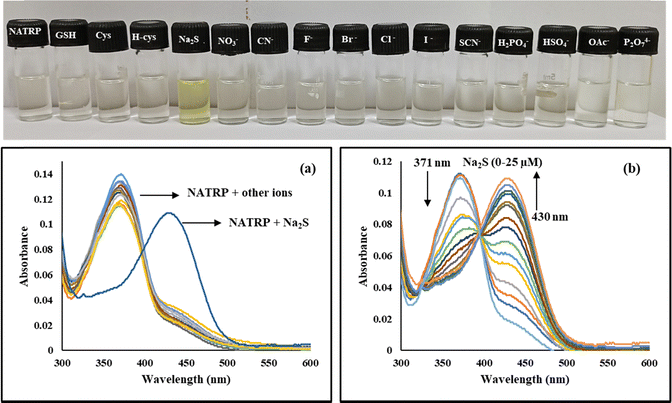
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and the detection of anions was examined by recording the UV-vis spectra of NATRP in the absence and presence of various anions (GSH, cysteine, homocysteine, S2−, NO3−, CN−, F−, Br−, Cl−, I−, SCN−, H2PO4−, HSO4−, OAc−, P2O74−) (Fig. 4a). NATRP exhibited an intense absorption band at 371 nm; upon addition of S2− ions, the absorption band of NATRP showed a red shift at 430 nm along with the development of light-yellow colour, visible by the naked eye (Fig. 4, inset). Whereas, no significant change in absorption band of NATRP was observed with the addition of other anions. These results implied that NATRP exhibited excellent selectivity towards S2− ions than other ions. Upon progressive addition of S2− ions (0–25 μM) to the solution of NATRP, the absorption band at 371 nm was diminished along with the development of a new band at 430 nm and an isosbestic point at 395 nm, indicating the existence of two species in equilibrium (Fig. 4b).
1), and the detection of anions was examined by recording the UV-vis spectra of NATRP in the absence and presence of various anions (GSH, cysteine, homocysteine, S2−, NO3−, CN−, F−, Br−, Cl−, I−, SCN−, H2PO4−, HSO4−, OAc−, P2O74−) (Fig. 4a). NATRP exhibited an intense absorption band at 371 nm; upon addition of S2− ions, the absorption band of NATRP showed a red shift at 430 nm along with the development of light-yellow colour, visible by the naked eye (Fig. 4, inset). Whereas, no significant change in absorption band of NATRP was observed with the addition of other anions. These results implied that NATRP exhibited excellent selectivity towards S2− ions than other ions. Upon progressive addition of S2− ions (0–25 μM) to the solution of NATRP, the absorption band at 371 nm was diminished along with the development of a new band at 430 nm and an isosbestic point at 395 nm, indicating the existence of two species in equilibrium (Fig. 4b).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
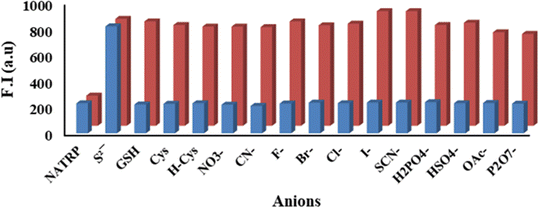
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), upon excitation at 375 nm, the compound displayed a weak fluorescence band at 530 nm with a quantum yield (Φf) of 0.27; while upon addition of S2− ions to the NATRP solution, the emission intensity enhanced dramatically, whereas no significant change in emission intensity of NATRP was observed upon the addition of other anions, implying the excellent selectivity of NATRP towards S2− ions over other ions (Fig. 5a). The visible color change was accompanied by an enhancement in fluorescence intensity seen under UV light (Fig. 5, inset). Spectrofluorimetric titrations were performed to observe the progressive variation in the emission response of the NATRP.S2− complex. The fluorescence intensity of NATRP was increased upon incremental addition of S2− ions (0–20 μM) into the solution of NATRP (Fig. 5b), with a quantum yield of 0.86, indicating the reduction of azide into amine.
1 v/v), upon excitation at 375 nm, the compound displayed a weak fluorescence band at 530 nm with a quantum yield (Φf) of 0.27; while upon addition of S2− ions to the NATRP solution, the emission intensity enhanced dramatically, whereas no significant change in emission intensity of NATRP was observed upon the addition of other anions, implying the excellent selectivity of NATRP towards S2− ions over other ions (Fig. 5a). The visible color change was accompanied by an enhancement in fluorescence intensity seen under UV light (Fig. 5, inset). Spectrofluorimetric titrations were performed to observe the progressive variation in the emission response of the NATRP.S2− complex. The fluorescence intensity of NATRP was increased upon incremental addition of S2− ions (0–20 μM) into the solution of NATRP (Fig. 5b), with a quantum yield of 0.86, indicating the reduction of azide into amine.
Furthermore, pH titrations of NATRP in the absence and presence of S2− ions were conducted in order to validate the sensing ability and practical applicability of NATRP towards S2− ions in physiological conditions. The emission intensities of NATRP alone and in the presence of S2− ions were recorded in different pH ranges (2.0–12.0), and the results are depicted in Fig. 7b. The fluorescence intensity of NATRP remained unchanged over a broad range of pH 2.0–12.0. The high stability of NATRP with varying pH ranges makes it beneficial for fast monitoring in environmental and biological samples. The reduction of azide into amine by S2− ions also showed excellent stability with varying pH, ranging from 2.0–12.0. These results indicated that NATRP could be capable of sensing S2− ions over a wide range of pH.
Time-dependent steady-state fluorescence measurements were performed on the NATRP probe to assess its photostability against photobleaching to ensure the experiment's accuracy and dependability. The probe in CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was stimulated at 365 nm, and the emission was recorded at 530 nm. To cause photobleaching, it was then exposed to the highest level of radiation for 60 minutes. Interestingly, as illustrated in Fig. 7c, the fluorescence intensity of the NATRP probe remained constant even after continuous exposure, indicating the excellent photostability.
1) was stimulated at 365 nm, and the emission was recorded at 530 nm. To cause photobleaching, it was then exposed to the highest level of radiation for 60 minutes. Interestingly, as illustrated in Fig. 7c, the fluorescence intensity of the NATRP probe remained constant even after continuous exposure, indicating the excellent photostability.
3.4 Time-correlated single photon counting (TCSPC) study
Furthermore, to get insight into the behaviour of NATRP towards S2− ions at 375 nm, a time-correlated single photon counting experiment was performed. NATRP and its complex with S2− ions are best fitted in bi-exponential mode, implying the existence of two different decay components (Fig. 8). For NATRP alone, the decay components exhibited lifetime values of 1.66 ns and 6.46 ns with a population of 72% and 28%, respectively. The average lifetime of NATRP was found to be 2.98 ns. Upon the addition of S2− to the solution of NATRP, two decay components were observed with lifetimes of 1.64 ns and 4.97 ns with populations of 87% and 13%, respectively. The average lifetime was calculated to be 2.05 ns, indicating the existence of a non-radiative pathway decay (Table 2). The decrease in decay time may also be due to the reduction of azide into an amine group. | ||
Fig. 8 Time-resolved spectra of NATRP (CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), pH = 7.3) in the absence and presence of S2− ions. 1, v/v), pH = 7.3) in the absence and presence of S2− ions. | ||
| τ 1 (ns) | τ 2 (ns) | α 1 | α 2 | τ av (ns) | χ 2 | |
|---|---|---|---|---|---|---|
| NATRP | 1.66 | 6.46 | 0.72 | 0.28 | 2.98 | 1.10 |
| NATRP + S2− | 1.64 | 4.97 | 0.87 | 0.13 | 2.05 | 1.10 |
3.5 Sensing mechanism with 1H NMR, HRMS and FTIR
Furthermore, to investigate the binding mechanism of S2− ions with NATRP and the reduction of azide into an amine group, we performed 1H NMR titrations and HRMS analysis. The 1H NMR titrations of NATRP were performed in CD3CN both in the absence and presence of S2− ions. The 1H NMR spectrum of NATRP exhibited 10 aromatic protons in the region of 8.51–6.95 ppm. Upon the addition of 0.2 equivalents of S2− ions, upfield shifts in naphthalimide protons were observed; the protons at 8.51 ppm (He), 8.46 ppm (Hb) and 8.38 ppm (Hc) were shifted upfield to 8.42 ppm (He), 8.21 ppm (Hb) and 8.33 ppm (Hc), respectively (Fig. 9a). Moreover, the proton (Hd) resonating at 7.75 ppm showed a slight upfield shift to 7.59 ppm, while the proton resonating at 7.54 ppm (Ha) showed a dramatic upfield shift to 6.38 ppm due to the reduction of the azide group into an amine in the presence of S2− ions. A transition state was also observed upon the addition of 0.2 eq. and 0.4 eq. S2− ions to the solution of NATRP, which clearly indicated the conversion of the azide group to an amine group and the presence of an azide form and amine form in the same solution. Furthermore, the complete reduction of the azide form into an amine was observed upon addition of 1.0 eq. of S2− ions, and the significant shifting in peaks of naphthalimide protons was observed, indicating the change in the electron density around the naphthalimide protons with reduction of the azide. Significant upfield shifting of Ha and Hb protons was observed, implying the complete reduction of the azide group into an amine.To corroborate the NMR findings, HRMS analysis of NATRP in the presence of sulphide ions was performed (Fig. S7, ESI†), which exhibited an m/z peak at 356.1396 a.m.u corresponding to the reduction of the azide group into an amine group (NATRP-N3 to NATRP-NH2) and m/z + Na peak at 378.1216 corresponding to NATRP-NH2 + Na+. These results, obtained from both 1H NMR titration and HRMS analysis, conclusively confirm the reduction of the azide group into an amine group in the presence of sulfide ions (Fig. 9b).
To further confirm the conversion of the azide group into an amine group in the presence of sulphide ions, we have recorded the FTIR spectrum of NATRP alone and in the presence of S2− ion. A characteristic peak of the azide group in the region 2100–2150 cm−1 was seen in the IR spectrum of NATRP alone (Fig. 9c). This peak was diminished in the presence of S2− ions, and a new peak in the region of 3300–3500 cm−1 was seen, which is characteristic of NH2. Hence, the results obtained from FTIR studies further confirmed the conversion of the azide group into an amine in the presence of sulphide ions, and the results were in accordance with NMR and HRMS findings.
3.6 Computational studies
As discussed above, we have optimized compound NATRP in the ground state (S0) at ωB97XD/6-311g(d) with no imaginary frequency, and three low-lying vertical excitations were calculated at the same level of theory to investigate the source of absorption spectra. For S0 → S1, excitation determined the orbital transition contribution from the HOMO−2 → LUMO and HOMO−1 → LUMO of 51% and 46%, respectively with an oscillation strength of 0.5583 at 332 nm for NATRP, which is near to the experimental absorption peak at 366 nm. Additionally, for S0 → S2 and S0 → S3, excitations were determined at 286 nm and 285 nm with oscillation strengths of 0.0191 and 0.0004, respectively. We have only taken into consideration S0 → S1 excitation for study because the second and the third excitations have weak oscillation strengths. The electron density analysis showed that HOMO−1 and HOMO−2 have a distribution over the molecule except for the linker ethyl unit, while the LUMO has a distribution over the naphthalimide-linked azide unit. This shift in density signified the intramolecular charge transfer from indole to the naphthalimide unit (Fig. 10a and b).Additionally, geometry optimization was done for NATRP's reduced form, and three low-lying vertical excitations that correspond to the ground state were calculated. For the reduced form of NATRP, the S0 → S1 excitation determined the orbital transition contribution from HOMO−1 → LUMO of 96% with an oscillation strength of 0.3856 at 352 nm. Furthermore, oscillation strengths of 0.0002 and 0.0003 were found for the S0 → S2 and S0 → S3 excitations at 286 nm and 266 nm, respectively. S0 → S1 exhibited the strongest and most notable oscillation; hence, it was further examined. According to electron density analysis, HOMO−1 and LUMO were distributed over the naphthalimide unit and indicated π → π* transition. However, the red shift in the absorption spectra, as observed in the experiment, was mimicked through a computational approach signifying the origin of the alteration of the absorption spectra (Fig. 10c and d).
3.7 Practical applications for the detection of S2− ions
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), and the fluorescence intensities were recorded at 530 nm (Fig. S8, ESI†). As shown in Table 3, the compound can successfully determine the sulphide ions in water samples, thus assisting the practical application of NATRP. The same studies were also performed for the detection of S2− ions in serum samples with a recovery rate >98%, In addition to this, the stability of the probe in serum medium was determined, and the results implied that the fluorescence intensity of NATRP was relatively stable in serum medium (Table S4 and Fig. S9, ESI†).
1 v/v), and the fluorescence intensities were recorded at 530 nm (Fig. S8, ESI†). As shown in Table 3, the compound can successfully determine the sulphide ions in water samples, thus assisting the practical application of NATRP. The same studies were also performed for the detection of S2− ions in serum samples with a recovery rate >98%, In addition to this, the stability of the probe in serum medium was determined, and the results implied that the fluorescence intensity of NATRP was relatively stable in serum medium (Table S4 and Fig. S9, ESI†).
| Spiked amount (μM) | Observed amount (μM) | Recovery (%) | RSD (%) | |
|---|---|---|---|---|
| RSD: relative standard deviation. | ||||
| Ganga river | 10 | 10.7 | 107 | 0.34 |
| 20 | 22.1 | 110.5 | 0.56 | |
| 30 | 31.3 | 104.3 | 0.39 | |
| 40 | 42.6 | 106.5 | 0.48 | |
| Tap water | 10 | 10.2 | 102 | 0.60 |
| 20 | 21.6 | 107 | 0.75 | |
| 30 | 32.4 | 108 | 0.52 | |
| 40 | 41.9 | 104.7 | 0.38 | |
| Ghaggar river | 10 | 11.7 | 117 | 0.69 |
| 20 | 21.5 | 107.5 | 0.75 | |
| 30 | 32.8 | 109.3 | 0.49 | |
| 40 | 39.9 | 99.7 | 0.42 | |
4. Conclusion
In the present work, we have successfully designed and synthesized a naphthalimide-based “turn-on” fluorescent NATRP sensor for precise, selective, and highly sensitive detection of S2− ions in CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O medium (1
H2O medium (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The NATRP displayed excellent selectivity and sensitivity towards S2− ions with an LOD value of 7.9 nM and limit of quantification of 26 nM. The compound exhibited aggregation-induced emission quenching properties, and in the presence of S2− ions, disaggregation along with enhanced fluorescence was observed. The response time of NATRP towards S2− ions was recorded to be less than 15 s. It displayed excellent pH stability, indicating that NATRP can detect sulfide ions over a wide range of pH. NMR titrations and HRMS results confirmed the reduction of azide into an amine group in the presence of S2− ions. Interference studies were also performed with other competitive metal ions, and compound NATRP can selectively detect S2− ions in the presence of other ions. Furthermore, the experimental outcomes are further endorsed by computational calculation, where the observed redshift in the presence of S2− ions was replicated through DFT/TD-DFT calculations. Furthermore, 1-to-2 decoder memory devices were constructed by considering the UV-visible and fluorescence results. Furthermore, the compound demonstrates the ability to detect S2− ions in a variety of real samples, including water, serum, and solid samples, in addition to its efficacy in live-cell imaging of bacterial cells. This versatility highlights its potential for practical applications in environmental monitoring, clinical diagnostics, and biological research.
1). The NATRP displayed excellent selectivity and sensitivity towards S2− ions with an LOD value of 7.9 nM and limit of quantification of 26 nM. The compound exhibited aggregation-induced emission quenching properties, and in the presence of S2− ions, disaggregation along with enhanced fluorescence was observed. The response time of NATRP towards S2− ions was recorded to be less than 15 s. It displayed excellent pH stability, indicating that NATRP can detect sulfide ions over a wide range of pH. NMR titrations and HRMS results confirmed the reduction of azide into an amine group in the presence of S2− ions. Interference studies were also performed with other competitive metal ions, and compound NATRP can selectively detect S2− ions in the presence of other ions. Furthermore, the experimental outcomes are further endorsed by computational calculation, where the observed redshift in the presence of S2− ions was replicated through DFT/TD-DFT calculations. Furthermore, 1-to-2 decoder memory devices were constructed by considering the UV-visible and fluorescence results. Furthermore, the compound demonstrates the ability to detect S2− ions in a variety of real samples, including water, serum, and solid samples, in addition to its efficacy in live-cell imaging of bacterial cells. This versatility highlights its potential for practical applications in environmental monitoring, clinical diagnostics, and biological research.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
KP thanks SERB, New Delhi (CRG/2023/004080), and CEEMS (Project No. TIET/CEEMS/Regular/2021/018), VT-India, for providing funds. SAI labs, Acal lab and TIET for NMR and DST-FIST (SR/FST/CS-II/2018/69) for HRMS analysis are also acknowledged. We would also like to thanks Central University of Punjab for Confocal Laser. SG thanks to CEEMS-TIET/VT/8365 for providing the fellowship.References
- J. Wu, C. Chan, J. Li, Y. Shi, Z. Xue and L. Zhao, A BODIPY-Based Fluorescent Chemosensor with 2, 6-Substitution for Visual and Highly Selective Detection of S2–, Spectrochim. Acta, Part A, 2023, 297, 122741 Search PubMed
.
- J. Fan, E. Wu, J. Dong, R. Zhu, M. Li, J. Gao, H. Han and L. Ding, A Minimalist Ratiometric Fluorescent Sensor Based on Non-Covalent Ternary Platform for Sensing H2S in Aqueous Solution and Serum, Colloids Surf., A, 2021, 616, 126299 Search PubMed
.
- C. Zhang, Y. Liang, W. Du, M. Kuang, Z. Meng, S. Gong, Z. Wang and S. Wang, A Novel BODIPY-Based Colorimetric Turn-on NIR Fluorescent Probe for Sensitive and Visual Detection of H2S in Food Samples with Smartphone Platform, J. Food Compos. Anal., 2024, 134, 106518 CrossRef
.
- G. Lu, L. Duan, S. Meng, P. Cai, S. Ding and X. Wang, Development of a Colorimetric and Turn-on Fluorescent Probe with Large Stokes Shift for H2S Detection and Its Multiple Applications in Environmental, Food Analysis and Biological Imaging, Dyes Pigm., 2023, 220, 111687 CrossRef
.
- G. Asaithambi and V. Periasamy, Hydrogen Sulfide Detection by ESIPT Based Fluorescent Sensor: Potential in Living Cells Imaging, J. Photochem. Photobiol., A, 2019, 369, 97–105 CrossRef
.
- D. Jothi and S. K. Iyer, A Highly Sensitive Naphthalimide Based Fluorescent “Turn-on” Sensor for H2S and Its Bio-Imaging Applications, J. Photochem. Photobiol., A, 2022, 427, 113802 CrossRef
.
- W. Lang, J. M. Qin and Q. Y. Cao, A Novel Polymer-Based Probe for Fluorescently Ratiometric Sensing of Hydrogen Sulfide with Multiple Applications, Anal. Chim. Acta, 2024, 1286, 342051 CrossRef PubMed
.
- Y. Mao, Q. Yu, T. Ye, M. Xi, W. Lai, Z. Chen, K. Chen, L. Li, H. Liu and J. Wang, New Rhodamine-Based Sensor for High-Sensitivity Fluorescence Tracking of Cys and Simultaneously Colorimetric Detection of H2S, Spectrochim. Acta, Part A, 2024, 306, 123589 CrossRef PubMed
.
- P. Sarkar, D. Das, S. Sutradhar and B. N. Ghosh, Selective Sensing of Sulphide Ion by a Simple Mercury (II) Complex of an Amino-Substituted Terpyridine in Aqueous Solution, J. Mol. Struct., 2024, 1301, 137392 Search PubMed
.
- J. Zheng, H. L. Noh, H. W. Chun, B. M. Oh, J. Lee, S. K. Choi, E. Kim, D. Jung, W. S. Lee and J. H. Kim, Highly Sensitive, Selective, and Rapid Response Colorimetric Chemosensor for Naked Eye Detection of Hydrogen Sulfide Gas under Versatile Conditions: Solution, Thin-Film, and Wearable Fabric, Sens. Actuators, B, 2021, 341, 130013 CrossRef
.
- V. Kavitha, P. Viswanathamurthi, J. Haribabu and C. Echeverria, An Active ESIPT Based Molecular Sensor Aided with Sulfonate Ester Moiety to Track the Presence of H2S Analyte in Realistic Samples and HeLa Cells, Microchem. J., 2023, 188, 108484 CrossRef CAS
.
- W. T. Guo, Y. F. Ding, X. Li, L. Tong, L. Dou and W. K. Dong, Highly Efficient and Selective Detection of Sulfur Ions and Picric Acid through Salamo-Cd(II) Coordination Polymer Chemosensor, Inorg. Chim. Acta, 2023, 557, 121704 CrossRef CAS
.
- C. Wang, Y. Gui, M. Wu, T. Wu, H. Wang, W. Gao, J. Zheng, N. Zhao, Y. Zhang, X. Shu and J. Shang, Design and Characterization of a Near-Infrared Fluorescent Probe SCN for Selective Detection of Hydrogen Sulfide (H2S) in Living Systems and Food Samples, J. Mol. Liq., 2024, 410, 125522 CrossRef CAS
.
- L. Liao, D. Guo, X. Luo, L. Meng and F. Wu, Facile Fabrication of Iron Porphyrin-Based Porous Organic Polymer with Excellent Oxidase-like Activity for Colorimetric Detection of Sulfide, Colloids Surf., A, 2022, 651, 129727 CrossRef CAS
.
- X. Yue, J. Wang, J. Han and B. Wang, A dual-ratiometric fluorescent
probe for individual and continuous detection of H2S and HClO in living cells, Chem. Commun., 2020, 56, 2849–2852 RSC
.
- Z. Guo, S. Park, J. Yoon and I. Shin, Recent Progress in the Development of Near-Infrared Fluorescent Probes for Bioimaging Applications, Chem. Soc. Rev., 2014, 43, 16–29 RSC
.
- X. Xiao, Y. Shen, X. Zhou, B. Sun, Y. Wang and J. Cao, Innovative Nanotechnology-Driven Fluorescence Assays for Reporting Hydrogen Sulfide in Food-Related Matrices, Coord. Chem. Rev., 2023, 480, 215012 CrossRef CAS
.
- D. P. Li, J. F. Zhang, J. Cui, X. F. Ma, J. T. Liu, J. Y. Miao and B. X. Zhao, A Ratiometric Fluorescent Probe for Fast Detection of Hydrogen Sulfide and Recognition of Biological Thiols, Sens. Actuators, B, 2016, 234, 231–238 CrossRef CAS
.
- Z. Ju, Y. Zhang and L. Kong, A Highly Selective Fluorescent Probe for Hydrogen Sulfide and Its Application in Living Cell, J. Fluoresc., 2025, 35, 1163–1169 CrossRef
.
- R. Kaushik, A. Ghosh and D. Amilan Jose, Recent Progress in Hydrogen Sulphide (H2S) Sensors by Metal Displacement Approach, Coord. Chem. Rev., 2017, 347, 141–157 CrossRef CAS
.
- L. Wang, W. Yang, Y. Song and Y. Hu, Novel Turn-on Fluorescence Sensor for Detection and Imaging of Endogenous H2S Induced by Sodium Nitroprusside, Spectrochim. Acta, Part A, 2020, 243, 118775 CrossRef CAS
.
- Y. M. Tian, S. S. Liu, W. N. Wu, X. L. Zhao, Y. Wang, Y. C. Fan, Z. H. Xu and T. D. James, A Mitochondria-Targeting Fluorescent Probe for the Dual-Emission Fluorescence-Enhanced Detection of Hydrogen Sulfide and Turn-on Detection of Hydrazine, Sens. Actuators, B, 2024, 409, 135496 CrossRef
.
- H. W. Chun, J. Zheng, E. H. Lee, B. M. Oh, C. B. Lee, J. S. Min, E. Kim, E. Kim, W. Lee and J. H. Kim, Pure-Water-Soluble Colorimetric Chemosensors for Highly Sensitive and Rapid Detection of Hydrogen Sulfide: Applications to Evaluation of on-Site Water Quality and Real-Time Gas Sensors, Sens. Actuators, B, 2024, 402, 134989 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ma00103j |
| This journal is © The Royal Society of Chemistry 2025 |