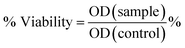Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hybrid silver–iron oxide nanoflowers: morphological tailoring, application as CT agents, and exploitation of induced apoptosis in glioblastoma treatment†
Sofia G.
Nikolopoulou
ab,
Beata
Kalska-Szostko
c,
Anna
Basa
c,
Giorgos
Papanastasiou
 de,
Adriana
Tavares
f,
Carlos Alcaide
Corral
de,
Adriana
Tavares
f,
Carlos Alcaide
Corral
 f,
Athina
Papadopoulou
f,
Athina
Papadopoulou
 ab,
Marios
Kostakis
ab,
Marios
Kostakis
 g,
Nikolaos S.
Thomaidis
g,
Nikolaos S.
Thomaidis
 g and
Eleni K.
Efthimiadou
g and
Eleni K.
Efthimiadou
 *ab
*ab
aA Inorganic Chemistry Laboratory, Chemistry Department, National and Kapodistrian University of Athens, Panepistimiopolis, Zografou 157 71, Greece. E-mail: efthim@chem.uoa.gr
bSol-Gel Lab, Institute of Nanoscience and Nanotechnology, NCSR “Demokritos”, 153 41 Aghia Paraskevi Attikis, Greece
cUniversity of Bialystok, Faculty of Chemistry, Bialystok 15-245, Poland
dArchimedes Unit, Athena Research Centre, Marousi, 15125, Greece
eSchool of Computer Science and Electronic Engineering, University of Essex, Colchester Campus, CO4 3SQ, UK
fEdinburgh Imaging Queen's Medical Research Institute, University of Edinburgh, Edinburgh EH16 4TJ, UK
gLaboratory of Analytical Chemistry, Department of Chemistry, National and Kapodistrian University of Athens, University Campus, Zografou, 15771, Athens, Greece
First published on 21st April 2025
Abstract
The ability of cancer to develop drug-resistance, in parallel with the undesired effects of chemotherapy, has led to the development of safe nanoparticles characterized by multi-sensitivity. Herein we focus on the synthesis and exploitation of the synthetic route of hybrid silver–iron oxide Nfs and their successful coating with citrate. The parameters of the synthetic route affecting the uniform formation of the Nfs are investigated to optimize the experimental route and the attained Nfs. Most importantly, the study focuses on the evaluation of the Nfs as theranostic agents in the case of glioblastoma. The results suggest that the Nfs are good candidates for CT contrast agents, as the contrast is enhanced after treatment. The in vitro evaluation shows that the Nfs exhibit cytotoxicity towards glioblastoma cells, whereas no significant toxicity towards red blood cells is reported. Finally, internalization studies provide insight information that helps unveil the exact mechanism of action of the Nfs.
1. Introduction
Glioblastoma (GBM) is the most common and aggressive malignant brain tumor, with an incidence of 3–5 cases per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people annually and a poor prognosis, as the median survival is only 12–18 months.1 Current treatment options, including surgery, radiotherapy, and chemotherapy, face significant limitations. GBM's high invasiveness makes complete surgical removal difficult, while the blood–brain barrier (BBB) restricts drug delivery. Additionally, tumor heterogeneity, therapeutic resistance, immune evasion, and rapid recurrence contribute to treatment failure. Future strategies, such as nanotechnology-based drug delivery, targeted molecular therapies, immunotherapy, and gene therapies, hold promise for improving patient outcomes.2 However, overcoming these challenges remains a major hurdle in GBM treatment. The current “gold standard” treatment combines chemotherapy, radiotherapy, and surgical interventions. Among chemotherapeutic agents, temozolomide remains the most widely used and effective, having been approved by the EMA and FDA in 1999.3 While this multimodal approach may extend patient life expectancy, it does not achieve a complete cure or long-term survival. Consequently, extensive research has been conducted to identify vulnerabilities in glioblastoma that could lead to the development of more effective therapies.4 In recent years, nanotechnology has emerged as a promising avenue for developing novel therapeutic agents aimed at reducing the chemotherapy side effects and overcoming cancer's drug resistance mechanisms. An increasing number of pharmaceutical products incorporate nanoparticles, underscoring their potential in cancer treatment5–7 Multifunctional nanostructures have gained significant attention in biomedical applications, particularly for cancer therapy. Ebrahimi et al. (2016) synthesized, characterized, and tested silver–iron nanoparticles for apoptosis induction using flow cytometry, while their influence on bax gene expression was analyzed via qRT-PCR. Nanoparticles demonstrated cytotoxicity and apoptosis, with the strongest effect observed in the nanoparticle synthesized using glucose as a reducing agent. Upregulation of bax gene expression suggested activation of the intrinsic apoptosis pathway, influenced by stress-related transcription regulators. These findings highlight the potential of silver–iron oxide hybrid nanoparticles for targeted cancer therapy by taking into advantage their dual nature.8 Iron oxide nanoparticles have emerged as promising agents for cancer diagnosis and treatment due to their magnetic properties and biocompatibility. These nanoparticles can be functionalized with drugs or phytochemicals for targeted delivery and have applications in thermoablation, hyperthermia, and magnetic resonance imaging (MRI).9 Das et al. (2016) report that Ag/Fe3O4 nanoflowers boost the specific absorption rate by one order of magnitude under combined magnetic and laser irradiation.10 Erdogan et al. (2021) demonstrate that silver nanoparticles produced via green chemistry reduce cell proliferation and migration in U87 glioblastoma cells, a change accompanied by lower Bcl-2 levels and higher Bax and caspase-3 activities.11 Gholami et al. (2018) describe chitosan nanoparticles loaded with doxorubicin that deliver the drug in a pH-sensitive manner and shorten T2 relaxation times in C6 glioma cells. Nikolopoulou et al. (2023) notes that hybrid Ag@SiO2 core–shell nanoparticles exert anticancer effects against glioblastoma cells while exhibiting only minor toxicity toward healthy cells,12 and Zhang et al. (2021) indicates that metal–phenolic network nanoparticles elevate intracellular reactive oxygen species and induce ferroptosis, along with enhanced MRI and photothermal responses.13 Reported particle sizes span approximately 141 to 184 nm, while synthesis methods range from green chemistry and ionic gelation to modified sol–gel techniques. Reaction time and the Ag/Fe ratio prove to influence nanoparticle morphology and dual imaging capability via silver or iron components. These findings support the view that silver-based and hybrid silver–iron nanoparticles can serve as multifunctional agents in glioblastoma theranostics. Other studies have explored combining iron oxide nanoparticles with other materials to enhance their functionality. Conjugated polymer nanoparticles incorporating iron oxide cores have been developed for simultaneous MRI and fluorescent imaging of brain tumors.14 Similarly, silver/iron oxide hybrid nano-popcorns have shown potential for imaging and therapy, demonstrating photothermal thrombolytic effects, anticancer activity, and MRI capabilities. These hybrid nanoparticles exhibited low toxicity and no long-term retention in mouse organs, suggesting their potential for diagnosing and treating various diseases.15 Generally, an approach for multi-sensitive nanomaterials has been recently reported in literature, where iron is contributing to the diagnostic potential of the nanomaterials, whilst the incorporation of noble metals such as silver improves the response to phototherapy.16
000 people annually and a poor prognosis, as the median survival is only 12–18 months.1 Current treatment options, including surgery, radiotherapy, and chemotherapy, face significant limitations. GBM's high invasiveness makes complete surgical removal difficult, while the blood–brain barrier (BBB) restricts drug delivery. Additionally, tumor heterogeneity, therapeutic resistance, immune evasion, and rapid recurrence contribute to treatment failure. Future strategies, such as nanotechnology-based drug delivery, targeted molecular therapies, immunotherapy, and gene therapies, hold promise for improving patient outcomes.2 However, overcoming these challenges remains a major hurdle in GBM treatment. The current “gold standard” treatment combines chemotherapy, radiotherapy, and surgical interventions. Among chemotherapeutic agents, temozolomide remains the most widely used and effective, having been approved by the EMA and FDA in 1999.3 While this multimodal approach may extend patient life expectancy, it does not achieve a complete cure or long-term survival. Consequently, extensive research has been conducted to identify vulnerabilities in glioblastoma that could lead to the development of more effective therapies.4 In recent years, nanotechnology has emerged as a promising avenue for developing novel therapeutic agents aimed at reducing the chemotherapy side effects and overcoming cancer's drug resistance mechanisms. An increasing number of pharmaceutical products incorporate nanoparticles, underscoring their potential in cancer treatment5–7 Multifunctional nanostructures have gained significant attention in biomedical applications, particularly for cancer therapy. Ebrahimi et al. (2016) synthesized, characterized, and tested silver–iron nanoparticles for apoptosis induction using flow cytometry, while their influence on bax gene expression was analyzed via qRT-PCR. Nanoparticles demonstrated cytotoxicity and apoptosis, with the strongest effect observed in the nanoparticle synthesized using glucose as a reducing agent. Upregulation of bax gene expression suggested activation of the intrinsic apoptosis pathway, influenced by stress-related transcription regulators. These findings highlight the potential of silver–iron oxide hybrid nanoparticles for targeted cancer therapy by taking into advantage their dual nature.8 Iron oxide nanoparticles have emerged as promising agents for cancer diagnosis and treatment due to their magnetic properties and biocompatibility. These nanoparticles can be functionalized with drugs or phytochemicals for targeted delivery and have applications in thermoablation, hyperthermia, and magnetic resonance imaging (MRI).9 Das et al. (2016) report that Ag/Fe3O4 nanoflowers boost the specific absorption rate by one order of magnitude under combined magnetic and laser irradiation.10 Erdogan et al. (2021) demonstrate that silver nanoparticles produced via green chemistry reduce cell proliferation and migration in U87 glioblastoma cells, a change accompanied by lower Bcl-2 levels and higher Bax and caspase-3 activities.11 Gholami et al. (2018) describe chitosan nanoparticles loaded with doxorubicin that deliver the drug in a pH-sensitive manner and shorten T2 relaxation times in C6 glioma cells. Nikolopoulou et al. (2023) notes that hybrid Ag@SiO2 core–shell nanoparticles exert anticancer effects against glioblastoma cells while exhibiting only minor toxicity toward healthy cells,12 and Zhang et al. (2021) indicates that metal–phenolic network nanoparticles elevate intracellular reactive oxygen species and induce ferroptosis, along with enhanced MRI and photothermal responses.13 Reported particle sizes span approximately 141 to 184 nm, while synthesis methods range from green chemistry and ionic gelation to modified sol–gel techniques. Reaction time and the Ag/Fe ratio prove to influence nanoparticle morphology and dual imaging capability via silver or iron components. These findings support the view that silver-based and hybrid silver–iron nanoparticles can serve as multifunctional agents in glioblastoma theranostics. Other studies have explored combining iron oxide nanoparticles with other materials to enhance their functionality. Conjugated polymer nanoparticles incorporating iron oxide cores have been developed for simultaneous MRI and fluorescent imaging of brain tumors.14 Similarly, silver/iron oxide hybrid nano-popcorns have shown potential for imaging and therapy, demonstrating photothermal thrombolytic effects, anticancer activity, and MRI capabilities. These hybrid nanoparticles exhibited low toxicity and no long-term retention in mouse organs, suggesting their potential for diagnosing and treating various diseases.15 Generally, an approach for multi-sensitive nanomaterials has been recently reported in literature, where iron is contributing to the diagnostic potential of the nanomaterials, whilst the incorporation of noble metals such as silver improves the response to phototherapy.16
Based on the recent silver–iron nanoparticles studies, our work investigates the theranostic potential of hybrid Nfs, focusing on understanding the mechanisms underlying their formation during synthesis. Key synthesis parameters are examined to determine the optimal conditions for producing Nfs with desirable properties. The physicochemical characteristics of the Nfs are analyzed using various techniques, including size measurement methods, FT-IR, UV-Vis spectroscopy, and pXRD. Additionally, the theranostic potential of the Nfs is evaluated through in vitro protocols, including cytotoxicity assays, internalization studies, reactive oxygen species (ROS) evaluation, and hemocompatibility tests. These biological assessments provide insights into the Nfs’ biocompatibility, ability to induce oxidative stress, and cellular localization, as observed via microscopy techniques. Additionally, the theranostic potential of the Nfs is evaluated through in vitro protocols, including cytotoxicity assays, internalization studies, reactive oxygen species (ROS) evaluation, and hemocompatibility tests. These biological assessments provide insights into the Nfs’ biocompatibility, ability to induce oxidative stress, and cellular localization, as observed via microscopy techniques.
The results indicate that uniform Nfs form under extended reaction times and low Ag/Fe ratios. Longer reaction times are associated with increased Nf size, while citrate coating has minimal impact on their physical properties. In vitro studies suggest that both coated and uncoated Nfs exhibit anticancer activity while maintaining hemocompatibility. Their fluorescence (due to silver content) and detectability through iron-based Prussian blue staining highlight their dual diagnostic and therapeutic capabilities.
The CT measurements along with apoptosis–necrosis evaluation and identification of endocytosis pathway verify the possible anticancer activity of the Nfs as well as their potential use as contrast agents in diagnostic techniques such as CT scan. In contrast to previously published studies, this research provides novel insights into the coating process of these nanoflowers (Nfs) and its impact on their biological activity, which, to the best of our knowledge, has not been previously reported. To date, biological applications of such Nfs are scarcely reported in the literature, underscoring the novelty and importance of this study. To our knowledge, this is the first investigation into their potential for glioblastoma diagnosis and treatment, paving the way for further research in this field.
It is also noteworthy that these exotic morphologies are rarely encountered in the literature, and increased documentation can significantly enhance the overall understanding of this category of materials.
2. Results and discussion
2.1 Synthesis of silver–iron oxide Nfs (SIONFs)
Hybrid SIONFs are synthesized via a solvothermal process, wherein silver (forming the core of the Nf) is reduced first, followed by the reduction of iron to create petal-like structures around the silver core. These complex nanoarchitectures of silver and iron oxide are not well-documented in the literature, with only a few studies published to date. Building on, previous work, such as Zhang et al. (2012), which explored the formation mechanism of Nfs with varying Ag/Fe ratios and reaction times,7 this study further investigates the effects of these synthetic parameters. Specifically, we evaluated Ag/Fe ratios of 0.15, 0.18, and 0.20, alongside reaction times of 6, 8, 12, and 24 hours, to determine their impact on the size, morphology, and structure of the Nfs. (Table 1). Since there is only one reference in literature so far concerning these parameters, we obtain results with different ratios of precursors and reaction times and are able to confirm the proposed conclusions regarding the effect of the synthetic parameters on the result of the size and shape of the obtained nanoparticles and support it with additional data (Table 2).17 From the TEM results as far as morphology is concerned, we notice that the increase of the reaction time leads to formation of uniform Nfs with district cores and petals. On the other hand, when comparing the results with the different ratios of silver and iron the changes in shape are not so obvious. While increasing the ratio of silver![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) iron there is a slight increase on the size of the Nfs, while the Nfs shape is more uniform (Fig. 1).
iron there is a slight increase on the size of the Nfs, while the Nfs shape is more uniform (Fig. 1).
| Nfs | Ag/Fe ratio | Reaction time | TEM size of Nfs |
|---|---|---|---|
| Nf 24H 0.2R | 0.2 | 24 h | 80.75 nm ± 10.12 |
| Nf 8H 0.15R | 0.15 | 8 h | 54.36 nm ± 13.40 |
| Nf 6H 0.18R | 0.18 | 6 h | 47.42 nm ± 12.87 |
| Nf 12H 0.2R | 0.2 | 12 h | 83.25 nm ± 15.89 |
| Nf 12H 0.18R | 0.18 | 12 h | 68.00 nm ± 13.28 |
| Case study | Ag/Fe ratio | Reaction time |
|---|---|---|
| Current study | 0.2 | 24 h |
| 0.15 | 8 h | |
| 0.18 | 6 h | |
| 0.2 | 12 h | |
| 0.18 | 12 h | |
| Literature available data | 0.15 | 4 h |
| 0.30 | 8 h | |
| 0.60 | 8 h |
 | ||
| Fig. 1 TEM images of (A) Nf 24H 0.2R, (B) Nf 8H 0.15R, (C) Nf 6H 0.18R, (D) Nf 12H 0.2R and (E) Nf 12H 0.18R. | ||
2.2 Physicochemical characterization
The colloidal stability of the Nfs is studied by DLS and Zeta potential measurements. The determination of size by the DLS can be different from the results of TEM, because DLS calculates the hydrodynamic diameter of the nanoparticles and not their actual size. These differences in size can be attributed to the fact that the nanoparticles exhibit intramolecular interactions with the dispersant, herein is water, that can cause an increase in the measured size by DLS18 (Table 3 and Fig. 2, Fig. S1–S3, ESI†). The structural characterization of the Nfs includes the UV-Vis study, the FT-IR and PXRD spectra of the synthesized Nfs. The hybrid nature of the Nfs results in two absorbance peaks in the UV-Vis study, which are accredited to silver and iron. Silver is known to appear SPR band at the UV-Vis region at around 00 nm.19,20 As shown in the obtained spectra, the presence of silver is confirmed by the appearance of these absorbance bands in all the synthesized Nfs. The shifting of the silver peak from one case to another is caused by the different sizes of the silver cores formed. A shift to the red region complies with an increase in the size of the silver core. In a similar way the shifting in the iron's peak at around 700 nm can be explained (Fig. 2).17 In the FT-IR spectra of the Nfs characteristic carboxyl group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O stretching and O–H stretching attributed to the sodium acetate stabilizing the Nfs is detected at 1634 cm−1, 1038 cm−1 and 3263 cm−1 respectively (Fig. 3), confirming the role of sodium acetate as a stabilizing agent.21
O, C–O stretching and O–H stretching attributed to the sodium acetate stabilizing the Nfs is detected at 1634 cm−1, 1038 cm−1 and 3263 cm−1 respectively (Fig. 3), confirming the role of sodium acetate as a stabilizing agent.21
| Nfs | d DLS (nm) | ζ (mV) | PdI index |
d
TEM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) size (nm) size (nm) |
|---|---|---|---|---|
| Nf 24H 0.2R | 220.3 | −21.4 | 0.300 | 80.8 |
| Nf 8H 0.15R | 971.4 | −16.1 | 0.359 | 54.4 |
| Nf 6H 0.18R | 1078 | −2.82 | 0.444 | 47.4 |
| Nf 12H 0.2R | 296 | −14.3 | 0.374 | 83.2 |
| Nf 12H 0.18R | 396.3 | −14.8 | 0.289 | 68 |
Powder X-ray diffraction (pXRD) is a key characterization method for determining the properties of nanoparticles. XRD measurements provide valuable insights into crystal structures, which can be crucial for evaluating their potential applications, such as use as contrast agents. In this study, the characteristic XRD peaks observed in all cases of the Nfs indicate that iron is present as magnetite, with notable planes at (220), (311), (400), (511), and (440). This finding aligns with the experimental observation of the Nfs’ magnetization during the washing process at the conclusion of the synthesis (Fig. 4). For silver, the characteristic planes observed at (111), (220), and (200) correspond to a face-centered cubic (fcc) structure, confirming the crystallinity of the Nfs.22
2.3 Coating of Nfs with citrate
As shown in Fig. 6, the peaks of silver and iron do not change significantly, indicating the stability of the Nfs after the coating process and the maintenance of these specific physicochemical properties.
The DLS results show the improvement of colloidal stability, which translates to lower PdI values and negatively higher Zeta Potential values (Table 4 and Fig. S4, S5, ESI†). As elaborated widely in literature, low PdI values are associated with increased uniformity of the obtained nanoparticles, which is crucial for the subsequent biological applications.23,24 Moreover, the FT-IR spectra of the coated Nfs (Fig. 7) confirms the presence of the coating, due to the stretching vibrations observed at 1350 cm−1 and 1600 cm−1, attributed to the stabilizing molecules of citrate.
| Nanoflowers | d H(DLS) (nm) | ζ (mV) | PdI index | d TEM (nm) |
|---|---|---|---|---|
| Nf@citrate 12H 0.18R | 147.0 | −18.8 | 0.156 | 98.6 |
| Nf@citrate 12H 0.2R | 246.4 | −19.1 | 0.236 | 52.4 |
| Nf@citrate 6H 0.18R | 190.4 | −17.8 | 0.293 | 107.3 |
2.4 Biological evaluation of hybrid Nfs and coated hybrid Nfs
While hemolysis results provide insights into toxicity toward red blood cells (RBCs), they may not reveal indirect toxicity, such as structural abnormalities, which can ultimately lead to cell death. However, as shown in Fig. 10(a)–(c), no structural alterations were observed for any of the tested Nfs, supporting their presumed high biocompatibility with RBCs.29
Regarding the statistical analysis that is performed for the cytotoxicity results obtained, it should be noted that One-way Anova is considered an appropriate statistical tool that is suitable for the analysis of the difference (if occurs) between the mean values of two or more groups. It is important to analyze and conclude the difference between the different nanoflowers and different concentrations, as those conclusions can lead to in depth understanding and linkage of the nanoparticle's toxicity with respects to their properties. Also, it should be noted that the study is repeated three times, in order to ensure the robustness of the findings, due to the high variability of the MTT assay. Moreover, the robustness of the method used has been ensured by the use of DMSO that increases the accuracy of the MTT assay, as suggested in literature.35 At this point it is important to clarify and justify the difference that is observed between healthy and cancer cells. As mentioned in literature due to the higher metabolic activity of tumour cells, there is higher ROS production and this leads to the susceptibility of cancer cells to present ferroptosis. On the contrary, this phenomenon does not take place in healthy cells. Given the increased iron availability inside glioblastoma cells, this explains why there is higher toxicity of the hybrid nanoflowers towards U87-MG cells than HaCat cells. The abovementioned rationale explains sufficiently the difference in the results of healthy and cancer cells.36–39 The abovementioned conclusion has been also confirmed by a recent study on iron–silver nanospikes and their effectiveness as dual imaging probes as well as synergestic chemotherapeutics.26
2.4.3.1 Investigation of internalization by Prussian blue protocol. The presence of iron oxide on the outer part of the Nfs enables their tracking using the Prussian blue staining protocol. Treatment with the Pearls reagent results in a blue coloration at the locations of the Nfs within the cells. This allows for confirmation of their internalization and investigation of their specific localization. In Fig. 13(a)–(c), the internalization inside healthy HaCat cells is observed. In all cases of Nfs, blue spots can be identified at the outer lining of the cells. No significant differences can be observed, except the cases of Nf 24H 0.2R and Nf 12H 0.18R where there seems to be higher internalization and can be attributed to their smaller size.
The same procedure is followed for the study regarding glioblastoma cells. As shown in Fig. 14(a)–(c), the internalization compared to healthy cells is much more intense. These results suggest that internalization is more favorable in glioblastoma cells compared to healthy cells.40,41 Regarding both cases (healthy and cancer cells) it is noted that the results of the staining are not in agreement with the cytotoxicity study results e.g. Nf 6H 0.18R. To clarify the light staining of the cells compared to the significant cytotoxicity levels, the first step is the literature review for any available data regarding this type of Nfs. Since these tests have not been reported so far, the next step is the careful examination of the protocol and the principles of the method. As it is extensively reported, the Prussian blue staining appears during the reduction of only Fe3+ ions by the Pearls reagent. In the present case of Nfs, the pXRD results show that the crystalline structure of all the Nfs is magnetite, which is found both as Fe3+ and Fe2+. Thus, the possible explanation behind some cases with no significant Prussian blue staining, lies in the quantitative ratio of the two oxidative states. Although, this is a logical justification of the observations, the possible explanation is confirmed by conduction of internalization studies by fluorescence microscopy.42 Furthermore, the results of the Prussian Blue staining demonstrate a greater internalization in glioblastoma cells compared to healthy cells. This observation aligns with the rationale discussed in the cytotoxicity section and is consistent with the findings of the MTT assay. Additionally, the size distribution and morphology of the nanoflowers are correlated with the cellular uptake results, as indicated by the Prussian Blue staining. As illustrated in the microscopy images, nanoflowers with lower PDI values exhibit higher accumulation within cells, resulting in more intense blue staining. This phenomenon can be attributed to the fact that more aggregated nanoflowers encounter difficulty in entering cells, thereby reducing observable staining. The aforementioned conclusion concurs with a recent study that demonstrated the PDI of nanoparticles exerts a greater influence on their internalization than their ζ potential39. Regarding the influence of shape, it is observed that more uniformly shaped nanoflowers exhibit more intense internalization. The presence of more “exotic” morphologies has been reported to cause higher internalization into cells due to an increased surface area that enhances interactions with cells. Nevertheless, given the similar shape of the nanoflowers across all synthetic procedures, no specific comparisons are applicable in the present report, as no significant differences in shape are observed.43–45
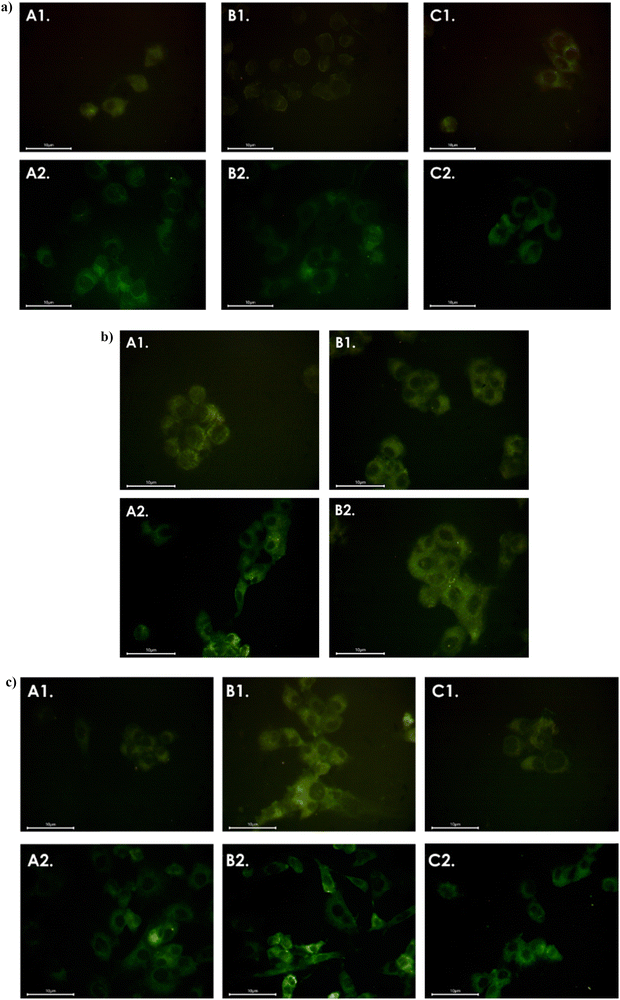
2.4.3.2 Investigation of internalization by fluorescence microscopy. As mentioned at the previous study, the duality of the Nfs’ nature offers monitoring of their action inside the cells by different protocols based on either silver or iron. It is known that silver nanoparticles often exhibit fluorescence due to their unique optical properties.12 The pictures of the fluorescence microscopy show intense green fluorescence of the Nfs, confirming the successful internalization of the Nfs and supporting the proposed explanation of the previous study results (Prussian blue staining). In detail, Fig. 15(a)–(c) depict the fluorescence of all Nfs coated inside healthy cells (HaCat).
The results regarding the glioblastoma cells show similar internalization of the Nfs. For both cases, fluorescence is located on the outer lining of the cell and shows possible accumulation of the Nfs at the cell membranes (Fig. 16(a)–(c)). It is important to note that the abovementioned protocols concern qualitive techniques of visualization. The conclusions that can be drawn are suggestions that should be further confirmed by a quantitative technique to ensure scientific plenitude of their biological perspective in theranostics. Thus, quantitative techniques were also used to verify and confirm the abovementioned results.
Finally, it is noteworthy that these Nfs have not, to our knowledge, been extensively studied using these protocols in the existing literature.
 | ||
| Fig. 20 Incubation of Nfs with inhibitors of specific endocytosis pathways in a (A) HaCat and in (B) U87-MG. | ||
In the case of HaCat cells (Fig. 20(B)), the results indicate the absence of a specific internalization mechanism; rather, the Nfs enter the cells via three distinct mechanisms, with pinocytosis being the predominant mode of cellular entry. As previously noted, the larger Nfs enter cells through pinocytosis, while smaller Nfs utilize a combination of clathrin and caveolin-mediated endocytosis. This conclusion has been corroborated in the literature for this particular cell line.56 The elucidation of the internalization pathway can serve as a foundation for the understanding the processes and steps involved into the observed biological activity of the Nfs.
3. Experimental
3.1 Equipment
Attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectra were obtained on PerkinElmer Spectrum 100 Spectrometer; the spectra were scanned over the range 4000–400 cm−1. Dynamic light scattering (DLS) measurements were performed on a Malvern Instruments Zetasizer Nano Series. In the data presented in this study, each measurement represents the average value of 3 measurements, with 11 to 15 runs for each measurement. Ultraviolet-visible (UV-Vis) absorption spectra in the wavelength range of 200–800 nm was obtained on a Jasco V-650 spectrometer. An ultrasonic bath was used for sonication (Elma Sonic, S 30H).3.2 Materials and methods
Silver nitrate 99% (AgNO3) was purchased from Fischer, ethylene glycol (EG) 99% and poly-L-lysine 0.01% were purchased from Merck, Polyvinylpyrrolidone (PVP) (MW = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were purchased from Alfa Aesar. Iron(III) nitrate·9H2O was purchased from Riedel-de Haen. Trisodium citrate (Citrate Potassium Dihydrogen Phosphate) and sodium acetate anhydrous was purchased from Lach-Ner. Ethanol 99% was purchased from Carlo Erba SA. DMEM high glucose, FBS 10%, Penicillin–Streptomycin 10%, Glutamine 5% were purchased from Biosera. MTT was purchased from Cayman Chemical Company. Adenine >99% was purchased from Apollo Scientific. 1,10-Phenanthroline hydrate was purchased from Fluorochem. Eosin was purchased from ROTH.
000) were purchased from Alfa Aesar. Iron(III) nitrate·9H2O was purchased from Riedel-de Haen. Trisodium citrate (Citrate Potassium Dihydrogen Phosphate) and sodium acetate anhydrous was purchased from Lach-Ner. Ethanol 99% was purchased from Carlo Erba SA. DMEM high glucose, FBS 10%, Penicillin–Streptomycin 10%, Glutamine 5% were purchased from Biosera. MTT was purchased from Cayman Chemical Company. Adenine >99% was purchased from Apollo Scientific. 1,10-Phenanthroline hydrate was purchased from Fluorochem. Eosin was purchased from ROTH.
3.3 Synthesis of silver–iron oxide Nfs (SIONFs)
The synthesized Nfs were prepared according to a solvothermal method with some modifications.10,17 In a spherical glass vial 10 mL of EG were added followed by the addition of different amounts of Fe(NO3)3·9H2O and stirred until totally dissolved. The synthesis continues by the addition of different amounts of silver nitrate and sodium acetate and the mixture is stirred for at least 30 min, until the solids are completely dissolved. Then, the mixture is transferred into a Teflon lined autoclave reactor and left at 200 °C for different reaction times (6 h, 8 h, 12 h, 24 h). The color of the mixture turns from intense orange into black-brown and the resulting solution was magnetically separated. The resulting precipitate was washed several times with ethanol and water to discard unreacted reagents. Then the precipitate was redispersed in water for further use.3.4 Physicochemical characterization
The size, morphology, and structural characteristics of the Nfs were determined by a combination of techniques (TEM, DLS, UV-Vis, FT-IR and PXRD). The morphology of the Nfs was confirmed by TEM and their size was estimated. DLS data were also acquired to verify the size of the Nfs as well as their surface charge, which is another important aspect of their characteristics, via Zeta Potential measurements. FT-IR spectra and PXRD confirmed the structural characteristics of the Nfs.3.5 Determination of iron concentration of the Nfs
The concentration of iron in the synthesized Nfs was determined by the colorimetric phenanthroline assay. Iron has the ability to form stable complexes with 1,10-phenanthroline resulting into strong orange red colored solutions that exhibit high absorbance values in the UV-Vis region and more specifically at 510 nm57. The Nfs are digested in HCl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) v/v at 70 °C for 30 min. The digested Nfs are diluted with water. Then the following reagents are added to eppendorfs (450 μL sodium acetate 125 mg mL−1, 50 μL hydroxylamine hydrochloride 10 mg mL−1, 200 μL of the sample and 300 μL, 1,10 phenanthroline 10 mg mL−1). Each sample is assessed in triplicates for repeatable results. For the quantification of the iron concentration prior to the samples, the procedure is repeated for known concentrations of iron to create a calibration curve.
1) v/v at 70 °C for 30 min. The digested Nfs are diluted with water. Then the following reagents are added to eppendorfs (450 μL sodium acetate 125 mg mL−1, 50 μL hydroxylamine hydrochloride 10 mg mL−1, 200 μL of the sample and 300 μL, 1,10 phenanthroline 10 mg mL−1). Each sample is assessed in triplicates for repeatable results. For the quantification of the iron concentration prior to the samples, the procedure is repeated for known concentrations of iron to create a calibration curve.
3.6 Determination of silver concentration of the Nfs
The amount of silver in the Nfs was quantified by a colorimetric method that is based on the absorbance of the pink complex between silver–adenine and eosin.58 First, the Nfs were digested with 1 M HNO3 at 50 °C until the solution is clear of color. Then, the Nfs are diluted with water to 1 mL. The following step was the addition of 300 μL of adenine solution 10−2 M, 500 μL of PVP 1% aqueous solution, 2.5 mL phosphate buffer pH 6, 500 μL eosin 10−3 M. and 595 μL of each sample of the Nfs. The same procedure was followed to create a standard curve using different concentrations of silver, which was used to calculate the amount of silver in the samples.3.7 Computed tomography imaging
A second generation Mediso microPET/CT scanner (nanoPET/CT, Mediso, Hungary) at the Edinburgh Preclinical Imaging Laboratory was employed to assess the computer tomography (CT) contrast enhancement capabilities of the silver Nfs and silver Nfs coated with citrate. The CT imaging and analysis method has been previously described.59–61 Imaging parameters included a tube voltage of 50 kVp, 300 ms exposure time, and 0.38 mm slice thickness. Attenuation coefficients were normalized to water, with subsequent conversion of CT image pixels to Hounsfield Units (HU) according to the following equation:where μ is the linear attenuation coefficient in each pixel measured by the CT scanner, and μwater and μair are the linear attenuation coefficients of water and air, respectively. CT images were analyzed using an in-house software.62,63 A cylindrical volume of interest was drawn at the center of each sample tube (excluding the boundary areas) and were used to quantify the mean HU values and standard deviation.
3.8 Hemocompatibility study
The primary evaluation of the synthesized Nfs’ toxicity was performed by use of the hemolysis assay. Clinical discarded blood sample was used for hemolysis assay. The procedure was followed according to existing protocol.12 First, RBCs were isolated from whole blood by centrifugation at 3000 rpm for 5 min and washed twice with DPBS. The samples of the Nfs were prepared at 15 μg mL−1 and 25 μg mL−1 concentrations for a final volume of 300 μL. In 2 mL eppendorfs the following were added in the mentioned order: 285 μL of each sample, 15 μL of RBCs and incubated for 3 h at 37 °C synthesized. For the isolation of the supernatant containing the released hemoglobin, the samples were centrifuged at 3000 rpm for 5 min and 100 μL of the supernatant of each sample were transferred in a 96-well plate. For the isolation of the supernatant containing the released hemoglobin, the samples were centrifuged at 3000 rpm for 5 min and 100 μL of the supernatant of each sample were transferred in a 96-well plate. As positive control was used water that cause full hemolysis of RBCs and releasw of hemoglobin and as negative control was used DPBS without Ca2+and Mg2+. The % Hemolysis was calculated by measuring the plate at 490 nm using an Eliza plate reader and the appropriate equation:where ODsample is the absorbance of the sample, ODpositive control is the absorbance of the positive control (Water) and ODnegative control is the absorbance of the negative control (DPBS).
For the completed evaluation of the hemocompatibility profile of the Nfs, the treated RBCs were morphologically evaluated by optical microscopy. In detail, the RBCs were seeded in 24-well plates and incubated for 3 h with the Nfs at the selected concentrations. At the end of the incubation, the RBCs were observed under the optical microscope for the examination of possible structure alterations induced by Nfs’ toxicity.
3.9 Cell culture
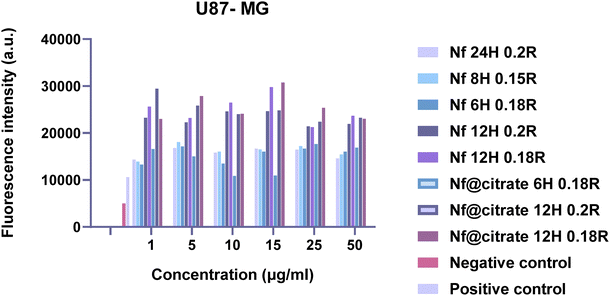
Cytotoxicity assessment of the synthesized nanoparticles as well as their cellular uptake was performed at two different cell lines: healthy (HaCat, human epidermal keratinocyte healthy cell line) and U87-MG: Uppsala 87 Malignant Glioma cancer cell line). The cells were cultured in a Dulbecco's Modified Eagle Medium high in glucose (DMEM) containing 10% Fetal Bovine Serum (FBS), 10% Penicillin–Streptomycin and 5% Glutamine inside a CO2 incubator (37 °C, 5% CO2).643.10 In vitro cytotoxicity study
The effect on the viability of the cells for the two different cell lines was measured with the MTT assay, according to the established protocol.65 The method is based on the ability of live cells to reduce MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium bromide) via their mitochondrial reductase into purple dissoluble formazan crystals. Those crystals are solubilized and based on the intensity of the absorption it is allowed to assess the viability of the cells. Briefly, in a 96-well plate cells were seeded and incubated for 24 h. The culture medium was removed and replaced by the samples in triplicates and different concentrations (50, 25, 15, 10, 5, 1 μg mL−1 of iron). After 24 h incubation, the medium was removed, 100 μL of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (1 mg mL−1) was added to each well and left for 3 h for the formation of the formazan crystals. The formazan crystals were dissolved in DMSO (Dimethyl sulfoxide), and their absorbance was measured at 540 nm (with reference filter at 620 nm) using an Eliza plate reader. The absorbance values were used for the calculation of the % Viability according to the equation:where OD(sample) is the average absorbance of the sample, OD(Control) is the average absorbance of the control samples containing only cells. From the measurements of the absorbance standard deviation is calculated for each concentration evaluated.
3.11 Internalization studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solution of 4% w/v K4Fe (CN)6·3H2O in PBS and 4 M HCl in PBS) for 30 min and washed again with PBS. Neutral red solution (0.02%) was added for 15 min and washed with PBS. The cells were immediately studied with optical microscopy.
1 solution of 4% w/v K4Fe (CN)6·3H2O in PBS and 4 M HCl in PBS) for 30 min and washed again with PBS. Neutral red solution (0.02%) was added for 15 min and washed with PBS. The cells were immediately studied with optical microscopy.
3.12 ROS assay
To assess oxidative stress induced by the Nfs, the DCFDA assay protocol was employed, with fluorescence measurements obtained using a plate reader. For both cell lines, cells were seeded in 96-well plates and grown to confluence. The culture media was then replaced with selected concentrations of coated and uncoated Nfs and incubated for 24 hours. Following incubation, the supernatant was discarded, and the cells were gently washed with PBS. Subsequently, 100 μL of DCFDA solution was added to each well and incubated in the dark for 30 minutes. For fluorescence intensity comparisons, PBS was used as the negative control, while hydrogen peroxide served as the positive control. Fluorescence intensity was then measured using a fluorescent plate reader.66–683.13 Apoptosis–necrosis evaluation (FACS, flow cytometry)
Both U87-MG and HaCat cells were seeded in 6 well plates or 25 mm2 culture flask for 24 h prior to their treatment with 15 μg mL−1 of Nfs 12H 0.2R. After 24 h of treatment, cells were harvested by trypsin and then centrifuged at 800 rpm for 3 min. For the Annexin-7AAD protocol, about 1 × 105 cells were well resuspended in 100 μL of binding buffer, then stained 5 μL of annexin-V and 5 μL of 7AAD and left to incubate for 20 min in the dark. After the incubation time was over, 400 μL of binding buffer was added and the sample was measured with a Becton Dickinson FACS Calibur flow cytometer (BD Bioscience, New Jersey, USA).3.14 Internalization mechanism
For the identification of the endocytosis pathway of Nfs, cells were seeded into 24-well plates for both cell lines studied until confluency reached 80–90%. The selected inhibitors were then added to the cells (a) Cytochalasin A 10 μg mL−1, (b) Chlorpromazine 10 μg mL−1 and (c) Genistein 25 μg mL−1 and were incubated for 1 h. The cells were subsequently washed with PBS and the Nfs were added for 24 h incubation at a concentration of 15 μg mL−1. At the end of the incubation time, cells were washed again with PBS, dettached with 200 μL trypsin and collected. Finally, 1 mL of HNO3 1 M and 1 mL 30% H2O2 were added and samples were left for 2 h at 95 °C. The results were obtained using ICP-MS.3.15 Statistical analysis
Statistical analyses were conducted utilizing GraphPad Prism as the statistical software. Based on the data input and the null hypothesis, which determines the parameters considered as well as the desired correlations and comparisons to be performed, the appropriate statistical tests were employed. For example, the statistical analysis of the MTT Assay involved the use of one-way ANOVA, which examines the variability of results by considering a single variable and is particularly useful when dealing with three or more groups of data. Conversely, for results corresponding to a single concentration tested but involving different parameters, such as apoptosis–necrosis, the results are suitable for t-test statistical analysis. Following this rationale, the statistical analysis was conducted by selecting the most appropriate statistical tool for each experiment implemented.4. Conclusions
Due to the limited literature on silver–iron oxide nanoflowers, this study provides valuable data supporting their potential use in glioblastoma treatment. By elucidating their synthesis parameters, physicochemical properties, and biological effects, this research enhances the understanding of these hybrid nanosystems, leading to future applications in theranostics and targeted cancer therapies. Recent investigations into the efficacy of hybrid nanoparticles for glioblastoma treatment have yielded promising results, particularly concerning silver–iron hybrid nanoparticles. The dual functionality of these nanoparticles positions them as ideal candidates for a versatile nanosystem with extensive applications in glioblastoma theranostics. Although morphologies such as nanoflowers have not been extensively documented, a limited number of studies have provided insights into their efficacy as photothermal therapy (PTT) agents. Furthermore, hybrid silver–iron nanoparticles have demonstrated suitability as thermo- and photo-sensitizers in glioblastoma cell testing.16,69–71The synthetic procedure of this study exploits the parameters affecting the development of hybrid silver – iron oxide Nfs and further demonstrates their successful coating with trisodium citrate. Critical parameters of this study including the reaction time and ratio of Ag/Fe demonstrate the impact on the size and formed flower shape of the Nfs. Furthermore, Nfs are characterized as far as their physicochemical properties are concerned, while also evaluated as potential multi-sensitive agents for the theranostic of glioblastoma. The in vitro biological evaluation suggests possible anticancer properties and high hemocompatibility, which are essential for the design and synthesis of an efficient and safe nanosystems. Their dual nature leads to their easy tracking by using different methods based on either silver or iron. The results of the CT measurements show that the Nfs are eligible nanosystems for diagnostic purposes, such CT contrast agents. Furthermore, the apoptosis–necrosis evaluation confirmed the hypothesis of the possible anticancer activity of the Nfs in glioblastoma, since the %apoptosis and %necrosis were significantly higher than the respective results of healthy cells. Lastly, the careful examination of the internalization of the Nfs offered a foundation for the detailed explanation and verification of their mechanism of action when interacting with both healthy and cancerous cells. Due to the limited literature data on the current Nfs it is notable to contribute to the literature with more data that support the possible use of these Nfs for biological applications.
Data availability
We submit a manuscript entitled:“Hybrid Silver–Iron Oxide Nanoflowers: Tailoring Morphology and Unlocking Theranostic Potential for Glioblastoma” authored by Sofia G. Nikolopouloua, Beata Kalska-Szostko, Anna Basa, Giorgos Papanastasiou, Adriana Tavares, Carlos Alcaide Corral, Marios Kostakis, Nikolaos S. Thomaidis, Athina Papadopoulou, Eleni K. Efthimiadou. The data supporting this article are available in case requested from the editorial team.Conflicts of interest
There are no conflicts to declare.References
- L. Rong, N. Li and Z. Zhang, Emerging therapies for glioblastoma: current state and future directions, J. Exp. Clin. Cancer Res., 2022, 41(1), 1–18 CrossRef PubMed.
- R. S. Angom, N. M. R. Nakka and S. Bhattacharya, Advances in Glioblastoma Therapy: An Update on Current Approaches, Brain Sci., 2023, 13(11), 1536, DOI:10.3390/brainsci13111536.
- J. Zhang, M. F. G. Stevens and T. D. Bradshaw, Temozolomide: Mechanisms of Action, Repair and Resistance, Curr. Mol. Pharmacol., 2012, 5(1), 102–114 CrossRef CAS PubMed.
- M. T. C. Poon, C. L. M. Sudlow, J. D. Figueroa and P. M. Brennan, Longer-term (≥2 years) survival in patients with glioblastoma in population-based studies pre- and post-2005: a systematic review and meta-analysis, Sci. Rep., 2020, 10(1), 1–10, DOI:10.1038/s41598-020-68011-4.
- A. Barzegar Behrooz, Z. Talaie and A. Syahir, Nanotechnology-Based Combinatorial Anti-Glioblastoma Therapies: Moving from Terminal to Treatable, Pharmaceutics, 2022, 14(8), 1–23 CrossRef PubMed.
- S. Ajith, F. Almomani, A. Elhissi and G. A. Husseini, Nanoparticle-based materials in anticancer drug delivery: Current and future prospects, Heliyon, 2023, 9(11), e21227, DOI:10.1016/j.heliyon.2023.e21227.
- F. Rodr, P. Caruana, N. Fuente De, P. Español and J. Balart, Nano-Based Approved Pharmaceuticals for Cancer Treatment: Present and Future Challenges, Biomolecules., 2022, 12(784), 1–27 Search PubMed.
- N. Ebrahimi, S. Rasoul-Amini, A. Niazi, N. Erfani, A. Moghadam and A. G. Y. Ebrahiminezhad, Cytotoxic and Apoptotic Effects of Three Types of Silver-Iron Oxide Binary Hybrid Nanoparticles, Curr. Pharm. Biotechnol., 2016, 17(12), 1049–1057 CAS.
- A. A. Hernández-Hernández, G. Aguirre-Álvarez, R. Cariño-Cortés, L. H. Mendoza-Huizar and R. Jiménez-Alvarado, Iron oxide nanoparticles: synthesis, functionalization, and applications in diagnosis and treatment of cancer, Chem. Pap., 2020, 74(11), 3809–3824, DOI:10.1007/s11696-020-01229-8.
- R. Das, N. Rinaldi-Montes, J. Alonso, Z. Amghouz, E. Garaio and J. A. García, et al., Boosted Hyperthermia Therapy by Combined AC Magnetic and Photothermal Exposures in Ag/Fe3O4 Nanoflowers, ACS Appl. Mater. Interfaces, 2016, 8(38), 25162–25169 CrossRef CAS PubMed.
- Ö. Erdoğan, M. Abbak, G. M. Demirbolat, M. Aksel, S. Paşa and G. Dönmez Yalçin, et al., Treatment of glioblastoma by photodynamic therapy with the aid of synthesized silver nanoparticles by green chemistry from citrus aurantium, J. Res. Pharm., 2021, 25(5), 641–652 Search PubMed.
- S. G. Nikolopoulou, B. Kalska, A. Basa, A. Papadopoulou and E. K. Efthimiadou, Novel Hybrid Silver − Silica Nanoparticles Synthesized by Modifications of the Sol − Gel Method and Their Theranostic Potential in Cancer, ACS Appl. Bio Mater., 2023, 6(12), 5125–5874 CrossRef PubMed.
- Y. Zhang, K. Xi, X. Fu, H. Sun, H. Wang and D. Yu, et al., Versatile metal-phenolic network nanoparticles for multitargeted combination therapy and magnetic resonance tracing in glioblastoma, Biomaterials, 2021, 278, 121163, DOI:10.1016/j.biomaterials.2021.121163.
- N. Arias-Ramos, L. E. Ibarra, M. Serrano-Torres, B. Yagüe, M. D. Caverzán and C. A. Chesta, et al., Iron oxide incorporated conjugated polymer nanoparticles for simultaneous use in magnetic resonance and fluorescent imaging of brain tumors, Pharmaceutics, 2021, 13(8), 1258, DOI:10.3390/pharmaceutics13081258.
- A. ur Rehman, Y. Wu, H. D. N. Tran, K. Vazquez-Prada, Y. Liu and H. Adelnia, et al., Silver/Iron Oxide Nano-Popcorns for Imaging and Therapy, ACS Appl. Nano Mater., 2021, 4(10), 10136–10147 CrossRef CAS.
- T. Grancharova, P. Zagorchev and B. Pilicheva, Iron Oxide Nanoparticles: Parameters for Optimized Photoconversion Efficiency in Synergistic Cancer Treatment, J. Funct. Biomater., 2024, 15(8), 207, DOI:10.3390/jfb15080207.
- Y. Zhang, H. Ding, Y. Liu, S. Pan, Y. Luo and G. Li, Facile one-step synthesis of plasmonic/magnetic core/shell nanostructures and their multifunctionality, J. Mater. Chem., 2012, 22(21), 10779–10786 RSC.
- T. Analysis, M. J. Jee, J. A. Tyson, E. Glikman and M. Bogosavljevi A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles.
- S. G. Nikolopoulou, N. Boukos, E. Sakellis and E. K. Efthimiadou, Synthesis of biocompatible silver nanoparticles by a modified polyol method for theranostic applications: Studies on red blood cells, internalization ability and antibacterial activity, J. Inorg. Biochem., 2020, 211, 111177, DOI:10.1016/j.jinorgbio.2020.111177.
- S. G. Nikolopoulou, B. Kalska, A. Basa, A. Papadopoulou and E. K. Efthimiadou, Novel Hybrid Silver-Silica Nanoparticles Synthesized by Modifications of the Sol–Gel Method and Their Theranostic Potential in Cancer, ACS Appl. Bio Mater., 2023, 6(12), 5235–5251 CrossRef CAS PubMed.
- A. L. P. Cruz, J. A. G. Cervantes, X. G. V. Anzaldo and G. G. Rivas, Synthesis and design of Ag – Fe bimetallic nanoparticles as antimicrobial synergistic combination therapies against clinically relevant pathogens, Sci. Rep., 2021, 1–11, DOI:10.1038/s41598-021-84768-8.
- M. H. Ali, M. A. K. Azad, K. A. Khan, M. O. Rahman, U. Chakma and A. Kumer, Analysis of Crystallographic Structures and Properties of Silver Nanoparticles Synthesized Using PKL Extract and Nanoscale Characterization Techniques, ACS Omega, 2023, 8(31), 28133–28142 CrossRef CAS PubMed.
- K. Srinivas and D. Press, A quality by design approach on polymeric nanocarrier delivery of gefitinib: formulation, in vitro, and in vivo characterization A quality by design approach on polymeric nanocarrier delivery of gefitinib: formulation, in vitro, and in vivo characte, Int. J. Nanomed., 2017, 12, 15–28, DOI:10.2147/IJN.S122729.
- M. Danaei, M. Dehghankhold, S. Ataei, F. H. Davarani, R. Javanmard and A. Dokhani, et al., Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems, Pharmaceutics, 2018, 10(57), 1–17 Search PubMed.
- M. Wang, Y. Liang, Z. Zhang, G. Ren, Y. Liu and S. Wu, et al., Ag@Fe3O4@C nanoparticles for multi-modal imaging-guided chemo-photothermal synergistic targeting for cancer therapy, Anal. Chim. Acta, 2019, 1086, 122–132, DOI:10.1016/j.aca.2019.08.035.
- S. S. Moonshi, K. X. Vazquez-Prada, J. Tang, N. J. Westra van Holthe, G. Cowin and Y. Wu, et al., Spiky Silver-Iron Oxide Nanohybrid for Effective Dual-Imaging and Synergistic Thermo-Chemotherapy, ACS Appl. Mater. Interfaces, 2023, 15(36), 42153–42169 CrossRef CAS PubMed.
- S. Yedgar and G. Barshtein, Hemolytic Activity of Nanoparticles as a Marker of Their Hemocompatibility, Micromachines, 2022, 13, 2091 CrossRef PubMed.
- S. Malehmir, M. A. Esmaili, M. K. Mahabady, A. Sobhani-nasab, A. Atapour and M. R. Ganjali, et al., A review: hemocompatibility of magnetic nanoparticles and their regenerative medicine, cancer therapy, drug delivery, and bioimaging applications, Front. Chem., 2023, 11, 1–14 Search PubMed.
- K. de la Harpe, P. Kondiah, Y. Choonara, T. Marimuthu, L. du Toit and V. Pillay, The Hemocompatibility of Nanoparticles: A Review of Cell–Nanoparticle Interactions and Hemostasis, Cells, 2019, 8(10), 1209 CrossRef CAS PubMed.
- D. Hernández-Moreno, M. Fernández-Díaz, I. Rucandio, J. M. Navas and M. L. Fernández-Cruz, Toxic Effects of Different Coating-Related Functionalized Nanoparticles on Aquatic Organisms, Toxics, 2024, 12(2), 142, DOI:10.3390/toxics12020142.
- I. Kim, B. T. Lee, H. A. Kim, K. W. Kim, S. D. Kim and Y. S. Hwang, Citrate coated silver nanoparticles change heavy metal toxicities and bioaccumulation of Daphnia magna, Chemosphere, 2016, 143, 99–105, DOI:10.1016/j.chemosphere.2015.06.046.
- J. K. Fard, S. Jafari and M. A. Eghbal, A review of molecular mechanisms involved in toxicity of nanoparticles, Adv. Pharm. Bull., 2015, 5(4), 447–454, DOI:10.15171/apb.2015.061.
- C. Nieves Lira, A. P. Carpenter, J. E. Baio, B. J. Harper, S. L. Harper and M. R. Mackiewicz, Size- and Shape-Dependent Interactions of Lipid-Coated Silver Nanoparticles: An Improved Mechanistic Understanding through Model Cell Membranes and In Vivo Toxicity, Chem. Res. Toxicol., 2024, 37(6), 968–980 Search PubMed.
- E. Mansouri, A. Mesbahi, H. Hamishehkar, S. Montazersaheb, V. Hosseini and S. Rajabpour, The effect of nanoparticle coating on biological, chemical and biophysical parameters influencing radiosensitization in nanoparticle-aided radiation therapy, BMC Chem., 2023, 17(1), 1–14, DOI:10.1186/s13065-023-01099-7.
- L. Khalef, R. Lydia, K. Filicia and B. Moussa, Cell viability and cytotoxicity assays: Biochemical elements and cellular compartments, Cell Biochem. Funct., 2024, 42(3), e4007, DOI:10.1002/cbf.4007.
- C. Hacioglu and F. Kar, Capsaicin induces redox imbalance and ferroptosis through ACSL4/GPx4 signaling pathways in U87-MG and U251 glioblastoma cells, Metab. Brain Dis., 2023, 38(2), 393–408, DOI:10.1007/s11011-022-00983-w.
- J. Shi, N. Yang, M. Han and C. Qiu, Emerging roles of ferroptosis in glioma, Front. Oncol., 2022, 12, 1–17 Search PubMed.
- K. Zhu, Y. Cai, L. Lan and N. Luo, Tumor Metabolic Reprogramming and Ferroptosis: The Impact of Glucose, Protein, and Lipid Metabolism, Int. J. Mol. Sci., 2024, 25(24), 1–23 Search PubMed.
- Y. Luo, G. Tian, X. Fang, S. Bai, G. Yuan and Y. Pan, Ferroptosis and Its Potential Role in Glioma: From Molecular Mechanisms to Therapeutic Opportunities, Antioxidants, 2022, 11(11), 2123, DOI:10.3390/antiox11112123.
- E. A. Salvanou, A. Kolokithas-Ntoukas, D. Prokopiou, M. Theodosiou, E. Efthimiadou, P. Koźmiński, S. Xanthopoulos, K. Avgoustakis and P. Bouziotis, 177Lu-Labeled Iron Oxide Nanoparticles Functionalized with Doxorubicin and Bevacizumab as Nanobrachytherapy Agents against Breast Cancer, Molecules, 2024, 29(5), 1030, DOI:10.3390/molecules29051030.
- E. D. Prokopiou, M. Pissas, G. Fibbi, F. Margheri, B. Kalska-Szostko, G. Papanastasiou, M. Jansen, J. Wang, A. Laurenzana and K. E. Efthimiadou, Toxicology in Vitro Synthesis and characterization of modified magnetic nanoparticles as theranostic agents: in vitro safety assessment in healthy cells, Toxicol. In Vitro, 2021, 72, 105094, DOI:10.1016/j.tiv.2021.105094.
- E. Science, Magnetite in the human body: Biogenic vs. anthropogenic, Proc. Natl. Acad. Sci. U. S. A., 2016, 113(39), 11986–11987 Search PubMed.
- A. Girigoswami, B. Deepika, S. Udayakumar, G. Janani, D. J. Mercy and K. Girigoswami, Peony-shaped zinc oxide nanoflower synthesized via hydrothermal route exhibits promising anticancer and anti-amyloid activity, BMC Pharmacol. Toxicol., 2024, 25(1), 101, DOI:10.1186/s40360-024-00830-x.
- L. Huang, X. Mao, J. Li, Q. Li, J. Shen and M. Liu, et al., Nanoparticle Spikes Enhance Cellular Uptake via Regulating Myosin IIA Recruitment, ACS Nano, 2023, 17(10), 9155–9166 CrossRef CAS PubMed.
- W. Sun, H. Xiao, J. Zhu, Z. Hao, J. Sun and D. Wang, et al., Multifunctional Oxygen-Generating Nanoflowers for Enhanced Tumor Therapy, ACS Appl. Bio Mater., 2023, 6(11), 4998–5008 CrossRef CAS PubMed.
- Y. Li, J. Yang and X. Sun, Reactive Oxygen Species-Based Nanomaterials for Cancer Therapy, Front. Chem., 2021, 9, 1–12 Search PubMed.
- A. A. Dayem, M. K. Hossain, S. B. Lee, K. Kim, S. K. Saha and G. Yang, et al., The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles, Int. J. Mol. Sci., 2017, 18(120), 1–21 Search PubMed.
- Z. Yu, Q. Li, J. Wang, Y. Yu, Y. Wang, Q. Zhou and P. Li, Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field, Nanoscale Res. Lett., 2020, 15(1), 115, DOI:10.1186/s11671-020-03344-7.
- H. Wu, J. Yin, W. G. Wamer, M. Zeng and Y. M. Lo, Reactive oxygen species-related activities of nano-iron metal and nano-iron oxides 5, J. Food Drug Anal., 2014, 22(1), 86–94, DOI:10.1016/j.jfda.2014.01.007.
- B. Lee, S. J. Yun and I. Choi, Silver nanoparticles induce reactive oxygen species-mediated cell cycle delay and synergistic cytotoxicity with 3-bromopyruvate in Candida albicans, but not in Saccharomyces cerevisiae, Int. J. Nanomed., 2019, 14, 4801–4816 CrossRef CAS PubMed.
- J. He, Y. Ma, X. Niu, J. Pei, R. Yan and F. Xu, et al., Silver nanoparticles induce endothelial cytotoxicity through ROS-mediated mitochondria-lysosome damage and autophagy perturbation: The protective role of N-acetylcysteine, Toxicology, 2024, 502, 153734, DOI:10.1016/j.tox.2024.153734.
- Z. Zhao, Iron and oxidizing species in oxidative stress and Alzheimer's disease, Aging Med., 2019, 2(2), 82–87 CrossRef PubMed.
- G. J. Doherty and H. T. McMahon, Mechanisms of endocytosis, Annu. Rev. Biochem., 2009, 78, 857–902 CrossRef CAS PubMed.
- W. Zhang, R. Taheri-Ledari, F. Ganjali, S. S. Mirmohammadi, F. S. Qazi and M. Saeidirad, et al., Effects of morphology and size of nanoscale drug carriers on cellular uptake and internalization process: a review, RSC Adv., 2022, 13(1), 80–114 RSC.
- M. Sousa De Almeida, E. Susnik, B. Drasler, P. Taladriz-Blanco, A. Petri-Fink and B. Rothen-Rutishauser, Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine, Chem. Soc. Rev., 2021, 50(9), 5397–5434 RSC.
- V. Laniosz, S. A. Dabydeen, M. A. Havens and P. I. Meneses, Human Papillomavirus Type 16 Infection of Human Keratinocytes Requires Clathrin and Caveolin-1 and Is Brefeldin A Sensitive, J. Virol., 2009, 83(16), 8221–8232 CrossRef CAS PubMed.
- L. G. Saywell and B. B. Cunningham, Determination of Iron: Colorimetric o-Phenanthroline Method, Ind. Eng. Chem., Anal. Ed., 1937, 9(2), 67–69 CrossRef CAS.
- Y. Fujita, I. Mori, M. Toyoda and T. Matsuo, Determination of Silver(I) by an Association Complex Formation between Silver-Adenine and Eosin, Anal. Sci., 1993, 9(6), 829–834 CrossRef CAS.
- A. P. Stavropoulou, M. Theodosiou, E. Sakellis, N. Boukos, G. Papanastasiou and C. Wang, et al., Bimetallic gold-platinum nanoparticles as a drug delivery system coated with a new drug to target glioblastoma, Colloids Surf., B, 2022, 214, 112463, DOI:10.1016/j.colsurfb.2022.112463.
- E. D. Prokopiou, M. Pissas, G. Fibbi, F. Margheri, B. Kalska-Szostko and G. Papanastasiou, et al., Synthesis and characterization of modified magnetic nanoparticles as theranostic agents: in vitro safety assessment in healthy cells, Toxicol Vitr, 2021, 72, 105094 CrossRef PubMed . Available from:.
- J. Kuhn, G. Papanastasiou, C. W. Tai, C. M. Moran, M. A. Jansen and A. A. S. Tavares, et al., Tri-modal imaging of gold-dotted magnetic nanoparticles for magnetic resonance imaging, computed tomography and intravascular ultrasound: An in vitro study, Nanomedicine, 2020, 15(25), 2433–2445 CrossRef CAS PubMed.
- G. Papanastasiou, M. A. Rodrigues, C. Wang, K. Heurling, C. Lucatelli and R. A. S. Salman, et al., Pharmacokinetic modelling for the simultaneous assessment of perfusion and 18F-flutemetamol uptake in cerebral amyloid angiopathy using a reduced PET-MR acquisition time: Proof of concept, Neuroimage, 2021, 225, 117482, DOI:10.1016/j.neuroimage.2020.117482.
- B. Bier, M. Unberath, T. Geimer, J. Maier, G. Gold and M. Levenston, et al., Motion compensation using range imaging in C-arm cone-beam CT, Communications in Computer and Information Science, 2017, vol. 723, pp. 561–570 Search PubMed.
- K. Phelan and K. M. May, Mammalian Cell Tissue Culture Techniques. 2016, pp. 1–23 Search PubMed.
- P. Kumar, A. Nagarajan and P. D. Uchil, Analysis of cell viability by the MTT assay, Cold Spring Harb. Protoc., 2018, 2018(6), 469–471 Search PubMed.
- J. Zhao and M. Riediker, Detecting the oxidative reactivity of nanoparticles: a new protocol for reducing artifacts, J. Nanoparticles Res., 2014, 16(7), 2493, DOI:10.1007/s11051-014-2493-0.
- M. P. Murphy, H. Bayir, V. Belousov, C. J. Chang, K. J. A. Davies and M. J. Davies, et al., Guidelines for measuring reactive oxxygen species and oxidative damage in cells and in vivo, Nat. Metab., 2022, 4, 651–662 CrossRef PubMed.
- C. P. Rubio and J. J. Cerón, Spectrophotometric assays for evaluation of Reactive Oxygen Species (ROS) in serum: general concepts and applications in dogs and humans, BMC Vet. Res., 2021, 8, 1–13 Search PubMed.
- V. Manescu, I. Antoniac, G. Paltanea, I. V. Nemoianu, A.G. Mohan and A. Antoniac, et al., Magnetic Hyperthermia in Glioblastoma Multiforme Treatment, Int. J. Mol. Sci., 2024, 25(18), 10065, DOI:10.3390/ijms251810065.
- A. Spoială, C. I. Ilie, L. Motelica, D. Ficai, A. Semenescu and O. C. Oprea, et al., Smart Magnetic Drug Delivery Systems for the Treatment of Cancer, Nanomaterials, 2023, 13(5), 876, DOI:10.3390/nano13050876.
- A. Van de Walle, A. Figuerola, A. Espinosa, A. Abou-Hassan, M. Estrader and C. Wilhelm, Emergence of magnetic nanoparticles in photothermal and ferroptotic therapies, Mater. Horiz., 2023, 10(11), 4757–4775 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ma00210a |
| This journal is © The Royal Society of Chemistry 2025 |