DOI:
10.1039/D5MA00334B
(Paper)
Mater. Adv., 2025, Advance Article
Crystalline structure modification of hydroxyapatite via a hydrothermal method using different modifiers
Received
7th April 2025
, Accepted 19th April 2025
First published on 8th May 2025
Abstract
In this study, hydroxyapatite (HAp) was synthesized using the hydrothermal method, where different modifiers, such as succinic acid, ascorbic acid, stearic acid, calcium phosphate, and silica, were used. The powdered HAp's morphological, compositional, and crystallographic features were explored using Fourier-transform infrared spectroscopy (FTIR), field emission scanning electron microscopy (FESEM), and X-ray diffraction (XRD) characterization techniques. Crystallographic characteristics, such as the lattice parameter, unit cell volume, and crystallinity degree, were calculated by utilizing XRD data. The crystallite size (<100 nm) was calculated using various models, including the Scherrer equation, the Sahadat–Scherrer model, the size-strain plot, the Halder–Wagner model, and different forms of Williamson–Hall plot. Williamson–Hall includes the uniform deformation model (UDM), the uniform stress deformation model (USDM), and the uniform deformation energy density model (UDEDM). Some models incorporate energy density, strain, and stress as additional components. The peaks at 962, 1026, and 1087 and 3000–3800 cm−1 in FTIR absorption spectra indicated the presence of phosphate and hydroxide groups in the synthesized HAp. Additionally, the optical bandgap energy of the synthesized Hap was estimated to range from 5.29 eV to 5.60 eV. Rietveld refinement analysis revealed that HAp is the predominant phase, with tricalcium phosphate (TCP) generated alongside.
1 Introduction
More than 2.2 million individuals undergo surgical procedures to address severe bone abnormalities resulting from accidents, illnesses, and trauma.1 The increased rate of surgery on human bone has led to relevant developments concerning synthetic biomaterials, which initially posed challenges due to infections caused by aseptic surgical techniques.2 Bioactive calcium phosphate ceramics, particularly hydroxyapatite (HAp), are highly biocompatible owing to their crystallographic and chemical comparability with bone and tooth mineral constituents.3 HAp bioceramics are known for their high level of biocompatibility and are commonly used for dense and porous bioceramic preparations. Owing to their low mechanical reliability in aqueous environments, they are unsuitable for heavy load-bearing applications. Instead, they are used as biocompatible phase reinforcements in composites, metal implant coatings, and human tissue incorporation.4,5 Additionally, HAp can be used as a protein delivery agent, as a drug delivery agent, and in chromatography.6 Numerous techniques have been listed for HAp synthesis, including hydrothermal,7,8 hydrolysis,9 combustion methods,10 solid-state methods,11 electrochemical deposition,12 and wet chemical precipitation methods.13 Frequent problems in synthesis methods include non-stoichiometric products, agglomeration, uncontrolled particle size, and long reaction times, highlighting the need for better morphological control.10 The hydrothermal process is a chemical precipitation method used to prepare HAp through chemical reactions using water as the solvent at elevated pressure and temperatures, resulting in the aging process within a pressure vessel or an autoclave.14 This process involves the transformation of liquids, gels, or slurries into crystalline phases under moderate conditions, producing needle-shaped powders with lengths between 100 and 160 nm and diameters between 20 and 40 nm.4,14 In recent years, studies have shown that the dimensions and shapes of HAp can be regulated by altering the hydrothermal reaction temperature and time.15 This method has led to the preparation of high-purity hydroxyapatite with various shapes, including spine-like, needle-like, rod-like, flake, nano-spherical, and hexagonal prisms.10,16,17 Modifiers are used to modify the crystalline structure of HAp based on their unique chemical properties.13,18,19 Succinic acid influences the growth and morphology of HAp crystals by interacting with calcium ions, thereby altering the crystallization process and affecting crystal size, shape, and surface properties.20,21 Ascorbic acid stabilizes the HAp structure and introduces functional groups that enhance biological activity, improving biocompatibility and bioactivity for applications like bone regeneration.22 Stearic acid acts as a surfactant, limiting growth and controlling particle size distribution, resulting in smaller, more uniform crystals with enhanced dispersibility.21 Calcium phosphate maintains the balance between calcium and phosphate ions, ensuring pure HAp with controlled crystallinity and phase composition.22 Silica is added to modify surface properties and improve mechanical strength, acting as a nucleation site for HAp crystal growth and enhancing bioactivity, osteoconductivity, and mechanical stability, making it suitable for bone tissue engineering.23 Each modifier tailors the structural and functional properties of HAp for specific applications. In this study, various modifiers like succinic acid, ascorbic acid, stearic acid, calcium phosphate, and silica were used to modify the morphological features (e.g., shape and size) of HAp via the hydrothermal method.
2 Experimental
2.1 Materials
Ca(OH)2 and H3PO4 were used as the sources of Ca2+ and PO43−, respectively. Additionally, several organic modifiers such as succinic acid (C4H6O4), ascorbic acid (C6H8O6), stearic acid (C18H36O2), calcium phosphate (Ca3(PO4)2), and silica (SiO2) were purchased from E-Merck, Germany. In addition, to maintain the solution pH (10–11), HNO3 and NH4OH were used.
2.2 Methods
2.2.1 HAp synthesis via hydrothermal method. In this synthesis process, a precalculated amount of Ca2+ and PO43− is dissolved in DI and continuously stirred for 2 h. Different organic modifiers like succinic acid (C4H6O4), ascorbic acid (C6H8O6), stearic acid (C18H36O2), calcium phosphate (Ca3(PO4)2), and silica (SiO2) at the amount of 0.1 g were dissolved into the solution to modify the crystalline structure of the synthesized HAp. Subsequently, the solution was delivered to an autoclave reactor made of stainless steel containing PTFE and then heated at 180 °C for 3 h (Fig. 1). After cooling, the mixture was separated and rinsed with DI water. The precipitate (ppt) was subjected to a temperature-controlled oven process (105 °C), where it was dried for 3 h before being cooled and analyzed.
 |
| | Fig. 1 Hydrothermal synthesis of hydroxyapatite. | |
2.2.2 Characterization. A sophisticated XRD instrument (Rigaku Smart Lab) was used to analyze the crystallographic features of as-synthesized nanocrystalline HAp using an X-ray diffraction machine, with scanning steps of 0.01 and scanning range at 2θ = 20–60°. The Ni-Kβ filter was placed to minimize Kβ-rays, and the experiment was calibrated using standard silicon. The optical bandgap was determined using a UV-2900 spectrophotometer, and the FTIR study was conducted using an IR prestige 21 spectrometer. Morphological scans were acquired using a field emission scanning electron microscopy (FESEM) device (Schottky JSM-7610F, Japan) while an accelerating voltage of 10 kV was maintained to obtain the images.24
3 Results and discussion
3.1 Crystallographic characterization
Fig. 2 shows the XRD pattern of the synthesized HAp. According to the output data, the reflected peak positions in 2θ values, including the plane, were 25.931° (002), 31.831° (211), 32.241° (112), 32.961° (300), 34.121° (202), 39.881° (130), 46.751° (222), and 49.531° (213). The HAp profiles were matched using standard criteria (ICDD database (card no: # 01-074-0565)).
 |
| | Fig. 2 XRD patterns of synthesized HAp using the Hydrothermal method. | |
The “preferential growth” in XRD data is a fundamental parameter that directly affects the “crystallite structure”.20,25 The obtained XRD patterns revealed preferences, with the sample's relative intensity estimated using the (211) plane in comparison with planes (002), (202), and (300), using an equation to calculate RI.26
| |
 | (1) |
To deduce the relative intensity (RI) regarding the considered crystal plane, ICDD card's intensities were examined, and the preference growth of the HAp samples was determined using mathematical
eqn (2), based on the measured RI of succinic acid_HAp, ascorbic acid_HAp, stearic acid_HAp, calcium phosphate_HAp, and silica_HAp (
Table 1),
25| |
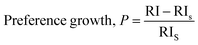 | (2) |
RI denotes the relative intensity of the plane, and RIs is the standard relative intensity of the plane.
Table 1 lists the preference growth of (211) planes for succinic acid_HAp, ascorbic acid_HAp, stearic acid_HAp, calcium phosphate_HAp, and silica_HAp. The synthesized HAp exhibits preference growth, as indicated by negative magnitudes, which indicate higher preferences in specific planes. Positive magnitudes indicate an increased concentration of atoms in the corresponding plane due to specimen agglomeration, resulting in dimensional stability and minimal surface energy in crystallites.
Table 1 Relative intensities and preference growth of HAp
| Synthesized HAp from |
Considered plane |
Intensities of the plane |
The relative intensity of the sample |
Standard relative intensity |
Preferential growth |
| Succinic acid_HAp |
002 |
21.27 |
0.13 |
0.18 |
−0.86 |
| 211 |
100 |
1.17 |
0.84 |
0.17 |
| 202 |
0 |
0 |
0.1 |
−1 |
| 300 |
63.48 |
0.52 |
0.39 |
−0.47 |
| |
| Ascorbic acid_HAp |
002 |
28.09 |
0.13 |
0.18 |
−0.86 |
| 211 |
91.97 |
0.63 |
0.84 |
−0.36 |
| 202 |
15.82 |
0.24 |
0.1 |
−0.75 |
| 300 |
100 |
0.73 |
0.39 |
−0.26 |
| |
| Stearic acid _HAp |
002 |
2.39 |
0.023 |
0.18 |
−0.97 |
| 211 |
100 |
41.84 |
0.84 |
4.84 |
| 202 |
0 |
0 |
0.1 |
−1 |
| 300 |
0 |
0 |
0.39 |
−1 |
| |
| Calcium phosphate_HAp |
002 |
5.7 |
0.14 |
0.18 |
−0.85 |
| 211 |
26.74 |
1.4 |
0.84 |
0.47 |
| 202 |
3.61 |
0.08 |
0.1 |
−0.91 |
| 300 |
8.79 |
0.24 |
0.39 |
−0.75 |
| |
| Silica_HAp |
002 |
20.81 |
0.14 |
0.18 |
−0.85 |
| 211 |
100 |
1.54 |
0.84 |
0.54 |
| 202 |
12.65 |
0.08 |
0.1 |
−0.91 |
| 300 |
31.21 |
0.23 |
0.39 |
−0.76 |
Data analysis revealed various crystallographic characteristics, including lattice parameters, crystalline index, crystallite dimensions, unit cell volume, dislocation density, micro-strain, crystallinity degree, and specific surface area, which were calculated using eqn (3)–(9) and listed in Table 2.27–29
| |
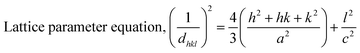 | (3) |
| |
 | (4) |
| |
Crystalline dimension, Dc = kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (5) |
| |
 | (6) |
| |
 | (7) |
| |
Microstrain: ε = β/4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tan tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (8) |
| |
 | (9) |
| |
 | (10) |
The plane (
h,
k,
l) identifies specific unit cells, and lattice parameters are expressed by
a,
b,
c, and the crystallinity degree is denoted as
Xc.
30 δ,
H(hkl), and CI
XRD are used to measure dislocation density, peak height, and the crystalline index of synthesized HAp.
Eqn (8) was used to compute the specific surface area, representing
Dc (crystallite size) and
ρ (density) (3.16 g cm
−3), respectively.
31 The crystallographic variables associated with hydrothermally synthesized HAp significantly influence the structural characteristics of the material. The lattice parameters (
a,
b,
c) remained relatively consistent across all samples, confirming the upholding of the hexagonal crystal structure, although silica incorporation slightly expanded the unit cell. Crystal size varies notably, with succinic acid_HAp exhibiting the smallest (8.69 nm) and silica_HAp having the largest (76.47 nm), indicating that inorganic modifiers promote larger crystals, while organic modifiers hinder growth. The unit cell volume follows a similar trend, with silica_HAp having the smallest volume, suggesting a denser lattice structure. The degree of crystallinity is highest for silica_HAp (10.97) and lowest for succinic acid_HAp, demonstrating that inorganic modifiers enhance crystallinity, whereas organic modifiers disrupt it. Microstrain, an indicator of lattice distortion, is highest in succinic acid_HAp (14.58 × 10
−3) and lowest in silica_HAp, indicating that organic modifiers induce more internal stress. The dislocation density, which reflects structural defects, is also highest for succinic acid_HAp and lowest for silica_HAp, supporting the observation that inorganic modifiers contribute to more stable defect-free crystals. The crystallinity index (CIXRD) indicates that ascorbic acid_HAp has the most well-ordered structure, whereas stearic acid_HAp exhibits the lowest crystallinity. Additionally, the specific surface area is highest for succinic acid_HAp and lowest for silica_HAp, correlating with crystal size and defect density. Overall, inorganic modifiers like silica and calcium phosphate promote larger crystal growth, higher crystallinity, and lower defect densities, leading to more stable HAp structures, whereas organic modifiers like succinic acid and stearic acid result in smaller crystals, lower crystallinity, and increased lattice strain. The preferred orientation of a polycrystalline material is determined by its texture coefficient, which is determined by its X-ray diffraction pattern and influences its crystallographic planes.
32 The value of the texture coefficient (TC) was estimated by fixing a distinct peak in the measured sample, and the peak in reference offers vital insights into the crystallographic orientation of a material, which significantly influences its performance in many applications.
33 The texture coefficient of the synthesized HAp can be calculated using
eqn (11)| |
 | (11) |
The term
I(hkl)/
I0(hkl) indicates the ratio of the relative intensity (RI) of the sample to the standard and

denotes the mean RI. The analysis reveals that succinic acid_HAp, ascorbic acid_HAp, calcium phosphate_HAp, and silica_HAp are grown in (002), (112), and (202) planes, while stearic acid_HAp is grown in (002) and (202) planes, respectively.
Table 2 Hydrothermally synthesized hydroxyapatite crystallographic variables
| Parameter |
Succinic acid_HAp |
Ascorbic acid_HAp |
Stearic acid_HAp |
Calcium phosphate_HAp |
Silica_HAp |
| Lattice parameter a = b (Å) |
9.38 ± 0.02 |
9.42 ± 0.01 |
9.39 ± 0.02 |
9.42 ± 0.01 |
9.45 ± 0.01 |
| Lattice parameter c (Å) |
6.88 ± 0.01 |
6.90 ± 0.01 |
6.86 ± 0.01 |
6.88 ± 0.01 |
6.87 ± 0.01 |
| Crystal size, Dc (nm) |
8.69 ± 0.5 |
33.51 ± 1.2 |
55.13 ± 2.0 |
63.62 ± 2.5 |
76.47 ± 3.0 |
| Unit cell volume, V (Å3) |
524.53 ± 1.5 |
530.25 ± 1.2 |
523.82 ± 1.3 |
529.36 ± 1.1 |
513.31 ± 1.0 |
| Degree of crystallinity, Xc |
(16.1 ± 0.5) × 10−3 |
1.49 ± 0.05 |
4.096 ± 0.1 |
6.30 ± 0.2 |
10.97 ± 0.3 |
| Microstrain, ε (×10−3) |
14.58 ± 0.2 |
10.42 ± 0.1 |
2.27 ± 0.05 |
1.98 ± 0.05 |
1.65 ± 0.05 |
| Dislocation density, δ (×1015 lines per m2) |
13.22 ± 0.5 |
0.89 ± 0.05 |
0.3289 ± 0.01 |
0.246 ± 0.01 |
0.17 ± 0.01 |
| Crystallinity index, CI_XRD |
1.85 ± 0.05 |
2.13 ± 0.05 |
1.25 ± 0.05 |
1.64 ± 0.05 |
1.88 ± 0.05 |
| Specific surface area, S (m2 g−1) |
0.21 ± 0.01 |
0.056 ± 0.005 |
0.034 ± 0.003 |
0.029 ± 0.002 |
0.024 ± 0.002 |
3.2 Crystal size estimation
3.2.1 Scherrer equation. The Scherrer equation is the first method to link the dimension of nano-crystallites with the broadening of peaks.34,35 Eqn (3) proposes a mathematical expression for spherical crystallite, ignoring instrumental broadening and crystal defects, using parameters such as crystallite size (Dc), Scherrer constant (K), X-ray source's wavelength (λ), the full width at half maxima (β), and Bragg's angle (θ). On average, the crystal dimensions of the synthesized HAp were 11.51, 16.11, 29.65, 23.25, and 25.12 nm for succinic acid_HAp, ascorbic acid_HAp, stearic acid_HAp, calcium phosphate_HAp and silica_Hap, respectively.
3.2.2 Williamson–Hall method. The Scherrer method, widely used for calculating crystallite size, has limitations in determining grain boundary triple junctions, stacking faults, and the intrinsic strain of point defects.36,37 Hence, the Williamsons–Hall method (W–H) and its revised version have been used, considering the inherent strain and particle size.38,39 The crystallite size and microstrain significantly influence the physical streak widening of the peak of X-ray diffraction, leading to a total broadening.| | |
βtotal = βhkl + βstrain
| (12) |
This study used revised W–H equations (e.g., UDM, USDM, and UEDM) to calculate crystallite dimension and microstrain, which are extensively mentioned in the subsequent segments.
3.2.3 Uniform deformation model (UDM). The UDM technique accounts for consistent deformation in nanocrystals caused by flaws in their crystal structure, leading to uniform strain in all directions.40 The XRD profile's physical broadening due to intrinsic strain (βstrain) was considered,| |
βstrain = 4ε·tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (13) |
where θ is the Bragg position. Rearranging eqn (5) and (13) yields…| |
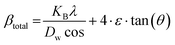 | (14) |
| |
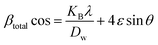 | (15) |
Fig. 3 shows the plot of eqn (15), with terms (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) on the horizontal axis and (βhkl × cos
θ) on the horizontal axis and (βhkl × cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) across the vertical axis, which assembles the obtained diffraction peaks for the HAp nanocrystals. Intrinsic strain for the crystal samples is provided via the slope of the as-generated linear graph, and the average size of the nanocrystal is estimated from the intercept. The lattice strain in nanocrystals originates from the expansion or contraction of the lattice, resulting in confinement to a small size. This alteration causes a small deviation in the atomic configuration compared to bulk crystals. Furthermore, the size constraint leads to imperfections in the lattice arrangement, causing strain inside the lattice. The average crystal size of the HAp is estimated as 4.20, 72.97, 29.50, 4.04, and 13.72 nm for succinic acid_HAp, ascorbic acid_HAp, stearic acid_HAp, calcium phosphate_HAp and silica_HAp, respectively.
θ) across the vertical axis, which assembles the obtained diffraction peaks for the HAp nanocrystals. Intrinsic strain for the crystal samples is provided via the slope of the as-generated linear graph, and the average size of the nanocrystal is estimated from the intercept. The lattice strain in nanocrystals originates from the expansion or contraction of the lattice, resulting in confinement to a small size. This alteration causes a small deviation in the atomic configuration compared to bulk crystals. Furthermore, the size constraint leads to imperfections in the lattice arrangement, causing strain inside the lattice. The average crystal size of the HAp is estimated as 4.20, 72.97, 29.50, 4.04, and 13.72 nm for succinic acid_HAp, ascorbic acid_HAp, stearic acid_HAp, calcium phosphate_HAp and silica_HAp, respectively.
 |
| | Fig. 3 UDM for (A) succinic acid_Hap, (B) ascorbic acid_Hap, (C) stearic acid_HAp, (D) calcium phosphate_HAp and (E) silica_HAp. | |
3.2.4 Uniform stress deformation model (USDM). In the UDM, the specimen is assumed to be isotropic, although this assumption is not valid for real crystals. The Williamson–Hall equation was modified to incorporate an anisotropic factor to account for anisotropic crystals, creating the USDM.41 Hooke's law holds that strain and stress are linearly connected, with stress represented asYhkl, also known as Young's modulus, is a crucial factor in determining the strain of a material. Applying the strain value in eqn (15) and with modification, we obtain| |
 | (17) |
The modified W–H model (eqn (17)), specifically the USDM, incorporates uniform stress along each crystallographic plane in the XRD pattern for HAp nanoparticles. The term (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/Yhkl) on the horizontal and (βhkl
θ/Yhkl) on the horizontal and (βhkl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) on the vertical axis (Fig. 4) correspond to each peak in the XRD pattern. The slope represents the stress value, whereas the intercept shows the average crystallite size (Table 3).
θ) on the vertical axis (Fig. 4) correspond to each peak in the XRD pattern. The slope represents the stress value, whereas the intercept shows the average crystallite size (Table 3).
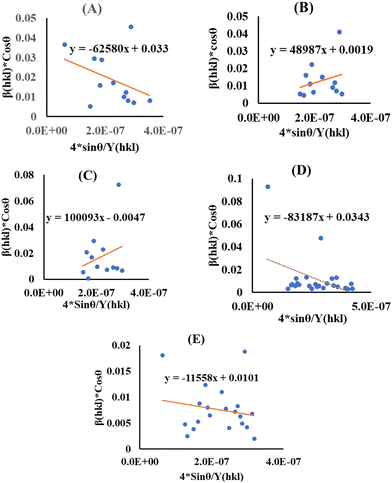 |
| | Fig. 4 USDM for (A) succinic acid_HAp, (B) ascorbic acid_HAp, (C) stearic acid _HAp, (D) calcium phosphate_HAp and (E) silica_HAp. | |
Table 3 Estimated microstructural features of HAp
| Model name |
Crystallite dimension (nm), stress (N m−2), energy density (J m−3) |
| Succinic acid_HAp |
Ascorbic acid_HAp |
Stearic acid _HAp |
Calcium phosphate_HAp |
Silica_HAp |
| Scherrer's equation |
DL = 11.51 |
16.11 |
29.65 |
23.24 |
25.12 |
| Williamson–Hall method |
UDM |
ε = 0.0104 |
ε = 0.0082 |
ε = 0.0167 |
ε = 0.0139 |
ε = 0.0019 |
| Dw = 4.201 |
Dw = 72.97 |
Dw = 29.50 |
Dw = 4.04 |
Dw = 13.72 |
| USDM |
σ = −62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 580 580 |
σ = 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 987 987 |
σ = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 093 093 |
σ = −83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 187 187 |
σ = −11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 558 558 |
| Dw = 4.20 |
Dw = 72.97 |
Dw = 29.50 |
Dw= 4.04 |
Dw= 13.72 |
| UDEM |
u = 326.344 |
u = 199.96 |
u = 864.86 |
u = 6.4 × 10−11 |
u = 11.13 |
| Dw = 4.20 |
Dw= 72.97 |
Dw = 29.50 |
Dw =4.04 |
Dw = 13.72 |
| Size-strain plot, Dw |
3.75 |
4.88 |
2.87 |
0.148 |
7.53 |
| Halder–Wagner method, Dw |
32.15 |
3.53 |
2.08 |
1.84 |
7.40 |
| Sahadat–Scherrer model, Ds–s |
15.93 |
20.6 |
138.6 |
29.50 |
33.81 |
3.2.5 Uniform deformation energy density model (UDEDM). The Williamson–Hall model was modified to account for real crystal defects by introducing the term energy density for estimating crystallite size and stress, known as the UDEDM. Hook's law, represented by eqn (18), describes the energy density.37,42| |
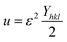 | (18) |
| |
 | (19) |
Eqn (19) was developed by a systematic modification of eqn (15), where the strain is integrated with the energy density, also known as UDEDM.43 The plot of the eqn (19) reveals the energy density value of crystalline HAp nanoparticles, with components (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ(√2u/Yhkl)) plotted horizontally and (βhkl·cos
θ(√2u/Yhkl)) plotted horizontally and (βhkl·cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) plotted vertically (Fig. 5), resembling each diffraction peak. All the estimated values of crystal size and energy density of the synthesized HAp nanoparticles are listed in Table 3.
θ) plotted vertically (Fig. 5), resembling each diffraction peak. All the estimated values of crystal size and energy density of the synthesized HAp nanoparticles are listed in Table 3.
 |
| | Fig. 5 UDEDM for (A) succinic acid_HAp, (B) ascorbic acid_HAp, (C) stearic acid _HAp, (D) calcium phosphate_HAp and (E) silica_HAp. | |
3.2.6 Size-strain plot method (SSPM). The SSPM, using both the Gaussian function (βG) and Lorentzian function (βL), effectively determines the size and strain of nano-crystallites in anisotropic crystal structures, as illustrated in eqn (20)44The SSPM is particularly useful because it analyzes lower angles, and it is ordinarily expressed as shown in eqn (21).45
| |
 | (21) |
By illustrating (dhklβhkl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ)2 and (dhkl2βhkl
θ)2 and (dhkl2βhkl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) along the Y and X axes, respectively, several mathematical expressions were generated, as shown in Fig. 6. These expressions were then fitted to the equation of a straight line. The slopes of these equations were used to calculate the crystallite size, whereas the intercept yields the value of strain. The estimated sizes of the nano-crystallites are listed in Table 3.
θ) along the Y and X axes, respectively, several mathematical expressions were generated, as shown in Fig. 6. These expressions were then fitted to the equation of a straight line. The slopes of these equations were used to calculate the crystallite size, whereas the intercept yields the value of strain. The estimated sizes of the nano-crystallites are listed in Table 3.
 |
| | Fig. 6 SSPM for (A) succinic acid_HAp, (B) ascorbic acid_HAp, (C) stearic acid _HAp, (D) calcium phosphate_HAp and (E) silica_HAp. | |
3.2.7 Halder–Wagner method (HWM). The SSP method perceives strain widening as a Gaussian function, whereas size widening is considered a Lorentzian function. The XRD peak is not subject to either of these two functions because its peak area approaches the Gaussian function, yet its tail quickly declines.46 The Halder–Wagner approach is used to address the issue of peak widening, assuming that it is a symmetric Voigt function, which can be denoted by eqn (22).47,48| | |
βhkl2 = βG2 + βLβhkl
| (22) |
Here, βG, βL = FWHM of the Gaussian and Lorentzian functions.
| |
 | (23) |
Here,  and
and 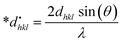 the crystal size was estimated from the slope (1/Dw) by plotting (*βhkl/*dhkl)2 and (*βhkl/*dhkl)2 on the vertical and horizontal axes, respectively, as shown in Fig. 7.
the crystal size was estimated from the slope (1/Dw) by plotting (*βhkl/*dhkl)2 and (*βhkl/*dhkl)2 on the vertical and horizontal axes, respectively, as shown in Fig. 7.
 |
| | Fig. 7 HWM for (A) succinic acid_HAp, (B) ascorbic acid_HAp, (C) stearic acid _HAp, (D) calcium phosphate_HAp and (E) silica_HAp. | |
3.2.8 Sahadat–Scherrer model (SSM). This model considers instrumental broadening and neglects intrinsic strain based on the Scherrer equation. It constructs a linear streak that passes through the (0,0) coordinate (origin), thereby allowing precise estimation of the crystallite dimension. Using this model, a varying range of crystal sizes can be calculated.24,49 The model's formula can be expressed in eqn (24).| |
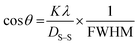 | (24) |
A straight line was constructed by scheming cos(θ) and 1/FWHM across the vertical and horizontal axes, with an additional line that precedes piercing the origin (Fig. 8). The aforementioned model can be used to deduce the sizes of HAp crystallites using the slope. Table 3 presents the measured crystalline dimensions of the synthesized HAp.
 |
| | Fig. 8 SSM for (A) succinic acid_HAp, (B) ascorbic acid_HAp, (C) stearic acid_HAp, (D) calcium phosphate_HAp and (E) silica_HAp. | |
3.2.9 Critical analysis of crystallite size calculation methods and the effect of modifiers. The discrepancies observed among the crystallite size calculation methods arise from fundamental differences in their assumptions and limitations. The Scherrer equation50,51 estimates size solely from peak broadening, ignoring lattice strain contributions, leading to overestimations in materials with significant defects or microstrains.13 Modified approaches like the Williamson–Hall (W–H) methods separate size and strain effects by assuming uniform deformation (UDM), stress (USDM), or energy density (UDEDM), but these oversimplify strain heterogeneity in real systems.13 The Sahadat–Scherrer model attempts to address linearity issues in earlier Scherrer-derived methods by enforcing origin-passing plots, yet its validity depends on idealized peak broadening behavior, which is rarely observed experimentally.49 Critically, instrumental broadening corrections introduce variability, as improper βhkl adjustments propagate errors across all methods. Furthermore, crystallite shape anisotropy52 and size-distribution effects differentially impact models—Scherrer assumes spherical crystals, whereas W–H methods require multiple diffraction peaks for reliable strain deconvolution.13,20 The Halder–Wagner and size-strain plot methods improve the accuracy of nano-crystallites but struggle with sub-10 nm systems where surface defects dominate.52 Ultimately, method selection must consider the dominant broadening mechanism (size vs. strain), crystallite morphology, and instrumental resolution because no universal model accounts for all material-specific variables.49
3.2.10 Systematic comparison of modifiers on HAp properties for practical applications. Stearic acid HAp has the most significant impact on HAp properties, with the largest crystallite size (138.6 nm), highest strain (0.0167), substantial tensile stress (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 093 N m−2), and highest energy density (864.86 J m−3) (Fig. 9). This makes it a strong candidate for high-stress applications requiring energy absorption, such as impact-resistant coatings and energy storage materials. Calcium phosphate HAp has the smallest crystallite size (0.148 nm) and negligible energy density (6.4 × 10−11 J m−3), but it induces high compressive stress (−83
093 N m−2), and highest energy density (864.86 J m−3) (Fig. 9). This makes it a strong candidate for high-stress applications requiring energy absorption, such as impact-resistant coatings and energy storage materials. Calcium phosphate HAp has the smallest crystallite size (0.148 nm) and negligible energy density (6.4 × 10−11 J m−3), but it induces high compressive stress (−83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 187 N m−2), limiting its practical utility. Succinic acid HAp has a moderate crystallite size (11.51 nm) and strain (0.0104), making it suitable for bioactive applications like bone implants and drug delivery systems.53 Ascorbic acid HAp has balanced properties, with a crystallite size of 16.11 nm, moderate tensile stress (48
187 N m−2), limiting its practical utility. Succinic acid HAp has a moderate crystallite size (11.51 nm) and strain (0.0104), making it suitable for bioactive applications like bone implants and drug delivery systems.53 Ascorbic acid HAp has balanced properties, with a crystallite size of 16.11 nm, moderate tensile stress (48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 987 N m−2), and stable energy density (199.96 J m−3), suggesting its potential for applications requiring both structural integrity and functional performance. Silica HAp exhibits minimal strain (0.0019) and low energy density (11.13 J m−3), making it suitable for stable, low-stress applications.
987 N m−2), and stable energy density (199.96 J m−3), suggesting its potential for applications requiring both structural integrity and functional performance. Silica HAp exhibits minimal strain (0.0019) and low energy density (11.13 J m−3), making it suitable for stable, low-stress applications.
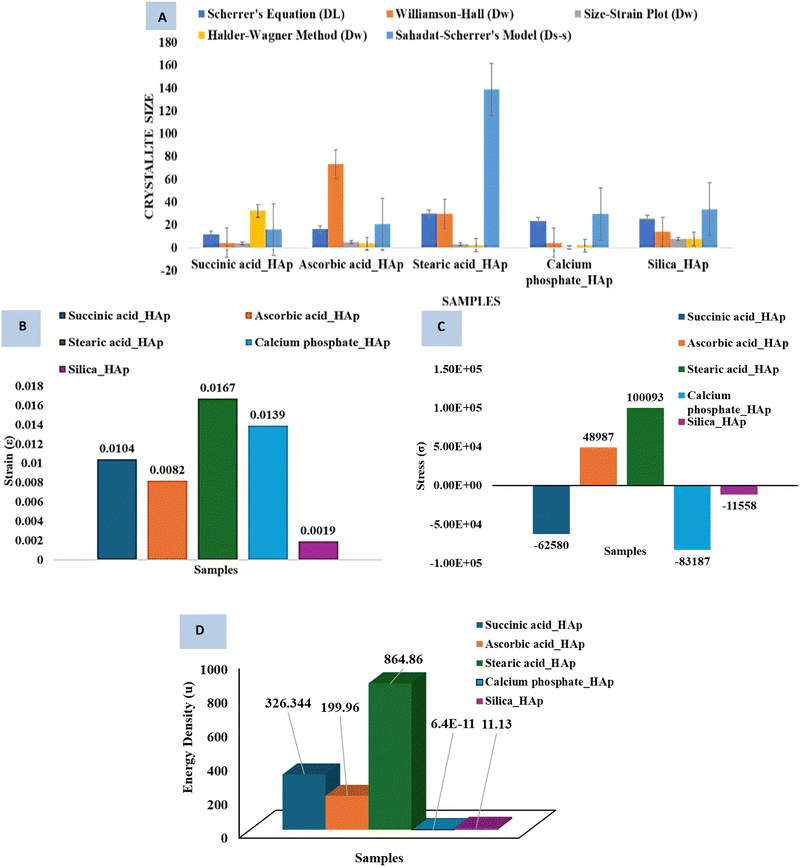 |
| | Fig. 9 Systematic comparison among (A) the crystallite size, (B) strain, (C) stress and (D) energy density. | |
3.2.11 Functional group analysis. The FTIR spectroscopy of hydrothermally generated hydroxyapatite (HAp) revealed characteristic peaks connected to the optically active groups, particularly PO43− and OH−. The peaks include v1 oscillation at 962 cm−1, v3 vibrations at 1026 and 1087 cm−1, v4 bending at 563 and 599 cm−1, and v2 bending at 473 cm−1. Peaks within the 3000–3800 cm−1 range indicate the occurrence of OH− groups.20,54–56 Silica-HAp spectra do not display separate Si–O–Si peaks in the 1000–1200 cm−1 region due to interference from a strong phosphate band (Fig. 10).57 The calcium phosphate-HAp control sample exhibited more identifiable spectrum features, suggesting negligible organic change. Stearic acid-HAp revealed a modest band at 2850–2960 cm−1, suggesting C–H stretching vibrations from the alkyl chain.58 The spectra of ascorbic acid-HAp validated the presence of HAp but did not reveal distinct peaks for ascorbic acid. Succinic acid-HAp spectra also indicated HAp without unequivocal evidence of the modifier. The peak near 2350 cm−1 was due to environmental carbon dioxide.20 Overall, the resolution of the spectra appears limited, likely due to the presence of functional groups from the modifiers and low concentrations, which hinder the definitive identification of the modifiers.
 |
| | Fig. 10 Functional group analysis of hydrothermally synthesized HAp. | |
3.2.12 Surface morphology study. The scanning electron microscopic technique (SEM) images (Fig. 11) of hydrothermally synthesized HAp exhibit distinct morphological characteristics. Succinic acid_HAp has an aggregated, cluster-like structure with densely packed irregular particles, indicating an enhanced surface area and improved bioactivity.53 Ascorbic acid_HAp has a fluffy, cloud-like texture, suggesting reduced particle agglomeration and improved dispersibility. Stearic acid_HAp has a well-defined rod-like or needle-like structure, promoting its mechanical stability. Calcium phosphate_HAp has a porous, sponge-like structure with visible voids, enhancing osteoconductivity and biointegration.59 Silica_HAp has a flaky, sheet-like texture with layered formations, which are linked to improved thermal stability and controlled drug delivery applications.60 These variations in morphology highlight the influence of organic acids and synthesis conditions on HAp's structural characteristics, which are crucial for different applications.
 |
| | Fig. 11 SEM images of hydrothermally synthesized (A) succinic acid_HAp, (B) ascorbic acid_HAp, (C) stearic acid _HAp, (D) calcium phosphate_HAp and (E) silica_HAp. | |
3.3 Assessment of optical properties
The optical bandgap is a fundamental parameter representing the required energy for a single electron to pass from the valence band to the conduction band. This energy was computed using Tauc plots, as expressed by eqn (25) (Fig. 12).61,62
 |
| | Fig. 12 Optical band gap estimation for (A) succinic acid_HAp, (B) ascorbic acid_HAp, (C) stearic acid _HAp, (D) calcium phosphate_HAp and (E) silica_HAp. | |
The formula comprises α as the absorption factor, hϑ as the photon energy, A as a constant value, and n as either a 2 (indirect) or ½ (direct) permitted band gap. The band gaps of the HAp specimens are listed in Table 4. The literature reveals a link between crystallite size and optical band gap energy, with bigger crystallites lowering the optical band gap and vice versa.63
Table 4 Measured band gaps of the synthesized HAp
| Samples |
Band gap (eV) |
| Succinic acid_HAp |
5.60 |
| Ascorbic acid_HAp |
5.29 |
| Stearic acid_HAp |
5.57 |
| Calcium phosphate_HAp |
5.35 |
| Silica_Hap |
5.42 |
3.4 Quantitative analysis of the XRD data
The Rietveld refinement data confirmed the key conclusions of the synthesized HAp samples (Fig. 13).
 |
| | Fig. 13 Rietveld refinement and phase analysis results for (A) succinic acid_HAp (B) ascorbic acid_HAp, (C) stearic acid _HAp, (D) calcium phosphate_HAp, (E) silica_HAp and (F) phase composition. | |
Calcium phosphate_HAp and silica_HAp exhibit superior phase purity with 94.7% and 94% HAp content, respectively, with low Rwp values. However, their low χ2 suggests potential over-parameterization in the refinement model. Stearic acid_HAp exhibited the poorest performance, with only 84.2% HAp and 15.8% TCP and the highest Rwp, indicating significant refinement challenges due to sample heterogeneity. Ascorbic and succinic acid_HAp exhibited moderate purity with acceptable refinement metrics (Table 5). Silica_HAp shows the most balanced refinement. The results highlight the critical relationship between synthesis conditions and refinement outcomes, with inorganic modifiers producing more phase-pure HAp with superior refinement characteristics. Organic acids, particularly stearic acid, introduce greater TCP impurities and compromise refinement quality, emphasizing the importance of modifier selection in HAp synthesis.
Table 5 Different phase estimation via the Rietveld refinement method
| Samples |
HAp phase (%) |
TCP phase (%) |
Rwp |
Rexp |
χ2 |
GoF |
| Succinic acid_HAp |
88.2 |
11.2 |
15.05 |
16.60 |
0.8220 |
0.9066 |
| Ascorbic acid_HAp |
92.5 |
7.5 |
16.00 |
17.05 |
0.8806 |
0.9384 |
| Stearic acid_HAp |
84.2 |
15.8 |
26.71 |
27.82 |
0.9218 |
0.9601 |
| Calcium phosphate_HAp |
94.7 |
5.3 |
15.26 |
19.11 |
0.6377 |
0.7985 |
| Silica_HAp |
94 |
6 |
14.63 |
16.96 |
0.7441 |
0.8626 |
4 Conclusion
This study investigated how the hydroxyapatite (HAp) structure changed under hydrothermal modification with organic modifiers (succinic acid, ascorbic acid, stearic acid) and inorganic modifiers (calcium phosphate, silica) by examining the crystallographic structure, morphology, and phase composition of HAp. The Williamson–Hall model measured crystallite sizes below 100 nm and microstructural parameters, showing stearic acid-modified HAp as having the largest crystallite size at 138.6 nm alongside a significant strain of 0.0167 and tensile stress of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 093 N m−2. Calcium phosphate modification yielded HAp with a minimum crystallite size of 0.148 nm but generated high compressive stress values reaching −83
093 N m−2. Calcium phosphate modification yielded HAp with a minimum crystallite size of 0.148 nm but generated high compressive stress values reaching −83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 187 N m−2. In comparison, the succinic and ascorbic acid modifications achieved moderate crystallite sizes of 11.51 and 16.11 nm, respectively, making them suitable for biomedical use despite their varying strain and tensile stress measurements. SEM analysis showed that succinic acid-HAp developed bioactive clusters, whereas ascorbic acid-HAp took on a dispersible, fluffy form. Stearic acid-HAp formed mechanically stable rod-like structures, calcium phosphate-HAp demonstrated osteoconductive porosity, and silica-HAp presented layered flakes that allowed controlled drug delivery. The Rietveld refinement analysis showed that calcium phosphate- (94.7% HAp) and silica-modified HAp (94% HAp) possessed higher phase purity in contrast to stearic acid-HAp, which contained significant tricalcium phosphate impurities (15.8% TCP), leading to reduced refinement reliability (Rwp: highest for stearic acid-HAp). The results demonstrate that selecting appropriate modifiers determines the properties of HAp that are suitable for biomedical, mechanical, and energy applications.
187 N m−2. In comparison, the succinic and ascorbic acid modifications achieved moderate crystallite sizes of 11.51 and 16.11 nm, respectively, making them suitable for biomedical use despite their varying strain and tensile stress measurements. SEM analysis showed that succinic acid-HAp developed bioactive clusters, whereas ascorbic acid-HAp took on a dispersible, fluffy form. Stearic acid-HAp formed mechanically stable rod-like structures, calcium phosphate-HAp demonstrated osteoconductive porosity, and silica-HAp presented layered flakes that allowed controlled drug delivery. The Rietveld refinement analysis showed that calcium phosphate- (94.7% HAp) and silica-modified HAp (94% HAp) possessed higher phase purity in contrast to stearic acid-HAp, which contained significant tricalcium phosphate impurities (15.8% TCP), leading to reduced refinement reliability (Rwp: highest for stearic acid-HAp). The results demonstrate that selecting appropriate modifiers determines the properties of HAp that are suitable for biomedical, mechanical, and energy applications.
Author contributions
Md. Kawsar synthesized and characterized the hydroxyapatites, executed the photocatalytic experiment, and wrote the draft and original manuscript. Md. Sahadat Hossain conceived and designed the experiment and analyzed the data. Dipa Islam conducted the SEM experiment. Newaz Mohammed Bahadur and Samina Ahmed supervised the findings of this work. Samina Ahmed supervised the overall work and managed the required facilities.
Data availability
Data will be made available upon request.
Conflicts of interest
No declaration of conflict is reported.
Acknowledgements
The authors are grateful to the Bangladesh Council of Scientific and Industrial Research (BCSIR) authority for financial support through the R&D project (ref. no. 39.02.0000.011.14.134.2021/900; Date: 30.12.2021). Md. Kawsar wishes to thank the Department of Applied Chemistry and Chemical Engineering, Noakhali Science and Technology University, Noakhali, Bangladesh, for approving the M. S. Thesis program.
References
- A. Szcześ, L. Hołysz and E. Chibowski, Synthesis of hydroxyapatite for biomedical applications, Adv. Colloid Interface Sci., 2017, 249, 321–330 CrossRef PubMed.
- D. F. Fitriyana, R. Ismail, Y. I. Santosa, S. Nugroho, A. J. Hakim and M. Syahreza Al Mulqi, Hydroxyapatite Synthesis from Clam Shell Using Hydrothermal Method: A Review 2019 International Biomedical Instrumentation and Technology Conference (IBITeC) 2019 International Biomedical Instrumentation and Technology Conference (IBITeC), 2019, vol. 1, pp. 7–11.
- P. Szterner and M. Biernat, The Synthesis of Hydroxyapatite by Hydrothermal Process with Calcium Lactate Pentahydrate: The Effect of Reagent Concentrations, pH, Temperature, and Pressure, in Bioinorganic Chemistry and Applications, ed. G. Mendoza-Diaz, 2022, p. 3481677 Search PubMed.
- R. E. Riman, W. L. Suchanek, K. Byrappa, C.-W. Chen, P. Shuk and C. S. Oakes, Solution synthesis of hydroxyapatite designer particulates, Solid State Ionics, 2002, 151, 393–402 CrossRef CAS.
- W. Suchanek and M. Yoshimura, Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants, J. Mater. Res., 1998, 13, 94–117 CrossRef CAS.
- L.-X. Yang, J.-J. Yin, L.-L. Wang, G.-X. Xing, P. Yin and Q.-W. Liu, Hydrothermal synthesis of hierarchical hydroxyapatite: preparation, growth mechanism and drug release property, Ceram. Int., 2012, 38, 495–502 CrossRef CAS.
- H. Nosrati, R. S. Mamoory, D. Q. S. Le and C. E. Bünger, Preparation of reduced graphene oxide/hydroxyapatite nanocomposite and evaluation of graphene sheets/hydroxyapatite interface, Diamond Relat. Mater., 2019, 100, 107561 CrossRef CAS.
- D. Smolen, T. Chudoba, I. Malka, A. Kedzierska, W. Lojkowski, W. Swieszkowski, K. J. Kurzydlowski, M. Kolodziejczyk-Mierzynska and M. Lewandowska-Szumiel, Highly biocompatible, nanocrystalline hydroxyapatite synthesized in a solvothermal process driven by high energy density microwave radiation, IJN, 2013, 653 CrossRef PubMed.
- O. Sinitsyna, A. Veresov, E. Kovaleva, Y. V. Kolen’ko, V. Putlyaev and Y. Tretyakov, Synthesis of hydroxyapatite by hydrolysis of α-Ca3(PO4)2, Russ. Chem. Bull., 2005, 54, 79–86 CrossRef CAS.
- H. Nosrati, R. S. Mamoory, D. Q. S. Le, C. E. Bünger, R. Z. Emameh and F. Dabir, Gas injection approach for synthesis of hydroxyapatite nanorods via hydrothermal method, Mater. Charact., 2020, 159, 110071 CrossRef CAS.
- X. Guo, H. Yan, S. Zhao, Z. Li, Y. Li and X. Liang, Effect of calcining temperature on particle size of hydroxyapatite synthesized by solid-state reaction at room temperature, Adv. Powder Technol., 2013, 24, 1034–1038 CrossRef CAS.
- M. Shirkhanzadeh, Direct formation of nanophase hydroxyapatite on cathodically polarized electrodes, J. Mater. Sci.: Mater. Med., 1998, 9, 67–72 CrossRef CAS PubMed.
- Md Kawsar, Md. S. Hossain, N. M. Bahadur and S. Ahmed, Synthesis of nano-crystallite hydroxyapatites in different media and a comparative study for estimation of crystallite size using Scherrer method, Halder–Wagner method size-strain plot, and Williamson–Hall model, Heliyon, 2024, 10, e25347 CrossRef CAS PubMed.
- T. Zhang and X. Xiao, Hydrothermal Synthesis of Hydroxyapatite Assisted by Gemini Cationic Surfactant, J. Nanomater., 2020, 2020, 1–7 Search PubMed.
- G. Ma, Three common preparation methods of hydroxyapatite, IOP Conf. Ser.: Mater. Sci. Eng., 2019, 688, 033057 CAS.
- S. Hussain and K. Sabiruddin, Effect of heat treatment on the synthesis of hydroxyapatite from Indian clam seashell by hydrothermal method, Ceram. Int., 2021, 47, 29660–29669 CrossRef CAS.
- Y. Yang, Q. Wu, M. Wang, J. Long, Z. Mao and X. Chen, Hydrothermal Synthesis of Hydroxyapatite with Different Morphologies: Influence of Supersaturation of the Reaction System, Cryst. Growth Des., 2014, 14, 4864–4871 CrossRef CAS.
- M. Jevtić, M. Mitrić, S. Škapin, B. Jančar, N. Ignjatović and D. Uskoković, Crystal Structure of Hydroxyapatite Nanorods Synthesized by Sonochemical Homogeneous Precipitation, Cryst. Growth Des., 2008, 8, 2217–2222 CrossRef.
- R. Rial, M. González-Durruthy, Z. Liu and J. M. Ruso, Advanced Materials Based on Nanosized Hydroxyapatite, Molecules, 2021, 26, 3190 CrossRef CAS PubMed.
- M. Kawsar, M. S. Hossain, S. Tabassum, D. Islam, N. Mohammed Bahadur and S. Ahmed, Crystal structure modification of nano-hydroxyapatite using organic modifiers and hydrothermal technique, RSC Adv., 2024, 14, 29665–29674 RSC.
- M. A. Fanovich, M. S. Castro and J. M. P. López, Structural analysis of modified hydroxyapatite powders, Mater. Res. Bull., 2001, 36, 487–496 CrossRef CAS.
- H. Zhu, D. Guo, H. Zang, D. A. H. Hanaor, S. Yu, F. Schmidt and K. Xu, Enhancement of hydroxyapatite dissolution through structure modification by Krypton ion irradiation, J. Mater. Sci. Technol., 2020, 38, 148–158 CrossRef CAS.
- W. Guan, N. Hayashi, S. Ihara, S. Satoh, C. Yamabe, M. Goto, Y. Yamaguchi and A. Danjoh, Modification of Hydroxyapatite Crystallization Using IR Laser, Free Electron Lasers 2003, Elsevier, 2004, pp. II-75–II-76 Search PubMed.
- S. Hossain Md, S. M. Tuntun, N. M. Bahadur and S. Ahmed, Enhancement of photocatalytic efficacy by exploiting copper doping in nano-hydroxyapatite for degradation of Congo red dye, RSC Adv., 2022, 12, 34080–34094 RSC.
- H. Zanin, E. Saito, F. R. Marciano, H. J. Ceragioli, A. E. C. Granato, M. Porcionatto and A. O. Lobo, Fast preparation of nano-hydroxyapatite/superhydrophilic reduced graphene oxide composites for bioactive applications, J. Mater. Chem. B, 2013, 1, 4947–4955 RSC.
- S. P. Muhoza, T. H. Taylor, X. Song and M. D. Gross, The Impact of Sintering Atmosphere and Temperature on the Phase Evolution of High Surface Area LSCF Prepared by In Situ Carbon Templating, J. Electrochem. Soc., 2021, 168, 034519 CrossRef CAS.
- S. Hossain Md, A. A. Shaikh Md, S. Rahaman Md and S. Ahmed, Modification of the crystallographic parameters in a biomaterial employing a series of gamma radiation doses, Mol. Syst. Des. Eng., 2022, 7, 1239–1248 RSC.
- M. Bin Mobarak, S. Hossain Md, Z. Yeasmin, M. Mahmud, M. Rahman Md, S. Sultana, S. M. Masum and S. Ahmed, Probing the photocatalytic competency of hydroxyapatite synthesized by solid state and wet chemical precipitation method, J. Mol. Struct., 2022, 1252, 132142 CrossRef CAS.
- A. A. Akl and A. S. Hassanien, Microstructure and crystal imperfections of nanosized CdS Se1− thermally evaporated thin films, Superlattices Microstruct., 2015, 85, 67–81 CrossRef CAS.
- A. A. Akl, S. A. Aly and M. A. Kaid, Microstructural and Electrical Properties of (WO3)1−x(MoO3)x Thin Films Synthesized by Spray Pyrolysis Technique, Research & Reviews: Journal of Material Sciences, 2017, 1, 10–19 Search PubMed.
- K. C. Vinoth Kumar, T. Jani Subha, K. G. Ahila, B. Ravindran, S. W. Chang, A. H. Mahmoud, O. B. Mohammed and M. A. Rathi, Spectral characterization of hydroxyapatite extracted from Black Sumatra and Fighting cock bone samples: a comparative analysis, Saudi J. Biol. Sci., 2021, 28, 840–846 CrossRef CAS PubMed.
- W. Tong, J. Chen, X. Li, J. Feng, Y. Cao, Z. Yang and X. Zhang, Preferred orientation of plasma sprayed hydroxyapatite coatings, J. Mater. Sci., 1996, 31, 3739–3742 CrossRef CAS.
- R. Stylianou, M. Tkadletz, N. Schalk, M. Penoy, C. Czettl and C. Mitterer, Effects of reference materials on texture coefficients determined for a CVD α-Al2O3 coating, Surf. Coat. Technol., 2019, 359, 314–322 CrossRef CAS.
- S. K. Sahoo, A. Madhuri, A. Saini, S. Jena, P. S. Devi, S. Laha and B. P. Swain, Correlation of microstructural and chemical bonding of FeNi-rGO nanocomposites, J. Alloys Metallurgical Systems, 2023, 4, 100035 CrossRef.
- S. Prosad Moulick, Md Sahadat Hossain, Md Zia Uddin Al Mamun, F. Jahan, Md Farid Ahmed, R. A. Sathee, Md Sujan Hossen, M. Ashraful Alam, Md Sha Alam and F. Islam, Characterization of waste fish bones (Heteropneustes fossilis and Otolithoides pama) for photocatalytic degradation of Congo red dye, Results Eng., 2023, 20, 101418 CrossRef CAS.
- R. Das and S. Sarkar, Determination of intrinsic strain in poly(vinylpyrrolidone)-capped silver nano-hexapod using X-ray diffraction technique, Curr. Sci., 2015, 109, 775–778 CrossRef.
- B. Himabindu, N. S. M. P. Latha Devi, G. Sandhya, T. Naveen Reddy, T. Saha, B. Rajini Kanth and Md Sarowar Hossain, Structure based photocatalytic efficiency and optical properties of ZnO nanoparticles modified by annealing including Williamson–Hall microstructural investigation, Mater. Sci. Eng., B, 2023, 296, 116666 CrossRef CAS.
- R. Jacob and J. Isac, X-ray diffraction line profile analysis of Ba0.6Sr0.4FexTi(1−x)O3−δ, (x = 0.4), Int. J. Chem. Stud., 2015, 2, 12–21 Search PubMed.
- W. H. Hall, X-Ray Line Broadening in Metals, Proc. Phys. Soc., London, Sect. A, 1949, 62, 741 CrossRef.
- M. A. Tagliente and M. Massaro, Strain-driven (002) preferred orientation of ZnO nanoparticles in ion-implanted silica, Nucl. Instrum. Methods Phys. Res., Sect. B, 2008, 266, 1055–1061 CrossRef CAS.
- S. Sarkar and R. Das, Determination of structural elements of synthesized silver nano-hexagon from X-ray diffraction analysis, IJPAP, 2018, 56(10), 765–772 Search PubMed.
- M. Kawsar, M. S. Hossain, S. Tabassum, N. Mohammed Bahadur and S. Ahmed, Different solvents and organic modifiers for the control of crystallographic parameters in nano-crystallite hydroxyapatite for amplification of photocatalytic activity, Nanoscale Adv., 2024, 6, 2682–2700 RSC.
- S. Nikmehr, M. Kazemzad, M. M. Sabzehmeidani, L. Nikzad, T. Ebadzadeh and M. Mousaei, Zn-based MOFs materials fabricated by mechanochemical ball milling and hydrothermal method and derived metal oxides, Results Chem., 2023, 5, 100973 CrossRef CAS.
- Md Sahadat Hossain and S. Ahmed, Sustainable synthesis of nano CuO from electronic waste (E-waste) cable: evaluation of crystallite size via Scherrer equation, Williamson–Hall plot, Halder–Wagner model, Monshi–Scherrer model, size-strain plot, Results Eng., 2023, 20, 101630 CrossRef CAS.
- A. Khorsand Zak, W. H. Abd. Majid, M. E. Abrishami and R. Yousefi, X-ray analysis of ZnO nanoparticles by Williamson–Hall and size–strain plot methods, Solid State Sci., 2011, 13, 251–256 CrossRef CAS.
- A. Hepp and C. Baerlocher, Learned Peak Shape Functions for Powder Diffraction Data, Aust. J. Phys., 1988, 41, 229–236 CrossRef CAS.
- L. Motevalizadeh, Z. Heidary and M. Ebrahimizadeh Abrishami, Facile template-free hydrothermal synthesis and microstrain measurement of ZnO nanorods, Bull. Mater. Sci., 2014, 37, 397–405 CrossRef CAS.
- N. C. Halder and C. N. J. Wagner, Separation of particle size and lattice strain in integral breadth measurements, Acta Crystallogr., 1966, 20, 312–313 CrossRef CAS.
- S. Hossain Md, M. Mahmud, M. B. Mobarak, S. Sultana, A. A. Shaikh Md and S. Ahmed, New analytical models for precise calculation of crystallite size: application to synthetic hydroxyapatite and natural eggshell crystalline materials, Chem. Pap., 2022, 76, 7245–7251 CrossRef.
- U. Holzwarth and N. Gibson, The Scherrer equation versus the “Debye-Scherrer equation”, Nat. Nanotechnol., 2011, 6, 534 CrossRef CAS PubMed.
- S. I. Yusuf, S. J. Mohammad and M. H. Ali, Scherrer andWilliamson-Hall estimated particle size using XRD analysis for cast aluminum alloys, J. Theor. Appl. Phys., 2024, 18, 012029 Search PubMed.
- M. E. Rodriguez-Garcia, S. M. Londoño-Restrepo, C. F. Ramirez-Gutierrez and B. Millan-Malo, Effect of the crystal size on the X-ray diffraction patterns of isolated orthorhombic starches: a-type, Cornel University, 2018, https://arxiv.org/abs/1808.02966 Search PubMed.
- S. Koutsopoulos, Synthesis and characterization of hydroxyapatite crystals: a review study on the analytical methods, J. Biomed. Mater. Res., 2002, 62, 600–612 CrossRef CAS PubMed.
- N. Kourkoumelis and M. Tzaphlidou, Spectroscopic Assessment of Normal Cortical Bone: Differences in Relation to Bone Site and Sex, Sci. World J., 2010, 10, 402–412 CrossRef PubMed.
- N. K. Nguyen, M. Leoni, D. Maniglio and C. Migliaresi, Hydroxyapatite nanorods: soft-template synthesis, characterization and preliminary in vitro tests, J. Biomater. Appl., 2013, 28, 49–61 CrossRef CAS PubMed.
- R. A. Ramli, R. Adnan, M. Abu Bakar and S. Masudi, Synthesis and characterisation of pure nanoporous hydroxyapatite, J. Phys. Sci., 2011, 22, 25–37 CAS.
- D. Davazoglou and V. Em Vamvakas, Arrangement of Si and O Atoms in Thermally Grown SiO[sub 2] Films, J. Electrochem. Soc., 2003, 150, F90 CrossRef CAS.
- C. Li, B. Zhao, Y. Lu and Y. Liang, Microstructure and Ion Exchange in Stearic Acid Langmuir–Blodgett Films Studied by Fourier Transform Infrared-Attenuated Total Reflection Spectroscopy, J. Colloid Interface Sci., 2001, 235, 59–65 CrossRef CAS PubMed.
- S. Raynaud, E. Champion, D. Bernache-Assollant and P. Thomas, Calcium phosphate apatites with variable Ca/P atomic ratio I. Synthesis, characterisation and thermal stability of powders, Biomaterials, 2002, 23, 1065–1072 CrossRef CAS PubMed.
- M. Manzano and M. Vallet-Regí, New developments in ordered mesoporous materials for drug delivery, J. Mater. Chem., 2010, 20, 5593 RSC.
- Md. S. Hossain, Md. A. A. Shaikh, Md. F. Ahmed and S. Ahmed, Synthesis and characterization of nano-crystallite triple superphosphate from waste Pila globosa shells for sustainable industrial production, Mater. Adv., 2023, 4, 2384–2391 RSC.
- M. Kawsar, M. S. Hossain, S. Tabassum, N. M. Bahadur and S. Ahmed, Synthesis of different types of nano-hydroxyapatites for efficient photocatalytic degradation of textile dye (Congo red): a crystallographic characterization, RSC Adv., 2024, 14, 11570–11583 RSC.
- S. Rehman, A. Mumtaz and S. K. Hasanain, Size effects on the magnetic and optical properties of CuO nanoparticles, J. Nanopart. Res., 2011, 13, 2497–2507 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a,
Newaz Mohammed Bahadurb,
Dipa Islamc and
Samina Ahmed
*a,
Newaz Mohammed Bahadurb,
Dipa Islamc and
Samina Ahmed *a
*a

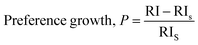
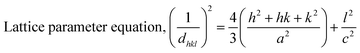

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ
θ


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tan
tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ
θ



 denotes the mean RI. The analysis reveals that succinic acid_HAp, ascorbic acid_HAp, calcium phosphate_HAp, and silica_HAp are grown in (002), (112), and (202) planes, while stearic acid_HAp is grown in (002) and (202) planes, respectively.
denotes the mean RI. The analysis reveals that succinic acid_HAp, ascorbic acid_HAp, calcium phosphate_HAp, and silica_HAp are grown in (002), (112), and (202) planes, while stearic acid_HAp is grown in (002) and (202) planes, respectively.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ
θ
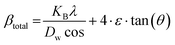
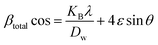
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) on the horizontal axis and (βhkl × cos
θ) on the horizontal axis and (βhkl × cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) across the vertical axis, which assembles the obtained diffraction peaks for the HAp nanocrystals. Intrinsic strain for the crystal samples is provided via the slope of the as-generated linear graph, and the average size of the nanocrystal is estimated from the intercept. The lattice strain in nanocrystals originates from the expansion or contraction of the lattice, resulting in confinement to a small size. This alteration causes a small deviation in the atomic configuration compared to bulk crystals. Furthermore, the size constraint leads to imperfections in the lattice arrangement, causing strain inside the lattice. The average crystal size of the HAp is estimated as 4.20, 72.97, 29.50, 4.04, and 13.72 nm for succinic acid_HAp, ascorbic acid_HAp, stearic acid_HAp, calcium phosphate_HAp and silica_HAp, respectively.
θ) across the vertical axis, which assembles the obtained diffraction peaks for the HAp nanocrystals. Intrinsic strain for the crystal samples is provided via the slope of the as-generated linear graph, and the average size of the nanocrystal is estimated from the intercept. The lattice strain in nanocrystals originates from the expansion or contraction of the lattice, resulting in confinement to a small size. This alteration causes a small deviation in the atomic configuration compared to bulk crystals. Furthermore, the size constraint leads to imperfections in the lattice arrangement, causing strain inside the lattice. The average crystal size of the HAp is estimated as 4.20, 72.97, 29.50, 4.04, and 13.72 nm for succinic acid_HAp, ascorbic acid_HAp, stearic acid_HAp, calcium phosphate_HAp and silica_HAp, respectively.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/Yhkl) on the horizontal and (βhkl
θ/Yhkl) on the horizontal and (βhkl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) on the vertical axis (Fig. 4) correspond to each peak in the XRD pattern. The slope represents the stress value, whereas the intercept shows the average crystallite size (Table 3).
θ) on the vertical axis (Fig. 4) correspond to each peak in the XRD pattern. The slope represents the stress value, whereas the intercept shows the average crystallite size (Table 3).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 580
580![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 987
987![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 093
093![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 187
187![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 558
558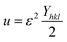

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ(√2u/Yhkl)) plotted horizontally and (βhkl·cos
θ(√2u/Yhkl)) plotted horizontally and (βhkl·cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) plotted vertically (Fig. 5), resembling each diffraction peak. All the estimated values of crystal size and energy density of the synthesized HAp nanoparticles are listed in Table 3.
θ) plotted vertically (Fig. 5), resembling each diffraction peak. All the estimated values of crystal size and energy density of the synthesized HAp nanoparticles are listed in Table 3.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ)2 and (dhkl2βhkl
θ)2 and (dhkl2βhkl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) along the Y and X axes, respectively, several mathematical expressions were generated, as shown in Fig. 6. These expressions were then fitted to the equation of a straight line. The slopes of these equations were used to calculate the crystallite size, whereas the intercept yields the value of strain. The estimated sizes of the nano-crystallites are listed in Table 3.
θ) along the Y and X axes, respectively, several mathematical expressions were generated, as shown in Fig. 6. These expressions were then fitted to the equation of a straight line. The slopes of these equations were used to calculate the crystallite size, whereas the intercept yields the value of strain. The estimated sizes of the nano-crystallites are listed in Table 3.
 and
and 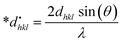 the crystal size was estimated from the slope (1/Dw) by plotting (*βhkl/*dhkl)2 and (*βhkl/*dhkl)2 on the vertical and horizontal axes, respectively, as shown in Fig. 7.
the crystal size was estimated from the slope (1/Dw) by plotting (*βhkl/*dhkl)2 and (*βhkl/*dhkl)2 on the vertical and horizontal axes, respectively, as shown in Fig. 7.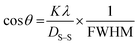
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 093 N m−2), and highest energy density (864.86 J m−3) (Fig. 9). This makes it a strong candidate for high-stress applications requiring energy absorption, such as impact-resistant coatings and energy storage materials. Calcium phosphate HAp has the smallest crystallite size (0.148 nm) and negligible energy density (6.4 × 10−11 J m−3), but it induces high compressive stress (−83
093 N m−2), and highest energy density (864.86 J m−3) (Fig. 9). This makes it a strong candidate for high-stress applications requiring energy absorption, such as impact-resistant coatings and energy storage materials. Calcium phosphate HAp has the smallest crystallite size (0.148 nm) and negligible energy density (6.4 × 10−11 J m−3), but it induces high compressive stress (−83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 187 N m−2), limiting its practical utility. Succinic acid HAp has a moderate crystallite size (11.51 nm) and strain (0.0104), making it suitable for bioactive applications like bone implants and drug delivery systems.53 Ascorbic acid HAp has balanced properties, with a crystallite size of 16.11 nm, moderate tensile stress (48
187 N m−2), limiting its practical utility. Succinic acid HAp has a moderate crystallite size (11.51 nm) and strain (0.0104), making it suitable for bioactive applications like bone implants and drug delivery systems.53 Ascorbic acid HAp has balanced properties, with a crystallite size of 16.11 nm, moderate tensile stress (48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 987 N m−2), and stable energy density (199.96 J m−3), suggesting its potential for applications requiring both structural integrity and functional performance. Silica HAp exhibits minimal strain (0.0019) and low energy density (11.13 J m−3), making it suitable for stable, low-stress applications.
987 N m−2), and stable energy density (199.96 J m−3), suggesting its potential for applications requiring both structural integrity and functional performance. Silica HAp exhibits minimal strain (0.0019) and low energy density (11.13 J m−3), making it suitable for stable, low-stress applications.


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 093 N m−2. Calcium phosphate modification yielded HAp with a minimum crystallite size of 0.148 nm but generated high compressive stress values reaching −83
093 N m−2. Calcium phosphate modification yielded HAp with a minimum crystallite size of 0.148 nm but generated high compressive stress values reaching −83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 187 N m−2. In comparison, the succinic and ascorbic acid modifications achieved moderate crystallite sizes of 11.51 and 16.11 nm, respectively, making them suitable for biomedical use despite their varying strain and tensile stress measurements. SEM analysis showed that succinic acid-HAp developed bioactive clusters, whereas ascorbic acid-HAp took on a dispersible, fluffy form. Stearic acid-HAp formed mechanically stable rod-like structures, calcium phosphate-HAp demonstrated osteoconductive porosity, and silica-HAp presented layered flakes that allowed controlled drug delivery. The Rietveld refinement analysis showed that calcium phosphate- (94.7% HAp) and silica-modified HAp (94% HAp) possessed higher phase purity in contrast to stearic acid-HAp, which contained significant tricalcium phosphate impurities (15.8% TCP), leading to reduced refinement reliability (Rwp: highest for stearic acid-HAp). The results demonstrate that selecting appropriate modifiers determines the properties of HAp that are suitable for biomedical, mechanical, and energy applications.
187 N m−2. In comparison, the succinic and ascorbic acid modifications achieved moderate crystallite sizes of 11.51 and 16.11 nm, respectively, making them suitable for biomedical use despite their varying strain and tensile stress measurements. SEM analysis showed that succinic acid-HAp developed bioactive clusters, whereas ascorbic acid-HAp took on a dispersible, fluffy form. Stearic acid-HAp formed mechanically stable rod-like structures, calcium phosphate-HAp demonstrated osteoconductive porosity, and silica-HAp presented layered flakes that allowed controlled drug delivery. The Rietveld refinement analysis showed that calcium phosphate- (94.7% HAp) and silica-modified HAp (94% HAp) possessed higher phase purity in contrast to stearic acid-HAp, which contained significant tricalcium phosphate impurities (15.8% TCP), leading to reduced refinement reliability (Rwp: highest for stearic acid-HAp). The results demonstrate that selecting appropriate modifiers determines the properties of HAp that are suitable for biomedical, mechanical, and energy applications.










