Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fluorescence switching via competitive ESIPT and spirolactam ring opening in a multifunctional rhodamine B probe for selective detection of Cu2+ and OCl−: theoretical insights with anticancer and biosensor activity†
Vishnu
S
a,
Avijit Kumar
Das
 *a,
Gouri
Karan
b and
Sujata Maiti
Choudhury
b
*a,
Gouri
Karan
b and
Sujata Maiti
Choudhury
b
aDepartment of Chemistry, Christ University, Hosur Road, Bangalore, Karnataka 560029, India. E-mail: avijitkumar.das@christuniversity.in
bCancer Nanotherapeutics Laboratory, Biochemistry, Molecular Endocrinology, and Reproductive Physiology Division, Department of Human Physiology, Vidyasagar University, Midnapore-721 102, West Bengal, India
First published on 27th May 2025
Abstract
A multifunctional ESIPT-based rhodamine-derived probe (BHS) was synthesized and developed as a colorimetric and fluorometric sensor for the selective detection of copper (Cu2+) and hypochlorite (OCl−) in aqueous solutions. Initially, BHS exhibits intense whitish blue fluorescence due to the active excited-state intramolecular proton transfer (ESIPT) mechanism within the molecule. However, upon interaction with Cu2+ and OCl−, noticeable changes in absorption and fluorescence occur, attributed to the inhibition of ESIPT resulting from analyte binding with BHS, leading to spirolactam ring opening. Furthermore, significant Stokes shifts in absorption (Δλ = 34 nm and 170 nm for Cu2+, and 163 nm for OCl−) and emission (Δλ = 67 nm for both Cu2+ and OCl−) further confirm this transformation. The spirolactam ring opening is induced by Cu2+ coordination, whereas for OCl−, it is triggered by oxidative cleavage. To explore potential biological applications, fluorescence titration experiments were conducted to study the interactions of the BHS–Cu2+ complex with ct-DNA and the transport protein bovine serum albumin (BSA). Additionally, molecular docking studies were performed to assess these interactions, while DFT calculations were employed to optimize the structures of BHS and its Cu2+ complex. The fluorescence changes of BHS in the presence of Cu2+ and OCl− in biological samples have been examined by the anticancer and biosensor activity of BHS in HCT-116 colorectal cancer cells.
1. Introduction
Metal ions and anions are essential for a variety of pathological and physiological functions, including catalysis, osmotic control, metabolism, and more. However, it is generally recognized that abnormalities in the concentrations of specific ions in organisms can have a negative impact on normal biological events.1 In this respect, metal ion copper is a micronutrient that is necessary for humans, plants and animals. Copper stands in the third position of the most abundant trace metals in earth's crust, behind iron and zinc. Numerous physiological functions, including red blood cell production, and maintenance and healthy development of brain tissues, the heart, kidneys, and other organs, rely on copper(II) ions.2,3 Research findings suggest that one of the variables raising the risk of coronary heart disease is copper misregulation. Furthermore, among the most prevalent contaminants, particularly in drinking water, Cu2+ in turn degrades water quality and causes a number of illnesses.4 Additionally, a number of genetic and metabolic diseases in humans, including Parkinson's disease, Alzheimer's disease, Wilson's disease, obesity, diabetes, and others, are brought on by copper dysregulation.5 The WHO states that the amount of copper ions in drinking water should not exceed more than 31.4 μM in order to safeguard human health.6Similarly, one among the most common ROS-reactive oxygen species is hypochlorite (ClO−). Endogenous hypochlorite is a physiologically significant ROS that is essential to many physiological and pathological processes.7 It is primarily produced by the innate immune system's myeloperoxidase (MPO)-mediated peroxidation of hydrogen peroxide (H2O2) and chloride (Cl−).8 It has been demonstrated that an excess of hypochlorite can be harmful and result in conditions including cancer, atherosclerosis, rheumatoid arthritis, fibrillation, and asthma. Cell well-being is majorly dependent on the optimum concentration of hypochlorite.9 Hence, the development of methods for copper ion and hypochlorite detection has been of utmost importance in environmental and food nutritional fields.
Several techniques have been established for the detection of metallic cations, such as ion chromatography,10 high-performance liquid chromatography,11 voltammetry,12 electrochemical,13 and ICP-AES - inductively coupled plasma atomic emission spectrometry,14etc. Although these methods prove to be efficient, they still suffer from certain limitations, such as expensive procedures and instrumentation, complicated operational protocols, and excessive sample pretreatment.15 In order to overcome these limitations, fluorescence chemosensors have attracted a lot of interest compared to alternative techniques due to their many benefits, which include ease of device manufacturing, low cost, excellent stability, and quick disposability.16 Fluorescent chemosensors have the ability to selectively detect several chemical species including cations,17 anions,18 and other compounds, by altering their fluorescence properties. These chemosensors have been widely developed and utilized in the domains of chemistry, life science, environmental research, and materials science due to their high detection sensitivity, rapid analyte response, ease of handling, and ability to facilitate real-time analysis.19 The rhodamine molecule is a notable example of an off–on type fluorescent probe among the known fluorescent compounds. First created in 1905 by Noelting and Dziewonsky, rhodamine is a class of xanthene-derived dye used in analytical research, initially used for colorimetric detection of a variety of substances, including zinc and silver.20 Since then, rhodamine as a scaffold has gained vast significance in analyte detection via colorimetric and fluorometric paths owing to its unique characteristics, such as high quantum yields of fluorescence, large molar extinction coefficients, and both long emission and absorption wavelengths.13 Rhodamine's structure includes a spirolactam ring, which can be observed in two structural forms. The ring opening is generally facilitated upon binding with cations, anions, and certain neutral molecules. While the ring closure spirolactam derivatives are nonfluorescent and colorless, opening of the spirolactam ring results in notable fluorescence changes and a noticeable brilliant pink color.21 Despite the fact that organic optoelectronic materials have employed a variety of binding mechanisms, the ESIPT-excited-state intramolecular proton transfer phenomenon, a photo-induced proton transfer via an intramolecular hydrogen bond, is of great interest because of its exciting photochemical and photophysical applications.22
The ESIPT process, initially reported in the 1950s by Weller for salicylic acid, has been extensively studied since then for various applications. ESIPT fluorescence occurs when molecules have intramolecular hydrogen bonding between donors and acceptors. ESIPT involves a fast enol-to-keto phototautomerization (kESIPT > 1012 s−1) followed by a radiative decay and reverse proton transfer (RPT) to regenerate the enol form (Scheme 1a). Its significant Stokes shift improves efficiency and helps prevent fluorophores from self-absorbing.23 Common ESIPT fluorophores include analogues of 2-(2′-hydroxyphenyl)benzimidazole, benzoxazole, and benzothiazole, along with quinoline, benzophenones, flavones, anthraquinones, benzotriazoles, and others.24 However, these ESIPT chromophores have rarely been coupled with rhodamine-based sensors for analyte detection (Scheme 1b).25 Therefore, we have designed and synthesized a multifunctional ESIPT based fluorescent probe 3′,6′-bis(diethylamino)-2-((4-(diethylamino)-2-hydroxybenzylidene)amino)spiro[isoindoline-1,9′-xanthen]-3-one (BHS) based on the Schiff base condensation reaction of rhodamine B hydrazide with 4-(diethylamino)salicylaldehyde derivatives for dual detection of cation Cu2+ and anion hypochlorite (OCl−). Probe BHS has been characterized by NMR and mass spectroscopy (Fig. S1 and S2, ESI†).
 | ||
| Scheme 1 (a) Diagrammatic representation of the ESIPT process. (b) Comparison of the ESIPT phenomenon with previous work and the current work. | ||
2. Experimental section
2.1. Synthesis of rhodamine B hydrazide (compound 1)
The synthesis procedure for compound 1 was adapted from Dujols et al.26 The process involved a single-step reaction between rhodamine B and hydrazine hydrate in methanol (Scheme 2a). In particular, rhodamine B (0.8 g) was solubilized in 30 mL of methanol, followed by the addition of excess hydrazine hydrate (1 mL). TLC was used to track the reaction mixture's progress and the refluxing continued until the disappearance of the pink color. After the completion of the reaction, the mixture was transferred into ice-cold water and a solid pink color precipitated out. The precipitate was then filtered and left to dry. A solid product yielding a total of 0.54 g (68%) of compound 1 was obtained.2.2. Synthesis of BHS
The compound BHS was synthesized by condensation of rhodamine B hydrazide (compound 1) (236.2 mg, 0.5173 mmol) and 4-(diethylamino)salicylaldehyde (compound 2) (100 mg, 0.5175 mmol) in methanol (20 mL) and the mixture was stirred at room temperature for 24 h. The reaction progress was monitored via TLC. A yellow-colored product was filtered, followed by drying, and it was further recrystallized from ethyl acetate (Scheme 2b). Yield: 70 mg, 21%, Mp: 275 °C. 1H NMR (CDCl3, 400 MHz): δ (ppm): 10.94 (s, 1H, –OH), 9.18 (s, 1H,![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.94 (t, 1H, J = 4 Hz), 7.49 (t, 2H, J = 3.6 Hz), 7.15 (t, 1H, J = 4 Hz), 6.19 (d, 1H, J = 8.4 Hz), 6.47 (q, 4H), 6.25 (q, 2H), 6.11 (d, 2H, J = 11.2), 3.31 (q, 12H), 1.41 (q, 18H). Mass (m/z): M+ calculated for C39H45N5O3 is 631.35; found: 633.49 (M + 2H)+. Elemental analysis: calculated value: C, 74.14; H, 7.18; N, 11.08. Observed value: C, 74.18; H, 7.17; N, 11.05.
CH), 7.94 (t, 1H, J = 4 Hz), 7.49 (t, 2H, J = 3.6 Hz), 7.15 (t, 1H, J = 4 Hz), 6.19 (d, 1H, J = 8.4 Hz), 6.47 (q, 4H), 6.25 (q, 2H), 6.11 (d, 2H, J = 11.2), 3.31 (q, 12H), 1.41 (q, 18H). Mass (m/z): M+ calculated for C39H45N5O3 is 631.35; found: 633.49 (M + 2H)+. Elemental analysis: calculated value: C, 74.14; H, 7.18; N, 11.08. Observed value: C, 74.18; H, 7.17; N, 11.05.
3. Results and discussion
3.1. Binding study with Cu2+ and OCl−
To gain insights into the sensing abilities of the synthesized probe BHS, emission and absorption studies were conducted with various analytes, including metal ions and anions. The colorimetric and fluorescence properties of BHS were analyzed with the addition of various metal ions, like Al3+, Cd2+, Fe2+, Fe3+, Cu2+, Ni2+, Pb2+, Hg2+, Co2+, Mn2+, and Zn2+, in a CH3CN-HEPES buffer (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4). The probe BHS initially produced an absorbance peak at 382 nm, which, over the addition of Cu2+, exhibited a bathochromic shift resulting in the generation of two strong absorption signals at 416 nm (Δλ = 34 nm) and 552 nm (Δλ = 170 nm) along with an isobestic point at 402 nm. This large spectral shift indicates the presence of the ESIPT phenomenon within the molecule. Incremental addition of Cu2+ led to a gradual increase in the absorption peak at 552 nm, followed by a noticeable naked-eye color transition, generating pink color from colorless (Fig. 1a). The absorbance of BHS changes with the concentration of Cu2+ up to the saturation level (Fig. 1b). A linear increase in Cu2+ concentration results in a corresponding linear rise in absorbance, which is attributed to the observed color change. The emission spectrum of BHS alone produced a strong signal at 509 nm when excited at 400 nm. However, after the introduction of Cu2+ into the probe solution, a significant decrease in fluorescence intensity at 509 nm was observed along with the emergence of a red-shifted emission signal at 576 nm (Δλ = 67 nm) at a high concentration of Cu2+, which corresponds to the emission signal appearance upon the spirolactam ring opening of rhodamine. Attributed to the BHS–Cu2+ binding, the fluorescence intensity gradually decreases with increasing concentrations of Cu2+ (Fig. 1d).
1, v/v, pH 7.4). The probe BHS initially produced an absorbance peak at 382 nm, which, over the addition of Cu2+, exhibited a bathochromic shift resulting in the generation of two strong absorption signals at 416 nm (Δλ = 34 nm) and 552 nm (Δλ = 170 nm) along with an isobestic point at 402 nm. This large spectral shift indicates the presence of the ESIPT phenomenon within the molecule. Incremental addition of Cu2+ led to a gradual increase in the absorption peak at 552 nm, followed by a noticeable naked-eye color transition, generating pink color from colorless (Fig. 1a). The absorbance of BHS changes with the concentration of Cu2+ up to the saturation level (Fig. 1b). A linear increase in Cu2+ concentration results in a corresponding linear rise in absorbance, which is attributed to the observed color change. The emission spectrum of BHS alone produced a strong signal at 509 nm when excited at 400 nm. However, after the introduction of Cu2+ into the probe solution, a significant decrease in fluorescence intensity at 509 nm was observed along with the emergence of a red-shifted emission signal at 576 nm (Δλ = 67 nm) at a high concentration of Cu2+, which corresponds to the emission signal appearance upon the spirolactam ring opening of rhodamine. Attributed to the BHS–Cu2+ binding, the fluorescence intensity gradually decreases with increasing concentrations of Cu2+ (Fig. 1d).
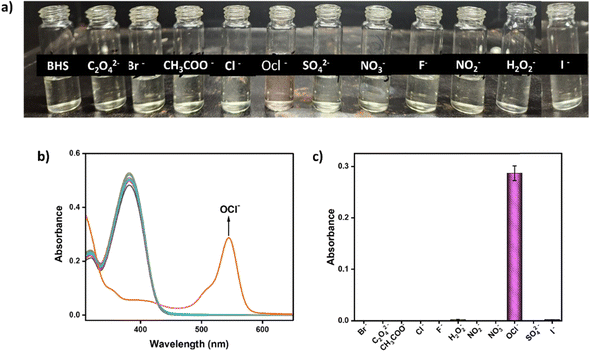
Similarly, the UV-vis and fluorescence behavior of BHS was studied with the addition of several anions, such as Br−, C2O4−, CH3COO−, Cl−, F−, I−, H2O2, NO2−, NO3−, OCl−, and SO42, in CH3CN-HEPES buffer (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4). The probe BHS itself exhibited a single distinct absorption maximum at 382 nm without the addition of any analytes. However, on exposure to OCl−, a new red-shifted absorption signal emerged at 545 nm (Δλ = 163 nm), which was gradually enhanced with increasing concentrations of OCl− (Fig. 2a and b). This transition was accompanied by a prominent, visually detectable color change from colorless to pink. As a result, a linear increase in absorbance was observed with increasing OCl− concentrations (Fig. 2b). In the fluorescence spectra, the probe BHS initially exhibited a strong whitish blue fluorescence with a prominent emission peak at 509 nm. Upon the addition of OCl−, the emission signal exhibited a red shift (Δλ = 67 nm) to 576 nm from 509 nm followed by the decrease of the emission intensity at 576 nm with the appearance of weak red fluorescence (Fig. 2c and d). The significant Stokes shift in the emission spectra also suggests the occurrence of the ESIPT phenomenon within the molecule.
1, v/v, pH 7.4). The probe BHS itself exhibited a single distinct absorption maximum at 382 nm without the addition of any analytes. However, on exposure to OCl−, a new red-shifted absorption signal emerged at 545 nm (Δλ = 163 nm), which was gradually enhanced with increasing concentrations of OCl− (Fig. 2a and b). This transition was accompanied by a prominent, visually detectable color change from colorless to pink. As a result, a linear increase in absorbance was observed with increasing OCl− concentrations (Fig. 2b). In the fluorescence spectra, the probe BHS initially exhibited a strong whitish blue fluorescence with a prominent emission peak at 509 nm. Upon the addition of OCl−, the emission signal exhibited a red shift (Δλ = 67 nm) to 576 nm from 509 nm followed by the decrease of the emission intensity at 576 nm with the appearance of weak red fluorescence (Fig. 2c and d). The significant Stokes shift in the emission spectra also suggests the occurrence of the ESIPT phenomenon within the molecule.
3.2. Interference study
Interference analysis using colorimetric and fluorometric methods was performed to evaluate the selectivity of BHS toward Cu2+ by testing various metal ions, including Al3+, Cd2+, Fe2+, Fe3+, Hg2+, Mn2+, Ni2+, Co2+, Pb2+, and Zn2+, in CH3CN-HEPES buffer (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4). The probe solution exhibited no notable absorption peak at 552 nm in the presence of different metal ions. Slight interference from Fe2+ was observed in the absorption spectra, indicated by the appearance of a pale pink color visible to the naked eye (Fig. 3a and b). However, upon the addition of Cu2+, a prominent signal appeared at 552 nm, attributed to the formation of a distinct pink color from the initially colorless probe solution. The naked eye visible color change appeared selectively in the presence of Cu2+ (Fig. 3a) with the appearance of the corresponding strong UV-vis absorption signal (Fig. 3b), while Fig. 3c presents a bar graph of the absorption intensities, with the red bar representing the solution containing Cu2+.
1, v/v, pH 7.4). The probe solution exhibited no notable absorption peak at 552 nm in the presence of different metal ions. Slight interference from Fe2+ was observed in the absorption spectra, indicated by the appearance of a pale pink color visible to the naked eye (Fig. 3a and b). However, upon the addition of Cu2+, a prominent signal appeared at 552 nm, attributed to the formation of a distinct pink color from the initially colorless probe solution. The naked eye visible color change appeared selectively in the presence of Cu2+ (Fig. 3a) with the appearance of the corresponding strong UV-vis absorption signal (Fig. 3b), while Fig. 3c presents a bar graph of the absorption intensities, with the red bar representing the solution containing Cu2+.
Similarly, fluorometric titration analysis revealed that no metal ion induced significant changes in the probe solution, except for Cu2+. This metal ion Cu2+, along with displaying a prominent quenching of fluorescence intensity at 509 nm, produced a secondary signal at 576 nm, which can be attributed to the binding interaction between BHS and Cu2+ (Fig. 4). The fluorescence quenching phenomena pave the way to the emergence of new fluorescence, as observed under a UV chamber (Fig. 4a). The emission spectrum of BHS with various metals ions has been shown in the interference study, wherein the signal of emission at 509 nm was unaffected with the presence of any other interfering cations except for Cu2+ but minor interference from Co2+ was detected in the fluorescence spectra (Fig. 4b). While bar graph representation displays the same observation on exposure to several interfering metal ions and the lower intensity red bar indicates the emission response of BHS in the presence of Cu2+ at 509 nm (Fig. 4c).
Similarly, the interference analysis of BHS with various anions, including Br−, C2O42−, CH3COO−, Cl−, F−, I−, H2O2−, NO2−, NO3− and SO42−, was conducted in CH3CN-HEPES buffer (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4). Only exposure to OCl− produced significant changes in absorbance and emission intensities at 545 nm and 576 nm, respectively. The absorption spectrum displayed a distinctive peak at 545 nm, while the fluorescence spectrum exhibited a new, selective signal at 576 nm exclusively with OCl−. No notable changes in absorbance or emission were observed in the presence of several other anions (Fig. 5 and 6). In the bar graph depiction of the same, the selective behavior of BHS toward OCl− is highlighted with a pink bar of higher intensity compared to the lower-intensity yellow bars for other anions in the absorption study (Fig. 5c). Similarly, in the fluorescence study, a lower-intensity bar (pink) represents OCl−, contrasting with higher-intensity bars (yellow) for other anions (Fig. 6c). All these results demonstrate the selective interaction of BHS with Cu2+ and OCl− against all the interfering metal ions and anions.
1, v/v, pH 7.4). Only exposure to OCl− produced significant changes in absorbance and emission intensities at 545 nm and 576 nm, respectively. The absorption spectrum displayed a distinctive peak at 545 nm, while the fluorescence spectrum exhibited a new, selective signal at 576 nm exclusively with OCl−. No notable changes in absorbance or emission were observed in the presence of several other anions (Fig. 5 and 6). In the bar graph depiction of the same, the selective behavior of BHS toward OCl− is highlighted with a pink bar of higher intensity compared to the lower-intensity yellow bars for other anions in the absorption study (Fig. 5c). Similarly, in the fluorescence study, a lower-intensity bar (pink) represents OCl−, contrasting with higher-intensity bars (yellow) for other anions (Fig. 6c). All these results demonstrate the selective interaction of BHS with Cu2+ and OCl− against all the interfering metal ions and anions.
3.3. Competition study
Furthermore, a competition study was conducted to analyze the selective binding abilities of BHS toward Cu2+ and OCl− against several other competing cations and anions, respectively, in CH3CN-HEPES buffer (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4). The absorption spectra demonstrate the selectivity of BHS for Cu2+ (red bars) and OCl− (pink bars), with the emergence of prominent peaks at 552 nm and 545 nm, respectively, even in a competing ionic environment (Fig. 7a and b). This indicates minimal competition effects on the efficiency of BHS towards selective detection of Cu2+ and OCl−. Furthermore, the fluorescence titrations showed that Cu2+ and OCl− selectively quenched the fluorescence of BHS. The high fluorescence intensity (yellow bars) represents the signal of BHS with individual ions, whereas, in the presence of Cu2+ (red bars) and OCl− (pink bars), a notable decrease in fluorescence intensity at 576 nm was observed, confirming the selective detection of target analytes. These results further assert the strong selectivity of BHS for Cu2+ and OCl− (Fig. 7c and d).
1, v/v, pH 7.4). The absorption spectra demonstrate the selectivity of BHS for Cu2+ (red bars) and OCl− (pink bars), with the emergence of prominent peaks at 552 nm and 545 nm, respectively, even in a competing ionic environment (Fig. 7a and b). This indicates minimal competition effects on the efficiency of BHS towards selective detection of Cu2+ and OCl−. Furthermore, the fluorescence titrations showed that Cu2+ and OCl− selectively quenched the fluorescence of BHS. The high fluorescence intensity (yellow bars) represents the signal of BHS with individual ions, whereas, in the presence of Cu2+ (red bars) and OCl− (pink bars), a notable decrease in fluorescence intensity at 576 nm was observed, confirming the selective detection of target analytes. These results further assert the strong selectivity of BHS for Cu2+ and OCl− (Fig. 7c and d).
On the basis of the fluorometric studies of BHS with Cu2+ and OCl−, the limits of detection of the probe BHS for Cu2+ and OCl− were determined as 6.3 μM and 8.21 μM, respectively, utilizing the formula DL = K × Sb1/S, where K = 3, Sb1 is the standard deviation of the blank solution, and S is the slope of the calibration curve (Fig. S5 and S6, ESI†).27 At 552 nm, the molar absorption coefficients for BHS and BHS–Cu2+ were calculated as 10.57 × 103 mol−1-liter-cm−1 and 2.48 × 103 mol−1-liter-cm−1. Through the Jobs plot analysis, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry for BHS–Cu2+ complexation was verified and from the fluorescence titration experiment, the association constant (Ka) of BHS with Cu2+ was calculated as 2.7 × 105 M−1 (error < 10%) (Fig. S9, ESI†).28,29 To further evaluate the probe's stability and sensing capabilities across different pH environments, UV-vis experiments were conducted. Notably, the sensor BHS exhibited an intense naked-eye-visible color in strongly acidic conditions, accompanied by a strong absorption signal at 552 nm, while no response was observed in neutral or basic media. This indicates that the spirolactam ring opens in acidic environments without the addition of metal ions, whereas it remains stable in neutral and basic media. However, in the presence of Cu2+, the spirolactam ring also opens under neutral and basic conditions (Fig. S11, ESI†). Therefore, to simulate physiological conditions, the sensing behavior of BHS with Cu2+ and OCl− was studied at pH 7.4 using HEPES buffer.
1 binding stoichiometry for BHS–Cu2+ complexation was verified and from the fluorescence titration experiment, the association constant (Ka) of BHS with Cu2+ was calculated as 2.7 × 105 M−1 (error < 10%) (Fig. S9, ESI†).28,29 To further evaluate the probe's stability and sensing capabilities across different pH environments, UV-vis experiments were conducted. Notably, the sensor BHS exhibited an intense naked-eye-visible color in strongly acidic conditions, accompanied by a strong absorption signal at 552 nm, while no response was observed in neutral or basic media. This indicates that the spirolactam ring opens in acidic environments without the addition of metal ions, whereas it remains stable in neutral and basic media. However, in the presence of Cu2+, the spirolactam ring also opens under neutral and basic conditions (Fig. S11, ESI†). Therefore, to simulate physiological conditions, the sensing behavior of BHS with Cu2+ and OCl− was studied at pH 7.4 using HEPES buffer.
3.4. Reversibility study
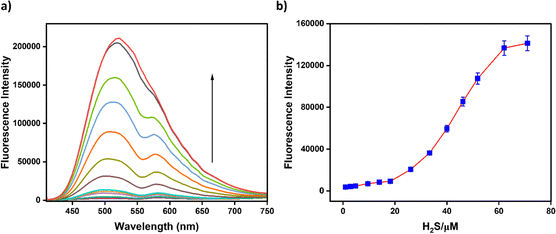
A fluorometric analysis was performed to assess the reversibility of the binding interaction between BHS and Cu2+, providing further insights into the binding properties of BHS with Cu2+. The intensity of the fluorescence gradually increased upon incremental addition of H2S to the BHS–Cu2+ complex, indicating the reversible binding behavior of BHS with Cu2+ (Fig. 8). The notable fluorescence enhancement suggests that H2S extracts Cu2+ from the BHS–Cu2+ complex, releasing the free probe BHS and confirming its reversible metal ion binding.3.5. Probable binding mode
Without the presence of analytes, the ligand BHS alone exhibits strong whitish blue fluorescence due to the conversion of the enol intermediate to the keto form through an ultrafast photo-induced tautomerization process via excited-state intramolecular proton transfer (ESIPT). This process involves the transfer of a proton from the acidic hydroxyl group to the basic imine nitrogen, facilitated by the formation of a six-membered transition state (pathway a, Scheme 3). As a result, BHS shows a strong absorbance signal at 382 nm and emission signal at 509 nm with notable Stokes shifts at about 127 nm. Moreover, large spectral shifts of absorption (Δλ = 34 nm and 170 nm for Cu2+ and 163 nm for OCl−) and emission signals (Δλ = 67 nm for Cu2+ and OCl−) of BHS on binding with Cu2+ and OCl− suggest the occurrence of the ESIPT phenomenon within the molecule.30 But, the changes in absorption and fluorescence upon exposure to Cu2+ and OCl− are attributed to the suppression of ESIPT due to analyte binding with BHS, followed by spirolactam ring opening. For Cu2+, this occurs through metal ion coordination, while for OCl−, it is driven by oxidative cleavage. During the interaction, a ring strain is influenced within the molecule due to the high Lewis acid strength of copper and its coordination with nitrogen and oxygen donor centers in BHS. The Cu2+ chelation with the rhodamine-linked derivative BHS triggers a chelation-enhanced fluorescence (CHEF) “off–on” colorimetric and fluorometric response (pathway b, Scheme 3). This transformation involves the translation of the colorless, non-fluorescent spirolactam structure BHS to the ring-open form, BHS1, which exhibits strong fluorescence and pink color. Spectroscopic analyses, including mass spectrometry and DFT studies, support this mechanistic transformation. The BHS1 and Cu2+ complex formation was confirmed by the appearance of a mass peak at m/z = 735.50 (Fig. S3, ESI†), corresponding to [BHS–Cu2+–CH3CN]+ (Fig. S4, ESI†). A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry has been confirmed through the Job's plot analysis and further corroborated by DFT analysis (Fig. S8, ESI†). The opening of the spirolactam ring of the rhodamine derivative is responsible for the changes of absorption and emission color of BHS upon the addition of OCl−. Coordination of metal ions such as Cu2+ with the rhodamine moiety usually results in ring opening without any loss of groups from the binding site of the rhodamine derivative. Nevertheless, in the presence of water, OCl− oxidizes the hydrazide group of BHS, leading to the formation of pink-colored, red fluorescent rhodamine-B itself (BHS2) (pathway c, Scheme 3). This transformation is confirmed by the presence of a mass peak at m/z = 444.02, corresponding to mass of rhodamine-B (Fig. S4, ESI†).
1 binding stoichiometry has been confirmed through the Job's plot analysis and further corroborated by DFT analysis (Fig. S8, ESI†). The opening of the spirolactam ring of the rhodamine derivative is responsible for the changes of absorption and emission color of BHS upon the addition of OCl−. Coordination of metal ions such as Cu2+ with the rhodamine moiety usually results in ring opening without any loss of groups from the binding site of the rhodamine derivative. Nevertheless, in the presence of water, OCl− oxidizes the hydrazide group of BHS, leading to the formation of pink-colored, red fluorescent rhodamine-B itself (BHS2) (pathway c, Scheme 3). This transformation is confirmed by the presence of a mass peak at m/z = 444.02, corresponding to mass of rhodamine-B (Fig. S4, ESI†).
3.6. Dipstick method
To analyze the real-time applicability of the probe, test strips were created by initially treating TLC plates with the synthesized receptor solution of BHS (c = 2 × 10−5 M). Furthermore, upon exposure of analytes OCl− and Cu2+ to the test kits that had been pre-treated with the BHS probe, significant color changes were observed attributed to the detection capabilities of the probe (Fig. 9). These test strips, often referred to as dipsticks, have proved efficacy in delivering prompt qualitative results without requiring any kind of complex instrumental examination. | ||
| Fig. 9 Color changes of the BHS-treated TLC plates (c = 2 × 10−5 M) with an exposure to Cu2+ and OCl− (c = 2 × 10−4 M). | ||
3.7. Biological applications
3.8. Theoretical applications
 | ||
| Fig. 13 Optimized geometrical molecular structure of both (a) BHS and (b) BHS–Cu2+ complex. (c) Frontier molecular orbital and the energy differences of the BHS and BHS–Cu2+ complex. | ||
On further analysis of the metal-complex it was found that, the π-electrons in the HOMO were predominantly confined near the metal center, while the LUMO was localized to the xanthene moiety. Following this the energy gap calculations were performed, wherein the gap between the HOMO (−4.650 eV) and LUMO (−0.957 eV) for BHS was calculated as 3.69 eV, whereas for the BHS–Cu complex, the energy gap was increased to 6.42 eV (HOMO = −6.901 eV, LUMO = −0.476 eV). From the TD-DFT study of the BHS–Cu complex, one of the experimental absorption signals appeared at 416 nm, which is so close to the theoretical TD-DFT calculated absorbance that appeared at 413.83 nm for S0 to S5 transition (energy: 2.996 eV, f = 0.0015).
4. Conclusion
In summary, the ESIPT-based rhodamine-derived sensor BHS was successfully developed as a multifunctional colorimetric and fluorescence probe for the selective detection of Cu2+ and OCl− in aqueous solutions. The sensing mechanism involves the suppression of ESIPT followed by spirolactam ring opening upon analyte interaction, leading to significant absorption and emission shifts. The distinct spectral responses and large Stokes shifts further validate the detection process. Additionally, the BHS–Cu2+ complex exhibited strong interactions with biomolecules such as ct-DNA and BSA, as demonstrated by fluorescence titration studies and molecular docking simulations. The structural optimization using DFT calculations provides further insights into the binding behavior and electronic properties of the system. The variations in fluorescence of BHS caused by Cu2+ and OCl− in biological samples were investigated through the anticancer and biosensing activities of BHS in HCT-116 colorectal cancer cells. These findings highlight the potential of BHS as an efficient chemosensor for environmental and biological applications, particularly in metal ion and oxidative stress detection.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors express their gratitude to Christ University, Bangalore, for providing research facilities, and Center for Research, Christ University for the seed money grant (grant approval number CU-ORS-SM-24/09). Avijit Kumar Das specially acknowledges State University Research Excellence (SERB-SURE) of the Science and Engineering Research Board (SERB) (File Number: SUR/2022/002461) under Anusandhan National Research Foundation (ANRF) and Department of Science and Technology (DST), Government of India, for the financial support by the research grant.References
- S.-H. Park, N. Kwon, J.-H. Lee, J. Yoon and I. Shin, Chem. Soc. Rev., 2020, 49, 143–179 RSC.
- N. Esfandiari and M. Aliofkhazraei, Talanta, 2024, 277, 126365 Search PubMed.
- L. Cui, J. Wu and H. Ju, Biosens. Bioelectron., 2015, 63, 276–286 CrossRef CAS PubMed.
- K. Sreenivasa Rao, T. Balaji, T. Prasada Rao, Y. Babu and G. R. K. Naidu, Spectrochim. Acta, Part B, 2002, 57, 1333–1338 Search PubMed.
- S. Khan, X. Chen, A. Almahri, E. S. Allehyani, F. A. Alhumaydhi, M. M. Ibrahim and S. Ali, J. Environ. Chem. Eng., 2021, 9, 106381 CrossRef CAS.
- R. Sivakumar and N. Y. Lee, BioChip J., 2021, 15, 216–232 Search PubMed.
- W. Ma, R. Chen, T. Hu, S. Xing, G. Zhou, X. Qin, H. Ren, Z. Zhang, J. Chen and Q. Niu, Talanta, 2023, 265, 124910 CrossRef CAS.
- R. Chen, S. Xing, T. Hu, Y. Li, J. Chen, Q. Niu and T. Li, Anal. Chim. Acta, 2023, 1237, 340557 CrossRef CAS PubMed.
- P. A. Kim, D. Choe, H. So, S. Park, B. Suh, S. Jeong, K.-T. Kim, C. Kim and R. G. Harrison, Spectrochim. Acta, Part A, 2021, 261, 120059 CrossRef CAS PubMed.
- B. Bansod, T. Kumar, R. Thakur, S. Rana and I. Singh, Biosens. Bioelectron., 2017, 94, 443–455 CrossRef CAS PubMed.
- Z. Yan, Y. Cai, J. Zhang and Y. Zhao, Measurement, 2022, 187, 110355 CrossRef.
- F. Abebe, P. Perkins, R. Shaw and S. Tadesse, J. Mol. Struct., 2020, 1205, 127594 CrossRef CAS.
- E. Karakuş, J. Mol. Struct., 2021, 1224, 129037 CrossRef.
- T. Chopra, S. Sasan, L. Devi, R. Parkesh and K. K. Kapoor, Coord. Chem. Rev., 2022, 470, 214704 CrossRef CAS.
- S. Khan, X. Chen, A. Almahri, E. S. Allehyani, F. A. Alhumaydhi, M. M. Ibrahim and S. Ali, J. Environ. Chem. Eng., 2021, 9, 106381 CrossRef CAS.
- S. B. Butt and M. Riaz, J. Liq. Chromatogr. Relat. Technol., 2009, 32, 1045–1064 CrossRef CAS.
- (a) S. Vishnu, A. Nag and A. K. Das, Anal. Methods, 2024, 16, 5263 RSC; (b) G. C. Das, A. K. Das, D. Das, T. R. Maity, A. Samanta, F. A. Alasmary, A. S. Almalki, A. Iqbal and M. Dolai, J. Photochem. Photobiol., A, 2023, 440, 114663 CrossRef CAS; (c) S. Goswami, A. K. Das and S. Maity, Dalton Trans., 2013, 42, 16259–16263 RSC; (d) S. Vishnu, S. Maity, A. C. Maity, S. K. Malavika, M. Dolai, A. Nag, Y. bylappa, G. Dutta, B. Mukherjee and A. K. Das, Spectrochim. Acta, Part A, 2024, 315, 124249 CrossRef CAS PubMed; (e) S. Maity, A. C. Maity, A. K. Das and N. Bhattacharyya, Anal. Methods, 2022, 14, 2739–2744 RSC; (f) M. S. Kumar, S. Pakrashy, S. Manna, S. M. Choudhury, B. Das, A. Ghosh, A. H. Seikh, M. Dolai and A. K. Das, Anal. Methods, 2025, 17, 2125–2133 RSC.
- (a) A. K. Das, Y. Bylappa, A. Nag and M. Dolai, Anal. Methods, 2024, 16, 8164–8178 RSC; (b) A. K. Das, S. Goswami, C. K. Quah and H. K. Fun, New J. Chem., 2015, 39, 5669–5675 RSC; (c) A. K. Das, N. Hayashi, Y. Shiraishi and T. Hirai, RSC Adv., 2017, 7, 30453–30458 RSC; (d) A. K. Das, S. Goswami, C. K. Quah and H. K. Fun, RSC Adv., 2016, 6, 18711–18717 RSC; (e) A. K. Das, S. Goswami, G. Dutta, S. Maity, T. K. Mandal, K. Khanra and N. Bhattacharyya, Org. Biomol. Chem., 2016, 14, 570–576 RSC; (f) A. K. Das, N. Hayashi, Y. Shiraishi and T. Hirai, RSC Adv., 2017, 7, 30453–30458 RSC; (g) T. Das, E. Joseph, M. S. Kumar, S. Vishnu, M. Dolai and A. K. Das, Microchem. J., 2024, 199, 110100 CrossRef CAS; (h) M. S. Kumar, M. Dolai, A. Nag, Y. Bylappa and A. K. Das, Anal. Methods, 2024, 16, 676–685 RSC.
- D. Xu, H. Jia, Y. Niu and S. Yin, Dyes Pigm., 2022, 200, 110185 CrossRef CAS.
- Y. Wang, X. Wang, W. Ma, R. Lu, W. Zhou and H. Gao, Chemosensors, 2022, 10, 399 CrossRef CAS.
- S. Sarkar, A. Chatterjee and K. Biswas, Crit. Rev. Anal. Chem., 2024, 54, 2351–2377 Search PubMed.
- A. Mondal, S. Mukhopadhyay, E. Ahmmed, S. Banerjee, E. Zangrando and P. Chattopadhyay, J. Phys. Chem. C, 2020, 124, 18181–18193 CrossRef CAS.
- J. Guan, Q. Tu, L. Chen, M.-S. Yuan and J. Wang, Spectrochim. Acta, Part A, 2019, 220, 117114 CrossRef CAS PubMed.
- (a) P.-T. Chou, Y.-C. Chen, W.-S. Yu, Y.-H. Chou, C.-Y. Wei and Y.-M. Cheng, J. Phys. Chem. A, 2001, 105, 1731–1740 CrossRef CAS; (b) M. T. Ignasiak, C. Houee-Levin, G. Kciuk, B. Marciniak and T. Pedzinski, Chem. Phys. Chem., 2015, 16, 628–633 Search PubMed; (c) D. Ghosh, S. Batuta, S. Das, N. A. Begum and D. Mandal, J. Phys. Chem. B, 2015, 119, 5650–5661 CrossRef CAS; (d) S. J. Schmidtke, D. F. Underwood and D. A. Blank, J. Am. Chem. Soc., 2004, 126, 8620–8621 Search PubMed; (e) M. J. Paterson, M. A. Robb, L. Blancafort and A. D. DeBellis, J. Am. Chem. Soc., 2004, 126, 2912–2922 Search PubMed; (f) E. Hadjoudis and I. M. Mavridis, Chem. Soc. Rev., 2004, 33, 579–588 CAS; (g) Y. Nakane, T. Takeda, N. Hoshino, K. Sakai and T. Akutagawa, J. Phys. Chem. A, 2015, 119, 6223–6231 CrossRef CAS PubMed.
- P. Majumdar and J. Zhao, J. Phys. Chem. B, 2015, 119, 2384–2394 CrossRef CAS PubMed.
- V. Dujols, F. Ford and A. W. Czarnik, J. Am. Chem. Soc., 1997, 119, 7386–7387 CrossRef CAS.
- M. Shortreed, R. Kopelman, M. Kuhn and B. Hoyland, Anal. Chem., 1996, 68, 1414–1418 Search PubMed.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703–2707 CrossRef CAS.
- Y. Shiraishi, Y. Kohno and T. Hirai, Ind. Eng. Chem. Res., 2005, 44, 847–851 CrossRef CAS.
- M. H. Lee, J. S. Kim and J. L. Sessler, Chem. Soc. Rev., 2015, 44, 4185–4191 RSC.
- R. Bhowmick, A. S. M. Islam, U. Saha, G. Suresh Kumar and M. Ali, New J. Chem., 2018, 42, 3435–3443 RSC.
- J. C. Stockert, R. W. Horobin, L. L. Colombo and A. Blázquez-Castro, Acta Histochem., 2018, 120, 159–167 CrossRef CAS PubMed; C. Kalaivanan, M. Sankarganesh, M. Y. Suvaikin, G. B. Karthi, S. Gurusamy, R. Subramanian and R. N. Asha, J. Mol. Liq., 2020, 320, 114423 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ma00517e |
| This journal is © The Royal Society of Chemistry 2025 |