Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis, characterization and biological activity of methotrexate-derived salts in lung cancer cells†
Dário Silvaabc,
Sandra Cordeirobc,
Pedro V. Baptista bc,
Alexandra R. Fernandes
bc,
Alexandra R. Fernandes *bc and
Luis C. Branco
*bc and
Luis C. Branco *a
*a
aLAQV-REQUIMTE, Nova School of Science and Technology, NOVA University Lisbon, 2829-516 Caparica, Portugal. E-mail: l.branco@fct.unl.pt
bAssociate Laboratory i4HB – Institute for Health and Bioeconomy, NOVA School of Science and Technology, NOVA University Lisbon, 2829-516 Caparica, Portugal. E-mail: ma.fernandes@fct.unl.pt
cUCIBIO – Applied Molecular Biosciences Unit, Department of Life Sciences, NOVA School of Science and Technology, NOVA University Lisbon, 2829-516 Caparica, Portugal
First published on 16th April 2025
Abstract
Lung cancer is one of the deadliest types of cancer, and is a public health problem worldwide. Methotrexate (MTX), a class IV drug in the biopharmaceutical classification system, is a folate antagonist that has demonstrated efficacy in cancer treatment. A suitable combination of MTX as a di-anion and biocompatible counter ions allowed the modulation of their physicochemical properties. In this work, twelve MTX salts were prepared and characterized by 1H NMR, 13C NMR, and elemental analysis. The antiproliferative effects of MTX salts were studied in A459 and H1975 (lung cancer cell lines) with three promising results: [C12mim]2[MTX] (IC50 = 0.55 ± 0.25) > [C10-3-picoline]2[MTX] (IC50 = 0.94 ± 0.03) > [C10mim]2[MTX] (IC50 = 1.71 ± 0.23) in A549. These three MTX salts also demonstrated intrinsic apoptosis, avoiding necrosis and the formation of reactive oxygen species.
1. Introduction
Cancer remains one of the main causes of death worldwide. In 2020, 9.9 million people died around the world and 18% of the deaths were due to lung cancer, which is one of the deadliest types of cancer.1 Lung cancer can be divided into two main histological types: small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC).2,3 NSCLC is the most prevalent form of lung cancer with 80–85% incidence. Platinum-based chemotherapy remains the foremost treatment for NSCLC but, due to its toxicity, new forms of treatment, such as targeted therapy or immune checkpoint inhibitors,3 have also been considered.Methotrexate (MTX) is a folate antagonist, which inhibits dihydrofolate reductase, which is essential for cellular replication. It is used in the treatment of different diseases, such as cancer, including NSCLC,4,5 rheumatoid arthritis,6 and psoriasis.7
As a folate antagonist, MTX has already shown efficacy in cancer treatment, especially in cancers with an abundance of folate receptors. Different reported studies showed a higher expression of folate receptors in lung cancer,8 which makes MTX a specific drug for lung cancer treatment. However, treatments with high doses of MTX cause significant side effects.9 Conversely, MTX also could induce resistance in cancer cells through folate cancer gene downregulation,10 leading to a reduction in cellular uptake of MTX as well as its treatment prospects. Additionally, MTX is a class IV drug in the biopharmaceutical classification system, showing low solubility and low permeability and, consequently, poor bioavailability.11 Recently, different approaches have been studied to target the delivery of MTX, involving nanocarriers or task-specific peptides, which selectively penetrate the cells linked to MTX.12,13
Ionic liquids (ILs) are low-melting organic salts with tunable physical–chemical properties and have found a large number of applications over the past two decades. Generally, it is possible to consider the evolution of ILs in three generations, with the third focusing on biological and pharmaceutical applications. In the last decade, different groups have reported the possibility of combining active pharmaceutical ingredients (APIs) with biocompatible counter ions forming API-ILs (also classified as active pharmaceutical organic salt & ionic liquids or API-OSILs and a group of uniform materials based on organic salts or GUMBOS). According to the cation–anion combinations, it is possible to tune their properties, such as solubility, permeability, and cytotoxicity14 as well as increasing their therapeutic activity. In fact, studies with MTX have already shown an enhancement in solubility and anti-proliferative activity in cancer cell lines when combined with ionic liquids (MTX-ILs).15,16 MTX has two ionizable carboxylic acids, meaning that it is a suitable pharmaceutical drug to combine with appropriate counter ions. Taking advantage of our previous experience in designing new API-OSILs with improved physical–chemical and pharmaceutical properties of the APIs, such as ciprofloxacin,17 mefloquine,18 levothyroxine,19 amphotericin B,20 valproate,21 hydroxyquinoline,22 penicillin,23 and amoxicilin,23 several biocompatible counter ions can be designed to develop new series of MTX salts. Additionally, the biocompatibility and low toxicity of some counter ions, combined with their bioavailability, can improve the original antitumoral activity of MTX while reducing side effects.
In this context, the main goal of the present study is focused on the development of MTX salts (Fig. 1) and the evaluation of their properties, including bioavailability and cytotoxicity studies, against lung cancer cell lines.
2. Experimental
2.1 Materials
Methotrexate hydrate (purity >98%) was purchased from TCI. Solvents were purchased from LabChem. The ion-exchange resin Amberlyst A26 (OH) (0.8 meq mL−1 ion-exchange capacity) was purchased from Alfa Aesar.2.2 MTX salts and counter ions: synthesis and characterization
General synthesis. Methotrexate (1.0 molar equiv.) was slightly dissolved in 5 mL of distilled water; then, a counter ion (2.0 molar equiv.), previously dissolved in 5 mL of water and treated with Amberlyst A-26 (1 h), was added and the reaction mixture was stirred at 40 °C for 2 h. The solvent was removed under reduced pressure to obtain the desired product.
[Na]2[MTX]. Following the general procedure, NaOH (17.6 mg; 0.44 mmol) was added to a methotrexate solution. Yellow solid (96% w/w).
1H NMR (400.13 MHz, D2O) δ (ppm): 8.45 (s, 1H); 7.63 (d, J = 8.90 Hz, 2H); 6.73 (d, J = 9.10 Hz, 2H); 4.58 (s, 2H); 4.29 (dd, J1 = 8.5 Hz, J2 = 4.70 Hz, 1H); 3.07 (s, 3H); 2.36–1.94 (m, 4H).
13C NMR (100.63 MHz, D2O) δ (ppm): 182.36; 179.19; 169.28; 162.85; 161.99; 153.36; 151.66; 149.10; 148.08; 128.78; 122.21; 120.58; 111.72; 55.83; 54.83; 38.62; 34.27; 28.57.
[Choline]2[MTX]. Following the general procedure, [choline][OH] (61.5 mg; 0.44 mmol) was added to a methotrexate solution. The final product was obtained as a yellow wax (128 mg; 88%).
1H NMR (400.13 MHz, D2O) δ (ppm): 8.54 (s, 1H); 7.68 (d, J = 7.20 Hz, 2H); 6.83 (d, J = 7.20 Hz, 2H); 4.72 (s, 1H); 4.29 (q, J1 = 7.20 Hz, J2 = 3.60 Hz, 1H); 4.05–4,02 (m, 4H); 3.50 (t, J = 4.40 Hz, 4H); 3.18 (s, 18H); 3.14 (s, 3H); 2.31–2.24 (m, 2H); 2.17–2.10 (m, 1H); 2.05–1.97 (m, 1H).
13C NMR (100.63 MHz, D2O) δ (ppm): 182.24; 179.08; 169.13; 162.83; 161.90; 153.11; 151.61; 149.11; 148.2; 128.78; 122.16; 120.52; 111.69; 67.38; 67.36; 67.33; 55.81; 55.54; 54.81; 53.83; 53.80; 53.77; 38.69; 34.27; 28.57.
IR (ATR) νmax (cm−1): 3328, 3218, 1589, 1556, 1449, 1380, 1208, 1087, 953, 826.
Elemental analysis calcd for C30H48N10O7·8.5H2O (%): C 44.27; H 8.05; N 17.21; found: C 44.58; H 7.89; N 17.08.
[N12,1,1,2OH]2 [MTX]. [N12,1,1,2OH][Br] was synthesized according to the literature.24
Following the general procedure, [N12,1,1,2OH][OH] (129 mg; 0.44 mmol) was added to a methotrexate solution. The final product was obtained as a yellow wax (175 mg; 95%).
1H NMR (400.13 MHz, D2O) δ (ppm): 8.50 (s, 1H); 7.74 (d, J = 8.3 Hz, 2H); 6.77 (d, J = 8.50 Hz, 2H); 4.33 (s, 1H); 3.97 (s, 4H); 3.40 (s, 4H); 3.24–3.13 (m, 6H); 3.05 (s, 12H); 2.31–1.96 (m, 3H); 1.55 (s, 4H) 1.33–1.01 (m, 40H); 0.89 (t, J = 7.0 Hz, 6H).
13C NMR (100.63 MHz, D2O) δ (ppm): 181.52; 178.26; 167.11; 162.79; 162.30; 154.14; 150.82; 146.98; 128.85; 122.06; 121.63; 111.51; 64.92; 55.52; 55.29; 51.35; 39.05; 34.24; 32.02; 29.90; 29.84; 29.71; 29.61; 29.55; 29.08; 26.06; 22.69; 22.25; 13.94.
IR (ATR) νmax (cm−1): 3302, 2921, 2853, 1628, 1556, 1449, 1374, 1208, 1087.
Elemental analysis calcd for C52H92N10O7·8H2O (%): C 56.09; H 9.78; N 12.58; found: C 55.92; H 10.49; N 12.57.
[N10,1,1,2OH]2[MTX]. [N10,1,1,2OH][Br] was synthesized according to the literature.24
Following the general procedure, [N10,1,1,2OH][OH] (136.6 mg; 0.44 mmol) was added to a methotrexate solution. The final product was obtained as a yellow wax (189 mg; 94%).
1H NMR (400.13 MHz, D2O) δ (ppm): 8.63 (s, 1H); 7.72 (d, J = 8.6 Hz, 2H); 6.90 (d, J = 8.70 Hz, 2H); 4.31 (dd, J1 = 8.6 Hz, J2 = 4.5 Hz, 1H); 4.03 (s, 4H); 3.46 (t, J = 6.2 Hz, 4H); 3.36–3.27 (m, 4H); 3.21 (s, 3H); 3.11 (s, 12H); 2.34–1.95 (m, 4H); 1.71 (s, 4H) 1.34–1.11 (m, 30H); 0.84 (t, J = 6.8 Hz, 6H).
13C NMR (100.63 MHz, D2O) δ (ppm): 181.88; 178.68; 168.26; 162.90; 162.05; 153.56; 151.34; 149.16; 147.87; 128.83; 122.19; 120.92; 111.71; 65.25; 64.90; 55.62; 55.29; 54.96; 51.29; 38.90; 34.22; 31.41; 28.89; 28.80; 28.77; 28.41; 25.61; 22.24; 21.96; 13.62.
IR (ATR) νmax (cm−1): 3309, 3182, 2924, 2853, 1628, 1585, 1553, 1445, 1377, 1208, 1090, 830, 761.
Elemental analysis calcd for C48H84N10O7·6H2O (%): C 56.45; H 9.47; N 13.71; found: C 56.60; H 9.77; N 14.13.
[MIMC12MIM][MTX]. [MIMC12MIM][Br] was synthesized according to the literature.25
Following the general procedure, [MIMC12MIM][OH] (102.2 mg; 0.22 mmol) was added to a methotrexate solution. The final product was obtained as a yellow wax (168 mg; 97%).
1H NMR (400.13 MHz, D2O) δ (ppm): 8.65 (s, 1H); 8.52 (s, 1H); 7.60 (d, J = 8.5 Hz, 2H); 7.38 (d, J = 1.8 Hz, 4H); 6.68 (d, J = 8.5 Hz, 2H); 4.71–4.62 (m, 2H); 4.31 (dd, J1 = 8.2 Hz, J2 = 4.6 Hz, 1H); 4.06 (t, J = 7.2 Hz, 4H); 3.86 (s, 4H); 3.12 (s, 3H); 2.35–1.96 (m, 4H); 1.781.63 (m, 4H); 1.14–0.91 (m, 16H).
13C NMR (100.63 MHz, D2O) δ (ppm): 181.66; 178.61; 168.10; 162.50; 160.74; 151.03; 148.68; 135.57; 128.60; 123.45; 123.41; 122.05; 122.00; 121.87; 120.37; 111.34; 55.55; 49.90; 49.37; 39.00; 35.61; 35.58; 34.02; 29.12; 28.90; 28.64; 28.54; 28.09; 25.32.
IR (ATR) νmax (cm−1): 3319, 3153, 2924, 2850, 1585, 1556, 1507, 1449, 1380, 1208, 1168, 1097, 826.
Elemental analysis calcd for C40H56N12O5·8.5H2O (%): C 51.21; H 7.84; N 17.92; found: C 51.07; H 7.43; N 18.14.
[C12MIM]2[MTX]. Following the general procedure, [C12MIM][OH] (146 mg; 0.44 mmol) was added to a methotrexate solution. The final product was obtained as a yellow wax (203 mg; 97%).
1H NMR (400.13 MHz, D2O) δ (ppm): 8.33 (s, 1H); 7.62 (d, J = 6.80 Hz, 2H); 7.30 (d, J = 1.60 Hz, 2H); 7.16 (d, J = 1.60 Hz, 2H); 6.59 (d, J = 6.80 Hz, 2H); 4.57 (s, 1H); 4.23 (t, J = 5.20 Hz, 1H); 3.74 (s, 6H); 3.0 (s, 3H); 2.14–1.88 (m, 4H); 1.46 (s br, 4H); 1.16–0.92 (m, 38H); 0.76 (t, J = 5.60 Hz, 3H).
13C NMR (100.63 MHz, D2O) δ (ppm): 181.46; 178.24; 167.0; 162.68; 162.22; 154.05; 150.78; 148.79; 146.92; 135.83; 135.58; 135.32; 128.76; 123.64; 121.91; 121.71; 121.59; 111.43; 55.54; 55.17; 49.19; 38.96; 35.73; 34.20; 31.98; 29.84; 29.82; 29.78; 29.59; 29.54; 29.49; 29.04; 26.03; 22.64; 13.89.
IR (ATR) νmax (cm−1): 3319, 2924, 2850, 1628, 1553, 1504, 1445, 1380, 1204, 1168, 1097, 826.
Elemental analysis calcd for C52H94N12O11·6H2O (%): C 58.73; H 8.91; N 15.81; found: C 58.56; H 9.07; N 15.85.
[C2OHMIM]2[MTX]. Following the general procedure, [C2OHMIM][OH] (71 mg; 0.44 mmol) was added to a methotrexate solution. The final product was obtained as a yellow wax (136 mg; 87%).
1H NMR (400.13 MHz, D2O) δ (ppm): 8.52 (s, 1H); 7.65 (d, J = 6.80 Hz, 2H); 7.45 (d, J = 1.60 Hz, 2H); 7.40 (d, J = 1.60 Hz, 2H); 6.8 (d, J = 7.20 Hz, 2H); 4.69 (s, 2H); 4.27 (t, J = 4.0 Hz, 4H); 3.90 (t, J = 3.20 Hz, 4H) 3.86 (s, 6H); 3.13 (s, 3H); 2.31–2.23 (m, 2H); 2.16–2.09 (m, 1H); 2.04–1.96 (m, 1H).
13C NMR (100.63 MHz, D2O) δ (ppm): 182.18; 179.02; 168.94; 162.72; 161.94; 153.32; 151.48; 149.16; 148.04; 136.03; 128.74; 123.48; 122.31; 122.02; 120.43; 111.63; 59.69; 55.82; 54.71; 51.46; 38.83; 35.62; 34.31; 28.67.
IR (ATR) νmax (cm−1): 3312, 3211, 1582, 1556, 1511, 1449, 1380, 1204, 1162, 1067, 826.
Elemental analysis calcd for C52H94N12O11·8.5H2O (%): C 44.70; H 6.92; N 19.55; found: C 44.81; H 7.07; N 19.59.
[C10MIM]2[MTX]. Following the general procedure, [C10MIM][OH] (133.4 mg; 0.44 mmol) was added to a methotrexate solution. The final product was obtained as a yellow wax (172 mg; 88%).
1H NMR (400.13 MHz, D2O) δ (ppm): 8.49 (s, 1H); 7.68 (d, J = 6.80 Hz, 2H); 7.39 (d, J = 1.60 Hz, 2H); 7.32 (d, J = 1.60 Hz, 2H); 6.73 (d, J = 6.80 Hz, 2H); 4.68 (s, 2H); 4.30 (q, J1 = 6.80 Hz, J2 = 4.0 Hz, 1H); 4.02 (t, J = 6.0 Hz, 4H); 3.84 (s, 6H); 3.10 (s, 3H); 2.28–2.17 (m, 2H); 2.14–2.07 (m, 1H); 2.03–1.95 (m, 1H); 1.64 (s br, 4H) 1.18–1.04 (m, 30H); 0.78 (t, J = 5.6 Hz, 6H).
13C NMR (100.63 MHz, D2O) δ (ppm): 181.67; 178.43; 167.54; 162.79; 162.24; 153.95; 151.10; 149.01; 147.31; 128.78; 123.61; 122.03; 121.89; 121.31; 111.50; 55.61; 49.32; 38.81; 35.71; 34.27; 31.67; 29.43; 29.26; 29.12; 29.05; 28.65; 25.76; 22.42; 13.77.
IR (ATR) νmax (cm−1): 3325, 3153, 2924, 2850, 1628, 1556, 1504, 1445, 1377, 1204, 1168, 1094, 826, 761.
Elemental analysis calcd for C48H74N12O5·6.5H2O(%): C 56.73; H 8.63; N 16.54; found: C 56.89; H 8.64; N 17.03.
[C10-3-Pic]2[MTX]. Following the general procedure, [C10-3-Pic][OH] (13 8 mg; 0.44 mmol) was added to a methotrexate solution. The final product was obtained as a yellow wax (183 mg; 90%).
1H NMR (400.13 MHz, D2O) δ (ppm): 8.57 (s, 2H); 8.53 (d, J = 4.80 Hz, 2H); 8.46 (s, 1H); 8.27 (d, J = 6.40 Hz, 2H); 7.86 (t, J = 5.20 Hz, 2H); 7.65 (d, J = 6.80 Hz, 2H); 4.65 (s, 2H); 4.42 (t. J = 6.0 Hz, 4H); 4.31 (q, J1 = 6.40 Hz, J2 = 4H.0z, 1H); 3.07 (s, 3H); 2.45 (s, 6H); 2.28–2.17 (m, 2H); 2.14–2.07 (m, 1H); 2.03–1.96 (m, 1H); 1.80–1.74 (m, 4H); 1.11–0.97 (m, 30H); 0.70 (t, J = 6.0 Hz, 6H).
13C NMR (100.63 MHz, DMSO-d6) δ (ppm): 181.67; 178.43; 167.54; 162.79; 162.24; 153.95; 151.00; 149.01; 147.31; 128.78; 123.61; 122.03; 121.89; 121.31; 111.50; 55.61; 49.32; 38.81; 35.71; 34.27; 31.67; 29.43; 29,26; 29.12; 29.05; 28.65; 25.76; 22.42; 13.77.
IR (ATR) νmax (cm−1): 3332, 3198, 2924, 2853, 1625, 1585, 1556, 1504, 1445, 1380, 1204, 1094, 826.
Elemental analysis calcd for C52H76N10O5·6H2O (%): C 60.68; H 8.62; N 13.61; found: C 60.40; H 8.51; N 13.29.
[C2OH-3-Pic]2[MTX]. Following the general procedure, [C2OH-3-Pic][OH] (96 mg; 0.44 mmol) was added to a methotrexate solution. The final product was obtained as a yellow wax (156 mg; 98%).
1H NMR (400.13 MHz, D2O) δ (ppm): 8.57 (s, 2H); 8.54 (d, J = 6.0 Hz, 2H); 8.45 (s, 1H); 8.23 (d, J = 8.0 Hz, 2H); 7.82 (t, J = 7.20 Hz, 2H); 7.58 (d, J = 6.80 Hz, 2H); 6.68 (d, J = 9.20 Hz, 2H); 4.60–4.57 (m, 6H); 4.27 (q, J1 = 8.40 Hz, J2 = 4.80 Hz, 1H); 4.01 (t, J = 5.20 Hz, 4H); 3,08 (s, 3H); 2.44 (s, 6H); 2.30–2.19 (m, 2H); 2.16–2.07 (m, 1H); 2.04–1.94 (m, 1H).
13C NMR (100.63 MHz, D2O) δ (ppm): 182.11; 178.94; 168.79; 162.61; 161.85; 153.23; 151.40; 149.21; 148.01; 146.17; 143.88; 141.57; 139.73; 128.73; 127.18; 121.91; 120.35; 111.62; 63.24; 60.28; 55.77; 54.59; 38.94; 34.27; 28.66; 17.56.
IR (ATR) νmax (cm−1): 3325, 3208, 1585, 1553, 1504, 1445, 1377, 1204, 1074, 761.
Elemental analysis calcd for C36H44N10O7·7H2O (%): C 50.58; H 6.84; N 16.38; found: C 50.93; H 6.82; N 15.85.
[C2MIM]2[MTX]. Following the general procedure, [C2MIM][OH] (84 mg; 0.44 mmol) was added to a methotrexate solution. The final product was obtained as a yellow wax (137 mg; 93%).
1H NMR (400.13 MHz, D2O) δ (ppm): 8.63 (s, 2H); 8.53 (s, 1H); 7.65 (d, J = 8.80 Hz, 2H); 7.37 (d, J = 22.40 Hz, 4H); 6.8 (d, J = 8.80 Hz, 2H); 4.69 (s, 2H); 4.28 (q, J1 = 8.80 Hz, J2 = 4.40 Hz, 1H); 4.15 (q, J1 = 15.20, J2 = 7.20 Hz, 4H); 3.83 (s, 6H); 3.13 (s, 3H); 2.33–2.21 (m, 2H); 2.18–2.08 (m, 1H); 2.04–1.94 (m, 1H); 1.44 (t, J = 7.20 Hz, 6H).
13C NMR (100.63 MHz, D2O) δ (ppm): 182.16; 178.99; 168.88; 162.72; 161.58; 152.81; 151.46; 149.18; 148.28; 135.38; 128.71; 123.33; 121.99; 121.72; 120.41; 111.61; 55.78; 54.68; 44.68; 44.66; 38.84; 35.50; 35.47; 34.26; 28.64; 14.35.
IR (ATR) νmax (cm−1): 3325, 1585, 1556, 1507, 1445, 1383, 1208, 1168, 1097, 826.
Elemental analysis calcd for C32H42N12O5·6.5H2O (%): C 48.54; H 7.0; N 21.23; found: C 48.48; H 6.73; N 20.75.
[C6MIM]2[MTX]. Following the general procedure, [C6MIM][OH] (109 mg; 0.44 mmol) was added to a methotrexate solution. The final product was obtained as a yellow wax (182 mg; 87%).
1H NMR (400.13 MHz, D2O) δ (ppm): 8.63 (s, 1H); 7.73 (d, J = 8.80 Hz, 2H); 7.42 (d, J = 8.0 Hz, 4H); 6.92 (d, J = 8.80 Hz, 2H); 4.31 (q, J1 = 8.40 Hz, J2 = 4.40 Hz, 1H); 4.14 (t, J = 6.80 Hz, 4H); 3.88 (s, 6H); 3.201 (s, 3H); 2.34–2.24 (m, 2H); 2.19–2.11 (m, 1H); 2.06–1.97 (m, 1H); 1.85–1.78 (m, 4H); 1.28–1.21 (m, 12H); 0.82 (t, J = 6.40 Hz, 6H).
13C NMR (100.63 MHz, D2O) δ (ppm): 182.24; 179.06; 169.23; 163.13; 162.18; 153.57; 151.79; 149.43; 148.34; 128.85; 123.37; 122.34; 122.05; 120.71; 111.99; 55.80; 54.94; 49.44; 38.89; 35.52; 34.28; 30.25; 29.04; 28.60; 24.91; 21.70; 13.14.
IR (ATR) νmax (cm−1): 3322, 3153, 2924, 1625, 1585, 1553, 1445, 1380, 1208, 1168, 1097, 830, 764.
Elemental analysis calcd for C40H58N12O5·9H2O (%): C 50.62; H 8.07; N 17.71; found: C 50.81; H 7.24; N 16.68.
[C8MIM]2[MTX]. Following the general procedure, [C8MIM][OH] (101 mg; 0.44 mmol) was added to a methotrexate solution. The final product was obtained as a yellow wax (196 mg; 91%).
1H NMR (400.13 MHz, D2O) δ (ppm): 8.62 (s, 1H); 7.71 (d, J = 8.40 Hz, 2H); 7.41 (d, J = 10.40 Hz, 4H); 6.91 (d, J = 8.400 Hz, 2H); 4.29 (q, J1 = 9.20 Hz, J2 = 4.0 Hz, 1H); 4.13 (t, J = 7.20 Hz, 4H); 3.86 (s, 6H); 3.20 (s, 3H); 2.29–2.22 (m, 2H); 2.18–2.08 (m, 1H); 2.03–1.94 (m, 1H); 1.83–1.75 (m, 4H); 1.27–1.13 (m, 22H); 0.82 (t, J = 6.40 Hz, 6H).
13C NMR (100.63 MHz, D2O) δ (ppm): 182.12; 178.94; 168.94; 163.01; 162.14; 153.53; 151.60; 149.39; 148.27; 128.90; 123.39; 122.26; 122.04; 120.58; 111.90; 55.71; 54.85; 49.43; 39.02; 35.54; 34.25; 30.92; 29.05; 28.72; 28.14; 27.93; 25.21; 21.93; 13.34.
IR (ATR) νmax (cm−1): 3315, 3149, 2924, 2856, 1631, 1553, 1507, 1445, 1377, 1208, 1168, 1097, 826.
Elemental analysis calcd for C44H66N12O5·7.5H2O (%): C 54.03; H 8.35; N 17.18; found: C 54.14; H 7.44; N 16.35.
2.3 MTX-salt characterization
To perform NMR, 5 mm borosilicate tubes were used, and the sample concentration was approximately 7 mg mL−1 for 1H NMR and 30 mg mL−1 for 13C NMR. Chemical shifts are reported in parts per million (ppm).
2.4 Biological studies
| Cell line | Gene | Driver mutation |
|---|---|---|
| A549 | KRAS | p.G12S |
| H1975 | EGFR | p.T790M, p.L858R |
3. Results and discussion
3.1 Synthesis and characterization of MTX-salts
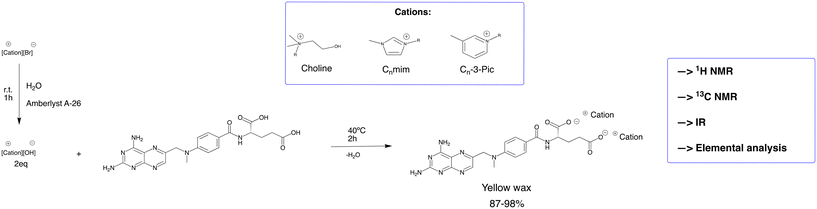
MTX salts were synthesized, in the first step, by an exchange reaction from bromide or chloride to hydroxide using the resin Amberlyst A-26 in an aqueous solution. Then, MTX (1.0 molar equivalent) was dissolved in water at 40 °C, and the desired counter ion (2.0 molar equivalents) was added. After 2 h without the presence of light, the solvent was removed under reduced pressure to obtain the desired product. The product was dried under vacuum for 24 h. MTX possesses two acidic protons (pKa = 2.9 and 4.6)29 in the carboxylic acids that can be deprotonated under suitable conditions. When the counter ions were treated with Amberlyst A-26, the pH of the solution was monitored to confirm adequate basicity to deprotonate the corresponding two acidic protons. Additionally, MTX salts were then dissolved in cellular medium DMEM (pH 7–7.6), which indicates that MTX seems to be deprotonated in DMEM solution. Fig. 2 illustrates the synthetic methodology for the preparation of different MTX salts.Choline, imidazolium, and picolinium derivatives were selected as cations for combination with the MTX di-anion, based on our previous knowledge of their biocompatibility and their effectiveness in modulating APIs properties like solubility or cytotoxicity.17,18,23 The similarities between counter ions and the different alkyl chain lengths are important parameters for understanding structure–activity correlation.
MTX salts were characterized by 1H NMR, 13C NMR, and elemental analysis. 1H NMR allowed us to elucidate the cation–anion ratio (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between counter ions and MTX) as well as the chemical stability of the desired product. In Fig. 3, the red boxes show the aromatic protons of MTX while the green boxes illustrate the methyl groups of choline (which by proton integration is proof of the 2
1 between counter ions and MTX) as well as the chemical stability of the desired product. In Fig. 3, the red boxes show the aromatic protons of MTX while the green boxes illustrate the methyl groups of choline (which by proton integration is proof of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cation–anion ratio, as expected).
1 cation–anion ratio, as expected).
3.2 Physicochemical properties
To study the solubility and stability of the MTX salts, two different assays were performed. To measure the solubility, 1 μL of water was added dropwise to 1 mg of MTX salt and the solubility was visually determined. Fig. 4 shows the results for the solubility of MTX salts in water. [Choline]2[MTX], as expected, increased 10-fold compared to original [Na]2[MTX]. The remaining MTX-salts also showed some improvement in solubility (between 1.3- and 5-fold) compared to original MTX and [Na]2[MTX].As biological assays were performed for a 48 h period of incubation in the presence of MTX and MTX salts, a UV-vis spectroscopy assay was performed to confirm that, despite its reduced solubility in aqueous-based solutions, MTX stability was retained in DMEM biological medium during the biological assays.
3.3 Biological assays
| MTX-salts | IC50 (μM) A549 | IC50 (μM) H1975 | IC50 (μM) fibroblasts | SI (A549) | SI (H1975) |
|---|---|---|---|---|---|
| MTX | >50 | n.p. | n.p. | — | — |
| [Na]2[MTX] | 3.82 ± 0.58 | >50 | >50 | — | — |
| [Choline]2[MTX] | >50 | n.p. | n.p. | — | — |
| [N10,1,1,2OH]2[MTX] | 19.15 ± 1.28 | n.p. | n.p. | — | — |
| [N12,1,1,2OH]2[MTX] | 8.55 ± 0.93 | n.p. | n.p. | — | — |
| [C2MIM]2[MTX] | >50 | n.p. | n.p. | — | — |
| [C2OHmim]2[MTX] | >50 | n.p. | n.p. | — | — |
| [C6mim]2[MTX] | >50 | n.p. | n.p. | — | — |
| [C8mim]2[MTX] | >50 | n.p. | n.p. | — | — |
| [C10mim]2[MTX] | 1.71 ± 0.23 | 14.90 ± 1.17 | 7.61 ± 0.88 | 4.5 | 0.5 |
| [C12mim]2[MTX] | 0.55 ± 0.25 | 2.72 ± 0.43 | 1.59 m ± 0.20 | 2.9 | 0.6 |
| [C2OH-3-picoline]2[MTX] | >50 | n.p. | n.p. | — | — |
| [C10-3-picoline]2[MTX] | 0.94 ± 0.03 | 29.12 ± 1.46 | 10.62 ± 1.03 | 11.3 | 0.4 |
| [MIMC12MIM][MTX] | 12.38 ± 1.09 | n.p. | n.p. | — | — |
| [Choline][Cl] | >50 | n.p. | n.p. | — | — |
| [N10,1,1,2OH][Br] | n.p. | n.p. | n.p. | — | — |
| [N12,1,1,2OH][Br] | n.p. | n.p. | n.p. | — | — |
| [C2MIM][Br] | >50 | n.p. | n.p. | — | — |
| [C2OHmim][Br] | >50 | n.p. | n.p. | — | — |
| [C6mim][Br] | n.p. | n.p. | n.p. | — | — |
| [C8mim][Br] | n.p. | n.p. | n.p. | — | — |
| [C10mim][Br] | 1.1 ± 0.02 | 19.25 ± 1.28 | >50 | 45.5 | 2.6 |
| [C12mim][Br] | 0.60 ± 0.21 | 10.22 ± 1.01 | 8.30 ± 0.92 | 13.83 | 0.8 |
| [C2OH 3-picoline][Br] | >50 | n.p. | n.p. | — | — |
| [C10-3-picoline][Br] | 1.03 ± 0.01 | 29.30 ± 1.47 | >50 | 48.5 | 1.7 |
| [MIMC12MIM][Br] | n.p. | n.p. | n.p. | — | — |
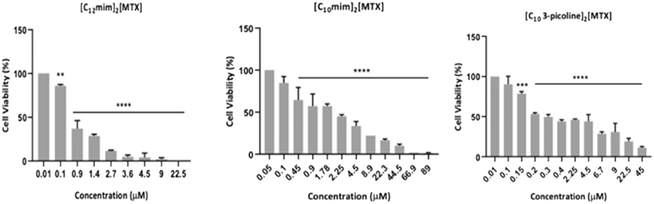
The following eight MTX salts, [Na]2[MTX], [choline]2[MTX], [C2MIM]2[MTX], [C2OHmim]2[MTX], [C10mim]2[MTX], [C12mim]2[MTX], [C2OH-3-picoline]2[MTX], [C10-3-picoline]2[MTX], were synthesized and characterized, and their antiproliferative profiles using the A549 cell line were evaluated. (see Table 2 and Fig. S27 to S33†). In general, [C12mim]2[MTX], [C10mim]2[MTX], and [C10-3-picoline]2[MTX] showed the highest antiproliferative activity in A549 (Table 2 and Fig. 5). The order of cytotoxicity is [C12mim]2[MTX] (IC50 = 0.55 ± 0.25) > [C10-3-picoline]2[MTX] (IC50 = 0.94 ± 0.03) > [C10mim]2[MTX] (IC50 = 1.71 ± 0.23).
Then, these selected MTX salts were tested in H1975 (Table 2 and Fig. S34 to S39†). As can be observed in Table 2, the cytotoxic effects of [C12mim]2[MTX], [C10mim]2[MTX], and [C10-3-picoline]2[MTX] were higher in A549 than those in H1975. This is an interesting result, as mutated EGFR seems to confer resistance to these MTX-salts, while KRAS codon 12 mutation seems to increase sensitivity. Indeed, RAS alterations are the most common activating lesions in human cancers, and KRAS is the most common oncogene-driven form of NSCLC, accounting for more than one-quarter of patients.30
Moreover, [C12mim]2[MTX], [C10mim]2[MTX] and [C10-3-picoline]2[MTX] showed a lower IC50 in A549 than [Na]2[MTX] (3.82 ± 0.58), which means that they are more cytotoxic compared to sodium MTX as a reference. Additionally, in most cases the bromide salts exhibited higher IC50 values than the respective MTX-counter ion salts in both cancer cell lines:
[C12mim][Br] (IC50 = 0.60 ± 0.21); [C10-3-picoline][Br] (IC50 = 1.03 ± 0.01); [C10mim][Br] (IC50 = 1.1 ± 0.02) in A549 and [C12mim][Br] (IC50 = 10.22 ± 1.01); [C10-3-picoline][Br] (IC50 = 29.30 ± 1.47); [C10mim][Br] (IC50 = 19.25 ± 1.28) in H1975.
These results showed that the synergetic effect between MTX and counter ions improves the solubility profile as well as increases its antiproliferative activity of the MTX salts.
Interestingly, these three MTX salts showed higher IC50 in fibroblasts, indicating their specificity to A549 NSCLC cells (Table 2). In particular, [C10-3-picoline]2[MTX] showed an IC50 in the cancer cell line 4.06-fold higher than that in [Na]2[MTX] and, in addition, it possesses a selectivity index (SI) of 11.3 (IC50 fibroblast/IC50 A549) (Table 2). The SI measures the specific cytotoxicity of a compound in a cancer cell line compared to that in normal cells (fibroblasts). The higher SI value means higher specificity of compounds to cancer cells.
Once again, a higher SI value was observed for A549 compared to that of H1975 NSCLC cells (Table 2), which indicating a higher therapeutic index for A549 cells in the case of these MTX salts. [C10-3-picoline]2[MTX] possesses a higher SI and it was considered the most promising for therapeutic applications.
Concerning the structure–activity relationship, the results showed that longer alkyl chains improve the antiproliferative effect when linked to an imidazole or picoline ring. Conversely, longer alkyl chains linked to choline do not change the original antiproliferative activity. Other counter ions such as [C6mim], [C8mim], [mimC12mim], or [C2OH-3-picoline] showed no significant improvement in the antiproliferative effect in A549 (Table 2). This observation can be attributed to the presence of shorter alkyl chain moieties.
Compared to other MTX salts reported in the literature, our MTX salts show higher antiproliferative activity.15 For instance, [TBP][MTX], [ProEt][MTX] and [AspEt][MTX]15 show an IC50 of around 50 μM in B16F10 cells, which indicates that the counter ions used in this work and the approach with two equivalents are more suitable. Nevertheless, [ProEt][MTX] demonstrated protection from chemical and enzymatic degradation, which improved the oral bioavailability.15 The authors demonstrated that [ProEt][MTX] has reduced side effects and better in vivo antitumor activity compared to that of [Na][MTX],15 using biodistribution studies.15
For a more complete characterization of the antiproliferative effect for the three most promising MTX-salts ([C12mim]2[MTX], [C10mim]2[MTX], and [C10-3-picoline]2[MTX]), cell death by necrosis and apoptosis were evaluated (Fig. 6).
| Compound | Live cells | Early apoptosis | Late apoptosis | Necrosis |
|---|---|---|---|---|
| DMSO | 90.2 ± 4.1 | 9.0 ± 4.2 | 0.6 ± 1.0 | 0.06 ± 0.07 |
| Untreated cells | 89.5 ± 3.8 | 9.3 ± 4.6 | 1.0 ± 0.5 | 0.06 ± 0.07 |
| DOX | 1.7 ± 1.8 | 9.3 ± 7.1 | 65.25 ± 30.4 | 23.7 ± 26.0 |
| [C12mim]2 [MTX] | 72.9 ± 4.1 | 16.7 ± 7.3 | 10.2 ± 8.3 | 0.1 ± 0.2 |
| [C10mim]2 [MTX] | 73.2 ± 5.0 | 14.9 ± 5.0 | 10.7 ± 8.1 | 1.1 ± 1.8 |
| [C10-3-picoline]2 [MTX] | 75.3 ± 3.0 | 14.9 ± 6.5 | 9.4 ± 5.6 | 0.02 ± 0.02 |
Moreover, almost no necrosis is observed in the presence of MTX-salts, contrary to the positive control (doxorubicin) (Table 3 and Fig. 6). This is a positive result because necrotic cells release content that includes molecules acting as signals to promote inflammation, which should be avoided in a cancer context.32
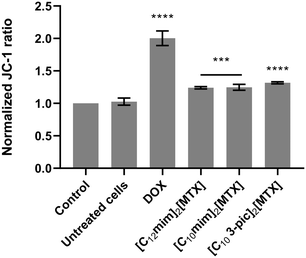
To elucidate the mechanisms underlying apoptotic cell death, the mitochondrial membrane potential (Fig. 7) was also evaluated.
4. Conclusions
Here, twelve MTX salts were prepared by sustainable acid–base neutralization reactions in high yields (87–98%) and then characterized by NMR and elemental analysis to elucidate their desired chemical structure. It is important to emphasize the large improvement in the water solubility profile of the novel MTX salts compared to initial MTX. Among the twelve MTX salts, three of them, [C12mim]2[MTX], [C10mim]2[MTX] and [C10-3-picoline]2[MTX], showed an antiproliferative effect in the KRAS-mutated A549 NSCLC cell line compared to the EGFR-mutated NSCLC cell line H1975. The order of cytotoxicity is [C12mim]2[MTX] (IC50 = 0.55 ± 0.25 μM) > [C10-3-picoline]2[MTX] (IC50 = 0.94 ± 0.03 μM) > [C10mim]2[MTX] (IC50 = 1.71 ± 0.23 μM). Moreover, the antiproliferative potential was higher than that of original MTX and sodium MTX salt. Interestingly, due to the structure–activity relationship, it is possible to conclude that longer alkyl chains (C10 and C12) conferred an antiproliferative effect but only when linked to an imidazole or picoline ring.The three MTX salts also showed a higher SI towards A549 NSCLC cells (lower IC50) compared to that in normal fibroblasts. In general, [C10-3-picoline]2[MTX] (IC50 = 0.940 μM) is the most promising candidate compared to IC50 in fibroblast (10.62 μM) with a SI of 11.3. These three MTX salts are able to trigger intrinsic apoptosis (via mitochondria) without increasing ROS levels.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
DV and SC contribute with experimental, validation and writing initial draft. PVB, ARF and LCB were involved in the supervision, validation, funding and revising final article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funding by national funds from FCT – Fundação para a Ciência e a Tecnologia, I. P., in the scope of the projects UID/QUI/50006/2019 (LAQV-REQUIMTE) and UIDB/04378/2020 (UCIBIO) and the project LA/P/0140/2020 of the Associate Laboratory Institute for Health and Bioeconomy – i4HB. D. Silva and S. Cordeiro are also thankful for doctoral grants (2020.09889.BD and 2021.08629.BD, respectively). We acknowledge Dra Catarina Rodrigues for technical support in MTS assays.Notes and references
- I. Population, M. Population and P. Sum, WHO Chron., 1969, 23, 323–326 Search PubMed.
- L. A. Byers and C. M. Rudin, Cancer, 2015, 121, 664–672 Search PubMed.
- H. Akamatsu, K. Ninomiya, H. Kenmotsu, M. Morise, H. Daga, Y. Goto, T. Kozuki, S. Miura, T. Sasaki, A. Tamiya, S. Teraoka, Y. Tsubata, H. Yoshioka, Y. Hattori, C. K. Imamura, Y. Katsuya, R. Matsui, Y. Minegishi, H. Mizugaki, K. Nosaki, Y. Okuma, S. Sakamoto, T. Sone, K. Tanaka, S. Umemura, T. Yamanaka, S. Amano, K. Hasegawa, S. Morita, K. Nakajima, M. Maemondo, T. Seto and N. Yamamoto, The Japanese Lung Cancer Society Guideline for non-small cell lung cancer, stage IV, Springer Singapore, 2019, vol. 24 Search PubMed.
- W. Y. Huang, P. M. Yang, Y. F. Chang, V. E. Marquez and C. C. Chen, Biochem. Pharmacol., 2011, 81, 510–517 CrossRef CAS PubMed.
- G. Subaiea, S. M. D. Rizvi, H. K. S. Yadav, T. Al Hagbani, M. H. Abdallah, E. S. Khafagy, H. V. Gangadharappa, T. Hussain and A. S. Abu Lila, Pharmaceuticals, 2023, 16, 230 CrossRef CAS PubMed.
- B. N. Cronstein, Pharmacol. Rev., 2005, 57, 163–172 CrossRef CAS PubMed.
- R. Auerbach, J. Am. Acad. Dermatol., 1988, 19, 145–156 CrossRef PubMed.
- D. J. O'shannessy, G. Yu, R. Smale, Y.-S. Fu, S. Singhal, R. P. Thiel, E. B. Somers and A. Vachani, Folate Receptor Alpha Expression in Lung Cancer: Diagnostic and Prognostic Significance, 2012, vol. 3 Search PubMed.
- A. Remesh, Int. J. Basic Clin. Pharmacol., 2012, 1, 2 CrossRef.
- A. Patiño-García, M. Zalacaín, L. Marrodán, M. San-Julián and L. Sierrasesúmaga, J. Pediatr., 2009, 154, 688–693 CrossRef PubMed.
- S. S. Abolmaali, A. M. Tamaddon and R. Dinarvand, Cancer Chemother. Pharmacol., 2013, 71, 1115–1130 CrossRef CAS PubMed.
- H. Abdelrady, R. M. Hathout, R. Osman, I. Saleem and N. D. Mortada, Eur. J. Pharm. Sci., 2019, 133, 115–126 CrossRef CAS PubMed.
- W. Yang, Y. Xia, Y. Fang, F. Meng, J. Zhang, R. Cheng, C. Deng and Z. Zhong, Adv. Healthcare Mater., 2018, 7, 1–8 CAS.
- R. Ferraz, L. C. Branco, C. Prudêncio, J. P. Noronha and Ž. Petrovski, ChemMedChem, 2011, 6, 975–985 CrossRef CAS PubMed.
- R. M. Moshikur, M. K. Ali, R. Wakabayashi, M. Moniruzzaman and M. Goto, Int. J. Pharm., 2021, 608, 121129 CrossRef CAS PubMed.
- R. M. Moshikur, M. R. Chowdhury, R. Wakabayashi, Y. Tahara, M. Moniruzzaman and M. Goto, J. Mol. Liq., 2019, 278, 226–233 CrossRef CAS.
- L. Filipe, T. de Sousa, D. Silva, M. M. Santos, M. Ribeiro Carrott, P. Poeta, L. C. Branco and S. Gago, Pharmaceutics, 2023, 15, 1–17 Search PubMed.
- D. Silva, M. V. C. Lopes, Ž. Petrovski, M. M. Santos, J. P. Santos, S. F. Yamada-Ogatta, M. L. F. Bispo, M. V. N. de Souza, A. R. C. Duarte, M. C. S. Lourenço, R. S. B. Gonçalves and L. C. Branco, Molecules, 2022, 27, 5167 CrossRef CAS PubMed.
- A. Barreira, A. F. M. Santos, M. Dionísio, A. R. Jesus, A. R. C. Duarte, Ž. Petrovski, A. B. Paninho, M. G. Ventura and L. C. Branco, Int. J. Mol. Sci., 2023, 24, 8822 CrossRef CAS PubMed.
- R. Ferraz, N. Santarém, A. F. M. Santos, M. L. Jacinto, A. Cordeiro-da-Silva, C. Prudêncio, J. P. Noronha, L. C. Branco and Ž. Petrovski, Antibiotics, 2022, 11, 1841 CrossRef CAS PubMed.
- A. R. Dias, R. Ferraz, J. Costa-Rodrigues, A. F. M. Santos, M. L. Jacinto, C. Prudêncio, J. P. Noronha, L. C. Branco and Ž. Petrovski, Future Pharmacol., 2022, 2, 320–329 CrossRef.
- F. Faísca, V. Correia, Ž. Petrovski, L. C. Branco, H. Rebelo-De-andrade and M. M. Santos, Pharmaceutics, 2022, 14, 877 CrossRef PubMed.
- R. Ferraz, D. Silva, A. R. Dias, V. Dias, M. M. Santos, L. Pinheiro, C. Prudêncio, J. P. Noronha, Ž. Petrovski and L. C. Branco, Pharmaceutics, 2020, 12, 221 CrossRef CAS PubMed.
- D. O. Hartmann, K. Shimizu, F. Siopa, M. C. Leitão, C. A. M. Afonso, J. N. C. Lopes and C. Silva Pereira, Green Chem., 2015, 17, 4587–4598 RSC.
- M. E. Mavis, C. Yolacan and F. Aydogan, Tetrahedron Lett., 2010, 51, 4509–4511 CrossRef CAS.
- O. A. Lenis-Rojas, R. Cabral, B. Carvalho, S. Friães, C. Roma-Rodrigues, J. A. A. Fernández, S. F. Vila, L. Sanchez, C. S. B. Gomes, A. R. Fernandes and B. Royo, Inorg. Chem., 2021, 60, 8011–8026 CrossRef CAS PubMed.
- S. J. Rice, X. Liu, H. G. Wang and C. P. Belani, PLoS One, 2019, 14, e0217657 CrossRef CAS PubMed.
- O. A. Lenis-Rojas, C. Roma-Rodrigues, B. Carvalho, P. Cabezas-Sainz, S. F. Vila, L. Sánchez, P. V. Baptista, A. R. Fernandes and B. Royo, Int. J. Mol. Sci., 2022, 23, 13594 CrossRef CAS PubMed.
- K. Mioduszewska, J. Dołżonek, D. Wyrzykowski, Ł. Kubik, P. Wiczling, C. Sikorska, M. Toński, Z. Kaczyński, P. Stepnowski and A. Białk-Bielińska, TrAC, Trends Anal. Chem., 2017, 97, 283–296 CrossRef CAS.
- R. Mendes, B. Carreira, P. V. Baptista and A. R. Fernandes, Expert Rev. Precis. Med. Drug Dev., 2016, 1, 155–168 CrossRef.
- A. M. Rieger, K. L. Nelson, J. D. Konowalchuk and D. R. Barreda, J. Visualized Exp., 2011, 50, 2597 Search PubMed.
- Z. G. Liu and D. Jiao, Cell Stress, 2020, 4, 1–8 CrossRef CAS PubMed.
- A. Perl, P. Gergely, G. Nagy, A. Koncz and K. Banki, Trends Immunol., 2004, 25, 360–367 CrossRef CAS PubMed.
- Section I Fundamentals, 2016 Search PubMed.
- C. C. Winterbourn, Nat. Chem. Biol., 2008, 4, 278–286 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4md00960f |
| This journal is © The Royal Society of Chemistry 2025 |