A reflection on ‘Controlled synthesis of conjugated random copolymers in a droplet-based microreactor’
Martin
Heeney
ab and
John C.
de Mello
c
aDivision of Physical Sciences & Engineering, King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Saudi Arabia. E-mail: martin.heeney@kaust.edu.sa
bDepartment of Chemistry, Molecular Sciences Research Hub, Imperial College London, London W12 0BZ, UK. E-mail: m.heeney@imperial.ac.uk
cDepartment of Chemistry, Norwegian University of Science and Technology (NTNU), NO-7491, Trondheim, Norway. E-mail: john.demello@ntnu.no
First published on 7th January 2025
Abstract
Flow chemistry offers an attractive route to making functional materials, providing a high level of reaction control and easy scalability to high volumes. In 2014, Bannock and co-workers reported a droplet-based flow reactor capable of controllably synthesising regioregular random copolymers of the semiconducting polymers poly(3-hexylthiophene) (P3HT) and poly(3-hexylselenophene) (P3HS) (J. H. Bannock, M. Al-Hashimi, S. H. Krishnadasan, J. J. M. Halls, M. Heeney and J. C. de Mello, Mater. Horiz., 2014, 1, 214–218, https://doi.org/10.1039/C3MH00066D). In this reflection, we discuss how the work came about, what followed from it, and the extent to which the advantages of droplet chemistry outlined in the paper have been confirmed by other researchers.
In 2014, in the second issue of Materials Horizons, we published an article entitled “Controlled synthesis of conjugated random copolymers in a droplet-based microreactor” (https://doi.org/10.1039/C3MH00066D).1 The paper built on earlier work from 20122 where we had shown that a particular kind of flow reactor, known as a droplet reactor, offered a highly controlled environment for synthesising conjugated polymers, capable of producing device-grade materials in industrially relevant quantities. At the time these papers came out, there were already a few published works describing the flow synthesis of polymers (including one relating specifically to conjugated polymers3), but these earlier papers had made use of conventional (single-phase) flow reactors, where reagents dissolved in a solvent are directly pumped through a length of heated tubing, bringing the growing polymer into contact with the reactor wall, with a high associated risk of reactor fouling.4
To prevent fouling, we had proposed the use of droplet-based flow reactors, where the reactants are loaded into small droplets of solvent that are then carried through the tubing by a flowing stream of an immiscible carrier liquid.5 The carrier liquid plays two important roles: firstly, it physically isolates the reagents from the reactor wall, preventing fouling, and secondly, it carries the reaction mixture through the tubing at a fixed speed, ensuring all reagents experience the same reaction time, something that is not true for single-phase reactors where drag at the channel wall causes a spread of velocities across the width of the channel.6
Our approach involved injecting monomer feedstock dissolved in tetrahydrofuran (THF) into a length of polytetrafluorethylene (PTFE) tubing through which a continuous stream of immiscible perfluorinated polyether was flowing. On entering the carrier liquid, the injected reaction mixture broke up into a series of near-identical sub-μL droplets that were carried downstream by the carrier liquid, each droplet acting as a self-contained microscale reaction vessel. Gentle heating of the tubing by immersing it in an oil-bath at 55 °C was sufficient to induce polymerisation inside the droplets, with the small droplet size ensuring rapid equilibration of composition and temperature, and so providing a highly uniform environment for polymer growth.
In our 2012 paper we used the Grignard metathesis (GRIM) route of McCullough et al.7 to synthesise the semiconducting polymer poly(3-hexylthiophene) (P3HT), which at the time was the work-horse material for polymer electronics. In brief, the GRIM method typically uses a nickel catalyst such as 1,3-bis[diphenyl-phosphinopropane]nickel(II) chloride (Ni(dppp)Cl2) to couple organomagnesium complexes of 3-hexylbromothiophene into P3HT via a quasi-living polymerisation reaction; see the upper path of Fig. 1A. For steric reasons, the monomers couple in a predominately head-to-tail manner, resulting in highly ordered material with regioregularities (RRs) of >97% and low dispersity (Đ) of <2. The GRIM technique is well-suited to small-scale (sub-gram) synthesis in a flask, but reaction control suffers at higher production volumes (≫1 g), leading to lower RRs and higher dispersity. By carrying out the reaction in a droplet-based flow reactor, we showed that high production rates of >60 g per day could be readily achieved without sacrificing reaction control (Đ = 1.7, RR > 98%), with easy tunability of the molecular weight via the catalyst to monomer ratio (the GRIM polymerisation is a quasi-living polymerisation where, loosely speaking, one catalyst molecule is associated with a single polymer chain, so increasing the catalyst to monomer ratio results in a higher number of shorter chains). Importantly, the droplet approach was found to enable the synthesis of high molecular weight polymers, which are challenging to synthesise in conventional single-phase flow reactors, where precipitation of product on the reactor walls can lead to blockage. Excellent sample-to-sample consistency was attained, with successive runs yielding material with very similar molecular weight distributions.
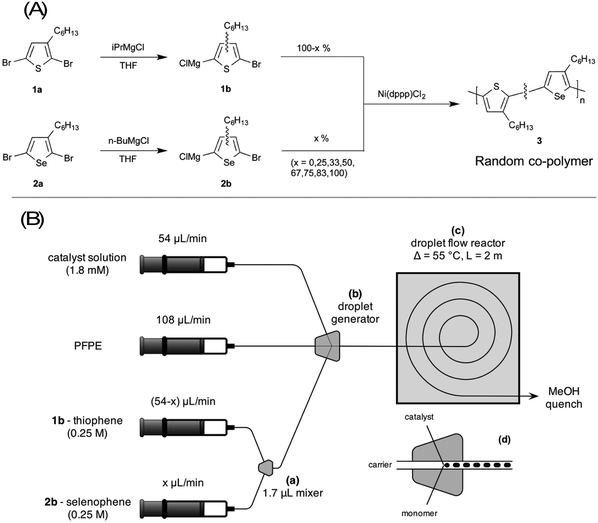 | ||
| Fig. 1 (A) Synthesis of poly(3-hexylthiophene)-co-poly(3-hexylselenophene) [P3HT-co-P3HS] (3). Synthesis of the homopolymers P3HT and P3HS proceeds in the same way, using 100% of 1b or 2b. (B) Schematic of droplet reactor, comprising: (a) a static y-shaped mixer for controlling the selenophene to thiophene ratio; (b) and (d) a three-input droplet generator into which the carrier fluid, catalyst solution and premixed monomer solution are injected; and (c) coiled PTFE tubing of inner diameter 1 mm in a temperature-stabilised oil-bath. Reproduced and adapted from ref. 1 with permission from the Royal Society of Chemistry. | ||
In our Materials Horizons paper, we extended the droplet method to the preparation of a series of statistical copolymers formed from 2,5-dibromo-3-hexylthiophene (HT) and its selenium-based analogue 2,5-dibromo-3-hexylselenophene (HS); see Fig. 1A. GRIM copolymerisation of the organomagnesium halides derived from HT and HS results in random copolymers with optical band-gaps intermediate between those of the two parent polymers,8,9 and hence provides a convenient means of tuning the optical band-gap to a given application. In the paper, we showed that carrying out the procedure in a droplet-based reactor (Fig. 1B and 2A, B) allows the copolymerisation reaction to be carried out in an unusually well-controlled manner. Equal-concentration solutions of the two monomers were loaded into separate syringes and injected into a y-shaped mixer. By controlling the relative injection rates of the two monomers while holding the total flow rate fixed, the composition of the reaction mixture could be accurately varied from 100% HT to 100% HS, providing tight control over the stoichiometry of the final copolymer. The optical band-gap varied linearly from 455 to 498 nm as the mole fraction of selenophene in the feedstock was increased from 0% to 100% (Fig. 2C and D), allowing the optical properties of the copolymers to be readily tuned to application requirements.
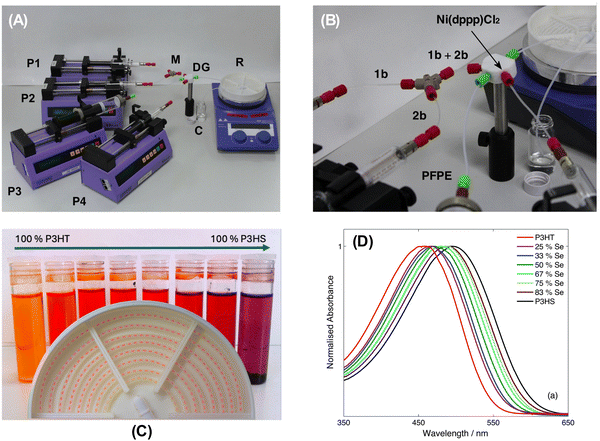 | ||
| Fig. 2 (A) A photograph of the full reactor setup and (B) a close-up photograph of the monomer-mixing and droplet-generation stages. A two-way y-shaped mixer (M) is used to merge the two monomer streams 1b and 2b, and a three-way droplet generator (DG) is used to form droplets of the pre-mixed monomers and the Ni(dppp)Cl2 catalyst solution in the PFPE carrier fluid. The polymerization takes place in the heated reaction zone R. (C, foreground) Photograph, showing part of the coiled flow reactor. Unreacted monomer enters the heated oil-bath close to the centre, and polymerisation takes place as the droplets spiral outwards through the coiled tubing, causing the color to shift from pale orange to deep red. (C, background) Vials of copolymer produced by the flow reactor, varying from 100% P3HT on the left to 100% P3HS on the right. The colour shift from orange (P3HT) to red (P3HS) corresponds to a progressive reduction in the optical band-gap. (D) Solution-phase absorption spectra in chlorobenzene for the series of droplet-synthesised P3HT-co-P3HS random copolymers. The legend denotes the mole fraction of selenophene in the initial reaction mixture. Reproduced and adapted from ref. 1 with permission from the Royal Society of Chemistry. | ||
While the Materials Horizons paper on P3HT-co-P3HS was in some respects a straightforward extension of our earlier paper on P3HT, employing the same Grignard approach of McCullough and co-workers, we improved on our original procedure in one important respect. We had previously found that direct dissolution of the Ni(dppp)Cl2 catalyst in THF caused ligand detachment, resulting in a bleaching of its red coloration and a severe loss of catalytic activity. This had led us to employ an unusual work-around in our first paper, where we dispersed the catalyst inside the carrier fluid, with the catalyst entering the reaction mixture diffusively via the droplet surface – an approach that worked but made it difficult to accurately control the catalyst-to-monomer ratio and hence difficult to control the molecular weight of the polymer. In our Materials Horizons paper, we described a simple but effective solution to the problem of catalyst de-activation in THF: co-dissolving excess dppp ligand alongside the Ni(dppp)Cl2 shifted the reaction equilibrium between ligated and un-ligated catalyst to the left, thus stabilising the catalyst in its active form. Using this simple strategy allowed us to dissolve all reagents within the same solvent (THF), which in turn allowed the molecular weight to be finely controlled by varying the relative flow rate of catalyst solution to monomer solution.
We made use of the same approach in a follow-up paper in 2016,10 where we replaced Ni(dppp)Cl2 with Ni(dppp)Br2 – a more soluble form of the catalyst that we formed in situ by combining nickel(II) bromide ethylene glycol dimethyl ether complex (Ni(dme)Br2) with dppp, again using a substantial excess of dppp to stabilise the catalyst in its active form. Switching to the bromide form of the catalyst permitted an approximate doubling of the catalyst concentration (and hence an increase in the catalyst to monomer ratio), which in turn made it possible to access weight-averaged molecular weights below 50 kg mol−1. Low-Mw polymers are especially important for printed electronics, where it is important to deposit semiconducting polymers from greener, non-chlorinated solvents that struggle to solvate higher weight material.11
By using Ni(dppp)Br2 in combination with the higher boiling-point, bio-derived THF-substitute 2-MeTHF and by raising the reaction temperature to 65 °C, an approximate four-fold increase in reaction rate was achieved compared to a standard THF-based synthesis at 55 °C, with full conversion achieved in just one minute. The purified, flow-synthesized polymer had an Mw of 46 kg mol−1, a very low Đ of 1.4, and a regioregularity of 93% – well suited to device applications. The high production rate attainable in a relatively green bio-derived solvent made the overall procedure a viable option for high-volume industrial production.
In the decade since these papers came out, droplet synthesis has established itself as a powerful technique for materials synthesis, especially for nanocrystal synthesis where the ability of the carrier fluid to isolate the droplets from the carrier wall is crucial for preventing reactor fouling.12 However, it remains the case that the use of droplet reactors for polymer synthesis is still relatively uncommon, especially for conjugated polymer synthesis. Where it has been used, though, the results have typically been very positive. Maes and co-workers, for instance, recently reported the use of a droplet reactor to produce high-performance push–pull polymers, specifically a low band-gap [1,2,5]thiadiazolo[3,4-g]quinoxaline-based alternating copolymer with promising performance as a near-infrared photosensor.13 Their work expanded the scope of droplet-based synthesis to include Stille copolymerisation and confirmed the advantages of droplet-based synthesis described above, namely resistance to fouling, compatibility with high-viscosity polymers, consistent product properties, and easy scalability to high production volumes. These conclusions were reaffirmed in a recent paper where the same group developed a droplet-flow protocol for the high-performance photovoltaic polymer PM6,14 achieving excellent sample-to-sample reproducibility.
A recent example of droplet-flow applied to non-conjugated polymers was reported by Chen and co-workers,15 who demonstrated the polymerisation of N,N-dimethylacrylamide by photo-mediated reversible-deactivation radical polymerization (RDRP). The approach used in that paper bears some resemblance to our original method for P3HT synthesis, with the carrier liquid serving as a host for the chain transfer agent that diffusively transferred into the reagent phase to initiate chain growth. The authors confirmed the utility of droplet methods for controlling molecular weight distributions and tuning the stoichiometry of copolymers, and noted the ease with which throughput could be scaled to higher volumes.
In finishing, we should mention that an unexpected outcome of our 2014 paper was a follow-up “Focus Article”, also published in Materials Horizons.16 One of the reviewers to the 2014 paper had pointed out that traditionally trained organic chemists lacking prior experience of flow chemistry might find it difficult to replicate the flow procedures we described, and had suggested that we add some instructional information to the Supporting Information, explaining how to assemble and use the flow reactors. We duly went ahead and tried to do this, but it soon became clear that we could not provide adequate guidance within the available space – indirectly confirming the very point the reviewer had made! In responding to their comment, we therefore offered to write a “nuts and bolts”-style introductory guide to flow chemistry, where we would provide practical advice on how to get started. The offer was warmly received by the Materials Horizons editors, who suggested the tutorial could take the form of a “Focus Article” – a new type of educational article, of which only one had been published at the time.
For reasons we can’t quite recall now (but may have had a little to do with laziness), we decided at a fairly early stage in the writing process that it would not be a thorough, deeply researched review-type article, but it should instead be a light, informal user guide that would educate but not intimidate. Having made this decision, we perhaps went a little further in the direction of informality than we had originally intended, and it was with some trepidation that we eventually submitted the finished article “A gentle introduction to the noble art of flow chemistry” for review. Half-expecting an outright rejection for its unserious tone, we were pleasantly surprised by the positive reaction from the Editorial Board, who agreed to publish it largely as written (rereading the handling editor's comments some ten years later we do, however, sense a small degree of apprehension: “we would like to suggest you make some minor revisions so a few of the more obscure phrases are a little more accessible to non-native speakers. Terms like “cut the mustard” and “swanky” may not translate particularly well…”). A few parts of the article are a little dated now – the reference to “cheque books” in the abstract seems particularly quaint in 2024 – but by and large we stand by the substance of the article, and hope it has succeeded in its aim of introducing synthetic chemists to what at heart is a simple, intuitive and surprisingly fun area of chemistry.
Ours was the second Focus Article to come out and, some ten years later, there are now at least forty-six of them, covering a vast swathe of materials science. In the years since its inception, Materials Horizons has firmly established itself as one of the leading journals in materials science, marked out by the breadth and consistent quality of the papers it publishes. As its anniversary year comes to an end, we look forward to the next decade of exciting and innovative science in Materials Horizons, and wish the Editorial Board every success in continuing to drive the journal forward.
Conflicts of interest
There are no conflicts to declare.References
- J. H. Bannock, M. Al-Hashimi, S. H. Krishnadasan, J. J. Halls, M. Heeney and J. C. de Mello, Mater. Horiz., 2014, 1, 214–218 RSC.
- J. H. Bannock, S. H. Krishnadasan, A. M. Nightingale, C. P. Yau, K. Khaw, D. Burkitt, J. J. Halls, M. Heeney and J. C. de Mello, Adv. Funct. Mater., 2013, 23, 2123–2129 CrossRef.
- H. Seyler, D. J. Jones, A. B. Holmes and W. W. H. Wong, Chem. Commun., 2012, 48, 1598–1600 RSC.
- S. Welzel and U. Nieken, Chem. Ing. Tech., 2024, 96, 1632–1641 CrossRef.
- A. M. Nightingale and J. C. de Mello, Adv. Mater., 2013, 25, 1813–1821 CrossRef PubMed.
- A. E. Rodrigues, Chem. Eng. Sci., 2021, 230, 116188 CrossRef.
- R. S. Loewe, S. M. Khersonsky and R. D. McCullough, Adv. Mater., 1999, 11, 250–253 CrossRef.
- M. Heeney, W. Zhang, D. J. Crouch, M. L. Chabinyc, S. Gordeyev, R. Hamilton, S. J. Higgins, I. McCulloch, P. J. Skabara, D. Sparrowe and S. Tierney, Chem. Commun., 2007, 5061 RSC.
- A. M. Ballantyne, L. Chen, J. Nelson, D. D. C. Bradley, Y. Astuti, A. Maurano, C. G. Shuttle, J. R. Durrant, M. Heeney, W. Duffy and I. McCulloch, Adv. Mater., 2007, 19, 4544–4547 CrossRef CAS.
- J. H. Bannock, W. Xu, T. Baïssas, M. Heeney and J. C. de Mello, Eur. Polym. J., 2016, 80, 240–246 CrossRef CAS.
- M. Koppe, C. J. Brabec, S. Heiml, A. Schausberger, W. Duffy, M. Heeney and I. McCulloch, Macromolecules, 2009, 42, 4661–4666 CrossRef CAS.
- T. W. Phillips, I. G. Lignos, R. M. Maceiczyk, A. J. deMello and J. C. deMello, Lab Chip, 2014, 14, 3172 RSC.
- O. Beckers, S. Gielen, F. Verstraeten, P. Verstappen, L. Lutsen, K. Vandewal and W. Maes, ACS Appl. Polym. Mater., 2020, 2, 4373–4378 CrossRef CAS.
- S. Smeets, Q. Liu, J. Vanderspikken, T. J. Quill, S. Gielen, L. Lutsen, K. Vandewal and W. Maes, Chem. Mater., 2023, 35, 8158–8169 CrossRef CAS.
- Y. Zhou, Y. Fu and M. Chen, Chin. J. Chem., 2022, 40, 2305–2312 CrossRef CAS.
- J. H. Bannock, S. H. Krishnadasan, M. Heeney and J. C. De Mello, Mater. Horiz., 2014, 1, 373 RSC.
| This journal is © The Royal Society of Chemistry 2025 |
