 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Lipid profiling: proving the geographical origin of strawberries (Fragaria × ananassa) using a non-targeted LC-IM-MS approach†
Johannes Brockelt ,
Felix Schmauder,
Marina Creydt and
Markus Fischer
,
Felix Schmauder,
Marina Creydt and
Markus Fischer *
*
Hamburg School of Food Science - Institute of Food Chemistry, University of Hamburg, Grindelallee 117, 20146 Hamburg, Germany. E-mail: Markus.Fischer@uni-hamburg.de; Fax: +49 40 42838-4342; Tel: +49-40 42838-4359
First published on 16th May 2025
Abstract
The strawberry, a globally traded fruit, is a prime example of how geographical origin has become an important marketing factor. Significant price differences between countries of origin make mislabeling a financially attractive form of food fraud, underlining the need for origin verification. In this context, the development of methods that can unequivocally prove the authenticity, i.e., the declared origin, is an absolute necessity. In this study, a non-targeted lipidomic approach using ion mobility combined with high resolution mass spectrometry was applied to clearly distinguish German strawberries from non-German strawberries. Using linear discriminant analysis (LDA), an accuracy of 90% was achieved. Furthermore, a detailed classification of strawberries from Central Europe (German, Dutch and Polish strawberries) as well as strawberries from Mediterranean regions (Spanish, Greek and Egyptian strawberries) was carried out with a classification accuracy of 74%. To further investigate the classification results, a total of 39 lipids were identified as relevant markers for German, Dutch and Polish strawberries as well as for Spanish, Greek and Egyptian strawberries using MS/MS measurements. A particular difficulty was the fact that the influence of climatic conditions on the metabolome is similar in countries that are geographically close to each other. Overall, the results of this study help to prevent food fraud and to confirm the authenticity of strawberries in terms of their origin.
1. Introduction
The geographical origin of food has become an increasingly important factor in consumer purchasing decisions in recent years.1,2 Furthermore, with the implementation of regulation (EC No 1169/2011) on the provision of food information to consumers in 2014, the indication of geographical origin also became mandatory in certain cases.3Strawberries are a fruit that is widely traded worldwide.4 This can be illustrated particularly well by the German import and export business. In 2023, approx. 2000 farms in Germany cultivated strawberries on an area of approximately 140 km2, producing about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of strawberries. During the same harvesting period, 114
000 tons of strawberries. During the same harvesting period, 114![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 010 tons of fresh strawberries were imported to Germany.5 The most important supplier countries for the German strawberry market are Spain, Greece, the Netherlands and Egypt. Spain exported 71
010 tons of fresh strawberries were imported to Germany.5 The most important supplier countries for the German strawberry market are Spain, Greece, the Netherlands and Egypt. Spain exported 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 tons, Greece 16
700 tons, Greece 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 tons, the Netherlands 13
900 tons, the Netherlands 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons, and Egypt 2200 tons to Germany.6 The average price of German strawberries in 2023 was 3.35 € per kg. In comparison, strawberries from non-German growing regions, including Spain and Greece, ranged between 1.36 and 2.46 € per kg.7 The higher prices for German strawberries are mainly the result of higher labor and production costs, shorter supply chains and a strong emphasis on freshness in regional marketing. Furthermore, a significant proportion of products are sold under regional quality labels, which communicate premium quality to consumers. Origin verification is therefore a crucial component in protecting both consumers and producers from economically motivated fraud.8–10 The significant price differences between the various countries of origin make the re-declaration of the origin of imported strawberries a financially beneficial endeavor. Therefore, the continuous development of analytical methods to ensure the authenticity of such products, particularly with regard to their ability to determine the geographical origin of strawberries, is crucial.
000 tons, and Egypt 2200 tons to Germany.6 The average price of German strawberries in 2023 was 3.35 € per kg. In comparison, strawberries from non-German growing regions, including Spain and Greece, ranged between 1.36 and 2.46 € per kg.7 The higher prices for German strawberries are mainly the result of higher labor and production costs, shorter supply chains and a strong emphasis on freshness in regional marketing. Furthermore, a significant proportion of products are sold under regional quality labels, which communicate premium quality to consumers. Origin verification is therefore a crucial component in protecting both consumers and producers from economically motivated fraud.8–10 The significant price differences between the various countries of origin make the re-declaration of the origin of imported strawberries a financially beneficial endeavor. Therefore, the continuous development of analytical methods to ensure the authenticity of such products, particularly with regard to their ability to determine the geographical origin of strawberries, is crucial.
The potential and limitations of metabolome analysis for determining the geographical origin of strawberries have been investigated in several studies. Various analytical approaches have been explored in the literature to determine the geographical origin of strawberries. For example, Covaciu et al. (2016) investigated elemental profiles and stable isotope ratios using ICP-MS and AAS, to assess environmental influence on strawberry composition.11 GC-MS was employed by Hakala et al. (2002) to characterize volatile compounds in selected cultivars, which are known to vary with both genotype and cultivation environment.12 Josuttis et al. (2013) used LC-MS to study the tannin composition of strawberries grown at different European locations, showing how metabolic profiles can reflect geographical and environmental influences. D’Urso et al. (2016) utilised a metabolomics approach, employing LC-MS/MS in conjunction with multivariate analysis to differentiate Fragaria vesca samples from diverse Italian regions, thereby identifying region-specific phenolic compounds.13,14 Khan et al. (2010) utilised a combination of fundamental physicochemical measurements, including pH and sugar content, in conjunction with multivariate analysis to differentiate Pakistani strawberries originating from distinct regions.13 Wu et al. (2023) employed GC-MS and HPLC to profile aroma compounds and phenolic acids, respectively thereby unveiling distinctive markers for strawberries from the Changping region in China.14 Although these studies demonstrate the potential of analytical methods for origin determination, they were often limited to a small number of countries, did not account for interannual or seasonal variability, and included only a few strawberry cultivars. This highlights the need for more comprehensive studies that integrate broader sampling strategies and analytical depth. In addition, in a previously published study, our group analyzed the origin of strawberries using a non-targeted approach based on Fourier-transform near-infrared (FT-NIR) spectroscopy. The results of this study suggest that it is possible to distinguish between German and non-German strawberries, including strawberries from Egypt, Spain and Greece, with 92% accuracy. However, it should be noted that due to the low resolution of FT-NIR spectroscopy measurements, the analysis only provides a general overview of various ingredients, but no detailed information on the exact metabolites that are relevant for differentiation according to geographical origin.15 A study on the geographical origin of asparagus showed that origin markers were present in both polar and non-polar extracts. Nevertheless, more relevant markers were predominantly detected in the lipidome.16 Therefore, in the present study, a lipidomic approach was used to determine the origin of strawberries using a non-polar extraction method.
The aim of this study was to develop a non-targeted liquid chromatography approach coupled to electrospray ionization ion mobility quadrupole time-of-flight mass spectrometry (LC-ESI-IM-qTOF) to classify German versus non-German strawberries according to their geographical origin based on their lipid profile. Moreover, the exact geographical origin of strawberries from six countries – namely Germany, Poland, the Netherlands, Spain, Greece and Egypt – was to be determined. The discrepancies between the sample populations, which could be due to external factors, were determined by applying multivariate statistical techniques, with a focus on comparing the relative lipid peak intensities. Furthermore, MS/MS experiments were conducted to identify the metabolites relevant for the different geographical origins. Marker identification was supported by the use of an ion mobility (IM) cell, which enabled the determination of the identification parameter collision cross section (CCS value). CCS-values can be used to separate isomers, isobars and conformers, which is particularly advantageous in case of very complex matrices such as strawberry extracts.17 The identification of markers represents an important step towards the development of simpler targeted authentication methods in the future. One example of this is the use of LC-MS techniques with a triple quadrupole (LC-ESI-QqQ-MS/MS) instrument in routine laboratories.18
2. Materials and methods
2.1. Reagents and chemicals
Isopropanol (LC-MS grade) was purchased from Merck KGaA (Darmstadt, Germany) and chloroform (HPLC grade), methanol (LC-MS grade), acetonitrile (LC-MS grade) as well as ammonium formate (LC-MS grade) were received from Carl Roth GmbH and Co. KG (Karlsruhe, Germany). Demineralized water was ultra-purified using a Direct-Q 3 UV-R system (Merck, Millipore, Darmstadt, Germany). Purine, hexakis(1H,1H,3H-tetrafluoropropoxy)phosphazine and the ESI tuning mix, hexamethoxyphosphazine were obtained from Agilent Technologies (City of Santa Clara, California, USA).2.2. Sample acquisition
In order to distinguish between German and non-German strawberries, a total of 195 strawberry samples with a balanced distribution of samples across the countries Germany, Poland, Netherlands, Greece, Egypt and Spain were used. An overview of sampling is provided in Table 1 and detailed sample information, including geographical origin, harvest year and variety, can be found in Table S1 in the ESI.† We collected the German samples directly from the fields and stored them at 4–5 °C during transport to maintain sample freshness. 500 g of strawberries were harvested from different rows and plants to cover location variances. The non-German samples were obtained from various traders in Germany and, where possible, directly from local retailers in Greece, Egypt, Spain, Poland, and the Netherlands. The collection of samples occurred between 2022 and 2024, contingent upon factors such as availability, seasonal access, and local logistics. Consequently, certain countries, such as Egypt, were represented in a single harvest year, whereas Germany was sampled more extensively across multiple years. This resulted in an imbalanced distribution of samples per country and year, reflecting realistic sourcing conditions.| Total sample amount | 2022 | 2023 | 2024 | |
|---|---|---|---|---|
| Germany | 50 | 32 | 12 | 6 |
| Poland | 28 | — | 12 | 16 |
| Netherlands | 33 | 4 | 10 | 19 |
| Greece | 22 | 3 | 13 | 6 |
| Spain | 33 | 7 | 18 | 8 |
| Egypt | 29 | — | 28 | 1 |
2.3. Sample preparation
For each sample, 500 g of strawberries were cleaned with demineralized water. The strawberries were quartered with a ceramic knife, discarding the stalk. The quarters were shock frozen in liquid nitrogen at −196 °C and stored at −20 °C. 300 g of the frozen strawberries were homogenized with the same amount of dry ice in a knife mill (Retsch, Haan, Germany). The frozen powders were freeze-dried (Christ, Osterode, Germany) and then stored at −80 °C.For analysis of non-polar metabolites, strawberry powder was extracted using a Bligh and Dyer extraction method, modified according to Creydt et al. (2018).16,19 For this purpose, 50.0 ± 0.5 mg of strawberry powder was weighed into a 2.0 mL reaction tube (Eppendorf, Hamburg, Germany) and mixed with 0.75 mL ice-cold chloroform/methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). Two steel balls (Φ = 3.25 mm) were added to each sample suspension. The extracts were ball-milled for 1 min at 3.1 m s−1 using a Bead Ruptor 24 equipped with a 2.0 mL microtube carriage kit (Biolabproducts, Bebensee, Germany). Thereafter, 0.5 mL of water and 0.25 mL of chloroform were added and the mixtures were again milled in a ball mill for 2 min. The remaining suspensions were centrifuged for 20 min at 14
2, v/v). Two steel balls (Φ = 3.25 mm) were added to each sample suspension. The extracts were ball-milled for 1 min at 3.1 m s−1 using a Bead Ruptor 24 equipped with a 2.0 mL microtube carriage kit (Biolabproducts, Bebensee, Germany). Thereafter, 0.5 mL of water and 0.25 mL of chloroform were added and the mixtures were again milled in a ball mill for 2 min. The remaining suspensions were centrifuged for 20 min at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g and 4 °C (Eppendorf 5420R, Eppendorf, Hamburg, Germany). The supernatants were diluted 1
000 g and 4 °C (Eppendorf 5420R, Eppendorf, Hamburg, Germany). The supernatants were diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (v/v) with chloroform and centrifuged again for 5 min under the same conditions. In order to prevent any changes in the metabolites during the process of extraction, the samples and solvents were kept on ice whenever possible.
4 (v/v) with chloroform and centrifuged again for 5 min under the same conditions. In order to prevent any changes in the metabolites during the process of extraction, the samples and solvents were kept on ice whenever possible.
2.4. Data acquisition
A 6560 Ion Mobility qToF LC-MS system (Agilent Technologies, City of Santa Clara, California, USA) was used for metabolomic profiling analyses. Liquid chromatography was performed on an Agilent 1290 Infinity II UHPLC system equipped with a high-speed pump (G7120A, 1290 High Speed Pump), a multisampler (G7167B, 1290 multisampler) and a temperature-controlled column tray (G7116B, 1290 MCT). Separation of non-polar metabolites was performed using an Accucore RP-MS UPLC column (150 mm × 2.1 mm i.d., 2.6 μm) equipped with a guard column of the same material (10 mm × 2.1 mm i.d., 2.6 μm) (Thermo Fisher Scientific, Braunschweig, Germany). The autosampler was temperature controlled at 5 °C. The column oven was set at 50 °C with a flow rate of 0.3 mL min−1. The mobile phase consisted of water (A) and acetonitrile/isopropanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (B) with the addition of 0.1 mMol L−1 ammonium formate. The chromatography gradient listed in Table S2 in the ESI† was used for the LC-MS measurements of the strawberry extracts. The injection volume was adjusted to 8 μL.
1, v/v) (B) with the addition of 0.1 mMol L−1 ammonium formate. The chromatography gradient listed in Table S2 in the ESI† was used for the LC-MS measurements of the strawberry extracts. The injection volume was adjusted to 8 μL.
The MS-analysis was performed in positive ionization mode in the mass range 50 to 1700 Da with the following ionization parameters: gas temperature 225 °C, drying gas flow rate 10 L min−1, nebulizer 40 psi, sheath gas temperature 375 °C, sheath gas flow rate 12 L min−1 and, capillary voltage 3500 V. The instrument was calibrated with a tuning mixture prior to the series of measurements. In addition, a lock mass calibration was performed using a secondary nebulizer with purine and hexakis(1H,1H,3H-tetrafluoropropoxy)phosphazine during the measurements. For the measurement in IM mode, the following parameters were used for the determination of the CCS values: drift gas nitrogen; drift gas pressure 3.95 Torr; frame rate 0.9 frames per s; IM transient rate 18 IM transients/frame; maximum drift time 60 ms; TOF transient rate 600 transients/IM transients; trap fill time 3900 μs; trap release time 250 μs; multiplexed pulse sequence length 4 bits. Drift times were calibrated by infusion of Agilent Technologies ESI tuning mix and hexamethoxyphosphazine under the same conditions for 1 min on a daily basis.
The QC sample was a mixture of 10 μL of the first 48 non-polar strawberry extracts used in the measurement sequence. The sample order was randomized with respect to origin and harvest year to avoid systematic bias and reduce potential instrumental drift. In addition, a blank sample was analyzed after every five measurements. MS/MS spectra were measured for the identification of metabolites. MS/MS fragment spectra were recorded at 10, 20 and 40 eV in QTOF mode. The acquisition rate/time for MS mode was 1.5 spectra per s with 666.7 ms per spectrum (transients/spectrum: 5452) and for MS/MS mode 1 spectra per s with 1000 ms per spectrum (transients/spectrum: 7999).
2.5. Data processing
Multiplexing of IM-TOF data files was conducted utilizing the PNNL preprocessor software (version 2020.03.23)20,21 with the following specified parameters: demultiplexing was enabled, moving average window size was five frames, moving average smoothing was enabled, m/z was not employed, drift three, chromatography/infusion three, and a lower signal intensity threshold of 20 counts. CCS values were calibrated utilizing the IM-MS Browser software, version 10.0 from Agilent. Subsequently, four-dimensional feature finding was conducted using Mass Profiler software (version 10.0) from Agilent with the following parameters: restriction of the retention time to the range of 0.0 to 28.0 min, ion intensity threshold of 200.0 counts, isotope model of common organic compounds (exclusive of halogens), charge state range of 1–2, report single-ion features with charge state z = 1, RT tolerance = ±10.0% + 0.50 min, DT tolerance = ±1.5%, mass tolerance = ±20.0 ppm + 2.0 mDa, Q-Score >70.0. The resulting bucket table was exported in the Excel.xlsx format (Microsoft Corporation, Washington, USA). To ensure statistical significance, each bucket had to be detectable in at least 10% of all samples within a single sample group (geographical origin), leaving 1523 variables for the dataset. The dataset was transferred to the MetaboAnalyst 6.0 software and missing values (proportion approx. 21%) were replaced by the smallest value at which a characteristic could still be detected. In addition, the dataset was sum-normalized and autoscaled using this software.22 Identification was based on the analysis of the high-resolution mass and fragment spectra, with additional confirmation provided by the Lipid Annotator software, the Agilent Masshunter Qualitative Analysis 10.0 software (Agilent Technologies), as well as the LipidMaps database23 and FoodDB.24 CCS values were cross-referenced with the LipidCCS database or LipidCCS Predictor.25,26 Lipid annotations follow the guidelines established by Liebisch et al. (2013).272.6. Statistical analysis
In order to identify significant metabolites based on the multivariate dataset (six countries), p-values were calculated using an analysis of variance (ANOVA, p-value < 0.01) according to Fisher's least significant difference (LSD). This statistical selection reduced the total number of LC-IM-MS features from 1523 to 379 variables that showed significant differences between the country-specific groups and were subsequently subjected to tentative identification via LC-MS/MS. The statistical methods and principal component analysis (PCA), an unsupervised approach, were performed using the MetaboAnalyst 6.0 software.22 PCA was utilised to ascertain preliminary grouping tendencies, both between German and non-German strawberries, as well as among the six countries of origin. In addition, a hierarchical cluster analysis using Euclidean distances and mean values was applied to the normalized and autoscaled peak intensities of the identified metabolites, alsousing the MetaboAnalyst 6.0 software to visualize their distribution patterns in relation to geographical origin.22 For the purpose of supervised classification, the algorithm linear discriminant analysis (LDA) was executed with MATLAB R2021b (The MathWorks Inc., Natick, MA, USA) to differentiate between German and non-German strawberries, in addition to distinguishing between the six distinct origin groups based on geographical origin. The LDA models were validated by a 5-fold cross-validation (CV) with 100 repetitions. The full dataset of 1523 preprocessed LC-IM-MS variables was used for both PCA and LDA analyses to explore data structure and to classify samples according to geographical origin.3. Results and discussion
3.1. Differentiation between German and non-German strawberries
In a first step, the comparability of the measurements after pre-processing was evaluated. For this purpose, the measurements of the 195 strawberry samples and 22 measurements of the QC sample were analyzed using PCA. Due to the small deviations of the QC sample measurement in the PCA plot, the measurements were considered comparable (Fig. S1, ESI†). An example of a total ion chromatogram (TIC) of a measurement from a QC sample is shown in Fig. S2 (ESI†).To investigate initial differences between German and non-German strawberry samples, the peak intensities obtained of the 1523 variables of the 195 samples were analyzed using PCA. For this purpose, the samples from Poland, the Netherlands, Spain, Greece and Egypt were summarized as “non-Germany”. Fig. 1 shows the PCA score plots of the first two main components (PC1 and PC2), which account for 14.8% of the total variance. The scores are displayed in different colors corresponding to the two classes. Based on the score plot, no clear separation of German samples and non-German samples is recognizable. The two sample classes are distributed in the negative and positive directions of PC1 and PC2. However, the score plot of PC2 and PC5, which explain a total variance of 9.5% (Fig. 2), shows a clustering of the two sample groups. The German samples show predominantly mostly negative values on PC2, while the non-German samples show a clustering with positive loadings on PC2.
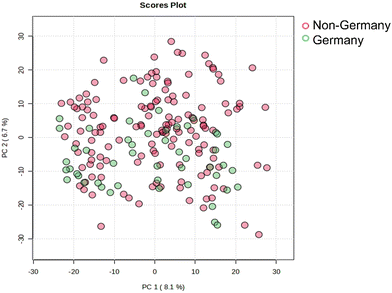 | ||
| Fig. 1 PCA score plots of PC1 and PC2 of the 195 strawberry samples. The German samples are colored green and all other samples red. | ||
 | ||
| Fig. 2 PCA score plots of PC2 and PC5 of the 195 strawberry samples. The German samples are colored green and all other samples red. | ||
Overall, the PCA results indicate initial trends suggesting that it is possible to distinguish between German and non-German strawberries based on the score plot of PC2 and PC5. Therefore, additional supervised methods such as LDA were used to classify German strawberries.
An LDA with a 5-fold cross-validation and 100 repetitions was carried out to create classification models to distinguish between German and non-German strawberries. To compensate for the imbalance in the class distribution of German samples compared to non-German samples, the sample size was adjusted by weighting. The weighting of the samples was inversely proportional to the class frequency and was only applied during training to compensate for class imbalances. The performance of the model was evaluated on unweighted validation datasets. The confusion matrix of the classification model is reported in Table 2.
| Predicted | ||||
|---|---|---|---|---|
| Non-Germany [%] | Germany [%] | Sensitivity [%] | ||
| True | Non-Germany [%] | 96.4 | 3.6 | 96.4 |
| Germany [%] | 22.3 | 77.7 | 77.7 | |
| Specificity [%] | 77.7 | 96.4 | 90.0 | |
The analysis with LDA resulted in a classification accuracy of 90.0 ± 5.1%. A correct classification of 96.4% was found for non-German samples and 77.7% for German samples. The results obtained show that it is possible to distinguish between German and non-German samples. Nevertheless, a misclassification of 22.3% of the samples for the classification of German samples can be observed. This result is due to the fact that the sample pool of the non-German class also included Polish and Dutch samples, which, due to their geographical proximity to Germany, may cause confusion in the classification of German samples. Climatic conditions of samples that are geographically close to each other are often analogous, especially if there are no significant geographical barriers such as mountain ranges or seas. This circumstance could influence the metabolome during the vegetation and fruit ripening phases in a similar way, resulting in similar chemical profiles. This thesis has already been proven in various studies.28,29 A previously published study by our institute determined the origin of strawberries using FT-NIR spectroscopy, showing that German strawberries can be distinguished from non-German strawberries with a classification accuracy of 91.9%. In contrast to this study, the pool of non-German strawberries included countries that are geographically further away from Germany, such as Spain, Greece and Egypt.15 The inclusion of geographically close neighboring countries, such as the Netherlands or Poland in the present study may influence the distinction between German and non-German strawberries. Consequently, the results of the classification indicate that a distinction between German and non-German samples is possible depending on the distance between the individual sample groups.
3.3. Differentiation of strawberries according to their countries of origin
The strawberries were then classified according to their countries of origin. Here, too, the datasets of the 195 strawberry samples were first analyzed using PCA. The score plot of PC1 and PC2 (Fig. 3, total variance of 14.8%) reveals that a clear separation regarding the six countries is not visible, using an unsupervised method. The Spanish and Greek strawberry samples form a cluster in the positive direction of PC2, while the samples from the Netherlands, Poland and Germany form a cluster with negative loadings of PC2. The Egyptian strawberries are located between the two clusters described previously. Based on the score plot of PC2 and PC5 (Fig. S3 in the ESI†), it can be shown that samples from the Netherlands, Poland and Germany are grouped in the negative direction of PC2 and are thus more distinct from the Egyptian, Spanish and Greek strawberries, which are grouped in the positive direction of PC2.The 6-class classification was again performed using LDA with a 5-fold CV and 100 repetitions. Based on the classification with the LDA algorithm, an accuracy of 74.2% ± 6.1% was obtained. The confusion matrix of the 6-group LDA classification is shown in Table 3.
| Predicted | ||||||||
| True | Class | Egypt [%] | Germany [%] | Greece [%] | Netherlands [%] | Poland [%] | Spain [%] | Specificity [%] |
| Egypt [%] | 86.2 | 3.4 | 3.5 | 0.0 | 3.4 | 3.5 | 96.3 | |
| Germany [%] | 0.0 | 89.8 | 0.0 | 6.1 | 4.1 | 0.0 | 92.4 | |
| Greece [%] | 4.7 | 0.0 | 47.6 | 4.8 | 4.8 | 38.1 | 95.8 | |
| Netherlands [%] | 3.1 | 6.3 | 3.1 | 78.1 | 6.3 | 3.1 | 94.9 | |
| Poland [%] | 7.7 | 23.1 | 0.00 | 11.5 | 61.5 | 7.7 | 95.9 | |
| Spain [%] | 3.0 | 3.0 | 15.2 | 3.0 | 3.0 | 72.8 | 93.3 | |
| Sensitivity [%] | 87.3 | 87.9 | 47.4 | 77.0 | 55.8 | 72.8 | 74.2 | |
The results of the classification demonstrate that the Egyptian samples were classified most accurately (86.2%) and the German strawberries with an accuracy of 89.8% were the most accurately classified. A small proportion of German samples (6.1% and 4.1%, respectively) were incorrectly assigned to the Netherlands and Poland. Polish samples were particularly often mistaken for samples from Germany (23.1%) and the Netherlands (11.5%), while the correct classification of Polish samples was achieved in 61.5% of cases. Misclassifications also occurred in the Dutch samples. Strawberries from the Netherlands were correctly classified with an accuracy of 78.1%. However, 6.3% of Dutch samples were incorrectly classified as samples from Poland and Germany. Due to the geographical proximity of Poland, the Netherlands and Germany, and the similar climatic conditions, confusion was to be expected. These three countries are located in a similar climate zone, which is characterized by cooler temperatures and more precipitation during the ripening period.30–33 The climatic conditions may have a similar exogenous influence on the metabolome of strawberry's metabolome, which could explain the mix-up between these countries.16,29,34–36
Greek strawberries were the worst performers, with an accuracy of only 47.6%. It is particularly striking that 38.1% of the Greek samples were assigned to Spain. The same trend was observed for Spanish samples. Although the Spanish samples were mostly correctly classified with an accuracy of 72.8%, 15.2% of the Spanish samples were attributed to Greece. The comparable climatic conditions in Spain and Greece are also the likely cause of these misclassifications and the reason for the similarity in the composition of the strawberries produced in both countries. The growing regions of Huelva in Spain and Pyrgos in Greece have a similar maritime climate zone, characterized by high temperatures and drought during the ripening period. The comparable climatic conditions in both countries lead to similar cultivation methods, in which irrigation systems are used to increase productivity.15,30,37,38 Prior studies have demonstrated that the metabolome can be influenced by exogenous factors such as temperature and rainfall.35,36 In neighboring countries of origin, it is therefore possible that similar influences, such as climatic conditions are reflected in the metabolome of these strawberries.
In addition, the influence of the strawberry varieties on the metabolome must also be taken into account. In order to minimize the influence of variety diversity, care was taken to include as many varieties as possible when selecting the samples to be analyzed (34 varieties in total, see Table S1 in the ESI†). Whenever possible, by purchasing the samples directly from the producers, the variety could be considered for about half of the samples, making Clery (eight samples) the most represented varieties. These were followed by Fortuna (four samples), Sonsation (four samples), Hademar (four samples), Asia (four samples) and Faith (four samples). The varieties Clery, Asia and Faith are exclusively represented in German samples, while Fortuna occurs in Egyptian, Greek and Spanish samples. The variety Hademar only contained samples from Poland and the samples of the variety Sonsation from the Netherlands and Germany. The PCA and the classification using LDA and a 5-fold CV with 100 repetitions were carried out according to the variety of the samples. At least four samples of one variety were considered. The results of the score plot of PC1 and PC2 in Fig. S4 in the ESI† demonstrated that the varieties Clery, Asia, Sonsation, Hademar and Faith cluster together in the positive range of PC2, while the variety Fortuna clusters in the negative range of PC2 and in the positive range of PC1. The PCA results indicate that there is only a differentiation between the variety Fortuna and the five other varieties.
The classification results shown in Table S3 in the ESI† describe an accuracy of 50.7%. The Clery variety, which exclusively contained samples from Germany, was accurately classified with a precision of 97.1%. The samples of the Asia and Faith varieties, which originated in Germany, were mostly incorrectly identified as the Clery variety and the Sonsation variety, which originated from Germany and the Netherlands, respectively. The variety Hademar, whose samples came exclusively from Poland, was correctly classified with 72.3%, but 22.3% were assigned to the variety Sonation, whose samples came from both Germany and the Netherlands. The variety Sonsation was primarily confused with the Clery variety (43.0%) and the Hademar variety (45.3%), while the Fortuna variety, whose samples could be traced back to Greece, Spain and Egypt, was correctly classified with 100%. This could be due to the similar climatic conditions in these countries. The results of the classification shows that the influence of geographical origin predominates, while the influence of variety plays a subordinate role. However, it is important to note that the findings must be interpreted with caution, as only a limited number of the samples (n = 28) from the dataset were suitable for chemometric testing to determine the influence of the variety. For the remaining samples, the variety was unknown, or the number of samples per variety was insufficient to draw statistically valid conclusions. Nevertheless, the dataset contains a total of 34 varieties, which suggests that it exhibits a high level of diversity. Given the limited and imbalanced number of samples per variety, the varietal classification should therefore be considered exploratory. Future studies should aim to include larger and more balanced sample sets across regions and seasons to better differentiate varietal from geographical effects. Consequently, a possible variety influence is also taken into account with regard to the classification by origin. Furthermore, discrepancies in post-harvest handling and ripeness stage among the samples must be taken into account as a potential source of variation. While German strawberries were collected directly from the field, several non-German samples were obtained from local retailers, which may have introduced variability due to transport and storage both of which are known to influence the strawberry metabolome.39 While this does represent a limitation, the classification of origin under such heterogeneous conditions highlights the robustness of the model and its applicability to realistic conditions. Indeed, recent studies have highlighted the sensitivity of the strawberry metabolome to postharvest handling conditions. In a recent study, Ma et al. (2023) reported significant changes in the lipid composition of strawberries due to postharvest fungal infections.39 This emphasizes the extent to which lipid profiles can be affected by storage duration, temperature, and transport conditions. In the present study, German samples were collected directly from the fields under controlled cooled conditions (4–5 °C). In contrast, non-German samples were obtained from various retailers, and their exact pre-analytical conditions could not be fully standardized. Notwithstanding this limitation, the approach successfully differentiated the samples according to geographical origin, thereby demonstrating robustness under these practical and realistic variations in sample handling.
To ensure that the differences are due to the origin of the individual countries and are not caused by different harvest years, the score plot of PC1 and PC2 was also colored according to the harvest years of the samples. Based on the score plot in Fig. S5 in the ESI,† no clusters could be identified by harvest year. Furthermore, LDA was applied with a 5-fold CV and 100 repetitions according to the harvest year of the samples (see Table S4 in the ESI†). The overall accuracy of the classification process was found to be 56.4%, with the 2022 harvest year demonstrating a correct classification rate of 50.4%, the 2023 harvest year achieving 51.6%, and the 2024 harvest year showing 44.5%. It was observed that the proportions of misclassifications were consistent across all harvest years, suggesting that the harvest year has no influence on the data.
3.4. Identification of important metabolites by LC-IM-MS/MS
The most important metabolites for determining geographical origin, corresponding to the six countries, were selected using an ANOVA (p-value <0.01). The analysis of the MS/MS spectra and CCS values allowed the identification of 45 metabolites from a total of 379 variables with a p-value <0.01 whose composition is influenced by geographical origin.A total of 23 of the significant signals were identified as phosphatidylcholines (PC). Since some of the PCs were detected in the form of several adducts ([M + H]+, [M + NH4]+ or [M + Na]+), the number was reduced to 19 PCs that were conspicuous as marker compounds. The identification of the PCs was based on the exact mass of the feature and the fragment ion at m/z 184.07, which is formed by cleavage of the phosphocholine group.
Furthermore, 17 triacylglycerides (TG) were identified as [M+H]+, [M+H−H2O]+ or [M+NH4]+ adducts. Again, some TGs were identified twice due to different adduct formations, so that the number was reduced to 15 TGs. Furthermore, five diacylglycerides (DG) were identified as [M+H]+, [M+H−H2O]+ or [M+NH4]+ adducts. The identification of TG and DG was based on the cleavage fragments of the respective fatty acids or the neutral loss of fatty acids as well as the exact mass. As an additional parameter for the identification of lipids, the obtained CCS values were compared with the calculated CCS values from the LipidCCS database and the LipidCCS predictor for the respective metabolite.25,26 The comprehensive list of all identified compunds with their associated identification parameters an p-values is provided in Table S3 (ESI†), and representative MS/MS spectra of the identified lipids are presented in Fig. S6 to S8 in the ESI.† All lipid annotations were performed in accordance with the lipid annotation guidelines provided by Liebisch et al. (2013), clearly indicating that positional (sn-position) isomers were not resolved.27
The identified lipids belong to the compounds with the lowest p-values in the ANOVA. Therefore, it can be deduced that these markers play a crucial role in differentiating strawberries according to their geographical origin. The scaled peak intensities of the identified markers show various trends that indicate which substance classes have higher peak intensities in the different countries. The heatmap in Fig. 4 shows the relationships between the normalized and scaled peak intensities of the identified metabolites and the geographical origin of the strawberries. In addition, Fig. 5 shows examples of marker substances with the corresponding autoscaled peak intensities in box plots.
The identified lipids can be divided into two groups: (i) the first group comprises markers for countries in temperate climate zones, which include Germany, Poland and the Netherlands (cluster I in Fig. 4).30–33 For example, TGs such as TG 18:0_18:3_22:0 (m/z 963.8346), and TG 18:0_18:2_22:0 (m/z 960.8949) and DGs such as DG 18:1_18:2 (m/z 601.5174) were identified for these countries (Fig. 5A–C). These TGs and DGs are esterified with stearidonic acid (FA 18:1), linoleic acid (FA 18:2) or linolenic acid (FA 18:3), among others. These are mono- or polyunsaturated fatty acids, which are increasingly found in plants in colder regions. This is due to the fact that they are required to maintain the fluidity of the cell membrane at cooler temperatures.40–43 The structure of unsaturated fatty acids leads to a reduction in van der Waals forces due to steric hindrance. This results in a higher fluidity of the cell membrane.44 Similar studies on hazelnuts and asparagus have already shown that fatty acids with a higher unsaturated character occur in higher concentrations in cold regions.16,29,40
Furthermore, highly unsaturated PC such as PC 36:6 (m/z 778.5414) (Fig. 5D) or PC 36:5 (m/z 780.5562) (Fig. 5E) were identified with high signal intensities in German, Polish and Dutch samples. PCs major constituents of the cell membrane and lipid bilayer and also influence the fluidity of the cell membranes.40–42
Cluster II in Fig. 4 shows the identified lipids for the group of countries in warmer climate zones such as Spain, Egypt and Greece. Mainly, different PCs were identified as markers for Spain, Greece and Egypt. Compared to the PCs identified for the Central European countries, they have an overall lower unsaturated character in their esterified fatty acids like PC 36:2 (m/z 786.5995) (Fig. 5F). The increased formation of PCs with a lower proportion of double bonds could be a reaction of the plant to prevent oxidative stress caused by high temperatures. This hypothesis is supported by results from a study on peanuts, which showed that heat stress triggers lipid remodeling, possibly to protect against oxidative damage.45
Nevertheless, some PCs and some TGs such as PC 40:7 (m/z 814.5713) (Fig. 5G), TG 60:5 (m/z 965.8538) (Fig. 5H) and TG 56:6 (m/z 907.7756) (Fig. 5I), were also identified as marker compounds for strawberries in these countries, which showed a higher proportion of unsaturated esterified fatty acids. The synthesis of highly unsaturated TGs and PCs is rather uncommon in plants growing in warmer regions. Therefore, it can only be speculated that they are formed to a greater extent in these countries to provide greater flexibility in response to changes in the cell membrane, such as those occurring during stress reactions e.g. when there is a lack of water.30,46–50
It is important to note that the number of identified lipids in our study is lower compared to some previous targeted lipidomic studies on strawberries. For instance, Bianco et al. (2020) applied a highly targeted approach using HILIC chromatography coupled to high-resolution mass spectrometry to quantify intact phospholipids, achieving greater lipid coverage. In a similar vein, Ma et al. (2023) identified extensive changes in the strawberry lipidome due to fungal stress. Conversely, the non-targeted LC-IM-MS approach utilized a modified Bligh and Dyer extraction method, as established by Creydt et al. (2018).16,39,51 This method was selected due to its proven robustness and reproducibility under realistic sampling conditions. Future investigations could benefit from integrating complementary targeted lipid extraction and analytical approaches to enhance the coverage of lipid subclasses and improve discrimination capabilities, particularly in geographically proximate regions.
Overall, the identified lipids of strawberries from countries with cooler temperatures are predominantly more unsaturated, while the identified markers of strawberries from countries in warmer regions tend to show a more saturated character (Cluster I, Cluster II in Fig. 4). Nevertheless, the identified markers show similar peak intensities for countries that are geographically close to each other and exposed to similar climate zones during fruit ripening. This is particularly true for German strawberries compared to Polish and to some extent Dutch strawberries, as well as for Spanish strawberries compared to Greek and Egyptian strawberries. This highlights the difficulties in distinguishing strawberries grown in geographically close countries and reflects the results of the 6-class classification.
4. Conclusion
In this study, a method for determining the origin of strawberries using an LC-ESI-IM-qTOF instrument is presented. The focus of the analyses was on the investigation of the lipidome, as previous studies had already shown that it is particularly suitable for distinguishing the geographical origin of food. The separation of German strawberries from non-German strawberries was successful, with an overall accuracy of 90.0%. However, the differentiation between the six countries proved to be much more challenging, with an overall accuracy of 74.21%. This result indicates that the lipidome of strawberries from countries that are geographically close to each other shows great similarities. Of particular relevance here are confusions between Spanish and Greek samples, as well as Polish, Dutch and German strawberries. MS/MS measurements also identified similar metabolites for German, Dutch and Polish strawberries, as well as similar metabolites for Spanish, Egyptian and Greek strawberries. To pinpoint the geographical origin of the strawberries, further investigations with a larger number of samples per country or further analyses from other disciplines could support this investigation. Furthermore, the identified marker compounds could be used in future work to validate targeted methods for determining the origin of strawberries. In this context, the use of triple quadrupole instruments, which are relatively widely used in state testing centers and commercial laboratories, is particularly suitable, enabling a successful transfer from research to industry. This approach is particularly suitable for protecting German strawberries from non-German strawberries, since the classification result of 90% is very promising, even though it is probably not possible to classify non-German strawberries very precisely according to their country of origin.Author contributions
Conceptualization, J. B. and M. F.; methodology, J. B.; software, J. B.; validation, J. B.; formal analysis, J. B.; investigation, J. B.; resources, M. F.; data curation, J. B.; writing – original draft preparation, J. B.; writing – review & editing F. S., M. C., and M. F.; visualization, J. B.; supervision, M. F.; project administration, M. F, M. C.Data availability
LC-MS dataset presented in this study is available in the Research data repository of the University of Hamburg at: https://doi.org/10.25592/uhhfdm.16687.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This IGF Project (01IF22909N) of the research association FEI, Godesberger Allee 125, 53175 Bonn, is/was funded as part of the program for promoting joint industrial research (IGF) by the Federal Ministry for Economic Affairs and Climate Protection based on a resolution of the German Bundestag. The authors thank, Christian Ahlers, Lina Cvancar, Kim Brettschneider and Soeren Wenck for their helpful discussions.Notes and references
- C. Stamatis, C. A. Sarri, K. A. Moutou, N. Argyrakoulis, I. Galara, V. Godosopoulos, M. Kolovos, C. Liakou, V. Stasinou and Z. Mamuris, What do we think we eat? Single tracing method across foodstuff of animal origin found in Greek market, Food Res. Int., 2015, 69, 151–155 Search PubMed.
- K. Katerinopoulou, A. Kontogeorgos, C. E. Salmas, A. Patakas and A. Ladavos, Geographical origin authentication of agri-food products: A review, Foods, 2020, 9, 489 Search PubMed.
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers and amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004 Text with EEA relevance. VO (EU) Nr. 1169/2011 LMIV, 2011.
- International Trade Center, List of exporters for the selected product Product: 081010 Fresh strawberries, (accessed 6 December 2024).
- Statistisches Bundesamt, Area under strawberries in Germany in the years 2012 to 2023, (accessed 4 December 2024).
- Statistisches Bundesamt, Most important supplier countries of strawberry imports to Germany by import volume in the years 2021 to 2023 (in tonnes), (accessed 4 December 2024).
- Statistisches Bundesamt, Sales price of strawberries in the European Union by country in 2022 and 2023, (accessed 4 December 2024).
- BMEL – Bundesministerium fur Ernährung und Landwirtschaft, Kennzeichnung regionaler Lebensmittel, https://www.bmel.de/DE/themen/ernaehrung/lebensmittel-kennzeichnung/freiwillige-angaben-und-label/kennzeichnung-regionale-lebensmittel.html, (accessed 6 April 2025).
- BZfE – Bundeszentrum für Ernährung, Regional einkaufen – gut für Umwelt und Klima, https://www.bzfe.de/inhalt/regional-einkaufen-31847.html, (accessed 6 April 2025).
- M. Bertelsmeier, B. Gerowitt and P. Kreins, Kosteneffekte und betriebliche Anpassungsmaßnahmen im deutschen Gartenbau nach Einführung des Mindestlohns, https://literatur.thuenen.de/digbib_extern/dn057665.pdf, (accessed 6 April 2025) Search PubMed.
- F. D. Covaciu, Z. Moldovan, A. A. Dehelean, D. A. Magdas, I. C. Feher, R. Puscas and M. Vlassa, Determination of pesticides, elements, and stable isotopes in strawberries, Anal. Lett., 2016, 49, 2560–2572 Search PubMed.
- M. A. Hakala, A. T. Lapveteläinen and H. P. Kallio, Volatile compounds of selected strawberry varieties analyzed by purge-and-trap headspace GC-MS, J. Agric. Food Chem., 2002, 50, 1133–1142 CrossRef CAS PubMed.
- M. N. Khan, A. Sarwar, S. Bhutto and M. F. Wahab, Physicochemical characterization of the strawberry samples on regional basis using multivariate analysis, Int. J. Food Prop., 2010, 13, 789–799 Search PubMed.
- L. Wu, X. Wang, J. Hao, N. Zhu and M. Wang, Geographical Indication Characteristics of Aroma and Phenolic Acids of the Changping Strawberry, Foods, 2023, 12, 3889 CrossRef CAS PubMed.
- J. Brockelt, F. Schmauder, K. Brettschneider, M. Creydt, S. Seifert and M. Fischer, Competing Technologies: Determining the Geographical Origin of Strawberries (Fragaria × ananassa) using laboratory based Near-Infrared Spectroscopy compared to a simple portable device, Mol. Omics, 2024, 21, 7–18 RSC.
- M. Creydt, D. Hudzik, M. Rurik, O. Kohlbacher and M. Fischer, Food authentication: Small-molecule profiling as a tool for the geographic discrimination of German white asparagus, J. Agric. Food Chem., 2018, 66, 13328–13339 Search PubMed.
- J. N. Dodds and E. S. Baker, Ion mobility spectrometry: fundamental concepts, instrumentation, applications, and the road ahead, J. Am. Soc. Mass Spectrom., 2019, 30, 2185–2195 CrossRef CAS PubMed.
- M. Creydt, B. Wegner, A. Gnauck, R. Hoerner, C. Hummert and M. Fischer, Food authentication in the routine laboratory: Determination of the geographical origin of white asparagus using a simple targeted LC-ESI-QqQ-MS/MS approach, Food Control, 2022, 135, 108690 CrossRef CAS.
- E. G. Bligh and W. J. Dyer, A rapid method of total lipid extraction and purification, Can. J. Biochem. Physiol., 1959, 66, 911–917 CrossRef.
- A. Bilbao and B. Gibbons, Pacific Northwest National Laboratory, PNNL Preprocessor software, https://pnnl-comp-mass-spec.github.io/PNNL-PreProcessor, (accessed December 2024) Search PubMed.
- A. Bilbao, B. C. Gibbons, S. M. Stow, J. E. Kyle, K. J. Bloodsworth, S. H. Payne, R. D. Smith, Y. M. Ibrahim, E. S. Baker and J. C. Fjeldsted, A preprocessing tool for enhanced ion mobility–mass spectrometry-based omics workflows, J. Proteome Res., 2021, 21, 798–807 CrossRef PubMed.
- Z. Pang, Y. Lu, G. Zhou, F. Hui, L. Xu, C. Viau, A. F. Spigelman, P. E. MacDonald, D. S. Wishart and S. Li, MetaboAnalyst 6.0: towards a unified platform for metabolomics data processing, analysis and interpretation, Nucleic Acids Res., 2024, gkae253 Search PubMed.
- M. Sud, E. Fahy, D. Cotter, A. Brown, E. A. Dennis, C. K. Glass, A. H. Merrill Jr, R. C. Murphy, C. R. H. Raetz and D. W. Russell, Lmsd: Lipid maps structure database, Nucleic Acids Res., 2007, 35, D527–D532 CrossRef CAS PubMed.
- The Metabolomics Innovation Centre (TMIC), FooDB, https://foodb.ca, (accessed 27 December 2024) Search PubMed.
- Z. Zhou, J. Tu, X. Xiong, X. Shen and Z.-J. Zhu, LipidCCS: prediction of collision cross-section values for lipids with high precision to support ion mobility–mass spectrometry-based lipidomics, Anal. Chem., 2017, 89, 9559–9566 CrossRef CAS PubMed.
- Zhu Lab, https://www.metabolomics-shanghai.org/LipidCCS/, (accessed 27 December 2024).
- G. Liebisch, J. A. Vizcaíno, H. Köfeler, M. Trötzmüller, W. J. Griffiths, G. Schmitz, F. Spener and M. J. O. Wakelam, Shorthand notation for lipid structures derived from mass spectrometry, J. Lipid Res., 2013, 54, 1523–1530 CrossRef CAS PubMed.
- M. Creydt, M. Arndt, D. Hudzik and M. Fischer, Plant metabolomics: evaluation of different extraction parameters for nontargeted UPLC-ESI-QTOF-mass spectrometry at the example of white Asparagus officinalis, J. Agric. Food Chem., 2018, 66, 12876–12887 CrossRef CAS PubMed.
- S. Klockmann, E. Reiner, R. Bachmann, T. Hackl and M. Fischer, Food fingerprinting: metabolomic approaches for geographical origin discrimination of hazelnuts (Corylus avellana) by UPLC-QTOF-MS, J. Agric. Food Chem., 2016, 64, 9253–9262 Search PubMed.
- H. E. Beck, N. E. Zimmermann, T. R. McVicar, N. Vergopolan, A. Berg and E. F. Wood, Present and future Köppen-Geiger climate classification maps at 1-km resolution, Sci. Data, 2018, 5, 1–12 CrossRef PubMed.
- D. Wetterdienst, Climate chart of Hamburg-Fuhlsbüttel (Airport)/Germany, (accessed 4 December 2024) Search PubMed.
- D. Wetterdienst, Climate chart of Poznan, Province of Wielkopolska/Poland, (accessed 4 December 2024).
- D. Wetterdienst, Climate chart of Utrecht-De Bilt (Central Obs.)/Netherlands, (accessed 4 December 2024).
- M. Meijón, I. Feito, M. Oravec, C. Delatorre, W. Weckwerth, J. Majada and L. Valledor, Exploring natural variation of Pinus pinaster Aiton using metabolomics: Is it possible to identify the region of origin of a pine from its metabolites?, Mol. Ecol., 2016, 25, 959–976 CrossRef PubMed.
- L. Zhang, Z. Wang, C. Zhang, S. Zhou and C. Yuan, Metabolomics analysis based on UHPLC-QqQ-MS/MS to discriminate grapes and wines from different geographical origins and climatological characteristics, Food Chem.: X, 2024, 22, 101396 CAS.
- E. M. Lee, S. J. Park, J.-E. Lee, B. M. Lee, B. K. Shin, D. J. Kang, H.-K. Choi, Y.-S. Kim and D. Y. Lee, Highly geographical specificity of metabolomic traits among Korean domestic soybeans (Glycine max), Food Res. Int., 2019, 120, 12–18 Search PubMed.
- D. Wetterdienst, Climate Table of Huelva, Andalusia, Spain, https://www.dwd.de/DWD/klima/beratung/ak/ak_083830_kt.pdf, (accessed 4 December 2024) Search PubMed.
- D. Wetterdienst, Climate Table Athen, Attika, Greece, https://www.dwd.de/DWD/klima/beratung/ak/ak_167160_kt.pdf, (accessed 4 December 2024) Search PubMed.
- Q. Ma, X. Wu, Z. Luo, Z. Ge, D. Li, D. Wu, X. Zhang, Y. Chen, L. Li and Y. Xu, Lipidomic analysis revealed dynamic changes of lipidic compounds of postharvest strawberry in response to gray mold, Postharvest Biol. Technol., 2023, 199, 112296 Search PubMed.
- S. Satyakam, G. Zinta, R. K. Singh and R. Kumar, Cold adaptation strategies in plants—An emerging role of epigenetics and antifreeze proteins to engineer cold resilient plants, Front. Genet., 2022, 13, 909007 Search PubMed.
- Z. D. Shomo, F. Li, C. N. Smith, S. R. Edmonds and R. L. Roston, From sensing to acclimation: The role of membrane lipid remodeling in plant responses to low temperatures, Plant Physiol., 2024, 196, 1737–1757 CrossRef CAS PubMed.
- Y. Wang, J. Wang, R. Sarwar, W. Zhang, R. Geng, K.-M. Zhu and X.-L. Tan, Research progress on the physiological response and molecular mechanism of cold response in plants, Front. Plant Sci., 2024, 15, 1334913 CrossRef PubMed.
- Q. Li, Q. Zheng, W. Shen, D. Cram, D. B. Fowler, Y. Wei and J. Zou, Understanding the biochemical basis of temperature-induced lipid pathway adjustments in plants, The plant cell, 2015, 27, 86–103 CrossRef CAS PubMed.
- N. Murata and D. A. Los, Membrane fluidity and temperature perception, Plant Physiol., 1997, 115, 875 CAS.
- Z. S. Zoong Lwe, R. Welti, D. Anco, S. Naveed, S. Rustgi and S. Narayanan, Heat stress elicits remodeling in the anther lipidome of peanut, Sci. Rep., 2020, 10, 22163 CrossRef CAS PubMed.
- S. K. Sah and A. Sofo, The role of lipids in abiotic stress responses, Front. Plant Sci., 2024, 15, 1378485 Search PubMed.
- R. G. Upchurch, Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress, Biotechnol. Lett., 2008, 30, 967–977 CAS.
- X. Wang, C. Yu, Y. Liu, L. Yang, Y. Li, W. Yao, Y. Cai, X. Yan, S. Li and Y. Cai, GmFAD3A, a ω-3 fatty acid desaturase gene, enhances cold tolerance and seed germination rate under low temperature in rice, Int. J. Mol. Sci., 2019, 20, 3796 CAS.
- J. Rubert, K. Hurkova, M. Stranska and J. Hajslova, Untargeted metabolomics reveals links between Tiger nut (Cyperus esculentus L.) and its geographical origin by metabolome changes associated with membrane lipids, Food Addit. Contam.,: Part A, 2018, 35, 1861–1869 Search PubMed.
- A. Zeb, Chemistry and liquid chromatography methods for the analyses of primary oxidation products of triacylglycerols, Free Radical Res., 2015, 49, 549–564 CAS.
- M. Bianco, C. D. Calvano, L. Huseynli, G. Ventura, I. Losito and T. R. I. Cataldi, Identification and quantification of phospholipids in strawberry seeds and pulp (Fragaria× ananassa cv San Andreas) by liquid chromatography with electrospray ionization and tandem mass spectrometry, J. Mass Spectrom., 2020, 55, e4523 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5mo00006h |
| This journal is © The Royal Society of Chemistry 2025 |



