Open Access Article
Open Access ArticleTrapping in situ generated CF3-nitrile imines with maleimides under solvent-free mechanochemical conditions†
Greta
Utecht-Jarzyńska‡
a,
Szymon
Jarzyński‡
b and
Marcin
Jasiński
 *a
*a
aUniversity of Lodz, Faculty of Chemistry, Department of Organic and Applied Chemistry, Tamka 12, 91-403 Łódź, Poland. E-mail: mjasinski@uni.lodz.pl
bUniversity of Lodz, Faculty of Chemistry, Department of Organic Chemistry, Tamka 12, 91-403 Łódź, Poland
First published on 7th November 2024
Abstract
A series of trifluoromethylated pyrrolo[3,4-c]pyrazoles was obtained via mechanochemical (3 + 2)-cycloaddition of in situ generated trifluoroacetonitrile imines with maleimide and its N-aliphatic/aromatic analogues. The presented work demonstrated that the aforementioned 1,3-dipoles can be efficiently trapped with electron-deficient dipolarophiles under solvent-free ball-milling conditions.
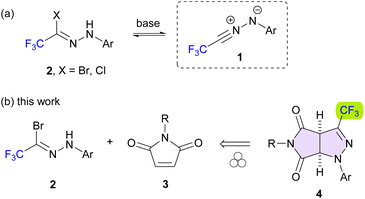
There is increasing interest in applications of fluorinated nitrile imines for the synthesis of both heteroatom and heterocyclic systems,1 and in this context, special attention has recently been paid to di- and trifluoroacetic acid analogues recognized as powerful building blocks for organofluorine synthesis.2 The latter CF3-nitrile imines 1 are readily available in situ by base-mediated dehydrohalogenation of the respective hydrazonoyl halides 2 (Scheme 1a), and they have been successfully applied for preparation of various five- and six-membered products including 1,3,4-thiadiazole,3 1,2,4-triazole,4 pyrazoline and pyrazole,5 as well as 1,3,4-thiadiazine6 and 1,2,4-triazine derivatives,7 available via formal (3 + 2)-cycloadditions or (3 + 3)-annulations, respectively.
 | ||
| Scheme 1 (a) Base-induced generation of CF3-nitrile imines 1 and (b) the mechanochemical (3 + 2)-cycloadditions of 1 with maleimides reported herein. | ||
In a series of recent reports, Huisgen cycloadditions of transient trifluoroacetonitrile imines 1 with suitable electron-deficient dipolarophiles leading to monocyclic as well as bicyclic (3 + 2)-cycloadducts, were demonstrated. For example, trapping of 1 with enones,8 quinones,9 nitro- and cyanoalkenes10 in organic solutions is known; however, the mentioned transformations required rather longer reaction times (up to several days) and/or elevated temperatures (up to 90 °C) to afford reasonable amounts of the desired products. Thus, despite remarkable progress in exploration of nitrile imines 1 in reactions performed in solutions, development of alternative mild approaches, e.g. under ball-milling activation, is of general interest. Taking into account the well documented significance of pyrrolo-pyrazole scaffolds for drug discovery (Fig. 1),11 here, we report (3 + 2)-cycloaddition reactions of CF3-nitrile imines 1 with maleimides 3 leading to trifluoromethylated pyrrolo[3,4-c]pyrazoles 4 under solvent-free mechanochemical conditions (Scheme 1b).
We commenced our study with N-phenylmaleimide (3a) selected as a model dipolarophile and N-(4-tolyl)-trifluoro-acetohydrazonoyl bromide (2a) applied as a precursor of the respective nitrile imine 1a (Scheme 2). First, based on our experience in (3 + 2)-cycloaddition reactions of 1 with electron-deficient dipolarophiles, the designed reaction was briefly examined in solutions, to afford the expected pyrrolo[3,4-c]pyrazole 4a in fair 81% yield under the optimized conditions (THF, 60 °C, 24 h, excess K2CO3). Notably, in contrast to previously reported cycloadducts of nitrile imines 1 with benzoquinones,9 no spontaneous air-aromatization of 4a could be observed and the final 4a was obtained exclusively. The structure of the isolated bicyclic product 4a was established on the basis of NMR data supplemented by MS measurements, while combustion analysis confirmed the molecular formula of 4a as C19H14F3N3O2 and the analytical purity of the sample. In 1H NMR (600 MHz, CDCl3) of 4a, a set of two diagnostic absorptions located at δ = 4.80 (dq, 4JH–F = 1.2 Hz, JH–H = 11.5 Hz) and δ = 5.41 (d, JH–H = 11.5 Hz), attributed to 3a-H and 6a-H, respectively, confirmed the relative cis-configuration of the obtained bicyclic product. As expected, two characteristic quartets at δ = 120.2 (1JC–F = 270.0 Hz) and δ = 131.3 (1JC–F = 39.8 Hz) attributed to the CF3 group and the C(3) atom of the core heterocycle were found in the 13C NMR (151 MHz, CDCl3) of 4a.
Initial mechanochemical experiments were carried out using equimolar amounts of starting materials 2a and 3a, in a ball-mill, using a 5 mL stainless steel vessel (one steel ball, ø 7 mm, 22 Hz), and a series of organic (Et3N and DABCO) and inorganic (KF, CsF, Na2CO3, K2CO3, and Cs2CO3) bases was checked to indicate nearly complete conversion (96%) and a high isolated yield of 85% in the case of K2CO3 (1.2 equiv.) used for the dehydrohalogenation step, after 90 min of grinding (see the ESI†). Further optimization with respect to diameter and number of milling balls (ø 3, 5 or 7 mm; up to 3 balls) showed no remarkable changes, whereas the use of a slight excess of the nitrile imine precursor 2a (1.1 equiv.) was found to be beneficial and provided the target cycloadduct 4a almost quantitatively (100% conversion; 93% isolated yield).§
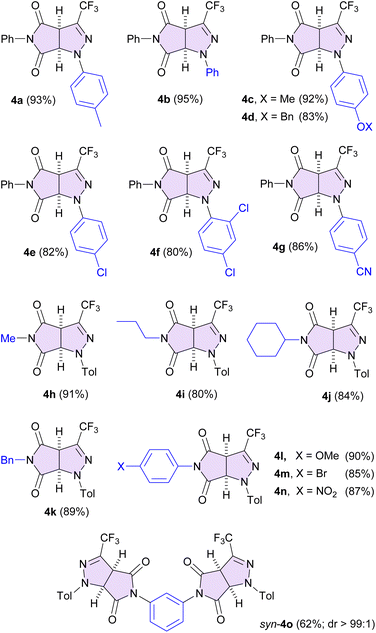
With optimized conditions in hand, a series of solid hydrazonoyl bromides 2b–2g was subjected to neat grinding with N-phenylmaleimide (3a) to provide the expected (3 + 2)-cycloadducts 4b–4g, which were generally isolated in high yield (80–95%; Fig. 2). However, in certain cases a prolonged reaction time was necessary to lead the reaction to completion (for details, see the ESI†); for example, in the case of the nitrile imine precursors 2f and 2g bearing strongly electron-withdrawing substituents (two Cl atoms and a CN group, respectively) attached to the phenyl ring, ball-milling for 10 h (for 2f) and 18 h (for 2g), assured complete consumption of starting materials. Noteworthily, neither 2f nor cycloadduct 2g could be obtained in solution according to Method A reported for model Tol-functionalized pyrrolo[3,4-c]pyrazole 4a. Only in the case of p-nitrophenyl-functionalized hydrazonoyl bromide of type 2 no desired product could be obtained under mechanochemical conditions; NMR analysis of the crude reaction revealed a low consumption of maleimide 3a (<10%) even after 48 h of ball-milling. In addition, partial decomposition of the starting nitrile imine precursor, leading to a complex mixture, was observed.
The scope of maleimides was also checked, and a series of selected solid N-(cyclo)alkyl (3b–3d) and N-aryl-substituted (3f–3h) analogues was examined in mechanochemical (3 + 2)-cycloaddition with bromide 2a (Fig. 2). Similar to the result noticed for the model compound 4a, in all reactions complete consumption of the starting materials was observed in a reasonable reaction time of 90 min, irrespective of the steric and electronic character of the N-substituent in maleimide. For example, Me- (4h, 91%) and cHex- (4j, 84%), as well as p-MeOC6H4- (4l, 90%) and p-NO2C6H4- (4n, 87%) analogues were isolated as spectroscopically pure materials by simple filtration through a short silica gel pad.
Next, 1,3-phenylene bis-maleimide 3i was involved in the study to provide the corresponding product 4o (62%) resulting from double (3 + 2)-cycloaddition, and the analysis of the 1H NMR (600 MHz, CDCl3) spectrum of the mother liquor revealed the formation of a single diastereomeric product (dr > 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). According to the literature, double cycloadditions of bis-imide 3i can either lead to products of C2-symmetry (anti-addition)12a,b or to syn-configured12c,d materials. To get more information about the structure of 4o, the isolated product was analysed by NMR spectroscopy in the presence of (–)-(R)-mandelic- and (+)-(R)-(tert-butyl)(phenyl)phosphonothioic acids selected as chiral solvating agents (in 1
1). According to the literature, double cycloadditions of bis-imide 3i can either lead to products of C2-symmetry (anti-addition)12a,b or to syn-configured12c,d materials. To get more information about the structure of 4o, the isolated product was analysed by NMR spectroscopy in the presence of (–)-(R)-mandelic- and (+)-(R)-(tert-butyl)(phenyl)phosphonothioic acids selected as chiral solvating agents (in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratios of 4o
2 ratios of 4o![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) additive, respectively).13 In all four measurements a single set of signals attributed to 4o was found in 1H NMR spectra. Furthermore, HPLC analysis of 4o by using a chiral stationary phase (Chiralcel OD) provided a single fraction of the product. Thus, based on the above experiments the meso structure of 4o resulting from syn-addition of the second nitrile imine molecule 1a was tentatively proposed (Fig. 2).
additive, respectively).13 In all four measurements a single set of signals attributed to 4o was found in 1H NMR spectra. Furthermore, HPLC analysis of 4o by using a chiral stationary phase (Chiralcel OD) provided a single fraction of the product. Thus, based on the above experiments the meso structure of 4o resulting from syn-addition of the second nitrile imine molecule 1a was tentatively proposed (Fig. 2).
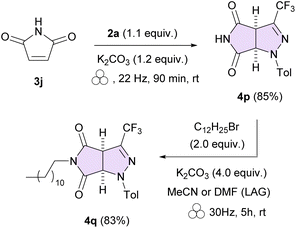
To check whether the N-unsubstituted pyrrolo[3,4-c]pyrazoles of type 4 can also be accessed by the devised mechanochemical approach, the model hydrazonoyl bromide 2a was treated with maleimide (3j) (Scheme 3). Gratifyingly, the desired product 4p was formed in a highly chemoselective manner, and was isolated in 85% yield, although the formation of small amounts of unidentified intermolecular by-products was also detected. Possibly, the competitive reaction initiated by nucleophilic attack of the N atom of maleimide onto the positively charged C-termini of the 1,3-dipole 1a takes place, analogous to a recent report by Madabhushi on reactions of classical C,N-diaryl nitrile imines with succinimide;14 however, attempted isolation of by-product(s) by standard column chromatography was unsuccessful.
Subsequent functionalization of 4p with dodecyl bromide, selected as an exemplary oleophilic electrophile, was carried out under standard alkylation conditions, i.e. in MeCN solution (K2CO3, 60 °C, 16 h), and provided the expected material 4q (87%) as a sole product. Furthermore, prompted by the work by Margetić dealing with mechanochemical alkylations of imides,15 we examined the solvent-free one-pot telescopic approach towards 4q. To our delight, treatment of the initially formed crude (3 + 2)-cycloadduct 4p with excess C12H25Br (2.0 equiv.) in the presence of K2CO3 (10.0 equiv.) opened up access to the final compound under exclusive mechanochemical activation; however, addition of either MeCN or DMF as a liquid assisted grinding solvent (η = 0.35 μL mg−1) was found to be essential as no desired product could be obtained under simple neat grinding.
Conclusions
In conclusion, an operationally simple and highly efficient protocol for the mechanochemical synthesis of trifluoromethylated pyrrolo[3,4-c]pyrazoles by trapping of CF3-nitrile imines with maleimides is reported. The presented results indicate that hydrazonyl halides can serve as suitable precursors for in situ generation of the corresponding nitrile imines under solvent-free ball-milling conditions. Of note, the remarkable decrease in reactivity with increasing electron-deficient character of the substituent located at the N-termini of the starting hydrazonoyl halide was observed. On the other hand, a series of N-functionalized maleimides bearing (cyclo)alkyl groups or variously substituted phenyl substituents reacted smoothly with the model 1,3-dipole. Finally, the parent maleimide lacking substituent at the N atom was successfully applied for one-pot telescopic (3 + 2)-cycloaddition followed by N-alkylation under mechanochemical conditions. The devised approach supplements hitherto reported classical methods for the synthesis of fluorinated and non-fluorinated pyrrolo[3,4-c]pyrazoles of interest in the context of chemical biology applications.11,12b,16Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
GUJ & SJ: conceptualization, methodology, investigation, and data processing. MJ: conceptualization, supervision, writing, review, and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the University of Lodz for financial support in the framework of the IDUB grant (MJ; no. 14/IGB/2024). Technical support by Mrs Dagmara Mrowiec is gratefully acknowledged.Notes and references
- (a) N. R. Yamaletdinova and R. R. Gataullin, Helv. Chim. Acta, 2024, 107, e202400058 CrossRef; (b) Z. Dongxu, Beilstein J. Org. Chem., 2023, 19, 1741 CrossRef; (c) C. Jamieson and K. Livingstone, The Nitrile Imine 1,3-dipole. Properties, Reactivity and Applications, Springer, Cham, Switzerland, 2020 CrossRef.
- (a) W.-J. Luo, X. Liang, M. Chen, K.-H. Wang, D. Huang, J. Wang, D.-P. Chen and Y. Hu, J. Org. Chem., 2024, 89, 10066 CrossRef CAS; (b) Y. Feng, Y. Ren, D. Tang, K.-H. Wang, J. Wang, D. Huang, X. Lv and Y. Hu, Org. Biomol. Chem., 2024, 22, 2797 RSC; (c) K. Świątek, G. Utecht-Jarzyńska, M. Palusiak, J.-A. Ma and M. Jasiński, Org. Lett., 2023, 25, 4462 CrossRef; (d) N. Zhang, H. Ma, C. W. Cheung, F.-G. Zhang, M. Jasiński, J.-A. Ma and J. Nie, Org. Biomol. Chem., 2023, 21, 5040 RSC; (e) A. Kowalczyk, G. Utecht-Jarzyńska and M. Jasiński, J. Fluorine Chem., 2023, 272, 110206 CrossRef CAS; (f) X. Liu, Y. Zhou, X. Li, K.-H. Wang, J. Wang, D. Huang, X. Lv and Y. Hu, Tetrahedron, 2024, 166, 134241 CrossRef CAS.
- (a) G. Utecht, J. Sioma, M. Jasiński and G. Mlostoń, J. Fluorine Chem., 2017, 201, 68 CrossRef; (b) G. Utecht-Jarzyńska, S. S. Mykhaylychenko, E. B. Rusanov, Y. G. Shermolovich, M. Jasiński and G. Mlostoń, J. Fluorine Chem., 2021, 242, 109702 CrossRef.
- (a) Y. Zhang, J.-L. Zeng, Z. Chen and R. Wang, J. Org. Chem., 2022, 87, 14514 CrossRef; (b) D. Wang, X. Wan, Y. Zhou, J. Liu, J. Cai and G.-J. Deng, Asian J. Org. Chem., 2023, 12, e202200674 CrossRef; (c) K. Cen, J. Wei, Y. Feng, Y. Liu, X. Wang, Y. Liu, Y. Yin, J. Yu, D. Wang and J. Cai, Org. Biomol. Chem., 2023, 21, 7095 RSC.
- (a) K. Tanaka, S. Maeno and K. Mitsuhashi, Chem. Lett., 1982, 11, 543 CrossRef; (b) K. Tanaka, S. Maeno and K. Mitsuhashi, J. Heterocycl. Chem., 1985, 22, 565 CrossRef; (c) G. Utecht, A. Fruziński and M. Jasiński, Org. Biomol. Chem., 2018, 16, 1252 RSC; (d) G. Utecht, G. Mlostoń and M. Jasiński, Synlett, 2018, 29, 1753 CrossRef CAS.
- G. Utecht-Jarzyńska, A. Michalak, J. Banaś, G. Mlostoń and M. Jasiński, J. Fluorine Chem., 2019, 222–223, 8 CrossRef.
- A. Kowalczyk, K. Świątek, M. Celeda, G. Utecht-Jarzyńska, A. Jaskulska, K. Gach-Janczak and M. Jasiński, Materials, 2023, 16, 856 CrossRef CAS.
- (a) Y.-C. Tian, J.-K. Li, F.-G. Zhang and J.-A. Ma, Adv. Synth. Catal., 2021, 363, 2093 CrossRef CAS; (b) A. Kowalczyk, G. Utecht-Jarzyńska, G. Mlostoń and M. Jasiński, Org. Lett., 2022, 24, 2499 CrossRef CAS; (c) G. Utecht-Jarzyńska, A. Kowalczyk and M. Jasiński, Molecules, 2022, 27, 8446 CrossRef.
- G. Utecht-Jarzyńska, K. Nagła, G. Mlostoń, H. Heimgartner, M. Palusiak and M. Jasiński, Beilstein J. Org. Chem., 2021, 17, 1509 CrossRef.
- (a) K.-H. Wang, H. Liu, X. Liu, C. Bian, J. Wang, Y. Su, D. Huang and Y. Hu, Asian J. Org. Chem., 2022, 11, e202200103 CrossRef CAS; (b) Y. Zhou, C.-F. Gao, H. Ma, J. Nie, J.-A. Ma and F.-G. Zhang, Chem.–Asian J., 2022, e202200436 CrossRef CAS.
- (a) N. M. Abunada, H. M. Hassaneen, N. G. Kandile and O. A. Miqdad, Molecules, 2008, 13, 1011 CrossRef CAS; (b) H.-J. Chen, Y. Liu, L.-N. Wang, Q. Shen, J. Li and F.-J. Nan, Bioorg. Med. Chem. Lett., 2010, 20, 2876 CrossRef; (c) X.-G. Bai, D.-K. Yu, J.-X. Wang, H. Zhang, H.-W. He, R.-G. Shao, X.-M. Li and Y.-C. Wang, Bioorg. Med. Chem. Lett., 2012, 22, 6947 CrossRef PubMed; (d) G.-N. Liu, R.-H. Luo, Y. Zhou, X.-J. Zhang, J. Li, L.-M. Yang, Y.-T. Zheng and H. Liu, Molecules, 2016, 21, 1198 CrossRef PubMed; (e) M. Golkowski, G. K. Perera, V. N. Vidadala, K. K. Ojo, W. C. Van Voorhis, D. J. Maly and S.-E. Ong, Mol. Omics, 2018, 14, 26 RSC ; see also review; (f) D. Raffa, B. Maggio, M. V. Raimondi, S. Cascioferro, F. Plescia, G. Cancemi and G. Daidone, Eur. J. Med. Chem., 2015, 97, 732 CrossRef PubMed.
- (a) Z.-J. Chen, W. Liang, Z. Chen and L. Chen, Eur. J. Org Chem., 2021, 2021, 788 CrossRef; (b) J.-N. Zhu, W.-K. Wang, Z.-H. Jin, Q.-K. Wang and S.-Y. Zhao, Org. Lett., 2019, 21, 5046 CrossRef PubMed; (c) J. Y. Hwang, A. Y. Ji, S. H. Lee and E. J. Kang, Org. Lett., 2020, 22, 16 CrossRef PubMed; (d) X. Zhou, J. Shi, J.-R. Song, W.-D. Pan, H. Ren and W. Wu, Eur. J. Org Chem., 2023, e202300563 CrossRef CAS.
- (a) S. C. Benson, P. Cai, M. Colon, M. A. Haiza, M. Tokles and J. K. Snyder, J. Org. Chem., 1988, 53, 5335 CrossRef CAS; (b) J. Szawkało, A. Zawadzka, K. Wojtasiewicz, A. Leniewski, J. Drabowicz and Z. Czarnocki, Tetrahedron: Asymmetry, 2005, 16, 3619 CrossRef.
- S. K. Chilaka, K. P. Chinthapally, A. K. Soda, R. K. Chellu, S. Kurva, J. B. Nanubolu and S. Madabhushi, Asian J. Org. Chem., 2023, 12, e202300041 CrossRef.
- A. Bris, M. Dud and D. Margetić, Beilstein J. Org. Chem., 2017, 13, 1745 CrossRef CAS.
- (a) B. Quiclet-Sire and S. Z. Zard, Chem. Commun., 2006, 1831 RSC; (b) M. P. Winters, C. A. Teleha and Z. Sui, Tetrahedron Lett., 2014, 55, 2150 CrossRef CAS; (c) E. Y. Slobodyanyuk, O. S. Artamonov, O. V. Shishkin and P. K. Mykhailiuk, Eur. J. Org Chem., 2014, 2487 CrossRef CAS; (d) B. Vonhören, O. Roling, C. Buten, M. Körsgen, H. F. Arlinghaus and B. J. Ravoo, Langmuir, 2016, 32, 2277 CrossRef; (e) J. Li, X.-L. Yu, J. Cossy, S.-Y. Lv, H.-L. Zhang, F. Su, P. K. Mykhailiuk and Y. Wu, Eur. J. Org Chem., 2017, 266 CrossRef; (f) J.-N. Zhu, Z.-H. Yang, M. Qi and S.-Y. Zhao, Adv. Synth. Catal., 2019, 361, 868 CrossRef CAS; (g) A. S. Carlson, E.-C. Liu and J. J. Topczewski, J. Org. Chem., 2020, 85, 6044 CrossRef CAS PubMed; (h) Y. Feng, B. Chang, Y. Ren, F. Zhao, K.-H. Wang, J. Wang, D. Huang, X. Lv and Y. Hu, Tetrahedron, 2023, 136, 133353 CrossRef CAS; (i) A. Ejjoummany, J. Elie, A. El Hakmaoui, M. Akssira, S. Routier and F. Buron, Molecules, 2023, 28, 5811 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4mr00075g |
| ‡ GUJ and SJ contributed equally. |
§ General procedure for synthesis of 4: solid hydrazonoyl bromide 2 (1.1 mmol), solid maleimide 3 (1.0 mmol), and solid K2CO3 (1.2 mmol, 166 mg) were placed in a 5 mL stainless steel grinding jar with one stainless steel ball (7 mm diameter). The jar was closed and ball-milled at 22 Hz until the starting maleimide was fully consumed. Then, CH2Cl2 (10 mL) was added, the precipitate was filtered, washed with CH2Cl2 (2 × 10 mL), and the solvent was removed in vacuo. The crude product 4 was purified by filtration through a short silica gel pad (FCC), standard column chromatography (CC) or recrystallized. 5-Phenyl-1-(p-tolyl)-3-trifluoromethyl-3a,6a-dihydropyrrolo[3,4-c]pyrazole-4,6(1H,5H)-dione (4a): reaction time 90 min; FCC (SiO2, petroleum ether/DCM 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); colorless solid, 347 mg (93%); mp 169–170 °C. 1H NMR (600 MHz, CDCl3) δ 2.33 (s, 3H), 4.80 (dq, J = 1.2, 11.5 Hz, 1H), 5.41 (d, J = 11.5 Hz, 1H), 7.16–7.19 (m, 2H), 7.29–7.31 (m, 2H), 7.42–7.50 (m, 5H). 13C{1H} NMR (151 MHz, CDCl3) δ 20.8, 52.2, 66.4, 115.2, 120.2 (q, 1JC–F = 270.0 Hz), 126.3, 129.4, 129.5, 130.0, 131.0, 131.3 (q, 2JC–F = 39.8 Hz), 133.1, 140.3, 169.1, 170.7. 19F NMR (565 MHz, CDCl3): δ −63.6 (s, CF3). IR (neat) ν 1722, 1514, 1498, 1379, 1320, 1193, 1122, 1077, 1040 cm−1. (−)-ESI-MS (m/z): 372.1 (100, [M − H]−). Anal. calcd for C19H14F3N3O2 (373.3): C 61.13, H 3.78, N 11.26; found: C 61.13, H 3.77, N 11.24. 1); colorless solid, 347 mg (93%); mp 169–170 °C. 1H NMR (600 MHz, CDCl3) δ 2.33 (s, 3H), 4.80 (dq, J = 1.2, 11.5 Hz, 1H), 5.41 (d, J = 11.5 Hz, 1H), 7.16–7.19 (m, 2H), 7.29–7.31 (m, 2H), 7.42–7.50 (m, 5H). 13C{1H} NMR (151 MHz, CDCl3) δ 20.8, 52.2, 66.4, 115.2, 120.2 (q, 1JC–F = 270.0 Hz), 126.3, 129.4, 129.5, 130.0, 131.0, 131.3 (q, 2JC–F = 39.8 Hz), 133.1, 140.3, 169.1, 170.7. 19F NMR (565 MHz, CDCl3): δ −63.6 (s, CF3). IR (neat) ν 1722, 1514, 1498, 1379, 1320, 1193, 1122, 1077, 1040 cm−1. (−)-ESI-MS (m/z): 372.1 (100, [M − H]−). Anal. calcd for C19H14F3N3O2 (373.3): C 61.13, H 3.78, N 11.26; found: C 61.13, H 3.77, N 11.24. |
| This journal is © The Royal Society of Chemistry 2025 |