 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reassessing mechanochemical processes in polyatomic systems for smart fabrication of nanocomposites
Mamoru Senna
 *a and
Adam A. L. Michalchuk
*a and
Adam A. L. Michalchuk
 b
b
aFaculty of Science and Technology, Keio University, Yokohoama, 223-8522, Japan. E-mail: senna@applc.keio.ac.jp
bSchool of Chemistry, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK
First published on 30th January 2025
Abstract
A brief review is given to reassess the integrity of the fundamentals and the application in mechanochemistry. The discussion starts from the chemical response of polyatomic systems (PAS) to the mechanical stress, in terms of the extended concept of the Jahn–Teller effects. The interplay between the deformation and the chemical properties was extended to larger scale units toward solid particles or crystallites, which comprise the ensemble and arrangements of PAS. Therefore, the role of disorder of PAS arrangement or defects in solids on the reactivity of solids was discussed. Since the mechanochemical process is based on the introduction and control of the defects in solids, the discussion abridges the fundamental understanding of deformation – chemical property relationship from the basis of molecules with the reactivity of solids and powder masses. By taking the subsequent steps of relaxation in different time scales, the affordability of functional materials via a mechanochemical route has been demonstrated to finalize the entire discussion. A short summary at the end emphasises the importance of learning from the exotic fields.
Introduction
Global environmental concerns are demanding that chemists find new, more sustainable routes to conduct chemistry and manufacture technologically important materials. Under this guise, mechanochemistry has seen immense renewed interest from a much broader community of chemists and materials scientists than ever before. Unsurprisingly, with ‘sustainability’ in mind, the majority of research into mechanochemistry today focuses on its use for molecular and materials synthesis, including the preparation of entirely new chemicals or the modification of materials to enhance/alter their properties.1–6 This has been accompanied by transformative developments in the way chemistry is being conducted, cementing mechanochemistry as a game-changing sustainability technology. Recently published excellent reviews further furnish the tendency.7–18Despite this immense interest in its applications, the development of mechanochemical processes remains largely serendipitous. This stems from the current lack in the understanding of exactly how mechanochemical transformations occur in the first place. Mechanochemical phenomena arise from the interplay between the generation and subsequent relaxation of a mechanical stress that is applied to various polyatomic systems (PAS). Other discussions have been described, aiming to understand ways to control mechanochemical reactivity in solids through the direct perturbation of nuclear geometry. Notably, Rana et al.19 referred to historical discussions based on transition-state theory, and summarized the role of normal and shear stresses on mechanochemical reaction kinetics. After thorough discussion on the role of shear stress, they concluded that the change in reaction rate depends on a modification of the activation volume that includes contributions from both the normal stress and sliding. Boldyrev discussed the related issues in detail.20 Therefore, an overall and unified concept, including anything from the simplest triatomic molecule to bulk solids and organic macromolecules is required. This is the motivation of the present review.
Though much can be learned from the long history of related processes, e.g., in mineral processing, metallurgy and metal physics,21–29 mechanochemistry has now extended to a much broader class of materials, including oxides or chalcogenides,23,30–32 and an immense number of organic compounds.3,33–39 This inevitably requires a reassessment of the phenomena associated with mechanochemical transformations at all scales. It remains to be seen to what degree we can expect the mechanochemical processes of metallurgical systems to inform on the behaviour of soft organic materials upon different types of external mechanical stimuli, with the aim being towards constructing a common basis for understanding mechanochemical processes.
With growing interest in understanding mechanochemical transformations, there has been a surge in the breadth and complexity of the analytical tools being used to probe them, spanning advanced analytical tools such as high-resolution electron microscopy,40–42 in situ structural analyses,43–45 and atomistic simulations.46–51 Despite this remarkable accumulation of knowledge, many gaps remain between experimental and theoretical understanding. This is generally attributed to the multi-length and -time scales associated with mechanochemical phenomena, which span many traditional disciplines of physical sciences from theoretical physics45,52–54 to large-scale process engineering.55–58
At one extreme, mechanochemistry is widely used for particle processing.3,59 Bulk powders are routinely processed by mechanochemical means for mixing and to modify particle size controlling the properties of complex formulations. However, the process of modifying particles at a macroscopic level has direct effects on the underlying atomistic behaviour. As particle size is reduced, continued mechanochemical processing begins to perturb the atomistic structure in the crystallite or particle, often by injecting defects into the crystallite or particle (i.e., plastic deformation). These defects subsequently affect the chemical properties of the material by directly changing the local electronic structure, by changing how a material responds to mechanical stress at the local atomic level, or by influencing the transport of chemical species within the defective lattice of a single crystal,60,61 for example via mechanically generated grain boundaries in polycrystals,62,63 or by promoting transport across the boundaries between particles or crystallites.64–66
Understanding the onset of chemical reactivity under mechanochemical conditions is unfortunately not the end of the complexity. Often, the products that are generated by mechanochemical processes do not coincide with those expected from conventional chemical intuition.67–69 This is exemplified by the mechanochemical control of crystal polymorphism,70–72 the synthesis of cluster-organic frameworks,48,73 and the high entropy stabilization of inorganic materials.74–76 Such unconventional products can make the interpretation of mechanochemical transformations a particular challenge. It follows that, to truly understand the effects of mechanochemical processing, we need to understand the impact of mechanochemical strains, and importantly, the feedback between processes across different lengths of scale.
This is the inspiration for the present review, which aims to compare, combine and reassess the various concepts associated with mechanochemistry from the laboratory to industry, and to find some commonality towards their rational application for understanding the fabrication of advanced materials. The present review begins by breaking down the mechanochemical process and demonstrating – with selected case studies – a set of atomic-level physico-chemical processes that can be initiated by mechanical strain. As we proceed from those concepts to more realistic materials systems, passing through the basics of solid-state chemistry, we inevitably need the ensemble of PAS in the solids and consequent disorder of PAS or defects in the solids. We will have to discuss this in the section between the fundamental PAS-based one and materials-science based application of mechanochemical processes to the fabrication of functional materials. Building upon this foundation, we then explore how mechanical strain alters properties at the scale of particles and powder. Finally, we attempt to break down the boundaries between ‘branches’ of the mechanochemical sciences by using selected case studies to demonstrate how these multi-scale phenomena link together and lead to mechanochemical transformations in functional solids.
Using mechanical stress to modify chemical reactivity at the molecular level
Before we can discuss the effect that mechanical stress has on chemical reactions, we must first agree how chemical reactions are initiated via ‘conventional’ routes. Chemical reactions inhibited by an energy barrier, Ea, are generally associated with thermally excited (temperature, T) translational, rotational, and vibrational energy states of the participating PAS. As these excited states are fully equilibrated, and the systems allow for spontaneous collisions, the reaction rates are generally predictable by classical Arrhenius theory, with kinetics described by a rate constant,
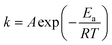 | (1) |
This is to say that Arrhenius theory is generally useful to understand the relation between thermal excitation and reactivity, with particular suitability for describing conventional fluid-phase chemical reactions.77,78 The thermal excitation of translational and rotational degrees of freedom leads to an increase in the number and energy of interatomic/molecular collisions, affecting the pre-exponential factor, A. Similarly, vibrational excitation decreases the effective activation energy, making the reaction more probable. In addition to describing the reaction rates, the existence of a physical model for conventional reactivity also allows us to understand the conditions needed to enhance chemical reactions through other external stimuli. For example, photochemical reactions take place by directly exciting electronic energy states. This has multiple effects, including to impose a ‘pressure’ on the nuclear positions that causes them to rearrange into a new (often more reactive) geometry. From this perspective, a photochemical reaction differs fundamentally from a thermochemical reaction in that the perturbation of the nuclear degrees of freedom occurs as a byproduct of the electronic excitation, not directly as a result of the (thermal) excitation.
This comparison between thermo- and photo-chemistry reminds us of an important reality in chemical systems: that the nuclear and electronic structures are intimately linked, and that the perturbation of either can lead to the onset of chemical changes. It is from this vantage point that we can now explore the link between the nuclear and electronic degrees of freedom and their response to mechanical stress.53
The interplay of mechanical stress and electronic energy states
Jahn and Teller were, in 1937, amongst the first to describe the interplay between the ‘mechanics’ of chemical bonds and electronic states.79 Their description is of course now known widely as the Jahn–Teller (JT) effect. The JT effect describes how the electronic structure can re-organise to enhance atomic stability, and in turn lead to distortion (symmetry breaking) of inter-nuclear interactions:80 i.e. it describes the interplay between the nuclear and electronic degrees of freedom at the atomic level.Gilman subsequently demonstrated an ‘inverse’ of the JT phenomenon by showing in a hypothetical 3-atom molecule that the strength of covalent bonds can be reduced by bending.81,82 This finding led to further studies on how elastic strains can modify the electronic structures of small molecules via symmetry break82 induced by shear stress. With recent advances in molecular simulation, it is now understood that shear deformation of molecules can cause the HOMO–LUMO gap (HLG) to close and ultimately enhance their reactivity.83–86 Though originally proposed for small molecules, this concept linking molecular deformation with enhanced chemical reactivity has been extended to larger molecules and extended solids.87–89
This ‘inverse’ JT effect – i.e. where internuclear distances are mechanically perturbed, causing a distortion of electronic energy states – provides an ideal basis for generalising mechanochemical reactions.47,90,91 The molecular crystal 1,1-diamino-2,2-dinitroethylene (FOX-7) provides an excellent example for this mechanism, Fig. 1. By applying a shear to the crystal structure of FOX-7, one can drive together molecules in adjacent layers. In doing so it was shown that the dissociation energy barrier for FOX-7 disappears, as the crystal is made ‘metallic’ through mechanical deformation (Fig. 1).92
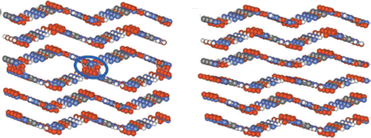 | ||
| Fig. 1 Demonstration of a shear-induced electronic interaction in molecular crystal, 1,1-diamino-2,2-dinitroethylene (FOX-7). (left) Strained (unrelaxed) and (right) unstrained (relaxed) state viewed at ab plane. The “bonds” between O atoms NO2 groups are located on the neighbouring molecules located at the interface at the strained state, as marked by a blue circle. They disappear after the stress was relaxed.92 Reproduced from with ref. 92 with permission from American Physical Society, copyright 2007. | ||
Internal routes to control the Jahn–Teller effect with mechanical stress
Having understood that the inverse JT effect provides a mechanism for mechanochemical reactivity, it is left to identify whether it is possible to control the interplay of mechanical deformation, nuclear displacement, and the eventual distortion of the electronic states and hence molecular stability. For the purpose of clarity, we will define JT type processes caused by mechanical stress as ‘internal’ channels, given that our perturbations influence directly the internal degrees of freedom of our system to induce the electronic response.This concept is shown schematically in Fig. 2 in the form of a contour diagram of the potential energy minima and the isomerization transition state energies.93,94 The ability to selectively pull apart covalent bonds – and hence directly probe the relation of bond length and energy states – has become a particularly vibrant area for the mechanochemistry of macromolecules,95,96 and for detailed studies into the strain-modification of small molecule decomposition.96,97 Though much can be achieved theoretically, this ‘single molecule mechanochemistry’ can be even experimentally probed, for example by attaching molecules to atomic force microscopy set-ups and pulling on individual bonds.
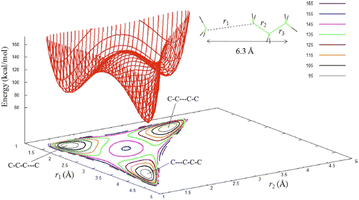 | ||
| Fig. 2 Two-dimensional potential energy surface and contours for n-butane stretched extensively past the C–C inflection points, with the C1 atoms displaced by 2.40 Å from the equilibrium separation of 3.9 Å. The structure at the top/right is for the C–C–C–C minimum.92,97 The numbers at the top/right define the energies in kcal mol−1 for the contour lines. Reproduced from ref. 93 with permission from American Institute of physics, copyright 2008. | ||
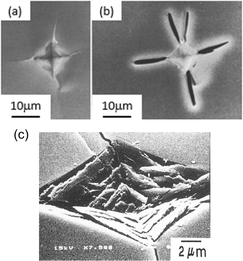
Concepts of single molecule mechanochemistry have been also explored under the guise of the ‘distortion-induced molecular electronic degeneracy (DIMED)’ scheme, proposed by Luty et al.,91 as a particular mechanochemical analogy to the inverse JT effect. In their theoretical study, deformations were applied to an energetic molecule to cause electronic degeneracies across the HLG and hence facilitate electronic excitation. Though not under the specific nomenclature of DIMED, similar concepts of mechanically induced molecular deformations have been also demonstrated for other simple molecules like anthracene and its derivatives, (Fig. 3).98 In related studies of a mechanically induced Diels–Alder reaction, mechanical stress induced changes in the dihedral angles of the reagent anthracene molecule. This mechanical distortion led to significant reduction in the HLG, causing marked enhancement of the reaction rates.99
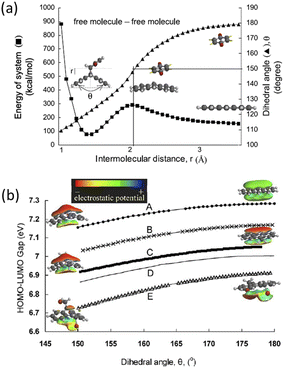 | ||
| Fig. 3 (a) Change in energy of system and dihedral angle of anthracene with decreasing intermolecular distance calculated using AM1 method. The dihedral angle of anthracene at the state of activated complex is 150°; (b) The intramolecular HOMO–LUMO gap of (A) anthracene, (B) 9-methyl anthracene, (C) 9,10-dimethylanthracene, (D) 9,10-bis(chloromethyl) anthracene, and (E) 9,10bis(bromomethyl)anthracene with electron density of (A), (C), and (E).98 Reproduced from ref. 98 with permission from Elsevier B.V., copyright 2004. | ||
It follows that, to understand mechanochemical reactions, we need to elucidate the link between nuclear and electronic degrees of freedom and identify how mechanical strains can affect the former. This necessity is becoming increasingly clear, with attempts to take the lessons learned from single molecule mechanochemistry and extend them to more general mechanochemistry, including for understanding material synthesis.100
Extending mechanochemical models to the solid state
Direct inverse JT effect in solids
Whilst it is relatively straightforward to envision mechanochemical processes applied to individual molecules, the vast majority of mechanochemical research is done on solids. An obvious extension is through the bulk mechanical stressing of solids, for example through the application of pressure (uni-axial or hydrostatic) or shear.37,101 This leads to an entire world of materials science (high pressure science) whose intricacies are beyond the scope of this manuscript, though it suffices to say that mechanical stress can exert enormous influence over atomic positions in solids and lead to a plethora of chemical and physical transformations. The major challenge in the solid state is the difficulty with controlling which strains will yield specific nuclear distortions (and hence which electronic effects), i.e. the solid-state equivalent of the aforementioned direct inverse JT effect. This has meant that our ability to designable mechanochemical reactions in solids is far behind our skill in e.g. single molecule polymer mechanochemistry.102,103This shortcoming has been noted, and recent efforts have shown exciting opportunity to control mechanochemistry in solids by controlling the flexibility in the crystal structure, and hence controlling which degrees of freedom are preferentially distorted by mechanical strain.104 There remains an enormous amount to study and understand with regard to controlling solid state mechanochemical response.
An early attempt to do so was put forward by Butyagin and coworkers.105–107 In their early work, they proposed the concept of energy yield, G,105 defined as the ratio of the amount of the product, ΔN (in moles) to the expended energy or the dose D,
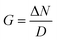 | (2) |
The inverse of the yield
 | (3) |
τ = τ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp([U0 − γσ]/RT) exp([U0 − γσ]/RT)
| (4) |
Indirect inverse JT effect in solids
When considering mechanochemical processes in the solid state, the inverse JT effect does not always occur in response to the direct distortion of the nuclear geometry by e.g. pulling/pushing on the system. Other unusual routes exist to incite this crossover between electronic and vibrational energy, most notably when we consider dynamic mechanical stressing.108 When stress is applied dynamically, one begins to discuss transiently strained geometries, for example through the mechanical excitation of nuclear vibrations. It is here that one begins to find parallels between mechanical and thermal excitations, and underpins major efforts to understand both the simultaneous and separate effects of thermal and mechanical energy.19,36,109,110To probe mechanistic aspects of mechanochemistry, it is desirable to separate the effects of mechanical stressing from the thermal effects. In this respect, explosive chemistry has proved to be a formidable playground. The concept of mechanochemical excitation has been introduced through an up-pumping model and has been explored through DFT simulations for simple PAS like LiN3.35 In this model, mechanical energy is directly absorbed by the solid through excitation of low frequency crystal vibrations. This excess energy then scatters through phonon–phonon collisions and transfers the energy into localised molecular vibrations, causing large amplitude nuclear distortions and hence perturbation of the ‘HLG’, (Fig. 4). Once the correct nuclear distortions are excited, this process can ultimately lead to chemical decomposition.111
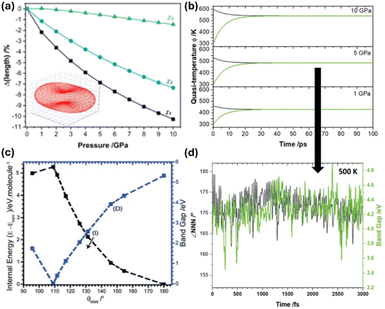 | ||
| Fig. 4 Dynamical mechanochemical excitation of crystalline LiN3.35 (a) The response of the principal strain axes to hydrostatic compression, and (b) the relative effect of different compression magnitudes on the quasi-temperature of mechanochemically excited crystalline LiN3. (c) Excitation of the N3 bending mode can induce transient metallisation of azides, with (d) this bending motion achieving high degrees of excitation following mechanochemical stimuli.35 Reproduced from ref. 35 with permission from Royal Society of Chemistry, copyright 2023. | ||
The excitation of electronic energy states by dynamic mechanical stressing is not unique to explosive chemistry, with such phenomena having been reported historically as triboluminescence113–116 or mechanoluminescence,117–121 (Fig. 5).112 There is growing needs to understand the similarities between ‘mainstream’ mechanochemical reactions and these more specific examples of mechanochemistry, with particular need to identify the general transferability of the underlying physical phenomena that cause them. Further discussion is to be done in view of the features of the solid-state of the materials.
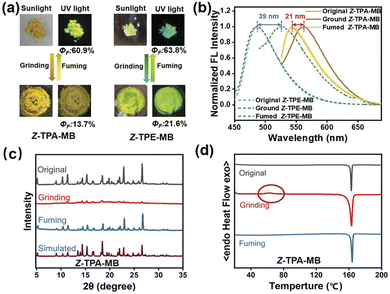 | ||
| Fig. 5 (a) Photographs of the as-prepared and grounded Z-TPA-MB and Z-TPE-MB taken under sunlight and UV light. (b) Emission spectra, (c) powder X-ray diffraction and (d) DSC profiles.112 Reproduced from ref. 112 with permission from Wiley-VCH, copyright 2022. | ||
Role of various defects in solids on the mechanochemical effects
External routes to mechanochemical effects in solids
We have thus far discussed the elementary, molecular level processes by which mechanical energy can incite a chemical response through indirect perturbation of the electronic structure (i.e. by mechanically distorting nuclear geometries). In doing so, we have identified a number of routes by which mechanical perturbations can cause chemical systems to react. We have termed those routes as being ‘internal’ mechanisms, as their effects occur by acting on the internal degrees of freedom of our molecules. However, within the solid state there exist many alternative ‘external’ channels, through which deformation around our molecules can influence mechanochemical reactivity. An obvious example of an external channel to mechanochemical reactivity is through the defect-chemistry of solids.122,123Hierarchical defect structure in realistic solid materials
Most solid-state chemical reactions, and much of solid state functional behaviour, is dominated by the type and quantity of defects within the solid, or the response of defects to mechanical stressing. These defects are hierarchical,124 and discussions related to their role in the reactivity of solids is often confused due to the mismatch of their species and scales. There are yet additional difficulties when discussing the relationship between defects and mechanochemical phenomena. For example, the generation and relaxation of defects in solids are kinetic processes.125 The time period between subsequent ‘pulses’ of mechanical stressing under mechanochemical conditions therefore plays an important role in addition to the hierarchical phenomena.While well-defined defects can be introduced in solids by ion-implantation,126,127 electromagnetic irradiation,128 or hydrostatic compression,129 such practice is generally restricted to model systems and fundamental studies. In most real mechanochemical studies, mechanical stressing is exerted by grinding mills and applied to an ill-defined bulk powder. The stresses are widely spread across the powder, and hence the consequent strain is widely distributed. Under such scenarios we have little understanding of what defects are generated, and in what quantities, despite enormous experimental and computational efforts being devoted to understanding this phenomenon.130–132 Studying the defects generated by mechanochemical processing is a real challenge owing again to their kinetic behaviour. Defects can relax or change once a mechanochemical reactor is stopped and before analysis can be done. It is therefore difficult to identify exactly what defects are generated in situ and their role in a mechanochemical process. This complexity has underpinned much of the recent development of novel methods to probe mechanochemical reactions in situ, for example using time resolved X-ray diffraction, X-ray spectroscopy and vibrational spectroscopies.45,125,133
Effects of microplasticity on mechanical transport
A key mechanism that affects the bulk mechanochemical reactivity of a solid is the efficacy of mass transport (electrons, ions, or molecules) throughout or atop the solid. While these mechanisms are generally well known for semiconductors134 and electroceramics,135 even with atomic-level detail in some cases,136 these processes are strongly affected by mechanical stressing. This is particularly important given the dynamic formation/annihilation of defects during mechanochemical processing, and the particle size-dependence of plastic deformation, and hence the rate of defect formation.137 This size dependence has been shown through numerical simulation, using representative volume element (RVE) methods, for example to probe how a small microstructure affects the broader sample volume.138 For example, an RVE analysis of mechanically stressed Ni–Nb alloy, Fig. 6, shows the strain distribution around different particles. A similar concept is understood under the more generalized concept coined as micro-plasticity,139–141 which provides a general mechanism for many mechanochemical processes induced by ball milling wherein a solid's reactivity is enhanced by particle size reduction and the onset of plasticity.139–141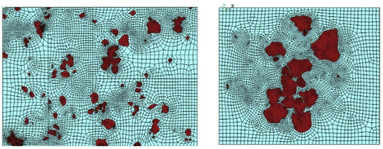 | ||
| Fig. 6 Strain distribution around the different particles in terms of the irregular meshes. The RVE analysis was based on the microstructural SEM images of mechanically strained Ni60Nb40/Mg composites.138 Reproduced from ref. 138, with permission from Elsevier B.V., copyright 2016. | ||
Having understood that mechanochemical processing can induce defect formation and strain in crystals, we are first tasked with understanding the localization of this excess mechanical energy within a bulk solid, and thus the potential consequences mechanical deformation has on solid state chemical reactivity.142,143
In deformable materials, the defects generated by mechanical stressing (causing mechanical energy to be stored) can be distributed throughout the bulk, but is instead localised at microcrack tips in brittle materials. This localisation in brittle materials occurs because the formation of cracks releases the energy that was previously stored locally at the crack tip.144,145 This phenomenon becomes very apparent when a well-defined single crystal surface is indented, (Fig. 7), and subsequently etched, Fig. 7(b). Within the indented deformation, we observe slip bands, where the dislocation density is concentrated. After etching, preferential activation at the slip bands becomes visible as shown in Fig. 7(c). This suggests that, to control mechanochemical excitations that cause sustained energy accumulation, we must find a way to control the local concentration of the lattice defects and amorphized area. Macroscopic deformation is mostly associated with dislocations, i.e., according to their species, concentration, and interactions.146,147 Each dislocation gives rise to inhomogeneous stress and deformation fields at the individual level.146 It is thus obvious that plastic deformation is size dependent and based on the size of the associated dislocation alleys.139 Stress-induced bulk energy change of the solid is initiated with irreversible or inelastic deformation via a slip. In a single crystal, slip occurs at a specific crystallographic plane of lower energy barriers and higher atomic density via dislocation, e.g., (111) in face-centred cubic single crystal, as Wijnen et al. indicated in their detailed computational study.148 In actual mechanical activation with repeated stressing such as in a ball mill, the dislocations are multiplied and entangled, and hence much more complicated, as detailed simulative studies indicated.149
 | ||
| Fig. 7 Scanning electron micrographs of the silicon surface as indented at 100 g, (a) as-indented. (b) Subsequently etched for 165 min in 0.1 N aqueous solution of KOH at 60 °C. (c) Aligned etch pits on the surface of Si (111) indent after etching by 1 N HF aqueous solution.50 Reproduced from ref. 50 with permission from Elsevier B.V., copyright 2020. | ||
To complete the picture, we can finally begin to correlate the effects of mechanical defects with the perturbation of electronic structure and the mobility of charged species within the crystal.142 This link can occur through two main mechanisms, (1) alter local chemical stability and charge density,150 (2) reduced diffusion/mobility barriers that hinder transport (c.f. decomposition of ammonium perchlorate, silver oxalate),151,152 and (3) by changing the local ‘concentration’ gradients of charged species and hence driving diffusion.153 As an example of the first mechanism, we can consider an analogy to the heterogeneous strains associated with the recrystallization of metallic species, as seen through finite elements (FE) simulations, (Fig. 8). There are various types of dislocations displayed here, associated with the recrystallization of metallic species. However, the nuclei-growth mechanisms are common to more general mechanochemical reactions. What is crucial with respect to mechanochemistry is the localized electron distribution, triggering rearrangement of the chemical bonding in a solid-state.50,68,154 Note that the dislocation induced structural change discussed above is not strictly separated from the effects of thermal excitation,155 as discussed previously.
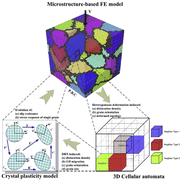 | ||
| Fig. 8 Illustration of the modelling approach of the full coupling 3D crystal plasticity finite element method (CACPFEM).149 Reproduced from ref. 149, with permission from Elsevier B.V., copyright 2016. | ||
Peculiarities of deformation near particle surfaces
Applying mechanical stressing on solids primarily changes the microstructure of the near surface region, including the specific surface area. Since penetration of any chemical species into solids begins from the surface into the interior, it is of utmost importance to examine first of all the chemical changes at the surface or near-surface region.36,156–160 However, the definition of solid surface is very ambiguous. Even at the well-defined surface of the single crystal, there are differences in the chemical bonds in the near surface area, primarily due to spontaneous surface relaxation.161–163 Such a relaxation process can be expedited and controlled, playing an important role for materials synthesis via a mechanochemical route. In addition, atomic movements and their energy states are highly restricted and dominated by those of phonons.Summary of single-particle reactivity
Despite the significant progress made in understanding mechanical deformation and its effect on solid reactivity, we are far from fully understanding the associated elementary mechanisms or obtaining complete experimental validation. There is currently significant effort being devoted to the development of novel methodology to follow mechanochemical reactions in real time, with excellent reviews on the state of the art.133,164,165 At the time of writing, these methods are limited to probing meso-scale phenomena and thus more detailed mechanisms are still to be elucidated.The physical response of solid materials is derived largely from their crystalline packing and both the strength and geometry of the interatomic or intermolecular interactions. Materials that are dominated by non-directional, delocalised interactions tend to be easily deformable (e.g. metals), whereas those with highly directed, strong interactions (e.g. quartz) tend to be more robust to mechanical strain. Molecular crystals are in a more complex situation, where both types of interactions are present. The mechanical response comes from a combination of the response of the weak intermolecular interactions and from the deformation of the molecular entity (comprised of stronger interatomic interactions) itself.
Understanding the mechanical response of a crystal is essential to the onset of mechanochemical reaction, as any perturbation of the atomic positions changes the underlying electronic density distribution, and hence the chemical reactivity itself.166,167 This is exemplified by a recent study that showed the specific mechanical response of a coordination polymer complex.168 Similar studies have also been reported for molecular crystals, in which mechanical deformations are known to decrease the dissociation barriers of covalent interactions.92,94
The direct effect of mechanical stressing of a solid to incite charge density redistribution is just one avenue that can affect material reactivity. Mechanical stressing can also cause imperfections in a crystal structure (mechanical activation) around which electron maldistributions are prominent.169–171 In non-ideal crystals, mechanical stressing can also affect reactivity by enhancing or hindering the mobility of chemical species, either by introducing or ‘clustering’ imperfections,28,172,173 or by introducing strain gradients along which defect or ion conduction is directed. The former is exemplified by the mechanical activation of ammonium perchlorate,174 whose thermal decomposition is greatly enhanced after mechanical treatment, and silver oxalate,175 where both the rate and product of thermal decomposition are sensitive to mechanical activation.
To understand when mechanical activation can be expected, and to what degree, it is important to first discuss various mechanical deformation regimes. In the simplest case, mechanical deformation caused by an external stress is entirely reversible once the stress is removed – i.e. the deformation is elastic. While elastic deformations can cause electronic redistribution during the mechanical stress and can hence transiently influence the material reactivity, there are no long-term effects to reactivity once the stress is released. Pure elastic deformation is relatively uncommon in real-world systems. Instead, mechanical stress tends to induce deformations that are only partly irreversible, known as inelastic deformation.176,177 Such deformations induce lattice imperfections like vacancies, dislocations or stacking faults, accompanied by local, microscopic strain energy. Macroscopically, this is identical with the calorimetrically measurable stored energy. This area was explored extensively by metallurgy and metal physics,178,179 and the concept have only recently expanded into our attempts to understand the reactivity of solid compounds more generally.180,181
Particulate processes
As we begin to extend our discussion towards bulk powder systems, we first consider the macroscopic processes associated with mechanochemical processes in bulk powders. When powders are placed into a milling vessel and the mill is actuated, many different processes take place simultaneously. Qualitatively, we can classify the dominating processes into three main categories: (i) the bulk mixing of the various ingredients and the macro- and meso-scales, (ii) the fracturing of solid particulates resulting in size reduction (which also leads to enhanced mixing), and (iii) the introduction of crystalline imperfection once a comminution threshold has been achieved.137From a chemical perspective, the importance of process (i) is self-evident: one cannot initiate a reaction unless the reagents come into contact with one another. However, unlike in conventional fluid-phase chemistry, these contacts do not occur spontaneously in bulk powders, and must be manufactured through mechanical agitation. The formation of contacts between reagents is facilitated by process (ii). As particles becomes smaller, their accessible surfaces contain a larger proportion of the starting materials and hence lead to more numerous reactive contacts being formed. Importantly, the process of particle size reduction also leads to enhanced reactivity of the powders, noting that surface species are inherently less stable than those residing in the bulk. Finally, as particle sizes are reduced, the associated fracture processes and inelastic deformations lead to permanent deformation of the particle structures, which themselves can enhance or alter the reactivity of solids.182 This very simplistic view of bulk mechanochemical reactivity already demonstrates the extreme complexity of the problem at hand, and the interdependence of processes across lengths of scale and time. From this point of view, we can now break down processes (ii) and (iii) in more detail. We then discuss briefly the mechanisms that underpin them and their role in mechanochemical reactivity, and refer readers to discussions elsewhere for further technical details.183,184
Materials transport across the boundary between crystallites or particles
Diffusion is the main transport mechanism within and between solid-state materials. The mechanisms of these diffusion processes have been studied in great detail for well-defined single crystals of metals, alloys or oxides.60,61,185,186 Studies related to mechanochemical processes are much more complicated. The challenge is exacerbated by the fact that most of the syntheses and functional materials being studied mechanochemically are composites of different components that start from a heterogeneous powder mixture. Understanding these multi-component reactions thus demands understanding beyond diffusion within a crystal, but rather across particle boundaries (grain boundaries and interparticulate interfaces).62,63Fortunately, diffusion across the boundary of heterogeneous solid particulates is well known within the field of mechanical alloying (MA), and much can be learned from the wealth of literature. Conventional explanations of MA are set out in extensive review articles elsewhere,188,189 with some of the key features associated with diffusion in mechanosynthesis and MA exemplified in Fig. 9. Structure evolution during molecular dynamics simulation of MA is shown for approximately equiatomic structure after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 700
000 to 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 time-steps, together with the corresponding radial distribution functions.187 In an ideal system, diffusion occurs along a well-defined grain boundary defined by a vertical line of numbered atom and interstitials denoted by I.190 In contrast to the traditional model of mechanical diffusion of metallic species in the case of MA, mechanistic studies of mechanosynthesis have developed with higher precision.
000 time-steps, together with the corresponding radial distribution functions.187 In an ideal system, diffusion occurs along a well-defined grain boundary defined by a vertical line of numbered atom and interstitials denoted by I.190 In contrast to the traditional model of mechanical diffusion of metallic species in the case of MA, mechanistic studies of mechanosynthesis have developed with higher precision.
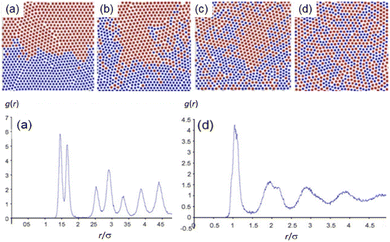 | ||
Fig. 9 Structure evolution during molecular dynamics simulation of mechanical alloying (MA) is shown for approximately equiatomic structure after (a) 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, (b) 150 000, (b) 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, (c) 300 000, (c) 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and (d) 700 000, and (d) 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 steps. Applied impact are random direction (axial and diagonal together). Visual change in the two different species, red and blue, are schematically illustrated in the upper case. Radial distribution functions for (a) and (d) are shown beneath their parent structure.187 Reproduced from ref. 187, with permission from Elsevier B.V., copyright 2008. 000 steps. Applied impact are random direction (axial and diagonal together). Visual change in the two different species, red and blue, are schematically illustrated in the upper case. Radial distribution functions for (a) and (d) are shown beneath their parent structure.187 Reproduced from ref. 187, with permission from Elsevier B.V., copyright 2008. | ||
In a real mechanosynthesis process, one must look more closely at the mechanisms of atomic transfer across the interface of solid compounds within the reacting bulk mixture. Conceptually, atomic transfer is initiated by some activation of the surface (e.g., removing passivating layers, heating surface layers, etc.). Following this activation, the process of atom transfer begins with the transfer of electronic charge from one surface to another.191,192 This transfer is followed rapidly by the shuttling of lighter cationic species in the same direction and across the same boundary, followed by mass transfer of heavier ionic and molecular species, as appropriate.193–195
The elementary mechanisms associated with atom transfer are much more complicated than the ideal model displayed in Fig. 8, and must account for the (1) simultaneous participation of multiple materials, (2) participation of molecular crystals, wherein the interplay of intra- and inter-molecular mechanical response must be understood, (3) continued deformation and restructuring of particle surfaces, (4) transport of chemical species across the boundary of solid particles in response to mechanical stress, and (5) relaxation of the products in the time scale from picoseconds to years.
Given this exceptional degree of complexity, it is not surprising that even today our most detailed views of diffusion during mechanochemical reactions still come from atomistic modelling efforts, which have shown that ionic diffusion across and between particle surfaces is essential for mechanochemical reactivity.196 Significant new developments in experimental methodology and capabilities will be required to truly unravel and confirm the complexity of these diffusion phenomena. However, the emerging trend of using atomic force microscopes and tribometers to carry out carefully controlled mechanochemical reactions, while allowing detailed structural analysis of the reacting surfaces, is particularly promising in this regard.102,197,198
Effects of mechanochemical stress in materials syntheses
To this point we have introduced the physical processes associated with mechanochemical processes in bulk powders, with particular focus on understanding how mass transport phenomena are initiated and proceed in response to mechanical stress. This of course does not account for the chemical phenomena themselves that lead to the onset of mechanochemical reactions, which becomes the focus of this section.54,109,158,199–213 A physical system will always attempt to stabilise itself and relax to its ground energy state via dissipation of energy.214 In realistic chemical process, however, the relaxation toward equilibrium is dominated by kinetics.214 There are many potential relaxation processes, each with their own rate constant, which can vary by orders of magnitude. The relaxation is often observable, such as by photoemission215–217 or through the generation of heat, which can have a feedback effect on the subsequent relaxation rates. Understanding these feedback phenomena and their relative rates is essential for understanding mechanochemical reactivity, given it is this relaxation that controls the properties of the final products of functional solids.216,218,219We must also remember that the rates of energy relaxation can be manipulated based on experimental conditions. This is best exemplified by considering the effects of heating on mechanically treated solids.220–223 Based on conventional thermodynamics, the mechanical stressing of crystalline solids automatically increases the concentration of crystal lattice imperfections and thus increases the Gibbs free energy. When a mechanically activated material is heated, recovery and recrystallization take place, leading to a lowering of the Gibbs free energy,224,225 as discussed in detail in thermal metallurgical processes. This occurs when the stored energy is released, including via the reduction of point defects, annihilation or rearrangement of dislocations, absorption of these defects by grain boundaries, or the reduction of grain boundary area.224–226
Cold working and subsequent annealing are the mainstream of metal industry to optimize the metallic materials for further treatment. Recovery and recrystallization during annealing are much common to the mechanical activation and subsequent heating.227,228 Nuclei-growth is of the largest importance since this predominates the microstructural properties.229,230
Function-oriented studies in this context are reported from different points of view.163,231,232 Most of the diffusion mechanisms in the solid state are based on thermal diffusion at high temperature, while different mechanisms are studied with mechanically driven diffusion.233–235 Note that there are many confusing steps when we realize that mechanochemical processes are automatically and inevitably coupled with thermal processes by local heating during mechanical activation.5,236
Even once we have a model for energy relaxation, we must remember that most mechanochemical processes are initiated under repetitive dynamic stresses. Under these conditions the reaction occurs through the successive excitation and relaxation of a solid. This dynamic behaviour has important implications for our description of thermodynamics in solid state mechanochemical processes. The equilibrium of a chemical reaction is well defined in terms of the law of mass action. In contrast, the terminology of mechanochemical equilibrium does not adhere to these same foundational principles. It is more accurate to discuss a mechanochemical ‘equilibrium’ as a kind of steady state. Size reduction stops at a certain point, which can be explained as a steady state between disintegration and reagglomeration. Another example is in the self-sustaining mechanochemical reaction including polymorphic transformation.237
Given that mechanochemical reactions do not reach a true thermodynamic equilibrium, but rather a (kinetic) steady state, it is important to mind the nature of ‘stability’ in these reactions. Since all solids except perfect single crystals of infinite dimension (which do not exist!) are imperfect, we have to think about the material stability. At a finite temperature, there are only a small number of thermodynamically stable defects.238,239 Since the products obtained from a mechanochemical route contains many crystallographic defects, it becomes important to discuss the concepts not of thermodynamic stability, but of kinetic stability.240–242 In the case of molecular crystals, kinetic stability can be discussed in terms of HLG. A higher HLG implies high kinetic stability and low chemical reactivity due to the lower probability of achieving an activated complex. The concept of molecular stability can be extended to the reactivity of deformed molecules for an organic reaction such as the Diels–Alder type.86 The reactivity of organic solids under mechanical stress has also been explained within the framework of HLG, such as fullerene oxygenation243 or cocrystal formation.244
Role of temperature in mechanochemical processing
From the early stage of mechanistic discussion of mechanochemical processes, the key word “hot spot” appeared. A new concept of a hotspot was recently proposed by Hamilton et al.155 Their proposal under the concept of extemporaneous or ad lib mechanism suggests the possibility of energy localization regions, hotspots, to govern shock initiation and run-to-detonation in energetic materials. Their definition of the hotspot is completely different from the traditional one based on frictional heat.246 Mechanochemical self-explosive reactions fall under a similar concept.247 Direct reactions between elemental metal powders are strongly exothermic and the product phases were formed in a self-sustaining manner.210 It is a mixture of mechanical and thermal reactions.245The technical process of thermal relaxation is called annealing, which is amongst the most important steps of metallurgical processing. A deformed metal stores energy as lattice defects, i.e. dislocations and point defects.24,169 The manner through which energy is released during subsequent heat treatment is controllable and crucial for material design. Processes or changes of state associated with thermal relaxation have been observed from various angles. Microscopically, the streaks of the electron diffraction of Al–3% Mg alloy become clear spots after annealing at 200 °C for 1 h. Spectral change of cubic-Li[InxLiy]Br4 was examined by 7Li NMR line width (full width at half maximum) as a function of temperature, as shown in Fig. 10.245 This exhibits the controlled structure via thermal relaxation. Structural control of LiFeSi2O6 by different combination of mechanical activation and thermal relaxation shown in Fig. 11 demonstrates the possibility of short and long-range ordering.
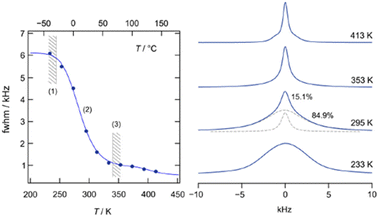 | ||
| Fig. 10 Relaxation and stabilization of deformed cubic-Li[InxLiy]Br4. 7Li NMR line width (full width at half maximum) as a function of temperature; regime (1) denotes the rigid lattice, (2) refers to the motional narrowing regime, and (3) represents the regime of extreme narrowing. Change of the shape of the 7Li NMR spectra with temperature (233, 295, 353, and 413 K). The spectrum at 295 K has been deconvoluted with a sum of a Gaussian and a Lorentzian function (see dashed lines).245 | ||
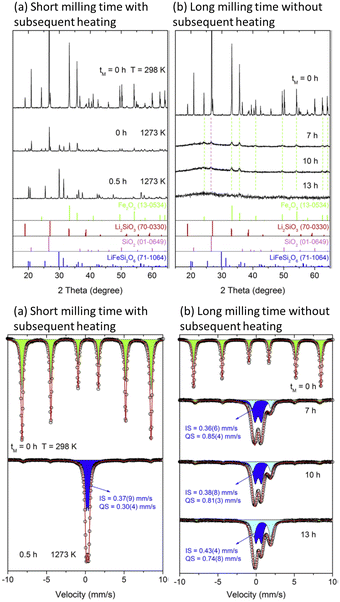 | ||
| Fig. 11 (Top) Difference in the products, LiFeSi2O6 via varying milling-heating conditions. Heating and cooling were carried out in air at the constant rate of 10 K min−1 beween room temperature and 1273 K, without holding at the maximum temperature. (a) Examined by XRD.248 (Bottom) Difference in the products, LiFeSi2O6 via varying milling-heating conditions. (b) Examined by Mössbauer spectra.248 Reproduced from ref. 248 with permission from Elsevier B.V., copyright 2017. | ||
Mechanophores
Mechanically labile molecular entities are coined as mechanophores (MPs).199 The concept belongs to an emerging area of interests in mechanochemistry, not only as a basic component of mechanochemical polymerization,200 but more generally for their potential applications in e.g. stress sensors. MPs are a class of PAS that exhibit chemical changes or reaction in response to external mechanical stimuli.199 Structural changes of PAS upon mechanical stressing like stretching, compression, shear are visible as simple colour change,201,202 photoemission109 or fluorescence,54,203 as a consequence of electron redistribution and molecular deformation including scission or ring deformation. The mechanophores are a visible example of the direct inverse JT effect.The concept of a mechanophore is not restricted to organic molecular crystals but is also observed in covalent crystals.204 For example, metal oxides exhibit 3D covalent networks of metal–oxygen units, typically MO4 tetrahedra or MO6 octahedra. When they are mechanically stressed, different states of the networks occur, leaving several dangling bonds with radicals.158,205,206 Sensitization of MPs is attempted via various routes.205,207,208 Applications of MPs are expanding to numerous technologies, including as smart materials with self-healing,209,210 pharmaceutics211 or medicine.212,213
The transfer of chemical species across a boundary between particles or crystallites (without heating) is achieved by local structural destruction and polarization, which effectively shorten the ‘jumping distance’ that the migrating species must overcome. After arrival of the migrating species on the edge of the neighbouring site (or structural island), they should migrate further into the interior of the new island. The concept of this migration corridor has been proposed in the field of solid electrolytes.249 Understanding what is the real structure of the corridor and how we can tune the corridor to favour the transport of a specific chemical species is a key open question. For the specific case of migrating ionic species, it is a local electric potential that helps tune this corridor. Elucidation and identification of the ionic species in the real crystalline materials with lattice defect by magnetic resonance spectroscopy will contribute to the progress.
Identification of the excited species is another important issue. For instance, singlet oxygen (SOX), the lowest excited electronic state of molecular oxygen and powerful oxidant could play an important role in many organic reactions.250,251 Indeed, in a mechanochemical reaction of fullerene, participation of SOX was verified by EPR.243,252
Choice of starting materials
Insufficient attention has been paid to the selection of starting materials for mechanosynthesis. For the mechanosynthesis of complex oxides, the initial stage is the formation of bridging bonds of two dissimilar cationic species abridged by an oxygen atom.191 For this indispensable step, water molecules play a decisive role.253,254 The use of hydroxides is in most cases beneficial than using conventional oxides for this reason.255–258 By the same token, use of oxyhydroxide of iron, goethite, α-FeOOH, is superior to conventional oxide, hematite, α-Fe2O3. As a matter of fact, replacing of hematite with goethite, α-FeOOH, enabled the product phase pure in the case of mechanosynthesis of LiFeSi2O6.259 For the mechanosynthesis of more complicated structures like the Sr2FeMoO6 double perovskite, it turned out that using metallic Fe powders instead of hematite brought about the higher phase purity.7 For a vast amounts of the research work of mechanosynthesis now days, a planetary mill is used. This is often due to their compact size and high mechanical intensity. However, a conventional planetary mill is not suitable for scaling up for industrial mass production. Efforts are paid for the scale up of the mechanochemical processing.196,260Use of temporary liquid states
Additives are important components of many mechanochemical reactions, particularly when considering the mechanochemical reactivity of molecular solids. It is extraordinarily significant when the additive produces low-melting eutectic compound, bringing about partial and temporal participation of liquid phase and facilitate mixing between reagent molecules at the molecular level (overcoming diffusion barriers of molecules within and between solids). We note that eutectics can also be formed directly between solid reagents without the need for additives, as shown in Fig. 12. Fig. 12A demonstrates an example of a Diels–Alder reaction.238 As shown in Fig. 12B, the starting mixture and the end product were dry powders, while the intermediates are partly wet, due to the formation of eutectics with their melting point close to or lower than room temperature. Autogenous fusion during mechanochemical organic synthesis has also been found in the quinone–phenol systems via charge transfer complex.261 Similar phenomena are reported elsewhere.187,262–264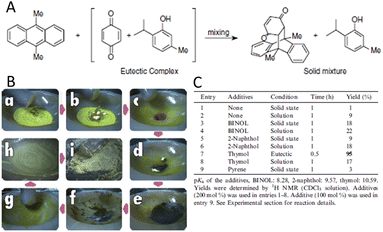 | ||
| Fig. 12 (A) Eutectic Diels–Alder reaction. Crystalline powders of TM (2 mmol), BQ (1 mmol), and DMA (1 mmol) were mixed in an agate mortar and pestle in an ambient condition. (B) Time-resolved observation: (a) quinone powder, (b) TM added, (c) autogenous fusion, (d) DMA added (0 min), (e) 1 min, (f) 5 min, (g) 10 min, (h) 20 min, and (i) reaction completed with solidification (30 min). (C) Effects of additives. Reproduced from ref. 243, with permission from Elsevier B.V., copyright 2006. | ||
A solvent-free process, typically dry grinding of a powder mixture, typically results in strong aggregation, which is detrimental for most applications. Therefore, efforts have been made to maintain or improve the colloidal dispersion states during the formulation process and to avoid post-redispersion treatment. The effects of dispersing surfactants are enhanced when the one co-mills the host particles with the dispersing medium.265
The combination of mechanochemical processing and colloidal dispersion is versatile. Kasprzak et al. succeeded in producing iron nanoparticles by planetary milling after carbon arc treatment with simultaneous surface modification and good dispersion state.157 An innovative process was proposed by combining mechanochemical and colloidal synthesis to produce well-dispersed metal nanoparticles.266–268 Similar methods are considered environmentally friendly as they do not require tedious redispersion steps.185,269,270
Selected case studies
Having demonstrated and discussed the hierarchical mechanisms associated with solid state mechanochemical reactions, we will finish by providing a brief overview of selected application fields of mechanochemical techniques in materials synthesis. It is not our aim to detail the associated chemistry, but rather provide a base for readers to begin exploring a wider scope of martials synthesis made possible by mechanochemistry, that is transformative for achieving global sustainability goals,271 and thus appreciating the sheer breadth of potential applications is of significant importance to the adoption of mechanochemistry across the chemical and materials sciences.Mechanosynthesis of complex oxides
There are many literature examples that report on inorganic mechanosynthesis, with a significant subset of these papers dedicated to the synthesis of oxide nanocomposites.26,195 Those with complicate structures like double perovskite are of particular interest with the initial process of phase emergence exclusively via a mechanochemical route.7,272 In the field of energy storage application, efforts are paid for rational syntheses of the tuneable materials for all solid rechargeable batteries.231,273–275 As mentioned in the previous sections, the milling process serves to homogenize the powder mass, and facilitate the incorporation of small amount of dopants.Organic synthesis for pharmaceutics and biocompatible materials
Scaling up organic synthesis is one of the game-changers in mechanochemistry, leading to significant expansion of the technology. Selected literature illustrates how to transfer from the small scale wet-chemical laboratory experiments to industrially affordable flow processing, and demonstrate the related scientific issues with detailed mechanistic elucidation.39,171,276–278 Twin screw extrusion is emerging as a particularly powerful approach to scaling up mechanochemical reactions for organic and pharmaceutical applications.279–281Metal–organic frameworks and cluster organic materials
Metal–organic frameworks (MOFs) are now spotlighted due to their extensive application capability. Fabrication techniques for those compounds used to be laborious until mechanosynthesis of MOFs has been developed. A number of mechanochemical routes toward MOFs were reported, where addition of small amount of liquids (liquid-assisted grinding, LAG), and ionic additives (ion and liquid-assisted grinding, ILAG), turned out to be effective, as displayed in Fig. 13.282 When metal sites of MOFs are replaced by metal clusters or compound particulates in sub-nanometre size, they are often called as cluster organic materials (COFs).283–285 By extending from MOFs to COFs, their functionality, e.g., as various sensors, will be enhanced.286–288 COFs are also prepared via a mechanochemical route.283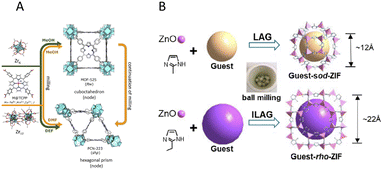 | ||
| Fig. 13 Mechanosynthesis of MOFs (A) mechanochemical reactions of M@TCPP and different zirconium precursors for controllable preparation of MOF-525 or PCN-223.260 Reproduced from ref. 260 with permission from American Chemical Society, copyright 2023. (B) Schematic illustration of the one-pot mechanochemical synthesis of guest-ZIF complexes.282 Reproduced from ref. 282, permission from Elsevier B.V., copyright 2021. | ||
High entropy compounds
High entropy compounds (HEC) are a new class of materials and defined as those with single-phase solid solutions containing more than four or five metallic species.196,289 The concept of entropy stabilization is based on the increasing the configurational entropy of the resulting compounds.290–292 There is a related, but a bit less strict definition of the similar compounds under the name of multiphase compositionally complex (MCC).293–295 Most HECs or MCCs are intermetallic compounds (high entropy alloys, HEA).296–298 However, oxides (HEO) or chalcogenides are also joined to HECs.299 The apparent stabilization of HECs is thought to originate from the high configurational entropy and slow diffusion, which increases the activation energy of nucleation for recrystallization.196,300 Thus, the concept of entropic stabilization is close to that of kinetic stabilization which we discussed above. It should be emphasized that mechanochemical technologies are understood to be very suitable for the preparation of HECs given the dominance of kinetic stabilisation.30 An example of mechanosynthesis of HEO, (NiMgCuZnCo)O, by milling an oxide mixture of ingredient metallic species is displayed in Fig. 14.222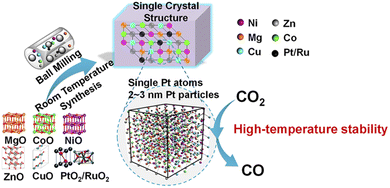 | ||
| Fig. 14 Mechanochemical Synthesis of Pt/Ru-(NiMgCuZnCo)O entropy-stabilized metal oxide solid solution.222 Reproduced from ref. 222, with permission from Am. Chem. Soc., copyright 2019. | ||
Non-firing ceramics and building materials
Several mechanochemical technologies for ceramics materials synthesis at room temperature are reported.301–303 Change in the surface silanol during milling the starting material is known to play a key role.304 Geopolymer is a kind of non-firing cements formed due to reaction between alumino-silicates and oxides with alkaline media, where mechanical activation plays an important role.305,306 Mechanochemical activation also serves positive role for the modification of geopolymers with polymeric materials as shown in Fig. 15.307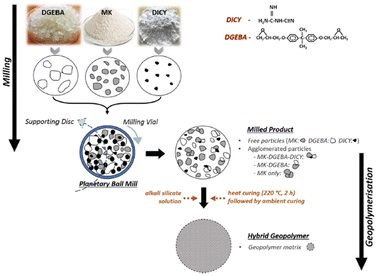 | ||
| Fig. 15 Mechanosynthesis scheme of geopolymer–epoxide composites.307 Reproduced from ref. 307, with permission from Elsevier B.V., copyright 2022. | ||
Biopolymers from biomass
Reduction of petroleum-derived polymeric species is another urgent problem in conjunction with SDGs. It could be done by replacing with biopolymers made from biomass resources or wastes.308,309 Energy intensive process of controlled disintegration of cellulose of chitosan for repolymerization is the main role of mechanical activation, as exemplified in Fig. 16.308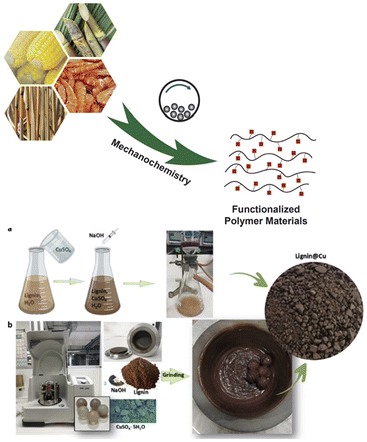 | ||
| Fig. 16 Mechanochemical conversion from biomass to functional composites. (a) Concept, and (b) explicit example.308Reproduced from ref. 308, with permission from Wiley-VCH, copyright 2022. | ||
Replacing rare-earth with ubiquitous metals
Efforts to replace precious or rare earth metals with more ubiquitous materials are to be recommended and respected. Rare-earth-free phosphors are complex oxide, nitride or chalcogenide with small amounts of dopants.310–312 The homogeneous incorporation of small amounts of dopants is suitable for a mechanochemical procedure. Care should be taken to avoid abrasion contamination during milling inducing brightness loss.Concluding remarks
In this brief review, an effort has paid to present an integrated concept abridging elementary processes of electronic excitation by mechanical stressing and green industrial processing via mechanochemical routes. Fundamental studies of the mechanisms of mechanochemical processes, both theoretical and experimental, are laborious and time-consuming, but indispensable to a fruitful applicational goal. By learning from “exotic fields”, science and technology in mechanochemistry, with its truly interdisciplinary nature, will continue to flourish.Data availability
The authors confirm that the data supporting the findings of this study are available within the article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge all of the colleagues for their contributions to developing this field.References
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friscic, F. Grepioni, K. D. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC.
- T. Friscic, Chem. Soc. Rev., 2012, 41, 3493–3510 RSC.
- L. Takacs, Chem. Soc. Rev., 2013, 42, 7649–7659 RSC.
- Z. Wang, Z. Li, M. Ng and P. J. Milner, Dalton Trans., 2020, 49, 16238–16244 RSC.
- P. Y. Butyagin, Colloid J., 2006, 68, 397–403 CrossRef CAS.
- M. K. Beyer, J. Chem. Phys., 2000, 112, 7307–7312 CrossRef CAS.
- E. Tothova, A. Duvel, R. Witte, R. A. Brand, A. Sarkar, R. Kruk, M. Senna, K. L. Da Silva, D. Menzel, V. Girman, M. Hegedus, M. Balaz, P. Makreski, S. Kubuki, M. Kanuchova, J. Valicek, H. Hahn and V. Sepelak, Front. Chem., 2022, 10, 846910 CrossRef CAS PubMed.
- N. Fantozzi, J. N. Volle, A. Porcheddu, D. Virieux, F. Garcia and E. Colacino, Chem. Soc. Rev., 2023, 52, 6680–6714 RSC.
- O. V. Lapshin, E. V. Boldyreva and V. V. Boldyrev, Russ. J. Inorg. Chem., 2021, 66, 433–453 CrossRef CAS.
- S. Mateti, M. Mathesh, Z. Liu, T. Tao, T. Ramireddy, A. M. Glushenkov, W. Yang and Y. I. Chen, Chem. Commun., 2021, 57, 1080–1092 RSC.
- R. T. O'Neill and R. Boulatov, Nat. Rev. Chem, 2021, 5, 148–167 CrossRef PubMed.
- X. Liu, Y. Li, L. Zeng, X. Li, N. Chen, S. Bai, H. He, Q. Wang and C. Zhang, Adv. Mater., 2022, 34, e2108327 CrossRef PubMed.
- V. Martinez, T. Stolar, B. Karadeniz, I. Brekalo and K. Uzarevic, Nat. Rev. Chem, 2023, 7, 51–65 CrossRef CAS PubMed.
- E. Boldyreva, Faraday Discuss., 2023, 241, 9–62 RSC.
- S. L. Craig, Faraday Discuss., 2023, 241, 485–491 RSC.
- S. Pagola, Crystals, 2023, 13, 124 CrossRef CAS.
- Y. S. Zholdassov, R. W. Kwok, M. A. Shlain, M. Patel, M. Marianski and A. B. Braunschweig, RSC Mechanochem., 2024, 1, 11–32 RSC.
- E. V. Boldyreva, in Ref. Module Chem Molec Sci Chem Eng, 2024, DOI:10.1016/b978-0-443-15742-4.00114-9.
- R. Rana, N. Hopper, F. Sidoroff, J. Cayer-Barrioz, D. Mazuyer and W. T. Tysoe, Tribol. Lett., 2024, 72, 76 CrossRef.
- V. V. Boldyrev, Russ. Chem. Rev., 2006, 75, 177–189 CrossRef CAS.
- V. Boldyrev and K. Meyer, Festkörperchemie – Beiträge aus Forshung und Praxis, VEB Deutcher Verlag für Grundstoffindustrie, Leipzig, 1973 Search PubMed.
- P. Baláž, A. Aláčová, M. Achimovičová, J. Ficeriová and E. Godočíková, Hydrometallurgy, 2005, 77, 9–17 CrossRef.
- V. Boldyrev and K. Tkacova, J. Mater. Synth. Process., 2000, 8, 121–132 CrossRef CAS.
- L. Takacs, J. Mater. Synth. Process., 2000, 8, 181–188 CrossRef CAS.
- A. Godfrey and Q. Liu, Scr. Mater., 2009, 60, 1050–1055 CrossRef CAS.
- P. Balaz, M. Achimovicova, M. Balaz, P. Billik, Z. Cherkezova-Zheleva, J. M. Criado, F. Delogu, E. Dutkova, E. Gaffet, F. J. Gotor, R. Kumar, I. Mitov, T. Rojac, M. Senna, A. Streletskii and K. Wieczorek-Ciurowa, Chem. Soc. Rev., 2013, 42, 7571–7637 RSC.
- N. Hansen and D. Juul Jensen, Mater. Sci. Technol., 2013, 27, 1229–1240 CrossRef.
- V. Boldyrev, Reactivity of Solids: Past, Present and Future, Blackwell Science, Oxford, U.K., 1996 Search PubMed.
- V. I. Levitas, Phys. Lett. A, 2004, 327, 180–185 CrossRef CAS.
- L. Lin, K. Wang, R. Azmi, J. Wang, A. Sarkar, M. Botros, S. Najib, Y. Cui, D. Stenzel, P. Anitha Sukkurji, Q. Wang, H. Hahn, S. Schweidler and B. Breitung, J. Mater. Sci., 2020, 55, 16879–16889 CrossRef CAS.
- H.-J. Choi, K.-M. Lee, G.-H. Kim and J.-G. Lee, J. Am. Ceram. Soc., 2001, 84, 242–244 CrossRef CAS.
- A. Moure, T. Hungría, A. Castro, J. Galy, O. Peña, I. Martínez, J. Tartaj and C. Moure, Ceram. Int., 2012, 38, 1507–1513 CrossRef CAS.
- K. Brandhorst and J. Grunenberg, Chem. Soc. Rev., 2008, 37, 1558–1567 RSC.
- A. A. L. Michalchuk, E. V. Boldyreva, A. M. Belenguer, F. Emmerling and V. V. Boldyrev, Front. Chem., 2021, 9, 685789 CrossRef CAS PubMed.
- A. A. L. Michalchuk, Faraday Discuss., 2023, 241, 230–249 RSC.
- F. H. Bhuiyan, Y. S. Li, S. H. Kim and A. Martini, Faraday Discuss., 2023, 241, 194–205 RSC.
- B. A. Steele, N. Goldman, I. W. Kuo and M. P. Kroonblawd, Chem. Sci., 2020, 11, 7760–7771 RSC.
- Z. Todres, Organic Mechanochemistry and its Practical Application, CRC Press, 2006 Search PubMed.
- J. Andersen and J. Mack, Green Chem., 2018, 20, 1435–1443 RSC.
- M. E. Rivas, C. Blakiston, R. T. Seljamae-Green, T. D. Tran, D. Thompsett, S. Day, E. Bilbe and J. Fisher, Faraday Discuss., 2023, 241, 341–356 RSC.
- D. Hasa, E. Carlino and W. Jones, Cryst. Growth Des., 2016, 16, 1772–1779 CrossRef CAS.
- W. Meng, W. Yuan, Z. Wu, X. Wang, W. Xu, L. Wang, Q. Zhang, C. Zhang, J. Wang and Q. Song, Powder Technol., 2019, 347, 130–135 CrossRef CAS.
- S. Lukin, L. S. Germann, T. Friscic and I. Halasz, Acc. Chem. Res., 2022, 55, 1262–1277 CrossRef CAS PubMed.
- M. Rautenberg, B. Bhattacharya, J. Witt, M. Jain and F. Emmerling, CrystEngComm, 2022, 24, 6747–6750 RSC.
- A. A. L. Michalchuk and F. Emmerling, Angew Chem. Int. Ed. Engl., 2022, 61, e202117270 CrossRef CAS PubMed.
- S. Okuda, Y. Inoue, T. Watanabe and T. Adachi, Interface Focus, 2015, 5, 20140095 CrossRef PubMed.
- Y. Li, R. K. Kalia, M. Misawa, A. Nakano, K. Nomura, K. Shimamura, F. Shimojo and P. Vashishta, Nanoscale, 2016, 8, 9714–9720 RSC.
- N. A. Alemasov, N. V. Ivanisenko, B. Taneja, V. Taneja, S. Ramachandran and V. A. Ivanisenko, J. Mol. Graphics Modell., 2019, 86, 247–255 CrossRef CAS PubMed.
- M. F. Sk, N. A. Jonniya, R. Roy, S. Poddar and P. Kar, Front. Mol. Biosci., 2020, 7, 590165 CrossRef CAS PubMed.
- S. Nordholm and G. Bacskay, Molecules, 2020, 25, 2667 CrossRef CAS PubMed.
- N. Hawthorne, S. Banerjee, Q. Moore, A. M. Rappe and J. D. Batteas, J. Phys. Chem. C, 2022, 126, 17569–17578 CrossRef CAS.
- J. Ribas-Arino, M. Shiga and D. Marx, Angew Chem. Int. Ed. Engl., 2009, 48, 4190–4193 CrossRef CAS PubMed.
- G. Subramanian, N. Mathew and J. Leiding, J. Chem. Phys., 2015, 143, 134109 CrossRef PubMed.
- H. Sugita, Y. Lu, D. Aoki, H. Otsuka and K. Mikami, Chemistry, 2023, 29, e202300230 CrossRef PubMed.
- J. F. Reynes, V. Isoni and F. Garcia, Angew Chem. Int. Ed. Engl., 2023, e202300819, DOI:10.1002/anie.202300819.
- P. Baláž and E. Dutková, Miner. Eng., 2009, 22, 681–694 CrossRef.
- C. F. Burmeister and A. Kwade, Chem. Soc. Rev., 2013, 42, 7660–7667 RSC.
- E. Colacino, M. Carta, G. Pia, A. Porcheddu, P. C. Ricci and F. Delogu, ACS Omega, 2018, 3, 9196–9209 CrossRef CAS PubMed.
- E. Ivanov and C. Suryanarayana, J. Mater. Synth. Process., 2000, 8, 235–244 CrossRef CAS.
- Y. Oishi and W. D. Kingery, J. Chem. Phys., 1960, 33, 480–486 CrossRef CAS.
- G. W. Tomlins, J. L. Routbort and T. O. Mason, J. Am. Ceram. Soc., 2005, 81, 869–876 CrossRef.
- R. E. Mistler and R. L. Coble, J. Appl. Phys., 1974, 45, 1507–1509 CrossRef CAS.
- R. W. Balluffi, Metall. Trans. B, 1982, 13, 527–553 CrossRef.
- T. Watanabe, N. Wakiyama, A. Kusai and M. Senna, Powder Technol., 2004, 141, 227–232 CrossRef CAS.
- K. R. Krishnadas, A. Baksi, A. Ghosh, G. Natarajan, A. Som and T. Pradeep, Acc. Chem. Res., 2017, 50, 1988–1996 CrossRef CAS PubMed.
- B. J. Henz, T. Hawa and M. R. Zachariah, J. Appl. Phys., 2010, 107, 024901 CrossRef.
- S. Baghel, H. Cathcart and N. J. O'Reilly, J. Pharm. Sci., 2016, 105, 2527–2544 CrossRef CAS PubMed.
- D. Ferro-Costas, A. M. Pendas, L. Gonzalez and R. A. Mosquera, Phys. Chem. Chem. Phys., 2014, 16, 9249–9258 RSC.
- O. I. Watanabe T, N. Wakiyama, A. Kusai and M. Senna, Int. J. Pharm., 2002, 241, 103–111 CrossRef PubMed.
- A. P. Amrute, Z. Łodziana, H. Schreyer, C. Weidenthaler and F. Schüth, Science, 2019, 366, 485–489 CrossRef CAS PubMed.
- A. M. Belenguer, T. Friščić, G. M. Day and J. K. M. Sanders, Chem. Sci., 2011, 2, 696–700 RSC.
- Y. Teoh, G. Ayoub, I. Huskic, H. M. Titi, C. W. Nickels, B. Herrmann and T. Friscic, Angew Chem. Int. Ed. Engl., 2022, 61, e202206293 CrossRef CAS PubMed.
- R. Mao, F. Zhan, N. Bu, Y. Cao, P. Hu, G. Gong and Q. Zhen, Mater. Lett., 2016, 173, 111–114 CrossRef CAS.
- A. Bonadio, C. A. Escanhoela, F. P. Sabino, G. Sombrio, V. G. de Paula, F. F. Ferreira, A. Janotti, G. M. Dalpian and J. A. Souza, J. Mater. Chem. A, 2021, 9, 1089–1099 RSC.
- M. Fu, X. Ma, K. Zhao, X. Li and D. Su, iScience, 2021, 24, 102177 CrossRef CAS PubMed.
- D. B. Miracle and O. N. Senkov, Acta Mater., 2017, 122, 448–511 CrossRef CAS.
- A. K. Galwey and M. E. Brown, Thermochim. Acta, 2002, 386, 91–98 CrossRef CAS.
- R. A. Luirink, M. C. A. Verkade-Vreeker, J. N. M. Commandeur and D. P. Geerke, Chembiochem, 2020, 21, 1461–1472 CrossRef CAS PubMed.
- E. Teller and H. A. Jahn, Proc. R. Soc. London, Ser. A, 1937, 161, 220–235 Search PubMed.
- E. Teller, An Historical Note, in The Jahn-Teller Effect in Molecules and Crystals, Wiley, London, 1972 Search PubMed.
- J. J. Gilman, Science, 1996, 274, 65 CrossRef CAS.
- J. J. Gilman, Mater. Sci. Technol., 2013, 22, 430–437 CrossRef.
- M. Jabbarzadeh Sani and A. H. Pakiari, Comput. Theor. Chem., 2018, 1136–1137, 18–28 CrossRef CAS.
- M. P. Kroonblawd and N. Goldman, Phys. Rev. B, 2018, 97, 184106 CrossRef CAS.
- B.-G. Kim, X. Ma, C. Chen, Y. Ie, E. W. Coir, H. Hashemi, Y. Aso, P. F. Green, J. Kieffer and J. Kim, Adv. Funct. Mater., 2013, 23, 439–445 Search PubMed.
- J. Aihara, J. Phys. Chem. A, 1999, 103, 7487–7495 CrossRef CAS.
- I. B. Bersuker, Chem. Rev., 2001, 101, 1067–1114 Search PubMed.
- I. B. Bersuker, Chem. Rev., 2020, 121, 1463–1512 CrossRef PubMed.
- I. B. Bersuker, Phys. Chem. Chem. Phys., 2023, 25, 1556–1564 Search PubMed.
- K. Shimamura, M. Misawa, Y. Li, R. K. Kalia, A. Nakano, F. Shimojo and P. Vashishta, Appl. Phys. Lett., 2015, 107, 231903 Search PubMed.
- T. Luty, P. Ordon and C. J. Eckhardt, J. Chem. Phys., 2002, 117, 1775–1786 CrossRef CAS.
- M. M. Kuklja and S. N. Rashkeev, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 104111 CrossRef.
- U. Lourderaj, J. L. McAfee and W. L. Hase, J. Chem. Phys., 2008, 129, 094701 Search PubMed.
- M. M. Kuklja and S. N. Rashkeev, Appl. Phys. Lett., 2007, 90, 151913 Search PubMed.
- C. Opera, F. Dan and F. Dan, Macromolecular Mechanochemistry, Cambridge Int. Sci. Publ., 2007 Search PubMed.
- H. Hu, Z. Ma and X. Jia, Mater. Chem. Front., 2020, 4, 3115–3129 RSC.
- A. Levy, H. Goldstein, D. Brenman and C. E. Diesendruck, J. Polym. Sci., 2020, 58, 692–703 CrossRef CAS.
- M. F. Pradipta, H. Watanabe and M. Senna, Solid State Ionics, 2004, 172, 169–172 CrossRef CAS.
- M. J. S. Dewar, E. F. Healy, A. J. Holder and Y. C. Yuan, J. Comput. Chem., 2004, 11, 541–542 CrossRef.
- S. H. Oh, S. H. Jeon, W. I. Cho, C. S. Kim and B. W. Cho, J. Alloys Compd., 2008, 452, 389–396 CrossRef CAS.
- J. A. Ciezak-Jenkins and T. A. Jenkins, J. Appl. Phys., 2018, 123, 085901 CrossRef.
- G. De Bo, Chem. Sci., 2018, 9, 15–21 RSC.
- I. M. Klein, C. C. Husic, D. P. Kovacs, N. J. Choquette and M. J. Robb, J. Am. Chem. Soc., 2020, 142, 16364–16381 CrossRef CAS PubMed.
- H. Yan, F. Yang, D. Pan, Y. Lin, J. N. Hohman, D. Solis-Ibarra, F. H. Li, J. E. P. Dahl, R. M. K. Carlson, B. A. Tkachenko, A. A. Fokin, P. R. Schreiner, G. Galli, W. L. Mao, Z. X. Shen and N. A. Melosh, Nature, 2018, 554, 505–510 CrossRef CAS PubMed.
- P. Y. Butyagin and A. N. Streletski, Phys. Solid State, 2005, 47, 856–862 CrossRef CAS.
- P. Y. Butyagin and A. Dubinskaya, Advances in Mechanochemistry: Physical and Chemical Processes under Deformation, Harwood Academic Publishers, Reading, UK, 1998 Search PubMed.
- P. Y. Butyagin, J. Mater. Synth. Process., 2000, 8, 205–211 CrossRef CAS.
- X. Hu, M. E. McFadden, R. W. Barber and M. J. Robb, J. Am. Chem. Soc., 2018, 140, 14073–14077 CrossRef CAS PubMed.
- T. Muramatsu, Y. Sagara, H. Traeger, N. Tamaoki and C. Weder, ACS Appl. Mater. Interfaces, 2019, 11, 24571–24576 CrossRef CAS PubMed.
- Y. S. Zholdassov, L. Yuan, S. R. Garcia, R. W. Kwok, A. Boscoboinik, D. J. Valles, M. Marianski, A. Martini, R. W. Carpick and A. B. Braunschweig, Science, 2023, 380, 1053–1058 CrossRef CAS PubMed.
- A. A. L. Michalchuk, S. Rudic, C. R. Pulham and C. A. Morrison, Phys. Chem. Chem. Phys., 2018, 20, 29061–29069 RSC.
- W. Luo, Y. Tang, X. Zhang, Z. Wu and G. Wang, Adv. Opt. Mater., 2022, 11, 2202259 CrossRef.
- Z. JI, Acc. Chem. Res., 1978, 11, 289–293 CrossRef.
- Y. Xie and Z. Li, Chem, 2018, 4, 943–971 Search PubMed.
- Y. Zhang, L. Ma, K. Wang, X. Xu, Y. Gao, S. Wen and J. Luo, J. Lumin., 2017, 182, 22–28 CrossRef CAS.
- H. Wu, K. Huang, J. Li, F. Jiang, X. Zhao, L. Wang and S. Jiang, RSC Adv., 2018, 8, 1436–1442 RSC.
- A. A. Tukhbatullin, G. L. Sharipov, A. M. Abdrakhmanov and M. R. Muftakhutdinov, Opt. Spectrosc., 2014, 116, 691–694 CrossRef CAS.
- S. Kato, K. Ishizuki, D. Aoki, R. Goseki and H. Otsuka, ACS Macro Lett., 2018, 7, 1087–1091 CrossRef CAS PubMed.
- W. Yuan, Y. Yuan, F. Yang, M. Wu and Y. Chen, Macromolecules, 2018, 51, 9019–9025 CrossRef CAS.
- F. Yang, Y. Yuan, R. P. Sijbesma and Y. Chen, Macromolecules, 2020, 53, 905–912 CrossRef CAS.
- W. Wang, Z. Wang, J. Zhang, J. Zhou, W. Dong and Y. Wang, Nano Energy, 2022, 94, 106920 CrossRef CAS.
- H. Schmalzried, Pure Appl. Chem., 2000, 72, 2137–2147 CrossRef CAS.
- J. Maier, Angew Chem. Int. Ed. Engl., 2003, 32, 313–335 CrossRef.
- V. Gutman and G. Resch, in Reactivity of Solids: Past, Present and Future, ed. V. V. Boldyrev, Blackwell Science, 1996, ch. 1, pp. 1–13 Search PubMed.
- R. P. Gupta, Phys. Rev. B: Condens. Matter Mater. Phys., 1981, 23, 6265 Search PubMed.
- M. Usman, A. Hallén and A. Nazir, J. Phys. D: Appl. Phys., 2015, 48, 455107 Search PubMed.
- M. J. Tadjer, J. L. Lyons, N. Nepal, J. A. Freitas, A. D. Koehler and G. M. Foster, ECS J. Solid State Sci. Technol., 2019, 8, Q3187–Q3194 CrossRef.
- I. J. Beyerlein, M. J. Demkowicz, A. Misra and B. P. Uberuaga, Prog. Mater. Sci., 2015, 74, 125–210 CrossRef CAS.
- F. T. Vas'ko and M. V. Strikha, J. Exp. Theor. Phys., 2008, 106, 562–568 CrossRef.
- W. Herrmann, H. Schubert and H. Rumpf, Powder Technol., 1973, 11, 121–131 Search PubMed.
- J. Bridgwater, Powder Technol., 1976, 15, 215–236 CrossRef.
- N. A. Fleck, J. Mech. Phys. Solids, 1995, 43, 1409–1430 Search PubMed.
- C. Weidenthaler, Crystals, 2022, 12, 345 CrossRef CAS.
- T. Y. Tan, U. Gosele and S. Yu, Crit. Rev. Solid State Mater. Sci., 1991, 17, 47–106 CrossRef CAS.
- J. L. M. Rupp, Solid State Ionics, 2012, 207, 1–13 CrossRef CAS.
- I. Chesser, R. K. Koju and Y. Mishin, Int. J. Mater. Res., 2024, 115, 85–105 CrossRef CAS.
- V. V. Boldyrev, S. V. Pavlov and E. L. Goldberg, Int. J. Miner. Process., 1996, 44–45, 181–185 CrossRef CAS.
- J. Nafar Dastgerdi, G. Marquis, B. Anbarlooie, S. Sankaranarayanan and M. Gupta, Compos. Struct., 2016, 142, 130–139 CrossRef.
- S. D. Mesarovic, S. Forest and J. P. Jaric, Proc. R. Soc. London, Ser. A, 2015, 471, 20140868 Search PubMed.
- K. Ariga, J. Li, J. Fei, Q. Ji and J. P. Hill, Adv. Mater., 2016, 28, 1251–1286 CrossRef CAS PubMed.
- R. Maaß and P. M. Derlet, Acta Mater., 2018, 143, 338–363 CrossRef.
- Y. Liu, P. Liu, H. X. Lei, Y. Y. Qu, Y. Tan and F. Chen, Appl. Surf. Sci., 2022, 599, 153940 Search PubMed.
- B. K. Zhang, R. Tan, L. Y. Yang, J. X. Zheng, K. C. Zhang, S. J. Mo, Z. Lin and F. Pan, Energy Storage Mater., 2018, 10, 139–159 Search PubMed.
- Y. Xu, W. Wan and F. P. E. Dunne, J. Mech. Phys. Solids, 2021, 146, 104209 Search PubMed.
- J. Shen, H. Fan, J. Wang, G. Zhang, R. Pan and Z. Huang, Int. J. Fatigue, 2023, 171, 179590 CrossRef.
- H. Y. Gao H, Scr. Mater., 2003, 48, 113–118 CrossRef.
- J. Amodeo, S. Merkel, C. Tromas, P. Carrez, S. Korte-Kerzel, P. Cordier and J. Chevalier, Crystals, 2018, 8, 240 Search PubMed.
- J. Wijnen, R. H. J. Peerlings, J. P. M. Hoefnagels and M. G. D. Geers, Int. J. Solids Struct., 2021, 228, 111094 CrossRef.
- H. Li, X. Sun and H. Yang, Int. J. Plast., 2016, 87, 154–180 CrossRef CAS.
- J. Wan, Y. Wang, H. Zhang and Y. Wang, Chem. Eng. J., 2023, 470, 144151 CrossRef CAS.
- M. Rossberg, E. F. Khairetdinov, E. Linke and V. V. Boldyrev, J. Solid State Chem., 1982, 41, 266–271 CrossRef CAS.
- V. V. Boldyrev, Thermochim. Acta, 2006, 443, 1–36 CrossRef CAS.
- R. W. Balluffi and A. L. Ruoff, Appl. Phys. Lett., 1962, 1, 59–60 CrossRef.
- A. M. Pendas and E. Francisco, Chemphyschem, 2019, 20, 2722–2741 CrossRef CAS PubMed.
- B. W. Hamilton, M. P. Kroonblawd and A. Strachan, J. Phys. Chem. Lett., 2022, 13, 6657–6663 CrossRef CAS PubMed.
- T. R. Huff, H. Labidi, M. Rashidi, M. Koleini, R. Achal, M. H. Salomons and R. A. Wolkow, ACS Nano, 2017, 11, 8636–8642 Search PubMed.
- A. Kasprzak, K. Fateyeva, M. Bystrzejewski, W. Kaszuwara, M. Fronczak, M. Koszytkowska-Stawinska and M. Poplawska, Dalton Trans., 2018, 47, 11190–11202 RSC.
- F. Delogu, J. Phys. Chem. C, 2011, 115, 21230–21235 CrossRef CAS.
- Y. Xie, Y. Jin and L. Xiang, ACS Appl. Mater. Interfaces, 2019, 11, 14796–14802 CrossRef CAS PubMed.
- R. Rana, R. Bavisotto, K. Hou, N. Hopper and W. T. Tysoe, Chem.: Methods, 2021, 1, 340–349 CAS.
- M. Gomes and R. F. Albuquerque, Physica A, 2002, 310, 377–383 Search PubMed.
- K. Kádas, Z. Nabi, S. K. Kwon, L. Vitos, R. Ahuja, B. Johansson and J. Kollár, Surf. Sci., 2006, 600, 395–402 Search PubMed.
- L. Qin, Z. Cheng, J. A. Fan, D. Kopechek, D. Xu, N. Deshpande and L.-S. Fan, J. Mater. Chem. A, 2015, 3, 11302–11312 Search PubMed.
- I. Akhmetova, K. Schutjajew, M. Wilke, A. Buzanich, K. Rademann, C. Roth and F. Emmerling, J. Mater. Sci., 2018, 53, 13390–13399 CrossRef CAS.
- K. J. Ardila-Fierro and J. G. Hernandez, Angew Chem. Int. Ed. Engl., 2024, 63, e202317638 Search PubMed.
- G. Will and R. Platzbecker, Z. Anorg. Allg. Chem., 2001, 627, 2207–2210 Search PubMed.
- T. Shang, D. Xiao, Q. Zhang, X. Wang, D. Su and L. Gu, Chin. Phys. B, 2021, 30, 100882 Search PubMed.
- G. K. Kole and J. J. Vittal, Chem. Soc. Rev., 2013, 42, 1755–1775 RSC.
- F. Zhou, X. Z. Liao, Y. T. Zhu, S. Dallek and E. J. Lavernia, Acta Mater., 2003, 51, 2777–2791 Search PubMed.
- Z. Jiang, Y. Li, Y. Shen, J. Yang, Z. Zhang, Y. You, Z. Lv and L. Yao, Molecules, 2021, 26, 2688 CrossRef CAS PubMed.
- L. Zhu, L. Deng, Y. Xie, L. Liu, X. Ma and R. Liu, Results Chem., 2023, 5, 100882 CrossRef CAS.
- H. Schmalzried, Solid State Reactions, Verlag Chemie, Weinheim, Germany, 1981 Search PubMed.
- K. Tkáčová, Mechanical Activation of Minerals, Elsevier, Amsterdam, 1989 Search PubMed.
- A. A. Shevchenko, A. Y. Dolgoborodov, V. G. Kirilenko and M. A. Brazhnikov, Combust., Explos. Shock Waves, 2017, 53, 461–470 CrossRef.
- M. Jose John, K. Muraleedharan, V. M. Abdul Mujeeb, M. P. Kannan and T. Ganga Devi, React. Kinet., Mech. Catal., 2012, 106, 355–367 CrossRef.
- J. J. C Lin, H. Ruan and X. Ma, Mater. Degrad., 2023, 7, 57 CrossRef.
- M. Senna and K. Schönert, Powder Technol., 1982, 32, 217–221 CrossRef CAS.
- E. Gautier, S. Diligenti, X. Lemoine and M. Berveiller, Acta Mater., 2001, 49, 4079–4088 CrossRef.
- A. Godfrey, N. Hansen and D. Juul Jensen, Metall. Mater. Trans. A, 2007, 38, 2329–2339 CrossRef.
- P. Pourghahramani and E. Forssberg, Powder Technol., 2007, 178, 30–39 CrossRef CAS.
- O. Lapshin, O. Shkoda, O. Ivanova and S. Zelepugin, Metals, 2021, 11, 1743 CrossRef CAS.
- M. Senna and K. Hamada, J. Mater. Sci., 1996, 31, 1725–1728 CrossRef.
- N. Yamamoto, Y. Matsuzaka, M. Kimura, H. Matsuki and Y. Yanagisawa, J. Biosci. Bioeng., 2009, 107, 464–470 CrossRef CAS PubMed.
- O. V. Lapshin, V. V. Boldyrev and E. V. Boldyreva, Russ. J. Phys. Chem. A, 2019, 93, 1592–1597 CrossRef CAS.
- S. Sayyar, J. Law, A. Golda, G. Ryder and G. Wallace, Mater. Chem. Front., 2022, 6, 2718–2728 RSC.
- R. M. Barrer, Trans. Faraday Soc., 1949, 45, 590–599 Search PubMed.
- G. Ali Nematollahi, E. Marzbanrad and A. R. Aghaei, Mater. Sci. Eng., A, 2008, 492, 455–459 CrossRef.
- C. Suryanarayana, Prog. Mater. Sci., 2001, 46, 1–184 CrossRef CAS.
- D. L. Zhang, Prog. Mater. Sci., 2004, 49, 537–560 CrossRef CAS.
- Y. Mishin and A. Suzuki, Interface Sci., 2003, 11, 131–148 CrossRef.
- M. Senna, ChemTexts, 2017, 3, 1–13 CrossRef CAS.
- M. Senna, Mater. Sci. Eng., A, 2001, 304–306, 39–44 CrossRef.
- M. Senna, E. Turianicová, V. Šepelák, M. Bruns, G. Scholz, S. Lebedkin, C. Kübel, D. Wang, M. Kaňuchová, M. Kaus and H. Hahn, Solid State Sci., 2014, 30, 36–43 CrossRef CAS.
- H. Rojas-Chavez, H. Cruz-Martinez, E. Flores-Rojas, J. M. Juarez-Garcia, J. L. Gonzalez-Dominguez, N. Daneu and J. Santoyo-Salazar, Phys. Chem. Chem. Phys., 2018, 20, 27082–27092 RSC.
- M. Senna, J. Alloys Compd., 2007, 434–435, 768–772 CrossRef CAS.
- S. S. Aamlid, M. Ohldah, J. Rottler and A. M. Hallas, J. Am. Chem. Soc., 2023, 145, 5991–6006 CrossRef CAS PubMed.
- R. Rana, N. Hopper, F. Sidoroff and W. T. Tysoe, Chem. Sci., 2022, 13, 12651–12658 RSC.
- A. Boscoboinik, D. Olson, H. Adams, N. Hopper and W. T. Tysoe, Chem. Commun., 2020, 56, 7730–7733 RSC.
- N. Deneke, M. L. Rencheck and C. S. Davis, Soft Matter, 2020, 16, 6230–6252 RSC.
- J. N. Brantley, K. M. Wiggins and C. W. Bielawski, Polym. Int., 2013, 62, 2–12 CrossRef CAS.
- Z. Ma, Z. Wang, M. Teng, Z. Xu and X. Jia, Chemphyschem, 2015, 16, 1811–1828 CrossRef CAS PubMed.
- W. Qiu, J. M. P. Scofield, P. A. Gurr and G. G. Qiao, Macromol. Rapid Commun., 2022, 43, e2100866 CrossRef PubMed.
- M. Tan, X. Wang, T. Xie, Z. Zhang, Y. Shi, Y. Li and Y. Chen, Macromolecules, 2022, 55, 6860–6865 CrossRef CAS.
- G. Kaupp, CrystEngComm, 2009, 11, 388–403 RSC.
- D. W. Kim, G. A. Medvedev, J. M. Caruthers, J. Y. Jo, Y.-Y. Won and J. Kim, Macromolecules, 2020, 53, 7954–7961 Search PubMed.
- Y. Sasai, M. Kuzuya, M. Mouri and S. Kondo, Thin Solid Films, 2002, 407, 144–150 CrossRef.
- T. Wang, N. Zhang, J. Dai, Z. Li, W. Bai and R. Bai, ACS Appl. Mater. Interfaces, 2017, 9, 11874–11881 Search PubMed.
- W. Qiu, P. A. Gurr and G. G. Qiao, Macromolecules, 2020, 53, 4090–4098 Search PubMed.
- M. Li, W. Liu, Q. Zhang and S. Zhu, ACS Appl. Mater. Interfaces, 2017, 9, 15156–15163 CrossRef CAS PubMed.
- R. Gunckel, B. Koo, Y. Xu, B. Pauley, A. Hall, A. Chattopadhyay and L. L. Dai, ACS Appl. Polym. Mater., 2020, 2, 3916–3928 CrossRef CAS.
- B. A. Versaw, T. Zeng, X. Hu and M. J. Robb, J. Am. Chem. Soc., 2021, 143, 21461–21473 CrossRef CAS PubMed.
- G. Kim, Q. Wu, J. L. Chu, E. J. Smith, M. L. Oelze, J. S. Moore and K. C. Li, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2109791119 Search PubMed.
- Y. Li, Y. Qin, Y. Shang, Y. Li, F. Liu, J. Luo, J. Zhu, X. Guo, Z. Wang and Y. Zhao, Adv. Funct. Mater., 2022, 32, 2112000 CrossRef CAS.
- V. V. Boldyrev, Solid State Ionics, 1993, 63–65, 37–543 Search PubMed.
- P. Y. Serdobintsev, A. S. Melnikov, A. A. Pastor, N. A. Timofeev and M. A. Khodorkovskiy, J. Chem. Phys., 2018, 148, 194301 Search PubMed.
- T. C. Lin, M. Sarma, Y. T. Chen, S. H. Liu, K. T. Lin, P. Y. Chiang, W. T. Chuang, Y. C. Liu, H. F. Hsu, W. Y. Hung, W. C. Tang, K. T. Wong and P. T. Chou, Nat. Commun., 2018, 9, 3111 Search PubMed.
- T. Naito, N. Watanabe, Y. Sakamoto, Y. Miyaji, T. Shirahata, Y. Misaki, S. Kitou and H. Sawa, Dalton Trans., 2019, 48, 12858–12866 Search PubMed.
- B. T. Mai, S. Fernandes, P. B. Balakrishnan and T. Pellegrino, Acc. Chem. Res., 2018, 51, 999–1013 CrossRef CAS PubMed.
- A. G. Ivanov, M. Y. Velitchkova, S. I. Allakhverdiev and N. P. A. Huner, Photosynth. Res., 2017, 133, 17–30 CrossRef CAS PubMed.
- P. M. Botta, E. F. Aglietti and J. M. Porto Lopez, Thermochim. Acta, 2000, 363, 143–147 CrossRef CAS.
- A. Monteiro, A. Afolabi and E. Bilgili, Drug Dev. Ind. Pharm., 2013, 39, 266–283 Search PubMed.
- H. Chen, W. Lin, Z. Zhang, K. Jie, D. R. Mullins, X. Sang, S.-Z. Yang, C. J. Jafta, C. A. Bridges, X. Hu, R. R. Unocic, J. Fu, P. Zhang and S. Dai, ACS Mater. Lett., 2019, 1, 83–88 CrossRef CAS.
- D. Guo, Z. Zhang, X. Hou, Y. Hu and Z. Liao, J. Polym. Res., 2022, 29, 501 CrossRef CAS.
- A. M. Kalinkin, E. V. Kalinkina, A. A. Politov, V. N. Makarov and V. V. Boldyrev, J. Mater. Sci., 2004, 39, 5393–5398 CrossRef CAS.
- M. V. Chaikina, N. V. Bulina, O. B. Vinokurova, I. Y. Prosanov and D. V. Dudina, Ceram. Int., 2019, 45, 16927–16933 CrossRef CAS.
- O. M. C Chrysochoos, G. Martin, H. Caumon and J. C. Chezeaux, Nucl. Eng. Des., 1989, 114, 323–333 CrossRef.
- U. K. G. Gottstein, Acta Metall., 1983, 31, 175–188 CrossRef.
- Z. Wu, H. Bei, F. Otto, G. M. Pharr and E. P. George, Intermetallics, 2014, 46, 131–140 CrossRef CAS.
- Q. Zhang and F. Saito, Adv. Powder Technol., 2012, 23, 523–531 CrossRef CAS.
- L. Zhang, M. Richardson and P. Mendis, Clin. Exp. Pharmacol. Physiol., 2012, 39, 706–710 CrossRef CAS PubMed.
- P. Heitjans and S. Indris, J. Phys.: Condens. Matter, 2003, 15, R1257–R1289 CrossRef CAS.
- R. Schlem, C. F. Burmeister, P. Michalowski, S. Ohno, G. F. Dewald, A. Kwade and W. G. Zeier, Adv. Energy Mater., 2021, 11, 2101022 CrossRef CAS.
- S. Chithra, K. D. Malviya and K. Chattopadhyay, Metall. Mater. Trans. A, 2013, 45, 1148–1160 CrossRef.
- M. S. El-Eskandarany, J. Alloys Compd., 1999, 284, 295–307 CrossRef.
- I. Olenych, L. Monastyrskii and P. Parandii, J. Cryst. Growth, 2001, 230, 314–317 CrossRef.
- A. A. L. Michalchuk, I. A. Tumanov, V. A. Drebushchak and E. V. Boldyreva, Faraday Discuss., 2014, 170, 311–335 RSC.
- M. S. Y Iguchi, Powder Technol., 1985, 43, 155–132 CrossRef.
- E. A. Lass, A. Zhu, G. J. Shiflet and S. Joseph Poon, Acta Mater., 2011, 59, 6341–6350 CrossRef CAS.
- H. Kum, H. K. Seong, W. Lim, D. Chun, Y. I. Kim, Y. Park and G. Yoo, Sci. Rep., 2017, 7, 40893 CrossRef CAS PubMed.
- Y. Yamane, K. Yamada and K. Inoue, Solid State Ionics, 2008, 179, 483–488 CrossRef CAS.
- Y. Zhou, F. Guo, C. E. Hughes, D. L. Browne, T. R. Peskett and K. D. M. Harris, Cryst. Growth Des., 2015, 15, 2901–2907 CrossRef CAS.
- Y. Lin, C.-C. Chang and S. L. Craig, Org. Chem. Front., 2019, 6, 1052–1057 RSC.
- H. Watanabe, E. Matsui, Y. Ishiyama and M. Senna, Tetrahedron Lett., 2007, 48, 4481–4484 CrossRef.
- K. L. Jyothi, R. Gautam, D. Swain, T. N. Guru Row and N. K. Lokanath, Cryst. Res. Technol., 2019, 54, 196016 CrossRef.
- M. Gombotz, D. Rettenwander and H. M. R. Wilkening, Front. Chem., 2020, 8, 100 CrossRef CAS PubMed.
- A. W. Tricker, G. Samaras, K. L. Hebisch, M. J. Realff and C. Sievers, Chem. Eng. J., 2020, 382, 122954 CrossRef CAS.
- M. H. Baradaran-Ghandi and S. A. Hassanzadeh-Tabrizi, Ceram. Int., 2018, 44, 5447–5452 CrossRef CAS.
- E. Turianicová, R. Witte, K. L. Da Silva, A. Zorkovská, M. Senna, H. Hahn, P. Heitjans and V. Šepelák, J. Alloys Compd., 2017, 707, 310–314 CrossRef.
- M. Botros and J. Janek, Science, 2022, 378, 1274 CrossRef PubMed.
- E. L. Clennan and A. Pace, Tetrahedron, 2005, 61, 6665–6691 CrossRef CAS.
- P. R. Ogilby, Chem. Soc. Rev., 2010, 39, 3181–3209 RSC.
- J. Moan and E. Wold, Nature, 1979, 279, 450–451 CrossRef CAS PubMed.
- J. Temuujin, K. Okada and K. J. D. MacKenzie, J. Solid State Chem., 1998, 138, 169–177 CrossRef CAS.
- S. Serraj, P. Boudeville, B. Pauvert and A. Terol, J. Biomed. Mater. Res., 2001, 55, 566–575 CrossRef CAS PubMed.
- T. Watanabe, J. Liao and M. Senna, J. Solid State Chem., 1995, 115, 390–394 CrossRef CAS.
- K. Hamada, T. Isobe and M. Senna, J. Mater. Sci. Lett., 1996, 15, 603–605 CrossRef CAS.
- T. Iwasaki, K. Kosaka, S. Watano, T. Yanagida and T. Kawai, Mater. Res. Bull., 2010, 45, 481–485 CrossRef CAS.
- W. Yuan, W. Solihin, Q. Zhang, J. Kano and F. Saito, Powder Technol., 2014, 260, 22–26 CrossRef CAS.
- O. Skurikhina, M. Senna, M. Fabián, R. Witte, R. Tarasenko, V. Tkáč, M. Orendáč, M. Kaňuchová, V. Girman, M. Harničárová, J. Valíček, V. Šepelák and E. Tóthová, Ceram. Int., 2020, 46, 14894–14901 CrossRef CAS.
- S. N. Madanayake, A. Manipura, R. Thakuria and N. M. Adassooriya, Org. Process Res. Dev., 2023, 27, 409–422 CrossRef CAS.
- R. Hiraoka and M. Senna, Chem. Eng. Res. Des., 2009, 87, 97–101 Search PubMed.
- K. Bialek, Z. Wojnarowska, M. Skotnicki, B. Twamley, M. Paluch and L. Tajber, Pharmaceutics, 2021, 13, 2125 Search PubMed.
- P. P. Mazzeo, M. Prencipe, T. Feiler, F. Emmerling and A. Bacchi, Cryst. Growth Des., 2022, 22, 4260–4267 Search PubMed.
- C. Winson and S. Varughese, CrystEngComm, 2023, 25, 3339–3346 RSC.
- C.-C. Li and C.-L. Huang, Colloids Surf., A, 2010, 353, 52–56 Search PubMed.
- P. F. M. de Oliveira, J. Quiroz, D. C. de Oliveira and P. H. C. Camargo, Chem. Commun., 2019, 55, 14267–14270 Search PubMed.
- M. Shahabuddin, N. A. Madhar, N. S. Alzayed and M. Asif, Materials, 2019, 12, 3044 CrossRef CAS PubMed.
- P. F. M. de Oliveira, R. M. Torresi, F. Emmerling and P. H. C. Camargo, J. Mater. Chem. A, 2020, 8, 16114–16141 RSC.
- A. V. Panko, I. G. Kovzun, V. A. Prokopenko, O. A. Tsyganovich, V. O. Oliinyk and O. M. Nikipelova, Appl. Nanosci., 2018, 9, 665–675 CrossRef.
- M. Toriyama, A. Ravagliolt, A. Krajewski, C. Falassi, E. Roncari and A. Piancastelli, J. Mater. Sci., 1995, 30, 3216–3221 CrossRef CAS.
- J. Alic, M. C. Schlegel, F. Emmerling and T. Stolar, Angew Chem. Int. Ed. Engl., 2024, e202414745, DOI:10.1002/anie.202414745.
- F. J. Garcia-Garcia, M. J. Sayagues and F. J. Gotor, Nanomaterials, 2021, 11, 3044 CrossRef PubMed.
- N. Muralidharan, C. N. Brock, A. P. Cohn, D. Schauben, R. E. Carter, L. Oakes, D. G. Walker and C. L. Pint, ACS Nano, 2017, 11, 6243–6251 CrossRef CAS PubMed.
- M. J. Muñoz-Batista, D. Rodriguez-Padron, A. R. Puente-Santiago and R. Luque, ACS Sustain. Chem. Eng., 2018, 6, 9530–9544 CrossRef.
- F. Wen, G. Xie, N. Chen, Q. Wu, M. Chaudhary, X. You, V. K. Michaelis, A. Mar and L. Sang, ACS Appl. Mater. Interfaces, 2023, 15, 40070–40079 CrossRef CAS PubMed.
- C. Espro and D. Rodríguez-Padrón, Curr. Opin. Green Sustainable Chem., 2021, 30, 100478 CrossRef CAS.
- R. R. A. Bolt, J. A. Leitch, A. C. Jones, W. I. Nicholson and D. L. Browne, Chem. Soc. Rev., 2022, 51, 4243–4260 RSC.
- A. Bodach, A. Portet, F. Winkelmann, B. Herrmann, F. Gallou, E. Ponnusamy, D. Virieux, E. Colacino and M. Felderhoff, ChemSusChem, 2023, e202301220, DOI:10.1002/cssc.202301220.
- P. R. Onffroy, E. V. Miu, W. J. Confer, C. M. Darkes-Burkey, W. C. Holler and K. Wakabayashi, Polym. Eng. Sci., 2019, 60, 503–511 CrossRef.
- H. Morrison, P. Fung, T. Tran, E. Horstman, E. Carra and S. Touba, Org. Process Res. Dev., 2018, 22, 1432–1440 CrossRef CAS.
- I. Brekalo, V. Martinez, B. Karadeniz, P. Orešković, D. Drapanauskaite, H. Vriesema, R. Stenekes, M. Etter, I. Dejanović, J. Baltrusaitis and K. Užarević, ACS Sustain. Chem. Eng., 2022, 10, 6743–6754 CrossRef CAS.
- S. Głowniak, B. Szczęśniak, J. Choma and M. Jaroniec, Mater. Today, 2021, 46, 109–124 CrossRef.
- W. H. Fang, L. Zhang, J. Zhang and G. Y. Yang, Chemistry, 2015, 21, 15511–15515 CrossRef CAS PubMed.
- W. H. Fang, L. Zhang, J. Zhang and G. Y. Yang, Chemistry, 2016, 22, 2611–2615 CrossRef CAS PubMed.
- L. Aboutorabi and A. Morsali, Coord. Chem. Rev., 2016, 310, 116–130 CrossRef CAS.
- X. Qiao, Y. Ge, Y. Li, Y. Niu and B. Wu, ACS Omega, 2019, 4, 12402–12409 CrossRef CAS PubMed.
- L. Li, B. Cai, D. Pang, X. Du, X. Yin, H. Wang, J. Yang, D. Li and J. Dou, CrystEngComm, 2020, 22, 8343–8352 RSC.
- J. Zhang, Q. Xiang, Q. Qiu, Y. Zhu and C. Zhang, J. Solid State Chem., 2021, 298, 122123 CrossRef CAS.
- S. A. Lee, J. Bu, J. Lee and H. W. Jang, Small Sci., 2023, 3, 2200109 CrossRef CAS.
- A. Sarkar, L. Velasco, D. Wang, Q. Wang, G. Talasila, L. de Biasi, C. Kubel, T. Brezesinski, S. S. Bhattacharya, H. Hahn and B. Breitung, Nat. Commun., 2018, 9, 3400 CrossRef PubMed.
- H. Xiang, Y. Xing, F.-Z. Dai, H. Wang, L. Su, L. Miao, G. Zhang, Y. Wang, X. Qi, L. Yao, H. Wang, B. Zhao, J. Li and Y. Zhou, J. Adv. Ceram., 2021, 10, 385–441 CrossRef CAS.
- Y. Ma, Y. Ma, Q. Wang, S. Schweidler, M. Botros, T. Fu, H. Hahn, T. Brezesinski and B. Breitung, Energy Environ. Sci., 2021, 14, 2883–2905 RSC.
- A. M. Manzoni and U. Glatzel, Mater. Charact., 2019, 147, 512–532 CrossRef CAS.
- Y. Qiu, Y. J. Hu, A. Taylor, M. J. Styles, R. K. W. Marceau, A. V. Ceguerra, M. A. Gibson, Z. K. Liu, H. L. Fraser and N. Birbilis, Acta Mater., 2017, 123, 115–124 CrossRef CAS.
- Y. Shang, Z. Lei, E. Alvares, S. Garroni, T. Chen, R. Dore, M. Rustici, S. Enzo, A. Schökel, Y. Shi, P. Jerabek, Z. Lu, T. Klassen and C. Pistidda, Mater. Today, 2023, 67, 113–126 CrossRef CAS.
- A. Ostovari Moghaddam, R. Fereidonnejad, D. Mikhailov and E. Trofimov, Mater. Lett., 2022, 318, 132161 CrossRef CAS.
- E. Trofimov, A. Ostovari Moghaddam, K. Litvinyuk and D. Mikhailov, J. Alloys Compd., 2023, 934, 168021 CrossRef CAS.
- N. Zhou, S. Jiang, T. Huang, M. Qin, T. Hu and J. Luo, Sci. Bull., 2019, 64, 856–864 CrossRef CAS PubMed.
- M. A. Buckingham, B. Ward-O'Brien, W. Xiao, Y. Li, J. Qu and D. J. Lewis, Chem. Commun., 2022, 58, 8025–8037 RSC.
- Z. Lei, X. Liu, R. Li, H. Wang, Y. Wu and Z. Lu, Scr. Mater., 2018, 146, 340–343 CrossRef CAS.
- M. D. Chermahini, H. A. Baghbaderani, M. M. Shahraki and M. Kazazi, Ceram. Int., 2019, 45, 5491–5495 CrossRef CAS.
- H. Razavi-Khosroshahi, H. Ishida and M. Fuji, Adv. Powder Technol., 2020, 31, 4672–4678 CrossRef CAS.
- Y. Miura, T. Kashiwagi, T. Fukuda, A. Shichiri, T. Shiobara and K.-i. Saitow, ACS Sustain. Chem. Eng., 2022, 10, 16159–16168 CrossRef.
- T. T. T. Hien, T. Shirai and M. Fuji, J. Ceram. Soc. Jpn., 2012, 120, 429–435 CrossRef CAS.
- S. Kumar and R. Kumar, Ceram. Int., 2011, 37, 533–541 CrossRef CAS.
- G. Mucsi, S. Kumar, B. Csőke, R. Kumar, Z. Molnár, Á. Rácz, F. Mádai and Á. Debreczeni, Int. J. Miner. Process., 2015, 143, 50–58 CrossRef CAS.
- R. Singla, M. Senna, T. Mishra, T. C. Alex and S. Kumar, Appl. Clay Sci., 2022, 221, 106459 CrossRef CAS.
- F. Hajiali, T. Jin, G. Yang, M. Santos, E. Lam and A. Moores, ChemSusChem, 2022, 15, e202102535 CrossRef CAS PubMed.
- H. Niu, K. Zhang, S. Myllymaki, M. Y. Ismail, P. Kinnunen, M. Illikainen and H. Liimatainen, ACS Appl. Mater. Interfaces, 2021, 13, 57841–57850 CrossRef CAS PubMed.
- X. Tong, J. Yang, P. Wu, X. Zhang and H. J. Seo, J. Alloys Compd., 2019, 779, 399–403 CrossRef CAS.
- Y. Hua, Y.-U. Seo, S. Yun Kim, H.-J. Kim and J. Su Yu, Chem. Eng. J., 2021, 412, 128633 CrossRef CAS.
- V. Rajagopal, R.-J. Xie and R. Nagaraj, J. Lumin., 2022, 252, 119356 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |


