Open Access Article
Open Access ArticleEmerging engineered nanozymes: current status and future perspectives in cancer treatments
Jiajia
Zheng†
ab,
Weili
Peng†
b,
Houhui
Shi
bc,
Jiaqi
Zhang
b,
Qinglian
Hu
 a and
Jun
Chen
a and
Jun
Chen
 *b
*b
aCollege of Biotechnology and Bioengineering, Zhejiang University of Technology, Hangzhou, Zhejiang, China. E-mail: huqinglian@zjut.edu.cn
bCancer Center, Department of Interventional Medicine, Zhejiang Provincial People's Hospital (Affiliated People's Hospital), Hangzhou Medical College, Hangzhou, Zhejiang, China. E-mail: chenjun@hmc.edu.cn
cCollege of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou, Zhejiang, China
First published on 28th January 2025
Abstract
Composite nanozymes are composed of enzymes with similar or different catalytic capabilities and have higher catalytic activity than a single enzyme. In recent years, composite nanozymes have emerged as novel nanomaterial platforms for multiple applications in various research fields, where they are used to produce oxygen, consume glutathione, or produce toxic reactive oxygen species (ROS) for cancer therapy. The therapeutic approach using composite nanozymes is known as chemo-dynamic therapy (CDT). Some composite nanozymes also show special photothermal conversion effects, enabling them to be combined with pioneering cancer treatments, such as photodynamic therapy (PDT), photothermal therapy (PTT) and sonodynamic therapy (SDT), and enhance the anti-cancer effects. In this study, the classification and catalytic performances of composite nanozymes are reviewed, along with their advantages and synthesis methods. Furthermore, the applications of composite nanozymes in the treatment of cancers are emphasized, and the prospective challenges in the future are discussed.
1. Introduction
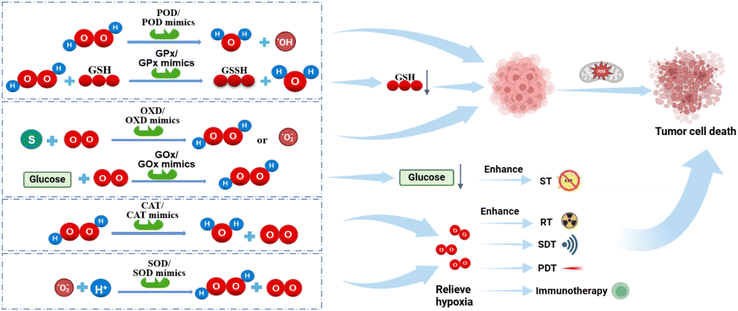
The incidence and mortality rates of cancer are rising, with close to 20 million newly diagnosed cases and 9.7 million deaths recorded in 2022.1 This data represent a significant increase of 41.84% and 18.29%, respectively, compared to the 14.1 million cases and 8.2 million deaths reported in 2012.2 Cancer has emerged as a grave threat to human life and well-being, ranking as the second leading cause of death worldwide.3 Conventional therapies, such as surgery, radiotherapy (RT), and chemotherapy (CTx), exhibit notable side effects, often encounter drug resistance, and frequently fall short of achieving the desired outcomes.4 Consequently, researchers have been exploring novel cancer treatment methods, with nanotechnology emerging as one of the most promising approaches.5The specificity of tumors makes their treatment difficult. The pro-angiogenic factors secreted by tumor cells lead to the formation of an abnormal tumor vascular network, which is disorganized, immature and poorly permeable, leading to poor tumor perfusion. At the same time, owing to the rapid growth of tumor tissues, a large amount of oxygen and nutrients are getting consumed, eventually leading to hypoxia in the tumor microenvironment (TME).6 However, in an anoxic environment, tumor cells alter the expression of glycolytic related proteins, such as GLUT1, GLUT3, LDHA, and PKM2, increasing glucose uptake to promote their growth, and an anoxic environment also induces epithelial–mesenchymal transformation, thereby increasing the production of matrix metalloproteinases (MMPs), which promote aggressive metastasis.7
The hypoxic tumor microenvironment not only promotes tumor growth, metastasis and the evasion of immunosuppression but also inhibits the efficacy of various oxygen-dependent therapies. For instance, the efficacy of radiotherapy is suppressed mainly because of hypoxia. In addition, most non-invasive new cancer therapies that have attracted much attention in recent years, such as PDT and SDT, are based on oxygen. Changing the anoxic microenvironment of the tumor is an effective prerequisite strategy to treat tumors.
The content of ROS in solid tumors is higher than that in normal cells (from 100 μM to 1 mM), especially for H2O2, which is the most prevalent and stable non-free radical ROS in cancer cells, and these ROS promote the occurrence and development of tumors as well as promote the metastasis of tumors.8 On the one hand, using endogenous H2O2 to generate oxygen and change the hypoxic microenvironment of tumors can improve the treatment efficiency of various oxygen-dependent therapies, which is conducive to overcoming cancer. On the other hand, the use of H2O2 to generate other types of ROS that cause fatal damage to cancer cells, such as hydroxyl radicals (·OH), can significantly enhance the oxidative damage to tumors and induce the apoptosis of cancer cells. In addition, some nanozymes can convert O2 into superoxide anion free radicals (·O2−) or H2O2, and some can convert ·O2− into H2O2, so as to supplement H2O2 and improve treatment efficiency.
Since the initial discovery of ferromagnetic nanozymes (Fe3O4 NPs) exhibiting peroxidase (POD) activity in 2007,9 nanozyme technology has been continuously developed, with many new nanozymes reported. Nanozymes are artificial enzyme that have a high enzyme-like catalytic activity and can regulate biochemical reactions. Nanozymes include several categories, such as metal-, metal oxide-, MXene-, carbon-, and metal–organic framework (MOF)-based nanozymes.10 They have attracted significant attention due to their high operational stability, work efficiency under extreme conditions, and resistance to protease digestion. Nanozymes can effectively replace natural enzymes in catalytic reactions, as they closely resemble natural enzymes in their active sites. Table 1 summarizes the advantages and limitation of nanozymes and conventional enzymes.11 For instance, most nanozymes can mimic the activities of oxidoreductases, such as POD,12 oxidase (OXD), catalase (CAT),13 and superoxide dismutase (SOD).14 Additionally, the nanomaterial characteristics of nanozymes provide them with enhanced stability surpassing that of natural enzymes. Moreover, their unique nanoscale dimensions facilitate efficient distribution within organisms and enable their accumulation at tumor sites through an enhanced permeability and retention (EPR) effect.15Fig. 1 and Table 2 summarize the types of nanozymes and their principle functions and characteristics in cancer therapy.
| Nanozymes | Enzymes | |
|---|---|---|
| Advantages | High stability, resistant to protease digestion | Highly selective about the targets |
| Enters the tumor through an EPR effect | Mild conditions | |
| Large specific surface area for surface modification and bioconjugation | Environmentally friendly | |
| Simple synthesis method, low cost | ||
| High stable biocatalytic activity | ||
| Limitations | Low specificity, accelerate the production of harmful free radicals in normal tissues | High cost |
| Low yield and storage challenges | ||
| Limited species of catalytic activity | Poor stability | |
| Toxicity in some nanozymes | Substrate restriction | |
| Low activity | Catalytic activity is easily inhibited |
| Type | Catalytic reaction | Example | Application in cancer therapy |
|---|---|---|---|
| POD | Catalyze H2O2 into H2O and ·OH, or H2O and GSSH in the presence of GSH | Fe2O3;16 iron chalcogenides;17 PB;18 MOF;19 and metallic compounds containing Pt, Ce,20 V, Zn,21 Co,21 Mn, Mo,22 W,23 Cu,24 Au,25 Ag,26 Pd,27 Ir,28 Os,29 Ru30 | POD-like nanozymes can convert hydrogen peroxide into hydroxyl free radicals (·OH) and water; hydroxyl free radicals are the most toxic ROS, which can kill tumor cells through chemokinetic treatment, thus treating cancer |
| CAT | Catalyze H2O2 into H2O and O2 | Carbon-based nanomaterials;31,32 Fe3O4;26 CeO2;33 Mn3O4;34 Co3O4;35 and metallic compounds containing Au,36 Ag,37 Pt,38 Pd27 | CAT-like nanozymes can use endogenous H2O2 to generate O2, relieve hypoxia at the tumor site, and improve the therapeutic effect of RT, CTx, PDT, and SDT39 |
| OXD | Catalyze O2 into H2O2 or ·O2− | CeO2;40 and metallic compounds containing Cu, Au, Ag, Pt, Ir15 | OXD-like nanozymes can use oxygen in tumors to resynthesize H2O2 for recycling or to produce toxic superoxide anion free radicals (·O2−) to further kill cancer cells |
| SOD | Catalyze ·O2− into H2O2 and O2 | CeO2;41 NiO;42 metallic compounds containing Cu, Zn, Fe, Mn | SOD-like nanozymes can catalyze ·O2− produced by OXD-like enzymes into H2O2 and O2. On the one hand, H2O2 can generate more toxic ·OH, and on the other hand, it can alleviate tumor hypoxia |
Nanozymes of a specific class may exhibit a spectrum of catalytic activities; for instance, copper nanoparticles (Cu NPs) typically possess POD and OXD activities. However, under specific conditions, such as variations in pH, particle size and morphology, or ion valence states, these nanozymes often manifest a single catalytic capability. To emulate the concatenated enzymatic reactions observed in natural systems, the development of composite nanozymes has been pursued as a feasible strategy.43 For instance, Hao et al. synthesized clusters of copper oxide nanoparticles (CuxO NPs), comprising a mixture of CuO and Cu2O, and demonstrated that these composite nanozymes could serve as antioxidants by functioning concurrently as CAT, GPx, and SOD analogs.44 Similarly, Ling et al. fabricated copper@copper(I) oxide aerogel networks (Cu@Cu2O) and identified their dual enzymatic mimicry, exhibiting activities analogous to both horseradish peroxidase (HRP) and NADH peroxidase.45 It is thus evident that the amalgamation of two or more nanozymes with analogous functionalities can result in an enhanced catalytic potency compared to the individual constituents of the nanozymes alone. For instance, Boruah et al. synthesized a composite nanozyme comprising Fe3O4, TiO2, and reduced graphene oxide (rGO), and observed that the POD activity of the resulting composite enzyme was significantly more robust than the intrinsic POD activities of the individual components, namely Fe3O4 NPs, TiO2 NPs, and rGO.46
There are many reviews that have extensively covered the topic of nanozymes;14,15,47 however, there is a lack of literature specifically summarizing the application of composite nanozymes in cancer treatment. Consequently, in this article we review the catalytic activity and therapeutic mechanisms of common nanozymes in cancer treatment. We also examine the classification and benefits of composite nanozymes, present various synthesis methods for these composite nanozymes, and describe their applications in combination with different therapeutic approaches for various cancers. The discussion concludes with an exploration of the remaining challenges and future directions for composite nanozymes.
2. Introduction to composite nanozymes
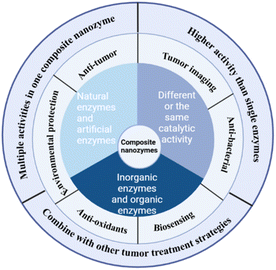
In order to improve the catalytic efficiency of nanozymes and optimize therapeutic outcomes, composite nanozymes have been proposed. These composite nanozymes can incorporate a combination of nanozymes with the same or different catalytic functions, including inorganic enzymes and organic enzymes, and artificial enzymes and natural enzymes. In addition to augmenting specific catalytic abilities or enabling multifunctional catalysis, some enzymes are also endowed with other properties after being made into composite nanozymes, such as magnetism, or photothermal properties, that were not originally available. Fig. 2 summarizes the composition and application of composite nanozymes in different fields and their various advantages.2.1 Classification of composite nanozymes
Composite nanozymes can be categorized into artificial enzymes and artificial–natural enzyme complexes in terms of their composition. Composite nanozymes can be classified as either purely synthetic metal-based enzymes, including metal-oxidases and monoatomic enzymes, MXene-based enzymes, or carbon-based enzymes, or as complexes that integrate both artificial and natural enzymes. Two notable natural enzymes in this context are GOx48,49 and HRP.In the study by Ma et al., MoS2 nanosheets (NSs) were decorated with Au NPs, and subsequently, Pt NPs were in situ grown on these nanosheets to create core–shell structured MoS2–Au@Pt NPs.50 DNA probes were then linked to the composite nanozyme through mercaptan groups, with HRP assembled into the composite nanozyme at the DNA probe level. This composite nanozyme was demonstrated to be capable of the ultra-sensitive electrochemical detection of gastric cancer-related microRNA. The presence of HRP increased the electrochemical signal of the MoS2–Au@Pt NPs by approximately 10.46 times. Cui et al. prepared Ce and Gd dual-rare earth-doped carbon dots (Ce–Gd@CDs) using a one-step hydrothermal method, and then conjugated these with GOx through an amine reaction to form the Ce–Gd@CDs-GOx complex nanozyme.51 This composite nanozyme possessed CAT activity for alleviating hypoxia in the tumor microenvironment. It could also generate highly toxic ·OH, inducing apoptosis or necrosis in cancer cells, and with GOx catalyzing the conversion of glucose into gluconic acid and H2O2, it could deprive cancer cells of glucose, leading to the starvation of cells and ultimately cancer cell death.
There have been extensive studies on entirely synthetic composite nanozymes.52–55 For instance, Wang et al. revealed that ZnMn2O4 NPs exhibited only SOD activity, while lithium-doped LiMn2O4 NPs possessed multiple enzyme activities, notably enhanced SOD activity, along with concurrent CAT and GPx activities.56 In Zhang et al.'s study, platinum was deposited onto gold bipyramids (Au NBPs) using two distinct approaches: tip-specific deposition to form ATP and full-body deposition to form ACP.57 They observed that ATP exhibited higher POD and CAT activities compared to ACP. Liu et al. fabricated N/P co-doped graphene quantum dots (NPGQDs) as metal-free nanozymes, which demonstrated POD activity exceeding that of traditional graphene quantum dots and graphene oxide by an order of magnitude.58 Yang et al. synthesized advanced composite nanozymes of FeSA-Ir@PFNSs through the conjugation of single-atom iron (FeSA) with strained-lattice iridium metallene nanoislands.59 The FeSA-Ir@PFNSs leveraged the POD activity of the FeSA and the photothermal effect of iridium to simultaneously generate ·OH and 1O2, while also showing enhanced CAT activity.
2.2 Advantages of composite nanozymes
Other than the aforementioned advantages, some composite nanozymes can also allow reducing production costs, such as doping non-noble metal nanoparticles (Cu, Mn) into precious metal nanozymes (Au, Ru). This approach not only lowers raw material expenses, but can also improve catalytic efficiency. Furthermore, some composite nanozymes can reduce the toxicity of single nanozymes and have higher levels of biosafety and bioavailability.
3. Synthesis of composite nanozymes
Composite nanozymes can be synthesized through solvent/hydrothermal doping, chemical deposition, covalent bonding, in situ growth, and other methods. Due to their excellent catalytic performance and other endowed properties, composite nanozymes are usually designed for use in combination with other treatment methods, such as PDT, PTT, SDT, ST,63 or CTx. In addition, composite nanozymes have also been designed as imaging probes or nano-platforms for diagnosis and treatment. However, this article is only focused on the application of composite nanozymes in cancer treatment. Table 3 summarizes and provides examples of the commonly used synthesis methods and the design of nanozymes in this field.| Synthesis method | Composite nanozyme system | Enzyme mimic | Enzyme/enzyme mimic activity | Combination therapy |
|---|---|---|---|---|
| Solvent/hydrothermal synthesis | CCCs24 | Cu–CeO2 | POD, CAT, SOD | CTx |
| Solvent/hydrothermal synthesis | Ce/ZnCo2O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
Ce/ZnCo2O4 | POD, OXD | — |
| Solvent/hydrothermal synthesis | Ox-POM@Cu22 | Ox-POM@Cu | GSH-PX, CAT | PTT, CDT |
| Solvent/hydrothermal synthesis | PtCu3-PEG64 | PtCu3 | POD, GSH-PX | SDT, CDT |
| Solvent/hydrothermal synthesis | Cu–CuFe2O4/DOX65 | Cu–CuFe2O4 | POD, GSH-PX | SDT, CDT, CTx |
| Solvent/hydrothermal synthesis | RuCu NSs30 | RuCu | POD, SOD, GSH-PX | CDT |
| Solvent/hydrothermal synthesis | MnWOx-PEG66 | MnWOx | POD, GSH-PX | SDT |
| Solvent/hydrothermal synthesis | PtFe@Fe3O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67 67 |
PtFe@Fe3O4 | POD, CAT | PTT |
| One-step synthesis | Au2Pt-PEG-Ce6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
Au2Pt | POD, CAT | PTT, PDT, CDT |
| Solvent/hydrothermal synthesis and coat | Co/La-PB@MOF-199/GOx61 | Co/La-PB@MOF-199 | POD, CAT, GOx | PTT, CDT |
| Solvent/hydrothermal synthesis | CMO-R@4T1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 68 68 |
CMO (Cu-doped MoOx) | POD, OXD, NADH-oxidase | PTT, immunotherapy |
| Chemical deposition | Au NBP/Pd69 | Au NBP/Pd | POD | PTT, PDT |
| Chemical deposition | CuS@CeO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70 |
CuS@CeO2 | CAT | PTT, RT |
| Liquid-phase deposition | PTZCs27 | Pd@TiO2 | CAT | PTT, PDT |
| Vacuum metal sputter deposition | Pt–CuS–P-TAPP71 | Pt–CuS | CAT | PTT, SDT |
| Vacuum metal sputter deposition and reduction | HPT-DOX72 | Pt–TiO2 | CAT | SDT |
| In situ growth | Ce6–Au–Pt@HPBs60 | Au–Pt@HPBs | CAT | PDT |
| In situ growth and thermal decomposition | MnFe2O4@MOF@PEG73 | MnFe2O4@MOF | GSH-PX, CAT | PDT |
| In situ growth | MnO2@PtCo74 | MnO2@PtCo | CAT, OXD | — |
| In situ growth | HAMF75 | HMSN@Au@MnO2 | CAT, OXD | PDT |
| In situ growth | Pt–carbon76 | Pt–carbon | CAT | PTT, PDT |
| In situ growth | ICG/Au/Pt@PDA–PEG77 | Au–Pt | POD, CAT, OXD | PTT, PDT, ST |
| In situ growth and adsorption | P@Pt@P-Au-FA78 | P@Pt@P–Au | CAT, OXD | PDT, ST |
| In situ growth and template method | PDAP-ICG-Pt79 | PDAP–Pt | GSH-PX, CAT | PDT |
| CM-MMNPs80 | MOF-MnO2# | CAT | PDT | |
| In situ growth and etching | Ti3C2Tx–Pt-PEG81 | Ti3C2Tx–Pt | POD | PTT |
| Solvent and hydrothermal synthesis, in situ growth | FAB NPs26 | Fe3O4/Ag/Bi2MoO6 | POD, GSH-PX, CAT, SOD | PTT, PDT, CDT |
| Template method | HMCGP82 | HMCuO2–GOD | CAT, GSH-PX, OXD | PTT, PDT, ST |
| Template method and reduction | Pt@HCS-Ce6 NPs83 | Pt@HCS | POD, OXD | PDT |
| Template method | FePc/HNCSs84 | FePc/HNCS | POD, CAT | PTT, PDT |
| Solvothermal galvanic replacement | Os–Te NRs29 | Os–Te | POD, CAT, SOD | PTT, PDT |
| Inverse microemulsion polymerization | RONS85 | Fc@Gox | OXD, GOx | CDT |
| Thermal decomposition | CFS@PF-CPT17 | CuFeS2 | POD, CAT, GSH-PX | PTT, PDT, CDT, CTx |
| Loading and etching | DFMC86 | Fe3O4–Mn | POD, GSH-OXD | CDT |
3.1 One-step solvent/hydrothermal synthesis
One-step solvent/hydrothermal synthesis is a simple and effective synthesis method that involves doping metal-based nanozymes into composite nanozymes with more catalytic capabilities. Yin et al. used a one-step solvent method to dope Ce into ZnCo2O4 NPs.21 The oxygen vacancies, superoxide radicals, and electron transfer of Ce/ZnCo2O4 jointly promoted the enhancement of the POD and OXD activities. Based on the dual enzyme-like activity of Ce/ZnCo2O4, H2O2 and GSH could be quickly and conveniently detected. Zhong et al. synthesized uniform PtCu3 nanocages using a one-pot solvothermal method (Fig. 3A).64 After PEG synthesis, the synthesized PtCu3-PEG nanocages could not only serve as a new sound sensitizer to generate ROS under ultrasonication (US), but could also serve as POD nanozymes to catalyze the decomposition of H2O2 into ·OH. At the same time, PtCu3 PEG nanocages, as GSH-Px nanozymes, could accelerate the process of GSH depletion in the presence of oxidizing molecules (H2O2, O2). Wang et al. designed a simpler and environmentally friendly one-pot method to synthesize a Au2Pt nanozyme at room temperature (Fig. 3B).25 Here, L-proline was introduced as a chelating agent to slow down the kinetics of the reduction reaction. Through the interaction of thiol groups with Au and Pt atoms, SH-PEG–NH2 was coupled to the surface of Au2Pt. Finally, Ce6 was connected to the surface of Au2Pt PEG–NH2 through an acylation reaction. After combining with Ce6, the nanozymes could not only simulate POD and CAT enzymes, but also combine with PTT and PDT to achieve better therapeutic effects. Similarly, Gong et al. synthesized a Cu-doped MOF (Cu–CuFe2O4) using a one-step hydrothermal method (Fig. 3C) and loaded this with the chemotherapy drug DOX and sonosensitizer agent Ce6.65 The nanozyme, while also possessing a catalytic function, could be combined with SDT and CTx for anti-cancer application.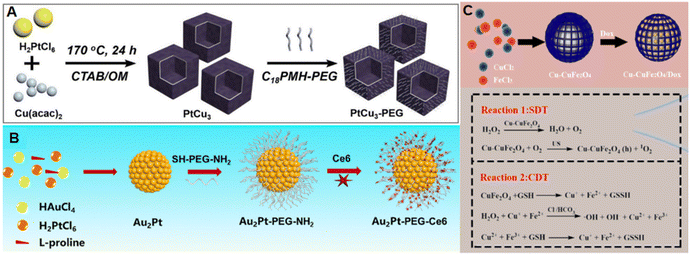 | ||
| Fig. 3 A) Schematic of the synthesis of PtCu3-PEG. This figure has been adapted/reproduced from ref. 64 with permission from WILEY, copyright 2019. (B) Schematic of the synthesis of Cu–CuFe2O4/DOX. This figure has been adapted/reproduced from ref. 25 with permission from ELSEVIER, copyright 2020. (C) Schematic of the synthesis of AuPt-PEG-Ce6. This figure has been adapted/reproduced from ref. 65 with permission from ELSEVIER, copyright 2022. | ||
3.2 Deposition
The deposition method is an effective synthesis method for obtaining composite nanozymes, and the deposition position of nanoparticles can also be selected. Yu et al. selectively deposited Pd NPs onto the tip of Au NBPs (Fig. 4A), and synthesized well-defined anisotropic photonanozymes, which exhibited peroxidase-like properties that were enhanced by 1.5 times under light excitation.69 At the same time, Au and Pd NPs exhibited strong PDT and PTT abilities. Liang et al. synthesized partially Pt deposited CuS Janus NPs through a vacuum metal sputter deposition method.71 The addition of Pt increased the temperature of CuS NPs by 30 °C after being irradiated with 808 nm laser. The hollow structure and negative surface potential of the Pt–CuS Janus NPs promoted the loading of the sound-sensitive agent tetra-(4-aminophenyl)porphyrin (TAPP). Triggering of the TAPP release mode by US was found to be particularly helpful for the cavitation effect of US (Fig. 4B), while at the same time, O2 could be generated in situ to promote PTT and SDT to kill cancers.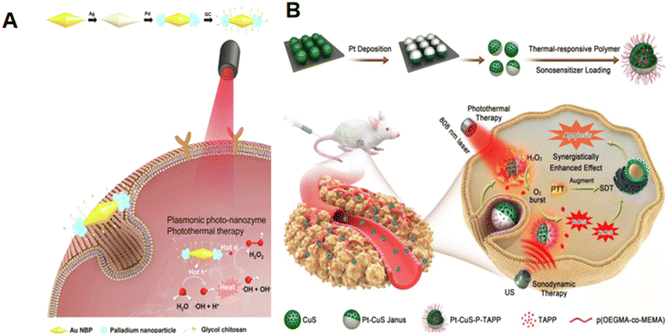 | ||
| Fig. 4 (A) Schematics of Au NBP/Pd synthesis, and in vitro plasma photo nuclease therapy for cancer based on POD activity and photothermal effect under near-infrared light irradiation. This figure has been adapted/reproduced from ref. 69 with permission from ELSEVIER, copyright 2021. (B) Schematics of Pt–CuS Janus NPs synthesis, and the CAT-like activity and the combination of PTT and SDT for cancer treatment. This figure has been adapted/reproduced from ref. 71 with permission from the American Chemical Society, copyright 2019. | ||
3.3 In situ growth
Metal nanoparticles, especially Au and Pt NPs, can be combined in larger nanoparticle platforms by reducing metal salt solutions in mixed solutions. Cold NaBH4 is often used to synthesize composite nanozymes in the in situ growth method. For example, Shen et al.60 mixed HAuCl4·4H2O and H2PtCl6·6H2O with etched HPB NPs, and then slowly added cold NaBH4 solution to the mixture. After stirring, APHPBs with Au NPs and Pt NPs on the surface were synthesized. APHPBs loaded with Ce6 could catalyze H2O2 to generate oxygen, which in turn generated 1O2 under the action of Ce6, enhancing the therapeutic effect of PDT. Similarly, in Chen et al.'s study,75 HAuCl4 was adsorbed on the surface of pre-synthesized mesoporous silica nanoparticles (HMSNs) with the help of cold NaBH4 and then grown into AuNPs. Subsequently, the MnO2 shell was covered by the in situ reduction, and finally, 4-FM, a TADF fluorescein derivative, was connected to the surface of the nanozyme through an amide reaction. It was found that H2O2 could be catalyzed into O2 by MnO2 in the HAMF nanozyme to alleviate TME hypoxia. Meanwhile, glucose was oxidized into gluconic acid and H2O2 by Au to promote cell starvation treatment and H2O2 circulation. At the same time, the generation of O2 could enhance PDT (Fig. 5A).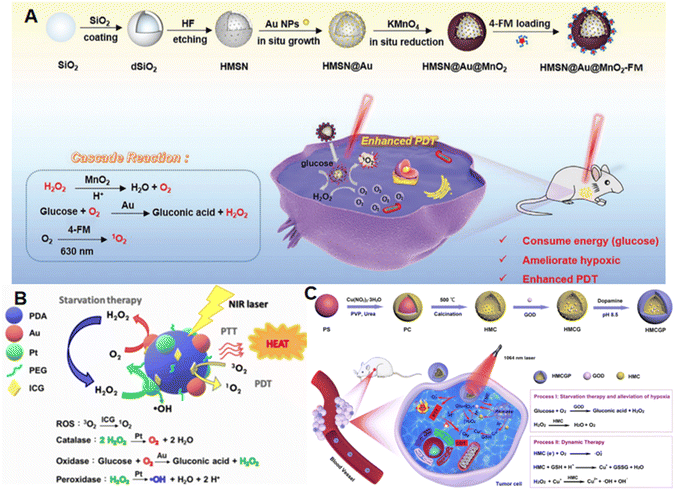 | ||
| Fig. 5 (A) Schematic of the synthesis process of HMSN@Au@MnO2-FM NPs and the mechanism for relieving cancer hypoxia and improving PDT by nanozyme-catalyzed cascade reactions. This figure has been adapted/reproduced from ref. 75 with permission from WILEY, copyright 2021. (B) Schematic for the proposed mechanism of ICG/Au/Pt@PDA–PEG NPs for the cooperative effect of ST/PTT/CDT. This figure has been adapted/reproduced from ref. 77 with permission from the American Chemical Society, copyright 2021. (C) Schematic of the synthesis of HMCGP and the mechanism of HMCGP for the starvation therapy-promoted alleviation of cancer hypoxia and combined photothermal–catalytic therapy by cascade enzymatic reactions. This figure has been adapted/reproduced from ref. 82 with permission from the American Chemical Society, copyright 2022. | ||
Polydopamine (PDA) has excellent photothermal conversion efficiency and multifunctional reactivity, making it often selected as a photothermal agent (PTA). At the same time, PDA can serve as a carrier for nanozymes and photosensitizers. In the work of Ciou et al., PDA was used as both a carrier for nanozymes and as a reducing agent, as it can form complexes with metal ions.77 HAuCl4 and K2PtCl6 were reduced to Au NPs and Pt NPs attached to the surface of PDA. Meanwhile PEG was connected to Au/Pt@PDA NPs through covalent interactions, and ICG was subsequently connected to the Au/Pt@PDA–PEG NPs through π–π interactions (Fig. 5B). The Pt NPs in the final synthesized nanozymes possessed the catalytic abilities of POD and CAT, catalyzing H2O2 into hydroxyl radicals and oxygen, respectively, and then glucose was oxidized into gluconic acid by O2, while H2O2 could be re-generated under the OXD activity of Au NPs, thereby allowing H2O2 circulation to be achieved. Under an NIR laser, a large amount of was heat generated for photothermal therapy, because of the ICG and PDA. In addition, singlet oxygen was generated in large quantities under the action of ICG, which, together with the hydroxyl radicals, induced cell apoptosis and promoted photodynamic therapy.
3.4 Template method
Hollow materials are usually synthesized through the template method, whereby nanozyme layers (such as metal oxides or carbon nanolayers) are deposited or reduced on hard templates (SiO2 is often used as a template), and then sacrificed by etching or by applying a high-temperature method to leave mesoporous nanozyme particles. Other active nanozymes may then be connected to them through different methods to form composite nanozymes.Polystyrene NPs were used as a template by Wang and colleagues,82 where, due to their opposite charges, CuO was adsorbed and reduced on the surface of the polystyrene NPs, followed by calcination at 500 °C to form HMC NPs. GOD was then loaded into the cavities and mesopores of HMC NPs to obtain GOD–CuO (HMCG) nanocomposites. Finally, PDA was coated on the surface of HMCG to improve its biosafety and bioavailability (Fig. 5C). After entering the cancer site, the nanozymes could be released under laser irradiation, playing the roles of CAT and OXD, respectively, and producing oxygen to alleviate TME hypoxia, while consuming glucose to promote starvation treatment, replenishing H2O2, or producing toxic ROS, and oxidizing GSH to prevent ROS from being disproportionated. Thus, the therapeutic effect of CDT could be greatly improved, and then combined with PTT to kill cancer cells.
3.5 Other methods
GOx was used as a template by Liu et al., and a nanozyme containing arginine and ferrocene was synthesized by reverse microemulsion polymerization.85 This nanozyme could consume glucose, produce nitric oxide and highly toxic hydroxyl radicals and superoxide anions. Then, a peroxynitrous acid radical anion was generated through the reaction between NO and ·O2−, thus stimulating reactive oxygen species and nitrogen (RONS) radical storms to effectively eradicate cancer cells in vitro and in vivo. Os is one of the group 8 elements, and nanostructures based on Os may have potential in various applications, including biomedical fields. However, it is difficult to synthesize Os NPs using traditional Ag nanotemplates under any conditions, thus Kang et al. tried to use TeNRs as templates to conduct solvothermal current displacement, and were able to finally successfully prepare OsTeNRs with both POD, CAT, SOD, and OXD activities.294. Factors affecting the catalytic activity of nanozymes
The catalytic efficacy of nanozymes is influenced by a multitude of parameters, including but not limited to, the morphological attributes, dimensional characteristics, elemental constituency, surface functionalization, and environmental perturbations, such as photic irradiation, sonication, thermal variations, and pH fluctuations.11,87,88 These factors can exert a significant control over the catalytic behavior of nanozymes and must be meticulously considered in the strategic design and fabrication of composite nanozyme systems to optimize their performance in biomedical applications.4.1 Dimensions
The dimensions of nanoparticles significantly influence their catalytic activity due to the direct correlation between the particle size and the number of surface-active sites. The distribution and density of these sites on the nanoparticle's surface are pivotal in determining the catalytic activity. Nanoparticles with smaller sizes demonstrate enhanced catalytic activity owing to their increased surface-area-to-volume ratio, which escalates the likelihood of substrate interaction. Precise synthetic manipulation of the diameter of nanoparticles allows fine-tuning their catalytic properties. In the case of CeO2 NPs, it has been established that a reduction in nanoparticle size is accompanied by an enlargement of the specific surface area, leading to an increased exposure of active sites and a heightened probability of substrate contact. Additionally, the downsizing of nanoparticles is linked to a higher density of surface oxidation vacancies, which can effectively accelerate Ce3+/Ce4+ redox cycling, thereby augmenting the catalytic activity.89 For this reason, Tang et al. integrated ultra-small CeO2 NPs with core–shell-structured Au@Pt NPs to fabricate CeO2/Au@Pt-PEG composite nanozymes, which demonstrated enhanced POD enzyme activity and augmented the generation of ROS and the efficacy of CDT in tumor therapy.904.2 Morphology
The catalytic activity of nanozymes is influenced by their morphology. Varying shapes, such as nanorods, nanowires, nanospheres, and nanofibers, possess distinct crystal facets, exposing different dangling bonds and atomic arrangements, which may increase the number of active sites, thereby enhancing the activity of the nanozymes. Qian et al. synthesized three morphologically distinct magnetite nanozymes (MNZs): spherical (SMNZ), ellipsoidal (EMNZ), and flaky (FMNZ). They observed that FMNZ exhibited the highest POD activity under weakly acidic conditions (pH 5.0), with a specific activity reaching 2.376 U mg−1, significantly outperforming SMNZ and EMNZ. Moreover, FMNZ demonstrated a faster and greater release of iron ions across all pH conditions, contributing to its enhanced POD activity. Similarly, FMNZ showed higher GPx activity compared to the other two MNZs tested. The flaky morphology also facilitated better internalization of the nanozyme by cancer cells. In vivo experiments revealed that FMNZ accumulated more in tumor tissues than SMNZ and EMNZ did.914.3 Composition
The composition of nanozymes is a critical factor influencing their activity. Composite nanozymes often exhibit higher catalytic activity than their single counterparts. Luo et al. constructed an Au@Pd composite nanozyme, which demonstrated a Km slightly higher than that of natural HRP, while its Vmax was significantly higher than that of both HRP and Au NPs.92 When the same nanozyme base was doped with different metal elements to form composite nanozymes, their catalytic efficiencies varied. Nguyen et al. doped various metal elements into cerium oxide nanoparticles, creating M–CeO2 NPs (where M represents a metal element, including Fe, Co, Ni, Cu, and Zn). They calculated the peroxidase activity of the different NPs and found significant differences in their catalytic activity depending on the doped element. Notably, Co–CeO2 NPs showed the highest enzyme activity, which was 627.5 times greater than that of CeO2 NPs.93 Changes in the valence state of the same metal nanozyme can also lead to alterations in catalytic action. For example, Zeng et al. synthesized four vanadium oxide nanozymes (Vnps-I, Vnps-II, Vnps-III, Vnps-IV) using a simple method, with the primary distinction among these Vnps being the ratio of V4+ to V5+. Vnps-III, containing a lower vanadium valence state (V4+), exhibited good POD and OXD activities, while Vnps-I, containing a higher vanadium valence state (V5+), possessed CAT activity.944.4 Surface modification
The surface modification of nanozymes can enhance their biocompatibility or enable them to achieve targeted localization. Since the majority of catalytic reactions occur on the surface of NPs, surface modification can not only improve the biocompatibility, aqueous dispersibility, and biological targeting capabilities of nanozymes but also influence their catalytic activity. Surface alterations encompass the incorporation of functional groups, inorganic ions, minute particles, and polymers, thereby modulating the characteristics of the nanozymes through the modification of their surface chemistry. For instance, He et al. synthesized Pt NPs decorated with carboxymethyl chitosan (CC–Pt NPs) and observed that these CC–Pt NPs exhibited a superior dispersion and maintained stable catalytic activity across a broader range of temperatures and pH values. Moreover, they demonstrated stronger ascorbate oxidase activity compared to unmodified Pt NPs.954.5 Environmental factors
The catalytic activity of nanozymes is not only determined by their intrinsic properties but may also be influenced by environmental stimuli, including exogenous and endogenous factors. Exogenous stimuli, such as light irradiation, ultrasound, temperature, and magnetism, can modulate the activity of nanozymes. Extensive research has revealed that certain noble metal nanozymes exhibit excellent photothermal conversion efficiency. Under NIR-II irradiation, such nanozymes convert light energy into heat, whereby the subsequent increase in temperature enhances the catalytic activity of the nanozymes.87 For instance, Au NPs are widely studied nanozymes with photothermal properties.57,90,96,97 Carbon-based nanozymes also possess photothermal characteristics. Feng et al. developed a mesoporous carbon nanozyme (MC-PEG) and observed that under NIR-II irradiation, it significantly increased the temperature of tumor cells and generated more ·OH, thereby demonstrating more effective and sustained glutathione GPx activity and more potent POD activity compared to graphene oxide nanoparticles.98 In another investigation, Saravanan et al. linked photothermal 2D material MoS2 nanoflowers with CeO2 NPs. Upon NIR-II irradiation, the temperature rose, which enhanced the POD enzyme activity of the CeO2 NPs.99 Recently, Dong et al. reported that US could significantly enhance the POD simulated catalytic activity of CaF2 nanozymes, and by increasing the collisions and interaction probability between H2O2 and the catalytic center, they could finally achieve efficient anti-tumor effects in 4T1 breast cancer and H22 liver cancer animal models.100Endogenous stimulation, primarily referring to pH and ROS within the TME, can play a significant role in modulating the activity of nanozymes. Among the four vanadium oxide nanozymes discussed above in Section 4.3, Vntp-III and VNTP-IV displayed heightened POD activity under acidic conditions (pH 6.0), effectively generating ROS for tumor treatment. Vnps-I exhibited more robust CAT activity than the other nanozymes tested under standard conditions and could maintain this activity across both pH 7.4 and pH 6.0, aiding amelioration of the hypoxic solid tumor environment.94 In a related study, Chen et al. illustrated that iron oxide nanoparticles (IONPs) presented POD activity under acidic conditions and CAT activity under neutral conditions (pH 7.4). They also demonstrated that these nanozymes retained high catalytic activity in the TME while being non-toxic to healthy tissues.101
5. Application of composite nanozymes in cancer treatment
Composite nanozymes fight cancer by producing oxygen, producing different reactive oxygen species, consuming glucose, or their cascades, or in combination with other therapies to overcome cancer. Table 4 summarizes some applications of composite nanozymes in different cancer treatments.| Cancer type | In vitro | In vivo | Nanozyme | Applications |
|---|---|---|---|---|
| Breast cancer | 4T1 | 4T1 | BDS–GOx@MnOx48 | CDT, ST |
| Breast cancer | 4T1 | 4T1 | PHMZCO-AT102 | CDT |
| Breast cancer | 4T1 | 4T1 | APHPBs60 | PDT |
| Breast cancer | 4T1 | 4T1 | Co/La-PB@MOF-199/GOx61 | CAT, PTT, ST |
| Breast cancer | 4T1 | 4T1 | CMO-R@4T1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 68 68 |
PTT, immunotherapy |
| Breast cancer | 4T1 | 4T1 | FAB NP26 | CDT, PDT, PTT |
| Breast cancer | 4T1 | 4T1 | P@Pt@P-Au-FA78 | PDT, ST |
| Breast cancer | 4T1 | 4T1 | PtCu3-PEG103 | CDT, SDT |
| Breast cancer | 4T1 | 4T1 | RuCu NSs30 | CDT |
| Breast cancer | 4T1 | 4T1 | FePc/HNCSs84 | PDT, PTT |
| Breast cancer | 4T1 | 4T1 | NC@GOx63 | CDT, PTT, ST |
| Breast cancer | MCF-7 | 4T1 | Fe3O4@MnO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 104 104 |
ST, RT |
| Breast cancer | MCF-7 | MCF-7 | PDAP-ICG-Pt79 | PDT, PTT |
| Breast cancer | MCF-7 | — | Cu–CuFe2O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 65 65 |
CDT, SDT, CTx |
| Breast cancer | MDA-MB-231 | MDA-MB-231 | CCCs24 | CAT, CTx |
| Cervical cancer | HeLa | U14 | Au2Pt-PEG-Ce6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
CDT, PDT, PTT |
| Cervical cancer | HeLa | U14 | HMCGP82 | CDT, PTT, ST |
| Cervical cancer | HeLa | — | CM-MMNPs80 | PDT |
| Cervical cancer | HeLa | 4T1 | MnFe2O4@MOF73 | PDT |
| Cervical cancer | HeLa | 4T1 | PTZCs27 | PDT, PTT |
| Colon cancer | CT26 | CT26 | Au1Pd3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 62 |
CDT |
| Colon cancer | CT26 | CT26 | PCPT71 | PTT, SDT |
| Colon cancer | CT26 | CT26 | HCS@Pt–Ce6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 83 83 |
PDT |
| Colon cancer | CT26 | CT26 | Pt–carbon76 | CDT, PDT |
| Colon cancer | CT26 | CT26 | DFMC86 | CDT |
| Melanoma | B16F1 | — | ICG/Au/Pt@PDA–PEG77 | CDT, PDT, PTT |
| Melanoma | B16F1 | — | CFS@PF17 | CDT, PDT, PTT, CTx |
| Liver cancer | HepG2 | HepG2 | MnO–N/C105 | CDT, PTT |
| Liver cancer | RIL-175 | RIL-175 | OsTeNRs29 | CDT, PDT, PTT, CTx, immunotherapy |
| Liver cancer | HepG2 | HepG2 | CuS@CeO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70 |
PTT, RT |
| Glioblastoma | U87MG | U87 MG | Au NBP/Pd69 | CDT, PTT |
| Glioblastoma | U87MG | — | MnFe2O4/C106 | PTT |
| Pancreatic cancer | SW1990 | SW1990 | PtFe@Fe3O4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67 67 |
CDT, PTT |
| Non-small cell lung cancer | A549 | A549 | Arg/Fc@GOx/HA85 | CDT, ST |
5.1 Different approaches to the treatment of cancer
PTT is an alternative modality of phototherapy, in which photosensitizers convert light energy into heat, leading to increased blood circulation in the tumor region. This temperature elevation enhances the penetration and effectiveness of nanozymes and can cause the demise of cancer cells due to hyperthermia. The combination of PTT with nanozymes to generate ROS for tumor treatment is a promising therapeutic approach. In the study by Hu et al., a manganese oxide/nitrogen-doped carbon composite nanozyme (MnO–N/C NPs) with photothermal conversion capability was developed. Upon irradiation with an 808 nm near-infrared laser, the POD, OXD, and CAT activities of MnO–N/C NPs were accelerated. The high temperature, in conjunction with the production of multiple ROS, led to apoptosis in cancer cells. Moreover, these nanozymes possessed T1-weighted MRI capabilities, enabling the MRI-guided efficient photothermal and enhanced catalytic synergistic treatment of tumors in mice.105
DOX is a potent and highly popular chemotherapeutic agent; however, it is commonly associated with cardiotoxicity. To mitigate the cardiac side effects of this drug, Xing et al. developed a novel auric ruthenium (AuRu) bimetallic cluster nanozyme conjugated with atrial natriuretic peptide (ANP), termed ATBMzyme. In co-administration studies, this enzyme has been shown to target the heart, demonstrating robust antioxidant activity within cardiomyocytes, as well as being able to effectively scavenge free radicals, and reduce DOX-induced ferroptosis in cardiac cells. Additionally, ATBMzyme has been observed to alleviate DOX-induced hepatotoxicity and nephrotoxicity without compromising the therapeutic efficacy of DOX against tumors.112
5.2 Application of composite nanozymes for the treatment of different cancers
Liu et al. developed a cascade catalyzed composite nanozyme with the formula P@Pt@P-Au-FA, in which the Pt nanoparticles in the interlayer and Au nanoparticles on the surface could function as effective catalase mimics and glucose oxidase mimics, respectively, to provide oxygen for PDT, recycle H2O2, and consume glucose to promote cancer cell starvation treatment.78 In the hypoxic environment, after 8 min light exposure, the nanoparticles reduced the survival rate of the mouse breast cancer cell 4T1 line to 40% (Fig. 6A), and the detection results for live and dead cells in Fig. 6B also well showed the good inhibition rate of NPs on cancer cells. It can be intuitively found from Fig. 6C and D that in the 4T1 breast cancer mouse model, P@Pt@P-Au-FA NPs combined with laser irradiation significantly inhibited the growth of tumors, in which the inhibition rate even reached 90.88%, while it was also clear that Au NPs could play a vital role in the starvation therapy of cancers.
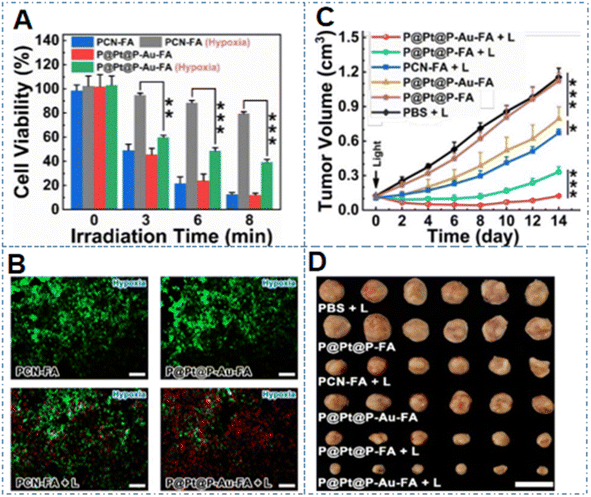 | ||
| Fig. 6 (A) Viability of 4T1 cells treated with different NPs. (B) Live–dead staining of 4T1 cells treated with different NPs (green indicates viable cells and red indicates dead cells). (C) Tumor growth curves of 4T1 tumor-bearing mice with different treatments; (D) tumors of the model mice after 14 days of treatment. This figure has been adapted/reproduced from ref. 78 with permission from the American Chemical Society, copyright 2019. | ||
Lyu et al. developed Fe3O4@MnO2 nanoparticles that integrated MR imaging and treatment capabilities, which could be used in combination with GOx and RT.104 The core Fe3O4 of this core–shell-structured nanozyme could trigger the Fenton reaction and generate a large amount of ROS. At the same time, Fe3O4 has a good magnetic targeting ability and could be used for T2 weighted MRI. The MnO2 in the shell has the ability to consume the GSH in the TME, which can not only consume the generated ROS but may also limit the RT effect. Meanwhile, Mn4+ could be used for T1 weighted MRI. Within the test concentration range, the inhibition rate of Fe3O4@MnO2 combined with GOx and RT on the human breast cancer cell line MCF-7 was 59% and 48.1% higher than that of GOx combined with RT and RT alone, respectively.
Wang et al. designed a composite biomimetic nanozyme called HMCGP. They loaded GOD into the cavity of hollow mesoporous copper oxide, and reported the system could achieve the combined treatment of ST, CDT, and PTT through a cascade enzyme method.82 This nanozyme could catalyze the conversion of endogenous H2O2 to O2, alleviate the problem of TME hypoxia, which then improved the effectiveness of GOD in catalyzing glucose decomposition. The Cu+ produced by HMC could catalyze the oxidation of endogenous GSH and promote the CDT effect and produce ROS to kill tumors. It was reported that when HMCGP was added to H2O2, oxygen quickly appeared from the liquid surface and produce more ·OH in a slightly acidic environment at 55 °C. Within the experimental concentration range tested, the HMCGP nanozyme alone showed no significant toxicity to normal mouse fibroblasts. However, after 1064 nm laser irradiation, it was found that the highest concentration of the HMCGP nanozyme could reduce the activity of HeLa cells to 11.6%. Through staining, it was also found that the mitochondria in most cells treated by HMCGP combined with laser irradiation were damaged, with 40.5% of the HeLa cells experiencing apoptosis. In U14 tumor-bearing mouse experiments, the tumor volume of the mice treated with the HMCGP nanozyme combined with laser irradiation was the smallest, and the tumors even disappeared in some mice. The tumor volume was the second smallest in the HMCG nanozyme combined with laser irradiation group, which was thanks to the cascade catalytic effect of the GOD and nanozyme combination.
The Pt–CuS Janus nanozyme (named PCPT) mentioned in Section 3.2 above not only had the function of catalase to promote the efficacy of SDT and PTT, but could also be used for photoacoustic imaging and near-infrared thermal imaging.71 It is worth mentioning that under US, the 1O2 production efficiency of the nanozyme was 1.85 times higher than that under 650 nm light irradiation (Fig. 7A). However, in cell experiments, the cell survival rates of the (PCPT + US) group and (PCPT + laser) group, respectively, were decreased to 59.5% and 40.1% (Fig. 7B). The combined treatment by US and laser irradiation could reduce the survival rate of CT26 cells to 19.8%, while in the CT26 tumor-bearing mice model, there were particularly significant reductions in the tumor volume and weight in the combined treatment group, indicating that the PCPT nanozyme plays an important role in inhibiting cancer (Fig. 7C and D).
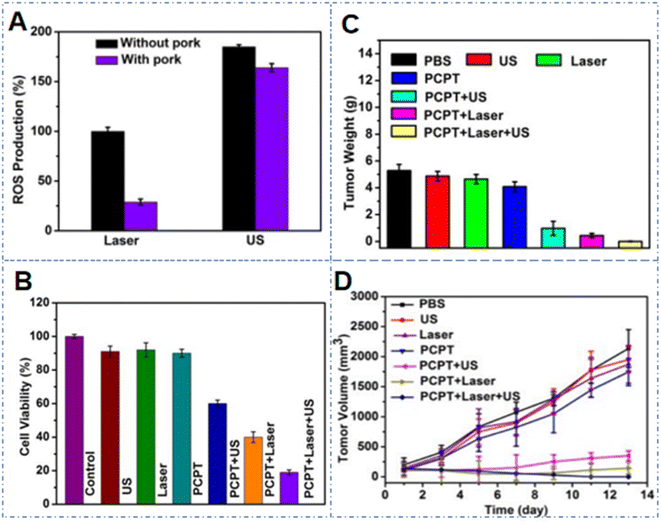 | ||
| Fig. 7 (A) Relative 1O2− production from PCPT after 650 nm NIR light or US irradiation, with or without the screening of pork. (B) Viability of CT26 cells with different treatments. (C) Tumor weight of mice after receiving different treatments. (D) Tumor volume of mice after receiving different treatments. This figure has been adapted/reproduced from ref. 71 with permission from the American Chemical Society, copyright 2019. | ||
Jiang et al. designed a CuS@CeO2 nanozyme with a special fusiform shape that could enhance the tumor-penetration ability, combining self-supplied oxygen, a good photothermal conversion ability, and that was RT sensitive and could be used in cancer therapy.70 When combined with PTT and RT, more than 80% of Hep-G2 cells were killed. After different treatments, the Hep-G2 tumor-bearing mice in the control group experienced rapid tumor growth, while the tumors of the group with CuS@CeO2 NPs-assisted RT/PTT combined treatment did not appear to have grown, and tumor regression was obvious. This design not only allows reducing the RT dose, but more importantly, enables the entire tumor to be treated without recurrence. The Os–Te nanozyme mentioned in Section 3.5 above could combine with PDT/PTT for the treatment of liver cancer.29 Under hypoxic conditions, it was found that the Os–Te nanozyme greatly improved the therapeutic efficiency of PDT, and the survival rate of RIL-175 cells after treatment was decreased to 10.7%, with a therapeutic efficiency 2.4 times that of Ce6.
Lung cancer is the malignant cancer with the highest mortality rate, with a mortality rate close to twice that of colorectal cancer, which ranks second. Lung cancer is mainly divided into non-small cell lung cancer and small cell lung cancer, of which 85% of cases are non-small cell lung cancer. The A549 cell line belongs to the typical non-small cell lung cancer. Liu et al. prepared a kind of nanozyme that was formed by absorbing various monomers with GOx as the core, including acrylamide, arginine-acrylamide (Arg), ferrocene-acrylate (Fc) and N,N′-bis(acryloyl)cystamine (BAC). Subsequently, Arg/Fc@GOx nanozymes were formed by an interfacial free radical polymerization.85 This composite nanozyme utilized the acceleration of enzymes and chemical reactions to induce intracellular RONS (reactive oxygen species and nitrogen) free radical storms, induce cancer cell apoptosis, inhibit tumor growth, and prolong survival rate. During the 17 days observation period, the Arg/Fc@GOx/HA nanozyme showed the most significant tumor inhibition, and in the subsequent 61 days survival experiment, the survival rate of this group of mice was also the highest, reaching 77.8%.
6. Conclusions and perspectives
Since their discovery, nanozymes have developed rapidly. Over the past decade, a variety of nanozymes with different catalytic activities have been developed, such as oxidoreductase including POD, CAT, OXD, and SOD, and hydrolases, including phosphatase, nuclease, esterase, and protease. Nanozymes are applied in various fields, such as anti-cancer, anti-bacterial, anti-biofilm, sensing, environmental governance, and antioxidant. In the pursuit of achieving better results, composite nanozymes have been developed, and a large number of studies have reported that composite nanozymes have better catalytic and therapeutic effects than individual nanozymes.This work summarizes and provides examples of the classification, advantages, and synthesis methods of many nanozymes, as well as their applications in cancer treatment. The anti-tumor principle of composite nanozymes can be summarized as follows: (1) reliance on CAT enzyme activity to consume endogenous H2O2 or on SOD enzyme activity to produce O2, thereby improving the hypoxia of TME and making other oxygen-dependent therapies feasible; (2) reliance on POD enzyme activity to consume H2O2 and produce toxic ROS; (3) reliance on GPx enzyme activity to consume endogenous GSH to prevent the consumption of generated ROS; (4) reliance on GOx enzyme activity to consume glucose and generate H2O2 for recycling, while achieving the effect of starvation therapy; (5) use in combination with other therapies, such as PDT, PTT, CTx, SDT, ST, and RT, where there is a significant synergistic effect that makes combined therapy much better than single therapy.
As mentioned before, composite nanozymes have already played a significant role in various cancers, but there is still a long way to go before the clinical implementation of composite nanozymes can be realized, including:119–122
(1) The therapeutic efficacy of nanozymes is contingent upon their in vivo stability and activity. Despite the superior catalytic efficiency and stability of composite nanozymes in vitro, their performance within the complex tumor microenvironment may be compromised. A profound understanding of the biodistribution and interaction profiles of nanomaterials within biological matrices is essential for the precise quantification of nanozymes' catalytic reactions in vivo, thereby facilitating the optimization of nanosystem designs to augment their therapeutic potency.
(2) In vivo safety research on nanozymes is inadequate, necessitating a meticulous and systematic dissection of nanozyme pharmacokinetics, encompassing their absorption, distribution, metabolism, and excretion, alongside the dosing regimens necessary for achieving an effective therapeutic impact. It is imperative to scrutinize the toxicity profile of nanozymes under these conditions, including potential hemolytic and coagulation effects, as well as adverse effects on non-target organs and tissues. The strategic incorporation of targeted action in nanozyme design is paramount for enhancing their tumor-specific accumulation. To bridge the gap between preclinical and clinical realms, an expansion of pharmacodynamic and toxicological investigations into larger animal models is imperative, transcending the current reliance on murine studies.
(3) Cancer cells possess a plethora of metabolic pathways, posing a great challenge for rapid metabolic reprogramming. The metabolic regulatory capabilities of extant synthetic nanozymes for oncological applications are relatively circumscribed, predominantly centered on oxidoreductases and often predicated on H2O2. Given the potential toxicity of H2O2 in excess, there is an urgent need to diversify the armamentarium of active nanozymes for cancer therapy, with particular emphasis on those independent of H2O2, such as transferases, isomerases, lyases, and synthases, to broaden the metabolic modulation spectrum of nanozymes in oncological interventions.
(4) The exploration of catalytic kinetics and mechanisms in composite nanozymes is currently lacking. The construction of a holistic theoretical framework for diverse nanozymes, one that amalgamates theoretical computations with empirical validations and harnesses sophisticated characterization methodologies, is imperative for elucidating the molecular underpinnings of nanozyme–cell interactions. This multidimensional approach is pivotal for unraveling the complex functional dynamics of nanozymes within biological systems and for laying the groundwork for the evolution of nanozyme-based therapeutics. By employing omics and single-cell analytical techniques, researchers can probe the molecular intricacies of nanozyme–cell interactions, thereby enhancing the precision and efficacy of nanozyme-mediated therapies. Despite all the challenges that lie ahead, it is strongly believed that through continuous in-depth research, composite nanozymes can be developed that will show excellent performance in clinical applications.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Nature Science Foundation of China (grant number: 81271677), and Zhejiang Provincial Natural Science Foundation of China (no. LY21E030011). In addition, this work was supported by the Medical Science and Technology Project of Zhejiang Province (grant number: 2024KY011, 2025KY561).References
- F. Bray, M. Laversanne, H. Sung, J. Ferlay, R. L. Siegel, I. Soerjomataram and A. Jemal, Ca-Cancer J. Clin., 2024, 74, 229–263 CrossRef.
- L. A. Torre, F. Bray, R. L. Siegel, J. Ferlay, J. Lortet-Tieulent and A. Jemal, Ca-Cancer J. Clin., 2015, 65, 87–108 CrossRef PubMed.
- W. Yin, J. Wang, L. Jiang and Y. James Kang, Exp. Biol. Med., 2021, 246, 1791–1801 CrossRef CAS.
- S. Zeng, Q. Tang, M. Xiao, X. Tong, T. Yang, D. Yin, L. Lei and S. Li, Mater. Today Bio, 2023, 20, 100633 CrossRef CAS.
- X. Li, S. Ai, X. Lu, S. Liu and W. Guan, RSC Adv., 2021, 11, 35392–35407 RSC.
- P. N. Navya, S. Mehla, A. Begum, H. K. Chaturvedi, R. Ojha, C. Hartinger, M. Plebanski and S. K. Bhargava, Adv. Healthcare Mater., 2023, 12, 2300768 CrossRef.
- X. Jiang, J. Wang, X. Deng, F. Xiong, S. Zhang, Z. Gong, X. Li, K. Cao, H. Deng, Y. He, Q. Liao, B. Xiang, M. Zhou, C. Guo, Z. Zeng, G. Li, X. Li and W. Xiong, J. Exp. Clin. Cancer Res., 2020, 39, 204 CrossRef.
- Y. Ding, Q. Pan, W. Gao, Y. Pu, K. Luo and B. He, Biomater. Sci., 2023, 11, 1182–1214 RSC.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed.
- S. Ahmadi, K. Rahimizadeh, A. Shafiee, N. Rabiee and S. Iravani, Process Biochem., 2023, 131, 154–174 CrossRef CAS.
- A. Deshwal, K. Saxena, G. Sharma, Rajesh, F. A. Sheikh, C. S. Seth and R. M. Tripathi, Int. J. Biol. Macromol., 2024, 256, 128272 CrossRef CAS.
- S. Thangudu and C.-H. Su, Biomolecules, 2021, 11, 1015 CrossRef CAS PubMed.
- D. Xu, L. Wu, H. Yao and L. Zhao, Small, 2022, 18, 2203400 CrossRef CAS PubMed.
- Y. Huang, J. Ren and X. Qu, Chem. Rev., 2019, 119, 4357–4412 CrossRef CAS.
- X. Zhang, X. Chen and Y. Zhao, Nano-Micro Lett., 2022, 14, 95 CrossRef CAS PubMed.
- C. Lu, X. Liu, Y. Li, F. Yu, L. Tang, Y. Hu and Y. Ying, ACS Appl. Mater. Interfaces, 2015, 7, 15395–15402 CrossRef CAS PubMed.
- W. B. Dirersa, G. Getachew, C. H. Hsiao, A. Wibrianto, A. S. Rasal, C. C. Huang and J. Y. Chang, Mater. Today Chem., 2022, 26, 101158 CrossRef CAS.
- X. Wang and L. Cheng, Coord. Chem. Rev., 2020, 419, 213393 CrossRef CAS.
- B. Xu, Z. Huang, Y. Liu, S. Li and H. Liu, Nano Today, 2023, 48, 101690 CrossRef CAS.
- H. Li, M. Wei, X. Lv, Y. Hu, J. Shao, X. Song, D. Yang, W. Wang, B. Li and X. Dong, J. Innovative Opt. Health Sci., 2022, 15, 2230009 CrossRef CAS.
- D. Yin, H. Yang, S. Wang, Z. Yang, Q. Liu, X. Zhang and X. Zhang, Colloids Surf., A, 2020, 607, 125466 CrossRef CAS.
- S. Wang, J. Zhao, L. Zhang, C. Zhang, Z. Qiu, S. Zhao, Y. Huang and H. Liang, Adv. Healthcare Mater., 2022, 11, e2102073 CrossRef.
- H. Xu, Z. Zhang, L. Zhang, Z. Chen and S. Wang, J. Colloid Interface Sci., 2022, 625, 544–554 CrossRef CAS PubMed.
- F. Cheng, S. Wang, H. Zheng, S. Yang, L. Zhou, K. Liu, Q. Zhang and H. Zhang, Colloids Surf., B, 2021, 205, 111878 CrossRef CAS.
- M. Wang, M. Chang, Q. Chen, D. Wang, C. Li, Z. Hou, J. Lin, D. Jin and B. Xing, Biomaterials, 2020, 252, 120093 CrossRef CAS PubMed.
- C. Cao, H. Zou, N. Yang, H. Li, Y. Cai, X. Song, J. Shao, P. Chen, X. Mou, W. Wang and X. Dong, Adv. Mater., 2021, 33, 2106996 CrossRef CAS.
- C. Wang, Y. Li, W. Yang, L. Zhou and S. Wei, Adv. Healthcare Mater., 2021, 10, e2100601 CrossRef.
- H. Wu, Q. Jiang, K. Luo, C. Zhu, M. Xie, S. Wang, Z. Fei and J. Zhao, J. Nanobiotechnol., 2021, 19, 203 CrossRef CAS PubMed.
- S. Kang, Y. G. Gil, G. Yim, D. H. Min and H. Jang, ACS Appl. Mater. Interfaces, 2021, 13, 44124–44135 CrossRef CAS.
- J. Yang, L. Fang, R. Jiang, L. Qi, Y. Xiao, W. Wang, I. Ismail and X. Fang, Adv. Healthcare Mater., 2023, e2300490, DOI:10.1002/adhm.202300490.
- B. Jiang, D. Duan, L. Gao, M. Zhou, K. Fan, Y. Tang, J. Xi, Y. Bi, Z. Tong, G. F. Gao, N. Xie, A. Tang, G. Nie, M. Liang and X. Yan, Nat. Protoc., 2018, 13, 1506–1520 CrossRef CAS.
- X. Hong, X. Xu, Z. Liu, S. Liu, J. Yu, M. Wu, Y. Ma and Q. Shuai, Nanotechnology, 2021, 32, 465701 CrossRef CAS.
- Y. Yan, Y. Hou, H. Zhang, W. Gao, R. Han, J. Yu, L. Xu and K. Tang, Colloids Surf., B, 2021, 208, 112103 CrossRef CAS PubMed.
- N. Singh, M. A. Savanur, S. Srivastava, P. D'Silva and G. Mugesh, Angew Chem. Int. Ed. Engl., 2017, 56, 14267–14271 CrossRef CAS PubMed.
- J. Mu, Y. Wang, M. Zhao and L. Zhang, Chem. Commun., 2012, 48, 2540–2542 RSC.
- Z. Zhu, Z. Guan, S. Jia, Z. Lei, S. Lin, H. Zhang, Y. Ma, Z. Q. Tian and C. J. Yang, Angew Chem. Int. Ed. Engl., 2014, 53, 12503–12507 CrossRef CAS.
- W. He, Y.-T. Zhou, W. G. Wamer, M. D. Boudreau and J.-J. Yin, Biomaterials, 2012, 33, 7547–7555 CrossRef CAS.
- Y. Li, K.-H. Yun, H. Lee, S.-H. Goh, Y.-G. Suh and Y. Choi, Biomaterials, 2019, 197, 12–19 CrossRef CAS PubMed.
- J. L. Qingqing Wang, L. He, S. Liu and P. Yang, Nanoscale, 2023, 15, 12455–12463 RSC.
- A. Asati, S. Santra, C. Kaittanis, S. Nath and J. M. Perez, Angew. Chem., Int. Ed., 2009, 48, 2308–2312 CrossRef CAS PubMed.
- I. Celardo, J. Z. Pedersen, E. Traversa and L. Ghibelli, Nanoscale, 2011, 3, 1411 RSC.
- J. Mu, X. Zhao, J. Li, E.-C. Yang and X.-J. Zhao, J. Mater. Chem. B, 2016, 4, 5217–5221 RSC.
- X. Yu, Y. Wang, J. Zhang, J. Liu, A. Wang and L. Ding, Adv. Healthcare Mater., 2023, 13, 2302023 CrossRef PubMed.
- C. Hao, A. Qu, L. Xu, M. Sun, H. Zhang, C. Xu and H. Kuang, J. Am. Chem. Soc., 2018, 141, 1091–1099 CrossRef PubMed.
- P. Ling, Q. Zhang, T. Cao and F. Gao, Angew. Chem., Int. Ed., 2018, 57, 6819–6824 CrossRef CAS.
- P. K. Boruah and M. R. Das, J. Hazard. Mater., 2020, 385, 121516 CrossRef CAS PubMed.
- S. Maddheshiya and S. Nara, Front. Bioeng. Biotechnol., 2022, 10, 880214 CrossRef.
- L. Li, Z. Lin, X. Xu, W. Wang, H. Chen, Z. Feng, Z. Yang and J. Hao, ACS Appl. Mater. Interfaces, 2023, 15, 41224–41236 CrossRef CAS PubMed.
- M. Wang, J. Li, J. Liu, Y. Huang, L. Yang, C. Zhu, Y. Zhang, X. Gui, H. Peng and M. Chu, J. Colloid Interface Sci., 2024, 676, 110–126 CrossRef CAS.
- J. Ma, Q. Yao, S. Lv, J. Yi, D. Zhu, C. Zhu, L. Wang and S. Su, J. Nanobiotechnol., 2024, 22, 596 CrossRef CAS PubMed.
- S. Cui, B. Wang, C. Zhai, S. Wei, H. Zhang and G. Sun, J. Mater. Chem. B, 2023, 11, 7986–7997 RSC.
- J. Liu, B. Yu, M. Rong, W. Sun and L. Lu, Nano Today, 2024, 54, 102113 CrossRef CAS.
- B. Li, H. Shen, Q. Liu, X. Liu, J. Cai, L. Zhang, D. Wu, Y. Xie, G. Xie and W. Feng, Sens. Actuators, B, 2023, 386, 133762 CrossRef CAS.
- X. Zhang, Y. Liu, J. Doungchawee, L. J. Castellanos-García, K. N. Sikora, T. Jeon, R. Goswami, S. Fedeli, A. Gupta, R. Huang, C.-M. Hirschbiegel, R. Cao-Milán, P. K. D. Majhi, Y. A. Cicek, L. Liu, D. J. Jerry, R. W. Vachet and V. M. Rotello, J. Controlled Release, 2023, 357, 31–39 CrossRef CAS PubMed.
- S. Zhang, Y. Liu, S. Sun, J. Wang, Q. Li, R. Yan, Y. Gao, H. Liu, S. Liu, W. Hao, H. Dai, C. Liu, Y. Sun, W. Long, X. Mu and X.-D. Zhang, Theranostics, 2021, 11, 2806–2821 CrossRef CAS PubMed.
- Q. Wang, C. Cheng, S. Zhao, Q. Liu, Y. Zhang, W. Liu, X. Zhao, H. Zhang, J. Pu, S. Zhang, H. Zhang, Y. Du and H. Wei, Angew. Chem., Int. Ed., 2022, 61, e202201101 CrossRef CAS.
- X. Zhang, X. Li, M. Fu, J. O. A. Machuki, W. Wang, L. Wu, Q. Zhao, N. Xin, L. Hua and F. Gao, Chem. Eng. J., 2024, 481, 148745 CrossRef CAS.
- H. Liu, Z. Deng, Z. Zhang, W. Lin, M. Zhang and H. Wang, Matter, 2024, 7, 977–990 CrossRef CAS.
- B. Yang, L. Cao, K. Ge, C. Lv, Z. Zhao, T. Zheng, S. Gao, J. Zhang, T. Wang, J. Jiang and Y. Qin, Small, 2024, 20, 2401110 CrossRef CAS.
- W. Shen, G. Han, L. Yu, S. Yang, X. Li, W. Zhang and P. Pei, Int. J. Nanomed., 2022, 17, 1397–1408 CrossRef PubMed.
- L. He, Q. Ji, B. Chi, S. You, S. Lu, T. Yang, Z. Xu, Y. Wang, L. Li and J. Wang, Colloids Surf., B, 2023, 222, 113058 CrossRef CAS PubMed.
- K. Nie, L. Zhu, Y. Chen, L. Yu, M. Chang, Y. Chen and H. Yu, Nano Today, 2023, 53, 102050 CrossRef CAS.
- K. Xu, X. Wu, Y. Cheng, J. Yan, Y. Feng, R. Chen, R. Zheng, X. Li, P. Song, Y. Wang and H. Zhang, Nanoscale, 2020, 12, 23159–23165 RSC.
- X. Zhong, X. Wang, L. Cheng, Y. A. Tang, G. Zhan, F. Gong, R. Zhang, J. Hu, Z. Liu and X. Yang, Adv. Funct. Mater., 2019, 30, 1907954 CrossRef.
- C. Gong, J. Zhao, X. Meng, Z. Yang and H. Dong, Chem. Eng. J., 2022, 435, 135083 CrossRef CAS.
- F. Gong, L. Cheng, N. Yang, O. Betzer, L. Feng, Q. Zhou, Y. Li, R. Chen, R. Popovtzer and Z. Liu, Adv. Mater., 2019, 31, e1900730 CrossRef PubMed.
- S. Li, L. Shang, B. Xu, S. Wang, K. Gu, Q. Wu, Y. Sun, Q. Zhang, H. Yang, F. Zhang, L. Gu, T. Zhang and H. Liu, Angew Chem. Int. Ed. Engl., 2019, 58, 12624–12631 CrossRef CAS PubMed.
- D. Jana, B. He, Y. Chen, J. Liu and Y. Zhao, Adv. Mater., 2024, 36, 2206401 CrossRef CAS.
- S. Yu, D. Jang, S. K. Maji, K. Chung, J. S. Lee, F. Marques Mota, J. Wang, S. Kim and D. H. Kim, J. Ind. Eng. Chem., 2021, 104, 106–116 CrossRef CAS.
- W. Jiang, X. Han, T. Zhang, D. Xie, H. Zhang and Y. Hu, Adv. Healthcare Mater., 2020, 9, e1901303 CrossRef.
- S. Liang, X. Deng, Y. Chang, C. Sun, S. Shao, Z. Xie, X. Xiao, P. Ma, H. Zhang, Z. Cheng and J. Lin, Nano Lett., 2019, 19, 4134–4145 CrossRef CAS PubMed.
- S. Liang, X. Deng, G. Xu, X. Xiao, M. Wang, X. Guo, P. A. Ma, Z. Cheng, D. Zhang and J. Lin, Adv. Funct. Mater., 2020, 30, 1908598 CrossRef CAS.
- S. Y. Yin, G. Song, Y. Yang, Y. Zhao, P. Wang, L. M. Zhu, X. Yin and X. B. Zhang, Adv. Funct. Mater., 2019, 29, 1901417 CrossRef.
- Z. Wang, Y. Zhang, E. Ju, Z. Liu, F. Cao, Z. Chen, J. Ren and X. Qu, Nat. Commun., 2018, 9, 3334 CrossRef.
- M. Chen, J. Song, J. Zhu, G. Hong, J. An, E. Feng, X. Peng and F. Song, Adv. Healthcare Mater., 2021, 10, 2101049 CrossRef CAS.
- Y. Yang, D. Zhu, Y. Liu, B. Jiang, W. Jiang, X. Yan and K. Fan, Nanoscale, 2020, 12, 13548–13557 RSC.
- T. Y. Ciou, C. Korupalli, T. H. Chou, C. H. Hsiao, G. Getachew, S. Bela and J. Y. Chang, ACS Appl. Bio Mater., 2021, 4, 5650–5660 CrossRef CAS PubMed.
- C. Liu, J. Xing, O. U. Akakuru, L. Luo, S. Sun, R. Zou, Z. Yu, Q. Fang and A. Wu, Nano Lett., 2019, 19, 5674–5682 CrossRef CAS.
- P. Dong, W. Wang, M. Pan, W. Yu, Y. Liu, T. Shi, J. Hu, Y. Zhou, S. Yu, F. Wang and X. Liu, ACS Appl. Mater. Interfaces, 2021, 13, 16075–16083 CrossRef CAS.
- D. Zhang, Z. Ye, L. Wei, H. Luo and L. Xiao, ACS Appl. Mater. Interfaces, 2019, 11, 39594–39602 CrossRef CAS.
- Y. Zhu, Z. Wang, R. Zhao, Y. Zhou, L. Feng, S. Gai and P. Yang, ACS Nano, 2022, 16, 3105–3118 CrossRef CAS PubMed.
- J. Wang, J. Ye, W. Lv, S. Liu, Z. Zhang, J. Xu, M. Xu, C. Zhao, P. Yang and Y. Fu, ACS Appl. Mater. Interfaces, 2022, 14, 40645–40658 CrossRef CAS.
- Z. Xu, P. Sun, J. Zhang, X. Lu, L. Fan, J. Xi, J. Han and R. Guo, Chem. Eng. J., 2020, 399, 125797 CrossRef CAS.
- H. Yang, B. Xu, S. Li, Q. Wu, M. Lu, A. Han and H. Liu, Small, 2021, 17, e2007090 CrossRef PubMed.
- X. Liu, W. Li, M. Wang, N. Liu, Q. Yang, Y. He, D. Hu, R. Zhu and L. Yin, Small Methods, 2023, 7, e2201641 CrossRef PubMed.
- F. Wu, Y. Du, J. Yang, B. Shao, Z. Mi, Y. Yao, Y. Cui, F. He, Y. Zhang and P. Yang, ACS Nano, 2022, 16, 3647–3663 CrossRef CAS PubMed.
- Q. Fu, C. Wei and M. Wang, ACS Nano, 2024, 18, 12049–12095 CrossRef CAS.
- X. Zhou, S. Feng, Q. Xu, Y. Li, J. Lan, Z. Wang, Y. Ding, S. Wang and Q. Zhao, Acta Biomater., 2025, 191, 1–28 CrossRef PubMed.
- N. Feng, Y. Liu, X. Dai, Y. Wang, Q. Guo and Q. Li, RSC Adv., 2022, 12, 1486–1493 RSC.
- M. Tang, Z. Zhang, C. Ding, J. Li, Y. Shi, T. Sun and C. Chen, J. Colloid Interface Sci., 2022, 627, 299–307 CrossRef CAS.
- Y. Qian, J. Zou, J. Zhang, X. Wang, X. Meng, Y. Lin, W. Lin, M. Zhang and H. Wang, Chem. Eng. J., 2024, 490, 151867 CrossRef CAS.
- M. Luo, F.-K. Zhao, Y.-M. Wang and J. Bian, J. Transl. Med., 2024, 22, 814 CrossRef CAS PubMed.
- P. T. Nguyen, J. Lee, A. Cho, M. S. Kim, D. Choi, J. W. Han, M. I. Kim and J. Lee, Adv. Funct. Mater., 2022, 32, 2112428 CrossRef CAS.
- X. Zeng, H. Wang, Y. Ma, X. Xu, X. Lu, Y. Hu, J. Xie, X. Wang, Y. Wang, X. Guo, L. Zhao and J. Li, ACS Appl. Mater. Interfaces, 2023, 15, 13941–13955 CAS.
- S.-B. He, L. Yang, Y. Yang, H. A. A. Noreldeen, G.-W. Wu, H.-P. Peng, H.-H. Deng and W. Chen, Carbohydr. Polym., 2022, 298, 120120 CrossRef CAS PubMed.
- J. Zhou, X.-J. Yang, Q. Yu, X.-L. Li, H.-Y. Chen and J.-J. Xu, ACS Appl. Nano Mater., 2022, 5, 7009–7018 CrossRef CAS.
- S. Li, X. Zhao, H. Ding, J. Chang, X. Qin, F. He, X. Gao, S. Gai and P. Yang, Chem. Eng. J., 2023, 473, 145414 CrossRef CAS.
- S. Feng, J. Wang, X. Mu, G. Gu, Y. Wang, J. Lu, S. Wang and Q. Zhao, Colloids Surf., B, 2023, 222, 113095 CrossRef CAS PubMed.
- N. Saravanan, P. Ganesh, S. Pitchaimuthu and A. Sundaramurthy, Surf. Interfaces, 2023, 41, 103225 CrossRef CAS.
- C. Dong, X. Dai, X. Wang, Q. Lu, L. Chen, X. Song, L. Ding, H. Huang, W. Feng, Y. Chen and M. Chang, Adv. Mater., 2022, 34, 2205680 CrossRef CAS.
- Z. Chen, J.-J. Yin, Y.-T. Zhou, Y. Zhang, L. Song, M. Song, S. Hu and N. Gu, ACS Nano, 2012, 6, 4001–4012 CrossRef CAS.
- S. Dong, Y. Dong, B. Liu, J. Liu, S. Liu, Z. Zhao, W. Li, B. Tian, R. Zhao, F. He, S. Gai, Y. Xie, P. Yang and Y. Zhao, Adv. Mater., 2022, 34, 2107054 CrossRef CAS.
- S. Dong, Y. Dong, T. Jia, S. Liu, J. Liu, D. Yang, F. He, S. Gai, P. Yang and J. Lin, Adv. Mater., 2020, 32, e2002439 CrossRef.
- M. Lyu, D. Zhu, X. Kong, Y. Yang, S. Ding, Y. Zhou, H. Quan, Y. Duo and Z. Bao, Adv. Healthcare Mater., 2020, 9, e1901819 CrossRef PubMed.
- Z. Hu, X. Zhou, W. Zhang, L. Zhang, L. Li, Y. Gao and C. Wang, J. Colloid Interface Sci., 2025, 679, 375–383 CrossRef CAS PubMed.
- X. Han, Y. Li, Y. Zhou, Z. Song, Y. Deng, J. Qin and Z. Jiang, Mater. Des., 2021, 204, 109646 CrossRef CAS.
- X. Meng, H. Fan, L. Chen, J. He, C. Hong, J. Xie, Y. Hou, K. Wang, X. Gao, L. Gao, X. Yan and K. Fan, Nat. Commun., 2024, 15, 1626 CrossRef CAS PubMed.
- X. Meng and J. Gao, Arabian J. Chem., 2024, 17, 105626 CrossRef CAS.
- S. Liang, X. Deng, G. Xu, X. Xiao, M. Wang, X. Guo, P. a. Ma, Z. Cheng, D. Zhang and J. Lin, Adv. Funct. Mater., 2020, 30, 1908598 CrossRef CAS.
- Z. Zhou, J. Huang, Z. Zhang, L. Zhang, Y. Cao, Z. Xu, Y. Kang and P. Xue, Chem. Eng. J., 2022, 435, 135085 CrossRef CAS.
- Y. Yin, L. Zhu, T. Jiang, R. Chai, Y. Zhang, T. Li, K. Wang, S. Wang and Q. Zhang, Chem. Eng. J., 2024, 501, 157762 CrossRef CAS.
- J. Xing, X. Ma, Y. Yu, Y. Xiao, L. Chen, W. Yuan, Y. Wang, K. Liu, Z. Guo, H. Tang, K. Fan and W. Jiang, Adv. Sci., 2024, 2024, 2405597 Search PubMed.
- M. Arnold, E. Morgan, H. Rumgay, A. Mafra, D. Singh, M. Laversanne, J. Vignat, J. R. Gralow, F. Cardoso, S. Siesling and I. Soerjomataram, Breast, 2022, 66, 15–23 CrossRef.
- M. Akram, M. Iqbal, M. Daniyal and A. U. Khan, Biol. Res., 2017, 50, 33 CrossRef PubMed.
- R. Fan, X. Tao, X. Zhai, Y. Zhu, Y. Li, Y. Chen, D. Dong, S. Yang and L. Lv, Biomed. Pharmacother., 2023, 161, 114444 CrossRef CAS.
- A. A. Ashkarran, Z. Lin, J. Rana, H. Bumpers, L. Sempere and M. Mahmoudi, Small, 2023, 20, 2301385 CrossRef.
- H. Rumgay, M. Arnold, J. Ferlay, O. Lesi, C. J. Cabasag, J. Vignat, M. Laversanne, K. A. McGlynn and I. Soerjomataram, J. Hepatol., 2022, 77, 1598–1606 CrossRef.
- M. Arnold, D. Singh, M. Laversanne, J. Vignat, S. Vaccarella, F. Meheus, A. E. Cust, E. de Vries, D. C. Whiteman and F. Bray, JAMA Dermatol., 2022, 158, 495–503 CrossRef.
- X. Xu, Y. Zhang, C. Meng, W. Zheng, L. Wang, C. Zhao and F. Luo, J. Mater. Chem. B, 2024, 12, 9111–9143 RSC.
- Q. Zhang, L. Song and K. Zhang, Mater. Chem. Front., 2023, 7, 44–64 RSC.
- N. Tagaras, H. Song, S. Sahar, W. Tong, Z. Mao and T. Buerki-Thurnherr, Adv. Sci., 2024, 11, 2407816 CrossRef CAS PubMed.
- X. Li, J. Hu, Q. Zhao, W. Yao, Z. Jing and Z. Jin, J. Transl. Med., 2024, 22, 1033 CrossRef PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |