Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cycloaddition reaction of NaN3 with nitriles toward the synthesis of tetrazoles catalyzed by a copper complex on boehmite nanoparticles†
Arida
Jabbari
 *a,
Bahman
Tahmasbi
*a,
Bahman
Tahmasbi
 b,
Elham
Mohseni
b and
Mitra
Darabi
b
b,
Elham
Mohseni
b and
Mitra
Darabi
b
aDepartment of Chemistry, Qe.C., Islamic Azad University, Qeshm, Iran. E-mail: aridajabbari@iau.ir
bDepartment of Chemistry, Faculty of Science, Ilam University, P. O. Box 69315516, Ilam, Iran. E-mail: b.tahmasbi@ilam.ac.ir
First published on 16th April 2025
Abstract
In the present study, the synthesis of boehmite nanoparticles was done using a hydrothermal method using an aluminum source in water solvent. The synthesized boehmite support was modified using (3-iodopropyl)trimethoxysilane (3-IPTMS), and then the modified boehmite was functionalized using a Schiff-base ligand. Finally, copper ions were immobilized on the functionalized boehmite denoted as a boehmite@Schiff-base-Cu nanocatalyst. The synthesized catalyst was identified and confirmed using SEM, FT-IR, TGA, EDXS, WDX, XRD, and BET techniques. The activity of boehmite@Schiff-base-Cu was investigated in preparing 5-substituted tetrazoles using nitrile derivatives and sodium azide, in which short reaction times and high yields were observed in described reactions. Also, the many advantages of the boehmite@Schiff-base-Cu nanocatalyst are ease of operation, compatibility with the environment, its easy separation from the reaction medium, and the ability to reuse it several times without significantly reducing its catalytic activity.
1. Introduction
An ideal catalytic system should have good selectivity and activity like homogeneous catalysts. It should also provide ease of recovery and reuse like heterogeneous catalysts. In this regard, in heterogeneous nanocatalysts, as the particle size decreases to the nanoscale, the surface area increases, and a high surface area will be available. Hence, nanocatalysts act as a bridge to the gap between conventional catalysts (homogeneous and heterogeneous). In other words, nanocatalysts provide the advantages of conventional catalysts (homogeneous and heterogeneous) simultaneously.1,2 In recent years, the immobilization of homogeneous catalysts on insoluble solid surfaces to recover catalysts from the reaction medium has attracted the attention of researchers in chemistry.3,4 The behavior of catalysts immobilized on the support is strongly influenced by the properties of the support. To solve this issue, various nanoheterogeneous supports such as boehmite nanoparticles, mesoporous silica, magnetic nanoparticles, carbon nanotubes, graphene oxide, metal–organic frameworks, zeolites, and ionic liquids have been used to heterogenize homogeneous catalysts.5–12 Among the supports mentioned, boehmite nanoparticles have outstanding physical and chemical properties such as high concentrations of surface hydroxyl groups, very high internal surface area, non-toxicity, being inexpensive, and high thermal and chemical stability. For this reason, these nanoparticles are considered one of the most attractive candidates for solid supports.13–16 Aluminum oxide has various phases which can be gibbsite [γ-Al(OH)3], bayrite [α-Al(OH)3], nordstrandite[β-Al(OH)3], diaspore[α-AlO(OH)], boehmite[γ-AlOOH] and alumina [Al2O3]. The most stable of them is α-Al2O3, and all these phases are intermediate and unstable phases, which after heating finally form an α-Al2O3 phase.17–19 Boehmite is actually one of the phases of aluminum oxide called aluminum oxy-hydroxide AlOOH, which has many applications in the fields of ceramics, petroleum, petrochemicals, and medicine. In addition, boehmite has been used as a catalyst, coating, membrane, optical material, water sweetener, abrasive, absorbent, and vaccine supplement.20–23 After alumina phase Al2O3, boehmite is the most stable aluminum oxide phase. This material starts to convert into an alumina phase in the temperature range of 250–450 °C, during which complete phase change occurs at a temperature of about 450 °C. Therefore, one of the most important applications of boehmite is as a precursor in the preparation of alumina.24 Today, there are many methods such as the electrochemical method, hydrothermal method, sol–gel method, and thermal decomposition method to prepare boehmite in nanodimensions. In the meantime, the hydrothermal method is controllable, has a high crystallization ability, and has been used more than other methods.25–28 According to the reported studies, boehmite is in the form of cubic structural units (orthorhombic) and the surface of these units contains many hydroxyl groups.29,30Tetrazoles are cyclic materials with a five-membered ring containing 4 nitrogen atoms and 1 carbon atom.31,32 Heterocyclic tetrazole derivatives have various applications in the synthesis of other organic compounds and pharmaceutical and biological industries. Because of having low sensitivity to impact and friction, high potential energy of heterocyclic tetrazole derivatives, and high explosion heat, they are good candidates for use in gas and explosives producers. Also, because of having a high percentage of nitrogen, they release a large amount of nitrogen gas after combustion, and for this reason, they have little pollution for the environment and are considered green explosives. In addition, tetrazole compounds play a significant role as ligands in coordination chemistry, so it is important to provide catalytic methods for the synthesis of this group of compounds.33–35
We have introduced a new protocol for the catalytic synthesis of tetrazoles using boehmite@Schiff-base@Cu as a heterogeneous nanocatalyst.
2. Experimental
2.1. Boehmite synthesis
First, 49.6 g of NaOH was dissolved in 50 ml of distilled water and poured into a burette. Then, in a 250 ml beaker, 20 g of Al(NO3)3·9H2O as the aluminum source was dissolved in 30 ml of water and stirred using a stirrer. The sodium hydroxide solution was added drop by drop to the aluminum solution under a mechanical stirrer. Sedimentation was allowed to proceed for twenty minutes with stirring. After sedimentation, the obtained sediment was dispersed using an ultrasonic bath (for 3 h). Then the sediment of the obtained gels was poured into a porcelain crucible and heated for 4 h at 220 °C. The obtained white solid powder is the boehmite crystal. To remove the nitrate impurity, the obtained boehmite was washed with distilled water and dried at 70 °C.362.2. Synthesis of 3-iodopropyltrimethoxysilane
For the synthesis of 3-IPTMS, potassium iodide (0.246 mmol) was first added to dry acetone in a 50 ml flask. Then, the same proportion of 3-chloropropyltrimethoxysilane (0.246 mmol) was refluxed dropwise under a N2 atmosphere at 50 °C overnight. After the completion of the reaction, the precipitate of potassium chloride (KI) was filtered, and the 3-iodopropyltrimethoxysilane product, which was in the form of a yellow liquid, was isolated (Scheme 1).372.3. Synthesis of the ligand
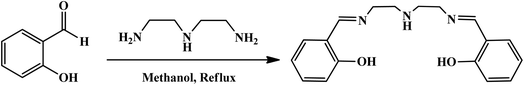
For the synthesis of the Schiff-base ligand, salicylaldehyde and di(ethylenetriamine) were used in a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in methanol solvent. For this purpose, salicylaldehyde (4 mmol) was dissolved in MeOH (methanol) solvent, and then diethylenetriamine (2 mmol) was added dropwise to the mixture. The mixture was stirred under reflux conditions for 4 h. After evaporation of the solvent, the obtained product was dried (Scheme 2).38 The final product was purified by recrystallization and characterized using FT-IR spectroscopy.
1 in methanol solvent. For this purpose, salicylaldehyde (4 mmol) was dissolved in MeOH (methanol) solvent, and then diethylenetriamine (2 mmol) was added dropwise to the mixture. The mixture was stirred under reflux conditions for 4 h. After evaporation of the solvent, the obtained product was dried (Scheme 2).38 The final product was purified by recrystallization and characterized using FT-IR spectroscopy.
2.4. Synthesis of (PMeOSi)DETA
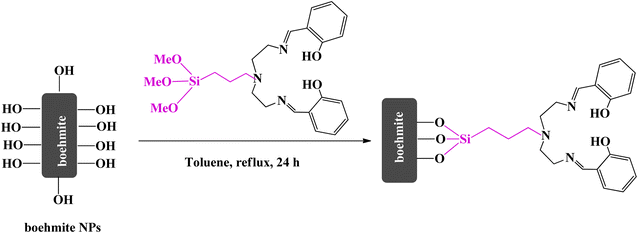
In a 100 ml flask, a mixture of 3-IPTMS (0.310 g) and the Schiff-base ligand (0.330 g) was prepared in THF solvent. Then K2CO3 (3 mmol) was added to the mixture and refluxed for 21 h at 65 °C. The obtained precipitate was filtered, washed several times with toluene, and dried at 70 °C for 4 h (Scheme 3).382.5. Functionalization of boehmite with (PMeOSi)DETA
In a 100 ml flask, 1 g of (MeO3Si)DETA was dissolved in toluene solvent and then 1.5 g of boehmite support was added to it. The mixture was refluxed for 24 h at 85 °C. After that, the obtained product (boehmite@Schiff-base) was isolated from the mixture using filter paper, washed several times with ethanol, and finally dried at 50 °C (Scheme 4).2.6. Preparation of copper catalysts (boehmite@Schiff-base-Cu)
In the final stage of nanocatalyst synthesis, 1 g of boehmite@Schiff-base and 3 mmol of Cu(NO3)2·9H2O were dissolved in ethanol solvent. The reaction mixture was refluxed for 24 h. After the reaction finished, the synthesized nanocatalyst (boehmite@Schiff-base-Cu) was filtered and washed with distilled water and ethanol. Finally, it was dried at 60 °C (Scheme 5).2.7. Synthesis of tetrazoles
In a 25 ml round bottom flask, a suspension was formed with a mixture of 35 mg of boehmite@Schiff-base-Cu nanocatalyst, 1 mmol of nitrile, 1.2 mmol of NaN3 (sodium azide), and a sufficient amount of PEG (2 ml) at a temperature of 120 °C under a magnetic stirrer. The progress of the reaction was checked by TLC in a mixture solvent of n-hexane and acetone at a ratio of (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). After the end of the reaction, the nanocatalyst was separated using filter paper. Then, 10 ml of 4N HCl and 7 ml of ethyl acetate were added to the mixture, and the organic phase extracted using a decanter was dried at ambient temperature (Scheme 6).
1). After the end of the reaction, the nanocatalyst was separated using filter paper. Then, 10 ml of 4N HCl and 7 ml of ethyl acetate were added to the mixture, and the organic phase extracted using a decanter was dried at ambient temperature (Scheme 6).
2.8. Spectral data
3. Results and discussion
As indicated in Scheme 1, we synthesized the boehmite@Schiff-base-Cu nanocatalyst for the first time and we investigated its catalytic performance in the synthesis of tetrazoles from various nitrile derivatives. The prepared nanocatalyst was identified by some techniques such as BET (Brunauer–Emmett–Teller), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDXS), thermogravimetric analysis (TGA), wavelength-dispersive X-ray spectroscopy (WDX), X-ray diffraction (XRD) and Fourier transform-infrared spectroscopy (FT-IR).3.1. N2 adsorption–desorption isotherm studies
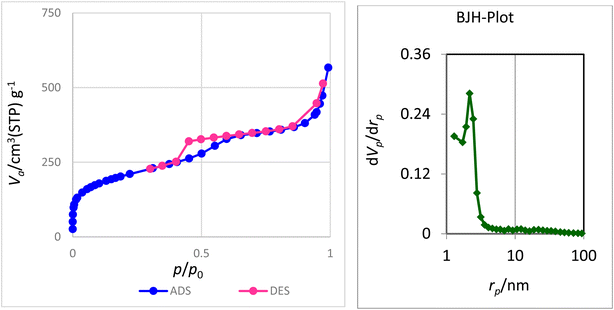
The nitrogen adsorption–desorption technique was used to determine the structural characteristics and examine the surface of nanocatalyst boehmite@Schiff-base-Cu. Fig. 1 shows the nitrogen adsorption–desorption analysis at 120 °C and the pore size distribution plot corresponding to the N2 adsorption–desorption for boehmite@Schiff-base-Cu. As it is clear in this figure, this diagram is a type IV isotherm (definition by IUPAC) in the region of relative pressure (P/P0) between 0.4 and 0.8, which is characteristic of mesoporous compounds.39,40 The structural parameters of nanocatalyst boehmite@Schiff-base-Cu, such as the average pore diameter, surface area, and total pore volume, are listed in Table 1. The average pore diameter, total pore volume, and specific surface area of the boehmite@Schiff-base-Cu nanocatalyst are 4.6495 nm, 0.8601 cm3, and 739.96 m2 g−1, respectively. Also, there is only one sharp peak at 2.3 nm as observed from the pore size distribution plot derived from this isotherm.| Sample | S BET (m2 g−1) | Pore diameter (nm) | Proe volume (cm3) |
|---|---|---|---|
| boehmite@Schiff-base-Cu | 739.96 | 4.6495 | 0.8601 |
3.2. Thermogravimetric analysis studies
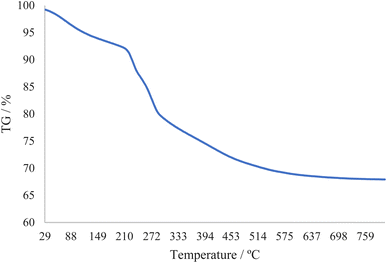
The thermal stability of catalyst boehmite@Schiff-base-Cu was investigated through thermogravimetric analysis. This TGA analysis was done in the heat range of 29 to 800 °C. The TGA diagram of boehmite@Schiff-base-Cu is shown in Fig. 2. The first weight loss (about 8%) at low temperatures is related to the evaporation of solvents. As indicated, except for the evaporation of the solvent, no weight loss occurred up to 210 °C, meaning that the boehmite@Schiff-base-Cu catalyst is stable up to 210 °C. The curve shows a weight loss of about 22% from the temperature range of 210–450 °C, which indicates the well stabilization of the copper complex on the boehmite nanoparticles.3.3. SEM photographs
The particle size and morphology of the boehmite@Schiff-base-Cu nanocatalyst were investigated by SEM. Fig. 3 shows the SEM images of the nanocatalyst. As shown in the images, this catalyst has been synthesized as cubic structural units (orthorhombic) with a uniform size between 10 and 25 nm.3.4. Energy dispersive X-ray analysis and elemental mapping
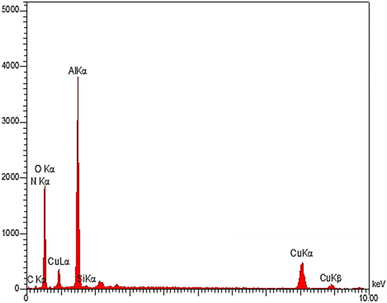
The EDS analysis is a method to determine the elemental composition of a sample. The EDS technique was used to determine the quality of the elements in the structure of boehmite@Schiff-base-Cu. The diagram obtained from this analysis is shown in Fig. 4. The obtained results showed that O, Al, C, Si, N, and Cu elements are present in the prepared nanocatalyst structure. This evidence confirms the successful synthesis of this nanocatalyst. Fig. 5 shows the distribution of the elements in boehmite@Schiff-base-Cu. In these images, the presence of aluminum, silicon, oxygen, carbon, nitrogen, and copper elements is visible. Also, the distribution of copper on the support surface was confirmed by this elemental analysis.3.5. FT-IR spectra
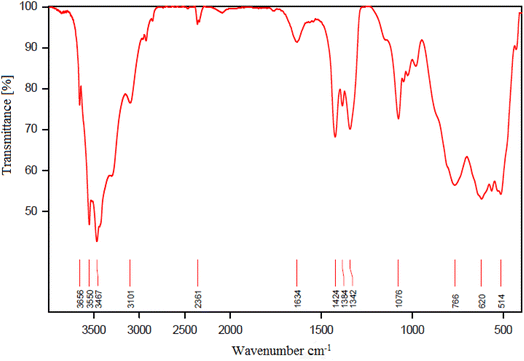
FTIR spectroscopy makes it possible to verify the functional group in the structure of the synthesized catalyst. Fig. 6 shows the FT-IR spectra of (a) boehmite support, (b) boehmite@Schiff-base, and (c) boehmite@Schiff-base-Cu. In spectrum a, the peaks at 3467 cm−1 (symmetric) and 3552 cm−1 (asymmetric) displayed in the spectrum of boehmite nanoparticles are related to the vibrations of surface O–H bonds that are attached to the surface of boehmite nanoparticles.41 The peaks appearing in all IR-FT spectra in the regions of 618 cm−1 and 764 cm−1 are attributed to the Al–O bond vibrations in the boehmite core.42 Also, the peak shown at 1638 cm−1 is related to the bending vibration of hydrogen bonds of surface hydroxyl groups.43 In the IR spectra of boehmite with a Schiff-base functionalized ligand (Fig. 6b), stretching vibrational bands C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and O–H were observed at 1660, 1394, and >3000 cm−1 respectively and the peak shown at 1080 cm−1 is attributed to the vibration of Si–O. The FTIR spectrum of the catalyst (Fig. 6c) shows the C
N, and O–H were observed at 1660, 1394, and >3000 cm−1 respectively and the peak shown at 1080 cm−1 is attributed to the vibration of Si–O. The FTIR spectrum of the catalyst (Fig. 6c) shows the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibrational band at a lower wave number (1637 cm−1). Based on these data, copper metal ions of C
N stretching vibrational band at a lower wave number (1637 cm−1). Based on these data, copper metal ions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups were coordinated.38
N groups were coordinated.38
 | ||
| Fig. 6 FT-IR spectra of (a) boehmite, (b) boehmite@Schiff-base and (c) boehmite@Schiff-base-Cu nanocatalyst. | ||
3.6. X-ray diffraction
The normal XRD patterns of boehmite and the boehmite@Schiff-base-Cu nanocatalyst are shown in Fig. 7. The XRD diffraction of boehmite (Fig. 7a) exhibits a series of peaks at different 2θ positions at 14.32° (0 2 0), 28.47° (1 2 0), 38.52° (0 3 1), 45.72° (1 3 1), 49.47° (0 5 1), 51.87° (2 0 0), 55.72° (1 5 1), 60.70° (0 8 0), 64.42° (2 3 1), 65.27° (0 0 2), 67.97° (1 7 1), and 72.52° (2 5 1), which are related to the standard pattern of boehmite nanoparticles in the orthorhombic unit cell (JCPDS-no. 00-049-0133 and JCPDS-no. 01-074-1895).44–49 All these peaks are also clearly observed in the XRD patterns of the boehmite@Schiff-base-Cu nanocatalyst, indicating that the crystal structure of boehmite nanoparticles remained stable after functionalization and stabilization of the copper complex in orthorhombic cells (Fig. 7b).Moreover, a broad peak of the 2θ value at the 18–26° position is related to the coated silica on boehmite nanoparticles,3,50 which is not observed in the XRD pattern of unmodified boehmite nanoparticles. This peak indicates that boehmite nanoparticles were successfully modified with (3-iodopropyl)trimethoxysilane.
3.7. Catalytic studies
Various experiments were conducted to investigate the boehmite@Schiff-base-Cu nanocatalyst and to optimize the conditions in the reaction related to 4-nitrobenzonitrile, including the type of solvent, the amount of the catalyst, and the temperature. To obtain optimal conditions for the synthesis of tetrazoles, the reaction of 4-nitrobenzonitrile (1 mmol) with sodium azide (1.2 mmol) in the vicinity of the boehmite@Schiff-base-Cu catalyst was selected as a sample reaction. The results related to the effect of different parameters on this reaction were investigated, which are summarized in Table 2. At first, the reaction was investigated in the presence of different amounts of the boehmite@Schiff-base-Cu catalyst; according to the results in the table, the shortest time and the highest yield were obtained in the presence of 35 mg of boehmite@Schiff-base-Cu nanocatalyst. Then the effects of different solvents such as DMSO, PEG-400, 1,4-dioxane, toluene, ethanol, and H2O were compared, and it was found that PEG-400 solvent provides the best results. Finally, the effect of temperature on the reaction of the model was investigated. The best results with excellent yield and low reaction time were obtained in PEG-400 solvent (as green solvent), for an amount of 35 mg of boehmite@Schiff-base-Cu nanocatalyst, and at a temperature of 120 °C (Table 2).| Entry | Solvent | Temp. (°C) | Catalyst (mg) | Time (min) | Yield (%) |
|---|---|---|---|---|---|
| 1 | PEG | 120 | 40 | 60 | 95 |
| 2 | PEG | 120 | 35 | 85 | 95 |
| 3 | PEG | 120 | 30 | 90 | 70 |
| 4 | PEG | 100 | 35 | 170 | Trace |
| 5 | PEG | 120 | — | 120 | N.R |
| 6 | H2O | 100 | 35 | 120 | Trace |
| 7 | EtOH | 77 | 35 | 120 | Trace |
After obtaining the conditions, the [3 + 2] cycloaddition reaction of nitrile derivatives and NaN3 was examined for the preparation of several types of tetrazoles (Table 3). All nitriles, including electron- (donating or accepting) functional groups, became related tetrazoles in the presence of boehmite@Schiff-base-Cu. Significantly, boehmite@Schiff-base-Cu exhibits a good homoselectivity in the tetrazole synthesis, when two similar types of cyano-substituted groups are present in two quite same positions of the benzonitrile ring, e.g. phthalonitrile and terephthalonitrile, that only mono-cycloaddition was observed (Table 3, entries 2 and 4). The homoselectivity of the boehmite@Schiff-base-Cu catalyst in the synthesis of tetrazoles was confirmed with 1H NMR spectroscopy, as 1H NMR (250 MHz, DMSO-d6): δH = 8.21–8.18 (d, J = 7.5 Hz, 2H), 8.07–8.04 (d, J = 7.5 Hz, 2H) ppm.
A reaction mechanism for the formation of tetrazoles catalyzed by boehmite@Schiff-base-Cu is shown in Scheme 7.51–53 In this suggested mechanism, at first, the nitrile group becomes susceptible to nucleophilic attack with the interaction of the cyano-functional group with the boehmite@Schiff-base-Cu catalyst. At this stage, intermediate I is formed. Then, intermediate II is formed through the [3 + 2] cycloaddition reaction with NaN3 and intermediate I as a sodium salt form. In the final stage, the salt form of the intermediate II is converted to a target molecule tetrazole compound through HCl addition in the work-up step.
3.8. Reusability of the catalyst
To investigate the recovery and reusability of the boehmite@Schiff-base-Cu nanocatalyst in the synthesis of tetrazoles under optimal conditions, the synthesis of 5-phenyl-1H-tetrazole was selected as a model reaction. After the end of the reaction in each cycle, the catalyst was separated using centrifugation and washed several times with HCl 4N and hot ethyl acetate and then it was reused in the next cycle after drying. The catalyst was recycled in 4 periods without a significant decrease in its activity. In Fig. 8, the activity results of the recycled boehmite@Schiff-base-Cu nanocatalyst are shown in the form of a diagram.To investigate the heterogeneous nature of the boehmite@Schiff-base-Cu nanocatalyst, a hot filtration test was carried out based on a published article.28 In this regard, in the synthesis of 5-phenyl-1H-tetrazole, the catalyst was removed after finishing the reaction and then the exact amount of probabilistic leached copper in the filtered reaction media was calculated by AAS analysis. In this analysis, a notable amount of leached copper was not detected. These results indicate that copper is not leached from the boehmite@Schiff-base-Cu catalyst and that this catalyst has a heterogeneous nature.
The structure of the recovered boehmite@Schiff-base-Cu nanocatalyst was identified using FT-IR analysis (Fig. 9). As it is clear from the FT-IR spectrum, there is no significant change in the recycled nanocatalyst compared to the original catalyst. These results indicate that the boehmite@Schiff-base-Cu nanocatalyst is stable under the reaction conditions for the formation of tetrazoles.
3.9. Comparison of the catalyst
The activity of the boehmite@Schiff-base-Cu catalyst was evaluated in comparison with other previously reported catalysts in the literature (Table 4). According to the results given in Table 4, the synthesis reaction of 4-(1H-tetrazol-5-yl)benzonitrile has been carried out in PEG-400 as a green solvent with low reaction time and high yield of the product. The results in Table 4 prove the superiority of the boehmite@Schiff-base-Cu catalyst in terms of efficiency or reaction time compared to other catalysts in the literature.| Entry | Catalyst | Solvent | Time (min) | Yield (%) |
|---|---|---|---|---|
| 1 | Boehmite@Schiff-base-Cu | PEG | 70 | 95 [This work] |
| 2 | Fe3O4@SBTU@Ni(II) | PEG | 7 h | 94 ref. (54) |
| 3 | Cu(II) immobilized on Fe3O4@SiO2@L-histidine | PEG | 90 | 95 ref. (55) |
| 4 | FeCl3–SiO2 | PEG | 20 h | 80 ref. (56) |
| 5 | Fe3O4-adenine-Zn | PEG | 140 | 91 ref. (57) |
| 6 | BNPs@Cur-Ni | PEG | 120 | 88 ref. (58) |
| 7 | Boehmite@SiO2@Tris-Cu(I) NPs | PEG | 110 | 89 ref. (59) |
| 8 | CdCl2 | PEG | 9 h | 79 ref. (60) |
| 9 | Fe3O4@boehmite | PEG | 24 h | 92 ref. (61) |
4. Conclusion
In this research, a new and green nanocatalyst (boehmite@Schiff-base-Cu) was prepared, whose catalytic activity was investigated in the important synthesis of tetrazoles using nitrile derivatives and NaN3, in PEG-400 solvent at 120 °C. This nanocatalyst was identified by SEM, FT-IR, TGA, EDXS, WDX, XRD, and BET. The advantages of tetrazole synthesis in the presence of boehmite@Schiff-base-Cu include short reaction time, high yield of products, stability of the catalyst, and inexpensive and availability of reagents. Also, the use of this nanocatalyst in this work compared to other catalysts has advantages such as low toxicity, transportation, easy storage, weighing and utilization, simple preparation of the catalyst from cheap and available raw materials, high catalytic activity due to the increased surface area, stability, and recyclability and reusability of the catalyst.Data availability
The data that support the findings of this study are available in the article file and ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the research facilities of Islamic Azad University Qeshm Branch and Ilam University for financial support for this research project.References
- V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J. M. Basset, Magnetically recoverable nanocatalysts, Chem. Rev., 2011, 111, 3036–3075 CrossRef CAS PubMed.
- W. Guo, G. Wang, Q. Wang, W. Dong, M. Yang, X. Huang, J. Yu and Z. Shi, A hierarchical Fe3O4@ P4VP@ MoO2 (acac) 2 nanocomposite: Controlled synthesis and green catalytic application, J. Mol. Catal. A:Chem., 2013, 378, 344–349 CrossRef CAS.
- A. Ghorbani-Choghamarani, M. Hajjami, B. Tahmasbi and N. Noori, Boehmite silica sulfuric acid: as a new acidic material and reusable heterogeneous nanocatalyst for the various organic oxidation reactions, J. Iran. Chem. Soc., 2016, 13, 2193–2202 CrossRef CAS.
- S. Molaei, T. Tamoradi, M. Ghadermazi and A. Ghorbani-Choghamarani, Synthesis and characterization of MCM-41@ AMPD@ Zn as a novel and recoverable mesostructured catalyst for oxidation of sulfur containing compounds and synthesis of 5-substituted tetrazoles, Microporous Mesoporous Mater., 2018, 272, 241–250 CrossRef CAS.
- Z. Yan, Z. Yang, Z. Xu, L. An, F. Xie and J. Liu, Enhanced room-temperature catalytic decomposition of formaldehyde on magnesium-aluminum hydrotalcite/boehmite supported platinum nanoparticles catalyst, J. Colloid Interface Sci., 2018, 524, 306–312 CrossRef CAS PubMed.
- F. Habeche, M. Hachemaoui, A. Mokhtar, K. Chikh, F. Benali, A. Mekki, F. Zaoui, Z. Cherifi and B. Boukoussa, Recent advances on the preparation and catalytic applications of metal complexes supported-mesoporous silica MCM-41, J. Inorg. Organomet. Polym. Mater., 2020, 30(11), 4245–4268 CrossRef CAS.
- A. Maleki, R. Taheri-Ledari, R. Ghalavand and R. Firouzi-Haji, Palladium-decorated o-phenylenediamine-functionalized Fe3O4/SiO2 magnetic nanoparticles: A promising solid-state catalytic system used for Suzuki–Miyaura coupling reactions, J. Phys. Chem. Solids, 2020, 136, 109200 CrossRef CAS.
- L. Zhang, J. Liang, L. Yue, Z. Xu, K. Dong, Q. Liu, Y. Luo, T. Li, X. Cheng, G. Cui, B. Tang, A. B. Ali, A. K. Ahmed, X. Guo and X. Sun, N-doped carbon nanotubes supported CoSe2 nanoparticles: A highly efficient and stable catalyst for H2O2 electrosynthesis in acidic media, Nano Res., 2022, 15(1), 304–309 CrossRef CAS PubMed.
- B. Tahmasbi, M. Darabi, P. Moradi, Y. A. Tyula and M. Nikoorazm, Gadolinium Schiff-base complex on nanocomposite of graphene oxide magnetic nanoparticles as a robust, reusable and chemoselective nanocatalyst in the C–C coupling reactions, Polyhedron, 2024, 258, 117038 CrossRef CAS.
- M. Koolivand, M. Nikoorazm, A. Ghorbani-Choghamarani and M. Mohammadi, A novel cubic Zn-citric acid-based MOF as a highly efficient and reusable catalyst for the synthesis of pyranopyrazoles and 5-substituted 1H-tetrazoles, Appl. Organomet. Chem., 2022, 36(6), e6656 CrossRef CAS.
- A. Velty and A. Corma, Advanced zeolite and ordered mesoporous silica-based catalysts for the conversion of CO 2 to chemicals and fuels, Chem. Soc. Rev., 2023, 52(5), 1773–1946 RSC.
- O. Bartlewicz, I. Dąbek, A. Szymańska and H. Maciejewski, Heterogeneous catalysis with the participation of ionic liquids, Catalysts, 2020, 10(11), 1227 CrossRef CAS.
- K. Bahrami, M. M. Khodaei and M. Roostaei, The preparation and characterization of boehmite nanoparticles-TAPC: a tailored and reusable nanocatalyst for the synthesis of 12-aryl-8, 9, 10, 12-tetrahydrobenzo [a] xanthen-11-ones, New J. Chem., 2014, 38(11), 5515–5520 RSC.
- E. Zarenezhad, R. Taghavi, P. Kamrani, M. Farjam and S. Rostamnia, Gold nanoparticle decorated dithiocarbamate modified natural boehmite as a catalyst for the synthesis of biologically essential propargylamines, RSC Adv., 2022, 12(49), 31680–31687 RSC.
- W. Lueangchaichaweng, B. Singh, D. Mandelli, W. A. Carvalho, S. Fiorilli and P. P. Pescarmona, High surface area, nanostructured boehmite and alumina catalysts: Synthesis and application in the sustainable epoxidation of alkenes, Appl. Catal., A, 2019, 571, 180–187 CrossRef CAS.
- A. Ghorbani-Choghamarani, Z. Seydyosefi and B. Tahmasbi, Tribromide ion supported on boehmite nanoparticles as a reusable catalyst for organic reactions, C. R. Chim., 2018, 21(11), 1011–1022 CrossRef CAS.
- A. A. Derakhshan and L. Rajabi, Review on applications of carboxylate–alumoxane nanostructures, Powder Technol., 2012, 226, 117–129 CrossRef CAS.
- L. Rajabi and A. Derakhshan, Room temperature synthesis of boehmite and crystallization of nanoparticles: effect of concentration and ultrasound, Sci. Adv. Mater., 2010, 2(2), 163–172 CrossRef CAS.
- E. Kumar, A. Bhatnagar, W. Hogland, M. Marques and M. Sillanpää, Interaction of anionic pollutants with Al-based adsorbents in aqueous media–A review, Chem. Eng. J., 2014, 241, 443–456 CrossRef CAS.
- V. K. Yadav and K. Ganesh Babu, Reactions on a solid surface. A simple, economical, and efficient acylation of alcohols and amines over Al2O3, J. Org. Chem., 2004, 69(2), 577–580 CrossRef CAS PubMed.
- D. Mishra, S. Anand, R. Panda and R. Das, Effect of anions during hydrothermal preparation of boehmites, Mater. Lett., 2002, 53(3), 133–137 CrossRef CAS.
- I. Vlassiouk, A. Krasnoslobodtsev, S. Smirnov and M. Germann, “Direct” detection and separation of DNA using nanoporous alumina filters, Langmuir, 2004, 20(23), 9913–9915 CrossRef CAS PubMed.
- Y.-w Jun, J.-H. Lee, J.-s Choi, and J. Cheon, Symmetry-controlled Colloidal Nanocrystals: Nonhydrolytic Chemical Synthesis and Shape Determining Parameters, ACS Publications, 2005, pp. 14795–14806 Search PubMed.
- M. Zhang, R. Zhang, G. Xi, Y. Liu and Y. Qian, From Sheets to Fibers: A Novel Approach to ˙-AlOOH and ˙-Al2O3 1D Nanostructures, J. Nanosci. Nanotechnol., 2006, 6(5), 1437–1440 CrossRef CAS PubMed.
- L. Zhang, B. Cheng, W. Shi and E. T. Samulski, In situ electrochemical synthesis of 1-dimensional alumina nanostructures, J. Mater. Chem., 2005, 15(46), 4889–4893 RSC.
- L. Qu, C. He, Y. Yang, Y. He and Z. Liu, Hydrothermal synthesis of alumina nanotubes templated by anionic surfactant, Mater. Lett., 2005, 59(29–30), 4034–4037 CrossRef CAS.
- Z. Q. Yu, C. X. Wang, X. T. Gu and C. Li, Photoluminescent properties of boehmite whisker prepared by sol-gel process, J. Lumin., 2004, 106(2), 153–157 CrossRef CAS.
- B. Tahmasbi, M. Darabi and M. Nikoorazm, A new Schiff-base complex of palladium nanoparticles on modified boehmite with di (pyridin-2-yl) methanone as a robust, reusable, and selective nanocatalyst in the C-C coupling reaction, Appl. Organomet. Chem., 2024, 38(3), e7348 CrossRef CAS.
- R. Kumar, M. Ehsan and M. Barakat, Synthesis and characterization of carbon/AlOOH composite for adsorption of chromium (VI) from synthetic wastewater, J. Ind. Eng. Chem., 2014, 20(6), 4202–4206 CrossRef CAS.
- E. Carbonell, E. Delgado-Pinar, J. Pitarch-Jarque, J. Alarcón and E. Garcia-Espana, Boehmite supported pyrene polyamine systems as probes for iodide recognition, J. Phys. Chem. C, 2013, 117(27), 14325–14331 CrossRef CAS.
- M. Nikoorazm, A. Ghorbani-Choghamarani and M. Khanmoradi, Application of Pd-2A3HP-MCM-41 to the Suzuki, Heck and Stille coupling reactions and synthesis of 5-substituted 1H-tetrazoles, Appl. Organomet. Chem., 2016, 30(8), 705–712 CrossRef CAS.
- D. R. Patil, M. B. Deshmukh and D. S. Dalal, Ammonium acetate mediated synthesis of 5-substituted 1 H-tetrazoles, J. Iran. Chem. Soc., 2012, 9, 799–803 CrossRef CAS.
- M.-S. Mashhoori and R. Sandaroos, New ecofriendly heterogeneous nano-catalyst for the synthesis of 1-substituted and 5-substituted 1 H-tetrazole derivatives, Sci. Rep., 2022, 12(1), 15364 CrossRef CAS PubMed.
- R. Uppadhayay, A. Kumar, J. Teotia and A. Singh, Multifaceted chemistry of tetrazole. Synthesis, uses, and pharmaceutical applications, Russ. J. Org. Chem., 2022, 58(12), 1801–1811 CrossRef CAS.
- Z. Du, C. Si, Y. Li, Y. Wang and J. Lu, Improved synthesis of 5-substituted 1 H-tetrazoles via the [3+ 2] cycloaddition of nitriles and sodium azide catalyzed by silica sulfuric acid, Int. J. Mol. Sci., 2012, 13(4), 4696–4703 CrossRef CAS PubMed.
- M. Mohammadi, M. Khodamorady, B. Tahmasbi, K. Bahrami and A. Ghorbani-Choghamarani, Boehmite nanoparticles as versatile support for organic–inorganic hybrid materials: Synthesis, functionalization, and applications in eco-friendly catalysis, J. Ind. Eng. Chem., 2021, 97, 1–78 CrossRef CAS.
- M. Nikoorazm, B. Tahmasbi, M. Darabi, Y. A. Tyula, S. Gholami, M. Khanmoradi and M. Koolivand, Synthesis of a new complex of lanthanum on MCM-41 as an efficient and reusable heterogeneous catalyst for the chemoselective synthesis of sulfoxides and tetrahydrobenzo [b] pyrans, J. Porous Mater., 2023, 1–16 Search PubMed.
- M. Akbari, M. Nikoorazm, B. Tahmasbi and A. Ghorbani-Choghamarani, The new Schiff-base complex of copper (II) grafted on mesoporous KIT-6 as an effective nanostructure catalyst for the homoselective synthesis of various tetrazoles, Appl. Organomet. Chem., 2023, 37, e7317 Search PubMed.
- D. Chandra, S. K. Das and A. Bhaumik, A fluorophore grafted 2D-hexagonal mesoporous organosilica: Excellent ion-exchanger for the removal of heavy metal ions from wastewater, Microporous Mesoporous Mater., 2010, 128(1–3), 34–40 CrossRef CAS.
- A. Dutta, A. K. Patra, S. Dutta, B. Saha and A. Bhaumik, Hierarchically porous titanium phosphate nanoparticles: an efficient solid acid catalyst for microwave assisted conversion of biomass and carbohydrates into 5-hydroxymethylfurfural, J. Mater. Chem., 2012, 22(28), 14094–14100 RSC.
- A. Ghorbani-Choghamarani, H. Rabiei, B. Tahmasbi, B. Ghasemi and F. Mardi, Preparation of DSA@ MNPs and application as heterogeneous and recyclable nanocatalyst for oxidation of sulfides and oxidative coupling of thiols, Res. Chem. Intermed., 2016, 42, 5723–5737 CrossRef CAS.
- A. Ghorbani-Choghamarani, B. Tahmasbi and P. Moradi, Synthesis of a new Pd (0)-complex supported on boehmite nanoparticles and study of its catalytic activity for Suzuki and Heck reactions in H 2 O or PEG, RSC Adv., 2016, 6(49), 43205–43216 RSC.
- A. Ghorbani-Choghamarani and B. Tahmasbi, The first report on the preparation of boehmite silica sulfuric acid and its applications in some multicomponent organic reactions, New J. Chem., 2016, 40(2), 1205–1212 RSC.
- A. Jabbari, P. Moradi, M. Hajjami and B. Tahmasbi, Tetradentate copper complex supported on boehmite nanoparticles as an efficient and heterogeneous reusable nanocatalyst for the synthesis of diaryl ethers, Sci. Rep., 2022, 12(1), 11660 CrossRef CAS PubMed.
- Y. Chen, D. Zhang, X. Wu, H. Wang, Y. Xue, R. Wu, Z. Zhang and Y. Chen, Epoxy/α-alumina nanocomposite with decreased dielectric constant and dielectric loss, Polym. Compos., 2018, 39(7), 2307–2319 CrossRef CAS.
- Y. Chen, D. Zhang, X. Wu, H. Wang, C. Zhang, W. Yang and Y. Chen, Epoxy/α-alumina nanocomposite with high electrical insulation performance, Prog. Nat. Sci.:Mater. Int., 2017, 27(5), 574–581 CrossRef CAS.
- M. Ortega-Franqueza, S. Ivanova, M. I. Domínguez and M. Á. Centeno, Mesoporous Carbon Production by Nanocasting Technique Using Boehmite as a Template, Catalysts, 2021, 11(9), 1132 CrossRef CAS.
- R. Raso, L. García, J. Ruiz, M. Oliva and J. Arauzo, Study of Ni/Al-Fe Catalyst Stability in the Aqueous Phase Hydrogenolysis of Glycerol, Catalysts, 2020, 10(12), 1482 CrossRef CAS.
- R. P. dos Santos, T. C. da Silva, M. L. A. Gonçalves, B. Louis, E. B. Pereira, M. H. Herbst and M. M. Pereira, Investigation of the nature of V-species on alumina modified by alkali cations: Development of multi-functional DeSOx catalysts, Appl. Catal., A, 2012, 449, 23–30 CrossRef CAS.
- T. Gholami, M. Salavati-Niasari, M. Bazarganipour and E. Noori, Synthesis and characterization of spherical silica nanoparticles by modified Stöber process assisted by organic ligand, Superlattices Microstruct., 2013, 61, 33–41 CrossRef CAS.
- M. Darabi, M. Nikoorazm, B. Tahmasbi and A. Ghorbani-Choghamarani, Homoselective synthesis of tetrazole derivatives using copper complex anchored on mesoporous KIT-6 as a reusable, highly efficient, and environmentally green nanocatalyst, Appl. Organomet. Chem., 2024, 38(4), e7392 CrossRef CAS.
- B. Tahmasbi, P. Moradi, F. Mohammadi, Y. Abbasi Tyula and T. Kikhavani, Synthesis of Sm-TADDbBP@MCM-41 as a Robust, Reusable, and Practical Nanocatalyst in the Homoselective [2 + 3] Cycloaddition Reaction, Appl. Organomet. Chem., 2025, 39(1), e7791 CrossRef CAS.
- E. Mohseni, A. Ghorbani-Choghamarani, B. Tahmasbi, M. Norouzi and M. Akbari, A new Nickel Complex of Lysine on Mesoporous KIT-6 as a Recoverable and Selective Catalyst in the Synthesis of Tetrazoles, J. Inorg. Organomet. Polym. Mater., 2025 DOI:10.1007/s10904-024-03532-3.
- A. Ghorbani-Choghamarani, Z. Moradi and G. Azadi, An efficient and recyclable catalytic system for carbon–sulfur coupling reaction and synthesis of 5-substituted 1H-tetrazoles, J. Sulfur Chem., 2018, 39(3), 237–251 CrossRef CAS.
- G. Azadi, A. Ghorbani-Choghamarani and L. Shiri, Copper (II) immobilized on Fe 3O4@ SiO2@ l-Histidine: A reusable nanocatalyst and its application in the synthesis of 5-substituted 1 H-tetrazoles. Transit, Met. Chem., 2017, 42, 131–136 CrossRef CAS.
- M. Nasrollahzadeh, Y. Bayat, D. Habibi and S. Moshaee, FeCl3–SiO2 as a reusable heterogeneous catalyst for the synthesis of 5-substituted 1H-tetrazoles via [2+ 3] cycloaddition of nitriles and sodium azide, Tetrahedron Lett., 2009, 50(31), 4435–4438 CrossRef CAS.
- T. Tamoradi, A. Ghorbani-Choghamarani and M. Ghadermazi, Fe3O4–adenine–Zn: a novel, green, and magnetically recoverable catalyst for the synthesis of 5-substituted tetrazoles and oxidation of sulfur containing compounds, New J. Chem., 2017, 41(20), 11714–11721 RSC.
- M. A. Jani and K. Bahrami, Synthesis of 5-substituted 1H-tetrazoles and oxidation of sulfides by using boehmite nanoparticles/nickel-curcumin as a robust and extremely efficient green nanocatalyst, Appl. Organomet. Chem., 2020, 34(12), e6014 CrossRef CAS.
- A. Ghorbani-Choghamarani, H. Aghavandi and M. Mohammadi, Boehmite@SiO2@ Tris (hydroxymethyl) aminomethane-Cu (I): a novel, highly efficient and reusable nanocatalyst for the C-C bond formation and the synthesis of 5-substituted 1H-tetrazoles in green media, Appl. Organomet. Chem., 2020, 34(10), e5804 CrossRef CAS.
- G. Venkateshwarlu, A. Premalatha, K. C. Rajanna and P. K. Saiprakash, Cadmium chloride as an efficient catalyst for neat synthesis of 5-substituted 1 H-tetrazoles, Synth. Commun., 2009, 39(24), 4479–4485 CrossRef CAS.
- P. Moradi, Investigation of Fe3O4@boehmite NPs as efficient and magnetically recoverable nanocatalyst in the homoselective synthesis of tetrazoles, RSC Adv., 2022, 12, 33459–33468 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5na00081e |
| This journal is © The Royal Society of Chemistry 2025 |