Open Access Article
Open Access ArticleComposite aerogel membranes with well dispersed nano M-TiO2@GO for efficient photocatalysis†
Xiaozhe
Zhang‡
 ,
Siqi
Zhang‡
,
Xiaohui
Mao
,
Yifan
Liu
,
Yaru
Li
,
Weilong
Meng
,
Liping
Zhu
,
Siqi
Zhang‡
,
Xiaohui
Mao
,
Yifan
Liu
,
Yaru
Li
,
Weilong
Meng
,
Liping
Zhu
 * and
Meifang
Zhu
* and
Meifang
Zhu

State Key Laboratory of Advanced Fiber Materials, College of Materials Science and Engineering, Donghua University, Shanghai 201620, China. E-mail: zhulp@dhu.edu.cn
First published on 23rd May 2025
Abstract
Photocatalytic technology plays a crucial role in addressing energy shortages and environmental pollution. TiO2 has been widely applied in photocatalysis but encounters several issues, such as the easy recombination of photoexcited electrons and holes, the poor dispersion uniformity, the wide band gap, and the complex recovery process. In this work, a recyclable M-TiO2@GO/modified chitosan composite aerogel membrane was designed and prepared for photocatalytic application, with TiO2 nanoparticles generated by MXene oxidation (M-TiO2) serving as the photocatalyst and modified chitosan acting as the matrix. The experimental results demonstrated that M-TiO2 nanoparticles were in situ generated on the surface of graphene oxide (GO) nanosheets, forming M-TiO2@GO nanoparticles with a 0D@2D structure. Due to the combined effects of M-TiO2 and carbon (another product resulting from the oxidation of MXene), the band gap of M-TiO2 nanoparticles was decreased from 3.10 eV to 2.06 eV, which significantly enhanced the photocatalytic efficiency of TiO2. When the photocatalytic degradation performance of the aerogel membrane containing M-TiO2@GO nanoparticles was evaluated, the sample C15M15G5 (containing modified chitosan 15 mg/M-TiO2 15 mg/GO 5 mg) demonstrated nearly complete degradation of Rhodamine B and Acid Blue 1 within 120 min, exhibiting excellent photocatalytic performance. In addition, the antibacterial properties of C15M15G5 were examined and found that the antibacterial rate reached 100% following ultraviolet irradiation. In summary, this study presents an innovative strategy to obtain well-dispersed nano TiO2, which can be used to prepare composite photocatalytic aerogel membranes with excellent catalytic performance and complete recyclability.
1. Introduction
Since TiO2 was first discovered for water photocatalytic splitting in 1972,1 it has drawn extensive attention due to its green, efficient, stable and reuseable features, and has been widely applied in numerous fields, such as photocatalytic hydrogen production,2–4 CO2 reduction,5,6 and organic pollutant degradation,7–9 to address the crisis of energy shortage and environmental pollution.10 The specific surface area, uniformity, and band gap of the photocatalyst will directly influence its photocatalytic performance,11 therefore, enhancing the porosity or dispersion, doping and hybridization can effectively enhance the photocatalytic efficiency.12,13 However, TiO2 still presents some issues, such as a tendency to agglomerate, difficulty in recovery, and the easy recombination of photoexcited electrons and holes.The high surface energy of TiO2 will lead to obvious agglomeration during the catalytic process, thereby significantly reducing the photocatalytic performance. By grafting KH570 onto the surface of TiO2, Qin et al. obtained modified TiO2 that could be evenly dispersed on the surface of the fiber.14 However, as the photocatalytic process mainly takes place on the surface of TiO2, the traditional surface modification technology will restrict the contact between TiO2 and other substances, thereby influencing the photocatalytic performance of TiO2. The in situ growth approach was frequently adopted to generate photocatalysts, thereby enhancing the distribution uniformity of photocatalysts. For example, Ćurković et al. fabricated the uniform nano-TiO2 film through the sol–gel method, which exhibited effective photocatalytic performance (solution with 7.3 mg L−1 Lissamine Green B was almost completely degraded within 120 min).15 This method leaves no substances on the surface of the catalyst and can mitigate the tendency of reduced catalytic performance. Nevertheless, the sol–gel method might sometimes yield Ti(OH)4 and other precipitates, affecting the stability of the system. The in situ oxidation of titanium sources represented by Ti3C2Tx MXene (such as high-temperature calcination,16 hydrothermal method,17 addition of an oxidizing agent,18etc.) provides a solution to avoid the aforementioned problems, which is considered as an effective strategy to stably obtain nano TiO2.
To enhance the photocatalytic activity of TiO2, the structure of the photocatalyst is frequently regulated through the addition of a cocatalyst or doping.19,20 The cocatalyst was often used to prevent the recombination of electrons and holes, thereby enhancing the photocatalytic efficiency of TiO2.21 Among them, graphene oxide (GO) is an oxidized derivative of graphene, inheriting the outstanding properties of graphene, such as excellent optical properties, mechanical flexibility, good thermal stability, and chemical stability.22,23 Moreover, it possesses excellent hydrophilicity, enabling GO nanosheets to form a stable dispersion in water or other solvents. Additionally, the specific surface area of GO nanosheets is large, and the surface is rich in active functional groups, thus it can effectively anchor the photocatalyst to form the composite photocatalytic structure, which is currently considered as one of the most popular strategies to enhance the catalytic performance.24–27 For example, Yang et al. fabricated a composite material for the efficient photocatalytic degradation of decabromodiphenyl ether (BDE209) by uniformly anchoring TiO2 nanoparticles onto GO nanosheets. The prepared TiO2/GO photocatalyst exhibited excellent photocatalytic performance owing to the intense coupling between GO nanosheets and TiO2 nanoparticles. Under the optimal photocatalytic degradation conditions, the degradation efficiency of BDE209 could exceed 90%, which offered an effective approach for designing highly active catalytic materials.28 Alternatively, by doping C, N and other elements to form heterojunctions with TiO2, the width of the band gap can be effectively reduced and the photocatalytic activity of TiO2 can be improved.29 For example, Wahyuni et al. employed a simple hydrothermal technique to prepare N-doped TiO2 with urea serving as the N source, and successfully decreased the band gap width from 3.20 eV to 3.06–2.96 eV.30
In addition, the recovery of TiO2 is also an issue that needs to be addressed. Ding et al. successfully incorporated Fe3O4 into the photocatalyst system to facilitate the magnetic recovery of the material.31 They synthesized a CS/Fe3O4/TiO2@MXene nanocomposite photocatalyst through a one-step hydrothermal method, which exhibited excellent bacteriostatic and degradation properties. Moreover, it can be readily recycled and reused through the external magnetic field by adding Fe3O4. Fernández-Ibáñez et al. employed flocculation agents to enhance the sedimentation of colloids in a pilot plant for catalyst recovery, resulting in an impressive 97% retrieval of TiO2.32 However, the recycling process of TiO2 described above is relatively complex. The preparation of composite materials of TiO2 with polymers in the form of aerogels or membranes represents another important solution for this issue. This approach can simultaneously achieve relatively efficient photocatalysis and comprehensive recovery of TiO2.33–36 Chitosan (CHs), being a natural organic macromolecule, has been extensively employed as the matrix in catalytic systems for water pollutant treatment due to its environmental friendliness, easy availability, and degradability.37–40
To achieve high photocatalytic performance, addressing the dispersion issue, the electron–hole recombination issue and the recycling issue of TiO2, in this study, a M-TiO2@GO/chitosan composite aerogel membrane with a 0D@2D in 3D structure (0D M-TiO2 nanoparticles @ 2D GO sheet dispersed in a 3D chitosan aerogel membrane) was successfully designed and fabricated. M-TiO2 was formed in situ through the oxidation of MXene, which was deposited on GO nanosheets to address the aggregation issue of TiO2. Employing GO nanosheets as the cocatalyst, which can effectively enhance the photocatalytic performance by transferring photoexcited electrons and preventing the recombination of electrons and holes. In the meantime, M-TiO2 could form the Schottky heterojunction with carbon (another product resulting from the oxidation of MXene), effectively narrowing the band gap. The complete recovery of M-TiO2@GO was achieved by using modified chitosan as the matrix to form a composite aerogel membrane. The excellent photocatalytic performance of M-TiO2@GO/modified chitosan composite aerogel membranes was demonstrated through the dye degradation experiments.
2. Experimental section
2.1. Materials
Chitosan (MW = 100 kDa, deacetylation degree 85%), LiF (99.9%) and Acid Blue 1 were purchased from Shanghai Macklin Biochemical Co. Ltd. Hydrochloric acid (HCl) solution (36.0–38.0%) and anhydrous ethanol (AR) were obtained from Sinopharm Chemical Reagent Co., Ltd. 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide (EDC, 98%) and 3,4-dihydroxyhydrocinnamic acid (HCA, 98%) were bought from TCI (Shanghai) Chemical Industry Development Co., Ltd. Ti3AlC2 was acquired from Jilin 11 Technology Co., Ltd. TiO2 (99.8%, 25 nm, anatase crystal type) (P25 TiO2) was purchased from Meryer (Shanghai) Biochemical Technology Co., Ltd. Graphene oxide (GO) dispersion (0.5–5.0 μm, 5 mg mL−1) was obtained from Nanjing XFNANO Materials Tech Co., Ltd. Hydrogen peroxide was bought from Shanghai Titan Scientific Co., Ltd. Rhodamine B was acquired from Shanghai Aladdin Biochemical Technology Co., Ltd. The qualitative filter paper was bought from Hangzhou Fuyang Beimu Jiangzhi Co., Ltd. All the reagents were used as received without further purification. Deionized (DI) water with a resistance of 18 MΩ cm was prepared via a lab water purification system and used in all experiments.2.2. In situ generation of M-TiO2 on GO nanosheets
2.3. Preparation of aerogel membranes with TiO2 and GO nanosheets
Modified chitosan, which is water-soluble and capable of being cross-linked, was prepared following a method modified from the literature-reported procedure.41,42 Chitosan (0.5 g) was dissolved in dilute hydrochloric acid to obtain a 1% chitosan solution, and the pH value was adjusted to approximately 5.2. A mixture of deionized water and ethanol with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was employed to dissolve EDC (0.36 g) and HCA (0.34 g), which were then added dropwise to the chitosan solution for the grafting reaction. The modified chitosan (CHs-HCA) was dialyzed using a dilute hydrochloric acid solution with a pH range of 4.6–4.8 as the dialysate, followed by freezing and freeze-drying.
1 was employed to dissolve EDC (0.36 g) and HCA (0.34 g), which were then added dropwise to the chitosan solution for the grafting reaction. The modified chitosan (CHs-HCA) was dialyzed using a dilute hydrochloric acid solution with a pH range of 4.6–4.8 as the dialysate, followed by freezing and freeze-drying.
A designed amount of CHs-HCA was dissolved in 1 mL deionized water, mixed with M-TiO2@GO dispersion, and stirred at 60 °C. Under the influence of the residual H2O2 in the solution, CHs-HCA was oxidized and cross-linked. Eventually, H2O2 that did not take part in the oxidation reaction was decomposed under heating conditions. Subsequently, the resulting solution was cast onto a substrate membrane (qualitative filter paper), followed by freeze-drying to obtain the aerogel membrane consisting of CHs-HCA/M-TiO2/GO (CMG), where the subscript of M represents the mass of TiO2 generated after oxidation of MXene by theoretical calculation.
Similarly, P25 TiO2 with a corresponding amount was uniformly dispersed along with GO nanosheets, and combined with CHs-HCA to produce the aerogel membrane consisting of CHs-HCA/TiO2/GO (CTG) using the same method described above. The respective component masses for different samples are summarized in Table 1. And the preparation process of composite aerogel membranes is shown in Fig. 1.
| Sample | CHs-HCA (mg) | MXene (mg) | Nano-TiO2 (mg) | GO (mg) |
|---|---|---|---|---|
| C20M10G5 | 20 | 7 | — | 5 |
| C15M15G5 | 15 | 10.5 | — | 5 |
| C10M20G5 | 10 | 14 | — | 5 |
| C20T10G5 | 20 | — | 10 | 5 |
| C15T15G5 | 15 | — | 15 | 5 |
| C10T20G5 | 10 | — | 20 | 5 |
2.4. Characterization
The Fourier transform infrared (FTIR) spectra of the chitosan before and after modification were recorded using a FTIR spectrophotometer (TENSOR II, BRUKER) with an ATR attachment. The grafting rate was calculated based on the integral of the corresponding peaks in the spectra collected from a Nuclear Magnetic Resonance (NMR) spectrometer (AVANCE NEO, BRUKER). The crystallographic structures of the MAX phase, MXene, and M-TiO2 were determined using X-ray diffraction (XRD) (X-ray dynamic 500, Anton Paar). The samples were run at a scan rate of 5° min−1 over an angular (2θ) range of 3°–90°. The morphology of GO nanosheets before and after loading M-TiO2 was characterized using a Transmission Electron Microscope (TEM) (JEM-F200, JEOL, Japan). The changes in elemental valence states in MXene before and after H2O2 treatment were analyzed by X-ray Photoelectron Spectroscopy (XPS) (K-Alpha, Thermo Scientific, USA). Raman spectra were obtained using an inVia-Reflex (Renishaw) with a 532 nm excitation laser. The electrical properties of M-TiO2 in water were tested using a zeta potential analyzer (Zetasizer Nano ZS90, Malvern, UK). The micromorphology of the composite aerogel membrane was observed using a Scanning Electron Microscope (SEM) (SU8010, Hitachi, Japan). The pore size distribution of the aerogel membrane was measured by mercury intrusion porosimetry (AutoPore V 9620, Micromeritics, USA). The absorbance of the dye solution after photocatalysis was measured using an ultraviolet-visible-near infrared (UV-vis-NIR) spectrophotometer (UV-3600, SHIMADZU), and the change in concentration was calculated.2.5. Photocatalytic performance
The composite aerogel membrane was immersed in a 100 mL, 5 ppm dye solution (Rhodamine B or Acid Blue 1) and irradiated with a 250 W ultraviolet high-pressure mercury lamp (with spectral energy centered at 365 nm) under constant temperature conditions maintained in a water bath. During this process, 5 mL aliquots of the solution were collected at 20 min intervals. UV-vis-NIR absorption spectra were recorded, and the change in dye concentration was calculated according to the principle of Lambert–Beer law, as shown in eqn (1). | (1) |
In addition, the obtained photocatalytic degradation data were analyzed, and the quasi-first-order reaction kinetics was used to describe the photocatalytic degradation process of Rhodamine B or Acid Blue 1 (eqn (2)),43 and the rate constant of the degradation reaction, kobs, was obtained.
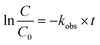 | (2) |
During the cycling photocatalytic performance test, the aerogel membrane after initial photocatalysis (recorded as Cycle 1) was taken out, washed with deionized water and freeze-dried. The cleaned and dried samples were tested again for photocatalysis, and the tests were recorded as Cycle x (x = 2, 3, …).
3. Results and discussion
3.1. Preparation and characterization of M-TiO2@GO
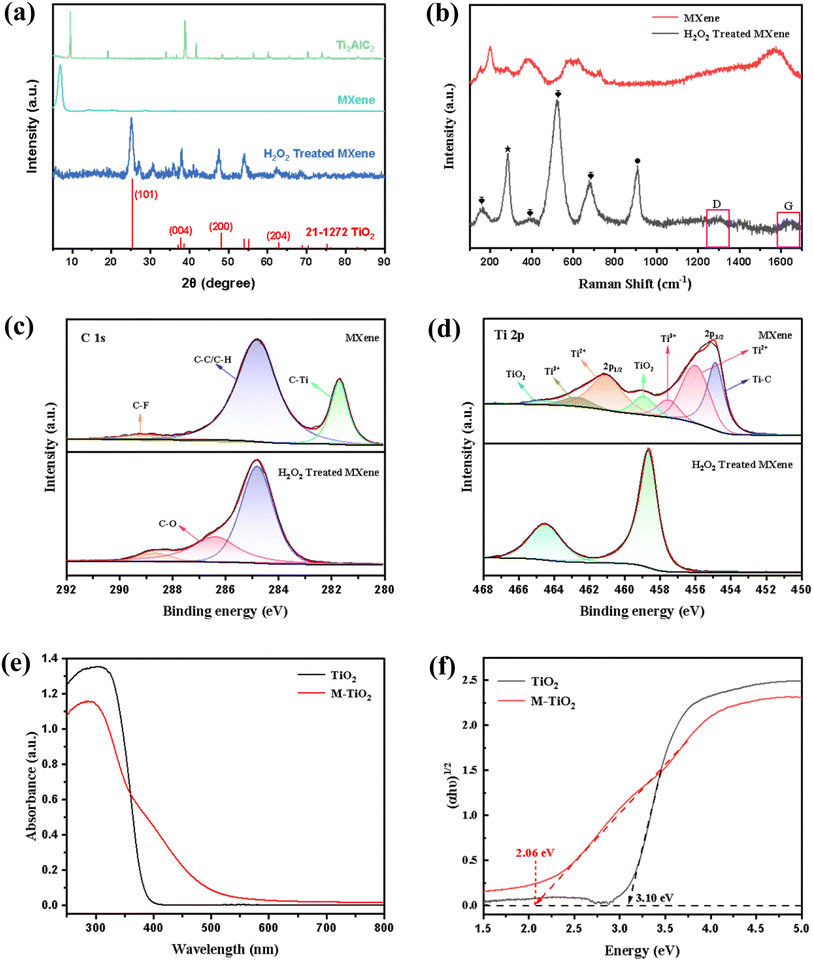
In this study, the dispersion of single-layer or few-layer MXene nanosheets was prepared by etching Ti3AlC2 in the LiF/HCl system and then freeze-dried for quantification. After the MXene and GO nanosheets were mixed and evenly dispersed, H2O2 was employed to oxidize the MXene attached to GO, thereby obtaining M-TiO2@GO nanocomposite structures. Among them, the few-layer MXene was the key to achieving rapid oxidation and obtaining nano-sized TiO2. Compared with the filtration-drying method for MXene preparation,18 the freeze-drying method adopted in this work could effectively prevent the restacking of the few-layer MXene, enabling the acquisition of M-TiO2 nanoparticles with smaller particle size and uniform distribution within a short time (30 min). In addition, GO nanosheets were designed as a carrier, which not only enhanced the structural stability of M-TiO2 but also provided an effective transfer path for photoexcited electrons. Since MXene was attached and oxidized on GO, the generated M-TiO2 could be anchored on the GO nanosheet surface, effectively avoiding the agglomeration of M-TiO2. In the meantime, M-TiO2 formed Schottky heterojunctions with carbon, another product resulting from the oxidation of MXene, which can effectively narrow the band gap.A series of characterization studies of MXene before and after oxidation were performed. Based on the XRD pattern (Fig. 2a), the characteristic peak of Ti3AlC2 at 38.76°44 became unobservable, whereas the peak at 9.51° associated with Ti3AlC2 shifted to 6.91°, which was attributed to the transformation of Ti3AlC2 to MXene.45 In conjunction with the morphological changes observed in Fig. 3b and S1,† these findings suggested that single- or few-layered MXene nanosheets have been successfully synthesized. In addition, the observed diffraction peaks at 25.2°, 38.0°, 47.5°, and 62.5° corresponded to the crystallographic planes (101), (004), (200), and (204), respectively, which are characteristic of anatase TiO2 according to PDF #21-1272.46,47 The peaks in the Raman spectrum of oxidized MXene powders (bottom spectrum in Fig. 2b) at approximately 150, 400, 520, and 676 cm−1 were assigned to the Eg, B1g, A1g & B1g, and Eg modes of anatase TiO2,48,49 respectively. The peaks observed at approximately 284 and 900 cm−1 in the Raman spectrum and 900 cm−1 in the FTIR spectrum (Fig. S2†) could be attributed to the oxidation of the sample induced by H2O2. This oxidation process resulted in the formation of oxygen-containing functional groups on the surface of the catalyst. The peak around 284 cm−1 corresponded to the surface hydroxyl related mode.50,51 The peak around 900 cm−1 was attributed to the combination of Ti and the surrounding hydroxyl groups.52 Ti atoms will form –TiOO groups after treatment with H2O2, and the peak around 900 cm−1 corresponded to the stretching vibration of the Oα–Oβ bond.53,54 Besides, the two broad peaks between 1200 and 1700 cm−1 were characteristic of the D and G modes of graphitic carbon.55
 | ||
| Fig. 3 Characterization of the morphology of M-TiO2@GO. The TEM images of (a) GO, (b) MXene and (c) sample M15G5; EDS elemental distribution maps of (d) C, (e) O, and (f) Ti on sample M15G5. | ||
XPS tests of the sample (Fig. 2c and d) further elucidated the evolution of the chemical composition and structure before and after H2O2 treatment. In Fig. 2c, the C 1s spectrum of MXene was deconvoluted into four peaks at binding energies of 289.2, 286.4, 284.8, and 281.7 eV, corresponding to C–F, C–O, C–C, and C–Ti bonds, respectively.56,57 The disappearance of the C–Ti peak and the emergence of the C–O peak in MXene following H2O2 treatment suggested that MXene has undergone a complete reaction. As illustrated in Fig. 2d and S3† (high resolution XPS spectra of the O 1s region), MXene treated with H2O2 exhibited only TiO2 peaks, confirming that MXene was oxidized by H2O2 to form TiO2. The above results might be due to the single-layer or few-layer structure allowing MXene to fully contact with H2O2, resulting in complete oxidation. The above results collectively indicated that the oxidation of MXene by H2O2 resulted in the formation of TiO2 and carbon.
To investigate the photocatalytic performance of the synthesized M-TiO2 catalyst, the corresponding characterization was conducted. Fig. 2e presents the diffuse reflectance absorption spectra of both P25 TiO2 and the prepared M-TiO2 samples. The bandgap absorption edges of M-TiO2 and P25 TiO2 were approximately 500 nm and 400 nm, respectively, indicating that both materials primarily absorbed ultraviolet light. Notably, M-TiO2 exhibited a broader absorption spectrum, attributed to the formed carbon during the oxidation process, which reduced the recombination of photogenerated electrons in TiO2.58 A plot of the transformed Kubelka–Munk function versus light energy59 is presented in Fig. 2f, from which the approximately determined band gaps were 2.06 and 3.10 eV for M-TiO2 and P25 TiO2, respectively. The main reason for the reduction of the band gap in M-TiO2 was that during the oxidation process of MXene, carbon (C), another oxidation product, formed a Schottky heterojunction with M-TiO2, thereby resulting in C-doped M-TiO2.60 The introduction of carbon atoms would create an “intermediate band” between the valence band and the conduction band of TiO2. The existence of these intermediate bands made it easier for electrons to transit from the valence band to the conduction band, thereby effectively reducing the width of the band gap. Owing to the better dispersion and wider light absorption range, M-TiO2 was anticipated to utilize the solar energy more effectively, thereby exhibiting superior photocatalytic performance, as will be evidenced in the photocatalytic experiment section.
Upon comparing Fig. 3a and c, it was evident that nanoparticles had uniformly formed on the lamellar structure, and the nanoparticles were thought to be TiO2. The EDS elemental distribution maps of M15G5 are shown in Fig. 3c–f. The particle size distribution of the synthesized M-TiO2 nanoparticles was characterized using a Nano Measurer, with a minimum of 30 individual particles analyzed to ensure statistical reliability. As shown in Fig. S4,† the particle size of the generated M-TiO2 was mainly distributed within the range of 1.0–5.5 nm. Similar functional groups on the surface of GO and MXene facilitated strong hydrogen bonding, leading to intimate interfacial interactions between them, consequently, MXene could effectively disperse on the surface of GO nanosheets.61,62 TiO2 nanoparticles were in situ generated on GO nanosheets by dispersing and adsorbing MXene onto GO, resulting in the formation of 0D@2D structured M-TiO2@GO nanoparticles. As a carrier, GO nanosheets not only enhanced the structural stability of M-TiO2 but also provided a transfer pathway for photoexcited electrons. As an intercalation agent, M-TiO2 produced via MXene oxidation significantly reduced the propensity of GO to aggregate.
3.2. Structure and morphology of CMG aerogel membranes
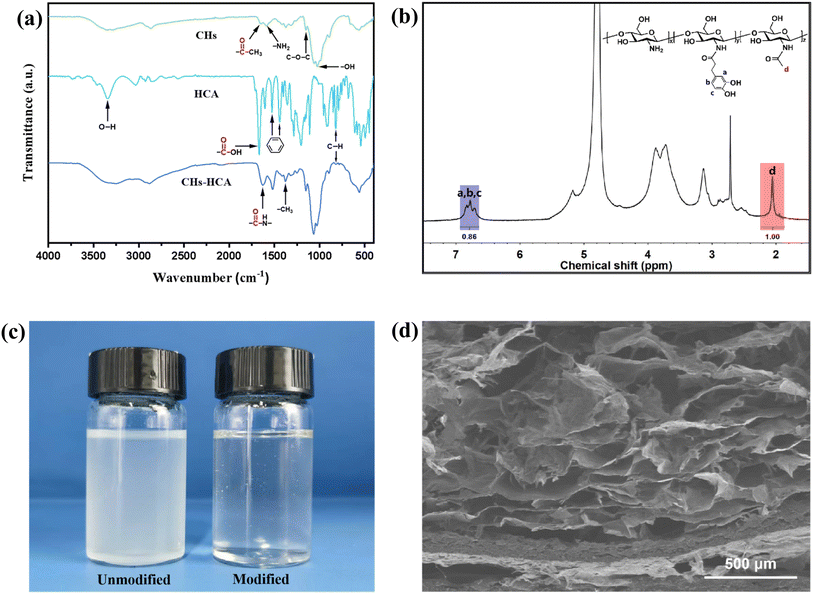
In order to ensure the complete recovery of M-TiO2@GO, a composite aerogel membrane was prepared by using non-toxic, green and biocompatible modified chitosan as the matrix. Chitosan is a polymer material soluble in dilute acid, but dilute acid solutions often react with fillers and would remain in the material to affect its properties. In this section, chitosan was grafted with HCA to render it water-soluble and enhance its processability. The infrared spectrum of chitosan after grafting was presented in Fig. 4a. Notably, the high abundance of O, N, and H within the system brought abundant hydrogen bonding sites to the system, thereby causing the redshift phenomenon of the characteristic peaks of the groups.63 The peaks at 1665, 1590, 1375, 1151, and 1025 cm−1 respectively corresponded to the absorption peaks of the carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), amino group (NH2), methyl group (CH3), C–O–C in the chitosan ring, and alcohol hydroxyl group (–OH). The peaks at 3342, 1668 and 820 cm−1 respectively corresponded to the absorption peaks of the O–H stretching vibration of the phenol hydroxyl group, the carboxyl group (–COOH), and the out-of-plane bending vibration of C–H in the 1,2,4-trisubstituted benzene ring in the HCA molecule. Meanwhile, the two peaks at 1442 and 1525 cm−1 respectively corresponded to the absorption peaks of the benzene ring skeleton. In the infrared spectrum of the prepared CHs-HCA, the peak at 1625 cm−1, corresponding to the secondary amide group (–CONH-), emerged compared with chitosan, and the out-of-plane bending vibration of C–H in the 1,2,4-trisubstituted benzene ring and the absorption peak corresponding to the benzene ring skeleton appeared. Additionally, due to the substitution of the –NH2 group in chitosan, the corresponding absorption peak transformed into the acromial peak in CHs-HCA.64,65 These alterations demonstrated the success of the grafting reaction.
O), amino group (NH2), methyl group (CH3), C–O–C in the chitosan ring, and alcohol hydroxyl group (–OH). The peaks at 3342, 1668 and 820 cm−1 respectively corresponded to the absorption peaks of the O–H stretching vibration of the phenol hydroxyl group, the carboxyl group (–COOH), and the out-of-plane bending vibration of C–H in the 1,2,4-trisubstituted benzene ring in the HCA molecule. Meanwhile, the two peaks at 1442 and 1525 cm−1 respectively corresponded to the absorption peaks of the benzene ring skeleton. In the infrared spectrum of the prepared CHs-HCA, the peak at 1625 cm−1, corresponding to the secondary amide group (–CONH-), emerged compared with chitosan, and the out-of-plane bending vibration of C–H in the 1,2,4-trisubstituted benzene ring and the absorption peak corresponding to the benzene ring skeleton appeared. Additionally, due to the substitution of the –NH2 group in chitosan, the corresponding absorption peak transformed into the acromial peak in CHs-HCA.64,65 These alterations demonstrated the success of the grafting reaction.
The modified chitosan was analyzed using 1H-NMR spectroscopy (Fig. 4b). The grafting degree of catechol was determined by comparing the relative peak areas of the catechol groups (3H, aromatic cyclic proton, δ 6.78, D2O) and the acetyl groups (3H, –COCH3, δ 2.06, D2O) on the chitosan backbone. The calculated grafting degree was approximately 15%. And images of the chitosan and CHs-HCA aqueous solution (Fig. 4c) demonstrated the excellent water solubility of the modified chitosan. Furthermore, a composite aerogel membrane featuring a porous structure and high specific surface area was synthesized using modified chitosan as the matrix and incorporated with M15G5 (as illustrated in Fig. 4d). The pore size distribution of the composite aerogel membrane was characterized using mercury intrusion porosimetry. The results indicated that the average pore size of the prepared aerogel membrane was approximately 71.5 μm, with a porosity of 93.2%, thereby confirming its significant porous structure (as shown in Fig. S5†), which was consistent with the porous morphology observed in the SEM image.
In addition, to explore the distribution of M-TiO2@GO within the modified chitosan matrix, cross-sections of the aerogel membranes with different components were observed by SEM. The results showed that the pore wall surface of the CHs-HCA aerogel membrane was smooth. As the relative content of CHs-HCA decreased, the surface of the pore wall became rougher, and no obvious agglomeration phenomenon emerged (as shown in Fig. 5b and c). Additionally, based on the EDS scanning results, it could be observed that M-TiO2 was evenly distributed in sample C15M15G5 (as shown in Fig. S6†). However, when the mass of CHs-HCA was decreased to 10 mg (sample C10M20G5), the nano-fillers agglomerated, leading to the uneven dispersion of M-TiO2@GO and forming the obvious granular structure (as shown in the red dashed line box in Fig. 5d). The uneven distribution of the catalyst resulting from this agglomeration structure might cause the decline of the photocatalytic performance of the composite system.
 | ||
| Fig. 5 Characterization of cross-section morphology of aerogel membranes with different M-TiO2 contents. SEM images of (a) CHs-HCA, (b) C20M10G5, (c) C15M15G5 and (d) C10M20G5. | ||
3.3. Photocatalytic performance of composite aerogel membranes
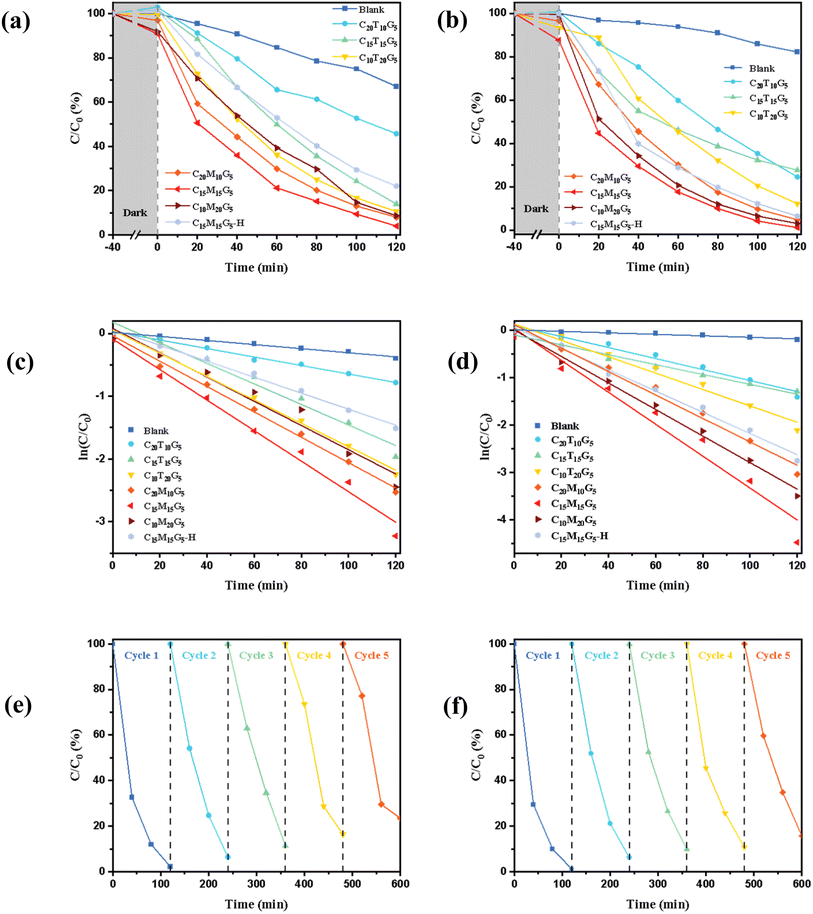
The photocatalytic degradation of Rhodamine B and Acid Blue 1 was used as model reactions to evaluate the photocatalytic performance of M-TiO2@GO/chitosan. The photocatalytic degradation performance of P25 TiO2@GO and M-TiO2@GO aerogel membranes was evaluated under identical conditions. Fig. 6a and b show the concentration–time curves of Rhodamine B and Acid Blue 1 degraded by different samples. UV-vis absorption spectra for the degradation of different dyes by C15M15G5 are shown in Fig. S7.† It was observed that the composite aerogel membrane exhibited a little adsorption effect on these dyes under dark conditions, and the adsorption curve is shown in Fig. S8.† The M-TiO2@GO composite aerogel membrane demonstrated superior photocatalytic activity compared to the P25 TiO2@GO composite aerogel membrane. After 120 min of ultraviolet irradiation, C15T15G5 degraded Rhodamine B by 86% and Acid Blue 1 by 72.36%, while C15M15G5 degraded Rhodamine B by 96.03% and Acid Blue 1 by 98.86%. This could be attributed primarily to the narrower band gap of M-TiO2 (2.06 eV), leading to enhanced catalytic degradation efficiency. Additionally, due to electrostatic interactions between MXene and GO, M-TiO2 was synthesized in situ on the surface of GO during the oxidation process, ensuring intimate contact between the catalyst and co-catalyst. This configuration facilitated the transfer of excited electrons generated during catalysis and inhibited the recombination of electron–hole pairs, thereby improving the photocatalytic performance. Additionally, the dye degradation efficiency of C15M15G5 samples prepared by heat drying in an oven (called C15M15G5-H) was evaluated. It was observed that the dye degradation efficiency of these samples was significantly lower than that of the aerogel membrane with the same composition. After 120 min of ultraviolet irradiation, C15M15G5-H degraded Rhodamine B by 77.94% and Acid Blue 1 by 93.62%, which was significantly lower compared to that of the C15M15G5 sample. This discrepancy arose because the dye degradation process occurs on the surface of the catalyst TiO2, and the porous structure (Fig. 4d) of the aerogel membrane provided a larger specific surface area, allowing for greater TiO2 exposure and thus increasing the number of active sites and overall catalytic efficiency.Through the quasi-first-order kinetic simulation of the degradation curves for various materials, the slope of the fitted line represents the degradation rate constant. As illustrated in Fig. 6c and d, it was evident that the chitosan aerogel membrane exhibited a strong linear relationship. Furthermore, the data indicated that sample C15M15G5 demonstrated the highest photocatalytic reaction rate constant for both dyes: the photocatalytic reaction rate constant of Rhodamine B was 0.02433 min−1, and that of Acid Blue 1 was 0.03367 min−1. This enhanced performance could be attributed to the formation of a Schottky heterojunction between M-TiO2 and carbon generated from oxidized MXene. This significantly improved the separation efficiency of photogenerated carriers and narrowed the band gap of TiO2,66 thereby enhancing photocatalytic efficiency.
In the field of photocatalytic technology, it was imperative to develop synthetic materials that could be efficiently recovered and reused for wastewater treatment. Fig. 6e and f illustrat the cycling performance of a composite aerogel membrane in dye degradation. Specifically, five consecutive photodegradation experiments were conducted using a C15M15G5 aerogel membrane. Upon initial application, the degradation efficiencies for Rhodamine B and Acid Blue 1 were 97.78% and 98.86%, respectively. After five successive uses, the degradation efficiencies decreased to 76.57% for Rhodamine B and 84.31% for Acid Blue 1. Despite this reduction, the C15M15G5 aerogel membrane retained a high level of catalytic activity, demonstrating its excellent cycling performance.
3.4. Antibacterial properties of composite aerogel membranes
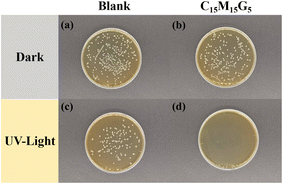
In addition to organic pollutants, pathogenic bacteria are also among the pollutant components in wastewater. Based on the efficient reaction mechanism of photocatalysis technology in degrading organic pollutants, it also offers a new idea for inactivating microorganisms in wastewater. We compared the antibacterial performance of sample C15M15G5 under dark and light conditions, as shown in Fig. 7. It can be seen that the number of colonies in the Petri dish with C15M15G5 added under dark conditions was slightly reduced compared with that in the blank sample. This is mainly due to the electrostatic interaction between the amino groups of CHs (positively charged) with the cell membranes (negatively charged) of bacteria.67 This interaction induces significant alterations to the cell surface, resulting in changes in permeability. This subsequently triggers osmotic imbalance and the efflux of intracellular components, ultimately leading to cell death.68After ultraviolet irradiation, the number of colonies in the blank sample significantly decreased due to the bactericidal properties of ultraviolet light. However, in the culture dish containing C15M15G5, no colonies were observed. This was primarily attributed to the fact that during irradiation, the photoelectron in the conduction band interacted with adsorbed O2 on the surface, generating reactive oxygen species (ROS).69 These ROS damaged bacterial cell membranes upon contact, penetrated the cells, and subsequently disrupted the protein structure or genetic material, ultimately leading to bacterial death.67,69
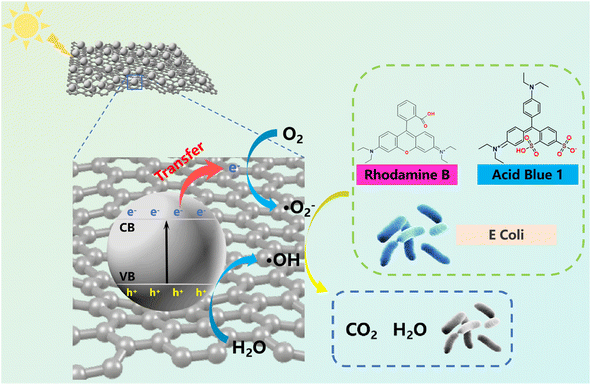
3.5. Mechanism for photocatalytic degradation of dyes and antibacterial activity by M-TiO2@GO
Based on the aforementioned findings, we built a schematic representation of the M-TiO2@GO catalytic degradation of dyes and sterilization, as illustrated in Fig. 8. When M-TiO2 nanoparticles absorb ultraviolet light or visible light containing a certain energy, electrons in the valence band (VB) would be excited to transition to the conduction band (CB), forming photogenerated electrons (e−) and leaving photogenerated holes (h+) in the valence band,70,71 as shown in eqn (3).| M-TiO2 + hν → M-TiO2(e−) + M-TiO2(h+) | (3) |
These holes could interact with water molecules on the surface of titanium dioxide to produce hydroxyl radicals (˙OH), as shown in eqn (4). However the photogenerated electrons migrated to the GO surface and reacted with dissolved oxygen in water to form superoxide radicals (˙O2−), as shown in eqn (5) and (6).
| H2O + M-TiO2(h+) → ˙OH + H | (4) |
| M-TiO2(e−) + GO → M-TiO2 + GO(e−) | (5) |
| O2 + GO(e−) → ˙O2− | (6) |
Subsequently, the ROS such as ˙OH and ˙O2− exhibited strong oxidation/reduction activities. These ROS could degrade organic dyes in water into inorganic small molecules such as CO2 and H2O, or destroy the cell structure of bacteria, thus achieving the effect of efficient water purification.
4. Conclusion
In this study, we presented a green and rapid method for preparing well-dispersed M-TiO2@GO photocatalysts and demonstrated that the composite aerogel membranes containing such photocatalysts exhibited excellent photocatalytic activity and recyclability. With MXene serving as the precursor of TiO2, the in situ generation of TiO2 was accomplished, effectively addressing the agglomeration issue of nano TiO2. The two oxidation products of MXene, TiO2 and carbon, successfully formed the Schottky heterojunction in the system, effectively reducing the band gap of TiO2 from 3.10 eV to 2.06 eV, which resolved the relatively wide band gap problem of TiO2 and thus significantly enhanced the photocatalytic efficiency. Additionally, GO was employed as the co-catalyst to alleviate the problem of photoexcited electron–hole recombination, and modified chitosan was prepared and used as a carrier to overcome the cumbersome issue of TiO2 recovery. When the fabricated M-TiO2@GO/chitosan composite aerogel membranes with a 0D@2D in 3D structure were applied to the dye degradation, after 120 min of ultraviolet light irradiation, the degradation rate of Rhodamine B reached 96.03%, and the degradation rate of Acid Blue 1 reached 98.86%. The composite membranes retained the ability to degrade 80% of the dye after five cycles. Moreover, after being exposed to ultraviolet light, the antibacterial rate of C15M15G5 reached 100%. Overall, this work provided an effective strategy for green, efficient and recyclable photocatalytic degradation of organic pollutants.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This study was financially supported by the National Key Research and Development Program of China (2022YFB3804905, 2022YFB3804900, and 2021YFA1201300) and National Natural Science Foundation of China (U23A20585).References
- A. Fujishima and K. Honda, Electrochemical Photolysis of Water at a Semiconductor Electrode, Nature, 1972, 238(5358), 37–38 CrossRef CAS PubMed
.
- J. Yang, W. Yu and C. Li,
et al., PtO nanodots promoting Ti3C2 MXene in-situ converted Ti3C2/TiO2 composites for photocatalytic hydrogen production, Chem. Eng. J., 2021, 420, 129695 CrossRef CAS
.
- S. Escobedo and H. de Lasa, Synthesis and Performance of Photocatalysts for Photocatalytic Hydrogen Production: Future Perspectives, Catalysts, 2021, 11, 1505 CrossRef CAS
.
- F. Bhom and Y. M. Isa, Photocatalytic Hydrogen Production Using TiO2-based Catalysts: A Review, Global Challenges, 2024, 8(11), 2400134 CrossRef PubMed
.
- J. Shen, Z. Wu and C. Li,
et al., Emerging applications of MXene materials in CO2 photocatalysis, FlatChem, 2021, 28, 100252 CrossRef CAS
.
- F. Wang, Z. Lu and H. Guo,
et al., Frontispiece: Plasmonic Photocatalysis for CO2 Reduction: Advances, Understanding and Possibilities, Chem.–Eur. J., 2023, 29(25), e202382561 CrossRef
.
- H. Zhang, Y. Wan and J. Luo,
et al., Drawing on Membrane Photocatalysis for Fouling Mitigation, ACS Appl. Mater. Interfaces, 2021, 13(13), 14844–14865 CrossRef CAS PubMed
.
- Z. Dong, Z. Zhang and Z. Li,
et al., 3D structure aerogels constructed by reduced graphene oxide and hollow TiO2 spheres for efficient visible-light-driven photoreduction of U(VI) in air-equilibrated wastewater, Environ. Sci.: Nano, 2021, 8(8), 2372–2385 RSC
.
- S. Nouacer and R. Djellabi, Easy-handling semi-floating TiO2-based aerogel for solar photocatalytic water depollution, Environ. Sci. Pollut. Res., 2023, 30(9), 22388–22395 CrossRef CAS PubMed
.
- G. Cheng, X. Liu and J. Xiong, Recent advances in coupling pollutants degradation with hydrogen production by semiconductor photocatalysis, Chem. Eng. J., 2024, 501, 157491 CrossRef CAS
.
- X. Qiu, Y. Zhang and Y. Zhu,
et al., Applications of Nanomaterials in Asymmetric Photocatalysis: Recent Progress, Challenges, and Opportunities, Adv. Mater., 2021, 33(6), 2001731 CrossRef CAS PubMed
.
- J. Pei, H. Li and D. Yu,
et al., g-C3N4-Based Heterojunction for Enhanced Photocatalytic Performance: A Review of Fabrications, Applications, and Perspectives, Catalysts, 2024, 14(11), 825 CrossRef CAS
.
- Y. Liu, H. Wu and Q. Wang, Strategies to improve the photocatalytic performance of covalent triazine frameworks, J. Mater. Chem. A, 2023, 11(40), 21470–21497 RSC
.
- X. Qin, X. Feng and T. Zhu,
et al., Preparation and application of heterojunction KH570-TiO2/MXene/PAN membranes with photocatalytic degradation and photothermal conversion properties, J. Solid State Chem., 2022, 312, 123142 CrossRef CAS
.
- L. Ćurković, D. Ljubas and S. Šegota,
et al., Photocatalytic degradation of Lissamine Green B dye by using nanostructured sol–gel TiO2 films, J. Alloys Compd., 2014, 604, 309–316 CrossRef
.
- L. Guo, Y. Liu and Q. Zeng,
et al., A self-driven solar coupling system with TiO2@MXene cathode for effectively eliminating uranium and organics from complex wastewater accompanying with electricity generation, J. Hazard. Mater., 2024, 465, 133415 CrossRef CAS PubMed
.
- Z. Zhuo, X. Wang and C. Shen,
et al., Construction of TiO2/SrTiO3 Heterojunction Derived from Monolayer Ti3C2 MXene for Efficient Photocatalytic Overall Water Splitting, Chem.–Eur. J., 2023, 29(12), e202203450 CrossRef CAS PubMed
.
- R. Han and P. Wu, High-performance graphene oxide nanofiltration membrane with continuous nanochannels prepared by the in situ oxidation of MXene, J. Mater. Chem. A, 2019, 7(11), 6475–6481 RSC
.
- M. Mohsin, I. A. Bhatti and M. Zeshan,
et al., Prospects, challenges, and opportunities of the metals-modified TiO2 based photocatalysts for hydrogen generation under solar light irradiation: a review, FlatChem, 2023, 42, 100547 CrossRef CAS
.
- K. Wang, K. Yoshiiri and L. Rosa,
et al., TiO2/Au/TiO2 plasmonic photocatalyst with enhanced photocatalytic activity and stability under visible-light irradiation, Catal. Today, 2022, 397–399, 257–264 CAS
.
- C. Ding, X. Qin and Y. Tian,
et al., PES membrane surface modification via layer-by-layer self-assembly of GO@TiO2 for improved photocatalytic performance, J. Membr. Sci., 2022, 659, 120789 CrossRef CAS
.
- X. Huang, X. Qi and F. Boey,
et al., Graphene-based composites, Chem. Soc. Rev., 2012, 41(2), 666–686 RSC
.
- R. Yang, Y. Fan and J. Hu,
et al., Photocatalysis with atomically thin sheets, Chem. Soc. Rev., 2023, 52(22), 7687–7706 RSC
.
- N. Thi Quynh Anh, H. M. Ngoc and N. Van Noi,
et al., Enhanced photocatalytic degradation of direct blue 71 dye using TiO2-PAA-GO composite in aqueous solution, Mater. Res. Express, 2023, 10(5), 55503 CrossRef CAS
.
- S. Liu, Z. Su and Y. Liu,
et al., Mechanism and Purification Effect of Photocatalytic Wastewater Treatment Using Graphene Oxide-Doped Titanium Dioxide Composite Nanomaterials, Water, 2021, 13(14), 1915 CrossRef CAS
.
- K. Jayaramulu, S. Mukherjee and D. M. Morales,
et al., Graphene-Based Metal–Organic Framework Hybrids for Applications in Catalysis, Environmental, and Energy Technologies, Chem. Rev., 2022, 122(24), 17241–17338 CrossRef CAS PubMed
.
- X. Li, J. Yu and M. Jaroniec,
et al., Cocatalysts for Selective Photoreduction of CO2 into Solar Fuels, Chem. Rev., 2019, 119(6), 3962–4179 CrossRef CAS PubMed
.
- M. Yang, Y. Zou and L. Ding,
et al., TiO2 nanoparticles anchored on graphene oxide nanosheets as a highly active photocatalyst for decabromodiphenyl ether degradation, Carbon Lett., 2023, 33(5), 1333–1341 CrossRef CAS
.
- Y. Pan, Y. Wang and S. Wu,
et al., One-Pot Synthesis of Nitrogen-Doped TiO2 with Supported Copper Nanocrystalline for Photocatalytic Environment Purification under Household White LED Lamp, Molecules, 2021, 26(20), 6221 CrossRef CAS PubMed
.
- E. T. Wahyuni, T. Rahmaniati and A. R. Hafidzah,
et al., Photocatalysis over N-Doped TiO2 Driven by Visible Light for Pb(II) Removal from Aqueous Media, Catalysts, 2021, 11(8), 945 CrossRef CAS
.
- H. Ding, M. Zhang and Y. Liu,
et al., Synthesis of CS/Fe3O4/TiO2@MXene nanocomposite photocatalyst with excellent degradation and bacteriostatic properties by one-step hydrothermal method, Ceram. Int., 2024, 50(22B), 46334–46346 CrossRef CAS
.
- P. Fernández-Ibáñez, J. Blanco and S. Malato,
et al., Application of the colloidal stability of TiO2 particles for recovery and reuse in solar photocatalysis, Water Res., 2003, 37(13), 3180–3188 CrossRef PubMed
.
- S. Y. Wu, L. Li and T. Jiang,
et al., Excellent self-cleaning surface achieved through the synergistic effect of superhydrophilicity and photocatalysis of PVDF-TiO2-HAP coating, Surf. Interfaces, 2024, 51, 104676 CrossRef CAS
.
- M. H. Zhang, Z. B. Han and G. B. Li,
et al., Promoted adsorption-photocatalysis synergistic removal of contaminants via surface regulation by a tree-like nanofiber membrane substrate, J. Environ. Chem. Eng., 2024, 12(3), 113034 CrossRef CAS
.
- Q. H. Li, M. Dong and R. Li,
et al., Enhancement of Cr(VI) removal efficiency via adsorption/photocatalysis synergy using electrospun chitosan/g-C3N4/TiO2 nanofibers, Carbohydr. Polym., 2021, 253, 117200 CrossRef CAS PubMed
.
- L. F. Liu, P. H. Zhang and F. L. Yang, Adsorptive removal of 2,4-DCP from water by fresh or regenerated chitosan/ACF/TiO2 membrane, Sep. Purif. Technol., 2010, 70(3), 354–361 CrossRef CAS
.
- Y. Liu, F. Wu and Y. Ding,
et al., Preparation and Characterization of Paclitaxel/Chitosan Nanosuspensions for Drug Delivery System and Cytotoxicity Evaluation In Vitro, Adv. Fiber Mater., 2019, 1(2), 152–162 CrossRef
.
- J. Yin, L. Xu and A. Ahmed, Batch Preparation and Characterization of Electrospun Porous Polylactic Acid-Based Nanofiber Membranes for Antibacterial Wound Dressing, Adv. Fiber Mater., 2022, 4(4), 832–844 CrossRef CAS
.
- Q. S. Zhu, X. J. Han and C. Z. Cheng,
et al., Study on Dissolubility of Chitosan in Four Kinds of Imidazole-Based Ionic Liquids, Acta Polym. Sin., 2011, 1173–1179 CrossRef CAS
.
- Y. C. Jin, Y. Lu and L. Yu,
et al., Synthesis of Chitosan-O-Poly(Ethylene Glycol) Through Diels-Alder Reaction, Acta Polym. Sin., 2013, 903–908 CAS
.
- J. H. Ryu, S. Jo and M. Koh,
et al., Bio-Inspired, Water-Soluble to Insoluble Self-Conversion for Flexible, Biocompatible, Transparent, Catecholamine Polysaccharide Thin Films, Adv. Funct. Mater., 2014, 24(48), 7709–7716 CrossRef CAS
.
- A. R. Narkar, E. Cannon and H. Yildirim-Alicea,
et al., Catechol-Functionalized Chitosan: Optimized Preparation Method and Its Interaction with Mucin, Langmuir, 2019, 35(48), 16013–16023 CrossRef CAS PubMed
.
- X. Guo, S. Hou and J. Chen,
et al., Transpiration-prompted Photocatalytic Degradation of Dye Pollutant with AuNPs/PANI Based Cryogels, Chin. J. Polym. Sci., 2022, 40(10), 1141–1153 CrossRef CAS
.
- P. Sarkar, K. Chatterjee and P. Pal,
et al., Exploring the molarity of Lithium Fluoride in minimally intensive layer delamination (MILD) method for efficient room temperature synthesis of high quality Ti3C2Tx free-standing film, Mater. Sci. Semicond. Process., 2025, 185, 108881 CrossRef CAS
.
- G. Cui, X. Sun and G. Zhang,
et al., Electromagnetic absorption performance of two-dimensional MXene Ti3C2Tx exfoliated by HCl + LiF etchant with diverse etching times, Mater. Lett., 2019, 252, 8–10 CrossRef CAS
.
- B. Li, D. Ji and A. K. Hamouda,
et al., MXene-derived TiO2 nanosheets/rGO heterostructures for superior sodium-ion storage, ChemPhysMater, 2025, 4(1), 48–55 CrossRef
.
- K. Chen, K. Yan and Q. Xie,
et al., MXene-derived C-doped TiO2/Ti3C2 heterojunction as a high-performance visible-light photocatalyst, Res. Chem. Intermed., 2022, 48(11), 4443–4458 CrossRef CAS
.
- J. Cai, Y. Peng and P. Hu,
et al., H2O2-assisted room temperature preparation of crystalline TiO2 and Ti3C2Tx-derived C-doped amorphous TiOx homojunction for photocatalytic degradation of tetracycline, Appl. Surf. Sci., 2025, 680, 161430 CrossRef CAS
.
- O. D. Saliu, M. Mamo and P. Ndungu,
et al., Starch built TiO2 nanoarchitecture with mixed anatase and rutile phase for high energy density supercapacitor electrode, J. Energy Storage, 2022, 49, 104155 CrossRef
.
- C. Zhao, J. Liu and B. Li,
et al., Multiscale Construction of Bifunctional Electrocatalysts for Long-Lifespan Rechargeable Zinc-Air Batteries, Adv. Funct. Mater., 2020, 30(36), 2003619 CrossRef CAS
.
- M. Song, K. Vijayarangamuthu and E. Han,
et al., Enhancement of photocatalytic disinfection of surface modified rutile TiO2 nanocatalyst, Korean J. Chem. Eng., 2016, 33(8), 2392–2395 CrossRef CAS
.
- E. M. Huseynov, R. R. Hakhiyeva and N. M. Mehdiyev, FTIR study of nanocrystalline titanium carbide (TiC) particles exposed to gamma radiation, Solid State Commun., 2024, 378, 115417 CrossRef CAS
.
- M. Li, X. Yan and M. Zhu,
et al., Theoretical investigation on the spectroscopic properties and catalytic activities of the Ti-Hydroperoxo intermediates in titanosilicate zeolites, Microporous Mesoporous Mater., 2020, 299, 110133 CrossRef CAS
.
- Y. Zhang, Y. Xin and J. Cao,
et al., Direct hydrothermal synthesis of Titanate Nanotubes with high selectivity for oxidative desulfurization, J. Porous Mater., 2023, 30(5), 1525–1532 CrossRef CAS
.
- J. Robertson and A. C. Ferrari, Interpretation of Raman spectra of disordered and amorphous carbon, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61(20), 14095–14107 CrossRef
.
- Z. Zhang, H. Fu and Z. Wang,
et al., Mixed-Dimensional Membranes with Double 0D@2D Structures Enable Efficient and Sustainable Water Treatment, Adv. Sustainable Syst., 2024, 8(7), 2300597 CrossRef CAS
.
- C. Peng, X. Yang and Y. Li,
et al., Hybrids of Two-Dimensional Ti3C2 and TiO2 Exposing {001} Facets toward Enhanced Photocatalytic Activity, ACS Appl. Mater. Interfaces, 2016, 8(9), 6051–6060 CrossRef CAS PubMed
.
- W. Gao, M. Wang and C. Ran,
et al., One-pot synthesis of Ag/r-GO/TiO2 nanocomposites with high solar absorption and enhanced anti-recombination in photocatalytic applications, Nanoscale, 2014, 6(10), 5498–5508 RSC
.
- C. Li, Z. Wei and Q. Lu,
et al., Photoelectrochemical and photo-Fenton mechanism of enhanced visible light-driven nanocatalyst synthesis of ZnFe2O4/BiOI, Environ. Sci. Pollut. Res., 2022, 29(23), 34930–34942 CrossRef CAS PubMed
.
- G. Jia, Y. Wang and X. Cui,
et al., Highly Carbon-Doped TiO2 Derived from MXene Boosting the Photocatalytic Hydrogen Evolution, ACS Sustain. Chem. Eng., 2018, 6(10), 13480–13486 CrossRef CAS
.
- B. Li, N. Wu and Y. Yang,
et al., Graphene Oxide-Assisted Multiple Cross-Linking of MXene for Large-Area, High-Strength, Oxidation-Resistant, and Multifunctional Films, Adv. Funct. Mater., 2023, 33(11), 2213357 CrossRef CAS
.
- X. Miao, Z. Li and S. Liu,
et al., Ionic bridging strengthened MXene/GO nanocomposite films with extraordinary mechanical and tribological properties, Appl. Surf. Sci., 2023, 625, 157181 CrossRef CAS
.
- B. Kordić, M. Kovačević and T. Sloboda,
et al., FT-IR and NIR spectroscopic investigation of hydrogen bonding in indole-ether systems, J. Mol. Struct., 2017, 1144, 159–165 CrossRef
.
- D. Xiang, K. Wang and F. Wang,
et al., Coagulopathy-independent injectable catechol-functionalized chitosan shape-memory material to treat non-compressible hemorrhage, Carbohydr. Polym., 2024, 346, 122648 CrossRef CAS PubMed
.
- D. Xiang, K. Wang and F. Wang,
et al., Coagulopathy-independent injectable catechol-functionalized chitosan shape-memory material to treat non-compressible hemorrhage, Carbohydr. Polym., 2024, 346, 122648 CrossRef CAS PubMed
.
- H. Ding, M. Zhang and Y. Liu,
et al., Synthesis of CS/Fe3O4/TiO2@MXene nanocomposite photocatalyst with excellent degradation and bacteriostatic properties by one-step hydrothermal method, Ceram. Int., 2024, 50(22B), 46334–46346 CrossRef CAS
.
- G. Tsai and W. Su, Antibacterial Activity of Shrimp Chitosan against Escherichia coli, J. Food Prot., 1999, 62(3), 239–243 CrossRef CAS PubMed
.
- M. Chandrasekaran, K. D. Kim and S. C. Chun, Antibacterial Activity of Chitosan Nanoparticles: A Review, Processes, 2020, 8(9), 1173 CrossRef CAS
.
- X. Gu, L. Hu and Z. Fu,
et al., Reactive TiO2 Nanoparticles Compatibilized PLLA/PBSU Blends: Fully Biodegradable Polymer Composites with Improved Physical, Antibacterial and Degradable Properties, Chin. J. Polym. Sci., 2021, 39(12), 1645–1656 CrossRef CAS
.
- M. Pelaez, N. T. Nolan and S. C. Pillai,
et al., A review on the visible light active titanium dioxide photocatalysts for environmental applications, Appl. Catal., B, 2012, 125, 331–349 CrossRef CAS
.
- Q. Guo, C. Zhou and Z. Ma,
et al., Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges, Adv. Mater., 2019, 31(50), 1901997 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5na00238a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |