Photoacoustic contrast agents: a review focusing on image-guided therapy
Xiao
Yang†
b,
Zeyu
Jiang†
b,
Jiayong
Dai
 *c,
Qinrui
Fu
*c,
Qinrui
Fu
 *b and
Shuhan
Pan
*a
*b and
Shuhan
Pan
*a
aDepartment of Emergency Medicine, The Affiliated Hospital of Qingdao University, Qingdao University, Qingdao, 266003, China. E-mail: panshe88@126.com
bInstitute of Chronic Disease, College of Medicine, Qingdao University, Qingdao 266021, China. E-mail: fuqinrui2022@qdu.edu.cn
cDepartment of Orthopaedic Surgery, Sir Run Run Shaw Hospital, Medical College of Zhejiang University, Hangzhou 310016, China. E-mail: daijy@zju.edu.cn
First published on 23rd April 2025
Abstract
Photoacoustic (PA) imaging is a burgeoning imaging modality that has a broad range of applications in the early diagnosis of cancer, detection of various diseases, and relevant scientific research. It is a non-invasive imaging modality that relies on the absorption coefficient of the imaging tissue and the injected PA-imaging contrast agent. Nevertheless, PA imaging exhibits weak imaging depth due to its exponentially decaying signal intensity with increasing tissue depth. To improve the depth and heighten the contrast of imaging, a series of PA contrast agents has been developed based on nanomaterials. In this review, we present a comprehensive overview of recent advancements in contrast agents for photoacoustic (PA) imaging, encompassing the emergence of first near-infrared region (NIR-I, 700–950 nm) PA contrast agents, second near-infrared region (NIR-II, 1000–1700 nm) PA contrast agents, and ratiometric PA contrast agents. Subsequently, the latest advances in PA image-guided cancer therapy were introduced, such as photothermal therapy (PTT), photodynamic therapy (PDT), sonodynamic therapy (SDT), and PTT-based synergistic therapy. Finally, the prospects of PA contrast agents and their biomedical applications were also discussed. This review provides a systematic summary of the development and utilization of the cutting-edge photoacoustic agents, which may inspire fresh thinking in the fabrication and application aspects of imaging agents.
1. Introduction
Photoacoustic (PA) imaging is a novel imaging modality that combines the advantages of ultrasound (US) imaging with the exceptional contrast of optical imaging and excellent spatial resolution.1–4 The light energy provided by the pulsed laser is converted into acoustic waves through thermoelastic expansion, and signal generation of PA imaging is achieved through the PA effect, which was proposed by Alexander Graham Bell in 1880.5 Due to its high sensitivity, high temporal resolution, and real-time monitoring, the use of PA imaging in studying organisms’ physiology and structure has gained tremendous research interest.6–8PA-imaging technology provides a unique advantage as the US wave signals obtained possess negligible scattering and dissipation in biological tissue, allowing for deeper tissue imaging compared to other optical imaging techniques.9–11 Nonetheless, the exponential decay of light intensity and the PA signal-to-noise ratio (SNR) with increasing tissue depth due to strong light absorption/scattering by the skin, blood, and tissue necessitates the development of suitable PA imaging contrast agents, which has become an immediate and significant challenge.12–14
Various nanomaterials can play a significant part in the early diagnosis, treatment, and monitoring of illnesses due to their particular physical and chemical features.15–17 With the acceleration of nanotechnology development, numerous nanomaterial-based contrast agents for PA imaging have been developed with high molar extinction coefficients, excellent photostability, and high biocompatibility.18,19 Diverse contrast agents for PA imaging, including the first near-infrared (NIR) region (NIR-I, 700–950 nm) PA contrast agents, the second NIR region (NIR-II, 1000–1700 nm) PA contrast agents, and ratiometric PA contrast agents, have been presented.20–22
Additionally, the integration of diagnosis and treatment into one nanocarrier is a hot topic of current research. Since theranostics integrates diagnostic and therapeutic functions, it has obvious advantages over a single diagnostic or therapeutic tool.23 Nanomaterials with integrated diagnosis and treatment functions can accurately diagnose the disease in real-time and treat it simultaneously, providing direct evidence for the early diagnosis, development, and progress of the disease and exhibiting broad clinical prospects.24,25
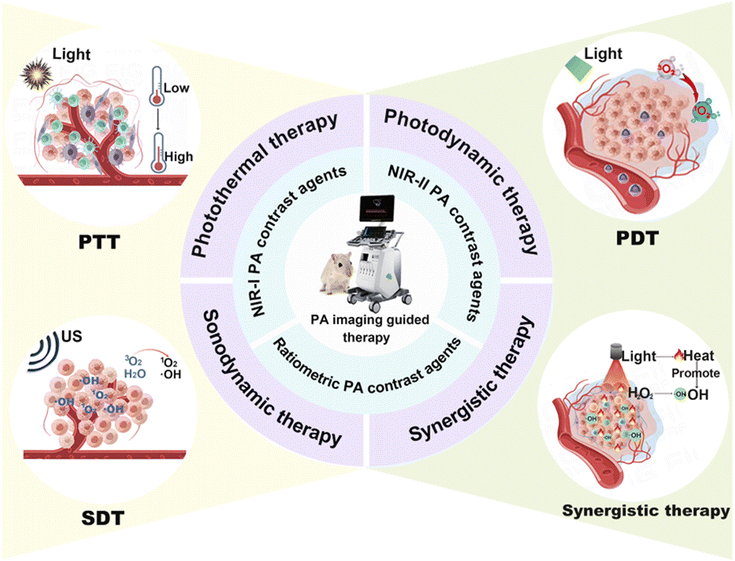
Despite the existence of numerous studies in the field of PA imaging, there is a dearth of comprehensive reviews that specifically focus on its application in guiding cancer treatment, as far as knowledge extends. Herein, this review systematically summarizes the PA contrast agents and their biomedical applications (Fig. 1). The usable PA contrast agents are comprehensively overviewed and classified into three different categories, NIR-I PA contrast agents, NIR-II PA contrast agents, and ratiometric PA contrast agents, based on their wavelengths and imaging capabilities for PA imaging. Additionally, attention has also been paid to their synthesis methods. Next, the latest advances in PA-imaging-guided cancer therapy are presented as well, which include photothermal therapy (PTT), photodynamic therapy (PDT), sonodynamic therapy (SDT), and PTT-based synergistic therapy. Lastly, the challenges and perspectives related to PA imaging and PA contrast agents’ development are discussed.
2. Photoacoustic imaging technology
2.1. Principle of photoacoustic imaging
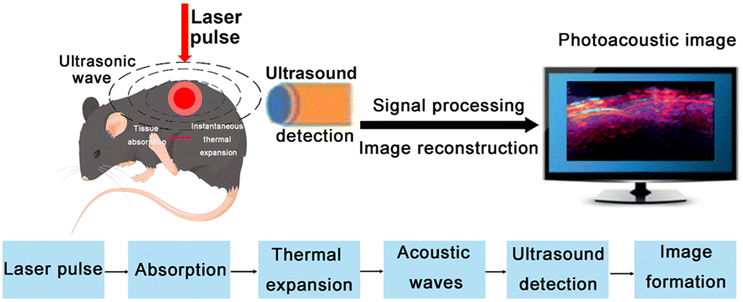
The PA effect was first reported by Alexander Graham Bell in 1880, and the noninvasive and nonionized biomedical PA imaging utilizing this effect was extensively exploited.5,26 However, it was not until Lloyd Barton Kreuzer introduced PA imaging to the domain of biological tissue imaging in the 1970s that PA imaging gradually attracted attention from the scientific community, and this set a solid stage for further molecular imaging and functional imaging applications.27 The rapid development of PA imaging in the 1990s sparked widespread interest in the biomedical field and offered great promise for future clinical applications.28,29 The PA effect occurs when biological tissues absorb the light energy from chromophores with light absorption properties under the excitation of a pulsed laser, causing transient thermoelastic expansion.30 Electrons in the excited state may jump to the ground state, releasing energy in the process, with partly transmitted into heat energy. The generated heat pressure is converted into the form of acoustic waves, producing US waves that are sensed by a US transducer on the surface of the tissue. These US waves are then processed to images, enabling the monitoring of the physiological and pathological conditions of biological tissues (Fig. 2).31–332.2. Main modes of PA imaging
PA-imaging technology is mainly comprised of photoacoustic microscopy (PAM) and photoacoustic tomography (PAT), both of which led to the further development of photoacoustic endoscopy (PAE).34–36 PAM can be further classified into optical-resolution PAM (OR-PAM) and acoustic-resolution PAM (AR-PAM), with the former providing sub-micron lateral resolution but limited imaging depth, while the latter provides less transverse resolution than OR-PAM but exhibits greater imaging depth.37–39 PAT is characterized by a reduced lateral resolution of several hundred microns due to lesser acoustic attenuation, and it has an imaging depth up to 6 cm.40,41 Another PA-imaging modality, PAE, is found more useful for gastrointestinal, digestive, and vascular examinations.42–44 The selection of each PA-imaging mode is dependent on the specific needs of the application, and the advantages and disadvantages in terms of imaging resolution and depth should be taken into consideration as well.3. PA contrast agents
Recently, employing PA-imaging technology for living biological tissue monitoring has been one of the most important research frontiers in new imaging technology development areas.45,46Although PA imaging is a promising biomedical imaging method, the lack of highly effective contrast agents drastically limited its further clinical application. For instance, some endogenous contrast agents, such as hemoglobin, chromophores, and lipids, have the ability to convert light energy into PA signals that carry characteristic information when exposed to laser irradiation. However, the endogenous contrast agents show considerable tissue background noise, limited tissue penetration ability, and restricted imaging site selection based on them.47–49 Exhilaratingly, the development of scientific instruments and imaging techniques in recent years enabled numerous exogenous contrast agents to generate enough PA signals in vivo or in specific pathological tissues, providing pathological information about diseases.50,51
It has been proved that NIR-II PA imaging (1000–1700 nm) is more suitable for improving the spatial resolution and penetration depth of in vivo imaging compared to NIR-I PA imaging (700–950 nm), as its longer wavelength reduces light scattering and minimizes thermal tissue damage.52–54 However, NIR-II PA imaging still faces significant challenges due to the absence of imaging contrast agents with NIR-II absorption and PA conversion capabilities. To address this challenge, this review article presents an overview of three categories of PA contrast agents that have been reported previously: (1) NIR-I PA contrast agents, (2) NIR-II PA contrast agents, and (3) ratiometric PA contrast agents (Table 1).
| Classification | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| NIR-I PA contrast agents | Improving PA-imaging contrast and resolution by locally enhancing tissue absorption performance compared with endogenous PA contrast agents | Limited tissue-penetration depth; the signal-to-noise ratio is relatively low | 55 and 56 |
| NIR-II PA contrast agents | Higher tissue-penetration depth; the scattering effect of biological tissue on photons is significantly inhibited | Potential toxicity; low quantum yields and lack of optimization of imaging systems | 57–60 |
| Ratiometric PA contrast agents | Built-in self-calibration, enabling more sensitive and reliable detection | Complex synthesis and fine molecular design | 61–63 |
3.1. NIR-I PA contrast agents
Gold nanomaterials are famous for their various nanostructures such as gold nanorods (AuNRs),66,67 gold nanospheres (AuNSp),68 gold nanostars (AuNSts),69 and gold nanocages (AuNCs),70 having been broadly used for biomedical applications such as PA-imaging contrast agents. One such example is that Cheng et al. prepared the AuNRs with a length of 40 ± 5 nm and a width of 8 ± 3 nm, which have near-infrared absorption at 800 nm and can be used as a contrast agent for PA imaging (Fig. 3A).71In vivo experiments conducted by Cheng and colleagues showed that this nanomaterial effectively targeted triple negative breast cancer sites and the process could be monitored through PA imaging. Changing the size of NPs, interparticle distance, etc., can adjust optical scattering and absorption, resulting in the enhancement of PA contrast.9 Li et al. reported poly(ethylene glycol) coated (PEGylated) hollow AuNSps with a size of 40–50 nm,72 whose optical absorption at 520 nm was shifted to 800 nm by adjusting the structure and size of AuNSps. These AuNSps have been verified to be highly efficient PA contrast agents for blood vessel imaging (Fig. 3B). AuNSts are another PA contrast agent with tunable LSPR in the visible-NIR range (Fig. 3C).73 Lu et al. designed long-chain amine/carboxy-terminated polyethylene glycol (PEG)-modified AuNSts with an LSPR band at 780 nm.74 These AuNSts have preeminent biocompatibility and can be used as a PA contrast agent. AuNCs are gold nanomaterials with hollow interiors and porous thin-walled structures that possess light absorption ability in the range of 600–1200 nm (Fig. 3D).75 Tao et al. constructed AuNC-based nanoagents for targeting lymph nodes (LNs), which can also act as a suitable contrast agent for PA imaging. Additionally, in vivo PA imaging allowed for the real-time visualization of AuNC build-up to track the immune process. Therefore, metallic and semimetallic nanomaterials, especially gold nanomaterials, have shown promising potential for use as contrast agents for PA imaging in biomedical applications.
 | ||
| Fig. 3 SEM and TEM images of different inorganic NIR-I PA contrast agents. (A) Nanorods, (B) nanospheres, (C) nanostars, (D) nanocages, (E) WS2-PEG, (F) CuS, (G) CuInS/ZnS, (H) CNTR@AuNPs, and (I) r-GO-AuNR. (A) Reproduced with permission.71 Copyright 2018, Wiley-VCH. (B) Reproduced with permission.72 Copyright 2010, Elsevier. (C) Reproduced with permission.73 Copyright 2012, IOP Publishing Ltd. (D) Reproduced with permission.75 Copyright 2008, American Chemical Society. (E) Reproduced with permission.76 Copyright 2014, Wiley-VCH. (F) Reproduced with permission.77 Copyright 2017, American Chemical Society. (G) Reproduced with permission.78 Copyright 2016, American Chemical Society. (H) Reproduced with permission.79 Copyright 2015, American Chemical Society. (I) Reproduced with permission.80 Copyright 2016, American Chemical Society. | ||
Transition metal chalcogenides are semiconductor nanocrystals with strong NIR absorption due to energy band transitions that have demonstrated great application potential in the fields of physics, chemistry, and materials science.81–83 Chalcogenides/MXene-based nanomaterials, such as copper sulfide (CuS), tungsten sulfide (WS2), and molybdenum sulfide (MoS2), are widely used in PA imaging as contrast agents, ascribed to their strong absorbance in the NIR region.84–86 For instance, Liu and colleagues fabricated a PEGylated WS2 nanosheet (WS2-PEG) with a strong absorbance in the NIR region (700–950 nm) for PA imaging (Fig. 3E).76 CuS has high photothermal conversion efficiency, low cytotoxicity, simple fabrication procedure, and is considerably cost-effective, thus becoming a contrast agent with high popularity for PA-imaging research (Fig. 3F).77 Additionally, the combination of two different transition metal compounds can also be used for PA imaging. Being well proved, CuInS/ZnS quantum dots (QDs) can achieve tumor site monitoring via PA imaging and meanwhile mediate photoinduced tumor ablation (Fig. 3G).78
Conversely, carbon nanomaterials, such as graphene-based nanomaterials and carbon nanotubes (CNTs), have been widely used as contrast agents in the biomedical field due to their superior photostability, biocompatibility, and ease of manufacture compared to gold nanomaterials.87–92 Carbon-based nanomaterials also have broad absorbance in the ultraviolet to NIR region, making them ideal candidates for PA imaging. For instance, Lim et al. prepared a carbon nanotube ring (CNTR)-based PA contrast agent (CNTR@AuNPs), whose PA intensity was about two orders of magnitude higher than that of CNTR due to the enhanced absorption of NIR light by the heterogeneous system, and the heat transfer resistance of gold nanoparticles to the surrounding signal generating medium was greatly reduced (Fig. 3H).79 In addition, Chen et al. designed a new type of rGO-coated AuNR (rGO-AuNR) PA contrast agent (Fig. 3I).80 Under laser irradiation, the interaction between the plasmonic AuNPs and the rGO led to an enhancement of the photocurrent of the rGO, thereby enhancing its photothermal properties and enabling strong NIR absorption and good PA-imaging capabilities.
Furthermore, quantum dots have garnered extensive attention due to their merits such as an ultrathin structure, high specific surface area, distinctive photoelectric characteristics, and favorable biological safety. Sun et al. fabricated titanium ligand (TiL4) coordinated black phosphorus quantum dots (BPQDs) as an efficient PA agent for cancer bioimaging.93 After the injection of TiL4@BPQDs, the PA signals in the tumor are conspicuously enhanced. The PA signal intensities in the tumor area are 21.49 ± 3.16 and 31.58 ± 2.42 a.u. At 4 h post-injection, robust PA signals as high as 120.80 ± 5.40 a.u. can be detected from the tumor.
Metal–organic frameworks (MOFs), composed of metal ions coordinated by organic linkers, have emerged as a promising category of functional materials and garnered extensive attention in numerous distinct areas including PA contrast agents. From the perspective of biomedicine, nanoscale MOFs with excellent biocompatibility, biodegradability, ultrahigh porosity, and tunable pore size are ideal nanocarriers for transporting chemotherapy drugs and photosensitizers. Zhou et al. developed a nanoscale porphyrin–palladium metal–organic framework (PdH-MOF) with highly dispersed Pd atoms serving as hydrogen carriers to effectively load highly reductive hydrogen for the tumor-targeted PA-imaging-guided hydrogenothermal therapy of cancer.94 After the intravenous injection of PdH-MOF nanoparticles for 1 h, an obvious PA signal emerged within the tumor region, and the signal intensity remained at a relatively high and stable level for 24 h, demonstrating great potential for the subsequent PA-imaging guidance of hydrogenothermal therapy.
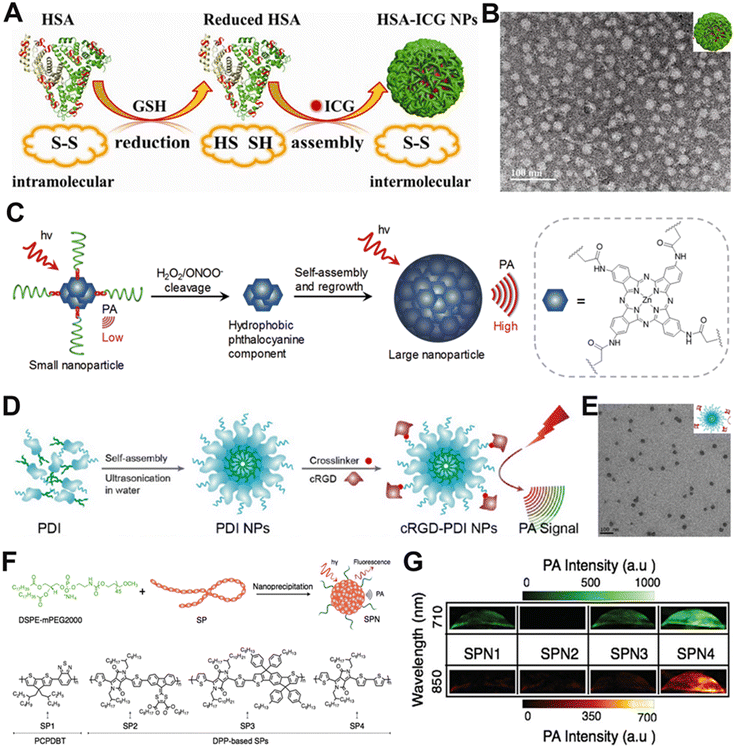 | ||
| Fig. 4 Schematic diagrams and representative characterization images of organic NIR-I PA contrast agents. (A) Schematic diagram for the preparation of HSA-ICG NPs. (B) TEM image of HSA-ICG NPs. (C) Schematic diagram of the self-assembled macromolecular probes (PCBP) for PA imaging. (D) Schematic diagram for the preparation of cRGD-PDI NPs. (E) TEM image of cRGD-PDI NPs. (F) Schematic illustration of the preparation of SPNs via nanoprecipitation. (G) PA images of SPNs in solution. (A and B) Reproduced with permission.98 Copyright 2014, American Chemical Society. (C) Reproduced with permission.100 Copyright 2017, Wiley-VCH. (D and E) Reproduced with permission.101 Copyright 2017, American Chemical Society. (F and G) Reproduced with permission.102 Copyright 2015, Wiley-VCH. | ||
Semiconducting polymers with conjugated structures have been developed for use as PA contrast agents. Compared to small molecule organic dyes, these semiconducting polymers have better photothermal stability and can be readily combined with other imaging modalities and therapeutic approaches.103–105 Formerly, Pu et al. reported a semiconductor macromolecular probe (PCBP), which had NIR-absorbing hydrophobic phthalocyanine and four hydrophilic PEG chains. The probe had a ROS-responsive linker, causing a significantly enhanced PA signal in the presence of ROS (Fig. 4C).100 Organic semiconductor NPs, such as perylene-diimide (PDI), are also favourable for PA imaging, benefiting from their high stability and excellent optoelectronic properties.106,107 Fan and his team designed cRGD-PDI NPs with an extinction coefficient of 2.58 × 108 M−1 cm−1, an organic semiconductor NP consisting of amphiphilic perylene-3,4,9,10-tetracarboxylic diimide molecules and cyclic Arg–Gly–Asp (cRGD) (Fig. 4D and E).101 These NPs were used for contrast-enhanced PA imaging of thrombus in living mice and produced a significant increase in PA intensity at the thrombus sites after intravenous injection.
Semiconductor polymer nanoparticles (SPNs) are a novel class of optically and electronically active nanomaterials consisting mainly of semiconductor polymers (SPs). SPNs have high absorption coefficients and controllable size,108,109 and are unique candidates for development as contrast agents for PA imaging. SPNs can expeditiously transform photonic energy into acoustic waves.110 For example, Pu et al. reported a series of low-bandgap diketopyrrolopyrrole-based SPNs for in vivo PA imaging (Fig. 4F).102 The differences in backbone structures among SPN1-4 lead to changes in the nature of the electron donor, which, in turn, affects the PA intensity variation. SPN4 showed the highest PA intensity, primarily due to the electron-donating ability of its corresponding structural unit (Fig. 4G), suggesting that SPN4 has great potential for in vivo tumor imaging.
3.2. NIR-II PA contrast agents
In contrast to PA imaging within the first near-infrared window (NIR-I, 700–1000 nm), PA imaging in the second near-infrared window (NIR-II, 1000–1700 nm) holds advantages such as lower background noise, deeper tissue penetration, and larger maximum permissible exposure, and has thus emerged as a research focus. Outperforming both the visible and NIR-I windows, the NIR-II window exhibits lower levels of tissue autofluorescence, facilitating high-quality bioimaging. Biological tissues possess varying degrees of light absorption and scattering capabilities, making it challenging for incident light to reach the depths of most tissues. Nevertheless, NIR-II light experiences significantly reduced scattering and absorption in biological tissues. The penetration depth of NIR-II light (5–20 mm) is notably deeper than that of visible light (≈1 mm) and NIR-I light (3.5 mm). Consequently, the NIR-II window offers superior light penetration, lower background signal, and higher sensitivity. More importantly, the NIR-II window, equipped with a longer wavelength excitation laser, possesses the advantages of superior tolerance of biological tissue. The maximum permissible exposure (MPE) in the NIR-II window is significantly higher than that in the NIR-I window, attributed to the lower tissue absorption and reduced energy of photons at longer wavelengths. Therefore, NIR-II light is more suitable for deep tissue imaging. Regarding applications, the MPE of 1064 and 808 nm lasers is 1 and 0.33 W cm−2, respectively. In conclusion, NIR-II light features less scattering, weak absorption, and high MPE, endowing PA imaging with high spatial resolution, deep tissue-penetration depth, and reduced background signal, which is conducive to expanding the applications of PA contrast agents in the biomedical field.The longer wavelength of NIR-I results in less absorption and scattering by biological tissues compared with visible light.111,112 As a result, NIR-I region PA-imaging contrast agents are suitable for imaging biological tissues like the skin and produce high-quality images. However, NIR-I region PA-imaging contrast agents have certain limitations, such as the presence of significant background noise that can adversely affect both imaging depth and contrast.55,113 In comparison to NIR-I PA contrast agents, NIR-II PA contrast agents demonstrate enhanced efficacy in mitigating the scattering and absorption of incident light by biological tissues.114 Thus, NIR-II PA contrast agents have become a current research hotspot since they can significantly improve imaging contrast.
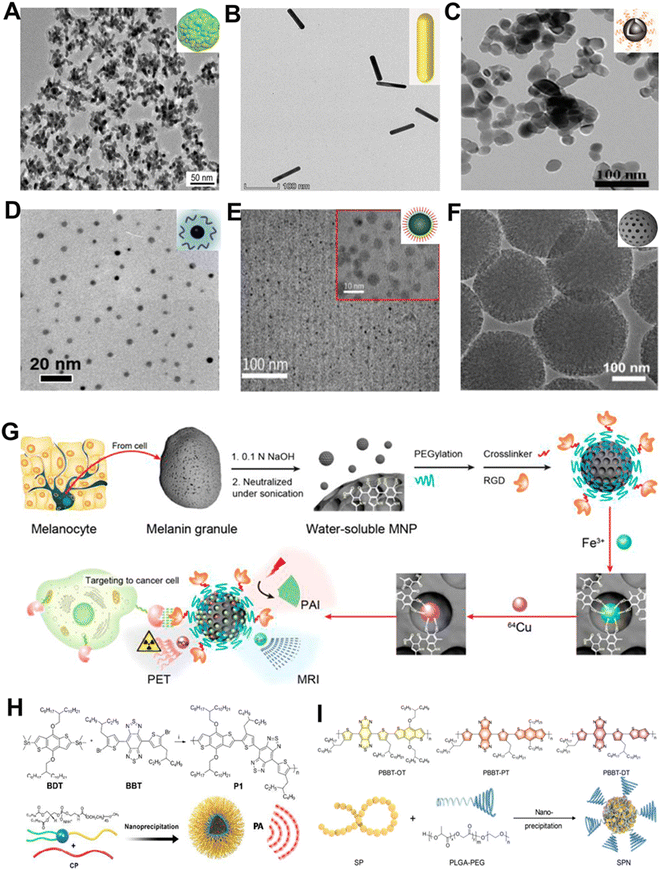 | ||
| Fig. 5 TEM images and schematic diagrams of different inorganic NIR-II PA contrast agents. (A) AuPBs, (B) AuNRs, (C) ZrO2-x-B@SiO2-HA, (D) Bi NPs, (E) CuS@BSA-RGD NPs, and (F) Meso-CNs. (G) Preparation process of MNPs and schematic diagram for multimodal imaging. (H) Schematic diagram of P1 formation. (I) Chemical structure of various NPs and preparation method of SPN. (A) Reproduced with permission.117 Copyright 2018, American Chemical Society. (B) Reproduced with permission.118 Copyright 2023, Wiley-VCH. (C) Reproduced with permission.119 Copyright 2020, Royal Society of Chemistry. (D) Reproduced with permission.120 Copyright 2017, American Chemical Society. (E) Reproduced with permission.121 Copyright 2018, Royal Society of Chemistry. (F) Reproduced with permission.122 Copyright 2018, Ivyspring. (G) Reproduced with permission.123 Copyright 2014, American Chemical Society. (H) Reproduced with permission.28 Copyright 2018, Wiley-VCH. (I) Reproduced with permission.124 Copyright 2019, Wiley-VCH. | ||
NIR-II PA contrast agents have outstanding potential for cancer diagnosis and treatment. Consequently, researchers have devoted a great amount of effort to exploring different types of contrast agents that can be used for PA-imaging-guided tumor-targeted phototherapy in the NIR-II window. Studies have shown that metallic element Zr and semimetallic element Bi are both effective PA contrast agents with low toxicity and few side effects. For instance, Shen et al. reported a ZrO2-based phototheranostic agent (ZrO2-x-B@SiO2-HA) that enables PA-imaging-guided tumor-targeted phototherapy in the NIR-II window (Fig. 5C).119 The ZrO2-x-B had a high NIR-II PA-imaging ability and photothermal conversion efficiency owing to its composition of oxygen vacancies and boron doping. Therefore, it had the potential to perform precise NIR-II radiation-activated PA-imaging-guided cancer treatment. For another example, Li and co-workers constructed ultrasmall semimetal NPs of bismuth (Bi-LyP-1 NPs) with high tumor aggregation (Fig. 5D).120 The Bi-LyP-1 NPs with brilliant photothermal conversion efficiency could be used for PA imaging and efficient treatment. The results not only indicated that Bi-based nanomaterials can also be used as contrast agents for PA imaging, but also demonstrated the great promise of semimetallic NPs in biomedical applications.
CuS NPs with high SNR are also an excellent PA contrast agent candidate due to their excellent NIR-II absorption property, low toxicity, and competent biodegradability.125–127 Zhang et al. designed CuS@BSA-RGD NPs by conjugating the ultrasmall CuS NPs with the cyclic arginine–glycine–aspartate (cRGD) peptide and extraordinary optical absorption in the NIR-II window could be achieved (Fig. 5E).121 After intravenous injection of the NPs, the area of hepatocellular carcinoma in situ in mice was visualized with highly sensitive PA images, indicating that CuS has great potential to be applied in cancer diagnosis and treatment.
In addition to metallic and semimetallic nanomaterials, as well as transition metal-based nanomaterials, carbon-based nanomaterials have also received much consideration in the biomedical field for their excellent optical properties.128,129 Wu et al. reported successful construction of mesoporous carbon nanospheres (Meso-CNs) with wideband and strong absorption in NIR-I and NIR-II windows (Fig. 5F).122 The Meso-CNs exhibited good photothermal conversion and PA signal generation ability and could potentially serve as a PA contrast agent for cancer diagnosis.
Additionally, conjugated polymers (CP) have been popularly used in PA-imaging-guided therapy as an attractive PA contrast agent, with superior optical features for photo-controlled drug delivery.130 Liu et al. synthesized a CP-based NIR-II PA contrast agent (P1) that showed good biocompatibility and superior PA stability in orthotopic brain tumor imaging (Fig. 5H).28 Furthermore, SPNs are a novel optical contrast agent with excellent photothermal conversion efficiency and photostability.131 Therefore, Pu and colleagues reported a kind of SPN-based metabolizable NIR-II nanoagent for in vivo biomarker detection and cancer therapy (Fig. 5I).124 The SPNs showed outstanding NIR-II PA-imaging performance for deep brain vasculature and subcutaneous tumors of live mice.
3.3. Ratiometric PA contrast agents
There are two acknowledged general design approaches for preparing ratiometric PA contrast agents. One method entails integrating a biomarker-insensitive signal as a reference signal and a biomarker-responsive sensing signal into a single probe. This approach eliminates the errors related to variations in the concentration of probes. The other method involves employing two biomarker-responsive reversible signal alterations to facilitate the measurement of ratiometry. Treatment with analytes leads to the specific increase or emergence of one signal, accompanied by the decrease or disappearance of the other, generating a significant ratio change between these two sensing signals. The ratiometric PA contrast agent offers a new option for preclinical disease diagnosis and has promising applications in exploring ClO− under pathological conditions. For instance, Song and colleagues synthesized an H2O2-responsive therapeutic nanoplatform, Ag shell coated Pd-tipped gold nanorods (Au–Pd@Ag NR), which was based on the LSPR effect of gold nanorods and can be used for in vivo detection of H2O2 (Fig. 6A and B).132 In the presence of H2O2, the Ag shell was etched to release Ag+, resulting in the increase and decrease of the PA signal of Au–Pd@Ag NR at 1260 and 700 nm, respectively, and a specific PA signal ratio was generated. These results verified that Au–Pd@Ag NR could be used as a ratiometric PA contrast agent for detecting H2O2 through PA imaging.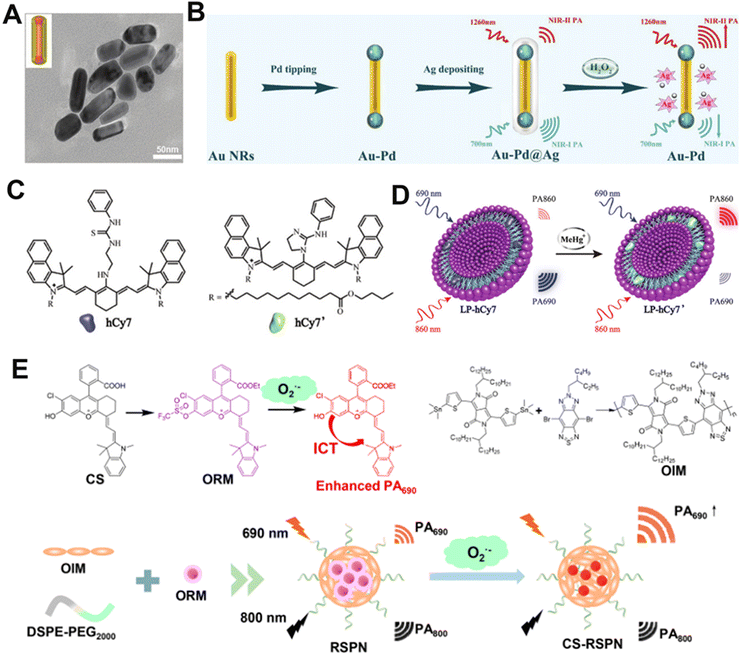 | ||
| Fig. 6 Representative ratiometric PA contrast agents. (A) TEM images of Au–Pd@Ag. (B) Proposed mechanism of Au–Pd@Ag for the detection of H2O2. (C) Chemical structures of hCy7 and hCy7′. (D) Proposed mechanism of LP-hCy7 for the detection of H2O2. (E) Chemical structure of the CS, ORM, OIM, and RSPN sensing mechanisms. (A) and (B) Reproduced with permission.132 Copyright 2020, Wiley-VCH. (C) and (D) Reproduced with permission.133 Copyright 2017, Wiley-VCH. (E) Reproduced with permission.134 Copyright 2021, American Chemical Society. | ||
Universally, dye-based nanomaterials have been used as one of the PA contrast agents because of their good biocompatibility and biodegradability.56 Huang et al. developed a PA sensor (LP-hCy7) consisting of liposomes (LP) and MeHg+-responsive NIR anabolic dye (hCy7) that exhibited high sensitivity and selectivity for MeHg+ (Fig. 6C).133 MeHg+ entered the lipid layer of LP-hCy7 and converted hCy7 to hCy7′ via a mercury-promoted cyclization reaction. The absorbance of the nanoprobe increased and decreased at 690 nm and 860 nm, respectively (Fig. 6D). Thus, the PA signal ratio (PA860/PA690) can be used as an indicator for MeHg+ detection in vitro and in vivo.
SPNs, being broadly explored as PA contrast agents for their good biocompatibility, can also be utilized as a ratiometric PA contrast agent.135 Zhang and co-workers synthesized a novel ratiometric semiconductor polymer nanoparticle (RSPN) (Fig. 6E), which provided a noninvasive tool for the diagnosis of atherosclerosis.134In vivo experiments showed that the PA signal ratio of the RSPN (PA690/PA800) was positively correlated with the level of oxidative stress in mice. These results proved that the RSPN enabled the accurate measurement of O2˙− levels in complex physiological environments.
4. PA-imaging-guided therapy
Imaging technologies provide the perfect medium for tracking nano-drugs and achieving personalized and specialized therapy results.24 PA-imaging-guided cancer treatment is set to be implemented to leverage the more precise localization data it provides.136 Traditional cancer treatment mainly involves surgical and non-surgical options.137,138 However, there are still limitations to the surgical treatments, such as high risk, low success rates, and the potential for postoperative complications.139 Non-surgical treatments such as radiotherapy and chemotherapy can result in the death of numerous normal cells and the disenabling of the body's immune system function.140 Additionally, they can inhibit the function of bone marrow hematopoietic stem cells and may lead to drug resistance in some people. In light of these limitations, emerging treatments are hoped to reduce the secondary effects caused by traditional treatments. In recent years, nanoagents based on molecular imaging and nanotechnology have been employed in emerging therapies such as PTT, PDT, SDT, and synergistic therapies (Table 2). These treatments provide an opportunity for minimally invasive tumor killing, optimize treatment protocols, and improve accuracy and efficiency.| Classification | Merits | Drawbacks | Advantages of PA image-guided therapies | Ref. |
|---|---|---|---|---|
| PTT | Oxygen independent; tumor tissue thermal ablation; spatiotemporal selectivity. | Heat-shock response; inadequate tissue-penetration of light results in an unsatisfactory therapeutic efficacy for deep tumors; potential for thermal damage to surrounding healthy tissues. | Provides rapid and efficient tumor ablation accompanied by real-time imaging monitoring. | 141–145 |
| PDT | High spatiotemporal precision; minimal damage to normal tissue; limited or no potential for resistance. | Limited light penetration; oxygen dependence and hypoxic in TME restrict treatment efficiency. | Provides real-time and high-resolution imaging of tumor oxygenation and photosensitizer distribution; facilitates precise treatment planning and monitoring of therapeutic response. | 146–150 |
| SDT | Compared with PDT, SDT has a deeper penetration depth; overcomes the oxygen dependence of PDT, rendering it effective for hypoxic tumors. | Lack of suitable sonosensitizers due to most sonosensitizers having low bioavailability. | Imaging information determines the starting time point of SDT. | 151–155 |
| Synergistic therapy | Improve the therapeutic effect; remove the limitations of using monotherapy. | The augmentation of complexity in treatment planning and optimization. | Imaging-guided synergistic therapy is beneficial for enhancing treatment efficiency. | 156–160 |
4.1. Photothermal therapy (PTT)
PTT, a highly effective and noninvasive therapy, has attracted increasing attention owing to its capacity to precisely target tumors and minimize damage to surrounding tissues.161–163 Under the irradiation of an external light source (usually NIR light), photothermal conversion nanomaterials harvested energy from light, which converts light energy into heat energy to ablate cancer cells.164,165 Recently, diverse types of nanotherapeutic agents have been extensively developed for PA-imaging-guided cancer therapy due to the easier entry and accumulation of nanomaterials in tumors. For example, Cheng et al. reported ultrasmall metal–organic coordination polymer nanodots Ru-Phen CPNs containing ruthenium ions (Ru3+) and phenanthroline (Phen) (Fig. 7A).166 The Ru3+/Phen loaded CPNs with high precision and effectiveness could be applied to highly effective PA-imaging-guided PTT elimination of tumors. The PA intensity was positively correlated with the concentration of Ru-Phen CPNs (0–200 μg mL−1) as shown in Fig. 7B. After intravenous administration, the contrast at the cancer cell site enhanced efficiently over time and reached a maximum at 12 h post-injection, suggesting a remarkable accumulation of Ru-Phen CPNs at the cancer cell site (Fig. 7C). PA-imaging results showed complete regression of tumors under PTT executed at 12 h post-injection. To generate hyperthermia to ablate tumor cells efficiently, the tumor site of tumor-bearing mice was irradiated under an 808 nm laser for 5 min, and the temperature increased to 60 °C after injection of Ru-Phen CPNs (Fig. 7D and E). The abovementioned results suggested that these Ru-Phen CPNs could well achieve the purpose of tumor ablation through PTT. Moreover, in vivo PA imaging could provide useful tumor information (such as tumor location and size information), especially the time-dependent tumor uptake of phototherapy nanoagents, so as to optimize the therapeutic plan.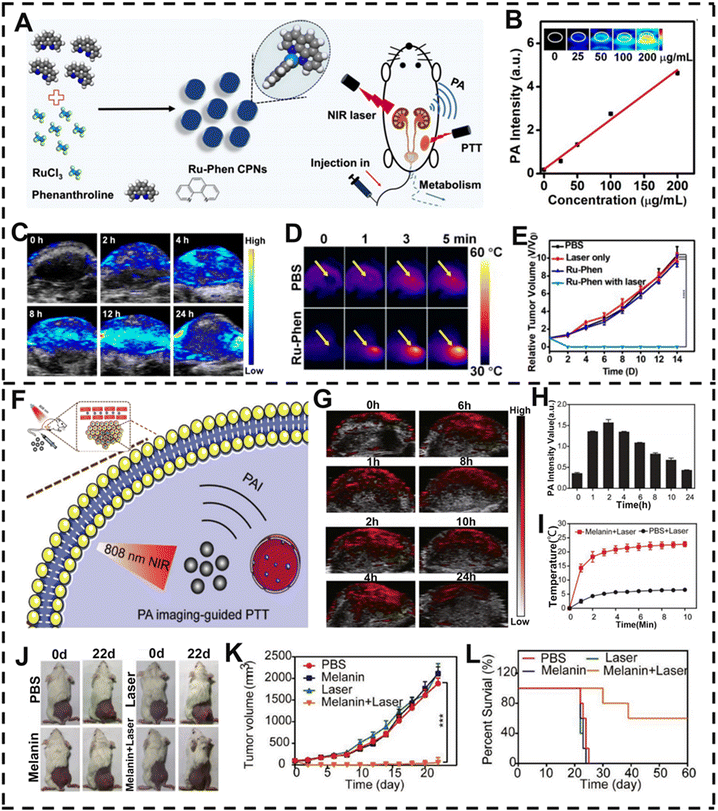 | ||
| Fig. 7 PA-imaging-guided PTT. (A) Synthesis pathway of Ru-Phen CPNs and schematic diagram of PTT therapy. (B) With the change of the concentration of Ru-Phen CPNs, the corresponding PA intensity changes. (C) PA images of tumors at various time points after intravenous administration of Ru-Phen CPNs in 4T1 tumor-bearing mice. (D) Infrared thermography of mice after intravenous injection of CPNs under 808 nm irradiation. (E) Changes of tumor volume in mice after various treatments. (F) Schematic diagram of biosynthetic melanin NPs for PA-imaging-guided PTT. (G) PA images of tumor areas in 4T1 tumor-bearing mice before and after intravenous administration of melanin NPs and (H) corresponding PA signal intensities at various time points. (I) The change of cancer tissue temperature with time after different treatments. (J) Images of tumor-bearing mice after different treatments and (K) corresponding tumor growth curves of 4T1 tumor-bearing mice. (L) Survival curve of 4T1 tumor-bearing mice. (A–E) Reproduced with permission.167 Copyright 2019, Ivyspring. (F–L) Reproduced with permission.168 Copyright 2022, Wiley-VCH. | ||
SPNs, which are also excellent PTT agents apart from their PA-imaging contrast agent property, are widely employed in PA-imaging-guided photothermal therapy.167,169 Lee et al. constructed a brand-new type of electron donor (5,5′-dibromo 4,4′-bis(2-octyldodecyl)-2,2′-dithiophene)–acceptor (5,6-difluoro-4,7-bis5-(trimethylstannyl)thiophen-2-ylbenzo-2,1,3-thiadiazole) conjugated SPN (PPorPEG NPs).170 The PA-imaging data showed that PPorPEG NPs accumulated efficiently at the tumor site, and reached a maximum signal intensity at 12 h post-injection, giving the optimal time to utilize their photothermal properties for PTT. Consequently, the tumors regressed completely due to the PA-imaging-guided PTT.
Personalized precision medicine using nanotherapeutic agents has attracted great interest and enthusiasm for its applications in the diagnosis of diseases. In addition, the use of melanin as a contrast agent in PA imaging is a significant development in the field of biomedicine.171 Yan et al. developed a melanin NP, which displayed high photothermal conversion efficiency (48.9%) and NIR region absorption for PA-imaging-guided PTT in vivo (Fig. 7F).172 Intravenous injection of melanin NPs followed by two hours of 808 nm laser irradiation resulted in excellent PA-imaging performance and PTT effects. As shown in Fig. 7G and H, the PA signal intensity of the cancer cell site was maximum after two hours of injection, indicating a maximum enrichment and the best time for PTT. The cancer cell region of the mouse was irradiated with an 808 nm laser at 2 h post-injection based on the location information provided by PA imaging. After 10 minutes of laser irradiation, the tumor temperature in the melanin + laser group was remarkably higher than that in the control group, and the cancer cell was entirely abated at 22 days after treatment (Fig. 7I–K). Moreover, the survival rate of mice improved significantly after intravenous injection of melanin NPs under NIR laser irradiation (Fig. 7L). Hence, melanin NPs offer us a new approach for developing phototherapeutic agents with excellent PA-imaging performance and PTT effect under 808 nm laser irradiation.
An ideal photothermal agent is expected to possess a high photothermal conversion effect, low biological toxicity, and degradability. The development of novel photothermal therapy agents featuring these properties is highly demanded. Guo et al. synthesized boron quantum dots (BQDs) with an ultrasmall hydrodynamic diameter for photoacoustic imaging-guided photothermal therapy. The BQDs demonstrate high photoacoustic imaging contrast performance due to their significant absorption in the near-infrared region. BQDs also exhibit good photothermal conversion capability, which can effectively convert near-infrared light into heat to eliminate cancer cells.173 Zhou et al. designed an activatable NIR-II plasmonic theranostic system founded on silica-encapsulated self-assembled gold nanochains (AuNCs@SiO2) for precise tumor diagnosis and efficacious treatment. This transformable chain configuration transcends the conventional molecular imaging window, and its absorption can be redshifted from the visible to the NIR-II region due to the fusion among adjacent gold nanoparticles within the restricted local space of AuNCs@SiO2, which is triggered by the elevated H2O2 level in the tumor microenvironment, resulting in the formation of a new string-like structure with robust NIR-II absorption. This is further confirmed by finite-difference-time-domain (FDTD) simulation. With the tumor microenvironment-activated transformable chain structure, AuNCs@SiO2 demonstrated outstanding properties for photoacoustic imaging and a high photothermal conversion efficiency of 82.2% at 1064 nm, leading to severe cell death and remarkable tumor growth inhibition in vivo.168
4.2. Photodynamic therapy (PDT)
PDT has emerged as a hopeful therapeutic strategy for the treatment of various malignancies and other disorders due to its spatiotemporal selectivity and noninvasive nature.174 The process involves the interaction of photosensitizers (PSs) and oxygen under light irradiation to produce ROS, which leads to tumor cell death.144,175 For instance, Yang and colleagues reported that iridium(III)-cyanine complex (IrCy) NPs (IrCy NPs) consisting of complex iridium(III) and a cyanine dye for 4T1 xenograft tumor treatment in vivo showed a maximum PA signal intensity at 24 h after injection (Fig. 8A and B),176 which was selected as the optimal time point for PDT treatment. When exposed to light irradiation, IrCy NPs generated a lot of 1O2, leading to apparent tumor cell necrosis and apoptosis (Fig. 8C). Based on PA-imaging information in the tumor region, IrCy NPs assisted by light irradiation effectively produced 1O2 to restrain the tumor volume, showing excellent tumor ablation ability (Fig. 8D).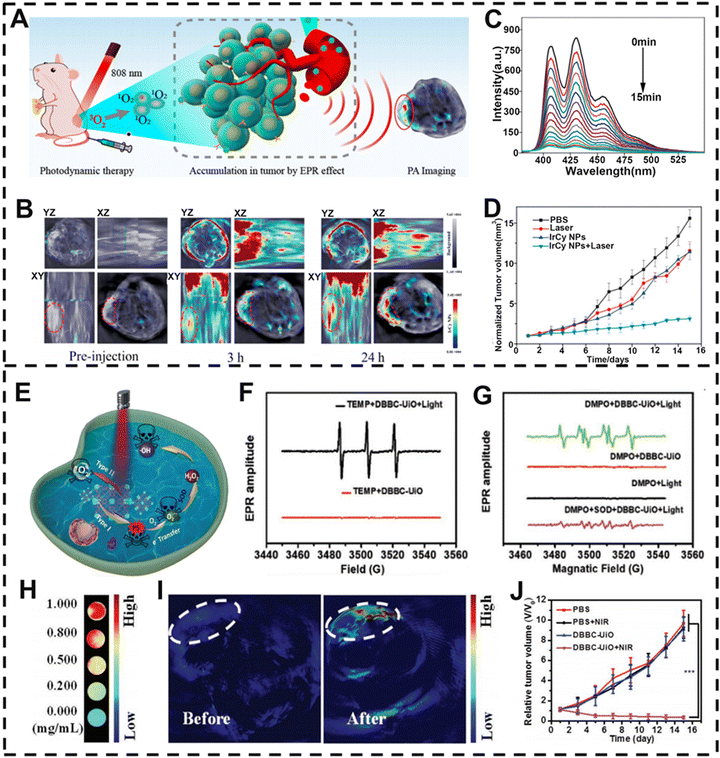 | ||
| Fig. 8 PA-imaging-guided PDT. (A) Schematic illustration of IrCy NPs for PA-imaging-guided PDT. (B) PA imaging in tumors at different time points before and after intravenous injection of IrCy NPs. (C) Fluorescence spectrum of ABDA changes in the presence of IrCy NPs under laser irradiation. (D) Changes in tumor volume with time after different treatments. (E) Proposed mechanism of DBBC-UiO for photoinduced PDT. (F) ESR spectra of TEMP for O2˙− detection. (G) ESR spectra of DMPO for 1O2 detection. (H) DBBC-UiO concentration is positively correlated with PA signal intensity. (I) Changes in PA imaging in vivo before and after DBBC-UiO injection. (J) Change curve of tumor volume in mice after 16 days of different treatments. (A–D) Reproduced with permission.176 Copyright 2019, Ivyspring. (E–J) Reproduced with permission.177 Copyright 2022, Wiley-VCH. | ||
However, one of the major characteristics of solid tumors in the tumor microenvironment (TME) is hypoxia, which can lead to unsatisfactory PDT outcomes.178,179 To address these drawbacks, Zhang et al. prepared a multifunctional metal–organic framework (MOF) nanosheet (DBBC-UiO) that could effectively alleviate the problem of hypoxia in the TME.177 DBBC-UiO acted as a photosensitizer (PS) to produce 1O2 under NIR light irradiation and could generate a large amount of O2˙− in the severe hypoxic microenvironment through the type I mechanism (Fig. 8E). Using 2,2,6,6-tetramethylpiperidine (TEMP) and 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) as 1O2 and O2˙− indicators, respectively, the ESR spectra showed good 1O2 and O2˙− generating ability of DBBC-UiO under light irradiation (Fig. 8F and G). After intravenous injection of DBBC-UiO, the PA signal intensity in the cancer tissue of mice was remarkably enhanced with the increasing concentration of the DBBC-UiO (Fig. 8H). Moreover, the peak value of the PA signal was reached at 12 h post-injection due to the accumulation of DBBC-UiO at the tumor site, which was considered as the optimal treatment time point (Fig. 8I). Moreover, given the PA-imaging result, the precise release of 1O2 and O2˙− and efficient reduction of tumor volume were accomplished by the DBBC-UiO + light treatment group (Fig. 8J), where the hypoxic environment was effectively mitigated and the tumor growth was obviously inhibited.
Boron dipyrromethene (Bodipy) dyes, as a type of representative organic small molecule, have been extensively utilized as fluorescent probes due to their high absorption coefficients and fluorescent quantum yields, low dark toxicity, and excellent photostability. Liu et al. developed an NIR excitable PS (Bodipy–Ir) with the PAI property by coupling a Bodipy derivative with Ir(III). Under 808 nm excitation, Bodipy–Ir could generate a considerable amount of singlet oxygen and exhibit photoacoustic properties. Bodipy–Ir NPs were intravenously administered into the A549 tumor-bearing mice via the tail vein. Two hours after injection, the PAI of the tumor was observed, and the signal increased over time, reaching a maximum at 12 h.180 Hypoxia, a prominent characteristic of hepatocellular carcinoma (HCC), undermines therapeutic outcomes, elevates recurrence rates, and promotes metastasis, particularly during PDT in clinical scenarios. Zeng et al. developed a biomimetic oxygen delivery system denoted as BLICP@O2, which employs hybrid tumor cell membranes and thermosensitive liposomes as oxygen carriers, incorporating the NIR-II dye IR1048, the photosensitizer Ce6, and perfluorohexane. Upon sequential irradiation at 1064 and 690 nm, BLICP@O2 demonstrates significant photothermal and photodynamic effects. Photothermal heating triggers oxygen release, enhancing the photodynamic effect of Ce6. Blood oxygen alterations during PDT are monitored by multispectral PA imaging. The enhanced PDT efficacy, mediated by hypoxia alleviation, is convincingly demonstrated both in vitro and in vivo. This work presents an imaging-guided, dual-wavelength programmed cascaded treatment strategy for tumor-targeted oxygen delivery and controlled release, with real-time efficacy surveillance using PA imaging, providing valuable insights for surmounting the challenges in PDT-based cancer therapy.181
4.3. Sonodynamic therapy (SDT)
Despite significant advances in PDT, its efficacy is still limited by the depth of light penetration and toxicity mediated by ROS. As a novel noninvasive therapy modality derived from PDT, SDT activates sonosensitizers through low-intensity ultrasound (US) to generate highly toxic ROS such as 1O2 and ˙OH, thereby inducing apoptosis and death of cancer cells.149,182,183 SDT overcomes the problems of shallow penetration and phototoxicity and has great advantages in deep tumor ablation, showing great clinical application prospects. For example, FA-HMME-MNPs-PLGA NPs (FHMP NPs) were successfully prepared by Li and co-workers.184 Melanin NPs (MNPs, PA contrast agent) and hematoporphyrin monomethyl ether (HMME, sonosensitizer) were encapsulated into NPs by poly(lactic-co-glycolic acid) (PLGA), and NPs were targeted to the tumor area through folate (FA), so as to achieve the purpose of PA-imaging-guided SDT (Fig. 9A). In vitro and in vivo studies were conducted to evaluate FHMP NPs as SDT sonosensitizers. The study showed that 1,3-diphenylisobenzofuran (DPBF) consumption can be used to monitor ROS production since the absorbance of DPBF at 410 nm is decreased when oxidized by ROS. Further study demonstrated that FHMP NPs produced a lot of ROS upon US irradiation (Fig. 9B), suggesting their potential as SDT sonosensitizers. In vitro PA-imaging studies revealed that the concentration of FHMP NPs was positively correlated with the intensity of the PA signal (Fig. 9C). In vivo PA imaging showed that FHMP NPs had maximal accumulation at the tumor site after 2 h of intravenous injection (Fig. 9D and E). Under the direction of PA imaging, FHMP NPs for SDT markedly reduced tumor volume after 15 days of treatment (Fig. 9F). The above results provided sufficient evidence that FHMP NPs can detect tumors as well as depict tumor regions by PA imaging. At the same time, under the action of US, FHMP NPs could generate a large amount of ROS, which realized the therapeutic effect on cancer cells and opened up a new way for tumor treatment.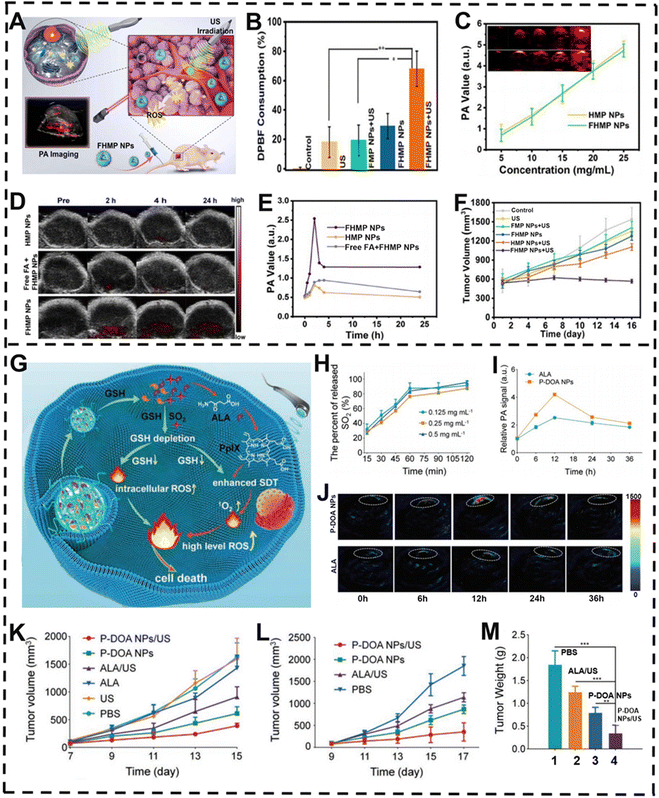 | ||
| Fig. 9 PA-imaging-guided SDT. (A) Schematic illustration of FHMP NPs for PA-imaging-guided SDT of cancer cells. (B) Changes in DPBT consumption under different treatments. (C) Relationship between PA signal intensity and different particle concentrations. (D) Changes in PA image of mouse tumor area with time after different treatments and (E) corresponding PA signal value. (F) Changes in tumor volume after different treatments. (G) Proposed mechanism of the nano-prodrug for PA imaging enhanced SDT against tumor cells. (H) Percentage of SO2 produced by different concentrations of P-DOA NPs. (I) PA images of tumors in mice at various time points after different treatments and (J) corresponding PA signal. (K) Changes in melanin volume in mice after different treatments. Changes in (L) tumor volume and (M) weight in squamous cell carcinoma mice after 17 days of different treatments. (A–F) Reproduced with permission.184 Copyright 2018, Ivyspring. (G–M) Reproduced with permission.185 Copyright 2022, Wiley-VCH. | ||
Reductive effect of the GSH content in the tumor region has become a primary problem that must be solved, as GSH can attenuate SDT-mediated ROS damage, which in turn reduces the efficacy of SDT. Inspired by this, Xiao et al. reported a nano-prodrug (P-DOA NPs) for PA-imaging-guided SDT of malignant melanoma and squamous cell carcinoma (SCC) (Fig. 9G).185 The nano-prodrug, consisting of dual prodrug molecules (DOA), was capable of releasing sulfide dioxide (SO2), 5-aminolevulinic acid (ALA), and methoxyl poly(ethylene glycol)-b-poly(L-lysine) (mPEG-b-PLL). In the TME, P-DOA NPs disintegrated and DOA could react with overexpressed GSH to release SO2 and ALA (Fig. 9H). On the one hand, ALA can be specifically transformed to protoporphyrin IX (PpIX) in the TME via the heme synthesis pathway in mitochondria for SDT and PA imaging. On the other hand, the release of SO2 and the consumption of GSH can dramatically enhance the intracellular ROS level in the tumor location, leading to enhanced SDT. After intravenous injection, P-DOA NPs efficiently accumulated at 12 h post-injection in tumor guided by PA imaging, which was selected as the optimal time point for SDT (Fig. 9I and J). Then, in vivo anti-tumor experiment results further proved that the P-DOA NPs + US treated group showed effectively suppressed tumor growth (Fig. 9K–M). Therefore, under the direction of real-time PA imaging, P-DOA NPs can achieve distinguished elimination of melanoma and SCC xenografts in mice, and ultimately realize the purpose of malignant cancer treatment.
SDT holds considerable promise as a therapeutic modality for treating atherosclerotic plaque. Nevertheless, the therapeutic efficacy of SDT is impeded by the limited tissue-penetration depth and the insufficient generation of reactive oxygen species (ROS) associated with conventional sonosensitizers. Moreover, determining the optimal timing for US irradiation subsequent to the administration of sonosensitizers poses a significant technical challenge. Addressing these issues is of paramount importance for enhancing the effectiveness of SDT in clinical applications. Fu et al. fabricated hyaluronic acid-modified US-propelled Janus mesoporous SiO2 partially coated gold nanorods loaded with 2,2-azobis[2-(2-imidazolin-2-yl)propane] dihydrochloride (AIPH), along with functionalized Ag/Ag2S nanoparticles (HA-JASAA), for NIR-II fluorescence imaging-guided SDT of atherosclerotic plaque.186 After intravenous administration of HA-JASAA, the hyaluronic acid modification enables specific targeting of proinflammatory macrophages within atherosclerotic plaques. Subsequently, upon reaction with H2O2 in the atherosclerotic microenvironment, the NIR-II fluorescence signal is activated. When the intensity of the NIR-II fluorescence signal reaches its peak, US irradiation is applied; the AIPH loaded in HA-JASAA is converted into nitrogen, propelling HA-JASAA to deeply penetrate into plaque tissue. Moreover, under US activation, two sonosensitizers, AIPH and Ag2S, respectively, generate oxygen-independent and oxygen-dependent ROS to induce the apoptosis of lesional macrophages, thereby significantly inhibiting the progression of atherosclerotic plaque. These results demonstrated the translational potential of HA-JASAA-mediated NIR-II fluorescence imaging-guided SDT for the treatment of atherosclerotic plaques.
4.4. Synergistic therapy
Due to its complexity, diversity, and heterogeneity, cancer is considered one of the deadliest diseases globally.187–189 While a variety of treatment methods, such as chemotherapy and radiotherapy, have been used in clinical practice, and clinical experiments, results have demonstrated that some cancer cells become resistant to monotherapy when present in heterogeneous tumor tissue, leading to unsatisfactory outcomes.190–192 Consequently, the current clinical treatment programs have achieved only limited success. To overcome the drawbacks of single therapy and achieve better therapeutic effects, the current trend of clinical research is the use of synergistic therapy, which involves the combination of two or more forms of treatment.193–195Phototherapy plays an important role in cancer treatment and includes PTT and PDT.196,197 However, the PSs used in PDT often have poor water solubility and photostability, and PTT hyperthermia can cause heat shock, causing unsatisfactory therapeutic efficacy and failure to achieve the intended treatment outcome.198,199 Facing these challenges, a growing number of researchers proposed that the combination of PTT and PDT using similar light-triggered conditions can significantly improve the therapeutic effect.200 Dong et al. demonstrated this synergistic effect by developing donor–acceptor–donor (D–A–D) structured NPs (DPP-TPA NPs), which take advantage of the thiophene group-containing diketopyrrolopyrrole (DPP) to enhance NIR-absorption and semiconducting functions, and also make use of triphenylamine (TPA) to improve bathochromic shift absorption and charge transport capacity by reprecipitation (Fig. 10A).201 Intriguingly, the DPP-TPA NPs exhibited enhanced PA-imaging ability and PA signal intensity (Fig. 10B), and demonstrated excellent 1O2 generation ability (ΦΔ = 33.6%) and photothermal conversion efficiency (η = 34.5%). After intravenous injection, the DPP-TPA NPs were found to accumulate at the tumor location, which was verified by PA-imaging information (Fig. 10C and D), enabling highly effective tumor therapy upon NIR light irradiation. The integration of PTT and PDT well reduced the tumor volume (Fig. 10E and F), illustrating the effectiveness, safety, and accuracy of the DPP-TPA NP platform in PA-imaging-guided phototherapy, along with the broad promise of DPP-TPA NPs for future use.
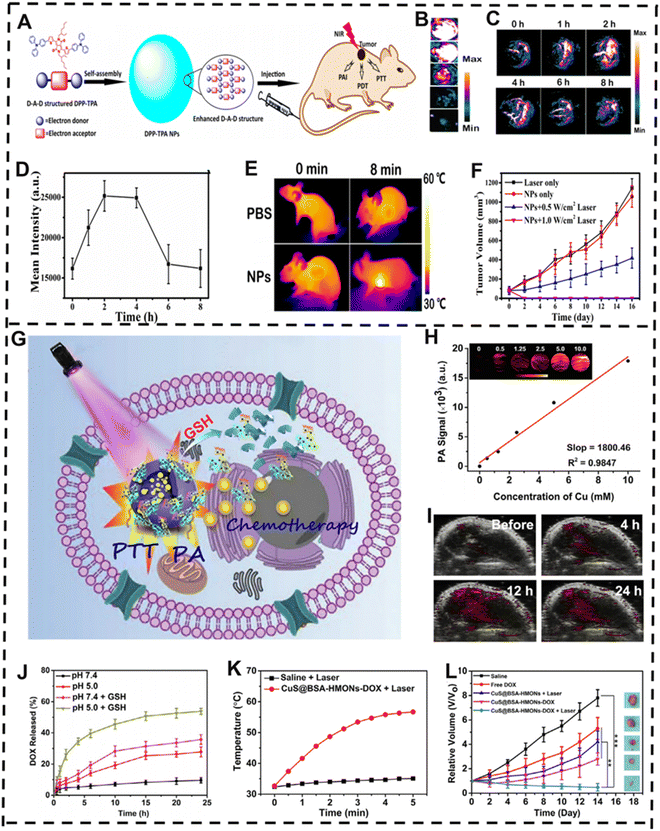 | ||
| Fig. 10 PA-imaging-guided synergistic therapy. (A) Schematic representation of DPP-TPA NP-guided PDT/PTT therapy via PA imaging. (B) PA image changes as the concentration of DPP-TPA NPs rises. (C) Following intravenous injection of DPP-TPA NPs, the PA images of tumor locations in tumor-bearing animals altered over time and (D) corresponding PA intensity. (E) Infrared thermal images of mice after different treatments. (F) Changes in tumor volume over time in different treatment groups. (G) Schematic diagram of CuS@BSA-HMONs-DOX in vivo PA-imaging-guided PTT/chemotherapy synergistic therapy. (H) Relationship between Cu concentration and PA signal intensity. (I) PA images of tumor-bearing animals taken before and after intravenous administration of CuS@BSA-HMONs-DOX NPs. (J) DOX release curves before and after GSH addition of CuS@BSA-HMONs-DOX at various pH levels. (K) Changes in tumor site temperature over time after intravenous administration of CuS@BSA-HMONs-DOX NPs. (L) Changes in tumor volume after different treatments. (A–F) Reproduced with permission.201 Copyright 2016, American Chemical Society. (G–L) Reproduced with permission.202 Copyright 2022, Elsevier. | ||
CDT represents an emerging anti-cancer strategy that has the ability to inhibit tumor growth and metastasis.203 Nonetheless, there are still limitations such as the high GSH content in the TME that leads to insufficient endogenous H2O2, and the unmatched optimal reaction pH of Fenton-like reaction with the TME, making it difficult to eliminate tumors.204,205 Consequently, the combination of CDT with other therapies has become a future direction in cancer treatment. It is noteworthy that Wei et al. developed a new kind of CoFeMn dichalcogenide nanosheet (CFMS NSs), which had excellent PA-imaging capability for guiding PTT/CDT synergistic therapy.206 CFMS NSs also exhibited brilliant photothermal properties, and the solutions’ temperature increased with the increase of material concentration under laser irradiation. Due to the conversion of overexpressed H2O2 to toxic ˙OH in tumor cells in the presence of Co and Fe and the reduction of intracellular GSH by Mn, cytotoxic ROS were massively produced in CFMS NS-treated tumor cells. Around 8 h after intravenous injection, the CFMS NSs reached maximal accumulation at the tumor site, as shown by in vivo PA imaging. Based on the PA-imaging results, the NIR and CFMS NS-treated group demonstrated notable tumor growth inhibition compared to other tested controls. This PA-imaging-guided-PTT/CDT synergistic therapy using CFMS NSs not only can significantly inhibit the proliferation of cancer cells, but also presents a new effective approach for cancer treatment.
In recent years, nanoplatforms for simultaneous cancer imaging and synergistic therapy have emerged as a new trend in cancer therapeutics.207 Even if chemotherapy has been widely used in many cancer therapeutic strategies, it also faces some drawbacks such as nonspecific distribution and rapid degradation of drugs,208,209 which may be eliminated by combining chemotherapy with other treatment modalities. For instance, studies have shown that when blood flow and oxygenation within the tumor were increased by mild hyperthermia treatments, tumor cells would become more sensitive to chemotherapy drugs, achieving better treatment effects.210 Gu and co-workers reported a biodegradable mesoporous organic silica NP (CuS@BSA-HMONs-DOX) based on biocompatible heterochemical (CuS@BSA) decorated mesoporous NPs for efficient PA-imaging-guided PTT/chemotherapy synergistic treatment of human osteosarcoma cancer (Fig. 10G).202 PA imaging showed that the PA signal was enhanced as the concentration of CuS@BSA-HMONs-DOX NPs increased (Fig. 10H). In particular, an acidic microenvironment will greatly increase the DOX release rate of CuS@BSA-HMONs-DOX NPs (Fig. 10J). Meanwhile, CuS@BSA-HMONs NPs have high photothermal conversion efficiency and could be used as a photothermal agent, which can obviously raise the temperature of the tumor site under 808 nm laser irradiation (Fig. 10K). This way, the PA signal of the tumor area was significantly brightened 4 h after the intravenous injection of CuS@BSA-HMONs-DOX NPs, reaching a maximum at 12 h after injection, which was clearly distinguishable from the surrounding normal tissues (Fig. 10I), indicating that CuS@BSA-HMONs-DOX NPs could accumulate in the tumor tissue via the enhanced permeability and retention (EPR) effect. In a Saos-2 tumor-bearing mouse model, the relative tumor volume has remarkably dwindled in the laser + CuS@BSA-HMONs NPs group when compared to the control group (Fig. 10L).
5. Conclusions
PA imaging plays a big part in early disease diagnosis due to its ability to provide real-time molecular information on the site of the disease and reflect the internal structure and function of the tissue. To achieve better imaging results, PA contrast agents have been developed as signal contrast enhancers that can significantly improve the resolution and contrast of PA imaging by changing the PA characteristics of local tissues. Multi-purpose nanomaterial PA contrast agents offer comprehensive diagnostic and therapeutic capabilities, which have led to their increasing use in the biomedical field. Various types of contrast agents, including NIR-I, NIR-II, and ratiometric PA contrast agents, have been developed and applied in tremendous biomedical research. The latest developments in PA image-guided therapy including PTT, PDT, SDT, and synergistic therapy opened up a brand-new area to achieve diagnosis and treatment integration.Despite the significant progress in disease diagnosis and treatment using PA contrast agents, considerable potential remains for further development. The development of PA contrast agents has ushered in a new era for the noninvasive diagnosis and treatment of diseases. By combining the advantages of different imaging technologies, PA-based multimodal contrast agents can non-invasively, in real-time, and specifically display complex biochemical processes in the body with high-resolution, providing more comprehensive and accurate information and achieving multimodal imaging-guided synergistic cancer treatment. Developing biomarker-targeted PA agents through specific biomarker optimization could enhance tumor precision while reducing nonspecific uptake by the reticuloendothelial system, particularly when utilizing TME biomarkers as responsive units. Endogenous biomarker-responsive PA agents show particular promise. Considering these developmental directions, attention must also be given to the biosafety, distribution, and biodegradability of PA contrast agents. Therefore, before clinical application, it is necessary to study the toxicity of PA contrast agents, improve their biocompatibility, and promote their clinical translation and regulatory approval.
In summary, with the extensive and systematic study of nanomaterials, we have reason to believe that PA contrast agents and PA-imaging-guided cancer therapy have broad application prospects in the biomedical field.
Author contributions
Xiao Yang: writing – original draft, review & editing; Zeyu jiang: writing – original draft; Jiayong Dai: supervision, writing – review & editing; Qinrui Fu: conceptualization, supervision, validation, visualization, writing – review & editing; Shuhan Pan: conceptualization, writing – review & editing.Data availability
No primary research results, software or code have been included, and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Taishan Scholar Youth Expert Program in Shandong Province (Grant Number: tsqnz20230608), Scientific Research of Distinguished Professor from Qingdao University, China (Grant Number: DC2200000953), grants from Natural Science Foundation of Shandong Province (Grant Number: ZR2023QB045), and grants from Natural Science Foundation of Qingdao Municipality, Shandong Province, China (Grant Number: 23-2-1-30-zyyd-jch).References
- Y. Jiang and K. Pu, Small, 2017, 13, 1700710 CrossRef.
- X. L. Deán-Ben, S. Gottschalk, B. Mc Larney, S. Shoham and D. Razansky, Chem. Soc. Rev., 2017, 46, 215 RSC.
- C. Wei, Z. Jiang, C. Li, P. Li and Q. Fu, Adv. Funct. Mater., 2023, 33, 2214655 CrossRef CAS.
- A. B. E. Attia, G. Balasundaram, M. Moothanchery, U. S. Dinish, R. Bi, V. Ntziachristos and M. Olivo, Photoacoustics, 2019, 16, 100144 CrossRef PubMed.
- A. G. Bell, Am. J. Sci., 1880, s3–20, 305 CrossRef.
- Y. Wang, K. Zhou, G. Huang, C. Hensley, X. Huang, X. Ma, T. Zhao, B. D. Sumer, R. J. DeBerardinis and J. Gao, Nat. Mater., 2014, 13, 204 CrossRef CAS PubMed.
- P. Zrazhevskiy and X. Gao, Nano Today, 2009, 4, 414 CrossRef CAS PubMed.
- Y. Xiao, J. Gateau, A. K. A. Silva, X. Shi, F. Gazeau, C. Mangeney and Y. Luo, View, 2021, 2, 20200176 CrossRef CAS.
- S. Wang, J. Lin, T. Wang, X. Chen and P. Huang, Theranostics, 2016, 6, 2394 CrossRef CAS PubMed.
- L. V. Wang, Nat. Photonics, 2009, 3, 50 CrossRef.
- X. Han, W. Fang, T. Zhang, X. Zhong, K. Qian, Z. Jiang, R. Hu, G. Shao, L. Zhang and Q. Zhang, J. Mater. Sci. Technol., 2022, 130, 208 CrossRef CAS.
- C. Kim, C. Favazza and L. V. Wang, Chem. Rev., 2010, 11, 2756 CrossRef PubMed.
- Y. I. Park, H. M. Kim, J. H. Kim, K. C. Moon, B. Yoo, K. T. Lee, N. Lee, Y. Choi, W. Park, D. Ling, K. Na, W. K. Moon, S. H. Choi, H. S. Park, S. Y. Yoon, Y. D. Suh, S. H. Lee and T. Hyeon, Adv. Mater., 2012, 24, 5755 CrossRef CAS.
- H. Wang, Z. Liu, S. Wang, C. Dong, X. Gong, P. Zhao and J. Chang, ACS Appl. Mater. Interfaces, 2014, 6, 3219 CrossRef CAS.
- A. Guerrero-Martínez, J. Pérez-Juste and L. M. Liz-Marzán, Adv. Mater., 2010, 22, 1182 CrossRef.
- P. Sondhi, M. H. U. Maruf and K. J. Stine, Biosensors, 2019, 10, 2 CrossRef.
- S. Wang, Y. Chen, S. Han, Y. Liu, J. Gao, Y. Huang, W. Sun, J. Wang, C. Wang and J. Zhao, Theranostics, 2022, 12, 388 Search PubMed.
- J. Weber, P. C. Beard and S. E. Bohndiek, Nat. Methods, 2016, 13, 639 CrossRef CAS PubMed.
- P. K. Upputuri and M. Pramanik, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2020, 12, e1618 Search PubMed.
- Z. Li, C. Zhang, X. Zhang, J. Sui, L. Jin, L. Lin, Q. Fu, H. Lin and J. Song, Bioconjugate Chem., 2022, 33, 67 CrossRef CAS PubMed.
- Y. Miao, C. Gu, B. Yu, Y. Zhu, W. Zou, Y. Shen and H. Cong, ChemBioChem, 2019, 20, 2793 CrossRef CAS PubMed.
- S. Yang, N. Li, H. Xiao, G. L. Wu, F. Liu, P. Qi, L. Tang, X. Tan and Q. Yang, Theranostics, 2022, 12, 7853 CrossRef CAS PubMed.
- Y. Ma, J. Huang, S. Song, H. Chen and Z. Zhang, Small, 2016, 12, 4936 CrossRef CAS PubMed.
- Z. Jiang, M. Zhang, P. Li, Y. Wang and Q. Fu, Theranostics, 2023, 13, 483 CrossRef CAS PubMed.
- S. C. Baetke, T. Lammers and F. Kiessling, Br. J. Radiol., 2015, 88, 2015020 Search PubMed.
- F. A. McDonald and G. C. Wetsel, J. Appl. Phys., 1978, 49, 2313 CrossRef CAS.
- L. B. Kreuzer and C. K. Patel, Science, 1971, 173, 45 CrossRef CAS PubMed.
- B. Guo, Z. Sheng, D. Hu, C. Liu, H. Zheng and B. Liu, Adv. Mater., 2018, 30, e1802591 CrossRef.
- C. Ou, W. Na, W. Ge, H. Huang, F. Gao, L. Zhong, Y. Zhao and X. Dong, Angew. Chem., Int. Ed., 2021, 60, 8157 CrossRef CAS PubMed.
- B. D. Musdal and M. Kurt, Photoacoustics, 2021, 21, 100214 CrossRef PubMed.
- J. Du, S. Yang, Y. Qiao, H. Lu and H. Dong, Biosens. Bioelectron., 2021, 191, 113478 CrossRef CAS PubMed.
- V. Ntziachristos and D. Razansky, Chem. Rev., 2010, 110, 2783 CrossRef CAS PubMed.
- Y. Zheng, M. Liu and L. Jiang, Front. Chem., 2022, 10, 1077937 CrossRef CAS PubMed.
- P. K. Upputuri and M. Pramanik, J. Biomed. Opt., 2017, 22, 41006 CrossRef PubMed.
- H. Zafar, M. Leahy, W. Wijns, M. Kolios, J. Zafar, N. Johnson and F. Sharif, Biomed. Phys. Eng. Express, 2018, 4, 032002 CrossRef.
- L. Li, H. C. Hsu, V. V. Verkhusha, L. V. Wang and D. M. Shcherbakova, Adv. Sci., 2021, 8, e2102474 CrossRef.
- M. Seong and S. L. Chen, Sci. China. Life Sci., 2020, 63, 179 Search PubMed.
- S.-L. Chen, L. J. Guo and X. Wang, Photoacoustics, 2015, 3, 143 CrossRef PubMed.
- Z. Zhang, H. Jin, Z. Zheng, A. Sharma, L. Wang, M. Pramanik and Y. Zheng, IEEE Trans. Med. Imaging, 2022, 41, 3636 Search PubMed.
- L. Lin and L. V. Wang, Nat. Rev. Clin. Oncol., 2022, 19, 365 CrossRef PubMed.
- L. V. Wang, Med. Phys., 2008, 35, 5758 CrossRef PubMed.
- Y. Qu, C. Li, J. Shi, R. Chen, S. Xu, H. Rafsanjani, K. Maslov, H. Krigman, L. Garvey, P. Hu, P. Zhao, K. Meyers, E. Diveley, S. Pizzella, L. Muench, N. Punyamurthy, N. Goldstein, O. Onwumere, M. Alisio, K. Meyenburg, J. Maynard, K. Helm, J. Slaughter, S. Barber, T. Burger, C. Kramer, J. Chubiz, M. Anderson, R. McCarthy, S. K. England, G. A. Macones, Q. Zhou, K. K. Shung, J. Zou, M. J. Stout, M. Tuuli and L. V. Wang, J. Biomed. Opt., 2018, 23, 1 Search PubMed.
- T. Zhao, A. E. Desjardins, S. Ourselin, T. Vercauteren and W. Xia, Photoacoustics, 2019, 16, 100146 CrossRef PubMed.
- Y. Li, G. Lu, J. J. Chen, J. C. Jing, T. Huo, R. Chen, L. Jiang, Q. Zhou and Z. Chen, Photoacoustics, 2019, 15, 100138 CrossRef PubMed.
- Q. Fu, R. Zhu, J. Song, H. Yang and X. Chen, Adv. Mater., 2019, 31, 1805875 CrossRef PubMed.
- X. Qin, H. Chen, H. Yang, H. Wu, X. Zhao, H. Wang, T. Chour, E. Neofytou, D. Ding, H. Daldrup-Link, S. C. Heilshorn, K. Li and J. C. Wu, Adv. Funct. Mater., 2018, 28, 201704939 Search PubMed.
- V. T. C. Tsang, X. Li and T. T. W. Wong, Sensors, 2020, 20, s20195595 CrossRef PubMed.
- P. Dey, I. Blakey and N. Stone, Chem. Sci., 2020, 11, 867 RSC.
- D. Wu, L. Huang, M. S. Jiang and H. Jiang, Int. J. Mol. Sci., 2014, 15, 23616 CrossRef PubMed.
- A. Farooq, S. Sabah, S. Dhou, N. Alsawaftah and G. Husseini, Nanomaterials, 2022, 12, 12030393 Search PubMed.
- T. Sowers and S. Emelianov, Phys. Med. Biol., 2018, 63, 22tr01 CrossRef CAS PubMed.
- X. Yang, C. Li, P. Li and Q. Fu, Theranostics, 2023, 13, 2632 CrossRef CAS PubMed.
- J. Chen, A. C. Sedgwick, S. Sen, Y. Ren, Q. Sun, C. Chau, J. F. Arambula, T. Sarma, L. Song, J. L. Sessler and C. Liu, Chem. Sci., 2021, 12, 9916 RSC.
- H. C. Zhou, J. Ren, Y. Lin, D. Gao, D. Hu, T. Yin, C. Qiu, X. Miao, C. Liu, X. Liu, H. Zheng, R. Zheng and Z. Sheng, J. Mater. Chem. B, 2021, 9, 3005 RSC.
- C. Yin, G. Wen, C. Liu, B. Yang, S. Lin, J. Huang, P. Zhao, S. H. D. Wong, K. Zhang, X. Chen, G. Li, X. Jiang, J. Huang, K. Pu, L. Wang and L. Bian, ACS Nano, 2018, 12, 12201 CrossRef CAS PubMed.
- Q. Fu, R. Zhu, J. Song, H. Yang and X. Chen, Adv. Mater., 2019, 31, e1805875 CrossRef PubMed.
- Y. Jiang, P. K. Upputuri, C. Xie, Y. Lyu, L. Zhang, Q. Xiong, M. Pramanik and K. Pu, Nano Lett., 2017, 17, 4964 CrossRef CAS PubMed.
- K. Homan, S. Kim, Y. S. Chen, B. Wang, S. Mallidi and S. Emelianov, Opt. Lett., 2010, 35, 2663 CrossRef PubMed.
- G. Hong, A. L. Antaris and H. Dai, Nat. Biomed. Eng., 2017, 1, 0010 CrossRef CAS.
- A. M. Smith, M. C. Mancini and S. Nie, Nat. Nanotechnol., 2009, 4, 710 CrossRef CAS PubMed.
- A. Choe, D. Qin, A. M. Yu, E. Chung, A. Jhunjhunwala, J. A. Rose and S. Y. Emelianov, Photoacoustics, 2023, 31, 100500 CrossRef PubMed.
- F. F. Faucher, K. J. Liu, E. D. Cosco, J. C. Widen, J. Sorger, M. Guerra and M. Bogyo, ACS Cent. Sci., 2023, 9, 1059 CrossRef CAS PubMed.
- W. Huang, R. Chen, Y. Peng, F. Duan, Y. Huang, W. Guo, X. Chen and L. Nie, ACS Nano, 2019, 13, 9561 CrossRef CAS.
- K. A. Homan, M. Souza, R. Truby, G. P. Luke, C. Green, E. Vreeland and S. Emelianov, ACS Nano, 2012, 6, 641 CrossRef CAS PubMed.
- X. Ge, Q. Fu, L. Bai, B. Chen, R. Wang, S. Gao and J. Song, New J. Chem., 2019, 43, 8835 RSC.
- P. Huang, L. Bao, C. Zhang, J. Lin, T. Luo, D. Yang, M. He, Z. Li, G. Gao, B. Gao, S. Fu and D. Cui, Biomaterials, 2011, 32, 9796 CrossRef CAS PubMed.
- J. Zhong, L. Wen, S. Yang, L. Xiang, Q. Chen and D. Xing, Nanomedicine, 2015, 11, 1499 CrossRef CAS PubMed.
- C. Bao, J. Conde, F. Pan, C. Li, C. Zhang, F. Tian, S. Liang, J. M. de la Fuente and D. Cui, Nano Res., 2016, 9, 1043 Search PubMed.
- S. Wang, P. Huang, L. Nie, R. Xing, D. Liu, Z. Wang, J. Lin, S. Chen, G. Niu, G. Lu and X. Chen, Adv. Mater., 2013, 25, 3055 Search PubMed.
- T. Sun, Y. Wang, Y. Wang, J. Xu, X. Zhao, S. Vangveravong, R. H. Mach and Y. Xia, Adv. Healthcare Mater., 2014, 3, 1283 CrossRef CAS PubMed.
- C. Xu, Q. Feng, H. Yang, G. Wang, L. Huang, Q. Bai, C. Zhang, Y. Wang, Y. Chen, Q. Cheng, M. Chen, Y. Han, Z. Yu, M. S. Lesniak and Y. Cheng, Adv. Sci., 2018, 5, 1800382 Search PubMed.
- W. Lu, Q. Huang, G. Ku, X. Wen, M. Zhou, D. Guzatov, P. Brecht, R. Su, A. Oraevsky, L. V. Wang and C. Li, Biomaterials, 2010, 31, 2617 Search PubMed.
- H. Yuan, C. G. Khoury, H. Hwang, C. M. Wilson, G. A. Grant and T. Vo-Dinh, Nanotechnology, 2012, 23, 075102 Search PubMed.
- S. Wang, Z. Teng, P. Huang, D. Liu, Y. Liu, Y. Tian, J. Sun, Y. Li, H. Ju, X. Chen and G. Lu, Small, 2015, 11, 1801 CrossRef CAS PubMed.
- S. E. Skrabalak, J. Chen, Y. Sun, X. Lu, L. Au, C. M. Cobley and Y. Xia, Acc. Chem. Res., 2008, 41, 1587 CrossRef CAS PubMed.
- L. Cheng, J. Liu, X. Gu, H. Gong, X. Shi, T. Liu, C. Wang, X. Wang, G. Liu, H. Xing, W. Bu, B. Sun and Z. Liu, Adv. Mater., 2014, 26, 1886 CrossRef CAS PubMed.
- G. Chen, B. Ma, Y. Wang, R. Xie, C. Li, K. Dou and S. Gong, ACS Appl. Mater. Interfaces, 2017, 9, 41700 CrossRef CAS PubMed.
- G. Lv, W. Guo, W. Zhang, T. Zhang, S. Li, S. Chen, A. S. Eltahan, D. Wang, Y. Wang, J. Zhang, P. C. Wang, J. Chang and X. J. Liang, ACS Nano, 2016, 10, 9637 CrossRef CAS PubMed.
- H. Moon, D. Kumar, H. Kim, C. Sim, J. H. Chang, J. M. Kim, H. Kim and D. K. Lim, ACS Nano, 2015, 9, 2711 CrossRef CAS PubMed.
- J. Song, F. Wang, X. Yang, B. Ning, M. G. Harp, S. H. Culp, S. Hu, P. Huang, L. Nie, J. Chen and X. Chen, J. Am. Chem. Soc., 2016, 13, 7005 CrossRef PubMed.
- X. Huang, Z. Zeng and H. Zhang, Chem. Soc. Rev., 2013, 42, 1934 RSC.
- H. Peng, K. Lai, D. Kong, S. Meister, Y. Chen, X. L. Qi, S. C. Zhang, Z. X. Shen and Y. Cui, Nat. Mater., 2010, 9, 225 CrossRef CAS PubMed.
- K. Yang, G. Yang, L. Chen, L. Cheng, L. Wang, C. Ge and Z. Liu, Biomaterials, 2015, 38, 1 Search PubMed.
- W. Yin, L. Yan, J. Yu, G. Tian, L. Zhou, X. Zheng, X. Zhang, Y. Yong, J. Li, Z. Gu and Y. Zhao, ACS Nano, 2014, 8, 6922 CrossRef CAS PubMed.
- J. Cui, R. Jiang, S. Xu, G. Hu and L. Wang, Small, 2015, 11, 4183 CrossRef CAS PubMed.
- C. M. Hessel, V. P. Pattani, M. Rasch, M. G. Panthani, B. Koo, J. W. Tunnell and B. A. Korgel, Nano Lett., 2011, 11, 2560 CrossRef CAS PubMed.
- L. Zhang, P. Rong, M. Chen, S. Gao and L. Zhu, Nanoscale, 2015, 7, 16204 RSC.
- J. Lin, X. Chen and P. Huang, Adv. Drug Delivery Rev., 2016, 105, 242 CrossRef CAS PubMed.
- J. Ge, Q. Jia, W. Liu, L. Guo, Q. Liu, M. Lan, H. Zhang, X. Meng and P. Wang, Adv. Mater., 2015, 27, 4169 CrossRef CAS.
- H. Gong, R. Peng and Z. Liu, Adv. Drug Delivery Rev., 2013, 65, 1951 CrossRef CAS PubMed.
- Y. Chen, C. Tan, H. Zhang and L. Wang, Chem. Soc. Rev., 2015, 44, 2681 RSC.
- A. De la Zerda, C. Zavaleta, S. Keren, S. Vaithilingam, S. Bodapati, Z. Liu, J. Levi, B. R. Smith, T. J. Ma, O. Oralkan, Z. Cheng, X. Chen, H. Dai, B. T. Khuri-Yakub and S. S. Gambhir, Nat. Nanotechnol., 2008, 3, 557 CrossRef CAS PubMed.
- Z. Sun, Y. Zhao, Z. Li, H. Cui, Y. Zhou, W. Li, W. Tao, H. Zhang, H. Wang, P. K. Chu and X. F. Yu, Small, 2017, 13, 1602896 CrossRef PubMed.
- G. Zhou, Y. Wang, Z. Jin, P. Zhao, H. Zhang, Y. Wen and Q. He, Nanoscale Horiz., 2019, 4, 1185–1193 RSC.
- E. Huynh, B. Y. Leung, B. L. Helfield, M. Shakiba, J. A. Gandier, C. S. Jin, E. R. Master, B. C. Wilson, D. E. Goertz and G. Zheng, Nat. Nanotechnol., 2015, 10, 325 CrossRef CAS PubMed.
- C. Yin, Y. Tang, X. Li, Z. Yang, J. Li, X. Li, W. Huang and Q. Fan, Small, 2018, 14, e1703400 CrossRef PubMed.
- R. J. Paproski, A. Heinmiller, K. Wachowicz and R. J. Zemp, Sci. Rep., 2014, 4, 5329 CrossRef CAS PubMed.
- J. F. Lovell, C. S. Jin, E. Huynh, H. Jin, C. Kim, J. L. Rubinstein, W. C. Chan, W. Cao, L. V. Wang and G. Zheng, Nat. Mater., 2011, 10, 324 CrossRef CAS PubMed.
- Z. Sheng, D. Hu, M. Zheng, P. Zhao, H. Liu, D. Gao, P. Gong, G. Gao, P. Zhang, Y. Ma and L. Cai, ACS Nano, 2014, 8, 12310 CrossRef CAS PubMed.
- C. Xie, X. Zhen, Y. Lyu and K. Pu, Adv. Mater., 2017, 29, 201703693 Search PubMed.
- C. Cui, Z. Yang, X. Hu, J. Wu, K. Shou, H. Ma, C. Jian, Y. Zhao, B. Qi, X. Hu, A. Yu and Q. Fan, ACS Nano, 2017, 11, 3298 CrossRef CAS PubMed.
- K. Pu, J. Mei, J. V. Jokerst, G. Hong, A. L. Antaris, N. Chattopadhyay, A. J. Shuhendler, T. Kurosawa, Y. Zhou, S. S. Gambhir, Z. Bao and J. Rao, Adv. Mater., 2015, 27, 5184 CrossRef CAS PubMed.
- I. B. Dimov, M. Moser, G. G. Malliaras and I. McCulloch, Chem. Rev., 2022, 122, 4356 CrossRef CAS PubMed.
- J. Yang, J. Choi, D. Bang, E. Kim, E. K. Lim, H. Park, J. S. Suh, K. Lee, K. H. Yoo, E. K. Kim, Y. M. Huh and S. Haam, Angew. Chem., Int. Ed., 2011, 50, 441 CrossRef CAS PubMed.
- J. Lin, M. Wang, H. Hu, X. Yang, B. Wen, Z. Wang, O. Jacobson, J. Song, G. Zhang, G. Niu, P. Huang and X. Chen, Adv. Mater., 2016, 28, 3273 CrossRef CAS PubMed.
- Q. Fan, K. Cheng, Z. Yang, R. Zhang, M. Yang, X. Hu, X. Ma, L. Bu, X. Lu, X. Xiong, W. Huang, H. Zhao and Z. Cheng, Adv. Mater., 2015, 27, 843 CrossRef CAS PubMed.
- Z. Yang, R. Tian, J. Wu, Q. Fan, B. C. Yung, G. Niu, O. Jacobson, Z. Wang, G. Liu, G. Yu, W. Huang, J. Song and X. Chen, ACS Nano, 2017, 11, 4247 CrossRef CAS PubMed.
- C. Wu and D. T. Chiu, Angew. Chem., Int. Ed., 2013, 52, 3086 CrossRef CAS PubMed.
- P. Howes, M. Green, J. Levitt, K. Suhling and M. Hughes, J. Am. Chem. Soc., 2010, 132, 3989 CrossRef CAS PubMed.
- K. Pu, A. J. Shuhendler, J. V. Jokerst, J. Mei, S. S. Gambhir, Z. Bao and J. Rao, Nat. Nanotechnol., 2014, 9, 233 CrossRef CAS PubMed.
- A. Sun, H. Guo, Q. Gan, L. Yang, Q. Liu and L. Xi, Opt. Express, 2020, 28, 9002 CrossRef PubMed.
- J. Cao, B. Zhu, K. Zheng, S. He, L. Meng, J. Song and H. Yang, Front. Bioeng. Biotechnol., 2019, 7, 487 CrossRef PubMed.
- S. Zhu, B. C. Yung, S. Chandra, G. Niu, A. L. Antaris and X. Chen, Theranostics, 2018, 8, 4141 CrossRef CAS PubMed.
- S. Jiang, J. Lin and P. Huang, Adv. Healthcare Mater., 2022, 11, e2202208 Search PubMed.
- J. N. Anker, W. P. Hall, O. Lyandres, N. C. Shah, J. Zhao and R. P. Van Duyne, Nat. Mater., 2008, 7, 442 CrossRef CAS PubMed.
- Y. Cui, K. H. Fung, J. Xu, H. Ma, Y. Jin, S. He and N. X. Fang, Nano Lett., 2012, 12, 1443 CrossRef CAS PubMed.
- J. Zhou, Y. Jiang, S. Hou, P. K. Upputuri, D. Wu, J. Li, P. Wang, X. Zhen, M. Pramanik, K. Pu and H. Duan, ACS Nano, 2018, 12, 2643 CrossRef CAS PubMed.
- Z. Li, S. Wang, J. Zhao, Y. Luo, H. Liang, S. Zhao and L. Zhang, Adv. Ther., 2023, 6, 2200350 CrossRef CAS.
- C. Zhu, Z. Ding, Z. Guo, X. Guo, A. Yang, Z. Li, B. P. Jiang and X. C. Shen, Biomater. Sci., 2020, 8, 6515 RSC.
- X. Yu, A. Li, C. Zhao, K. Yang, X. Chen and W. Li, ACS Nano, 2017, 11, 3990 CrossRef CAS PubMed.
- H. Yan, J. Chen, Y. Li, Y. Bai, Y. Wu, Z. Sheng, L. Song, C. Liu and H. Zhang, Biomater. Sci., 2018, 7, 92 RSC.
- L. Zhou, Y. Jing, Y. Liu, Z. Liu, D. Gao, H. Chen, W. Song, T. Wang, X. Fang, W. Qin, Z. Yuan, S. Dai, Z. A. Qiao and C. Wu, Theranostics, 2018, 8, 663 CrossRef CAS PubMed.
- Q. Fan, K. Cheng, X. Hu, X. Ma, R. Zhang, M. Yang, X. Lu, L. Xing, W. Huang, S. S. Gambhir and Z. Cheng, J. Am. Chem. Soc., 2014, 136, 15185 CrossRef CAS PubMed.
- Y. Jiang, P. K. Upputuri, C. Xie, Z. Zeng, A. Sharma, X. Zhen, J. Li, J. Huang, M. Pramanik and K. Pu, Adv. Mater., 2019, 31, e1808166 CrossRef PubMed.
- G. Ku, M. Zhou, S. Song, Q. Huang, J. Hazle and C. Li, ACS Nano, 2012, 6, 7489 CrossRef CAS PubMed.
- M. Zhou, J. Li, S. Liang, A. K. Sood, D. Liang and C. Li, ACS Nano, 2015, 9, 7085 CrossRef CAS PubMed.
- L. Guo, I. Panderi, D. D. Yan, K. Szulak, Y. Li, Y. T. Chen, H. Ma, D. B. Niesen, N. Seeram, A. Ahmed, B. Yan, D. Pantazatos and W. Lu, ACS Nano, 2013, 7, 8780 CrossRef CAS PubMed.
- C. Liu, Z. Wang, H. Jia and Z. Li, Chem. Commun., 2011, 47, 4661 RSC.
- H. Hong, Y. Zhang, J. W. Engle, T. R. Nayak, C. P. Theuer, R. J. Nickles, T. E. Barnhart and W. Cai, Biomaterials, 2012, 33, 4147 CrossRef CAS PubMed.
- Y. Lyu and K. Pu, Adv. Sci., 2017, 4, 160048 Search PubMed.
- J. Li and K. Pu, Chem. Soc. Rev., 2019, 48, 38 RSC.
- J. Ye, Z. Li, Q. Fu, Q. Li, X. Zhang, L. Su, H. Yang and J. Song, Adv. Funct. Mater., 2020, 30, 2001771 CrossRef CAS.
- Y. Liu, S. Wang, Y. Ma, J. Lin, H. Y. Wang, Y. Gu, X. Chen and P. Huang, Adv. Mater., 2017, 29, 201606129 Search PubMed.
- Y. Ma, L. Xu, B. Yin, J. Shang, F. Chen, J. Xu, Z. L. Song, B. Nan, G. Song and X. B. Zhang, Nano Lett., 2021, 21, 4484 CrossRef CAS PubMed.
- H. Zhu, Y. Fang, X. Zhen, N. Wei, Y. Gao, K. Q. Luo, C. Xu, H. Duan, D. Ding, P. Chen and K. Pu, Chem. Sci., 2016, 7, 5118 RSC.
- Y. Zhang, X. Zhang, H. Yang, L. Yu, Y. Xu, A. Sharma, P. Yin, X. Li, J. S. Kim and Y. Sun, Chem. Soc. Rev., 2021, 50, 11227 RSC.
- F. Zaina, C. Tomkins-Lane, E. Carragee, S. Negrini and D. B. Cochrane, Syst. Rev., 2016, 2016, Cd010264 Search PubMed.
- M. D. Redden, T. Y. Chin, M. L. van Driel and D. B. Cochrane, Syst. Rev., 2017, 3, Cd010651 Search PubMed.
- J. Kleeff, C. Stöβ, J. Mayerle, L. Stecher, M. Maak, P. Simon, U. Nitsche and H. Friess, Dtsch, Arztebl. Int., 2016, 113, 489 Search PubMed.
- M. F. Mulcahy, A. O. Wahl and W. Small, Jr., J. Natl. Compr. Canc. Netw., 2005, 3, 637 Search PubMed.
- Z. Liu, X. Zhao, B. Yu, N. Zhao, C. Zhang and F.-J. Xu, ACS Nano, 2021, 15, 7482 CrossRef CAS PubMed.
- Y. Wang, H.-M. Meng, G. Song, Z. Li and X.-B. Zhang, ACS Appl. Polym. Mater., 2020, 2, 4258 CrossRef CAS.
- Z. Jiang, C. Zhang, X. Wang, M. Yan, Z. Ling, Y. Chen and Z. Liu, Angew. Chem., Int. Ed., 2021, 60, 22376 CrossRef CAS PubMed.
- X. Huang, Y. Lu, M. Guo, S. Du and N. Han, Theranostics, 2021, 11, 7546 CrossRef CAS.
- W. Bian, Y. Wang, Z. Pan, N. Chen, X. Li, W.-L. Wong, X. Liu, Y. He, K. Zhang and Y.-J. Lu, ACS Appl. Nano Mater., 2021, 4, 11353 CrossRef CAS.
- J. Li, Z. Zhuang, Z. Zhao and B. Z. Tang, View, 2022, 3, 20200121 CrossRef CAS.
- X. Li, J. F. Lovell, J. Yoon and X. Chen, Nat. Rev. Clin. Oncol., 2020, 17, 657 CrossRef PubMed.
- Y. Lu and W. Wu, Adv. Ther., 2022, 5, 2200165 CrossRef CAS.
- M. Lan, S. Zhao, W. Liu, C.-S. Lee, W. Zhang and P. Wang, Adv. Healthcare Mater., 2019, 8, 1900132 CrossRef PubMed.
- N. Maeding, T. Verwanger and B. Krammer, Cancers, 2016, 8, 41 CrossRef PubMed.
- C. Xu and K. Pu, Chem. Soc. Rev., 2021, 50, 1111 RSC.
- J. Ouyang, Z. Tang, N. Farokhzad, N. Kong, N. Y. Kim, C. Feng, S. Blake, Y. Xiao, C. Liu, T. Xie and W. Tao, Nano Today, 2020, 35, 100949 CrossRef CAS.
- A. Bunevicius, S. Pikis, F. Padilla, F. Prada and J. Sheehan, J. Neurooncol., 2022, 156, 1 CrossRef PubMed.
- J. Guo, X. Pan, C. Wang and H. Liu, Bioconjugate Chem., 2022, 33, 993 CrossRef CAS PubMed.
- X. Pan, H. Wang, S. Wang, X. Sun, L. Wang, W. Wang, H. Shen and H. Liu, Sci. China: Life Sci., 2018, 61, 415 CrossRef PubMed.
- X. Meng, X. Zhang, M. Liu, B. Cai, N. He and Z. Wang, Appl. Mater. Today, 2020, 21, 100864 CrossRef.
- X. Lyu, J. Yu, L. Zhang, Y. Zhao, Z. Qiu, Y. Chen, Z. Zhao and B. Z. Tang, J. Mater. Chem. B, 2023, 11, 5953–5975 RSC.
- W. Fan, B. Yung, P. Huang and X. Chen, Chem. Rev., 2017, 117, 13566 CrossRef CAS.
- L. Zeng, K. Huang, Y. Wan, J. Zhang, X. Yao, C. Jiang, J. Lin and P. Huang, Sci. China Mater., 2020, 63, 611 CrossRef CAS.
- C. Lan and S. Zhao, J. Mater. Chem. B, 2018, 6, 6685 RSC.
- J. Beik, Z. Abed, F. S. Ghoreishi, S. Hosseini-Nami, S. Mehrzadi, A. Shakeri-Zadeh and S. K. Kamrava, J. Controlled Release, 2016, 235, 205 CrossRef CAS PubMed.
- J. Xu, X. Cheng, F. Chen, W. Li, X. Xiao, P. Lai, G. Xu, L. Xu and Y. Pan, J. Mater. Sci. Technol., 2021, 63, 97 CrossRef CAS.
- C. Yin, X. Lu, Q. Fan and W. Huang, View, 2021, 2, 20200070 CrossRef CAS.
- N. Fernandes, C. F. Rodrigues, A. F. Moreira and I. J. Correia, Biomater. Sci., 2020, 8, 2990 RSC.
- L. Cheng, C. Wang, L. Feng, K. Yang and Z. Liu, Chem. Rev., 2014, 114, 10869 CrossRef CAS PubMed.
- R. Zhang, X. Fan, Z. Meng, H. Lin, Q. Jin, F. Gong, Z. Dong, Y. Li, Q. Chen, Z. Liu and L. Cheng, Theranostics, 2019, 9, 8266 CrossRef CAS PubMed.
- Y. Lyu, Y. Fang, Q. Miao, X. Zhen, D. Ding and K. Pu, ACS Nano, 2016, 10, 4472 CrossRef CAS PubMed.
- C. Zhou, L. Zhang, T. Sun, Y. Zhang, Y. Liu, M. Gong, Z. Xu, M. Du, Y. Liu, G. Liu and D. Zhang, Adv. Mater., 2021, 33, 2006532 CrossRef CAS PubMed.
- X. Liang, Y. Li, X. Li, L. Jing, Z. Deng, X. Yue, C. Li and Z. Dai, Adv. Funct. Mater., 2015, 25, 1451 CrossRef CAS.
- J. Zhang, C. Yang, R. Zhang, R. Chen, Z. Zhang, W. Zhang, S. H. Peng, X. Chen, G. Liu, C. S. Hsu and C. S. Lee, Adv. Funct. Mater., 2017, 27, 1605094 CrossRef PubMed.
- K. Y. Ju, J. W. Lee, G. H. Im, S. Lee, J. Pyo, S. B. Park, J. H. Lee and J. K. Lee, Biomacromolecules, 2013, 14, 3491 CrossRef CAS PubMed.
- M. Fu, Y. Yang, Z. Zhang, Y. He, Y. Wang, C. Liu, X. Xu, J. Lin and F. Yan, Small, 2022, 18, e2205343 Search PubMed.
- T. Guo, Q. Tang, Y. Guo, H. Qiu, J. Dai, C. Xing, S. Zhuang and G. Huang, ACS Appl. Mater. Interfaces, 2021, 13(1), 306–311 CrossRef CAS PubMed.
- X. Li, S. Lee and J. Yoon, Chem. Soc. Rev., 2018, 47, 1174 RSC.
- D. Cui, J. Huang, X. Zhen, J. Li, Y. Jiang and K. Pu, Angew. Chem., Int. Ed., 2019, 58, 5920 CrossRef CAS PubMed.
- Q. Yang, H. Jin, Y. Gao, J. Lin, H. Yang and S. Yang, ACS Appl. Mater. Interfaces, 2019, 11, 15417 CrossRef CAS PubMed.
- K. Zhang, Z. Yu, X. Meng, W. Zhao, Z. Shi, Z. Yang, H. Dong and X. Zhang, Adv. Sci., 2019, 6, 1900530 CrossRef PubMed.
- W. Piao, K. Hanaoka, T. Fujisawa, S. Takeuchi, T. Komatsu, T. Ueno, T. Terai, T. Tahara, T. Nagano and Y. Urano, J. Am. Chem. Soc., 2017, 139, 13713 CrossRef CAS PubMed.
- Y. Y. Zhao, L. Zhang, Z. Chen, B. Y. Zheng, M. Ke, X. Li and J. D. Huang, J. Am. Chem. Soc., 2021, 143, 13980 CrossRef CAS PubMed.
- C. Liu, Y. Jiang, J. Xiang, C. Xiang, H. Li, H. Li, F. Wei, J. Huang, R. Li, K. M.-C. Wong and P. Gong, Dyes Pigm., 2023, 209, 110900 CrossRef CAS.
- S. Zeng, J. Chen, R. Gao, R. Chen, Q. Xue, Y. Ren, L. Liu, C. Tang, H. Hu, N. Zeng, S. Wen, H. Zhang, C. Liu and C. Fang, Adv. Mater., 2024, 36, 2308780 CrossRef CAS PubMed.
- P. Zhu, Y. Chen and J. Shi, ACS Nano, 2018, 12, 3780 CrossRef CAS PubMed.
- C. Dai, S. Zhang, Z. Liu, R. Wu and Y. Chen, ACS Nano, 2017, 11, 9467 CrossRef CAS PubMed.
- J. Huang, F. Liu, X. Han, L. Zhang, Z. Hu, Q. Jiang, Z. Wang, H. Ran, D. Wang and P. Li, Theranostics, 2018, 8, 6178 CrossRef CAS PubMed.
- S. Sun, D. Wang, R. Yin, P. Zhang, R. Jiang and C. Xiao, Small, 2022, 18, e2202558 CrossRef PubMed.
- Z. Jiang, C. Wei, S. Cai and Q. Fu, Adv. Funct. Mater., 2025, 35, 2503885 CrossRef.
- I. Dagogo-Jack and A. T. Shaw, Nat. Rev. Clin. Oncol., 2018, 15, 81 CrossRef CAS PubMed.
- Q. Jia, A. Wang, Y. Yuan, B. Zhu and H. Long, Exp. Hematol. Oncol., 2022, 11, 24 CrossRef CAS PubMed.
- W. Fan, B. Yung, P. Huang and X. Chen, Chem. Rev., 2017, 117, 13566 CrossRef CAS PubMed.
- M. Dean, T. Fojo and S. Bates, Nat. Rev. Cancer, 2005, 5, 275 CrossRef CAS PubMed.
- J. Gao, S.-S. Feng and Y. Guo, Nanomedicine, 2012, 7, 465 CrossRef CAS PubMed.
- D. Ling, W. Park, S.-J. Park, Y. Lu, K. S. Kim, M. J. Hackett, B. H. Kim, H. Yim, Y. S. Jeon, K. Na and T. Hyeon, J. Am. Chem. Soc., 2014, 136, 5647 CrossRef CAS.
- C. He, J. Lu and W. Lin, J. Controlled Release, 2015, 219, 224 CrossRef CAS PubMed.
- S. R. Lucena, N. Salazar, T. Gracia-Cazaña, A. Zamarrón, S. González, Á. Juarranz and Y. Gilaberte, Int. J. Mol. Sci., 2015, 16, 25912 CrossRef CAS PubMed.
- J. Huo, Q. Jia, H. Huang, J. Zhang, P. Li, X. Dong and W. Huang, Chem. Soc. Rev., 2021, 50, 8762 RSC.
- E. Boisselier and D. Astruc, Chem. Soc. Rev., 2009, 38, 1759 RSC.
- J. P. Celli, B. Q. Spring, I. Rizvi, C. L. Evans, K. S. Samkoe, S. Verma, B. W. Pogue and T. Hasan, Chem. Rev., 2010, 110, 2795 CrossRef CAS PubMed.
- S. S. Lucky, K. C. Soo and Y. Zhang, Chem. Rev., 2015, 115, 1990 CrossRef CAS PubMed.
- Y. Zhang, T. T. Shen, A. M. Kirillov, W. S. Liu and Y. Tang, Chem. Commun., 2016, 52, 7939 RSC.
- S. Fang, J. Lin, C. Li, P. Huang, W. Hou, C. Zhang, J. Liu, S. Huang, Y. Luo, W. Fan, D. Cui, Y. Xu and Z. Li, Small, 2017, 13, 1602580 CrossRef PubMed.
- Y. Cai, P. Liang, Q. Tang, X. Yang, W. Si, W. Huang, Q. Zhang and X. Dong, ACS Nano, 2017, 11, 1054 CrossRef CAS PubMed.
- D. Li, T. Zhang, C. Min, H. Huang, D. Tan and W. Gu, Chem. Eng. J., 2020, 388, 124253 CrossRef CAS.
- H. Ranji-Burachaloo, P. A. Gurr, D. E. Dunstan and G. G. Qiao, ACS Nano, 2018, 12, 11819 CrossRef CAS PubMed.
- C. Liu, D. Wang, S. Zhang, Y. Cheng, F. Yang, Y. Xing, T. Xu, H. Dong and X. Zhang, ACS Nano, 2019, 13, 4267 CrossRef CAS PubMed.
- N. Gong, X. Ma, X. Ye, Q. Zhou, X. Chen, X. Tan, S. Yao, S. Huo, T. Zhang, S. Chen, X. Teng, X. Hu, J. Yu, Y. Gan, H. Jiang, J. Li and X. J. Liang, Nat. Nanotechnol., 2019, 14, 379 CrossRef CAS PubMed.
- Y. Zhu, Y. Wang, G. R. Williams, L. Fu, J. Wu, H. Wang, R. Liang, X. Weng and M. Wei, Adv. Sci., 2020, 7, 2000272 CrossRef CAS PubMed.
- C. Ren, Z. Wang, X. Zhang, J. Gao, Y. Gao, Y. Zhang, J. Liu, C. Yang and J. Liu, Chem. Eng. J., 2021, 405, 127008 CrossRef CAS.
- Y. Tang, T. Lei, R. Manchanda, A. Nagesetti, A. Fernandez-Fernandez, S. Srinivasan and A. J. McGoron, Pharm. Res., 2010, 27, 224 CrossRef PubMed.
- P. T. Wong and S. K. Choi, Chem. Rev., 2015, 115, 3388 CrossRef CAS PubMed.
- B. K. Jung, Y. K. Lee, J. Hong, H. Ghandehari and C. O. Yun, ACS Nano, 2016, 10, 10533 Search PubMed.
Footnote |
| † Xiao Yang and Zeyu Jiang contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |