Spillover of active oxygen intermediates of binary RuO2/Nb2O5 nanowires for highly active and robust acidic oxygen evolution†
Linqing
Liao‡
ab,
Wangyan
Gou‡
c,
Mingkai
Zhang
d,
Xiaohe
Tan
ab,
Zening
Qi
e,
Min
Xie
e,
Yuanyuan
Ma
*ab and
Yongquan
Qu
 *ab
*ab
aResearch & Development Institute of Northwestern Polytechnical University in Shenzhen, Shenzhen, 518057, China. E-mail: yyma@nwpu.edu.cn; yongquan@nwpu.edu.cn
bKey Laboratory of Special Functional and Smart Polymer Materials of Ministry of Industry and Information Technology, School of Chemistry and Chemical Engineering, Northwestern Polytechnical University, Xi’an, 710072, China
cSchool of Materials Engineering, Xi’an Aeronautical University, Xi'an, 710077, China
dSchool of Science, Xi’an University of Technology, Xi’an, 710048, China
eXi'an Yiwei Putai Environmental Protection Co., Ltd, Xi’an, 710065, China
First published on 4th January 2025
Abstract
Over-oxidation of surface ruthenium active sites of RuOx-based electrocatalysts leads to the formation of soluble high-valent Ru species and subsequent structural collapse of electrocatalysts, which results in their low stability for the acidic oxygen evolution reaction (OER). Herein, a binary RuO2/Nb2O5 electrocatalyst with abundant and intimate interfaces has been rationally designed and synthesized to enhance its OER activity in acidic electrolyte, delivering a low overpotential of 179 mV at 10 mA cm−2, a small Tafel slope of 73 mV dec−1, and a stabilized catalytic durability over a period of 750 h. Extensive experiments have demonstrated that the spillover of active oxygen intermediates from RuO2 to Nb2O5 and the subsequent participation of lattice oxygen of Nb2O5 instead of RuO2 for the acidic OER suppressed the over-oxidation of surface ruthenium species and thereby improved the catalytic stability of the binary electrocatalysts.
New conceptsThe lattice oxygen mechanism (LOM) can break the scale relationship of Ru-based electrocatalysts for the acidic oxygen evolution reaction (OER) through the involvement of lattice oxygen, which can greatly enhance the activity. However, the generation of surface high-valent ruthenium species accompanied by the formation of oxygen vacancies leads to dissolution of surface Ru species and then decay of catalytic activity. Herein, we designed and synthesized a binary RuO2/Nb2O5 nanowire catalyst with intimate interfaces, which enabled the spillover of the reactive intermediate *O from RuO2 to Nb2O5 and thereby delivered significantly improved activity and durability for the acidic OER. Combination of various in situ and ex situ techniques demonstrated the occurrence of the spillover of active oxygen intermediates from RuO2 to Nb2O5 and the subsequent participation of lattice oxygen of Nb2O5 instead of RuO2 for the acidic OER, thereby suppressing the over-oxidation of surface ruthenium species. We anticipate that this work can be beneficial in stimulating the study of oxygen spillover at the interface of binary oxide catalysts for designing high performance OER electrocatalysts. |
Introduction
The rational design and synthesis of Ru-based acidic OER electrocatalysts with low overpotential and high durability are essential for the development of electrocatalytic hydrogen generation from water electrolysis in acidic media.1,2 Previous investigations have suggested that the surface Ru species of RuO2 tend to be over-oxidized during the acidic OER and generate the soluble high valent Ru species under external potentials of over 1.4 V.1,3,4 Especially, defective RuOx has been reported to deliver high catalytic activity for the acidic OER, which has been recognized to undergo a lattice oxygen mediated (LOM) pathway.3,5–7 Participation of lattice oxygen leads to the formation of oxygen vacancies and subsequent production of high valent Ru species, thereby unavoidably inducing the serious dissolution of surface electroactive high-valent Ru species and consequent collapse of the crystal structure of the highly defective RuOx-based electrocatalysts during the long-term operation. Thus, it's highly expected that new strategies to realize high performance Ru-based electrocatalysts for acidic OER can be developed.To address this challenge, various interfacial and structural engineering strategies have been developed to modulate the valence state of Ru and suppress the over-oxidation of surface Ru species, including the hybridization of Ru-based catalysts with other components, chemical doping, formation of solid solution, etc.8–11 Moreover, alternating between the LOM pathway of the defective RuOx-based electrocatalysts and adsorbate evolution mechanism (AEM) pathway or oxide path mechanism (OPM) also showed the potential to enhance the catalytic activity and durability of Ru-based catalysts for the acidic OER.3,8,12,13 However, the catalytic performances were still far below the expectations for the practical applications, and part of the above-mentioned methodologies improved the catalytic stability of Ru-based electrocatalysts at the expense of activity.14–16 Recently, the spillover of active oxygen intermediates has been proposed as a promising strategy to simultaneously enhance the activity and stability of IrOx-based OER electrocatalysts in acidic electrolyte.17 We recently reported the spillover of active oxygen intermediates from RuO2 to MoO3 in the binary RuO2/MoO3 electrocatalysts for the enhanced OER activity and stability in acidic electrolyte.18 In this approach, MoO3 with a low solubility in acidic media was integrated with RuO2, in which the active oxygen species was initially generated on the surface active sites of RuO2 and then spilled over towards MoO3 for the release of oxygen. The spillover of the active oxygen species from RuO2 to MoO3 for the subsequent release of oxygen can avoid the generation of oxygen vacancies on RuO2 and consequently suppresses the over-oxidation of surface Ru species, which theoretically improves the catalytic activity and stability. To date, only a few electrocatalysts have been reported to realize the simultaneously improved activity and stability for the acidic OER through the oxygen spillover strategy. To investigate the universal applicability of the oxygen spillover strategy for enhancing the acidic OER performance of RuO2, the exploration of new binary electrocatalysts is anticipated.
Herein, a binary electrocatalyst of ruthenium oxide and niobium oxide nanowire (RuO2/Nb2O5) was synthesized via a facile electrospinning/thermal treatment approach. RuO2/Nb2O5 delivered a remarkable low overpotential of 176 mV at a current density of 10 mA cm−2, a Tafel slope of 73 mV dec−1, and a mass activity of 296 A g−1, suggesting substantially superior catalytic performance in comparison to commercial RuO2. Furthermore, RuO2/Nb2O5 demonstrated an exceptional stability, enduring for at least 750 h in 0.5 M H2SO4. The S-numbers of RuO2/Nb2O5 (3.01 × 105) and C-RuO2 (1.07 × 103) indicated a significantly reduced dissolution rate of ruthenium in RuO2/Nb2O5. Extensive experiments demonstrated that the integration of Nb2O5 and RuO2 effectively inhibited the over-oxidation of surface Ru species and avoided the dissolution of Ru of the binary electrocatalysts for the acidic OER. Catalytic mechanism studies suggested the occurrence of spillover of the active oxygen species from RuO2 to Nb2O5 during the acidic OER. Such a binary electrocatalyst broke the dilemma between the activity and stability of Ru-based electrocatalysts by preserving lower oxidation states of surface Ru species for a long period in the acidic OER.
Synthesis and characterization of RuO2/Nb2O5
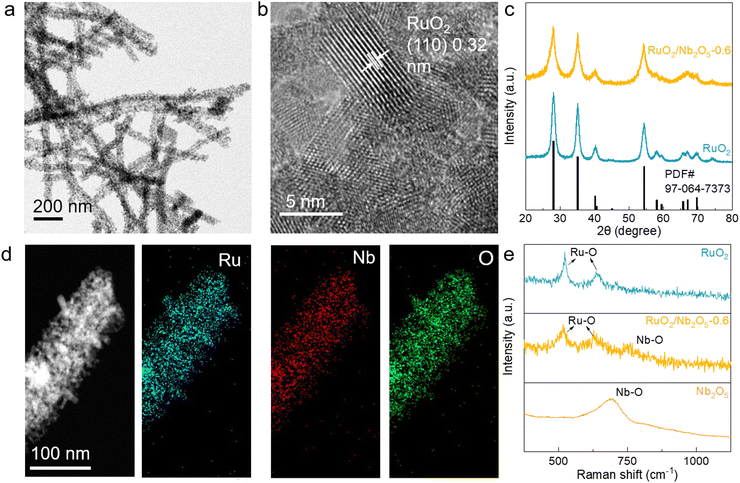
The synthetic process of the RuO2/Nb2O5 binary nanowire electrocatalysis involves electron-spinning and subsequent thermal treatment at various temperatures. The details can be found in the ESI.† Typically, the nanowire-like precursors were prepared by electro-spinning of a polyvinyl pyrrolidone (PVP) solution containing RuCl3 and NbCl5 in the desired molar ratios. Then, calcination under a flow of air removed the polymers and transformed the wire-like precursors into binary oxide catalysts.19Three RuO2/Nb2O5 electrocatalysts were synthesized with the Ru/(Ru + Nb) molar ratios of 0.7, 0.6, and 0.5, which were determined using inductively coupled plasma emission spectrometry (ICP-ES, Table S1, ESI†). The catalysts were named RuO2/Nb2O5-x, where x represented the molar ratio of Ru/(Ru + Nb). The transmission electron microscopy (TEM) image demonstrated the nanowire-like morphology of the binary RuO2/Nb2O5-0.6 electrocatalysts (treated at 400 °C) with an average diameter of ∼100 nm (Fig. 1a). The electrocatalysts were composed of small nanoparticles, indicating the highly accessible surface and largely exposed active sites for electrocatalysis. High-resolution TEM (HRTEM) images of RuO2/Nb2O5-0.6 revealed a crystal structure with a lattice fringe spacing of 0.32 nm, which corresponded to the (110) lattice plane of rutile phase RuO2 (Fig. 1b),20 while no lattice fringe information of Nb2O5 was found, which could be attributed to the poor crystalline structure and small size of Nb2O5 in the catalysts. Fig. 1c showed the X-ray diffraction (XRD) patterns of RuO2/Nb2O5-0.6 and RuO2. RuO2 was synthesized through the identical approach of RuO2/Nb2O5 in the absence of the Nb precursor (Fig. S1, ESI†). The characteristic peaks of RuO2 and RuO2/Nb2O5 electrocatalysts matched well with the database profile of RuO2 (rutile phase, PDF#: 97-064-7373), suggesting the formation of rutile phase RuO2 in the catalysts.21 The absence of characteristic XRD peaks of Nb2O5 further indicated the poorly crystallized and/or small Nb2O5 particles in the electrocatalysts. Energy-dispersive X-ray spectroscopy (EDS) elemental mapping images suggested the uniform distribution of Ru, Nb and O in a single nanowire of RuO2/Nb2O5-0.6, demonstrating the presence of Nb in the electrocatalyst (Fig. 1d). As shown in Table S2 (ESI†), the atomic and weight ratios of Ru/(Ru + Nb) in RuO2/Nb2O5-0.6 derived from EDS elemental mapping images were 0.63 and 0.65, respectively, which were close to the results of ICP (0.59 and 0.62). Similar EDS and ICP results further indicated that the distribution of elements within the catalyst was uniform. To further determine the formation of a binary structure of the electrocatalyst, Raman spectra of RuO2, Nb2O5 and RuO2/Nb2O5-0.6 were recorded and analyzed. In the Raman spectrum of RuO2/Nb2O5-0.6, the characteristic peaks at 517 and 624 cm−1 corresponded to the Eg and A1g vibrational modes of RuO2, respectively (Fig. 1e).22 The broad peak surrounding at 748 cm−1 was attributed to the Nb–O vibrational peak of Nb2O5. In comparison with the characteristic peak of highly crystalline commercial Nb2O5 appearing at 693 cm−1, the blueshift and broadening of the characteristic peak of the RuO2/Nb2O5-0.6 catalysts by nearly 50 cm−1 suggested the poor crystalline and small size of Nb2O5 in the binary electrocatalysts.23
Catalytic performance
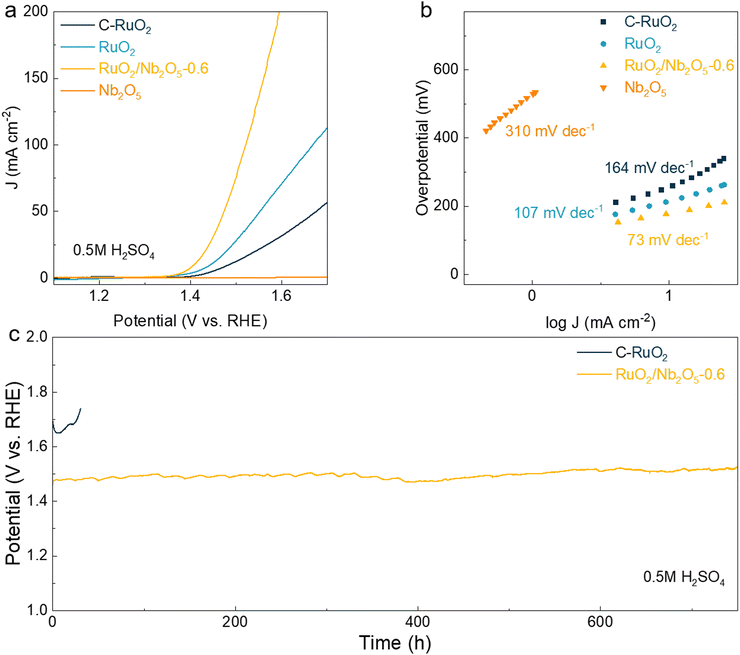
Initially, the catalytic performance of a series of RuO2/Nb2O5 electrocatalysts was evaluated in a three-electrode electrolytic cell with 0.5 M H2SO4 as the electrolyte, in which graphite rod and Hg/Hg2Cl2/KCl electrode were employed as the counter and reference electrodes, respectively. All electrode potentials were calibrated and normalized to the reversible hydrogen electrode (RHE). After calcination under different temperatures, various RuO2/Nb2O5 electrocatalysts were synthesized, exhibiting similar morphological features and the same crystal phase (Fig. 1 and Fig. S2–S5, ESI†). Afterwards, the catalytic performance of the prepared catalysts was analyzed to screen out the optimal composition and treatment temperature. As shown in Fig. S6 and S7 (ESI†), the RuO2/Nb2O5-0.6 electrocatalyst treated at 400 °C delivered the best OER activity with the lowest overpotential of 179 mV at 10 mA cm−2 and the smallest Tafel slope of 73 mV dec−1, which was identified as the optimal electrocatalyst in this work. Then, RuO2, the commercially available Nb2O5 and RuO2 (C-RuO2) catalysts, as well as the electron-spun RuO2 electrocatalysts synthesized through the identical process of RuO2/Nb2O5-0.6 were selected as the control catalysts to highlight the importance and roles of Nb2O5 in improving catalytic performance of RuO2/Nb2O5-0.6. As shown in their linear voltammetry scanning (LSV) curves (Fig. 2a), the bare OER activity of Nb2O5 was observed, indicating the catalytic inertness of Nb2O5. Comparatively, the RuO2/Nb2O5-0.6 catalysts delivered the lowest overpotential of 179 mV at a current density of 10 mA cm−2, which was much lower than those of RuO2 (215 mV) and C-RuO2 (256 mV). The catalytic current density of RuO2/Nb2O5-0.6 at 1.5 V was 75.2 mA cm−2, which was 2.7 times higher than that of the electron-spun RuO2 electrocatalysts (27.9 mA cm−2) and 6.1 times higher than that of the commercial C-RuO2 electrocatalysts (12.3 mA cm−2). The reaction kinetics were further evaluated by the derived Tafel slopes. The Tafel slope of RuO2/Nb2O5-0.6 (73 mV dec−1) was significantly lower than that of RuO2 (107 mV dec−1) and C-RuO2 (164 mV dec−1), indicating that the combination of ruthenium oxide and niobium oxide significantly enhanced the OER kinetics of the binary electrocatalysts (Fig. 2b).In order to explore the intrinsic activity of the catalysts, the electrochemically active surface area (ECSA) was calculated from the electrochemical double layer capacitance (CDL) by cyclic voltammetry (CV) tests (Fig. S8, ESI†).24 After analyzing and fitting the data, the CDL values of various electrocatalysts were calculated to be 2.73, 5.18 and 11.89 mF for the commercial C-RuO2, RuO2 and RuO2/Nb2O5-0.6, respectively. CS was the capacitance of an atomically smooth planar surface, which was 0.06 mF cm−2 in acidic media.25 According to ECSA = CDL/CS, the RuO2/Nb2O5-0.6 electrocatalysts possessed the highest ECSA of 198.2 cm2, which was significantly higher than that of RuO2 (86.4 cm2) and commercial RuO2 (45.5 cm2).24 By normalizing with the respective ECSA, the RuO2/Nb2O5-0.6 electrocatalysts delivered the highest intrinsic OER activity of 200 mV at 0.1 mA cmECSA−2 under the acidic electrolyte, in comparison with that of RuO2 (210 mV at 0.1 mA cmECSA−2) and commercial C-RuO2 (217 mV at 0.1 mA cmECSA−2).
In addition, the turnover frequency (TOF) served as a pivotal metric for assessing catalyst activity. At an applied potential of 1.5 V, the TOF value of RuO2/Nb2O5-0.6 reached 7.7 s−1, representing an almost sevenfold increase compared to RuO2 (1.7 s−1) and tenfold enhancement in comparison to C-RuO2 (0.7 s−1), as illustrated in Fig. S9a (ESI†). Moreover, the mass activity of RuO2/Nb2O5-0.6 was 296 A g−1 at 1.5 V, surpassing that of RuO2 (73 A g−1) and C-RuO2 (30 A g−1), as depicted in Fig. S9b (ESI†). These significantly elevated TOF values and mass activities illustrated the enhanced intrinsic activity of RuO2/Nb2O5-0.6 through the incorporation of Nb2O5.
The d-band of various catalysts, which was directly related to the strength of interaction between the Ru and guest molecules, was also determined by high-resolution valence-band (VB) XPS spectra. As shown in Fig. S10 (ESI†), our findings revealed that the d-band center of RuO2/Nb2O5-0.6 was located at −1.53 eV, positioning it midway between C-RuO2 (−1.55 eV) and RuO2 (−1.48 eV). It was noteworthy that the closer proximity of the d-band center to the Fermi level implied a stronger binding strength between the catalyst and the adsorbed intermediates, which could be inconducive to their desorption.26 Therefore, RuO2/Nb2O5-0.6 showed a balanced adsorption capacity for these intermediates, ultimately contributing to its high activity.
Stability was another key parameter of electrocatalysts. The chronopotentiometry (CP) test was employed to assess the OER stability of RuO2/Nb2O5-0.6, which exhibited high catalytic durability during a period of 750 h at a constant current density of 10 mA cm−2 (Fig. 2c). The performance of RuO2/Nb2O5-0.6 was superior to that of the majority of recently reported Ru-based electrocatalysts in acidic electrolytes (Table S3, ESI†). Negligible decay was observed for RuO2/Nb2O5-0.6. Based on the results of the ICP-ES tests, the S-numbers (S = nO2/nRu) of various catalysts at 10 mA cm−2 were calculated according to a previous report.27 Compared to that of C-RuO2 (1.07 × 103), the S-number of RuO2/Nb2O5-0.6 was as high as 3.01 × 105, indicating that the dissolution of Ru was significantly suppressed during the long-term operation. Characterization studies on the spent catalysts indicated the preserved structural features of RuO2/Nb2O5-0.6 during the OER (Fig. S11–S13, ESI†).
These results demonstrated the high chemical and structural stability and suppressed Ru dissolution of RuO2/Nb2O5-0.6 for the acidic OER.
Chemical state analysis
To understand the influence of Nb on the electronic structures of RuO2/Nb2O5-0.6 and their roles in the catalytic activity, chemical states of Ru were analyzed by using X-ray photoelectron spectroscopy (XPS, Fig. 3). For RuO2/Nb2O5-0.6, the peak with a binding energy of 462.6 eV could be categorized as the 3p XPS characteristic peak of Ru4+, which was shifted to the lower binding energy by 0.2 eV compared with those of RuO2 and C-RuO2 (at 462.8 eV).28,29 Niobium 3d XPS spectroscopy showed that the RuO2/Nb2O5-0.6 shifted to the lower binding energy by 0.5 eV (from 207.1 to 206.6 eV) compared to Nb2O5. The fitted deconvolution spectra of Nb contained 3d5/2 and 3d3/2 peaks in Nb2O5, which represented the Nb5+ states at 207.1 and 209.9 eV, respectively.30 The Nb4+ peaks were also observed for RuO2/Nb2O5-0.6 with a derived Nb4+/Nb5+ ratio of 0.92 for RuO2/Nb2O5-0.6, indicating the presence of abundant oxygen vacancy in the as-synthesized RuO2/Nb2O5-0.6 electrocatalysts. In addition, the O 1s XPS spectra of various electrocatalysts were deconvoluted into lattice oxygen (OL), oxygen atoms adjacent to defects (OV) and hydroxyls (OH).31 The binding energy for lattice oxygen of the RuO2/Nb2O5-0.6 was located at 530 eV, which was shifted towards higher binding energy by 0.5 eV compared to the synthetic RuO2 and commercial C-RuO2 (529.5 eV). The positive shift of the lattice oxygen indicated the transfer of electrons from lattice oxygen to metal sites, which reduced the oxidation state of Ru and Nb. The characteristic peaks located at 530.7 eV and 532.1 eV were attributed to OV and OH, respectively. The proportion of OH of RuO2/Nb2O5-0.6 (OH/OV = 1.49) was considerably higher than that of commercial C-RuO2 (OH/OV = 0.94) and RuO2 (OH/OV = 1.02).32 Furthermore, the OH/OV value of RuO2/Nb2O5-0.6 was higher than that of RuO2/Nb2O5-0.5 (0.68) and RuO2/Nb2O5-0.7 (1.08). This finding illustrated that the binary electrocatalysts exhibited higher catalytic activity, accompanied by a larger OH/OV ratio, among catalysts with varying amounts of Nb2O5 introduced (Fig. S14 and Table S4, ESI†). The enhanced hydroxyl adsorption with defects at the active sites modulated the adsorption energy of the oxygen intermediates and enhanced the catalytic activity and stability of RuO2/Nb2O5-0.6.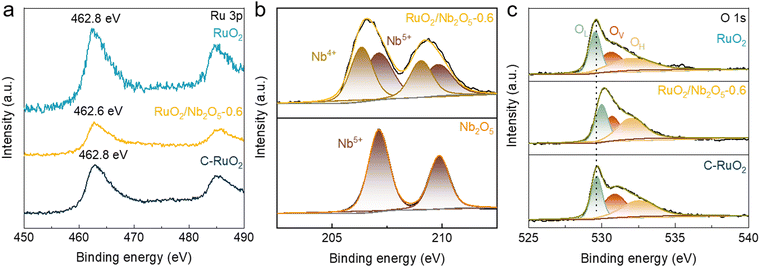 | ||
| Fig. 3 XPS spectroscopies of catalysts. (a) Ru 3p XPS spectra of RuO2/Nb2O5-0.6, RuO2, C-RuO2. (b) Nb 3d XPS spectra of RuO2/Nb2O5-0.6, Nb2O5. (c) O 1s XPS spectra of RuO2/Nb2O5-0.6, RuO2, C-RuO2. | ||
Catalytic mechanism
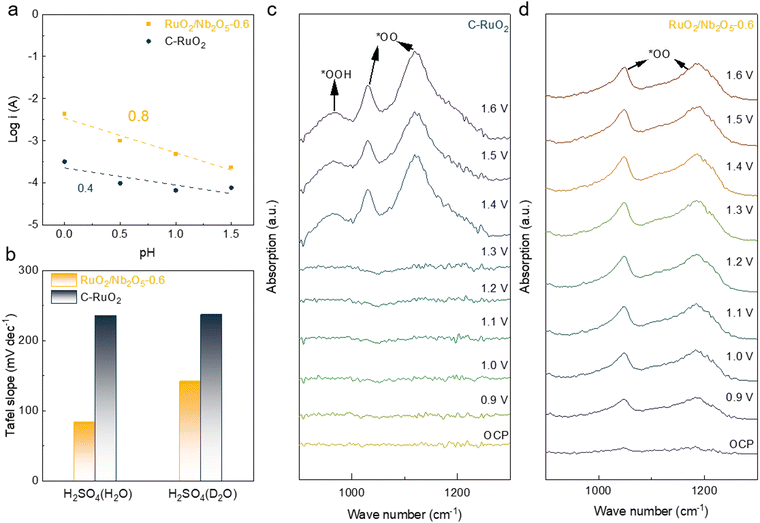
To investigate the reaction mechanism of the binary RuO2/Nb2O5 electrocatalysts, the pH-dependent tests of various catalysts were performed and compared (Fig. 4a). In contrast to the coupled proton–electron transfer step in the AEM pathway of bare RuO2 with good crystallinity, the LOM pathway breaks this scaling relationship, where the proton transfer step becomes the potential-determining step (PDS).33 Therefore, the pH-dependent kinetics of RuO2/Nb2O5-0.6 sufficiently reflected the participation of lattice oxygen in the OER procedure, indicating the LOM pathway for the OER. When the relationship was further compared at 1.5 V, a linear relationship was fitted by a linear fit between the logarithm of the current density and the pH values. The proton reaction orders derived from the slope (ρ = (∂(log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i)/∂pH)E) were 0.8 and 0.4 for RuO2/Nb2O5 and C-RuO2, respectively, confirming the dominant LOM pathway of RuO2/Nb2O5-0.6 for the acidic OER.34 In order to probe the PDS of the binary electrocatalysts, isotope experiments with 0.5 M H2SO4 in the H2O and D2O as solvents were performed. The Tafel slope of RuO2/Nb2O5 significantly increased from 84 mV dec−1 in H2O to 142 mV dec−1 in D2O, suggesting that the deprotonation process was PDS of the binary catalysts,35 while the Tafel slopes of the commercial RuO2 showed a minor change for the isotope experiments, indicating that the AEM pathway was dominant (Fig. 4b).
i)/∂pH)E) were 0.8 and 0.4 for RuO2/Nb2O5 and C-RuO2, respectively, confirming the dominant LOM pathway of RuO2/Nb2O5-0.6 for the acidic OER.34 In order to probe the PDS of the binary electrocatalysts, isotope experiments with 0.5 M H2SO4 in the H2O and D2O as solvents were performed. The Tafel slope of RuO2/Nb2O5 significantly increased from 84 mV dec−1 in H2O to 142 mV dec−1 in D2O, suggesting that the deprotonation process was PDS of the binary catalysts,35 while the Tafel slopes of the commercial RuO2 showed a minor change for the isotope experiments, indicating that the AEM pathway was dominant (Fig. 4b).
To further reveal the OER reaction mechanism of the binary catalysts, operando Fourier transform infrared (FTIR) spectroscopy was employed to monitor the reaction intermediates. When the applied potential exceeded the reaction onset potential of the C-RuO2 electrocatalysts (>1.3 V), a pair of the distinguishable absorption peaks located at 1050 cm−1 and 1150 cm−1 were observed (Fig. 4c). The pair absorption peaks were identified as OO*, which was an intermediate prior to the release of oxygen in the LOM approach. Generally, the absorption peak at 948 cm−1 was attributed to OOH* as a reaction intermediate in the AEM.7 The dynamically enhanced peak intensity was observed, suggesting that the increase in the applied potential accelerated the generation of the reaction intermediates. The presence of the absorption peaks of OOH* and OO* in the C-RuO2 catalysts was attributed to the coexistence of the LOM pathway and the AEM pathway in the acidic OER (Fig. 4c). However, only OO* adsorption peaks located at 1050 cm−1 and 1180 cm−1 were detected in the FTIR spectra of RuO2/Nb2O5-0.6, suggesting that the catalytic pathway of the binary electrocatalysts was dominated by the LOM pathway for the acidic OER (Fig. 4d). The observation of OO* peaks at a lower potential of 0.9 V suggested high catalytic OER performance of the binary electrocatalysts with more reactive intermediates generated on the surface of RuO2/Nb2O5-0.6. It has been widely recognized that surface Ru species of RuO2 are converted to the higher oxidation state through the LOM pathway for the acidic OER, therefore leading to the formation of the soluble ruthenium species and subsequent decay of catalytic activity.
Next, the charge transfer that occurred between catalyst surfaces and electrolyte was investigated by electrochemical impedance spectroscopy (EIS) to understand the roles of Nb incorporated with RuO2 for enhanced catalytic stability as well as activity. The Bode point plots of RuO2/Nb2O5-0.6 and commercial C-RuO2 with potentials from 1.1 V to 1.6 V are shown in Fig. S15 (ESI†), respectively. The characteristic peaks in the high frequency region (101–103 Hz) were attributed to the surface oxidation of the catalyst. The characteristic peaks in the low-frequency region (10−1–101 Hz) reflected the adsorption of reaction intermediates on the surface of the catalyst.36 For the C-RuO2 catalysts, the phase angle decreased slowly in the low-frequency range with increasing potential, indicating that C-RuO2 had insufficient capacity to adsorb intermediates37,38 whereas the significantly decreased phase angle of RuO2/Nb2O5-0.6 in the low frequency region suggested that the binary electrocatalysts showed less resistance to adsorb intermediates at the same voltage. The peak shape of C-RuO2 significantly changed over 1.4 V, suggesting the unstable surface of C-RuO2 with the increased potentials. In contrast, the barely changed characteristic peaks of RuO2/Nb2O5-0.6 in the high-frequency region with the increased potentials revealed more stable surface oxidation states of Ru at higher potentials. The comparative results demonstrated high catalytic activity and stability of the binary RuO2/Nb2O5-0.6 electrocatalysts for the acidic OER.
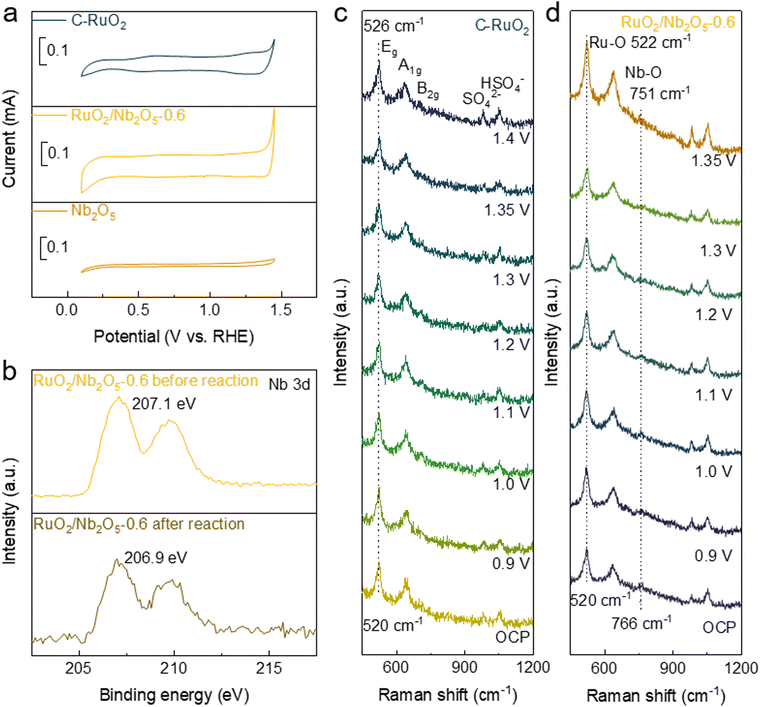
To further elucidate the enhanced catalyst stability, CV curves were recorded to examine the oxidation states of the surface Ru species during the acidic OER (Fig. 5a). Two sets of redox peaks located around 0.6 V and 1.25 V were observed for the C-RuO2 electrocatalysts, which represented Ru3+/4+ and Ru4+/6+ redox pairs, respectively.39 Thus, the surface Ru species of C-RuO2 could be oxidized into high valent Ru species, leading to the serious over-oxidation of catalysts and formation of soluble Ru species during the acidic OER.40 Thus, it's not surprising to observe the poor catalytic stability of C-RuO2. Comparatively, only the redox peaks of Ru3+/4+ was observed for RuO2/Nb2O5-0.6, indicating that the introduction of Nb effectively inhibited the over-oxidation of Ru in the catalyst and stabilized the binary electrocatalysts for the acidic OER. Afterwards, the chemical states of two catalysts before and after the acidic OER were analyzed by XPS. For the RuO2/Nb2O5-0.6 catalyst, the binding energy of Ru 3p barely changed after 10 h of reaction at a current density of 10 mA cm−2. In contrast, the Ru 3p binding energy of C-RuO2 increased from 462.8 eV of the fresh electrocatalysts to 463 eV of the spent ones, indicating the over-oxidation of Ru species of C-RuO2 (Fig. S16, ESI†). As shown in the XPS spectra of Nb 3d of RuO2/Nb2O5-0.6 before and after the acidic OER, the peaks of Nb were negatively shifted by 0.2 eV, suggesting the formation of oxygen vacancies at the Nb site (Fig. 5b).41 The electron transfer pathway in the LOM was based on a redox-catalyzed reaction of oxygen, in which lattice oxygen was electrochemically activated in the form of released oxygen intermediates, following the pathway OH−–(OO)2−–O2, essentially a hybridization of neighbouring non-bonded oxygen atoms (O−), with no transfer of electrons to the external circuit.42 Thus, it's logical to predict the occurrence of spillover of O* intermediates from RuO2 to Nb2O5 and the participation of lattice oxygen in Nb2O5 for the acidic OER, like in the previous reports.18 In this way, the OO* intermediate was formed on Nb2O5 instead of RuO2 for the steps of the release of oxygen, generation of oxygen vacancies and rehabilitation of lattice oxygen. Therefore, the over-oxidation and dissolution of surface Ru species of the binary RuO2/Nb2O5 electrocatalysts could be suppressed, which was consistent with the EIS profile (Fig. S15, ESI†) and CV results (Fig. 5a).
To investigate the potential of oxygen spillover in the binary electrocatalysts, oxygen temperature-programmed desorption (O2-TPD) profiles were recorded for Nb2O5 and RuO2 (Fig. S17, ESI†).43 The desorption peak of oxygen from RuO2 was observed at a significantly higher temperature of 431 °C compared to 303 °C for Nb2O5, suggesting that RuO2 requires more energy for O2 desorption than Nb2O5. The O2-TPD results indicated that the formation of oxygen vacancies on Nb2O5 was more facile than RuO2. Based on these findings, the thermodynamic feasibility of oxygen spillover involving the transfer of intermediate O* species from RuO2 to Nb2O5 and the participation of lattice oxygen in Nb2O5 for the acidic OER, was established.
To experimentally confirm the occurrence of oxygen spillover, in situ Raman spectroscopy was used to dynamically monitor the surface M–O species of the RuO2/Nb2O5-0.6 and C-RuO2 catalysts at different external potentials. The characteristic peaks of Ru–O in C-RuO2 were located at 520 cm−1, 640 cm−1 and 705 cm−1, which represented the Eg, A1g and B2g vibrational modes of Ru–O, respectively (Fig. 5c).22,44 Since the characteristic peak of Eg was more obvious and the peak position was easier to be distinguished, the characteristic Eg peak of Ru–O of C-RuO2 was mainly analyzed. As shown in Fig. 5c, the Eg peak of C-RuO2 gradually shifted to the higher wavenumber by 6 cm−1 (from 520 cm−1 to 526 cm−1) with the increased external applied voltage from the open-circuit potential to 1.6 V, indicating that the surface Ru–O bonds in C-RuO2 were gradually compressed and the valence state of Ru was elevated progressively with the increased potentials.45 As for RuO2/Nb2O5-0.6, the characteristic peak of Ru–O was barely shifted with the increased potential, whereas the characteristic peak of Nb–O showed a redshift of 15 cm−1 (from 766 cm−1 to 751 cm−1), indicating the significantly stretched Nb–O of the binary electrocatalysts (Fig. 5d).17 The reduced oxidation state of Nb combined with the LSV and CV curves showed that Nb suppressed the elevation of the oxidation state of Ru during the oxygen evolution reaction through the modification of bonding with oxygen. Therefore, compared to C-RuO2, the over-oxidation of Ru in RuO2/Nb2O5-0.6 was noticeably suppressed, leading to the stabilized surface Ru species. Significantly, as the circuit was disconnected after applying 1.6 V for a period of time, the characteristic Eg peak of Ru–O in RuO2/Nb2O5-0.6 gradually recovered to the initial state (522 cm−1). Compared with the pristine binary electrocatalyst, the characteristic Raman peak of Nb–O also gradually restored to the same position before the reaction (763 cm−1, Fig. S18, ESI†). These shifts further illustrated the structural stability of RuO2/Nb2O5-0.6 in the acidic OER. Combined with the results of CV curves, XPS and in situ FT-IR, in situ Raman spectra strongly suggested the reactive oxygen species spilled over from RuO2 to Nb2O5, consistent with the preserved Eg of Ru–O and shifted 15 cm−1 of Nb–O in the binary RuO2/Nb2O5 electrocatalysts.
Based on all of the above analysis and experimental evidence, a catalytic pathway is proposed for RuO2/Nb2O5-0.6 with high catalytic activity and stability through the LOM pathway (Fig. 6). Initially, water molecules are absorbed and activated on the surface Ru sites of the binary electrocatalysts to generate OH* species. After the deprotonation of OH*, the O* intermediates spill over from Ru sites to Nb sites. With the participation of the lattice oxygen of Nb2O5, OO* intermediates are formed on Nb sites, followed by the release of O2 associated with the generation of oxygen vacancies and rehabilitation of lattice oxygen on Nb2O5. Such a catalytic pathway of RuO2/Nb2O5 effectively suppresses the over-oxidation of Ru species and avoids the formation of soluble high valent Ru species, leading to the high catalytic activity and stability for the acidic OER despite undergoing the LOM mechanism.
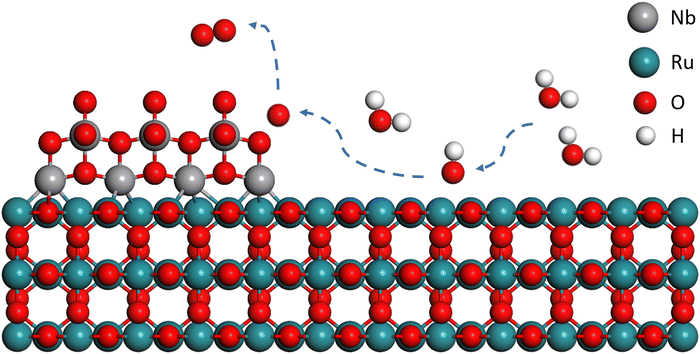 | ||
| Fig. 6 Schematic overview of the proposed oxygen spillover for the OER on the surface of RuO2/Nb2O5-0.6 catalysts. | ||
Conclusions
In summary, we presented the highly active and robust binary RuO2/Nb2O5 electrocatalysts for the acidic OER, delivering a low overpotential of 176 mV at 10 mA cm−2 and a high stability of at least 750 h in 0.5 M H2SO4. Incorporation of Nb2O5 with RuO2 enabled the spillover of active O* intermediate from RuO2 to Nb2O5. Thus, the participation of lattice oxygen of Nb2O5 instead of RuO2 gave the OO* intermediate for the release of oxygen, which effectively inhibited the over-oxidation of surface Ru species and thereby significantly improved the catalytic stability of the binary electrocatalysts. Extensive experimental investigations verified the proposed oxygen spillover reaction pathway for the enhanced catalytic performance of the binary electrocatalysts. This work provided seminal insights into solving the stability problem of Ru-based catalysts and offered a methodology for the rational design of high performance electrocatalysts for the acidic OER.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors acknowledge the Basic and Applied Basic Research Foundation of Guangdong Province (2023A1515012288) and the Fundamental Research Funds for the Central Universities (D5000210829). Yongquan Qu acknowledges the Fundamental Research Funds for the Central Universities (D5000210601).References
- Q. Wang, Y. Cheng, H. B. Tao, Y. Liu, X. Ma, D. S. Li, H. B. Yang and B. Liu, Angew. Chem., Int. Ed., 2023, 62, e202216645 CrossRef CAS.
- L. Li, G. Zhang, C. Zhou, F. Lv, Y. Tan, Y. Han, H. Luo, D. Wang, Y. Liu, C. Shang, L. Zeng, Q. Huang, R. Zeng, N. Ye, M. Luo and S. Guo, Nat. Commun., 2024, 15, 4974 CrossRef CAS PubMed.
- X. Rong, J. Parolin and A. M. Kolpak, ACS Catal., 2016, 6, 1153–1158 CrossRef CAS.
- L. Li, P. Wang, Q. Shao and X. Huang, Adv. Mater., 2021, 33, 2004243 CrossRef CAS PubMed.
- C. Roy, R. R. Rao, K. A. Stoerzinger, J. Hwang, J. Rossmeisl, I. Chorkendorff, Y. Shao-Horn and I. E. L. Stephens, ACS Energy Lett., 2018, 3, 2045–2051 CrossRef CAS.
- N. Hodnik, P. Jovanovič, A. Pavlišič, B. Jozinović, M. Zorko, M. Bele, V. S. Šelih, M. Šala, S. Hočevar and M. Gaberšček, J. Phys. Chem. C, 2015, 119, 10140–10147 CrossRef CAS.
- C. Lin, J.-L. Li, X. Li, S. Yang, W. Luo, Y. Zhang, S.-H. Kim, D.-H. Kim, S. S. Shinde, Y.-F. Li, Z.-P. Liu, Z. Jiang and J.-H. Lee, Nat. Catal., 2021, 4, 1012–1023 CrossRef CAS.
- R. Y. Fan, Y. S. Zhang, J. Y. Lv, G. Q. Han, Y. M. Chai and B. Dong, Small, 2024, 20, e2304636 CrossRef PubMed.
- L. Zhou, Y. Shao, F. Yin, J. Li, F. Kang and R. Lv, Nat. Commun., 2023, 14, 7644 CrossRef CAS.
- Z. Niu, Z. Lu, Z. Qiao, S. Wang, X. Cao, X. Chen, J. Yun, L. Zheng and D. Cao, Adv. Mater., 2024, 36, 2310690 CrossRef CAS.
- W. Gou, Y. Wang, M. Zhang, X. Tan, Y. Ma and Y. Qu, Chin. J. Catal., 2024, 60, 68–106 CrossRef CAS.
- Z. Feng, C. Dai, P. Shi, X. Lei, R. Guo, B. Wang, X. Liu and J. You, Chem. Eng. J., 2024, 485, 149992 CrossRef CAS.
- Z. Wang, W. A. Goddard and H. Xiao, Nat. Commun., 2023, 14, 4228 CrossRef CAS.
- Y. Wu, R. Yao, Q. Zhao, J. Li and G. Liu, Chem. Eng. J., 2022, 439, 135699 CrossRef CAS.
- Y. Xue, J. Fang, X. Wang, Z. Xu, Y. Zhang, Q. Lv, M. Liu, W. Zhu and Z. Zhuang, Adv. Funct. Mater., 2021, 31, 2101405 CrossRef CAS.
- J. Wang, C. Cheng, Q. Yuan, H. Yang, F. Meng, Q. Zhang, L. Gu, J. Cao, L. Li, S.-C. Haw, Q. Shao, L. Zhang, T. Cheng, F. Jiao and X. Huang, Chem, 2022, 8, 1673–1687 CAS.
- Z. Shi, J. Li, J. Jiang, Y. Wang, X. Wang, Y. Li, L. Yang, Y. Chu, J. Bai, J. Yang, J. Ni, Y. Wang, L. Zhang, Z. Jiang, C. Liu, J. Ge and W. Xing, Angew. Chem., Int. Ed., 2022, 61, e202212341 CrossRef CAS PubMed.
- W. Gou, S. Zhang, Y. Wang, X. Tan, L. Liao, Z. Qi, M. Xie, Y. Ma, Y. Su and Y. Qu, Energy Environ. Sci., 2024, 17, 6755–6765 RSC.
- X. Tan, M. Zhang, D. Chen, W. Li, W. Gou, Y. Qu and Y. Ma, Small, 2023, 19, e2303249 CrossRef PubMed.
- J. Okal and M. Zawadzki, Appl. Catal., B, 2009, 89, 22–32 CrossRef CAS.
- K. V. K. Rao and L. Iyengar, Acta Crystallogr., Sect. A, 1969, 25, 302–303 CrossRef CAS.
- A. V. Korotcov, Y. S. Huang, K. K. Tiong and D. S. Tsai, J. Raman Spectrosc., 2007, 38, 737–749 CrossRef CAS.
- A. L. Paulsen, F. Borup, R. W. Berg and S. Boghosian, J. Phys. Chem. A, 2010, 114, 7485–7493 CrossRef CAS.
- M.-R. Gao, M. K. Y. Chan and Y. Sun, Nat. Commun., 2015, 6, 7493 CrossRef PubMed.
- J. Chen, G. Liu, Y.-Z. Zhu, M. Su, P. Yin, X.-J. Wu, Q. Lu, C. Tan, M. Zhao, Z. Liu, W. Yang, H. Li, G.-H. Nam, L. Zhang, Z. Chen, X. Huang, P. M. Radjenovic, W. Huang, Z.-Q. Tian, J.-F. Li and H. Zhang, J. Am. Chem. Soc., 2020, 142, 7161–7167 CrossRef CAS PubMed.
- J. He, X. Zhou, P. Xu and J. Sun, Adv. Energy Mater., 2021, 11, 2102883 CrossRef CAS.
- S. Geiger, O. Kasian, M. Ledendecker, E. Pizzutilo, A. M. Mingers, W. T. Fu, O. Diaz-Morales, Z. Li, T. Oellers, L. Fruchter, A. Ludwig, K. J. J. Mayrhofer, M. T. M. Koper and S. Cherevko, Nat. Catal., 2018, 1, 508–515 CrossRef CAS.
- P. Singh and M. S. Hegde, Chem. Mater., 2009, 21, 3337–3345 CrossRef CAS.
- J. Fu, K. Yang, C. Ma, N. Zhang, H. Gai, J. Zheng and B. H. Chen, Appl. Catal., B, 2016, 184, 216–222 CrossRef CAS.
- K. Islam, R. Sultana, B. Satpati and S. Chakraborty, Vacuum, 2022, 195, 110675 CrossRef CAS.
- Y. Zhang, Y. Zhang, Z. Zeng and D. Ho, Mater. Horiz., 2023, 10, 2904–2912 RSC.
- H. Liu, Z. Zhang, J. Fang, M. Li, M. G. Sendeku, X. Wang, H. Wu, Y. Li, J. Ge, Z. Zhuang, D. Zhou, Y. Kuang and X. Sun, Joule, 2023, 7, 558–573 CrossRef CAS.
- N. Zhang and Y. Chai, Energy Environ. Sci., 2021, 14, 4647–4671 RSC.
- D. Antipin and M. Risch, Electrochem. Sci. Adv., 2023, 3, e2100213 CrossRef CAS.
- W. Li, F. Li, H. Yang, X. Wu, P. Zhang, Y. Shan and L. Sun, Nat. Commun., 2019, 10, 5074 CrossRef CAS.
- W. Gou, Z. Xia, X. Tan, Q. Xue, F. Ye, S. Dai, M. Zhang, R. Si, Y. Zou, Y. Ma, J. C. Ho and Y. Qu, Nano Energy, 2022, 104, 107960 CrossRef CAS.
- R. Šimpraga, G. Tremiliosi-Filho, S. Y. Qian and B. E. Conway, J. Electroanal. Chem., 1997, 424, 141–151 CrossRef.
- B. Zhou, Y. Li, Y. Zou, W. Chen, W. Zhou, M. Song, Y. Wu, Y. Lu, J. Liu, Y. Wang and S. Wang, Angew. Chem., Int. Ed., 2021, 60, 22908–22914 CrossRef CAS PubMed.
- N. Cong, Y. Han, L. Tan, C. Zhai, H. Chen, J. Han, H. Fang, X. Zhou, Y. Zhu and Z. Ren, J. Electroanal. Chem., 2021, 881, 114955 CrossRef CAS.
- K. Klyukin, A. Zagalskaya and V. Alexandrov, J. Phys. Chem. C, 2019, 123, 22151–22157 CrossRef CAS.
- K. K. Rahangdale and S. Ganguly, Phys. B, 2022, 626, 413570 CrossRef CAS.
- X. Wang, H. Zhong, S. Xi, W. S. V. Lee and J. Xue, Adv. Mater., 2022, 34, 2107956 CrossRef CAS.
- Y. Wang, J. Wu, G. Wang, D. Yang, T. Ishihara and L. Guo, Appl. Catal., B, 2021, 285, 119873 CrossRef CAS.
- Y. S. Huang and F. H. Pollak, Solid State Commun., 1982, 43, 921–924 CrossRef CAS.
- A. Rahman, S. Mondal, M. Modak, A. Singh, N. S. Thayat, H. Singh, J. K. Clegg, H. K. Poswal, V. Haridas and S. P. Thomas, Small, 2024, 2402120 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nh00437j |
| ‡ These authors contributed equally to this study. |
| This journal is © The Royal Society of Chemistry 2025 |