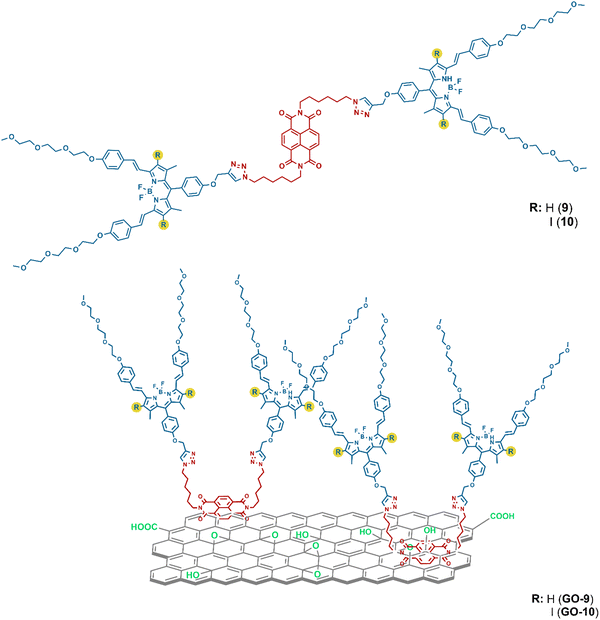
NDI-BODIPY-graphene oxide nanosized photocatalysts for LED irradiated organic synthesis†
Hande
Eserci Gürbüz‡
,
Ezel
Öztürk Gündüz‡
 ,
Ümmügülsüm
Büyükpolat
and
Elif
Okutan
,
Ümmügülsüm
Büyükpolat
and
Elif
Okutan
 *
*
Department of Chemistry, Faculty of Science, Gebze Technical University, P.O. Box: 141, Gebze, 41400, Kocaeli, Turkey. E-mail: eokutan@gtu.edu.tr; Fax: +90 262 6053105; Tel: +90 262 6053091
First published on 15th November 2024
Abstract
Rational design and synthesis of visible-light-absorbing triplet photosensitizers are of great importance for efficient and sustainable photocatalytic utilizations. Herein, we explored two distyryl-NDI-BODIPY systems (9 and 10) with strong visible-light-absorbing antennas and a bridging aromatic core to enable π–π interactions with graphene oxide (GO), resulting in NDI-BODIPY-GO nanocomposites (GO-9 and GO-10). The presence of iodine moieties on 10 empowered the formation of a long-lived triplet state for efficient singlet oxygen generation. The relaxation pathway of the heavy-atom-free system (9 and GO-9) did not permit efficient population of the triplet manifold, whereas the iodine-bearing compounds 10 and GO-10 nanocomposite exhibited good performance to generate singlet oxygen and could be applied effectively in the photocatalysis of 1,5-dihydroxynaphthalene (DHN) to juglone under mild conditions. The singlet oxygen quantum yield of 10 (ΦΔ = 0.7) reached the value of 0.81 for GO-10, and the photooxidation of DHN to juglone with 10 was enhanced with the use of the GO-based nanocomposite.
1. Introduction
As one of the most known representatives of boron complexes-based fluorophores, dipyrromethene boron difluoride (BODIPY) is currently of interest to researchers due to its various usages in fluorescent sensors,1–3 light-emitting diodes4,5 and as an optical component in solar photovoltaics.6,7 Constructions of new and diverse BODIPY-based combinations, including chromophores and nanomaterials, are pursued to increase the understanding of their photophysical and photochemical characteristics, particularly for photocatalysis applications.Since the isolation of graphene and then the emergence of the complete graphene derivatives family, researchers have tremendously studied them for a vast array of utilizations.8,9 As one of the widely explored graphene derivatives, graphene oxide (GO) can form stable colloidal suspensions owing to its 2D honeycomb structure bearing various oxygen functionalities (epoxide, hydroxyl, etc.). Especially, GO-based fluorescence quenching of a wide variety of molecules, including polymers, fluorophores and quantum dots, has been reported in detail.10,11 In fact, BODIPY-graphene and graphene oxide-based nanocarrier systems led to the fluorescence quenching of adsorbed dyes12,13 and enhanced vibrational spectroscopic signals.14,15 Very recently, we developed BODIPY-GO-based nanocarriers for light-irradiated photodynamic therapy in which the quenching effect of GO on the emission of the chromophore was clearly observed.12,16 Despite their excellent photochemical and photophysical properties, bare BODIPY sometimes shows poor stability in water media, especially while irradiated with light, which hinders its applicability.17 It has been reported that photobleaching of a triplet photosensitizer can be prevented via adsorbing it on to GO.18 In this context, a growing interest has emerged to tune derivatization strategies for coherent combinations of BODIPY and GO pairings to increase their biocompatibility and for photocatalysis applications under mild conditions.
Herein, we report two new NDI-BODIPY-based systems (9 and 10) and the adsorption of these dyads onto the surface of GO through noncovalent approach to use these as photocatalysts. We found that the dye 10 bearing iodine moieties and the related GO-based nanocomposite (GO-10) have efficient catalytic properties while producing a high yield of singlet oxygen (1O2). The 10 and GO-10 nanocomposite demonstrated good singlet oxygen quantum yields, which can oxidize organic reactions under mild conditions within a reliable time. Moreover, the efficient generation of 1O2 allowed realizing the photocatalytic oxidation of DHN to juglone. The formation of 1O2 was demonstrated via an indirect method by applying 1,3-diphenylisobenzofuran as a trap molecule (Scheme 1).
2. Experimental
2.1. Materials
The deuterated solvent (CDCl3) used for NMR spectroscopy, silica gel (230–400 mesh) for column chromatography, 1,3-diphenylisobenzofuran (DPBF), methylene blue (MB), dimethyl sulfoxide (DMSO), sodium ascorbate, and copper(II) sulfate pentahydrate (CuSO4·5H2O) were obtained from Merck. The following chemicals were acquired from Sigma Aldrich; DHN, methanol (MeOH), tetrahydrofuran (THF), dichloromethane (DCM), benzene, toluene, sodium sulfate (Na2SO4), and glacial acetic acid. Piperidine was obtained from Acros Organics.2.2. Equipment
Analytical thin-layer chromatography (TLC) was used to follow the reactions and was performed on silica gel plates (Merck, Kieselgel 60 Å, 0.25 mm thickness) with the F254 indicator. Column chromatography was used for purification with silica gel (Merck, Kieselgel 60 Å, 230–400 mesh). Infrared spectra were recorded on a Perkin Elmer Spectrum100 FT-IR spectrophotometer. Mass spectra were obtained in linear mode on a Bruker Daltonics Microflex mass spectrometer equipped with a nitrogen UV-laser at 337 nm. The 1H and 13C NMR spectra were acquired in deuterated solutions (CDCl3) with a Bruker Avance Neo 500 system (500 MHz). The absorption spectra of the compounds were recorded with a Shimadzu 2101 UV spectrophotometer in the UV-vis region. Fluorescence emission spectra were obtained with a Varian Eclipse spectrofluorometer (1 cm path-length cuvette, RT). The fluorescence lifetime measurements were performed using a Horiba Jobin-Yvon-SPEX Fluorolog 3-2iHR instrument at excitation wavelengths with a time-correlated single photon counting (TCSPC) module for signal acquisition. AFM images were recorded using a Digital Instruments NanoScope IV AFM device. XRD analyses were carried out using a Bruker D8 Advance diffractometer using Cu Kα (1.54 Å) radiation, with scanning performed between 5°–90° with a step size of 0.02°, scanning rate of 2° per min, and electrical parameters set at 40 mA and 40 kV.2.3. Synthesis
Compounds 1–6 were synthesized according to the prior literature (Scheme S1, ESI†).19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (99
MeOH (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent (yield: 150 mg, 66%).
1) as the eluent (yield: 150 mg, 66%).
Spectral data of compound 7. MS (MALDI-TOF) (DIT) (m/z): Calc.: 878.82; found: 878.092 [M]+ (Fig. S1, ESI†). 1H NMR (500 MHz, CDCl3, 298 K): δ = 7.60 (d, J = 14.8 Hz, 2H, trans-CH), 7.56 (d, J = 8.7 Hz, 4H, Ar–CH), 7.23 (d, J = 8.7 Hz, 2H, Ar–CH), 7.19 (d, J = 16.4 Hz, 2H, trans-CH), 7.09 (d, J = 8.7 Hz, 2H, Ar–CH), 6.94 (d, J = 8.8 Hz, 4H, Ar–CH), 6.60 (s, 2H, pyrrole-CH), 4.76 (d, J = 2.4 Hz, 2H, H2C
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C), 4.17 (t, J = 4.8 Hz 4H, –OCH2), 3.88 (t, J = 4.8 Hz 4H, –OCH2), 3.76–3.74 (m, 4H, –OCH2), 3.71–3.69 (m, 4H, –OCH2), 3.68–3.65 (m, 4H, –OCH2), 3.57–3.55 (m, 4H, –OCH2), 3.39 (s, 6H, –OCH3), 2.56 (t, J = 2.4 Hz, 1H, C
C), 4.17 (t, J = 4.8 Hz 4H, –OCH2), 3.88 (t, J = 4.8 Hz 4H, –OCH2), 3.76–3.74 (m, 4H, –OCH2), 3.71–3.69 (m, 4H, –OCH2), 3.68–3.65 (m, 4H, –OCH2), 3.57–3.55 (m, 4H, –OCH2), 3.39 (s, 6H, –OCH3), 2.56 (t, J = 2.4 Hz, 1H, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH), 1.47 (s, 6H, –CH3) (Fig. S2, ESI†). 13C NMR (126 MHz, CDCl3, 298 K): δ = 159.62, 158.08, 152.65, 141.78, 137.87, 135.70, 133.51, 129.79, 129.71, 129.01, 128.32, 117.49, 117.30, 115.52, 114.94, 78.11, 75.91, 71.96, 70.89, 70.69, 70.60, 69.71, 67.52, 59.07, 56.05, 14.82. (Fig. S3, ESI†).
CH), 1.47 (s, 6H, –CH3) (Fig. S2, ESI†). 13C NMR (126 MHz, CDCl3, 298 K): δ = 159.62, 158.08, 152.65, 141.78, 137.87, 135.70, 133.51, 129.79, 129.71, 129.01, 128.32, 117.49, 117.30, 115.52, 114.94, 78.11, 75.91, 71.96, 70.89, 70.69, 70.60, 69.71, 67.52, 59.07, 56.05, 14.82. (Fig. S3, ESI†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (99
MeOH (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent (yield: 80 mg, 45%).
1) as the eluent (yield: 80 mg, 45%).
Spectral data of compound 8. MS (MALDI-TOF) (DIT) (m/z): Calc.: 1130.61; found: 1131.2131 [M + H]+ (Fig. S4, ESI†). 1H NMR (500 MHz, CDCl3, 298 K): δ = 8.13 (d, J = 16.6 Hz, 2H, trans-CH), 7.60 (d, J = 8.7 Hz, 4H, Ar–CH), 7.58 (d, J = 16.7 Hz, 2H, trans-CH),7.20 (d, J = 8.7 Hz, 2H, Ar–CH), 7.12 (d, J = 8.7 Hz, 2H, Ar–CH), 6.96 (d, J = 8.7 Hz, 4H, Ar–CH), 4.79 (d, J = 2.4 Hz, 2H, H2C ≡ C), 4.19 (t, J = 4.8 Hz, 4H, –OCH2), 3.89 (t, J = 4.8 Hz, 4H, –OCH2), 3.77–3.75 (m, 4H, –OCH2), 3.71–3.69 (m, 4H, –OCH2), 3.68–3.66 (m, 4H, –OCH2), 3.57–3.55 (m, 4H, –OCH2), 3.39 (s, 6H, –OCH3), 2.58 (t, J = 2.4 Hz, 1H, C ≡ CH), 1.49 (s, 6H, –CH3) (Fig. S5, ESI†). 13C NMR (126 MHz, CDCl3, 298 K): δ = 158.94, 157.42, 149.45, 144.65, 138.08, 137.19, 132.16, 128.70, 128.22, 127.25, 115.75, 114.91, 113.96, 81.68, 76.88, 75.06, 70.93, 69.87, 69.66, 69.58, 68.67, 66.54, 58.05, 55.06, 16.66 (Fig. S6, ESI†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (16 mL, 3
water (16 mL, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v
1; v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v). A solution of a catalytic amount of CuSO4·5H2O and sodium ascorbate in THF
v). A solution of a catalytic amount of CuSO4·5H2O and sodium ascorbate in THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (4 mL, 3
water (4 mL, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v
1; v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) was added (after 10 min. sonication) and the reaction mixture was refluxed for 24 h at 60 °C. Once TLC indicated the complete consumption of compound 7, the mixture was cooled to room temperature and extracted with DCM:water (100 mL, 3 times). The combined organic layers were dried over anhydrous Na2SO4 and then concentrated under reduced pressure. Compound 9 was purified by silica gel (230–400 mesh) column chromatography using DCM
v) was added (after 10 min. sonication) and the reaction mixture was refluxed for 24 h at 60 °C. Once TLC indicated the complete consumption of compound 7, the mixture was cooled to room temperature and extracted with DCM:water (100 mL, 3 times). The combined organic layers were dried over anhydrous Na2SO4 and then concentrated under reduced pressure. Compound 9 was purified by silica gel (230–400 mesh) column chromatography using DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (99
MeOH (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent (yield: 50 mg, 38%).
1) as the eluent (yield: 50 mg, 38%).
Spectral data of compound 9. FT-IR (ATR, cm−1): 2931.34 (C–H, str), 2865.67 (C–H, str), 1711.94 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, str), 1596.24 (C
O, str), 1596.24 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, str), 1482.84 (B–N, str), 1370.89 (C–H, bending), 1197.01 (C–N, str), 1052.98 (C–O, str). MS (MALDI-TOF) (DIT) (m/z): Calc.: 2274.20; found: 2274.0609 [M]+ (Fig. S7, ESI†). 1H NMR (500 MHz, CDCl3, 298 K): δ = 8.68 (s, 4H, Ar–CH), 7.64 (s, 2H, N–CH), 7.53 (d, 8H, J = 8.7 Hz, Ar–CH), 7.52 (d, 4H, J = 16.3 Hz, trans-CH), 7.21 (d, J = 8.5 Hz, 4H, Ar–CH), 7.15 (d, J = 16.2 Hz, 4H, trans-CH), 7.10 (d, J = 8.6 Hz, 4H, Ar–CH), 6.93 (d, J = 8.7 Hz, 8H, Ar–CH), 6.56 (s, 4H, pyrrole-CH), 5.26 (s, 4H, O–CH2), 4.38 (t, J = 7.1 Hz, 4H, N–CH2), 4.17 (t, J = 4.8 Hz, 8H, –OCH2), 4.12 (t, J = 7.5 Hz, 4H, N–CH2), 3.88 (t, J = 4.8 Hz, 8H, –OCH2), 3.76–3.74 (m, 8H, –OCH2), 3.71–3.69 (m, 8H, –OCH2), 3.67–3.66 (m, 8H, –OCH2), 3.57–3.55 (m, 8H, –OCH2), 3.38 (s, 12H, –OCH3), 1.95 (q, J = 7.2 Hz, 4H, –CH2–), 1.73 (q, J = 7.4 Hz, 4H, –CH2–), 1.45 (m, 20H, –CH3 + –CH2–) (Fig. S8, ESI†). 13C NMR (126 MHz, CDCl3, 298 K): δ = 162.94, 159.75, 158.89, 152.69, 143.78, 141.82, 138.03, 135.79, 133.60, 130.99, 129.96, 129.78, 129.12, 128.04, 126.82, 126.66, 122.76, 117.54, 117.32, 115.53, 115.06, 72.08, 71.00, 70.81, 70.72, 69.82, 67.65, 62.27, 59.19, 50.47, 40.69, 30.21, 27.80, 26.50, 26.16, 14.96 (Fig. S9, ESI†).
C, str), 1482.84 (B–N, str), 1370.89 (C–H, bending), 1197.01 (C–N, str), 1052.98 (C–O, str). MS (MALDI-TOF) (DIT) (m/z): Calc.: 2274.20; found: 2274.0609 [M]+ (Fig. S7, ESI†). 1H NMR (500 MHz, CDCl3, 298 K): δ = 8.68 (s, 4H, Ar–CH), 7.64 (s, 2H, N–CH), 7.53 (d, 8H, J = 8.7 Hz, Ar–CH), 7.52 (d, 4H, J = 16.3 Hz, trans-CH), 7.21 (d, J = 8.5 Hz, 4H, Ar–CH), 7.15 (d, J = 16.2 Hz, 4H, trans-CH), 7.10 (d, J = 8.6 Hz, 4H, Ar–CH), 6.93 (d, J = 8.7 Hz, 8H, Ar–CH), 6.56 (s, 4H, pyrrole-CH), 5.26 (s, 4H, O–CH2), 4.38 (t, J = 7.1 Hz, 4H, N–CH2), 4.17 (t, J = 4.8 Hz, 8H, –OCH2), 4.12 (t, J = 7.5 Hz, 4H, N–CH2), 3.88 (t, J = 4.8 Hz, 8H, –OCH2), 3.76–3.74 (m, 8H, –OCH2), 3.71–3.69 (m, 8H, –OCH2), 3.67–3.66 (m, 8H, –OCH2), 3.57–3.55 (m, 8H, –OCH2), 3.38 (s, 12H, –OCH3), 1.95 (q, J = 7.2 Hz, 4H, –CH2–), 1.73 (q, J = 7.4 Hz, 4H, –CH2–), 1.45 (m, 20H, –CH3 + –CH2–) (Fig. S8, ESI†). 13C NMR (126 MHz, CDCl3, 298 K): δ = 162.94, 159.75, 158.89, 152.69, 143.78, 141.82, 138.03, 135.79, 133.60, 130.99, 129.96, 129.78, 129.12, 128.04, 126.82, 126.66, 122.76, 117.54, 117.32, 115.53, 115.06, 72.08, 71.00, 70.81, 70.72, 69.82, 67.65, 62.27, 59.19, 50.47, 40.69, 30.21, 27.80, 26.50, 26.16, 14.96 (Fig. S9, ESI†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (16 mL, 3
water (16 mL, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v
1; v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v). A solution of a catalytic amount of CuSO4·5H2O and sodium ascorbate in THF
v). A solution of a catalytic amount of CuSO4·5H2O and sodium ascorbate in THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (4 mL, 3
water (4 mL, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v
1; v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) was added (after sonication for 30 min) and the reaction mixture was heated for 24 h at 60 °C. Once TLC indicated the complete consumption of compound 8, the mixture was cooled to room temperature and extracted with DCM:water. The combined organic layers were dried over anhydrous Na2SO4 and then concentrated under reduced pressure. Compound 10 was purified by silica gel column chromatography using DCM
v) was added (after sonication for 30 min) and the reaction mixture was heated for 24 h at 60 °C. Once TLC indicated the complete consumption of compound 8, the mixture was cooled to room temperature and extracted with DCM:water. The combined organic layers were dried over anhydrous Na2SO4 and then concentrated under reduced pressure. Compound 10 was purified by silica gel column chromatography using DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (99
MeOH (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (230–400 mesh) (yield: 40 mg, 39%).
1) (230–400 mesh) (yield: 40 mg, 39%).
Spectral data of compound 10. FT-IR (ATR, cm−1): 2923.13 (C–H, str), 2863.43 (C–H, str), 1701.49 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, str), 1661.19 (C
O, str), 1661.19 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, str), 1596.27 (C
C, str), 1596.27 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, str), 1508.95 (B–N, str), 1243.28 (C–N, str), 1100.75 (C–O, str). MS (MALDI-TOF) (DIT) (m/z): Calc.: 2777.78; found: 2758.040 [M-F]+ (Fig. S10, ESI†). 1H NMR (500 MHz, CDCl3, 298 K): δ = 8.70 (s, 4H, Ar–CH), 8.08 (d, J = 16.6 Hz, 4H, trans-CH), 7.66 (s, 2H, N–CH), 7.57 (d, J = 8.6 Hz, 8H, Ar–CH), 7.53 (d, J = 16.6 Hz, 4H, trans-CH), 7.19 (d, J = 8.4 Hz, 4H, Ar–CH), 7.14 (d, J = 8.6 Hz, 4H, Ar–CH), 6.96 (d, J = 8.6 Hz, 8H, Ar–CH), 5.29 (s, 4H, O–CH2), 4.40 (t, J = 7.0 Hz, 4H, N–CH2), 4.19 (t, J = 4.7 Hz, 8H, –OCH2), 4.12 (t, J = 7.5 Hz, 4H, N–CH2), 3.89 (t, J = 4.7 Hz, 8H, –OCH2), 3.77–3.75 (m, 8H, –OCH2), 3.71–3.69 (m, 8H, –OCH2), 3.68–3.65 (m, 8H, –OCH2), 3.57–3.55 (m, 8H, –OCH2), 3.38 (s, 12H, –OCH3), 1.96 (q, J = 7.1 Hz, 4H, –CH2–), 1.73 (q, J = 7.1 Hz, 4H, –CH2–), 1.47 (m, 20H, –CH3 + –CH2–) (Fig. S11, ESI†). 13C NMR (126 MHz, CDCl3, 298 K): δ = 161.77, 158.95, 158.14, 149.35, 144.59, 142.49, 138.02, 137.28, 132.15, 129.91, 128.65, 128.21, 126.87, 125.54, 121.65, 115.68, 114.78, 113.96, 81.65, 70.93, 69.87, 69.66, 69.58, 68.67, 66.54, 61.16, 58.05, 49.36, 39.57, 29.08, 26.68, 25.39, 25.03, 16.67 (Fig. S12, ESI†).
C, str), 1508.95 (B–N, str), 1243.28 (C–N, str), 1100.75 (C–O, str). MS (MALDI-TOF) (DIT) (m/z): Calc.: 2777.78; found: 2758.040 [M-F]+ (Fig. S10, ESI†). 1H NMR (500 MHz, CDCl3, 298 K): δ = 8.70 (s, 4H, Ar–CH), 8.08 (d, J = 16.6 Hz, 4H, trans-CH), 7.66 (s, 2H, N–CH), 7.57 (d, J = 8.6 Hz, 8H, Ar–CH), 7.53 (d, J = 16.6 Hz, 4H, trans-CH), 7.19 (d, J = 8.4 Hz, 4H, Ar–CH), 7.14 (d, J = 8.6 Hz, 4H, Ar–CH), 6.96 (d, J = 8.6 Hz, 8H, Ar–CH), 5.29 (s, 4H, O–CH2), 4.40 (t, J = 7.0 Hz, 4H, N–CH2), 4.19 (t, J = 4.7 Hz, 8H, –OCH2), 4.12 (t, J = 7.5 Hz, 4H, N–CH2), 3.89 (t, J = 4.7 Hz, 8H, –OCH2), 3.77–3.75 (m, 8H, –OCH2), 3.71–3.69 (m, 8H, –OCH2), 3.68–3.65 (m, 8H, –OCH2), 3.57–3.55 (m, 8H, –OCH2), 3.38 (s, 12H, –OCH3), 1.96 (q, J = 7.1 Hz, 4H, –CH2–), 1.73 (q, J = 7.1 Hz, 4H, –CH2–), 1.47 (m, 20H, –CH3 + –CH2–) (Fig. S11, ESI†). 13C NMR (126 MHz, CDCl3, 298 K): δ = 161.77, 158.95, 158.14, 149.35, 144.59, 142.49, 138.02, 137.28, 132.15, 129.91, 128.65, 128.21, 126.87, 125.54, 121.65, 115.68, 114.78, 113.96, 81.65, 70.93, 69.87, 69.66, 69.58, 68.67, 66.54, 61.16, 58.05, 49.36, 39.57, 29.08, 26.68, 25.39, 25.03, 16.67 (Fig. S12, ESI†).
2.4. Preparation of GO based nanocomposites GO-9 and GO-10
Commercial GO was used for the preparation of NDI-BODIPY-GO nanocomposites according to a previously reported procedure.20 Here, 10 mg of the commercial GO was dispersed in 30 mL of distilled water by ultrasonication for 1 h to obtain a homogeneous suspension of GO. Afterwards, 10 mg of NDI-BODIPY derivative (9, 10) was dissolved in 3 mL DMF and added to the GO suspension. Then, the mixture was stirred for 40 h for efficient dye adsorption on GO at room temperature. The resulting mixture was filtered through a polycarbonate membrane with 0.2 mm pores and the obtained solid material was washed with water several times to remove the excess of compounds 9 and 10 and then dried in a vacuum oven for 48 h at 45 °C.2.5. Parameters for determining the fluorescence quantum yield
The fluorescence quantum yield values of the compounds (9 and 10) and nanocomposites [(GO-9) and (GO-10)] were determined in the appropriate solvent by a comparitive method. Cresyl violet (ΦF = 0.56 in ethanol) and zinc phtalocynanine (ΦF = 0.20 in DMSO) were employed as standard fluorophores. The fluorescence quantum yield values (ΦFsample) were calculated using eqn (1).21,22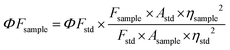 | (1) |
The average lifetime values of the photosensitizers 7, 9, and GO-9 were determined according to eqn (2), where τi is the decay time constant and αi is the pre-exponential factor.
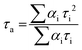 | (2) |
2.6. Parameters for determining the singlet oxygen quantum yield
The singlet-oxygen-generation abilities of 9, 10 and GO-9, GO-10 were determined via an indirect method by using the singlet-oxygen-trapping molecule DPBF. Solutions containing the photosensitizers and DPBF were prepared in DCM and then irradiated with red LED (630 nm, 4.0 mW cm−2) at regular intervals. After each irradiation, the absorbance spectrum was recorded to monitor the spectral changes of DPBF at 414 nm. The singlet oxygen quantum yields were calculated from a comparative method according to eqn (3). MB was employed as a reference triplet photosensitizer with a 0.57 singlet oxygen quantum yield in DCM.23 In the equation, k is the slope value, calculated from the absorbance value of DPBF at 414 nm versus irradiation time as plotted graphically; F is the absorption correction factor, which is given by F = 1 − 10−O.D. (O.D. is the optical density at the irradiation wavelength); and PF refers to the light intensity (energy flux, mW cm−2).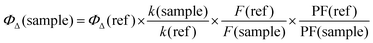 | (3) |
2.7. Photooxidation of DHN
The photooxidation of DHN was perfomed in 10 mL DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (9
MeOH (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v
1; v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) solution in a flask. Solutions containing both DHN (2 × 10−4 M) and photosensitizers 9, 10, GO-9, and GO-10 (5 mol% with respect to DHN) were prepared in a flask and irradiated by 630 nm LED for 30 min and the absorbance spectra were recorded at 5 min intervals. The photooxidation of DHN was tracked by the absorbance decrease at 301 nm. Furthermore, the production of 5-hydroxy-1,4-naphthoquinone (juglone) from DHN was determined by the increase in absorbance at 427 nm. The juglone production yield was calculated by dividing the concentration of juglone (Cjuglone) by the initial concentration of DHN (CDHN) (eqn (4)). The concentrations of DHN and juglone were calculated from their molar absorption coefficients (εDHN = 7664 M−1 cm−1; εjuglone =3811 M−1 cm−1).
v) solution in a flask. Solutions containing both DHN (2 × 10−4 M) and photosensitizers 9, 10, GO-9, and GO-10 (5 mol% with respect to DHN) were prepared in a flask and irradiated by 630 nm LED for 30 min and the absorbance spectra were recorded at 5 min intervals. The photooxidation of DHN was tracked by the absorbance decrease at 301 nm. Furthermore, the production of 5-hydroxy-1,4-naphthoquinone (juglone) from DHN was determined by the increase in absorbance at 427 nm. The juglone production yield was calculated by dividing the concentration of juglone (Cjuglone) by the initial concentration of DHN (CDHN) (eqn (4)). The concentrations of DHN and juglone were calculated from their molar absorption coefficients (εDHN = 7664 M−1 cm−1; εjuglone =3811 M−1 cm−1).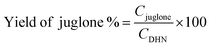 | (4) |
3. Results and discussion
3.1. Synthesis and structural characterization
Details on the synthesis and characterization of compounds 1–6 are reported in our previous work and are provided in the ESI† (Scheme S1).19 In the present study, the NDI-BODIPY derivative was combined with GO, driven by non-covalent π–π interactions via a simple method. The NDI-BODIPY-GO junction effectively bridged the unique properties of the GO nanosheets and NDI-BODIPY dyads with light-harvesting antennae and the obtained system was then used for the light-driven transformation of DHN to juglone via photocatalysis. In this molecular design, NDI was utilized for the non-covalent π–π interactions and binding point for two BODIPY units.Distyryl BODIPY derivatives 8 and 9 were prepared via Knoevenagel condensation in benzene to enhance the π-conjugation and to tune the light absorption. The bis BODIPY-functionalized NDI-BODIPY compounds were obtained via a Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC) in THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mixture with moderate yields. After purification of all the compounds, mass, 1H, and 13C NMR spectra confirmed the proposed structures, where the [M]+ peak for compound 9 and both [M]+ and [M-F]+ peaks for compound 10 were observed. Distyryl trans C
water mixture with moderate yields. After purification of all the compounds, mass, 1H, and 13C NMR spectra confirmed the proposed structures, where the [M]+ peak for compound 9 and both [M]+ and [M-F]+ peaks for compound 10 were observed. Distyryl trans C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H protons were distinguished as doublets with ∼16 Hz coupling constant in the aromatic region. A peak was also observed related to the proton on the triazole ring resonating around 7.60 ppm as a singlet for both 9 and 10. Singlet peaks were also observed at 6.60 and 6.56 ppm attributed to the protons on the pyrrole rings for compounds 7 and 9, respectively. These peaks vanished for compounds 8 and 10 due to the introduction of iodine atoms into the structures. Furthermore, the methylene bridge protons between the BODIPY and triazole ring resonated as singlets at 5.26 and 5.29 ppm for 9 and 10, respectively (Table S1, ESI†). The peaks of all the carbon atoms in the 13C NMR spectra were observed in the expected regions (see ESI†).
H protons were distinguished as doublets with ∼16 Hz coupling constant in the aromatic region. A peak was also observed related to the proton on the triazole ring resonating around 7.60 ppm as a singlet for both 9 and 10. Singlet peaks were also observed at 6.60 and 6.56 ppm attributed to the protons on the pyrrole rings for compounds 7 and 9, respectively. These peaks vanished for compounds 8 and 10 due to the introduction of iodine atoms into the structures. Furthermore, the methylene bridge protons between the BODIPY and triazole ring resonated as singlets at 5.26 and 5.29 ppm for 9 and 10, respectively (Table S1, ESI†). The peaks of all the carbon atoms in the 13C NMR spectra were observed in the expected regions (see ESI†).
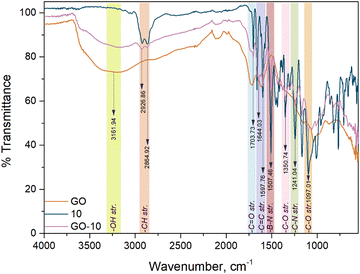
NDI-BODIPY-GO (GO-9 and GO-10) nanocomposites as photocatalysts were obtained from the loading of NDI-BODIPY dyes on GO by the one-pot synthesis method via noncovalent interactions. The carbon network of GO can form π–π interactions with the NDI unit and strong dipole–dipole, van der Waals, etc. interactions with the dyad. FT-IR analysis confirmed the expected functional groups on the prepared nanocomposites. Broad peaks at 3172.39 cm−1 (for GO-9) and 3161.94 cm−1 (for GO-10) were observed attributed to the –OH stretching of GO. The C–H bonds of BODIPY 10 were responsible for the peaks observed at 2923.13 and 2863.43 cm−1. Carbonyl groups on the both GO and compound 10 led to the stretching band at ∼1701.49 cm−1. The peak at 1508.95 cm−1 could be ascribed to the stretching of the B–N bond in BODIPY. Other FT-IR peaks were observed that were compatible with the structures of the nanocomposites (Fig. 1, Fig. S13 and Table S2, ESI†).
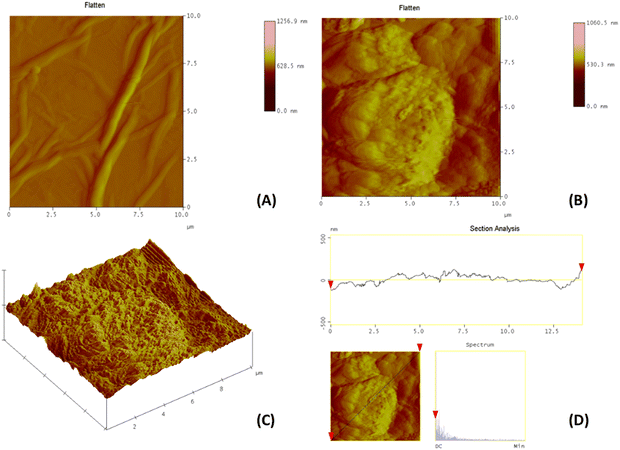
AFM images (2D and 3D) were obtained to investigate the morphologies of the GO-9 and GO-10 and compared with the AFM image of GO. It could be clearly seen that the topography of GO was changed with the adsorption of BODIPY dyes on the GO (Fig. 2 and Fig. S14, ESI†). With the addition of BODIPY molecules, the sheet-like morphology corresponding to the layers of GO disappeared and cluster-like shapes emerged in the AFM image of the NDI-BODIPY-GO nanocomposites. The XRD patterns of 9, 10, GO-9, and GO-10 as well as commercial GO are given in Fig. S15 (ESI†). For the commercial GO powder, there was an intense peak located at 2θ = 10.35° corresponding to the (002) reflection with an average d-spacing of 8.53 Å, and a peak at 2θ = 42.6° corresponding to the (100) reflection with a d-spacing of 2.13 Å, illustrating the typical XRD pattern of GO. The peak observed at 2θ = 25.53° coincided with the graphite (002) reflection and was most probably due to residual graphite remaining in the GO powder.24,25 On the other hand, a broad peak around 22° was observed in the diffractograms of compounds 9 and 10. The GO-9 and GO-10 composites contained crystalline GO phase based on the peak observed at 2θ = 10.74° with an average d-spacing of 8.27 Å. An additional broad peak located around 22° was also observed in the diffractograms due to presence of BODIPY moieties. The (002) reflection in GO-9 and GO-10 showed a slight shift compared with the commercial GO powder.
3.2. Photophysical properties
One of the hallmarks of BODIPY dyes is their tunable photophysical properties via various functionalization procedures.16 Therefore, the optical properties of the new derivatives (9 and 10) and nanocomposites (GO-9 and GO-10) were evaluated for assessing the possible application areas of the compounds. Specifically, the photophysical properties of compounds 9, 10 and their nanocomposites GO-9 and GO-10 were investigated by steady-state UV-vis absorption and fluorescence spectroscopy techniques in several solvents, such as DCM, DMSO, THF, MeOH, toluene, and water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (95
DMSO (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; v
5; v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) mixture. As can be seen in Fig. S16 and S20 (ESI†), 9 and 10 demonstrated low solubility in methanol and water
v) mixture. As can be seen in Fig. S16 and S20 (ESI†), 9 and 10 demonstrated low solubility in methanol and water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (95
DMSO (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; v
5; v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) but were soluble in the other tested solvents. Also, 9 and 10 displayed multiple absorption bands centered between 330–425 and 530–675 nm for 9, and 350–500 and 550–720 nm for 10. The absorption bands of the distyryl BODIPYs were observed in the red region due to the extension of π conjugation of the BODIPY core (Fig. 3). Hence, compound 9 exhibited a sharp absorption band at ∼640 nm in DCM, THF, and toluene, as expected, with an accompanied shoulder approximately at 590 nm for each solvent. The absorption peak of 9 was slightly shifted in both DMSO and toluene. The absorption profiles showed similar behaviors in the solvents with medium polarity, wherein the intensity optical density was clearly decreased in MeOH and water
v) but were soluble in the other tested solvents. Also, 9 and 10 displayed multiple absorption bands centered between 330–425 and 530–675 nm for 9, and 350–500 and 550–720 nm for 10. The absorption bands of the distyryl BODIPYs were observed in the red region due to the extension of π conjugation of the BODIPY core (Fig. 3). Hence, compound 9 exhibited a sharp absorption band at ∼640 nm in DCM, THF, and toluene, as expected, with an accompanied shoulder approximately at 590 nm for each solvent. The absorption peak of 9 was slightly shifted in both DMSO and toluene. The absorption profiles showed similar behaviors in the solvents with medium polarity, wherein the intensity optical density was clearly decreased in MeOH and water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (95
DMSO (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; v
5; v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
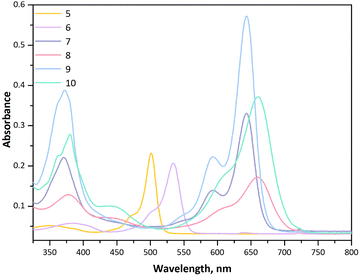
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) mixture, corresponding to the low solubility of 9. It is well known that for BODIPY dyes the primary peak is due to the S0–S1 transition and so the peaks around 370 nm in all the solvents were attributed to the S0–S2 transition.26 Compounds 6, 8, and 10 bore two iodine moieties at the 2- and 6-positions of the core as a result of the functionalization via electrophilic substitution with iodine without disturbing the planarity, and we could observe the effect of this derivatization on the electronic profiles of 6, 8, and 10 compared with the iodine-free derivatives 5, 7, and 9, respectively. Via further functionalization on the BODIPY core at the 3- and 5-positions, the maximum absorption was shifted to the far-red region at 650–660 nm for compounds 7 and 8. As given in Table 1 although 9 and 10 demonstrated characteristic transitions of distyryl-BODIPY and NDI units in the same wavelength range as 7 and 8, the absorbance values of 9 and 10 increased due to the enhanced number of BODIPY groups on the core (Fig. S16 and S20, ESI†).
v) mixture, corresponding to the low solubility of 9. It is well known that for BODIPY dyes the primary peak is due to the S0–S1 transition and so the peaks around 370 nm in all the solvents were attributed to the S0–S2 transition.26 Compounds 6, 8, and 10 bore two iodine moieties at the 2- and 6-positions of the core as a result of the functionalization via electrophilic substitution with iodine without disturbing the planarity, and we could observe the effect of this derivatization on the electronic profiles of 6, 8, and 10 compared with the iodine-free derivatives 5, 7, and 9, respectively. Via further functionalization on the BODIPY core at the 3- and 5-positions, the maximum absorption was shifted to the far-red region at 650–660 nm for compounds 7 and 8. As given in Table 1 although 9 and 10 demonstrated characteristic transitions of distyryl-BODIPY and NDI units in the same wavelength range as 7 and 8, the absorbance values of 9 and 10 increased due to the enhanced number of BODIPY groups on the core (Fig. S16 and S20, ESI†).
| Compound | λ ab (nm) | λ em (nm) | Δ Stokes (cm−1) | ε | Φ F | τ F (ns) | Φ Δ | k obs | ν i | % Yield of juglone |
|---|---|---|---|---|---|---|---|---|---|---|
| a Molar extinction coefficients (×104 M−1 cm−1) in DCM. b Fluorescence quantum yield in DCM. c Fluorescence lifetime in DCM. d Singlet oxygen quantum yield. e Pseudo-first-order rate constant (10−3 min−1), ln(At/A0) = –kobst. f Initial consumption rate (10−7 m min−1) of DHN, νi = kobs [DHN]. | ||||||||||
| 6 | 371, 643 | 657 | 331 | 12.2 | 0.51 | 3.60 | — | — | — | — |
| 8 | 376, 661 | 683 | 487 | 6.9 | 0.13 | 1.96 | — | — | — | — |
| 9 | 372, 642 | 659 | 402 | 24.1 | 0.17 | 1.87 | 0.007 | — | — | 2.4 |
| 10 | 379, 660 | 695 | 763 | 17.0 | 0.05 | 1.42 | 0.70 | 6.6 | 1.3 | 34 |
| GO-9 | 372, 642 | 659 | 402 | — | 0.14 | 1.75 | 0.10 | — | — | 6.5 |
| GO-10 | 379, 660 | 695 | 763 | — | 0.04 | 1.42 | 0.81 | 15.6 | 3.12 | 64 |
The same solvents were employed to examine the fluorescence emission properties of NDI-BODIPYs and NDI-BODIPY-GO nanocomposites (ESI,† Fig. S17, S19, and S21, S23, ESI†). For this purpose, a 1.0 μM solution of compound 9 was excited at 610 nm, and the recorded fluorescence spectra exhibited strong emission in DCM, DMSO, and THF at around 660 nm, with an approximate 285 cm−1 Stokes’ shift. As can be seen from Fig. S17 (ESI†) the fluorescence was completely quenched in MeOH and water:DMSO. On the other hand, the emission of compound 10 drastically decreased due to the presence of iodine atoms due to the enhanced intersystem crossing (ESI,† Fig. S21).
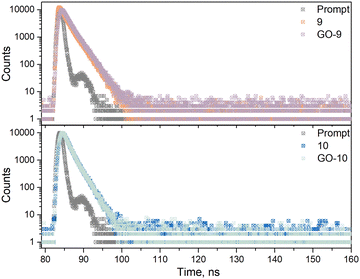
The photophysical properties of the GO-based nanocarriers were investigated under the same conditions with the same solvents. Both GO-9 and GO-10 displayed predominantly similar absorption profiles to the NDI-BODIPYs. After the noncovalent functionalization of 9 and 10 onto the π-conjugated GO, the absorption profiles corresponding to the distyryl-BODIPY core (S0–S1 transitions) did not exhibit a change (the quantity of the dye was quantified by using these peaks) but the S0–S2 vibration area was slightly altered. This observation was important evidence of the interactions between the two units (Fig. 4a). Also, the quenching effect of GO could be indisputably observed from the fluorescence spectra, since there was a distinguishable decline in the emission compared to BODIPYs 9 and 10 (Fig. 4b). This was possibly because of energy or electron transfer from the dye to GO.27 The optical behaviors were investigated via time-resolved fluorescence spectroscopy, and the obtained fluorescence lifetime decays are shown in Fig. 5. The fluorescence lifetimes of 9, 10, GO-9, and GO-10 were calculated to be 1.87, 1.42, 1.75, and 1.42 ns, respectively, via appropriate exponential calculations (Table 1). As an important photophysical parameter, the fluorescence quantum yields of 9, 10 and their related nanocomposites were calculated to be 0.17, 0.05, 0.14, and 0.04, respectively, viaeqn (1) under identical conditions.
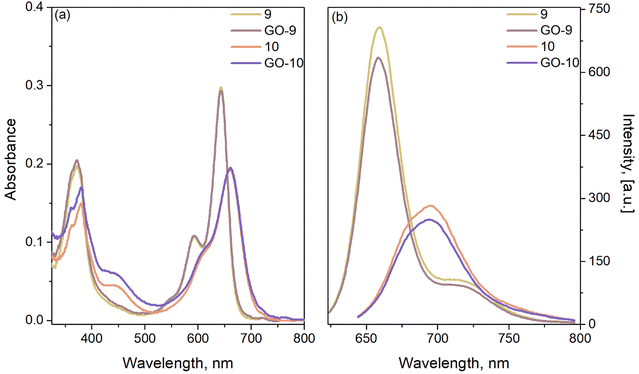 | ||
| Fig. 4 (a) UV-vis absorption, and (b) florescence spectra of compounds 9, 10 and nanocarriers GO-9 and GO-10 at 1 μM in DCM. | ||
3.3. Photochemical properties
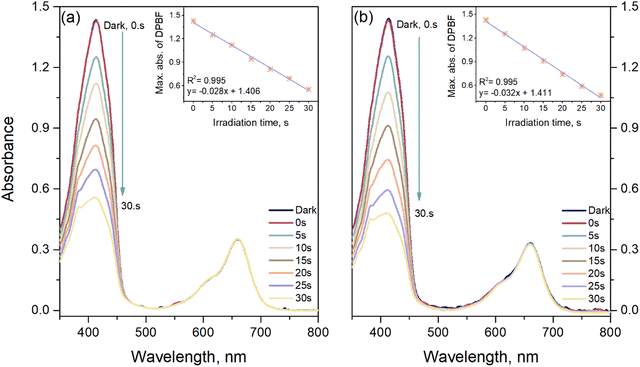
Singlet oxygen (1O2) can photochemically be produced and is an important oxidant that is pervasive in aquatic environments. It is also known to be an important reactive intermediate in many applications, such as hetero Diels–Alder, [2 + 2] cycloaddition reactions and photodynamic therapy (PDT).28,29 Both in natural and engineered circumstances, singlet oxygen is created via energy transfer from the triplet excited state of a chromophore, which is able to absorb light.30 A comparative study of singlet oxygen generation in dichloromethane solutions was realized to determine the ability of the materials to generate singlet oxygen. Here, 2 μM dichloromethane solutions or the materials were irradiated with 625 nm red LED at 4.0 mW cm−2. Singlet oxygen generation was estimated experimentally via an indirect method using DPBF, which is recognized as a singlet oxygen indicator.31 The expected decrease in the 414 nm absorbance band was monitored, caused by the transformation of DPBF to endoperoxide via oxidation.28 The inset of Fig. 4 shows the time profiles of the absorption spectra with red LED irradiation in dichloromethane, in which NDI-BODIPY derivatives and the related GO-based nanocomposites were the photosensitizer while DPBF was the substrate. DPBF absorbs with a peak maximum at 414 nm and shows no absorption between 600–700 nm. The absorption bands of the dyads and nanocomposites were not changed by the LED irradiation; thus, it would be safe to conclude the lack of dark side reactions in the environment. It was found that DPBF absorption was not noticeably decreased with irradiation in the presence of the non-iodine-functionalized NDI-BODIPY derivative 9 or related composite GO-9 (ESI,† Fig. S24). Hence the effect of the incorporation of GO caused an increase in the slope of the plot of DPBF absorbance against time relative to the bare NDI-BODIPY 9. It is well known that to produce 1O2 efficiently, the S1 excited state of the chromophore must display an efficient ISC process to populate the formation of a triplet excited state (T1).32 The nonfluorescent dyad (10) and nanocomposite (GO-10) were tested to assess the generation of 1O2. As the red LED specifically irradiated compound 10 and GO-10 at regular intervals, the efficient formation of singlet was detected in the absorption spectra of DPBF. The iodinated materials (10 and GO-10) displayed an improved singlet-oxygen-generating ability compared to the heavy-atom-free derivatives having the highest ΦΔ of 0.70 and 0.81, respectively (Fig. 6).3.4. Photocatalytic properties
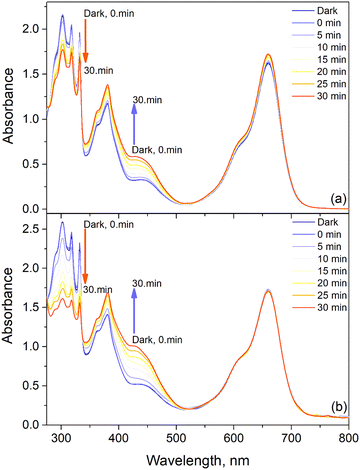
In the past few years, the application of triplet photosensitizers as photocatalysts has become desirable.33 Light-harvesting photosensitizers (PSs) with efficient ISC and strong absorption in the visible range have been used both in natural and artificial photosynthetic conversions.34,35 The high bioactivity of juglone is important for various applications,36 thus we applied the prepared NDI-BODIPYs and nanocomposites to drive the photooxidation of DHN to juglone and analyzed their photocatalysis ability (Fig. 7, Fig. S26 (ESI†) and Table 1). The photooxidation of DHN was monitored by following the change in the absorption of DHN at 301 nm in the presence of 5% mole BODIPYs and the nanocomposites as PSs. Upon the addition of 9 and GO-9 to the reaction media (without a noticeable amount of singlet oxygen), the photolysis of DHN did not occur, indicating that an effective PS is required for photooxidation. Upon irradiation with a red LED, compound 10 and the GO-10 nanocomposite, the absorption at 301 nm of DHN decreased and the juglone absorption at 427 nm increased. The photooxidation rates using 10 and GO-10 as triplet PSs were compared by plotting the ln(A/A0) − t, where A is the absorption and the t is the irradiation time (Fig. S27, ESI†). Distinct differences could be observed between the slopes (kobs) of the photooxidation reactions when using different systems. Naturally the slopes of 9 and GO-9 were much smaller than those of 10 and GO-10 (Table 1). Notably, the photooxidation ability of GO-10 was much potent than that of bare 10 as a photocatalyst, indicating the preparation of GO-based nanocarriers has wide applicability for the photocatalysis of organic reactions (Fig. 8). The initial consumption rate of DHN (νi) and also the yield of juglone were also calculated and were found to be adequate for GO-10 (νi = 3.12 × 10−7 M min−1) and the yield of juglone in 30 min was up to 64%. For 10, nevertheless, the reaction was slower (νi = 1.3 × 10−7 M min−1) with a lesser yield of 34%. These results were in accordance with the light-harvesting/photophysical abilities and singlet oxygen quantum yields. Furthermore, the photocatalytic performance of GO-10 with a moderate yield of juglone was comparable to the photocatalysts reported before with respect to the molar amount of the photocatalyst and/or energy flux of the irradiation source.19,26,37,38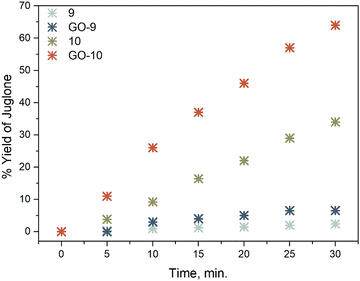 | ||
| Fig. 8 Percentage yield of juglone vs. irradiation time of compounds 9, 10 and the nanocomposites GO-9 and GO-10. | ||
Conclusion
We prepared NDI-BODIPY dyads and NDI-BODIPY-GO-based nanocomposites as triplet photosensitizers to investigate their 1O2-generation quantum yield and photosensitizing applicability in the photooxidation reaction of DHN. Upon visible light irradiation, the prepared systems exhibited strong absorbing peaks between ∼350–450 nm and ∼550–670 nm with high molar extinction coefficients corresponding to the S0–S2 and S0–S1 transition of BODIPY, respectively. The excitation energy of the BODIPY light-harvesting antennas was efficiently transferred to the triplet state (T1) via the ISC process to further switch the formation of singlet oxygen from molecular oxygen. The catalytic performance of GO-10 was much higher than that of compound 10 and showed comparable catalytic activity to metal-based catalysts.Data availability
The authors declare that all other data supporting the findings of this study are available within the article and ESI† files and from the corresponding author, upon reasonable request.Conflicts of interest
The authors declare that the is no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the GTU-BAP project no: 2022-A-105-36.References
- H.-C. Xia, X.-H. Xu and Q.-H. Song, Anal. Chem., 2017, 89, 4192–4197 CrossRef CAS PubMed.
- J. Xu, Q. Li, Y. Yue, Y. Guo and S. Shao, Biosens. Bioelectron., 2014, 56, 58–63 CrossRef CAS PubMed.
- L.-Y. Niu, Y.-S. Guan, Y.-Z. Chen, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, J. Am. Chem. Soc., 2012, 134, 18928–18931 CrossRef CAS PubMed.
- A. Zampetti, A. Minotto, B. M. Squeo, V. G. Gregoriou, S. Allard, U. Scherf, C. L. Chochos and F. Cacialli, Sci. Rep., 2017, 7, 1611 CrossRef.
- Y. H. Jung, D. Karthik, H. Lee, J. H. Maeng, K. J. Yang, S. Hwang and J. H. Kwon, ACS Appl. Mater. Interfaces, 2021, 13, 17882–17891 CrossRef CAS.
- R. Rajagopalan, S. Shankar S, N. Balasubramaniyan and G. D. Sharma, ACS Appl. Mater. Interfaces, 2023, 15, 13405–13414 CrossRef CAS PubMed.
- O. García, R. Sastre, D. del Agua, A. Costela and I. García-Moreno, Chem. Mater., 2006, 18, 601–602 CrossRef.
- S. Roy, N. Soin, R. Bajpai, D. S. Misra, J. A. McLaughlin and S. S. Roy, J. Mater. Chem., 2011, 21, 14725–14731 RSC.
- C. Anichini and P. Samorì, Small, 2021, 17, 2100514 CrossRef CAS PubMed.
- Y. Wang, D. Kurunthu, G. W. Scott and C. J. Bardeen, J. Phys. Chem. C, 2010, 114, 4153–4159 CrossRef CAS.
- Y. Liu, C. Liu and Y. Liu, Appl. Surf. Sci., 2011, 257, 5513–5518 CrossRef CAS.
- E. Öztürk Gündüz, R. Atajanov, M. E. Gedik, E. Tanrıverdi Eçik, G. Günaydın and E. Okutan, Dalton Trans., 2023, 52, 5466–5477 RSC.
- X. Wu, Y. Xing, K. Zeng, K. Huber and J. X. Zhao, Langmuir, 2018, 34, 603–611 CrossRef CAS PubMed.
- E. de la O-Cuevas, I. Badillo-Ramírez, S. R. Islas, C. Araujo-Andrade and J. M. Saniger, RSC Adv., 2019, 9, 12269–12275 RSC.
- E. de la O-Cuevas, V. Alvarez-Venicio, I. Badillo-Ramírez, S. R. Islas, M. del P. Carreón-Castro and J. M. Saniger, Spectrochim. Acta, Part A, 2021, 246, 119020 CrossRef CAS PubMed.
- E. Öztürk Gündüz, B. Tasasız, M. E. Gedik, G. Günaydın and E. Okutan, ACS Omega, 2023, 8, 8320–8331 CrossRef PubMed.
- R. Xing, T. Jiao, Y. Liu, K. Ma, Q. Zou, G. Ma and X. Yan, Polymers, 2016, 8(5), 181 CrossRef PubMed.
- G. Reina, G. M. Beneventi, R. Kaur, G. Biagiotti, A. Cadranel, C. Ménard-Moyon, Y. Nishina, B. Richichi, D. M. Guldi and A. Bianco, Chem. – Eur. J., 2023, 29, e202300266 CrossRef CAS.
- Ü. Büyükpolat, E. Öztürk Gündüz, H. Eserci Gürbüz and E. Okutan, J. Fluoresc., 2023, 33, 2305–2313 CrossRef.
- M. Wojtoniszak, D. Rogińska, B. Machaliński, M. Drozdzik and E. Mijowska, Mater. Res. Bull., 2013, 48, 2636–2639 CrossRef CAS.
- A. Ogunsipe, D. Maree and T. Nyokong, J. Mol. Struct., 2003, 650, 131–140 CrossRef CAS.
- A. M. Brouwer, Pure Appl. Chem., 2011, 83, 2213–2228 CrossRef CAS.
- L. Huang, X. Cui, B. Therrien and J. Zhao, Chem. – Eur. J., 2013, 19, 17472–17482 CrossRef CAS PubMed.
- X. Jiao, Y. Qiu, L. Zhang and X. Zhang, RSC Adv., 2017, 7, 52337–52344 RSC.
- Q. T. Ain, S. H. Haq, A. Alshammari, M. A. Al-Mutlaq and M. N. Anjum, Beilstein, J. Nanotechnol., 2019, 10, 901–911 CrossRef CAS PubMed.
- E. Öztürk Gündüz, H. Eserci Gürbüz, B. Tasasız, E. Yıldız Gül, E. Tanrıverdi Eçik and E. Okutan, New J. Chem., 2022, 46, 19702–19711 RSC.
- V. A. Povedailo, B. V. Ronishenko, V. I. Stepuro, D. A. Tsybulsky, V. V. Shmanai and D. L. Yakovlev, J. Appl. Spectrosc., 2018, 85, 605–610 CrossRef CAS.
- S. G. Awuah, J. Polreis, V. Biradar and Y. You, Org. Lett., 2011, 13, 3884–3887 CrossRef CAS.
- A. G. Leach and K. N. Houk, Chem. Commun., 2002, 1243–1255 RSC.
- R. Ossola, O. M. Jönsson, K. Moor and K. McNeill, Chem. Rev., 2021, 121, 4100–4146 CrossRef CAS.
- E. A. Lissi, M. V. Encinas, E. Lemp and M. A. Rubio, Chem. Rev., 1993, 93, 699–723 CrossRef CAS.
- R. Prieto-Montero, A. Prieto-Castañeda, R. Sola-Llano, A. R. Agarrabeitia, D. García-Fresnadillo, I. López-Arbeloa, A. Villanueva, M. J. Ortiz, S. de la Moya and V. Martínez-Martínez, Photochem. Photobiol., 2020, 96, 458–477 CrossRef CAS.
- K.-K. Chen, S. Guo, H. Liu, X. Li, Z.-M. Zhang and T.-B. Lu, Angew. Chem., Int. Ed., 2020, 59, 12951–12957 CrossRef CAS PubMed.
- T. Mirkovic, E. E. Ostroumov, J. M. Anna, R. van Grondelle, G. Govindjee and G. D. Scholes, Chem. Rev., 2017, 117, 249–293 CrossRef CAS.
- A. S. Weingarten, A. J. Dannenhoffer, R. V. Kazantsev, H. Sai, D. Huang and S. I. Stupp, J. Am. Chem. Soc., 2018, 140, 4965–4968 CrossRef CAS PubMed.
- K.-K. Chen, C.-C. Qin, M.-J. Ding, S. Guo, T.-B. Lu and Z.-M. Zhang, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2213479119 CrossRef CAS.
- K.-K. Chen, S. Guo, H. Liu, X. Li, Z.-M. Zhang and T.-B. Lu, Angew. Chem., Int. Ed., 2020, 59, 12951–12957 CrossRef CAS PubMed.
- S. Takizawa, R. Aboshi and S. Murata, Photochem. Photobiol. Sci., 2011, 10, 895–903 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nj03634d |
| ‡ Both these authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2025 |