DOI:
10.1039/D4NJ03858D
(Paper)
New J. Chem., 2025,
49, 2040-2049
Synthesis of benzylidene amino arylpyridinones and their AIE studies: dual metal sensing of Fe3+ and Hg2+†
Received
1st September 2024
, Accepted 10th October 2024
First published on 23rd October 2024
Abstract
Our research journey began with the successful synthesis and reporting of benzylidene amino aryl-pyridinone derivatives (4a–e) from corresponding pyranones. This process was conducted with meticulous attention to detail, ensuring the highest quality of results. These derivatives, treated with benzaldehyde and hydrazine hydrate under these controlled conditions, yielded an excellent yield. The benzylidene amino aryl-pyridinone, which contains electron-donor and acceptor groups, exhibited remarkable photophysical properties. We further evaluated the changes in the fluorescence properties of compound 4e in the presence of various cations. Our findings revealed compound 4e can be a highly sensitive and selective chemosensor for detecting Fe3+ and Hg2+ in DMSO solvent. The aggregated compound A-4e detected Fe3+ and Hg2+ with LODs of 1.03 × 10−7 M−1 and 1.71 × 10−7 M−1, which were lower than those in recent sensor reports. Presenting a novel approach to metal sensing will surely interest the scientific community. The application of compound 4e for the determination of Fe3+ and Hg2+ in spiked samples in different environmental water samples showed a satisfactory result with good recovery.
Introduction
In recent years, Fe3+ and Hg2+ have attracted significant attention due to their widespread applications in various fields, including environmental monitoring, biomedical sensors, and chelator development. Iron deficiency is associated with conditions such as anaemia,1 liver2 and kidney damage,3 and diabetes,4 when excess iron can lead to hepatitis, malignancies, organ degradation, neurodegenerative diseases5,6 and Parkinson's illnesses,7etc. Also, Fe3+ plays a vital role in biological systems and metabolism. On the other hand, mercury is more stable and highly toxic8 and exists in multiple forms, including (metallic, ionic, inorganic salts, and complex organic molecules).9 Interactions between metal ions and biological or environmental biomolecules have been the subject of extensive research. As an illustration, the microbial biomethylation of mercuric ions produces methyl mercury, which enters the body through the food chain and is known to induce chronic illnesses and brain damage.10 Fluorescence signaling is one of the best choices due to its high detection sensitivity. There are many fluorescence chemo-sensors to detect Zn,11 Cu, and Ni12 however, only a few reports available in the literature for sensing Fe3+ and Hg2+ in a water medium (the allowed concentration of both Fe3+ and Hg2+ ions in drinking water are 20 mM) The current research focuses on developing highly discriminating selective fluorescent chemo-sensors for Fe3+ and Hg2+ due to their importance in various applications, including environmental monitoring,13 biomedical14 biosensors15 and chelator development. A structure–property investigation shows that the standard hydrazone switch can generate enduring negative photochromic compounds by substituting a phenyl group for the rotational pyridyl one.16 In this context, our group has reported successful attempts to detect Fe3+ in the indeno-phenanthridine scaffold.17 In the present work, a rational design and synthetic approach were employed to yield a dihydropyridine-based compound (E)-1-((4-methylbenzylidene)amino)-4-(methylthio)-6-(naphthalen-2-yl)-2-oxo-1,2-dihydropyridine-3-carbonitrile 4a.18–21 The synthesis of target molecule 4 involving pyranones22–24 and hydrazine monohydrate25 in ethanol at reflux conditions yielded excellent outcomes.26 The electron withdrawing and donating groups were identified as an effective fluorescent sensor for Hg2+ even in the presence of other metal cations, including Fe3+, which might interfere with its detection in the aqueous medium. Notably, the heterocyclic compound 3,4-dihydro-2H-pyran has a prominent theme in medicinal chemistry,27 and it may be found in several naturally occurring physiologically active substances with wound-healing properties.28 The hydrazide-hydrazone significant motif has been reported to have diverse pharmacological activities, especially anticancer activity with low detection limits. Over the past decade, aggregation-induced emission luminogens (AIEgens) have gained prominence for their applications in chemical sensor and theragnostic probes for biomedical contexts due to their brightness and excellent photo-stability29 and also their wide range of uses in fluorescence probes, memory storage, and photoelectric devices, In several electrical and photonic applications, such as semiconductor lasers, fluorescence sensors, organic light-emitting diodes (OLEDs),30 stimuli-responsive sensing, bioimaging,31 and optoelectronic devices, owing to their ability to adjust and regulate fluorescence hues.32
Results and discussion
This work describes a simple and effective method for replacing oxygen atoms with amines pyrone 1a molecule. The strategy was highly effective, resulting in a minimum yield of 20% of the desired product 4a by involving a mixture of 1a, 2, and 3avia a one-pot multi-component approach under reflux condition in methanol (Table 1, entry 2). NMR and mass spectroscopy confirmed the product 4a (Schemes 1–3).
Table 1 Optimized solvent screening
| Entry |
Conditions |
Solvents |
Time (h) |
Isolated yield (%) |
| 1 |
RT |
Ethanol |
2 h |
0 |
| 2 |
Reflux |
Ethanol |
2 h |
20 |
| 3 |
Reflux |
Methanol |
2 h |
40 |
| 4 |
Reflux |
DMSO |
2 h |
50 |
| 5 |
Reflux |
DMF |
2 h |
30 |
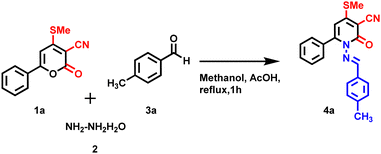 |
| | Scheme 1 Optimization of reaction condition to synthesis of 4a-one-pot multicomponent reaction. | |
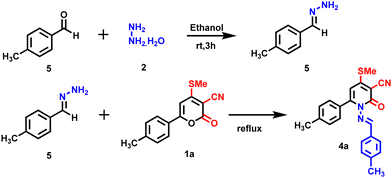 |
| | Scheme 2 Control reaction for the synthesis of 4a. | |
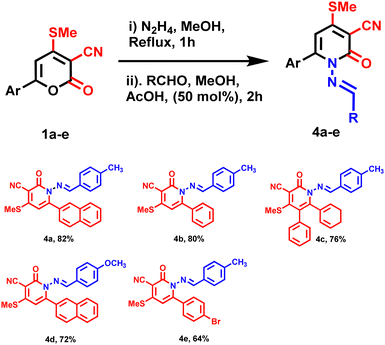 |
| | Scheme 3 Synthesis of (E)-1-((4-methylbenzylidene)amino)-4-(methylthio)-6-(naphthalene-2-yl)-2-oxo-1,2-dihydropyridine-3-carbonitrile (4a–e). | |
Due to the poor yield of product 4avia a one-pot multicomponent reaction, the step-wise method was tried to achieve a high yield. Initially, compound 1a and hydrazine monohydrate 2 were treated separately under reflux conditions in methanol for one hour. After compound 1a was consumed completely (TLC), 4-methyl benzaldehyde 3a was added dropwise, leaving the reaction for another 2 h under reflux conditions. Interestingly, the reaction was completed, and the reaction mixture was purified and obtained product 4a in excellent yield 40%) (Table 1, entry 3). The same method was tried with various solvents such as DMSO (50%), and DMF (30%), which obtained moderate yield (Table 1, entries 4 and 5). When the reaction was performed in the presence of acetic acid (50 mole %) for 2 h under reflux conditions obtained, product 4a had an excellent yield (80%) (Table 1, entry 6).
Further, several controlled reactions were attempted to improve the yield of 4a (Table 2). Initially, compound 1a was treated with hydrazone 5 (prepared in situ by reacting hydrazine with benzaldehyde) in ethanol under reflux conditions; however, no product 4a was observed. Continually, the reaction with various basic conditions, including triethyl amine (TEA), NaOH, KOH, and KHCO3 (Table 2, entries 1–4), was tested to optimize the reaction condition, but no product was observed. Subsequently, the product 4a was obtained with lower yield when a catalytic amount of trifluoroacetic acid (TFA) (40%) and PTSA/acetic acid (20%) (Table 2, entries 5 & 7) in the presence of acetic acid under reflux condition. After several attempts, the reaction condition was optimized to obtain the product 4a in 80% yield in the presence of acetic acid under reflux condition in a step-wise manner (Table 1, entry 6)
Table 2 Optimization of reaction conditions of various catalysts to synthesis of 4a
| Entry |
Catalysts |
Solvents |
Time (h) |
Isolated yield (%) |
| 1 |
TEA |
Methanol |
2 h |
Nil |
| 2 |
NaOH |
Methanol |
2 h |
Nil |
| 3 |
KOH |
Ethanol |
2 h |
Nil |
| 4 |
KHCO3 |
Ethanol |
2 h |
Nil |
|
5
|
AcOH
|
Methanol
|
2 h
|
80
|
| 6 |
TFA |
Acetic acid |
2 h |
40 |
| 7 |
PTSA |
Acetic acid |
2 h |
50 |
Following the optimized reaction condition, compound 4a–e was synthesized with an excellent yield (80–90%) (Table 2 entry 5). All the products underwent thorough characterization through various NMR spectroscopies (1H, 13C) and exact mass spectrometry (HRMS). In previous findings, the aim was to optimize the reaction conditions to attain the maximum yield. Both electron-donating and withdrawing substituents gave corresponding yields under the optimized reaction conditions. Column chromatography purified all the derivatives 4a–e which were characterized with 1H, 13C NMR, and HRMS.
The plausible reaction mechanism was proposed for synthesizing 4a–e under optimized reaction conditions (Scheme 4). Nucleophilic for constructing compound 4a, the first step is the formation of I by the reaction of substituted pyrones 1 and hydrazine monohydrate 2. The nucleophilic attack of hydrazine hydrate to a more electrophilic carbonyl lactone ring causes an opening of the lactone ring (I) and the transfer of a hydrogen atom to form II. The nucleophilic attack of lone pair of electrons on nitrogen to carbonyl via cyclization to form intermediate IV followed by removal of a water molecule to form amide groups to obtain VI (1-amino-4-(methylthio)-6-(naphthalen-2-yl)-2-oxo-1,2-dihydropyridine-3-carbonitrile) derivatives. Further, reactions with different aromatic aldehydes proceeded via condensation to form 4. The other method was followed by reacting 1 and 5 to form 4via intermediate IV.
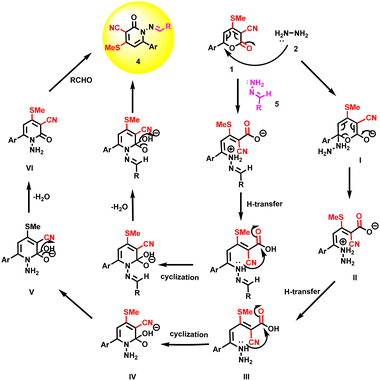 |
| | Scheme 4 Plausible mechanism pathway of compound 4. | |
Photophysical properties
UV-vis absorption and fluorescent properties of compounds 4a–e were studied in DMSO solvent, as shown in Fig. S21 (ESI†) and Fig. 2, and summarized in Table 3. The initial solvatochromism studies of all five (4a–e) derivatives were screened using various solvents in DMSO, ACN, methanol, THF, DCM, and hexane (50 μL each) with different polarities. There were no changes in the wavelength, but a notable change in increasing fluorescence intensity (4a–e in DMSO) was observed in the UV/Emission spectrum while increasing the solvent polarity, the maximum absorption of 4a–e in DMSO (50 μL) was found to be around 300–390 nm, shown in Fig. S21 (ESI†). Which may be attributed to the π–π* or n–π* transitions. The compounds 4b and 4d showed two absorption peaks with maxima in the range of 300–380 nm, with the highest absorption band at 315 and 370 nm due to π–π* transitions and the lowest absorption band at 370 and 315 nm corresponding to n−π* transitions respectively shown in Fig. S21 (ESI†) (4b & 4d). However, the bromo-substituted compound 4e causes a red shift of the absorption spectra compared to 4a–d. The fluorescence spectrum of 4a–e was recorded in DMSO (5 × 10−5 M) with an excitation maximum wavelength of 390 nm, and maximum emission intensity was observed at 360–450 nm (Fig. 2 and Table 3). Interestingly, dual emission was observed for compounds 4c and 4d due to the excited state's intramolecular charge transfer (ICT). The emission maxima of compound 4a, located at 360 nm with a Stokes shift of 5556 cm−1, leads to a moderate blue shift of a shorter wavelength compared to all the other derivatives. Due to the presence of the bulky aryl group and the methoxy group more strongly electron-donating in compound 4d, a higher Stokes shift of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 594 cm−1 was obtained compared to the present in methyl group electron-donating of all the other derivatives. Relative fluorescence quantum yields were measured in DMSO solvent (Table 3).
594 cm−1 was obtained compared to the present in methyl group electron-donating of all the other derivatives. Relative fluorescence quantum yields were measured in DMSO solvent (Table 3).
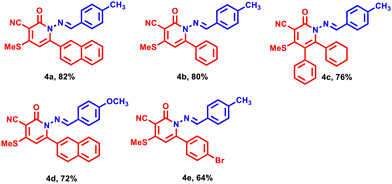 |
| | Fig. 1 Benzylidene amino arylpyridinones derivatives 4a–e. | |
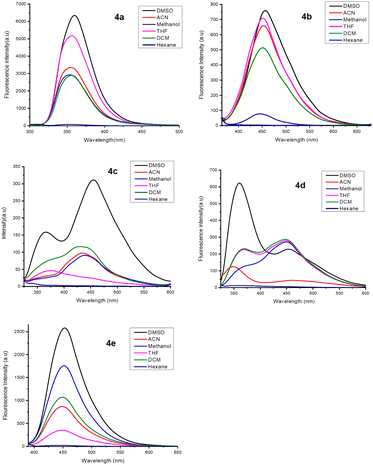 |
| | Fig. 2 Fluorescence spectra of 4a–e (5 × 10−5 M) in various solvents. | |
Table 3 Photophysical properties of compounds 4a–e in DMSO solvent
| S. No |
Compound |
∑/mol−1 dm3 cm−1 |
λ
abs maxa (nm) |
λ
Em maxa (nm) |
Stokes shiftb cm−1 (nm) |
ΦSflc (%) |
|
The absorbance and fluorescence spectra of DHP molecules were recorded at 5 × 10−5 M concentration dissolved in DMSO solvent.
Stokes shift = UV − Fl.
The fluorescence quantum yields (ΦSfl).
|
| 1 |
4a
|
180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
300 |
360 |
5556 (060) |
0.78 |
| 2 |
4b
|
84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
320 |
450 |
9027 (130) |
0.02 |
| 3 |
4c
|
64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
320 |
450 |
9027 (130) |
0.05 |
| 4 |
4d
|
392![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
300 |
460 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 594 (160) 594 (160) |
0.13 |
| 5 |
4e
|
108![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
370 |
450 |
4804 (080) |
0.06 |
| 6 |
A-4e
|
192![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
350 |
480 |
7738 (130) |
0.30 |
Compound 4e showed strong red fluorescence in the solid state due to the nonbonding interaction of the lone pair of N and O electrons in the DHP scaffold, favoring a bathochromic shift. Compound 4e shows the emission wavelength 450 nm in the solution state. Interestingly, the 4e exhibits a longer wavelength of 530 nm in the solid state, which shows a bathochromic shift by 80 nm compared to the solution state (Fig. 3).
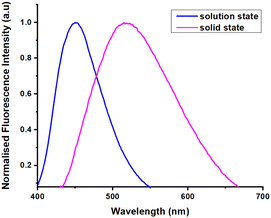 |
| | Fig. 3 The fluorescence spectrum of 4e in solution state (5 × 10−5 M) in DMSO and solid state. | |
Fluorescence was characterized using a spectrofluorometer in 3D scanning mode, measuring fluorescence intensity across emission and excitation wavelengths. The 3D fluorescence spectra of compound 4e revealed green fluorescence in the 380–480 nm range (Fig. 4(a)), while compound A-4e exhibited red fluorescence between 350–460 nm (Fig. 4(b)). As the water ratio increased, a significant enhancement in fluorescence emission intensity was observed. In the solid state, 4e displayed a broad red fluorescence, ranging from 420 nm to 530 nm. The fluorescence intensity was visualized as contour maps in excitation-emission wavelength space, providing a detailed representation of the spectral behavior. The 3D fluorescence spectra were visualized as contour maps, providing detailed insight into the excitation-emission behavior of the compounds.
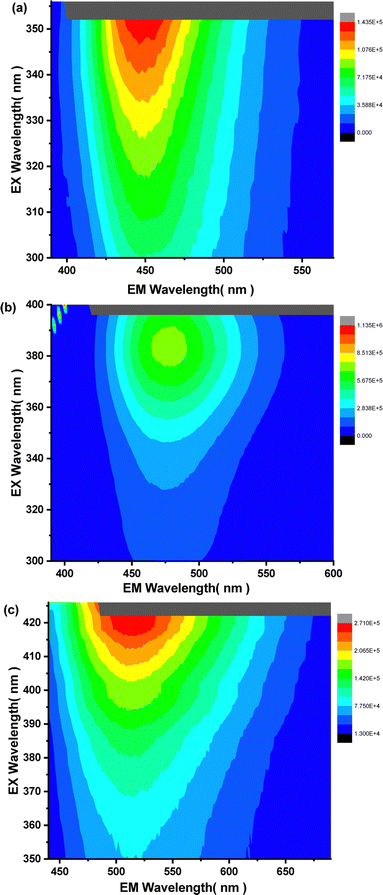 |
| | Fig. 4 (a) 3D-spectrum of 4e (5 × 10−5 M) in DMSO (b) 3D-spectrum of A-4e (5 × 10−5 M) in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (7 water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) solvent mixtures (c) 3D-spectrum of 4e in solid state. 3) solvent mixtures (c) 3D-spectrum of 4e in solid state. | |
The presence of a pyridine unit in 4e and its exhibit of longer wavelength at 530 nm in solid state and insoluble characteristics in water encouraged us to study the aggregation-induced emission property of 4e in DMSO Water solvent mixtures. The 100% DMSO solution 4e is emissive with λmax at 450 nm. The photoluminescence feature of 4e is gradually increased by adding water fraction. It was observed that upon the addition of 40% of water fraction, the emission spectra of 4a–e progressively bathochromic-shift or red-shifted to 460 nm with increasing the emission intensity. With the addition of 70% water fractions, the increasing the emission intensity with luminescence maximum exhibited green solid emission in the aggregated state, with red-shifted to 480 nm with high fluorescence quantum yield (Φf = 30.88%). The absence of luminescence for 4a–e in the diluted DMSO solution might be attributed to intramolecular rotations of N–N single bonds in the molecules. Intermolecular face-to-face π–π interactions between two neighboring DHPs are still found in the aggregates of the cross-stacking (j-type aggregation) 4a–e scaffold. The generation of nanoparticles is confirmed by the scanning electron microscopy (SEM) pictures of the combined samples 4e (Fig. 5b). Thus, enhanced fluorescence intensity was observed for 4e in the aggregated state.
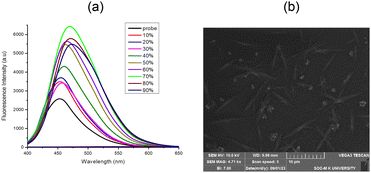 |
| | Fig. 5 (a) The fluorescence spectra 4e (5 × 10−5 M) in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solvent mixtures. (b) SEM image of 4e in DMSO/water mixture with 70% water fraction. water solvent mixtures. (b) SEM image of 4e in DMSO/water mixture with 70% water fraction. | |
Interestingly, the fluorescence intensity of A-4e was affected based on low temperatures at 5 °C to −5 °C in the solution state. The emission intensity of probe A-4e increased maxima at −5 °C low temperature, and there was no other change in wavelength. High-temperature fluorescence (0 °C to 90 °C) also studied A-4e, which was found to be the emission peak maximum at 480 nm, was found to remain affected by temperature. As shown in Fig. 7, one observes the emission spectrum of A-4e showing a drastic decrease towards blue shifted to the fluorescence intensity at λmax = 470 nm with increasing temperature. Therefore, the probe's low temperature-dependent properties could lead to a wide range of potential future applications in low-temperature thermal sensing.
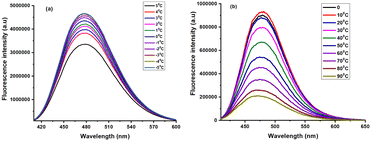 |
| | Fig. 6 Temperature dependant of 4e and A-4e Fluorescence properties (5 × 10−5 M) in DMSO. | |
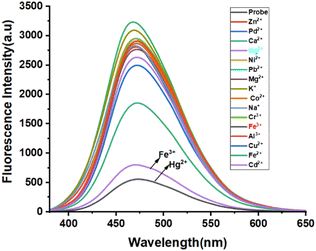 |
| | Fig. 7 Metal selectivity and fluorescence spectrum of 4e (5 × 10−5 M) in DMSO upon addition of various cations (1 × 10−2 M). | |
To evaluate the metal cation selective nature of 4e, the sensing abilities of compound 4e (50 μM) were studied in DMSO solvent through the addition of various cations, such as Zn2+, Pd2+, Ca2+, Hg2+, Ni2+, Pb2+, Mg2+, K+, Co2+, Na+, Cr3+, Fe3+, Al3+, Cu2+, Fe2+, Cd2+ (1 × 10−2 M in water) at room temperature. No significant changes in the UV-vis absorption were noted upon adding various metal ions to the probe solution (Fig. 5). However, a significant blue shift at 320 and 350 nm was observed upon adding Fe3+ and Hg2+, respectively (Fig. S3, ESI†). Surprisingly, compassionate and good selective fluorescence change of 4e occurred in the presence of (300 μM) of these cations (Fig. 5) for Fe3+ and Hg2+ detection in DMSO solvent. At the same time, other metal ions did not cause any significant changes under identical conditions. Significant strong fluorescence in the monomer emission (Fig. 5) was observed, but there was a small revival of fluorescence “off” quenching emission with the addition of Fe3+ and Hg2+. The selectivity observed for Fe3+ over other ions is remarkably low fluorescence. Further, the UV and emission spectrum of probe A-4e (5 × 10−5) with various concentrations of Fe3+ and Hg2+ are equivalent from 0–300 μM. In addition, the Fluorescence quenching intensity resulting from adding Fe3+ and Hg2+ is not influenced by the subsequent addition of other metal ions. The addition of Fe3+ and Hg2+A-4e for the quenching efficiency and the binding constants detection of Fe3+ and Hg2+ were observed 2.6 × 10−5 M−1 and 4.31 × 10−5 M−1 respectively, by plotting the fluorescence intensity against the concentration of Fe3+ and Hg2+ displayed an excellent linear correlation. Using the Stern–Volmer equation, the displayed low limits of detection (LODs) of 1.03 × 10−7 M and 1.71 × 10−7 M were obtained, respectively, using the 3σ/S formulae. The binding stoichiometric ratios of 4e to Fe3+ and Hg2+ were obtained from Job's plot measurements based on the fluorescence intensity, and the result suggested that the stoichiometric ratio of compound 4e to Fe3+ and Hg2+ was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S4, ESI†). An interference study of probe A-4e (5 × 10−5 M) in DMSO solvent presence of various metal ions was carried out (Fig. S4 and S5, ESI†) (Fig. 8 and 9).
1 (Fig. S4, ESI†). An interference study of probe A-4e (5 × 10−5 M) in DMSO solvent presence of various metal ions was carried out (Fig. S4 and S5, ESI†) (Fig. 8 and 9).
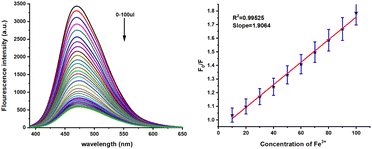 |
| | Fig. 8 Metal sensitivity of 4e (5 × 10−5 M) in various concentrations of Fe3+ (0–300 μL) in DMSO (left) and the linear plot of the fluorescence intensity versus concentration of Fe3+ (right). | |
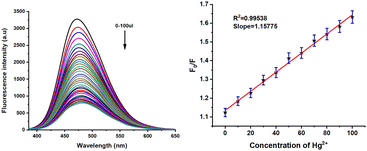 |
| | Fig. 9 Metal sensitivity of 4e (5 × 10−5 M) in various concentrations of Hg2+ (0–300 μL) in DMSO (left) and the linear plot of the fluorescence intensity versus concentration of Hg2+ (right). | |
FTIR studied the sensing mechanism of 4e toward Fe3+ and Hg2+ the sensing process. As illustrated in Fig. S29 (ESI†), in the absence of Fe3+ and Hg2+, 4e exhibited an intense peak at 1647.2739 cm−1, which was attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations. 1665.89096 cm−1 However, with the addition of Fe3+ and Hg2+, this peak shifted to 1639.1986 cm−1 and 1653.7396 cm−1 which provides evidence for the participation of the C
O stretching vibrations. 1665.89096 cm−1 However, with the addition of Fe3+ and Hg2+, this peak shifted to 1639.1986 cm−1 and 1653.7396 cm−1 which provides evidence for the participation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of 4e involved in the ionic interaction with Fe3+ and Hg2+ ions, respectively shown in (Fig. S29, ESI†). To elucidate the fluorescence quenching mechanism of the probe in response to Fe3+ and Hg2+ metal cations, density functional theory (DFT) calculations were performed using Gaussian 09 for the probe, probe + Fe3+, and probe + Hg2+ with appropriate basis sets (Fig. S28, ESI†). Frontier molecular orbital analysis revealed distinct electronic distributions: in probe 4e, the HOMO predominantly resides over the amino and cyano hydrazone aroyl groups, while the LUMO extends across the entire aryl pyridine structure. Contrastingly, in 4e + Fe3+, the HOMO shifts primarily to the pyridine hydrazone moiety, with the LUMO localized over the pyridine unit and iron. Similarly, in 4e + Hg2+, the HOMO localizes over the mercury atom. At the same time, the LUMO spans the entire molecule (Fig. S28, ESI†). Moreover, the calculated energy gap differences of HOMO–LUMO transitions for 4e + Fe3+ (−0.25 eV) and 4e + Hg2+ (−0.30 eV) were found to be narrower compared to probe 4e (−0.31 eV), suggesting favorable coordination between probe 4e and Fe3+, as well as Hg2+. These findings indicate an internal charge transfer (ICT) process between the electron-donating aryl group in the benzylidene amino ring and the electron-withdrawing CN group in the pyridine ring D–A system, which induces fluorescence enhancement in probe 4e. This ICT process is disrupted upon complexation with Fe3+ and Hg2+, leading to fluorescence quenching (Scheme 5).
O bond of 4e involved in the ionic interaction with Fe3+ and Hg2+ ions, respectively shown in (Fig. S29, ESI†). To elucidate the fluorescence quenching mechanism of the probe in response to Fe3+ and Hg2+ metal cations, density functional theory (DFT) calculations were performed using Gaussian 09 for the probe, probe + Fe3+, and probe + Hg2+ with appropriate basis sets (Fig. S28, ESI†). Frontier molecular orbital analysis revealed distinct electronic distributions: in probe 4e, the HOMO predominantly resides over the amino and cyano hydrazone aroyl groups, while the LUMO extends across the entire aryl pyridine structure. Contrastingly, in 4e + Fe3+, the HOMO shifts primarily to the pyridine hydrazone moiety, with the LUMO localized over the pyridine unit and iron. Similarly, in 4e + Hg2+, the HOMO localizes over the mercury atom. At the same time, the LUMO spans the entire molecule (Fig. S28, ESI†). Moreover, the calculated energy gap differences of HOMO–LUMO transitions for 4e + Fe3+ (−0.25 eV) and 4e + Hg2+ (−0.30 eV) were found to be narrower compared to probe 4e (−0.31 eV), suggesting favorable coordination between probe 4e and Fe3+, as well as Hg2+. These findings indicate an internal charge transfer (ICT) process between the electron-donating aryl group in the benzylidene amino ring and the electron-withdrawing CN group in the pyridine ring D–A system, which induces fluorescence enhancement in probe 4e. This ICT process is disrupted upon complexation with Fe3+ and Hg2+, leading to fluorescence quenching (Scheme 5).
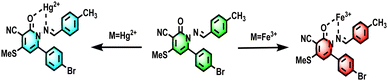 |
| | Scheme 5 Sensing mechanism of 4e with Fe3+and Hg2+ in aqueous solution. | |
Electrochemical studies
A cyclic voltammetry (CV) experiment was carried out to determine the energy potentials for the electrochemical reduction/oxidation of probe A-4e. Electrochemical measurements were done using a CV in a three-electrode system. The CV experiment was carried out in 0.1 M acetonitrile solvent containing tetrabutylammonium per bromide (TBAPB) as the supporting electrolyte, shown in Fig. 6. The reference electrode, Ag/AgCl, was directly immersed in the reaction cell, and the working electrode was a glassy carbon electrode. The counter electrode was a platinum wire. The scan rate was commonly 50 mV S−1. CV measurements were measured for the probe A-4e at a concentration of 5 × 10−5 M. The Fe3+ and Hg2+ ion concentrations of 10−2 M were gradually added to A-4e, and their electrochemical changes were monitored at room temperature. The electrochemical response of A-4e with diverse metal ions in acetonitrile was tested using cyclic voltammetry in the potential window of 0 V to −2 V.
The electrochemical responses of the highest occupied molecular orbital (HOMO)/lowest unoccupied molecular orbital (LUMO) and the band gap energy potentials of compounds A-4e, A-4e + Fe3+, A-4e + Hg2+ the data obtained are summarized (in Fig. 10 and Table 4). Cyclic voltammograms are shown (in Fig. S27, ESI†) in the ESI† section. The appearance of a new reduction peak showed that E1/2 values shifted to a more negative potential (−1.20 V) range from −0.95 to −1.99 V, as shown in (Fig. 10 and Table 4 entry A-4e). The peak current value was enhanced (−0.06 mA), ranging from −0.011 to −0.054 mA, whereas the reduction peak was shown by adding Fe3+ ion (Fig. 10 and Table 4 entry A-4e + Fe3+). E1/2 values shifted to a more negative potential (−1.52 V) range from −0.95 to −1.99; the peak current value was enhanced (−0.07 mA) range from −0.01 to −0.06 mA. The reduction peak was shown by adding the Hg2+ ion (Fig. 10 and Table 4 entry A-4e + Hg2+). E1/2 values shifted to less negative potential (−1.45 V), ranging from low current values compared to probe Fe3+. The peak current value was enhanced (−0.08 mA), ranging from −0.011 to −0.073 mA. The negative potential shift value from −1.20 to −1.52 V indicates the sharing of electrons from the nitrogen and oxygen atoms of A-4e to the metal ions Fe3+ and Hg2+. Overall, the electrochemical results of compound 4e are in good agreement with those from the absorption wavelength.
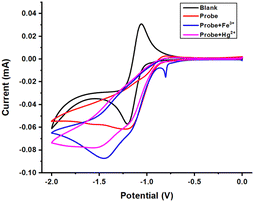 |
| | Fig. 10 Cyclic voltammograms for compounds probe, probe + Fe3+, and probe + Hg2+ were recorded in acetonitrile, with glassy carbon as a working electrode, Ag/AgCl as a reference electrode, and Pt as a counter electrode. | |
Table 4 Electrochemical Data of A-4e, A-4e + Fe3+, A-4e + Hg2+ in Acetonitrile Solvent with 0.1 M at a Scan Rate of 50 mV S−1
| Entry |
E
oxd
|
E
red
|
HOMO (ev) |
LUMO (ev) |
Band gap (Eg) |
|
A-4e
|
−0.79 |
−1.20 |
2.94 |
2.53 |
0.41 |
|
A-4e + Fe3+ |
−0.73 |
−1.52 |
3.02 |
2.21 |
0.81 |
|
A-4e + Hg2+ |
−0.73 |
−1.45 |
3.02 |
2.28 |
0.74 |
Fluorescence lifetime
Additionally, the mechanism behind the selective sensing of Fe3+ and Hg2+ over other metal ions in aqueous conditions was confirmed by time-correlated single photon counting (TCSPC). One useful method for differentiating between these quenching mechanisms is to measure the fluorescence lifetime. The analysis and display of 4eA-4e, A-4e + Fe3+, and A-4e + Hg2+ findings in DMSO were done in (Fig. 11). The decay time of probe 4e (0.96 ns) A-4e (1.54 ns) A-4e + Fe3+ (1.47 ns) A-4e + Hg2+ (0.89). The results revealed that τ0/τ1 = 1, indicating that a static quenching occurs between probe A-4e and Fe3+ and Hg2+ ions.
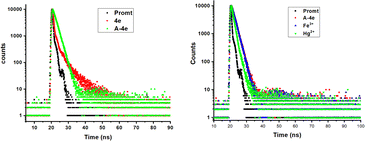 |
| | Fig. 11 TCSPC-time correlated single photon counting spectrometer for probe 4e, A-4e (left), A-4e, A-4e + Fe3+, and A-4e + Hg2+(right). | |
Real sample analysis
To assess the practical application of compound 4e for detecting Fe3+ and Hg2+, an environmental water sample analysis was conducted using the spiking method for quantitative measurement. Sensor 4e demonstrated sensitivity to Fe3+ and Hg2+ detection in various water sources, including groundwater, river water, and seawater. For the analysis, these water samples were spiked with different concentrations of Fe3+ and Hg2+ analyzed using the proposed method. The results, summarized in a table, showed recovery rates ranging from 94.2% to 104.3% (Tables 5 and 6) (Fig. 12 and 13).
Table 5 Determination of Fe3+ and Hg2+ in different water samples
| Sample |
Analyte |
Spiked analyte concentration |
Recovery (%) |
| Ground water |
Fe3+ |
3 × 10−4 |
99.5 |
| River water |
Fe3+ |
3 × 10−4 |
103.4 |
| Sea water |
Fe3+ |
3 × 10−4 |
100.4 |
| Ground water |
Hg2+ |
3 × 10−4 |
104.3 |
| River water |
Hg2+ |
3 × 10−4 |
94.2 |
| Sea water |
Hg2+ |
3 × 10−4 |
97.2 |
Table 6 Comparison of the reported Fe3+ and Hg2+ sensors with hydrazide–hydrazone molecule
| Structure of Chemo sensor |
Sensing medium |
Selectivity |
Limit of detection (LOD) |
Sensing mechanism/fluorescence response |
Ref. |
|
H2O/CH2OH |
Hg3+ |
3 × 10−7 M |
Turn-off |
33
|
|
CH3CN:H2O |
Hg2+ |
— |
Turn off–on |
34
|
|
DMSO |
Fe3+ |
3.87 μM |
Turn-on |
35
|
|
CH3CN-Tris |
Fe3+ |
9.83 × 10−8 M |
Turn-off/on |
36
|
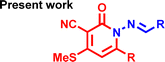
|
DMSO:H2O |
Fe3+, Hg3+ |
1.03 × 10−7 and 1.71 × 10−7 M |
Turn-off |
— |
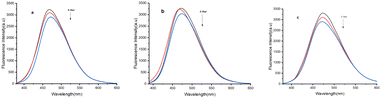 |
| | Fig. 12 Emission spectra of A-4e involved detection of Fe3+ in real water samples (a) ground water (b) river water (c) sea water. | |
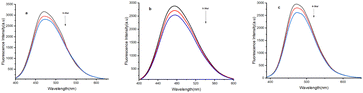 |
| | Fig. 13 Emission spectra of A-4e involved the detection of Hg2+ in real water samples (a) ground water (b) river water (c) sea water. | |
Experimental details
Materials and reagents
The chloride and nitrate salts of Fe2+, Cu2+, Zn2+, Pd2+, Ca2+, Hg2+, Ni2+, Ag2+, Pb2+, Mg2+, K+, Co2+, Na+, Cr3+, Fe3+, Al3+, and all other reagents were purchased from Sigma-Aldrich. The analytical grade solvents were purchased from Avra Chemicals and used without further purification. Aromatic aldehydes and methyl cyanoacetate were purchased from TCI Chemicals and Alfa Aesar and used without purification.
General information
The NMR spectra were recorded using a Bruker 40a Pt wire counter electrode, and a d6 as the solvent with the chemical shift referring to tetramethyl silane (TMS), (1H and 13C NMR) as internal standard Splitting patterns were designated as follows: s, singlet; bs, broad singlet; d, doublet; dd, doublet of doublet; t, triplet; m, multiplet. Melting points were determined on the melting point apparatus equipped with a thermometer, which was uncorrected. FTIR spectra of the synthesized organic compounds were recorded using a Jasco 4100 spectrometer. UV-visible and fluorescence spectra in the solution state were recorded using a spectramax m2e spectrophotometer, and solid states were measured using a TCSPC-fluorescence spectrophotometer. The fluorescence quantum yields (Φfl) were measured by using HORIBA-Fluoromax. Analytical thin-layer chromatography (TLC) was performed on precoated plates (Merck, silica gel 60F254). Silica gel-G plates (Merck) were used for TLC analysis with a mixture of distilled petroleum ether (60–80 °C) and ethyl acetate as eluent. Silica gel (60–120 mesh) was used for column chromatography. Electrochemical measurements were made using an SP-150 Biologic electrochemical analyzer. A conventional three-electrode configuration consisted of a glassy carbon working electrode, a Pt wire counter electrode, and a non-aqueous Ag/AgCl reference electrode. The supporting electrolyte was 0.1 M tetrabutylammonium bromide (TBAB). The fluorescence lifetime measurements were carried out using a single-photon-counting method. HORIBA-Fluoromax TCSPC measured the fluorescence decay curves measured the fluorescence decay curves.
Determine the limit of detection (LOD)
Based on absorption titration, the limit of detection was determined. Five further measurements were taken to determine the standard deviation of the probes' absorption spectra obtained. The slopes of the calibration plot between the absorbance intensity and the concentration of the analytes (Fe3+ and Hg2+) were utilized in the following equation to get the LOD.
The detection threshold is 3σ/s. Where s is the slope value obtained from the calibration plot and is the probe's standard deviation.
Preparation of stock solution
Probe 4a–e stock solution (5 × 10−5 M) was prepared in HPLC-grade DMSO solvent. Analytes chloride and nitrate salts of Fe2+, Cu2+, Zn2+, Pd2+, Ca2+, Hg2+, Ni2+, Ag2+, Pb2+, Mg2+, K+, Co2+, Na+, Cr3+, Fe3+, Al3+, Cu2+ Fe2+and Cd2+ ions were dissolved in double-distilled water.
General procedure for the synthesis of ((E)-1-((4-methylbenzylidene)amino)-4-(methylthio)-6-(naphthalen-2-yl)-2-oxo-1,2-dihydropyridine-3-carbonitrile)(4a). Initially, pyrones 3 treated with hydrazine monohydrate in methanol as a solvent under reflux condition. After one hour, aldehyde was added to the reaction mixture, and acetic acid was added to the mixture and stirred. We evaluated the reaction using TLC and monitored its completion. Then, the reaction mixture was quenched with an ice cube and stirred for 10 minutes. A pale brown precipitate was formed, which was collected and filtered off. Recrystallized to obtain pure ((E)-1-((4-methylbenzylidene)amino)-4-(methylthio)-6-(naphthalen-2-yl)-2-oxo-1,2-dihydropyridine-3-carbonitrile 4a.
(E)-1-((4-methylbenzylidene)amino)-4-(methylthio)-6-(naphthalen-2-yl)-2-oxo-1,2-dihydropyridine-3-carbonitrile 4a pale brown, yield 90%, m.p.-above 200 °C.
1H NMR (400 MHz, DMSO) δ 12.36 (s, 1H), 7.20 (s, 2H), 6.80 (s, 6H), 6.40 (s, 3H), 5.68 (s, 1H), 2.96 (s, 3H), 2.54–2.17 (m, 1H) (Fig. 1) 13C NMR (101 MHz, DMSO) δ 186.65, 167.96, 161.70, 144.13–143.93, 143.69, 133.54, 133.54, 133.03, 133.03, 129.04, 129.04, 128.53, 128.48, 128.32, 128.15, 127.19, 127.19, 126.84, 126.84, 124.18, 124.18, 123.92, 123.92, 118.91, 118.91, 102.49, 102.49, 17.07, 17.07 (Fig. 2). FTIR/cm−1 (KBr): 1604 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2357 (–C Ξ N), 1659 (–C
O), 2357 (–C Ξ N), 1659 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1242 (C
N), 1242 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1518 (C–C), 769 (C–H) (Fig. S20, ESI†). HRMS (ES MS TOF) m/z: [M − 2H + K]− Calcd for C21H16BrN3OS 410.1282, found 446.1282 (Fig. S11, ESI†).
C), 1518 (C–C), 769 (C–H) (Fig. S20, ESI†). HRMS (ES MS TOF) m/z: [M − 2H + K]− Calcd for C21H16BrN3OS 410.1282, found 446.1282 (Fig. S11, ESI†).
(E)-6-(cyclohexane-1,5-dien-1-yl)-1-((4-methylbenzylidene)amino)-4-(methylthio)-2-oxo-1,2-dihydropyridine-3-carbonitrile 4b brown solid, yield 80%, m.p.-above 200 °C.
1H NMR (400 MHz, DMSO) δ 12.07 (s, 1H), 6.82 (d, J = 8.1 Hz, 1H), 6.53 (d, J = 8.0 Hz, 3H), 6.42–6.34 (m, 2H), 6.22 (t, J = 7.5 Hz, 3H), 6.14 (s, 1H), 5.45 (s, 1H), 2.81 (s, 3H), 2.16 (s, 3H) (Fig. S3, ESI†).13C NMR (101 MHz, DMSO) δ 172.00, 169.65, 161.46, 157.65, 133.32, 133.16, 130.23, 129.70, 129.42, 129.02, 128.63, 127.45, 127.34, 125.59, 118.81, 114.60, 102.02, 100.01, 89.64, 16.71, 14.78 (Fig. S4, ESI†). FTIR/cm−1 (KBr): 1606 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2360 (–C Ξ N), 1686 (–C
O), 2360 (–C Ξ N), 1686 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1515 (–C–C),1252 (C
N), 1515 (–C–C),1252 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) 769 (–C–H) (Fig. S17, ESI†). HRMS m/z: [M + Na]+ Calcd for C21H17N3OSNa 359.1092. found 382.0938 (Fig. S12, ESI†).
C) 769 (–C–H) (Fig. S17, ESI†). HRMS m/z: [M + Na]+ Calcd for C21H17N3OSNa 359.1092. found 382.0938 (Fig. S12, ESI†).
(E)-6-(cyclohexane-1,5-dien-1-yl)-1-((4-methylbenzylidene)amino)-4-(methylthio)-2-oxo-5-phenyl-1,2-dihydropyridine-3-carbonitrile 4c pale yellow solid, yield 75%, m.p.-above 200 °C.
1H NMR (400 MHz, DMSO-d6) δ 11.91 (s, 0H), 6.67 (d, J = 8.1 Hz, 0H), 6.38 (d, J = 8.0 Hz, 1H), 6.23 (dt, J = 15.3, 7.2 Hz, 1H), 6.07 (t, J = 7.5 Hz, 1H), 5.97 (t, J = 7.0 Hz, 1H), 5.86 (s, 0H), 5.30 (s, 0H), 2.65 (s, 1H), 2.01 (s, 1H) (Fig. S5, ESI†). 13C NMR (101 MHz, DMSO) δ 169.78, 166.53, 164.05, 163.47, 142.21, 139.62, 130.80, 130.25, 130.17, 128.82, 128.50, 128.23, 128.06, 127.90, 127.73, 127.59, 127.50, 127.28, 126.65, 126.50, 126.41, 126.20, 116.76, 116.31, 61.66, 14.26, 12.44 (Fig. S6, ESI†). FTIR/cm−1 (KBr): 1606 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2359 (–C Ξ N), 1678 (–C
O), 2359 (–C Ξ N), 1678 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1513 (–C–C), 1252 (C
N), 1513 (–C–C), 1252 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) 772 (C–H) (Fig. S18, ESI†). HRMS (ES MS TOF) m/z: [M + H]+ Calcd for C27H21N3OS 437.1605, found fragmentation peak [M + H]+ Calcd for C19H15N3OS at 334.10 (Fig. S13, ESI†).
C) 772 (C–H) (Fig. S18, ESI†). HRMS (ES MS TOF) m/z: [M + H]+ Calcd for C27H21N3OS 437.1605, found fragmentation peak [M + H]+ Calcd for C19H15N3OS at 334.10 (Fig. S13, ESI†).
(E)-1-((4-methoxybenzylidene)amino)-4-(methylthio)-6 (naphthalen-2-yl)-2-oxo-1,2-dihydropyridine-3-carbonitrile 4d brown solid, yield 72%, m.p. above 200 °C.
1H NMR (400 MHz, DMSO) δ 11.03 (s, 1H), 8.04 (s, 2H), 7.92 (s, 2H), 7.63 (d, J = 8.8 Hz, 2H), 7.57 (d, J = 8.7 Hz, 2H), 6.97 (d, J = 2.8 Hz, 3H), 6.94 (d, J = 2.8 Hz, 1H), 2.17 (s, 3H), 1.94 (s, 3H) (Fig. S7, ESI†).13C NMR (101 MHz, DMSO) δ 173.01 (d, J = 15.7 Hz), 166.80 (s), 161.20 (s), 160.98 (s), 158.67 (s), 157.77 (s), 146.63 (s), 143.63 (s), 131.86 (s), 129.20 (s), 128.73 (s), 128.46 (s), 128.08 (s), 127.87 (s), 127.49–126.11 (m), 114.67 (s), 114.26 (d, J = 4.6 Hz), 55.64 (s), 21.73 (s), 20.47 (s) (Fig. S8, ESI†). FTIR/cm−1 (KBr): 1604 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2357 (–C Ξ N), 1659 (–C
O), 2357 (–C Ξ N), 1659 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1507 (–C–C), 1242 (C
N), 1507 (–C–C), 1242 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) 769 (C–H) (Fig. S19, ESI†). HRMS (ES MS TOF) m/z: [M]+Calcd for C25H19N3O2S 425.1298, found 425.1293 (Fig. S14, ESI†).
C) 769 (C–H) (Fig. S19, ESI†). HRMS (ES MS TOF) m/z: [M]+Calcd for C25H19N3O2S 425.1298, found 425.1293 (Fig. S14, ESI†).
(E)-6-(4-bromophenyl)-1-((4-methylbenzylidene)amino)-4-(methylthio)-2-oxo-1,2-dihydropyridine-3-carbonitrile 4e yellow solid, yield 70%, m.p. above 200 °C.
1H NMR (400 MHz, DMSO) δ 11.94 (s, 1H), 7.27 (dd, J = 20.6, 8.6 Hz, 2H), 6.88 (d, J = 8.5 Hz, 1H), 6.79–6.16 (m, 1H), 6.09 (d, J = 14.9 Hz, 1H), 2.86 (s, 1H), 2.20 (s, 1H). 13C NMR (101 MHz, DMSO) δ 164.32 (d, J = 16.1 Hz), 160.85 (d, J = 3.9 Hz), 150.28 (s), 149.83 (s), 143.62 (s), 132.27 (s), 131.90 (s), 130.42 (s), 128.65 (s), 125.81 (s), 125.49 (s), 115.81 (d, J = 18.2 Hz), 101.78 (s), 100.91 (s), 95.24 (s), 94.59 (s), 14.58 (s), 14.37 (s). FTIR/cm−1 (KBr): 1605 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2362 (–C Ξ N), 1556 (C
O), 2362 (–C Ξ N), 1556 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1254 (C
N), 1254 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1513 (C–C), 771 (C–H), 699 (C–Br) (Fig. S20, ESI†). HRMS (ES MS TOF) m/z: [M + Na]+ Calcd for C21H16BrN3OS 437.0200, found 460.0514 (Fig. S15, ESI†).
C), 1513 (C–C), 771 (C–H), 699 (C–Br) (Fig. S20, ESI†). HRMS (ES MS TOF) m/z: [M + Na]+ Calcd for C21H16BrN3OS 437.0200, found 460.0514 (Fig. S15, ESI†).
Conclusions
In conclusion, we rationally designed and synthesized 4a–e based small molecules in concise and easy steps in high yield, derivatives were synthesized successfully using the reaction of pyrone and hydrazine monohydrates and then reacted with a variety of ortho and para-substituted benzaldehydes and with Glacial acetic acid as a catalyst which demonstrated for the first time remarkable aggregation-induced emission (AIE) properties in water as well as in solids. The synthesized compound 4e exhibited strong red emission in the solution state, with higher Stokes shift values. Moreover, Aggregated compound A-4e detected Fe3+ and Hg2+ with LODs of 1.03 × 10−7 M−1 and 1.71 × 10−7 M−1, which were lower than those in recent sensor reports. Cyclic voltammetric studies were carried out for the synthesized aggregated compound A-4e and its sensing metal ions. The compounds showed more negative and positive potential values, representing the occurrence of both metal ions in the synthesized hydrazide hydrazone. Compound 4e can be used to detect Fe3+ and Hg2+ for environmental real water sample analysis with satisfactory results.
Author contributions
SS supervision and conceptual difficulties and wrote some extensive checks to approve the final version of the manuscript. Murugesan Preethi's conceptual plan performed the experiment, collected all the data, and prepared the manuscript draft. Ajay Dev Ramesh Babu helps to study cyclic voltammetry and DFT studies−done at the School of Chemistry, Madurai Kamaraj University, Madurai, India.
Data availability
Data is contained within the article and ESI.†
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
S. S. thank RUSA 2.0 for financial assistance. M. P., thank RUSA for the fellowship. Also, we thank DST-FIST-PURSE and TANSCHE for the joint funding and instruments facility.
Notes and references
- S. Sidhu, K. Kumari and M. Uppal, J. Hum. Eco., 2007, 21, 265–267 CrossRef.
- D. Pectasides, E. Gkamprela and M. Deutsch, Hellenic Soc. Gastroenterol., 2017, 30, 405–413 Search PubMed.
- B. Zhao, T. Liu, Y. Fang, L. Wang, B. Song and Q. Deng, Tetrahedron Lett., 2016, 57, 4417–4423 CrossRef CAS.
- P. S. Hardikar, S. M. Joshi, D. S. Bhat, D. A. Raut, P. A. Katre, H. G. Lubree, A. Jere, A. N. Pandit, C. H. D. Fall and C. S. Yajnik, Diabetes Care, 2013, 36, e24–e24 CrossRef CAS PubMed.
- S. Dornelles, V. A. Garcia, M. N. M. de Lima, G. Vedana, L. A. Alcalde, M. R. Bogo and N. Schröder, Neurochem. Res., 2009, 35, 564–571 CrossRef PubMed.
- B. Sui, S. Tang, T. Liu, B. Kim and K. D. Belfield, ACS Appl. Mater. Interfaces, 2014, 6, 18408–18412 CrossRef CAS PubMed.
- J. Beard, Am. Soc. Nutr. Sci., 2003, 133, 1468S–1472S CAS.
- M. Korbas, S. R. Blechinger, P. H. Krone, I. J. Pickering and G. N. George, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 12108–12112 CrossRef CAS PubMed.
- D. Wu, W. Huang, Z. Lin, C. Duan, C. He, S. Wu and D. Wang, Inorg. Chem., 2008, 47, 7190–7201 CrossRef CAS PubMed.
- I. Onyido, A. R. Norris and E. Buncel, Chem. Rev., 2004, 104, 5911–5930 CrossRef CAS PubMed.
- D. Ashley Johnson, M. Rose Curtis and J. Karl Wallace, Chemosensors, 2019, 7, 22 CrossRef.
- P. Goutam Kumar, S. Meman, M. Amit Kumar and C. Shubhamoy, RSC Adv., 2020, 10, 44860–44875 RSC.
- S. Machado, W. Stawiński, P. Slonina, A. R. Pinto, J. P. Grosso, H. P. A. Nouws, J. T. Albergaria and C. Delerue-Matos, Sci. Total Environ, 2013, 461–462, 323–329 CrossRef CAS PubMed.
- I. Ullah, W. Li, S. Lei, Y. Zhang, W. Zhang, U. Farooq, S. Ullah, M. W. Ullah and X. Zhang, Ceram. Int., 2018, 44, 21338–21348 CrossRef CAS.
- T. D. Nguyen, A. Labed, R. El Zein, S. Lavandier, F. Bedu, I. Ozerov, H. Dallaporta, J.-M. Raimundo and A. M. Charrier, Biosens. Bioelectron., 2014, 54, 571–577 CrossRef CAS PubMed.
- H. Qian, S. Pramanik and I. Aprahamian, J. Am. Chem. Soc., 2017, 139, 9140–9143 CrossRef CAS PubMed.
- K. Jamuna, S. Thimmarayaperumal, M. K. Aravind, S. Sivakumar and B. Ashokkumar, New J. Chem., 2022, 46, 9207–9213 RSC.
- Y. M. Zhang, X. P. Chen, G. Y. Liang, K. P. Zhong, H. Yao, T. B. Wei and Q. Lin, Can. J. Chem., 2018, 96, 363–370 CrossRef CAS.
- M. Cacic, M. Trkovnik, F. Cacic and E. Has-Schon, Molecules, 2006, 11, 134–147 CrossRef CAS PubMed.
- M. Cacic, M. Molnar, T. Balic, N. Draca and V. Rajkovic, Molecules, 2009, 14, 2501–2513 CrossRef CAS PubMed.
- M. Sahu, A. K. Manna, S. Chowdhury and G. K. Patra, RSC Adv., 2020, 10, 44860–44875 RSC.
- V. Mahendran, K. Pasumpon, S. Thimmarayaperumal, P. Thilagar and S. Shanmugam, J. Org. Chem., 2016, 81, 3597–3602 CrossRef CAS PubMed.
- V. Ramesh, N. Savitha Devi, R. Ajaydev and S. Sivakumar, Tetrahedron, 2023, 131, 154765 CrossRef.
- S. Thimmarayaperumal and S. Shanmugam, ACS Omega, 2017, 2, 4900–4910 CrossRef CAS PubMed.
- A. Widiyana, G. Satrio Putra, M. Sulistyowaty, S. Hardjono and T. Budiati, J. Chem. Pharm. Res., 2017, 9, 24–28 CAS.
- S. Patil, S. Pandey, A. Singh, M. Radhakrishna and S. Basu, Chem. – Eur. J., 2019, 25, 8229–8235 CrossRef CAS PubMed.
- S. Omura, F. Kuno, K. Otoguro, T. Sunazuka, K. Shiomi, R. Mamuma and Y. Iwai, J. Antibiot., 1995, 48, 745 CrossRef CAS PubMed.
- N. Ozturk, S. Korkmaz, Y. Ozturk and K. H. C. Baser, Planta Med., 2005, 72, 289–294 CrossRef PubMed.
- J. Zhang and J. Liu, Luminescence, 2020, 35, 1185–1194 CrossRef CAS PubMed.
- N. Zhao, M. Li, Y. Yan, J. W. Y. Lam, Y. L. Zhang, Y. S. Zhao, K. S. Wong and B. Z. Tang, J. Mater. Chem. C, 2013, 1, 4640 RSC.
- J. Yang, Z. Chi, W. Zhu, B. Z. Tang and Z. Li, Sci. China: Chem., 2019, 62, 1090–1098 CrossRef CAS.
- S. Seenan and S. Kulathu Iyer, J. Org. Chem., 2020, 85, 1871–1881 CrossRef PubMed.
- M. Honglei, G. Rui, M. Qiao, S. Yimin and F. Enqin, Tetrahedron Lett., 2007, 48, 5525–5529 CrossRef.
- K. Ha Na, N. Seong-Won, K. Swamy, J. Yan, C. Xiaoqiang, K. Yonugmee, K. Sung-Jin, P. Sungsu and Y. Juyoung, Analyst, 2011, 136, 1339–1343 RSC.
- A. Sharmin, M. Abdul, G. William Emmanuel, A. Adams, U. Royhan, M. Mayez, H. Aminul, U. Jamal and K. Mohsin, RSC Adv., 2023, 13, 23819–23828 RSC.
- L. Bai, G. Xin, W. Minghui, L. Xuejun and X. Kuoxi, Dyes Pigm., 2021, 194, 109637 CrossRef.
|
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  *
*

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 594 cm−1 was obtained compared to the present in methyl group electron-donating of all the other derivatives. Relative fluorescence quantum yields were measured in DMSO solvent (Table 3).
594 cm−1 was obtained compared to the present in methyl group electron-donating of all the other derivatives. Relative fluorescence quantum yields were measured in DMSO solvent (Table 3).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 594 (160)
594 (160)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (7
water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) solvent mixtures (c) 3D-spectrum of 4e in solid state.
3) solvent mixtures (c) 3D-spectrum of 4e in solid state.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solvent mixtures. (b) SEM image of 4e in DMSO/water mixture with 70% water fraction.
water solvent mixtures. (b) SEM image of 4e in DMSO/water mixture with 70% water fraction.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S4, ESI†). An interference study of probe A-4e (5 × 10−5 M) in DMSO solvent presence of various metal ions was carried out (Fig. S4 and S5, ESI†) (Fig. 8 and 9).
1 (Fig. S4, ESI†). An interference study of probe A-4e (5 × 10−5 M) in DMSO solvent presence of various metal ions was carried out (Fig. S4 and S5, ESI†) (Fig. 8 and 9).

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations. 1665.89096 cm−1 However, with the addition of Fe3+ and Hg2+, this peak shifted to 1639.1986 cm−1 and 1653.7396 cm−1 which provides evidence for the participation of the C
O stretching vibrations. 1665.89096 cm−1 However, with the addition of Fe3+ and Hg2+, this peak shifted to 1639.1986 cm−1 and 1653.7396 cm−1 which provides evidence for the participation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of 4e involved in the ionic interaction with Fe3+ and Hg2+ ions, respectively shown in (Fig. S29, ESI†). To elucidate the fluorescence quenching mechanism of the probe in response to Fe3+ and Hg2+ metal cations, density functional theory (DFT) calculations were performed using Gaussian 09 for the probe, probe + Fe3+, and probe + Hg2+ with appropriate basis sets (Fig. S28, ESI†). Frontier molecular orbital analysis revealed distinct electronic distributions: in probe 4e, the HOMO predominantly resides over the amino and cyano hydrazone aroyl groups, while the LUMO extends across the entire aryl pyridine structure. Contrastingly, in 4e + Fe3+, the HOMO shifts primarily to the pyridine hydrazone moiety, with the LUMO localized over the pyridine unit and iron. Similarly, in 4e + Hg2+, the HOMO localizes over the mercury atom. At the same time, the LUMO spans the entire molecule (Fig. S28, ESI†). Moreover, the calculated energy gap differences of HOMO–LUMO transitions for 4e + Fe3+ (−0.25 eV) and 4e + Hg2+ (−0.30 eV) were found to be narrower compared to probe 4e (−0.31 eV), suggesting favorable coordination between probe 4e and Fe3+, as well as Hg2+. These findings indicate an internal charge transfer (ICT) process between the electron-donating aryl group in the benzylidene amino ring and the electron-withdrawing CN group in the pyridine ring D–A system, which induces fluorescence enhancement in probe 4e. This ICT process is disrupted upon complexation with Fe3+ and Hg2+, leading to fluorescence quenching (Scheme 5).
O bond of 4e involved in the ionic interaction with Fe3+ and Hg2+ ions, respectively shown in (Fig. S29, ESI†). To elucidate the fluorescence quenching mechanism of the probe in response to Fe3+ and Hg2+ metal cations, density functional theory (DFT) calculations were performed using Gaussian 09 for the probe, probe + Fe3+, and probe + Hg2+ with appropriate basis sets (Fig. S28, ESI†). Frontier molecular orbital analysis revealed distinct electronic distributions: in probe 4e, the HOMO predominantly resides over the amino and cyano hydrazone aroyl groups, while the LUMO extends across the entire aryl pyridine structure. Contrastingly, in 4e + Fe3+, the HOMO shifts primarily to the pyridine hydrazone moiety, with the LUMO localized over the pyridine unit and iron. Similarly, in 4e + Hg2+, the HOMO localizes over the mercury atom. At the same time, the LUMO spans the entire molecule (Fig. S28, ESI†). Moreover, the calculated energy gap differences of HOMO–LUMO transitions for 4e + Fe3+ (−0.25 eV) and 4e + Hg2+ (−0.30 eV) were found to be narrower compared to probe 4e (−0.31 eV), suggesting favorable coordination between probe 4e and Fe3+, as well as Hg2+. These findings indicate an internal charge transfer (ICT) process between the electron-donating aryl group in the benzylidene amino ring and the electron-withdrawing CN group in the pyridine ring D–A system, which induces fluorescence enhancement in probe 4e. This ICT process is disrupted upon complexation with Fe3+ and Hg2+, leading to fluorescence quenching (Scheme 5).







![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2357 (–C Ξ N), 1659 (–C
O), 2357 (–C Ξ N), 1659 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1242 (C
N), 1242 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1518 (C–C), 769 (C–H) (Fig. S20, ESI†). HRMS (ES MS TOF) m/z: [M − 2H + K]− Calcd for C21H16BrN3OS 410.1282, found 446.1282 (Fig. S11, ESI†).
C), 1518 (C–C), 769 (C–H) (Fig. S20, ESI†). HRMS (ES MS TOF) m/z: [M − 2H + K]− Calcd for C21H16BrN3OS 410.1282, found 446.1282 (Fig. S11, ESI†).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2360 (–C Ξ N), 1686 (–C
O), 2360 (–C Ξ N), 1686 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1515 (–C–C),1252 (C
N), 1515 (–C–C),1252 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) 769 (–C–H) (Fig. S17, ESI†). HRMS m/z: [M + Na]+ Calcd for C21H17N3OSNa 359.1092. found 382.0938 (Fig. S12, ESI†).
C) 769 (–C–H) (Fig. S17, ESI†). HRMS m/z: [M + Na]+ Calcd for C21H17N3OSNa 359.1092. found 382.0938 (Fig. S12, ESI†).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2359 (–C Ξ N), 1678 (–C
O), 2359 (–C Ξ N), 1678 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1513 (–C–C), 1252 (C
N), 1513 (–C–C), 1252 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) 772 (C–H) (Fig. S18, ESI†). HRMS (ES MS TOF) m/z: [M + H]+ Calcd for C27H21N3OS 437.1605, found fragmentation peak [M + H]+ Calcd for C19H15N3OS at 334.10 (Fig. S13, ESI†).
C) 772 (C–H) (Fig. S18, ESI†). HRMS (ES MS TOF) m/z: [M + H]+ Calcd for C27H21N3OS 437.1605, found fragmentation peak [M + H]+ Calcd for C19H15N3OS at 334.10 (Fig. S13, ESI†).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2357 (–C Ξ N), 1659 (–C
O), 2357 (–C Ξ N), 1659 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1507 (–C–C), 1242 (C
N), 1507 (–C–C), 1242 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) 769 (C–H) (Fig. S19, ESI†). HRMS (ES MS TOF) m/z: [M]+Calcd for C25H19N3O2S 425.1298, found 425.1293 (Fig. S14, ESI†).
C) 769 (C–H) (Fig. S19, ESI†). HRMS (ES MS TOF) m/z: [M]+Calcd for C25H19N3O2S 425.1298, found 425.1293 (Fig. S14, ESI†).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 2362 (–C Ξ N), 1556 (C
O), 2362 (–C Ξ N), 1556 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1254 (C
N), 1254 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1513 (C–C), 771 (C–H), 699 (C–Br) (Fig. S20, ESI†). HRMS (ES MS TOF) m/z: [M + Na]+ Calcd for C21H16BrN3OS 437.0200, found 460.0514 (Fig. S15, ESI†).
C), 1513 (C–C), 771 (C–H), 699 (C–Br) (Fig. S20, ESI†). HRMS (ES MS TOF) m/z: [M + Na]+ Calcd for C21H16BrN3OS 437.0200, found 460.0514 (Fig. S15, ESI†).









