Ionic liquid-mediated Pd-free biaryl synthesis catalysed by in situ generated nickel nanoparticles†
Samprity
Sarmah
a,
Bidyutjyoti
Dutta
 ab and
Diganta
Sarma
ab and
Diganta
Sarma
 *a
*a
aDepartment of Chemistry, Dibrugarh University, Dibrugarh-786004, Assam, India. E-mail: dsarma22@dibru.ac.in
bDepartment of Chemical Sciences, Indian Institute of Science Education and Research Mohali, Mohali-140306, Punjab, India
First published on 23rd January 2025
Abstract
For the first time, an ionic liquid-mediated nickel-catalysed mild yet efficient protocol has been developed for biaryl synthesis via Suzuki–Miyaura cross coupling (SMC) reactions. The protocol gives access to a wide variety of substituted biaryls with good to excellent yields. The nickel pre-catalyst under the reaction condition is reduced to Ni nanoparticles (Ni NPs). Formation of Ni NPs is confirmed and characterized by PXRD, HRTEM, EDAX and XPS analyses. The XPS analysis reveals that Ni NPs are mostly in the zero oxidation state, which largely explains the efficiency of the catalyst to promote the SMC reaction. The key highlights of the protocol are the use of Ni NPs as a catalyst generated in situ from an inexpensive and easily available precursor, short reaction time, mild reaction conditions and operation in the absence of expensive metals and ligands. This ligand-free methodology also works well for heteroaryl boronic acids.
Introduction
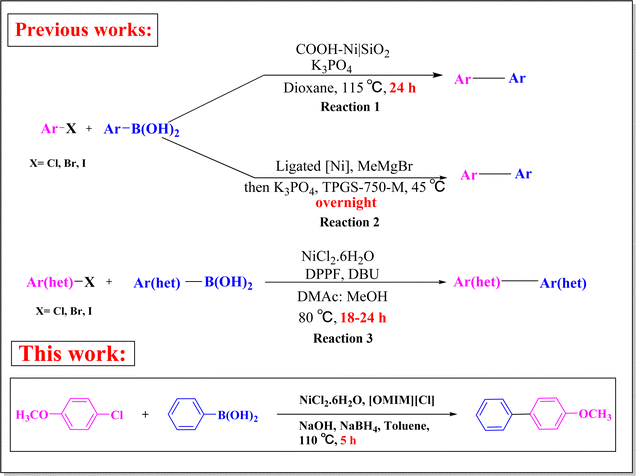
Transition metal-catalysed biaryl synthesis has provided access to a wide range of compounds that have potential applications in medicinal chemistry and drug discovery.1 Palladium-catalysed Suzuki–Miyaura cross coupling (SMC) reaction is the most widely utilized reaction for the synthesis of biaryls. Even though the use of Pd-catalysed biaryl synthesis is the most common, nowadays alternative methodologies employing Pd-free conditions have become attractive choices.2 Ni as a catalyst for SMC reactions has attracted the attention of researchers over Pd catalysts owing to its high abundance, low cost and less toxic nature. In addition, Ni has become a more preferable catalyst as compared to other metals such as Pd, Pt, Cu etc. because of its other properties like smaller size which tends to increase its nucleophilic character and enhance the reactivity towards aromatic chlorides as well as Ni being stable even in ligand-free conditions.3 To address the drawbacks of Pd-catalysed SMC reactions, such as slow reactions with aryl chlorides, requirement of expensive ligands and self-coupled product formation, Ni catalysts have emerged as superior alternatives for SMC reactions.4For the past few decades, researchers have been continuously trying to establish green substitutes in the fields of organic syntheses due to the volatile nature of organic solvents. In this regard, ionic liquids (ILs) have emerged as green alternatives for chemical transformations. ILs are organic salts that contain organic cations with organic or inorganic anions and generally occur in liquid form below 100 °C.5,6 They are considered as green and eco-friendly solvents over conventional organic solvents because of some exceptional physicochemical properties such as low volatility, large range of solubility, low flammability, high thermal stability etc.7–9 In recent years, nanotechnology has gained much attention as one of the most attractive and interesting research areas. Nanoparticles (NPs) can possess novel and advanced attributes, offering numerous applications to the scientific community due to their small particle size which results in increased surface area per unit mass and improves some of their physicochemical properties including catalytic activities.10 Contemporarily nanosized transition metal particles have become one of the best tools to develop methodologies for organic synthesis by virtue of their higher catalytic activity.11 ILs have also been employed for the synthesis of nanostructures, including many transition metal NPs.12 With all their merits, ILs are becoming multifunctional reagents of choice for synthetic chemistry.
Considering the advantages of nickel as a catalyst, for the first time Miyaura et al. conducted Ni-catalysed synthesis of biaryls using inert aryl chloride and aryl boronic acid via SMC reaction.13 Subsequently, the use of nickel catalysts has begun to increase in organic syntheses. After Miyarura et al., several research groups such as those of Indolese,14 Genet,15 Garg,16 Shi,17 Miyaura and Inada,18 and Hu19 satisfactorily developed Ni-catalysed methods for biaryl syntheses. In 2019, Key et al. prepared SiO2 supported [(2,2′:6′,2′′-terpyridine-4′-benzoic acid)Ni(II)]Cl2 (COOH–Ni) catalyst and employed it in SMC reaction at 115 °C for 24 h. They separately prepared a Ni(COOH)/SiO2 complex which led to the formation of biaryls by reacting aryl halides with arylboronic acids20 (Scheme 1, reaction (1)). Goldfogel et al. developed a catalytic system for the synthesis of pharmaceutically significant heterocycles via SMC reaction.21 Handa et al. also improved a methodology for the nano-nickel-catalysed synthesis of biaryls by employing commercially available designer surfactant TPGS-750-M2722 (Scheme 1, reaction (2)). But in their reaction conditions, nickel was not in the zero oxidation state. Also, pre-activation of the catalyst was also required, unlike our protocol where Ni(0) NPs were simply formed during the course of the reaction. They used 1,1′-bis(diphenylphosphino)ferrocene (DPPF) and dimethylacetamide (DMAc) as ligand and solvent respectively along with NiCl2·6H2O as pre-catalyst and DBU as base (Scheme 1, reaction (3)). Nan et al. focused on nano-catalytic carbonylative SMC reaction where the nanocatalyst (Ni@Pd/CNT) was based on carbon nanotubes (CNT).23
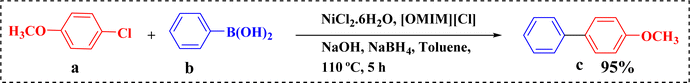
Herein, our study was instigated with ionic liquid-mediated in situ generated Ni(0) NPs as a catalyst for SMC reaction. In situ formation of stable heterogeneous Ni(0) NPs was observed while using a homogenous pre-catalyst, i.e. NiCl2·6H2O. The NPs were stable even after extraction from the reaction medium. For initial screening of suitable reaction conditions, 4-chloroanisole and phenylboronic acid were chosen as model substrates for the synthesis of biaryls. The reaction was carried out in toluene at 110 °C using a simple nickel precursor, NiCl2·6H2O, in the presence of an IL, 1-methyl-3-octylimidazolium chloride ([OMIM][Cl]) (Scheme 2). NaOH was added as base and the mixture was stirred for 5 h under air. The IL-mediated Ni NPs were formed in situ by the reduction of nickel(II) chloride in the presence of sodium borohydride during the SMC reaction. NaBH4 is required for the reduction of nickel ion to its zero oxidation state.24 This in situ generated Ni NPs-catalysed biaryl synthetic protocol is a less time-consuming yet effective way of biaryl synthesis as compared to existing Pd-free SMC reactions which require drastic conditions and tedious catalyst preparation steps. To the best of our knowledge, this is the first in situ generated nano Ni(0) catalyst for SMC reaction under sustainable conditions.
The initial study was carried out by taking the specimen substrates under various reaction parameters, including the solvent, IL, base and metal precursor. Replacing NiCl2.6H2O with other nickel salts such as NiSO4 and Ni(OAc)2 afforded the required biaryl in 30% and 50% yields respectively (Table 1, entries 1–3).
| Entry | Catalyst | Time (h) | Temperature (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: (i) 4-chloroanisole (0.5 mmol, 1 equiv.), (ii) phenylboronic acid (0.75 mmol, 1.5 equiv.), (iii) IL (0.25 mmol, 0.5 equiv.), (iv) Ni precursor (5 mol%), (v) NaBH4 (0.25 mmol, 0.5 equiv.), (vi) toluene (1.5 ml), (vii) NaOH (1 mmol, 2 equiv.), (viii) time (5 h), (ix) temperature (110 °C). b Isolated yield. | ||||
| 1 | NiCl 2 ·6H2O | 5 | 110 | 95 |
| 2 | Ni(OAc)2·4H2O | 5 | 110 | 30 |
| 3 | NiSO4 | 5 | 110 | 50 |
After optimizing the catalyst and ILs, various inorganic and organic bases, e.g. K2CO3, Na2CO3, NaOH, KOH, Cs2CO3, NH4OH, Et2NH, EtNH2, Et3N etc., were tested (Table 2, entries 1–10). No significant difference in product yield was observed while using inorganic bases. However, NaOH gives a more satisfactory result with low loading of catalyst (5 mol%), while other inorganic bases give comparable results only when catalyst loading was doubled (10 mol%). Conversely, organic bases failed to produce any convincing result. Hence considering the environmental as well as economic advantages, NaOH was chosen as the base for our reaction (Table 2, entry 5).
| Entry | Base | Time (h) | Temperature (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: (i) 4-chloroanisole (0.5 mmol, 1 equiv.), (ii) phenylboronic acid (0.75 mmol, 1.5 equiv.), (iii) IL (0.25 mmol, 0.5 equiv.), (iv) NiCl2·6H2O (5 mol%), (v) NaBH4 (0.25 mmol, 0.5 equiv.), (vi) toluene (1.5 ml), (vii) base (1 mmol, 2 equiv.), (viii) time (5 h), (ix) temperature (110 °C). b Isolated yield. c Precursor NiCl2·6H2O (5 mol%). | ||||
| 1 | K2CO3 | 5 | 110 | 90 |
| 2 | Na2CO3 | 5 | 110 | 90 |
| 3 | Cs2CO3 | 5 | 110 | 90 |
| 4 | CaCO3 | 5 | 110 | 90 |
| 5 | NaOH | 5 | 110 | 95 |
| 6 | KOH | 5 | 110 | 92 |
| 7 | Diethylamine | 5 | 110 | 40 |
| 8 | Triethylamine | 5 | 110 | Trace |
| 9 | Ethylamine | 5 | 110 | — |
| 10 | NH4OH | 5 | 110 | Trace |
To determine the best IL for our catalytic system, a series of ILs have been examined (Table 3, entries 1–8). It is worth mentioning that this protocol for Ni-catalysed biaryl synthesis succeeded only in the presence of 1-octyl-3-methylimidazolium chloride ([OMIM][Cl]). While in the presence of 1-butyl-3-methylimidazolium chloride ([BMIM][Cl]) satisfactory results were not observed. Remarkably, other ILs we have tested did not promote any product formation. It is assumed that the length of the alkyl chain in the cationic part of the IL has a major significance in the catalytic activity of the IL. Imidazolium ILs having moderate alkyl chain length, as for example carrying eight carbons in an alkyl chain, are more favoured to improve the catalytic activity which is well documented with other organic transformations such as the epoxy addition reaction. The viscosity of ILs increases with increasing alkyl chain length of the cation.25 Moreover, ILs containing cations with alkyl chain length greater than C8H17 are believed to form clusters as well as possess a hydrophobic nature resulting in a decrease in catalytic performance.26 Alkyl chain length of the cationic part of the IL influences the size or size distributions of NPs. Throughout the reaction Ni(0) NPs are uniformly surrounded by the IL that stabilizes the NPs in their metallic state, which is further confirmed by TEM analysis.27
| Entry | Ionic liquid | Time (h) | Temperature (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: (i) 4-chloroanisole (0.5 mmol, 1 equiv.), (ii) phenylboronic acid (0.75 mmol, 1.5 equiv.), (iii) IL (0.25 mmol, 0.5 equiv.), (iv) NiCl2·6H2O (5 mol%), (v) NaBH4 (0.25 mmol, 0.5 equiv.), (vi) toluene (1.5 ml), (vii) NaOH (1 mmol, 2 equiv.), (viii) time (5 h), (ix) temperature (110 °C). b Isolated yield. c 1-Butyl-3-methylimidazolium bromide. d 1-Butly-3-methylimidazolium hydroxide. e DABCO (1,4-diazabicyclo[2.2.2]octane). f 1-Dodecyl-3-methylimidazolium chloride. g 1-Dodecyl-3-methylimidazolium hydroxide. | ||||
| 1 | [OMIM][Cl] | 5 | 110 | 95 |
| 2 | [BMIM][Cl] | 5 | 110 | 30 |
| 3c | [BMIM][Br] | 5 | 110 | — |
| 4d | [BMIM][OH] | 5 | 110 | — |
| 5e | [C4DABCO][Br] | 5 | 110 | — |
| 6 | Triethylamine acetate | 5 | 110 | — |
| 7f | [C12MIM][Cl] | 5 | 110 | — |
| 8g | [C12MIM][OH] | 5 | 110 | — |
In the optimization study for solvents (Table 4), toluene was found to be the best solvent for the protocol (Table 4, entry 1). Polar solvents like DMF, 1,4-dioxane, DCM, THF, water, and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mixture of water and tBuOH did not afford promising results (Table 4, entries 2–7). With Pd catalysts, SMC reactions in polar aprotic solvents like DCM, THF along with water show good reactivity. However, these low-boiling solvents cannot provide the required thermal activation to the Ni-catalysed SMC reaction which is the cause of their poor reactivity. Moreover, water and tBuOH may lead to phase separation of base with reactants leading to no product formation. Use of green solvents like PEG does not give any encouraging result (Table 4, entry 8).28
3 mixture of water and tBuOH did not afford promising results (Table 4, entries 2–7). With Pd catalysts, SMC reactions in polar aprotic solvents like DCM, THF along with water show good reactivity. However, these low-boiling solvents cannot provide the required thermal activation to the Ni-catalysed SMC reaction which is the cause of their poor reactivity. Moreover, water and tBuOH may lead to phase separation of base with reactants leading to no product formation. Use of green solvents like PEG does not give any encouraging result (Table 4, entry 8).28
| Entry | Solvent | Temperature (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: (i) 4-chloroanisole (0.5 mmol, 1 equiv.), (ii) phenylboronic acid (0.75 mmol, 1.5 equiv.), (iii) IL (0.25 mmol, 0.5 equiv.), (iv) NiCl2·6H2O (5 mol%), (v) NaBH4 (0.25 mmol, 0.5 equiv.), (vi) solvent (1.5 ml), (vii) NaOH (1 mmol, 2 equiv.), (viii) time (5 h), (ix) temperature (110 °C). b Isolated yields. | ||||
| 1 | Toluene | 110 | 5 | 95 |
| 2 | DMF | 150 | 5 | 90 |
| 3 | 1,4-Dioxane | 110 | 5 | 85 |
| 4 | DCM | 60 | 5 | — |
| 5 | THF | 60 | 5 | — |
| 6 | Water | 100 | 5 | — |
| 7 | Water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tBuOH (1 tBuOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
100 | 5 | — |
| 8 | PEG (400) | 110 | 5 | 60 |
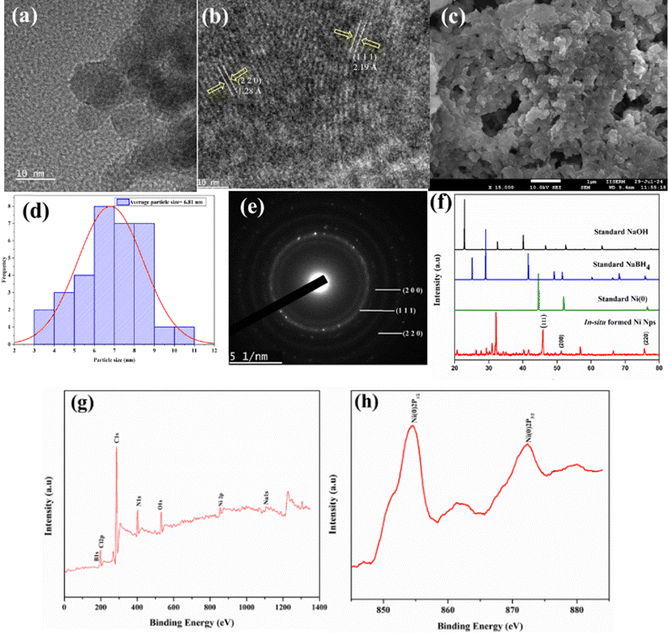
After completion of the reaction, it was observed that reduced NiCl2 became settled at the bottom of the reaction vessel. The nature of the in situ generated active Ni catalyst was investigated by performing various analytical techniques. Powder X-ray diffraction (PXRD) was used to determine the crystallographic structure of Ni(0) NPs. Fig. 1(f) illustrates the typical PXRD pattern of Ni(0). The characteristic peaks of Ni appeared at 2θ values of 45.2°, 51.78° and 76° which are associated with (111), (200) and (220) planes of orientation respectively and almost coincide with standard data.29 Some additional peaks were also observed in the XRD patterns, which are believed to be the peaks of NaBH4 and NaOH.30 Again to confirm the oxidation state of nickel NPs, XPS analysis was performed. The survey-scan XPS spectrum of the sample (Fig. 1g) shows the presence of nickel along with other elements (C, N, Na, B, Cl, O). The high-resolution XPS spectrum of Ni 2p of Ni(0) NPs is shown in Fig. 1(h). The XPS spectrum illustrates two prominent bands which are observed at 854.6 and 872.3 eV of Ni(0) 2p3/2 and Ni(0) 2p1/2, respectively. The binding energies of Ni(0) 2p3/2 and 2p1/2 as compared to the values for bulk nickel are marginally shifted to higher values which is characteristic of the matrix effect along with the quantum size effect.31,32 The morphology and particle size distribution of Ni(0) NPs were examined by transmission electron microscopy (TEM). The HRTEM image (Fig. 1a) indicates that Ni(0) NPs are homogenously distributed into the surface of the IL. Fig. 1(b) shows the inter-planer distances of Ni NPs. It can be observed from Fig. 1(d) that the Ni NPs exhibited an average particle size of 6.81 nm and an average diameter of 7.92 nm with nearly spherical shape. Many diffraction spot circles could be observed in the SAED pattern (Fig. 1e), which clearly signifies the corresponding lattice planes (200), (111) and (220) of Ni NPs (d spacing calculation is provided in Table S1 in ESI†).33 The elemental mapping of the catalyst is shown in Fig. S4 in ESI.† The surface morphology of the in situ formed catalyst was investigated by SEM analysis. NPs with nearly spherical shape were observed in SEM images (Fig. 1c). (EDAX spectrum of the sample is shown Fig. S3 in ESI†).
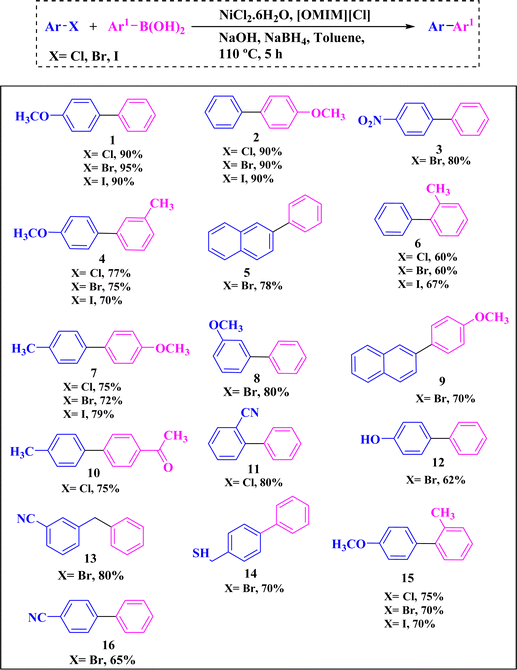
To demonstrate synthetic utility of the optimized conditions, the study was extended to explore the substrate scope for this reaction. A series of aryl halides were tested with arylboronic acids and good to excellent yields of the products (60–95%) were obtained (Table 5, entries 1–16). Both electron-rich and electron-deficient aryl bromides, as well as electron-rich and electron-deficient arylboronic acids, showed good to excellent reactivity and provided moderate to high yields of products. Interestingly a wide range of functional groups, such as –OMe, –CH3, –NO2, –CN, –CHO and –COCH3, are well tolerated by this catalytic system.
| a Reaction conditions: (i) aryl halide (0.5 mmol, 1 equiv.), (ii) arylboronic acid (0.75 mmol, 1.5 equiv.), (iii) [OMIM][Cl] (0.25 mmol, 0.5 equiv), (iv) NiCl2.6H2O (5 mol%), (v) NaBH4 (0.25 mmol, 0.5 equiv.), (vi) NaOH (1 mmol, 2 equiv.), (vii) toluene (1.5 mL), (viii) time (5 h), (ix) temperature (110 °C). |
|---|
|
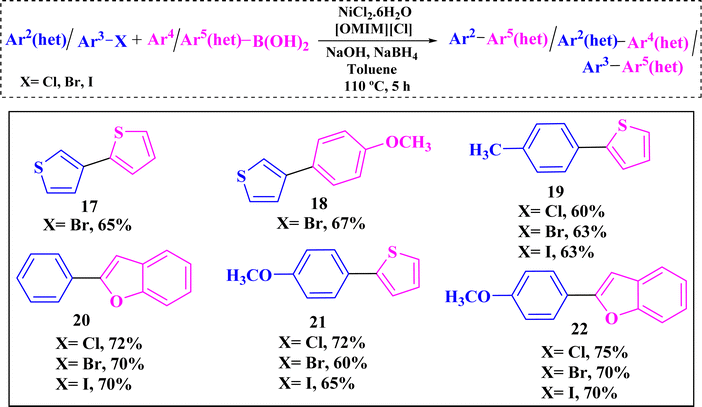
It was satisfying to observe that the amount of unwanted homo coupling product was also very much less under the reaction conditions. Additionally, the developed reaction conditions work well with the heterocyclic substrates. To ensure superiority of the catalytic protocol, various heteroaryl halides as well as heteroarylboronic acids were employed as coupling partners for SMC reaction. To our delight all the heterocyclic substrates provided good yields (60–75%) (Table 6, entries 17–22).
| a Reaction conditions: (i) aryl/heteroaryl halide (0.5 mmol, 1 equiv.), (ii) arylboronic acid (0.75 mmol, 1.5 equiv.), (iii) [OMIM][Cl] (0.25 mmol, 0.5 equiv), (iv) NiCl2·6H2O (5 mol%), (v) NaBH4 (0.25 mmol, 0.5 equiv.), (vi) NaOH (1 mmol, 2 equiv.), (vii) toluene (1.5 mL), (viii) time (5 h), (ix) temperature (110 °C). |
|---|
|
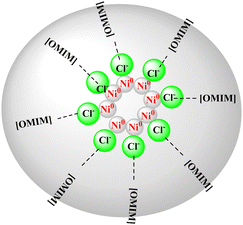
On the basis of literature reports,32,34–37 we have proposed a plausible mechanism for this protocol. Sodium borohydride reduces the nickel precursor from Ni(II) to Ni(0). It is believed that the nickel NPs were stabilized by the imidazolium-based IL via electrostatic and steric interactions. The anion of the IL, i.e. chloride anion, interacts with Ni(0) metal surface through an electrostatic interaction by forming an ionic double layer while the cation of the IL forms the outer part of the ionic double layer which then enhances a steric stabilization. DFT binding energy (BE) calculation has proved that chloride anions have high binding energy to bind the nickel NPs with strong binding which further improves the stabilization effects (Fig. 2).38 Consequently, the basic and simple catalytic cycle was initiated with oxidative addition of nano Ni(0) with aryl halide by producing in situ generated organonickel intermediate (ii). Furthermore, intermediate (iii) was formed on addition of a base, this intermediate reacting with activated arylboronic acid (iv) to give an intermediate (v) via transmetalation. The main function of base in the SMC reaction is to convert arylboronic acids to organoborate which is more reactive to assist transmetalation with intermediate36,39 (iii). Subsequently, reductive elimination of intermediate (v) yields the biaryl product along with regeneration of the Ni-based nanocatalyst (Scheme 3). The base plays a crucial role in this reaction. It converts poorly reactive boronic acid to most reactive borate anion Ar–B(OH)3− and thereby enhances the rate of the subsequent transmetalation step. It is assumed that a stronger base like NaOH is helpful to increase the concentration of borate in solution by furnishing more OH− ions.39
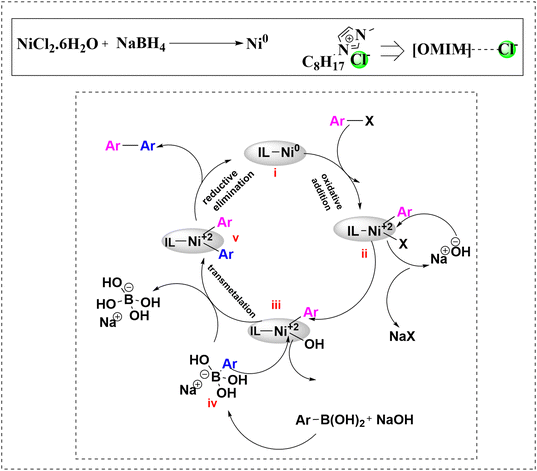 | ||
| Scheme 3 Proposed mechanism for the Suzuki–Miyaura cross coupling reactions in the presence of IL mediated Ni(0) NPs. | ||
Conclusion
To summarise, a simple IL-mediated in situ generated nano Ni catalysed SMC reaction protocol has been developed to construct biaryls in ligand-free conditions. The developed Pd-free protocol is very effective, affording biaryls in good to excellent yields. Apart from commonly used simple aryl halides and benzyl halides, sterically hindered substrates also performed proficiently under this protocol. Moreover, the in situ formed Ni NPs stabilized by the IL in a metallic state can efficiently catalyse the SMC reaction of many heteroaryl halides and heteroarylboronic acids.Experimental
All starting materials and solvents were purchased from common commercial sources and were used without additional purification: NiCl2.6H2O, CAS no. 7791-20-0, Merck; 1-methylimidazole, CAS no. 616-47-7, Sigma-Aldrich; NaBH4, CAS no. 16940-66-2, Sigma-Aldrich; 1-chlorooctane, CAS no. CAS RN: 111-85-3, TCI; NaOH pellets, CAS no. 1310-73-2, Merck; toluene, CAS no. 108-88-3, Merck. To maintain a palladium-free environment, each and every apparatus such as glassware, spatula, round bottomed flask, magnetic beads used for the reaction was subjected to acid washing and oven drying. All reactions were carried out under atmospheric conditions and all reported yields are isolated yields. Aluminium sheets pre-coated with silica gel 60F254 (Merck) were used for thin-layer chromatography (TLC) to visualize products under 254 nm UV light. Column chromatography was executed on silica gel (120–230 mesh) to obtain isolated yields. All 1H NMR spectra were recorded at 500 MHz and 13C NMR spectra at 125 MHz using TMS as an internal standard. Chemical shifts are reported in parts per million (ppm, δ). Transmission electron microscopy (TEM) analysis was performed by using a JEOL JEM-F200 TEM model, scanning electron microscopy (SEM) was performed by using FESEM (JEOL, JSM-7600F), X-ray photoelectron spectrometry (XPS) was performed by using an ESCALAB Xi+ model, and X-ray diffraction (XRD) analysis was performed using a Bruker D8 Advance model. Both 1H NMR and 13C NMR spectra were recorded on a Bruker Advance 500 MHz. Mass spectra were recorded with a Thermo-Scientific mass spectrometer (TSQ Endura model).Author contributions
S. S.: conceptualization, data curation, formal analysis, methodology, software, writing original draft. B. D.: visualization, investigation, data curation. D. S.: visualization, supervision, resources, methodology, investigation, conceptualization.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank CSIR, New Delhi, for a research grant [no. 02 (0399)/21/EMR-II]. The authors thank CSIC, Dibrugarh University for NMR analysis. The authors also acknowledge DST-FIST for financial assistance to the Department of Chemistry, Dibrugarh University (grant no. SR/FST/CS-I/2020/152). The authors are grateful to the Department of Science and Technology (DST), India for financial assistance under PURSE programme [no. SR/PURSE/2022/143 (C)].References
-
(a)
N. Miyaura, in Metal-Catalyzed Cross-Coupling Reactions, ed. A. de Meijere and F. Diederich, Wiley-VCH, New York, 2004 Search PubMed
; (b) J. Hassan, M. Sevignon, C. Gozzi, E. Schulz and M. Lemaire, Chem. Rev., 2002, 102, 1359–1469 CrossRef CAS PubMed
; (c) J. Corbet and G. Mignani, Chem. Rev., 2006, 106, 2651–2710 CrossRef CAS PubMed
; (d) E. Negishi, Bull. Chem. Soc. Jpn., 2007, 80, 233–257 CrossRef CAS
.
- S. D. Ramgren, L. Hie, Y. Ye and N. K. Garg, Org. Lett., 2013, 15, 3950–3953 CrossRef CAS PubMed
.
- M. Yadav and R. K. Sharma, Curr. Opin. Green Sustainable Chem., 2019, 15, 47–59 CrossRef
.
- S. Kotha, K. Lahiri and D. Kashinath, Tetrahedron, 2002, 58, 9633–9695 CrossRef CAS
.
- D. R. MacFarlane, J. Golding, S. Forsyth, M. Forsyth and G. B. Deacon, Chem. Commun., 2001, 1430–1431 RSC
.
- J. S. Wilkes, Green Chem., 2002, 4, 73–80 RSC
.
-
(a) R. Guterman, H. Miao and M. Antonietti, J. Org. Chem., 2018, 83, 684–689 CrossRef CAS PubMed
; (b) P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Weinheim, Germany, 2008, 1–776 Search PubMed
.
-
(a) K. N. Ganesh, D. Zhang, S. J. Miller, K. Rossen, P. J. Chirik, M. C. Kozlowski, J. B. Zimmerman, B. W. Brooks, P. E. Savage, D. T. Allen and A. M. Voutchkova-Kostal, Org. Lett., 2021, 23, 4935–4939 CrossRef CAS PubMed
; (b) T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef CAS PubMed
; (c) J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS PubMed
.
- M. D. Coney, D. C. Morris, A. Gilbert, S. W. Prescott, R. S. Haines and J. B. Harper, J. Org. Chem., 2022, 87, 1767–1779 CrossRef CAS PubMed
.
- H. Zhang and H. Cui, Langmuir, 2009, 25, 2604–2612 CrossRef CAS PubMed
.
-
(a)
B. Ward, Development, synthesis and characterization of multifunctional nanomaterials, KU Leuven, Belgium, 2014 Search PubMed
; (b) K. Manojkumar, A. Sivaramakrishna and K. Vijayakrishna, J. Nanopart. Res., 2016, 18, 1–22 CrossRef CAS
.
- L. Wu, J. Ling and Z. Q. Wu, Adv. Synth. Catal., 2011, 353, 1452–1456 CrossRef CAS
.
-
(a) S. Saito, M. Sakai and N. Miyaura, Tetrahedron Lett., 1996, 37, 2993 CrossRef CAS
; (b) S. Saito, S. Oh-tani and N. Miyaura, J. Org. Chem., 1997, 62, 8024 CrossRef CAS PubMed
; (c) F. S. Han, Chem. Soc. Rev., 2013, 42, 5270–5298 RSC
.
- A. F. Indolese, Tetrahedron Lett., 1997, 38, 3513–3516 CrossRef CAS
.
- J. C. Galland, M. Savignac and J. P. Genet, Tetrahedron Lett., 1999, 40, 2323–2326 CrossRef CAS
.
- K. W. Quasdorf, M. Riener, K. V. Petrova and N. K. Garg, J. Am. Chem. Soc., 2009, 131, 17748–17749 CrossRef CAS PubMed
.
- B. T. Guan, Y. Wang, B. J. Li, D. G. Yu and Z. J. Shi, J. Am. Chem. Soc., 2008, 130, 14468–14470 CrossRef CAS PubMed
.
- K. Inada and N. Miyaura, Tetrahedron, 2000, 56, 8657–8660 CrossRef CAS
.
- Z. Y. Tang and Q. S. Hu, J. Org. Chem., 2006, 71, 2167–2169 CrossRef CAS PubMed
.
- R. J. Key, J. M. M. Tengco, M. D. Smith and A. K. Vannucci, Organometallics, 2019, 38, 2007–2014 CrossRef CAS
.
- M. J. Goldfogel, X. Guo, J. L. Melendez Matos, J. A. Gurak Jr, M. V. Joannou, W. B. Moffat and S. R. Wisniewski, OPR, 2021, 26, 785–794 Search PubMed
.
- S. Handa, E. D. Slack and B. H. Lipshutz, Angew. Chem., 2015, 54, 11994 CrossRef CAS PubMed
.
- L. Nan, C. Yalan, L. Jixiang, O. Dujuan, D. Wenhui, J. Rouhi and M. Mustapha, RSC Adv., 2020, 10, 27923–27931 RSC
.
- J. Wagner, T. R. Tshikhudo and J. M. Köhler, CEJ, 2008, 135, S104–S109 CAS
.
-
(a) H. Kawanami, A. Sasaki, K. Matsui and Y. Ikushima, Chem. Commun., 2003, 896–897 RSC
; (b) E. H. Lee, J. Y. Ahn, M. M. Dharman, D. W. Park, S. W. Park and I. Kim, Catal. Today, 2008, 131, 130–134 CrossRef CAS
.
- L. Deng, Q. Su, X. Tan, Y. Wang, L. Dong, H. He and W. Cheng, Mol. Catal., 2022, 519, 112153 CrossRef CAS
.
- B. H. Park, S. E. Hyeon, J. H. Cha, Y. K. Hong, J. W. Kang and K. S. Kim, J. Nanosci. Nanotechnol., 2016, 16, 11005–11008 CrossRef CAS
.
- J. Sherwood, J. H. Clark, I. J. Fairlamb and J. M. Slattery, Green Chem., 2019, 21, 2164–2213 RSC
.
- C. Jayaseelan, A. Rahuman, R. Ramkumar, P. Perumal, G. Rajakumar, A. V. Kirthi and S. Marimuthu, Ecotoxicol. Environ., 2014, 107, 220–228 CrossRef CAS PubMed
.
-
(a) J. Mao, Q. Gu, Z. Guo and H. K. Liu, J. Mater. Chem. A, 2015, 3, 11269–11276 RSC
; (b) A. K. F
![[i with combining dot above]](https://www.rsc.org/images/entities/char_0069_0307.gif) gen and S. Pişkin, Bull. Mater. Sci., 2012, 35, 203–209 CrossRef
gen and S. Pişkin, Bull. Mater. Sci., 2012, 35, 203–209 CrossRef ; (c) W. Chen, L. Z. Ouyang, J. W. Liu, X. D. Yao, H. Wang, Z. W. Liu and M. Zhu, J. Power Sources, 2017, 359, 400–407 CrossRef CAS
; (d) D. Rohendi, N. Syarif and E. K. Wati, IOP Conf. Ser. Earth Environ. Sci., 2019, 248, 012008 CrossRef
; (e) X. Min, D. Chai, K. Ding, R. Li and X. Zhang, Fuel, 2023, 350, 128777 CrossRef CAS
; (f) J. Mao, Q. Gu and D. H. Gregory, Materials, 2015, 8, 2191–2203 CrossRef CAS
.
- D. Özhava, N. Z. Kılıçaslan and S. Özkar, Appl. Catal., B, 2015, 162, 573–582 CrossRef
.
- I. Dindarloo Inaloo, S. Majnooni, H. Eslahi and M. Esmaeilpour, Appl. Organomet. Chem., 2020, 34, 5662 CrossRef
.
- F. Alonso, J. J. Calvino, I. Osante and M. Yus, J. Exp. Nanosci., 2006, 1, 419–433 CrossRef CAS
.
- H. Wang, S. Zhang, M. Xü and G. Zou, Molecules, 2023, 28, 636 CrossRef CAS PubMed
.
- J. El-Maiss, T. Mohy El Dine, C. S. Lu, I. Karamé, A. Kanj, K. Polychronopoulou and J. Shaya, Catalysts, 2020, 10, 296 CrossRef CAS
.
- K. Matos and J. A. Soderquist, J. Org. Chem., 1998, 63, 461–470 CrossRef CAS PubMed
.
-
L. Kurti, Strategic Applications of Named Reactions in Organic Synthesis, Academic Press, 2005 Search PubMed
.
-
(a) S. Wegner and C. Janiak, Top. Curr. Chem., 2017, 375, 1–32 CrossRef CAS PubMed
; (b) M. M. Seitkalieva, D. E. Samoylenko, K. A. Lotsman, K. S. Rodygin and V. P. Ananikov, Coord. Chem. Rev., 2021, 445, 213982 CrossRef CAS
; (c) Y. Zhao, G. Cui, J. Wang and M. Fan, Inorg. Chem., 2009, 48, 10435–10441 CrossRef CAS PubMed
.
- C. F. Lima, A. S. Rodrigues, V. L. Silva, A. M. Silva and L. M. Santos, ChemCatChem, 2014, 6, 1291–1302 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nj05058d |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2025 |