Open Access Article
Open Access ArticleUnraveling boron oxidation and structure in 2D Mo4/3B2−xTz via solid-state NMR†
Yuan Zenga,
Hong Maa,
Zhongfu Taia,
Yan Tonga,
Yan Wangb and
Xiaolong Liu *a
*a
aSchool of Materials, Sun Yat-sen University, Shenzhen, Guangdong 518107, China. E-mail: liuxlong9@mail.sysu.edu.cn
bInstrumental Analysis and Research Center, Sun Yat-sen University, Guangzhou, Guangdong 510275, China
First published on 19th February 2025
Abstract
Atoms from i-MAB phases are selectively etched for boridene synthesis. However, water exposure severely hinders 2D boridene formation. By using NMR techniques, we precisely uncover the oxidation mechanism of boron and gain in-depth insights into the atomic-scale properties. Our findings offer a new avenue for advancing 2D boridene research.
Since the exfoliation of graphene from graphite, two-dimensional (2D) materials have attracted extensive interest due to their inherent attributes, such as a high surface area-to-volume ratio, unique electronic structure, and diverse physicochemical properties, which make them highly suitable for applications in electronics, energy storage, and catalysis.1 Scientists are actively growing the 2D material family to explore quantum confinement and low dimensionality.2 Since 2011, MXenes (2D transition metal carbides, nitrides, and carbonitrides) have stood out because of their rich configurations and tunable properties.3,4 Transition metal borides known as MBenes, formed by replacing the X element of MXenes with the neighboring B, naturally aroused increasing attention.3,5 Because it can form multicentered bonds to overcome its electron deficiency, boron, a light element with three valence electrons, reacts strongly with metal ions.6 Consequently, boron provides an excellent opportunity to form many intricate, multidimensional boride structures in materials. Unlike MXenes, MBenes may have notable bioactivity due to harmless boron, making them promising post-MXene materials for biotechnological applications. Although 2D MBenes are relatively new and in the early exploration stage, they are emerging as a promising class of nanomaterials.7
The 2D MBene family has orthorhombic and hexagonal structures, originating from MAB phases (M: transition metal, A: A-group element, and B: boron). Similar to MAX phases (layered ternary carbides and nitrides, X = C and/or N), the metallic M–A bonds in MAB phases are weaker, enabling active liquid-phase delamination.8 The 2D material is obtained by selectively etching the A element (usually Al) out. Its one-dimensional formula is MnB2n−2 (n = 2, 3, 4), with structures of alternating transition metal and B layers. Besides these typical MBenes, there are also fourteen predicted (M′2/3M′′1/3)2B2 MBenes, where M′ includes Ti, Cr, Mn, Fe, Mo, and W and M′′ includes Sc, Y, Zr, Nb, and Hf,9 along with high-entropy MAB/MAX phases10 and M2AlB2 structures.11,12
Finding a suitable precursor susceptible to deintercalation has proven difficult in the search for 2D MBenes. Recently, quaternary 3D i-MAB (in-plane chemical ordering) phases like Mo4/3Y2/3AlB2 and Mo4/3Sc2/3AlB2 were synthesized.13 Binary MBenes with ordered vacancies can be fabricated by etching light transition metals and Al from i-MAB phases. In the resultant molybdenum boride, vacancies emerge at the Y or Sc atom sites of the parent structure. These vacancies alter the electronic band and optical properties, impacting conductivity, bandgap, and absorption.14 Ordered vacancies can influence the diffusion and transport of ions within molybdenum borides, making them promising candidates for applications in energy storage devices, such as batteries and supercapacitors.15 2D MBenes are 2D honeycomb boron layers doped with transition metals. Electron localization functions (ELFs) clearly show electron localization between neighboring B–B atomic pairs, suggesting covalent bonding interactions among boron atoms in the planar layer of molybdenum boride.16 2D boridene in molybdenum boride resembles graphene to an extent. As a single-layer boron material, it's less stable than graphene and other 2D counterparts, hampering its development. Boridene oxidizes rapidly in air due to high oxygen reactivity, degrading its properties. It also reacts with water, yielding boron oxides. To obtain boridene, selective etching extracts specific atoms from i-MAB phases. Water exposure during etching is almost inevitable, especially with aqueous solutions. The reactive 2D boron atoms readily react with water, changing the boridene structure. Since 2D molybdenum boride is in the early development phase, its properties demand further study. Thus, this work utilized SSNMR, XPS, and IR for a comprehensive composition analysis.
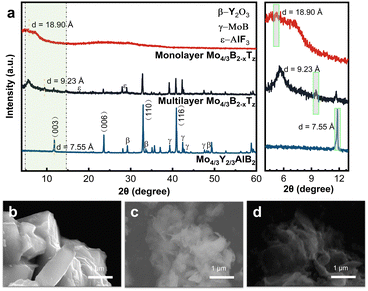
(Mo2/3Y1/3)2AlB2 compounds were prepared according to ref. 14 by a solid-state reaction of elemental powders. Through HF-etching, 2D Mo4/3B2−xTz, where Tz is fluorine, oxygen, or hydroxide surface terminations, was obtained by removing the Al and Y/Sc atoms and a small amount of B atoms.14 Generally, the relative intensity of the newly formed lower angle (00l) peak increased with the increasing HF concentration and etching time. Because 2D Mo4/3B2−xTz materials are sensitive to the environment, especially in concentrated HF solutions, shorter etching times and lower HF concentrations are preferred to obtain 2D ribbons with higher quality and stability. The corresponding XRD results of the etched powders are shown in Fig. 1a. After the HF treatment, there has been a significant decrease in the peak intensities originating from the (Mo2/3Y1/3)2AlB2 precursor. The (001) peak downshifts to a lower angle of 2θ = 9.35°, corresponding to an enlarged d-spacing value (d) of 9.23 Å, from the original 7.55 Å for (Mo2/3Y1/3)2AlB2. This additional low-angle (001) peak is frequently seen for MXenes that are made by selectively etching a MAX phase in HF.3,5 To investigate the morphology of the synthesized samples, the microstructure of 2D Mo4/3B2−xTz was observed by SEM and is shown in Fig. 1c and d. The morphology of monolayer Mo4/3B2−xTz films appears to be ribbon-like. The transition from a bulk phase to a layered material shows notable crystal structure and morphology changes due to selective etching.
A microscopic investigation (Fig. S1, ESI†) has been performed to study the topochemical deintercalation of Al and Y from (Mo2/3Y1/3)2AlB2 crystals by using transmission electron microscopy (TEM). An isolated 2D Mo4/3B2−xTz monolayer was observed in Fig. S1c (ESI†), suggesting that the synthesis and exfoliation of MBenes are feasible. The pristine 2D molybdenum boride should be Mo4/3B2 without surface termination. TEM images of Mo4/3Y2/3AlB2 demonstrate that each 2D Mo4/3B2 monolayer consists of boron layers sandwiched with molybdenum layers, i.e., three atomic layers stacked as Mo−B−Mo.17 Scheme S1 (ESI†) demonstrates the sandwich structure of 2D Mo4/3B2. Raman spectroscopy is recognized as a robust, nondestructive technique for characterizing low-dimensional materials. Raman spectroscopy is employed to reveal the remarkable structure of monolayer Mo4/3B2−xTz. Raman spectral measurements of 2D Mo4/3B2−xTz are shown in Fig. S2 (ESI†). Multiple Raman peaks are found at around 188, 337, 808, and 967 cm−1 for monolayer Mo4/3B2−xTz. The two intense peaks at 808 and 967 cm−1 could be assigned to the covalent B–B vibration of the boridene sheet in monolayer Mo4/3B2−xTz.18 Meanwhile, these peaks might be related to other types of vibrational modes in the material. The intense peaks at 188 cm−1 and 337 cm−1 could be attributed to B–O and Mo–B vibrations, respectively.19 Due to the electron deficiency, the boron-based graphene-like honeycomb materials cannot exist stably. The above observations indicate that covalent bonds between B atoms create a particular 2D borophene-like B layer that is bonded to MoB2 frameworks and does not exist in isolated forms.
The experimentally determined chemical formula of molybdenum boride, Mo4/3B2−xTz, indicates the possible existence of boron vacancies, and the concentration of the unknown surface termination group (T) remains undetermined. As our experimental investigations have shown, these surface groups play a crucial role in the stability and significantly influence many other materials applications. The XPS measurements have been employed to identify the chemical states (binding energies (BEs)) of those constituting elements in the (Mo2/3Y1/3)2AlB2 precursor and the filtered Mo4/3B2−xTz film.14 The XPS spectrum of monolayer Mo4/3B2−xTz is shown in Fig. 2. In Fig. 2a, four peaks fit the Mo 3d region for the Mo4/3B2−xTz film, and the peaks correspond to Mo species belonging to the Mo4/3B2−xTz sheets with oxidized states of Mo4+, Mo5+, and Mo6+. Fig. 2b presents the XPS spectrum of the B 1s region for the film, where the spectrum is fitted by two species: one belonging to Mo4/3B2−xTz sheets and the other one corresponding to B2O3 surface oxide. Based on the XPS measurements and analysis, it can be found that almost all of the Y atoms are removed, in addition to the complete removal of Al atoms during etching. Furthermore, in Fig. S3 (ESI†), surface terminations Tz were a mixture of –O, –OH, and –F based on the XPS spectra of O 1s and F 1s of the Mo4/3B2−xTz sheets. A previous XPS analysis of a freestanding film also suggests that the three termination types O, F, and OH are present.14,20 Due to the synthesis of MBenes through wet etching approaches, the existence of surface terminations on the outer MBenes’ surface and between the layers is inevitable, and the surface terminations including –O, –OH, and –F groups will be present for MBenes.20 Although the surface chemistry's effect is considered minor for most macroscopic materials, it is crucial for 2D nanomaterials due to their massive lateral diameters and unusual surface areas.6
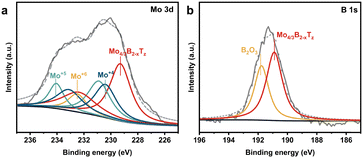 | ||
| Fig. 2 High-resolution XPS spectra of monolayer Mo4/3B2−xTz: (a) the Mo 3d region and (b) the B 1s region, and their fittings representing various species. | ||
Our XRD and XPS observations are consistent with the findings reported in Science.10 In that paper, XPS offered valuable surface chemistry and bonding data, especially for boron and molybdenum. As a surface analysis technique, XPS details the chemical composition, oxidation states, and electronic states of surface elements, probing only the top 1–10 nm. XPS spectra show elemental composition and chemical states at specific surface points or regions. Solid-state NMR, in contrast, is a bulk characterization method. It covers the whole sample, both surface and interior, and is great for studying atomic-scale nucleus structure, dynamics, and chemical environments. Thus, it gives comprehensive bulk details about a material's atomic-scale structural and dynamic properties.
Indeed, these oxides may localize on the surface. Such surface localization can cause high intensity in the XPS spectrum, perhaps masking the underlying boride material. So, though boron oxide seems to dominate the surface chemistry seen in XPS, the underlying monolayer hydrogenated boride may still exist beneath the oxide layer. Solid-state NMR is valuable for studying bulk information, especially useful for crystalline, amorphous materials, surfaces, interfaces, and heterogeneous systems. It can also offer details about local environments, chemical bonding, and atomic-scale structure. Since the bond nature of MAB phases is crucial for MBene synthesis, thorough experimental research on it is urgently needed.
Due to the ability of 11B and 27Al solid-state NMR spectroscopy to provide diverse chemical information, 11B and 27Al NMR spectroscopies are indispensable tools for characterizing MAB phases. Since 11B and 27Al are quadrupolar nuclei with half-integral spin (3/2 and 5/2), operation at high magnetic field strength causes only the central (1/2, −1/2) transition to be observed. Fig. 3(a) and Fig. S4 (ESI†) show the 11B and 27Al MAS NMR spectra of Mo4/3Y2/3AlB2, multilayer Mo4/3B2−xTz and monolayer Mo4/3B2−xTz. To eliminate interference from the probe components (rotors), the 11B MAS NMR spectra of the boride materials comprise different spectra of them and the spectrum of an empty rotor, all obtained using the same experimental conditions. The 27Al chemical shift of Mo4/3Y2/3AlB2 is around 184 ppm, which indicates that aluminum atoms form metallic bonds within the layered structure. Only a few aluminum oxides with −5.0 ppm were observed after the deintercalation process of Al from Mo4/3Y2/3AlB2 crystals.
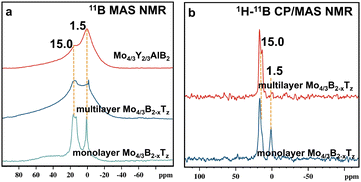 | ||
| Fig. 3 (a) 11B MAS NMR spectra of Mo4/3Y2/3AlB2, multilayer Mo4/3B2−xTz, and monolayer Mo4/3B2−xTz. (b) 1H–11B CP/MAS NMR spectra of multilayer Mo4/3B2−xTz and monolayer Mo4/3B2−xTz. | ||
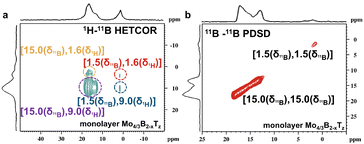
According to the reference, the 15 ppm and 1.5 ppm signals in 11B MAS NMR spectra correspond to BO3 and BO4 units in boron-containing materials, respectively.21 Relying solely on chemical shifts often doesn’t provide convincible assignments, especially in complex materials. To overcome the limitations of 11B MAS for signal assignment, 1H–11B CP/MAS, 1H–11B (HETeronuclear CORrelation) HETCOR, and 11B–11B proton-driven spin diffusion (PDSD) techniques have been employed to study structural correlations between boron and hydrogen, as well as between different boron species. HETCOR often gives a more detailed picture of heteronuclear proximities (e.g., boron and protons), whereas PDSD excels in exploring homonuclear couplings (e.g., 11B–11B). HETCOR reveals heteronuclear interactions (e.g., 1H–11B), which provides more detailed insights into the local bonding environment, particularly the relationship between boron centers and surrounding protons. PDSD reveals spatial proximities and connects nearby atoms within the same spin system, making it valuable for understanding short- and medium-range structures. In this case, PDSD can be a valuable technique to explore the spatial proximity between boron atoms, especially B–B interactions.
The 1H–11B CP/MAS and 1H–11B HETCOR NMR techniques have been used to characterize the hydrogen–boron interactions, providing valuable insights into the chemical environment of boron atoms in hydroxylated terminations. The 11B resonance peak in Fig. 3b and the correlated signals in Fig. S5 (ESI†) provide valuable information about a sample's spatial proximity and interactions between hydrogen and boron atoms in multilayer Mo4/3B2−xTz and monolayer Mo4/3B2−xTz. The correlated contour in Fig. 3b demonstrates the spatial correlation between a proton with a chemical shift around +9 ppm and B with a chemical shift around +15 ppm. The correlated signal indicates that B–OH⋯OB hydrogen bonds spatially connect BO3 units. The correlated signals in Fig. 4a indicate that the protons in both B–OH⋯OB hydrogen bonds and B–OH groups are spatially close to the BO3 and BO4 units in monolayer Mo4/3B2−xTz, respectively. The protons in monolayer Mo4/3B2−xTz created an H-bonded network, which chemically bonded or structurally integrated the oxidized boron species into the primary 2D Mo4/3B2−xTz structure. The first-principles calculations showed that surface functionalization improves the dynamical stability of the boridene sheets.16 Oxidized boron stabilized the 2D Mo4/3B2−xTz material under ambient conditions, preventing further degradation or oxidation of the Mo or B atoms.
Besides HETCOR, proton-driven spin diffusion (PDSD) is highly effective for probing spatial proximity, especially in B–B interactions in boridene. However, no B–B correlated peaks have been observed in Fig. 4b. The self-correlated signals in Fig. 4b between the same type of boron nuclei (e.g., BO3 or BO4 groups) suggest that these boron atoms are in close spatial proximity within the same phase or structural environment. The boron atoms in boridene are in close spatial proximity within the same structural environment. However, the absence of correlated peaks other than BO3 or BO4 groups in Fig. 4b suggests that there are no B–B interactions within the detectable range of the experiment. The lack of inter-site interactions between different boron environments, i.e., BO3 vs. BO4, implies that little spatial interaction exists between these distinct boron environments in the material. NMR spectra show that borate-like or borax-like species were formed when boron sources partially oxidized and created discrete layers or clusters during synthesis or post-processing. Then, multiple 11B NMR techniques have been performed on boric acid (H3BO3) in Fig. S6 (ESI†) and borax (sodium borate, Na2B4O7·10H2O) in Fig. S7 (ESI†) further to clarify the oxidation species in 2D Mo4/3B2−xTz. Fig. S6 (ESI†) demonstrates that boric acid primarily consists of boron in a trigonal BO3 configuration. The three-coordinate trigonal planar structure of boron (BO3) has a broadened line shape due to quadrupolar coupling for environments with lower symmetry. Fig. S7 (ESI†) exhibits similarities to the NMR spectra of monolayer Mo4/3B2−xTz. The 11B chemical shifts around 15.0 ppm correspond to BO3 units, and the 11B resonance peak at 1.5 ppm could be attributed to BO4 units. Based on the NMR spectra of H3BO3 and borax, it is observed that during the etching process of light transition metal atoms and Al from the i-MAB phases, boron–oxygen bonds (such as BO3 or BO4 units) are formed. The formation mechanism proceeds from Mo4/3Y2/3AlB2 and Mo4/3Sc2/3AlB2 to the formation of Mo4/3B2−x(OH)z, alongside the generation of AlF3·xH2O and YF3·xH2O. This process demonstrates the role of boron–oxygen bond formation in stabilizing the structure during oxidation, preventing further degradation of the Mo or B atoms in the 2D material. The similarity between the 11B NMR spectra of borax and monolayer Mo4/3B2−xTz is that they both have boron in trigonal (BO3) and tetrahedral (BO4) habitats. Multiple 11B NMR spectra suggest that most boron atoms in Mo4/3B2−xTz could be oxidized into BO3 and BO4 units. Boridene is inherently sensitive to oxidation, thermal conditions, and environmental factors, which can lead to degradation over time. Advances in controlled etching environments, protective strategies, and alternative techniques are essential to overcome these challenges and achieve stable boride with desirable properties. The observed 11B MAS NMR signals mainly come from borate-like species, helping to clarify the actual structure of the 2D Mo4/3B2−xTz materials.
Bulk boron does not have a simple layered structure like graphite (the precursor to graphene). Instead, boron forms complex, three-dimensional structures with strong covalent bonds between atoms, making it different from materials like graphite or molybdenum disulfide (MoS2), where layers can be easily separated into 2D sheets.22 Boridene's metastability arises from the nonlayered structure of bulk boron, making it inherently less stable than materials like graphene. This metastability requires careful synthesis and handling to maintain boridene in its 2D form. Boridene's synthesis often requires careful control of experimental conditions, such as temperature and the choice of substrate, to stabilize the 2D form.23,24 Typically, 2D materials are synthesized by exfoliating van der Waals solids, but boridene is an exception, produced through the selective etching of aluminum and yttrium (or scandium) from chemically ordered 3D compounds like (Mo2/3Y1/3)2AlB2 and (Mo2/3Sc1/3)2AlB2. The etching process in aqueous hydrofluoric acid selectively removes Al and Y/Sc, leaving Mo4/3B2−x sheets terminated with fluorine, oxygen, or hydroxide (Tz). The possibility of synthesizing a wide variety of 2D transition metal borides through chemical exfoliation of laminated materials may expand the toolkit of 2D materials beyond conventional van der Waals solids or MXenes. However, NMR results confirm the formation of boron–oxygen bonds (e.g., BO3 or BO4 units), which prevents further oxidation or degradation of the underlying Mo or B atoms in the 2D material. By preventing deep oxidation or corrosion of the Mo and B atoms, the oxidized boron species help maintain the overall structural integrity of the 2D material. The etching process to produce “boridene” from i-MAB phases is delicate and dramatically influences the resulting material's stability.
In summary, 2D Mo4/3B2−x MBenes with ordered vacancies were made by etching light transition metals and Al from i-MAB phases. Combining solid-state NMR and other methods, we probed the structural and chemical properties of MAB phases and their exfoliated products. Results show that materials change greatly during wet-chemical etching and delamination. Most B atoms in multilayer and monolayer Mo4/3B2−x oxidize. Grasping boridene-environment interactions is key for applications. These findings expand our understanding of synthesis and lay a foundation for exploring atomically thin materials.
Author contributions
Yuan Zeng: writing – original draft, investigation; Hong Ma: data curation and analysis; Zhongfu Tai and Yan Tong: reviewing and editing; Yan Wang: visualization; and Xiaolong Liu: writing – reviewing and editing, methodology, funding acquisition.Data availability
Data are available on reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (NSFC) (22075322).Notes and references
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- S. Z. Butler, S. M. Hollen, L. Cao, Y. Cui, J. A. Gupta, H. R. Gutiérrez, T. F. Heinz, S. S. Hong, J. Huang, A. F. Ismach, E. Johnston-Halperin, M. Kuno, V. V. Plashnitsa, R. D. Robinson, R. S. Ruoff, S. Salahuddin, J. Shan, L. Shi, M. G. Spencer, M. Terrones, W. Windl and J. E. Goldberger, ACS Nano, 2013, 7, 2898–2926 CrossRef CAS PubMed.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 16098 CrossRef CAS.
- M. Naguib, O. Mashtalir, J. Carle, V. Presser, J. Lu, L. Hultman, Y. Gogotsi and M. W. Barsoum, ACS Nano, 2012, 6, 1322–1331 CrossRef CAS PubMed.
- H. Braunschweig and M. Colling, Coord. Chem. Rev., 2001, 223, 1–51 CrossRef CAS.
- M. Jakubczak, A. Szuplewska, A. Rozmysłowska-Wojciechowska, A. Rosenkranz and A. M. Jastrzębska, Adv. Funct. Mater., 2021, 31, 2103048 CrossRef CAS.
- X. Liu, X. Ge, Y. Dong, K. Fu, F. Meng, R. Si, M. Zhang and X. Xu, Mater. Chem. Phys., 2020, 253, 123334 CrossRef CAS.
- Y. Yao, N. Miao, Y. Gong and J. Wang, Nanoscale, 2021, 13, 13208–13214 RSC.
- Z. Du, C. Wu, Y. Chen, Q. Zhu, Y. Cui, H. Wang, Y. Zhang, X. Chen, J. Shang, B. Li, W. Chen, C. Liu and S. Yang, Adv. Energy Mater., 2022, 12, 2103228 CrossRef CAS.
- L. T. Alameda, R. W. Lord, J. A. Barr, P. Moradifar, Z. P. Metzger, B. C. Steimle, C. F. Holder, N. Alem, S. B. Sinnott and R. E. Schaak, J. Am. Chem. Soc., 2019, 141, 10852–10861 CrossRef CAS PubMed.
- T. Yilmaz, O. G. Yildiz, N. S. Peighambardoust, M. Baitinger and U. Aydemir, Inorg. Chem., 2025, 64, 1139–1145 CrossRef CAS PubMed.
- J. Zhou, J. Palisaitis, J. Halim, M. Dahlqvist, Q. Tao, I. Persson, L. Hultman, P. O. Å. Persson and J. Rosen, Science, 2021, 373, 801–805 CrossRef CAS PubMed.
- K. Kim, C. Chen, D. Nishio-Hamane, M. Okubo and A. Yamada, Chem. Commun., 2019, 55, 9295–9298 RSC.
- B. Chen, H. Liu, T. Bai, Z. Song, J. Xie, K. Wu, Y. Cheng and B. Xiao, Nanoscale, 2022, 14, 17955–17975 RSC.
- H. Wu, Z. Gao, F. Ma, Z. Tian, Y. Liu, Y. Jiao and A. Du, Appl. Surf. Sci., 2022, 602, 154374 CrossRef CAS.
- M. Dahlqvist, Q. Tao, J. Zhou, J. Palisaitis, P. O. Å. Persson and J. Rosen, J. Am. Chem. Soc., 2020, 142, 18583–18591 CrossRef CAS PubMed.
- A. Sarycheva and Y. Gogotsi, Chem. Mater., 2020, 32, 3480–3488 CrossRef CAS.
- S. Sahoo and S. K. Singh, Ceram. Int., 2017, 43, 15561–15566 CrossRef CAS.
- M. Khazaei, J. Wang, M. Estili, A. Ranjbar, S. Suehara, M. Arai, K. Esfarjani and S. Yunoki, Nanoscale, 2019, 11, 11305–11314 RSC.
- S. Geng, F. U. Shah, P. Liu, O. N. Antzutkin and K. Oksman, RSC Adv., 2017, 7, 7483–7491 RSC.
- X. Sun, X. Liu, J. Yin, J. Yu, Y. Li, Y. Hang, X. Zhou, M. Yu, J. Li, G. Tai and W. Guo, Adv. Funct. Mater., 2017, 27, 1603300 CrossRef.
- B. Feng, J. Zhang, Q. Zhong, W. Li, S. Li, H. Li, P. Cheng, S. Meng, L. Chen and K. Wu, Nat. Chem., 2016, 8, 563–568 CrossRef CAS PubMed.
- B. Kiraly, X. Liu, L. Wang, Z. Zhang, A. J. Mannix, B. L. Fisher, B. I. Yakobson, M. C. Hersam and N. P. Guisinger, ACS Nano, 2019, 13, 3816–3822 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and other data. See DOI: https://doi.org/10.1039/d5nj00282f |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2025 |