 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Blue to yellow-emitting neutral Cu(I) complexes with μ-bridging 1,2,4-triazole: synthesis, photophysical characterization and DFT calculations†
Valentina Ferraro‡
 a,
Xuemin Gan‡
a,
Xuemin Gan‡ a,
Florian R. Rehak
a,
Florian R. Rehak b,
Philipp Ralle
b,
Philipp Ralle c,
Martin Nieger
c,
Martin Nieger d,
Andreas Steffen
d,
Andreas Steffen c,
Wim Klopper
c,
Wim Klopper *be and
Stefan Bräse
*be and
Stefan Bräse *af
*af
aInstitute for Organic Chemistry (IOC), Karlsruhe Institute of Technology (KIT), Kaiserstrasse 12, 76131 Karlsruhe, Germany. E-mail: braese@kit.edu
bInstitute of Physical Chemistry (IPC), Karlsruhe Institute of Technology (KIT), Kaiserstrasse 12, 76131 Karlsruhe, Germany. E-mail: klopper@kit.edu
cDepartment of Chemistry and Chemical Biology, TU Dortmund University, Otto-Hahn-Strasse 6, 44227 Dortmund, Germany
dDepartment of Chemistry, University of Helsinki, A.I. Virtasen Aukio 1, P.O. Box 55, FI 00014 Helsinki, Finland
eInstitute of Nanotechnology (INT), Karlsruhe Institute of Technology (KIT), Kaiserstrasse 12, 76131 Karlsruhe, Germany
fInstitute of Biological and Chemical Systems-Functional Molecular Systems (IBCS-FMS), Karlsruhe Institute of Technology (KIT), Kaiserstrasse 12, 76131 Karlsruhe, Germany
First published on 9th April 2025
Abstract
A series of Cu(I) complexes having 1,2,4-triazole as bridging ligand and aminophosphanes as chelating [NP]-donors were successfully isolated and spectroscopically characterized. The structure of the Cu(I) derivatives was ascertained through single-crystal X-ray diffraction confirming the formation of dimers. The Cu(I) species were characterized by absorptions below 400 nm, with no metal-to-ligand charge transfer (MLCT) band observable in the visible region of the spectrum. Upon excitation with UV light, the Cu(I) complexes exhibited appreciable blue to yellow emissions with lifetimes in the 2–9 μs range and photoluminescent quantum yields up to 0.42. The photophysical properties were investigated in the solid state and polymeric matrix both at room temperature and 77 K. The increased lifetimes τ at low temperatures and the two orders of magnitude different radiative decay constants kr at 297 and 77 K suggest the presence of two different decay channels (fluorescence and phosphorescence), thus indicating thermally activated delayed fluorescence (TADF). The experimental data were supported by computational studies, further confirming the presence of TADF properties.
Introduction
1,2,4-Triazole and its derivatives have gained particular attention as ligands for transition metal elements since they combine the coordination geometry of both pyrazoles and imidazoles, and they can act as bridging ligands between two metal centres.1,2 As highlighted in Fig. 1a, 1,2,4-triazole is an electron-rich heterocycle containing one acidic pyrrole-like NH group in position 1 and a basic pyridine-like nitrogen in position 2. While the NH group can easily donate its proton, the basic pyridine-like nitrogen can accept protons even more readily; hence, the μ-bridging coordination mode is usually found for many anionic triazoles, leaving the nitrogen atom in 4-position unsubstituted (see Fig. 1b).3 In this bridging mode, the two metal ions are located at distances of about 400 pm, and the M–N–N angles are close to 126°. This peculiar feature and the relatively easy synthesis make them suitable for designing new transition metal complexes.1 | ||
| Fig. 1 (a) Structure of 1,2,4-triazole emphasizing the nitrogen atoms in position 1 and 2; (b) bridging coordination mode of 1,2,4-triazole. | ||
On the other hand, NP-ligands such as aminophosphanes are used with Cu(I) halides to prepare luminescent derivatives due to their tuneable electronic and steric properties.4–6 For instance, Yersin et al. described the use of 2-diphenylphosphanyl-N,N-dimethylaniline and diphenyl-(2-pyrrolidin-1-ylphenyl)phosphine for the synthesis of blue- and green-emitting Cu(I) dimers exhibiting thermally activated delayed fluorescence (TADF) to be applied for the preparation of organic light-emitting diodes (OLEDs).7,8 Similarly, the use of bulkier tridentate NPP- and tetradentate PNNP-ligands afforded emissive Cu(I) complexes characterized by long-lived excited states lifetimes and high quantum yields due to the low degree of reorganization between the ground and the excited state.9–11 Other types of NP ligands instead of aminophosphane are constituted by 2-diphenylphosphinopyridine and its derivatives.12–19 This type of chelating [NP]-donor ligands was deeply investigated by our research group in combination with Cu(I) halides for the preparation of derivatives to be employed for OLED applications.20–24
Given our interest in the preparation of Cu(I) complexes exhibiting TADF,6,20–25 we deemed it interesting to investigate the effect of a bridging 1,2,4-triazole ligand in the photophysical properties of the corresponding Cu(I) dimers. Herein, we report the synthesis and characterization of a series of luminescent neutral Cu(I) complexes having bridging 1,2,4-triazoles and aminophosphanes as coordinated [NP]-donor ligands. The molecular structure of the derivatives was ascertained through single-crystal X-ray diffraction. The photophysical properties were investigated at room temperature and 77 K both in the solid state and in a poly(methyl)methacrylate (PMMA) matrix, and supported by density functional theory (DFT) and time-dependent DFT (TD-DFT) calculations confirming the presence of TADF properties.
Results and discussion
Synthesis and characterization of the complexes
The NP-ligands were synthesized by first functionalizing the amino group with methyl iodide or 1,4-dibromobutane, as observable in Scheme 1. Then the phosphine group was introduced by phosphorylation reaction with the corresponding chloro(diaryl)phosphine in the presence of n-butyllithium (2.5 M in hexane). 2-(Diphenylphosphanyl)-N,N-dimethylpyridin-3-amine (NpyP) was synthesized as previously reported by Lu et al. employing diphenylphosphine, palladium(II) acetate and potassium acetate.4 For the preparation of the corresponding Cu(I) complexes, 1,2,4-triazole was deprotonated with tBuOK in dry dichloromethane (DCM) under Ar atmosphere, and the mixture was added to a solution containing CuCl and the NP-ligand in dry DCM. After filtering off the KCl, the Cu(I) complexes were isolated in good yields (43–68%) as pale solids. The derivatives were stable as solids under air, but rapidly oxidated once dissolved in chlorinated solvents, e.g. CDCl3.Crystals suitable for X-ray diffraction were obtained by slow diffusion of diethyl ether vapours in dichloromethane solutions under argon atmosphere. Crystallographic and refinement data are collected in the ESI† (Tables S1 and S3), together with selected distances and angles (Tables S2 and S4–S6, ESI†). The X-ray structures are collected in Fig. 2.
 | ||
| Fig. 2 X-ray structures of [Cu(μ-trz)(NP)]2, [Cu(μ-trz)(NPF)]2, [Cu(μ-trz)(NPAr)]2, [Cu(μ-trz)(NpyrP)]2, and [Cu(μ-trz)(NpyP)]2. Displacement parameters drawn at 30% probability level. | ||
In all the complexes, two Cu(I) atoms are bridged with the two anionic 1,2,4-triazole ligands, forming a six-membered ring structure. Both the metal centres adopt a distorted tetrahedral geometry, coordinated by one N of μ-trz1 and one of μ-trz2, and the respective chelating NP-ligand. [Cu(μ-trz)(NPAr)]2 crystallized with a molecule of water and dichloromethane in the asymmetric unit. Except for [Cu(μ-trz)(NPAr)]2, the tetrahedral environment around the metal centre is highly distorted, with the angles N–Cu1–P1 and N–Cu1–Ntrz respectively around 75–80° and 127–132°. Two different symmetric arrangements were found for the investigated substances: complexes [Cu(μ-trz)(NP)]2, [Cu(μ-trz)(NPF)]2, [Cu(μ-trz)(NpyrP)]2 and [Cu(μ-trz)(NpyP)]2 show Ci symmetry, with a centre of inversion at the midpoint of the Cu–N bond of the six-membered moiety, while complex [Cu(μ-trz)(NPAr)]2 displays Cs symmetry. The six-membered ring is formed by the four nitrogen atoms of the two anionic 1,2,4-triazole ligands and the two coplanar Cu(I) atoms. The central hexagonal core is distorted, being the N–Cu1–N angle in the range of 109–111°, instead of the expected 120°. On the other hand, all the other angles of the hexagon are comprised between 123° and 127°. The distortion is mainly induced by the more rigid NPAr ligand. Similarly to what is observed in other binuclear [Cu(μ-X)(NP)]2 complexes (X = Cl, Br, I),7 the N–Cu–P bite angles are comprised between 121.85 and 131.89°, whereas the corresponding Cu–P and Cu–N bond lengths are around 2.18 Å and 1.98 Å, respectively.
The μ-bridging mode of the anionic 1,2,4-triazole leads to a Cu–Cu distance between 3.60 and 3.69 Å for all the measured complexes. This distance is significantly longer than the sum of the van der Waals radii (2.80 Å), ruling out any possible cuprophilic interaction.26 The highest value was measured with NpyP as chelating ligand, but the Cu–Cu distance remained almost constant for all the other NP ligands, suggesting a negligible influence of the alkyl N-substituents.
Differently from previously reported binuclear butterfly-shaped Cu(I) complexes having chelating NP-ligands,7 the interplanar distance between the centroids originating from the six-membered rings is found between 9.23 and 11.91 Å for [Cu(μ-trz)(NP)]2, [Cu(μ-trz)(NPF)]2, [Cu(μ-trz)(NpyrP)]2 and [Cu(μ-trz)(NPAr)]2, suggesting the absence of intermolecular interactions. Instead, complex [Cu(μ-trz)(NpyP)]2 exhibits a highly distorted and asymmetric configuration and disordered molecular packing arrangement. The different molecular packing of [Cu(μ-trz)(NP)]2 and [Cu(μ-trz)(NpyP)]2 is observable in Fig. S1 (ESI†). Intra- and intermolecular hydrogen bonds are in this case expected, being the distances H15⋯N2 and H5⋯N24 of 2.62 and 2.65 Å, respectively. The related H-bond parameters in complex [Cu(μ-trz)(NpyP)]2 are summarized in Table S7 (ESI†).
Photophysical properties
The UV-vis spectra in DCM of all the Cu(I) complexes are collected in Fig. 3 and are characterized by absorptions below 400 nm. No band associated with the MLCT transition in the visible range could be detected in line with the pale colour of the Cu(I) complexes as powder samples. The only observable band has maximum molar coefficients ε below 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L mol−1 cm−1 and can be ascribed to the π → π* of the coordinated phenyl fragments. This band is shifted more towards 300 nm for [Cu(μ-trz)(NP)]2 and [Cu(μ-trz)(NpyP)]2 (see black and blue lines in Fig. 3).
000 L mol−1 cm−1 and can be ascribed to the π → π* of the coordinated phenyl fragments. This band is shifted more towards 300 nm for [Cu(μ-trz)(NP)]2 and [Cu(μ-trz)(NpyP)]2 (see black and blue lines in Fig. 3).
Photoluminescent measurements were carried out on powder samples and in a 1 wt% doped PMMA matrix (see Experimental section for details on the sample preparation). After exciting the Cu(I) complexes with wavelengths between 330 and 380 nm, appreciable emissions in the 480–570 nm range were observed.
The broad and featureless properties suggest the involvement of charge transfer mechanisms in the emission (see Fig. 4 for the emission spectra at 297 and 77 K both in the solid sample and PMMA matrix). It is worth mentioning that, in some of the PL spectra collected in PMMA matrix both at 297 and 77 K, a band in the 400–475 nm range was detected, as observable for instance in Fig. 4 for [Cu(μ-trz)(NP)]2, [Cu(μ-trz)(NpyrP)]2 and [Cu(μ-trz)(NpyP)]2. This band is not due to the emission of the Cu(I) derivatives, but to a minor impurity originating during the polymerization process of PMMA. The complete photoluminescence data is provided in Table 1, and the excitation and emission spectra at different temperatures and in different matrices are collected in Fig. S2–S5 (ESI†). Compared to the Cu(I) iodo-complexes reported by Yersin et al. in combination with NP and NpyrP,7 the emission maxima appear to be more red-shifted passing from around 465 nm to 480 and 499 nm for [Cu(μ-trz)(NP)]2 and [Cu(μ-trz)(NpyrP)]2, respectively. The introduction of substituents in the phenyl rings of the NP ligand determined a bathochromic shift in the emission spectra in the 20–40 nm range in [Cu(μ-trz)(NPF)]2 and[Cu(μ-trz)(NPAr)]2. As previously observed for similar compounds,20–24 the introduction of the pyridine fragment in the skeleton of the NP ligand provoked the most significant red-shift of the emission maxima, reaching the yellow region with λem = 570 nm.
| Medium | T [K] | λmax [nm] | τ〈amp〉 [μs] | ϕ | kr [s−1] | |
|---|---|---|---|---|---|---|
| [Cu(μ-trz)(NP)]2 | Solid | 297 | 480 | 6.7 | 0.25 | 3.73 × 104 |
| 77 | 476 | 188.2 | — | — | ||
| PMMA | 297 | 480 | 5.4 | 0.11 | 2.03 × 04 | |
| 77 | 539 | 251.3 | 0.24 | 9.56 × 101 | ||
| [Cu(μ-trz)(NPF)]2 | Solid | 297 | 522 | 9.4 | 0.33 | 3.51 × 104 |
| 77 | 539 | 252.0 | — | — | ||
| PMMA | 297 | 513 | 4.9 | 0.10 | 2.04 × 104 | |
| 77 | 523 | 235.5 | 0.12 | 5.10 × 101 | ||
| [Cu(μ-trz)(NPAr)]2 | Solid | 297 | 503 | 7.4 | 0.42 | 5.71 × 104 |
| 77 | 509 | 218.0 | — | — | ||
| PMMA | 297 | 521 | 5.3 | 0.20 | 3.75 × 104 | |
| 77 | 520 | 242.3 | 0.37 | 1.53 × 102 | ||
| [Cu(μ-trz)(NpyrP)]2 | Solid | 297 | 499 | 4.4 | 0.16 | 3.64 × 104 |
| 77 | 491 | 179.2 | — | — | ||
| PMMA | 297 | 532 | 6.4 | 0.10 | 1.56 × 104 | |
| 77 | 530 | 307.5 | 0.14 | 4.55 × 101 | ||
| [Cu(μ-trz)(NpyP)]2 | Solid | 297 | 570 | 2.3 | 0.06 | 2.61 × 104 |
| 77 | 591 | 120.9 | — | — | ||
| PMMA | 297 | 563 | 2.0 | 0.02 | 1.02 × 104 | |
| 77 | 558 | 131.3 | 0.05 | 3.81 × 101 | ||
Although a bathochromic shift in the emission maximum due to the stabilization of a low-lying triplet state at low temperatures is a common qualitative hint for TADF materials,6,27 for the Cu(I) complexes here investigated it was observed only for [Cu(μ-trz)(NPF)]2, [Cu(μ-trz)(NPAr)]2 and [Cu(μ-trz)(NpyP)]2. For the other Cu(I) complexes the opposite trend was detected.In general, for all the other Cu(I) species investigated, a hypsochromic shift of the excitation spectrum was noticeable due to the stabilization of the polar ground state once the complexes were embedded in the PMMA matrix.
As observable in Fig. 5, in the case of [Cu(μ-trz)(NpyP)]2 an additional excitation band at lower energy was detected around 390 nm, which disappeared in the PMMA matrix. Despite the nature of this band not being fully understood, we can assume that it is due to the direct S0 → T1 transition since it is observable only in the solid state, when the high concentration of the molecules enables the detections of transitions which are normally forbidden.28 This hypothesis is further supported by the absence of this excitation band in the PMMA matrix.
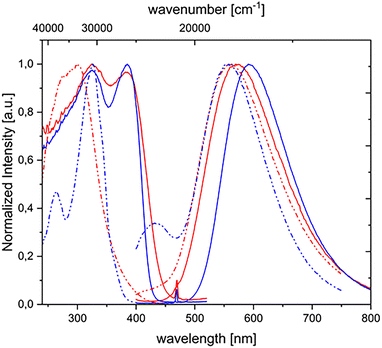 | ||
| Fig. 5 Excitation and emission spectra of [Cu(μ-trz)(NpyP)]2 in the solid state (solid lines) and in the PMMA matrix (dashed lines) at room temperature (red) and 77 K (blue). | ||
As concerns the lifetimes τ, all the Cu(I) derivatives exhibit a lifetime between 2 and 9 μs at room temperature (see Table 1). Except for [Cu(μ-trz)(NpyrP)]2, the estimated lifetime is normally shorter once the Cu(I) complexes are embedded in the polymeric matrix. The lifetime is significantly increased in the range of hundreds of μs at 77 K, as expected for TADF materials due to the freezing of the prompt fluorescence.6,27,29 The emission decays both at the solid state and in 1 wt% PMMA at different temperatures are provided in Fig. S6–S15 (ESI†). Since the emission decay curves were not monoexponential, the preexponential B-factors and the related lifetimes τ are collected in Table S8 (ESI†).
The photoluminescent quantum yields Φ were measured for all the Cu(I) derivatives in the solid state at 297 K and in a 1 wt% PMMA matrix both at 297 and 77 K (see Table 1). As observed also for the lifetimes, a general decrease of Φ was detected after the embedding in PMMA at room temperature. In all cases, [Cu(μ-trz)(NPAr)]2 exhibited the highest values, being Φ = 0.42 at the solid state, Φ = 0.20 and Φ = 0.37 in 1 wt% PMMA at 297 K and 77 K, respectively. The radiative rate constant kr was estimated based on the equation Φ = kr/(kr + knr).8,30 The comparison between the values at 297 and 77 K indicates the involvement of fluorescence (kr ≈ 104 s−1) and phosphorescence (kr ≈ 101–102 s−1) channels, supporting the TADF mechanism.
DFT calculations
The singlet ground state S0 was optimized starting from the crystal structures of the Cu(I) derivatives, and the corresponding structures are collected in Fig. S16 (ESI†). The cutting angles θ and ϕ highlighted in Fig. S17 (ESI†) were used to compare the optimized S0 states with the crystal structures (see Table S9, ESI†).The computed UV-vis spectra of the Cu(I) complexes are presented in Fig. S18 (ESI†) and indicate only a minor influence of the substituents on the skeleton of the NP-ligand. For all the complexes the UV-vis spectra are characterized by a main band around 260 nm and a small shoulder at 285 nm. In contrast, for [Cu(μ-trz)(NpyP)]2 both bands are red-shifted by about 10 nm to 15 nm, as also observed in the experimental UV-vis spectrum (see Fig. 3 for comparison).
To evaluate the suitability of investigating Cu(I) complexes as TADF emitters, the first S1 state with MLCT character (SMLCT1) was determined starting from S0, whereas the T1 state with MLCT character (TMLCT1) was determined from SMLCT1. As highlighted in Table S9 (ESI†), there is a change in the plane intersection angle θ of 4° to 7° for the excited states compared to the S0 states. The bond intersection angle ϕ shows even a much stronger change, increasing from 5° to 9° for the S0 states to 25° to 30° for the excited states. The described MLCT character is similar for all the described Cu(I) complexes, and it is illustrated in the non-relaxed difference densities in Fig. S19 (ESI†). This is because the fluorine and aryl substituents are not directly involved in the excitation, in contrast to the pyridinic nitrogen of [Cu(μ-trz)(NpyP)]2.
The similarity of the excitations for SMLCT1 and TMLCT1 is also supported by the analysis of the natural orbitals. As observable in Tables S10 and S13 (ESI†), for the TMLCT1 of [Cu(μ-trz)(NP)]2 the contribution of C(6) is 0.06 and for Npy in [Cu(μ-trz)(NpyP)]2 it is 0.10. For SMLCT1 the influence is even weaker with 0.06 for C(6) and 0.07 for Npy. The natural population analysis of [Cu(μ-trz)(NPF)]2 and [Cu(μ-trz)(NPAr)]2 are similar to the other two complexes (see Tables S11 and S12 for completeness, ESI†).
To determine the amount of charge transfer, the overlap between the natural transition orbitals SNTO was calculated and the data is provided in Table S14 (ESI†). The NTOs show the same character as the non-relaxed difference densities illustrated in Fig. S19 (ESI†). The SNTO analysis indicates that [Cu(μ-trz)(NpyP)]2 has a slightly higher charge transfer character for TMLCT1 and SMLCT1 compared to the other Cu(I) complexes. The lower SNTO is also reflected in the calculated ΔEadiaST, which is 179 meV for [Cu(μ-trz)(NpyP)]2 and between 234 and 242 meV for the other Cu(I) complexes. These values are in a reasonable range for TADF.6
As observable in Table S15 (ESI†), HOMO → LUMO and HOMO−1 → LUMO+1 are the dominant excitation contributions, being the HOMO and HOMO−1 as well as the LUMO and LUMO+1 nearly degenerate due to symmetry.
A similar trend was observed in the transition dipole moment μT for the excited states, where [Cu(μ-trz)(NpyP)]2 has both the lowest SNTO and the lowest μT (see Table S14, ESI†). This suggests a lower radiation rate for [Cu(μ-trz)(NpyP)]2 compared to the other Cu(I) derivatives, as supported also by the experimental data (see Table 1).31
It should be noted that an additional T1 state with ligand-centred (LC) character was found in all the Cu(I) complexes, as illustrated in Fig. 6 and Fig. S20 (ESI†). An S1 state with LC character could not be determined starting from TLC1. Instead, the optimization terminated again in SMLCT1, and TLC1 was obtained from the S0 state. As highlighted in Table S16 (ESI†), due to the local character of the excitation for TLC1, SNTO is larger compared to TMLCT1, which is also energetically higher compared to the former (12 vs. 19 kJ mol−1). The local character in TLC1 probably results from the increased distance between the Cu atom and the nitrogen atom of the ligand, d(Cu⋯NL). However, TMLCT1 is occupied despite the increased energy enabling the TADF mechanism probably due to environmental effects, which led to a stabilization and made it more energetically favourable compared to TLC1.
Experimental
Materials and methods
The solvents were purchased from Fisher, if not stated otherwise. Dichloromethane, toluene and THF were dried with the solvent purification system (SPS) from MBraun (model MB-SPS-800) and degassed with argon before usage. Dry DMF and N,N-dimethylacetamide (DMAC) were purchased from Acros Organics, whereas n-pentane or diethyl ether employed for the precipitation of the Cu(I) complexes were acquired from Merck. CuCl pellets, tBuOK, methyl iodide, 1,4-dibromobutane, N,N-diisopropylethylamine (DIPEA), sodium hydride (60% in mineral oil), n-butyllithium (2.5 M in hexane), palladium(II) acetate, diphenylphosphine and all the chloro(diaryl)phosphine were purchased from Merck. 2-Bromoaniline was purchased from chemPUR, while 3-amino-2-bromopyridine and 1,2,4-triazole were obtained respectively from BLDPharm and abcr. All chemicals were used without any further purification. The heteronuclear NMR spectra of the Cu(I) complexes were recorded in CDCl3 purchased from Eurisotop. All reactions were carried out using Schlenk techniques under Ar atmosphere.General information concerning NMR, mass spectrometry, IR and elemental analyses, and melting points is detailed in the ESI.† Additional information on the experimental procedure is available via the Chemotion Repository.32
Crystal structure determination
Single-crystal X-ray diffraction was carried out on a Bruker D8 Venture diffractometer with a PhotonII detector at 298(2) K using Cu-Kα radiation (λ = 1.54178 Å). Dual space methods SHELXT were used for the structure solution and the refinement was carried out using SHELXL-2014 (full-matrix least-squares on F2).33,34 The hydrogen atoms were refined using a riding model (H(O) free). Semi-empirical absorption corrections were applied. For [Cu(μ-trz)(NPAr)]2 an extinction correction was applied. In [Cu(μ-trz)(NPF)]2 disordered water molecules were squeezed out (see cif-files for details).35,36CCDC 2380060–2380064 contain the ESI† crystallographic data for this paper.
Photoluminescent measurements
The UV-vis spectra were collected in dichloromethane solutions employing an Analytik Jena Specord 50 instrument. All the solid-state photophysical measurements were performed under rigorous exclusion of air and moisture. The dichloromethane (DCM) used for steady-state absorption spectroscopy was dried and degassed prior to the measurement. Polymethylmethacrylate (PMMA) films were prepared by drop casting method with 1 wt% of complexes dissolved in DCM, followed by evaporation of the solvent and drying of polymeric films under high vacuum. Excitation and emission spectra were recorded on an Edinburgh Instrument FLSP920 or FLS1000 spectrometer, equipped with a 450 W Xenon arc lamp. The emission was collected at right angle to the excitation source with the emission wavelength selected using a double-grated monochromator for the excitation and emission pathways and detected by a red-sensitive photomultiplier (PMT-R928 or 980) as detector. The excitation and emission spectra were corrected using the standard corrections supplied by the manufacturer for the spectral power of the excitation source and the sensitivity of the detector. Quantum yields of polymeric matrices were measured using an integrating cryosphere (Microstat N2) from Oxford Instruments. Alternatively, quantum yields of the solid samples were recorded using a quantaurus-QY absolute PL quantum yield spectrometer C11347 by Hamamatsu. The luminescence lifetimes were measured using a VPLED 320/380 nm, with 1 to 5 μs pulse width and an MCS module, depending on the time range.DFT calculations
All calculations were carried out with Turbomole37 and the resolution of identity approximation using symmetry.38 CAM-B3LYP was employed as functional.39 For Cu, P, N, and the carbon atoms in the triazoles the def2-SVPD basis was used, whereas for the remaining atoms, def2-SVP was employed. This combination is abbreviated as SVPD. The numerical integration for the calculation of the energy was carried out with the lattice of size four.40 Ci symmetry for [Cu(μ-trz)(NP)]2, [Cu(μ-trz)(NPF)]2 and [Cu(μ-trz)(NpyP)]2, and Cs symmetry for [Cu(μ-trz)(NPAr)]2 were respectively utilized. Weight derivatives were accounted for during the optimizations41 and the structures were converged when the change in energy was below 10−7Eh and the change in the cartesian gradients was below 10−5Eh/a0. The singlet ground state (S0) optimizations were started from the crystal structure. The Tamm–Dancoff approximation (TDA) within time-dependent DFT (TD-DFT)42 was used starting from S0 to obtain the first singlet excited state S1 with metal-to-ligand charge transfer (MLCT) character, and the first triplet excited state T1 with ligand-centred (LC) character. The T1 with MLCT character was obtained with the respective S1 as the starting point. The singlet–triplet energy gap (ΔEadiaST) was calculated as the difference in electronic energy of S1 and T1 with MLCT character. For the computed UV-vis spectra Gaussian broadening (FWHM = 2500 cm−1) and oscillator strengths in length representation were used.Conclusions
1,2,4-Triazole was successfully employed as bridging ligand for the preparation of blue- to yellow-emitting Cu(I) dimeric complexes having aminophosphanes as chelating NP-ligands. The molecular structure of the Cu(I) complexes was unambiguously confirmed through single-crystal X-ray diffraction. The photoluminescence data conducted at room temperature and 77 K in the solid state and in a 1 wt% doped PMMA matrix support the TADF mechanism due to the appreciable increase in the observed lifetime τ and the presence of two significantly different radiative rate constants kr at 297 and 77 K.From a computational point of view, the excited states with MLCT characters are structurally very similar to each other, and these similarities could be observed also by analysing the natural transition orbitals (NTOs). For the Cu(I) complexes investigated, all these aspects lead to a ΔEadiaST in the 179–242 meV range, with the complex with the lowest ΔEadiaST also showing the lowest transition dipole moment μT for the related excited states.
To conclude, the experimental and computed data here presented support that the emission properties are due to TADF. However, compared to the similar Cu(I) complexes with NP-ligands, the absence of halides in the coordination sphere significantly enhances the solubility of these compounds in common organic solvents without significantly affecting the photoluminescent properties, thus making them suitable candidates to be applied in solution-processed OLEDs.
Author contributions
Valentina Ferraro: data curation, formal analysis, investigation, methodology, validation, writing – original draft, writing – review & editing. Xuemin Gan: conceptualization, investigation, methodology. Florian R. Rehak: formal analysis, investigation, methodology, writing – original draft. Philipp Ralle: formal analysis, investigation, methodology, writing – original draft, writing – review & editing. Martin Nieger: data curation, formal analysis, investigation, methodology, validation, writing – original draft, writing – review & editing. Andreas Steffen: funding acquisition, validation, writing – original draft, writing – review & editing. Wim Klopper: funding acquisition, validation, writing – original draft, writing – review & editing. Stefan Bräse: funding acquisition, validation, writing – original draft, writing – review & editing.Data availability
The details on the chemical synthesis and original analytical data were added to the repository Chemotion https://www.chemotion.net/home.32Conflicts of interest
There are no conflicts to declare.Acknowledgements
X. G. acknowledges the support provided by the China Scholarship Council and V. F. thanks Luca Schichtel for performing some of the experimental procedures. The authors thank the Karlsruhe School of Optics and Photonics (KSOP) and the DFG for financial support through Germany's Excellence Strategy 3D Matter Made to Order (3DMM2O, Grant No. EXC-2082/1-390761711).Notes and references
- J. G. Haasnoot, Coord. Chem. Rev., 2000, 200–202, 131–185 CrossRef CAS.
- L. Bergmann, C. Braun, M. Nieger and S. Bräse, Dalton Trans., 2018, 47, 608–621 RSC.
- M. Freund, Ber. Dtsch. Chem. Ges., 2006, 29, 2483 CrossRef.
- X.-L. Chen, R. Yu, X.-Y. Wu, D. Liang, J.-H. Jia and C.-Z. Lu, Chem. Commun., 2016, 52, 6288–6291 RSC.
- C. E. Housecroft and E. C. Constable, J. Mater. Chem. C, 2022, 10, 4456–4482 RSC.
- V. Ferraro, C. Bizzarri and S. Brase, Adv. Sci., 2024, 11, 2404866 CrossRef CAS PubMed.
- M. J. Leitl, F. R. Kuchle, H. A. Mayer, L. Wesemann and H. Yersin, J. Phys. Chem. A, 2013, 117, 11823–11836 CrossRef CAS PubMed.
- H. Yersin, Highly Efficient OLEDs: Materials Based on Thermally Activated Delayed Fluorescence, Wiley-VCH, 2019 Search PubMed.
- S. B. Harkins and J. C. Peters, J. Am. Chem. Soc., 2005, 127, 2030–2031 CrossRef CAS PubMed.
- M. Klein, N. Rau, M. Wende, J. Sundermeyer, G. Cheng, C.-M. Che, A. Schinabeck and H. Yersin, Chem. Mater., 2020, 32, 10365–10382 CrossRef CAS.
- J.-H. Jia, X.-L. Chen, J.-Z. Liao, D. Liang, M.-X. Yang, R. Yu and C.-Z. Lu, Dalton Trans., 2019, 48, 1418–1426 RSC.
- T. Hofbeck, T. A. Niehaus, M. Fleck, U. Monkowius and H. Yersin, Molecules, 2021, 26, 3415 CrossRef CAS PubMed.
- A. Schinabeck, M. J. Leitl and H. Yersin, J. Phys. Chem. Lett., 2018, 9, 2848–2856 CrossRef CAS PubMed.
- T. Hofbeck, U. Monkowius and H. Yersin, J. Am. Chem. Soc., 2015, 137, 399–404 CrossRef CAS PubMed.
- A. V. Artem’ev, M. P. Davydova, A. S. Berezin, M. R. Ryzhikov and D. G. Samsonenko, Inorg. Chem., 2020, 59, 10699–10706 CrossRef PubMed.
- M. P. Davydova, A. S. Berezin, D. G. Samsonenko and A. V. Artem'ev, Inorg. Chim. Acta, 2021, 521, 120347 CrossRef CAS.
- A. Kobayashi, T. Hasegawa, M. Yoshida and M. Kato, Inorg. Chem., 2016, 55, 1978–1985 CrossRef CAS PubMed.
- A. Y. Baranov, A. S. Berezin, D. G. Samsonenko, A. S. Mazur, P. M. Tolstoy, V. F. Plyusnin, I. E. Kolesnikov and A. V. Artem’ev, Dalton Trans., 2020, 49, 3155–3163 RSC.
- B. Goswami, T. J. Feuerstein, R. Yadav, S. Lebedkin, P. J. Boden, S. T. Steiger, G. Niedner-Schatteburg, M. Gerhards, M. M. Kappes and P. W. Roesky, Chem. – Eur. J., 2021, 27, 15110–15119 CrossRef PubMed.
- D. M. Zink, M. Bachle, T. Baumann, M. Nieger, M. Kuhn, C. Wang, W. Klopper, U. Monkowius, T. Hofbeck, H. Yersin and S. Brase, Inorg. Chem., 2013, 52, 2292–2305 CrossRef CAS PubMed.
- D. M. Zink, D. Volz, T. Baumann, M. Mydlak, H. Flügge, J. Friedrichs, M. Nieger and S. Bräse, Chem. Mater., 2013, 25, 4471–4486 CrossRef CAS.
- L. Bergmann, G. J. Hedley, T. Baumann, S. Bräse and I. D. W. Samuel, Sci. Adv., 2016, 2, e1500889 CrossRef PubMed.
- J. M. Busch, D. M. Zink, P. Di Martino-Fumo, F. R. Rehak, P. Boden, S. Steiger, O. Fuhr, M. Nieger, W. Klopper, M. Gerhards and S. Bräse, Dalton Trans., 2019, 48, 15687–15698 RSC.
- J. M. Busch, D. S. Koshelev, A. A. Vashchenko, O. Fuhr, M. Nieger, V. V. Utochnikova and S. Bräse, Inorg. Chem., 2021, 60, 2315–2332 CrossRef CAS PubMed.
- J. M. Busch, F. R. Rehak, V. Ferraro, M. Nieger, M. Kemell, O. Fuhr, W. Klopper and S. Bräse, ACS Omega, 2024, 9, 2220–2233 CrossRef CAS PubMed.
- N. V. S. Harisomayajula, S. Makovetskyi and Y.-C. Tsai, Chem. – Eur. J., 2019, 25, 8936–8954 CrossRef CAS PubMed.
- G. U. Mahoro, J. Fernandez-Cestau, J. L. Renaud, P. B. Coto, R. D. Costa and S. Gaillard, Adv. Opt. Mater., 2020, 8, 2000260 CrossRef CAS.
- M. Gernert, U. Müller, M. Haehnel, J. Pflaum and A. Steffen, Chem. – Eur. J., 2017, 23, 2206–2216 CrossRef CAS PubMed.
- A. Steffen and B. Hupp, Comprehensive Coordination Chemistry III, Elsevier, 2021, pp. 466–502 Search PubMed.
- H. Yersin, A. F. Rausch, R. Czerwieniec, T. Hofbeck and T. Fischer, Coord. Chem. Rev., 2011, 255, 2622–2652 CrossRef CAS.
- H. Yersin, R. Czerwieniec, M. Z. Shafikov and A. F. Suleymanova, ChemPhysChem, 2017, 18, 3508–3535 CrossRef CAS PubMed.
- V. Ferraro, X. Gan and S. Bräse, Chemotion Repository, 2024 DOI:10.14272/collection/VF_2024-03-21.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3–8 CrossRef PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- A. L. Spek, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2009, 65, 148–155 CrossRef CAS PubMed.
- A. L. Spek, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 9–18 CrossRef CAS PubMed.
- TURBOMOLE V7.5 2020: a development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, Turbomole GmbH, 2007. https://www.turbomole.org Search PubMed.
- R. A. Kendall and H. A. Früchtl, Theor. Chem. Acc., 1997, 97, 158–163 Search PubMed.
- T. Yanai, D. P. Tew and N. C. Handy, Chem. Phys. Lett., 2004, 393, 51–57 CrossRef CAS.
- O. Treutler and R. Ahlrichs, J. Chem. Phys., 1995, 102, 346–352 CrossRef CAS.
- P. Deglmann, F. Furche and R. Ahlrichs, Chem. Phys. Lett., 2002, 362, 511–518 CrossRef CAS.
- S. Hirata and M. Head-Gordon, Chem. Phys. Lett., 1999, 314, 291–299 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2380060–2380064. See DOI: https://doi.org/10.1039/d5nj01056j |
| ‡ Valentina Ferraro and Xuemin Gan equally contributed to this work. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2025 |




