Open Access Article
Open Access ArticleThe cytochalasans: potent fungal natural products with application from bench to bedside†
Mohamed A. Tammam
 a,
Florbela Pereira
a,
Florbela Pereira
 b,
Elizabeth Skellam
b,
Elizabeth Skellam
 c,
Stefan Bidula
d,
A. Ganesan
c,
Stefan Bidula
d,
A. Ganesan
 d and
Amr El-Demerdash
d and
Amr El-Demerdash
 *def
*def
aDepartment of Biochemistry, Faculty of Agriculture, Fayoum University, Fayoum 63514, Egypt
bLAQV REQUIMTE, Department of Chemistry, NOVA School of Science and Technology, Universidade Nova de Lisboa, 2829516 Caparica, Portugal
cDepartment of Chemistry and BioDiscovery Institute, University of North Texas, 1155 Union Circle, Denton, TX 76201, USA
dSchool of Chemistry, Pharmacy and Pharmacology, University of East Anglia, Norwich Research Park, Norwich NR4 7TJ, UK. E-mail: A.Eldemerdash@uea.ac.uk
eDivision of Organic Chemistry, Department of Chemistry, Faculty of Sciences, Mansoura University, Mansoura 35516, Egypt
fDepartment of Biochemistry and Metabolism, The John Innes Centre, Norwich Research Park, Norwich NR4 7UH, UK. E-mail: Amr.El-Demerdash@jic.ac.uk
First published on 24th February 2025
Abstract
Covering: 2000–2023
Cytochalasans are a fascinating class of natural products that possess an intricate chemical structure with a diverse range of biological activities. They are known for their complex chemical architectures and are often isolated from various fungi. These compounds have attracted attention due to their potential pharmacological properties, including antimicrobial, antiviral, and anticancer effects. For decades, researchers have studied these molecules to better understand their mechanisms of action and to explore their potential applications in medicine and other fields. This review article aims to shed light over the period 2000–2023 on the structural diversities of 424 fungal derived cytochalasans, insights into their biosynthetic origins, pharmacokinetics and their promising therapeutic potential in drug discovery and development.
1. Introduction
Microbial natural products have long been recognized as a valuable source of bioactive compounds with diverse chemical structures and potent biological activities. These organic compounds, produced by microorganisms such as bacteria and fungi, play a crucial role in drug discovery and development.1,2 Indeed, they have evolved intricate biosynthetic pathways, allowing them to produce an array of secondary metabolites that serve various ecological functions, including defence against competitors and communication within microbial communities.3,4Moreover, microbial natural products have demonstrated unique chemical scaffolds with a wide range of biological activities, including antimicrobial, anticancer, immunomodulatory, and antiviral properties making them promising candidates for drug discovery.5 These compounds have served as the basis for the development of numerous clinically important drugs, such as antibiotics (e.g., Penicillin, Erythromycin), immunosuppressants (e.g., Cyclosporine), and anticancer agents (e.g., Doxorubicin).6,7
Fungi, a diverse group of microorganisms, have long captured the interest of scientists and researchers for their ability to produce an astonishing variety of natural products.8,9 These fungal natural products, also known as secondary metabolites, exhibit a wide range of chemical structures and biological activities, making them valuable resources for drug discovery.10–12
Fungal natural products have played a significant role in the development of therapeutic agents. Many well-known approved drugs as well as these under preclinical or clinical investigations, such as antibiotics [e.g. Penicillin and Cephalosporins],13,14 immunosuppressants [e.g. Cyclosporine A and Mycophenolic acid],15,16 anticancers [e.g. Taxol],17,18 anti-inflammatories [e.g. Cordycepin and Gliotoxin],19,20 and cholesterol-lowering agents [e.g. Lovastatin and Pravastatin],21,22 owe their origins to fungal sources. These compounds have revolutionized medicine and have been instrumental in combating various diseases.23–25 Indeed, the remarkable diversity of fungal species offers an extensive repertoire of natural products waiting to be explored.26,27 Fungi possess unique biosynthetic pathways and have the capacity to produce complex molecules with intricate chemical scaffolds.28–30 This structural complexity often translates into a wide array of biological activities, including antimicrobial, anticancer, anti-inflammatory, and antiviral properties.31–34
One of the key advantages of fungal natural products in drug discovery is their novelty. Fungi can produce compounds that are not found in other organisms, making them a rich source of untapped chemical diversity.35–37 This inherent novelty opens new avenues for developing innovative therapeutics and addressing unmet medical needs.13,38,39 Furthermore, the increasing threat of drug-resistant pathogens has underscored the urgent need for new antimicrobial agents.40–42 Additionally, fungal natural products have shown promise in combating multidrug-resistant bacteria, providing potential solutions to the global health challenge posed by antibiotic resistance.43–45
Cytochalasans are a class of fungal metabolites that have a wide range of biological activities. The name “cytochalasans” comes from the Greek words “cytos” meaning cell and “chalasis” meaning relaxation, referring to their ability to disrupt actin filaments in cells.46,47 Historically, the first cytochalasans, namely phomin and cytochalasans A–D were discovered in the 1960s from cultures of Helminthosporium dematioideum and Metarrhizium anisopliae.48,49 Since then, they have been isolated mainly from various fungal genera including Aspergillus,50 Chaetomium,51 and Phomopsis.52
Chemically, cytochalasans have a unique tricyclic core structure consisting of a polyketide-derived 11, 13 or 14-membered macrocyclic ring fused to various aromatic or heteroaromatic rings (e.g. an isoindolone moiety). The unique chemistry of cytochalasans arises from their incorporation of numerous amino acids and the presence of functional groups, such as hydroxyl, methyl, and acyl groups, which further enhance their chemical complexity, structural diversity, and biomedical potentialities. Additionally, the presence of these functional groups allows for potential modifications and derivatizations to fine-tune the biological activity of cytochalasans.53,54 Furthermore, the stereochemistry of cytochalasans plays a crucial role in their biological properties, with specific configurations at key positions influencing their interactions with biological targets.55–57
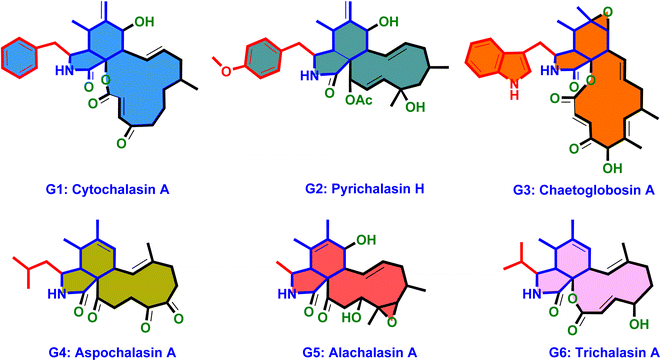
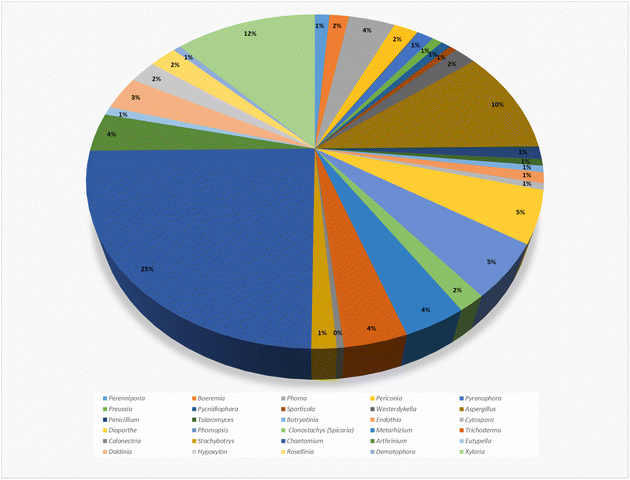
Structurally, cytochalasans are generally classified into six main groups (G1–G6) based on the specific amino acids involved in their formation (Chart 1). These groups include “Cytochalasins” which contain a phenylalanine residue, “Pyrichalasins” which incorporate a tyrosine or a related derivative residue, “Chaetoglobosins” which feature a tryptophan residue, “Aspochalasins” which include a leucine residue, “Alachalasins” which have an alanine residue, and “Trichalasin” which involves α-valine residue.58–60 To date, over 400 different naturally occurring cytochalasans have been identified, particularly from fungal species of The Ascomycota and Basidiomycota.53,58
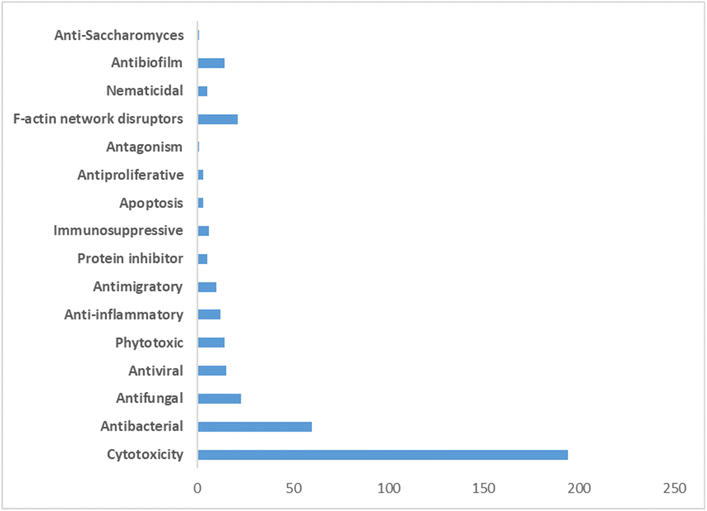
The pharmacological profile of cytochalasans encompasses a wide range of biological activities, making them promising candidates for various therapeutic applications. These compounds have demonstrated cytotoxic effects, meaning they can inhibit the growth and proliferation of cancer cells. This property has garnered significant interest in the development of cytochalasans as potential anticancer agents.61–63
In addition to their cytotoxicity, cytochalasans have also shown antimicrobial activity against bacteria, fungi, and parasites. They can inhibit the growth and reproduction of these microorganisms, making them valuable in the search for new antimicrobial agents.64
Furthermore, certain cytochalasans exhibit antiviral properties, with the ability to interfere with viral replication and infection processes.65 This makes them potential candidates for the development of antiviral therapies.66 The anti-inflammatory effects of cytochalasans have also been investigated. These compounds can modulate inflammatory responses by inhibiting the production of inflammatory mediators and reducing the activation of immune cells.67 Such anti-inflammatory properties may have implications for the treatment of inflammatory disorders and autoimmune diseases.68
Moreover, cytochalasans have demonstrated immunosuppressive effects. This property can be beneficial in cases where immune hyperactivity needs to be controlled, such as following organ transplantation or in autoimmune conditions.69,70
Biosynthetically, cytochalasans are synthesized through polyketide synthase–nonribosomal peptide synthetase (PKS–NRPS) hybrid pathways. These pathways play a pivotal role in constructing the intricate molecular architecture of these metabolites. PKS–NRPS pathways represent distinctive modular enzymatic systems that orchestrate the integration of both polyketide and peptide building blocks.
Additionally, within these pathways, the PKS components are responsible for assembling the polyketide backbone, while the NRPS components incorporate amino acid residues into the growing PKS chain. This hybrid nature of PKS–NRPS systems enables the incorporation of diverse building blocks, fostering structural and biological diversification.60,71–74
Previously, Hertweck and his co-workers,58 outlined a comprehensive overview of the chemistry and biology of fungal cytochalasans. They explored the diverse chemical structures, biological activities, biosynthesis, structure–activity relationships, modes of action, with focused examination of their impact on cellular processes and their potential as therapeutic agents.
Additionally, Zhu et al., presented a book chapter discussing the recent progress in the chemistry of fungal-derived cytochalasans. They provided an updated overview on isolation, structural determination, biological activities, biosynthesis and the recent strategies employed in the total synthesis and modifications of these fungal natural products.53
Furthermore, Lambert et al., reported on the impact of cytochalasans on actin filament remodelling, a crucial process in cell motility and shape changes. They highlighted the structural diversity of a total of 136 cytochalasans, their capacity to modulate actin dynamics and organization in eukaryotic cells, and their potential as future therapeutic tools, particularly in the context of employing click chemistry.75
In line with our continuous research efforts on identifying biologically active natural products,76,77 particularly focusing on pharmacologically active fungal-derived natural products (FNPs),78–83 herein we present a comprehensive and up-to-date literature review on cytochalasans exclusively isolated from different fungal strains.
The review encompasses the period from 2000–2023 and aims to provide a thorough comprehensive examination of the chemical and structural diversities, pharmacological activities, biosynthesis, pharmacokinetics and druglikeness properties associated with these fungal derived metabolites. Throughout the review, we systematically documented the distribution of a total of 424 cytochalasans among various fungal genera, shedding light on their unique chemical profiles and therapeutic potentialities. This in-depth analysis allows us to gain insights into the remarkable diversity of cytochalasans derived from fungal sources and their significance in drug discovery and development.
Additionally, the review discusses recent advances in the biogenesis of cytochalasans, offering insights into the mechanisms underlying their formation within fungal strains. Indeed, we hope that this comprehensive literature review serves as a valuable resource for researchers and scientists interested in the field of fungal cytochalasans and their potential applications in the development of novel therapeutic agents.
2. Recent advances in cytochalasan biosynthesis
2.1. Biosynthetic gene clusters (BGCs)
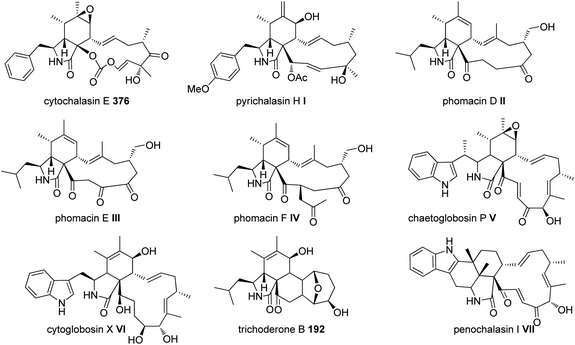
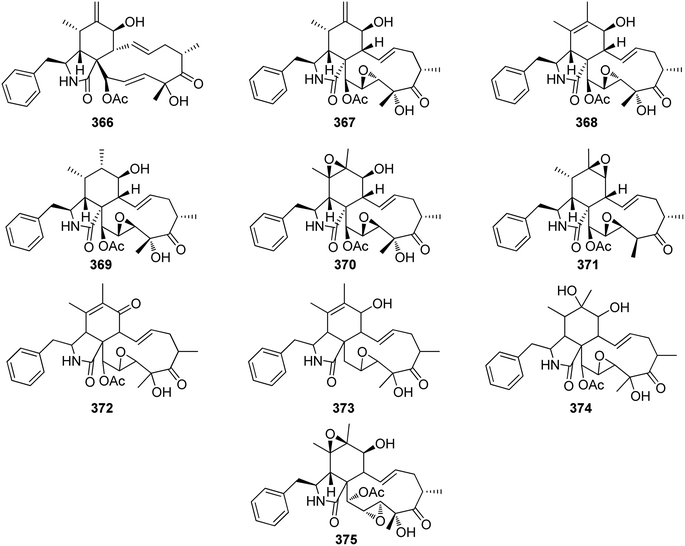
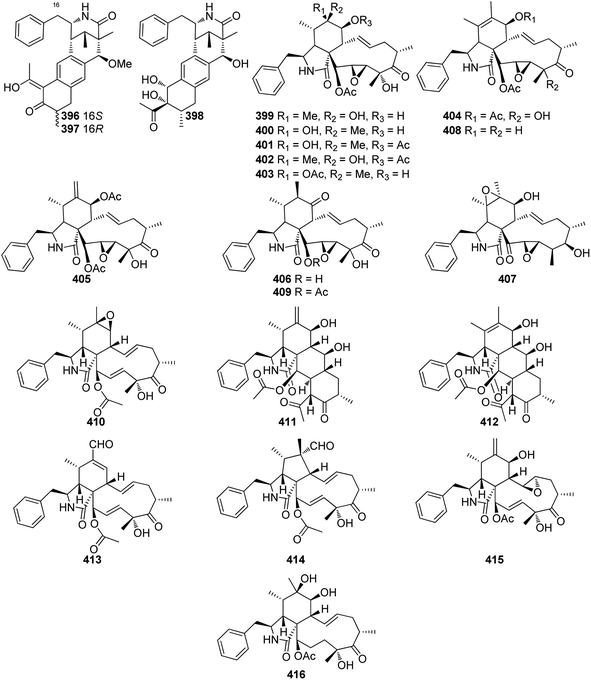
Extensive isotope-labelled feeding studies performed during the 1970–1990s indicated that cytochalasans are biosynthesized from acetate/malonate, S-adenosylmethionine, L-amino acids, and molecular oxygen.84–88 In fungi, polyketide–peptide hybrids are typically synthesized by giant multi-functional multi-domain-containing polyketide synthase/non-ribosomal peptide synthetase (PKS–NRPS) enzymes.74,89 The understanding of PKS–NRPS enzymes in fungi led to the identification of biosynthetic gene clusters (BGCs) encoding chaetoglobosin A (199),90 cytochalasans E (376)/K (329),91 and cryptic cytochalasans,92–96 in quick succession (Chart 2).BGCs encoding pyrichalasin H (I),97 phomacins D/E/F (II, III and IV),98 aspochalasins C, E, M and flavichalasines F and G (77), (69), (70), (79) and (80),99 cytochalasin H (116) and related cyctochalasins,100 aspergillin PZ (81),101 chaetoglobosin P (V),102 cytoglobosin X (VI),103 have also been identified recently. These BGCs share a high level of homology, where the PKS–NRPS, trans-acting enoylreductase (trans-ER), α,β-hydrolase (α,β-HYD), and Diels–Alder (DA) genes are considered to be the core genes,104 yet all BGCs identified to date also encode several additional tailoring enzymes such as: cytochrome P450 monooxygenases (P450); oxidoreductases (OXR); Baeyer–Villiger monooxygenases (BVMO); and transferases (Chart 3). Typically, BGCs also encode transcription factors (TF) which have mostly been shown to function as positive regulators of cytochalasan biosynthesis.91,98,105,106 The precise role of the major facilitator superfamily (MFS) transporter is unknown.
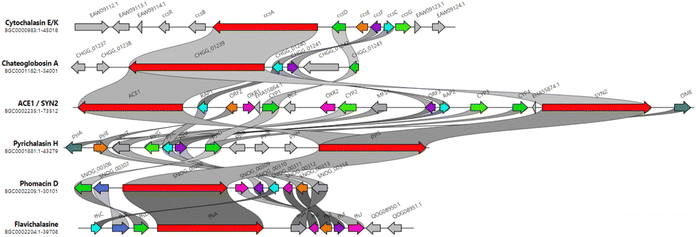 | ||
| Chart 3 Clinker comparison of experimentally validated cytochalasan BGCs.107 Abbreviations: OMeT = O-methyltransferase; α,β-HYD = α,β-HYD and trans-ER; MFS = major facilitator superfamily transporter; trans-ER = trans-acting enoyl reductase; DA = Diels–Alderase; P450 = cytochrome P450 monooxygenase; PKS–NRPS = polyketide synthase/non-ribosomal peptide synthetase; OXR = oxidoreductase; TF = transcription factor. | ||
2.2. Enzymes required for the synthesis of the perhydroisoindolone core
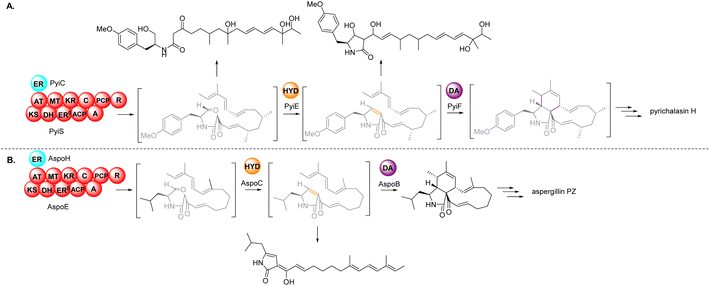
Based on the chemical structures of cytochalasans, biosynthetic proposals suggested that after the polyketide chain had reached the length and level of reduction defined by the PKS module, it is condensed with a specific amino acid, dictated by the NRPS module, and released from the PKS–NRPS via reduction to generate an aldehyde.91,108 The resulting aldehyde undergoes Knoevenagel condensation, followed by [4 + 2] cycloaddition to generate the characteristic perhydroisoindolone core (Scheme 1). Gene disruption (also knock-out; KO) and/or heterologous expression experiments determined that the PKS–NRPS gene was essential for biosynthesis of the carbon backbone during the biosynthesis of cytochalasans.90,91,97–99,101 Similarly, KO of the trans-ER confirmed that the PKS–NRPS cannot function successfully without its partner,90,97 analogous to other studies of fungal PKS–NRPS systems.In-depth studies of the terminal reductase (R) domain of PyiS, the PKS–NRPS required for pyrichalasin H (I) biosynthesis, determined the precise chain release mechanism is a two-electron NADPH-mediated reduction of the covalently bound polyketide thiolester.109 Although this mechanism had long been suspected, several in vivo investigations had led to identification of an alcohol thought to result from over-reduction of the aldehyde by unknown enzymes in the fungal host,72,95 however a four-electron reduction mechanism could not be ruled out until now.
Additionally, studies of PyiS indicated that the adenylation (A) domain of the NRPS can be quite flexible towards a range of amino acid substrates.73,97 Pyrichalasin H (I) possesses a para-methoxy group, presumed to arise via methylation of tyrosine. KO experiments determined that a dedicated O-methyltransferase (–OMeT) methylated tyrosine which is then condensed with the growing polyketide chain.97 Indeed, in the absence of the –OMeT, a range of unnatural para-substituted phenylalanine analogues can be fed to the fungal host generating a library of new-to-nature functionalized cytochalasans.73 In vivo experiments determined the role of the α,β-HYD in facilitating formation of the pyrrolinone moiety.110 Synthetic aldehyde analogues were shown to be reactive and spontaneously hydrolyze to give a pyrrolinone, however the double bond was not in the position required for a subsequent [4 + 2] cyclization.110 This tautomerization was also observed during heterologous co-expression of the PKS–NRPS and trans-ER genes in certain fungal hosts.98,101 Therefore, the α,β-HYD appears to prevent reduction of the aldehyde and ensures correct tautomerization of the resulting five-membered heterocyclic ring. Similarly, KO experiments of the DA gene confirmed its role as facilitating [4 + 2] cycloaddition of the polyketide terminal diene and the correctly tautomerized pyrrolinone core.105 Heterologous expression of the PKS–NRPS, trans-ER, α,β-HYD, and DA from the aspergillin PZ (72) into Aspergillus nidulans led to the production of the characteristic cytochalasan core.101
2.3. Oxidative modifications by tailoring enzymes and non-enzymatic processes
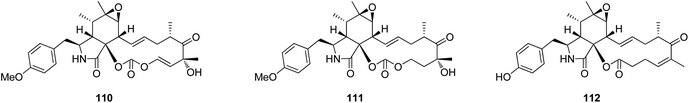
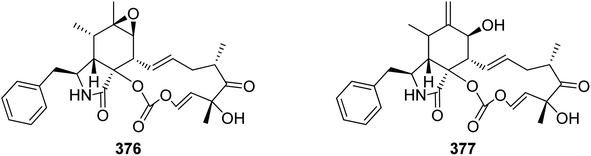
The oxidative modifications associated with cytochalasans are typically observed around the cyclohexene moiety such as epoxidation or hydroxylation (e.g. 115–117), and around the macrocycle including oxygen insertion, hydroxylation, and epoxidation (e.g. 119, 73, 314). Additionally, there are numerous examples of cytochalasans that possess more elaborate modifications and lead to significant structural divergence including halogenation (e.g. xylarichalasin A (387)), penta- and hexacyclic fused ring systems tricoderone B (192) and penochalasin I (VII), spiro-cytochalasans (e.g. trichodermone (193)), merocytochalasans (e.g. 57–60, 64, and 74–76), and open-chain cytochalasans (e.g. 109, 297, 298, 310). Of the cyctochalasan BGCs interrogated to date, each contains one or more P450s (Chart 3); one family epoxidizes the cyclohexene moiety, and the other hydroxylates the macrocycle.90,97,100,101 In some instances, the P450 that hydroxylates the macrocycle is known to be, or suspected to be, iterative introducing two consecutive hydroxyl groups at adjacent carbon atoms (e.g. 376, 81, 199, 329).90,91,101 There are various examples of cytochalasans that exhibit either single or double oxygen atom insertion into the macrocycle to form a lactone (e.g. 119, 95, 3), or carbonate (e.g. 376, 110, 111, 329, 330), respectively, and typically both are co-isolated from the same culture. The mechanism of oxygen insertion was investigated in the biosynthesis of E (376)/K (329) and found to be mediated by CcsB, a flavin-dependent monooxygenase (FMO) designated as a Baeyer–Villiger Monooxygenase (BVMO).111 In vitro studies of CcsB confirmed that this single enzyme could catalyze both single and double oxygen insertion on the substrate ketocytochalasin (328) and demonstrated that a vinylogous 1,5-diketo system is essential for carbonate formation (Scheme 2).111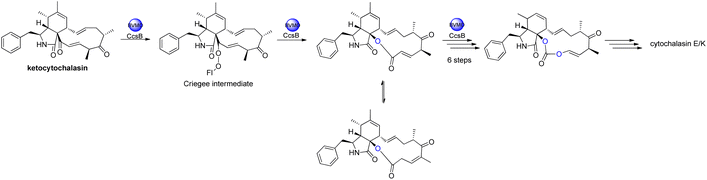 | ||
| Scheme 2 Overview of oxygen insertion into the cyctochalasan backbone via a Baeyer–Villiger monooxygenase. | ||
Aside from P450s and BVMOs, cytochalasan BGCs often contain one or more oxidoreductases (OXR) that are either FAD- or NAD(P)H dependent. PyiH, the NAD(P)H-dependent short-chain dehydrogenase (SDR) involved in pyrichalasin H (I) biosynthesis simply reduces a ketone to an alcohol, enabling acetyl transfer by the acetyltransferase PyiB.97 Similarly, AspoD, the NAD(P)H-dependent SDR involved in flavichalasine G (80) biosynthesis, also reduces a ketone to an alcohol. In contrast, the flavin-dependent monooxygenases (FMO) CHGG_01242-1 and AspoA, required for chaetoglobosin A (199) and aspergillin PZ (81) biosynthesis respectively, convert specific hydroxyl groups to ketones.90,101 However, AspoA displays a rather unusual mechanism as the oxidase is involved in keto–enol tautomerization. Without isomerization of the C-19/C-20 double bond, acid-conditions mediate formation of intramolecular cyclizations, oxygen-bridges, and merocytochalasans.101 AspoA therefore represents a significant enzymatic strategy for controlling metabolic flux between intended and competing (i.e., non-enzymatic) modifications. Exploiting acid-catalyzed intramolecular conversions, a series of novel cytochalasans e.g., cytochalasans J1 to J5 and H1/H2 were rapidly generated in a separate study.55,112
3. Structural diversity and biological activities of cytochalasans derived from fungi
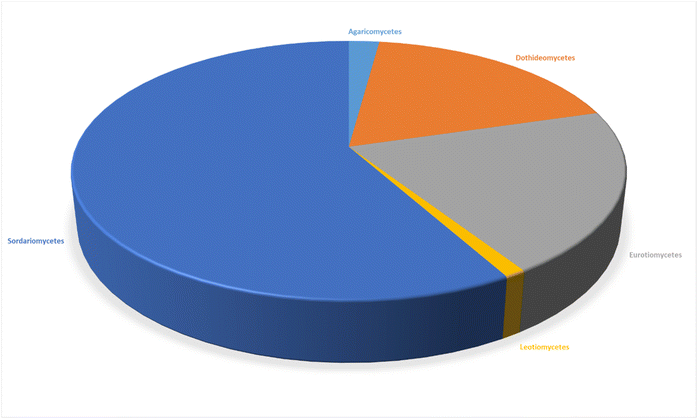
3.1. Fungi of the class Agaricomycetes
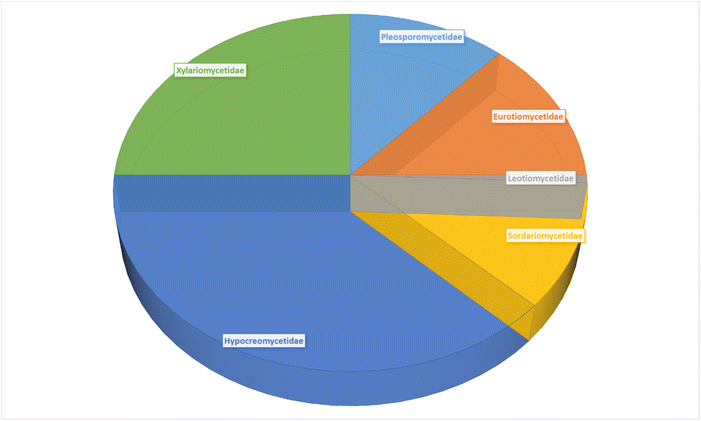
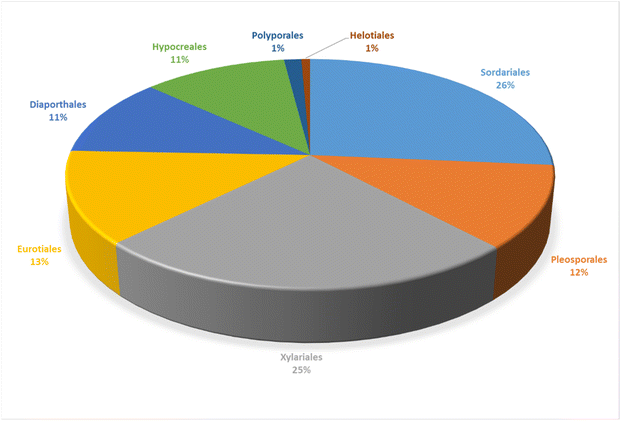
3.1.1.1. Fungi of the order Polyporales.
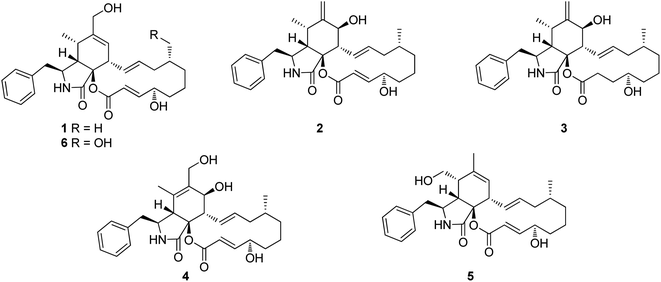
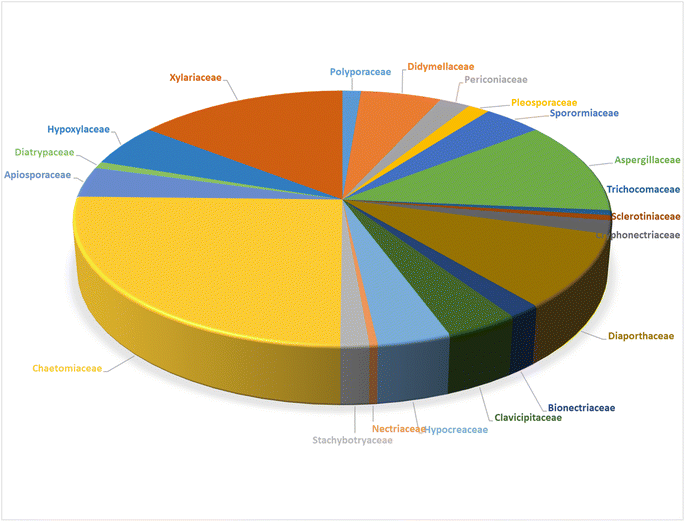
3.1.1.1.1. Fungi of the family Polyporaceae. 3.1.1.1.1.1. Genus Perenniporia (MycoBank ID 18204). A chemical investigation of the organic extract of the fungus Perenniporia subacida, collected from Jinlin Province, China, in the mountain of Changbai, afforded the isolation of the previously reported cytochalasans Z2 (1), B (2), and dihydrocytochalasin B (3), along with three previously undescribed analogues namely perenniporins A (4), B (5) and C (6) (Fig. 1). Compounds 4–6 were examined for their cytotoxic effects against SMMC-7721, A549 and MCF-7 cancer cell lines, using Taxol as positive control with IC50 value of >0.008 μM, among them, 4 and 6 showed weak effect only against SMMC-7721 and A549 with IC50 values of 23.3 and 37.6 μM, respectively. Additionally, they were examined for their anti-inflammatory effect through the examination for their ability to inhibit NO production in LPS-activated macrophages. Only 6 displayed an inhibition effect with IC50 value of 18.4 μM, when compared to MG-132 (Sigma) as positive control which displayed IC50 value of 0.14 μM.113 Even though the successful trial of the Guo et al., led to the isolation of the above mentioned cytochalasin derivatives from the genus of Perenniporia, but we must express our doubts regarding the ability of this genus to produce such compounds.
3.2. Fungi of the class Dothideomycetes
3.2.1.1. Fungi of the order Pleosporales.
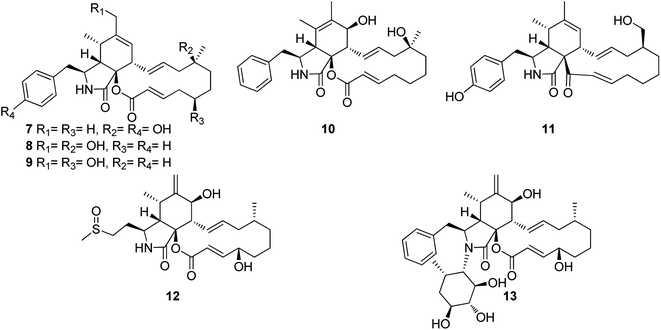
3.2.1.1.1. Fungi of the family Didymellaceae. 3.2.1.1.1.1. Genus Boeremia (MycoBank ID 515621). Seven previously undescribed analogues namely boerechalasins A–G (7–13) (Fig. 2), along with the previously mentioned (2), were isolated from the EtOAc extract of the plant derived fungus Boeremia exigua, isolated from the healthy potato. Compounds 7–13 were examined for their cytotoxicity against MCF-7 cancer cell line, among them, boerechalasin F (12), displayed moderate cytotoxic activity with IC50 value of 22.8 μM, when compared to the positive control Paclitaxel (IC50 < 0.008 μM). Moreover, they were examined for their ani-inflammatory activity, where boerechalasins A (7) and E (11), showed an ani-inflammatory potential through the inhibition of NO production with IC50 values of 21.9 and 5.7 μM, respectively.114
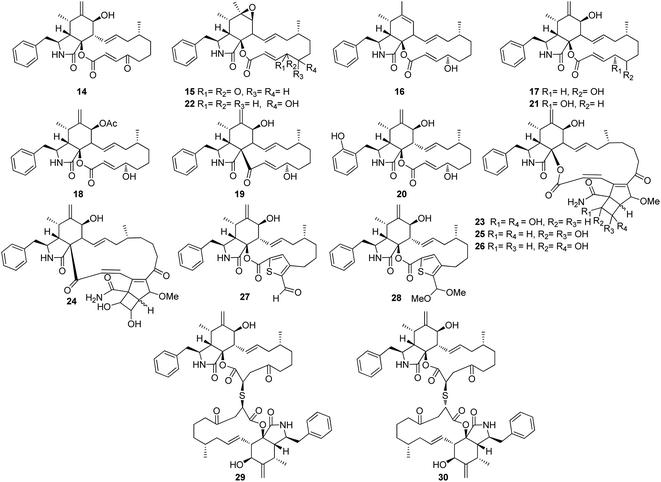
3.2.1.1.1.2. Genus Phoma (MycoBank ID 9358). A chemical investigation of the plant derived fungus Phoma exigua var. heteromorpha [taxonomically updated as Boeremia exigua]. isolated from Nerium oleander L. leaves necrotic spots, collected in Italy from Bari, led to the isolation of the previously mentioned (1) and (2), along with the previously reported cytochalasans A (14), F (15), T (16), Z3 (17), 7-O-acetylcytochalasin B (18), and deoxaphomin (19), together with three previously unreported cytochalasans Z4 (20), Z5 (21), and Z6 (22), (Fig. 3). Whilst 20 and 21, were proven as inactive phytotoxins, compound 22, displayed weak inhibitory activity of root elongation on tomato seedlings at concentration of 10−4 M. Moreover, only 21 caused 20% mortality of brine shrimp.115 Additionally, the previously mentioned cytochalasans derivatives 1, 2, 15, 17, and 19 were isolated from the organic extract of the plant derived fungus P. exigua var exigua, isolated from the lesions that exist on the leaves of C. arvense and S. arvensis, which was collected from different areas. Whilst 1, 2, 15 and 17 exhibited lower phytotoxic activity on C. arvense leaves, deoxaphomin (19) showed the highest level of toxicity on S. arvensis leaves.116 Further four previously unreported analogues namely phomachalasins A (23), B (24), C (25), and D (26), (Fig. 3), were isolated from the organic extract of the plant derived fungus P. exigua var. exigua, obtained from the necrotic lesions of C. arvense and S. arvensis leaves, collected from Norway (Oslo) and Russia (St. Petersburg). Phomachalasins A–D (23–26) represent the first examples of cytochalasans which possessing 1,2,3,4,6,7-hexasubstituted bicycle[3.2.0]heptene connected to the macrocyclic ring. None of the isolated metabolites was proven active neither as a phytotoxic agent when tested on L. esculentum, C. arvense, and E. repens, nor antifungal against C. tropicalis.117 Additional chemical investigation of the endophytic derived fungus P. multirostrata XJ-2-1, isolated from Parasenecio albus collected in China, Xinning County Province, Hunan, led to the isolation of four previously undescribed sulphur-containing cytochalasans derivatives namely thiocytochalasans A (27), B (28), C (29) and D (30), (Fig. 3). Structurally, it is worth to mention that thiocytochalasans A (27) and B (28), represent the first cytochalasan derivatives containing a thiophene moiety, possessing a novel 5/6/14/5 tetracyclic scaffold. Whilst thiocytochalasans C (27) and D (28), are homodimers epimers, formed through a thioether bridge. Compounds 27–30 were examined for their cytotoxic effects against HepG2, MCF-7, A549, CT26 and HT-29 cancer cell lines. Whilst 27 and 28, exhibited moderate cytotoxic effect against the examined cell lines with IC50 values ranging from 4.17 to 23.41 μmol L−1. Additionally, 29 and 30 displayed potent cytotoxicity against the tested cells with IC50 values ranging from 0.76 to 7.52 μmol L−1, when compared to Doxorubicin hydrochloride as positive control displayed IC50 values of 1.37, 1.07, 1.05, 1.01 and 0.48 μmol L−1, against the tested tumour cells, respectively. Furthermore, the investigation of 28–30 examined their impact on cell cycle progression in CT26, A549, and HT29 cells. The findings suggested that these compounds have the potential to induce concentration-dependent G2/M cell cycle arrest in these cells. Notably, 29 and 30 exhibited significant G2/M phase arrest in CT26 cells at a concentration of 1 μM L−1.118
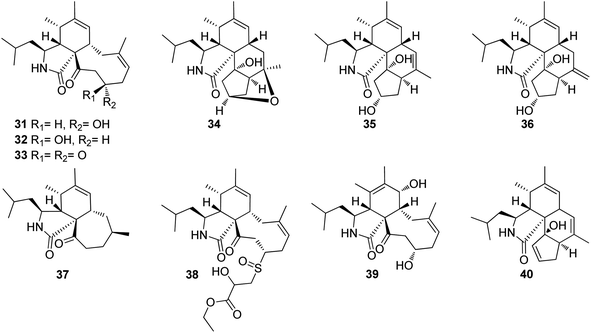
3.2.1.1.2. Fungi of the family Periconiaceae. 3.2.1.1.2.1. Genus Periconia (MycoBank ID 9263). Three previously unreported cytochalasans derivatives possessing an unusual 9/6/5 tricyclic ring system, namely periconiasins A–C (31–33) (Fig. 4), were isolated from the endophytic derived fungus Periconia sp., isolated from the leaves of A. muricata, a medicinal plant, collected in China, Hainan Province. Compounds 31–33 were investigated for their cytotoxic effects against A549, BGC-823, Bel-7402, HCT-8, and A2780 human cancer cell lines, using Camptothecin as positive control (IC50 values of 0.001, 0.04, 6.3, 3.6, and 0.9 μM, respectively), where 33 was inactive against all the examined cells with IC50 values of >10 μM, 31 displayed potent selective cytotoxicity against HCT-8 and BGC-823 tumour cell lines with IC50 values of 0.9 and 2.1 μM, respectively. Whilst 32, displayed significant cytotoxic activity towards HCT-8, Bel-7402 and BGC-823, with IC50 values of 0.8, 5.1 and 9.4 μM. Indeed, 31 and 32, might considered to be lead cytotoxic agents against HCT-8 tumour cell line, due to their prominent cytotoxic activity in comparison with the positive control with IC50 value of 3.6 μM.119
Later, further investigation of the same fungal strain by the same group resulted in the isolation of three additional previously undescribed cytochalasans derivatives namely periconiasins D–F (34–36) (Fig. 4). Compounds 34–36 were found inactive when examined for their cytotoxic potential against HCT-8, Bel-7402, BGC-823, A549 and A2780 tumour cell lines. Additionally, these compounds were found inactive when evaluated for their anti-inflammatory activity. Furthermore, only 35 showed a weak anti-HIV activity when compared with the positive control Efavirenz, with IC50 values of 29.2 and 1.4 nM, respectively.120 Moreover, a re-examination of the same fungus by the same group afforded the isolation of two additional previously unreported cytochalasan derivatives namely periconiasins G–H (37–38) (Fig. 3). Structurally, periconiasin G (37) possess an unusual 7/6/5 tricyclic ring system. Whilst periconiasin H (38) bears unpredicted sulfoxide group. Compounds 37–38 were found inactive when examined for their cytotoxic activity against A549, A2780, Bel-7402, HCT-8, and BGC 823 cell lines, at concentration of 10−5 M, compared with Camptothecin as positive control. Furthermore, when both compounds were examined for their antiviral activity against HIV, using Efavirenz as the positive control, only 37 exhibited weak activity with IC50 value of 67.0 μM.121 Additionally, the same group through a further chemical investigation managed to isolate further two previously unreported analogues namely periconiasins I–J (39–40) (Fig. 4), which possesses 9/6/5 tricyclic ring and 5/6/6/5 tetracyclic ring systems, respectively. Whilst 40 displayed no cytotoxicity against MCF-7 cells, 39 displayed potent cytotoxic effect against the same cancer cell line when compared with Paclitaxel as positive control with IC50 values of 4.8 and 0.0002 μM, respectively. Moreover, 39 showed no antiviral activity towards HIV, but 40 was found active as antiviral agent when compared to the positive control Efavirenz with IC50 values of 25.0 and 0.0014 μM, respectively.122
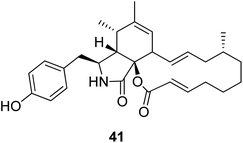
3.2.1.1.3. Fungi of the family Pleosporaceae. 3.2.1.1.3.1. Genus Pyrenophora (MycoBank ID 4596). A chemical examination of the wheat derived fungus Pyrenophora semeniperda, led to the isolation of the previously mentioned cytochalasans derivatives 1, 2, 15, 16, 17 and 19, along with the previously unreported cytochalasin Z1 (41) (Fig. 5). Cytochalasans B (2), F (15) and deoxaphomin (19), displayed a remarkable inhibition activity towards root elongation, when compared to cytochalasans Z1 (41), Z2 (1), and Z3 (17).123
3.2.1.1.4. Fungi of the family Sporormiaceae. 3.2.1.1.4.1. Genus Preussia (MycoBank ID 4363). Chemical investigation of the medicinal plant derived fungus Preussia similis, isolated from the roots of Globularia alypum, collected in Algeria from the city of Batna, resulted in the isolation of the previously mentioned metabolites 1, 2, 15, and 19. While 2 and 19 caused complete actin disruption at a concentration of 1 μg mL−1, this effect was reversible for 2 and irreversible for 19. The effects of 1 and 15 were found to be incomplete and reversible at a concentration of 5 μg mL−1.124
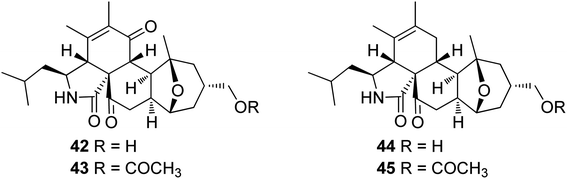
3.2.1.1.4.2. Genus Pycnidiophora (MycoBank ID 4563). Four previously undescribed cytochalasans derivatives sharing the rare 5/6/6/5/6 pentacyclic skeleton, namely pycnidiophorones A–D (42–45) (Fig. 6) were reported from the wetland derived fungus Pycnidiophora dispersa, isolated from the soil sample collected in China, at Hebei Province, from the Lake of Baiyangdian. Compounds 42–45 were examined for their cytotoxicity against the HeLa, PC-3, A549, HepG-2 and HL-60 cells. Whilst no cytotoxicity was observed for 43 against A549 cells, 44 showed no cytotoxicity against PC-3 and HepG-2 cells, and 45 displayed no cytotoxicity against PC-3, HepG-2, and HL-60 cells. However, the examined compounds exhibited weak to mild cytotoxic effects against the tested cells, with IC50 values ranging from 7.0 to 99.0 μM, when compared to the positive control, Cis-platin (IC50 values of 11.7, 5.6, 11.8, 9.3 and 15.7 μM, respectively).125
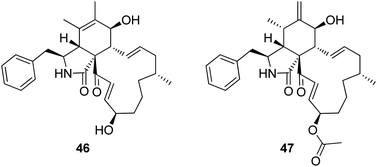
3.2.1.1.4.3. Genus Sparticola (MycoBank ID 551921). The previously mentioned cytochalasin derivative (2), along with the previously described deoxaphomin B (46) together with the previously unreported triseptatin (47) (Fig. 7) were isolated from the decayed branch derived fungus, Sparticola triseptata obtained from Tofieldia calyculata (L.) Wahlenb. Compounds 2, 46 and 47, were examined for their antiproliferative ability against HUVEC and K562 cells using Imatinib as positive control (GI50 values of 18.5 and 0.17 μM, respectively), where they displayed significant antiproliferative activity against the examined cells with GI50 values ranging from 1.08 to 8.31 μM. Furthermore, 46 and 47 were tested against L929, HeLa, MCF-7, A549, PC-3 SKOV-3 and A431 cancer cell lines, using Epothilone B as positive control (IC50 values of. 0.0014, 0.000089, 0.00024, 0.000065, 0.0016, 0.00028 and 0.000079 μM, respectively). Both compounds displayed strong cytotoxicity with IC50 values of 1.55 to 6.91 and 1.80 to 11.28 μM, respectively. Additionally, 46 and 47 displayed an inhibition effect towards F-actin network.126
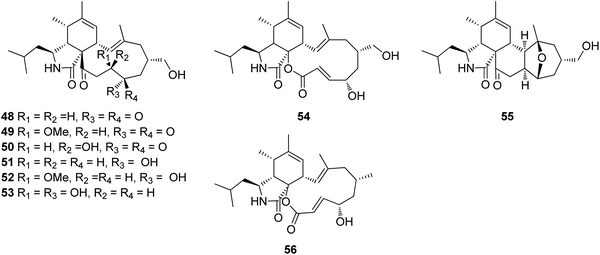
3.2.1.1.4.4. Genus Westerdykella (MycoBank ID 5772). The chemical examination of the marine derived fungus Westerdykella dispersa, obtained from the marine sediment, collected from Guangdong Province, China, from the South China Sea, afforded the isolation of six previously unreported cytochalasans analogues, namely 18-oxo-19,20-dihydrophomacin C (48), 18-oxo-19-methoxy-19,20-dihydrophomacin C (49), 18-oxo-19-hydroxyl-19,20-dihydrophomacin C (50), 19,20-dihydrophomacin C (51), 19-methoxy-19,20-dihydrophomacin C (52), and 19-hydroxyl-19,20-dihydrophomacin C (53) along with the previously reported analogue phomacin B (54) (Fig. 8). Compounds 48–54 were examined for their cytotoxicity effect against MCF-7, HepG2, A549, HT-29 and SGC-7901 tumour cancer lines using 5-Flurorouracil as positive control (IC50 values of 63.98, 58.10, 66.82, 67.13 and >100 μM, respectively). Compounds 48–50 showed no cytotoxicity against the examined cancer lines, however 51 and 53 displayed a selective mild cytotoxicity against HT-29 with IC50 values of 55.5 and 49.1 μM, respectively. Furthermore, 52 and 54 exhibited moderate cytotoxicity with IC50 values ranging from 25.6 to 83.7 μM. Additionally, all the isolated compounds were found inactive when tested for their antibacterial activity against the Gram-negative bacterial strains (S. typhimurium, P. vulgaris, E. aerogenes and E. coli) and the Gram-positive bacteria strains (S. enterica, B. anthracis, M. luteus and B. subtilis),127 when compared to Ciprofloxacin as positive control (MIC value of 0.78125 μg mL−1 against the tested bacterial strains). Further examination for the same fungal strain by the same group resulted in the isolation of another two previously unreported cytochalasans derivatives, namely 16-hydroxymethylaspergillin PZ (55) and, 16α-methylaspochalasin J (56) (Fig. 8). Both compounds were examined for their antibacterial activity against Proteus vulgaris, Enterobacter aerogenes, Escherichia coli, Salmonella enterica, Micrococcus luteus, and Bacillus subtilis, using Ciprofloxacin as positive control (MIC value of 0.78125 μg mL−1 against the tested bacterial strains), where they displayed moderate antibacterial effect against (P. vulgaris and E. aerogenes) and B. subtilis, respectively with MIC values of 50 μg mL−1.128 The previously mentioned cytochalasin derivatives (53), (54), and (55), were reported from the mangrove derived fungus W. nigra, isolated from the roots of Avicennia marina, collected in the Red Sea, Egypt from the port of Safaga. Compounds 53–55 were examined for their inhibition effect towards the activity of acetylcholine esterase using Donepezil (IC50 0.035 μM) as positive control, where 53 exhibited the most promising activity with IC50 value of 0.056 μM, followed by 55 and 56, with IC50 values of 0.088 and 0.140 μM, respectively.129
3.3. Fungi of the class Eurotiomycetes
3.3.1.1. Fungi of the order Eurotiales.
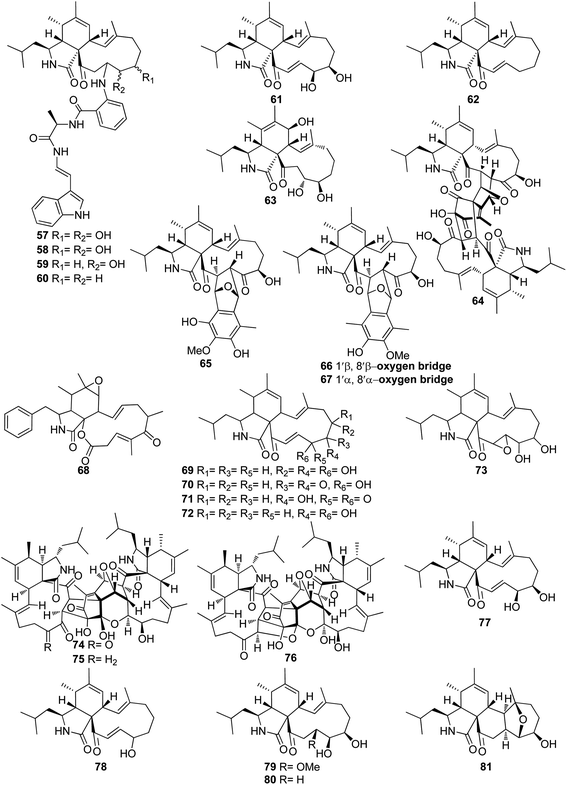
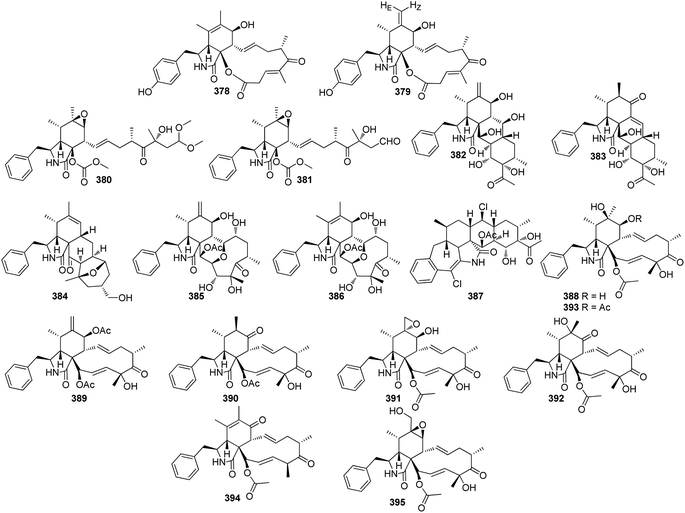
3.3.1.1.1. Fungi of the family Aspergillaceae. 3.3.1.1.1.1. Genus Aspergillus (MycoBank ID 7248). Chemical examination of the endosymbiotic derived fungus Aspergillus niveus LU 9575, isolated from woodlouse, collected near Konigsheide in Germany, led to the isolation of the previously undescribed aspochalamins A (57), B (58), C (59) and D (60) along with the previously reported aspochalasins D (61), and Z (62) (Fig. 9). Compounds 57–62 were examined for their cytotoxic effects against HMO2, MCF7, HepG2 and Huh7 cells, where 57–60 and 62, displayed weak to mild cytotoxic effect against the examined cells. Additionally, these compounds were evaluated for their antimicrobial activity against A. globiformis DSM 20124, A. aurescens DSM 20116, A. oxydans DSM 6612, A. pascens DSM 20545, B. subtilis DSM 10, B. brevis DSM 30, R. erythropolis DSM 1069, and S. aureus DSM 20231, where 57–60, showed weak antibacterial effect against the examined Gram-positive bacteria.130,131 Aspochalasin U (63), a previously unreported cytochalasin derivative was isolated from the marine derived fungus Aspergillus sp., obtained from Dongshi Saltern, Fujian Province (China), (Fig. 9). Aspochalasin U (63), showed mild effect against the necrotic cell death induced by TNF-α.132 A chemical investigation of methanolic extract of the river derived fungus A. flavipes-507, resulted in the isolation of a previously unprecedented cytochalasan dimer featuring the decacyclic ring system, namely asperchalasine A (64), along with asperchalasines B (65), C (66) and D (67), a three related biogenetically intermediates (Fig. 9), compounds 64–67, were examined for their cytotoxic effects against (HL-60, SMMC-7721, A-549, MCF-7, and SW-480), cancer cell lines, where all of them displayed weak antitumour effect against all the examined cancer cell lines, when compared to Cis-platin (IC50 values of 1.16, 8.08, 7.10, 10.45, and 8.88 μM) and Taxol (IC50 values of <0.0008 μM) as positive controls. Additionally, compound 64 was proven to be effective cytoskeletal inhibitor, when examined for its ability to disturb F-actin microfilaments. Furthermore, 64 exhibited a reasonable G1-phase cell cycle arrest in the four tested cancer cell lines and interestingly with no effect on the normal cell lines.133 The chemical exploration of the marine derived fungus A. flavipes co-cultured with the actinomycete Streptomyces sp., isolated from Nanji Islands, sediments, (China), led to the detection of six previously reported cytochalasin derivatives, namely rosellichalasin (68) aspochalasin E (69), aspochalasin M (70), aspochalasin P (71), 19,20-dihydro-aspochalasin D (72), and aspochalasin H (73) (Fig. 9). Compounds 68–73 displayed strong inhibition ability against Streptomyces sp, growth with an inhibition rate of 50–80% at concentration ranged from 2 to 16 μg mL−1, it is worth mention that the above-mentioned cytochalasans derivatives support the fungus A. flavipes when competing with Streptomyces sp.134 The aforementioned findings regarding the antibacterial activity of the obtained cytochalasin derivatives must be considered with great caution, as they contradict all other reports in the literature. Additionally, three previously unreported heterotrimers cytochalasan analogues namely amichalasines A (74), B (75), and C (76) (Fig. 9), together with the previously mentioned analogues (61) and (64), were obtained through the chemical examination of the organic extract of the endophytic fungus A. micronesiensis PG-1, isolated from Phyllanthus glaucus roots. Compounds 74–76 were examined for their cytotoxicity against HL60, U87MG, MDA-MB-231, A549, Hep3B, and SW480 cancer cell lines, where they displayed mild to potent cytotoxic effect against the examined cancer cell lines with IC50 values ranging from 1.71 to 35.84 μM. It is worth noting that amichalasine B (75), was more active than the model compounds, cytochalasans B (2) and D (366).135 A chemical investigation of the marine derived fungus A. flavipes CNL-338, isolated from the red alga Laurencia sp. collected from the Bahamas, led to the isolation of the previously mentioned derivatives (69) and (71), along with five previously reported analogues namely aspochalasin C (77), TMC-169 (78), flavichalasines F (79) and G (80) and aspergillin PZ (81) (Fig. 9). These isolated compounds were not tested for any relevant biological activity.99
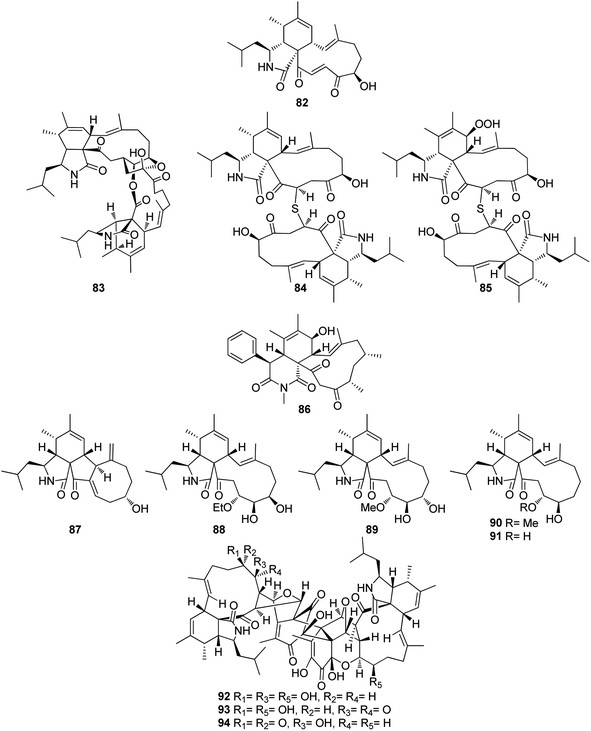
The previously mentioned cytochalasan monomer (61) and its previously reported 18-keto analogue aspochalasin B (82), together with three unusual homodimers cytochalasans derivatives previously undescribed namely bisaspochalasins A (83), B (84) and C (85), (Fig. 10), were obtained from the organic extract of the plant derived fungus A. flavipes, isolated from Hevea brasiliensis steams, collected from Yunnan Province, China, in Banna Prefecture, it is worth mention that 85 might be an artifact of 84, through a Schenckene photooxygenation. Compounds 83–85 were tested for their immunosuppressive effect, among them only 83 displayed an inhibition effect towards human T cell proliferation activated by anti CD3/anti-CD28 antibodies, with IC50 value of 15.8 μM.136 Asporychalasin (86) (Fig. 10) a further cytochalasin derivative with an unprecedented 6/6/11 skeleton, was isolated from the Red Sea derived fungus A. oryzae isolated from the sediments collected in Saudi Arabia from Jeddah. Asporychalasin (86) displayed mild antiproliferative properties against three human cell lines, A549, HepG2, and MCF7 cells with IC50 values of 8.8, 7.4, and 8.3 μg mL−1, respectively, when compared to Doxorubicin (IC50 values of 0.3, 0.2, and 0.4 μg mL−1, respectively) as positive control, additionally compound 86, showed no toxicity towards zebrafish embryos.137
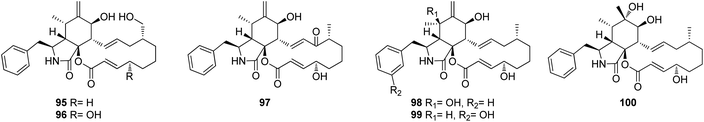
Further five previously unreported cytochalasan monomers namely aspermichalasines A (87), B (88), C (89), D (90), and E (91), aspermichalasine A (87), represent the first example of cytochalasan monomer possesses a 5/6/5/8 skeleton, along with three previously undiscovered cytochalasan heterotetramers namely asperflavipines C (92), D (93), and E (94) (Fig. 10), were obtained through the chemical examination of the EtOAc extract of the medicinal plant derived fungus A. micronesiensis, isolated from Phyllanthus glaucus roots, collected in China, Jiangxi Province, in the mountain of LuShan. Compounds 88–90 and 92–94 displayed potent cytotoxic effect against the HL60 cells with IC50 values ranging from 5.67 to 18.75 μM. Furthermore, 88 and 94 exhibited strong cytotoxicity against Hep3B cells with IC50 values of 7.99 and 5.60 μM, respectively, when compared to Cis-platin (IC50 value of 2.15 μM) as positive control. Additionally, 92 was found as potent apoptosis inducer in HL60 cells.138 A chemical examination of the medicinal plant derived fungus Aspergillus sp., isolated from Lonicera japonica Thunb, stems and flowers, led to the isolation of eight previously unreported cytochalasan derivatives, namely aspergicytochalasans A (95), B (96), C (97), D (98), E (99) and F (100), (Fig. 11) along with the previously mentioned analogues (1), (2), (3), (14), and (20). Compounds 95–100 were examined for their antibacterial properties towards E. coli ATCC25922 and S. aureus subsp. aureus ATCC29213, where aspergicytochalasans C (97) and D (98) showed antibacterial activity against S. aureus subsp. aureus ATCC29213 with MIC values of 128 and 64 μg mL−1, respectively. Additionally, 95–100 were evaluated for their anti-inflammatory effects by examining their ability to inhibit NO production in LPS-induced RAW 264.7 macrophages. Whilst compounds 95, 97, 99 and 100 exhibited an inhibition effect towards the NO production with IC50 values of 26.8, 17.1, 31.2 and 12.7 μM, respectively. Compounds 96 and 98 were found inactive.139
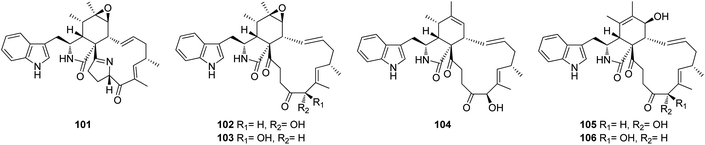
3.3.1.1.1.2. Genus Penicillium (MycoBank ID 9257). Chemical investigation of the marine alga derived fungus Penicillium sp. OUPS-79, isolated from Enteromorpha intestinalis, led to the isolation of five previously unreported cytochalasan derivatives namely, penochalasins D (101), E (102), F (103), G (104), and H (105), along with the previously reported analogue chaetoglobosin O (106) (Fig. 12). Compounds 101–106 displayed potent cytotoxic effect towards P388 cancer cell line with ED50 values of 3.2, 2.1, 1.8, 1.9, 2.8, and 2.4 μg mL−1, respectively, when compared to 5-Flurorouracil as positive control (ED50 value of 0.08 μg mL−1).140
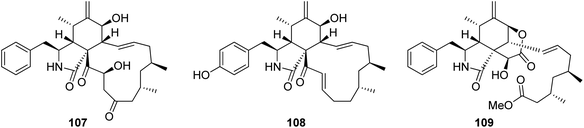
3.3.1.1.2. Fungi of the family Trichocomaceae. 3.3.1.1.2.1. Genus Talaromyces (MycoBank ID 5347). Three previously unreported cytochalasans namely talachalasins A (107), B (108) and C (109), possessing an unusual 16β-methyl group (Fig. 13), were isolated from the marine derived fungus Talaromyces muroii sp. SCSIO 40439, isolated from the deep sea, sediment of the South China sea. Compounds 107–109 were examined for their cytotoxic effects towards SF-268, MCF-7, HepG2 and A549 cancer cell lines. Whilst 107 and 108, showed mild and weak effects with IC50 values ranging from 3.40 to 10.02 and 17.30 to 30.58 μM, respectively. Compound 109 displayed no activity against the examined cancer cell lines. Additionally, they were tested for their antiviral effect towards HSV-1 (herpes simplex virus) and RSV (respiratory syncytial virus). Whilst 107 and 109 showed no antiviral activity, 108 displayed moderate and potent antiviral activity with IC50 values of 20.0 and 12.5 μM when compared to Ribavirin (IC50 value of 8.25 μM) as positive control, and selectivity index 1.50 and 3.20, respectively.141
3.4. Fungi of the class Leotiomycetes
3.4.1.1. Fungi of the order Helotiales.
3.4.1.1.1. Fungi of the family Sclerotiniaceae. 3.4.1.1.1.1. Genus Botryotinia (MycoBank ID 638). A chemical manipulation of the plant derived fungus Botryotinia fuckeliana A-S-3, isolated from the roots of Ajuga decumbens, collected in China, Fujian Province, led to the isolation of three previously reported cytochalasan derivatives phenochalasin B (110), 1,3-dioxacyclotridecino (111) and 12-cytochalasin (112) (Fig. 14). Compounds 110–112 were examined for their cytotoxic effects against SMMC-7721, A549, HepG2 and MCF-7 cancer cell lines. Whilst 111, displayed no cytotoxicity against the examined cancer cell lines with IC50 values of >50 μM, 110 and 112 showed potential anticancer effects with IC50 values ranging from 0.10 to 7.43 and 0.59 to 0.88 μM, respectively. Furthermore, 110 and 112 were found to induce apoptosis in HepG2 tumour cell line.142
3.5. Fungi of the class Sordariomycetes
3.5.1.1. Fungi of the order Diaporthales.
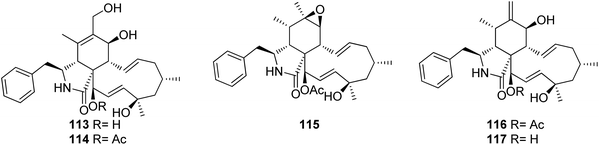
3.5.1.1.1. Fungi of the family Cryphonectriaceae. 3.5.1.1.1.1. Genus Endothia (MycoBank ID 1810). Chemical examination of the endophytic fungus Endothia gyrosa IFB-E023, isolated from the healthy leaf of Vatica mangachapo, collected from botanical garden in the south of China, led to the isolation of the previously undescribed cytochalasans Z10 (113) and Z11 (114) along with the previously reported epoxycytochalasin H (115) cytochalasin H (116), and cytochalasin J (117) (Fig. 15). Compounds 113–117 were examined for their anti-tumour effects against human leukaemia K562 cancer cell line using 5-Fluorouracil (IC50 value of 33.0 μM), as positive control, where they displayed cytotoxicity with IC50 values of 28.3, 24.4, 24.5, 10.1 and 1.5 μM, respectively.143
3.5.1.1.1.2. Genus Cytospora (MycoBank ID 7904). Chemical investigation of the MeOH extract of the plant derived fungus Cytospora chrysosperma, isolated from the aerial part of Hippophae rhamnoides, collected from Qinghai Province (China), resulted in the isolation of three previously undescribed cytochalasans derivatives namely cytochrysins A (118), B (119) and C (120) (Fig. 16). Compounds 118–120 were tested for their antibacterial effects towards carbapenem-resistant Pseudomonas aeruginosa, methicillin resistant S. aureus, multi-drug-resistant Enterococcus faecalis, multi-drug-resistant Enterococcus faecium, carbapenem-resistant Acinetobacter baumannii, multi-drug-resistant Staphylococcus epidermidis, carbapenem-resistant Klebsiella pneumoniae, and carbapenem-resistant E. coli using Ciprofloxacin, as positive control. Whilst 119 showed no antibacterial effects, 118 displayed significant antibacterial activity against E. faecium with MIC value of 25 μg mL−1, as well as 120 showed potent antibacterial activities against S. aureus with MIC value of 25 μg mL−1. Furthermore, they were examined for their antifungal activity against the plant pathogenic fungi Physalospora piricola, Fusarium oxysporum, Diplodia maydis, Verticillium dahliae, Sclerotinia sclerotiorum, Rhizoctonia solani, and Aphelenchoides fragriae, using Ketoconazole as positive control. Indeed, none of the isolated compounds displayed antifungal activity against these plant pathogenic fungi.144
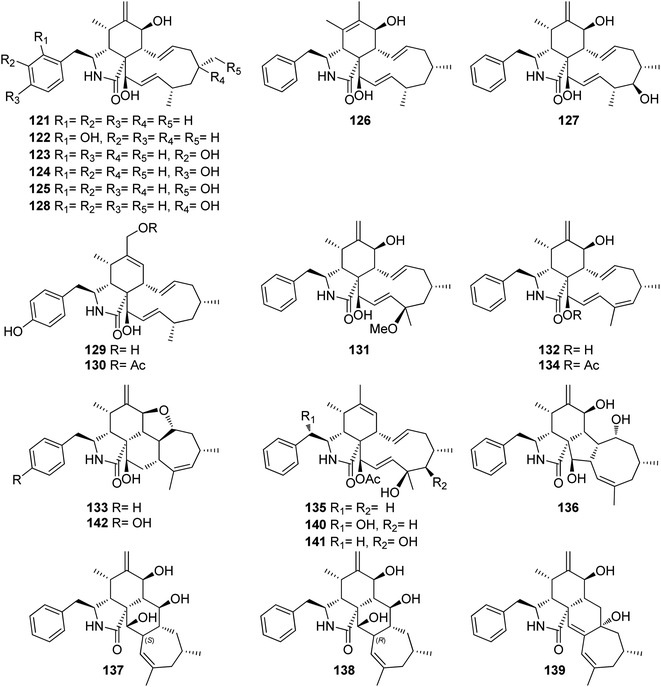
3.5.1.1.2. Fungi of the family Diaporthaceae. 3.5.1.1.2.1. Genus Diaporthe (MycoBank ID 839358). Ten cytochalasans derivatives including the previously reported 21-O-deacetyl-L-696,474 (121), phomopsichalasin G (122), diaporthichalasins A (123), B (124) and C (125), along with the previously undescribed diaporthichalasins D (126), E (127), F (128), G (129) and H (130) (Fig. 17), were recorded from the EtOAc extract of the endophytic fungus Diaporthe sp. SC-J0138, obtained from fresh leaves of Cyclosorus parasiticus, collected in Guangdong Province, (China). Whilst diaporthichalasin G (129) displayed no cytotoxicity against A549, HeLa, HepG2 and MCF-7 cancer cell lines, diaporthichalasin H (130) was proven to be active against these cells, with IC50 values ranging from 9.9 to 32.1 μM. Furthermore, all the other compounds had a demonstrable cytotoxicity against these cells, i.e., 21-O-deacetyl-L-696,474 (121) displayed cytotoxicity against A549, HeLa and HepG2 cells, with IC50 values of 17.3, 14.7 and 26.2 μM, respectively. However, 121 displayed no cytotoxicity towards the MCF-7 cell line.
Similarly, phomopsichalasin G (122) was found to be active against HeLa and HepG2 cells with IC50 values of 24.5 and 11.0 μM, respectively, but no activity was reported against A549 and MCF-7 cancer cells. Diaporthichalasin A (123) only showed cytotoxicity against HepG2 cells with IC50 value of 10.1 μM. Whilst diaporthichalasin B (124) displayed moderate cytotoxicity against HeLa and HepG2 cells with IC50 values of 22.1 and 21.2 μM, respectively. Diaporthichalasin C (125) showed anti-tumour effects against A549, HeLa and HepG2, with IC50 values of 12.0, 15.2 and 38.1 μM, respectively. However, diaporthichalasin D (126), displayed potent cytotoxicity against A549, HeLa and HepG2 cells, with IC50 values of 13.7, 15.3 and 8.8 μM, respectively. Additionally, diaporthichalasin E (127) showed selective cytotoxicity towards HepG2 cell line with IC50 value of 30.6 μM. Furthermore, diaporthichalasin F (128) only displayed selective cytotoxicity against HeLa and HepG2 cells with IC50 values of 34.7 and 16.5 μM, respectively, when compared to Adriamycin (IC50 values ranged from 0.31 to 1.02 μM).145 A chemical investigation of the organic extract of the endophytic derived fungus D. ueckerae SC-J0123, isolated from Pteris vittata L. leaves collected from Shatoujiao forestry centre, Guangdong Province, China, led to the isolation of the previously mentioned cytochalasan derivatives (116) and (117), along with the previously described cytochalasans J1 (131), J2 (132), J3 (133), longichalasin B (134), RKS-1778 (135), and phomopchalasin A (136), together with the previously unreported ueckerchalasins A (137), B (138), C (139), D (140), E (141) and 4′-hydroxycytochalasin J3 (142) (Fig. 17). It is worth mentioning that 137–139, possesses an unusual 5/6/6/7-fused heterocycle core. Compounds 116–117, and 133–142, were examined for their antibacterial abilities against Staphylococcus aureus (SA) and methicillin-resistant S. aureus (MRSA) using Kanamycin and Vancomycin (MIC values of 0.625 and 1.25 μg mL−1, respectively), as positive control. Only two compounds 136 and 139, displayed weak antibacterial effects against the examined bacterial strains with MIC values of (20 and 20 μg mL−1) and (20 and 40 μg mL−1), against SA or MRSA, respectively. Additionally, 137–142, were tested for their anti-tumour effect towards A549, MCF-7, HepG2, and HeLa cancer cells and non-cancerous Vero cells using MTT method to quantify cell viability and Adriamycin (IC50 values ranged from 0.11 to 0.80 μM), as positive control. Whilst 139–141 displayed weak cytotoxicity towards HepG2 and/or HeLa cells with IC50 values ranging from 18.0 to 36.4 μM, 137, 138, and 142 showed no cytotoxicity against these cells.146 Additionally, the previously mentioned cytochalasan analogues (116), and (117), were obtained from an organic extract of the endophytic derived fungus D. cf. ueckeri, isolated from Pittosporum mannii, collected from the west of Cameroon in Tonga.55
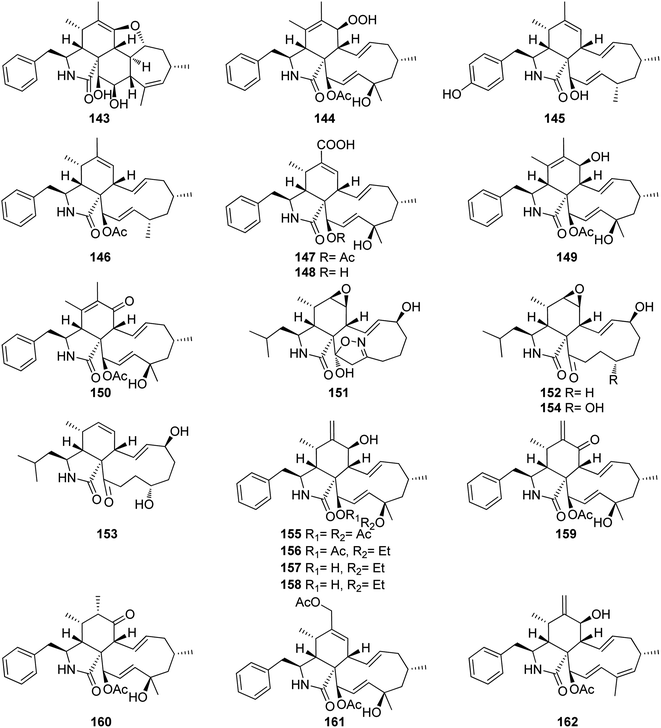
3.5.1.1.2.2. Genus Phomopsis (MycoBank ID 9365) [taxonomically updated to Diaporthe]. The aforementioned cytochalasan derivatives (117) and (136), along with two previously undescribed analogues namely phomopchalasins B (143) and C (144) (Fig. 18), were identified from an organic extract of the medicinal plant derived fungus Phomopsis sp. shj2, isolated from I. eriocalyx var. laxiflora. It is worth mentioning that phomopchalasin A (144) possesses an unusual hydroperoxyl motif in position C-7. Compounds 136, 143 and 144 were examined for their in vitro anti-inflammatory, cytotoxicity and antimigratory abilities using MG132, Cis-platin (IC50 values ranged from 1.1 to 12.7 μM), and cytochalasin D (366), respectively as the positive controls. Compounds 143–144 disrupted migrations of MDA-MB-231 cells with IC50 values of 19.1 and 12.7 μM, respectively. Additionally, 144 exhibited a mild cytotoxic effect towards A-549, HL-60, and SMMC-7721, cancer cells with IC50 values of 21.1, 14.9, and 22.7 μM, respectively. Moreover, 144 showed a potent inhibitory activity on nitric oxide (NO) production with IC50 value of 11.2 μM, when compared to MG-132 (IC50 value of 0.2 μM) as positive control.147 The chemical examination of the mangrove derived fungi Phomopsis sp. xy21 and Phomopsis sp. xy22, isolated from Xylocarpus granatum leaves, collected from Trang Province in Thailand, led to the isolation of the aforementioned derivatives (115–117) and (121–123), along with the previously unreported phomopsichalasin F (145) as well as the previously described 18-deoxycytochalasin H (7, L-696,474) (146) together with the previously undescribed phomopsichalasins D (147) and F (148) (Fig. 18). All the isolated compounds were examined for their cytotoxicity using the MTT method and Cis-platin (IC50 values of 8.5, 6.3, 18.1, 22.0, 28.2, 12.1, 10.7, and 15.4 μM, respectively) as positive control, against A2780, MDA-MB-231, A375, HCT-8, HCT-8/T, A549, SMMC-7721, and AGS cancer cell lines. Whilst (122) showed potent cytotoxic effect towards A2780, MDA-MB-231, A549, HCT-8, and HCT-8/T with IC50 values of 7.1, 3.4, 6.4, 7.5 and 8.6 μM, respectively. Moreover, (147) displayed no cytotoxic activity against the examined tumour cell lines with IC50 value of >100 μM.148 The previously described cytochalasin N (149), along with the previously unreported phomocytochalasin (150) (Fig. 18), together with the previously mentioned cytochalasan derivatives (116), and (135), were isolated from the endophytic fungus P. theicola, isolated from L. hypophaea leaves, collected in Mutan, Taiwan. Compounds 116, 149 and 150 were examined for their inhibitory effect towards NO and IL-6 production using Quercetin (IC50 values of 36.8, and 31.3 μM, respectively), as positive control. Among them, cytochalasin N (149) exhibited weak inhibitory ability with IC50 value of 77.8 μM. Furthermore, they were examined against the progesterone receptor (PR) antagonism using RU486 (IC50 values 0.000063 μM) as positive control. Among the isolated compounds, cytochalasin H (116), displayed significant antagonism effect with the IC50 value of 1.42 μM.149 Three previously unreported cytochalasan derivatives namely phomopsisins A (151), B (152) and C (153), along with the previously reported once xylarisin (154) (Fig. 18), were obtained through the chemical examination of the endophytic fungus Phomopsis sp. sh917, isolated from I. eriocalyx var. laxiflora stems, collected in China, from Kunming botanical garden. Compounds 151–152 displayed no significant cytotoxic effect when examined for their cytotoxicity against SW480, MCF-7, SMMC-7721, A549, and HL-60 cells using the MTT method and Taxol & Cis-platin, as positive controls. Moreover, among the isolated compounds, phomopsisin C (153) showed moderate inhibition effect towards NO-production when compared with the positive control L-NMMA, with IC50 values of 32.38 and 42.34 μM, respectively.150 Chemical investigation of the endophytic fungus Phomopsis sp. shj2, isolated from the stems of I. eriocalyx var. laxiflora, collected in China from Kunming botanical garden; led to the isolation of the previously mentioned derivatives (116), (131), and (135) along with the previously undescribed 18-acetoxycytochalasin H (155), 18-ethoxycytochalasin H (156), 18-acetoxycytochalasin J (157), 18-ethoxycytochalasin J (158), 7-oxocytochalasin H (159), cytochalasin H3 (160), cytochalasin H4 (161), together with the previously reported 21-acetoxycytochalasin J2 (162) (Fig. 18). Compounds 116, 131, 135, 155–157 and 161–162 were examined for their in vitro antimigratory abilities against MDA-MB-231, using cytochalasin D (366) (IC50 value of 0.78 μM) as positive control. Whilst cytochalasin H4 (161) displayed no antimigratory effect with IC50 value >25 μM, the rest of the examined compounds were found to be active with IC50 values ranging from 1.01–10.42 μM.151
3.5.2.1. Fungi of the order Hypocreales.
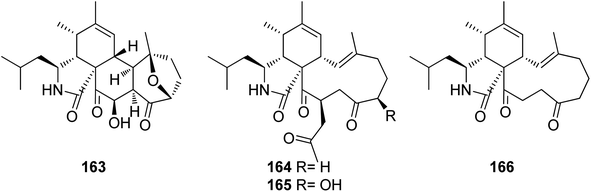
3.5.2.1.1. Fungi of the family Bionectriaceae. 3.5.2.1.1.1. Genus Clonostachys (MycoBank ID 7701) (Spicaria, MycoBank ID 22355). Four previously unreported derivatives, spicochalasin A (163), aspochalasins N (164), O (165) and Q (166) (Fig. 19), along with the previously mentioned derivatives, (61), (70), (71) and (82), were isolated from the marine derived fungus Spicaria elegans, isolated from the marine sediment of Jiaozhou Bay, China. All compounds were examined for their cytotoxicity against MOLT4, A549, HL-60 and BEL-7402 cell lines. Whilst 70 and 164–166 were found to be inactive with IC50 values >100 μM, 61 displayed mild to potent cytotoxicity with IC50 values ranging from 2.3 to 16.3 μM. Aspochalasin B (82) exhibited cytotoxicity against MOLT4, A549, and HL60 with IC50 value of 13.0, 19.6, and 11.6 μM, respectively. Moreover 71 and 163 showed moderate cytotoxicity against HL-60 with IC50 values of 20.0 and 19.9 μM, respectively.152
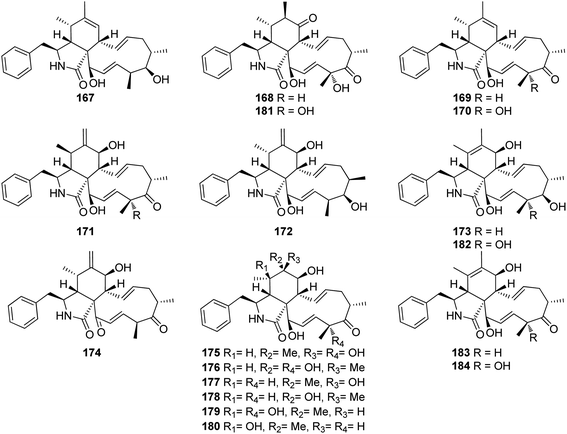
3.5.2.1.2. Fungi of the family Clavicipitaceae. 3.5.2.1.2.1. Genus Metarhizium (MycoBank ID 8912). Fourteen previously unreported cytochalasans derivatives namely brunnesins A (167), B (168), C (169), D (170), E (171), F (172), G (173), H (174), I (175), J (176), K (177), L (178), M (179), N (180) along with the previously reported analogues, 6,7-dihydro-7-oxo-deacetylcytochalasin C (181), zygosporin D (182), deacetylcytochalasin C (183) and 18-deshydroxyl-deacetylcytochalasin C (184), (Fig. 20) were isolated from the organic extract of the pathogenic derived fungus Metarhizium brunneum TBRC-BCC 79240, isolated from the dead insect (Lepidoptera), collected in Thailand from the Province of Kalasin. Compounds 167–173 and 175–184 were evaluated for their antibacterial using Isoniazid, Rifampicin, Vancomycin and Erythromycin (MIC values ranged from 0.0063 to 25.0 μg mL−1) as positive controls, against Mycobacterium tuberculosis, Staphylococcus aureus, Acinetobacter baumannii and for their antifungal activity using Amphotericin (MIC value ranged from 0.781 to 1.56 μg mL−1) as positive control, towards the phytopathogenic fungi, Alternaria brassicicola, Colletotrichum acutatum, and Curvularia lunata, as well as their cytotoxicity using Doxorubicin, Ellipticine and Tamoxifen (IC50 values ranged from 0.077 to 9.14 μg mL−1) as positive controls, against the mammalian cells MCF-7, NCI-H187 and Vero.
Additionally, the examined compounds displayed neither antibacterial nor antifungal against (S. aureus and A. baumannii), and (A. brassicicola, C. acutatum, and C. lunata), with MIC values of >50 μg mL−1. However, 168, 170–173 and 181–184 displayed weak to mild anti-bacterial activity against M. tuberculosis with MIC values ranging from 25 to 50 μg mL−1. Furthermore, whilst 167–169, 171, 176–177, 181 and 183–184, showed no cytotoxic effect against the examined cell lines with IC50 values of >50 μg mL−1, compound 170 displayed significant cytotoxicity against all the examined cells with IC50 values of 16.8, 3.71 and 2.09 μg mL−1, respectively.
Additionally, 172 and 182, showed a selective remarkable effect against the Vero cell lines with IC50 values of 4.03 and 0.637 μg mL−1, respectively. Moreover, 173 and 179–180 showed cytotoxic ability against NCI-H187 and Vero cells with IC50 values ranging from 3.98 to 44.81 μg mL−1. Furthermore, 175 and 178 exhibited a selective activity against NCI-H187 cancer cell line with IC50 values of 18.59 and 18.54 μg mL−1, respectively.153
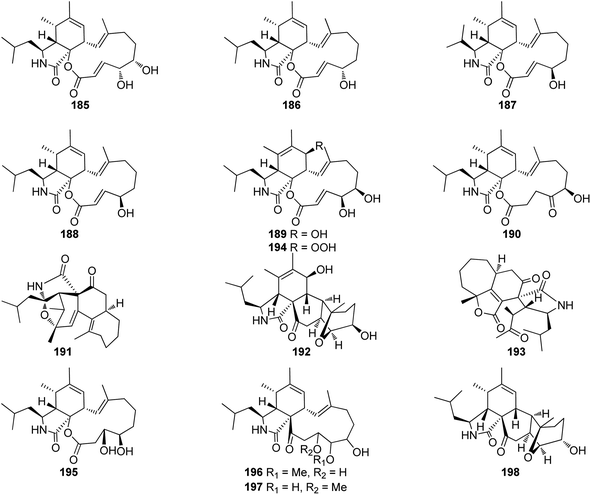
3.5.2.1.3. Fungi of the family Hypocreaceae. 3.5.2.1.3.1. Genus Trichoderma (MycoBank ID 10282). A chemical exploration of the medicinal plant fungus Trichoderma gamsii, isolated from Panax notoginseng, led to the isolation of the previously reported cytochalasin derivatives aspochalasins I (185), and J (186), together with two previously unreported analogues namely trichalasins A (187) and B (188) (Fig. 21). Compounds 185–188 were tested for their effect against HeLa cancer cell lines using Taxol and VP-16 as positive controls (EC50 value of 0.0025 and 0.0057 μM, respectively). Among them, only aspochalasin J (186) showed weak cytotoxic effect with IC50 value of 27.8 μM.59 Further examination of the same endophytic fungus by the same group resulted in the isolation of the previously mentioned analogues (61), (70) and (71), together with the two previously unreported trichalasins C (189) and D (190) (Fig. 21). All the isolated compounds were examined for their cytotoxic activity towards the HeLa cancer cell line, using VP-16 and Taxol as positive controls.
Whilst aspochalasin D (61) exhibited cytotoxicity with EC50 value of 5.72 μM, all the other compounds showed no cytotoxicity with EC50 values of >40 μM.154 Further investigation of the same fungal strain by the same group yielded in the isolation of the previously mentioned cytochalasan derivatives (61), (185) and (186), along with two additional previously unreported analogues trichoderones A (191) and B (192) (Fig. 21). All isolated compounds were examined for their cytotoxicity against HeLa cell line using VP-16 and Taxol as positive controls. Whilst aspochalasins D (61) and J (186), showed cytotoxic effect with IC50 value of 5.72 and 27.4 μM, respectively, aspochalasin I (185), trichoderones A (191) and B (192) were found inactive.155
Later, the same group reported the isolation of a further previously unreported trichodermone (193) (Fig. 21), along with (61), which might be considered as its possible biosynthetic precursor. Whilst trichodermone (193), displayed no cytotoxicity against HeLa cell line, aspochalasin D (61) showed mild cytotoxic effect with IC50 value of 5.72 μM.156 Furthermore, three previously unreported analogues namely trichalasins E (194), F (195), and H (198), along with two previously reported derivatives namely, aspochalasin K (196), and trichalasin G (197), (Fig. 21), together with the previously mentioned analogues (81) and (189) were isolated during a re-examination of the same fungal strain by the same group.
Compounds 81, 189, and 194–198, were examined for their cytotoxic effects against A549, MDA-MB-231 and PANC-1 tumour cell lines using 5-Fluorouracil (IC50 values of 0.47, 0.12 and 0.67 μM, respectively) as positive control. Among the reported metabolites only trichalasin G (197) displayed weak cytotoxicity against MDA-MB-231 with IC50 value of 60.6 μM.157
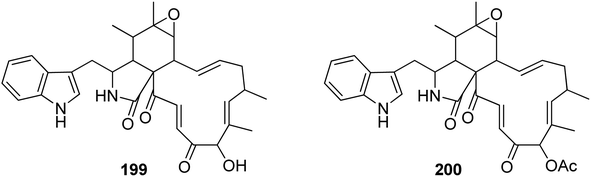
3.5.2.1.4. Fungi of the family Nectriaceae. 3.5.2.1.4.1. Genus Calonectria (MycoBank ID 746). Chemical examination of the plant derived fungus Calonectria morganii (synonym Cylindrocladium scoparium), led to the detection of two previously unreported analogues namely chaetoglobosin A (199) and 19-O-acetylchaetoglobosin A (200) (Fig. 22).158
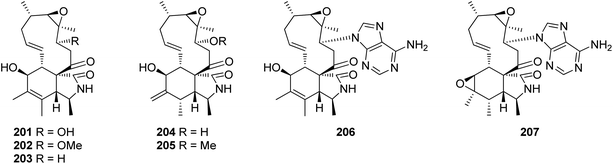
3.5.2.1.5. Fungi of the family Stachybotryaceae. 3.5.2.1.5.1. Genus Stachybotrys (MycoBank ID 10052). Chemical examination of the mountain derived fungus Stachybotrys charatum, collected at hight of 3600 m of Tibet isolated from an unnamed glacier, led to the isolation of seven previously unreported analogues namely alachalasins A (201), B (202), C (203), D (204), E (205), F (206), and G (207) (Fig. 23). Alachalasin A (201) displayed anti-HIV-1 activity and in vitro activity against HIV-1LAI in C8166 cells, with CC50 and EC50 values of 101.55 and 8.01 μM, respectively, when compared to Indinavir (EC50 value of 0.00818 μM). Additionally, all the isolated compounds were tested for their antimicrobial activity against different bacterial strains including Streptococcus mutans (ATCC 25175), Staphylococcus aureus (ATCC 6538), Enterococcus faecalis (ATCC 19433), Micrococcus luteus (ATCC 9431), and Sarcina lutea (CMCC B28001), as well as a panel of fungi including Candida albicans (ATCC 10231), Geotrichum cadidum (AS2.498), and Aspergillus fumigatus (ATCC 10894), while none of them showed antimicrobial effect against S. lutea, S. mutans, E. faecalis, G. candidum, A. fumigatus, and C. albicans, at a concentration of 100 μg per disk; alachalasin D (204) displayed activity against S. aureus and M. luteus at a concentration of 100 μg per disk with an inhibition zones of 7 and 9 mm, respectively. Furthermore, alachalasin G (207) displayed mild effect towards S. aureus, exhibiting an inhibition zone of 15 mm at a concentration of 100 μg per disk as well as showed an MIC value of 99 μM.65
3.5.2.2. Fungi of the order Sordariales.
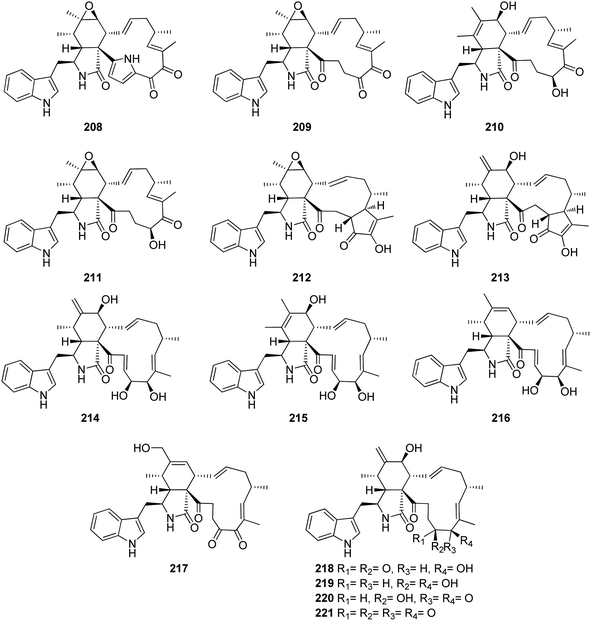
3.5.2.2.1. Fungi of the family Chaetomiaceae. 3.5.2.2.1.1. Genus Chaetomium (MycoBank ID 953). Five previously described cytochalasan derivatives namely penochalasin A (208), chaetoglobosins C (209), E (210), F (211) and chaetoglobosin U (212) (Fig. 24) were isolated from the plant derived fungus Chaetomiun globosum IFB-E019, isolated from Imperata cylindrica, collected in China, from Yancheng seashore, from the Province of Jiangsu. Compound 212 displayed strong cytotoxic effect against KB cancer cell line with IC50 value of 16.0 μM, with almost an identical value reported for the positive control 5-Fluorouracil (i.e., IC50 value of 14.0 μM). Additionally, 208–211 showed mild cytotoxicity against KB cancer cell with IC50 values of 34.0, 40.0, 52.0 and 48.0 μM, respectively.159 Chemical inspection of the organic extract of the marine green alga derived fungus C. globosum QEN-14, isolated from Ulva pertusthe fresh tissue, collected in China from the coastline of Qingdao Province, led to the isolation of seven previously unreported cytochalasan derivatives namely cytoglobosins A–G (213–219), along with the previously described analogues chaetoglobosin Fex (220) and isochaetoglobosin D (221) (Fig. 24). Compounds 213–217 and 219, were tested for their cytotoxic effects against KB, A549, and P388 cell lines. Whilst 213, 214, 217, and 219, were found inactive with IC50 values of >10 μM, 215 and 216, exhibited a selective cytotoxicity against A549 with IC50 values of 2.26 and 2.55 μM, respectively.160
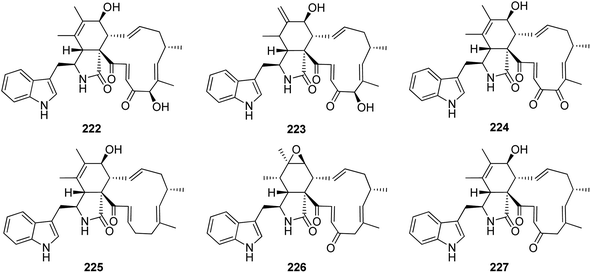
Dou et al. isolated the previously mentioned analogue (220) from the marine-derived fungus C. globosum QEN-14. Subsequently, they investigated its biological activity and proposed that its anti-inflammatory potential might be attributed to its ability to negatively regulate phosphorylation of ERK1/2, JNK1/2, and p38, as well as inhibit NF-kB. On the other hand, these inhibitory activities may also be attributed to the blocking of mCD14 expression.161 Further nine cytochalasan derivatives were reported from the soil derived fungus C. elatum ChE01, isolated from a soil sample collected in Thailand from the Province of Yala, including the previously mentioned derivatives (209), (211), and (221), along with the previously reported chaetoglobosins B (222), D (223), and G (224), together with three previously undescribed analogues namely chaetoglobosin V (225), prochaetoglobosin III (226), and prochaetoglobosin IIIed (227) (Fig. 25). Compounds 209, 211, and 221–227, exhibited potent cytotoxicity against the cholangiocarcinoma cells with IC50 values ranging from 3.41 to 86.95 μM when compared to 5-Fluorouracil (IC50 value 348 μM), as positive control. Additionally, all the examined compounds displayed cytotoxic effect against the human breast cancer with IC50 values ranging from 2.54 to 21.29 μM, when compared to Ellipticine (IC50 value 1.06 μM), as positive control.162
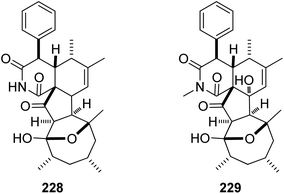
Additional two previously undescribed cytochalasan derivatives exhibited unprecedented 6/6/5/5/7 pentacyclic ring system, namely chaetoconvosins A & B (228 & 229) (Fig. 26) were recorded from the plant derived fungus C. convolutum cib-100, isolated from the roots of wheat, collected in China, Sichuan Province. Compounds 228–229 were examined for their cytotoxicity against SMMC-7721, A549, HEPG2, PC-3, and A375 tumour cell lines using Cis-platin as positive control. Whilst 229 exhibited moderate cytotoxicity against the examined cells with IC50 values of 43.0, 26.12, 44.60, 49.74 and 47.93 μM, respectively, 228 was found inactive. Furthermore 216, displayed an inhibitory activity towards root elongation.163
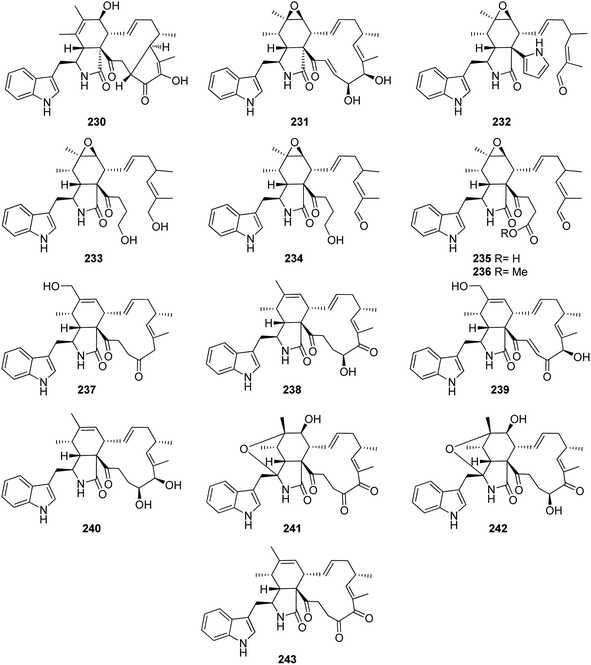
Chaetoglobosin Vb (230) (Fig. 27), a previously undescribed cytochalasan derivative along with the previously mentioned analogue (224), were reported from the plant derived fungus C. globosum, isolated from G. biloba leaves. Compounds 224 and 230 were tested for their antibacterial properties towards E. coli, S. aureus, B. cereusi, B. subtilis and P. aeruginosa, using Ampicillin as positive control. Whilst 230 showed no activity against all the examined bacterial strains with MIC values of >100 μg mL−1. Compound 224 exhibited weak antibacterial ability against S. aureus, B. cereusi, and P. aeruginosa with MIC values of 50 μg mL−1. Additionally, both compounds were checked for their activity against selected phytopathogens, using Carbendazim and Hymexazol as positive controls. Whilst 224 showed weak antifungal activity against F. graminearum, A. solani, and A. alternate, with MIC values of 50, 25, and 25 μg mL−1, respectively. Additionally, 230 was found inactive against all the examined phytopathogens with MIC values of >100 μg mL−1.164 The chemical inspection of the medicinal plant derived fungus C. globosum, isolated from G. biloba leaves, collected in Shandong Province, China afforded the isolation of five previously reported derivatives including 20-dihydrochaetoglobosin A (231) (Fig. 27) along with the previously mentioned analogues (209), (210), (211), and (220). Compounds 209–211 and 220, displayed phytotoxic effect towards radish seedlings when compared to Glyphosate as positive control. Additionally, 210, 211, 220 and 231 were examined for their antitumour activity against the HCT116 tumour cell line using Etoposide (IC50 2.13 μM), as positive control. Compound 210 was found inactive with IC50 value of >100 μM, however 211, 220, and 231 displayed mild to strong activity with IC50 values of 17.8, 4.43, and 8.44 μM, respectively. These findings reflect crucial role of the epoxide ring as well as the double bond at C-6(12), which enhance the cytotoxicity.165 A chemical exploration of the methanolic extract of the arthropods derived fungus C. globosum TW1-1, isolated from pillbug (Armadillidium vulgare), collected in China Hubei Province, yielded ten previously undescribed cytochalasan analogues namely armochaetoglobins A–J (232–241), along with the previously reported chaetoglobosin W (242) and isochaetoglobosin J (243) (Fig. 27) together with the previously mentioned cytoglobosin derivatives (215) and (217). Compounds 233–241 were examined for their cytotoxicity against HL-60, SMMC-7721, A549, MCF-7, and SW480, tumour cell lines using Paclitaxel and Cis-platin as positive controls (IC50 values between <0.0008–0.58 and 1.33–13.3 μM, respectively). Compounds 237 and 240 were found inactive with IC50 values of >40 μM, however 236, 238, 239 and 241, exhibited strong to weak cytotoxicity with IC50 values ranging from (10.84–38.17 μM), (12.58–20.55 μM), (3.31–9.83 μM), and (17.43–35.28 μM), respectively. Furthermore, 233 and 234 displayed a selective moderate cytotoxicity against HL-60, SMMC-7721, and A549 cancer cell lines with IC50 values ranging from (16.47–30.88 μM), and (10.62–16.32 μM), respectively. Additionally, 235 showed a selective cytotoxicity against SMMC-7721 cell line with IC50 value of 24.99 μM.166
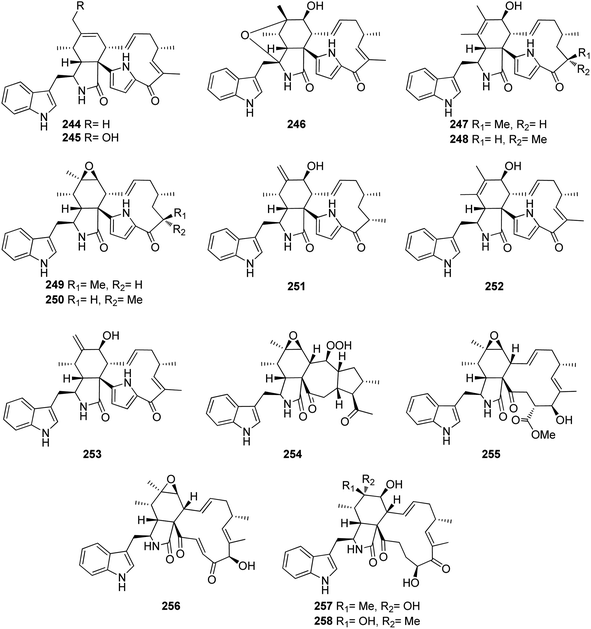
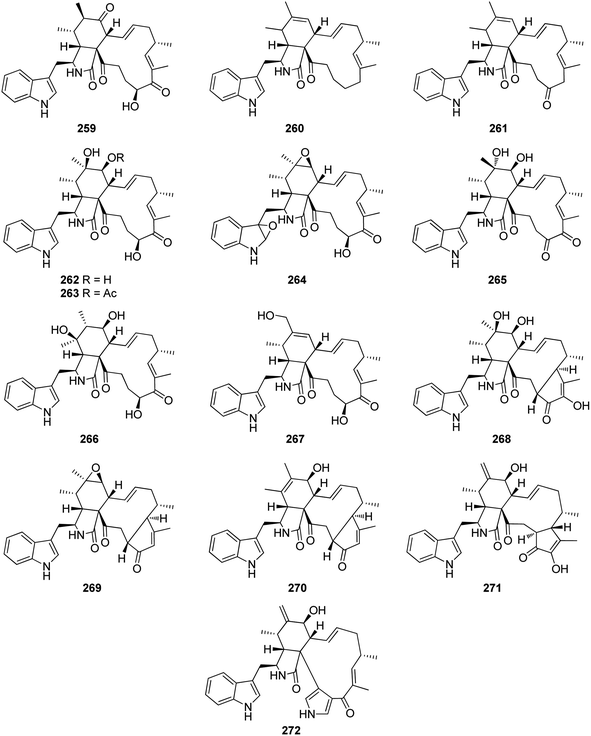
Further examination of the same fungal strain by the same group, resulted in the isolation armochaetoglobins K–R (244–251), additional eight previously undescribed derivatives resembled the unusual pyrrole-based cytochalasans, along with the previously described analogues penochalasins B–C (252–253) (Fig. 28), together with the previously mentioned analogue (208). All the isolated compounds were examined for their antiviral activity against the HIV using the cytopathic effect and p24 assays, and AZT (EC50 0.012 μM), as positive control. Among them, 245–247 and 250–252 displayed strong anti-HIV activity with EC50 values of 0.48, 0.55, 0.25, 0.31, 0.34 and 0.11 μM, respectively and with a selectivity index ranging from 12.33 to 75.42, however 208, 244, 248, 249, and 253, were found to be less potent with EC50 values of 6.12, 1.23, 0.61, 0.68, and 4.92 μM, respectively.167 Further investigation by the same groups for the same fungal strain led to the isolation of three previously undescribed analogues namely armochaeglobines A–C (254–256) (Fig. 28), along with the previously mentioned derivatives (199) and (212). Compounds 254–256 were examined for their cytotoxicity against SW480, HL-60, MCF-7, SMMC-7721, and A549 cancer cell lines, using MTS method and Cis-platin (IC50 values of 10.1, 2.2, 12.2, 10.2, and 7.3 μM, respectively), as positive control. Among them, 255 displayed a mild cytotoxicity with IC50 values ranging from 3.4 and 14.7 μM, against the examined cancer cell lines.168 The previously mentioned cytochalasan derivatives (199) and C (209), were reported from the ethyl acetate extract of the aquatic plant derived fungus C. globosum, obtained from Nymphaea nouchali, collected in Sri Lanka from the Province of Gampaha. Both compounds were examined for their antimicrobial abilities against the Gram-positive bacteria, methicillin-resistant S. aureus (MRSA, ATCC 33591), S. aureus (ATCC 43300) and B. subtilis (UBC 344). Whilst 199 displayed moderate antibacterial activity against the examined Gram-positive bacteria with MIC values of 32, 32 and 16 μg mL−1, respectively. Compound 209 was found to be inactive.169 A chemical inspection of the marine derived fungus C. globosum MCCC 3A00607, isolated from the sediments collected from the Indian Ocean led to the isolation of the previously mentioned analogues 210–211, 214–215, and 220–222, along with two previously undescribed analogues namely cytoglobosins H–I (257–258) (Fig. 28). All the isolated compounds were evaluated for their cytotoxicity against MDA-MB-231, LNCaP, and B16F10 cancer cell lines using Cis-platin (IC50 values of 2.48, 1.04 and 2.80 μM, respectively), as positive control. None of them showed cytotoxicity towards MDA-MB-231B. Furthermore, 258 was found inactive against LNCaP, and B16F10 cancer cell lines as well. Interestingly, 210 displayed the highest cytotoxicity against B16F10 and LNCaP tumour cell line with IC50 values of 2.78 and 0.63 μM, respectively.170
A chemical exploration of the pillbug derived fungus C. globosum TW1-1, isolated from R. vulgare, collected from Hubei Province, China, afforded the isolation of the previously mentioned derivatives 211–213, 225, and 231, along with the previously described chaetoglobosin Y (259), prochaetoglobosins I–II (260–261), together with nine previously undescribed polyoxygenated cytochalasan derivatives namely armochaetoglobin S (262), 7-O-acetylarmochaetoglobin S (263), armochaetoglobins T–Z (264–270) (Fig. 29). Compounds 262–270 were examined for their cytotoxicity against the cancer cell lines SMMC-7721, HL-60, A549, SW480, MCF-7, and BEAS-2B using Cis-platin (IC50 values of 10.21, 1.93, 6.59, 12.16, 8.20, and 9.07 μM, respectively) as positive control. Whilst 262–268 exhibited no cytotoxicity, 269 displayed moderated activity against all the examined cell lines except MCF-7 line with IC50 values ranging from 14.13 to 30.42 μM. Moreover, 270 displayed a selective cytotoxic effect against HL-60, A549, and BEAS-2B cells with IC50 values of 10.45, 23.11 and 10.45 μM, respectively.171 The previously mentioned analogues 209–211, 213–215, 220, 224, 221, and 230 along with two previously unreported analogues namely cytoglobosin Ab (271) and isochaetoglobosin Db (272) (Fig. 29) were reported from the organic extract of the fungus C. globosum SNSHI-5. Compounds 271–272 were tested for their cytotoxic activity against H292 cells, where 271 displayed no cytotoxic effect, 272 displayed promising cytotoxicity with IC50 value of 3.5 μM.172,173
A chemical inspection of an organic extract of the arthropod A. vulgare derived fungus C. globosum TW1-1, grown in a L-tyrosine rich media, afforded the isolation of nine previously undescribed cytochalasans namely armochaetoglasins A–I (273–281), along with the previously described derivatives armochaetoglobin E (282) and chaetoglobosin J (283) (Fig. 30) together with the previously mentioned analogue 225. Compounds 225 and 273–283 were tested for their cytotoxicity against SW480, HT1080, SW1990, MM231, HL60, U87MG, HEP3B and A549 cells, and NCM460 (normal colonic epithelial cell), using the MTS method and Cis-platin (IC50 values of 1.42, 2.47, 3.75, 4.62, 1.63, 2.13, 2.96 2.79 and 0.85 μM, respectively) as positive control. Compounds 273, and 275–282 were found inactive with IC50 values of >40 μM. On the other hand, 225, 274 and 283, displayed weak selective cytotoxicity with IC50 values ranging from 19.5 and 34.72 μM. Additionally, all the isolated compounds were found inactive against the drug-resistant microbial pathogens, K. pneumonia, E. coli, and S. aureus.174 Further re-examination by the same group on the same fungal strain grown in a cultural medium rich in 1-methyl-L-tryptophan (1-MT) instead of L-tyrosine, resulted in the isolation of three previously unreported cytochalasans namely armochaetoglosins A–C (284–286), along with the previously undescribed chaetoglobosin T (287) (Fig. 30), together with the previously mentioned derivatives 209, 230, and 269. All compounds were examined for their antibacterial activity against five drug-resistant bacterial strains methicillin-resistant S. aureus ATCC 43300; ESBL-producing E. coli ATCC 35218; E. faecalis ATCC 29212; NDM-1-producing K. pneumoniae ATCC BAA2146; and P. aeruginosa ATCC 15542. Compound 209 was found inactive. On the other hand, 286 displayed potent antibacterial effect against K. pneumoniae when compared to the positive control Meropenem with MIC values of 4 and 8 μg mL−1, respectively.175 Chamiside A (288) (Fig. 30), a previously undescribed cytochalasans analogue possessing an unusual 6/6/5-Flurorouracilsed tricyclic ring system, bearing the rare lactone seven membered and a benzene ring was recorded from the medicinal plant derived fungus C. nigricolor F5, isolated from Mahonia fortunei roots, collected from Qingdao Province, China. Compound 288 showed moderate antibacterial effect towards S. aureus (ATCC 6538), with MIC value of 25 μg mL−1.176
A chemical examination of the soil derived fungus C. madrasense 375, isolated from a soil sample of Sinkiang Province desert, China, afforded the isolation of the previously undescribed chaetomadrasins A–B (289–290) (Fig. 31), along with the previously mentioned derivatives 213, 221, 224, 230, 265, and 271. Compounds 289–290 were evaluated for their cytotoxicity towards HepG2 tumour cell line, where they displayed mild activity when compared to the positive control Cis-platin, with IC50 values of 8.7, 19.4 and 3.14 μM, respectively.177 The HPLC-DAD-guided separation of the soft coral derived C. globosum C2F17, led to the isolation of the previously mentioned derivatives 104, 199, 209–211, 220–222, 223–224, 230, 238 and 259, along with the previously reported aureochaeglobosin B (291) and the previously unreported 6-O-methyl-chaetoglobosin Q (292) (Fig. 31). Compounds 104, 209–211, 220–224, 230, 238, 259, and 291, were tested for their cytotoxicity against U937, H1975, K562, MOLT-4, BGC823, MCF-7, HeLa, A549, Huh-7 and HL60 tumour cell lines. Compounds 104, 209, 211, 221–224, 230, 238, 259, and 291, were found inactive with IC50 values >10 μM, but 210 displayed significant activity against all the examined cancer cells with IC50 values ranging from 1.4 to 9.2 μM. Furthermore, 220 exhibited a selective cytotoxicity against MOLT-4, Huh7, U937, and MCF-7 cells, with IC50 values of ranging from 2.9 to 7.5 μM, respectively. Moreover, 104, 209–211, 220–224, 230, 238, 259, and 291, were found inactive when tested for their anti-tuberculosis activity.178 Additionally, four previously unreported cytochalasans derivatives namely pchaeglobolactone A (293), spiropchaeglobosin A (294), pchaeglobosals A–B (295–296) (Fig. 31), were reported from the endophytic derived fungus C. globosum P2-2-2, isolated from a Ptychomitrium plant, collected in China from Hubei Province. Compound 296 exhibited potent cytotoxicity against A549, HepG2, MCF-7, CT26, and HT29 with IC50 values ranging from 1.04 to 9.90 μM, when compared to Doxorubicin hydrochloride (IC50 values ranging from 0.48 to 1.37 μM) as positive control. Furthermore, it displayed apoptosis-inducing and cell cycle arrest activities in HT26 and CT29 cells.179 The aforementioned cytochalasan derivatives 210, 222 and 252, were recorded from the endophytic derived fungus C. globosum TY1, isolated from the medicinal plant Ginkgo biloba fresh bark. Compounds 210, 222 and 252, haven't been examined for any relevant biological activity.180
Two previously unreported 19,20-seco-chaetoglobosins namely salchaetoglobosins A–B (297–298) (Fig. 31), along with the previously mentioned analogues 210, 220, and 230, were reported from the plant derived fungus C. globosum D38. All the isolated compounds were examined for their cytotoxicity against HCT-116 and PC-3 cells. Only 297 exhibited a mild cytotoxicity against PC-3 tumour cells with IC50 value of 62.21 μM. Additionally, the isolated compounds were examined for the anti-inflammatory activity. None of them was active.181 The previously mentioned cytochalasan derivatives 199, 209, 210, 212, 215, 230, 240, 260 and II 261, along with the previously undescribed aureochaeglobosin A (299) (Fig. 31), were documented from the plant derived fungus C. globosum, collected in China, Yunnan Province. All the isolated compounds were examined for their antibacterial activity against B. subtilis, S. typhimurium, and S. aureus and for their antifungal activity toward Fusarium graminearum and Gibberella saubinetii, using Vancomycin and Nystatin as positive controls respectively. All the isolated compounds displayed weak to potent antimicrobial effect against all the examined microbial strains with MIC values ranging from 4 and 512 μg mL−1.182
A chemical investigation of the mangrove derived fungus C. globosum kz-19, isolated from Ceriops tagal twigs, collected in China from Hainan Province, afforded the isolation of the previously mentioned cytochalasan analogues 104, 209, 210, 220, 221, 223, 224, 225, 227, and 283, along with the previously described armochaetoglobosin G (300) penochalasin J (301), together with four previously undescribed cytochalasans namely phychaetoglobins A–D (302–305) (Fig. 32). They were examined for their cytotoxicity against HeLa and A549 tumour cell lines using the MTT methods and Adriamycin (IC50 values of 0.8 and 2.9 μM, respectively), as positive control. Whilst phychaetoglobin A (302) was found inactive against HeLa cell line with IC50 value of >40 μM, all the other compounds displayed notable cytotoxicity with IC50 values ranging from 3.7 and 39.1 μM. Additionally, 302–303 were found inactive against A549 tumour cells with IC50 values of >40 μM, however all other compounds displayed weak to moderate cytotoxicity with IC50 values ranging from 7.3 and 32.3 μM.183 A chemical inspection of the genetically engineered soil derived fungus C. madrasense 375, isolated from a soil sample collected from the desert of XinJiang Province, China, afforded the isolation of the previously mentioned derivatives 210, 213, 222 and 223, along with three previously undescribed analogues namely chaetoglobosins B1–B3 (306–308) (Fig. 32). The isolated compounds were tested for their cytotoxicity against A549, HCC827, SW620, and MDA-MB-231 tumour cell lines. Compounds 307 and 308 were found inactive with IC50 values of >20 μM, however, 210 displayed potent cytotoxicity with IC50 values of 2.0, 1.7, 2.9, and 9.9 μM, respectively when compared to the positive control Cis-platin with IC50 values of 6.4, 4.5, 4.2 and 47.7 μM, respectively. Additionally, 210 showed strong cytotoxicity against two drug-resistant HCC827 cells (Osimertinib-resistant, Gefitinib-resistant) with IC50 values of 2.8 and 5.1 μM, respectively.184 The chemical investigation of the plant derived fungus C. tectifimeti S104, isolated from Rubia podantha roots collected from Kunming Province China, led to the isolation of the previously mentioned analogues 210, 220–224, 230, 259, and 281 along with a previously unreported rubichaetoglobin A (309) (Fig. 32). They were tested for their cytotoxicity against MDA-MB-231, HCT116, and HepG2 using Cis-platin (IC50 values of 27.89, 10.31, and 12.75 μM, against the tested cells respectively), as positive control. Compounds 210 and 221 displayed mild cytotoxicity against MDA-MB-231 cell line with IC50 values of 10.27 and 11.43 μM, respectively. Additionally, none of them exhibited antibacterial activity against S. aureus, B. subtilis, and E. faecalis. Moreover, all the compounds showed no anti-inflammatory activity.185 Armochaetoglasins L (310) and M (311) (Fig. 32), two previously undescribed cytochalasans analogues were reported from the arthropod derived fungus C. globosum, isolated from Armadillidium vulgare, collected in China Hubei Province. Both compounds showed moderate inhibitory activity against NO production when compared to the positive control Indomethacin with IC50 values of 7.06, 9.97, and 3.15 μg mL−1, respectively. Additionally, none of the obtained compounds displayed antibacterial effect against K. pneumonia, E. coli and S. aureus with MIC values of ≥100 μg mL−1.186
Five unusual heterodimers cytochalasan derivatives namely ergochaeglobosins A–E (312–316) (Fig. 33), were reported from the plant derived fungus C. globosum P2-2-2, isolated from Ptychomitrium plant, collected in China, from Hubei Province. Whilst 312–316 displayed mild immunosuppressive effect towards LPS-induced B cells with IC50 ranging from 14.10 to 32.10 μmol L−1 when compared to Mycophenolate mofetil (MMF, IC50 value 0.01 μmol L−1), as positive control, only 312–314 showed an inhibitory activity against ConA-induced T cells, with IC50 ranging from 19.20 and 47.81 μmol L−1 when compared to Cyclosporin A (CsA, IC50 value 0.01 μmol L−1), as positive control. Furthermore, only 312 showed weak cytotoxic effect against CT26 tumour cell lines with IC50 value of 39.1 μmol L−1, when compared to Doxorubicin hydrochloride (IC50 value 1.01 μmol L−1), as positive control. Additionally, 314 displayed significant TRAIL-resistance-over-coming activity.187 A chemical manipulation of the medicinal plant derived fungus C. nigricolor F5, isolated from Mahonia fortunei roots, afforded the isolation of five previously undescribed cytochalasans derivatives namely chamisides B–F (317–321) along with two previously reported analogues chaetoconvosins C–D (322–323) (Fig. 33). All the isolated compounds were found inactive when examine for their antibacterial and anticandidal abilities. Additionally, the crude extract, along with pure 317 and 321 were examine for their ability to inhibit A. thaliana root elongation. Only 317, displayed a mild inhibition effect. Furthermore, all the isolated compounds as well as the previously mentioned analogue (288) were evaluated for their interaction with cholesterol transporter protein Niemann-Pick C1-like 1 (NPC1L1), using molecular docking calculations. Compound 321 showed the highest binding affinity to NPC1L1.51
3.5.3.1. Fungi of the order Xylariales.
3.5.3.1.1. Fungi of the family Apiosporaceae. 3.5.3.1.1.1. Genus Arthrinium (MycoBank ID 7214). A chemical investigation of the marine sponge P. fusca, derived fungus Arthrinium arundinis ZSDS1-F3, collected from the Island of Xisha (China) afforded the isolation of the previously mentioned analogue 68, along with five previously undescribed analogues namely arthriniumnins A–D (324–327) and ketocytochalasin (328), together with the previously described cytochalasin K (329), Cytocalasin Z16 (330), 10-phenyl-[12]-cytochalasin Z16 (331), and cytotochalasin Z17 (332) (Fig. 34). Compounds 68, 324, 325, and 327–332 were examined for their cytotoxicity against K562, A549, Huh-7, H1975, MCF-7, U937, BGC823, HL60, HeLa, and MOLT-4 cancer cell lines using CCK8 (DOjinDo) and Trichostatin A as the positive controls. Whilst 68, 324, 325, 327, 328, 330, and 332, displayed no cytotoxicity against all the examined cells, 329 displayed weak cytotoxicity against K562, A549, Huh-7, H1975, HL60, HeLa, and MOLT-4 with IC50 values ranging from 10.5 to 47.4 μM, respectively.
Furthermore, 331 displayed a mild cytotoxic effect against all the examined cells except of Huh-7 cell, with IC50 values ranging from 1.1 and 18.8 μM. Additionally, none of these compounds exhibited antituberculosis activity.188
A chemical inspection of the plant derived fungus A. arundinis DJ-13, isolated from Aconitum brevicalcaratum, roots collected from the mountain of Dongjia, Yunnan Province (China), afforded the isolation of the previously mentioned analogues 68, and 324–327, along with six previously unreported analogues namely arundisins A–F (333–338), together with two previously described derivatives namely cytochalasin Z12 (339) and cytochalasin Z21 (340) (Fig. 34).
Compounds 333–336 and 338 were examined for their cytotoxic effects against HL-60, A549, SMMC-7721, MCF-7, and SW480 tumour cell lines using Cis-platin (IC50 values ranged from 2.256 to 23.96 μM), and Taxol (IC50 values ranged from <0.008 to 0.196 μM), as positive controls. Whilst 336 and 338, displayed no cytotoxicity against all the examined cells, the rest of compounds exhibited weak to potent cytotoxicity with IC50 values ranging from 9.59 to 30.31 μM.
Additionally the antimicrobial activity of these compounds was evaluated against E. coli and C. albicans using Kanamycin (MIC 2.0 μg mL−1), and Nystatin (MIC 1.0 μg mL−1), as positive controls, respectively. Only, 336 and 338, displayed weak antimicrobial activity against E. coli and C. albicans with MIC values of 8 and 32 μg mL−1, respectively.189
3.5.3.1.2. Fungi of the family Diatrypaceae. 3.5.3.1.2.1. Genus Eutypella (MycoBank ID 1951). Chemical investigation of the soil derived fungus Eutypella sp. D-1, isolated from the soil sample collected from the District of Alesund, Arctic, led to the isolation of three previously undescribed cytochalasans derivatives namely, cytochalasans Z24 (341), Z25 (342) and Z26 (343), along with the previously described analogue scoparasin B (344) (Fig. 35). When they tested for their cytotoxicity against U251, SW-1990, SG7901, MCF-7, Huh-7, HeLa, and H460 cells. Indeed, 343 was found inactive with IC50 value of >100 μM, 341 exhibited weak to mild cytotoxicity against the examined cells with IC50 values ranging from 9.33 and 73.21 μM, respectively. Regarding 342, it displayed weak selective cytotoxicity against SW-1990 and SG7901 cells with IC50 values of 50.1 and 50.87 μM respectively. For 344, it was found inactive only against H460 cells with IC50 value of 3.9 μM.190,191
3.5.3.1.3. Fungi of the family Hypoxylaceae. 3.5.3.1.3.1. Genus Daldinia (MycoBank ID 1408). Chemical inspection of the EtOAc extract obtained from Daldinia concentrica fruit bodies, isolated from Fraxinus excelsior trunks collected in North Rhine Westphalia, Germany, in the Neandertal near Haan-Gruiten, resulted in the isolation of the previously mentioned analogue 110.192
Chemical examination of the South China Sea derived fungus D. eschscholtzii HJ001, isolated from the mangrove Brguiera sexangula var.rhynchopetala, led to the isolation of one previously unreported cytochalasan analogue namely [11]-cytochalasa-5(6),13-diene-1,21-dione-7,18-dihydroxy-16,18-dimethyl-10-phenyl-(7S*,13E,16S*,18R*) (345), along with its previously reported analogue [11]-cytochalasa-6(12),13-diene-1,21-dione-7,18-dihydroxy 16,18-dimethyl-10-phenyl-(7S*,13E,16S*,18R*) (346), (Fig. 36). Both 345–346 were examined for their cytotoxic effects against HepG2, A549, and HeLa tumour cell lines using MTT method and Epirubicin as positive control, both compounds displayed no cytotoxicity towards the tested cell lines. Furthermore, both compounds were tested for their antibacterial effect against the pathogenic bacterial strains B. cereus (ATCC 11778), E. coli (ATCC 25922), S. aureus (ATCC 25923), Methicillin-resistant S. aureus MRSA (ATCC 33591), V. alginolyticus (ATCC 17749), and V. parahaemolyticus (ATCC 17802), using Ciprofloxacin as positive control, among them 345, displayed weak antibacterial effect against B. cereus, E. coli, S. aureus, V. alginolyticus and V. parahaemolyticus with MIC values of 50 μg mL−1.193
Chemical investigation of the stomata of the sugarcane derived fungus D. sacchari, led to the isolation of two novel cytochalasans analogues namely saccalasins A (347) and B (348), along with the previously described (16S,17R,18S)-16,18-dimethyl-17-hydroxy-10-phenyl[11]cytochalasa-6,13,19-triene 1,21-dione (349) (Fig. 36), together with the previously mentioned analogue 328. Compounds 328, and 347–349, were tested for their antimicrobial effect against several bacterial and fungal strains. Whilst 347 was found to be inactive against all the examined microorganisms, the rest of the compounds displayed antimicrobial effect against Mucor hiemalis DSM2656 using Nystatin (MIC 16.66 μg mL−1) as positive control, Bacillus subtilis DSM10, Staphylococcus aureus DSM346 using Oxytetracycline (MIC values of 8.33 and 0.41 μg mL−1, respectively) as positive control, with MIC value ranged from 16.66 to 66.66 μg mL−1. Additionally, 328 and 349 displayed mild to potent antimicrobial ability against Schizosaccharomyces pombe DSM70572, when compared to Nystatin as positive control with MIC values of 33.33, 16.66 and 16.66 μg mL−1, respectively.
Moreover, only 349 showed weak antibacterial effect against Micrococcus luteus DSM1790, when compared to the positive control Oxytetracycline with MIC values of 66.66 and 0.41 μg mL−1, respectively. Furthermore, the isolated compounds were tested for their cytotoxic effects towards A431, A549, KB3.1, L929, MCF-7, PC-3 and SKOV-3 tumour cell lines using the positive control Epothilon B (IC50 values of 0.0001, 0.002, 0.00006, 0.0008, 0.00004, 0.0011, and 0.00012 μg mL−1, respectively), where all the isolated compounds displayed moderate to strong cytotoxic effect against all the examined cells with IC50 values ranged from 0.14 to 6.1 μg mL−1.194
The previously mentioned 349, along with four previously reported analogues (350–353) (Fig. 36), were isolated from the stomata of D. eschscholtzii BBH42278. The isolated compounds along with previously mentioned 347, were examined for their antibacterial effect against Staphylococcus aureus, as well as to check their ability to inhibit the biofilm formation. Despite they displayed weak antibacterial effect with MIC values of >256 μg mL−1, 351 showed no effect against the biofilm formation, 347 and 349 showed mild effect toward the biofilm formation with 20–40% inhibition effect, and interestingly 350, 352 and 353, showed strong inhibition effect against the biofilm formation with potency effect of 70–90%.195
Daldinin (354), a previously undescribed cytochalasin analogue, along with the previously described [11]-cytochalasa-6(12),13-diene-1,21-dione-7,18,19-trihy droxy-16,18-dimethyl-10-phenyl-(7S*,13E,16S*,18S*,19R*) (355) and the previously mentioned (346) (Fig. 36), were isolated from the organic extract of D. concentrica fruiting bodies, collected in Vietnam from the Pumat National Park of Nghe, compounds 346, 354 and 355, were tested for their cytotoxic effects against SK-LU-1, HepG2, Hep3B, SW480, and MCF7 cancer cell lines using Ellipticine (IC50 values of 0.4, 0.4, 0.4, 0.5, and 0.4 μM, respectively) as positive control, all of them displayed weak to mild cytotoxic effect against the tested cells with IC50 values ranged from 11.4 to 58.2 μM.196
Kretz et al.,124 2019, examined the previously mentioned analogues 347, 349, and 350–353, along with (7S,13E,16S,18S,19E)16,18-dimethyl-6,7-epoxy-18-hydroxy-10-phenyl-[11]-cytochalasa–13,19-diene-1,21-dione (356), isolated from D. eschscholtzii, as well as phenochalasins C (357) and D (358) (Fig. 36), obtained from D. kretzschmarioides, which originally described as Hypoxylon kretzschmarioides,195 based on the taxonomic study by Wongkanoun et al.197 While 352–353, and 356 showed the ability to disrupt the F-actin network incompletely at a concentration of 5 μg mL−1, 351 and 357 caused complete disruption at 5 μg mL−1, and 350 caused complete disruption at 1 μg mL−1.124
3.5.3.1.3.2. Genus Hypoxylon (MycoBank ID 2456). Chemical investigation of the fungus Hypoxylon fragiforme, led to the isolation of the previously mentioned derivative 116, along with two previously undescribed analogues namely fragiformins A (359) and B (360) (Fig. 37). The discovered compounds along with the previously mentioned derivatives 2, 14, 110, 117, 199, 346, 350 and 355–365, were tested for their nematocidal effect against Caenorhabditis elegans. All compounds displayed antinematode effect with LD90 and LD50 of ranged from 10 to >100. Additionally, the above-mentioned compounds were examined for their antimicrobial effect against B. subtilis, Y. lipolytica, M. hiemalis, P. griseofulvum, S. chartarum, T. atroviride, and T. harzianum where all of them showed weak to potent effect with MIC values ranged from 1 to 50 μg mL−1.198
The previously mentioned analogues 116, 359 and 360, were isolated from H. howeanum, where these compounds were evaluated for their antibacterial effect against B. subtilis, using Penicillin G (inhibition zone of 23 mm) as positive control, where they showed antibacterial effect with an inhibition zone of 15, 16 and 18 mm, respectively. Additionally, they were tested for their antifungal effect against Y. lipolytica using Actinomycin D (inhibition zone of 17 mm), as positive control where they showed effect with an inhibition zone of 18, 19 and 21 mm, respectively; interestingly, all of them were found to be inactive when they were undergoes for MIC evaluation against B. subtilis, T. atroviride, and Y. lipolytica. Furthermore, none of the obtained compounds showed nematocidal effect against Caenorhabditis elegans.199
Chemical investigation of the fungus H. fragiforme, collected from Harz Mountains, led to the isolation of the previously mentioned analogues 116, and 123, along with the previously described L-696,474 (361), as well as the previously mentioned 357 and 358, that were isolated from H. cf. kretzschmarioides BBH42276. The isolated compounds were examined for their antibacterial effect against Staphylococcus aureus, as well as to check their ability to inhibit the biofilm formation, even though they displayed weak antibacterial effect with MIC values of >256 μg mL−1, while compound 116 displayed no effect toward biofilm formation, the rest of the compounds displayed mild effect toward the biofilm formation with potency of 20–70%.195 Fragiformins C (362) and D (363), (Fig. 37), along with the previously mentioned 346, were obtained through the chemical investigation of H. fragiforme, isolated from Fagus sylvatica. While 362 caused a partial reversible and incomplete actin disruption at 5 μg mL−1, 363 caused a complete irreversible disruption at 1 μg mL−1.124 Chemical examination of H. fuscum complex, led to the isolation of pseudofuscochalasin A (364), a structurally related analogue to cytochalasin C (365) (Fig. 37). Compound 364, when compared to cytochalasin C (365) in an actin disruption assay using fluorescence microscopy of human osteosarcoma U2OS cells, demonstrated comparable activity towards F-actin, but its effect was irreversible, in contrast to cytochalasin C (365).200
3.5.3.1.4. Fungi of the family Xylariaceae. 3.5.3.1.4.1. Genus Rosellinia (MycoBank ID 4785). The previously mentioned analogue 365, along with three previously reported analogues namely cytochalasin D (366), 19,20-epoxycytochalasin D (367), and 19,20-epoxycytochalasin C (368), together with the previously unreported jammosporin A (369) (Fig. 38), were isolated from the medicinal plant derived fungus Rosellinia sanctae-cruciana, isolated from Albizia lebbeck leaves, collected in India, Jammu. All the compounds as well as the crude extract were examined for their cytotoxicity against MOLT-4, A549, MIAPaCa-2, and MDAMB-231 cancer cell lines, all of them displayed selective cytotoxicity against MOLT-4 tumour cell line with IC50 values of 6, 25, 10, 8, and 20 μmol L−1, respectively and 10 μg mL−1 for the crude extract, when compared to Flavopiridol (0.5 μmol L−1).201
The previously mentioned cytochalasin analogues 367 and 368, along with two previously reported derivatives namely 19,20-epoxycytochalasin N (370) and 18-deoxy-19,20-epoxycytochalasin Q (371) (Fig. 38) were isolated from the culture of R. rickii. Compounds 367–368 and 370–371 were examined for their antibacterial effect against Staphylococcus aureus, as well as to check their ability to inhibit the biofilm formation. All of them displayed weak antibacterial effect with MIC values of >256 μg mL−1, and only 368, showed a mild effect toward the biofilm formation with potency of 40–70%.195 Through semipreparative HPLC, seven cytochalasans were isolated from the crude extract of R. sanctae-cruciana, including the previously mentioned 365, 366, 367, and 368 along with other three analogues i.e., oxidized 19,20-epoxycytochalasin C (372), deacetyl 19,20-epoxycytochalasin C (373), and cytochalasin P1 (374) (Fig. 38). The isolated compounds were examined for their antitumour activity against FR-2, A549, PC3, HCT-116, MCF-7, HT-29, and SW-620 tumour cell lines, where all of them showed cytotoxicity against the different tested cells with IC50 values ranged from 0.658 to >100 μM, respectively.
Additionally, 368, were tested in vivo against colon induced cancer, where it was found to be an effective agent with IC50 value of 866 nM, when compared to Flavopiridol (IC50; 290 nM), as positive control.202 Further examination of the same fungus by the same group led to the successful isolation of the previously undescribed optical isomer of compound 370, namely 19,20-epoxycytochalasin N1 (375) (Fig. 38). Compound 375 displayed weak to potent cytotoxicity against FR-2, A549, PC3, HCT-116, MCF-7, HT-29, and SW-620 tumour cell lines with IC50 values ranged from 1.34 to 36.85 μM, when compared to Paclitaxel (IC50 < 0.01–0.140 μM) and 5-Fluorouracil (IC50 14.76–82.49 μM) as positive controls.203
3.5.3.1.4.2. Genus Dematophora (MycoBank ID 216282). Chemical examination of Dematophora necatrix (MUCL 57709), led to the isolation of cytochalasin E (376) and its related analogue Δ6,12-cytochalasin E (377) (Fig. 39), along with their previously mentioned analogue 329. Compounds 329 and 376–377 were examined for their antimicrobial effect against a wide panel of microorganisms, among them only compound 376, showed an inhibition effect against Saccharomyces pombe, with MIC value of 16.8 μM. Additionally, the obtained compounds were tested for their cytotoxic effects towards L929, KB3.1, PC-3, MCF-7, SKOV-3, A431 and A549, where all of them displayed moderate effect against all the examined cells, with IC50 values ranged from 0.026 and 14.5 μM. Furthermore, they were examined for their F-actin network disruptors, where compound 376 and 377 caused collapse of the F-actin network, totally at a concentration of >0.3 μg mL−1, while compound 329, showed its effect only at a higher concentration of 10 μg mL−1.204
3.5.3.1.4.3. Genus Xylaria (MycoBank ID 5832). Cytochalasans Z27 (378), and Z28 (379), two previously undescribed analogues along with two previously reported analogues namely cytochalasin Z18 (380), and seco-cytochalasin E (381) (Fig. 40), together with the previously mentioned analogue 376, were isolated from the EtOAc extract of endophytic fungus Xylaria sp. XC16 isolated from Toona sinensis healthy leaves, collected in China from Shaanxi Province. All the obtained compounds were tested for their toxicity against the brine shrimp, where all compounds displayed very weak toxic effect with lethality below 10%. Apart from 376, it showed strong toxic effect with lethality of 100%, at a concentration of 50 μM, in a concentration dependent manner when compared to Toosendanin as positive control.
Additionally, the abovementioned compounds were examined for their phytotoxicity towards Lactuca sativa and Raphanus sativus, seedling growth. While 378–381, were inactive at a concentration of 100 μM, 376, showed strong phytotoxicity even was higher than that of the positive control Glyphosate. Furthermore, they were examined for their activity against four different pathogens, including Gibberella saubinetti, Fusarium solani, Alternaria solani and Botrytis cinerea, where they showed weak to mild effect with MIC values ranged from 12.5 to >100 μM, when compared to Carbendazim (MIC 3.13–6.25 μM) and Hymexazol (MIC 12.5–50 μM), as positive controls.205
Curtachalasins A–B (382–383) (Fig. 40), two previously undescribed cytochalasans, possessing an unusual pyrrolidine/perhydroanthracene tetracyclic ring system, were reported from the potato derived fungus X. curta E10, isolated from the stem tissues collected in China, Dali Province. Both compounds displayed weak antifungal activity against Microsporum gypseum, with inhibition percentage of 70.3 and 68.4%, respectively.206
A chemical examination of the plant derived fungus X. striata, isolated from Sophora japonica trunk base, collected from Sichuan Province, (China) afforded the isolation of a previously unreported xylastriasan A (384) (Fig. 40), a cytochalasin featuring an unusual pentacyclic ring system. Compound 384 displayed weak cytotoxicity against HEPG2, A549, and B16 cells with IC50 values of 93.61, 91.58 and 85.61 μM, respectively when compared to Paclitaxel as positive control.207 Two previously undescribed cytochalasan derivatives namely, cytochalasans D1 (385) and C1 (386) (Fig. 40), were reported from the potato derived fungus X. cf. curta, isolated from Solanum tuberosum stem tissue. Both 385–386 displayed mild cytotoxicity against HL-60 cancer cells with IC50 values of 12.7 and 22.3 μM, respectively. Additionally, only 385 showed a weak cytotoxicity against SMMC-7721 with IC50 value of 37.2 μM,208 when compared to Cis-platin (IC50 values of 4.86 and 25.61 μM, respectively) as positive control.
Further examination of the same fungal strain by the same group, afforded isolation of a previously unreported halogenated hexacyclic cytochalasan, namely xylarichalasin A (387) (Fig. 40). it showed moderate to potent cytotoxicity against HL-60, A549, SMMC-7721, MCF-7, and SW480 cells with IC50 values of 17.3, 11.8, 8.6, 6.3 and 13.2 μM, respectively,209 when compared to Cis-platin (IC50 values of 2.0, 13.2, 12.7, 23.3 and 18.0 μM, respectively) as positive control. The previously mentioned analogues 181, 182, 365, and 366 along with the previously described cytochalasans P (388), 7-O-acetylcytochalasin D (389), and cytochalasin C (390), together with five previously undescribed analogues namely 6,12-epoxycytochalasin D (391), 6-epi-cytochalasin P (392), 7-O-acetylcytochalasin P (393), 7-oxo-cytochalasin C (394) and 12-hydroxylcytochalasin Q (395) (Fig. 40), were reported from mountain derived fungus X. longipes, isolated from a sample collected from the mountain of Ailao, China. The abovementioned compounds were examined for their cytotoxicity against HL-60, A549 SMMC-7721, MCF-7, and SW480 cells (IC50 values of 4.86, 25.61, 21.13, 23.59 and 27.7 μM, respectively) using Cis-platin as positive control. Potentially, 181, 182, 366, 388 and 390 displayed selective cytotoxicity with IC50 values 4.17–37.18 μM.210
A chemical inspection of the plant derived fungus X. cf. curta E10, isolated from Solanum tuberosum stem tissues, collected from Yunnan Province (China), afforded the isolation of three previously undescribed cytochalasan analogue, featuring an unusual bridged 6/6/6/6 ring fused system, namely curtachalasins C–E (396–398) (Fig. 41). Compounds 396 and 398, were examined for their antifungal effect against fluconazole-resistant C. albicans, whereas 396 displayed significant improvement when compared to Fluconazole as positive control. On the contrary, 398 exhibited weak antifungal effect.211 Chemical inspection of the potato derived fungus X. cf. curta, isolated from Solanum tuberosum steam tissue, resulted in the isolation of nine previously undescribed cytochalasans namely 19-epi-cytochalasin P1 (399), 6-epi-19,20-epoxycytochalasin P (400), 7-O-acetyl-6-epi-19,20-epoxycytochalasin P (401), 7-O-acetyl 19-epi-cytochalasin P1 (402), 6-O-acetyl-6-epi-19,20-epox cytochalasin P (403), 7-O-acetyl-19,20-epoxycytochalasin C (404), 7-O-acetyl 19,20-epoxycytochalasin C (exomethylene analogue) (405), deacetyl-5,6-dihydro-7-oxo-19,20-epoxycytochalasin C (406), and 18-deoxy-21-oxo-deacetyl-19,20-epoxycytochalasin N (407) along with two previously described analogues namely 18-desoxy-19,20 epoxycytochalasin C (408) and 5,6-dihydro-7-oxo-19,20-epoxycytochalasin C (409), (Fig. 41) together with the previously mentioned derivatives 367 and 368. All the abovementioned compounds were tested for their cytotoxicity against five cancer cell lines HL-60, A549, SMM-7721 MCF-7 and SW480 using (IC50 values of 4.86, 25.61, 21.13, 23.59, and 27.70 μM, respectively) Cis-platin as positive control. Among them, 368, 399, 401, 405, and 409 displayed cytotoxic effect against HL-60 tumour cells with IC50 values of 1.11, 13.31, 37.16, 25.83, and 10.04 μM, respectively. Additionally, 401 and 405 showed weak cytotoxicity towards MCF-7 cell lines with IC50 values of 26.64, and 34.03 μM, respectively.212 da Silva et al., examined the crude extract of the endophytic fungus Xylaria sp, isolated from Turnera ulmifolia flowers collected Manaus, Brazil, that resulted in the isolation of the previously described cytochalasin Q (410) (Fig. 41), along with the previously mentioned analogues 2, 252, and 367.213 Four previously unreported cytochalasans namely arbuschalasins A–D (411–414), along with the previously 13,14-epoxid-cytochalasin D (415), and cytochalasin O (416), and (Fig. 41), together with the previously mentioned 181, 182, 183, 355, 365, 366, 367, 368, 388, 390, 395, were reported from the mangrove derived fungus X. arbuscula GZS74, isolated from Bruguiera gymnorrhiza fruits, collected from Guangzhou Province, China. All the isolated compounds were examined for their cytotoxicity against HCT-15 cells. Whilst 411–414, displayed weak cytotoxic effect with 50% inhibition rate at concentration of 100 μM, 181, 183, 365–368, 388, 390, 410 and 415–146, displayed moderate cytotoxicity with IC50 values ranging from 50 and 100 μM. Moreover, 182 and 395, exhibited potent cytotoxicity with IC50 values of 13.5 and 13.4 μM, respectively when compared with the positive control 5-Fluorouracil (IC50 103.1 μM).214
A chemical investigation of the plant derived fungus Xylaria sp. WH2D4, isolated from Palicourea elata leaves collected in Costa Rica, afforded the isolation of four previously undescribed cytochalasans analogues namely lagambasines A–D (417–420) (Fig. 42). It is worth noting that 418–419 were obtained as an inseparable mixture. None of them exhibited antifungal ability against Trichoderma longibrachiatum UAMH 7956 and T. reesei QM6a, when compared to Hygromycin B as positive control.215
The previously undescribed karyochalasin (421), along with three previously reported analogues namely deacetyl 19,20-epoxycytochalasin C (422), engleromycin (423), and 19,20-epoxycytochalasin Q (424) (Fig. 42), together with the previously mentioned derivatives 367, 368, 370, 374, 400 and 409, were isolated from the culture of X. karyophthora NRRL 66613. These compounds were tested for their antimicrobial ability against a several microorganisms, among them only compounds 367–368 displayed weak effect towards S. pombe (MIC values of 127 and 64 μM, respectively), while 367 showed weak effect against B. subtilis (MIC value of 127 μM) and 368 showed weak effect against C. albicans (MIC value of 127 μM).216
Additionally, the authors studied and evaluated the ability of these compounds to inhibit biofilm formation and disperse preformed biofilms produced by S. aureus and C. albicans. Compounds 367–368, 409, and 424 were effective in inhibiting and dispersing biofilms of both organisms at varying concentrations, with no lethal effects observed, as their concentrations were below the toxicity levels determined in the MIC assay. Compound 421 showed activity only in dispersing preformed biofilms of C. albicans, while 400 was inactive in both biofilm inhibition and dispersal. None of the compounds exhibited significant activity against P. aeruginosa biofilms.216
Furthermore, the authors evaluated the cytotoxic effects of all compounds against a panel of murine (fibroblast) and human cancer cell lines. The bioactivity showed variability, with 370, 374, 400, and 421 displaying no or weak cytotoxicity against L929 fibroblasts (>25 μM). Cytotoxicity against other human cell lines was weak (2, 20.3 μM against KB3.1 cells) or moderately reduced compared to other cytochalasans (1 and 3 vs. 5 and 10, 3.5 and 3.4 μM vs. 0.1 and 0.5 μM against KB3.1 cells). Interestingly, 367–368 and 422–424 exhibited the strongest overall cytotoxicity.216
Moreover, these compounds showed induced changes to the F-actin network, with the severity of these changes correlating with their cytotoxicity in the MTT assay (except for 370 and 421). At low doses, 367–368, 409, and 424 caused the loss of lamellipodia and the formation of F-actin-rich aggregates in the cell body. High-dose treatments led to complete collapse of the F-actin network, characterized by the reduction of stress fibers, retraction of the cell periphery, and the formation of knot-like aggregates resembling a tree root network. Indeed, cytochalasans 400, 409, and 421 had less pronounced effects, even at high doses, with 400 and 421 showing no visible difference compared to the DMSO control. In contrast, 367 exhibited a high-dose-like effect even at low doses. Compound 423 showed a stronger impact on F-actin organization than 368, 370, 409, 422, and 424, even at lower concentrations, causing the loss of visible stress fibers and F-actin aggregation. Finally, recovery experiments demonstrated that all compounds tested 367–368, 370, 409 and 422–424 fully reversed the F-actin network disruption after high-dose treatment for 1 hour, with a recovery time of 1 hour.216
4. Pharmacokinetics and ADMET of cytochalasans derived from fungi
Overall, a list of 424 cytochalasans were characterized from 27 fungal genera. These include ten major genera, namely Arthrinium, Aspergillus, Chaetomium, Daldinia, Endothia, Metarhizium, Phoma, Phomopsis, Trichoderma, and Xylaria. In addition to these ten genera, another class called “Others” was created, comprising the remaining 17 minority genera, namely Boeremia, Botryotinia, Calonectria, Cytospora, Dematophora, Eutypella, Hypoxylon, Penicillium, Perenniporia, Periconia, Pyrenophora, Rosellinia, Sparticola, Spicaria, Stachybotrys, Talaromyces, and Westerdykella. The identified fungal-derived cytochalasans had comprised diverse biological and pharmacological properties including anticancer, antiviral, antimicrobial, antioxidant, and anti-inflammatory activity. However, the biological activity of approximately 17% of reported cytochalasan derivatives has not been assessed. To assess the pharmacokinetics and ADMET (absorption, distribution, metabolism, excretion, and toxicity) properties of these 424 cytochalasans, two free online tools were used, SwissADME (http://www.swissadme.ch/) and pkCSM (https://biosig.lab.uq.edu.au/pkcsm/).217,218 Of these 424 cytochalasans, it was only possible to predict these properties for 410 cytochalasan derivatives, the remaining 14 derivatives gave errors in both tools (Table S1 in the ESI†). Here, we intended to highlight their potential as drug candidates by exploiting their drug-likeness using several molecular descriptors, including five drug-likeness rules such as Muegge, Ghose, Veber, Egan, and Lipinski (Fig. 43, 44 and Table S1†). Its pharmacokinetic properties and ADMET will also be explored with greater focus on predicting its toxicity (Table S1†). Its pharmacokinetic properties and ADMET will also be explored with greater focus on predicting its toxicity (Table S1†). There were 69 cytochalasans that satisfied all the parameters of the five tested drug-likeness rules (2, 17, 1, 4, 14, 4, and 27 cytochalasans from Arthrinium, Aspergillus, Endothia, Phomopsis, Trichoderma, Xylaria, and Others, respectively). On the other hand, there were 22 cytochalasans that violated the five tested drug-likeness rules (2–4 and 6–7 cytochalasans from Aspergillus, Chaetomium, Phoma, Xylaria, and Others, respectively), see (Fig. 43 and 44). In each of the five tested drug-likeness rules, ranges were established for each of the physicochemical properties established in each of the drug-likeness rules, for example topological polar surface area (TPSA) which is the sum of the surfaces of all the polar atoms present in a molecule, appears in three of the five tested drug-likeness rules namely Muegge, Veber and Egan. TPSA appears to be related to the potential of a compound to penetrate cell membranes and the blood–brain barrier. Egan, Veber and Muegge rules highlighted that those compounds with TPSA less than or equal to 130 Å2, 140 Å2 and 150 Å2, respectively, tend to be well absorbed and able to reach their molecular target within cells. All cytochalasans from Arthrinium, Daldinia, Endothia, Metarhizium, Phomopsis and Trichoderma complied the Egan, Veber and Muegge drug-likeness rules in terms of TPSA and therefore had TPSA less than or equal to 130 Å2 (Fig. 43).Conversely, 12%, 31% and 10% of cytochalasans derived from Aspergillus, Phoma and Xylaria, respectively, fail to satisfy the Egan, Veber and Muegge drug-likeness rules with respect to TPSA (Fig. 43). Octanol–water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P), another measure, is related to the drug lipophilicity, which is a key property for solubility, absorption, membrane penetration, plasma protein binding, distribution, and tissue penetration. log
P), another measure, is related to the drug lipophilicity, which is a key property for solubility, absorption, membrane penetration, plasma protein binding, distribution, and tissue penetration. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P appears in four of the five tested drug-likeness rules namely Muegge, Ghose, Egan and Lipinski. All cytochalasans from Arthrinium, Endothia, Metarhizium, Trichoderma, and Xylaria complied with the Muegge, Ghose, Egan and Lipinski drug-likeness rules in terms of log
P appears in four of the five tested drug-likeness rules namely Muegge, Ghose, Egan and Lipinski. All cytochalasans from Arthrinium, Endothia, Metarhizium, Trichoderma, and Xylaria complied with the Muegge, Ghose, Egan and Lipinski drug-likeness rules in terms of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, which is not greater than 5 (Fig. 43). Some cytochalasan derivatives from Aspergillus, Chaetomium, Daldinia, Phoma, Phomopsis and Others do not comply with the drug-likeness rules of Muegge, Ghose, Egan and Lipinski in terms of log
P, which is not greater than 5 (Fig. 43). Some cytochalasan derivatives from Aspergillus, Chaetomium, Daldinia, Phoma, Phomopsis and Others do not comply with the drug-likeness rules of Muegge, Ghose, Egan and Lipinski in terms of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P (Fig. 43), e.g. seven [aspochalamins A–D (57–60)] and [bisaspochalasin A–C (83–85)], twenty six some examples, [armochaetoglobin A (232)], [isochaetoglobosin J (243)], [armochaetoglobins N–R (247–251)], penochalasin B (252), [prochaetoglobosins I–II (260–261)], armochaetoglasin C (275), [armochaetoglosins A–C (284–285)], [chaetoglobosin T (287)], one [saccalasin A (347)], one [thiocytochalasin A (27)], one [18-deoxycytochalasin H (7, L-696,474) (146)], and one cytochalasin Z1 (41) cytochalasan derivatives violate Lipinski's drug-likeness rule in terms of log
P (Fig. 43), e.g. seven [aspochalamins A–D (57–60)] and [bisaspochalasin A–C (83–85)], twenty six some examples, [armochaetoglobin A (232)], [isochaetoglobosin J (243)], [armochaetoglobins N–R (247–251)], penochalasin B (252), [prochaetoglobosins I–II (260–261)], armochaetoglasin C (275), [armochaetoglosins A–C (284–285)], [chaetoglobosin T (287)], one [saccalasin A (347)], one [thiocytochalasin A (27)], one [18-deoxycytochalasin H (7, L-696,474) (146)], and one cytochalasin Z1 (41) cytochalasan derivatives violate Lipinski's drug-likeness rule in terms of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, respectively (Fig. 43). The oral bioavailability, bioavailability score (BS), is another parameter that indicates the possibility of a compound to be more than 10% bioavailable in the absorption assays. Molecules complying with the Lipinski's drug-likeness rule and with a BS of 0.55 are considered orally bioavailable. There are 222 cytochalasan derivatives (17, 31, 10, 10, 26, 18, 6, 13, 14, 16, and 61), which showed a BS of 0.55 and with no Lipinski's drug-likeness rule violation from Arthrinium, Aspergillus, Chaetomium, Daldinia, Endothia, Metarhizium, Phoma, Phomopsis, Trichoderma, Xylaria, and Others, respectively. All Arthrinium, Metarhizium and Trichoderma derived cytochalasans showed a BS of 0.55 and no Lipinski's rule failure. Three cytochalasan derivatives, phomopsichalasin F (148) and curtachalasins C–D (396–397) from Phomopsis and Xylaria, respectively, are of special interest as they showed a good BS of 0.56 and no Lipinski's rule failure. The curtachalasins C–D (396–397) are particularly interesting as they exhibit the unusual bridged 6/6/6/6 ring fused system (Fig. 41). The water solubility, log
P, respectively (Fig. 43). The oral bioavailability, bioavailability score (BS), is another parameter that indicates the possibility of a compound to be more than 10% bioavailable in the absorption assays. Molecules complying with the Lipinski's drug-likeness rule and with a BS of 0.55 are considered orally bioavailable. There are 222 cytochalasan derivatives (17, 31, 10, 10, 26, 18, 6, 13, 14, 16, and 61), which showed a BS of 0.55 and with no Lipinski's drug-likeness rule violation from Arthrinium, Aspergillus, Chaetomium, Daldinia, Endothia, Metarhizium, Phoma, Phomopsis, Trichoderma, Xylaria, and Others, respectively. All Arthrinium, Metarhizium and Trichoderma derived cytochalasans showed a BS of 0.55 and no Lipinski's rule failure. Three cytochalasan derivatives, phomopsichalasin F (148) and curtachalasins C–D (396–397) from Phomopsis and Xylaria, respectively, are of special interest as they showed a good BS of 0.56 and no Lipinski's rule failure. The curtachalasins C–D (396–397) are particularly interesting as they exhibit the unusual bridged 6/6/6/6 ring fused system (Fig. 41). The water solubility, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S, is another essential measure for drug bioavailability. Although all cytochalasans were predicted to have adequate water solubility characteristics, there were different solubility orders as Chaetomium derived cytochalasans were the most soluble (mean value of −3.82), followed by Endothia (mean value of −3.94), but Arthrinium were the most poorly soluble (mean value of −4.95) (Table S1†). Intestinal absorption (human), blood–brain barrier (BBB) permeability, P-glycoprotein (P-gp) substrate and P-gp I and II inhibition potential were also surveyed to draw insights into the pharmacokinetic behaviour of the reviewed cytochalasans. Only twenty cytochalasans from Aspergillus (1), Chaetomium (2), Daldinia (1), Endothia (2), Phomopsis (1), Trichoderma (2), Xylaria (4) and Others (7) showed high intestinal absorption, passively crossed BBB and no potential for P-gp inhibition (Table S1†), with Xylaria lagambasines A–C (417–419) emerging as particularly noteworthy. Some toxicological properties were also evaluated, such as AMES toxicity (related to mutagenicity), hepatotoxicity, hERG I and II inhibition (related to cardio toxicity), and skin sensitisation. A total of 106 cytochalasan derivatives were predicted without any toxicity alert for the parameters evaluated, 4, 11, 9, 4, 8, 2, 3, 6, 12, 19, and 28 cytochalasans from Arthrinium, Aspergillus, Chaetomium, Daldinia, Endothia, Metarhizium, Phoma, Phomopsis, Trichoderma, Xylaria, and Others, respectively (Table S1†). Surprisingly, the three cytochalasans, lagambasines A–C (417–419), obeyed all the surveyed parameters (five drug-likeness rules, log
S, is another essential measure for drug bioavailability. Although all cytochalasans were predicted to have adequate water solubility characteristics, there were different solubility orders as Chaetomium derived cytochalasans were the most soluble (mean value of −3.82), followed by Endothia (mean value of −3.94), but Arthrinium were the most poorly soluble (mean value of −4.95) (Table S1†). Intestinal absorption (human), blood–brain barrier (BBB) permeability, P-glycoprotein (P-gp) substrate and P-gp I and II inhibition potential were also surveyed to draw insights into the pharmacokinetic behaviour of the reviewed cytochalasans. Only twenty cytochalasans from Aspergillus (1), Chaetomium (2), Daldinia (1), Endothia (2), Phomopsis (1), Trichoderma (2), Xylaria (4) and Others (7) showed high intestinal absorption, passively crossed BBB and no potential for P-gp inhibition (Table S1†), with Xylaria lagambasines A–C (417–419) emerging as particularly noteworthy. Some toxicological properties were also evaluated, such as AMES toxicity (related to mutagenicity), hepatotoxicity, hERG I and II inhibition (related to cardio toxicity), and skin sensitisation. A total of 106 cytochalasan derivatives were predicted without any toxicity alert for the parameters evaluated, 4, 11, 9, 4, 8, 2, 3, 6, 12, 19, and 28 cytochalasans from Arthrinium, Aspergillus, Chaetomium, Daldinia, Endothia, Metarhizium, Phoma, Phomopsis, Trichoderma, Xylaria, and Others, respectively (Table S1†). Surprisingly, the three cytochalasans, lagambasines A–C (417–419), obeyed all the surveyed parameters (five drug-likeness rules, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S, intestinal absorption, BBB, Pgp, AMES toxicity, hepatotoxicity, hERG I and II inhibition, and skin sensitisation).
S, intestinal absorption, BBB, Pgp, AMES toxicity, hepatotoxicity, hERG I and II inhibition, and skin sensitisation).
5. Applications of cytochalasans in imaging and drug discovery
In the vast realm of biomedical research, cytochalasans, a distinctive group of natural compounds derived from fungi have shown significant potential in various applications, including imaging and drug discovery. Renowned for their diverse biological activities and unique chemical structures, cytochalasans have not only demonstrated their efficacy as antitumor and antimicrobial agents but have also opened new horizons in the realms of imaging and drug discovery. Herein we summarize whenever applicable the application of different members of cytochalasans in imaging, drug discovery and biomedical applications.5.1. Cytochalasans as illuminating probes
Indeed, within advances in the intricate world of cellular and molecular imaging, cytochalasans have emerged as captivating tools, owing to their inherent fluorescent and structural properties. Their unique molecular characteristics enable them to serve as remarkable imaging probes, shedding light on various biological processes. In imaging, cytochalasans have been utilized as fluorescent probes due to their ability to selectively label specific cellular structures or proteins, enabling the visualization and tracking of biological processes. Their unique chemical structures and diverse modes of action make cytochalasans valuable naturally occurring molecules for the development of novel therapeutic agents.63,219–2245.2. The epic quest in drug discovery
Driven by their captivating chemical structures and diverse biological activities, cytochalasans have become protagonists in the epic quest for novel therapeutics. Their potential stretches beyond their direct use as therapeutic agents, serving as treasured lead compounds and scaffolds in the pursuit of groundbreaking drugs.63,234–236These compounds were further investigated for their antifungal towards Schizosaccharomyces pombe, Pichia anomala, Mucor hiemalis, Candida albicans, and Rhodotorula glutinis, cytotoxicity against mouse (L929) and human (KB3.1,PC3, A549, MCF-7, A431 and SKOV-3) cell lines, and actin disruption properties in vivo using osteosarcoma U2OS cells. Concerning the antifungal activity, most of the compounds were inactive as antifungal, however the coumarin-based 425 exhibited remarkable activity with IC50 of approximately 5 mM against the opportunistic pathogen C. albicans. However, it showed only moderate or weak activity against the other organisms. With respect to the cytotoxicity, most of the compounds were inactive, however the dimeric analogues exhibited cytotoxicity that surpassed expectations, such as 426 with IC50 of 120 nM against KB3.1 cells. Acetylene 427 and borate 428 also demonstrated relatively good potency, with examples like 428 showing an IC50 of 300 nM against KB3.1 cells. However, the red dyed 429 displayed the most potent cytotoxicity of all, with remarkably low IC50 values as low as 20 nM against the KB3.1 cell line.
Regarding the actin disruption activity, the fluorescence analysis using the coumarin channel indicated that of 425 prominently co-localized with actin aggregates induced at different concentrations. In contrast, the perylene-linked 430 showed a more diffuse localization within cells and less prominent co-localization with F-actin. This difference in localization may be attributed to the increased hydrophobicity of the perylene unit, leading to stronger association with membranes. The specific association of certain actin-inhibitory compounds with actin aggregates during or after cell treatment, as well as their co-localization with phalloidin, suggests that these derivatives could serve as simple staining tools for fixed, non-treated cells. For example, staining with the hydrophilic Texas-red-linked 429 revealed selective binding to F-actin, highlighting key structures of the actin cytoskeleton at sub-micrometre resolution.
Surprisingly, staining with 429 also revealed prominent sub-compartments of the actin cytoskeleton, such as microspikes, stress fibres, and lamellipodia, with staining patterns consistent with those obtained using phalloidin. These findings indicate the continuous distribution of actin filament barbed ends within these structures composed of filaments with variable lengths, assuming the barbed end specificity of actin filament binding by this novel compound.
5.3. Current stage of clinical development of cytochalasans
As of today, the clinical application of cytochalasans is still in its infancy, with most advancements confined to preclinical and early-phase studies. However, the following points summarize the broader clinical development of cytochalasans in different biomedical applications.6. Conclusion, future directions opportunities and challenges
Fungal natural products have played a significant role in the development of therapeutic agents. Many well-known drugs, such as antibiotics, immunosuppressants, anticancers, anti-inflammation, and cholesterol-lowering agents, owe their origins to fungal sources. These compounds have revolutionized medicine and have been instrumental in combating various diseases. Among the extraordinary classes of secondary metabolites are cytochalasans.Cytochalasans are a class of fungal metabolites displaying unique tricyclic core structure consisting of a polyketide-derived 11, 13 or 14-membered macrocyclic ring fused to various aromatic or heteroaromatic rings (e.g. an isoindolone moiety), where they are biosynthesized through polyketide synthase–nonribosomal peptide synthetase (PKS–NRPS) hybrid pathways. These pathways play a pivotal role in constructing the intricate molecular architecture of these metabolites. Indeed, cytochalasans were found to be a powerful natural product to serve not only as a cytotoxic agent but also as antimicrobial, antiviral, anti-inflammatory and immunosuppressive agents. Within this documentation, we tried to present a comprehensive-up-to-date literature review focused on the chemistry of 424 cytochalasans isolated over the period 2000–2023 from different fungal natural sources, along with shedding light on their biosynthetic pathways, pharmacokinetics and ADMET properties, and their application in drug discovery.
Collectively, 424 fungal derived cytochalasans were listed thoroughly by fungal class, subclass, order, family, genera and their different assigned therapeutic activities. Through the statistical analysis of the collected data, it can be concluded that the fungal class (Sordariomycetes) with 165 different cytochalasans analogues considered to be the main source of cytochalasans compounds followed by Eurotiomycetes and Dothideomycetes (Fig. 46). While according to the subclass level, Hypocreomycetidae and Xylariomycetidae represent the richest source of the targeted metabolites followed by Eurotiomycetidae, Pleosporomycetidae and Sordariomycetidae with 167, 110, 58, 52, and 50 different cytochalasan derivatives, respectively (Fig. 47).
Based on the order level fungal orders (Sordariales) and (Xylariales) with 118 and 110 different isolated cytochalasans, respectively, emerges as the richest source of cytochalasan representing 26 and 25%, respectively of the total reported cytochalasans. Furthermore, the fungal orders Diaporthales, Eurotiales, Hypocreales, and Pleosporales, were almost identical in their contribution to the cytochalasans chemistry (Fig. 48).
Based on the family level, the family (Chaetomiaceae) represents the primary source of cytochalasans with 118 different derivatives being isolated comprising 28% of the total reported cytochalasans, followed by (Xylariaceae), (Aspergillaceae), and (Diaporthaceae) with contribution of 16%, 13% and 10% of the recorded cytochalasans, respectively (Fig. 49). The fungal species Chaetomium is emerging as the prevailing source of the reported compounds (Fig. 50), where a total of 118 different cytochalasans were solely isolated from this fungal species representing 25% out of the reported cytochalasans over the specified 23 years-period of the review. Regarding the therapeutic potentials of the discovered cytochalasans, a broad array of multiple biological activities was reported.
These were primarily concerned with cytotoxicity, but they also had antibacterial, antifungal, antiviral, phytotoxic, anti-inflammatory, antimigratory, enzyme inhibitory, immunosuppressive, antagonistic, F-actin network disruptors, nematocidal, and antibiofilm activities. Moreover, it is worthy to emphasise that the cytotoxicity activity was the most profoundly studied biological assay followed by the antibacterial, antifungal and phytotoxic assays.
In total 194 cytochalasans were tested for their cytotoxicity against different tumour cell lines, and a total number of 60, 23, 15, and 14 cytochalasans were evaluated for their antibacterial and antifungal, antiviral, and phytotoxic activities, respectively (Fig. 51). Furthermore, 424 cytochalasan derivatives were extensively investigated for their pharmacokinetics and ADMET using two online platforms, SwissADME and pkCSM, respectively.
Among these, three derivatives, phomopsichalasin F (148) (Fig. 18) and curtachalasins C–D (396–397) (Fig. 41) from Phomopsis and Xylaria, respectively, caught our attention. They boasted a promising bioavailability score of 0.56 and no Lipinski's rule failure (see Table S1†). The curtachalasins C–D (396–397), were particularly interesting, showcasing an uncommon bridged 6/6/6/6 ring fused system (Fig. 41).
Remarkably, lagambasines B (418) and C (419) (Fig. 42) complied with all the assessed parameters. These parameters encompassed five drug-likeness rules, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S values, intestinal absorption, BBB permeability, Pgp interactions, AMES toxicity, hepatotoxicity, hERG I and II inhibition, and skin sensitisation (Table S1†).
S values, intestinal absorption, BBB permeability, Pgp interactions, AMES toxicity, hepatotoxicity, hERG I and II inhibition, and skin sensitisation (Table S1†).
Appealingly, as we shown before that cytochalasans offer promising potential in cancer therapy, immune modulation, and antimicrobial treatments. Their ability to disrupt actin polymerization and inhibit metastasis makes them valuable candidates for combination cancer therapies. Additionally, their antimicrobial properties against bacteria, fungi, and viruses, along with their ability to inhibit biofilm formation and HIV-related pathways, highlight their broad-spectrum potential. Advances in targeted drug delivery systems may further enhance their therapeutic efficacy while reducing side effects. As we continue to unravel the mysteries of cytochalasans, optimizing their structures, elucidating their mechanisms, and refining their formulations, scaling up their production through the transient expression of their biosynthetic routes, we set sail toward a future where these remarkable compounds could transform clinical applications, offering new hope to those in need.
However, despite their biomedical potential, cytochalasans current research faces several challenges, including their off-target effects and cytotoxicity as actin inhibitors, can disrupt essential cellular processes. The complexity of their mechanisms complicates understanding and clinical application. Moreover, the transition from preclinical models to human use is hindered by insufficient clinical data and safety profiles. Regulatory hurdles and the need for extensive clinical trials further delay their development for widespread therapeutic use. Nonetheless, with continued research and refinement, cytochalasans could revolutionize medicine, addressing diseases that are currently difficult to treat.235,257–263
7. Abbreviations
| A2780 | Human ovarian cell line |
| A375 | Human melanoma cell line |
| A431 | Squamous cell carcinoma |
| A549 | Human lung cell line |
| ADMET | Absorption, distribution, metabolism, excretion, and toxicity |
| AspoA | This is an enzyme name – an FMO from the aspergillin PZ pathway |
| AspoD | This is an enzyme name – an SDR from the aspergillin PZ pathway |
| B16F10 | Mouse melanoma cells |
| BBB | Blood–brain barrier |
| BEL-7402 | Human liver cancer cell line |
| BGC-823 | Human gastric cell line |
| BGCs | Biosynthetic gene clusters |
| BS | Bioavailability score |
| BVMO | Baeyer–Villiger monooxygenases |
| CcsB | This is an enzyme name – a BVMO from the cytochalasin E/K pathway |
| CHGG_01242-1 | This is an enzyme name – an FMO from the chaetoglobosin A pathway |
| CT26 | Murine colon carcinoma |
| DA | Diels–Alder |
| EC50 | Half-maximal effective concentration |
| EtOAc | Ethyl acetate |
| FAD | Flavin adenine dinucleotide |
| FMO | Flavin-dependent monooxygenase |
| FNPs | Fungal-derived natural products |
| H292 | Human lung mucoepidermoid carcinoma cells |
| HCT-8 | Human colorectal adenocarcinoma cell line |
| HCT-8/T | Hematocrit |
| HeLa | Human cervical cell line |
| Hep3B | Human hepatoma cell line |
| HepG2 | Human hepatocellular cell line |
| HepG2 | Hepatocellular carcinoma |
| hERG | Human ether-a-go-go-related gene |
| HIV | Human immunodeficiency viruses |
| HL-60 | Human promyelocytic leukemia cells |
| HMO2 | Gastric adenocarcinoma |
| HSV-1 | Herpes simplex virus |
| HT-29 | Human colon carcinoma |
| Huh7 | Hepatocellular carcinoma |
| HUVEC | Human umbilical vein epithelial cell |
| IC50 | Inhibitory concentration that causes a 50% reduction in cell viability |
| K562 | Myelogenous leukaemia |
| KB | Human nasopharyngeal epidermoid tumor |
| KO | Knock-out |
| L929 | Mouse fibroblast |
| LNCaP | Human prostate cancer cells |
| L-NMMA | NG-Monomethyl-L-arginine acetate |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P | Octanol–water partition coefficient |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S S | Water solubility |
| MCF-7 | Human breast cells |
| MDA-MB-231 | Melanoma cell line |
| MeOH | Methanol |
| MFS | Major facilitator superfamily |
| MOLT4 | T lymphoblast cell line |
| MTT assay | Colorimetric assay for assessing cell metabolic activity |
| MW | Molecular weight |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NCI-H187 | Small cell lung cancer |
| NO | LPS-induced nitric oxide |
| NRPS | Nonribosomal peptide synthetase |
| OMeT | O-methyltransferase |
| OXR | Oxidoreductases |
| P388 | Lymphocytic leukaemia |
| P450 | Cytochrome P450 monooxygenases |
| PANC-1 | Human pancreatic cancer cell line |
| PC-3 | Human prostate cancer |
| P-gp | P-glycoprotein |
| PKS | Polyketide synthase |
| PKS–NRPS | Polyketide synthase–nonribosomal peptide synthetase |
| PyiH | This is an enzyme name – an SDR from the pyrichalasin H pathway |
| PyiS | This is an enzyme name – the PKS–NRPS from the pyrichalasin H pathway |
| RSV | Respiratory syncytial virus |
| SDR | Short-chain dehydrogenase |
| SF-268 | Human glioblastoma cell line |
| SGC-7901 | Human gastric cancer cell |
| SKOV-3 | Ovarian carcinoma |
| SMMC-7721 | Human hepatocarcinoma |
| SW480 | Colon cancer |
| TF | Transcription factors |
| TNF-α | Tumour necrosis factor-alpha |
| TPSA | Topological polar surface area |
| trans-ER | trans-acting enoylreductase |
| U87MG | Human glioblastoma cell line |
| α,β-HYD | α,β-hydrolase |
8. Data availability
This is a review article, and no new data were generated or analyzed in this study. All data discussed are based on published literature cited within the manuscript.9. Author contributions
Conceptualization: Amr El-Demerdash. Validation: Amr El-Demerdash. Formal analysis: Mohamed A. Tammam, Florbela Pereira, Elizabeth Skellam and Amr El-Demerdash. Investigation: Mohamed A. Tammam, Florbela Pereira, Elizabeth Skellam, A. Ganesan and Amr El-Demerdash. Resources: Mohamed A. Tammam, Florbela Pereira, Elizabeth Skellam and Amr El-Demerdash. Data curation: Mohamed A. Tammam and Amr El-Demerdash. Writing – original draft: Mohamed A. Tammam, Florbela Pereira, Elizabeth Skellam, Stefan Bidula, A. Ganesan and Amr El-Demerdash. Writing – review & editing: Mohamed A. Tammam, Florbela Pereira, Elizabeth Skellam, Stefan Bidula, A. Ganesan and Amr El-Demerdash.10 Conflicts of interest
There are no conflicts to declare.11. Acknowledgements
Amr El-Demerdash is thankful to his home universities, University of East Anglia (UK) and Mansoura University (Egypt) for the unlimited support, inside and outside. Elizabeth Skellam is thankful for the National Science Foundation (NSF) for a financial support (Grant number 2048347). Mohamed A. Tammam is humbly dedicating this work to the soul of his sister Dr Mai A. Tammam who passed away on 19 March 2022, was always a kind supporter in all aspects of his life.12. References
- A. S. Abdel-Razek, M. E. El-Naggar, A. Allam, O. M. Morsy and S. I. Othman, Processes, 2020, 8, 470 CrossRef CAS.
- H. Bansal, R. K. Singla, S. Behzad, H. Chopra, A. S. Grewal and B. Shen, Curr. Top. Med. Chem., 2021, 21, 2374–2396 CrossRef CAS PubMed.
- I. A. Stringlis, H. Zhang, C. M. J. Pieterse, M. D. Bolton and R. De Jonge, Nat. Prod. Rep., 2018, 35, 410–433 RSC.
- D. J. Kenny and E. P. Balskus, Chem. Soc. Rev., 2018, 47, 1705–1729 RSC.
- P. J. Rutledge and G. L. Challis, Nat. Rev. Microbiol., 2015, 13, 509–523 CrossRef CAS PubMed.
- J. W. H. Li and J. C. Vederas, Science, 2009, 325, 161–165 CrossRef PubMed.
- G. L. Challis, J. Med. Chem., 2008, 51, 2618–2628 CrossRef CAS PubMed.
- Fungal Biomolecules: sources, applications and recent developments, ed. V. K. Gupta, R. L. Mach and S. Sreenivasaprasad, 2015, 978-1-118-95829-2 Search PubMed.
- P. Spiteller, Nat. Prod. Rep., 2015, 32, 971–993 RSC.
- A. H. Aly, A. Debbab and P. Proksch, Fungal Divers., 2011, 50, 3–19 CrossRef.
- J. J. Zhong and J. H. Xiao, Adv. Biochem. Eng. Biotechnol., 2009, 113, 79–150 CAS.
- Drug Design: Novel Advances in the Omics Field and Applications, ed. A. A. Parikesit and A. A. Parikesit, 2021, 118-95829-2 Search PubMed.
- J. Correia, A. Borges, M. Simões and L. C. Simões, Antibiotics, 2023, 12, 1250 CrossRef CAS PubMed.
- E. Martinez-Klimova, K. Rodríguez-Peña and S. Sánchez, Biochem. Pharmacol., 2017, 134, 1–17 CrossRef CAS PubMed.
- N. Suwannarach, J. Kumla, K. Sujarit, T. Pattananandecha, C. Saenjum and S. Lumyong, Molecules, 2020, 25, 1800 CrossRef CAS PubMed.
- A. Ganjoo, N. Sharma, H. Shafeeq, N. A. Bhat, K. K. Dubey and V. Babu, Biotechnol. Bioeng., 2022, 119, 3339–3369 CrossRef CAS PubMed.
- S. Chandra, Appl. Microbiol. Biotechnol., 2012, 95, 47–59 CrossRef CAS PubMed.
- S. K. Deshmukh, V. Prakash and N. Ranjan, Front. Microbiol., 2018, 8, 2536 CrossRef PubMed.
- E. A. Elsayed, H. El Enshasy, M. A. M. Wadaan and R. Aziz, Mediators Inflammation, 2014, 2014, 805841 Search PubMed.
- J. Xu, M. Yi, L. Ding, S. He, L. Dak, S. Yip, Y. Chin and K. Li, Mar. Drugs, 2019, 17, 636 CrossRef CAS PubMed.
- M. Manzoni and M. Rollini, Appl. Microbiol. Biotechnol., 2002, 58, 555–564 CrossRef CAS PubMed.
- V. V. Pandey, V. K. Varshney and A. Pandey, J. Biol. Act. Prod. Nat., 2019, 9, 162–178 Search PubMed.
- N. P. Keller, Nat. Rev. Microbiol., 2018, 17, 167–180 CrossRef PubMed.
- C. Greco, N. P. Keller and A. Rokas, Curr. Opin. Microbiol., 2019, 51, 22–29 CrossRef CAS PubMed.
- A. Singh, D. K. Singh, R. N. Kharwar, J. F. White, S. K. Gond, A. Singh, D. K. Singh, R. N. Kharwar, J. F. White and S. K. Gond, Microorganisms, 2021, 9, 197 CrossRef CAS PubMed.
- A. Schueffler and T. Anke, Nat. Prod. Rep., 2014, 31, 1425–1448 RSC.
- Y. M. Chianga, K. H. Lee, J. F. Sanchez, N. P. Keller and C. C. C. Wang, Nat. Prod. Commun., 2009, 4, 1510 Search PubMed.
- Y. Matsuda and I. Abe, Nat. Prod. Rep., 2015, 33, 26–53 RSC.
- C. Schmidt-Dannert, Adv. Biochem. Eng. Biotechnol., 2015, 148, 19–61 CrossRef CAS PubMed.
- E. Skellam, Trends Biotechnol., 2019, 37, 916 CrossRef CAS PubMed.
- R. Linnakoski, D. Reshamwala, P. Veteli, M. Cortina-Escribano, H. Vanhanen and V. Marjomäki, Front. Microbiol., 2018, 9, 412077 Search PubMed.
- A. A. Brakhage, J. Schuemann, S. Bergmann, K. Scherlach, V. Schroeckh and C. Hertweck, Prog. Drug Res., 2008, 66, 1–12 Search PubMed.
- D. H. Demers, M. A. Knestrick, R. Fleeman, R. Tawfik, A. Azhari, A. Souza, B. Vesely, M. Netherton, R. Gupta, B. L. Colon, C. A. Rice, M. A. Rodríguez-Pérez, K. H. Rohde, D. E. Kyle, L. N. Shaw and B. J. Baker, Mar. Drugs, 2018, 16, 376 CrossRef CAS PubMed.
- G. Niu, T. Annamalai, X. Wang, S. Li, S. Munga, G. Niu, Y. C. Tse-Dinh and J. Li, PeerJ, 2020, 8, e10392 CrossRef PubMed.
- T. A. K. Prescott, R. Hill, E. Mas-Claret, E. Gaya and E. Burns, Biomolecules, 2023, 13, 986 CrossRef CAS PubMed.
- H. B. Tiwari, P. Tiwari and H. Bae, Microorganisms, 2022, 10, 360 Search PubMed.
- F. Kempken, PLoS Pathog., 2023, 19, e1011624 CrossRef CAS PubMed.
- L. Marquez and C. L. Quave, Antibiotics, 2020, 9, 150 Search PubMed.
- M. Barbero, E. Artuso and C. Prandi, Curr. Med. Chem., 2017, 25, 141–185 CrossRef PubMed.
- K. Sułkowska-Ziaja, M. Trepa, A. Olechowska-Jarząb, P. Nowak, M. Ziaja, K. Kała and B. Muszyńska, Pharmaceuticals, 2023, 16, 1200 CrossRef PubMed.
- A. N. Wethalawe, Y. V. Alwis, D. N. Udukala and P. A. Paranagama, Molecules, 2021, 26, 3901 CrossRef CAS PubMed.
- D. Jakubczyk and F. Dussart, Molecules, 2020, 25, 911 Search PubMed.
- K. M. G. O'Connell, J. T. Hodgkinson, H. F. Sore, M. Welch, G. P. C. Salmond and D. R. Spring, Angew. Chem. Int. Ed., 2013, 52, 10706–10733 CrossRef PubMed.
- P. Pasrija, M. Girdhar, M. Kumar, S. Arora and A. Katyal, Phytomed. Plus, 2022, 2, 100249 CrossRef.
- T. O. Lawal, N. Raut, S. M. Wicks, G. B. Mahady and G. B. Mahady, Med. Res. Arch., 2018, 6(1), 1–22 Search PubMed.
- S. Banerjee and S. B. Paruthy, Fungal Metabolites, 2017, pp. 669–700 Search PubMed.
- J. A. Cooper, J. Cell Biol., 1987, 105, 1473–1478 CrossRef CAS PubMed.
- M. Binder and C. Tamm, Angew. Chem. Int. Ed., 1973, 12, 370–380 CrossRef CAS PubMed.
- A. A. Ismaiel and J. Papenbrock, Agriculture, 2015, 5, 492–537 CrossRef.
- J. Zhu, L. Song, S. Shen, W. Fu, Y. Zhu and L. Liu, Molecules, 2023, 28, 7789 CrossRef CAS PubMed.
- C. C. Gu, J. Zeng, X. P. Peng, Y. J. Sun, S. Z. Yuan, X. N. Wang, R. S. Zhang, H. X. Lou and G. Li, J. Org. Chem., 2023, 88, 3185–3192 CrossRef CAS PubMed.
- R. Chen, L. J. Guo, X. D. Li, X. R. Li, K. Hu, J. W. Tang, Z. N. Ye, B. C. Yan and P. T. Puno, Org. Chem. Front., 2023, 10, 2218–2225 RSC.
- H. Zhu, et al., Progress in the Chemistry of Cytochalasans, in Progress in the Chemistry of Organic Natural Products 114, ed. A. D. Kinghorn, H. Falk, S. Gibbons, J. Kobayashi, Y. Asakawa and J. K. Liu, Springer, Cham, 2021, vol. 114, DOI:10.1007/978-3-030-59444-2_1.
- D. C. Aldridge, J. J. Armstrong, R. N. Speake and W. B. Turner, Chem. Commun., 1967, 26–27 RSC.
- B. M. Kemkuignou, C. Lambert, K. Schmidt, L. Schweizer, E. G. M. Anoumedem, S. F. Kouam, M. Stadler, T. Stradal and Y. Marin-Felix, Fitoterapia, 2023, 166, 105434 CrossRef CAS PubMed.
- B. B. Shi, C. Tian, X. Lv, J. Schinnerl, K. Ye, H. Guo, F. Xu, Y. He, H. L. Ai and J. K. Liu, J. Org. Chem., 2023, 88, 13926–13933 CrossRef CAS PubMed.
- X. Hu, X. Li, S. Yang, X. Li, B. Wang and L. Meng, Chin. Chem. Lett., 2023, 34, 107516 CrossRef CAS.
- K. Scherlach, D. Boettger, N. Remme and C. Hertweck, Nat. Prod. Rep., 2010, 27, 869–886 RSC.
- G. Ding, H. Lou Wang, L. Chen, A. J. Chen, J. Lan, X. D. Chen, H. W. Zhang, H. Chen, X. Z. Liu and Z. M. Zou, J. Antibiot., 2012, 65, 143–145 CrossRef CAS PubMed.
- E. Skellam, Nat. Prod. Rep., 2017, 34, 1252–1263 RSC.
- X. Peng, Y. He, Y. Gao, F. Duan, J. Chen and H. Ruan, Bioorg. Chem., 2020, 104, 104317 CrossRef CAS PubMed.
- M. Zaghouani, O. Gayraud, V. Jactel, S. Prévost, A. Dezaire, M. Sabbah, A. Escargueil, T. L. Lai, C. Le Clainche, N. Rocques, S. Romero, A. Gautreau, F. Blanchard, G. Frison and B. Nay, Chem.–Eur. J., 2018, 24, 16686–16691 CrossRef CAS PubMed.
- M. Trendowski, Anticancer Agents Med. Chem, 2015, 15, 327–335 CrossRef CAS PubMed.
- X. Y. Hu, X. M. Li, S. Q. Yang, B. G. Wang and L. H. Meng, Chem. Biodivers., 2022, 19, e202200550 CrossRef CAS PubMed.
- Y. Zhang, R. Tian, S. Liu, X. Chen, X. Liu and Y. Che, Bioorg. Med. Chem., 2008, 16, 2627–2634 CrossRef CAS PubMed.
- K. J. S. Farias, P. R. L. Machado, R. F. d. A. Júnior and B. A. L. da Fonseca, Access Microbiol., 2019, 1, e000041 Search PubMed.
- D. S. Chulpanova, Z. E. Gilazieva, S. K. Kletukhina, A. M. Aimaletdinov, E. E. Garanina, V. James, A. A. Rizvanov and V. V. Solovyeva, Biology, 2021, 10, 141 CrossRef CAS PubMed.
- Y. Liu, Q. Ruan, S. Jiang, Y. Qu, J. Chen, M. Zhao, B. Yang, Y. Liu, Z. Zhao and H. Cui, Fitoterapia, 2019, 137, 104187 CrossRef CAS PubMed.
- Z. Feng, X. Zhang, J. Wu, C. Wei, T. Feng, D. Zhou, Z. Wen and J. Xu, Mar. Drugs, 2022, 20, 526 CrossRef CAS PubMed.
- W. X. Wang, G. G. Cheng, Z. H. Li, H. L. Ai, J. He, J. Li, T. Feng and J. K. Liu, Org. Biomol. Chem., 2019, 17, 7985–7994 RSC.
- M. Zhao, Y. Ge, C. Zhou, X. Liu and B. Wu, Fitoterapia, 2023, 168, 105523 CrossRef CAS PubMed.
- R. Fujii, A. Minami, K. Gomi and H. Oikawa, Tetrahedron Lett., 2013, 54, 2999–3002 CrossRef CAS.
- C. Wang, C. Lambert, M. Hauser, A. Deuschmann, C. Zeilinger, K. Rottner, T. E. B. Stradal, M. Stadler, E. J. Skellam and R. J. Cox, Chem.–Eur. J., 2020, 26, 13578–13583 CrossRef CAS PubMed.
- K. M. Fisch, RSC Adv., 2013, 3, 18228–18247 RSC.
- C. Lambert, K. Schmidt, M. Karger, M. Stadler, T. E. B. Stradal and K. Rottner, Biomolecules, 2023, 13, 1247 CrossRef CAS PubMed.
- M. A. Tammam and A. El-Demerdash, Curr. Res. Biotechnol., 2023, 6, 100145 CrossRef CAS.
- J. Reed, A. Orme, A. El-Demerdash, C. Owen, L. B. B. Martin, R. C. Misra, S. Kikuchi, M. Rejzek, A. C. Martin, A. Harkess, J. Leebens-Mack, T. Louveau, M. J. Stephenson and A. Osbourn, Science, 2009, 325, 161–165 CrossRef PubMed.
- M. A. Tammam, M. I. Gamal El-Din, A. Abood and A. El-Demerdash, RSC Adv., 2023, 13, 8049–8089 RSC.
- M. A. Tammam, M. Sebak, C. Greco, A. Kijjoa and A. El-Demerdash, J. Mol. Struct., 2022, 1268, 133711 CrossRef CAS.
- M. Sebak, F. Molham, C. Greco, M. A. Tammam, M. Sobeh and A. El-Demerdash, RSC Adv., 2022, 12, 24887–24921 RSC.
- A. El-Demerdash, C. Borde, G. Genta-Jouve, A. Escargueil and S. Prado, Nat. Prod. Res., 2022, 36, 1273–1281 CrossRef CAS PubMed.
- A. El-Demerdash, J. Fungi, 2018, 4, 130 CrossRef CAS PubMed.
- A. El-Demerdash, G. Genta-Jouve, M. Bärenstrauch, C. Kunz, E. Baudouin and S. Prado, Phytochemistry, 2019, 166, 112056 CrossRef CAS PubMed.
- M. Binder, J. -R Kiechel and C. Tamm, Helv. Chim. Acta, 1970, 53, 1797–1812 CrossRef CAS PubMed.
- J. -L Robert and C. Tamm, Helv. Chim. Acta, 1975, 58, 2501–2504 CrossRef PubMed.
- J. C. Vederas and C. Tamm, Helv. Chim. Acta, 1976, 59, 558–566 CrossRef CAS PubMed.
- A. Probst and C. Tamm, Helv. Chim. Acta, 1981, 64, 2065–2077 CrossRef CAS.
- H. Oikawa, Y. Murakami and A. Ichihara, J. Chem. Soc., Perkin Trans. 1, 1992, 2955–2959 RSC.
- D. Boettger and C. Hertweck, Chem. Biol. Chem., 2013, 14, 28–42 CrossRef CAS PubMed.
- K. Ishiuchi, T. Nakazawa, F. Yagishita, T. Mino, H. Noguchi, K. Hotta and K. Watanabe, J. Am. Chem. Soc., 2013, 135, 7371–7377 CrossRef CAS PubMed.
- K. Qiao, Y. H. Chooi and Y. Tang, Metab. Eng., 2011, 13, 723–732 CrossRef CAS PubMed.
- J. Collemare, M. Pianfetti, A. E. Houlle, D. Morin, L. Camborde, M. J. Gagey, C. Barbisan, I. Fudal, M. H. Lebrun and H. U. Böhnert, New Phytol., 2008, 179, 196–208 CrossRef CAS PubMed.
- H. U. Böhnert, I. Fudal, W. Dioh, D. Tharreau, J. L. Notteghem and M. H. Lebrun, Plant Cell, 2004, 16, 2499–2513 CrossRef PubMed.
- N. Khaldi, J. Collemare, M. H. Lebrun and K. H. Wolfe, Genome Biol., 2008, 9, 1–10 CrossRef PubMed.
- Z. Song, W. Bakeer, J. W. Marshall, A. A. Yakasai, R. M. Khalid, J. Collemare, E. Skellam, D. Tharreau, M. H. Lebrun, C. M. Lazarus, A. M. Bailey, T. J. Simpson and R. J. Cox, Chem. Sci., 2015, 6, 4837–4845 RSC.
- M. L. Nielsen, T. Isbrandt, L. M. Petersen, U. H. Mortensen, M. R. Andersen, J. B. Hoof and T. O. Larsen, PLoS One, 2016, 11, e0161199 CrossRef PubMed.
- C. Wang, V. Hantke, R. J. Cox and E. Skellam, Org. Lett., 2019, 21, 4163–4167 CrossRef CAS PubMed.
- H. Li, H. Wei, J. Hu, E. Lacey, A. N. Sobolev, K. A. Stubbs, P. S. Solomon and Y. H. Chooi, ACS Chem. Biol., 2020, 15, 226–233 CrossRef CAS PubMed.
- S. C. Heard, G. Wu and J. M. Winter, J. Antibiot., 2020, 73, 803–807 CrossRef CAS PubMed.
- C. Wang, K. Becker, S. Pfütze, E. Kuhnert, M. Stadler, R. J. Cox and E. Skellam, Org. Lett., 2019, 21, 8756–8760 CrossRef CAS PubMed.
- J. M. Zhang, X. Liu, Q. Wei, C. Ma, D. Li and Y. Zou, Nat. Commun., 2022, 13, 1–10 Search PubMed.
- B. Perlatti, C. B. Nichols, N. Lan, P. Wiemann, C. J. B. Harvey, J. A. Alspaugh and G. F. Bills, Front. Microbiol., 2020, 11, 564149 Search PubMed.
- J. Qi, L. Jiang, P. Zhao, H. Chen, X. Jia, L. Zhao, H. Dai, J. Hu, C. Liu, S. H. Shim, X. Xia and L. Zhang, Appl. Microbiol. Biotechnol., 2020, 104, 1545–1553 CrossRef CAS PubMed.
- G. G. Moore, J. Collemare, M. H. Lebrun and R. E. Bradshaw, Natural Products: Discourse, Diversity, and Design, 2014, pp. 341–356 Search PubMed.
- V. Hantke, E. J. Skellam and R. J. Cox, Chem. Commun., 2020, 56, 2925–2928 RSC.
- M. Cheng, S. Zhao, H. Liu, Y. Liu, C. Lin, J. Song, C. Thawai, S. Charoensettasilp and Q. Yang, Fungal Biol., 2021, 125, 201–210 CrossRef CAS PubMed.
- C. L. M. Gilchrist and Y. H. Chooi, Bioinformatics, 2021, 37, 2473–2475 CrossRef CAS PubMed.
- J. Schümann and C. Hertweck, J. Am. Chem. Soc., 2007, 129, 9564–9565 CrossRef PubMed.
- H. Heinemann, H. Zhang and R. J. Cox, Chem.–Eur. J., 2023, e202302590 Search PubMed.
- H. Zhang, V. Hantke, P. Bruhnke, E. J. Skellam and R. J. Cox, Chem.–Eur. J., 2021, 27, 3106–3113 CrossRef CAS PubMed.
- Y. Hu, D. Dietrich, W. Xu, A. Patel, J. A. J. Thuss, J. Wang, W. B. Yin, K. Qiao, K. N. Houk, J. C. Vederas and Y. Tang, Nat. Chem. Biol., 2014, 10, 552–554 CrossRef CAS PubMed.
- Z. Shang, R. Raju, A. A. Salim, Z. G. Khalil and R. J. Capon, J. Org. Chem., 2017, 82, 9704–9709 CrossRef CAS PubMed.
- H. Guo, Q. P. Diao, B. Zhang and K. H. Wang, Phytochem. Lett., 2021, 46, 162–165 CrossRef CAS.
- S.-Q. Teng, X.-F. Zhang, H.-F. Li, X.-W. Luo, Y.-S. Zhou, H. Liu, J.-K. Liu and T. Feng, Phytochemistry, 2023, 215, 113861 CrossRef CAS PubMed.
- A. Evidente, A. Andolfi, M. Vurro, M. C. Zonno and A. Motta, J. Nat. Prod., 2003, 66, 1540–1544 CrossRef CAS PubMed.
- A. Cimmino, A. Andolfi, A. Berestetskiy and A. Evidente, J. Agric. Food Chem., 2008, 56, 6304–6309 CrossRef CAS PubMed.
- A. Evidente, A. Cimmino, A. Andolfi, A. Berestetskiy and A. Motta, Tetrahedron, 2011, 67, 1557–1563 CrossRef CAS.
- X. Peng, J. Chang, Y. Gao, F. Duan and H. Ruan, Chin. Chem. Lett., 2022, 33, 4572–4576 CrossRef CAS.
- D. Zhang, H. Ge, D. Xie, R. Chen, J. H. Zou, X. Tao and J. Dai, Org. Lett., 2013, 15, 1674–1677 CrossRef CAS PubMed.
- D. Zhang, X. Tao, R. Chen, J. Liu, L. Li, X. Fang, L. Yu and J. Dai, Org. Lett., 2015, 17, 4304–4307 CrossRef CAS PubMed.
- D. Zhang, X. Tao, J. Liu, R. Chen, M. Zhang, L. Li, X. Fang, L. Y. Yu and J. Dai, Tetrahedron Lett., 2016, 57, 796–799 CrossRef CAS.
- J. Liu, D. Zhang, M. Zhang, X. Liu, R. Chen, J. Zhao, L. Li, N. Wang and J. Dai, Tetrahedron Lett., 2016, 57, 5794–5797 CrossRef CAS.
- A. Evidente, A. Andolfi, M. Vurro, M. C. Zonno and A. Motta, Phytochemistry, 2002, 60, 45–53 CrossRef CAS PubMed.
- R. Kretz, L. Wendt, S. Wongkanoun, J. J. Luangsa-Ard, F. Surup, S. E. Helaly, S. R. Noumeur, M. Stadler and T. E. B. Stradal, Biomolecules, 2019, 9, 73 CrossRef CAS PubMed.
- C. Zhao, G. Liu, X. Liu, L. Zhang, L. Li and L. Liu, RSC Adv., 2020, 10, 40384–40390 RSC.
- K. Y. M. Garcia, M. T. J. Quimque, C. Lambert, K. Schmidt, G. Primahana, T. E. B. Stradal, A. Ratzenböck, H. M. Dahse, C. Phukhamsakda, M. Stadler, F. Surup and A. P. G. Macabeo, J. Fungi, 2022, 8, 560 CrossRef CAS PubMed.
- D. Xu, M. Luo, F. Liu, D. Wang, X. Pang, T. Zhao, L. Xu, X. Wu, M. Xia and X. Yang, Sci. Rep., 2017, 7, 1–9 CrossRef PubMed.
- D. Xu, X. Zhang, X. Shi, P. J. Xian, L. Hong, Y. D. Tao and X. L. Yang, Phytochem. Lett., 2019, 32, 52–55 CrossRef CAS.
- A. Sallam, M. A. Sabry and A. A. Galala, Chem. Biodivers., 2021, 18, e2000957 CrossRef CAS PubMed.
- A. Höltzel, D. G. Schmid, G. J. Nicholson, P. Krastel, A. Zeeck, K. Gebhardt, H. P. Fiedler and G. Jung, J. Antibiot., 2004, 57, 715–720 CrossRef PubMed.
- K. Gebhardt, J. Schimana, A. Höltzel, K. Dettner, S. Draeger, W. Beil, J. Rheinheimer and H. P. Fiedler, J. Antibiot., 2004, 57, 707–714 CrossRef CAS PubMed.
- J. Liu, Z. Hu, H. Huang, Z. Zheng and Q. Xu, J. Antibiot., 2011, 65, 49–52 CrossRef PubMed.
- H. Zhu, C. Chen, Y. Xue, Q. Tong, X. N. Li, X. Chen, J. Wang, G. Yao, Z. Luo and Y. Zhang, Angew. Chem. Int. Ed., 2015, 54, 13374–13378 CrossRef CAS PubMed.
- L. Yu, W. Ding and Z. Ma, Nat. Prod. Res., 2016, 30, 1718–1723 CrossRef CAS PubMed.
- Z. Wu, Q. Tong, X. Zhang, P. Zhou, C. Dai, J. Wang, C. Chen, H. Zhu and Y. Zhang, Org. Lett., 2019, 21, 1026–1030 CrossRef CAS PubMed.
- L. Wang, J. Yang, J. P. Huang, J. Li, J. Luo, Y. Yan and S. X. Huang, Org. Lett., 2020, 22, 7930–7935 CrossRef CAS PubMed.
- R. Orfali, S. Perveen, M. F. Khan, A. F. Ahmed, S. Tabassum, P. Luciano, G. Chianese and O. Taglialatela-Scafati, Phytochemistry, 2021, 192, 112952 CrossRef CAS PubMed.
- X. Zhang, Z. Wu, A. Bao, Z. Zhao, Y. Chen, H. Zhao, J. Wang, C. Chen, Q. Tong, H. Zhu and Y. Zhang, Org. Chem. Front., 2022, 9, 2585–2592 Search PubMed.
- Y. C. Li, J. Yang, Y. Z. Zhao, Q. Di Ma, B. G. Liu and J. L. Sun, J. Fungi, 2023, 9, 374 CrossRef CAS PubMed.
- C. Iwamoto, T. Yamada, Y. Ito, K. Minoura and A. Numata, Tetrahedron, 2001, 57, 2997–3004 CrossRef CAS.
- X. Ding, W. Ye, B. Tan, Q. Song, Y. Chen, W. Liu, L. Sun, W. Tang, Y. Qiao, Q. Zhang, H. Zhang, Y. Wang, W. Zhang, C. Zhang and W. Zhang, Chin. J. Chem., 2023, 41, 915–923 CrossRef CAS.
- T. Lin, G. Wang, D. Zeng and H. Chen, Phytochem. Lett., 2015, 13, 206–211 CrossRef CAS.
- S. Xu, M. G. Hui, C. S. Yong, Y. Shen, H. Ding and X. T. Ren, Chem. Biodivers., 2009, 6, 739–745 CrossRef CAS PubMed.
- Q. L. Mou, S. X. Yang, T. Xiang, W. W. Liu, J. Yang, L. P. Guo, W. J. Wang and X. L. Yang, Tetrahedron Lett., 2021, 87, 153207 CrossRef CAS.
- X. Yang, P. Wu, J. Xue, H. Li and X. Wei, Fitoterapia, 2020, 145, 104611 CrossRef CAS PubMed.
- S. Gao, P. Wu, J. Xue, H. Li and X. Wei, Phytochemistry, 2022, 202, 113295 CrossRef CAS PubMed.
- B. C. Yan, W. G. Wang, D. B. Hu, X. Sun, L. M. Kong, X. N. Li, X. Du, S. H. Luo, Y. Liu, Y. Li, H. D. Sun and J. X. Pu, Org. Lett., 2016, 18, 1108–1111 CrossRef CAS PubMed.
- Y. F. Luo, M. Zhang, J. G. Dai, P. Pedpradab, W. J. Wang and J. Wu, Phytochem. Lett., 2016, 17, 162–166 CrossRef CAS.
- Y. Hsiao, H.-S. Chang, T.-W. Liu, S.-Y. Hsieh, G.-F. Yuan, M.-J. Cheng and I.-S. Chen, Rec. Nat. Prod., 2016, 10, 189–194 CAS.
- J. wei Tang, K. Hu, X. zheng Su, X. nian Li, B. chao Yan, H. dong Sun and P. tenzin Puno, Tetrahedron, 2020, 76, 131475 CrossRef.
- B. C. Yan, W. G. Wang, L. M. Kong, J. W. Tang, X. Du, Y. Li and P. T. Puno, J. Fungi, 2022, 8, 543 CrossRef CAS PubMed.
- Z. Lin, T. Zhu, H. Wei, G. Zhang, H. Wang and Q. Gu, Eur. J. Org Chem., 2009, 2009, 3045–3051 CrossRef.
- J. Kornsakulkarn, P. Auncharoen, A. Khonsanit, N. Boonyuen and C. Thongpanchang, RSC Adv., 2023, 13, 10564–10576 RSC.
- G. Ding, L. Chen, A. Chen, X. Tian, X. Chen, H. Zhang, H. Chen, X. Z. Liu, Y. Zhang and Z. M. Zou, Fitoterapia, 2012, 83, 541–544 CrossRef CAS PubMed.
- G. Ding, H. Wang, L. Li, A. J. Chen, L. Chen, H. Chen, H. Zhang, X. Liu and Z. Zou, Eur. J. Org Chem., 2012, 2012, 2516–2519 CrossRef CAS.
- G. Ding, H. Wang, L. Li, B. Song, H. Chen, H. Zhang, X. Liu and Z. Zou, J. Nat. Prod., 2014, 77, 164–167 CrossRef CAS PubMed.
- L. Chen, Y. T. Liu, B. Song, H. W. Zhang, G. Ding, X. Z. Liu, Y. C. Gu and Z. M. Zou, Fitoterapia, 2014, 96, 115–122 CrossRef CAS PubMed.
- C. Von Wallbrunn, H. Luftmann, K. Bergander and F. Meinhardt, J. Gen. Appl. Microbiol., 2001, 47, 33–38 CrossRef CAS PubMed.
- G. Ding, Y. C. Song, J. R. Chen, X. Chen, H. M. Ge, X. T. Wang and R. X. Tan, J. Nat. Prod., 2006, 69, 302–306 CrossRef CAS PubMed.
- C. M. Cui, X. M. Li, C. S. Li, P. Proksch and B. G. Wang, J. Nat. Prod., 2010, 73, 729–733 CrossRef CAS PubMed.
- H. Dou, Y. Song, X. Liu, W. Gong, E. Li, R. Tan and Y. Hou, Biol. Pharm. Bull., 2011, 34, 1864–1873 CrossRef CAS PubMed.
- S. Thohinung, S. Kanokmedhakul, K. Kanokmedhakul, V. Kukongviriyapan, O. Tusskorn and K. Soytong, Arch Pharm. Res., 2010, 33, 1135–1141 CrossRef CAS PubMed.
- G. B. Xu, L. M. Li, T. Yang, G. L. Zhang and G. Y. Li, Org. Lett., 2012, 14, 6052–6055 CrossRef CAS PubMed.
- M. Xue, Q. Zhang, J. M. Gao, H. Li, J. M. Tian and G. Pescitelli, Chirality, 2012, 24, 668–674 CrossRef CAS PubMed.
- H. Li, J. Xiao, Y. Q. Gao, J. J. Tang, A. L. Zhang and J. M. Gao, J. Agric. Food Chem., 2014, 62, 3734–3741 CrossRef CAS PubMed.
- C. Chen, J. Wang, J. Liu, H. Zhu, B. Sun, J. Wang, J. Zhang, Z. Luo, G. Yao, Y. Xue and Y. Zhang, J. Nat. Prod., 2015, 78, 1193–1201 CrossRef CAS PubMed.
- C. Chen, H. Zhu, J. Wang, J. Yang, X. N. Li, J. Wang, K. Chen, Y. Wang, Z. Luo, G. Yao, Y. Xue and Y. Zhang, Eur. J. Org Chem., 2015, 2015, 3086–3094 CrossRef CAS.
- C. Chen, H. Zhu, X. N. Li, J. Yang, J. Wang, G. Li, Y. Li, Q. Tong, G. Yao, Z. Luo, Y. Xue and Y. Zhang, Org. Lett., 2015, 17, 644–647 CrossRef CAS PubMed.
- R. K. Dissanayake, P. B. Ratnaweera, D. E. Williams, C. D. Wijayarathne, R. L. C. Wijesundera, R. J. Andersen and E. D. de Silva, Mycology, 2016, 7, 1 CrossRef CAS PubMed.
- Z. Zhang, X. Min, J. Huang, Y. Zhong, Y. Wu, X. Li, Y. Deng, Z. Jiang, Z. Shao, L. Zhang and F. He, Mar. Drugs, 2016, 14, 233 CrossRef PubMed.
- C. Chen, Q. Tong, H. Zhu, D. Tan, J. Zhang, Y. Xue, G. Yao, Z. Luo, J. Wang, Y. Wang and Y. Zhang, Sci. Rep., 2016, 6, 18711–18719 CrossRef CAS PubMed.
- X. Yan, L. J. Wang, Z. Wu, Y. L. Wu, X. X. Liu, F. R. Chang, M. J. Fang and Y. K. Qiu, J. Chromatogr. B, 2016, 1033–1034, 1–8 CrossRef CAS PubMed.
- X. Y. Wang, X. Yan, M. J. Fang, Z. Wu, D. Wang and Y. K. Qiu, Nat. Prod. Res., 2017, 31, 1669–1675 CrossRef CAS PubMed.
- W. Gao, W. Sun, F. Li, C. Chai, Y. He, J. Wang, Y. Xue, C. Chen, H. Zhu, Z. Hu and Y. Zhang, Phytochemistry, 2018, 156, 106–115 CrossRef CAS PubMed.
- W. Gao, Y. He, F. Li, C. Chai, J. Zhang, J. Guo, C. Chen, J. Wang, H. Zhu, Z. Hu and Y. Zhang, Bioorg. Chem., 2019, 83, 98–104 CrossRef CAS PubMed.
- H. H. Wang, G. Li, Y. N. Qiao, Y. Sun, X. P. Peng and H. X. Lou, Org. Lett., 2019, 21, 3319–3322 CrossRef CAS PubMed.
- Q. F. Guo, Z. H. Yin, J. J. Zhang, W. Y. Kang, X. W. Wang, G. Ding and L. Chen, Molecules, 2019, 24, 3240 CrossRef CAS PubMed.
- X. W. Luo, C. H. Gao, H. M. Lu, J. M. Wang, Z. Q. Su, H. M. Tao, X. F. Zhou, B. Yang and Y. H. Liu, Molecules, 2020, 25, 1237 CrossRef CAS PubMed.
- X. G. Peng, J. Liu, Y. Gao, F. Cheng, J. L. Chang, J. Chen, F. F. Duan and H. L. Ruan, Org. Lett., 2020, 22, 9665–9669 CrossRef CAS PubMed.
- J. Qi, D. Wang, X. Yin, Q. Zhang and J. M. Gao, Nat. Prod. Commun., 2020, 15, 7 CrossRef.
- J. Ji, C. Liu, X. He, G. Chen, L. Qin and C. Zheng, Fitoterapia, 2021, 151, 104874 CrossRef CAS PubMed.
- J. F. Wang, R. Huang, S. S. Liu and S. H. Wu, Chem. Nat. Compd., 2021, 57, 1169–1174 CrossRef CAS.
- T. Li, Y. Wang, L. Li, M. Tang, Q. Meng, C. Zhang, E. Hua, Y. Pei and Y. Sun, Mar. Drugs, 2021, 19, 438 CrossRef CAS PubMed.
- Q. Guo, J. Chen, Y. Ren, Z. Yin, J. Zhang, B. Yang, X. Wang, W. Yin, W. Zhang, G. Ding and L. Chen, Front. Chem., 2021, 9, 620589 CrossRef CAS PubMed.
- A. X. Zhang, L. Feng, J. Wang, N. H. Tan and Z. Wang, J. Asian Nat. Prod. Res., 2022, 24, 769–776 CrossRef CAS PubMed.
- W. Gao, R. Jiang, H. Zeng, J. Cao, Z. Hu and Y. Zhang, Nat. Prod. Res., 2024, 38, 1599–1605 CrossRef CAS PubMed.
- X. Peng, J. Liu, C. Qin, Q. Wu, W. Li, F. Mohammadipanah and H. Ruan, Chin. J. Chem., 2022, 40, 1909–1916 CrossRef CAS.
- J. Wang, Z. Wang, Z. Ju, J. Wan, S. Liao, X. Lin, T. Zhang, X. Zhou, H. Chen, Z. Tu and Y. Liu, Planta Med., 2015, 81, 160–166 CrossRef CAS PubMed.
- Y. Shu, J. P. Wang, B. X. Li, J. L. Gan, H. Ding, R. Liu, L. Cai and Z. T. Ding, Phytochemistry, 2022, 194, 113009 CrossRef CAS PubMed.
- J. T. Liu, B. Hu, Y. Gao, J. P. Zhang, B. H. Jiao, X. L. Lu and X. Y. Liu, Chem. Biodivers., 2014, 11, 800–806 CrossRef CAS PubMed.
- Y. Zhou, Y. X. Zhang, J. P. Zhang, H. B. Yu, X. Y. Liu, X. L. Lu and B. H. Jiao, Nat. Prod. Res., 2017, 31, 1676–1681 CrossRef CAS PubMed.
- D. N. Quang, T. Hashimoto, M. Tanaka, M. Baumgartner, M. Stadler and Y. Asakawa, J. Nat. Prod., 2002, 65, 1869–1874 CrossRef CAS PubMed.
- L. J. Yang, H. X. Liao, M. Bai, G. L. Huang, Y. P. Luo, Y. Y. Niu, C. J. Zheng and C. Y. Wang, Nat. Prod. Res., 2018, 32, 208–213 CrossRef CAS PubMed.
- A. Narmani, S. Pichai, P. Palani, M. Arzanlou, F. Surup and M. Stadler, Mycol. Prog., 2019, 18, 175–185 CrossRef.
- K. T. Yuyama, L. Wendt, F. Surup, R. Kretz, C. Chepkirui, K. Wittstein, C. Boonlarppradab, S. Wongkanoun, J. Luangsa-Ard, M. Stadler and W. R. Abraham, Biomolecules, 2018, 8, 129 CrossRef PubMed.
- H. Van Trung, P. C. Kuo, N. N. Tuan, N. T. Ngan, N. Q. Trung, N. T. Thanh, H. V. Hai, D. L. Phuong, B. L. Giang, Y. C. Li, T. S. Wu and T. D. Thang, Nat. Prod. Commun., 2019, 14, 5 Search PubMed.
- S. Wongkanoun, L. Wendt, M. Stadler, J. Luangsa-ard and P. Srikitikulchai, Mycol. Prog., 2019, 18, 553–564 CrossRef.
- M. Stadler, D. N. Quang, A. Tomita, T. Hashimoto and Y. Asakawa, Mycol. Res., 2006, 110, 811–820 CrossRef CAS PubMed.
- M. Stadler, J. Fournier, D. N. Quang and A. Y. Akulov, Nat. Prod. Commun., 2007, 2, 287–304 CrossRef CAS.
- C. Lambert, M. J. Pourmoghaddam, M. Cedeño-Sanchez, F. Surup, S. A. Khodaparast, I. Krisai-Greilhuber, H. Voglmayr, T. E. B. Stradal and M. Stadler, J. Fungi, 2021, 7, 131 CrossRef CAS PubMed.
- N. Sharma, M. Kushwaha, D. Arora, S. Jain, V. Singamaneni, S. Sharma, R. Shankar, S. Bhushan, P. Gupta and S. Jaglan, J. Appl. Microbiol., 2018, 125, 111–120 CrossRef CAS PubMed.
- M. Kushwaha, A. Qayum, S. K. Jain, J. Singh, A. K. Srivastava, S. Srivastava, N. Sharma, V. Abrol, R. Malik, S. K. Singh, R. A. Vishwakarma and S. Jaglan, ACS Omega, 2021, 6, 3717–3726 CrossRef CAS PubMed.
- M. Kushwaha, A. Qayum, N. Sharma, V. Abrol, P. Choudhary, M. Murtaza, S. K. Singh, R. A. Vishwakarma, U. Goutam, S. K. Jain and S. Jaglan, ACS Omega, 2022, 7, 29135–29141 CrossRef CAS PubMed.
- M. J. Pourmoghaddam, G. Ekiz, C. Lambert, F. Surup, G. Primahana, K. Wittstein, S. A. Khodaparast, H. Voglmayr, I. Krisai-Greilhuber, T. E. B. Stradal and M. Stadler, Mycol. Prog., 2022, 21, 1–14 CrossRef.
- Q. Zhang, J. Xiao, Q. Q. Sun, J. C. Qin, G. Pescitelli and J. M. Gao, J. Agric. Food Chem., 2014, 62, 10962–10969 CrossRef CAS PubMed.
- W. X. Wang, Z. H. Li, T. Feng, J. Li, H. Sun, R. Huang, Q. X. Yuan, H. L. Ai and J. K. Liu, Org. Lett., 2018, 20, 7758–7761 CrossRef CAS PubMed.
- C. W. Lei, Z. Q. Yang, Y. P. Zeng, Y. Zhou, Y. Huang, X. S. He, G. Y. Li and X. H. Yuan, Nat. Prod. Res., 2018, 32, 7–13 CrossRef CAS PubMed.
- W. X. Wang, T. Feng, Z. H. Li, J. Li, H. L. Ai and J. K. Liu, Tetrahedron Lett., 2019, 60, 150952 CrossRef CAS.
- W. X. Wang, X. Lei, Y. L. Yang, Z. H. Li, H. L. Ai, J. Li, T. Feng and J. K. Liu, Org. Lett., 2019, 21, 6957–6960 CrossRef CAS PubMed.
- W. X. Wang, Z. H. Li, J. He, T. Feng, J. Li and J. K. Liu, Fitoterapia, 2019, 137, 104278 CrossRef CAS PubMed.
- W. X. Wang, X. Lei, H. L. Ai, X. Bai, J. Li, J. He, Z. H. Li, Y. S. Zheng, T. Feng and J. K. Liu, Org. Lett., 2019, 21, 1108–1111 CrossRef CAS PubMed.
- W. X. Wang, Z. H. Li, H. L. Ai, J. Li, J. He, Y. S. Zheng, T. Feng and J. K. Liu, Fitoterapia, 2019, 137, 104253 CrossRef CAS PubMed.
- P. H. F. da Silva, F. M. A. da Silva and H. H. F. Koolen, Chem. Nat. Compd., 2019, 55, 592–593 CrossRef CAS.
- J. H. Su, M. Q. Wang, Y. Z. Li, Y. S. Lin, J. Y. Gu, L. P. Zhu, W. Q. Yang, S. Q. Jiang, Z. X. Zhao and Z. H. Sun, Fitoterapia, 2022, 157, 105124 CrossRef CAS PubMed.
- W. Hinterdobler, M. Bacher, B. B. Shi, D. Baurecht, I. Krisai-Greilhuber, M. Schmoll, L. Brecker, K. Valant-Vetschera and J. Schinnerl, Nat. Prod. Res., 2023, 37, 85–92 CrossRef CAS PubMed.
- C. Lambert, L. Shao, H. Zeng, F. Surup, P. Saetang, M. C. Aime, D. R. Husbands, K. Rottner, T. E. B. Stradal and M. Stadler, Mycologia, 2023, 115, 277–287 CrossRef CAS PubMed.
- A. Daina, O. Michielin and V. Zoete, Sci. Rep., 2017, 7(1), 1–13 CrossRef PubMed.
- D. E. V. Pires, T. L. Blundell and D. B. Ascher, J. Med. Chem., 2015, 58, 4066–4072 CrossRef CAS PubMed.
- E. Urbanik and B. R. Ware, Arch. Biochem. Biophys., 1989, 269, 181–187 CrossRef CAS PubMed.
- L. Pampanella, G. Petrocelli, P. M. Abruzzo, C. Zucchini, S. Canaider, C. Ventura and F. Facchin, Cells, 2024, 13, 400 CrossRef CAS PubMed.
- C. Hua, Y. Yang, L. Sun, H. Dou, R. Tan and Y. Hou, Immunobiology, 2013, 218, 292–302 CrossRef CAS PubMed.
- L. Pampanella, P. M. Abruzzo, R. Tassinari, A. Alessandrini, G. Petrocelli, G. Ragazzini, C. Cavallini, V. Pizzuti, N. Collura, S. Canaider, F. Facchin and C. Ventura, Pharmaceuticals, 2023, 16, 289 CrossRef CAS PubMed.
- M. Valli, J. M. Souza, R. C. Chelucci, C. R. Biasetto, A. R. Araujo, V. da Silva Bolzani and A. D. Andricopulo, PLoS One, 2022, 17, e0275002 CrossRef CAS PubMed.
- M. Trendowski, T. D. Christen, C. Acquafondata and T. P. Fondy, BMC Cancer, 2015, 15, 1–14 CrossRef CAS PubMed.
- S. MacLean-Fletcher and T. D. Pollard, Cell, 1980, 20, 329–341 CrossRef CAS PubMed.
- P. A. Theodoropoulos, A. Gravanis, A. Tsapara, A. N. Margioris, E. Papadogiorgaki, V. Galanopoulos and C. Stournaras, Biochem. Pharmacol., 1994, 47, 1875–1881 CrossRef CAS PubMed.
- J. H. Hartwig and T. P. Stossel, J. Mol. Biol., 1979, 134, 539–553 CrossRef CAS PubMed.
- A. Forer, J. Emmersem and O. Behnke, Science, 1972, 175, 774–776 CrossRef CAS PubMed.
- N. K. Wessells, B. S. Spooner, J. F. Ash, M. O. Bradley, M. A. Luduena, E. L. Taylor, J. T. Wrenn and K. M. Yamada, Science, 1971, 171, 135–143 CrossRef CAS PubMed.
- D. W. Goddette and C. Frieden, J. Biol. Chem., 1986, 261, 15974–15980 CrossRef CAS PubMed.
- J. S. Choy, X. Lu, J. Yang, Z. Du Zhang and G. S. Kassab, Am. J. Physiol. Heart Circ. Physiol., 2014, 306, H69 CrossRef CAS PubMed.
- Z. A. Schiller, N. R. Schiele, J. K. Sims, K. Lee and C. K. Kuo, Stem Cell Res. Ther., 2013, 4, 1–10 CrossRef PubMed.
- J. F. Casella, M. D. Flanagan and S. Lin, Nature, 1981, 293(5830), 302–305 CrossRef CAS PubMed.
- K. Kobayashi, D. Matsuda, H. Tomoda and T. Ohshiro, Drug Discov. Ther., 2022, 16, 148–153 CrossRef CAS PubMed.
- B. B. Khomtchouk, Y. S. Lee, M. L. Khan, P. Sun, D. Mero and M. H. Davidson, Expet Opin. Drug Discov., 2022, 17, 443–460 CrossRef CAS PubMed.
- S. Bräse, F. Gläser, C. Kramer, S. Lindner, A. M. Linsenmeier, K.-S. Masters, A. C. Meister, B. M. Ruff and S. Zhong, The Chemistry of Mycotoxins, Springer, Wien Heidelberg New York Dordrecht London, 2013 Search PubMed.
- D. G. Menter, B. F. Sloane, B. W. Steinert, J. Onoda, R. Craig, C. Harkins, J. D. Taylor and K. V Honn, J. Natl. Cancer Inst., 1987, 79, 1077–1090 CAS.
- B. H. Choi, J. A. Park, K. R. Kim, G. I. Lee, Y. T. Lee, H. Choe, S. H. Ko, M. H. Kim, Y. H. Seo and Y. G. Kwak, Am. J. Physiol. Cell Physiol., 2005, 289, 425–436 CrossRef PubMed.
- Y. Takanezawa, R. Nakamura, Y. Kojima, Y. Sone, S. Uraguchi and M. Kiyono, Biochem. Biophys. Res. Commun., 2018, 498, 603–608 CrossRef CAS PubMed.
- G. Van Goietsenoven, V. Mathieu, A. Andolfi, A. Cimmino, F. Lefranc, R. Kiss and A. Evidente, Planta Med., 2011, 77, 711–717 CrossRef CAS PubMed.
- S. Delebassée, L. Mambu, E. Pinault, Y. Champavier, B. Liagre and M. Millot, Fitoterapia, 2017, 121, 146–151 CrossRef PubMed.
- M. S. Reifenberger, L. Yu, H. F. Bao, B. J. Duke, B. C. Liu, H. P. Ma, A. A. Alli, D. C. Eaton and A. A. Alli, Am. J. Physiol., 2014, 307, 86–95 Search PubMed.
- T. Udagawa, J. Yuan, D. Panigrahy, Y.-H. Chang, J. Shah and R. J. D'Amato, J. Pharmacol. Exp. Ther., 2000, 249, 421–428 CrossRef.
- K. C. Tjandra, N. McCarthy, L. Yang, A. J. Laos, G. Sharbeen, P. A. Phillips, H. Forgham, S. M. Sagnella, R. M. Whan, M. Kavallaris, P. Thordarson and J. A. McCarroll, J. Med. Chem., 2020, 63, 2181–2193 CrossRef CAS PubMed.
- K. E. Fischer, G. Nagaraj, R. Hugh Daniels, E. Li, V. E. Cowles, J. L. Miller, M. D. Bunger and T. A. Desai, Biomaterials, 2011, 32, 3499–3506 CrossRef CAS PubMed.
- R. E. Serda, J. Gu, R. C. Bhavane, X. W. Liu, C. Chiappini, P. Decuzzi and M. Ferrari, Biomaterials, 2009, 30, 2440–2448 CrossRef CAS PubMed.
- A. W. Baird, C. T. Taylor and D. J. Brayden, Adv. Drug Deliv. Rev., 1997, 23, 111–120 CrossRef CAS.
- A. Nair, J. Bu, P. A. Rawding, S. C. Do, H. Li and S. Hong, Nanomaterials, 2022, 12, 3 CrossRef CAS PubMed.
- T. T. Morgan, H. S. Muddana, E. I. Altinoǧlu, S. M. Rouse, A. Tabaković, T. Tabouillot, T. J. Russin, S. S. Shanmugavelandy, P. J. Butler, P. C. Eklund, J. K. Yun, M. Kester and J. H. Adair, Nano Lett., 2008, 8, 4108–4115 CrossRef CAS PubMed.
- K. G. Lehmann, J. J. Popma, J. A. Werner, A. J. Lansky and R. L. Wilensky, J. Am. Coll. Cardiol., 2000, 35, 583–591 CrossRef CAS PubMed.
- M. Trendowski, J. M. Mitchell, C. M. Corsette, C. Acquafondata and T. P. Fondy, Invest. New Drugs, 2015, 33, 290–299 CrossRef CAS PubMed.
- F. Y. Huang, W. L. Mei, Y. N. Li, G. H. Tan, H. F. Dai, J. L. Guo, H. Wang, Y. H. Huang, H. G. Zhao, S. L. Zhou, L. Li and Y. Y. Lin, Eur. J. Cancer, 2012, 48, 2260–2269 CrossRef CAS PubMed.
- J. Hwang, M. Yi, X. Zhang, Y. Xu, J. H. Jung and D. K. Kim, Oncol. Rep., 2013, 30, 1929–1935 CrossRef CAS PubMed.
- M. Y. Kim, J. H. Kim and J. Y. Cho, Biol. Ther., 2014, 22, 295–300 CAS.
- J. Bylund, S. Pellmé, H. Fu, U. H. Mellqvist, K. Hellstrand, A. Karlsson and C. Dahlgren, BMC Cell Biol., 2004, 5, 1–14 CrossRef PubMed.
- V. Betina, D. Mičeková and P. Nemec, Microbiology, 1972, 71, 343–349 CrossRef CAS.
- J. He, Q. Zou, H. Deng, S. He, D. Yan, K. Pan, Y. Zhou, Z. Zhao, H. Cui and Y. Liu, Fitoterapia, 2024, 173, 105804 CrossRef CAS PubMed.
- Z. Wu, W. Wang, J. Li, C. Ma, L. Chen, Q. Che, G. Zhang, T. Zhu and D. Li, J. Nat. Prod., 2024, 26, 87–91 Search PubMed.
- A. Ali, B. A. Malla, N. Sehar, S. B. Ahmad, Z. Imtiyaz, A. Arafah, M. U. Rehman and A. Nadeem, J. Biol. Regul. Homeost. Agents, 2023, 4555–4569 CAS.
- K. Bhattarai, M. E. Kabir, R. Bastola and B. Baral, Adv. Genet., 2021, 107, 193–284 CAS.
- I. S. Asogwa, C. Osita Eze, A. I. Onah, C. A. Agbo, S. A. Evurani and A. A. Attama, Int. J. Sci. Res. Arch., 2024, 2024, 302–321 CrossRef.
- X.-H. Si, R.-Y. Lyu, Z.-G. Sun, H. Dong and X.-Y. Liu, Cancer Adv., 2023, 6, e23021 CrossRef.
- P. B. Devi, R. Jayaseelan, P. B. Devi and R. Jayaseelan, Drug Design – Novel Advances in the Omics Field and Applications, 2020, pp. 117–125 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4np00076e |
| This journal is © The Royal Society of Chemistry 2025 |