Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dietary substances and their glucuronides: structures, occurrence and biological activity
Andrew V. Stachulski
 *a,
Edwin A. Yates
*a,
Edwin A. Yates
 b,
Aleksandra Teriosina
c,
Lesley Hoyles
b,
Aleksandra Teriosina
c,
Lesley Hoyles
 d and
Simon McArthur
d and
Simon McArthur
 e
e
aDepartment of Chemistry, University of Liverpool, Liverpool L69 7ZD, UK. E-mail: stachuls@liv.ac.uk
bDepartment of Biochemistry, Cell and Systems Biology, ISMIB, University of Liverpool, Crown St., Liverpool L69 7ZB, UK
cSchool of Biological Sciences, University of Liverpool, Crown Street, Liverpool, L69 7ZB, UK
dDepartment of Biosciences, School of Science and Technology, Nottingham Trent University, Nottingham, NG11 8NS, UK
eInstitute of Dentistry, Faculty of Medicine & Dentistry, Queen Mary University London, Blizard Institute, 4, Newark Street, London E1 2AT, UK
First published on 4th June 2025
Abstract
Covering up to 2025.
Plant-derived polyphenols of various chemical classes are widely distributed in dietary substances, e.g. fruits, nuts, vegetables and teas. Such phenolic derivatives are natural antioxidants and have been linked with numerous health benefits, notably anti-cancer and anti-inflammatory properties. Additionally, they may behave as mild estrogens, as in the case of genistein. However, there has often been no clear correlation between in vitro properties, as measured in cell lines for instance, and in vivo performance. Moreover, it is not always clear what the true active species might be, as most phenols are readily subject to phase II metabolism, generating predominantly glucuronides and sulfates. In this highlight, we seek to address the question of whether dietary substance metabolites, especially glucuronides, which have been more widely studied, do indeed possess distinct activities in their own right compared to their parent substances. In most cases this will refer to enzyme inhibition and/or interaction with cell lines. General observations concerning glucuronidation are provided, accompanied by practical comments concerning the synthesis of glucuronides, which are not always available or marketed in useful quantities. The main structural classes of natural polyphenols are introduced, with comments including synthetic details and biological properties for important members of each class.
1. Introduction
Monophenolic and polyphenolic organic compounds, especially stilbenes and flavonoids, are frequent constituents of a wide variety of common foodstuffs.1 For some time, various health benefits including anti-cancer properties have been claimed for these molecules, notably resveratrol and its stilbene relatives. However, even if a health benefit can be demonstrated, it is not clear what the causative substance might be, as many such molecules have a complex in vivo metabolism, generating O-glucuronides and O-sulfates in particular with, in some cases, multiple sites of metabolism. Indeed, the simple derivative p-cresyl sulfate, formed in response to p-cresol, a metabolite of tyrosine metabolism, is known to be a uremic toxin2 and has recently been shown to impact the function of the blood–brain barrier, acting via EGF receptor3 also providing a direct link between products of gut microbial activity and functions relating to the brain.Over the last decade, we and others have shown that glucuronides are indeed biologically active in their own right4–6 and one potential application may be their use in cases where the parent substances exhibit toxicity at high dose. An interesting link between the glucuronides of ethanol,7 morphine (morphine-3 glucuronide) as well as a variety of other glucuronides8,9 has been made, showing that they induce increased pain via binding to Toll-like receptor (TLR4) and, together with a recent report that quercetin 3-O-glucuronide was the probable cause of red wine hangovers,10 serve as a reminder that less beneficial effects might also result.
In this highlight, we set out to examine the evidence for biological activity, concentrating on a comparison between the parent substances and their glucuronides. We focus predominantly on glucuronides, which have been the most studied metabolites, but where O-sulfates have been assayed alongside them, we also include their activity.
2. Glucuronidation
Formerly, glucuronides were often dismissed as waste products of (mostly) phase II metabolism,11 though their potential for biological activity per se in some cases was recognized, morphine-6-glucuronide being the best-known example.11,12 In the case of the phenolic substances covered in this highlight, the relatively insoluble parent compounds are rendered more water-soluble by glucuronidation and excreted, but glucuronidation is now understood to be a more complex and nuanced process; both the formation of glucuronides, mediated by mammalian UDP-glucuronosyl transferases (UGTs, Scheme 1), and their removal from cells by efflux transporters must be considered.13–15 Enzymatic glucuronidation involves the incorporation of D-glucuronic acid via a β-glycosidic linkage onto the hydroxyl group of the parent phenol by uridine 5′-diphosphoglucuronosyl transferase (UGT) enzymes (EC 2.4.17). The human UGT enzyme family (comprehensively classified in the CAZy database; https://www.cazy.org/) is divided into 4 sub-families (UGT1, UGT2, UGT3 and UGT8) with 22 members exhibiting specificity among dietary phenols but with some overlap. Thus, resveratrol is a substrate for UGT1A1, pterostilbene (both resveratrol and pterostilbene are stilbenes; Section 4) is acted on by both UGT1A1 and UGT1A3, while p-cresol (formed largely by bacterial action on dietary tyrosine in the intestine) is predominantly modified by UGT1A6 and UGT1A9, although several other enzymes can act, albeit at lower levels. A further major dietary polyphenol, quercetin (Section 5), can be glucuronidated by UGT1A1, UGT1A8 and UGT1A9.The range of biological activities against which polyphenols, phenols and their glucuronides are being tested continues to expand to include manifold effects on the brain,16 as well as antioxidant action. Hepatocytes can clear glucuronides via the biliary system efficiently and there can be subsequent re-hydrolysis of the glucuronides by microbiota-associated glucuronidases (mGUS)17 in the large intestine followed by re-absorption, as well as subsequent processing of the aglycone component. Thus, the fate of glucuronides in the gut is linked to the health status of the host, enterohepatic recycling of (non-)toxic substances and the functional potential of the microbiota, and is the subject of sometimes over-stated claims regarding health effects or benefits.18
The activity of mGUS in the gastrointestinal tract influences processing of xenobiotics and endogenous compounds in mammalian systems. Inhibiting mGUS has been shown to reduce intestinal side-effects (e.g. inflammation, diarrhea, gastritis, constipation) of medications, increase systemic efficacy of drugs (e.g. opioids, non-steroidal anti-inflammatory drugs), and modulate whole-body exposure to bilirubin, steroid hormones (e.g. estrone, estradiol, testosterone, androstanediol) and the neurotransmitters dopamine and serotonin.19 The estrobiome is the collection of all microbial genes within a microbiota encoding products capable of metabolizing or reactivating conjugated estrogens, or affecting the reabsorption of free estrogen. Imbalances in the estrobiome have been identified as a potential link to hormone-associated hepatocellular adenomas.20
Metabolism of over 100 drugs is known to be influenced by mGUS activity.21 Among them, the anti-cancer drug irinotecan (Fig. 1) has received most attention because conversion of SN-38 glucuronide to SN-38, the active metabolite of irinotecan, damages the gut epithelium and leads to diarrhea, contributing to poor quality of life in almost 90% of patients given the drug.19,22,23 Aglycones generated via the action of mGUS on intestinal glucuronides are increasingly implicated in hormone-driven cancers (e.g. breast, prostate, ovarian, hepatocellular adenoma), with the suggestion that an imbalance in the estrobolome of the small intestine contributes to evolution of diseases associated with sex hormones.20,22
 | ||
| Fig. 1 Irinotecan, a topoisomerase I inhibitor. In vivo glucuronidation occurs at O(10), highlighted, after cleavage of the carbamoyl group; SN-38 is the free phenol/active metabolite. | ||
In a survey spanning geographically widespread subjects, almost 300 distinct enzymes with β-glucuronidase activity were predicted to be encoded by the human gut microbiota, falling into seven classes, two of which (L1 and mL1) focus on small glucuronide substrates.23 These are present predominantly in the bacteria Bacteroides ovatus, Phocaeicola (formerly Bacteroides) dorei, Bacteroides fragilis, Escherichia coli, Lachnospira (formerly Eubacterium) eligens, Faecalibacterium prausnitzii and Mediterraneibacter (formerly Ruminococcus) gnavus. Wide variation in bacterial populations has also been noted between individuals, providing a possible explanation for variations in the behaviour and efficacy of drugs between patients.24–26 An interesting, but as yet largely unexplored, question is the extent to which the release of aglycone components by particular bacterial species affects other bacterial species, as well as the surrounding cells of the host.
Carefully defined experimental systems will be required to accurately unpick the complex interactions of dietary-derived glucuronides with the host and microbiota. Before surveying dietary substances and their glucuronides by structural class, we summarise briefly, methods of phenolic glucuronide synthesis by both chemical and enzyme-mediated means.
2.1 Chemical synthesis of O-aryl glucuronides
Here we consider only the glucuronidation of phenols: a broader discussion of O-glucuronidation has been published.11 Although the traditional Koenigs–Knorr synthesis using anomeric bromide 1 is still used,11 the desirability of avoiding heavy metal catalysts, typically Ag, Hg or Cd salts, is now generally recognised. Glucuronidation using anomeric bromide 1 under phase transfer catalysis conditions is also possible.11 We and others4,27 have effected glucuronidation of phenols using the β-tetraester 2, and this frequently provides good yields, though the pKa of the phenol is critical. Empirically, the method is applicable for phenols of pKa > ∼9.5, but shuts off below that value. The Schmidt anomeric trichloroacetimidate 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11,28 generally gives high yields for a wide range of phenols (7.5 < pKa < 10) and was used for the isomeric resveratrol glucuronides, v. i.: the related (N-phenyl)trifluoro derivative 4 is also a good donor.29 In general, the imidate 3 is the most reliable donor, but since β-tetraester 2 is commercially available, inexpensive and highly stable at 20 °C, its suitability – based on a knowledge of pKa values – should always be assessed.
11,28 generally gives high yields for a wide range of phenols (7.5 < pKa < 10) and was used for the isomeric resveratrol glucuronides, v. i.: the related (N-phenyl)trifluoro derivative 4 is also a good donor.29 In general, the imidate 3 is the most reliable donor, but since β-tetraester 2 is commercially available, inexpensive and highly stable at 20 °C, its suitability – based on a knowledge of pKa values – should always be assessed.The acetates, or in some cases higher esters, used in the carbohydrate intermediates are removed from the primary adducts using base hydrolysis. Mild hydrolysis using Na2CO3 is favoured (Fig. 2) to minimise the risk of base-catalysed by-products. By suitable pH adjustment, either the free acid form or the sodium salt of the final product may be isolated.
Although a universal approach to glucuronide synthesis by conventional means does not exist, a modular strategy incorporating a ‘self-immolative’ linker has been explored (Fig. 3) with the aim of enabling universal glucuronidation, via a series of glucuronide-containing pro-drugs active against HeLa cells.30 Here, the desired phenol was released from the conjugate by the action of a glucuronidase.
 | ||
| Fig. 3 A glucuronide conjugate used as a phenolic prodrug. A range of bacterial β-glucuronidases was screened.30 | ||
2.2 Enzyme-mediated synthesis
In addition to the extensive synthetic efforts summarized briefly above, glucuronidation has also been carried out using liver microsomes, rich in UGTs31,32 and both microbiological33,34 and enzymatic approaches,35,36 although the former seem best suited to the production of relatively low levels of material and may require considerable optimization of conditions.The question of whether all polyphenols are glucuronidated at each hydroxyl function equally effectively, is beginning to be addressed. For quercetin 5 (Section 5), considered one of the most heavily consumed flavonoids on a daily basis (10–100 mg per day)37 and possessing five hydroxy groups, not all glycosylated forms were observed following incubation with cell or tissue extracts.37,38
2.3 Occurrence and isolation of glucuronides
From a practical perspective, it was also noted that it is much easier to extract the phenolic form than the glucuronide from tissues, since the aqueous solubility of the latter carries through many other water-soluble components.39 The broader question of whether all dietary polyphenols are dealt with through glucuronidation has also been examined. Ingestion of a soya preparation by healthy volunteers and subsequent analysis of their plasma, identified numerous 4′- and 7-monoglucuronides and 4′,7-di-glucuronides as well as sulfates of daidzein and genistein40 (see Section 6), but only low levels of the intact isoflavones. Many other, often larger (potentially presenting more difficult substrates for glucuronosyltransferase enzymes) polyphenolic compounds remain to be studied. Distinct selectivity among the UGTs that add the GlcA moiety has been observed between animal species. Thus, in oxyresveratrol (Fig. 4; cf. Section 4), human enzymes preferentially add GlcA to stilbene molecules at the 2′-OH group whereas those from rats favour the 3-OH,41 suggesting the possibility of evolutionary adaptation that will be interesting to pursue.Novel glucuronides also continue to be unearthed, including an S-bridged pyranonaphthoquinone dimer 6 to which a modified GlcA is appended.42 Unsaturated glucuronic acid residues such as in 6 may be formed as by-products from the hydrolysis step in chemical synthesis,11 cf. Fig. 2.
2.4 Glucuronides and glucosides
Since glucosides are generally easier to prepare than glucuronides, glucuronide synthesis via a final oxidative step from a glucoside (viz. CH2OH to CO2H using e.g. TEMPO) is a good alternative.11 Indeed, phenolic glucosides may themselves be naturally occurring, e.g. diadzin, a glucoside of daidzein (Section 6). In particular, this offers an attractive route to 13C-labelled glucuronides to aid mass spectrometric or 13C NMR spectroscopic detection. Deuterium labelling may be similarly introduced and the use of 19F labelled glucuronides allows specific NMR detection of both the glucuronide and parent substance.43 A recent report collates the preparation of a diverse collection of coumarin derivatives, including some β-O-glycosides, some of which may provide access to the glucuronide by this direct oxidation route.443. Phenol carboxylic acids
Phenolic carboxylic acids, especially cinnamic acids, are widely distributed. Both the O-aryl 7 and O-acyl glucuronide 8 of coumaric acid are known; the further oxygenated cinnamic acids caffeic acid 9 and ferulic acid 10 can form both isomeric 3- and 4-O-aryl glucuronides, in the case of 9, and O-acyl glucuronides. Ferulic acid 10 is commonly used as a skin care additive and is regarded as a safe, mild anti-inflammatory agent.45 Rigorous identification of glucuronide and sulfate metabolites of hydroxycinnamic acids following coffee consumption identified dihydroisoferulic acid 3-O-glucuronide, caffeic acid 3-O-sulfate, and both the sulfate and glucuronide adducts of 3,4-dihydroxyphenylpropionic acid.46 A large number of glucuronide and sulfate metabolites of this class have been synthesised as standards.47Differences between the antioxidant properties of caffeic and ferulic acids suggest that the methyl ether in the latter has a significant effect and that, while some adducts showed reduced antioxidant activity compared to their parent compound, including ferulic acid 4-O-sulfate and 4-O-glucuronide, others such as ferulic acid acyl glucuronide and caffeic acid 3-O-glucuronide, retained significant activity.48
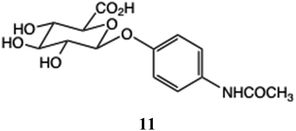
The glucuronides of hydroxycinnamic acid exhibit activity against the mammalian enzyme heparanase,49 which is linked to the regulation of many processes including inflammation. Numerous established drugs, including the very widely used phenolic analgesic and antipyretic agent, paracetamol (N-acetyl p-aminophenol), bind to human serum albumin, the most abundant serum protein present at around 40 mg mL−1, which affects both its pharmacokinetics and efficacy. Paracetamol O-glucuronide 11 is the major in vivo metabolite,50 (60%) with the O-sulfate (30%) also important.
It is reasonable to assume that glucuronide forms of other phenolic compounds may also bind albumin. Interactions of hydroxycinnamic glucuronides with human serum albumin, including affinity measurements, employing absorbance and fluorescence spectroscopy have been conducted51 which confirm binding and suggest that similar measurements will be relevant for other glucuronides, especially in assessment of their health-related effects.
4. Stilbenes
Resveratrol (3,5,4′-trihydroxystilbene), has long been studied for its potential medicinal benefits including anti-HIV activity52 and there is accumulating evidence that its two monoglucuronides 12 and 13 have activity in their own right, notably a cholesterol lowering effect.53 Resveratrol, its 4′-O-glucuronide, 13, and its 3-O-sulfate derivative have been shown to have subtly different delipidating effects on both maturing and mature adipocytes.54,55 As a stilbene, resveratrol can exist in both E and Z configurations, and we recently showed that the 3-glucuronide 12 undergoes isomerisation under remarkably mild conditions (diffuse light, D2O solution, 20 °C) to afford up to a 9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Z
1 Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E mixture. Under the same conditions, however, isomerism of the 4′-glucuronide 13 was negligible:56 the implications for biological activity are currently unclear. Thus, new features continue to be uncovered and distinct properties are emerging for distinct glucuronides of the same polyphenol. The glucuronide of structurally related oxyresveratrol (scire licet 2,3′,4,5′-tetrahydroxystilbene, Fig. 4) is known57 and the glucuronidated forms of further hydroxylated analogues, formed with varying efficiency, have been examined; some also exhibit anti-inflammatory effects.41
E mixture. Under the same conditions, however, isomerism of the 4′-glucuronide 13 was negligible:56 the implications for biological activity are currently unclear. Thus, new features continue to be uncovered and distinct properties are emerging for distinct glucuronides of the same polyphenol. The glucuronide of structurally related oxyresveratrol (scire licet 2,3′,4,5′-tetrahydroxystilbene, Fig. 4) is known57 and the glucuronidated forms of further hydroxylated analogues, formed with varying efficiency, have been examined; some also exhibit anti-inflammatory effects.41The glucuronidation of the dimethyl ether pterostilbene 14, likewise a common dietary substance, is much less efficient than that of resveratrol58 and it would be interesting to evaluate the glucuronide per se. The glucuronide and sulfate derivative of 14 have been shown to prevent triglyceride accumulation in a mouse hepatocyte model.59 The 3-glucuronide of deoxyrhapontigenin 15, scire licet resveratrol 4-O-Me ether, is also known to cross the blood–brain barrier.16
5. Flavonoids including anthocyanidins
Flavonoids 16, or 2-arylchromene-4-ones, are positional isomers of 3-arylchromene-4-ones, or isoflavonoids (Section 6): both classes are abundantly present in many foodstuffs. Quercetin 5, vide supra, is a pentahydroxy derivative; other examples may contain fewer OH groups, or partial O-methylation, e.g. kaempferide 17. Partially reduced forms, e.g. naringenin 18 (a flavanone) and (epi)-catechins 19 and 20 are also important and introduce chirality at C(2) and C(3).
The synthesis of the simpler o-, m- and p-cresyl glucuronides using the anomeric tetraester method (vide supra) and subsequent toxicity testing revealed subtle differences, especially for the o-isomer.4 These findings also suggest it is likely that regioisomeric glucuronides of individual polyphenols, such as quercetin, the major dietary flavanol, will also exhibit distinct properties but this remains to be tested; cf. the association of the red wine hangover effect with specifically quercetin 3-glucuronide.10 The related anthocyanidins 21 are true flavonoids and form the colour principals of many plants: like uncharged flavonoids, they are strong antioxidants, and typically occur as glycosides called anthocyanins.60
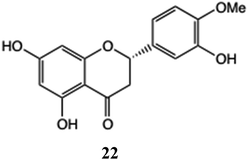
Several syntheses have been reported for quercetin glucuronides. One of the synthetic challenges is to enable appropriate selection of one of the five hydroxyl groups of 5 to act as acceptor and this has been met through selective protection to provide the 3-O-glucuronide,61 as well as both the 3-O- and 3′-O-forms via a 4′,7- di-O-benzyl protected derivative.62a,b Other approaches include the generation of the 3′-O-glucuronide starting from the readily available quercetin-3-O-rutinoside and 3′,4′,5,7-tetra-O-benzyl quercetin intermediates.63 The tissue distribution and pharmacokinetics of a novel derivative of hesperetin 22 and its glycoside and sulfate, have been explored62 revealing that, following a single dose, while the free hesperetin persisted in all organs assayed, the glucuronidated and sulfated forms predominated in plasma, liver, kidney and in the intestine.
Direct comparisons of the biological activities of the phenolic component and their respective glucuronide forms remain comparatively rare. Quercetin 5 is an inhibitor of xanthine oxidase, and is one example for which comparisons of the activities of the aglycone and glucuronide forms of these molecules can be made. The aglycone inhibits both xanthine oxidase64 and lipoxygenase65 activity.
Xanthine oxidase catalyses oxidation of hypoxyxanthine to xanthine, and to uric acid, generating superoxide radicals that are implicated in pathologies which include inflammation and atherosclerosis, but are also involved in the process of ageing.66 It has been reported that the different hydroxyl functions of quercetin67 exhibit distinct susceptibilities to glucuronidation by UDP-glucuronosyl transferase.
The preference for glucuronidation, referring to the hydroxyl groups of quercetin, follows the order 4′ > 3′ > 7 > 3 (Fig. 5) with no evidence for glucuronide formation at the 5-position;68 likewise, the glucosides of quercetin lack those formed at the 5-position.69 Other naturally occurring glycosides of quercetin are typically conjugated at the 3- and 4′-position.69 The order of inhibition with xanthine oxidase follows the same pattern, with a Ki of 0.25 μM for the most potent (the 4′-O-glucuronide form). All forms, with the exception of the 3-glucuronide, are capable of inhibiting lipoxygenases which catalyse the oxidation of polyunsaturated fatty acids.69 The specific attribution of the red wine hangover effect to the 3-O-glucuronide was noted earlier.10
Quercetin is a potent competitive inhibitor of both the oxidative activity of xanthine oxidase and its dehydrogenase activity,70 with Ki values of 0.13 and 0.2 μM respectively. Those authors suggest that, since this represents similar levels of activity to allopurinol, the anti-uremic drug, then quercetin may have some therapeutic potential. Quercetin also possesses free-radical scavenging activity71 and could conceivably act to quench radical intermediates, although other, unrelated antioxidants lacked this inhibitory capability. These observations illustrate the multi-faceted nature of these in vivo activities and emphasize that both caution and careful experimental design need to be exercised when investigating them. Two quercetin derivatives, the chiral 2,3-dihydroquercetin and the 3-L-rhamnoside glycoside, quercitrin, both failed to show any inhibitory effect70 providing further evidence that this activity is independent of antioxidative potency per se, and that subtle structural requirements are at play in the inhibition of xanthine oxidase.
A further study72 examined the activities of a series of common flavonoids and their 3-O- and 7-O-glucuronides for anti-inflammatory effects against polymorphonuclear leukocyte (PMN) and human umbilical vein endothelial (HUVEC) cells. Only the aglycone forms exhibited anti-inflammatory effects, and most strikingly in HUVEC cells, in which ICAM, VCAM and E-selectin were downregulated. Additionally, IL-6 (against which the most effective were kaempferide 17, apigenin and luteolin) and IL-8 (apigenin and luteolin) secretion were reduced. β-Glucuronidase activity was also detected at sites of inflammation and, overall, flavones as a class exhibited the most effective anti-inflammatory activity. Interestingly, kaempferide 17 (the methyl ether of kaempferol) was more effective than kaempferol in reducing IL-6 secretion.
Anti-inflammatory activities of compounds from Bergamot (Citrus bergamia), including the 3′-O-glucuronide of hesperetin 22 have been noted,73 employing a zebra fish model. Hesperetin 7-glucuronide has been reported to protect pancreatic cells against stress induced by cholesterol metabolism.74,75 The 8-O-glucuronide of a plant-based flavone (from Malva verticillate) was found to be an effective antioxidant in a zebra fish pancreatic islet model,76 highlighting that structural specificity deserves attention in this class of compounds.
6. Isoflavonoids
The glucuronides of the common isoflavones (3-arylchromene-3-ones) daidzein 23 and genistein 24 have been fairly well studied. Efficient chemical synthesis of derivatives requires differentiation of the hydroxyl groups, though the 5-OH of genistein, being hydrogen bonded, is difficult to react.77,78a,b Both 23 and 24 are estrogenic.Here, partially reduced forms, lacking the pyran ring functionality are also important. Thus, equol 25 (ref. 79) is the eventual product of oxidative metabolism of daidzein80 and its glucuronides are also significant.81 The existence of a chiral centre introduces additional complexity and the properties of both (R)- and (S)-equol and their glucuronides merit study. Similarly, O-desmethylangolensin 26 and its glucuronide are products of the bacterial metabolism of 23, reportedly produced in some, but not all, individuals.82
While the activities of daidzein and related isoflavones have been reviewed,83 it has been reported that the 7-O-glucuronide of (R/S) equol, the racemic mixture of 25, modulates migration and tubulogenesis in human aortic endothelial cells via VEGF (vascular endothelial growth factor), a property that is not shared by the glucuronides of daidzein, 23, or genistein, 24.84 A pharmacokinetic study found that the glucuronides were well absorbed in rats85 and the glucuronides of daidzein and genistein activated human natural killer cells (of the innate immune system) and were mildly estrogenic.86 The enzymatic synthesis of the glycosides of daidzein has been reported87 and these could serve as intermediates for the respective glucuronides: indeed, daidzin, the 7-O-glucoside of daidzein, is itself naturally occurring in soy leaves. Health benefits that have been claimed for 25 include broad measures of health such as lowered blood pressure79 and, on the basis of the higher affinity of equol for the estrogen receptor and its improved anti-oxidant activities compared to daidzein, some claims for improved bone health and other factors have been made although, as noted, the number of well-controlled studies into these effects is low.79
7. Coumarins
Coumarins, especially when 3-substituted, have proved interesting through their potential as inhibitors of β-glucuronidases.88 The glucuronide of 7-hydroxycoumarin 27 (7-HCG) is the usual end in vivo metabolite of coumarin, present in many foods and cosmetics, and is readily accessed via imidate 3.89 There is relatively little information concerning the biological activity of the glucuronide, beyond in vivo measurements of its hydrolysis by liver enzymes90 although a recent paper showed that 7-HCG could protect against nephrotoxicity induced by cisplatin in mice.91
8. Conclusions: towards the application of dietary glucuronides?
Once thought of solely as a means of rendering relatively insoluble phenolic compounds more soluble and more easily excreted as part of phase II metabolism, glucuronidation is now understood to be a more complex and nuanced process. While the rate of formation of glucuronides is governed by substrate susceptibility to UGTs (of which there are 22 in humans), their removal depends on their rate of egress from cells by efflux transporters. Hepatocytes can clear glucuronides via the biliary system efficiently and it is clear that there can be re-hydrolysis of the glucuronides by glucuronidase enzymes from bacteria in the large intestine as well as subsequent processing of the aglycone component.13 The availability of a wider variety of glucuronides through synthesis is enabling their more detailed study.5The relatively poor uptake of glucuronides compared to the corresponding polyphenols makes using glucuronide prodrugs an attractive mode of delivery.92 There is some precedence, in that the drug-derived (gemfibrozil and clopidogrel) glucuronides are substrates for human CYP450 2C8,93 and as inhibitors of Apaf-1, an enzyme involved in ischaemia.94 Given the considerable levels of polyphenols consumed, up to 0.5 g daily,51 the extent to which these enter the blood and, in keeping with well-studied pharmaceuticals such as paracetamol,95 bind human serum albumin, is also likely to be relevant to the extent and duration of any subsequent effects.
It is clear that, despite the once presumed neutralising effect of glucuronidation of phenolic compounds, their glucuronides possess a range of distinct biological activities in their own right. Subtle differences in cell toxicity have been shown to exist between the glucuronides of the isomeric cresols, o-, m- and p-methylphenol, the latter being the microbial glucuronide derived from the metabolism of tyrosine.4 Given that similar observations also extend to the various glucuronides of quercetin 5 (ref. 68) and caffeic acid 7,68 combined with differences in physicochemical properties, for example, between the 3-O- and 4′-O-glucuronides of resveratrol,56 this seems likely to be a general property. Further work is therefore required to provide a more complete picture of the variation in the biological activities of glucuronides of particular polyphenols.
Recent findings reveal that the 7-O-glucuronide of equol (the racemic R/S forms of 25), in contrast to other circulating isoflavone adducts, targets the VEGF signalling pathway to alter endothelial cell migration and tubulogenesis (important processes in angiogenesis) at comparable levels to free genistein, daidzein and equol.81 This represents one of the most clear-cut examples of an individual glucuronide possessing selective activity, and is an important step in understanding the oft-quoted, but often ambiguous, beneficial effects attributed to these compounds. One biological activity that repeatedly emerges among many of the polyphenol classes, their glucuronides and, in some cases, also their sulphated adducts, is their ability to act as anti-inflammatory agents, and an emerging target is TLR4.7–9
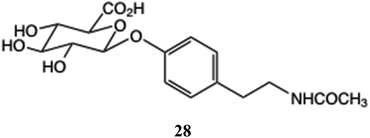
A further application of glucuronides is their potential as markers of disease. Two reports describing the use of N-acetyl tyramine glucuronide 28 (NATOG) to act as a diagnostic marker of river blindness (Onchocerciasis), caused by the nematode, Onchocerca volvulus have been published.96,97 This illustrates a potential use for synthetic glucuronides as standards in biologically and medically important investigations, which dietary glucuronides could follow, especially in relation to the human microbiome and dysbiosis. In the large intestine, bacterial glucuronidases are capable of releasing the phenolic and glucuronic acid components with the potential for subsequent toxic or beneficial effects to bacteria or host cells. The status of the gut microbiome has also been linked to the efficacy of anti-cancer agents, although the relationship is complex98 and there is future scope for action tailored towards individuals employing dietary glucuronides, either introduced directly or induced by providing food supplements containing selected polyphenols.
Despite the known specificities of some UGT enzymes, examples of which were mentioned above (Section 2), broad questions remain unanswered, or only partially answered, including whether mammalian enzymes are able to glucuronidate all (e.g. all dietary) polyphenols and to the same extent. The recent, but incomplete, evidence would suggest not41,99 – and what is the ultimate capacity of this system?
9. Outlook
We are starting to understand the wider roles that glucuronides (and sulfates) of dietary phenols play, and how their effects can differ not only from those of the parent phenols but also from each other. The rates of formation, and even the possibility of forming all possible glucuronide derivatives for a polyphenol, are now being addressed. Glucuronides formed from positional isomers within the aglycone moiety, such as the o-, m- and p-cresyl glucuronides, exhibit different biological activities,4 and unexpected structural subtleties are emerging, such as the varying rates of E/Z isomerization of resveratrol 3-O- and 4′-O-glucuronides,56 that suggest the possibility of distinct activities for the E/Z isoforms, in addition to those of each glucuronide.Increased access to some synthetic glucuronides, and emerging approaches for the preparation of a wider section of dietary glucuronides, will continue to enable more detailed studies of their metabolic roles. Isotopically labelled glucuronides, either in the phenolic or glucuronic acid component, present an attractive possibility for future studies of metabolism. One key challenge is to unravel the interplay between glucuronides, the various bacterial species of the microbiota possessing glucuronidase capabilities, the parent polyphenols and host cells. Significant synthetic challenges also remain, for example, the selective synthesis of the four possible glucuronides of the chiral product equol 25, which is the product of the oxidative metabolism of daidzein 23.
A universally applicable synthetic approach still appears distant, although the less demanding route of glucosidation, either chemically by established routes or enzymatically employing hydrolases such as β-glucosidase (a member of the GH1 family in the CAZy nomenclature) from almonds driven in reverse (EC. 3.2.1.21)100 or other glucosidases,101 followed by direct oxidation of the primary hydroxyl in situ to generate the glucuronic acid moiety, as well as some enzyme-based methods, merit further research. Nevertheless, aside from bespoke synthesis in the research laboratory, many derivatives are likely to continue to remain unavailable for some time.
One interesting development has been the growing establishment of a causal link between the microbiome status and the brain (the so-called gut–brain axis), in which secondary metabolites such as cresol sulfate act as mediators, and in which EGF receptor is emerging as a target. A second significant trend is the emerging role of TLR4 (linked to the inflammatory response) as a target for a wide range of derivatives including those of morphine, ethanol, and some endogenous compounds but for which, on occasion, the effect can be counter to the intended purpose of the parent compound; increasing, rather than reducing, pain.
The complex interplay between host, bacterial populations mediated by polyphenols and their glucuronides is now beginning to be appreciated and surely represents an area ripe for future investigation.
10. Author contributions
AVS originally proposed the highlight and jointly wrote the manuscript; EAY jointly wrote the manuscript; AT collated references and contributed to the manuscript; LH and SM added relevant references, commented on and enhanced the manuscript.11. Conflicts of interest
No conflicts of interest.12. Acknowledgements
LH is in receipt of funding from the European Union's Horizon 2020 research and innovation programme under grant agreement no. 874583. This publication reflects only the authors' views, and the European Commission is not responsible for any use that may be made of the information it contains.13. References
- H. Zhang and R. Tsao, Curr. Opin. Food Sci., 2016, 8, 33–42 CrossRef.
- T. Gryp, R. Vanholder, M. Vaneechoutte and G. Glorieux, Toxins, 2017, 9, 52 CrossRef PubMed.
- S. N. Shah, T. B.-A. Knausenberger, M. G. Pontifex, E. Connell, G. Le Gall, T. A. J. Hardy, D. W. Randall, K. McCafferty, M. M. Yaqoob, E. Solito, M. Muller, A. V. Stachulski, R. C. Glen, D. Vauzour, L. Hoyles and S. McArthur, Gut Microbes, 2024, 16, 2431651 CrossRef PubMed.
- J. A. London, E. C. S. Wang, I. L. Barsukov, E. A. Yates and A. V. Stachulski, Carbohydr. Res., 2021, 499, 108225 CrossRef CAS PubMed.
- A. Gorecka, H. Schacht, M. K Fraser, A. Teriosina, J. A. London, I. L. Barsukov, A. K. Powell, A. Cartmell, A. V. Stachulski and E. A. Yates, ACS Omega, 2024, 10, 1419–1428 CrossRef PubMed.
- A. V. Stachulski, T. B.-A. Knausenberger, S. N. Shah, L. Hoyles and S. McArthur, Tissue Barriers, 2023, 11, e2073175 CrossRef PubMed.
- S. S. Lewis, M. R. Hutchinson, Y. Zhang, D. K. Hund, S. F. Maier, K. C. Rice and L. R. Watkins, Brain, Behav., Immun., 2013, 30, 24–32 CrossRef CAS PubMed.
- S. S. Lewis, M. R. Hutchinson, N. Rezvani, L. C. Loram, Y. Zhang, S. F. Maier, K. C. Rice and L. R. Watkins, Neurosci, 2010, 165, 569–583 CrossRef CAS PubMed.
- S. S. Lewis, M. R. Hutchinson, M. M. Frick, Y. Zhang, S. F. Maier, T. Sammakia, K. C. Rice and L. R. Watkins, Brain, Behav., Immun., 2015, 44, 128–136 CrossRef CAS PubMed.
- A. Devi, M. Levin and A. L. Waterhouse, Sci. Rep., 2023, 13, 19503 CrossRef PubMed.
- A. V. Stachulski and X. L. Meng, Nat. Prod. Rep., 2013, 30, 806–848 RSC.
- R. Klimas and G. Mikus, Br. J. Anaesth., 2014, 113, 935–944 CrossRef CAS PubMed.
- G. Yang, S. Ge, R. Singh, S. Basu, K. Shatzer, M. Zen, J. Liu, Y. Tu, C. Zhang, J. Wei, J. Shi, L. Zhu, Z. Liu, Y. Wang, S. Gao and M. Hu, Drug Metab. Rev., 2017, 49, 105–138 CrossRef CAS PubMed.
- P. I. Mackenzie, K. W. Bock, B. Burchell, C. Guillemette, S.-I. Ikushiro, T. Iyanagi, J. O. Miners, I. S. Owens and D. W. Nebert, Pharmacogenet. Genomics, 2005, 15, 677–685 CrossRef CAS PubMed.
- P. M. Coutinho, E. Deleury, G. J. Davies and B. Henrissat, J. Mol. Biol., 2003, 328, 307–317 CrossRef CAS PubMed.
- A. de Fatima, M. Docampo-Palacios, A. Alvarez-Hernandez, G. M. Pasinetti and R. A. Nixon, ACS Omega, 2019, 4, 8222–8230 CrossRef CAS PubMed.
- S. J. Pellock and M. R. Redinbo, J. Biol. Chem., 2017, 292, 8569–8578 CrossRef CAS PubMed.
- A. W. Walker and L. Hoyles, Nat. Microbiol., 2023, 8, 1392–1396 CrossRef CAS PubMed.
- S. Gao, R. Sun, R. Singh, S. Y. So, L. T. Y. Chan, T. Savidge and M. Hu, Drug Discovery Today, 2022, 27, 103316 CrossRef CAS PubMed.
- S. Bucurica, M. Lupanciuc, F. Ionita-Radu, I. Stefan, A. E. Munteanu, D. Anghel, M. Jinga and E. L. Garman, Int. J. Mol. Sci., 2023, 24, 16034 CrossRef CAS PubMed.
- M. M. Elmassry, S. Kim and B. Busby, PLoS One, 2021, 0244876 Search PubMed.
- A. N. Chamseddine, M. Ducreux, J.-P. Armand, X. Paoletti, T. Satar, A. Paci and O. Mir, Pharmacol. Ther., 2019, 199, 1–15 CrossRef CAS PubMed.
- B. Yue, R. Gao, Z. Wang and W. Dou, Front. Cell. Infect. Microbiol., 2021, 11, 710945 CrossRef CAS PubMed.
- M. L. Fernandez-Murga, F. Gil-Ortiz, L. Serrano-Garcia and A. Llombart-Cussac, Pathogens, 2023, 12, 1086 CrossRef CAS PubMed.
- R. M. Pollet, E. H. D'Agostino, W. G. Walton, Y. Xu, M. S. Little, K. A. Biernat, S. J. Pellock, L. M. Patterson, B. C. Creekmore, H. N. Isenberg, R. R. Bahethi, A. P. Bhatt, J. Liu, R. Z. Gharaibeh and M. R. Redinbo, Structure, 2017, 25, 967–977 CrossRef CAS PubMed.
- F. Candeliere, S. Raimondi, R. Ranieri, E. Musmeci, A. Zambon, A. Amaretti and M. Rossi, Front. Microbiol., 2022, 13, 826994 CrossRef PubMed.
- B. Werschkun, K. Gorziza and J. Thiem, J. Carbohydr. Chem., 1999, 18, 629–637 CrossRef CAS.
- R. R. Schmidt, Angew. Chem., Int. Ed. Engl., 1986, 25, 212–235 CrossRef.
- B. Yu and J. Sun, Chem. Commun., 2010, 46, 4668–4679 RSC.
- R. Walther, M. T. Jaristad Olesen and A. N. Zelikin, Org. Biomol. Chem., 2019, 17, 6970–6974 RSC.
- M. Docampo, A. Olubu, X. Wang, G. Pasinetti and R. A. Dixon, J. Agric. Food Chem., 2017, 65, 7607–7623 CrossRef CAS PubMed.
- G. M. Woodward, P. W. Needs and C. D. Kay, Mol. Nutr. Food Res., 2011, 5, 378–386 CrossRef PubMed.
- C. Marvalin and R. Azerad, J. Mol. Catal. B: Enzym., 2011, 73, 43–52 CrossRef CAS.
- T. Ohnuki, M. Ejiri, M. Kizuka, M. Fujiwara and T. Nishi, Bioorg. Med. Chem. Lett., 2019, 29, 199–203 CrossRef CAS PubMed.
- J. W. Blount, B. W. Redan, M. G. Ferruzzi, B. L. Reuhs, B. R. Cooper, J. S. Harwood, V. Shulaev, G. Pasinetti and R. A. Dixon, J. Agric. Food Chem., 2015, 63, 2233–2240 CrossRef CAS PubMed.
- S. A. Robotham and J. S. Broadbelt, J. Agric. Food Chem., 2013, 61, 1457–1463 CrossRef CAS PubMed.
- M. Kajjout and C. Rolando, Tetrahedron, 2011, 67, 4731–4741 CrossRef CAS.
- H. van der Woude, M. G. Boersma, J. Vervoort and I. M. C. M. Rietjens, Chem. Res. Toxicol., 2004, 17, 1520–1530 Search PubMed.
- A. Boumendjel, M. Blanc, G. Williamson and D. Barron, J. Agric. Food Chem., 2009, 57, 7264–7267 CrossRef CAS PubMed.
- K. Hosoda, T. Furuta, A. Yokokawa and K. Ishii, Anal. Bioanal. Chem., 2010, 397, 1563–1572 CrossRef CAS PubMed.
- R. Hornedo-Ortega, M. Jourdes, G. Da Costa, A. Courtois, J. Gabaston, P.-L. Teissedre, T. Richard and S. Krisa, J. Agric. Food Chem., 2022, 70, 13082–13092 CrossRef CAS PubMed.
- Q. Che, H. Tan, X. Han, X. Zhang, Q. Gu, T. Zhu and D. Li, Org. Lett., 2016, 18, 3358–3361 CrossRef CAS PubMed.
- A. Teriosina, I. L. Barsukov, A. Cartmell, A. K. Powell, A. V. Stachulski and E. A. Yates, Anal. Methods, 2025, 17, 2015–2020 RSC.
- S. Dwivedi, S. Dey and A. Sau, Carbohydr. Res., 2024, 544, 109244 CrossRef CAS PubMed.
- A. Alam, Front. Nutr., 2019, 6, 00121 CrossRef PubMed.
- R. Fumeaux, C. Menozzi-Smarrito, A. Stalmach, C. Munari, K. Kraehenbuehl, H. Steiling, A. Crozier, G. Williamson and D. Barron, Org. Biomol. Chem., 2010, 8, 5199–5211 RSC.
- A. Filipa Almeida, C. N. Santos and M. Rita Ventura, J. Agric. Food Chem., 2017, 65, 6460–6466 CrossRef PubMed.
- A. Piazzoni, U. Vrhovesk, D. Masuero, F. Mattivi, F. Mandori and M. Nardini, J. Agric. Food Chem., 2012, 60, 12312–12323 CrossRef PubMed.
- A. G. Pearson, M. J. Kiefel, V. Ferro and M. von Itzstein, Carbohydr. Res., 2005, 340, 2077–2085 CrossRef CAS PubMed.
- L. Zhao and G. Pickering, Drug Metab. Rev., 2011, 43, 41–52 CrossRef CAS PubMed.
- S. Galland, N. Rakotomanomana, C. Dufour, N. Mora and O. Dangles, Org. Biomol. Chem., 2008, 6, 4253–4260 RSC.
- L.-X. Wang, A. Hereida, H. Song, Z. Zhang, B. Yu, C. Davis and R. Redfield, J. Pharm. Sci., 2004, 93, 2448–2457 CrossRef CAS PubMed.
- D. Shao, Y. Wang, Q. Huang, J. Shi, H. Yang, Z. Pan, M. Jin, H. Zhao and X. Xu, J. Food Sci., 2016, 81, H2841–H2848 CrossRef CAS PubMed.
- A. Lasa, I. Churruca, I. Eseberri, C. Andres-Lacueva and M. P. Portillo, Mol. Nutr. Food Res., 2012, 56, 1559–1568 CrossRef CAS PubMed.
- I. Eseberri, A. Lasa, I. Churruca and M. P. Portillo, PLoS One, 2013, 8, e63918 CrossRef PubMed.
- M. G. Fraser, A. Gorecka, E. A. Yates, J. A. Iggo, K. Baj and A. V. Stachulski, Org. Chem. Front., 2024, 11, 2720–2726 RSC.
- N. Hu, M. Mei, J. Ruan, W. Wu, Y. Wang and R. Yan, Drug Metab. Pharmacokinet., 2014, 29, 229–236 CrossRef CAS PubMed.
- R. W. Dellinger, A. M. Gomez Garcia and F. L. Meyskens Jr, Drug Metab. Pharmacokinet., 2014, 29, 112–119 CrossRef CAS PubMed.
- J. Trepiana, S. Krisa and M. P. Portillo, Molecules, 2020, 25, 5444 CrossRef CAS PubMed.
- H. Gui, L. Sun, R. Lui, X. Si, D. Li, Y. Wang, C. Shu, X. Sun, Q. Jiang, Y. Qiao, B. Li and J. Tian, Crit. Rev. Food Sci. Nutr., 2023, 63, 5953–5966 CrossRef CAS PubMed.
- M. Boubktaib, A. Atmani and C. Rolando, Tetrahedron Lett., 2002, 43, 6263–6266 CrossRef.
- (a) C. Shen, Z. Qian, R. Chen, X. Mang, T. Hu, Z. Chen, Y. Li, C. Huang, C. Hu and J. Li, Eur. J. Drug Metab. Pharmacokinet., 2016, 41, 675–688 CrossRef PubMed; (b) P. W. Needs and P. A. Kroon, Tetrahedron, 2006, 62, 6862–6868 CrossRef CAS.
- M. Kajjout, R. Zemmouri and C. Rolando, Tetrahedron Lett., 2011, 52, 4738–4740 CrossRef CAS.
- P. Cos, L. Ying, M. Calomme, J. P. Hu, K. Cimanga, B. Van Poel, L. Pieters, A. J. Vlietinck and D. Van den Berghe, J. Nat. Prod., 1998, 61, 71–76 CrossRef CAS PubMed.
- E. L. Da Silva, T. Tsushida and J. Terao, Arch. Biochem. Biophys., 1998, 349, 313–320 CrossRef CAS PubMed.
- B. Halliwell, J. M. Gutteridge and C. E. Cross, J. Lab. Clin. Med., 1992, 119, 598–620 CAS.
- J. Terao, Antioxidants, 2023, 12, 258 CrossRef CAS PubMed.
- A. J. Day, Y. Bao, M. R. A. Morgan and G. Williamson, Free Radical Biol. Med., 2000, 29, 1234–1243 CrossRef CAS PubMed.
- A. Murakami, H. Ashida and J. Terao, Cancer Lett., 2008, 269, 315–325 CrossRef CAS PubMed.
- A. Bindoli, M. Valente and L. Cavallini, Pharmacol. Res. Commun., 1985, 17, 831–839 CrossRef CAS PubMed.
- B. Havsteen, Biochem. Pharm., 1983, 32, 1141–1148 CrossRef CAS PubMed.
- B. Zyzynska-Granica, B. Gierlikowska, A. Parazonko, A. K. Kiss and S. Granica, Food Chem. Toxicol., 2020, 135, 110929 CrossRef CAS PubMed.
- V. Spigoni, P. Mena, F. Fantuzzi, M. Tassotti, F. Brighenti, R. C. Bonadonna, D. del Rio and A. Dei Cas, Nutrients, 2017, 9, 1328 CrossRef PubMed.
- S. L. Anacleto, D. Milenkovic, P. A. Kroon, P. W. Needs, F. M. Lajolo and N. M. A. Hassimotto, Food Funct., 2020, 11, 8612–8624 RSC.
- L. N. Fraga, S. L. Anacleto, D. Milenkovic, F. M. Lajolo and N. M. A. Hassimotto, Food Funct., 2022, 13, 12983–13001 RSC.
- J.-H. Ko, Y. H. Nam, S.-W. Joo, H.-G. Kim, Y.-G. Lee, T. H. Kang and N.-I. Baek, Molecules, 2018, 23, 833 CrossRef PubMed.
- P. W. Needs and G. Williamson, Carbohydr. Res., 2001, 330, 511–515 CrossRef CAS PubMed.
- (a) N. Al-Maharik and N. P. Botting, Tetrahedron Lett., 2006, 47, 8703–8706 CrossRef CAS; (b) N. Al-Maharik and N. P. Botting, Eur. J. Org. Chem., 2008, 5622–5629 CrossRef CAS.
- P. Magee, Proc. Nutr. Soc., 2011, 70, 10–18 CrossRef CAS PubMed.
- K. Noda, Y. Hattori, H. Murata, Y. Kokubo, A. Higashiyama and M. Ihara, Nutrients, 2024, 16, 3377 CrossRef CAS PubMed.
- J. A. Gimenez-Bastida, M. A. Avila-Galvez, A. Martinez-Lopez, D. Garcia-Moreno, J. Carlos Espin and A. Gonzalez-Sarrias, Food Funct., 2024, 15, 7387–7399 RSC.
- C. L. Frankenfeld, Adv. Nutr., 2011, 2, 317–324 CrossRef CAS PubMed.
- M. M. Alshehri, J. Sharifi-Rad, J. Herrera-Bravo, E. L. Jara, L. A. Salazar, D. Kriegel, Y. Uprety, M. Akram, M. Iqbal, M. Martorell, M. Torrens-Mas, D. G. Pons, S. D. Dastan, N. Cruz-Martins, F. A. Ozdemir, M. Kumar and W. C. Cho, Oxid. Med. Cell. Longevity, 2021, 6331630 CrossRef CAS PubMed.
- J. A. Gimenez-Bastida, M. A. Avila-Galvez, A. Martinez-Lopez, D. Garcia Moreno, J. C. Espin and A. Gonzalez-Sarrias, Food Funct., 2024, 15, 7387–7399 RSC.
- F. Qiu, X.-Y. Chen, B. Song, D.-F. Zhong and C.-X. Li, Acta Pharmacol. Sin., 2005, 26, 1145–1152 CrossRef CAS PubMed.
- Y. Zhang, T. T. Song, J. A. Cunnick, P. A. Murphy and S. Hendrich, J. Nutr., 1999, 129, 399–405 CrossRef CAS PubMed.
- Y. Fujitaka, H. Hamada, D. Uesugi, A. Kuboki, K. Shimoda, T. Iwaki, Y. Kiriake and T. Saikawa, Molecules, 2019, 24, 2975 CrossRef CAS PubMed.
- N. Arif, Z. Shafiq, K. Mahmood, M. Rafiq, S. Naz, S. A. Shahzad, U. Farooq, A. H. Bahkali, A. M. Elgorban, M. Yaqub and A. El-Gokha, ACS Omega, 2022, 7, 28605–28617 CrossRef CAS PubMed.
- R. T. Brown, F. Scheinmann and A. V. Stachulski, J. Chem. Res., 1997, 370–371 RSC.
- A. J. Killard, R. O'Kennedy and D. P. Bogan, J. Pharm. Biomed. Anal., 1996, 14, 1585–1590 CrossRef CAS PubMed.
- H. J. Wu, Life Sci., 2023, 327, 121864 CrossRef CAS PubMed.
- R. Walther, J. Rautio and A. N. Zelikin, Adv. Drug Delivery Rev., 2017, 118, 65–77 CrossRef CAS PubMed.
- Y. Ma, Y. Fu, S. Cyrus Khojasteh, D. Dalvie and D. Zhang, J. Med. Chem., 2017, 60, 8691–8705 CrossRef CAS PubMed.
- Y. Wang, Y. Cao, Q. Zhu, X. Gu and Y. Z. Zhu, Sci. Rep., 2016, 6, 29820 CrossRef CAS PubMed.
- S. H. Rutherford, G. M. Greetham, M. Towrie, A. W. Parker, S. Kharratian, T. F. Krauss, A. Nordon, M. J. Baker and N. T. Hunt, Analyst, 2022, 147, 3464–3469 RSC.
- D. Globisch, A. Y. Moreno, M. S. Hixon, A. A. K. Nunes, J. R. Denery, S. Specht, A. Hoerauf and K. D. Janda, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 4218–4223 CrossRef CAS PubMed.
- D. Globisch, L. M. Eubanks, R. J. Shirey, K. M. Pfarr, S. Wanji, A. Y. Debrah, A. Hoerauf and K. D. Janda, Bioorg. Med. Chem. Lett., 2017, 27, 3436–3440 CrossRef CAS PubMed.
- L.-Y. Zhao, J.-X. Mei, G. Yu, L. Lei, W.-H. Zhang, K. Liu, X.-L. Chen, D. Kolat, K. Yang and J.-K. Hu, Signal Transduction Targeted Ther., 2023, 8, 201 CrossRef PubMed.
- M. Kajjout and C. Rolando, Tetrahedron, 2011, 67, 4731–4741 CrossRef CAS.
- G. Vic and D. H. G. Crout, Carbohydr. Res., 1995, 279, 315–319 CrossRef CAS.
- M. Kotik, L. Kulik and K. Valentova, J. Agric. Food Chem., 2023, 71, 14890 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |