Open Access Article
Open Access ArticleChirality generation on carbon nanosheets by chemical modification
Ryo
Sekiya
 *ab,
Saki
Arimura
a,
Haruka
Moriguchi
a and
Takeharu
Haino
*ab,
Saki
Arimura
a,
Haruka
Moriguchi
a and
Takeharu
Haino
 *ac
*ac
aDepartment of Chemistry, Graduate School of Advanced Science and Engineering, Hiroshima University, 1-3-1 Kagamiyama, Higashi-Hiroshima, Hiroshima, 739-8526, Japan
bDepartment of Frontier Materials Chemistry, Graduate School of Science and Technology, Hirosaki University, 3 Bunkyo-cho, Hirosaki, Aomori 036-8561, Japan. E-mail: csekiya@hirosaki-u.ac.jp
cInternational Institute for Sustainability with Knotted Chiral Matter (SKCM2), Hiroshima University, 1-3-1 Kagamiyama, Higashi-Hiroshima, Hiroshima, 739-8526, Japan. E-mail: haino@hiroshima-u.ac.jp
First published on 15th November 2024
Abstract
Chirality is an intriguing property of molecules, and an exciting area of study involves the generation of chirality in nanographenes (NGs), also known as graphene quantum dots. Unlike those synthesized through stepwise carbon–carbon bond formation by organic reactions (bottom-up method), NGs obtained by cutting parent carbons (top-down method) pose challenges in precisely regulating their three-dimensional structures by post-synthesis. This includes the incorporation of non-hexagonal rings and helicene-like structures in carbon frameworks. Currently, edge functionalization is the only method for generating chirality in NGs produced by the top-down method. While various chiral NGs have been synthesized through organic methods, examples of chemical modification remain rare due to limited structural information and the substantial size of NGs. However, these problems can be mitigated by disclosing the structures of NGs, particularly their edge structures. This mini-review focuses on recently published papers that have addressed the structural characterization of NGs and their chirality generation by edge modification. Comparing these NGs with those synthesized by organic synthesis will help to develop reasonable strategies for creating sophisticated chiral NGs. We hope this mini-review contributes to the advancement of NG–organic hybrid materials.
Introduction
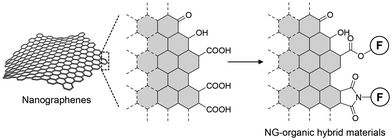
Chirality significantly impacts homochirality. A molecule becomes chiral when it lacks an improper axis of symmetry, such as a plane of symmetry or a center of inversion. Helically curved polycyclic aromatic hydrocarbons (PAHs), known as helicenes,1 exhibit exceptional chiroptical properties compared to their linear counterparts. Recently, chiral nonplanar PAHs, also known as chiral nanocarbons or chiral nanographenes (NGs), have become a significant area of study in synthetic organic chemistry. Researchers have developed a diverse range of nonplanar chiral NGs through manipulating their three-dimensional structures by incorporation of helicene-like frameworks and/or non-hexagonal rings.These NGs were synthesized through stepwise carbon–carbon bond formation and aromatization (bottom-up method). However, the production of NGs is not limited to organic synthesis. NGs can also be obtained through the cleavage of parent carbons, such as graphite and carbon fibers (top-down method). These NGs typically have diameters ranging from a few to tens of nanometers and can be considered graphene fragments.2,3 Although these NGs are mixtures of various sizes and shapes, their gram-scale production within a few reaction steps is attractive for developing NG–organic hybrid materials. During the oxidative cleavage of the parent carbons, oxygen-containing functional groups, such as carboxy and hydroxy groups, are generated on the edges, enabling the chemical modification of NGs (Fig. 1).4–13 For example, the installation of naphthalene-1,8-diamine derivatives into the edges, allowed by covalent linkages between the edge and the installed small aromatic systems, facilitates electronic interactions between them.14 This connection of the two π systems influences their optical properties, resulting in photoluminescence (PL) in the near-infrared region. Edge functionalization with triphenylamines has offered electrochromic materials functioning in the near-infrared region.8 Recently, our group6,9 and Martín's group15 independently reported the controlled self-assembly of NGs. These examples demonstrated the potential of NGs in creating functional carbon materials.4
A remaining and underdeveloped topic is the generation of chirality in NGs through chemical modification. The combination of chirality and PL properties of NGs could yield excellent chiroptical properties, comparable to those produced by organic synthesis. However, chiral NGs synthesized by chemical modification are rare compared to those produced by organic synthesis. This scarcity is partly due to limited structural information on NGs, as they are composed of a nonstoichiometric mixture of graphene fragments of various sizes and shapes, and their structures are not fully disclosed. This lack of detailed structural information complicates the development of effective strategies for generating chirality in NGs. Additionally, the large surface area of NGs with diameters ranging from a few to tens of nanometers makes it challenging to rigidify and amplify the induced chirality. While the latter issue persists with top-down method-based NGs, the former can be mitigated by disclosing NG structures, particularly their edge structures. Hence, information from recent studies on the structural characterization of NGs and their chirality generation is crucial.
Based on this background, this mini-review is structured as follows: we first provide three representative production methods for NGs. We then focus on the generation of structures and chirality, discussing computational approaches that address the structural characterization of NGs. This information is valuable for developing effective strategies to create sophisticated chiral NGs. We hope this mini-review will contribute to advancing the field of NG–organic hybrid materials.
Discussions
Production methods can be categorized into bottom-up and top-down methods (Fig. 2). Bottom-up methods are further subdivided into organic synthesis and graphitization. As the NGs produced by these methods have distinct structures, their chirality generation strategies completely differ from each other. Briefly, the bottom-up methods embed chirality in carbon frameworks to realize chiral NGs, while the top-down method installs chiral sources into the edge of NGs to generate chirality on NGs.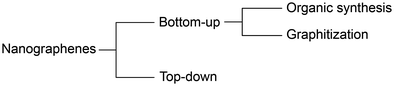 | ||
| Fig. 2 Classification of NG production methodologies: organic synthesis, graphitization, and top-down method. | ||
Organic synthesis
Organic synthesis involves constructing carbon frameworks through organic reactions. Stepwise carbon–carbon bond formation and cyclodehydrogenation with suitable oxidants, such as those through Pd-catalyzed cross-coupling and Scholl reactions, produce NGs. Fig. 3a shows a typical example of an NG produced by organic synthesis.16 The formation of carbon–carbon bonds and aromatization with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone yielded helically twisted NGs.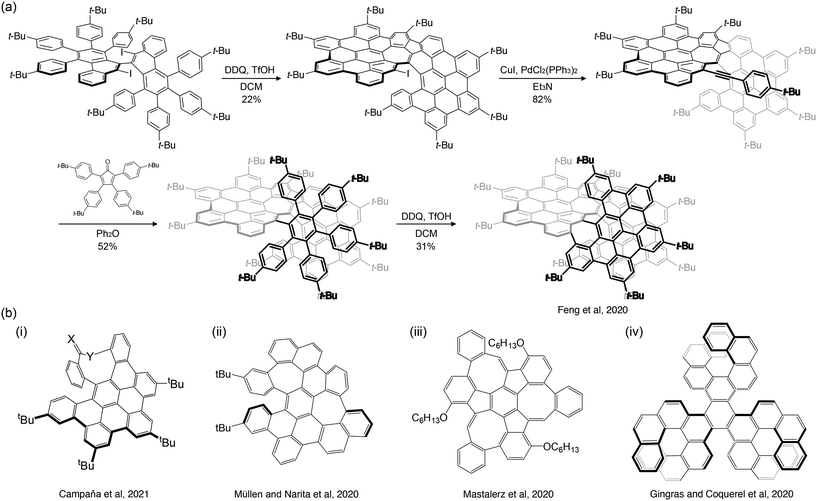 | ||
| Fig. 3 (a) Example of the synthesis of NG by organic synthesis (ref. 16). (b) Non-planar chiral NGs synthesized by organic synthesis as reported by (i) Campaña et al. (ref. 17), (ii) Müllen and Narita et al. (ref. 18), (iii) Mastalerz et al. (ref. 28), and (iv) Gingras and Coquerel et al. (ref. 20). | ||
Well-defined carbon frameworks allow for an in-depth analysis of their physical properties using spectroscopic methods, such as 1H- and 13C{1H}-nuclear magnetic resonance (NMR) spectroscopy, and absorption, circular dichroism (CD), and PL spectroscopy. Cyclic voltammetry also offers valuable information. Furthermore, these chiral NGs can yield single crystals, enabling single-crystal X-ray diffraction analysis. This analysis can reveal three-dimensional structures of NGs with angstrom-level accuracy. Computational approaches are also useful for assessing electronic structures.
Non-planar PAHs can be achieved by incorporating non-hexagonal ring(s) and/or helicene-like structure(s) into their carbon frameworks.21,22 A five- or four-membered ring can induce a positive curvature, whereas a seven- or eight-membered ring can induce a negative curvature in the carbon framework. The structures with the former curvature include fullerenes and corannulenes, while the latter curvature results in saddle-shaped carbon frameworks. Combining non-six-membered rings and helicene-like frameworks has led to numerous nonplanar chiral NGs.16–20,23–34 Due to limited space, only a few nonplanar chiral NGs reported in 2020–23 are briefly introduced in the following section.
Campaña et al. reported interesting chiral saddle–helix hybrid NGs (Fig. 3b-i),17 containing a nine-membered ring at their periphery, resulting in a sizeable interplanar angle (134.8°). Due to the rigidification of the carbon framework by the nonagonal ring, these NGs showed excellent tolerance for racemization. The same group also reported octagon-embedded NGs,26 which, like nonagonal-ring-embedded NGs, exhibited a significant twist in the carbon framework. A negatively curved NG containing a heptagon ring was reported by Müllen and Narita et al. (Fig. 3b-ii).18 The combination of the five- and eight-membered rings resulted in unique carbon frameworks. For instance, Mastalerz et al. reported monkey saddle-like NGs by embedding five- and eight-membered rings surrounding a central benzene ring in an alternate fashion (Fig. 3b-iii).28 In addition to these non-hexagonal ring-containing NGs, fully hexagonal chiral NGs have also been actively constructed. Gong et al. realized four hexabenzocoronene-fused helical NGs.23 Coquerel et al. developed NGs with three helicene units (Fig. 3b-iv).20
An important feature of chiral NGs is their chiroptical properties. Fig. 4 shows the molecular structures of three chiral NGs (NGG, NGB, and NGR) and their absorption (solid line) and PL (dashed line) spectra, as well as the CD and circularly polarized luminescence (CPL) spectra of NGR and NGG in dichloromethane as reported by Campaña et al.26 The structurally rigid carbon frameworks allowed for optical resolution by HPLC. The resulting homochiral NGs exhibited well-resolved CD (250–450 nm) and CPL spectra (450–700 nm).
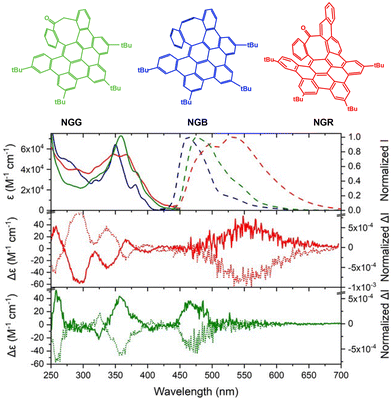 | ||
| Fig. 4 Molecular structures of NGG, NGB, and NGR. (Top) UV-vis absorption and PL spectra of NGG (green), NGB (blue), and NGR (red) in dichloromethane. (Middle and bottom) ECD (250–450 nm) and CPL (450–700 nm) spectra of (middle) NGR and (bottom) NGG in dichloromethane (ref. 26). Copyright 2021, Wiley-VCH. | ||
As demonstrated by Campaña et al., the structural rigidity of carbon frameworks is crucial for realizing excellent chiroptical properties. To achieve optical resolution, sufficient activation energy is required to suppress racemization at room temperature. Martín et al. categorized chiral NGs into four classes according to the energy barrier of chiral inversion:35 flexible (<5 kcal mol−1), detectable (5–20 kcal mol−1), isolable (20–35 kcal mol−1), and rigid (>35 kcal mol−1) (Fig. 5). These chiral NGs are classified as isolable or rigid NGs. Martín et al.'s classification is helpful for assessing the current stages of chiral NGs realized by edge modification.
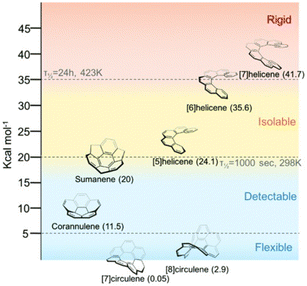 | ||
| Fig. 5 Classification of NGs based on the energy barrier (ref. 35). Copyright 2022, Royal Society of Chemistry. | ||
Graphitization
Although graphitization also employs small molecules as carbon sources, the procedures differ completely from organic synthesis. A typical method is the solvothermal reaction of small organic molecules. Under high temperatures and high-pressure conditions, organic molecules decompose and reorganize to produce graphitic materials. A well-known example is the graphitization of citric acid.36 Chi et al. reported that the heating of citric acid at 200 °C for 30 min produces NGs. Prolonged heating resulted in large graphitic sheets, indicating that the basal planes gradually grew during the reaction.Graphitization inevitably produces a mixture of NGs of distinct sizes and shapes, as demonstrated by Chi et al. These NGs, or more precisely, mixtures of NGs, often display excitation wavelength-dependent PL. Experimental and theoretical approaches have been devoted to explaining this phenomenon, such as the PL from isolated aromatic areas (sp2 domains).37 Lim and Chen et al. computed sp2 domains surrounded by sp3 carbons and suggested that these domains are responsible for the excitation-wavelength-dependent PL.37 Urban et al. supported the latter hypothesis based on the PL spectra of PAH mixtures.38 Column chromatographic separation of NGs further supported the latter hypothesis; the size separation of nitrogen-doped NGs yielded size-separated NGs emitting various colors.39 Excitation-wavelength-dependent PL properties of NGs can be found in other studies.40,41
Because the physical properties of NGs, such as those producing their PL spectra, can be influenced by their size distribution, controlling the size or narrowing the size distribution of NGs is crucial for enhancing the reproducibility of these physical properties. Müllen et al.42 provided an excellent example by demonstrating the synthesis of mono-dispersed disk-like NGs with ∼60 nm diameter and 2–3 nm thickness through the pyrolysis of hexa-peri-hexabenzocoronene aggregates followed by oxidative cleavage, functionalization, and reduction with hydrazine.
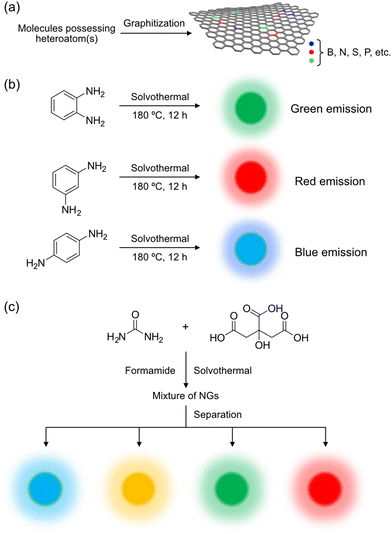
Graphitization offers the advantage of utilizing various organic molecules as carbon sources. When these carbon sources contain heteroatom(s), such as boron,43 nitrogen,39,44,45 sulfur,46 and phosphorous,47 heteroatom-doped NGs can be produced (Fig. 6a).48 The presence of these heteroatoms, with their three (B), five (N, P), and six (S) valence electrons, can modify the electronic structures of the carbon framework. For instance, nitrogen-doped NGs were synthesized from o-, m-, and p-phenylenediamine via solvothermal reactions (Fig. 6b),44 each showing distinct optical properties. Nachtigallová and Zbořil et al.,39 similarly employed citric acid and urea as carbon sources in formamide, resulting in NGs emitting blue, green, yellow, and red light after separation on a preparative anion-exchange column (Fig. 6c). The emission color was influenced by the nitrogen content and its positioning within the carbon framework, as suggested by time-dependent density functional theory (TD-DFT) calculations that indicated narrowing of the HOMO–LUMO gap by nitrogen atoms.
Given the versatility of graphitization in utilizing diverse organic molecules as starting materials, researchers have explored employing chiral molecules to produce chiral NGs. Despite the limited number of examples for producing chiral NGs by graphitization,49 Đorđević and Prato et al. reported the microwave-assisted hydrothermal synthesis of chiral carbon nanodots, which are generally considered carbon nanoparticles with carbon cores covered by oxygen-containing functional groups,49 from (R,R)- or (S,S)-cyclohexane diamine and arginine (Fig. 7), yielding products with mirrored CD spectra. This preservation of chirality from the starting chiral sources after graphitization suggests that chiral information may persist in ungraphitized areas (e.g., sp3 carbons) of the carbon frameworks and/or on the edge, even as graphitization primarily produces sp2 carbons and diminishes chirality.
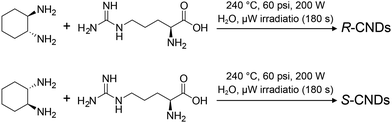 | ||
| Fig. 7 Chiral carbon nanodots (CNDs) synthesized from (R,R)- and (S,S)-1,2-cyclohexane diamine and arginine under microwave-assisted hydrothermal synthesis (ref. 49). | ||
Although graphitization is appealing for its straightforwardness and, therefore, is an attractive method, ensuring the purity of the products is essential. When unreacted starting materials and/or chiral byproducts coexist with the products, the observed CD and CPL spectra can be contaminated by chiral molecular species. This contamination should be removed from products, especially when the coexisting chiral molecular species have high molar extinction coefficients and/or PL quantum yields. Prato et al. reported this possibility in three case studies.50 They demonstrated that the observed CD spectra did not originate from NGs but from coexisting starting materials or byproducts. They suggested that the 1H-NMR spectroscopy could effectively verify the purity of the products. Coexisting molecular species (starting materials and byproducts) show sharp signals, whereas graphitized species show broad signals.
Oxidative cleavage
The top-down method offers NGs through the cleavage of carbon sources,41 such as graphite,13 graphene obtained by thermal deoxidation of GOs, fullerene,52 and carbon nanofibers.53Fig. 8a shows an example of the production of NGs by acid-assisted oxidative cleavage of graphite developed by our group. Neutralization followed by deionization with dialysis membranes (pore size: 2 kDa) and acidification with acid chloride provides carboxy-group-terminated NGs, which can be used as starting materials for NG–organic hybrid materials.51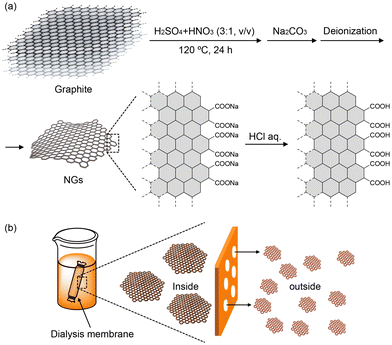 | ||
| Fig. 8 (a) Production of NGs by oxidative cleavage of graphite with a mixture of nitric acid and sulfuric acid. Note: the surfaces of NGs are drawn with single lines. (b) Size separation of NGs with dialysis membranes (ref. 51). | ||
One advantage of this protocol is the production of NGs with minimally oxidized surfaces. 13C{1H}-NMR spectra of the NGs in D2O showed no signals assignable to oxidized sp3 carbons. This contrasts sharply with GOs produced by modified Hummers methods.54 Solid-state CP/MAS NMR spectra of 13C-enriched GOs showed strong peaks assignable to oxidized sp3 carbons.55 A possible explanation for the lack of signals in oxidized carbons is that surface oxidation does not occur randomly but linearly. A theoretical calculation of the unzipping of GOs suggests that the strain generated by the cooperative alignment of epoxy groups can initiate cracks in GOs.56 This calculation provides insight into the mechanism of oxidative cleavage. When oxidation occurs linearly on the surface, the cleavage of the oxidized parts leaves NGs with minimally oxidized surfaces.
Similar to graphitization, the top-down method produces NGs as mixtures of various sizes and shapes. Several groups have conducted size separation of NGs. Matsuda et al. reported the size separation of as-produced NGs by HPLC.57 Guo and Zhang et al. separated NGs by gel electrophoresis.58 Our group conducted the size separation of edge-functionalized NGs by size-exclusion chromatography.59 A convenient and inexpensive procedure is the utilization of dialysis membranes, which allows gram-scale size separation of NGs (Fig. 8b).51,60 When dialysis membranes with a pore size of 50 kDa are used, NGs smaller than the pores leak out, while those larger than the pore size remain inside. Repeated utilization of dialysis membranes with distinct pore sizes offers separation of NGs with a narrow size distribution; however, this procedure is time-consuming and needs improvement. Furthermore, because this protocol relies on the pores in the dialysis membranes, the accuracy of size separation is inevitably inferior to that of HPLC.
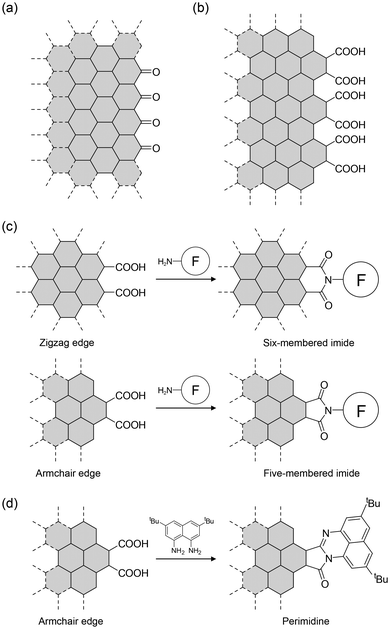
The structure of NGs depends on the carbon source and production method. For example, Ajayan et al. suggested that NGs produced from carbon fibers have carbonyl-terminated zigzag edges (Fig. 9a).53 We showed that acid-assisted oxidative cleavage of graphite yields NGs with the carboxy group-terminated armchair edge as the dominant edge structure (Fig. 9b).13 Subsequently, we reported that distinct carbon sources (graphite, finely crushed graphite powders, and artificial graphite) provided NGs with similar edge structures, although their optical properties differed slightly.60 The characterization of our examples was based on comparing IR spectra.
Characterization of edges is crucial because their structure and the existing functional groups on the edge determine the procedures for edge modification. The reaction of carboxy-terminated edges with organic amines yielded three types of structures. The isolated carboxyl groups on the zigzag and armchair edges provide amides. When two carboxyl groups are arranged along the zigzag edge, amides and/or six-membered imides are formed (Fig. 9c). The two carboxyl groups on the armchair edge provide five-membered imides. Although it is difficult to distinguish between amides and five- and six-membered imides using 13C{1H}-NMR spectroscopy, IR spectroscopy can be used. The amides exhibited both C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H stretching vibrations. In contrast, the six- and five-membered imides show weak and strong pairs of C
O and N–H stretching vibrations. In contrast, the six- and five-membered imides show weak and strong pairs of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations caused by symmetric and asymmetric vibrations, respectively, and no N–H stretching vibrations. Because the ring size of the imide influences the wavenumbers of the C
O stretching vibrations caused by symmetric and asymmetric vibrations, respectively, and no N–H stretching vibrations. Because the ring size of the imide influences the wavenumbers of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations,61 six- and five-membered imides are distinguishable using IR spectroscopy.
O stretching vibrations,61 six- and five-membered imides are distinguishable using IR spectroscopy.
A more reliable characterization method is to compare the IR spectra of functionalized NGs with those of structurally known model compounds. One example is shown in Fig. 10.63 The comparison of the observed IR spectrum of functionalized NGs (GQD-2 in Fig. 10a) with those of model compounds (M1–M3 in Fig. 10a) demonstrated that the two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of the functionalized NGs were in good agreement with those of M1 which has a five-membered imide. Hence, the dominant edge structure was an armchair edge with two carboxyl groups. Comparing the spectroscopic data of functionalized NGs with those of structurally known model compounds can be widely utilized to characterize the edge structures of graphitic materials, including NGs produced by graphitization. This edge structure was supported by the reaction of NGs with 1,8-naphthalene diamine derivatives, which produced perimidine at the edges (Fig. 9d).14
O stretching vibrations of the functionalized NGs were in good agreement with those of M1 which has a five-membered imide. Hence, the dominant edge structure was an armchair edge with two carboxyl groups. Comparing the spectroscopic data of functionalized NGs with those of structurally known model compounds can be widely utilized to characterize the edge structures of graphitic materials, including NGs produced by graphitization. This edge structure was supported by the reaction of NGs with 1,8-naphthalene diamine derivatives, which produced perimidine at the edges (Fig. 9d).14
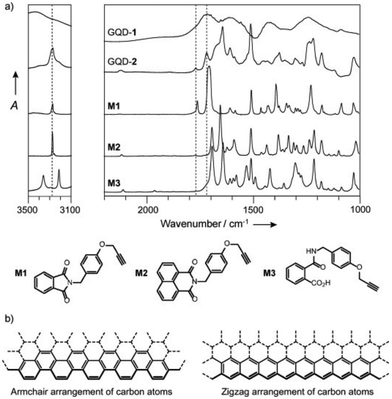 | ||
| Fig. 10 (a) IR spectra of NG (GQD-1), edge-functionalized NG (GQD-2), and model compounds (M1–M3). (b) Armchair (left) and zigzag (right) edges of NGs (ref. 13). Copyright 2014, Wiley-VCH. | ||
The above observations, specifically the presence of an armchair edge with two carboxyl groups on the edge, provide a rough image of the NG edge structures. Fig. 11a shows a model NG composed of 174 carbon atoms, which was designed based upon experimental observations.62 Although the size of the model NG is much smaller than the actual NGs, DFT calculations provided valuable information. The calculations predict an up-and-down puckered edge. The flat structure of the core originates from the fully hexagonal rings in the carbon framework. The puckered edge results from the steric contact between neighboring carboxy groups. The puckered structure is predicted to be retained after the edge functionalization (Fig. 11c). Hence, regulating edge distortion is the first step in realizing chiral NGs through chemical modification.
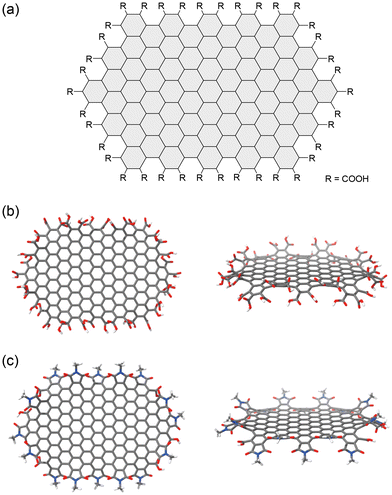 | ||
| Fig. 11 (a) Chemical structure of a model NG used for the DFT calculation. Optimized structures of (b) carboxy-terminated and (c) N-methyl cyclic imide-terminated model NGs at the B3LYP/6-31G(d,p) level of functionality. Color scheme: gray (carbon), white (hydrogen), blue (nitrogen), red (oxygen) (ref. 62). Copyright 2023, Wiley-VCH. | ||
The installed functional groups can adopt various orientations at the edges. A computational study predicted that up-and-down and twisted orientations were possible orientations of the five-membered imides on the edge (Fig. 12a).64 A model of the edge extracted from a NG with N-methyl five-membered imides predicted twisted surfaces when the functional groups pointed in distinct directions (up-and-down orientation) or when the functional groups were twisted relative to the edge (twisted orientation) (Fig. 12b and c).
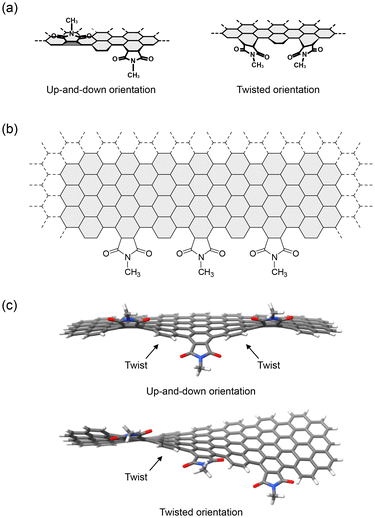 | ||
| Fig. 12 (a) Up-and-down (left) and twisted orientations (right). (b) Chemical structure of the model edge used for the DFT calculations. (c) Optimized structures of the model edge carrying N-methyl five-membered imides on the edge. Color scheme: gray (carbon), white (hydrogen), blue (nitrogen), red (oxygen) (ref. 5). Copyright 2023, Wiley-VCH. | ||
Because this NG has a vast surface area and a long annular edge, twists are likely to be generated at various sites on the edge. Thus, one strategy for chirality generation is to bias the twist direction by installing chiral functional groups and rigidifying and amplifying the induced chirality. Chirality information is conveyed from the installed chiral source to the NGs through steric contacts and/or intermolecular interactions, such as π–π stacking interactions (Fig. 13a). These contacts and/or interactions can bias the direction of twisting. The following section introduces chiral NGs produced by the chemical modification of NGs.
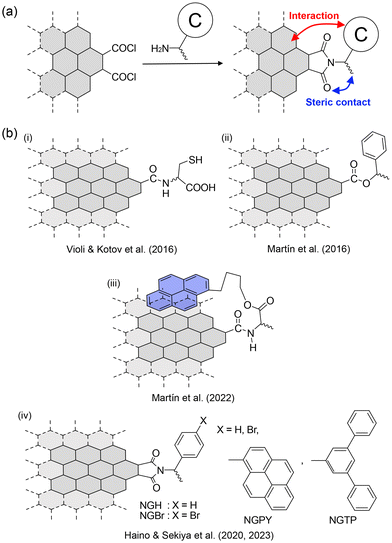 | ||
| Fig. 13 (a) Strategy of edge functionalization of NGs produced by the top-down method. (b) Examples of chirality generation on NGs by the edge functionalization reported by (i) Violi and Kotov et al. (ref. 65), (ii) and (iii) Martín et al. (ref. 66 and 15), (iv) Haino and Sekiya et al. (ref. 10 and 5). | ||
The earliest example was reported by Kotov et al. in 2016.65 They conducted edge functionalization with D- and L-cysteine (Fig. 13b-i). CD spectra and computational calculations indicate the generation of left- and right-handed helicity on the basal plane. Non-covalent interactions between the installed D- and L-cysteine and the edge are likely to bias the twist direction, resulting in chiral NGs. A similar procedure was reported by Martín et al. in 2016 (Fig. 13b-ii).66 Interestingly, they reported that the aggregation of chiral NGs carrying (R)- or (S)-phenylethyl alcohol and pyrene conveyed chirality from NGs to pyrene, allowing for the CPL emission of pyrene. Subsequently, the same group reported an interesting protocol for chirality transfer. They installed pyrene-terminated chiral functional groups at the edge (Fig. 13b-iii), and the π–π stacking interaction between the pyrene unit and the surface secured chirality transfer.
Our group has also contributed to the development of chiral NGs.10 (R)- and (S)-phenylethylamines and (R)- and (S)-p-bromophenylethylamine were installed at the edges to realize chiral NGs (NGH and NGBr in Fig. 13b-iv). The CD spectra of (R)- and (S)-NGH (Fig. 14a) were similar in shape to those of the model compounds. More concentrated solutions showed weak CD signals over 350 nm, indicating chiral transfer from the chiral functional groups to the NGs. In the case of (R)- and (S)-NGBr, complex CD spectra were observed (Fig. 14b), caused by exciton coupling67–69 between neighboring chiral functional groups on the edge. This suggests that the functional groups on the edges are close enough to interact with each other.
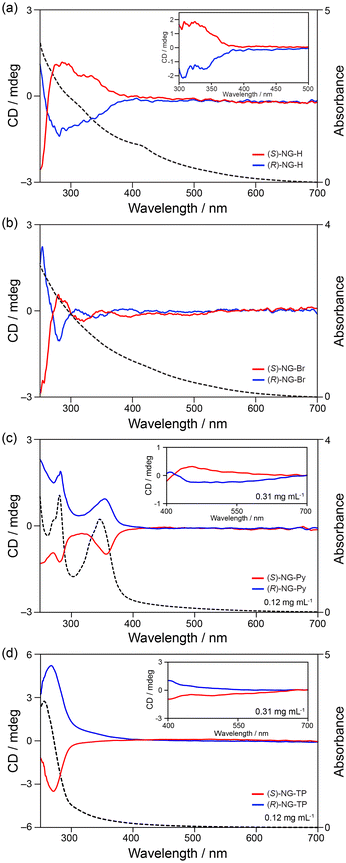 | ||
| Fig. 14 Observed CD spectra of (a) NG-H, (b) NG-Br, (c) NG-pyrene, and (d) NG-terphenyl in chloroform (ref. 10 and 5). Red lines represent the (R)-enantiomers and blue lines represent the (S)-enantiomers. Black dotted lines are the UV-vis absorption spectra of the (S)-enantiomers. Copyright 2020 & 2023, Wiley-VCH. | ||
The X-ray crystal structure analysis of a model compound provided clues for chirality transfer (Fig. 15a).10 The contact between the methyl group on the stereogenic center and the carboxyl group on the phthalimide induced a twist of approximately 2° between the benzene ring and the five-membered imide ring. This suggests that steric contacts between the methyl group on the stereogenic center, the carbonyl group on the imide, and the edge are routes for chirality transfer (Fig. 15b).
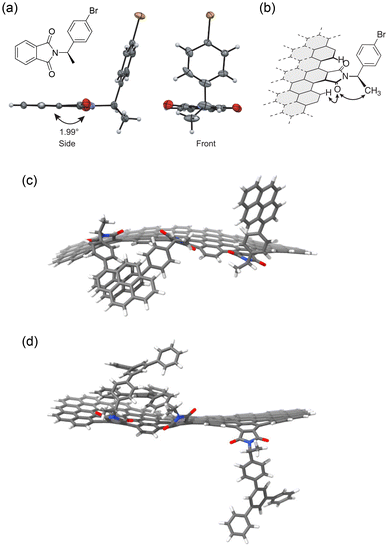 | ||
| Fig. 15 X-ray crystal structure of a model compound carrying an (R)-p-bromophenylethyl group. (b) Possible mechanism of chirality transfer from the stereogenic center to the edge of the NG (ref. 10). (c) and (d) Optimized structures of the model edges of NGPY and NGTP (ref. 5). In (a), (c), and (d) Color scheme: gray (carbon), white (hydrogen), blue (nitrogen), red (oxygen), brown (bromine). Copyright 2020 & 2023, Wiley-VCH. | ||
Compared to chiral NGs synthesized by organic methods, the above examples show very weak CD spectra, and their CD spectra are not perfect mirror images. One possible explanation for the former is that the steric contacts and/or intermolecular interactions between NG and the chiral functional group are insufficient to rigidify the induced chirality, resulting in a slight twist bias on the edge. This is because the installed functional groups protrude from the edge and the rotatable C–N single bond connects the functional groups and NGs. Imperfect mirror-image CD spectra may arise from the nonstoichiometric mixture of these chiral NGs. Based on the classification by Martín et al. (Fig. 5), these NGs can be categorized as flexible to detectable, although the energy barriers of chirality inversion are difficult to estimate. Hence, the generation of chirality in NGs by chemical functionalization is still in the early stages of development.
Steric contacts between the functional groups and edges can rigidify the induced chirality. This can be achieved by installing bulky chiral functional groups on the edges. Bulky substituents would also hinder the rotation of C–N single bonds through steric contact between them. This strategy is supported by the edge modification of NGs with bulky and less bulky substituents (Fig. 16),12 although this example involves non-chiral NGs. NGs carrying third-generation dendritic wedges had smaller effective conjugated areas than those with less bulky substituents. One reason is that the effective π conjugation was narrowed to avoid steric contact between neighboring dendritic wedges.
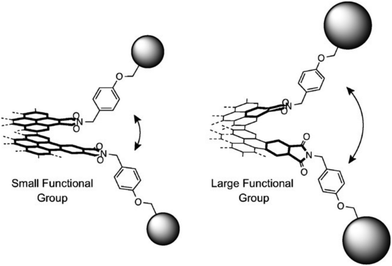 | ||
| Fig. 16 Schematic representation of (left) small and (right) large substituents on the edge. The latter may cause a larger twist on the edge (ref. 12). Copyright 2016, Wiley-VCH. | ||
Recently, an example was reported70 where the bromine atoms on NGBr were substituted with pyrene (NGPY) and p-terphenyl (NGTP) through Pd-catalyzed cross-coupling reactions. The CD spectra of (R)- and (S)-NGPY and NGTP showed improved CD signals (Fig. 14c and d). CD signals were observed in the visible region, although the signal intensities were weak. Interestingly, the CD signals of (R)- and (S)-NGPY and NGTP were opposite to each other, likely due to the induction of opposite chirality despite having the same absolute configuration on the stereogenic centers. DFT calculations suggested that the pyrene and p-terphenyl groups interacted with the surface through π–π stacking interactions (Fig. 15c and d). The interactions between the surface and the pyrene groups and those between the surface and the p-terphenyl groups may differ, resulting in an opposite twist on the edge. These observations indicate that not only the chirality of the stereogenic centers but also the interactions between the functional group and the surface and/or neighboring functional groups on the edge can influence the chirality of NGs.
Advantages and disadvantages
We briefly summarize the advantages and disadvantages of the three methods for developing chiral NGs. The advantage of the bottom-up method by organic synthesis is undoubtedly structurally well-defined carbon frameworks. Their structures and functions, such as optical properties and chirality, can be tuned precisely. A disadvantage might be a multi-step synthesis to construct carbon frameworks, although this is not severe. An advantage of the bottom-up method by graphitization is that various chiral sources can be used as starting materials. However, the contamination of starting materials and/or byproducts can lead to wrong conclusions. Also, the characterization, for example, where chiral sources exist and how chirality is generated on NGs, would be troublesome. Hence, this method may suffer from the tunability of functions. By contrast, the top-down method can tune their functions by edge modification. The gram-scale production of NGs is also attractive from the perspective of practical applications. However, the non-stoichiometry of NGs can be troublesome. Further, we must overcome inefficient chirality transfer and rigidify the induced chirality on the surface by developing sophisticated chiral functional groups.Chirality and structural analysis
This section presents a structural analysis of NGs based on chirality. A recent method to analyze the edge structures of NGs was reported.71Exciton coupling was used to determine the absolute configurations of chiral molecules. This coupling occurs when chiral chromophores are in close proximity.69 The exciton coupling is defined as Rij(μi0a × μj0a)·Vij, where Rij is an interchromophoric distance vector from chromophores i to j, μi0a and μj0a are the electronic transition moments of chromophores i and j from the ground state to the excited state a, and Vij is the interaction energy. When two chromophores approach the edge at a certain distance, the spectral shape changes (Fig. 17a). For example, p-bromo- and p-methoxy benzoate chromophores on a cyclohexane ring68 produce bisignate CD signals depending on their relative orientation.
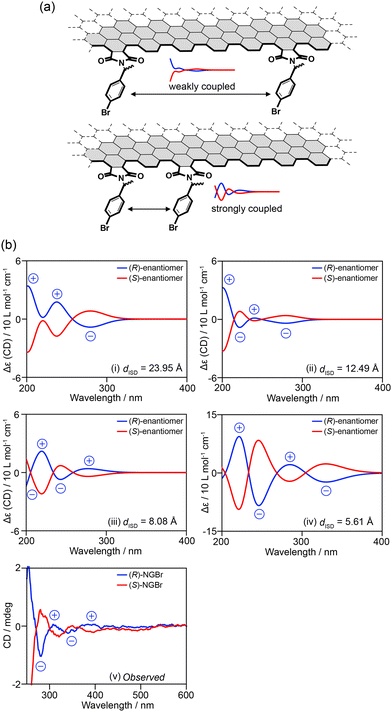 | ||
| Fig. 17 (a) Concept of using exciton coupling for the structural analysis of the edge structures of NGs. (b) (i)–(iv) Calculated CD signals of model edge NGs with interchromophoric distances of (i) 23.95 Å, (ii) 12.49 Å, (iii) 8.08 Å, and (iv) 5.61 Å, and (v) observed CD signals of NGBr (ref. 71). Copyright 2024 Wiley-VCH. | ||
This equation provides information on the distance and relative orientation of the interacting chromophores. When TD-DFT calculations of model structures carrying two or more chromophores reproduce the observed CD signals, particularly their signal patterns, the model structures should closely resemble real structures in terms of the distance and relative orientation of the chromophores. Hence, information regarding the distance and orientation of the chromophores on the edge can be obtained from the model structures.
This concept has been applied to the structural analysis of chiral NGs. First, the edge structures of the model were optimized. The chromophores were then extracted from the optimized structures and subjected to TD-DFT calculations to obtain the CD spectra of the given distance (dISD) and orientation of the two chromophores (Fig. 17b). By removing the edge from the TD-DFT calculations, the contribution of π–π* transitions on the surface was eliminated. The calculations demonstrated that when two p-bromophenyl groups are separated by dISD = 8.08 Å (Fig. 17b-iii), the model edge structure produced CD spectra consistent in sign with the observed ones (Fig. 17b-v). TD-DFT calculations of the model edge structures carrying three, four, and six chromophores demonstrated that when the p-bromophenylethyl chromophores adopt up–up–down–down alternating orientations, the model edge can reproduce the observed CD signs (Fig. 18). These observations suggest that the p-bromophenylethyl groups on the edge of NGBr were installed on every other armchair edge and adopted up–up–down–down alternating orientations. Moreover, the information on dISD allows us to estimate the number of functional groups attached to the NGs using the equation of 2πr/dISD, where r is the radius of a given NG.
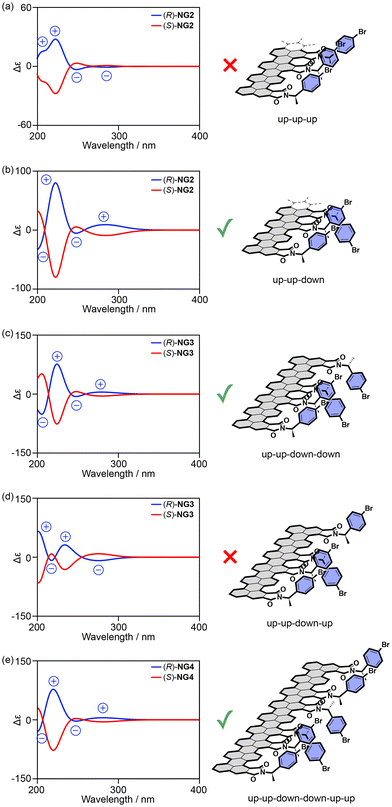 | ||
| Fig. 18 (a)–(e) Calculated CD spectra of model edge structures with (a) up–up–up, (b) up–up–down, (c) up–up–down–down, (d) up–up–down–up, and (e) up–up–down–down–up–up orientations (ref. 71). Copyright 2024 Wiley-VCH. | ||
Structural characterization is crucial for any chemical. The nonstoichiometric mixture of top-down method-produced NGs and vast surface areas complicates the structural analysis of NG–organic hybrid materials. Although spectroscopic methods employed in the characterization of graphitic materials, such as Raman spectroscopy, XPS, NMR, and IR spectroscopy, provide evidence of the installation of functional groups, their number and orientation are difficult to determine. While the above procedure is limited to chiral NGs, combining CD spectroscopy with computational calculations provides “average” edge structures of NG–organic hybrid materials.
Conclusions
Currently, chiral NGs realized by chemical modification are still in their early stages of development. This is because these carbon frameworks are formed by oxidative cleavage of parent carbons rather than step-by-step organic synthesis, limiting the methods for inducing chirality. Some reported approaches involve introducing chiral sources at the edges to convey chiral information to the surface through steric and/or intermolecular interactions. However, the weak CD signals suggest that the induced chirality is not well rigidified and/or the chirality transfer from chiral sources anchored on the edge to the surface is inefficient. Therefore, the next stages are (1) the rigidification of the induced chirality on the surface, similar to that achieved with chiral NGs synthesized by organic synthesis, in which the carbon frameworks have a higher tolerance for racemization, and (2) the improvement of the efficiency of the chirality transfer from chiral sources to the surface. Solving these issues should allow amplification of the induced chirality and eventually realize chiral NGs with excellent chiroptical properties.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Nippon Sheet Glass Foundation, Iketani Science and Technology Foundation, The Urakami Scholarship Foundation, ENEOS Tonen General Research/Development Encouragement & Scholarship Foundation, JSPS KAKENHI, Grants-in-Aid for Transformative Research Areas, “Condensed Conjugation” grant number JP21H05491 and “Materials Science of Meso-Hierarchy” grant number JP23H04873, and Grant-in-Aid for Scientific Research (A) grant number JP21H04685. We also acknowledge support from KEIRIN JKA, grant number 2023 M-419.References
- Y. Shen and C.-F. Chen, Chem. Rev., 2012, 112, 1463–1535 CrossRef CAS PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- R. Sekiya and T. Haino, Chem. Rec., 2022, 22, e202100257 CrossRef CAS PubMed.
- S. Arimura, I. Matsumoto, R. Sekiya and T. Haino, Angew. Chem., Int. Ed., 2024, 63, e202315508 CrossRef CAS.
- H. Moriguchi, R. Sekiya and T. Haino, Small, 2023, 19, 202207475 Search PubMed.
- S. Takahashi, R. Sekiya and T. Haino, Angew. Chem., Int. Ed., 2022, 61, e202205514 CrossRef CAS.
- I. Matsumoto, R. Sekiya, H. Fukui, R. D. Sun and T. Haino, Angew. Chem., Int. Ed., 2022, 61, e202200291 CrossRef CAS PubMed.
- I. Matsumoto, R. Sekiya and T. Haino, Angew. Chem., Int. Ed., 2021, 60, 12706–12711 CrossRef CAS.
- S. Nishitani, R. Sekiya and T. Haino, Angew. Chem., Int. Ed., 2020, 59, 669–673 CrossRef CAS.
- Y. Uemura, K. Yamato, R. Sekiya and T. Haino, Angew. Chem., Int. Ed., 2018, 57, 4960–4964 CrossRef CAS PubMed.
- R. Sekiya, Y. Uemura, H. Naito, K. Naka and T. Haino, Chem. – Eur. J., 2016, 22, 8198–8206 CrossRef CAS PubMed.
- R. Sekiya, Y. Uemura, H. Murakami and T. Haino, Angew. Chem., Int. Ed., 2014, 53, 5619–5623 CrossRef CAS.
- K. Yamato, R. Sekiya, K. Suzuki and T. Haino, Angew. Chem., Int. Ed., 2019, 58, 9022–9026 CrossRef CAS PubMed.
- M. Vázquez-Nakagawa, L. Rodríguez-Pérez, N. Martín and M. A. Herranz, Angew. Chem., Int. Ed., 2022, 61, e202211365 CrossRef.
- J. Ma, Y. Fu, E. Dmitrieva, F. Liu, H. Komber, F. Hennersdorf, A. A. Popov, J. J. Weigand, J. Liu and X. Feng, Angew. Chem., Int. Ed., 2020, 59, 5637–5642 CrossRef CAS PubMed.
- M. A. Medel, C. M. Cruz, D. Miguel, V. Blanco, S. P. Morcillo and A. G. Campaña, Angew. Chem., Int. Ed., 2021, 60, 22051–22056 CrossRef CAS PubMed.
- Z. Qiu, S. Asako, Y. Hu, C.-W. Ju, T. Liu, L. Rondin, D. Schollmeyer, J.-S. Lauret, K. Müllen and A. Narita, J. Am. Chem. Soc., 2020, 142, 14814–14819 CrossRef CAS PubMed.
- M. Krzeszewski, L. Dobrzycki, A. L. Sobolewski, M. K. Cyrański and D. T. Gryko, Angew. Chem., Int. Ed., 2021, 60, 14998–15005 CrossRef CAS PubMed.
- M. Roy, V. Berezhnaia, M. Villa, N. Vanthuyne, M. Giorgi, J. V. Naubron, S. Poyer, V. Monnier, L. Charles, Y. Carissan, D. Hagebaum-Reignier, J. Rodriguez, M. Gingras and Y. Coquerel, Angew. Chem., Int. Ed., 2020, 59, 3264–3271 CrossRef CAS PubMed.
- Chaolumen, I. A. Stepek, K. E. Yamada, H. Ito and K. Itami, Angew. Chem., Int. Ed., 2021, 60, 23508–23532 CrossRef CAS PubMed.
- M. A. Majewski and M. Stępień, Angew. Chem., Int. Ed., 2019, 58, 86–116 CrossRef CAS PubMed.
- Y.-J. Shen, N.-T. Yao, L.-N. Diao, Y. Yang, X.-L. Chen and H.-Y. Gong, Angew. Chem., Int. Ed., 2023, 62, e202300840 CrossRef CAS PubMed.
- J.-K. Li, X.-Y. Chen, W.-L. Zhao, Y.-L. Guo, Y. Zhang, X.-C. Wang, A. C.-H. Sue, X.-Y. Cao, M. Li, C.-F. Chen and X.-Y. Wang, Angew. Chem., Int. Ed., 2023, 62, e202215367 CrossRef CAS PubMed.
- D. Reger, P. Haines, K. Y. Amsharov, J. A. Schmidt, T. Ullrich, S. Bönisch, F. Hampel, A. Görling, J. Nelson, K. E. Jelfs, D. M. Guldi and N. Jux, Angew. Chem., Int. Ed., 2021, 60, 18073–18081 CrossRef CAS.
- M. A. Medel, R. Tapia, V. Blanco, D. Miguel, S. P. Morcillo and A. G. Campaña, Angew. Chem., Int. Ed., 2021, 60, 6094–6100 CrossRef CAS PubMed.
- Y. Chen, C. Lin, Z. Luo, Z. Yin, H. Shi, Y. Zhu and J. Wang, Angew. Chem., Int. Ed., 2021, 60, 7796–7801 CrossRef PubMed.
- T. Kirschbaum, F. Rominger and M. Mastalerz, Angew. Chem., Int. Ed., 2020, 59, 270–274 CrossRef CAS PubMed.
- M. Navakouski, H. Zhylitskaya, P. J. Chmielewski, T. Lis, J. Cybinska and M. Stępień, Angew. Chem., Int. Ed., 2019, 58, 4929–4933 CrossRef CAS PubMed.
- C. M. Cruz, I. R. Márquez, S. Castro-Fernández, J. M. Cuerva, E. Maçôas and A. G. Campaña, Angew. Chem., Int. Ed., 2019, 58, 8068–8072 CrossRef CAS.
- K. Kato, Y. Segawa, L. T. Scott and K. Itami, Angew. Chem., Int. Ed., 2018, 57, 1337–1341 CrossRef CAS PubMed.
- U. Hahn, E. Maisonhaute and J.-F. Nierengarten, Angew. Chem., Int. Ed., 2018, 57, 10635–10639 CrossRef CAS PubMed.
- P. J. Evans, J. K. Ouyang, L. Favereau, J. Crassous, I. Fernández, J. Perles and N. Martín, Angew. Chem., Int. Ed., 2018, 57, 6774–6779 CrossRef CAS PubMed.
- C. M. Cruz, S. Castro-Fernández, E. Maçôas, J. M. Cuerva and A. G. Campaña, Angew. Chem., Int. Ed., 2018, 57, 14782–14786 CrossRef CAS PubMed.
- J. M. Fernández-García, P. Izquierdo-García, M. Buendía, S. Filippone and N. Martín, Chem. Commun., 2022, 58, 2634–2645 RSC.
- Y. Dong, J. Shao, C. Chen, H. Li, R. Wang, Y. Chi, X. Lin and G. Chen, Carbon, 2012, 50, 4738–4743 CrossRef CAS.
- M. A. Sk, A. Ananthanarayanan, L. Huang, K. H. Lim and P. Chen, J. Mater. Chem. C, 2014, 2, 6954–6960 RSC.
- M. Fu, F. Ehrat, Y. Wang, K. Z. Milowska, C. Reckmeier, A. L. Rogach, J. K. Stolarczyk, A. S. Urban and J. Feldmann, Nano Lett., 2015, 15, 6030–6035 CrossRef CAS PubMed.
- K. Holá, M. Sudolská, S. Kalytchuk, D. Nachtigallová, A. L. Rogach, M. Otyepka and R. Zbořil, ACS Nano, 2017, 11, 12402–12410 CrossRef.
- L. Li, G. Wu, G. Yang, J. Peng, J. Zhao and J.-J. Zhu, Nanoscale, 2013, 5, 4015–4039 RSC.
- S. Kim, S. W. Hwang, M.-K. Kim, D. Y. Shin, D. H. Shin, C. O. Kim, S. B. Yang, J. H. Park, E. Hwang, S.-H. Choi, G. Ko, S. Sim, C. Sone, H. J. Choi, S. Bae and B. H. Hong, ACS Nano, 2012, 6, 8203–8208 CrossRef CAS PubMed.
- R. Liu, D. Wu, X. Feng and K. Müllen, J. Am. Chem. Soc., 2011, 133, 15221–15223 CrossRef CAS.
- T. Van Tam, S. G. Kang, K. F. Babu, E.-S. Oh, S. G. Lee and W. M. Choi, J. Mater. Chem. A, 2017, 5, 10537–10543 RSC.
- K. Jiang, S. Sun, L. Zhang, Y. Lu, A. Wu, C. Cai and H. Lin, Angew. Chem., Int. Ed., 2015, 54, 5360–5363 CrossRef CAS PubMed.
- C. Zhu, S. Yang, G. Wang, R. Mo, P. He, J. Sun, Z. Di, N. Yuan, J. Ding, G. Ding and X. Xie, J. Mater. Chem. C, 2015, 3, 8810–8816 RSC.
- C. Shen, S. Ge, Y. Pang, F. Xi, J. Liu, X. Dong and P. Chen, J. Mater. Chem. B, 2017, 5, 6593–6600 RSC.
- A. Ananthanarayanan, Y. Wang, P. Routh, M. A. Sk, A. Than, M. Lin, J. Zhang, J. Chen, H. Sun and P. Chen, Nanoscale, 2015, 7, 8159–8165 RSC.
- N. Sohal, B. Maity and S. Basu, RSC Adv., 2021, 11, 25586–25615 RSC.
- L. Đorđević, F. Arcudi, A. D'Urso, M. Cacioppo, N. Micali, T. Bürgi, R. Purrello and M. Prato, Nat. Commun., 2018, 9, 3442 CrossRef.
- B. Bartolomei, A. Bogo, F. Amato, G. Ragazzon and M. Prato, Angew. Chem., Int. Ed., 2022, 61, e202200038 CrossRef CAS PubMed.
- I. Matsumoto, R. Sekiya and T. Haino, RSC Adv., 2019, 9, 33843–33846 RSC.
- C. K. Chua, Z. Sofer, P. Šimek, O. Jankovský, K. Klímová, S. Bakardjieva, S. H. Kučková and M. Pumera, ACS Nano, 2015, 9, 2548–2555 CrossRef CAS PubMed.
- J. Peng, W. Gao, B. K. Gupta, Z. Liu, R. Romero-Aburto, L. H. Ge, L. Song, L. B. Alemany, X. Zhan, G. Gao, S. A. Vithayathil, B. A. Kaipparettu, A. A. Marti, T. Hayashi, J.-J. Zhu and P. M. Ajayan, Nano Lett., 2012, 12, 844–849 CrossRef CAS PubMed.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339–1339 CrossRef CAS.
- W. Cai, R. D. Piner, F. J. Stadermann, S. Park, M. A. Shaibat, Y. Ishii, D. Yang, A. Velamakanni, S. An, M. Stoller, J. An, D. M. Chen and R. S. Ruoff, Science, 2008, 321, 1815–1817 CrossRef CAS.
- J.-L. Li, K. N. Kudin, M. J. McAllister, R. K. Prud'homme, I. A. Aksay and R. Car, Phys. Rev. Lett., 2006, 96, 176101 CrossRef PubMed.
- N. Fuyuno, D. Kozawa, Y. Miyauchi, S. Mouri, R. Kitaura, H. Shinohara, T. Yasuda, N. Komatsu and K. Matsuda, Adv. Opt. Mater., 2014, 2, 983–989 CrossRef CAS.
- F. Zhang, F. Liu, C. Wang, X. Xin, J. Liu, S. Guo and J. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 2104–2110 CrossRef CAS PubMed.
- K. Yamato, R. Sekiya, M. Abe and T. Haino, Chem. – Asian J., 2019, 14, 1786–1791 CrossRef CAS.
- I. Matsumoto, R. Sekiya and T. Haino, Bull. Chem. Soc. Jpn., 2021, 94, 1394–1399 CrossRef CAS.
- T. Matsuo, Bull. Chem. Soc. Jpn., 1964, 37, 1844–1848 CrossRef CAS.
- S. Takahashi, R. Sekiya and T. Haino, ChemPhysChem, 2022, 24, e202200465 CrossRef.
- R. Sekiya and T. Haino, Chem. – Eur. J., 2021, 27, 187–199 CrossRef CAS PubMed.
- S. Arimura, I. Matsumoto, S. Nishitani, R. Sekiya and T. Haino, Chem. – Asian J., 2023, 18, e202300126 CrossRef CAS PubMed.
- N. Suzuki, Y. C. Wang, P. Elvati, Z. B. Qu, K. Kim, S. Jiang, E. Baumeister, J. Lee, B. Yeom, J. H. Bahng, J. Lee, A. Violi and N. A. Kotov, ACS Nano, 2016, 10, 1744–1755 CrossRef CAS PubMed.
- M. Vázquez-Nakagawa, L. Rodríguez-Pérez, M. A. Herranz and N. Martín, Chem. Commun., 2016, 52, 665–668 RSC.
- R. V. Person, K. Monde, H. U. Humpf, N. Berova and K. Nakanishi, Chirality, 1995, 7, 128–135 CrossRef CAS.
- W. T. Wiesler, J. T. Vazquez and K. Nakanishi, J. Am. Chem. Soc., 1987, 109, 5586–5592 CrossRef CAS.
- K. Nakanishi, M. Kuroyanagi, H. Nambu, E. M. Oltz, R. Takeda, G. L. Verdine and A. Zask, Pure Appl. Chem., 1984, 56, 1031–1048 CrossRef CAS.
- S. Arimura, I. Matsumoto, S. Nishitani, R. Sekiya and T. Haino, Chem. – Asian J., 2023, 18, e202300126 CrossRef CAS.
- R. Sekiya and T. Haino, ChemPhysChem, 2024, 63, e202315508 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |