 Open Access Article
Open Access ArticleExfoliation of triazole-based C3N4.8, C3N6, and C3N7 nanosheets for efficient photocatalytic ammonia production†
Ayoung
Yoon‡
a,
Taehoon
Kim‡
b,
Dokyung
Kim
c,
Young Joo
Lee
 cd,
Seong-Ju
Hwang
cd,
Seong-Ju
Hwang
 *b and
In Young
Kim
*b and
In Young
Kim
 *a
*a
aDepartment of Chemistry and Nanoscience, College of Natural Sciences, Ewha Womans University, Seoul 03760, Republic of Korea. E-mail: iykim@ewha.ac.kr
bDepartment of Materials Science and Engineering, College of Engineering, Yonsei University, Seoul 03722, Republic of Korea. E-mail: hwangsju@yonsei.ac.kr
cMetropolitan Seoul Center, Korea Basic Science Institute, Seoul 03759, Republic of Korea
dDepartment of Chemistry, Chung-Ang University, Seoul 06974, Republic of Korea
First published on 18th December 2024
Abstract
Atomically thin two-dimensional nanosheets of nitrogen-rich C3N4.8, C3N6, and C3N7 are synthesized by sonochemical process. Despite their high nitrogen content, their triazole-based crystal structures remain intact after exfoliation. Among the present materials, the nitrogen-richest C3N7 nanosheets display the highest photocatalytic activity for ammonia production, highlighting the synergetic effect of composition control and exfoliation.
Although carbon nitrides of triazole-based C3N4.8, C3N6, and C3N7 have recently been synthesized, their inherent properties have remained largely unknown.1 Whereas g-C3N4 has a heptazine-based framework,2 the C3N4.8, C3N6, and C3N7 systems are stabilised through triazole frameworks together with triazine or tetrazine,1 as indicated by density functional theory (DFT) calculations and supported by a range of techniques including X-ray diffraction (XRD), X-ray absorption spectroscopy (XAS), Fourier-transform infrared (FT-IR) spectroscopy, and transmission electron microscopy (TEM).1 The nitrogen-rich character of carbon nitrides plays important roles in various reactions. For example, introducing nitrogen into the sp2 carbon framework opens a bandgap on the carbon, enabling its use as a visible-light-sensitive g-C3N4 semiconductor for photocatalysis.3 Triazole-based C3N4.8 shows enhanced basicity over g-C3N4, facilitating CO2 feedstock reactions.4,5 Our previous studies reported promising activities of triazole-based C3N4.8, C3N6, and C3N7, which outperform g-C3N4 in the oxygen reduction reaction (ORR)1 and the carbon dioxide reduction reaction (CO2RR)5 and in applications in lithium- and sodium-ion batteries.6 In this context, nitrogen-rich carbon nitrides have become a focus of research.1,3–7 Triazole-based C3N7 is particularly noteworthy as it shows the highest nitrogen content of the carbon nitrides reported to date.
The exfoliation of g-C3N4 has been attempted using various strategies, including sonochemical,8 thermal expansion,9 intercalation,10 and oxidation–reduction methods.11 Exfoliated g-C3N4 nanosheets often exhibit enhanced performance in catalytic applications owing to their expanded surface area and changed electronic band positions.12–14 However, exfoliation of the triazole-based C3N4.8, C3N6, and C3N7 has not been achieved. Because they consist of not only conjugated C–N bonds but also N–N bonds,1 it is uncertain whether these thermodynamically unstable N–N bonds retain their integrity in triazole-based C3N4.8, C3N6, and C3N7 upon exfoliation.
In this study, we successfully exfoliated bulk triazole-based C3N4.8, C3N6, and C3N7 into nanosheets via a fast and efficient sonochemical process using isopropyl alcohol. XAS confirmed the retention of the triazole-based crystal structures of C3N4.8, C3N6, and C3N7 upon sonochemical exfoliation. These materials have been employed as photocatalysts for nitrogen reduction reactions (NRRs). Considering the many reports on the photocatalytic NRR activities of g-C3N4,15–19 triazole-based carbon nitrides provide the opportunity to enhance the NRR activities of carbon nitrides. Because the appropriate conduction band position and specific element-deficient characteristics of carbon nitrides make them suitable photocatalysts for the NRR,18 it is important to clarify the band structures of exfoliated triazole-based C3N4.8, C3N6, and C3N7 nanosheets and study the relationships between their band structures and NRR properties.
The XRD patterns of bulk g-C3N4, C3N4.8, C3N6, and C3N7 are shown in Fig. S1 (see the ESI†). All samples show a strong peak between 26° and 28° corresponding to the (002) reflection of carbon nitride. As the nitrogen content in the carbon nitride increases, the (002) reflection appears at lower 2θ angles. The d(002) spacings of bulk g-C3N4, C3N4.8, C3N6, and C3N7 are 0.328, 0.330, 0.330, and 0.330 nm, respectively. The expansion of d(002) spacing is due to the repulsion of nitrogen and nitrogen.1 For bulk g-C3N4, a weak peak is observed at 13°, which accounts for the in-plane reflection of the heptazine units. The peak is displaced to higher 2θ angles for bulk C3N4.8, C3N6, and C3N7, indicating that their core structures differ from those of bulk g-C3N4.
Because the XRD results do not provide information on the in-plane crystal structures of the triazole-based carbon nitrides, we analysed their structures for the first time using solid-state 13C magic-angle spinning (MAS) nuclear magnetic resonance (NMR) spectroscopy. Fig. 1 shows the solid-state 13C MAS NMR spectra of bulk C3N4.8, C3N6, and C3N7 as well as their crystal motifs together with the crystal motif of g-C3N4. It has been reported that g-C3N4 shows two well-resolved signals in the solid-state 13C MAS NMR spectrum. The two signals at approximately 155 ppm and approximately 162 ppm correspond to different carbon environments, denoted as C1 and C2 in Fig. 1(b), respectively.20,21 Interestingly, the 13C NMR spectra of C3N4.8, C3N6, and C3N7 present broad overlapping signals that can be deconvoluted into at least three signals at 148–151, 155–158, and 161–165 ppm, as shown in Fig. 1(a). Compared to C3N4.8, the 13C NMR signals of C3N6 and C3N7 appear at slightly higher frequencies. Interestingly, C3N4.8, C3N6, and C3N7 commonly display additional signals at 148–151 ppm, presumably due to the effect of the triazole motif. The relatively weakened signal at 151 ppm for C3N7 might be due to the altered bonding environment of carbon in the triazole motif such as the neighbouring guanidine group in C3N7 that is different from C3N4.8 and C3N6. Triazole exhibits 13C NMR signals that are typically 20 ppm lower than those of triazine. The 13C NMR spectra of the triazole-based carbon nitrides demonstrate that the in-plane crystal structures of C3N4.8, C3N6, and C3N7 differ, providing additional support for the crystal structures of C3N4.8, C3N6, and C3N7 proposed in Fig. 1(b).
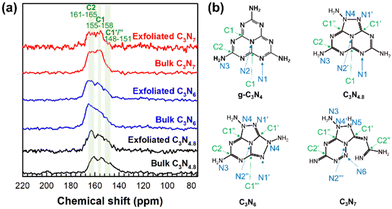 | ||
| Fig. 1 (a) Solid-state 13C MAS NMR spectra of bulk and exfoliated C3N4.8, C3N6, and C3N7. (b) Crystal motifs of g-C3N4, C3N4.8, C3N6, and C3N7. | ||
Photographs of the colloidal suspensions of exfoliated nanosheets are shown in Fig. 2. The colloidal suspensions of the g-C3N4, C3N4.8, and C3N6 nanosheets exhibit faint ivory colours, suggesting visible-light-sensitive bandgaps. The colloidal suspension of C3N7 nanosheets was white, indicating a large bandgap for UV light absorption. The Tyndall phenomenon induced by exfoliated nanosheets is observed for colloidal suspensions of g-C3N4, C3N4.8, C3N6, and C3N7 nanosheets, confirming their incorporation into nanostructures. The negative zeta potential values ranging from −60 to −40 mV for the g-C3N4, C3N4.8, C3N6, and C3N7 nanosheets (Table S1, see the ESI†) demonstrate that they can form stable dispersions in isopropyl alcohol.
 | ||
| Fig. 2 (a) Photographs and (b) Tyndall phenomenon of colloidal suspensions of exfoliated g-C3N4, C3N4.8, C3N6, and C3N7 nanosheets. | ||
In terms of the crystal morphology observed by TEM, g-C3N4, C3N4.8, C3N6, and C3N7 exhibit exfoliated two-dimensional nanosheets with lateral sizes of approximately 100 nm (Fig. 3(a)). Their thicknesses are evaluated to be 0.47–0.98 nm based on their atomic force microscopy (AFM) height profile (Fig. 3(b) and (c)), which is slightly larger than the theoretical thickness of a monolayer of carbon nitrides (ca. 0.33 nm). Considering the presence of an adsorbed water layer on the surface of the carbon nitrides, it is believed that g-C3N4, C3N4.8, C3N6, and C3N7 are exfoliated into mono- or bi-layer flakes. The surface area of the exfoliated nanosheets was investigated by N2 sorption analysis. The g-C3N4, C3N4.8, C3N6, and C3N7 nanosheets have 3–6-fold expanded Brunauer–Emmett–Teller (BET) surface area as compared to their parent bulk materials (Fig. S2, see the ESI†). The expanded surface area and better N2 adsorption ability of exfoliated carbon nitride nanosheets could contribute to increased catalytic performance of g-C3N4, C3N4.8, C3N6, and C3N7 upon exfoliation.
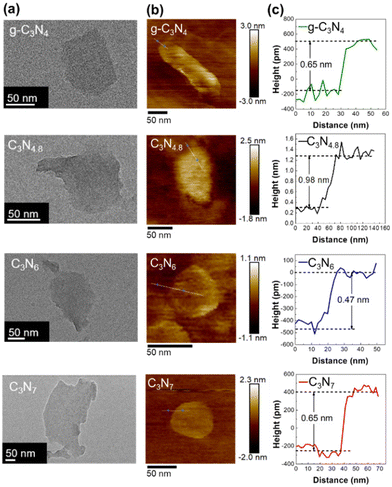 | ||
| Fig. 3 (a) TEM and (b) AFM images and (c) height profiles for exfoliated g-C3N4, C3N4.8, C3N6, and C3N7 nanosheets. | ||
The structural retention of triazole-based carbon nitrides upon exfoliation are confirmed by solid-state 13C MAS NMR spectroscopy as shown in Fig. 1(a). The 13C MAS NMR spectral features of exfoliated C3N4.8, C3N6, and C3N7 nanosheets are identical to those of their parent bulk materials. X-ray photoelectron spectroscopy (XPS) analysis results also support the structural retention of the triazole-based carbon nitrides after exfoliation. The C 1s and N 1s XPS spectra of the exfoliated g-C3N4, C3N4.8, C3N6, and C3N7 nanosheets are shown in Fig. S3† and Fig. 4(a), respectively. The overall N 1s XPS spectral features of the exfoliated C3N4.8, C3N6, and C3N7 nanosheets are the same as the XPS spectral features of bulk C3N4.8, C3N6, and C3N7 (Fig. S4, see the ESI†), indicating a retention of the triazole-based structure after exfoliation. In both bulk and exfoliated nanosheet systems, g-C3N4 samples display an intense binding energy of C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (N1) and a weaker binding energy of N–(C)3 (N2), which are typical N 1s XPS spectral features of g-C3N4.24,25 In contrast, exfoliated C3N4.8, C3N6, and C3N7 nanosheets and their bulk samples have an additional component at a binding energy of approximately 400.0 eV in their N 1s XPS spectra, which correspond to the binding energy of N–N (N4) in the triazole-based structure. The terminal N–H2 (N3) feature at approximately 404.6 eV is weak or negligible in N 1s XPS spectra of all present carbon nitride samples due to their low portion in materials.
C (N1) and a weaker binding energy of N–(C)3 (N2), which are typical N 1s XPS spectral features of g-C3N4.24,25 In contrast, exfoliated C3N4.8, C3N6, and C3N7 nanosheets and their bulk samples have an additional component at a binding energy of approximately 400.0 eV in their N 1s XPS spectra, which correspond to the binding energy of N–N (N4) in the triazole-based structure. The terminal N–H2 (N3) feature at approximately 404.6 eV is weak or negligible in N 1s XPS spectra of all present carbon nitride samples due to their low portion in materials.
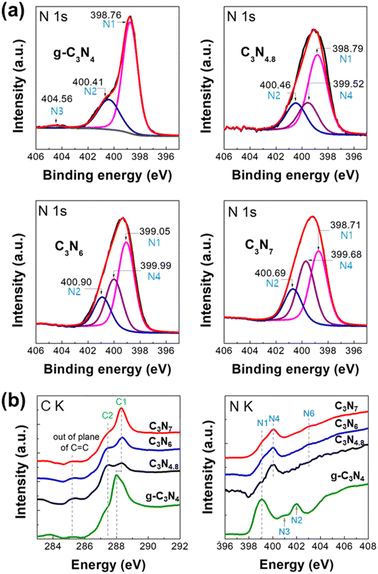 | ||
| Fig. 4 (a) N 1s XPS spectra and (b) (left) C K- and (right) N K-edge NEXAFS spectra of exfoliated g-C3N4, C3N4.8, C3N6, and C3N7 nanosheets. | ||
Detailed local atomic structures of exfoliated g-C3N4, C3N4.8, C3N6, and C3N7 nanosheets were further examined via near-edge X-ray absorption fine structure (NEXAFS) analysis at the C K- and N K-edges (Fig. 4(b)). In the C K-edge spectra, the g-C3N4 nanosheets show π* excitations from the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C plane, HN–C
C plane, HN–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (C2), and N–C
N (C2), and N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (C1) at 285.2, 287.3, and 288.0 eV, respectively.22,23 While the π* positions of C
N (C1) at 285.2, 287.3, and 288.0 eV, respectively.22,23 While the π* positions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C for g-C3N4 and triazole-based C3N4.8, C3N6, and C3N7 nanosheets are the same, the triazole-based C3N4.8, C3N6, and C3N7 nanosheets exhibit π* of N–C
C for g-C3N4 and triazole-based C3N4.8, C3N6, and C3N7 nanosheets are the same, the triazole-based C3N4.8, C3N6, and C3N7 nanosheets exhibit π* of N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (C1′ and C1′′) at higher energy compared to g-C3N4. The π* blueshift of N–C
N (C1′ and C1′′) at higher energy compared to g-C3N4. The π* blueshift of N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (C1) is an intrinsic spectral feature for triazole-based carbon nitrides, which arises from reinforcement of N–C
N (C1) is an intrinsic spectral feature for triazole-based carbon nitrides, which arises from reinforcement of N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (C1′ and C1′′) by neighbouring N–N (N4) bonds.1 In the N K-edge spectra, unlike g-C3N4 nanosheets, the triazole-based C3N4.8, C3N6, and C3N7 nanosheets display π* of heterocyclic N–N (N4) bonds at 400.2 eV. This is an additional unique spectral feature of triazole-based carbon nitrides, which is associated with the emergence of the N–N (N4) component at a binding energy of approximately 400.0 eV in the N 1s XPS spectra of triazole-based C3N4.8, C3N6, and C3N7 samples. The C K- and N K-edge NEXAFS spectral features of exfoliated C3N4.8, C3N6, and C3N7 nanosheets are in good agreement with those of highly ordered mesoporous C3N4.8, C3N6, and C3N7 reported previously,1 highlighting that all the triazole-based materials maintained their crystal structure after exfoliation into nanosheets.
N (C1′ and C1′′) by neighbouring N–N (N4) bonds.1 In the N K-edge spectra, unlike g-C3N4 nanosheets, the triazole-based C3N4.8, C3N6, and C3N7 nanosheets display π* of heterocyclic N–N (N4) bonds at 400.2 eV. This is an additional unique spectral feature of triazole-based carbon nitrides, which is associated with the emergence of the N–N (N4) component at a binding energy of approximately 400.0 eV in the N 1s XPS spectra of triazole-based C3N4.8, C3N6, and C3N7 samples. The C K- and N K-edge NEXAFS spectral features of exfoliated C3N4.8, C3N6, and C3N7 nanosheets are in good agreement with those of highly ordered mesoporous C3N4.8, C3N6, and C3N7 reported previously,1 highlighting that all the triazole-based materials maintained their crystal structure after exfoliation into nanosheets.
The spectroscopic analyses support the conclusion that the triazole-based atomic structures of C3N4.8, C3N6, and C3N7 remain unchanged after the sonochemical process, which is contrary to the general expectation that nitrogen-rich compounds can easily decompose under high external energy.26 When additional TEM analyses were conducted to confirm whether the nitrogen-rich triazole-based C3N4.8, C3N6, and C3N7 nanosheets decomposed or were retained, the exfoliated C3N4.8, C3N6, and C3N7 nanosheets maintained the nanosheet morphology after 30 d in isopropyl alcohol, indicating their excellent long-term stability (Fig. S5, see the ESI†).
Because band structural features, including the bandgap and band position, are critical for carbon nitrides as photocatalysts,27 the band structures of the triazole-based C3N4.8, C3N6, and C3N7 nanosheets were examined by UV–Vis spectroscopy and ultraviolet photoelectron spectroscopy (UPS). As shown in Fig. 5(a), the bandgaps of the triazole-based C3N4.8, C3N6, and C3N7 nanosheets increased upon exfoliation, with bulk g-C3N4, C3N4.8, C3N6, and C3N7 having optical bandgaps of 2.63, 2.00, 2.70, and 3.00 eV, respectively. The optical bandgaps of g-C3N4, C3N4.8, C3N6, and C3N7 nanosheets were 2.72, 2.26, 2.84, and 3.18 eV, respectively. All the exfoliated nanosheets show visible-light absorption below the band edge termed the Urbach tail owing to their low crystallinity.28 The UPS provides information on the top position of the valence bands of carbon nitrides.29 Based on the UPS spectra of the triazole-based C3N4.8, C3N6, and C3N7 nanosheets plotted in Fig. 5(b), the band structures of the corresponding nanosheets are shown in Fig. 5(c). Their valence band top position does not significantly differ from that of the g-C3N4 nanosheet in the range of 1.80–1.90 eV vs. NHE;29 however, the conduction band position shifts toward negative potentials as the nitrogen content increases for the triazole-based carbon nitrides. The conduction band positions of triazole-based C3N4.8, C3N6, and C3N7 nanosheets are −0.36, −0.94, and −1.38 eV vs. NHE, respectively. As compared to g-C3N4 nanosheets, triazole-based C3N7 nanosheets have a more negative conduction band position, indicating higher potential energy to reduce nitrogen to ammonia at 0.55 eV vs. NHE.30 This characteristic of C3N7 nanosheets could help lower the activation energy of N2 for the NRR.
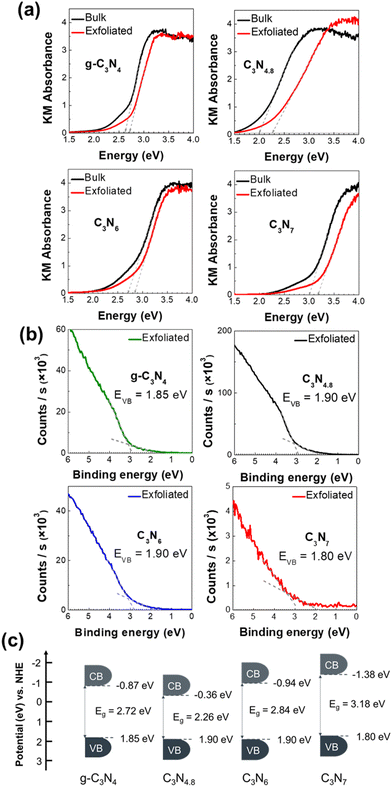 | ||
| Fig. 5 (a) UV–Vis absorption spectra, (b) UPS spectra, and (c) schematic illustration of the band position for exfoliated g-C3N4, C3N4.8, C3N6, and C3N7 nanosheets. | ||
Prior to examining the photocatalytic NRR activities of the triazole-based nanosheets, those of bulk carbon nitrides were investigated. The NRR performances of bulk g-C3N4, C3N4.8, C3N6, and C3N7 are shown in Fig. S6 (see the ESI†). Bulk C3N7 exhibits the highest activity for ammonia production, underscoring the usefulness of nitrogen enrichment in improving the NRR activity of carbon nitride. As presented in Fig. 6(a), all present carbon nitride materials commonly show a significant improvement of photocatalyst performance upon exfoliation. Based on N2 sorption analysis, the improvement in the NRR activity upon exfoliation is ascribed to enhanced N2 adsorption efficiency on the expanded surface of exfoliated nanosheets of carbon nitrides. The ammonia production rates of exfoliated g-C3N4, C3N4.8, C3N6, and C3N7 nanosheets are approximately 242.1, 60.8, 265.8, and 472.4 μmol gcat−1 h−1, respectively. In the triazole-based carbon nitride system, the ammonia production rates are enhanced as the nitrogen content in carbon nitride increases. Furthermore, triazole-based C3N6 and C3N7 nanosheets outperform exfoliated g-C3N4 nanosheets as catalysts for the photocatalytic NRR. These results highlight the importance of the nitrogen-enrichment of triazole-based carbon nitrides in developing efficient NRR photocatalysts.
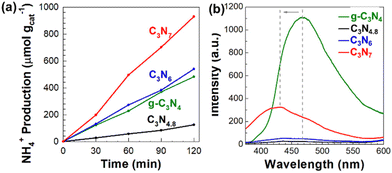 | ||
| Fig. 6 (a) Amount of generated ammonia under a N2 atmosphere and (b) PL spectra for exfoliated g-C3N4, C3N4.8, C3N6 and C3N7 nanosheets. | ||
The NRR performance of carbon nitride increases in the order of C3N4.8 < g-C3N4 < C3N6 < C3N7 in both bulk and exfoliated nanosheet systems, which is an inverse trend with an expectation on NRR performance based on the bandgap size of photocatalysts. Generally, it is expected that a broader bandgap results in reduced photocatalytic performance owing to the reduced light harvesting ability.31 Our opposite observation to the expectation indicates that light harvest ability is not the most critical factor affecting NRR performance in the present triazole-based carbon nitrides system.
The other affecting factors for the NRR performance of carbon nitride catalysts are their surface area and N2 adsorption efficiency. Given that the NRR performance of g-C3N4 nanosheets with the largest surface area is lower than those of triazole-based C3N6 and C3N7 nanosheets, the surface area and N2 adsorption efficiency are not the primary factors for the NRR.
The increasing NRR performance trend in the order of C3N4.8 < g-C3N4 < C3N6 < C3N7 nanosheets is exactly matched with the more negative conduction band position trend of C3N4.8, g-C3N4, C3N6, and C3N7 nanosheets. This good agreement strongly demonstrates that carbon nitride nanosheets with a more negative conduction band position exhibit better NRR activity due to their higher potential energy to reduce nitrogen to ammonia at 0.55 eV vs. NHE.
We also investigated the charge recombination behaviour of all exfoliated carbon nitride nanosheets by photoluminescence (PL) spectroscopy. In general, the low photocatalytic activity of g-C3N4 nanosheets is due to their rapid charge recombination.32,33 As shown in Fig. 6(b), the g-C3N4 nanosheets display a strong PL signal at 468.6 nm, suggesting significant charge recombination. In contrast, triazole-based C3N4.8, C3N6, and C3N7 nanosheets exhibit either negligible or very depressed PL signals, highlighting good charge separation within them. Compared to g-C3N4 nanosheets, the C3N7 nanosheets represent a blue-shifted PL at 432.2 nm, which is associated with their bandgap broadening. In addition to the optimization effect of the conduction band position, the efficient charge separation in the C3N7 nanosheets evidenced by their weak PL signal synergistically contributes towards enhancing their photocatalytic NRR performance.
As present carbon nitrides are nitrogen-rich, cautious ammonia quantification is required when using them as a photocatalyst in the NRR.34–36 To confirm that the present carbon nitride nanosheets are not decomposed to contribute to ammonia production, the photocatalytic NRR under an Ar atmosphere and cyclability test of a photocatalyst were performed for triazole-based C3N7 nanosheets. As shown in Fig. S7 of the ESI,† negligible ammonia production on C3N7 nanosheets under an Ar atmosphere obviously demonstrates the fixation of atmospheric N2 into ammonia on the C3N7 nanosheets. The excellent photocatalytic stability of the C3N7 nanosheets is confirmed by the consecutive NRR activity test, showing no significant degradation in the continuous 5 cycles; see Fig. S8 of the ESI.†
Conclusions
We developed a sonochemical exfoliating process for triazole-based C3N4.8, C3N6, and C3N7 nanosheets with thicknesses of 0.47–0.98 nm. The crystal structures and band structures of the triazole-based C3N4.8, C3N6, and C3N7 nanosheets were revealed by systematic spectroscopic analyses. The exfoliated triazole-based C3N4.8, C3N6, and C3N7 nanosheets retain the parent triazole-based crystal structures and possess more negative conduction band positions with respect to the reduction potential of N2 to NH3 (0.55 V vs. NHE at pH 0), while their valence band positions do not differ significantly. The NRR performance of the carbon nitrides increases in the order of C3N4.8 < g-C3N4 < C3N6 < C3N7 in both bulk and exfoliated nanosheet systems, which is exactly matched with the conduction band position trend of g-C3N4, C3N4.8, C3N6, and C3N7 nanosheets. Additionally, PL analysis of all present carbon nitride nanosheets shows that charge recombination is alleviated within triazole-based carbon nitride nanosheets rather than g-C3N4 nanosheets. Given all these factors, it is concluded that the optimized conduction band position and suppressed charge recombination make triazole-based C3N7 nanosheets the best catalyst in the photocatalytic NRR. The nitrogen-richest C3N7 nanosheets highlight the high efficacy of the simultaneous composition control and exfoliation approach for optimising the photocatalytic activity of triazole-based carbon nitrides.Author contributions
A. Y.: investigation, visualisation, and writing – original draft; T. K.: investigation and formal analysis; D. K.: investigation and formal analysis; Y. J. L.: methodology and writing – review and editing; S.-J. H.: conceptualisation, methodology, writing – review and editing, and funding acquisition; I. Y. K.: supervision, conceptualisation, writing – review and editing, and funding acquisition.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (no. 2021R1C1C1008941, RS-2024-00350241, and RS-2024-00411134), the Seoul R&D Program supported by the Seoul Business Agency (SBA) (BT230224), and a Korea Basic Science Institute (National Research Facilities and Equipment Center) grant funded by the Ministry of Education (2020R 1A 6C 101B194).References
- I. Y. Kim, S. Kim, S. Premkumar, J.-H. Yang, S. Umapathy and A. Vinu, Small, 2020, 16, 1903572 Search PubMed.
- S. Lee, E. Y. Shin, D. Jang, S. Choi, H. Park, J. Kim and S. Park, Bull. Korean Chem. Soc., 2022, 43, 1124 CrossRef CAS.
- G. R. Dillip, T. V. M. Sreekanth and S. W. Joo, Ceram. Int., 2017, 43, 6437 CrossRef CAS.
- P. Kumar, E. Vahidzadeh, U. K. Thakur, P. Kar, K. M. Alam, A. Goswami, N. Mahdi, K. Cui, G. M. Bernard, V. K. Michaelis and K. Shankar, J. Am. Chem. Soc., 2019, 141, 5415 CrossRef CAS.
- S. Kim, G. Singh, C. Sathish, P. Panigrahi, R. Daiyan, X. Lu, Y. Sugi, I. Y. Kim and A. Vinu, Chem. – Asian J., 2021, 16, 3999 CrossRef CAS.
- S. Kim, M. Hankel, W. Cha, G. Singh, J. M. Lee, I. Y. Kim and A. Vinu, Nano Energy, 2020, 72, 104702 CrossRef CAS.
- S. M. Ruban, C. I. Sathish, K. Ramadass, S. Joseph, S. Kim, V. D. B. C. Dasireddy, I. Y. Kim, A. H. Al-Muhtaseb, Y. Sugi and A. Vinu, ChemCatChem, 2021, 13, 468 CrossRef CAS.
- W. Wang, G. Li, T. An, D. K. L. Chan, J. C. Yu and P. K. Wong, Appl. Catal., B, 2018, 238, 126 Search PubMed.
- L. Yang, X. Liu, Z. Liu, C. Wang, G. Liu, Q. Li and X. Feng, Ceram. Int., 2018, 44, 20613 CrossRef CAS.
- C. Hu, W.-F. Tsai, W.-H. Wei, K.-Y. A. Lin, M.-T. Liu and K. Nakagawa, Carbon, 2021, 175, 467 CrossRef CAS.
- P. Xia, B. Zhu, B. Cheng, J. Yu and J. Xu, ACS Sustainable Chem. Eng., 2018, 6, 965 CrossRef CAS.
- C. Wu, S. Xue, Z. Qin, M. Nazari, G. Yang, S. Yue, T. Tong, H. Ghasemi, F. C. R. Hernandez, S. Xue, D. Zhang, H. Wang, Z. M. Wang, S. Pu and J. Bao, Appl. Catal., B, 2021, 282, 119557 CrossRef CAS.
- X. Wu, X. Wang, F. Wang and H. Yu, Appl. Catal., B, 2019, 247, 70 CrossRef CAS.
- Y. Li, M. Yang, Y. Xing, X. Liu, Y. Yang, X. Wang and S. Song, Small, 2017, 13, 1701552 CrossRef.
- N. H. Kwon, J. Park, X. Jin, S.-J. Kim, H. Kim and S.-J. Hwang, ACS Nano, 2023, 17, 23732 CrossRef CAS PubMed.
- N. H. Kwon, S.-J. Shin, X. Jin, Y. Jung, G.-S. Hwang, H. Kim and S.-J. Hwang, Appl. Catal., B, 2020, 277, 119191 CrossRef CAS.
- G. Liu, Z. Tang, X. Gu, N. Li, H. Lv, Y. Huang, Y. Zeng, M. Yuan, Q. Meng, Y. Zhou and C. Wang, Appl. Catal., B, 2022, 317, 121752 CrossRef CAS.
- G. Dong, W. Ho and C. Wang, J. Mater. Chem. A, 2015, 3, 23435 RSC.
- Y. Xue, Y. Guo, Z. Liang, H. Cui and J. Tian, J. Colloid Interface Sci., 2019, 556, 206 CrossRef CAS.
- W. Li, Z. Guo, L. Jiang, L. Zhong, G. Li, J. Zhang, K. Fan, S. Gonzalez-Cortes, K. Jin, C. Xu, T. Xiao and P. P. Edwards, Chem. Sci., 2020, 11, 2716 RSC.
- D. Vidyasagar, S. G. Ghugal, S. S. Umare and M. Banavoth, Sci. Rep., 2019, 9, 7186 CrossRef PubMed.
- I. Y. Kim, S. Kim, X. Jin, S. Premkumar, G. Chandra, N.-S. Lee, G. P. Mane, S.-J. Hwang, S. Umapathy and A. Vinu, Angew. Chem., Int. Ed., 2018, 57, 17135 CrossRef CAS.
- Y. Zheng, Y. Jiao, Y. Zhu, L. H. Li, Y. Han, Y. Chen, A. Du, M. Jaroniec and S. Z. Qiao, Nat. Commun., 2014, 5, 3783 CrossRef PubMed.
- K. Akaike, K. Aoyama, S. Dekubo, A. Onishi and K. Kanai, Chem. Mater., 2018, 30, 2341 CrossRef CAS.
- S. Gu, J. Xie and C. M. Li, RSC Adv., 2014, 4, 59436 RSC.
- W. Sun, A. Holder, B. Orvañanos, E. Arca, A. Zakutayev, S. Lany and G. Ceder, Chem. Mater., 2017, 29, 6936 CrossRef CAS.
- D. Zhao, C.-L. Dong, B. Wang, C. Chen, Y.-C. Huang, Z. Diao, S. Li, L. Guo and S. Shen, Adv. Mater., 2019, 31, 1903545 CrossRef CAS.
- K. J. Archana, A. C. Preetha and K. Balasubramanian, Opt. Mater., 2022, 127, 112245 CrossRef.
- Y. Kang, Y. Yang, L.-C. Yin, X. Kang, G. Liu and H.-M. Cheng, Adv. Mater., 2015, 27, 4572 CrossRef CAS.
- Y. Huang, N. Zhang, Z. Wu and X. Xie, J. Mater. Chem. A, 2020, 8, 4978 RSC.
- B. S. Reghunath, S. Rajasekaran, S. Mathew, D. Pinheiro, S. Devi K. R, S. Jung, T. Jayaraman and M. Y. Choi, Bull. Korean Chem. Soc., 2023, 44, 969 CrossRef CAS.
- S. Choe, S. M. Kim, Y. Lee, J. Seok, J. Jung, J. S. Lee and Y. J. Jang, Nano Convergence, 2021, 8, 22 CrossRef CAS PubMed.
- C. Liang, H. Y. Niu, H. Guo, C. G. Niu, D. W. Huang, Y. Y. Yang, H. Y. Liu, B. B. Shao and H. P. Feng, Chem. Eng. J., 2020, 396, 125395 CrossRef CAS.
- Y. Zhao, R. Shi, X. Bian, C. Zhou, Y. Zhao, S. Zhang, F. Wu, G. I. N. Waterhouse, L.-Z. Wu, C.-H. Tung and T. Zhang, Adv. Sci., 2019, 6, 1802109 CrossRef.
- D. Li, Y. Zhao, Y. Miao, C. Zhou, L.-P. Zhang, L.-Z. Wu and T. Zhang, Adv. Mater., 2022, 34, 2207793 CrossRef CAS PubMed.
- A. C. Nielander, J. M. McEnaney, J. A. Schwalbe, J. G. Baker, S. J. Blair, L. Wang, J. G. Pelton, S. Z. Andersen, K. Enemark-Rasmussen, V. Čolić, S. Yang, S. F. Bent, M. Cargnello, J. Kibsgaard, P. C. K. Vesborg, I. Chorkendorff and T. F. Jaramillo, ACS Catal., 2019, 9, 5795 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr03639e |
| ‡ These authors equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2025 |

