 Open Access Article
Open Access ArticleSustainable nanofibrous membranes for air filtration, water purification and oil removal
Nayli Erdeanna Binte
Surat'man
a,
Xin Lin
Quek
a,
Nannan
Wang
a,
Enyi
Ye
 b,
Jianwei
Xu
b,
Jianwei
Xu
 a,
Zibiao
Li
a,
Zibiao
Li
 *abc and
Bofan
Li
*abc and
Bofan
Li
 *a
*a
aInstitute of Sustainability for Chemicals, Energy and Environment (ISCE2), Agency for Science, Technology, and Research (A*STAR), 1 Pesek Road, Jurong Island, Singapore 627833, Republic of Singapore. E-mail: lizb@isce2.a-star.edu.sg; li_bofan@isce2.a-star.edu.sg
bInstitute of Materials Research and Engineering (IMRE), Agency for Science, Technology, and Research (A*STAR), 2 Fusionopolis Way, Innovis #08-03, Singapore 138634, Republic of Singapore
cDepartment of Materials Science and Engineering, National University of Singapore, Singapore 117576, Republic of Singapore
First published on 13th February 2025
Abstract
The increasing demand for sustainable solutions to address environmental and energy challenges has driven the development of advanced materials. Among them, nanofibrous membranes have emerged due to their high surface area, tunable porosity and versatile mechanical properties. However, traditional nanofibrous membranes, made from petroleum-based synthetic polymers, pose significant environmental concerns due to their non-biodegradability and reliance on fossil resources. This paper reviews recent advancements in the development of sustainable nanofibrous membranes, focusing on the use of biobased and biodegradable materials, and circular design approaches aimed at reducing environmental impact throughout the membrane life cycle. Challenges associated with improving the mechanical strength and stability of biopolymer-based nanofibers and expanding application areas are discussed. By highlighting strategies to overcome these limitations, this review aims to provide insights into the future direction of sustainable nanofibrous membranes, paving the way for their broader adoption in eco-friendly technological solutions.
1. Introduction
The world today faces unprecedented environmental and energy challenges, such as water scarcity, air pollution, and the depletion of non-renewable resources.1,2 As urbanisation and industrial activities continue to intensify, the demand for clean water, breathable air, and sustainable energy solutions is becoming increasingly critical. Water scarcity, exacerbated by climate change and pollution, threatens not only the availability of drinking water, but also the agricultural and industrial processes that sustain economies worldwide.3,4 Additionally, air pollution, driven by emissions from vehicles, factories, and power plants, contributes to a host of respiratory diseases and environmental degradation.5,6 Addressing these challenges requires the development of innovative materials and technologies that are not only effective, but also environmentally sustainable.Nanofibrous membranes have emerged as a promising class of materials with the potential to mitigate these environmental issues. Due to their unique properties, such as a high surface area-to-volume ratio, controllable pore size, and excellent mechanical strength, nanofibrous membranes are widely applied in water purification, air filtration and energy storage.7–9 Traditionally, nanofibrous membranes have been produced from synthetic polymers such as polyacrylonitrile (PAN), polyvinylidene fluoride (PVDF), and polystyrene (PS).10–12 These materials are favoured for their chemical resistance, thermal stability, and ease of processing, making them suitable for a wide range of industrial applications. However, the environmental impact of these traditional materials cannot be overlooked. The production of synthetic polymers often involves the use of non-renewable petroleum resources, energy-intensive processes, and hazardous chemicals.13,14 Furthermore, disposal of non-renewable materials at the end of their life cycle poses significant environmental challenges, as they persist in the environment for hundreds of years, contributing to the growing problem of plastic pollution and microplastics in our oceans.15,16
Given these drawbacks, there is an urgent need to transition towards more sustainable nanofibrous membrane technologies.13 This shift involves the development of nanofibers from renewable, biodegradable, and recyclable materials such as biopolymers. Biopolymers such as cellulose, chitosan, and polylactic acid (PLA) have gained attention as alternatives to traditional synthetic polymers.17,18 These biobased materials not only reduce the reliance on fossil fuels, but also offer the potential for complete biodegradability, thus minimising their environmental footprint.19 Sustainable transformation of nanofibrous membranes extends beyond the choice of materials and production methods. It requires design considerations to endow the membranes with easy recyclability and safe degradability at the end of their use. By incorporating circular economy principles into the life cycle of membranes, creating recyclable nanofibrous membranes for environmental sustainability without performance compromise is possible. This transformation is essential to ensure that membranes can play a role in addressing the environmental and energy challenges of the 21st century.
The use of polymeric nanomaterials such as nanofibers in membrane technology allows for excellent membrane performance due to intrinsic features including their high surface-to-volume ratio and interconnected porous structure. Electrospinning is the most established technique in nanofiber preparation. A general electrospinning set-up includes a syringe with a nozzle, an electric field source, a counter electrode and a pump. Using electrostatic repulsion forces in a high electrical field, a large electrical field is generated between the nozzle and counter electrode and a solution in the syringe nozzle is ejected. Ultimately, as the solvent in the solution evaporates, the nanofiber solid is formed. Besides electrospinning, nanofibers can also be prepared using other techniques, including centrifugal spinning, melt-blowing and recently, electro-centrifugal spinning.20
In this review, we aim to provide a comprehensive overview of the advancements in sustainable nanofibrous membranes in the last three years, including biobased, biodegradable and recyclable nanofibrous membranes. Sustainable nanofibrous membranes and the sustainable polymers used in their preparation are discussed in sections 2 and 3. The properties and performance of these materials, and their applications in gas separation, water purification and oil remediation are examined in section 4. An outlook of sustainable nanofibrous membranes that may catalyse the shift towards sustainability in the membrane industry will be provided. By exploring these aspects, this paper seeks to highlight the potential of sustainable nanofibers to contribute to a more resilient and sustainable future.
2. Biobased & biodegradable nanofibers
2.1. Polymers
Polymers are broadly classified into biobased polymers and fossil-based or fuel-based polymers. Biobased polymers are derived from biomass sources while fuel-based polymers are derived from non-renewable fossil fuel sources. The broad classification can be further specified into 4 categories according to its biodegradability: (1) biobased and biodegradable polymers, (2) biobased and non-biodegradable polymers, (3) fuel-based and biodegradable polymers, and (4) fuel-based and non-biodegradable polymers (Fig. 1). Currently, industries including the membrane industry are largely using the last category (fuel-based and non-biodegradable polymers) as raw materials.21–23 Due to the non-renewable nature and the unsustainable practices caused by these non-renewable materials, there is an increasing necessity to reverse the damage done to the environment and to shift towards a green and circular economy using renewable and biodegradable resources with sustainable industrial practices.23–26 In this review, categories (1), (2) and (3) will be briefly discussed in sections 2.1.1, 2.1.2 and 2.1.3, respectively.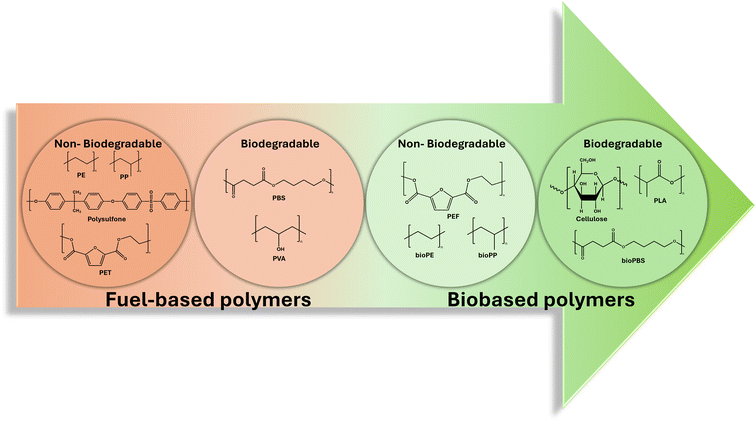 | ||
| Fig. 1 The classification of bio-based polymers and fossil-based polymers based on biodegradability. | ||
Notably, it is not sufficient to rely on the replacement of non-renewable polymers with biobased alternatives as the preparation of biobased nanofibers using conventional methods may have negative effects on the environment due to the inevitable utilisation of toxic and harsh chemicals or high energy consumption.27,28 On top of replacing conventional non-renewable polymers with their biobased or degradable counterparts, additional considerations such as reduction of non-renewable waste or by-products and minimal usage of toxic materials for a reduced carbon footprint are necessary to eventually move forward to a circular and sustainable nanofibrous membrane industry in the future.29–31
Cellulose nanofibers have been widely applied in water purification due to their good mechanical properties and low cost. Despite the extensive use of cellulose nanofibers in water treatment applications, they lack sufficient chemical groups on their surface for the adsorption of pollutants such as heavy metal ions.36 Bymbatsogt and team utilized TEMPO to generate carboxylate groups on the cellulose surface in TEMPO-oxidised cellulose nanofiber water filters.37 A reusable water filter with high water permeance and excellent mechanical properties was retrieved at the end of their investigation. The cast-coated TEMPO-oxidised cellulose nanofiber filters exhibited enhanced adsorption efficiency as its adsorption of copper(II) ions, a heavy ion water pollutant, reached 280 mg m−2. Other than TEMPO, Khan et al. utilised oil palm empty fruit bunch, filter paper and denim cotton waste to enhance the adsorption efficiency of cellulose nanofibers.38 The cellulose nanofibers demonstrated abundant negative charges which interacted strongly via electrostatic interactions and hydrogen bonding with positively charged methylene blue of molecular size 1.382 nm. PLA is a biobased and biodegradable polymer used in industries such as packaging, textile and biomedicine. PLA was combined with TEMPO-oxidised cellulose nanofibers to obtain PLA/nanocellulose biocomposites with excellent stiffness and thermal stability for packaging applications.39 In this novel study, the investigators fabricated the biocomposites via the Pickering emulsion method followed by cold crystallization. As compared to the pristine PLA film with Young's modulus of 2.8 GPa, Young's modulus of the modified PLA/nanocellulose film is 4.0 GPa.
Another biobased and biodegradable polymer group used in the development of nanofibers is PHAs. PHAs are naturally occurring and include polymers such as poly(3-hydroxybutyrate) (PHB), poly(3-hydroxybutyrate-co-valerate) (PHBV) and polyhydroxybutyrate-co-hexanoate (PHBH). Obtained from microorganisms and equipped with intrinsic biocompatibility, PHAs are commonly studied in biomedical applications. It was reported that PHAs formed by 3-hydroxybutyrate and 4-hydroxybutyrate monomers were fabricated into antibacterial nanofibrous membranes for drug delivery and cell culture (Fig. 2a).40 The membrane was loaded with ceftazidime and doripenem antibiotics and successfully inhibited the development of S. aureus and E. coli. An electrospun PHB was modified by atmospheric plasma to enhance its surface roughness and high hydrophobicity.41 Plasma surface modification is a fast, cheap and environmentally friendly method to improve PHB-based nanofibers. The modified PHB nanofiber scaffolds displayed enhanced cell–nanofiber scaffold interaction as apatite-like depositions covered the scaffolds, thus enabling a high calcium-to-phosphorus ratio, suitable for the intended application of the work.
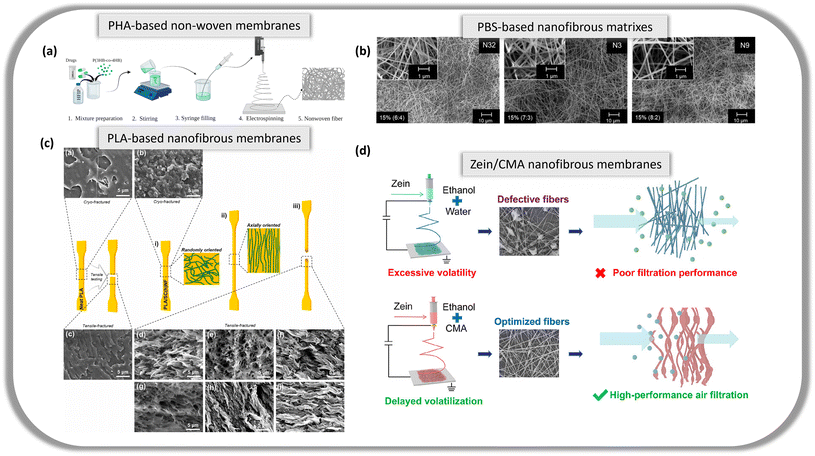 | ||
Fig. 2 (a) Schematic diagram of the process of obtaining PHA-based non-woven membranes loaded with antibiotic drugs via the electrospinning method. Reproduced from ref. 40 with permission from MDPI copyright 2023. (b) SEM images of electrospun biobased 15% (w/v) PBS fibrous matrices from CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCOOH solutions. N32, N3 and N9 with solvent ratios of 6 HCOOH solutions. N32, N3 and N9 with solvent ratios of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 7 4, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 8 3 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 respectively. Reproduced from ref. 42 with permission from Elsevier Ltd. Copyright 2023. (c) SEM images of cryo-fractured cross-sections of (a) pristine PLA, (b) PLA–chitin reinforced nanofibers, and tensile-fractured cross-sections of (c) pristine PLA, (d–f) PLA–cellulose reinforced nanofibers, (g–i) PLA–chitin reinforced nanofibers. Reproduced from ref. 43 with permission from Elsevier Ltd. Copyright 2024. (d) Schematic illustration of the optimized electrospun zein/CMA nanofibers using the solution volatility optimization strategy compared to conventional pure zein nanofibers using ethanol and water for air filtration. Reproduced from ref. 44 with permission from American Chemical Society copyright 2023. 2 respectively. Reproduced from ref. 42 with permission from Elsevier Ltd. Copyright 2023. (c) SEM images of cryo-fractured cross-sections of (a) pristine PLA, (b) PLA–chitin reinforced nanofibers, and tensile-fractured cross-sections of (c) pristine PLA, (d–f) PLA–cellulose reinforced nanofibers, (g–i) PLA–chitin reinforced nanofibers. Reproduced from ref. 43 with permission from Elsevier Ltd. Copyright 2024. (d) Schematic illustration of the optimized electrospun zein/CMA nanofibers using the solution volatility optimization strategy compared to conventional pure zein nanofibers using ethanol and water for air filtration. Reproduced from ref. 44 with permission from American Chemical Society copyright 2023. | ||
BioPBS is a biobased and biodegradable polyester that is structurally similar to PBS and is derived from succinic acid and 1,4-butanediol. PBS is a fuel-based polymer, which will be discussed in section 2.1.3. Unlike PBS, succinic acid is derived from plant materials. Cooper et al. fabricated porous bioPBS nanofibers with enhanced mechanical properties.45 The electrospun nanofibers with the least bead defects displayed excellent tensile strength and Young's modulus.
BioPE and bioPP are analogous to conventional PE and PP respectively. These biobased but non-biodegradable alternatives are derived from bioethanol from glucose in biomass such as sugarcane. As per our knowledge, currently, bioPE and bioPP are less focused on the development of nanofibers and the fabrication of sustainable nanofibrous membranes. One probable reason could be the rising cost of the production of bioethylene, a crucial building block in the production of both biopolymers.47 However, being completely derived from natural resources, bioPE and bioPP eliminate the need for the use of fossil fuel in nanofibrous membrane fabrication and undoubtedly hold great potential towards a sustainable and circular future in the industry.
2.2. Reinforcing materials
Despite being the most promising alternatives to replace non-renewable conventional polymers, biobased nanofibrous membranes still face challenges for long-term durability such as chemical stability, low permeability and poor antifouling performance due to weaker intrinsic mechanical properties of most biobased materials. To improve the mechanical properties, there are several reinforcement strategies, such as blending or crosslinking by reinforcement materials, post-treatment using thermal treatment and optimizing nanofiber morphology. Among them, additives or reinforcement agents used alongside biobased materials are most investigated and discussed here. In the following section, additives or reinforcing agents used alongside biobased materials are discussed. Some examples of biobased nano-fillers such as chitosan, cellulose, chitin, lignin and alginate and other techniques used to reinforce the mechanical properties and to enhance the functionalities of biobased nanofibers for their desired application in industries are discussed in this section.Cellulose has also been reportedly utilized as a reinforcement material. A dye removal study reported a biobased hydrogel reinforced with wastepaper-derived modified cellulose nanofibers.57 The surface-modified cellulose nano-reinforcement enhanced the water absorption, swelling rate and dye removal efficiency of the biobased hydrogel. The maximum dye removal capacity was demonstrated at 414 mg g−1 for methylene blue dye with a molecular weight of 319.85 g mol−1. In a separate study, a cellulose nanofibril-alginate hydrogel was investigated for its architectural flexibility for a range of industrial applications.58 Cellulose acetate and Desmodesmus sp. microalgae combined in acetic acid/water solutions to fabricate nanofibrous mats via electrospinning was previously reported.59 Fully biobased cellulose acetate films and nanofiber mats were reinforced using fungal chitin nanofibrils in a study.60 Addition of fungal chitin nanofibrils in the electrospinning process endowed the developed film with improved water affinity, reduced surface roughness and tailored the diameter of the nanofiber mats. Additionally, the ultimate tensile strength of the reinforced biobased film reached up to approximately 15.1 MPa. Notably, the fungal chitin was obtained from a common white mushroom, not from crustacean sources as the former does not require harsh pre-treatment methods. High-performance, antibacterial and mosquito-repelling nanofibrous membranes were also reportedly constructed using cellulose acetate and cinnamaldehyde (CMA) for air filtration. When compared to an N95 mask, a filtration efficiency of up to 99.91% was achieved.61 The reinforcement, CMA, is a naturally occurring compound found in the bark of cinnamon trees.
Some reinforcers used with PLA nanofibers to improve mechanical strength and functionalities include cellulose and chitin. Kim et al. demonstrated PLA-based nanocomposites with cellulose and chitin nanofibers as reinforcing nanofillers.43 The cellulose and chitin reinforcing nanofillers possess excellent mechanical properties, abundantly obtained from natural sources, are biodegradable and carbon-neutral. Hence, the PLA-based nanocomposite is completely biobased, biodegradable and renewable. The biobased nanofiber fillers endowed the PLA-based nanocomposites with an incredible mechanical toughness of 151.2 MJ m−3 and an impact strength of 123 J m−1. The cryo-fractured and tensile-fractured SEM images demonstrated the morphological structure of the reinforced PLA-based nanofibers (Fig. 2c).
The aforementioned green materials including cellulose and its derivatives, PVA, PLA, CMA and SA as reinforcement materials for nanofibrous membranes can equip the membranes with excellent separation performance and sufficient mechanical strength. In addition to adding reinforcing materials, using post-treatment and optimizing the nanofiber morphology, such as thermal annealing and control of the alignment of nanofibers, could also improve the mechanical strength of the nanofibrous membrane. However, the performance may be compromised due to the change in the packing of nanofibers.
2.3. Biodegradability studies
In this section, reports on the reusability and biodegradability of biobased and/or biodegradable nanofibers in multiple applications are discussed. The demonstration of biodegradability is usually conducted via enzymatic and natural degradation. In the former demonstration, enzymes such as proteases are utilized to break down the polymer chain in the nanofibers while in the latter demonstration, the nanofibers are left isolated in soil or compost for an extended period for degradation to naturally occur. However, due to the elaborate duration of natural degradation, some studies utilise accelerated processes to degrade the nanofibers at a quicker rate to gather data within a feasible timeframe.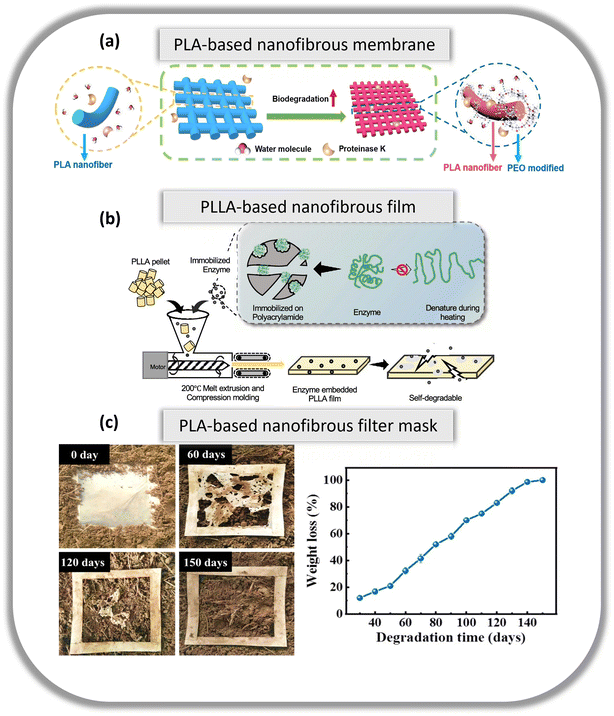 | ||
| Fig. 3 (a) Illustration of the degradation of PLA-based nanofibrous membranes using roteinase K. Reproduced from ref. 64 with permission from American Association for the Advancement of Science copyright 2023. (b) Illustration of the self-degradable PLLA fim embedded with proteinase K using heat. Reproduced from ref. 66 with permission from the American Chemical Society copyright 2020. (c) Photos of the soil burial degradation of the PLA-based mask filter media (3/3, 40 mg) under natural conditions and its corresponding weight loss as a function of time. Reproduced from ref. 67 with permission from Elsevier Ltd. Copyright 2021. | ||
All in all, plenty of investigations have been conducted to demonstrate the potential of biobased and/or biodegradable polymers in the development of nanofibers for industrial applications. However, some challenges still stand – reduced mechanical properties which compromise the long-term durability of the product and the replacement of harmful solvents or materials which can be more costly or less practical for industrial scale-up. Notably, for established industries with pre-existing procedures, the switch to more renewable methods may not necessarily be an easy process. Despite these facts, many studies have also proven that these hurdles can be overcome, as discussed earlier in this section.
3. Recyclable nanofibers
Polymeric nanofibrous membranes are commonly used in filtration applications because of their reliable mechanical properties, chemical resistance and excellent filtration capabilities. However, disposal problems arise when they reach the end of their life cycle as nanofibrous membranes made of conventional non-renewable polymers cannot be easily reused or recycled. Previously, biobased and/or biodegradable nanofibrous membranes obtained from renewable sources were discussed. Henceforth, recyclable nanofibrous membranes which may consist of synthetic polymers from non-renewable sources are discussed and considered for the development of the circular nanofibrous membrane industry. In this section, current common methods of recycling nanofibrous membranes include (1) transforming existing materials to be reused in a suitable alternate process; (2) recovering existing material to be reused in the same process with similar mechanical properties; (3) synthesis of material that can be easily recycled or broken down back into its monomer units.An example of transforming existing materials for reuse in a suitable alternate process was demonstrated by Varanasi et al. where cellulose nanofiber composite membranes were fabricated, with the addition of polyamide–amine–epichlorohydrin (PAE) and silica nanoparticles of 22 nm for pore size control.72 PAE was added to enhance membrane wet strength by allowing negatively charged nanoparticles to adhere to the nanofiber network. The cellulose nanofiber composite membranes were prepared by either direct addition or controlled and simultaneous addition methods. It was reported that a high flux of 80 L m−2 h−1 and an improved MWCO of 200 kDa were observed. Furthermore, the cellulose nanofiber composite membranes displayed biodegradability and recyclability to be used as a feedstock for paper making. The soaked membrane was initially disintegrated with a hand blender to obtain a suspension with a similar consistency to the original suspension used in the fabrication of fresh cellulose nanofiber composite membranes. However, as the layers in the recycled suspension separate unevenly, it cannot be reused to prepare new membranes but is only added as a recycled paper feedstock.
Single-use face masks have low efficiency in trapping PM0.3/pathogens, poor air permeability and poor reusability or recyclability. Xiong et al. tackled this issue by developing a light weight PAN-based nanofiber carbon nanotube network (NF/CNT) with a high filtration efficiency of >99.994% for PM0.3 removal, a low resistance of <0.05% atmospheric pressure and efficient photo-thermal self-sterilization under 1 sun of >99.986% in 5 min or electrothermally driven self-sterilization of >99.9999% in 2 min under sunless conditions.73 Its durability was also evaluated when it underwent a wet–dry cycle for up to 50 times with only a slight decrease in its filtration efficiency from 99.999% to 99.993% Notably, its photothermal-driven self-sterilization properties were maintained throughout. The NF/CNT filter demonstrated excellent recyclability for the proposed use in desalinating seawater as a high-performance solar vapour generator. The authors depict a route for recycling discarded NF/CNT filters where abandoned filters are self-sterilized under the Sun to be used for seawater desalination based on solar-driven evaporation. When performing water evaporation tests under simulated solar conditions, due to its ultra-high photothermal conversion rate, the NF/CNT filter mask achieved a fast evaporation rate of 1.49 kg m−2 h−1. These results were verified with outdoor experiments where 90.52 g of purified water was collected in 8 h. This measurement is equivalent to a harvesting rate of 3.56 L m−2 d−1. Hence, the NF/CNT filter demonstrated excellent potential to be used as a high-performance solar vapour generator in the desalination of seawater.
Aside from reusing existing materials in an appropriate process, research has also been done on trying to recover and reuse existing materials in the same process. Xu et al. reported how easily this can be done by the simple dissolution of their membrane in the solvent.74 A high-performance superhydrophobic strain sensor was fabricated via electrospinning of recyclable thermoplastic elastomer styrene ethylene butylene styrene copolymer (SEBS) reinforced by multiwalled carbon nanotubes (MWCNTs), followed by ultrasonic anchoring of modified superhydrophobic MWCNT (with fluorine-free octadecyltrichlorosilane (OTS)). These flexible and highly sensitive strain sensors demonstrated their versatility and durability for various applications such as human motion detection, weather monitoring, underwater sensing, and emulsion separation of multiple cycles. More importantly, its recyclability was successfully demonstrated after dissolution in cyclohexane. After complete dissolution in cyclohexane, the recovered materials were put through the fabrication process again for membrane formation. Notably, the recovered modified MWCNTs still maintained their superhydrophobic properties.
Liu et al. also fabricated a biodegradable and recyclable film that can be simply recovered by dissolving in hot water.75 Cellulose nanofibers (CNFs) extracted from Sargasso were modified with 3-glycidyloxypropyltrimethoxysilane (KH560) to form KCNFs (Fig. 4a). PVA has weak water resistance, but when combined with KCNF, the biodegradable cross-linked KCNF/PVA film exhibited increased hydrophobicity with a water contact angle of 119°, an improved dry and wet tensile strength, as compared to CNF/PVA films, outstanding thermal stability and good transmittance. With these properties, KCNF/PVA films display good potential for mass manufacturing and applications under humid and watery conditions. KCNF/PVA films were easily recycled by dissolving in distilled water at 95° and refabricated by casting in molds to dry naturally and to regenerate new films (Fig. 4b). The mechanical properties of recycled films were maintained and were even studied after recycling for 5 cycles. With each cycle, the cross-linked network of KCNF and PVA was partially destroyed, causing a noticeable level of decline in mechanical performance. However, after the 5th cycle, good mechanical performance was achieved. Thus, KCNF/PVA films displayed good potential for mass manufacturing and applications under humid and watery conditions.
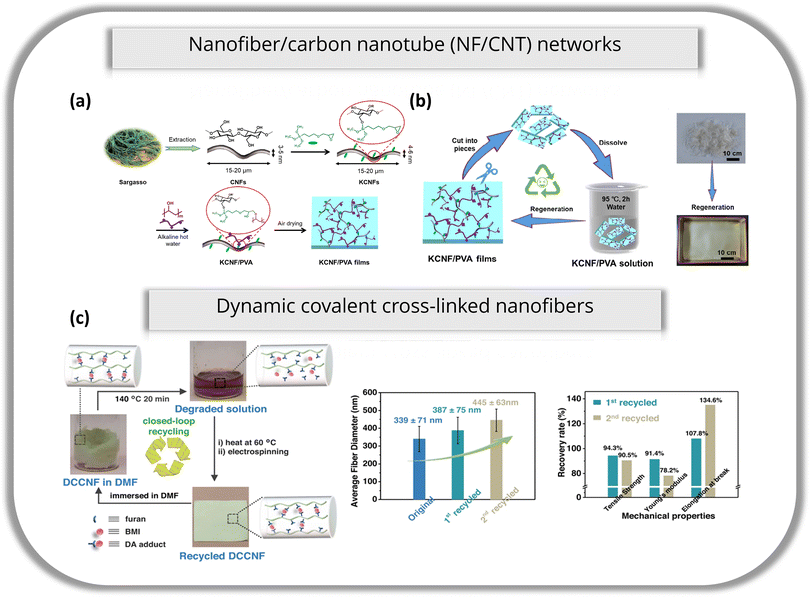 | ||
| Fig. 4 (a) Schematic illustration of the fabrication of the KCNF/PVA film from Sargasso. (b) Schematic diagram of recycling KCNF/PVA films and photos of KCNF/PVA film regeneration after mold casting. Reproduced from ref. 75 with permission from Elsevier Ltd. Copyright 2022. (c) Left to right: Schematic representation of the one-pot closed-loop recycling process of DCCNF-60C; average fiber diameters of original and recycled DCCNF-60C; recovery rate of mechanical properties after each recycling cycle of DCCNF-60C. Reproduced from ref. 76 with permission from Springer Nature Limited. Copyright 2024. | ||
Aramid fibre is a heat-resistant and strong synthetic fibre and its products are widely used in the military and defense industries. However, when damaged, it can be very difficult to be reused or recycled. Chen et al. developed a cost-effective method of recycling Kevlar and aramid fibre products by breaking them down into aramid nanofiber (ANF) dispersions.77 These dispersions were prepared based on the principle of dissolution–precipitation, where potassium hydroxide (KOH) fine powders were produced in situ when mixed in dimethyl sulfoxide (DMSO) and KOH aqueous solutions. The aqueous KOH/DMSO system formed ANF dispersions of 0.2 wt% at ambient temperature in just 26 min, which can be further reduced to 15 min at 40 °C. Additionally, ANF dispersions were utilised to produce thermally stable ANF aerogels with high decomposition temperatures of more than 500 °C. ANF aerogels were also recycled back into ANF dispersions using the same aqueous KOH/DMSO system, ultimately developing a promising method in the closed loop recycling of aramid fibres and ANF-based materials. Aramid nanofibers can also demonstrate excellent performance in organic solvent systems. An ANF/PEI organic solvent nanofiltration membrane with high flux and excellent selectivity was previously developed.78 The membrane successfully demonstrated ultrafast organic solvent permeance for THF and acetone at 20.5 L m−2 h−1 bar−1 and 11.2 L m−2 h−1 bar−1 respectively. In 2019, Li et al. demonstrated a lab-scale solvent-resistant nanofibrous hydrogel thin-film composite (TFC) membrane with aramid nanofibers.79 The membrane exhibited an impressively high pure solvent permeance for methanol and ethanol (polar solvents) and acetone and dimethyl formamide (DMF, harsh organic solvents) at 54.0 L m−2 h−1 bar−1, 13.6 L m−2 h−1 bar−1, 70.0 L m−2 h−1 bar−1 and 34.0 L m−2 h−1 bar−1, respectively. It also displayed high rejections of methyl orange at >90%, and Rose Bengal at approximately 100% with demonstrated long-term stability.
The final example of synthesizing a material that can be easily broken down was demonstrated by Wang et al.76 They utilized covalent adaptable networks (CANs) to produce dynamic covalently crosslinked nanofibers (DCCNFs) that can undergo a one-pot closed-loop recycling process (Fig. 4c). Using poly[(furfuryl methacrylate)-co-(butyl methacrylate)] (FMA-co-BMA) copolymer with bismaleimide (BMI) as the crosslinker, the thermally reversible Diels–Alder (DA) cycloaddition reaction can occur between furan and maleimide. The mixed solution was electrospun and crosslinked before the formation of nanofibrous membranes. Apart from possessing excellent mechanical properties and thermal stability, good flexibility, hydrophobicity and solvent resistance, these DCCNF membranes can be easily repaired or welded back together when encountering wear-and-tear or fractures and undergo closed-loop recycling to be refabricated into a new DCCNF membrane without a significant change in morphology or performance. Further research was done on DCCNFs when Li et al.80 developed DCCNF membranes for use in efficient oil/water separation. Membranes electrospun from furfuryl methacrylate and hexafluorobutyl methacrylate (FMA-co-HFBMA) copolymers displayed excellent chemical resistance, high porosity and hydrophobicity upon in situ crosslinking with BMI. After numerous cycles of filtration and removal of contaminants, the synthesized DCCNF membranes can be decrosslinked in DMF solvent at 140 °C. Using this solution, recycled membranes with very similar morphologies, physical and filtration properties can easily be refabricated via the same electrospinning process.
In the works discussed in this section, the authors successfully demonstrated the potential for nanofibrous membranes to be fabricated using recyclable materials with minimal to no compromise to their mechanical and filtration properties. The methods used include transforming existing materials for other purposes, reusing the recyclable materials for the same purpose after disintegration or dissolution or synthesizing reusable materials such as DCCNFs as part of a closed-loop recycling process. Table 1 summarises the sustainable nanofibrous membranes discussed in sections 2 and 3.
| No. | Membrane | Remarks | Ref. |
|---|---|---|---|
| 1 | Cellulose acetate/chitosan biocomposite nanofibrous membranes | • Woven porous structure | 36 |
| • Low cost | |||
| • Facile operation | |||
| • Optimum Cu2+ adsorption capacity: 86.4 mg g−1 | |||
| 2 | Foam-coated TEMPO cellulose nanofibrous films | • Water permeance: ∼200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 dm3 h−1 m−2 MPa−1 at 1 bar 000 dm3 h−1 m−2 MPa−1 at 1 bar |
37 |
| • Optimum Cu2+ adsorption capacity: ∼60 mg g−1 | |||
| • Optimum Ca2+ adsorption capacity: 63.6 mg g−1 | |||
| 3 | Poly(L-lactic acid)/tempo-oxidised nanocellulose biocomposite films | • Thermally stable and mechanically durable | 39 |
| • Optimum YM: 2.8 GPa | |||
| • Optimum coefficient thermal expansion (CTE): 12, 923 ppm K−1 | |||
| 4 | P(3HB-co-4HB) nanomembranes | • Exhibited antibacterial activity when loaded with ceftazidime, doripinem, and actovegin antibiotics; inhibit the development of S. aureus and E. coli | 40 |
| 5 | BioPBS nanofibrous mats | • Sufficient mechanical properties for applications in wound healing and tissue engineering | 45 |
| 6 | PEF/carboxylated cellulose nanofiber films | • Excellent mechanical and barrier properties | 46 |
| • Optimum tensile strength: 69.0 MPa | |||
| • Optimum YM: 4.3 GPa | |||
| • Optimum toughness: 1.26 MJ m3 | |||
| 7 | PBS nanofibrous mats | • Non-woven nanofibrous mats with potential applications in sustainable solutions | 42 |
| 8 | PBS/CTAB nanofibrous membranes | • Excellent mechanical properties and thermal stability | 55 |
| • Optimum tensile strength: 5 MPa | |||
| • Optimum crystallinity: 38.6% | |||
| 9 | Wastepaper derived modified-cellulose nanofiber hydrogel | • Wastepaper derived cellulose nanofiber reinforcement | 57 |
| • Optimum water absorption capacity: 754 g g−1 | |||
| • Highest dye removal capacity: 414, 405, 377 and 323 mg g−1 for MB, MG, MV and CR dyes respectively | |||
| 10 | Cellulose nanofibril/sodium alginate hydrogel | • An architecturally lightweight product | 58 |
| • Potential application in bio-based and sustainable interior building systems | |||
| 11 | Cellulose acetate/Desmodesmus. sp. nanofibrous mats | • Addition of microalgae waste Desmodesmus. sp. reduced nanofibers sizes from 122 ± 55.2 to ∼50 nm | 59 |
| 12 | Cellulose acetate/fungal chitin nanofibril films | • Increased mechanical property | 60 |
| • Optimum YM: 359 ± 99 MPa | |||
| • Increased elongation at break by ∼45% | |||
| 13 | CA/CMA/PHMB nanofibrous membrane | • Improved antibacterial activity | 61 |
| • Bacteriostatic rates against E. coli and S. aureus 99.65% and 99.99% | |||
| 14 | PVA/AMPS–LMA nanofibrous hydrogel | • High mechanical strength: 24.9 MPa | 62 |
| • Strong thermal stability at 170 °C with no shrinkage | |||
| • Electrolyte uptake: 661.4% | |||
| 15 | PLA/cellulose–chitin nanofibrous membranes | • Excellent mechanical properties | 43 |
| • Mechanical toughness: 151.2 MJ m−3 | |||
| • Elongation at break: 377.5% | |||
| 16 | Zein/CMA nanofibrous membranes | • Air filtration efficiency: 99.25% (PM0.3) | 44 |
| • Quality factor: 0.084 Pa−1 | |||
| 17 | Guar gum/sodium alginate/carboxylated cellulose nanofibrous membranes | • Enhanced mechanical strength | 63 |
| • Improved thermal stability | |||
| • High barrier capacity | |||
| 18 | PLA/polyethylene oxide nanofibrous hydrogel | • Potential alternative to commercial ultrafiltration membranes for oily wastewater separation | 64 |
| • Excellent antifouling performance | |||
| • Degradable using proteinase K | |||
| 19 | PLA/cellulose nanocrystal composite | • Enzymatically degraded using lipase and proteinase K | 65 |
| 20 | PLA nanofibrous membrane | • Resultant mask filter exhibited a high filtration efficiency of 99.996% (PM0.3) | 67 |
| 21 | Cellulose acetate/thermoplastic polyurethanes nanofibrous filter | • Filtration efficiency: 99.8% | 68 |
| • Maintained optimal performance up to 10 cycles | |||
| 22 | Bio-based nanofibrous hydrogel filter | • Flux: 90.6 g cm−2 h−1 | 69 |
| • Rejection of ultrafine suspended solids (10 nm): ∼100% | |||
| 23 | Poly(L-lactic acid) nanofibrous filter | • Filtration efficiency: >99% for PM2.5 and >91% for PM1.0 | 71 |
| 24 | Cellulose/polyamide–amine–epichlorohydrin nanofibrous membrane | • Enhanced membrane wet strength | 72 |
| • Flux: 80 L m−2 h−1 | |||
| • MWCO: 200 kDa | |||
| 25 | PAN/CNT nanofibrous filter | • High removal efficiency: 99.994% for PM0.3 at a low air resistance 49 Pa | 73 |
| • High QF: 0.1984 Pa−1 | |||
| 26 | SEBS/MWCNT nanofibrous membrane | • Resultant strain sensor is versatile and durable | 74 |
| 27 | KCNF/PVA nanofibrous film | • Biodegradable and recyclable | 75 |
| • Increased hydrophobicity; water contact angle 119 °C | |||
| • Enhanced tensile strength | |||
| 28 | ANF/PEI nanofibrous membrane | • Permeance: 20.5 L m−2 h−1 bar−1 (THF) and 11.2 L m−2 h−1 bar−1 (acetone) | 78 |
| 29 | FMA-co-BMA nanofibrous membrane | • Dynamic covalent crosslinking feature endows membrane with long-term reusability | 76 |
| 30 | FMA-co-HFBMA nanofibrous membrane | • Dynamic covalent crosslinking feature endows membrane with long-term reusability | 80 |
4. Applications
Nanofibrous membranes have a wide range of applications and can be easily fabricated for specialized use in various industries and fields, from electronics, energy, textiles, apparel and environmental remediation with high importance in the biomedical and healthcare sectors.81 However, not being able to reuse or recycle these membranes at the end of their usage remains a problem throughout many industries as high volumes of waste are being constantly generated. The following examples will elaborate on the novelty of environmentally friendly materials used in nanofibrous membranes for air filtration, water purification and oil removal applications.4.1. Air filtration
During the COVID-19 pandemic, the demand and usage of face masks surged exponentially with the need to filter airborne particulate matter (PM) and to intercept airborne and infectious particles as a form of biological protection. Conventional face masks made of PP, PE and polyester commonly used in manufacturing surgical face masks, are single-use masks which means they are disposed of after each use. Conventional air filter membranes can be improved in terms of filtration efficiencies and additional capabilities such as antibacterial/antiviral functionalities by utilizing sustainable materials which are renewable, biodegradable and/or recyclable. To ease the burden on landfills and incinerators, methods such as the incorporation of biodegradable materials into current technologies can be applied. With a simple biodegradable polymer derived from renewable organic sources such as corn or sugarcane, Li et al. used PLLA in electrospinning to form a membrane with multi-structured networks (MSN) comprising of micro-sized ribbon-like fibres and ultrafine nanofibers that are tens of nanometers in diameter.82 PLLA MSN membrane demonstrates highly efficient filtration capabilities of >99.9% for PM2.5 and >99.5% for PM0.3 and a low pressure drop of 20 Pa. Similarly, Yang et al.83 designed multi-scale PLA micro-/submicron-fibers and bacterial cellulose (BC) nanofibrous membranes which were fabricated via electrospinning and electro-spraying techniques respectively (Fig. 5a and b). These techniques allowed the multi-layer membrane to have a low pore size of 1.27 μm and provided a delicate balance of filtration efficiency and air resistance. The prepared filter exhibits efficient air filtration of 99.89% PM0.3 removal with a low pressure drop of 104 Pa and a durable filtration efficiency of >99.6% under various airflow rates or after being exposed to 90% humidity for 120 h. The multi-scale nanofibrous filter was fully biodegradable via enzymatic degradation within 2 h.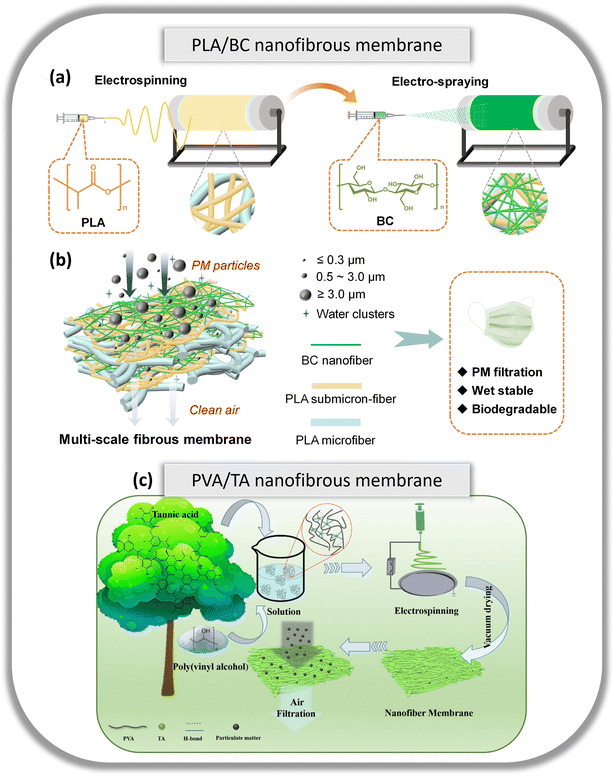 | ||
| Fig. 5 (a) Schematic diagram of the electrospinning and electro-spraying process for the PLA/BC multi-scale fibre membrane. (b) Schematic representation of the PM filtration process of the multilayer multi-scale PLA/BC membrane under a humid environment and its characteristics. Reproduced from ref. 83 with permission from Elsevier Ltd. Copyright 2024. (c) Schematic illustration on the preparation and application of the PVA–TA nanofibrous membrane. Reproduced from ref. 84 with permission from Elsevier Ltd. Copyright 2021. | ||
The use of biobased and biodegradable synthetic polymers in membrane fabrication has also been of interest because of their better mechanical and filtration properties. Zhan et al. used polyamide-based polybutyrolactam (PA4) to fabricate membranes with high porosity of >80% and 99.85% PM2.5 filtration performance, and highly stable and excellent mechanical properties with a tensile strength of ≥4.25 MPa and Young's modulus of ≥34.82 MPa.85 The biodegradability was also investigated where a weight loss of 88% was reported within 49 days. Chen et al. also utilized a biosynthetic polymer, PHBV to fabricate a hierarchical nanoweb-structured membrane via electrospinning.86 Respectively, excellent filtration efficiencies of PM0.3 and PM2.5 of 99.999% and 100% with 0.077% standard atmospheric pressure of 5.3 cm s−1 airflow speed were reported. Notably, the membranes were easily biodegradable under composting conditions where complete disintegration was achieved within 1 week.
If a green polymer of interest is not able to achieve the required properties on its own, additional materials may aid in the fabrication of an improved membrane. Cui et al. managed to fabricate durable biodegradable nanofibrous membranes via green electrospinning and physical crosslinking of PVA and tannic acid (TA) (Fig. 5c).84 The PVA–TA nanofibrous membranes have a removal efficiency of 99.5% for PM1.0 at a pressure drop of 35 Pa, with a quality factor of almost 0.15 Pa−1. It was also proven to be durable when its filtration efficiency was >99% at a low pressure drop of 35.5 Pa even after 10 filtration test cycles. The presence of intermolecular hydrogen bonds provided the PVA–TA nanofibrous membrane with improved mechanical properties i.e. increased tensile strength and elongated nanofibrous membrane by 20% and 50% respectively. Utilizing a synthetic biodegradable polymer, polybutylene adipate terephthalate (PBAT), Cho et al. produced a core–shell nanofibrous membrane (NFM) via electrospinning PBAT with modified montmorillonite (MMT) clay and cetyltrimethylammonium bromide (CTAB).87 Encasing PBAT with CTAB–MMT allows for various significant improvements as compared to pure PBAT NFM. Enhanced mechanical properties and an improvement in the handling process were demonstrated as compared to pure PBAT. Notably, the core–shell membrane was tested to have antibacterial activity against S. aureus, at 99.8%, antiviral properties against influenza at 99.79%, and human coronavirus at 99.99%, and enhanced surface triboelectric properties that provided more durable and stronger electrostatic filtration capabilities of 98.3%, for PM0.3 even at a low differential pressure of ≤40 Pa.
In an attempt to utilize a by-product of naturally occurring materials, Fan et al. electrospun aligned zein nanofibers (zNFs) using zein Pickering emulsion onto a uniquely designed collector plate.88 Before electrospinning, the silver nanoparticle (AgNP)–paper towel (PT) microfiber substrate was fabricated by in situ reduction to ultimately acquire the zNFs–Ag@PT filter. AgNPs endowed the zNFs–Ag@PT filter with an effective broad-spectrum of antimicrobial activity, excellent hydrophobicity and the ability to form thinner nanofibers by forming an anisotropic electric field to stretch and align zein fibres during fabrication, thus enhancing the overall filtration performance of a zNFs–Ag@PT filter by up to 99.3% for PM0.3. Also using zein, Shen et al. developed a green solution of phloretin (PL) and chitosan hydrochloride (CS) in water/ethanol/CMA.89 The solution was utilized to electrospin a fully biobased bimodal fibrous membrane with antibacterial activity and efficient air filtration ability. Advantageously, in the green solution which consists of zein, PL and CS, PL plays a role in the enhancement of zein and CS chain structures which allows better electrospinnability, while CS cations improved jet splitting to form a bimodal structure. The bimodal structure consists of a combination of coarse and fine fibres which endows the nanofibrous membrane with an excellent air filtration performance as coarse fibres allow for sufficient space for airflow passage while fine fibres ensure filtration of fine particles is sustained. An optimal ratio of 3% PL and 1% CS in the green solvent was reported to boost air filtration and antibacterial performances, where air filtration efficiency reached 99.65%, at a pressure drop of 57.7 Pa and a quality factor of 0.098 Pa−1. Here, highly effective, efficient and antibacterial properties were demonstrated with inhibition rates of 99.9% and 98.1% against E. coli and S. aureus respectively. The inhibition rates were maintained even after 30 days.
With these examples, the utilisation of alternative synthetic but biodegradable polymers in nanofibrous membranes has shown promising results in air filtration applications due to comparable performance and functionalities to conventional nanofiber filter masks. However, more research can be focused on scaling up and manufacturing processes to allow biodegradable nanofibrous membranes to be used as improved and sustainable versions of respiratory face masks.
4.2. Water purification
Conventional nanofiltration (NF) or reverse osmosis (RO) membranes used in water treatment are commonly derived from fossil fuel and typically not recyclable or reusable which ultimately end up in landfills or incinerators. Interest and research in alleviating the environmental burden have been picking up in recent years. The development of biodegradable membranes that incorporate renewable, biobased or recyclable polymers with similar mechanical and filtration properties to conventional nanofiltration membranes is gaining attention and is currently sought after to provide a solution towards making the membrane industry sustainable and circular. The following examples elaborate on how the use of natural and/or biodegradable polymers can be applied in this field of water treatment.In a desalination investigation, Sui et al. fabricated biobased nanofibrous foam for high efficiency solar interface evaporation to produce clean water.90 Via electrospinning, a hybrid membrane comprising of cellulose nanofibers and graphene oxide (GO) was obtained via in situ self-assembly and layer-by-layer assembly (Fig. 6a). When reacted with NaBH4, it was transformed into a 3D foam that enhanced the photothermal conversion efficiency, supported water transport at the gas–water interface and reduced GO to reduced graphene oxide (rGO). One side of the foam is altered to be hydrophobic by spray-coating with a fluorocarbon resin (FR), resulting in a Janus type 3D foam (FR@EC/rGO). The evaporation rate in a 3.5 wt% NaCl solution reached 1.83 kg m−2 h−1, with an excellent solar vapour conversion efficiency of 94.2%.
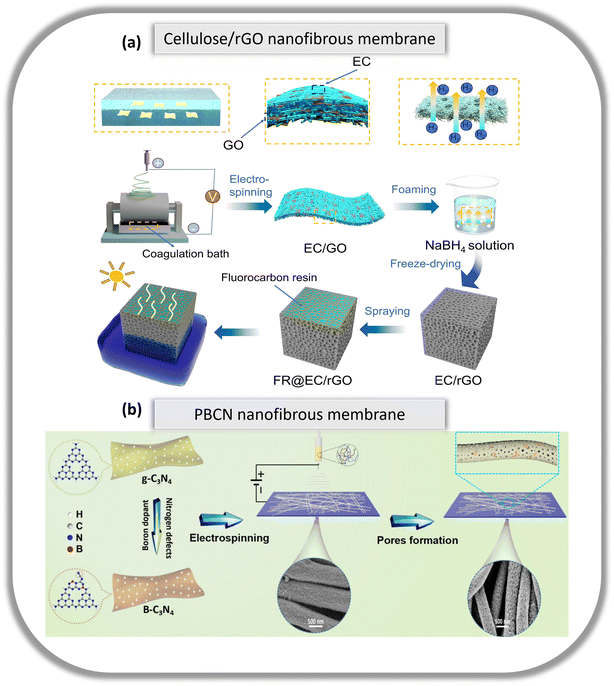 | ||
| Fig. 6 (a) Schematic illustration of the fabrication process of Janus FR@EC/rGO foam. Reproduced from ref. 90 with permission from Elsevier Ltd. Copyright 2024. (b) Schematic illustration of the preparation of superhydrophilic and polyporous nanofibrous membrane (PBCN) via the combination of loading modified photocatalyst (B–C3N4) and constructing hydrophilic channels in nanofibers. Reproduced from ref. 91 with permission from Elsevier Ltd. Copyright 2021. | ||
PLA being a well-known biopolymer derived from natural and renewable sources, is a popular choice of material in the fabrication of nanofibrous membranes. Aijaz et al. electrospun PLA membrane fibres with an average diameter of 700 nm.92 The PLA membrane was subjected to heat treatment at 70 °C for 45 min to allow fibres to fuse and overlap, thus, improving its structural integrity while maintaining its hydrophobicity, porosity and pore size for potential use in membrane distillation. Heat treating hierarchically structured PLA membranes increased the liquid entry pressure (LEP) of up to 25 kPa and enhanced the tensile strength by 1.5 times. More importantly, the PLA membranes can be completely degraded via alkaline hydrolysis with 0.4 M NaOH after 30 min. When the membranes were reinforced with PVDF and mesh spacers, a maximum LEP of 32 kPa and an increased tensile strength of 230% were achieved. The optimized membrane successfully rejected 99% of salt and ∼2 kg m−2 h−1 flux in the air gap membrane distillation. However, it is important to note that the PLA-based nanofibrous membranes demonstrated in this study were unable to withstand high temperatures and extreme flow rates despite improved membrane performance. Hence, further investigation is to be conducted to optimise its properties for membrane distillation applications. In a separate study, the pore size of PLA nanofibrous membranes was minimized using green solvents and plant-based materials, genipin and priamine.93 A TFC membrane on a PLA biodegradable nanofibrous support was fabricated which comprises gelatin as an interlayer to increase hydrophilicity and adhesion of genipin and priamine. In this process, citric acid was used as a green catalyst. By varying concentrations of genipin and priamine, the optimized TFC membrane achieved a high acetone permeance of 10 L m−2 h−1 bar−1, a low MWCO of 281 Da, a high oil removal efficiency of 99.6% and water permeance of 5.6 L m−2 h−1 bar−1. The PLA-based nanofibrous membrane is robust and durable as consistent performance was observed when it was subjected to continuous crossflow filtration under 30 bar for 7 days. Additionally, the PLA nanofibrous support was entirely biodegraded using proteinase K within 10 h.
It is also possible to fabricate durable water purification membranes from naturally derived materials, as demonstrated by Li et al.94 A nanofibrous membrane from natural silk nanofibers was developed on dopamine-modified cellulose acetate membrane for dye/salt fractionation with potential use in the treatment of textile wastewater. Silk nanofibers were extracted from silkworm cocoons using a green deep eutectic solvent which consists of choline chloride and oxalic acid. Using the green deep eutectic solvent, minimal damage is done to the silk fibres and thus, they can be recycled for future use to prepare silk nanofibers. The silk nanofibers were self-assembled onto the dopamine-modified cellulose acetate membrane via a pressure-assisted deposition process. By optimizing the loading and thickness of the layer, the final composite membrane obtained a water permeance of 26 L m−2 h−1 bar−1 and a high rose bengal rejection of 99%. This composite membrane exhibited excellent durability by withstanding 100 h of continuously fractioning high concentrations of dye and inorganic salts with a separation factor of 110.7.
The treatment of organic contaminants in wastewater using photocatalysts is a promising effort in environmental remediation due to its simplicity in operation. Typically, solar energy is used to convert organic contaminants to biodegradable compounds, H2O, CO2 and other inorganic ions. However, under low light conditions, this process is poorly efficient. On top of that, easy aggregation is observed and recycling of powder photocatalyst materials remains a challenge. Xu et al.91 loaded a modified photocatalyst within membrane nanofibers to demonstrate excellent performance under low light conditions (Fig. 6b). They first synthesized a novel graphitic carbon nitride photocatalyst with a boron-doped and nitrogen-deficient structure (B–C3N4). The photocatalyst was then blended with polyethersulfone (PES) and poly(vinylpyrrolodone) (PVP) followed by the removal of PVP to obtain a superhydrophilic and polyporous nanofibrous membrane (PBCN). In this investigation, the photocatalytic activity of PBCN was enhanced by exposing more B–C3N4 sites to diffuse contaminant molecules in wastewater under low light. PBCN demonstrated sustained improvement of photocatalytic activity, thus, displaying its reusability and stability. A rejection of methylene blue of almost 100% was achieved after 5 cycles, with an increased removal rate from 24.85% to 80.6% as each cycle progressed. Even after 5 cycles, SEM images showed that the PBCN membrane maintained satisfactory fibrous morphology, while the FTIR results concluded no change in its chemical structure. The recent research works discussed in this section demonstrated upward interest in efforts to reduce further environmental pollution by incorporating renewable, biodegradable, bio-derived polymers and green solvents in fabricating NF membranes in water treatment and purification, thus, offering a promising road ahead towards building a more sustainable membrane industry.
4.3. Oil removal
Wastewater containing oil is typically discharged from industries such as petrochemical, textile, metallurgical, and food. Along with frequent oil spills, detrimental effects are imposed on the environment and aquatic species. Polymeric membranes such as PAN, PVDF and PES are typically used for their excellent mechanical properties, high separation efficiency and antifouling capabilities. However, these polymers are derived from non-renewable fossil fuels, are neither recyclable nor biodegradable, and are not economically viable to be cleaned and reused for other purposes. Hence, research is needed to produce eco-friendly membranes that have comparable mechanical and filtration properties to conventional membranes.PAN is a conventional thermosetting polymer used in the fabrication of nanofibrous membranes, however, despite its superior mechanical properties, it cannot be recycled. On the other hand, PET is a more economical commodity polymer than specialty plastics such as PAN, PES and PVDF, especially in large-scale production for industries. More importantly, in comparison to PAN, PES and PVDF, PET can be easily recycled. Doan et al. demonstrated the economical and recyclability of PET by electrospinning recycled PET (rPET) in a solution of dichloromethane (DCM)/trifluoroacetic acid (TFA).95 This solution is easily collected via vapour condensation for recycling. The rPET membranes were functionalized with polydimethylsiloxane (PDMS) using a dip-coating method (Fig. 7a). This enhanced its anti-fouling properties and water intrusion pressure, while its mechanical and physical properties could be altered by varying the concentration of PDMS. The rPET@PDMS membrane also had a high flux of ∼20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L m−2 h−1 when testing its filtration efficiency of oil/water mixtures via gravity. The rPET membranes maintained a high separation efficiency of >98% for up to 10 cycles, thus, making it durable and reliable for extended use.
000 L m−2 h−1 when testing its filtration efficiency of oil/water mixtures via gravity. The rPET membranes maintained a high separation efficiency of >98% for up to 10 cycles, thus, making it durable and reliable for extended use.
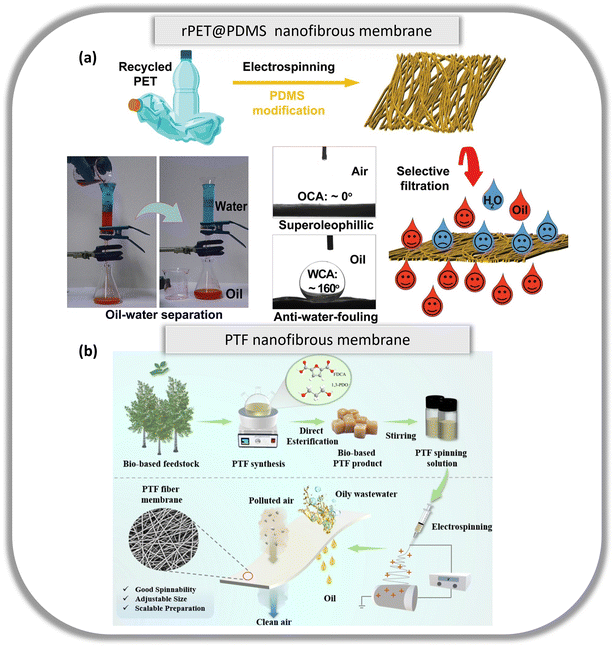 | ||
| Fig. 7 (a) Schematic diagram of fabricating fibrous membranes from recycled PET. Reproduced from ref. 95 with permission from Elsevier Ltd. Copyright 2020. (b) Schematic illustration of the overall production of PTF nanofibrous membranes. Reproduced from ref. 96 with permission from Elsevier Ltd. Copyright 2024. | ||
Petroleum-based polyesters are another example of a polymer obtained through non-renewable sources. This has led Li et al. to synthesize a high molecular weight polyester from renewable biomass and to fabricate a nanofibrous membrane from a sustainable alternative source.96 Biomass-based poly(trimethylene 2,5-furanicarboxylate) (PTF) was synthesized through a simple and economical direct esterification method. PTF nanofibrous membranes were fabricated via electrospinning (Fig. 7b), with excellent mechanical properties and customizable fibre diameters. Additionally, these membranes can be easily scaled up to produce a large membrane of 100 cm × 50 cm. When tested in a gravity-based filtration set-up, PTF membranes successfully separate oil–water mixed solutions of carbon tetrachloride (CCl4) with water, saturated NaCl solution, HCl solution (pH = 1), and NaOH solution (pH = 13), with a 99.88% separation rate even after 15 cycles.
In an interesting paper published by Zhuang et al., with the help of cultured bacteria (Gluconacetobacter xylinus), bio-cellulose nanofibers (bio-CNFs) were synthesized in situ and used in the testing of separating oily wastewater.97 Utilizing 0.75 M NaOH to purify bio-CNFs via alkali treatment, the surface's hydrophilicity was increased and a distinct nanofiber network structure was achieved. When used for separating a water/n-hexadecane emulsion stabilized with tween 60, a high separation efficiency of >99%, a high permeate flux recovery ratio of >94% after 10 h cycles of filtration, and good anti-oil fouling abilities against nanosized oil droplets were observed in this stable bio-CNF membrane.
Synthetic petroleum-derived polymeric membranes are often difficult and expensive to clean after oil removal, thus, it is frequently disposed of. To combat this issue Mizan et al. used a biodegradable polycaprolactone (PCL) and sulfonated kraft lignin (SKL) based membrane prepared via electrospinning using an acetic acid solution.98 When modified with 5 and/or 10 wt% SKL, membranes demonstrated superhydrophilicity and underwater superoleophobicity, due to their surface roughness and hydrophilic chemical functionality. These membranes demonstrated excellent pure water flux of 800–900 L m−2 h−1 and emulsion flux of 170–480 L m−2 h−1 when subjected to gravity-driven filtration of three surfactant-stabilized oil-in-water emulsions (mineral oil/water, gasoline/water and n-hexadecane/water). Its anti-oil-fouling performance was also found to produce great results with a high separation efficiency of 97–99% and a high flux recovery ratio of >98%. By incorporating 10 wt% of SKL, the membrane was proven to be reliable and stable as its separation performance remained consistent after 10 cycles while maintaining its wettability from pH 1–10.
Apart from flat membranes, aerogels can also be efficient in separating oil and water due to their large surface area in their open-cell or open-pore structure. Dong et al. used a gas-inflating technique to fabricate sustainable 3D PCL aerogels from electrospun 2D PCL nanofibrous membranes.99 They were then modified with CH3SiCl3 through a chemical vapour deposition process, to increase its hydrophobicity with a contact angle of ∼145°, to efficiently trap oil from water with a sorption range of 25.6–42.13 g g−1, and to achieve a high separation efficiency of oil mixtures and emulsions of >96.4%. Additionally, it can be reused by squeezing out excess oil due to its mechanically robust aerogel structure. After cleaning with ethanol and re-dissolving in DCM/DMF, it can be refabricated into reconstituted PCL aerogels to be utilized as new oil absorbents for 5 repeated cycles with insignificant oil absorption change. Furthermore, the PCL aerogels can be completely biodegraded due to the hydrolysis of ester bonds. Ye et al. also developed an ultra-light weight, super elastic and biobased aerogel using chitosan, chitin nanofibril (ChNF) by directional freezing, and glutaraldehyde (GA) crosslinking.100 To enhance its hydrophobicity, its surface was modified with methyltrimethoxysilane (MTMS) by chemical vapour deposition. The optimum mass fraction of chitosan was determined to be 12%, where optimum physical properties were observed. After 500 compression-release cycles, it maintained high resilience with a deformation recovery rate of 88% in air. Its ability to absorb a list of organic solvents, such as DCM, chloroform, DMF and hexane was also demonstrated, thus, making the nanofibrous membrane chemically resistant. Additionally, it is highly efficient with adsorption capacities of 52–114 g g−1 with a retention rate of >90% of its initial capacity even after 20 cycles. Its adaptable pore size under various degrees of compression allows for the effective separation of water/oil emulsions of different particle sizes. The aforementioned papers demonstrated that with more research efforts, a wider range of biobased polymer materials with biodegradable functionalities can be utilized in fabricating membranes for efficient separation of oily wastewater, while still using conventional manufacturing methods, and without compromising filtration properties or durability. Table 2 summarises the sustainable nanofibrous membranes discussed in section 4.
| No. | Membrane | Application | Performance markers | Ref. |
|---|---|---|---|---|
| 1 | PLLA multi-structured network fibrous membranes | Air filtration | • Filtration capabilities: >99.9% for PM2.5 and >99.5% for PM0.3 at a low pressure drop of 20 Pa | 82 |
| 2 | PLA/BC nanofibrous membranes | Air filtration | • Low pore size: 1.27 μm | 83 |
| • Filtration capability: 99.89% for PM0.3 at a low pressure drop of 104 Pa | ||||
| • Durable filtration efficiency: >99.6% after being exposed to 90% humidity for 120 h | ||||
| 3 | PA4-based nanofibrous membrane | Air filtration | • High porosity: >80% | 85 |
| • Filtration efficiency: 99.85% for PM2.5 | ||||
| 4 | PHBV-based nanofibrous membrane | Air filtration | • Excellent filtration efficiency: PM0.3 and PM2.5 of 99.999% and 100% with 0.077% standard atmospheric pressure of 5.3 cm s−1 airflow speed | 86 |
| 5 | PVA/TA nanofibrous membrane | Air filtration | • Removal efficiency: 99.5% for PM1.0 at a pressure drop of 35 Pa | 84 |
| • QF: ∼0.15 Pa−1 | ||||
| 6 | PBAT/MMT–CTAB nanofibrous membrane | Air filtration | • Electrostatic filtration: 98.3% for PM0.3 even at a low differential pressure of ≤40 Pa | 87 |
| 7 | Zein-based nanofibrous membrane | Air filtration | • Filtration performance: 99.3% for PM0.3 | 88 |
| 8 | Zein/PL/chitosan nanofibrous membrane | Air filtration | • Filtration efficiency: 99.65%, at a pressure drop of 57.7 Pa | 89 |
| • QF: 0.098 Pa−1 | ||||
| 9 | Cellulose/GO nanofibrous membrane | Water purification | • Evaporation rate in a 3.5 wt% NaCl solution: 1.83 kg m−2 h−1 | 90 |
| • Excellent solar vapour conversion efficiency of 94.2% | ||||
| 10 | PLA-based nanofibrous membrane | Water purification | • Salt rejection 99% of salt | 92 |
| • Flux: ∼2 kg m−2 h−1 | ||||
| 11 | PLA/genipin/priamine nanofibrous membrane | Water purification | • Acetone permeance: 10 L m−2 h−1 bar−1 | 93 |
| • Low MWCO: 281 Da | ||||
| • High oil removal efficiency: 99.6% | ||||
| • Water permeance: 5.6 L m−2 h−1 bar−1 | ||||
| 12 | Silk-based nanofibrous membrane | Water purification | • Water permeance: 26 L m−2 h−1 bar−1 | 94 |
| • High rose bengal rejection: 99% | ||||
| 13 | PBCN nanofibrous membrane | Water purification | • MB rejection: ∼100% after 5 cycles | 91 |
| • Increased removal rate from 24.85% to 80.6% | ||||
| 14 | rPET-based nanofibrous membrane | Oil removal | • High flux: ∼20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L m−2 h−1 000 L m−2 h−1 |
95 |
| • High separation efficiency: >98% for up to 10 cycles | ||||
| 15 | PTF-based nanofibrous membrane | Oil removal | • Separation rate: 99.88% even after 15 cycles | 96 |
| 16 | Bio-cellulose nanofibrous membrane | Oil removal | • High separation efficiency: >99% | 97 |
| • High permeate flux recovery ratio of >94% after 10 h filtration cycles | ||||
| 17 | PCL/SKL nanofibrous membrane | Oil removal | • Pure water flux: 800–900 L m−2 h−1 | 98 |
| • Emulsion flux: 170–480 L m−2 h−1 | ||||
| • High separation efficiency: 97–99% | ||||
| • High flux recovery ratio of >98% | ||||
| 18 | 3D PCL aerogel | Oil removal | • Sorption range: 25.6–42.13 g g−1 | 99 |
| • High separation efficiency: >96.4% | ||||
| 19 | CS/ChNF/GA biobased aerogel | Oil removal | • Adsorption capacities: 52–114 g g−1 | 100 |
| • Retention rate: >90% of its initial capacity even after 20 cycles |
5. Outlook
The development of sustainable nanofibrous membranes represents a promising frontier in addressing the pressing environmental challenges. As the demand for clean water, fresh air, and sustainable energy continues to grow, these advanced materials offer a path toward achieving environmental sustainability without performance compromise. However, realizing the full potential of sustainable nanofibrous membranes will require continued innovation and interdisciplinary collaboration. One key area of focus is the advancement of properties and performance of sustainable nanofibrous membranes. Current approaches, such as reinforcements and blending, are significant steps forward. However, further research is needed to enhance the mechanical strength and stability of sustainable nanofibrous membranes. While biopolymers and other sustainable materials offer environmental benefits, they often face challenges in matching the mechanical and thermal properties of traditional synthetic polymers. Enhancing the durability and long-term stability of these membranes is crucial for their successful implementation in demanding applications such as water purification and air filtration. This may involve the development of novel composite materials, the incorporation of reinforcing agents, or the optimization of the fibre architecture to achieve the desired scalability, efficiency, and cost-effectiveness and the balance between sustainability and performance. In addition, methods should ensure that sustainable nanofibrous membranes can be produced at industrial scales without sacrificing their environmental benefits. Another critical consideration is the life cycle management of nanofibrous membranes. As these materials move from the laboratory to real-world applications, it is essential to design end-of-life scenarios. This includes developing membranes that can be easily recycled, safely biodegraded, or repurposed for other uses, thereby reducing waste and contributing to a circular economy. The integration of life cycle assessments in the design and manufacturing process will help identify potential environmental impacts and guide the development of more sustainable products. Furthermore, there is an opportunity to expand the range of applications for sustainable nanofibrous membranes. While significant progress has been made in environmental remediation processes such as air filtration, water purification and oil removal, these materials have the potential to play a transformative role in other sectors, including energy harvesting and storage, medical devices, and biomedical applications. The properties of sustainable nanofibrous membranes can be manipulated by implementing renewable additives and specific preparation techniques to unlock new functionalities while maintaining desirable performance and mechanical properties.In conclusion, sustainable nanofibrous membranes have demonstrated excellent potential in global sustainability efforts. By continued innovations in materials science, manufacturing processes, and application development, and by fostering collaboration across disciplines and sectors, we can pave the way for a new generation of sustainable technologies to address the critical challenges of energy, environment and sustainability.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the financial support from the Agency for Science, Technology and Research (A*STAR) under RIE2025 Manufacturing, Trade and Connectivity (MTC) Young Individual Research Grants (YIRG) (Grant No. M22K3c0103), RIE2025 Manufacturing, Trade and Connectivity (MTC) Programmatic Funding (Grant No. M22K9b0049) and RIE2025 I&E IEO Decentralised GAP (Grant No. I24D1AG068).References
- M. Z. Jacobson, Energy Environ. Sci., 2009, 2, 148–173 RSC.
- A. Siddique, A. Shahzad, J. Lawler, K. A. Mahmoud, D. S. Lee, N. Ali, M. Bilal and K. Rasool, Environ. Res., 2021, 193, 110443 CrossRef CAS PubMed.
- C. He, Z. Liu, J. Wu, X. Pan, Z. Fang, J. Li and B. A. Bryan, Nat. Commun., 2021, 12, 4667 CrossRef CAS PubMed.
- L. Rosa, D. D. Chiarelli, M. C. Rulli, J. Dell'Angelo and P. D'Odorico, Sci. Adv., 2020, 6, eaaz6031 CrossRef PubMed.
- J. González-Martín, N. J. R. Kraakman, C. Pérez, R. Lebrero and R. Muñoz, Chemosphere, 2021, 262, 128376 CrossRef PubMed.
- J. Rentschler and N. Leonova, Nat. Commun., 2023, 14, 4432 CrossRef CAS PubMed.
- J. Cui, F. Li, Y. Wang, Q. Zhang, W. Ma and C. Huang, Sep. Purif. Technol., 2020, 250, 117116 CrossRef CAS.
- Kenry and C. T. Lim, Prog. Polym. Sci., 2017, 70, 1–17 CrossRef CAS.
- X. Yan, X. Xiao, C. Au, S. Mathur, L. Huang, Y. Wang, Z. Zhang, Z. Zhu, M. J. Kipper, J. Tang and J. Chen, J. Mater. Chem. A, 2021, 9, 21659–21684 RSC.
- X. Cheng, Z. Sun, X. Yang, Z. Li, Y. Zhang, P. Wang, H. Liang, J. Ma and L. Shao, J. Mater. Chem. A, 2020, 8, 16933–16942 RSC.
- J. Wu, Y. Ding, J. Wang, T. Li, H. Lin, J. Wang and F. Liu, J. Mater. Chem. A, 2018, 6, 7014–7020 RSC.
- T.-D. Lu, B.-Z. Chen, J. Wang, T.-Z. Jia, X.-L. Cao, Y. Wang, W. Xing, C. H. Lau and S. P. Sun, J. Mater. Chem. A, 2018, 6, 15047–15056 RSC.
- S. P. Nunes, P. Z. Culfaz-Emecen, G. Z. Ramon, T. Visser, G. H. Koops, W. Jin and M. Ulbricht, J. Membr. Sci., 2020, 598, 117761 CrossRef CAS.
- B. Li, S. Japip and T. S. Chung, Nat. Commun., 2020, 11, 1198 CrossRef CAS PubMed.
- B. Li, S. Wang, X. J. Loh, Z. Li and T. S. Chung, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2301009120 CrossRef CAS PubMed.
- W. Xie, T. Li, A. Tiraferri, E. Drioli, A. Figoli, J. C. Crittenden and B. Liu, ACS Sustainable Chem. Eng., 2021, 9, 50–75 CrossRef CAS.
- F. Galiano, K. Briceño, T. Marino, A. Molino, K. V. Christensen and A. Figoli, J. Membr. Sci., 2018, 564, 562–586 CrossRef CAS.
- B. Doshi, M. Sillanpää and S. Kalliola, Water Res., 2018, 135, 262–277 CrossRef CAS PubMed.
- Y. Bian, C. Zhang, H. Wang and Q. Cao, Sep. Purif. Technol., 2023, 306, 122642 CrossRef CAS.
- H. Xu, S. Yagi, S. Ashour, L. Du, M. E. Hoque and L. Tan, Macromol. Mater. Eng., 2023, 308, 2200502 CrossRef CAS.
- N. Singh, O. A. Ogunseitan, M. H. Wong and Y. Tang, Sustainable Horiz., 2022, 2, 100016 CrossRef.
- U. Kong, N. F. Mohammad Rawi and G. S. Tay, Polymers, 2023, 15, 2399 CrossRef CAS PubMed.
- M. Zhang, G. M. Biesold, W. Choi, J. Yu, Y. Deng, C. Silvestre and Z. Lin, Mater. Today, 2022, 53, 134–161 CrossRef CAS.
- C. Maraveas, Polymers, 2020, 12, 1127 CrossRef CAS PubMed.
- K. R. Kunduru, R. Hogerat, K. Ghosal, M. Shaheen-Mualim and S. Farah, Chem. Eng. J., 2023, 459, 141211 CrossRef CAS.
- K.-T. Huang, C.-C. Chueh and W.-C. Chen, Mater. Today Sustainability, 2021, 11–12, 100057 CrossRef.
- A. Pellis, M. Malinconico, A. Guarneri and L. Gardossi, New Biotechnol., 2021, 60, 146–158 CrossRef CAS PubMed.
- J. R. A. Pires, V. G. L. Souza, P. Fuciños, L. Pastrana and A. L. Fernando, Polymers, 2022, 14, 1359 CrossRef CAS PubMed.
- S. M. Ioannidou, C. Pateraki, D. Ladakis, H. Papapostolou, M. Tsakona, A. Vlysidis, I. K. Kookos and A. Koutinas, Bioresour. Technol., 2020, 307, 123093 CrossRef CAS PubMed.
- D. Briassoulis, A. Pikasi, M. Hiskakis, A. Arias, M. T. Moreira, S. M. Ioannidou, D. Ladakis and A. Koutinas, Curr. Opin. Green Sustain. Chem., 2023, 41, 100818 CrossRef.
- B. von Vacano, H. Mangold, G. W. M. Vandermeulen, G. Battagliarin, M. Hofmann, J. Bean and A. Künkel, Angew. Chem., Int. Ed., 2023, 62, e202210823 CrossRef CAS PubMed.
- D. Trache, A. F. Tarchoun, M. Derradji, T. S. Hamidon, N. Masruchin, N. Brosse and M. H. Hussin, Front. Chem., 2020, 8, 392 CrossRef CAS PubMed.
- K. J. Nagarajan, N. R. Ramanujam, M. R. Sanjay, S. Siengchin, B. Surya Rajan, K. Sathick Basha, P. Madhu and G. R. Raghav, Polym. Compos., 2021, 42, 1588–1630 CrossRef CAS.
- A. Isogai and L. Bergström, Curr. Opin. Green Sustain. Chem., 2018, 12, 15–21 CrossRef.
- M. Totani, T. Anai and J. I. Kadokawa, Carbohydr. Polym., 2024, 335, 122086 CrossRef CAS PubMed.
- J. Wu, Q. Li, G. Su, R. Luo, D. Du, L. Xie, Z. Tang, J. Yan, J. Zhou, S. Wang and K. Xu, Cellulose, 2022, 29, 5745–5763 CrossRef CAS.
- K. Byambatsogt, Q. Jiang, A. K. Jaiswal, V. Kunnari, A. Bismarck and A. Mautner, Monatsh. Chem., 2024, 156, 91–101 CrossRef.
- M. J. Khan, Z. Karim, B. Charnnok, T. Poonsawat, P. Posoknistakul, N. Laosiripojana, K. C. W. Wu and C. Sakdaronnarong, Int. J. Mol. Sci., 2023, 24, 6030 CrossRef CAS PubMed.
- C.-N. Wu and K. C. W. Wu, Cellulose, 2023, 31, 1017–1037 CrossRef.
- T. G. Volova, A. V. Demidenko, A. V. Murueva, A. E. Dudaev, I. Nemtsev and E. I. Shishatskaya, Technologies, 2023, 11, 106 CrossRef.
- M. Mohammadalipour, M. Asadolahi, Z. Mohammadalipour, T. Behzad and S. Karbasi, Int. J. Biol. Macromol., 2023, 230, 123167 CrossRef CAS PubMed.
- G. Masionė, D. Čiužas, E. Krugly, I. Stasiulaitenė, L. Pečiulytė, M. Tichonovas and D. Martuzevičius, Mater. Today Commun., 2023, 37, 107212 CrossRef.
- J.-K. Kim, S. H. Oh, M.-O. Song, S. Jang, S. J. Kang, S. K. Kwak and J. Jin, Composites, Part B, 2024, 281, 111563 CrossRef CAS.
- R. Shen, Z. Shao, J. Xie, H. Li, Z. Zeng, J. Jiang, X. Wang, W. Li, Y. Liu, S. Guo and G. Zheng, ACS Appl. Polym. Mater., 2023, 5, 8559–8569 CrossRef CAS.
- C. J. Cooper, A. K. Mohanty and M. Misra, ACS Omega, 2018, 3, 5547–5557 CrossRef CAS PubMed.
- M. R. Miah, J. Ding, H. Zhao, H. Wang, Q. Chu, B. Fang, L. Fan, J. Wang and J. Zhu, Mater. Today Commun., 2024, 38, 108538 CrossRef CAS.
- G. Fredi and A. Dorigato, Adv. Ind. Eng. Polym. Res., 2021, 4, 159–177 CAS.
- L. Cicero, M. Licciardi, R. Cirincione, R. Puleio, G. Giammona, G. Giglia, P. Sardo, G. Edoardo Vigni, A. Cioffi, A. Sanfilippo and G. Cassata, J. Biomed. Mater. Res., Part B, 2022, 110, 125–134 CrossRef CAS PubMed.
- M. E. Ostheller, N. K. Balakrishnan, K. Beukenberg, R. Groten and G. Seide, Polymers, 2023, 15, 2936 CrossRef CAS PubMed.
- M. Pedroni, E. Vassallo, M. Aloisio, M. Brasca, H. Chen, R. Donnini, G. Firpo, S. Morandi, S. M. Pietralunga, T. Silvetti, G. Speranza and T. Virgili, Surf. Coat. Technol., 2023, 471, 129828 CrossRef CAS.
- M. Nabels-Sneiders, A. Barkane, O. Platnieks, L. Orlova and S. Gaidukovs, Foods, 2023, 12, 4136 CrossRef CAS PubMed.
- P. Nuamduang, R. Auras, C. Winotapun, B. Hararak, W. Wanmolee and P. Leelaphiwat, Int. J. Biol. Macromol., 2024, 267, 131185 CrossRef CAS PubMed.
- M. A. Bonakdar, H. Kazemi and D. Rodrigue, Polym. Eng. Sci., 2024, 64, 1083–1095 CrossRef CAS.
- M. Zarei, M. J. Żwir, E. Wiśniewska, B. Michalkiewicz and M. El Fray, Polym. Adv. Technol., 2023, 34, 3586–3602 CrossRef CAS.
- M. A. Bonakdar, O. Hamdi, Y. Nazarenko, P. A. Ariya and D. Rodrigue, Polymer, 2023, 280, 126045 CrossRef CAS.
- D. Minguez-García, I. Montava, M. Bonet-Aracil, J. Gisbert-Payá and P. Díaz-García, Polymers, 2023, 15, 4480 CrossRef PubMed.
- A. Bora and N. Karak, J. Polym. Res., 2023, 30, 452 CrossRef CAS.
- M. A. Zboinska, S. Sämfors and P. Gatenholm, Mater. Des., 2023, 236, 112472 CrossRef CAS.
- R. M. González-Balderas, M. T. Orta Ledesma, I. Santana, M. Felix and C. Bengoechea, J. Environ. Chem. Eng., 2023, 11, 110621 CrossRef.
- A. Kramar, J. González-Benito, N. Nikolić, A. Larrañaga and E. Lizundia, Int. J. Biol. Macromol., 2024, 269, 132046 CrossRef CAS PubMed.
- Z. Shao, G. Kang, J. Xie, R. Shen, H. Li, Z. Zeng, J. Jiang, X. Wang, W. Li, S. Guo, Y. Liu and G. Zheng, Sep. Purif. Technol., 2023, 327, 124920 CrossRef CAS.
- Y. Dou, S. Li, S. Wang, M. E. Gibril and F. Kong, Composites, Part B, 2024, 281, 111537 CrossRef CAS.
- Z. Liu, Q. Liu, L. Lin, Q. Wang, W. Ma, Q. Cheng, J. Yang, F. Tang, M. Xu, X. Yang, H. Shang and H. Wu, Food Hydrocolloids, 2024, 156, 110266 CrossRef CAS.
- X. Cheng, T. Li, L. Yan, Y. Jiao, Y. Zhang, K. Wang, Z. Cheng, J. Ma and L. Shao, Sci. Adv., 2023, 9, eadh8195 CrossRef CAS PubMed.
- N. Hegyesi, Y. Zhang, A. Kohári, P. Polyák, X. Sui and B. Pukánszky, Ind. Crops Prod., 2019, 141, 111799 CrossRef CAS.
- Q. Huang, M. Hiyama, T. Kabe, S. Kimura and T. Iwata, Biomacromolecules, 2020, 21, 3301–3307 CrossRef CAS PubMed.
- L. Wang, Y. Gao, J. Xiong, W. Shao, C. Cui, N. Sun, Y. Zhang, S. Chang, P. Han, F. Liu and J. He, J. Colloid Interface Sci., 2022, 606, 961–970 CrossRef CAS PubMed.
- J. Wang, S. Liu, X. Yan, Z. Jiang, Z. Zhou, J. Liu, G. Han, H. Ben and W. Jiang, Membranes, 2021, 12, 23 CrossRef PubMed.
- M. Jiang, C. Jing, C. Lei, X. Han, Y. Wu, S. Ling, Y. Zhang, Q. Li, H. Yu, S. Liu, J. Li, W. Chen and G. Yu, Nat. Sustainability, 2024, 7, 168–178 CrossRef.
- C.-J. Cho, Y.-S. Chang, Y.-Z. Lin, D.-H. Jiang, W.-H. Chen, W.-Y. Lin, C.-W. Chen, S.-P. Rwei and C.-C. Kuo, J. Taiwan Inst. Chem. Eng., 2020, 106, 206–214 CrossRef CAS.
- T. T. Le, E. J. Curry, T. Vinikoor, R. Das, Y. Liu, D. Sheets, K. T. M. Tran, C. J. Hawxhurst, J. F. Stevens, J. N. Hancock, O. R. Bilal, L. M. Shor and T. D. Nguyen, Adv. Funct. Mater., 2022, 32, 2113040 CrossRef CAS.
- S. Varanasi, Z.-X. Low and W. Batchelor, J. Chem. Eng., 2015, 265, 138–146 CrossRef CAS.
- J. Xiong, A. Li, Y. Liu, L. Wang, X. Qin and J. Yu, Small, 2022, 18, 2105570 CrossRef CAS PubMed.
- L. Xu, W. Wang, L. Zhang, D. Wang and A. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 21623–21635 CrossRef CAS PubMed.
- Y. Liu, Y. Chen and H. Qi, Carbohydr. Polym., 2022, 293, 119729 CrossRef CAS PubMed.
- S. Wang, N. Wang, D. Kai, B. Li, J. Wu, J. C. C. Yeo, X. Xu, J. Zhu, X. J. Loh, N. Hadjichristidis and Z. Li, Nat. Commun., 2023, 14, 1182 CrossRef CAS PubMed.
- H.-J. Chen, Q.-Y. Bai, M.-C. Liu, G. Wu and Y.-Z. Wang, Green Chem., 2021, 23, 7646–7658 RSC.
- Y. Li, S. Yuan, C. Zhou, Y. Zhao and B. Van der Bruggen, J. Mater. Chem. A, 2018, 6, 22987–22997 RSC.
- Y. Li, E. Wong, A. Volodine, C. Van Haesendonck, K. Zhang and B. Van der Bruggen, J. Mater. Chem. A, 2019, 7, 19269–19279 RSC.
- B. Li, C. Qu, S. Wang, J. C. C. Yeo, N. E. B. Surat'man, X. J. Loh, Z. Li and T.-S. Chung, J. Membr. Sci., 2024, 693, 122378 CrossRef CAS.
- J. Zhou, X. Li, Z. Zhang, T. Hou, J. Xu, Y. Wang, H. Ye and B. Yang, J. Chem. Eng., 2024, 491, 152105 CrossRef CAS.
- L. Li, Y. Gao, G. Nie, X. Yan, S. Wang, T. Zhang, S. Ramakrishna, Y.-Z. Long and W. Han, Small, 2024, 2402317 CrossRef CAS PubMed.
- Y. Yang, M. Zhong, W. Wang, N. Lu, Y. Gou, W. Cai, J. Huang and Y. Lai, Sep. Purif. Technol., 2025, 352, 128143 CrossRef CAS.
- J. Cui, Y. Wang, T. Lu, K. Liu and C. Huang, J. Colloid Interface Sci., 2021, 597, 48–55 CrossRef CAS PubMed.
- F. Zhan, Z. Wang, J. Shi, D. Zhang, M. Wang, Z. Yi and L. Zhao, ACS Appl. Polym. Mater., 2024, 6, 1215–1223 CrossRef CAS.
- J. Chen, B. Yu, J. Zhu, Y. Gao, W. Deng, R. Chen and H.-L. Wang, ACS Appl. Mater. Interfaces, 2023, 15, 35507–35515 CrossRef CAS PubMed.
- Y. Cho, Y. Son, J. Ahn, H. Lim, S. Ahn, J. Lee, P. K. Bae and I.-D. Kim, ACS Nano, 2022, 16, 19451–19463 CrossRef CAS PubMed.
- X. Fan, L. Rong, L. Kong, Y. Li, J. Huang, Y. Cao and W.-H. Zhong, ACS Appl. Mater. Interfaces, 2021, 13, 8736–8744 CrossRef CAS PubMed.
- R. Shen, Z. Shao, R. Chen, Q. Wang, Z. Gui, Y. Qi, W. Song, Y. Liu and G. Zheng, Sep. Purif. Technol., 2024, 341, 126893 CrossRef CAS.
- Z. Sui, X. Xue, Q. Wang, M. Li, Y. Zou, W. Zhang and C. Lu, Carbohydr. Polym., 2024, 331, 121859 CrossRef CAS PubMed.
- Y. Xu, D. Yuan, Y. Guo, S. Chen, W. Lin, Y. Long, J. Bao, C. He, C. Cheng, C. Deng, Y. Zhang, Y. Wu, W. Zhao and C. Zhao, J. Chem. Eng., 2022, 427, 131685 CrossRef CAS.
- M. O. Aijaz, M. H. D. Othman, M. R. Karim, A. Ullah Khan, A. Najib, A. K. Assaifan, H. F. Alharbi, I. A. Alnaser and M. H. Puteh, Desalination, 2024, 586, 117825 CrossRef CAS.
- C. Yang, F. Topuz, S.-H. Park and G. Szekely, Green Chem., 2022, 24, 5291–5303 RSC.
- B. Li, C. H. T. Chai, X. Q. Koh, K. Y. Tang, C. Y. Chan, J. Z. X. Heng, S. Wang, N. Wang, E. Ye and Z. Li, J. Membr. Sci., 2024, 701, 122741 CrossRef CAS.
- H. N. Doan, P. Phong Vo, K. Hayashi, K. Kinashi, W. Sakai and N. Tsutsumi, J. Environ. Chem. Eng., 2020, 8, 103921 CrossRef CAS.
- Y. Li, L. Wan, J. Geng, Z. Wang, G. Wang, X. Qiao, D. Yuan, Z. Zhao and W. Chen, Sep. Purif. Technol., 2024, 343, 127165 CrossRef CAS.
- G.-L. Zhuang, S.-Y. Wu, Y.-C. Lo, Y.-C. Chen, K.-L. Tung and H.-H. Tseng, J. Membr. Sci., 2020, 605, 118091 CrossRef CAS.
- M. M. H. Mizan, P. M. Gurave, M. Rastgar, A. Rahimpour, R. K. Srivastava and M. Sadrzadeh, ACS Appl. Mater. Interfaces, 2023, 15, 41961–41976 CrossRef CAS PubMed.
- T. Dong, H. Ye, W. Wang, Y. Zhang, G. Han, F. Peng, C.-W. Lou, S. Chi, Y. Liu, C. Liu and J.-H. Lin, J. Hazard. Mater., 2023, 454, 131474 CrossRef CAS PubMed.
- W. Ye, J. Xi, Y. Sun, L. Meng, H. Bian, H. Xiao and W. Wu, Int. J. Biol. Macromol., 2023, 249, 125958 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |


