Nanoparticle-assembled interconnected PbO1.44 hollow spheres enabled by PVP-driven transformation of β-PbO2 and self-sacrificial templating for superior lithium storage†
Xiaoxu
Bo‡
*a,
Jiatong
Zhang‡
b,
Qian
Zhang
b,
Ruijie
Wu
b,
Sheng
Wang
a,
Shiqiang
Zhao
 *b and
Shun
Wang
*b and
Shun
Wang
 *b
*b
aDepartment of Agriculture and Biotechnology, Wenzhou Vocational College of Science and Technology, Wenzhou 325006, P. R. China. E-mail: boxxsci@163.com
bKey Laboratory of Advanced Energy Storage and Conversion of Wenzhou, Key Laboratory of Carbon Materials of Zhejiang Province, College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou 325035, P. R. China. E-mail: zhaosq@wzu.edu.cn; shunwang@wzu.edu.cn
First published on 17th December 2024
Abstract
Lead oxides (PbOx, 1 ≤ x ≤ 2) are promising high-capacity and low-cost anodes for lithium ion batteries (LIBs). However, the huge lithiation-induced volume expansion of conventional large-sized PbOx particles leads to severe electrode pulverization with poor cycling stability. Herein, a rare mixed-valence PbO1.44 with a unique hierarchical architecture of nanoparticle-assembled interconnected hollow spheres (denoted PbO1.44 NAHSs) is crafted by introducing polyvinylpyrrolidone (PVP) into the solution of generating β-PbO2 microspheres (MSs), which is exploited for the first time as a potential advanced anode material for LIBs. Notably, an intriguing PVP-driven dissolution–recrystallization transformation process converting β-PbO2 MSs into PbO1.44 NAHSs is revealed by PVP-concentration and reaction-time control experiments, demonstrating the dual function of PVP as a mild reducing agent combined with it being a morphology regulator for the construction of PbO1.44 NAHSs. Furthermore, a self-sacrificial templating mechanism is demonstrated for yielding the interconnected hollow structure of PbO1.44 NAHSs. Remarkably, PbO1.44 NAHSs deliver stable capacities of 561 and 453 mA h g−1 after 100 and 200 cycles at 50 and 500 mA g−1, respectively, in sharp contrast to the performance of β-PbO2 MSs (52 and 43 mA h g−1). Structural and electrochemical measurements of the cycled electrodes indicate that the hollow and nanoarchitectural structure of PbO1.44 NAHSs enables their superior cycling and rate capabilities, benefiting from the effectively buffered volume expansion and shortened lithium storage distance, respectively. As such, this work highlights a robust PVP-assisted strategy to fabricate rare mixed-valence PbO1.44 NAHSs with outstanding electrochemical reactivity and mechanical robustness for lithium storage and various potential applications.
1. Introduction
The ever-increasing demand for portable electronics with long service life and high power density has triggered the rapid development of high-performance energy storage technologies.1,2 Among all of the commercial electrochemical power sources, lithium ion batteries (LIBs) have achieved great success due to their higher energy density, longer cycle life, and better environmental friendliness in contrast to most of the popular secondary batteries.3,4 However, the low theoretical mass and volume capacities of commercial graphite anodes (i.e., 372 mA h g−1 and 830 mA h cm−3) seriously restrict the property improvement of next-generation LIBs.5,6 In recent decades, elements in Group IVA (i.e., Si, Ge, Sn and Pb) and their compounds have attracted wide attention as promising high-capacity anodes for LIBs based on their alloying–dealloying reaction mechanisms with the advantages of suitable low-voltage plateaus (∼0.5 V vs. Li+/Li), low cost and abundance.7,8 Therein, lead oxides (PbOx, 1 ≤ x ≤ 2) possess higher theoretical mass and especially volumetric specific capacities (e.g., PbO (769 mA h g−1 and 7329 mA h cm−3) and PbO2 (941 mA h g−1 and 8827 mA h cm−3)) than graphite, based on the conversion-combined alloying–dealloying reactions of PbOx + 2xLi+ + 2xe− ⇌ Pb + xLi2O (1 ≤ x ≤ 2) and Pb + yLi+ + ye− ⇌ LiyPb (0 ≤ y ≤ 4.4), respectively.9–12 Nevertheless, due to the sluggish electrochemical reaction kinetics and huge volume expansion, the conventionally fabricated PbOx particles with large-sized bulk structures usually exhibit poor cycling stability and rate capability.13–15 Therefore, rational approaches to the design of structures and compositions of PbOx-based materials are crucial to enhance their lithium storage performances for realizing their practical application as anodes of LIBs.16–19As a post-transition metal with the electronic configuration of 6s26p2, Pb has many types of oxides including PbO, PbO2 and various mixed-valence compounds (i.e., Pb2O3, Pb3O4 and non-stoichiometric PbOx (1 < x < 2)). Due to their outstanding properties in optical, ferroelectric, dielectric, energy-storage and oxygen-sensing fields, PbOx-based materials have been widely used in the industrial fields of lead acid batteries, paints, glasses and so on.20–22 As a result, the fabrication strategies and crystallization behaviors of PbOx have always been hot research topics.23,24 For electrochemical syntheses, orthorhombic α-PbO2 and tetragonal β-PbO2 can be generally obtained in a strong acidic medium and an alkaline or neutral medium, respectively.25 In contrast to electrochemical methods, solution-based chemical routes for synthesizing PbOx are more controllable in terms of the structure–composition design and easier to scale up.26–28 However, up to now, the preparation of phase-pure non-stoichiometric PbOx (1 < x < 2) by the more popular solution-based methods has rarely been reported, limiting their structural analysis, property investigation and application exploration.20–28
As for the mixed-valence oxides of PbOx (e.g., Pb2O3, Pb3O4 and non-stoichiometric PbOx (1 < x < 2)), the non-integral x value can be regarded as an incomplete oxidation of Pb2+ or a partial reduction of Pb4+ in the crystallization process of PbOx (1 < x < 2), occasionally found in the heat-treatment process of solid-state precursors of PbO2 or PbO. For example, PbO1.55 has been captured during the heat-treatment of chemically produced β-PbO2 at 400 °C, which is recognized as one of the crystalline intermediates of partially reduced β-PbO2.29 Similarly, serial crystalline phases of PbOx (1 < x < 2) have also been detected during the air-oxidizing of tetragonal PbO, resulting in PbO1.548 and PbO1.333 at 286 and 351 °C, respectively.30 Therein, the created PbOx series (1.41 < x < 1.57) exhibited similar X-ray diffraction (XRD) patterns to that of cubic PbO1.44 (a = 4.9525 Å, JCPDS no. 27-1201) obtained after annealing PbO at 293 °C for 180 h in an air atmosphere. A thin film of well-defined PbO1.44 was prepared by reactive direct current magnetron sputtering from a metallic lead target onto Si (100) wafers and glass substrates in a precisely controlled flow of an oxygen–argon mixture.31 Nevertheless, there has been no report concerning the fabrication of PbO1.44 with high purity, high crystallinity and fine structure via a facile solution-based method until now. The solution-based method is widely used for the synthesis of ultra-fine powders in research and industry due to its significant advantages of high controllability, low cost and high reaction efficiency.3,5,7,8 First, the solution-based method can control the reaction rate, particle size distribution and morphology by adjusting the reaction conditions and additives, thus achieving precise control of the products. Second, compared with other synthesis methods (e.g. gas-phase method and solid-phase method), the equipment cost for solution reaction is relatively low with simple operation, so it is easier to achieve large-scale industrial production. Third, the reaction speed of solution-based synthesis is generally fast with high reaction efficiency to quickly obtain the desired products.
In this work, a facile polyvinyl pyrrolidone (PVP)-assisted hydrothermal route has been rationally developed to produce pure-phase cubic PbO1.44 particles (∼1 μm in diameter) with high crystallinity and a unique structure of nanoparticles (∼50 nm in diameter) assembled into interconnected hollow spheres (denoted PbO1.44 NAHSs), which have been studied for the first time as promising anode materials for LIBs. As a polymeric surfactant, PVP generally acts as a soft-templating and capping agent for the crystallization and self-assembly of inorganic particles.32–34 Besides, PVP has also been successfully used for the controllable synthesis of noble metal particles (e.g., Ag, Au, Pd and Pt) by serving as a mild reductant for the slow reduction of water-soluble metal salts.35–37 As expected, by introducing PVP as a mild reductant into the hydrothermal reaction solution for the preparation of β-PbO2 microspheres (denoted β-PbO2 MSs), an intriguing PVP-driven dissolution–recrystallization transformation process converting β-PbO2 MSs into PbO1.44 NAHSs has been discovered and well proven by a PVP-concentration control experiment. More importantly, time-dependent experiments demonstrate a self-sacrificial templating mechanism for the formation of the unique interconnected structure of PbO1.44 NAHSs. Therefore, PVP serves as both a mild reductant and a morphology regulator for the formation of nanostructured PbO1.44 NAHSs.
Notably, PbO1.44 NAHSs exhibit outstanding cycling and rate capabilities with stable capacities of 561 and 453 mA h g−1 after 100 and 200 cycles at 50 and 500 mA g−1, respectively, in sharp contrast to the performance of β-PbO2 MSs (52 and 43 mA h g−1). As uncovered by systematic structural and electrochemical investigations, the superior electrochemical properties of PbO1.44 NAHSs can be attributed to the unique interconnected hollow structure assembled from nanoparticles. First, the secondary nanoparticles shorten the lithium storage distance and enlarge the contact area with the electrolyte for sufficient lithium storage reaction and fast electrochemical kinetics, giving rise to the high capacity and excellent rate capability, respectively. Second, the interconnected hollow structure effectively buffers the lithiation–delithiation-induced volume expansion–shrinkage of PbO1.44 NAHSs, enabling the ultra-high structrual robustness of the electrode and thus the outstanding cycling performance. As such, this study underscores a robust PVP-assisted hydrothermal route to prepare high-quality PbO1.44 NAHSs with excellent electrochemical reactivity and mechanical robustness for a wide range of diverse potential applications including lithium-ion batteries, lead-acid batteries, catalysis, sensing, and so on.
2. Results and discussion
2.1. PVP-driven transformation of β-PbO2 MSs into PbO1.44 NAHSs
Scanning electron microscopy (SEM, Fig. 1a–d and S1†) and transmission electron microscopy (TEM, Fig. 1e–h) images reveal that the 7 h hydrothermal reaction of lead acetate, sodium hydroxide and ammonium persulfate in the presence of polyvinylpyrrolidone K30 (PVP, 3 g L−1) at 120 °C gave rise to a product with a unique structure of nanoparticle-assembled hollow spheres (NAHSs). Notably, all the hollow spheres are interconnected with adjacent ones, as distinctly demonstrated by the TEM images of Fig. 1e–g. The high magnification SEM images (Fig. 1d and S1c–f†) clearly show that the hollow spheres are assembled from ultra-fine nanoparticles. The high-resolution TEM (HRTEM) image (Fig. 1i), selected area electron diffraction (SAED) pattern (Fig. 1j) and powder X-ray diffraction (XRD) pattern (Fig. 1k) of the product indicate the pure-phase, high-crystallinity and polycrystalline character of cubic PbO1.44 crystals (a = 5.469 Å, JCPDS no. 27-1201). In the X-ray photoelectron spectroscopy (XPS) spectrum of Pb 4f of the PbO1.44 NAHSs (Fig. 1l), the Pb 4f5/2 and Pb 4f7/2 peaks can be divided into two pairs of peaks at 142.6 and 137.8 eV corresponding to Pb2+ and at 142.1 and 137.2 eV corresponding to Pb4+, respectively. According to the relative area percentages of the Pb 4f peaks of PbO1.44 NAHSs (i.e., Pb2+ (55.6%) and Pb4+ (44.4%)), a chemical formula of PbO1.444 can be estimated, which is almost identical to the stoichiometric ratio of PbO1.44. From the particle size statistics based on the SEM and TEM images (Fig. 1a–h and S1†), the average diameters of PbO1.44 NAHSs and secondary nanoparticles that are assembled into PbO1.44 NAHSs are 1.1 ± 0.2 μm and 49.7 ± 6.8 nm (Fig. 1m), respectively.As a well-known dispersant or colloidal stabilizer of nanoparticles, the polymeric surfactant PVP has been popularly involved in the size and morphology controllable syntheses of a variety of functional micro/nano particles.38–42 Besides, it has been proved that the abundant carbonyl groups of PVP can be oxidized to carboxyl or ester groups, endowing PVP with mild reducibility.35 In order to clarify the crucial roles of PVP in the formation of PbO1.44 NAHSs, a series of control experiments that involve regulating PVP concentrations (i.e., 0, 0.5, 1.0, 1.5, 2.0 and 2.5 g L−1) were performed (Fig. 2 and S2–S8†). As a blank experiment in the absence of PVP, the reaction of lead acetate, sodium hydroxide and ammonium persulfate resulted in the generation of inhomogeneous large-sized solid β-PbO2 microspheres (denoted β-PbO2 MSs) (Fig. 2a, b, l and S2†) with an average diameter of 3.9 ± 1.5 μm (Fig. S2f†). The Pb 4f XPS spectrum of β-PbO2 MSs (Fig. S3†) shows Pb 4f5/2 and Pb 4f7/2 peaks at 142.1 and 137.2 eV, corresponding to Pb4+ in β-PbO2.
Interestingly, after introducing a small amount of PVP (0.5 g L−1) into the reaction solution for producing β-PbO2, a mixture of β-PbO2 MSs (primary) and PbO1.44 NAHSs (secondary) was created (Fig. 2c, l and S4†). Moreover, along with the gradually increased concentrations of PVP to 1.0, 1.5, 2.0, and 2.5 g L−1, the amount ratios of PbO1.44 NAHSs in the mixed products keep on increasing (Fig. 2d–k and S5–S8†), in accordance with the continuously strengthening XRD peaks of PbO1.44 and weakening XRD peaks of β-PbO2 (Fig. 2l). It is noteworthy that some PbO1.44 NAHSs grow in situ on the β-PbO2 MSs, as indicated by the white arrows in Fig. 2d and e, implying the close relationship between PbO1.44 NAHSs and β-PbO2 MSs. In addition, the fragmentary particles of β-PbO2 MSs can be observed in the mixtures as indicated by the white arrows in Fig. 2g, i and k, suggesting a PVP-driven reduction combined dissolution process of β-PbO2 causing the transformation of β-PbO2 MSs into PbO1.44 NAHSs. Therefore, it can be concluded that PVP serves as a dual-functional additive acting as a mild reducing agent combined with being a morphology regulator to induce the conversion of β-PbO2 MSs into PbO1.44 NAHSs.
2.2. Self-sacrificial templating formation of the interconnected hollow structure of PbO1.44 NAHSs
To elucidate the formation mechanism of the unique architecture of nanoparticle-assembled interconnected hollow spheres of PbO1.44 NAHSs, time-dependent experiments were performed over designated incubation times of 1, 3 and 5 h (Fig. 3 and S9–S11†). After reaction for 1 h, amorphous mixed particles were obtained (Fig. 3a–c, l and S9†), including a large amount of randomly aggregated solid spheres assembled from nanoparticles (Fig. 3c and S9b†) and a small amount of large irregular particles (as indicated by the white arrows in Fig. 3a and b). Then, after 3 h of reaction (Fig. 3d–h, l and S10†), the hydrothermal solution-mediated dissolution–recrystallization took place from the inside to the outside of the amorphous solid spheres formed after 1 h, generating interconnected PbO1.44 hollow spheres with thick shells (Fig. 3f and g). Notably, the SAED pattern of a solid microsphere from the 3 h product confirms that the microspheres in the intermediate mixtures are β-PbO2. Due to the gradual PVP-driven reduction and dissolution of β-PbO2 MSs and hydrothermal-induced dissolution–recrystallization from the inside out of PbO1.44 NAHSs, a mixture of highly crystalline PbO1.44 NAHSs (primary) with thinner shells and a small amount of small irregular crumbs of β-PbO2 MSs (secondary, as indicated by the white arrows in Fig. 3i and j) were obtained after 5 h (Fig. 3i–l and S11†). Finally, pure phase PbO1.44 NAHSs were produced after 7 h of the hydrothermal reaction (Fig. 1 and S1†).SEM images of the representative intermediate particles obtained after 1, 3 and 5 h visually demonstrate the structural evolution of PbO1.44 NAHSs (Fig. 4a–c) and β-PbO2 MSs (Fig. 4d–f). A typical hydrothermal-driven Ostwald ripening combined with a self-sacrificial templating mechanism accounts for the formation of PbO1.44 NAHSs.43 Aggregated amorphous solid spheres assembled from nanoparticles (denoted amorphous PbO1.44 NASs) were formed after 1 h (Fig. 4a). Then, the hydrothermal environment induced the dissolution of the inner smaller nanoparticles, which were initially deposited from the solution, and then the recrystallization on the surfaces of later deposited larger nanoparticles, giving rise to the formation of PbO1.44 NAHSs with thick shells (∼250–320 nm) after 3 h of reaction (Fig. 4b).43,44 As the Ostwald ripening process continued, the shell thicknesses of the PbO1.44 NAHSs continued to decrease to ∼110–140 nm for the 5 h intermediate (Fig. 4c) and then ∼50 nm for the 7 h product (Fig. 1d). Therein, due to the dissolution of the nanoparticles at the contact areas of randomly aggregated spheres formed after 1 h, the unique interconnected hollow structure is created.44 As for the β-PbO2 MSs, a PVP-driven progressive dissolution process was observed along with extended reaction times, that is, from complete MSs (1 h, Fig. 4d) to incomplete MSs (3 h, Fig. 4e) and then to irregular fragments (5 h, Fig. 4f), which finally disappeared at 7 h (Fig. 1a, k and 3l). The structure formation mechanism of PbO1.44 NAHSs is concisely illustrated in Fig. 4g. The weak reducing agent of PVP induces the progressive reduction and dissolution of β-PbO2 MSs and simultaneous recrystallization on the PbO1.44 NAHSs. Furthermore, the Ostwald ripening process from the inside outwards of the aggregated spheres generated after 1 h triggers a self-sacrificial templating mechanism that constructs the interconnected hollow structure of PbO1.44 NAHSs.43,44
2.3. Superior lithium storage capability of PbO1.44 NAHSs
The electrochemical properties of PbO1.44 NAHSs and β-PbO2 MSs were examined using lithium cell models within a potential window of 0.01–3.0 V vs. Li+/Li.45,46 As an anode material of LIBs, PbO1.44 NAHSs exhibited outstanding cycling stability at 50 (Fig. 5a) and 500 mA g−1 (Fig. 5b) with ultra-stable capacities of 561 and 453 mA h g−1 after 100 and 200 cycles, respectively, in sharp contrast to the β-PbO2 MSs (52 and 43 mA h g−1). It is noteworthy that the capacities of PbO1.44 NAHSs at the high current rate (C-rate) of 500 mA g−1 show a continuously increasing trend, from 412 mA h g−1 at the second discharge to 453 mA h g−1 at the 200th discharge. This can reasonably be attributed to the electrochemical activation of the electrode along with increased cycle numbers, due to the gradual perfection of the SEI layer and the progressive full penetration of the electrolyte with improved ionic conductivity for enhanced reaction kinetics.5–8 As expected, PbO1.44 NAHSs also exhibited markedly enhanced rate capability at various C-rates compared with that of β-PbO2 MSs (Fig. 5c). At a C-rate of 50 mA g−1, PbO1.44 NAHSs yielded an initial Coulomb efficiency (ICE) of 69.5%, with initial discharge/charge capacities of 893/621 mA h g−1, respectively, which is much higher than the ICE of β-PbO2 (54.4%). Even at a high C-rate of 1000 mA g−1, PbO1.44 NAHSs delivered a satisfactory capacity of 351 mA h g−1 on the 50th cycle, 4.4 times higher than that of β-PbO2 MSs (79 mA h g−1). When the C-rate was returned to 50 mA g−1 after 60 cycles, the capacity of PbO1.44 NAHSs successfully recovered to a high value of 623 mA h g−1 on the 90th cycle, which is almost the same as the first charge capacity (i.e., 621 mA h g−1), demonstrating the excellent capacity retention ability of PbO1.44 NAHSs even after cycling at various C-rates.To understand the electrochemical behaviors of PbO1.44 NAHSs and β-PbO2 MSs, the charge–discharge profiles (Fig. 5d and e) and cyclic voltammetry (CV) curves (Fig. 5f and g) were comparatively investigated. During the first cycles, the discharge capacities being higher than the charge capacities for both PbO1.44 NAHSs and β-PbO2 MSs can be primarily attributed to the irreversible formation of solid electrolyte interphase (SEI) layers on the electrodes, in agreement with the significant degradation of the discharge plateaus at 1.3 and 0.1 V (Fig. 5d) and the corresponding anodic peaks at 1.22 and 0.11 V (Fig. 5f) of PbO1.44 NAHSs in the first lithiation process.47 Similar decay trends in the related discharge plateaus and anodic peaks also occurred in the case of β-PbO2 MSs (Fig. 5e and g). Remarkably, after the first discharge process, the charge–discharge profiles (Fig. 5d) and CV curves (Fig. 5f) of PbO1.44 NAHSs are almost overlapped with each other, indicating the excellent reversibility of the lithiation–delithiation reactions on the PbO1.44 NAHSs electrode. According to the reports about PbOx-based anodes for LIBs, the three pairs of discharge/charge plateaus (0.5/0.6, 0.4/0.5 and 0.3/0.4 V) and anodic/cathodic peaks below 0.7 V (i.e., 0.53/0.63, 0.35/0.51 and 0.28/0.42 V) for PbO1.44 NAHSs can be assigned to the reversible alloying/dealloying reactions in Pb.9,10 In addition, the discharge plateau (1.5 V)/anodic peak (1.52 V) and charge plateaus (1.2 and 1.6 V)/cathodic peaks (1.26 and 1.61 V) in the 0.7–1.7 V range (Fig. 5d and f) can be attributed to the conversion reactions between Pb and PbO1.44.11,12
In stark contrast to the outstanding electrochemical reversibility of PbO1.44 NAHSs, the lengths of the discharge/charge plateaus (Fig. 5e) and the intensities of the anodic/cathodic peaks (Fig. 5g) of β-PbO2 MSs continue to decay with increasing cycle numbers. Finally, there is almost no voltage plateau on the 50th cycle at 50 mA g−1 for β-PbO2 MSs (Fig. 5e). More importantly, the decay rate of the discharge/charge plateaus of β-PbO2 MSs (Fig. 5i) is much faster than that of PbO1.44 NAHSs (Fig. 5h) along with increased C-rates, as obviously exhibited in the contrasting differential capacity versus voltage (dQ/dV) curves of the discharge profiles of PbO1.44 NAHSs (Fig. 5j) and β-PbO2 MSs (Fig. 5k). Notably, for both β-PbO2 MSs and PbO1.44 NAHSs, a faster degradation rate can be observed for the plateaus in the voltage range of 0.7–1.7 V than that in the 0.01–0.7 V range along with increased C-rates, corresponding to the conversion reaction of PbOx and the alloying–dealloying reaction of Pb, respectively. This suggests that the kinetics of the conversion reaction is slower than that of the alloying–dealloying reaction.7,9 The capacities at different C-rates of the discharge profiles in the two voltage ranges of 0.01–0.7 V and 0.7–1.7 V are statistically analyzed as shown in Fig. 5l. The long Li+/e− transport distance of large-sized bulk-structured β-PbO2 MSs leads to the poor kinetics in both conversion and alloying–dealloying reactions with rapidly decaying capacities along with increasing C-rates (Fig. 5l). Remarkably, PbO1.44 NAHSs demonstrate an apparently improved stability in the discharge capacities from the plateaus over the voltage range of 0.01–0.7 V, corresponding to the alloying reaction of Pb, which contributes the major capacity at low and especially high C-rates. Namely, the superior lithium storage capability of PbO1.44 NAHSs can be primarily attributed to the much higher alloying–dealloying reaction kinetics than that of β-PbO2 MSs, benefiting from the nanoparticle-assembled hollow structure with rich channels and short distances for the fast Li+ transfer.
To clarify the performance enhancement mechanisms of PbO1.44 NAHSs, systematic characterization studies of the cycled electrodes were performed, such as galvanostatic intermittent titration (GITT) measurements (Fig. 6a–d), electrochemical impedance spectroscopy (EIS) measurements (Fig. 6e), capturing SEM (Fig. 6f, k, S12 and S13†) and TEM (Fig. 6g and h) images, an SAED pattern (Fig. 6i) and the XPS spectrum (Fig. 6j). Fig. 6a and c show the potential response curves of PbO1.44 NAHSs and β-PbO2 MSs from the GITT measurements taken during the 10th discharge–charge processes at 50 mA g−1 as anodes of LIBs, respectively. Notably, the overpotentials of PbO1.44 NAHSs remain at a lower level than those of β-PbO2 MSs at the same discharge/charge state, especially in the high voltage ranges for the conversion reaction, suggesting a higher Li+ ion conductivity and a lower polarization of PbO1.44 NAHSs than those of β-PbO2 MSs. Assuming that the Li+ transfer satisfies Fick's second law, the Li+ diffusion coefficient (DLi+) can be calculated by the equation of 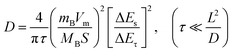 , where τ, L and S represent the constant current titration time, diffusion length and electrode surface area, ΔEs and ΔEτ refer to the steady-state voltage change after a current disturbance and voltage change during the constant current titration, and mB, Vm and MB are the molar mass, volume and molecular mass of PbO1.44 or β-PbO2, respectively.48 The average DLi+ values of 1.2 × 10−11 cm2 s−1 and 1.7 × 10−11 cm2 s−1 of PbO1.44 NAHSs for the alloying and dealloying reaction processes (Fig. 6b and d) are approximately one order of magnitude higher than those of β-PbO2 MSs (1.6 × 10−12 and 1.9 × 10−12 cm2 s−1), respectively. This firmly demonstrates the excellent alloying–dealloying reaction kinetics of PbO1.44 NAHSs, which accounts for the high capacity and outstanding rate capability. The Nyquist plots of the freshly assembled cells of PbO1.44 NAHSs and β-PbO2 MSs are fitted according to the equivalent circuit (inset in Fig. 6e), where Rs, Rct, Zw and CPE represent the electrolyte resistance, charge transfer resistance, Warburg impedance and constant phase element, respectively.49 The estimated Rct of PbO1.44 NAHSs (118 Ω) is about a quarter of that of β-PbO2 MSs (426 Ω), demonstrating the strengthened charge transfer conductivity of PbO1.44 NAHSs with their special hierarchical hollow nanoarchitecture.43,44
, where τ, L and S represent the constant current titration time, diffusion length and electrode surface area, ΔEs and ΔEτ refer to the steady-state voltage change after a current disturbance and voltage change during the constant current titration, and mB, Vm and MB are the molar mass, volume and molecular mass of PbO1.44 or β-PbO2, respectively.48 The average DLi+ values of 1.2 × 10−11 cm2 s−1 and 1.7 × 10−11 cm2 s−1 of PbO1.44 NAHSs for the alloying and dealloying reaction processes (Fig. 6b and d) are approximately one order of magnitude higher than those of β-PbO2 MSs (1.6 × 10−12 and 1.9 × 10−12 cm2 s−1), respectively. This firmly demonstrates the excellent alloying–dealloying reaction kinetics of PbO1.44 NAHSs, which accounts for the high capacity and outstanding rate capability. The Nyquist plots of the freshly assembled cells of PbO1.44 NAHSs and β-PbO2 MSs are fitted according to the equivalent circuit (inset in Fig. 6e), where Rs, Rct, Zw and CPE represent the electrolyte resistance, charge transfer resistance, Warburg impedance and constant phase element, respectively.49 The estimated Rct of PbO1.44 NAHSs (118 Ω) is about a quarter of that of β-PbO2 MSs (426 Ω), demonstrating the strengthened charge transfer conductivity of PbO1.44 NAHSs with their special hierarchical hollow nanoarchitecture.43,44
 | ||
| Fig. 6 Performance enhancement mechanism of PbO1.44 NAHSs. (a and c) GITT voltage profiles and (b and d) the calculated Li+ chemical diffusion coefficients based on stoichiometry from GITT of PbO1.44 NAHSs and β-PbO2 MSs during the 10th cycle at 50 mA g−1. (e) Nyquist plots (inset, equivalent circuit) of cells with PbO1.44 NAHSs and β-PbO2 MSs used as anodes. (f) SEM and (g and h) TEM images, (i) SAED pattern and (j) XPS spectrum of (f–i) cycled PbO1.44 NAHSs and (k) SEM image of cycled β-PbO2 MSs after the 90th cycle in the rate performance tests. (l) Nitrogen adsorption–desorption isotherms and (inset) the pore size distributions of PbO1.44 NAHSs and β-PbO2 MSs. Schematic illustrations of (m) lithiation-induced volume expansion and pulverization of β-PbO2 MSs and (n) effectively buffered volume changes combined with shortened ion transfer distance of PbO1.44 NAHSs. (o) Performance comparison of PbO1.44 NAHSs and reported PbOx-based anodes as listed in Table S1.† | ||
To verify the electrode stability enhancement mechanism, the contrasting structures of cycled electrodes of PbO1.44 NAHSs and β-PbO2 MSs (Fig. 6f–k, S12 and S13†) were investigated after the rate performance tests (Fig. 5c). Encouragingly, the cycled electrode of PbO1.44 NAHSs is flat and compact (Fig. 6f, S12, S14a and S15a†). The interconnected hollow interior of PbO1.44 NAHSs can robustly buffer the volume expansion and shrinkage induced by the repeated lithiation and delithiation processes, respectively, to stabilize the structure of PbO1.44 NAHSs (Fig. 6f and g) and enhance the robustness of the whole electrode. The HRTEM image (Fig. 6h) and SAED pattern (Fig. 6i) of the cycled PbO1.44 NAHSs show the reversible generation of the PbO1.44 phase after the 90th charging process, with distinguished lattice fringes of the PbO1.44-based (111) facet (d = 3.16 Å) and diffraction rings of the (111), (200), (220), (222) and (311) facets of the PbO1.44 crystal (JCPDS card no. 27-1201). The Pb 4f XPS spectrum of the cycled PbO1.44 NAHSs (Fig. 6j) is similar to that of the pristine PbO1.44 NAHSs (Fig. 1l). Therein, the Pb 4f5/2 and Pb 4f7/2 peaks can be split into two pairs of peaks at 142.7 and 137.9 eV corresponding to Pb2+ and at 142.0 and 137.2 eV corresponding to Pb4+, respectively. A chemical formula of PbO1.563 can be estimated from the relative area percentages of the Pb 4f XPS peaks (i.e., Pb4+ (56.3%) and Pb2+ (43.7%)) of cycled PbO1.44 NAHSs, which is close to the stoichiometric ratio of PbO1.44.
In sharp contrast, severe pulverization of the cycled β-PbO2 MSs electrode (Fig. 6k, S13, S14b and S15b†) was visually detected, as revealed in the upper right inset SEM image of representative cracked β-PbO2 MSs. More seriously, the huge volume expansion of lithiated β-PbO2 MSs caused the peeling off of active materials from the current collector (i.e., Cu foil), as indicated by the yellow arrows in Fig. 6k for the exposed areas of the current collector, resulting in the rapid degradation of the capacities of β-PbO2 MSs with a short cycle life (Fig. 5a–c). As shown in the cross-sectional view SEM images of the pristine and cycled electrodes, the electrode of β-PbO2 MSs (Fig. S14b and S15b†) underwent a thickness variation of 40% (i.e., from ∼30 to 42 μm), which is much higher than the 14% (i.e., from ∼28 to 32 μm) of the electrode of PbO1.44 NAHSs (Fig. S14a and S15a†). To clarify the larger specific surface area of PbO1.44 NAHSs compared to that of β-PbO2 MSs, nitrogen adsorption–desorption measurements were performed. The adsorption–desorption isotherms of PbO1.44 NAHSs and β-PbO2 MSs (Fig. 6l) can be classified as type IV isotherms with a type H3 hysteresis loop. The calculated specific surface area (i.e., Brunauer–Emmett–Teller (BET) surface area) and pore volume of PbO1.44 NAHSs (32 m2 g−1 and 0.12 cm3 g−1) are significantly higher than those of β-PbO2 MSs (2 m2 g−1 and 0.01 cm3 g−1), respectively. More importantly, in contrast to β-PbO2 MSs, the higher specific surface area of PbO1.44 NAHSs can induce their more homogeneous mixing with the conductive agent (acetylene black) and binder (sodium alginate), thus allowing them to attach more firmly to the current collector (copper foil) (Fig. S14†). This improves the robustness of the macrostructure of the PbO1.44 NAHSs electrode.
As illustrated comparatively in the schematic image (Fig. 6m), the drastic increase in electrode thickness, caused by the severe volume expansion of lithiated β-PbO2 MSs, induces the electrode to pulverize (Fig. S15b†) and the active material to peel off from the current collector (Fig. 6k), resulting in a rapid capacity decline. In sharp contrast, the superior cycling stability and rate capability of PbO1.44 NAHSs (Fig. 6n) can be reasonably assigned to the excellent structural features described as follows: (1) the interconnected hollow interior (Fig. 1e–g, 6f and g) with excellent volume change buffering efficiency enhances the electrode robustness for splendid cycling stability (Fig. 5a and b); (2) the short Li+/e− transfer distance of the secondary nanoparticles (∼50 nm in diameter, Fig. 1d, h and S1a–S1f†) promotes the fast and adequate electrochemical reaction for high capacity at various C-rates (Fig. 5c); (3) the thin shells constructed from ultra-fine nanoparticles (Fig. 1d) effectively reduce the volume expansion–shrinkage of the particles caused by lithium insertion–extraction, to decrease the electrode thickness variation and improve the structural stability of the entire electrode. Owing to the excellent structural features of the nanoparticle assembly and its interconnected hollow interior, the PbO1.44 NAHSs manifests enhanced lithium storage capability in contrast to the recently reported PbOx-based materials (Fig. 6o and Table S1†).10–19
3. Conclusions
In summary, we have developed a robust strategy for the PVP-driven transformation of β-PbO2 microspheres (MSs) to craft unique nanoparticle-assembled interconnected PbO1.44 hollow spheres (NAHSs), which have been explored for the first time as an advanced anode material for LIBs. In particular, an intriguing PVP-driven reduction combined dissolution–recrystallization transformation process of converting β-PbO2 MSs into PbO1.44 NAHSs is demonstrated by PVP concentration regulation experiments. Moreover, PVP also serves as a capping agent to stabilize the PbO1.44 solid spheres aggregated from the nanoparticles formed after 1 h of reaction and guides their inside-out Ostwald ripening to construct the interconnected hollow structure. Remarkably, PbO1.44 NAHSs demonstrated dramatically enhanced cycling stability and rate capability in stark contrast to the performance of β-PbO2 MSs. Based on the electrochemical analyses of the voltage plateaus and CV peaks, the superior lithium storage capability of PbO1.44 NAHSs is primarily attributed to the effectively enhanced alloying–dealloying reaction kinetics of Pb, which is dramatically boosted by the short ion/electron transfer distance of the ultra-fine secondary PbO1.44 nanoparticles (∼50 nm in diameter). Furthermore, the ample space of the interconnected hollow interiors of PbO1.44 NAHSs provides superb volume expansion buffering efficiency to reinforce the structural robustness of the electrode for distinguished cycling durability. As such, PbO1.44 NAHSs with excellent lithiation–delithiation reaction kinetics and reversibility may stand out as promising low-cost and high mass/volume capacity (i.e., 561 mA h g−1/5262 mA h cm−3) anode candidates for LIBs. Moreover, the proposed reductant and capping dual functions of PVP and the self-sacrificial templating mechanism in the construction of the rare mixed-valence PbO1.44 NAHSs provide new insights into the rational design of novel hollow-structured functional materials with excellent mechanical and electrochemical characteristics for various potential applications.Author contributions
X. B. and J. Z. contributed equally to this work. All authors have given approval to the final version of the manuscript.Data availability
All relevant data are within the manuscript and its ESI,† which are also available from the corresponding author on reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support from the Natural Science Foundation of Zhejiang Province (LY23B030001), the Basic Public Welfare Research Project of Wenzhou City (G20240025, S20240025), and the National Natural Science Foundation of China (21905208, 52331009).References
- Y. Li, S. Song, H. Kim, K. Nomoto, H. Kim, X. Sun, S. Hori, K. Suzuki, N. Matsui, M. Hirayama, T. Mizoguchi, T. Saito, T. Kamiyama and R. Kanno, Science, 2023, 381, 50–53 CrossRef CAS.
- A. Kondori, M. Esmaeilirad, A. M. Harzandi, R. Amine, M. T. Saray, L. Yu, T. Liu, J. Wen, N. Shan, H.-H. Wang, A. T. Ngo, P. C. Redfern, C. S. Johnson, K. Amine, R. Shahbazian-Yassar, L. A. Curtiss and M. Asadi, Science, 2023, 379, 499–505 CrossRef CAS.
- S. Zhao, Z. Wang, Y. He, H. Jiang, Y. Harn, X. Liu, C. Su, H. Jin, Y. Li, S. Wang, Q. Shen and Z. Lin, Adv. Energy Mater., 2019, 9, 1901093 CrossRef.
- X. Yuan, B. Liu, M. Mecklenburg and Y. Li, Nature, 2023, 620, 86–91 CrossRef CAS PubMed.
- S. Zhao, C. D. Sewell, R. Liu, S. Jia, Z. Wang, Y. He, K. Yuan, H. Jin, S. Wang, X. Liu and Z. Lin, Adv. Energy Mater., 2020, 10, 1902657 CrossRef CAS.
- X. Bo, Q. Zhang, G. Li, J. Zhang, R. Wu, S. Wang, I. K. Tiwalade, S. Wang, Z. Lin and S. Zhao, Chem. Eng. J., 2024, 487, 150559 CrossRef CAS.
- S. Zhao, Y. He, Z. Wang, X. Bo, S. Hao, Y. Yuan, H. Jin, S. Wang and Z. Lin, Adv. Energy Mater., 2022, 12, 2201015 CrossRef CAS.
- F. Xie, S. Zhao, X. Bo, G. Li, J. Fei, E.-A. M. A. Ahmed, Q. Zhang, H. Jin, S. Wang and Z. Lin, J. Mater. Chem. A, 2023, 11, 53–67 RSC.
- J. Han, J. Park, S.-M. Bak, S.-B. Son, J. Gim, C. Villa, X. Hu, V. P. Dravid, C. C. Su, Y. Kim, C. Johnson and E. Lee, Adv. Funct. Mater., 2021, 31, 2005362 CrossRef CAS.
- M. Martos, J. Morales, R. A. L. Sánchez, D. Leinen, F. Martin and J. R. R. Barrado, Electrochim. Acta, 2001, 46, 2939–2948 CrossRef CAS.
- S. H. Ng, J. Wang, K. Konstantinov, D. Wexler, J. Chen and A. H. K. Liu, J. Electrochem. Soc., 2006, 153, A787–A793 CrossRef CAS.
- K. Konstantinov, S. H. Ng, J. Z. Wang, G. X. Wang, D. Wexler and H. K. Liu, J. Power Sources, 2006, 159, 241–244 CrossRef CAS.
- Q. Pan, Z. Wang, J. Liu, G. Yin and M. Gu, Electrochem. Commun., 2009, 11, 917–920 CrossRef CAS.
- H. Wang, Y. Li, Y. Wang, J. Ma, S. Hu, H. Hou and J. Yang, Ceram. Int., 2017, 43, 12442–12451 CrossRef CAS.
- C.-H. Li, P. Sengodu, D.-Y. Wang, T.-R. Kuo and C.-C. Chen, RSC Adv., 2015, 5, 50245–50252 RSC.
- A. Guo, E. Chen, B. R. Wygant, A. Heller and C. B. Mullins, ACS Appl. Energy Mater., 2019, 2, 3017–3020 CrossRef CAS.
- Z. Chen, X. Cheng, H. Yu, H. Zhu, R. Zheng, T. Liu, J. Zhang, M. Shui and J. Shu, Ceram. Int., 2018, 44, 17094–17101 CrossRef CAS.
- F. B. F. R. Lipparoni, S. Panero and B. Scrosati, Ionics, 2002, 8, 177–182 CrossRef CAS.
- L. Zhan, X. Xiang, B. Xie and B. Gao, Powder Technol., 2017, 308, 30–36 CrossRef CAS.
- P. He, Y. Yang, H. Huang, J. Huang, H. Wang, Y. He and Z. Guo, J. Energy Chem., 2024, 97, 486–497 CrossRef CAS.
- H. Wang, J. Yu, Y. Zhao and Q. Guo, J. Power Sources, 2013, 224, 125–131 CrossRef CAS.
- J. P. Carr and N. A. Hampson, Chem. Rev., 1972, 72, 679–703 CrossRef CAS.
- Y. Kwon, H. Lee and J. Lee, Nanoscale, 2011, 3, 4984–4988 RSC.
- R. Inguanta, F. Vergottini, G. Ferrara, S. Piazza and C. Sunseri, Electrochim. Acta, 2010, 55, 8556–8562 CrossRef CAS.
- I. Sirés, C. T. J. Low, C. Ponce-de-León and F. C. Walsh, Electrochem. Commun., 2010, 12, 70–74 CrossRef.
- G. Xi, Y. Peng, L. Xu, M. Zhang, W. Yu and Y. Qian, Inorg. Chem. Commun., 2004, 7, 607–610 CrossRef CAS.
- M. Bervas, M. Perrin, S. Geniès and F. Mattera, J. Power Sources, 2007, 173, 570–577 CrossRef CAS.
- M. Cao, C. Hu, G. Peng, Y. Qi and E. Wang, J. Am. Chem. Soc., 2003, 125, 4982–4983 CrossRef CAS PubMed.
- I. Petersson and E. Ahlberg, J. Power Sources, 2000, 91, 137–142 CrossRef CAS.
- C. A. Sorrell, J. Am. Ceram. Soc., 1973, 56, 613–618 CrossRef CAS.
- S. Venkataraj, J. Geurts, H. Weis, O. Kappertz, W. K. Njoroge, R. Jayavel and M. Wuttig, J. Vac. Sci. Technol., A, 2001, 19, 2870–2878 CrossRef CAS.
- W. Shi, S. Song and H. Zhang, Chem. Soc. Rev., 2013, 42, 5714–5743 RSC.
- Z. Zhuang, Q. Peng and Y. Li, Chem. Soc. Rev., 2011, 40, 5492–5513 RSC.
- Y. Xiong, J. McLellan, J. Chen, Y. Yin, Z. Li and Y. Xia, J. Am. Chem. Soc., 2005, 127, 17118–17127 CrossRef CAS.
- Y. Xiong, I. Washio, J. Chen, H. Cai, Z. Li and Y. Xia, Langmuir, 2006, 22, 8563–8570 CrossRef CAS PubMed.
- Y. Xiong, J. Chen, B. Wiley, Y. Xia, Y. Yin and Z. Li, Nano Lett., 2005, 5, 1237–1242 CrossRef CAS.
- A. Ali Umar and M. Oyama, Cryst. Growth Des., 2006, 6, 818–821 CrossRef.
- B. Xi, S. Xiong, H. Fan, X. Wang and Y. Qian, Cryst. Growth Des., 2007, 7, 1185–1191 CrossRef CAS.
- S. Coskun, B. Aksoy and H. E. Unalan, Cryst. Growth Des., 2011, 11, 4963–4969 CrossRef CAS.
- Y. Zhang and J. Lu, Cryst. Growth Des., 2008, 8, 2101–2107 CrossRef CAS.
- Y. Wang, J. Ren, K. Deng, L. Gui and Y. Tang, Chem. Mater., 2000, 12, 1622–1627 CrossRef CAS.
- L. Guo, Q.-J. Huang, X.-Y. Li and S. Yang, Langmuir, 2006, 22, 7867–7872 CrossRef CAS.
- J. Fei, S. Zhao, X. Bo, F. Xie, G. Li, E.-A. M. A. Ahmed, Q. Zhang, H. Jin and Z. Lin, Carbon Energy, 2023, 5, e333 CrossRef CAS.
- S. Zhao, Z. Wang, Y. He, B. Jiang, Y. Harn, X. Liu, F. Yu, F. Feng, Q. Shen and Z. Lin, ACS Energy Lett., 2017, 2, 111–116 CrossRef CAS.
- B. Jiang, Y. He, B. Li, S. Zhao, S. Wang, Y.-B. He and Z. Lin, Angew. Chem., Int. Ed., 2017, 56, 1869–1872 CrossRef CAS.
- S. Zhao, Y. Wang, R. Liu, Y. Yu, S. Wei, F. Yu and Q. Shen, J. Mater. Chem. A, 2015, 3, 17181–17189 RSC.
- M. Y. Yang, S. V. Zybin, T. Das, B. V. Merinov, W. A. Goddard, E. K. Mok, H. J. Hah, H. E. Han, Y. C. Choi and S. H. Kim, Adv. Energy Mater., 2022, 13, 2202949 CrossRef.
- W. Weppner and R. A. Huggins, J. Electrochem. Soc., 1997, 124, 1569 CrossRef.
- X. Xu, F. Xu, C. Qu, G. Jiang, H. Yu, H. Repich, H. Han, F. Cao, L. Li and H. Wang, Adv. Funct. Mater., 2021, 31, 2101059 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr04763j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |






