Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nanomaterial-enabled anti-biofilm strategies: new opportunities for treatment of bacterial infections
Yijia
Xie†
a,
Huanhuan
Liu†
a,
Zihao
Teng†
b,
Jiaxin
Ma
*a and
Gang
Liu
 *a
*a
aState Key Laboratory of Vaccines for Infectious Diseases, Center for Molecular Imaging and Translational Medicine, Xiang An Biomedicine Laboratory, National Innovation Platform for Industry-Education Integration in Vaccine Research, School of Public Health, Xiamen University, Xiamen, 361102, China. E-mail: jiaxinma@xmu.edu.cn; gangliu.cmitm@xmu.edu.cn
bSchool of Pharmaceutical Sciences, Xiamen University, Xiamen, 361102, China
First published on 20th January 2025
Abstract
Biofilms play a pivotal role in bacterial pathogenicity and antibiotic resistance, representing a major challenge in the treatment of bacterial infections. The limited diffusion and inactivation efficacy of antibiotics within biofilms hinder their clearance, and while increasing dosage may enhance effectiveness, it also promotes antibiotic resistance. Nano-delivery systems that target antimicrobial agents directly to biofilms offer a promising strategy to overcome this challenge. This review summarizes the resistance mechanisms and therapeutic challenges associated with biofilms, with a focus on recent advances in nano-delivery systems such as liposomes, nanoemulsions, cell membrane vesicles (CMVs), polymers, dendrimers, nanogels, inorganic nanoparticles, and metal–organic frameworks (MOFs). Furthermore, the review explores the potential applications and challenges of nano-delivery systems in biofilm treatment and provides recommendations to guide future research and development in this field.
1. Introduction
Biofilms are critical in bacterial pathogenicity and resistance, complicating the treatment of bacterial infections. Approximately 65–80% of microbial infections and 80% of chronic human infections are linked to biofilm formation.1,2 Conditions such as endocarditis, osteomyelitis, sinusitis, urinary tract infections, chronic prostatitis, periodontitis, otitis media, chronic pneumonia, and cystic fibrosis of the lungs are all associated with biofilm development. Furthermore, biofilms can form on surgical implants, leading to device failure and, in severe cases, patient mortality.3 Protected by the biofilm matrix, these infections exhibit remarkable resistance to antibiotics and evade the immune system, often resulting in persistent or recurrent infections that present significant therapeutic challenges.Current strategies for biofilm removal include surgical debridement and antibiotic therapy. Surgical debridement typically utilizes ultrasound to disrupt the biofilm structure, followed by physical removal.4 However, this method can cause patient discomfort, and residual bacteria may lead to reinfection. Antibiotic treatment is another common strategy, but the extracellular polymeric substances (EPS) of biofilms hinder antibiotic penetration, rendering the bacteria 1000 to 1500 times more resistant than their planktonic counterparts.5 While conventional antibiotics can eliminate planktonic bacteria released from biofilms, they fail to eradicate bacteria embedded within biofilms, resulting in suboptimal treatment outcomes. Moreover, traditional antibiotics lack specificity, and systemic administration often results in reduced drug concentrations at the infection site due to metabolic degradation. Although high-dose antibiotics may temporarily suppress biofilm infections, they can cause severe side effects.6 The overuse and misuse of antibiotics have contributed to the emergence of multidrug-resistant bacteria, complicating infectious disease control. According to the World Health Organization, antimicrobial resistance accounts for approximately 7 million deaths annually, a figure projected to rise to 10 million by 2050.7 Therefore, biofilm-associated infections have become one of the most pressing challenges in global healthcare, highlighting the urgent need for innovative treatment strategies.
Nano-delivery systems present significant advantages in addressing biofilm-related infections. While emerging strategies, such as novel antibiotics, biofilm disruption, quorum sensing inhibition, and biofilm dispersal, show some promise, they still face limitations. In contrast, nanomaterials, due to their small size, large surface area, and high reactivity, offer more effective treatment options.8,9 Certain nano-delivery systems exhibit intrinsic antibacterial properties and enable precise drug delivery, optimizing drug concentration, minimizing adverse effects, and enhancing therapeutic efficacy. Their nanoscale size facilitates penetration into biofilms, increasing drug concentration and uniform distribution, thereby improving delivery efficiency. Additionally, nano-delivery systems can be designed as smart responsive platforms that react to changes in the infection microenvironment (e.g., pH, enzymes, and hydrogen peroxide) or to physical stimuli (e.g., light, ultrasound, and magnetic field). These systems can also be combined with photothermal therapy (PTT), photodynamic therapy (PDT), and gas therapy to effectively eradicate bacteria and biofilms.10 Nano-delivery systems offer a novel therapeutic approach to biofilm-related infections by overcoming drug resistance and minimizing side effects.
Nano-delivery systems show great potential in the treatment of biofilm infections due to their unique physicochemical properties, especially when antibiotic efficacy is limited. This review aims to provide a comprehensive overview of the design strategies and recent advancements in commonly used nano-delivery systems for biofilm treatment, including liposomes, nanoemulsions, CMVs, polymers, dendrimers, nanogels, inorganic nanoparticles, and MOFs. The mechanisms, features, and advantages of these systems in combating bacterial biofilms are discussed, along with current challenges in biofilm management, existing treatment strategies, and future directions for the development of nano-delivery systems.
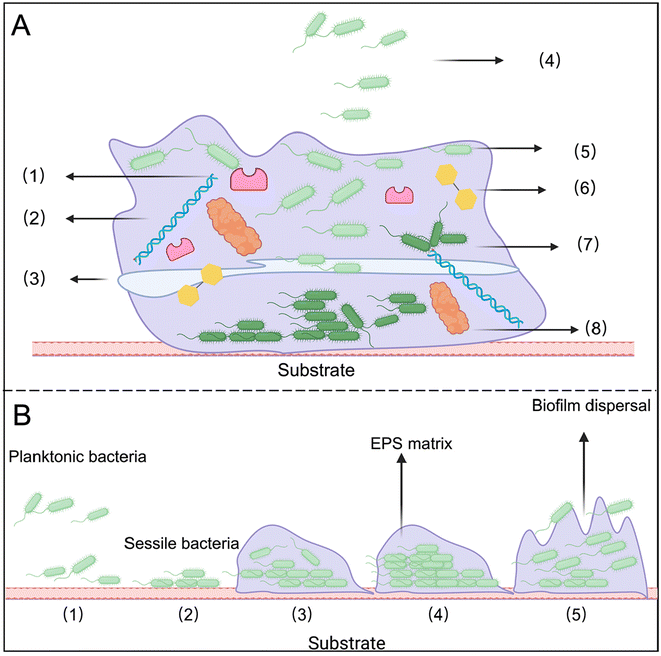
2. Overview of biofilms
Biofilms refer to the adhesion of extracellular viscous substances, such as polysaccharide matrices, fibrin, and lipoproteins secreted by microorganisms, to the surfaces of living or inanimate objects under external environmental stimuli, resulting in the formation of microbial aggregates.11,12 These EPS protect the bacteria within the biofilm (Fig. 1A). A mature biofilm structure consists of a matrix layer, conditional layer, connecting layer, and biofilm layer, arranged from the innermost to the outermost.2.1 Properties of bacterial biofilms
1. Electronegativity: most substances within bacterial biofilms are anionic, resulting in a negatively charged surface.132. Hydrophobicity: the outer layer of the biofilm typically contains lipids, methylated and acetylated polysaccharides, and proteins, contributing to its hydrophobic nature. This hydrophobic zone helps protect bacterial cells from external influences and the invasion of foreign molecules.14
3. Acidity: the biofilm creates an anoxic, malnourished, and acidic microenvironment. Bacteria at the surface rapidly consume oxygen, leading to relative hypoxia within the biofilm. Additionally, anaerobic processes produce numerous acidic metabolites, resulting in a low pH environment.15
4. Abundant enzymes: when bacteria colonize and form biofilms, they secrete various enzymes, including those capable of degrading or modifying antibiotics. These enzymes can alter the molecular structure of antibiotics before they reach bacterial cells, reducing or eliminating their effectiveness and contributing to antibiotic resistance.
5. Variety of toxins: toxins in bacterial biofilms primarily include exotoxins and endotoxins, both of which can harm the host. Exotoxins are proteins or peptides secreted by bacteria that can directly damage host cells or disrupt their normal functions. Endotoxins mainly refer to lipopolysaccharides (LPS) found in the cell walls of Gram-negative bacteria. When bacteria die and break down, LPS is released, triggering an immune response that can cause inflammation and fever. For example, Pseudomonas aeruginosa (P. aeruginosa) biofilms contain significant amounts of endotoxins, which can elicit strong inflammatory reactions upon release.
2.2 The process of bacterial biofilm formation
The formation of biofilms is a complex and dynamic process that can be divided into five distinct stages (Fig. 1B).16 Notably, even dead biofilms can facilitate the adhesion of other microbial cells and promote biofilm regeneration.The biofilm lifestyle begins with bacterial attachment to a surface, starting with the reversible attachment stage. In this phase, floating bacteria temporarily adhere to the substrate through electrostatic, van der Waals, and hydrophobic interactions. The second stage is the irreversible attachment stage, during which bacteria secrete EPS, promote colony growth, and form a nanogel layer that envelops the bacterial cells. The third stage involves the increment of microcolonies, characterized by the early formation and proliferation of small colonies. In the fourth stage, known as the full maturity stage, the biofilm develops a mature, three-dimensional structure. Finally, during the aging and diffusion stage, certain enzymes degrade the substrate, allowing bacteria to revert to their planktonic form. These planktonic bacteria can then seek out new nutrients and surfaces, perpetuating the biofilm cycle.
2.3 Challenges in treating bacterial biofilm infections
First, biofilms serve as physical barriers that restrict the penetration of antibiotics, making it challenging to achieve sufficient drug concentrations within the biofilm to effectively kill or inhibit bacteria.17 Only a few antibiotics, such as gentamicin, cefotaxime, and certain fluoroquinolones, demonstrate effective activity against biofilms, as they can penetrate the EPS. However, in some cases, these antibiotics can still lead to clinical treatment failure, such as failure to treat intracellular bacterial infections, development of drug resistance, biological toxicity, and so on.3,18 Second, the microenvironment within the biofilm promotes bacterial entry into dormant or slow-growing states, rendering these non-proliferating bacteria particularly tolerant to antibiotics, many of which target actively growing cells.19 Third, biofilms facilitate the transfer of drug-resistant genes, resulting in the formation of highly resistant bacterial populations. Additionally, bacteria within biofilms can evade the host immune system, achieving immune escape, while toxins secreted by these bacteria can directly damage immune cells. Consequently, the protective nature of biofilms allows some bacteria to survive treatment and re-establish the biofilm, leading to recurrent infections.3. Lipid-based nanoparticles
Lipid molecules are synthesized and self-assembled to form lipid-based nanoparticles, with liposomes being among the most widely studied and promising antimicrobial nanocarriers. Composed primarily of double-layer vesicles formed by phospholipids and cholesterol or other additives, liposomes feature one hydrophilic end and one hydrophobic end, resembling the structure of cell membranes. This amphiphilic property facilitates the fusion of liposomes with bacterial cell membranes, enhancing their encapsulation capacity for both water-soluble and lipophilic drugs. Liposomes offer advantages such as prolonged drug efficacy, reduced biological toxicity, and altered drug delivery pathways.20 Based on their functions, liposomes can be categorized into conventional liposomes and functional liposomes.3.1 Liposomes
Traditional liposomes are simple in structure, are usually unmodified, and have good carrying capacity and biocompatibility. However, they often lack targeting ability and are quickly cleared by the mononuclear phagocytic system (RES), making long circulation in the body difficult.21 Although they can optimize their distribution in biofilms by regulating particle size and surface charge, they still have the problem of poor specificity (Fig. 2A).22,23 Moreover, differences in antimicrobial delivery are not solely related to liposome adsorption.24 For instance, uncharged liposomes can enhance their antibacterial effects by fusing with bacterial membranes, releasing drugs into the surrounding media to interact with bacteria and biofilms.25 This lack of targeting limits the efficacy of conventional liposomes in complex biofilm environments.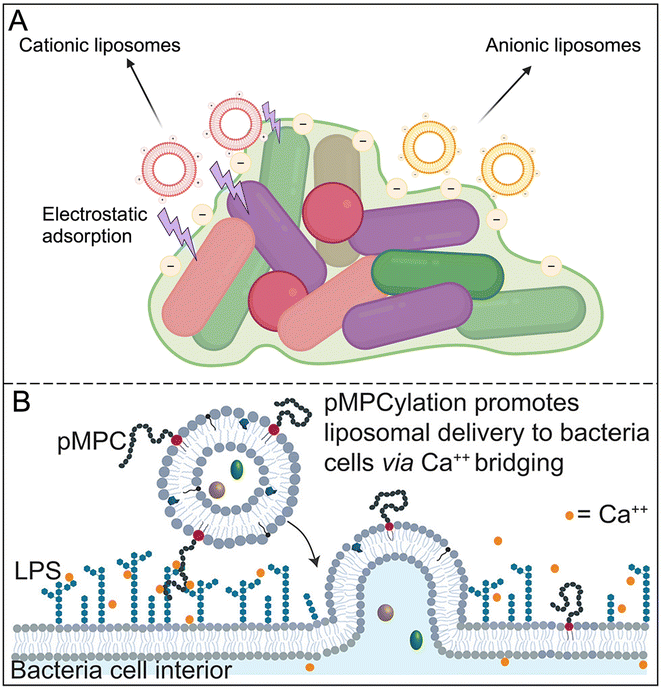 | ||
| Fig. 2 (A) Different effects of surface charges of traditional liposomes on biofilms. Created with https://www.BioRender.com. (B) Schematic of proposed two-stage mechanism for calcium-mediated adhesion and fusion between pMPC-LUVs and P. aeruginosa membrane. Reproduced from ref. 27 with permission from Monika Kluzek, copyright 2022. | ||
To overcome the limitations of traditional liposomes, researchers have developed engineered liposomes, covering a variety of types such as temperature-sensitive, pH-sensitive, targeting and immune liposomes. For example, PEG-modified liposomes significantly extend blood circulation time, but at the same time reduce interaction with target cells, thus weakening the targeting effect.26 To solve this problem, some novel materials such as pMPC were introduced into the liposome membrane to enhance the adsorption of negatively charged bacteria, which significantly improved the biofilm ablation efficiency (Fig. 2B).27
Stimulus-responsive liposomes enable targeted drug release by modifying molecules on the surface of the liposome to respond to specific internal or exogenous stimuli (such as temperature, pH, or magnetic field). This technique is particularly useful for antimicrobial therapy in complex microenvironments such as biofilms. For example, temperature-sensitive liposomes can release drugs at specific temperatures, further enhancing the effectiveness of treatment. Munaweera et al. explored the potential of temperature-sensitive liposomes to deliver ciprofloxacin to the site of infection for treating metal implant biofilms (Fig. 3A).25 Zhou et al. effectively packaged doxorubicin into quaternary ammonium chitosan liposome nanoparticles with pH-triggered drug release, demonstrating higher anti-biofilm properties and high biosafety.28 By binding to monoclonal antibodies, immune liposomes achieve efficient recognition and binding to target bacteria or cells, thereby reducing the distribution of drugs in healthy tissues and reducing side effects (Fig. 3B).
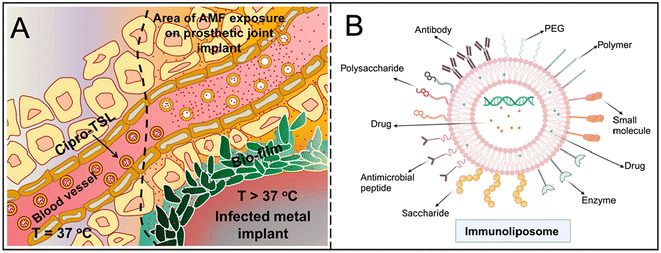 | ||
| Fig. 3 (A) Schematic of ciprofloxacin release from temperature-sensitive liposomes in the vicinity of an infected metal implant heated by exposure to an alternating magnetic field. Reproduced from ref. 25 with permission from Int J Hyperthermia, copyright 2018. (B) Multiple antibody modifications of immunoliposomes. Created with https://www.BioRender.com. | ||
In combination therapy, liposomes further demonstrated synergistic effects with techniques such as phototherapy and ultrasound. Through PTT and PDT, liposomes can accurately release antibacterial components in the biofilm, and use reactive oxygen species (ROS) or heat energy to destroy the extracellular matrix, enhancing the killing effect on the biofilm (Fig. 4A).29,30 Liposomes can also act as oxygen carriers to alleviate the anoxic microenvironment, thereby improving antibiotic efficacy and reducing drug resistance.31 In addition, ultrasound stimulates liposomes to release ROS and drugs, improves the penetration of liposomes to bacteria and biofilms, and increases the efficacy of anti-biofilms (Fig. 4B).32
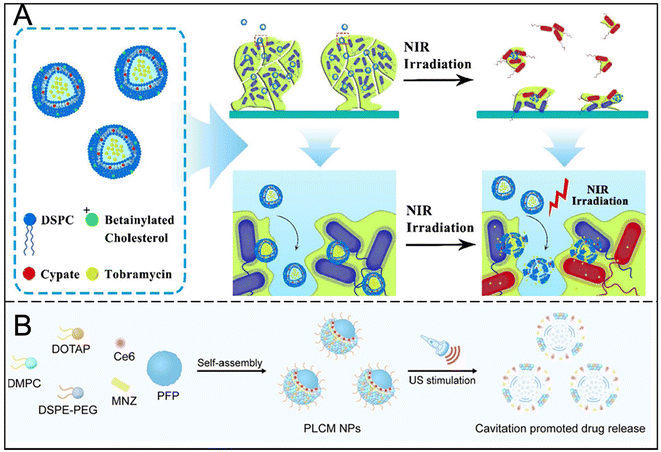 | ||
| Fig. 4 (A) Structure and function of nanoparticles and mechanism of near-infrared light-activated thermosensitive liposomes for the treatment of biofilms. Reproduced from ref. 29 with permission from ACS Appl Mater Interfaces, copyright 2018. (B) Schematic illustration of the preparation and US-stimulated cavitation of PFP@Lip-Ce6/MNZ nanoparticles. Reproduced from ref. 32 with permission from ACS Nano, copyright 2024. | ||
The development of liposomes has gradually evolved from simple drug carriers to multi-functional combination therapy systems. Although the traditional liposomes have strong biocompatibility, there are some problems such as insufficient targeting and short cycle time. Engineered and stimulus-responsive liposomes provide effective solutions in terms of specificity and controlled release. With advancements in technology, the application of liposomes is expected to achieve personalized and precise treatment by combining multiple stimulus-response functions and precise targeting, and ultimately improve the application value in anti-biofilm therapy.
3.2 Solid lipid nanoparticles (SLNs)
SLNs have been developed based on liposome technology. Unlike liposomes, SLNs do not possess a bilayer structure, but they offer higher loading capacity and bioavailability, as well as long-term stability; aquatic SLNs can be stored for up to three years. Typically composed of solid lipids with the addition of surfactants, SLNs are easy to mass-produce and do not require the use of organic solvents. Notably, even without encapsulated antibiotics, core–shell SLNs can effectively eradicate bacteria and reduce bacterial adhesion.33SLNs present rich possibilities for intravenous, oral, and ocular drug therapy, achieving the best encapsulation rates for water-soluble antibiotics, prolonging drug action time, and enhancing penetration through biofilm matrices. For instance, tobramycin encapsulated in SLNs and nanostructured lipid carriers can remain in the body for up to 34 hours, maintaining antibacterial activity against planktonic bacteria while also preserving or enhancing the ability to eradicate pre-formed biofilms.34 Similarly, various SLNs containing rifampicin have shown increased effectiveness in reducing the number of biofilms and live bacterial residues in Staphylococcus epidermidis. Additionally, some SLNs exhibit triple activity, for example, SLN-Nisin can significantly inhibit the growth of the oral pathogen Treponema denticola, disrupt oral biofilms, and reduce the viability of oral squamous cell carcinoma cells.35
While liposomes have stable physical properties, adjustable particle sizes, and good cellular compatibility, many liposomal formulations have entered clinical trials. Liposomes coated with antibiotics, such as amikacin, have been approved for use in biofilm-related lung infections.36Table 1 summarizes the types and characteristics of liposomes. In addition to treating biofilm infections, liposomes are utilized for various diseases, including cancer and ocular conditions. However, liposomes also have disadvantages, such as high costs and complex production technologies.
| Liposome types | Antibacterial element | Characteristics | Ref. |
|---|---|---|---|
| Anionic liposomes | Antibiotics | Bind to cationic antibiotics | 37 |
| Cationic liposomes | Electrostatic interaction | Combine with negatively charged biofilms | 37 |
| PEGylated liposomes | Antibiotics | Avoid nanoparticle aggregation and be engulfed by the body | 38 |
| pMPCylation liposomes | Antibiotics | Calcium-mediated adhesion for efficiently delivery of antibiotics | 27 |
| pH-sensitive liposomes | Antibiotics | pH-responsive drug release | 28 |
| Targeted liposome | Antibiotics | Attracted to N-acetylglucosamine residues in bacterial cell walls | 39 |
| Thermosensitive liposomes | Antibiotics | Heat-triggered drug release upon entering the microchannels of biofilms | 29 |
| Immunoliposomes | Antibiotics | Active targeting, increasing efficiency, reducing antibiotic usage and reducing toxicity | 40 |
| SLNs | Fatty acid | Obstructing biofilm formation and reducing bacterial adhesion to tissues and surfaces | 41 |
| SLNs | Quorum sensing inhibitor and alginate lyase | Site-specific biofilm-targeted interventional therapy | 42 |
4. CMVs
Although liposomes excel in drug encapsulation and protection, their synthetic nature can provoke immune responses in the host. In contrast, natural cell membranes derived from the host offer a gentler, more effective, and stable drug delivery route. CMVs possess a "core–shell" structure, consisting of membrane vesicles that encapsulate the nanoparticle core, thereby mimicking the properties of natural cell membranes (Fig. 5A).43 This design retains the complexity of cell membranes and overcomes the limitations of traditional surface modifications in nano-delivery.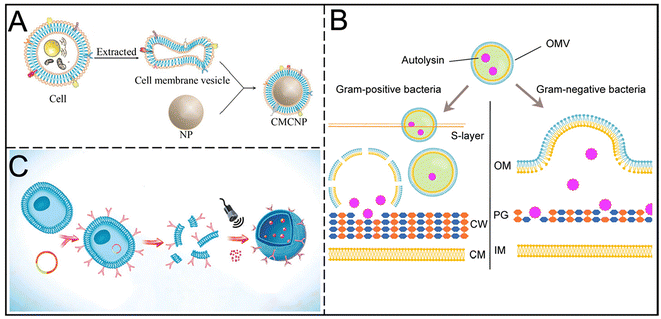 | ||
| Fig. 5 (A) Structural representation of CMCNPs. Reproduced from ref. 43 with permission from Journal of Shanghai Jiao Tong University (Medical Science), copyright 2021. (B) Schematic illustration of the antibacterial mechanisms of autolysin-loaded OMVs against Gram-positive and Gram-negative bacteria. Reproduced from ref. 46 with permission from Acta Biomaterialia, copyright 2022. (C) Schematic illustration of the budding process of ANVs nanocapturer from antibody-overexpressed cells. Reproduced from ref. 48 with permission from Adv Mater, copyright 2019. | ||
Both eukaryotic and prokaryotic cells can actively or passively generate membrane vesicles (MVs). These MVs inherit the membrane proteins and bioactivity of their parent cells, enabling them to perform similar biological functions and making them ideal platforms for drug delivery and gene therapy. Living bacteria can also produce MVs, which can passively accumulate at infection sites or actively target pathogens and macrophages, effectively inducing host immune responses and demonstrating unique advantages.44,45 Further modifications through physical, chemical, or biological methods can reduce vesicle toxicity and enhance targeting capabilities.
4.1 Targeting bacteria
CMVs can be designed to target bacteria, carrying therapeutic agents within the vesicles or coating the surfaces of drug-loaded core nanoparticles.45 Antibiotics encapsulated in cell membrane vesicles not only enhance specificity and binding but also facilitate direct delivery to biofilms, increasing drug concentration. Nanoparticles coated with bacterial membrane vesicles (BMVs) exhibit stability, uniformity, and enhanced antibacterial efficacy. Huang et al. demonstrated that Bacillus subtilis-derived outer membrane vesicles loaded with levofloxacin show superior antibacterial effects in mice infected with intestinal bacteria compared to free levofloxacin. Additionally, outer membrane vesicles (OMVs) secreted by Gram-negative bacteria can transport hydrolytic proteins, leading to the breakdown of peptidoglycan in both Gram-positive and Gram-negative bacteria (Fig. 5B). OMVs serve as effective carriers for delivering antibiotics such as fluoroquinolones, which easily fuse with Gram-negative bacteria.46 Further studies employed a "toxin-for-toxin" strategy, enhancing biofilm disruption through the synergistic effects of membrane characteristics and antibiotics. Researchers encapsulated triclosan (TCS) in poly(lactic-co-glycolic acid) (PLGA) to significantly inhibit S. aureus biofilms.47Targeted delivery of bacterial toxin monoclonal antibodies can be achieved by modifying the surface of cell membranes through genetic engineering. Liu et al. first demonstrated the combined application of antibacterial sonodynamic therapy and antitoxin immunotherapy using nanovesicles synthesized from engineered cell membranes. This method effectively neutralizes α-toxin secreted by Methicillin-resistant Staphylococcus aureus (MRSA), while ultrasound activation generates ROS that disrupt bacterial cell membranes, leading to depolarization of the membrane potential and accelerated bacterial cell death, along with promoting toxin removal (Fig. 5C).48 On this basis, cell membrane vesicles with mutated penicillin-binding protein PBP2a on the surface of MRSA were designed by genetic engineering to target the delivery of nano-antibiotics, showing better drug targeted delivery ability in MRSA-induced pneumonia, keratitis, muscle abscess models, and overcoming the alveolar barrier. This enhanced the accumulation of drugs at the MRSA infection site and helped inhibit the formation of MRSA biofilms.49 Gong et al. developed nanoparticles combining naftifine, hemoglobin (Hb), and erythrocyte membrane coatings. Naftifine disrupts carotenoid biosynthesis, Hb reduces hydrogen sulfide levels in bacteria, and the erythrocyte membrane alters bacterial lipid composition, collectively exerting destructive effects on S. aureus biofilms.50
4.2 Targeting the infection microenvironment
CMVs can be designed to target the infectious microenvironment, providing a carefully engineered approach for antibacterial treatment. Peng et al. developed neutrophil–bacteria hybrid membrane vesicle (HMV)-coated biocompatible lipid nanoparticles (LNP@HMVs) aimed at specifically delivering antibiotics to bacterial cells at infection sites. HMVs exhibit dual-targeting capabilities, accumulating in inflammatory endothelial cells and homologous Gram-negative bacterial cells, enhancing the in vitro inhibitory effects of levofloxacin-loaded LNP@HMVs on both planktonic bacteria and biofilms.51 Gao et al. found that BMV-coated nanoparticles demonstrated significant targeting capabilities both in vitro and in vivo, with increased drug accumulation in S. aureus infected mice compared to healthy controls, particularly in macrophages and major organs (kidney, lung, spleen, and heart).52By integrating the natural targeting mechanisms of cell membranes with the antibacterial properties of specific drugs, CMVs offer a promising strategy for targeted and effective antibacterial therapy. This approach has the potential to transform the fight against antibiotic-resistant bacterial infections. Attenuated vaccines can also be delivered by fusing cell membranes for antimicrobial treatment.49Table 2 summarizes the types and characteristics of MVs. However, it is crucial to consider the immunogenicity and pathogenicity of OMVs used for antibacterial treatment. Challenges remain in the large-scale production of uniform membrane vesicles, effective encapsulation of drugs with varying physicochemical properties, and ensuring safety, all of which require further investigation.
| Membrane vesicles types | Antibacterial element | Characteristics | Ref. |
|---|---|---|---|
| CMV | Sonosensitizer | Sonodynamic therapy and antitoxin immunotherapy | 48 |
| OMV | Antibiotics | Interference with biofilm formation and reduction of the virulence factors | 47 |
| OMV | Antibiotics | Targeted delivery and immune regulation | 53 |
| OMV | PDT and metal | Targeted delivery | 54 |
| HMV | Antibiotics | Targeting specific bacterial infection microenvironments | 51 |
5. Nanoemulsions
Nanoemulsions are systems that encapsulate active substances through nanodroplets of oil to stabilize and control drug release. Despite lacking the biocompatibility of cell membrane vesicles, nanoemulsions offer greater flexibility in drug encapsulation and release characteristics. They exhibit significant antibiofilm activity, exceeding that of commercially available antibiotics,55 and their surfactant properties prevent phase separation.56,57The natural antibacterial nanoemulsions include water-in-oil (W/O), oil-in-water (O/W) and double continuous types (Fig. 6A).56 Essential oils, such as cloves, thyme and peppermint, have poor solubility and stability, but their conversion into nanoemulsions can improve their bioavailability and ability to inhibit biofilms. For example, clove oil nanoemulsions showed enhanced antimicrobial properties,58 with peppermint nanoemulsions also showing inhibition of biofilm formation.59 The phenolic hydroxyl groups of thyme and clove also enhanced their hydrophilicity and improved membrane permeability.60 Nanoemulsions combined with cashew gum and clove essential oil showed antioxidant and antibiofilm activity.61
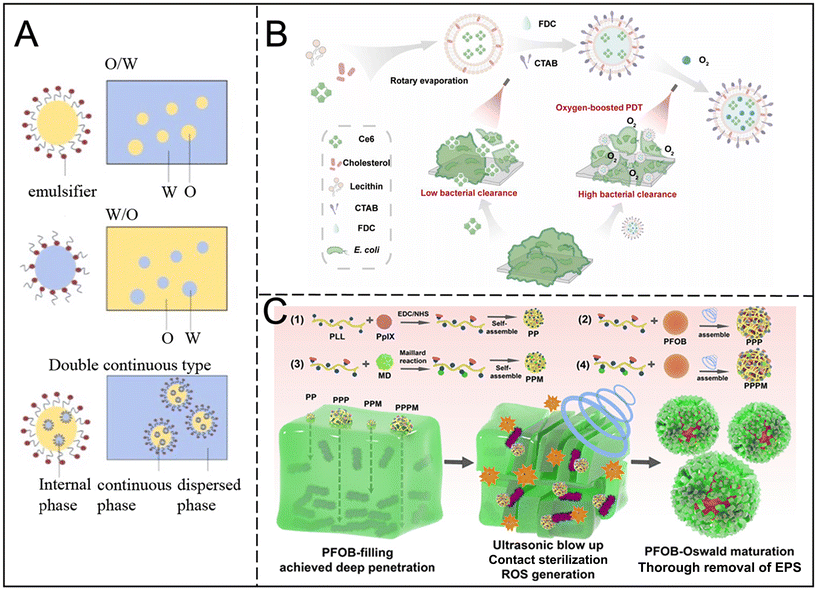 | ||
| Fig. 6 (A) O/W, W/O, and double continuous type nanoemulsions. Reproduced from ref. 56 with permission from Meat Research, copyright 2022. (B) Schematic illustration of the fabrication process of Ce6@FDC nanoemulsion and its oxygen delivery for enhanced photodynamic antibacterial efficiency. Reproduced from ref. 64 with permission from Springer Nature, copyright 2018. (C) Schematic illustration of fabricating various nanoagents and the potential advantages of PFOB in biofilm treatment. Reproduced from ref. 65 with permission from ACS Appl. Mater Interfaces, copyright 2024. | ||
Nanoemulsions can also achieve synergistic treatment, by encapsulating essential oils and antibiotics, improving the efficacy. For example, the combination of levofloxacin with clove oil can effectively remove biofilms.62 In addition, porphyrin-based nanoemulsions have a good photosensitizer loading capacity, can directly target microbial cells, and enhance sensitivity to Gram-negative bacteria.63 Oxygen, crucial to PDT, enhances the sensitivity of Gram-negative bacteria to treatment. Niu et al. synthesized a photodynamic perfluorocarbon nanoemulsion (Ce6@FDC) with oxygen transport capabilities, achieving a five-log reduction in planktonic bacteria and biofilm removal compared to free Ce6 treatment (Fig. 6B).64 Combined with ultrasound, the biofilm can be further damaged. Low-intensity ultrasound is used to enhance the penetration of nanoemulsions and improve the destruction ability of biofilms (Fig. 6C).65
Nanoemulsions can encapsulate drugs or imaging probes and achieve precise delivery through targeted modifications, making them suitable for multiple drug delivery routes.59,66 They exhibit low biotoxicity, do not promote resistant strains, and have a storage life of up to two years, facilitating transportation and clinical use.67 However, the synthetic surfactants used in the preparation may present a risk of toxicity, so research into natural alternatives is particularly important. Scaling up the continuous production of nanoemulsions remains a challenge.
6. Polymers
Polymers, due to their modifiability, provide great flexibility in biofilm treatment. They can be classified as natural or artificial based on their composition source.6.1 Natural polymers
Natural polymers have the advantages of diverse structure, extensibility and biocompatibility, and are effective drug carriers for inhibiting or eliminating bacterial biofilms. Among them, CS has good biocompatibility and antibacterial activity; through its positive charge interaction with bacterial cells, it effectively destroys the bacterial cell membrane and inhibits the formation of biofilm and bacterial growth. Although CS has mild bactericidal action and limited solubility,68 studies have shown that improving the structure of CS can enhance its water solubility and antibacterial effect.69 For example, CS nanoparticles (CSNPs) improve antibacterial potency by increasing the surface charge density and volume ratio. Combined with antibiotics (such as gentamicin)70 or metal salts (such as silver, zinc, and copper),71 the anti-biofilm effect of CS is significantly enhanced.CSNPs can be used in different forms to achieve targeted antimicrobial therapy: nanospheres are used to adsorb or encapsulate drugs on the surface (Fig. 7A),72 while nanocapsules encapsulate antimicrobials through core–shell structures (Fig. 7B).73 Nanofibers, due to their needle-like physical structure, can penetrate the EPS matrix, delivering drugs into bacterial cells and eliminating biofilms (Fig. 7C).74,75 It has been found that the combination of positively charged CSNPs with DNA enzymes or antimicrobial peptides has a highly efficient cleaning effect on Gram-positive and Gram-negative bacterial biofilms.73 Core–shell nanocapsules and penetrating nanofibers can further enhance therapeutic effectiveness through deep delivery of effective drugs and are suitable for local infection control. In addition, sheet- and rod-like nanostructures have a "nanoknife" effect that can pierce bacterial membranes and increase antibacterial action.
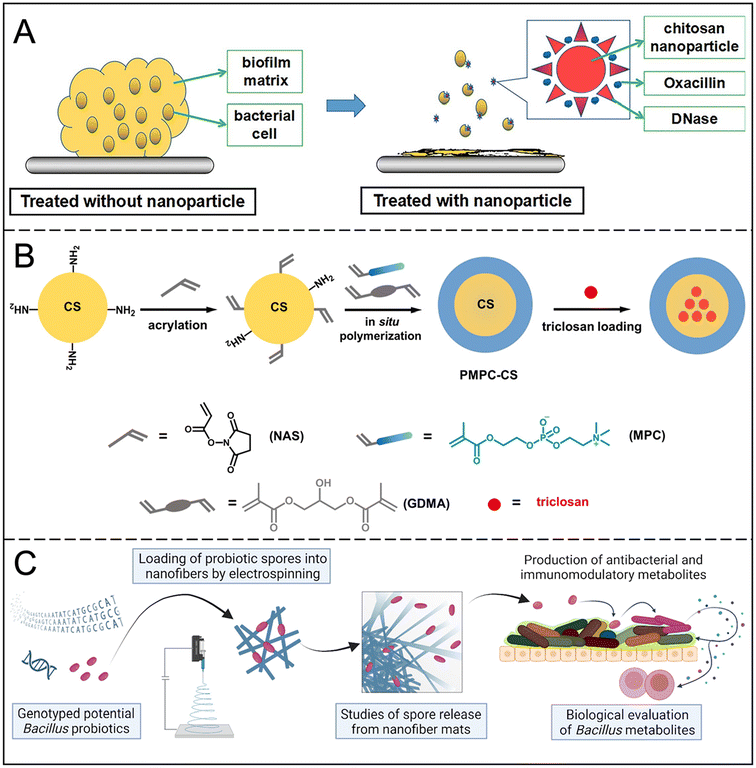 | ||
| Fig. 7 (A) Schematic diagram of the composition and therapeutic mechanism of CSNP DNase Oxa. Reproduced from ref. 72 with permission from Carbohydrate Polymers, copyright 2018. (B) Sequential steps for the preparation of triclosan-loaded nanocapsules. Reproduced from ref. 73 with permission from ACS Macro Lett, copyright 2019. (C) Schematic diagram of the composition and therapeutic mechanism of nanofibers. Reproduced from ref. 75 with permission from J Control Release, copyright 2023. | ||
6.2 Artificial polymers
Compared to natural polymers, synthetic polymers offer greater consistency and repeatability, and can be chemically modified to introduce specific functional groups for more precise drug release and targeting. Examples include polylactic acid-glycolic acid (PLGA) and micelles.PLGA is an FDA-approved medicinal carrier material whose degradation products are safe for humans. PLGA nanoparticles can effectively encapsulate hydrophobic and hydrophilic drugs for sustained release and have been shown to be effective in removing S. aureus biofilms.76 Xylitol in PLGA nanoparticles can enhance penetration into the biofilm matrix and overcome antibiotic resistance associated with biofilms.77 The cationic PLGA nanopolymer can inhibit the growth of Streptococcus mutans within 24 hours, and can significantly destroy the biofilm at high concentrations.78 In addition, polymers that respond to internal and external stimuli can increase antibacterial activity, such as pH-activated micelles that release drugs on demand (Fig. 8).79 Polymers combined with PTT and PDT enhance the ability to eliminate biofilms under near-infrared irradiation. This multi-treatment strategy not only improves the antibacterial effect, but also provides a new idea for the treatment of biofilm-associated infections. Huang et al. have improved the loading efficiency of antibiotics by designing carbon quantum dots mixed with PLGA nanoparticles to effectively fight bacterial biofilms.80 These results show that PLGA and its derived materials have broad application prospects in the field of antibacterial therapy.
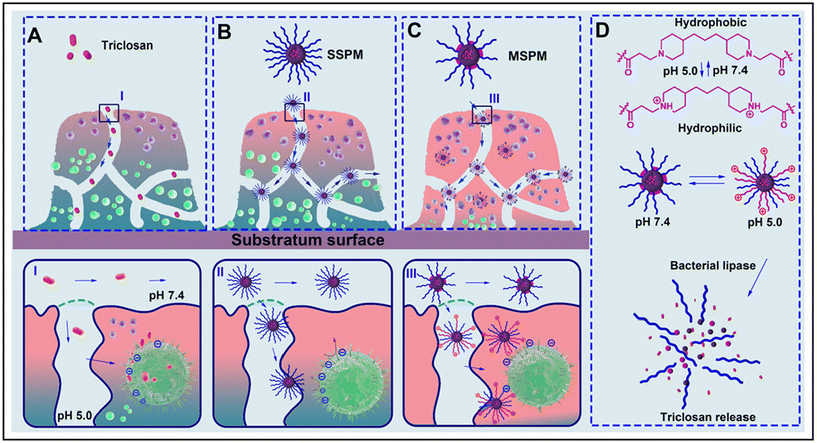 | ||
| Fig. 8 Schematic diagram of the mechanism of action of MSPM. (A) Nonencapsulated antimicrobials penetrate to a limited degree into a biofilm and kill only bacteria on the outside of the biofilm. (B) Antimicrobials encapsulated in an SSPM nanocarrier with stealth properties will show better penetration into a biofilm and thus kill bacteria in deeper layers of the biofilm, provided sufficient antimicrobial release. (C) MSPM target the bacterial cell surface and expose their micelle core, which subsequently becomes hydrolyzed by bacterial lipases to release its antimicrobial content. (D) Summary of the surface adaptability of MSPMs under the influence of pH changes and lipase degradation. Reproduced from ref. 79 with permission from ACS Nano, copyright 2016. | ||
Table 3 summarizes the types and characteristics of polymers. However, the preparation process for polymers is relatively complex, and quality control and economic feasibility must be further considered. The characteristics of polymer nanoparticles can be significantly influenced by changes in polymer monomers’ properties, especially in vivo, which can result in performance variations, including circulation time, biological distribution, metabolic behavior, and other pharmacological effects. Improving drug loading capacity, achieving precise and controllable drug release, and developing polymers capable of tracking infections in the body are crucial development directions.
| Delivery types | Antibacterial element | Characteristics | Ref. |
|---|---|---|---|
| Natural CS | CS | Reduces the vitality of initially adhering bacteria | 96 |
| CS derivatives | DNase and CS and antibiotics | Degradation of eDNA | 72 |
| Engineering CS | Natural polyphenol | Mucoadhesion profile | 97 |
| PLGA | Xylitol | Enhance penetration of biofilm matrix | 77 |
| Micelle | Enzymes and electrostatic interaction | pH-responsive drug release and charge reversal | 79 |
| h-PAMAM, D < 1 | NO | Enhance penetration of biofilm matrix and NO release | 90 |
| PAMAM, D = 1 | PTT and NO | Increased local temperature and the released NO | 98 |
6.3 Dendrimers
Hyperbranched and dendritic macromolecules show great potential as delivery carriers of fungicides and nano-antimicrobials due to their highly branched properties, nanoscale size and abundant terminal functional groups. Dendritic macromolecules have significant advantages over conventional polymers, with their three-dimensional structure offering unique nanoscale spherical properties and internal hydrophobic or hydrophilic cavities.81 This structure makes them highly responsive to microorganisms, and multiple surface functional groups can be easily chemically modified for use in combination with chemotherapy drugs to regulate antimicrobial properties.82,83Dendrimers can interfere with biofilm formation. For example, the glycopeptide dendritic macromolecules synthesized by Bergmann et al. can significantly inhibit the formation and dispersion of biofilms.84 When used in combination with antibiotics, dendrimers can enhance the efficacy of antibiotics and reduce drug resistance. The AZM-DA nanoparticles developed by Gao et al. decomposed in an acidic microenvironment and released azithromycin, which significantly improved the killing effect on biofilms (Fig. 9A).85 Pamukçu et al. enhanced the bactericidal effect of curcumin and successfully inhibited the formation of MRSA biofilms by grafting mesoporous silica nanoparticles onto hyperbranched polyethyleneimine.86 In addition, dendrimers also show advantages in targeted therapy. For example, the Cur-DA NPs prepared by Chen et al. using polyamide tree-like macromolecules enhance the targeting of infected tissues through interaction with biotin, thus improving the therapeutic effect of antibiotics.87 Polyamides are dendritic macromolecules most widely studied in the field of biomedicine, which can be divided into hyperbranched polyamides (h-PAMAM, D < 1) and dendritic polyamides (PAMAM, D = 1) according to different synthesis methods and branching degrees (D). h-PAMAM with different terminations has different effects on biofilms, and h-PAMAM with amine termination has a better eradication effect.88,89 In addition, dendritic macromolecules can further enhance their antibacterial properties through functionalization. For example, the introduction of NO into polyamides can significantly increase their bactericidal activity. h-PAMAM-PO-2/NO can not only reduce the metabolic activity of biofilms, but also kill the bacteria isolated from biofilms, and has good water solubility, which has broad application prospects in mouthwash, gel, ointment and other fields.90 Yang et al. grafted Fe3O4@PDA as a photoconverter and core, with PAMAM and NO donors on the surface to obtain a multifunctional Fe3O4@PDA@PAMAM@NONOates nanocomposite, which can activate both photothermal and non-release properties after laser irradiation. This leads to more effective antimicrobial effects and eradication of bacterial biofilms (Fig. 9B).91
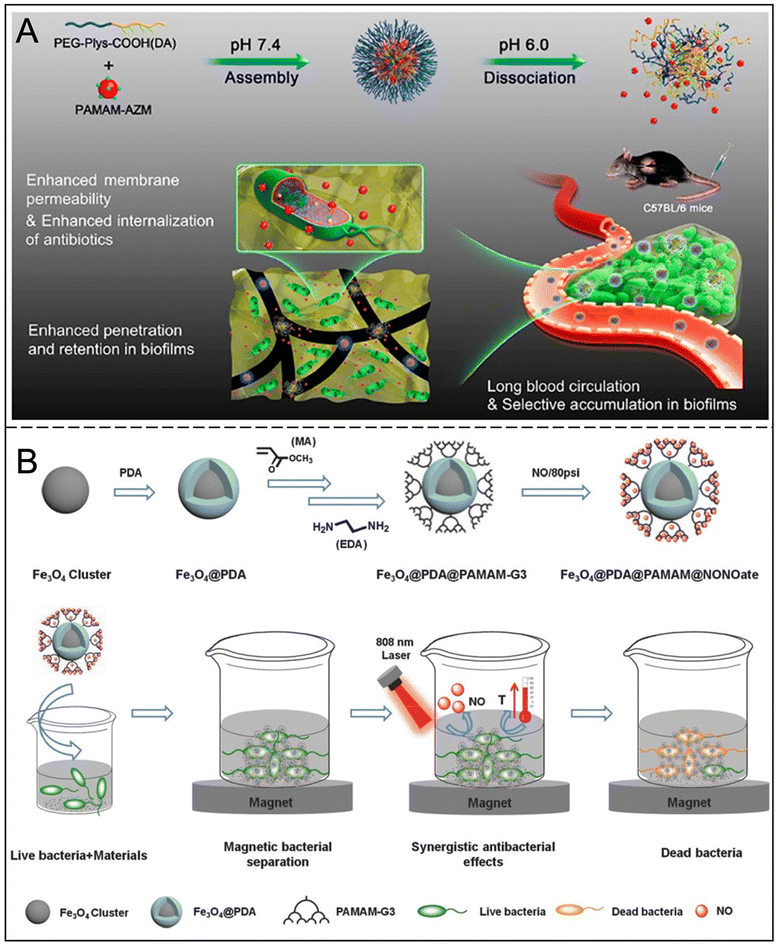 | ||
| Fig. 9 (A) Illustration of the self-assembly of AZM-DA NPs at pH 7.4 and release of secondary PAMAM-AZM NPs in an acidic biofilm microenvironment and the accumulation of AZM-DA NPs in biofilms and subsequent release of PAMAM-AZM NPs for enhanced biofilm penetration, permeabilization of the bacterial membrane, and increased AZM internalization. Reproduced from ref. 85 with permission from ACS Nano, copyright 2020. (B) Fe3O4@PDA@PAMAM@NONOate synthetic route for magnetic separation, synergistic photothermal and NO killing bacteria. Reproduced from ref. 91 with permission from Advanced Functional Materials, copyright 2018. | ||
Dendrimers enhance bactericidal activity, reduce cell toxicity, and have a lower synthetic burden, providing a new platform for anti-biofilm therapies. Table 3 summarizes the types and characteristics of dendrimers. Some dendrimer-based nanodrugs have already been commercialized or are in clinical development,92 such as the gene transfection reagents Superfect® (Qiagen)and Priostar™ (Starpharma),93 and the vaginal microbicide Vivagel® (SPL7013).94 However, dendrimer-based nanomedicines still face challenges, including the need for expanded production, improved analytical characterization methods, and more promising clinical trial results.95
7. Nanogels
Nanogels have gained considerable attention for applications in drug-controlled release, temperature sensing, and various sensing devices. Their porous three-dimensional mesh structure, excellent adhesion properties, and biodegradability make them promising materials for wound dressings.95 These characteristics enable gas exchange, absorption of wound exudate, maintenance of a moist microenvironment, prevention of bacterial adhesion and biofilm formation, and promotion of wound healing. Consequently, they hold significant potential in anti-biofilm applications. Some antibacterial nanogels have inherent antibacterial properties. For instance, CS possesses intrinsic antibacterial properties due to its abundant amino groups.99 Zhang et al. utilized the peptide QP5 in CS nanogels, leveraging the interaction between the positively charged CS and bacterial cell walls. This approach significantly impacted biofilm models, resulting in low colony-forming unit counts, reduced lactic acid production, and diminished metabolic activities after seven days of treatment.997.1 Loading antibacterial agents
Another design approach for nanogels is the incorporation of antibacterial agents, such as metal NPs and antimicrobial peptides. These nanogels typically comprise highly biocompatible materials, such as natural polysaccharides and collagen, minimizing adverse reactions and tissue damage in vivo.Nanogels can achieve continuous release of antibacterial agents. Haidari et al. proposed a design that utilizes F-127 polymers as AgNPs carriers, facilitating targeted transport to infected wounds while maintaining safe concentrations to mitigate toxic effects on cells. The ultra-small size of AgNPs enables them to penetrate the dense extracellular matrix of the bacterial biofilm, while the hydrogel enables the continuous release of silver particles, enhancing interactions with the bacterial membrane and promoting the elimination of pathogens (Fig. 10A).100 Nanogels can not only improve the sensitivity of bacteria to antibiotics, but also have a high drug loading capacity due to their large pores and surface area, improving the efficiency of drug loading and release. A smart hydrogel developed by Zhang et al. is able to release pectinase and antibiotics when an infection occurs, effectively eliminating biofilms.101 A novel nanogel consists of cationic peptide pools from curds that self-assemble into nanogels that are subsequently cross-linked with zinc ions to inhibit bacterial flagellar movement and exhibit antimicrobial and antibiofilm properties.102
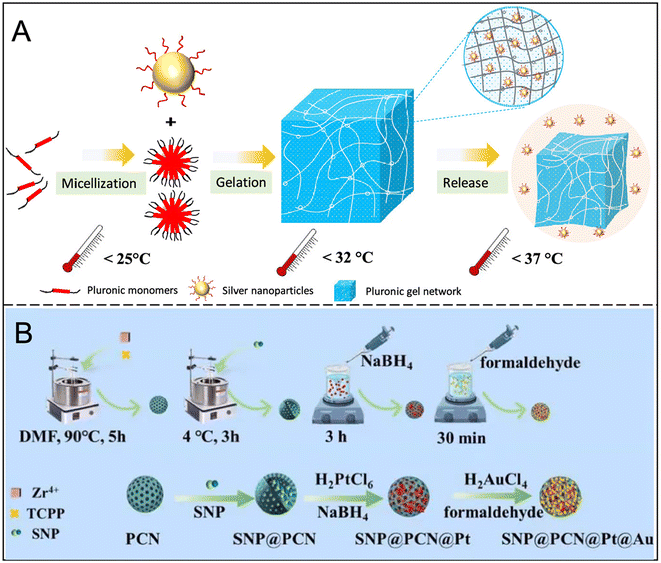 | ||
| Fig. 10 (A) Schematic illustration of AgNPs@MSA-loaded pluronic hydrogel preparation. Reproduced from ref. 100 with permission from ACS Appl. Mater. Interfaces, copyright 2020. (B) Schematic diagram of the synthetic procedure for SNP@PCN@Pt@Au. Reproduced from ref. 104 with permission from Acta Biomater, copyright 2023. | ||
7.2 Combination treatment strategy
Nanogels have shown great potential as drug carriers in anti-biofilm combination therapy. Their unique structure allows the loading and simultaneous delivery of multiple therapeutic ingredients, facilitating targeted drug release and synergies. For example, CS nanogels combined with antimicrobial peptides and hydrogen peroxide can significantly reduce biofilms,103 while multifunctional nanogel devices based on PDT, PTT and NO gases can alleviate the hypoxic microenvironment of wound infection and further inhibit biofilm formation (Fig. 10B).104 By co-delivering antibiotics and biofilm dispersants, nanogels effectively accelerate wound healing.105 In addition, the precise tunability of nanogels makes them particularly effective in controlling drug release. For example, by adjusting crosslinking density and acid-sensitive bonds, on-demand responsive release of antibiotics can be achieved, reducing side effects on healthy tissues. pH-switched antibacterial nanogels release antimicrobials in acidic pathological environments, protecting healthy tissue from damage.106 Local delivery capabilities further enhance therapeutic effectiveness: modified dextran bismuth selenide nanoparticles and iron-responsive antimicrobials both target biofilm removal while avoiding damage to normal tissue.107,108 These properties make nanogels widely used in infection control and targeted drug delivery.The high biocompatibility of nanogels minimizes adverse effects on normal cells and enhances drug penetration within biofilms. Their three-dimensional network structure protects encapsulated drugs from enzymatic degradation and allows for the adjustment of physical and chemical properties to meet drug delivery needs, achieving continuous and controlled release while reducing treatment frequency. Nonetheless, challenges remain, including potential immune responses, difficulty in controlling degradation rates, and limited loading capacity for hydrophobic drugs. However, the versatility of nanogels offers opportunities for integrating novel antibacterial strategies, such as magnetothermal therapy, immunotherapy, and metabolic interference therapy, to expand their applications and develop more diverse formulations.
8. Inorganic nanoparticles
Inorganic nanoparticle carriers are increasingly utilized for drug delivery due to their ability to disrupt biofilms and interfere with cellular processes. The positive charge of metal ions allows these nanoparticles to attract negatively charged biofilms, impairing their protective effects. They can bind to thiol groups on the surface of proteins, leading to protein coagulation and disruption of enzyme function, which hinders cell division and proliferation. Inorganic nanoparticles deliver drugs through two main methods: encapsulation, where drugs are stored in the nanoparticle pores for controlled release, and surface modification, where drugs are attached to the nanoparticle surface via degradable chemical bonds.8.1 Metal nanoparticles
Metal nanoparticles, particularly gold nanoparticles (AuNPs) and silver nanoparticles (AgNPs), have demonstrated significant potential in the treatment of biofilms and drug delivery. AuNPs can inhibit the synthesis of intracellular adenosine triphosphate (ATP) and affect the binding of transfer RNA, thereby enhancing the permeability of antibiotics to bacterial cells.109 Studies have shown that the combination of AuNPs with antibiotics significantly improves antibacterial efficacy;110 for instance, Hasoon et al. found that the combination of AuNPs with ciprofloxacin effectively prevents the formation of bacterial biofilms (Fig. 11A).111 Furthermore, Zhang et al. developed a glyconjugate-based imaging and therapeutic strategy that integrates PDT and PTT, successfully eradicating biofilms caused by P. aeruginosa.112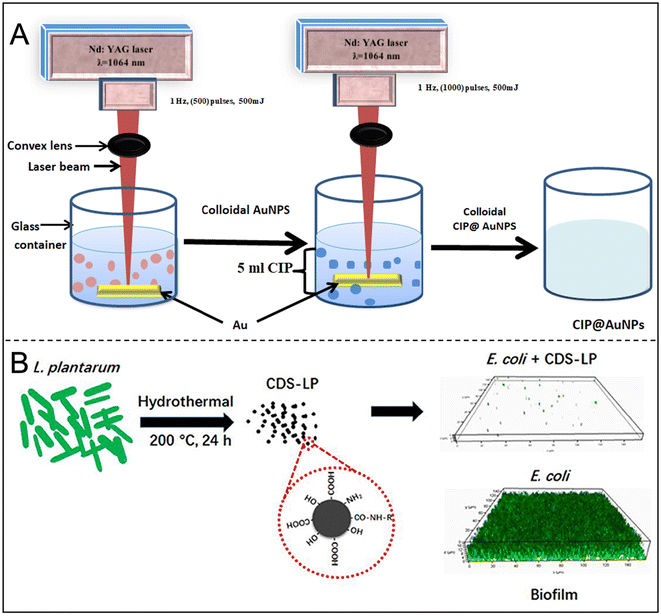 | ||
| Fig. 11 (A) Synthesis of ciprofloxacin-conjugated gold nanoparticles and their study antibacterial effects on growth and biofilm formation through nebulizer mask against respiratory infections. Reproduced from ref. 111 with permission from Plasmonics, copyright 2023. (B) Schematic of the synthetic route and anti-biofilm activity of CDs-LP. Reproduced from ref. 128 with permission from Lin, Li and Chen, copyright 2018. | ||
On the other hand, AgNPs can effectively penetrate bacterial biofilms and release a substantial amount of silver ions to enhance antibacterial activity.113 By forming chelates with antibiotics, AgNPs can selectively target and eliminate biofilms that are resistant to traditional single antibiotics.114 Metal nanoparticles can be endowed with various shapes, such as nanospheres,115–117 nanorods,118–121 nanostars,122,123 nanoshells,124 and nanocages.125,126 Among them, gold-silver nanocages (GSNCs) are regarded as effective drug delivery systems for near-infrared PTT; however, the limited silver release from these structures remains a challenge that needs to be addressed. Qin et al. synthesized GSNC-Cyh using cysteine hydrochloride, successfully enhancing bacterial adhesion to the nanoparticles and promoting the effective release of ultra-small AgNPs, which significantly increased intracellular ROS levels and completely eradicated multidrug-resistant biofilms. Additionally, metal oxides such as ZnO,127 TiO2, and SiO2 also exhibit the ability to inhibit resistant bacterial strains and prevent biofilm formation.
8.2 Non-metal nanoparticles
Other inorganic nanoparticles, such as CQDs, calcium phosphate, and mesoporous silica nanoparticles, have been effectively employed for in vitro delivery of various molecules. CQDs, with sizes under 10 nm, are notable for their modifiable functional groups and ability to generate ROS via photodynamic mechanisms, making them effective against bacterial biofilms. Wan et al. designed CQD and PLGA mixed nanoparticles encapsulating azithromycin and tobramycin, utilizing a combination of chemotherapy and photothermal effects to combat biofilms. Lin et al. used CQDs synthesized by Lactobacillus plantarum (LP) to inhibit E. coli biofilm formation without cytotoxic effects (Fig. 11B).128 Ching et al. functionalized curcumin (Cur) with calcium phosphate, finding that while the release of Cur was low, it effectively inhibited biofilm maturation. They proposed using pH-sensitive linkers to enhance Cur release from hydroxyapatite surfaces.129 Barros et al. synthesized antibacterial Cur-conjugated silica nanoparticles that improved Cur solubility and disrupted mature biofilms by reducing biofilm adhesion protein production.130 Hydrophilic antibiotics, such as vancomycin, can bind to amine-functionalized silica nanoparticles for tandem transport and bacterial killing. Mesoporous silica nanoparticles encapsulated with chlorhexidine, averaging approximately 140 nm, demonstrated promising antimicrobial effects against planktonic S. mutans, as well as monospecies and multispecies bacterial biofilms.131Most inorganic nanoparticles exhibit good biocompatibility and stability, addressing the decreased stability often associated with organic materials and macromolecules in drug delivery. Their low cost, ease of manufacturing, prolonged in vivo residence time, and improved pharmacokinetics make them attractive options. Table 4 summarizes the types and characteristics of inorganic nanoparticles. However, the potential toxicity of metal and inorganic particles and their distribution and metabolism within the body remain poorly understood, limiting their clinical applications. In research contexts, it is essential to consider the metabolic capacity of cells and the impact of the delivery carriers, especially those containing heavy metals.132 Addressing the toxic effects of carriers on delivery targets is crucial for advancing the clinical application of inorganic nano-delivery systems.
| Delivery types | Antibacterial element | Characteristics | Ref. |
|---|---|---|---|
| Metal nanoparticles | Antibiotics and Au | Strong antioxidant properties and synergistic enhancement of antibiotic efficacy | 111 |
| Metal oxide nanoparticles | Zn and ROS | ROS and free radical production, synergistic CIP | 127 |
| CDs | Antibiotics and PTT | Stimuli-responsive release of the cargos and chemo-photothermally synergistic anti-biofilm effects | 149 |
| Metal nanoparticles | Antibiotics and Au | Strong antioxidant properties and synergistic enhancement of antibiotic efficacy | 111 |
| MOFs | Zn and antibiotics | Inhibition of biofilm formation and controlled release of Zn | 136 |
| ZIF-8 | Antibiotics and PDT | pH-responsive drug release and PDT | 139 |
| Simulation enzyme MOFs | Enzyme | Cutting eDNA and hydrolyzing DNA | 142 |
| PCN-224 | PDT and enzyme | Bactericidal and anti-inflammatory synchronous treatment mode | 150 |
9. MOFs
MOFs are crystalline porous materials characterized by periodic network structures formed through the self-assembly of metal ions or clusters with organic ligands via coordination bonds. MOFs can inhibit the formation and development of biofilms through several mechanisms: (i) increased membrane permeability and leakage of cellular contents, (ii) deficiency in proton motive force, (iii) ROS generation, and (iv) metabolic dysregulation (Fig. 12A).133,134 By rationally designing the structure and composition of MOFs, precise control over the release of antibacterial agents can be achieved, enhancing antibacterial efficacy while minimizing side effects.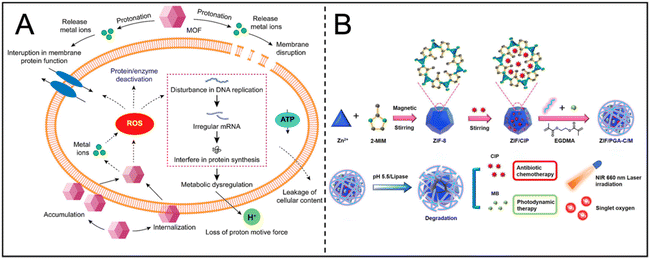 | ||
| Fig. 12 (A) Proposed model illustrating the antimicrobial mode of action by MOF. Reproduced from ref. 134 with permission from ACS Appl. Bio Mater, copyright 2021. (B) Schematic illustration of the preparation of ZIF/PGA-C/M hybrid nanocomposite. Reproduced from ref. 139 with permission from Materials & Design, copyright 2023. | ||
9.1 Metal antibacterial properties
MOFs inherently contain antibacterial components, with many metal ions and organic ligands shown to possess antibacterial properties. These frameworks can release metal ions (such as Ag+, Zn2+, Co2+, Cu2+), organic ligands, or other antibacterial agents that interact with bacterial cell membranes, disrupt metabolic activity, cause leakage of cellular components, and ultimately lead to bacterial death.135 For instance, the Zn-MOF synthesized by Akbarzadeh et al. demonstrates controlled ligand release and inhibits biofilm formation in various bacteria, including E. coli, Bacillus subtilis, S. aureus, and Klebsiella pneumoniae.136 The Ag-MOF developed by Arenas-Vivo et al. utilizes multiple mechanisms to inhibit biofilms, including the intrinsic bactericidal activity of the MOFs, the biological killing ability of silver nanoparticles, and photoactivity following ultraviolet A irradiation.137 The antibacterial effectiveness of Ni-MOFs likely stems from the synergistic release of Ni2+ and organic linkers; positively charged Ni2+ ions attract to the negatively charged bacterial cell envelope, resulting in ROS production and bacterial cell death.138 Compared to traditional antibiotics, the release of metal ions may mitigate the development of bacterial resistance due to differing mechanisms of action. Furthermore, the antibacterial performance of these MOFs often surpasses that of conventional antibiotics.9.2 Small molecule antibacterial properties
With a diverse spatial structure and adjustable composition, MOFs can respond to the microenvironment of biofilms and dynamically release bioactive substances, including antibiotics and photosensitizers in addition to metal ions. Represented by Zeolitic Imidazolate Framework-8 (ZIF-8) nanoparticles, MOFs can degrade in an acidic environment (pH 5.5–6.8), but remain stable under physiological conditions, providing a platform for controlled release of antibacterial drugs. Ciprofloxacin (CIP) and methylene blue (MB) were loaded into ZIF-8 nanocomposites to form a dual response system, which promoted the synergistic release of CIP and MB under low pH and high lipase conditions in the bacterial microenvironment, and enhanced the chemical-photodynamic therapeutic effect (Fig. 12B).139 At the same time, MOFs can also improve the stability and solubility of small molecules, which is conducive to the effective release of ROS at the infected site.140 Through charge conversion and pH response mechanisms, MOFs further enhance the permeability of nanocomposites to biofilms, facilitating drug delivery to the infection core.141 Based on this function, MOFs show great application prospects in the rapid control of infection and improving the stability of antimicrobial agents.9.3 Enzyme activity antibacterial properties
Some MOFs exhibit enzyme-like activity, capable of cutting extracellular DNA, disrupting biofilm stability and inhibiting bacterial growth. For example, bimetallic layered macroporous MOFs (HMUiO-66 (Zr/Ce)) effectively promote DNA hydrolysis and weaken biofilms through the synergistic interaction of Zr-OH and Ce-OH sites.142,143 Ce-MOF nanoenzymes simulate the dual activity of DNase and peroxidase, using Ce(IV) complexes to hydrolyze eDNA to destroy mature biofilms, and peroxidase activity further removes dispersed bacteria in the presence of H2O2.144 Due to their ultra-small size, Ce-MOF nanofibers have 3–15 times the hydrolytic activity of conventional MOFs, which make them excellent for biofilm prevention and removal.145 In addition, the gold-cluster-modified Au@ZIF-8 showed enhanced peroxidase activity and photothermal responsiveness under near-infrared irradiation, and had a significant bactericidal effect on MRSA.146 In response to natural enzyme instability and the complexity of artificial enzyme synthesis, Qiu et al. developed CeO2 modified porphyrin MOFs that synergistically inhibit biofilm formation through ATP deprivation and ROS production.147 These approaches demonstrate the innovative potential of MOFs in dynamic biofilm therapy.Given these advantages, MOFs hold significant potential for applications in antibacterial therapy and medical devices. For instance, antibacterial coatings or membrane materials based on MOFs can be designed for surface modification of medical devices to inhibit bacterial adhesion and colonization. Zang et al. prepared an MOF polymer coating that effectively prevents bacterial attachment and colonization, significantly inhibiting biofilm formation.148Table 4 summarizes the types and characteristics of MOFs. However, despite the considerable promise of MOFs in anti-biofilm applications, practical implementation faces challenges. Key issues include ensuring the stability and biocompatibility of MOFs in complex biological environments and achieving precise regulation of MOF structure and composition to meet specific application requirements. Therefore, further in-depth research on the preparation, properties, and mechanisms of MOFs is essential to advance their practical applications in the field of anti-biofilm strategies.
10. Future perspectives and conclusions
The complex structure and drug resistance of biofilms make the effective delivery of drugs within the membrane a major problem in the field of anti-biofilm therapy. The nano-delivery system not only has the advantages of nanosize and can penetrate the biofilm more easily, but also can increase solubility, stability, and blood circulation time, while reducing the dosage. Precision therapy can also be achieved through active or passive targeting to maximize the local effective concentration of the drug. In addition, through personalized design, intelligent responsive drug release can be achieved, ensuring drug release on demand. More importantly, nano-delivery systems can also achieve combined therapy, including PTT, PDT, etc., which not only shows excellent results in inhibiting biofilm formation and accelerating the degradation of biofilm, but also reduces the risk of drug resistance. The versatility and adaptability of nano-delivery systems make them show great prospects in the field of anti-biofilm therapy, making targeted therapy, combination therapy and immunotherapy possible. We summarize the advantages and disadvantages of several types of nano-delivery systems for antimicrobial and anti-biofilm applications that have been mainly reported in the current literature (Table 5).| Nano-delivery systems | Advantages | Disadvantages |
|---|---|---|
| Liposomes | Enhance drug efficacy, reduce biological toxicity, alter delivery pathways | Rapid clearance affects drug concentration; need to extend retention time in biofilms |
| CMVs | Can evade immune clearance | Production challenges; limited scalability for application |
| Nanoemulsions | Multiple delivery routes; enhance penetration through biofilms | Low drug encapsulation efficiency; complex preparation processes |
| Polymers | Accommodate various drug types; numerous modification options suitable for controlled release | Physiological stability and clearance efficiency in vivo need improvement |
| Dendrimers | Lower synthetic burden | Often exhibit poorer biocompatibility |
| Nanogels | Favorable biocompatibility and biodegradability; facilitate sustained release of antibiotics | Stability challenges |
| Inorganic nanoparticles | High drug loading capacity; low immunogenicity | Potential toxicity warrants careful consideration |
| MOFs | High surface area; ease of functionalization; suitable for effective antibiotic storage and release | Frequently lack stability |
Although nano-delivery systems show significant advantages in anti-biofilm therapy, most research is still at the laboratory stage, and clinical applications have not been widely realized. In order to accelerate the development of this field, we suggest that future research should focus on the following areas:
1. Clinical translation potential: although many nano-delivery systems have shown promise at the laboratory stage, clinical translation remains challenging. More preclinical and clinical studies are needed to validate their safety and efficacy. Establishing in vitro and in vivo models that closely simulate real pathological environments could lay the groundwork for their application.
2. Combination therapy: given the complexity of biofilms, future studies could explore the co-administration of various agents (e.g., antibiotics and anti-biofilm drugs) for synergistic treatment at multiple targets. Integrating multidisciplinary approaches may enhance therapeutic outcomes.
3. Personalized treatment: with advancements in precision medicine, nano-delivery systems customized according to patient characteristics (e.g., infection type and immune status) may offer higher efficacy.
4. Long-term efficacy and resistance: investigating ways to mitigate antibiotic resistance through nano-delivery systems is crucial. For instance, modulating release rates to avoid peak drug concentrations may help reduce resistance pressure on bacteria.
5. Biocompatibility assessment: although certain materials (e.g., nanogels and CMVs) have shown good biocompatibility, systematic evaluation of their potential side effects in long-term use remains essential.
6. Development of new nano-delivery systems: increasing the specificity and sensitivity of these delivery platforms could enable real-time monitoring, early intervention, and cost-effective treatment.
In conclusion, as a nanotechnology, nano-delivery systems enhance their function in treating biofilm diseases through specific modifications. This article provides a review of the application progress of nano-delivery systems in the treatment of biofilm diseases. In addition to developing new antibiotics, nano-delivery systems may be a new strategy for the future treatment of bacterial infections and biofilm diseases. Although there are still challenges in preparation, quality control, personalized application, and regulation, future research and development will help address these issues and promote the clinical application of nano-delivery systems.
Abbreviations
| MOFs | Metal–organic frameworks |
| DNA | Deoxyribonucleic acid |
| EPS | Extracellular polymeric substances |
| PTT | Photothermal therapy |
| PDT | Photodynamic therapy |
| PEG | Polyethylene glycol |
| pMPC | Poly[2-(methacryloyloxy)ethyl phosphorylcholine] |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| NIR | Near-infrared |
| ILs | Immunoliposomes |
| S. aureus | Staphylococcus aureus |
| SLNs | Solid lipid nanoparticles |
| MVs | Membrane vesicles |
| BMVs | Bacterial membrane vesicles |
| OMVs | Outer membrane vesicles |
| CMVs | Cell membrane vesicles |
| HMV | Hybrid membrane vesicle |
| O/W | Oil in water |
| W/O | Water in oil |
| CL | Clove oil |
| Hb | Hemoglobin |
| TCS | Triclosan |
| LP | Lactobacillus plantarum |
| PLGA | Poly(lactic-co-glycolic acid) |
| NPs | Nanoparticles |
| CS | Chitosan |
| Oxa | Oxacillin |
| CSNPs | Chitosan nanoparticles |
| DNase | Deoxyribonuclease |
| PMPC | Poly(2-methacryloxyethyl phosphate choline) copolymer |
| ROS | Reactive oxygen species |
| CQDs | Carbon quantum dots |
| h-PAMAM | Hyperbranched polyamide |
| PAMAM | Branched polyamide |
| NO | Nitric oxide |
| AZM | Azithromycin |
| Cur | Curcumin |
| DA | 2,3-Dimethyl maleic anhydride |
| MSN | Mesoporous silica nanoparticles |
| PEI | Polyethyleneimine |
| P. aeruginosa | Pseudomonas aeruginosa |
| Am | α-Amylase |
| Cef | Cefepime |
| E. coli | Escherichia coli |
| CDs | Carbon nanodots |
| KCDs | Carbon nanodots derived from kanamycin |
| GSNC | Gold and silver nanocages |
| Cyh | Cysteine hydrochloride |
| ZIF-8 | Zeolitic imidazolate framework-8 |
| CIP | Ciprofloxacin |
| MB | Methylene blue |
| DA | Dopamine |
| ATP | Adenosine triphosphate |
Author contributions
Yijia Xie: writing – original draft & editing. Jiaxin Ma: writing – review & editing, formal analysis. Huanhuan Liu: writing – review & editing, formal analysis. Zihao Teng: writing – formal analysis. Gang Liu: writing – review & editing, supervision, conceptualization.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This study was financially supported by the Postdoctoral Fellowship Program of CPSF (GZC20231414), the Major State Basic Research Development Program of China (No. 2017YFA0205201), the National Natural Science Foundation of China (81925019 and U1705281), the Fundamental Research Funds for the Central Universities (20720190088 and 20720200019), the Science Foundation of Fujian Province (2020Y4003 and 201812631010), and the Program for New Century Excellent Talents in University, China (No. NCET-13-0502).References
- A. Y. An, K. G. Choi and A. S. Baghela, et al., An Overview of Biological and Computational Methods for Designing Mechanism-Informed Anti-biofilm Agents, Front. Microbiol., 2021, 12, 640787 CrossRef PubMed.
- A. Singh, A. Amod and P. Pandey, et al., Bacterial biofilm infections, their resistance to antibiotics therapy and current treatment strategies, Biomed. Mater., 2022, 17(2), 35105823 CrossRef PubMed.
- J. L. del Pozo, Biofilm-related disease, Expert Rev. Anti-Infect. Ther., 2018, 16(1), 51–65 CrossRef CAS PubMed.
- Y. Kataoka, M. Kunimitsu and G. Nakagami, et al., Effectiveness of ultrasonic debridement on reduction of bacteria and biofilm in patients with chronic wounds: A scoping review, Int. Wound J., 2021, 18(2), 176–186 CrossRef PubMed.
- A. G. Goswami, S. Basu and T. Banerjee, et al., Biofilm and wound healing: from bench to bedside, Eur. J. Med. Res., 2023, 28(1), 157 CrossRef PubMed.
- X. N. Chen, Y. N. Shen and P. Y. Li, et al., Bacterial biofilms:Characteristics and combat strategies, Acta Pharm. Sin., 2018, 12, 103–112 Search PubMed.
- J. O'neill, Tackling drug-resistant infections globally: final report and recommendations, F, 2016.
- Y.-C. Yeh, T.-H. Huang and S.-C. Yang, et al., Nano-Based Drug Delivery or Targeting to Eradicate Bacteria for Infection Mitigation: A Review of Recent Advances, Front. Chem., 2020, 8, 286 CrossRef CAS PubMed.
- J. M. V. Makabenta, A. Nabawy and C.-H. Li, et al., Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections, Nat. Rev. Microbiol., 2021, 19(1), 23–36 CrossRef CAS PubMed.
- S. Mura, J. Nicolas and P. Couvreur, Stimuli-responsive nanocarriers for drug delivery, Nat. Mater., 2013, 12(11), 991–1003 CrossRef CAS PubMed.
- M. Jamal, W. Ahmad and S. Andleeb, et al., Bacterial biofilm and associated infections, J. Chin. Med. Assoc., 2018, 81(1), 7–11 CrossRef PubMed.
- A. Penesyan, I. T. Paulsen and S. Kjelleberg, et al., Three faces of biofilms: a microbial lifestyle, a nascent multicellular organism, and an incubator for diversity, npj Biofilms Microbiomes, 2021, 7(1), 80 CrossRef CAS PubMed.
- S. Sharma, J. Mohler and S. D. Mahajan, et al., Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment, Microorganisms, 2023, 11(6), 1614 CrossRef CAS PubMed.
- H.-C. Flemming and J. Wingender, The biofilm matrix, Nat. Rev. Microbiol., 2010, 8(9), 623–633 CrossRef CAS PubMed.
- S. Çam and İ. Badilli, The effect of NaCl, pH, and phosphate on biofilm formation and exopolysaccharide production by high biofilm producers of Bacillus strains, Folia Microbiol., 2024, 69(3), 613–624 CrossRef PubMed.
- Z. Shanshan, S. Jing and W. Jiajun, et al., Formation of Bacterial Biofilm and Anti-Biofilm Activity of Antimicrobial Peptides, Chin. J. Anim. Nutr., 2021, 33(12), 6631–6640 Search PubMed.
- X. N. Chen, Y. N. Shen and P. Y. Li, et al., Bacterial biofilms: Characteristics and combat strategies, Acta Pharm. Sin., 2018, 53, 2040–2049 Search PubMed.
- C. Mottola, J. J. Mendes and J. M. Cristino, et al., Polymicrobial biofilms by diabetic foot clinical isolates, Folia Microbiol., 2016, 61(1), 35–43 CrossRef CAS PubMed.
- W. Xiu, L. Wan and K. Yang, et al., Potentiating hypoxic microenvironment for antibiotic activation by photodynamic therapy to combat bacterial biofilm infections, Nat. Commun., 2022, 13(1), 3875 CrossRef CAS PubMed.
- J. D. Chambless, S. M. Hunt and P. S. Stewart, A three-dimensional computer model of four hypothetical mechanisms protecting biofilms from antimicrobials, Appl. Environ. Microbiol., 2006, 72(3), 2005–2013 CrossRef CAS PubMed.
- M. L. Immordino, F. Dosio and L. Cattel, Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential, Int. J. Nanomed., 2006, 1(3), 297–315 CrossRef CAS PubMed.
- Y. Tang, X. Wang and J. Li, et al., Overcoming the Reticuloendothelial System Barrier to Drug Delivery with a “Don't-Eat-Us” Strategy, ACS Nano, 2019, 13(11), 13015–13026 CrossRef CAS PubMed.
- V. S. Solleti, M. Alhariri and M. Halwani, et al., Antimicrobial properties of liposomal azithromycin for Pseudomonas infections in cystic fibrosis patients, J. Antimicrob. Chemother., 2015, 70(3), 784–796 CrossRef CAS PubMed.
- H. Nsairat, W. Alshaer and F. Odeh, et al., Recent advances in using liposomes for delivery of nucleic acid-based therapeutics, OpenNano, 2023, 11, 100132 CrossRef CAS.
- I. Munaweera, S. Shaikh and D. Maples, et al., Temperature-sensitive liposomal ciprofloxacin for the treatment of biofilm on infected metal implants using alternating magnetic fields, Int. J. Hyperthermia, 2018, 34(2), 189–200 CrossRef CAS PubMed.
- J. J. Verhoef and T. J. Anchordoquy, Questioning the Use of PEGylation for Drug Delivery, Drug Delivery Transl. Res., 2013, 3(6), 499–503 CrossRef CAS PubMed.
- M. Kluzek, Y. Oppenheimer-Shaanan and T. Dadosh, et al., Designer Liposomic Nanocarriers Are Effective Biofilm Eradicators, ACS Nano, 2022, 16(10), 15792–15804 CrossRef CAS PubMed.
- Z. Zhou, F. Hu and S. Hu, et al., pH-Activated nanoparticles with targeting for the treatment of oral plaque biofilm, J. Mater. Chem. B, 2018, 6(4), 586–592 RSC.
- Y. Zhao, X. Dai and X. Wei, et al., Near-Infrared Light-Activated Thermosensitive Liposomes as Efficient Agents for Photothermal and Antibiotic Synergistic Therapy of Bacterial Biofilm, ACS Appl. Mater. Interfaces, 2018, 10(17), 14426–14437 CrossRef CAS PubMed.
- S. Afrasiabi, A. Partoazar and N. Chiniforush, In vitro study of nanoliposomes containing curcumin and doxycycline for enhanced antimicrobial photodynamic therapy against Aggregatibacter actinomycetemcomitans, Sci. Rep., 2023, 13(1), 11552 CrossRef CAS PubMed.
- P. Cressey, L. G. Bronstein and R. Benmahmoudi, et al., Novel liposome-like assemblies composed of phospholipid-porphyrin conjugates with photothermal and photodynamic activities against bacterial biofilms, Int. J. Pharm., 2022, 623, 121915 CrossRef CAS PubMed.
- W. Xiu, H. Dong and X. Chen, et al., Metabolic Modulation-Mediated Antibiotic and Immune Activation for Treatment of Chronic Lung Infections, ACS Nano, 2024, 18(23), 15204–15217 CrossRef CAS PubMed.
- E. N. Taylor, K. M. Kummer and D. Dyondi, et al., Multi-scale strategy to eradicate Pseudomonas aeruginosa on surfaces using solid lipid nanoparticles loaded with free fatty acids, Nanoscale, 2014, 6(2), 825–832 RSC.
- R. Tenchov, R. Bird and A. E. Curtze, et al., Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement, ACS Nano, 2021, 15(11), 16982–17015 CrossRef CAS PubMed.
- A. Radaic, E. Malone and P. Kamarajan, et al., Solid Lipid Nanoparticles Loaded with Nisin (SLN-Nisin) are More Effective Than Free Nisin as Antimicrobial, Antibiofilm, and Anticancer Agents, J. Biomed. Nanotechnol., 2022, 18(4), 1227–1235 CrossRef CAS PubMed.
- Z. Ehsan and J. P. Clancy, Management of Pseudomonas aeruginosa infection in cystic fibrosis patients using inhaled antibiotics with a focus on nebulized liposomal amikacin, Future Microbiol., 2015, 10(12), 1901–1912 CrossRef CAS PubMed.
- M. Alhajlan, M. Alhariri and A. Omri, Efficacy and safety of liposomal clarithromycin and its effect on Pseudomonas aeruginosa virulence factors, Antimicrob. Agents Chemother., 2013, 57(6), 2694–2704 CrossRef CAS PubMed.
- K. Ahmed, P. W. Muiruri and G. H. Jones, et al., The effect of grafted poly(ethylene glycol) on the electrophoretic properties of phospholipid liposomes and their adsorption to bacterial biofilms, Colloids Surf., A, 2001, 194, 287–296 CrossRef CAS.
- Y. Meng, X. Hou and J. Lei, et al., Multi-functional Liposomes Enhancing Target and Antibacterial Immunity for Antimicrobial and Anti-Biofilm Against Methicillin-Resistant Staphylococcus aureus, Pharm. Res., 2016, 33(3), 763–775 CrossRef CAS PubMed.
- Y. Zhang, Y. Zhao and D. Dong, et al., Effects of isosorbide mononitrate loaded nanoparticles conjugated with anti-Staphylococcus aureus α-toxin on Staphylococcus aureus biofilms, Exp. Ther. Med., 2020, 19(2), 1267–1274 Search PubMed.
- E. N. Taylor, K. M. Kummer and D. Dyondi, et al., Multi-scale strategy to eradicate Pseudomonas aeruginosa on surfaces using solid lipid nanoparticles loaded with free fatty acids, Nanoscale, 2014, 6(2), 825–832 RSC.
- N. Nafee, D. M. Gaber and A. Abouelfetouh, et al., Enzyme-Linked Lipid Nanocarriers for Coping Pseudomonal Pulmonary Infection. Would Nanocarriers Complement Biofilm Disruption or Pave Its Road?, Int. J. Nanomed., 2024, 19, 3861–3890 CrossRef PubMed.
- T. Shi and Y. Chen, Preparation of cell membrane-coated nanoparticles and its application to antimicrobial, J. Shanghai Jiaotong Univ., Med. Sci., 2021, 41(7), 953–958 Search PubMed.
- J. Xie, X. Liu and G. Liu, A new track for antibacterial treatment: Progress and challenges of using cytomembrane-based vesicles to combat bacteria, Particuology, 2023, 78, 136–146 CrossRef CAS.
- Q. V. Le, J. Lee and H. Lee, et al., Cell membrane-derived vesicles for delivery of therapeutic agents, Acta Pharm. Sin. B, 2021, 11(8), 2096–2113 CrossRef CAS PubMed.
- W. Huang, L. Meng and Y. Chen, et al., Bacterial outer membrane vesicles as potential biological nanomaterials for antibacterial therapy, Acta Biomater., 2022, 140, 102–115 CrossRef CAS PubMed.
- L. Weng, L. Wu and R. Guo, et al., Lactobacillus cell envelope-coated nanoparticles for antibiotic delivery against cariogenic biofilm and dental caries, J. Nanobiotechnol., 2022, 20(1), 356 CrossRef CAS PubMed.
- X. Pang, X. Liu and Y. Cheng, et al., Sono-Immunotherapeutic Nanocapturer to Combat Multidrug-Resistant Bacterial Infections, Adv. Mater., 2019, 31(35), e1902530 CrossRef PubMed.
- L. Ding, X. Liang and J. Ma, et al., Sono-Triggered Biomimetically Nanoantibiotics Mediate Precise Sequential Therapy of MRSA-Induced Lung Infection, Adv. Mater., 2024, e2403612 CrossRef PubMed.
- J. Zhu, R. Xie and R. Gao, et al., Multimodal nanoimmunotherapy engages neutrophils to eliminate Staphylococcus aureus infections, Nat. Nanotechnol., 2024, 19(7), 1032–1043 CrossRef CAS PubMed.
- X. Peng, J. Chen and Y. Gan, et al., Biofunctional lipid nanoparticles for precision treatment and prophylaxis of bacterial infections, Sci. Adv., 2024, 10(14), eadk9754 CrossRef CAS PubMed.
- F. Gao, L. Xu and B. Yang, et al., Kill the real with the fake: eliminate intracellular Staphylococcus aureus using nanoparticle coated with its extracellular vesicle membrane as active-targeting drug carrier, ACS Infect. Dis., 2018, 5(2), 218–227 CrossRef PubMed.
- W. Huang, Q. Zhang and W. Li, et al., Development of novel nanoantibiotics using an outer membrane vesicle-based drug efflux mechanism, J. Controlled Release, 2020, 317, 1–22 CrossRef CAS PubMed.
- X. Wei, B. Xue and S. Ruan, et al., Supercharged precision killers: Genetically engineered biomimetic drugs of screened metalloantibiotics against Acinetobacter baumanni, Sci. Adv., 2024, 10(12), eadk6331 CrossRef CAS PubMed.
- D. S. Raj, D. Dhamodharan and S. Thanigaivel, et al., Nanoemulsion as an Effective Inhibitor of Biofilm-forming Bacterial Associated Drug Resistance: An Insight into COVID Based Nosocomial Infections, Biotechnol. Bioprocess Eng., 2022, 27(4), 543–555 CrossRef CAS PubMed.
- S. Zhao, L. Zhang, Q. Liu, Q. Chen and B. Kong, Preparation, Antibacterial Mechanism of Natural Antimicrobial-Loaded Nanoemulsions and Their Application in Meat Preservation: A Literature Review, Meat Research, 2022, 36(4), 48–56 Search PubMed.
- Q. Liu, Y. Gao and X. Fu, et al., Preparation of peppermint oil nanoemulsions: Investigation of stability, antibacterial mechanism and apoptosis effects, Colloids Surf., B, 2021, 201, 111626 CrossRef CAS PubMed.
- H. Sun, D. Luo and S. Zheng, et al., Antimicrobial behavior and mechanism of clove oil nanoemulsion, J. Food Sci. Technol., 2022, 59(5), 1939–1947 CrossRef CAS PubMed.
- M. E. El-Naggar, A. M. Abdelgawad and R. Abdel-Sattar, et al., Potential antimicrobial and antibiofilm efficacy of essential oil nanoemulsion loaded polycaprolactone nanofibrous dermal patches, Eur. Polym. J., 2023, 184, 111782 CrossRef CAS.
- A. H. Hashem, A. S. Doghish and A. Ismail, et al., A novel nanoemulsion based on clove and thyme essential oils: Characterization, antibacterial, antibiofilm and anticancer activities, Electron. J. Biotechnol., 2024, 68, 20–30 CrossRef CAS.
- T. D. S. Araujo, J. da Costa and F. de Oliveira Silva Ribeiro, et al., Nanoemulsion of cashew gum and clove essential oil (Ocimum gratissimum Linn) potentiating antioxidant and antimicrobial activity, Int. J. Biol. Macromol., 2021, 193(Pt A), 100–108 CrossRef PubMed.
- K. Razdan, V. S. Gondil and S. Chhibber, et al., Levofloxacin loaded clove essential oil nanoscale emulsion as an efficient system against Pseudomonas aeruginosa biofilm, J. Drug Delivery Sci. Technol., 2022, 68, 103039 CrossRef CAS.
- H. H. Buzzá, F. Alves and A. J. B. Tomé, et al., Porphyrin nanoemulsion for antimicrobial photodynamic therapy: effective delivery to inactivate biofilm-related infections, Proc. Natl. Acad. Sci. U. S. A., 2022, 119(46), e2216239119 CrossRef PubMed.
- P. Niu, J. Dai and Z. Wang, et al., Sensitization of Antibiotic-Resistant Gram-negative Bacteria to Photodynamic Therapy via Perfluorocarbon Nanoemulsion, Pharmaceuticals, 2022, 15(2), 156 CrossRef CAS PubMed.
- R. Yang, H. Zhang and Z. Marfavi, et al., Infiltrating Perfluorocarbon Nanoemulsion and Sensitizing Ultrasound Cavitation to Eradicate Biofilms, ACS Appl. Mater. Interfaces, 2024, 16(3), 3126–3138 CrossRef CAS PubMed.
- A. Eqbal, V. A. Ansari and A. Hafeez, et al., Recent Applications of Nanoemulsion Based Drug Delivery System: A Review, Res. J. Pharm. Technol., 2021, 14, 2852–2858 Search PubMed.
- K. Ramalingam, N. C. Frohlich and V. A. Lee, Effect of nanoemulsion on dental unit waterline biofilm, J. Dent. Sci., 2013, 8(3), 333–336 CrossRef.
- P. Sahariah, M. Másson and R. L. Meyer, Quaternary Ammoniumyl Chitosan Derivatives for Eradication of Staphylococcus aureus Biofilms, Biomacromolecules, 2018, 19(9), 3649–3658 CrossRef CAS PubMed.
- F. Wu, G. Meng and J. He, et al., Antibiotic-loaded chitosan hydrogel with superior dual functions: antibacterial efficacy and osteoblastic cell responses, ACS Appl. Mater. Interfaces, 2014, 6(13), 10005–10013 CrossRef CAS PubMed.
- F. Wu, G. Meng and J. He, et al., Antibiotic-loaded chitosan hydrogel with superior dual functions: antibacterial efficacy and osteoblastic cell responses, ACS Appl. Mater. Interfaces, 2014, 6(13), 10005–10013 CrossRef CAS PubMed.
- R. C. Gonçalves, D. P. da Silva and R. Signini, et al., Inhibition of bacterial biofilms by carboxymethyl chitosan combined with silver, zinc and copper salts, Int. J. Biol. Macromol., 2017, 105(Pt 1), 385–392 CrossRef PubMed.
- Y. Tan, S. Ma and M. Leonhard, et al., Enhancing antibiofilm activity with functional chitosan nanoparticles targeting biofilm cells and biofilm matrix, Carbohydr. Polym., 2018, 200, 35–42 CrossRef CAS PubMed.
- J. Cao, Y. Zhao and Y. Liu, et al., Phosphorylcholine-Based Polymer Encapsulated Chitosan Nanoparticles Enhance the Penetration of Antimicrobials in a Staphylococcal Biofilm, ACS Macro Lett., 2019, 8(6), 651–657 CrossRef CAS PubMed.
- Y. Su, V. L. Mainardi and H. Wang, et al., Dissolvable Microneedles Coupled with Nanofiber Dressings Eradicate Biofilms via Effectively Delivering a Database-Designed Antimicrobial Peptide, ACS Nano, 2020, 14(9), 11775–11786 CrossRef CAS PubMed.
- N. K. Grilc, A. Zidar and P. Kocbek, et al., Nanofibers with genotyped Bacillus strains exhibiting antibacterial and immunomodulatory activity, J. Controlled Release, 2023, 355, 371–384 CrossRef CAS PubMed.
- N. Thomas, C. Thorn and K. Richter, et al., Efficacy of Poly-Lactic-Co-Glycolic Acid Micro- and Nanoparticles of Ciprofloxacin Against Bacterial Biofilms, J. Pharm. Sci., 2016, 105(10), 3115–3122 CrossRef CAS PubMed.
- A. Anjum, P. Y. Chung and S. F. Ng, PLGA/xylitol nanoparticles enhance antibiofilm activity via penetration into biofilm extracellular polymeric substances, RSC Adv., 2019, 9(25), 14198–14208 RSC.
- R. A. Harper, G. H. Carpenter and G. B. Proctor, et al., Diminishing biofilm resistance to antimicrobial nanomaterials through electrolyte screening of electrostatic interactions, Colloids Surf., B, 2019, 173, 392–399 CrossRef CAS PubMed.
- Y. Liu, H. J. Busscher and B. Zhao, et al., Surface-Adaptive, Antimicrobially Loaded, Micellar Nanocarriers with Enhanced Penetration and Killing Efficiency in Staphylococcal Biofilms, ACS Nano, 2016, 10(4), 4779–4789 CrossRef CAS PubMed.
- Z. Huang, T. Zhou and Y. Yuan, et al., Synthesis of carbon quantum dot-poly lactic-co-glycolic acid hybrid nanoparticles for chemo-photothermal therapy against bacterial biofilms, J. Colloid Interface Sci., 2020, 577, 66–74 CrossRef CAS PubMed.
- V. Gajbhiye, V. K. Palanirajan and R. K. Tekade, et al., Dendrimers as therapeutic agents: a systematic review, J. Pharm. Pharmacol., 2009, 61(8), 989–1003 CrossRef PubMed.
- A. Santos, F. Veiga and A. Figueiras, Dendrimers as Pharmaceutical Excipients: Synthesis, Properties, Toxicity and Biomedical Applications, Materials, 2019, 13(1), 65 CrossRef PubMed.
- R. Kharwade, S. More and A. Warokar, et al., Starburst pamam dendrimers: Synthetic approaches, surface modifications, and biomedical applications, Arabian J. Chem., 2020, 13(7), 6009–6039 CrossRef CAS.
- M. Bergmann, G. Michaud and R. Visini, et al., Multivalency effects on Pseudomonas aeruginosa biofilm inhibition and dispersal by glycopeptide dendrimers targeting lectin LecA, Org. Biomol. Chem., 2016, 14(1), 138–148 RSC.
- Y. Gao, J. Wang and M. Chai, et al., Size and Charge Adaptive Clustered Nanoparticles Targeting the Biofilm Microenvironment for Chronic Lung Infection Management, ACS Nano, 2020, 14(5), 5686–5699 CrossRef CAS PubMed.
- A. Pamukçu, N. Erdoğan and D. Şen Karaman, Polyethylenimine-grafted mesoporous silica nanocarriers markedly enhance the bactericidal effect of curcumin against Staphylococcus aureus biofilm, J. Biomed. Mater. Res., Part B, 2022, 110(11), 2506–2520 CrossRef PubMed.
- Y. Chen, Y. Gao and Y. Huang, et al., Inhibiting Quorum Sensing by Active Targeted pH-Sensitive Nanoparticles for Enhanced Antibiotic Therapy of Biofilm-Associated Bacterial Infections, ACS Nano, 2023, 17(11), 10019–10032 CrossRef CAS PubMed.
- Y. Zhou, W. Huang and J. Liu, et al., Self-assembly of hyperbranched polymers and its biomedical applications, Adv. Mater., 2010, 22(41), 4567–4590 CrossRef CAS PubMed.
- A. Labena, K. I. Kabel and R. K. Farag, One-pot synthesize of dendritic hyperbranched PAMAM and assessment as a broad spectrum antimicrobial agent and anti-biofilm, Mater. Sci. Eng., C, 2016, 58, 1150–1159 CrossRef CAS PubMed.
- L. Yang, F. Teles and W. Gong, et al., Antibacterial action of nitric oxide-releasing hyperbranched polymers against ex vivo dental biofilms, Dent. Mater., 2020, 36(5), 635–644 CrossRef CAS PubMed.
- S. Yu, G. Li and R. Liu, et al., Dendritic Fe3O4@Poly(dopamine)@PAMAM Nanocomposite as Controllable NO-Releasing Material: A Synergistic Photothermal and NO Antibacterial Study, Adv. Funct. Mater., 2018, 28(20), 1707440 CrossRef.
- J. M. Caster, A. N. Patel and T. Zhang, et al., Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2017, 9(1), 27312983 Search PubMed.
- S. Akhtar, B. Al-Zaid and A. Z. El-Hashim, et al., Impact of PAMAM delivery systems on signal transduction pathways in vivo: Modulation of ERK1/2 and p38 MAP kinase signaling in the normal and diabetic kidney, Int. J. Pharm., 2016, 514(2), 353–363 CrossRef CAS PubMed.
- C. F. Price, D. Tyssen and S. Sonza, et al., SPL7013 Gel (VivaGel®) retains potent HIV-1 and HSV-2 inhibitory activity following vaginal administration in humans, PLoS One, 2011, 6(9), e24095 CrossRef CAS PubMed.
- Y. Kim, E. J. Park and D. H. Na, Recent progress in dendrimer-based nanomedicine development, Arch. Pharmacal Res., 2018, 41(6), 571–582 CrossRef CAS PubMed.
- N. Hazime, Y. Belguesmia and A. Barras, et al., Enhanced Antibacterial Activity of Dermaseptin through Its Immobilization on Alginate Nanoparticles-Effects of Menthol and Lactic Acid on Its Potentialization, Antibiotics, 2022, 11(6), 787 CrossRef CAS PubMed.
- L. Spósito, D. Fonseca and S. Gonçalves Carvalho, et al., Engineering resveratrol-loaded chitosan nanoparticles for potential use against Helicobacter pylori infection, Eur. J. Pharm. Biopharm., 2024, 199, 114280 CrossRef PubMed.
- L. Yang, F. Teles and W. Gong, et al., Antibacterial action of nitric oxide-releasing hyperbranched polymers against ex vivo dental biofilms, Dent. Mater., 2020, 36(5), 635–644 CrossRef CAS PubMed.
- M. C. Jennings, L. E. Ator and T. J. Paniak, et al., Biofilm-eradicating properties of quaternary ammonium amphiphiles: simple mimics of antimicrobial peptides, ChemBioChem, 2014, 15(15), 2211–2215 CrossRef CAS PubMed.
- H. Haidari, Z. Kopecki and R. Bright, et al., Ultrasmall AgNP-Impregnated Biocompatible Hydrogel with Highly Effective Biofilm Elimination Properties, ACS Appl. Mater. Interfaces, 2020, 12(37), 41011–41025 CrossRef CAS PubMed.
- J. Hu, C. Zhang and L. Zhou, et al., A smart hydrogel for on-demand delivery of antibiotics and efficient eradication of biofilms, Sci. China Mater., 2021, 64(4), 1035–1046 CrossRef CAS.
- S. Manna, A. K. Ghosh and S. M. Mandal, Curd-Peptide Based Novel Hydrogel Inhibits Biofilm Formation, Quorum Sensing, Swimming Mortility of Multi-Antibiotic Resistant Clinical Isolates and Accelerates Wound Healing Activity, Front. Microbiol., 2019, 10, 951 CrossRef PubMed.
- V. O. Fasiku, C. A. Omolo and N. Devnarain, et al., Chitosan-Based Hydrogel for the Dual Delivery of Antimicrobial Agents Against Bacterial Methicillin-Resistant Staphylococcus aureus Biofilm-Infected Wounds, ACS Omega, 2021, 6(34), 21994–22010 CrossRef CAS PubMed.
- T. Du, Z. Xiao and G. Zhang, et al., An injectable multifunctional hydrogel for eradication of bacterial biofilms and wound healing, Acta Biomater., 2023, 161, 112–133 CrossRef CAS PubMed.
- S. Liang, L. Xiao and Y. Fang, et al., A nanocomposite hydrogel for co-delivery of multiple anti-biofilm therapeutics to enhance the treatment of bacterial biofilm-related infections, Int. J. Pharm., 2024, 649, 123638 CrossRef CAS PubMed.
- H. Chen, J. Cheng and X. Cai, et al., pH-Switchable Antimicrobial Supramolecular Hydrogels for Synergistically Eliminating Biofilm and Promoting Wound Healing, ACS Appl. Mater. Interfaces, 2022, 14(16), 18120–18132 CrossRef CAS PubMed.
- Z. Liu, K. Guo and L. Yan, et al., Janus nanoparticles targeting extracellular polymeric substance achieve flexible elimination of drug-resistant biofilms, Nat. Commun., 2023, 14(1), 5132 CrossRef CAS PubMed.
- K. Richter, N. Thomas and J. Claeys, et al., A Topical Hydrogel with Deferiprone and Gallium-Protoporphyrin Targets Bacterial Iron Metabolism and Has Antibiofilm Activity, Antimicrob. Agents Chemother., 2017, 61(6), e00481–e00417 Search PubMed.
- S. M. Dizaj, F. Lotfipour and M. Barzegar-Jalali, et al., Antimicrobial activity of the metals and metal oxide nanoparticles, Mater. Sci. Eng., C, 2014, 44, 278–284 CrossRef CAS PubMed.
- H. Motamedi, S. Khademi Mazdeh and A. Akbarzadeh Khiavi, et al., Optimization of Gold Nanoparticle Biosynthesis by Escherichiacoli DH5α and its Conjugation with Gentamicin, J. Nano Res., 2015, 32, 105–193 Search PubMed.
- B. A. Hasoon, K. H. Jawad and S. S. Abdulsahib, Synthesis of Ciprofloxacin-Conjugated Gold Nanoparticles and their Study Antibacterial Effects on Growth Biofilm Formation Through Nebulizer Mask Against Respiratory Infection, Plasmonics, 2023, 1–15 Search PubMed.
- C. Zhang, D. T. Shi and K. C. Yan, et al., A glycoconjugate-based gold nanoparticle approach for the targeted treatment of Pseudomonas aeruginosa biofilms, Nanoscale, 2020, 12(45), 23234–23240 RSC.
- B. Le Ouay and F. Stellacci, Antibacterial activity of silver nanoparticles: A surface science insight, Nano Today, 2015, 10(3), 339–354 CrossRef CAS.
- K. Zheng, M. I. Setyawati and D. T. Leong, et al., Antimicrobial silver nanomaterials, Coord. Chem. Rev., 2018, 357, 1–17 CrossRef CAS.
- G. A. Pang, J. Laufer and R. Niessner, et al., Photoacoustic Signal Generation in Gold Nanospheres in Aqueous Solution: Signal Generation Enhancement and Particle Diameter Effects, J. Phys. Chem. C, 2016, 120, 27646–27656 CrossRef CAS.
- Z. Wang, L. Chen and Z. Chu, et al., Gemcitabine-loaded gold nanospheres mediated by albumin for enhanced anti-tumor activity combining with CT imaging, Mater. Sci. Eng., C, 2018, 89, 106–118 CrossRef CAS PubMed.
- W. Li, H. Zhang and X. Guo, et al., Gold Nanospheres-Stabilized Indocyanine Green as a Synchronous Photodynamic-Photothermal Therapy Platform That Inhibits Tumor Growth and Metastasis, ACS Appl. Mater. Interfaces, 2017, 9(4), 3354–3367 CrossRef CAS PubMed.
- L. An, Y. Wang and Q. Tian, et al., Small Gold Nanorods: Recent Advances in Synthesis, Biological Imaging, and Cancer Therapy, Materials, 2017, 10(12), 1372 CrossRef PubMed.
- Y.-S. Chen, Y. Zhao and S. J. Yoon, et al., Miniature gold nanorods for photoacoustic molecular imaging in the second near-infrared optical window, Nat. Nanotechnol., 2019, 14(5), 465–472 CrossRef CAS PubMed.
- O. B. Knights, S. Ye and N. Ingram, et al., Optimising gold nanorods for photoacoustic imaging in vitro, Nanoscale Adv., 2019, 1(4), 1472–1481 RSC.
- F. Ratto, S. Centi and C. Avigo, et al., A Robust Design for Cellular Vehicles of Gold Nanorods for Multimodal Imaging, Adv. Funct. Mater., 2016, 26(39), 7178–7185 CrossRef CAS.
- Y. Liu, H. Yuan and A. Fales, et al., Multifunctional gold nanostars for molecular imaging and cancer therapy, Front. Chem., 2015, 3(3), 51 Search PubMed.
- F. Xia, J. Niu and Y. Hong, et al., Matrix metallopeptidase 2 targeted delivery of gold nanostars decorated with IR-780 iodide for dual-modal imaging and enhanced photothermal/photodynamic therapy, Acta Biomater., 2019, 89, 289–299 CrossRef CAS PubMed.
- W. Shang, C. Zeng and Y. Du, et al., Core-Shell Gold Nanorod@Metal-Organic Framework Nanoprobes for Multimodality Diagnosis of Glioma, Adv. Mater., 2017, 29(3), 1604381 CrossRef PubMed.
- Z. Qin, Y. Zheng and T. Du, et al., Cysteamine: A key to trigger aggregation-induced NIR-II photothermal effect and silver release booming of gold-silver nanocages for synergetic treatment of multidrug-resistant bacteria infection, Chem. Eng. J., 2021, 414, 128779 CrossRef CAS.
- B. Pang, X. Yang and Y. Xia, Putting gold nanocages to work for optical imaging, controlled release and cancer theranostics, Nanomedicine, 2016, 11(13), 1715–1728 CrossRef CAS PubMed.
- K. H. Jawad, Laser ablation mediated ZnO nanoparticles inhibit growth and biofilm forming potential of urinary tract bacterium Proteus mirabilis, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2023, 14(1), 015002 Search PubMed.
- F. Lin, C. Li and Z. Chen, Bacteria-Derived Carbon Dots Inhibit Biofilm Formation of Escherichia coli without Affecting Cell Growth, Front. Microbiol., 2018, 9, 259 CrossRef PubMed.
- W. H. Lee, R. Rohanizadeh and C. Y. Loo, In situ functionalizing calcium phosphate biomaterials with curcumin for the prevention of bacterial biofilm infections, Colloids Surf., B, 2021, 206, 111938 CrossRef CAS PubMed.
- C. H. N. Barros, H. Devlin and D. W. Hiebner, et al., Enhancing curcumin's solubility and antibiofilm activity via silica surface modification, Nanoscale Adv., 2020, 2(4), 1694–1708 RSC.
- H. Yan, H. Yang and K. Li, et al., Effects of Chlorhexidine-Encapsulated Mesoporous Silica Nanoparticles on the Anti-Biofilm and Mechanical Properties of Glass Ionomer Cement, Molecules, 2017, 22(7), 1225 CrossRef PubMed.
- Y. Wang, Z. Wang and K. Xie, et al., High-Efficiency Cellular Reprogramming by Nanoscale Puncturing, Nano Lett., 2020, 20(7), 5473–5481 CrossRef CAS PubMed.
- R. Li, T. Chen and X. Pan, Metal-Organic-Framework-Based Materials for Antimicrobial Applications, ACS Nano, 2021, 15(3), 3808–3848 CrossRef CAS PubMed.
- S. A. Polash, T. Khare and V. Kumar, et al., Prospects of Exploring the Metal–Organic Framework for Combating Antimicrobial Resistance, ACS Appl. Bio Mater., 2021, 4(12), 8060–8079 CrossRef CAS PubMed.
- C. Pettinari, R. Pettinari and C. Di Nicola, et al., Antimicrobial MOFs, Coord. Chem. Rev., 2021, 446, 214121 CrossRef CAS.
- F. Akbarzadeh, M. Motaghi and N. P. S. Chauhan, et al., A novel synthesis of new antibacterial nanostructures based on Zn-MOF compound: design, characterization and a high performance application, Heliyon, 2020, 6(1), e03231 CrossRef PubMed.
- A. Arenas-Vivo, G. Amariei and S. Aguado, et al., An Ag-loaded photoactive nano-metal organic framework as a promising biofilm treatment, Acta Biomater., 2019, 97, 490–500 CrossRef CAS PubMed.
- P. Raju, T. Ramalingam and T. Nooruddin, et al., In vitro assessment of antimicrobial, antibiofilm and larvicidal activities of bioactive nickel metal organic framework, J. Drug Delivery Sci. Technol., 2020, 56, 101560 CrossRef CAS.
- Y.-L. Xiang, S.-H. Huang and D.-Y. Tang, et al., MOF/polypeptide hybrid nanocomposites with pH/lipase dual responsive drug release and photodynamic effect for combination treatment of drug-resistant bacterial infection, Mater. Des., 2023, 232, 112180 CrossRef CAS.
- M. Ding, W. Zhao and X. Zhang, et al., Charge-switchable MOF nanocomplex for enhanced biofilm penetration and eradication, J. Hazard. Mater., 2022, 439, 129594 CrossRef CAS PubMed.
- Q. Deng, P. Sun and L. Zhang, et al., Porphyrin MOF Dots–Based, Function–Adaptive Nanoplatform for Enhanced Penetration and Photodynamic Eradication of Bacterial Biofilms, Adv. Funct. Mater., 2019, 29(30), 1903018 CrossRef.
- J. Yang, K. Li and J. Gu, Hierarchically Macro-Microporous Ce-Based MOFs for the Cleavage of DNA, ACS Mater. Lett., 2022, 4(2), 385–391 CrossRef CAS.
- F. Xia, K. Li and J. Yang, et al., DNase-mimetics based on bimetallic hierarchically macroporous MOFs for the efficient inhibition of bacterial biofilm, Sci. China Mater., 2024, 67(1), 343–354 CrossRef CAS.
- Z. Liu, F. Wang and J. Ren, et al., A series of MOF/Ce-based nanozymes with dual enzyme-like activity disrupting biofilms and hindering recolonization of bacteria, Biomaterials, 2019, 208, 21–31 CrossRef CAS PubMed.
- X. Yuan, J. Xiong and X. Wu, et al., Ultrasmall Ce-based metal–organic frameworks nanozyme with hydrolytic activity for boosting antibiofilm therapy, Chem. Eng. J., 2024, 480, 148246 CrossRef CAS.
- X. Ren, L. Chang and Y. Hu, et al., Au@MOFs used as peroxidase-like catalytic nanozyme for bacterial infected wound healing through bacterial membranes disruption and protein leakage promotion, Mater. Des., 2023, 229, 111890 CrossRef CAS.
- H. Qiu, F. Pu and Z. Liu, et al., Depriving Bacterial Adhesion-Related Molecule to Inhibit Biofilm Formation Using CeO(2) -Decorated Metal-Organic Frameworks, Small, 2019, 15(36), e1902522 CrossRef PubMed.
- Y. Zang, T. R. Roberts and A. I. Batchinsky, et al., Metal-Organic Framework Polymer Coating Inhibits Staphylococcus aureus Attachment on Medical Circulation Tubing under Static and Dynamic Flow Conditions, ACS Appl. Bio Mater., 2020, 3(6), 3535–3543 CrossRef CAS PubMed.
- Z. Wang, P. Zhang and C. Yin, et al., Antibiotic–Derived Carbon–Nanodot–Decorated Hydrogel for Reactive Oxygen Species–Enhanced Anti–Infection Through Biofilm Damage, Adv. Funct. Mater., 2023, 33(29), 2300341 CrossRef CAS.
- Y. Peng, S. Pang and Y. Zeng, et al., Antibiotic-free ocular sterilization while suppressing immune response to protect corneal transparency in infectious keratitis treatment, J. Controlled Release, 2024, 374, 563–576 CrossRef CAS PubMed.
Footnote |
| † These authors have contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |