Fluffy mesoporous Al2O3 supported Ag–In2O3 schottky junction catalysts for selective hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e001.gif) O of α,β-unsaturated aldehydes†
O of α,β-unsaturated aldehydes†
Jiasheng
Wang
 *ab,
Tianyu
Zhang
ab,
Jiliang
Song
ab,
Fengxin
Zhang
ab,
Hong
Liu
ab,
Wan-Hui
Wang
*ab,
Tianyu
Zhang
ab,
Jiliang
Song
ab,
Fengxin
Zhang
ab,
Hong
Liu
ab,
Wan-Hui
Wang
 ab and
Ming
Bao
ab and
Ming
Bao
 *ab
*ab
aState Key Laboratory of Fine Chemicals, Dalian University of Technology, Dalian, 116024, China. E-mail: jswang@dlut.edu.cn; mingbao@dlut.edu.cn
bSchool of Chemical Engineering, Ocean and Life Sciences, Dalian University of Technology, Panjin, 124221, China
First published on 5th March 2025
Abstract
Unsaturated alcohols (UOLs) are important fine chemical intermediates. Thus, it is of great significance to design and prepare catalysts for highly selective hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of α,β-unsaturated aldehydes (UALs). In this paper, a fluffy mesoporous Al2O3-supported Ag–In2O3 catalyst (Ag-In2O3/f-m-Al2O3) was synthesized by employing a two-solvent method, in which Ag and In2O3 form a Mott–Schottky junction and lead to electron transfer from In2O3 to Ag. Electron-rich Ag repels the C
O of α,β-unsaturated aldehydes (UALs). In this paper, a fluffy mesoporous Al2O3-supported Ag–In2O3 catalyst (Ag-In2O3/f-m-Al2O3) was synthesized by employing a two-solvent method, in which Ag and In2O3 form a Mott–Schottky junction and lead to electron transfer from In2O3 to Ag. Electron-rich Ag repels the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond owing to “four-electron repulsion”, and electron-deficient In2O3 acts as the electrophilic site to adsorb the O atom of the C
C bond owing to “four-electron repulsion”, and electron-deficient In2O3 acts as the electrophilic site to adsorb the O atom of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, thus improving the selectivity towards UOLs. In addition to a larger specific surface area and smaller mass transfer resistance, the fluffy mesopore Al2O3 exhibits a large number of Lewis acid sites, which can further improve UOL selectivity. With the help of Ag–In2O3/f-m-Al2O3, high UOL selectivity can be obtained from UALs containing aliphatic, aromatic and heterocyclic groups. This elaborate design of the catalyst could contribute to the highly selective hydrogenation of UALs to UOLs.
O bond, thus improving the selectivity towards UOLs. In addition to a larger specific surface area and smaller mass transfer resistance, the fluffy mesopore Al2O3 exhibits a large number of Lewis acid sites, which can further improve UOL selectivity. With the help of Ag–In2O3/f-m-Al2O3, high UOL selectivity can be obtained from UALs containing aliphatic, aromatic and heterocyclic groups. This elaborate design of the catalyst could contribute to the highly selective hydrogenation of UALs to UOLs.
1. Introduction
Unsaturated alcohols (UOLs), as important organic intermediates, are widely used to synthesize organic chemicals, such as resins, spices, medicines, surfactants and food additives.1–3 Selective hydrogenation of α,β-unsaturated aldehydes (UALs) is one of the important methods to prepare unsaturated alcohols.4–6 However, because the activation energy of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond is lower than that of the C
C bond is lower than that of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, it is difficult to achieve the preferential hydrogenation of the C
O bond, it is difficult to achieve the preferential hydrogenation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond.7–9
O bond.7–9
To achieve the selective hydrogenation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, it is necessary to design catalysts that can inhibit the hydrogenation of the C
O bond, it is necessary to design catalysts that can inhibit the hydrogenation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and promote the hydrogenation of the C
C bond and promote the hydrogenation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond.10–13 The adsorption mode of UALs determines the selectivity of the product. According to Delbecq and Sautet's work,14,15 there are nine possible adsorption modes for UALs on the catalyst surface, as shown in Fig. S1.† Among them, the η2 diσCO adsorption mode, in which the C and O of the C
O bond.10–13 The adsorption mode of UALs determines the selectivity of the product. According to Delbecq and Sautet's work,14,15 there are nine possible adsorption modes for UALs on the catalyst surface, as shown in Fig. S1.† Among them, the η2 diσCO adsorption mode, in which the C and O of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond are simultaneously adsorbed, is considered the best adsorption mode for the selective hydrogenation of UALs to UOLs.
O bond are simultaneously adsorbed, is considered the best adsorption mode for the selective hydrogenation of UALs to UOLs.
Because the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond is a polar bond, to realize the η2 diσCO adsorption mode, catalysts with both electrophilic and nucleophilic sites are more advantageous.16 Therefore, frustrated Lewis pairs (FLPs) with Lewis acids and bases, which fail to form classical Lewis acid–base sites owing to steric hindrance, have been used in recent years to catalyse this reaction to obtain high selectivity but usually need complex procedures.17–19 Theoretically, catalysts with oppositely charged sites can also simultaneously adsorb C and O of C
O bond is a polar bond, to realize the η2 diσCO adsorption mode, catalysts with both electrophilic and nucleophilic sites are more advantageous.16 Therefore, frustrated Lewis pairs (FLPs) with Lewis acids and bases, which fail to form classical Lewis acid–base sites owing to steric hindrance, have been used in recent years to catalyse this reaction to obtain high selectivity but usually need complex procedures.17–19 Theoretically, catalysts with oppositely charged sites can also simultaneously adsorb C and O of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and meanwhile induce heterolysis of hydrogen to polar active hydrogen ions, which is conducive to the hydrogenation of polar C
O and meanwhile induce heterolysis of hydrogen to polar active hydrogen ions, which is conducive to the hydrogenation of polar C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds.20–22 Therefore, bimetallic catalysts have drawn much attention, where platinum group metals (PGMs) with high electronegativity are often needed.23–26 To avoid the use of expensive PGMs,27,28 Schottky junctions (M-MOX) formed by the contact and electron transfer between metals and semiconductors (such as metal oxides) have become an ideal candidate owing to their oppositely charged components.29 Among the two types of Schottky junctions, metal–n-type semiconductors and metal–p-type semiconductors, the former is a better choice because it can create an electron-enriched metal centre via electron transfer from the n-type semiconductor to the active metal.30 The electron-enriched active metal strengthens the four-electron repulsion to C
O bonds.20–22 Therefore, bimetallic catalysts have drawn much attention, where platinum group metals (PGMs) with high electronegativity are often needed.23–26 To avoid the use of expensive PGMs,27,28 Schottky junctions (M-MOX) formed by the contact and electron transfer between metals and semiconductors (such as metal oxides) have become an ideal candidate owing to their oppositely charged components.29 Among the two types of Schottky junctions, metal–n-type semiconductors and metal–p-type semiconductors, the former is a better choice because it can create an electron-enriched metal centre via electron transfer from the n-type semiconductor to the active metal.30 The electron-enriched active metal strengthens the four-electron repulsion to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C.31,32 Meanwhile, the electron-deficient metal oxide serves as an electrophilic site promoting C
C.31,32 Meanwhile, the electron-deficient metal oxide serves as an electrophilic site promoting C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O adsorption.33 For metal–p-type semiconductor Schottky catalyst without such a repulsion, the hydrogenation of the C
O adsorption.33 For metal–p-type semiconductor Schottky catalyst without such a repulsion, the hydrogenation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond is preferential, as reported by Ren et al.34
C bond is preferential, as reported by Ren et al.34
Ag was chosen as the active metal because it has shown unique high selectivity for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond hydrogenation of UAL.35–37 Tian et al. also found that Ag had a good adsorption and activation effect on the C
O bond hydrogenation of UAL.35–37 Tian et al. also found that Ag had a good adsorption and activation effect on the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond.38 In2O3 was chosen as the promoter because it is a typical n-type semiconductor that is easy to donate electrons.30,39–41
O bond.38 In2O3 was chosen as the promoter because it is a typical n-type semiconductor that is easy to donate electrons.30,39–41
Theoretically, Lewis acidic support can improve the selectivity of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O owing to the electrophilicity of the empty orbitals.42 Thus, Lewis acidic oxide Al2O3 was selected as the support. Furthermore, it should be better to make the Al2O3 fluffy,43 mesoporous,44 and amorphous.45 First, the specific surface area and pore size can be greatly increased, which can reduce the mass transfer resistance. Second, more defects (mainly oxygen vacancies) can be created when crystallinity is destroyed. Oxygen vacancies tend to adsorb the O in oxygen-containing functional groups.46 Moreover, the absence of O produces coordination unsaturated Al, increasing the number of empty orbitals and thus enhancing Lewis acidity, which further enhances electrophilicity.
O owing to the electrophilicity of the empty orbitals.42 Thus, Lewis acidic oxide Al2O3 was selected as the support. Furthermore, it should be better to make the Al2O3 fluffy,43 mesoporous,44 and amorphous.45 First, the specific surface area and pore size can be greatly increased, which can reduce the mass transfer resistance. Second, more defects (mainly oxygen vacancies) can be created when crystallinity is destroyed. Oxygen vacancies tend to adsorb the O in oxygen-containing functional groups.46 Moreover, the absence of O produces coordination unsaturated Al, increasing the number of empty orbitals and thus enhancing Lewis acidity, which further enhances electrophilicity.
Therefore, an Ag–In2O3 Schottky junction supported on fluffy mesoporous Al2O3 (f-m-Al2O3) was designed and synthesized for the selective hydrogenation C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of UAL. Electron-rich Ag reduces the probability of adsorption of the C
O of UAL. Electron-rich Ag reduces the probability of adsorption of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, while electron-deficient In2O3 improves the adsorption probability of the C
C bond, while electron-deficient In2O3 improves the adsorption probability of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. The fluffy Al2O3 with many electrophilic sites can also enhance the adsorption of the C
O bond. The fluffy Al2O3 with many electrophilic sites can also enhance the adsorption of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, thus improving the selectivity of UAL hydrogenation to UOL. The preparation process is facile, and the catalyst cost is reduced. The combination of the Schottky effect and the fluffy structure could provide new ideas for the highly selective hydrogenation of UAL to UOL.
O bond, thus improving the selectivity of UAL hydrogenation to UOL. The preparation process is facile, and the catalyst cost is reduced. The combination of the Schottky effect and the fluffy structure could provide new ideas for the highly selective hydrogenation of UAL to UOL.
2. Materials and methods
2.1 Materials
P123 (the ratio of PEO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPO
PPO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEO = 20
PEO = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70
70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20), γ-Al2O3, 1,4-dioxane, and aluminium nitrate 9-hydrate were analytically pure and purchased from Shanghai Macklin Biochemical Co., Ltd. Anhydrous ethanol, isopropyl alcohol, and cyclohexane were analytically pure and purchased from Sinopharm Chemical Reagent Co., Ltd. Anhydrous citric acid and indium nitrate hydrate were analytically pure and purchased from Shanghai Aladdin Biological Reagent Co., Ltd. Silver nitrate with the purity of 99.8% was purchased from Tianjin Tiangan Chemical Co., Ltd. All chemicals were used directly without further purification. Deionized water was used for the experiment.
20), γ-Al2O3, 1,4-dioxane, and aluminium nitrate 9-hydrate were analytically pure and purchased from Shanghai Macklin Biochemical Co., Ltd. Anhydrous ethanol, isopropyl alcohol, and cyclohexane were analytically pure and purchased from Sinopharm Chemical Reagent Co., Ltd. Anhydrous citric acid and indium nitrate hydrate were analytically pure and purchased from Shanghai Aladdin Biological Reagent Co., Ltd. Silver nitrate with the purity of 99.8% was purchased from Tianjin Tiangan Chemical Co., Ltd. All chemicals were used directly without further purification. Deionized water was used for the experiment.
2.2 Synthesis of catalysts
2.3 Catalytic hydrogenation reaction
First, 100 mg of Ag–In2O3/f-m-Al2O3 and 10 mL of 0.1 mol L−1 reaction substrate in isopropyl alcohol solution were added to the autoclave. The reactor was replaced five times with H2 and then charged with H2 to 2.5 MPa. After reacting at 160 °C for 3 h, the reactor was placed into an ice bath to cool down. After the reaction system was reduced to room temperature, the gas was released, and the reaction liquid was filtered. Conversion and selectivity were determined by GC-FID with 1,4-dioxane as an internal standard.2.4 Characterization
Transmission electron microscopy (TEM) results were obtained by field emission transmission electron microscopy (FEI Tecnai G2 F30). High resolution transmission electron microscopy (HRTEM) was performed using a JEOL JEM-2000 EX transmission electron microscope with an accelerating voltage of 120 kV. The X-ray diffractometer used was Shimadzu's instrument (XRD-7000S), and the test conditions were as follows: the X-ray source was Al-Kα (λ = 1.542 Å), the scanning angle was 30–80°, and the scanning speed was 5° min−1. The nitrogen adsorption–desorption test results were obtained at 77 K using an automatic mesoporous micropore analyzer (BK100C), and the specific surface area was calculated using the Brunauer–Emmett–Teller (BET) equation. According to the desorption data in N2 adsorption−desorption, the pore size information of the material was calculated by applying the BJH method. The pore volume of the material is obtained, corresponding to a relative pressure P/P0 of 0.2. XPS data were acquired by X-ray photoelectron spectroscopy (Thermo Fisher, ESCALAB 250Xi). The X-ray source is Al-Kα. Binding energy values were corrected for C 1s (284.8 eV). ICP–MS was used to determine the elemental content in the samples, which was detected by applying an inductively coupled plasma mass spectrometer (Agilent, 7900). UPS data were acquired by UV photoelectron spectroscopy (Thermo Fisher, ESCALAB 250Xi). The radiation source is a He I light source calibrated with Au. Raman data were acquired by applying a laser microscope Raman spectrometer (Thermo Fisher DXR, inVia). The instrument used for NH3-TPD is Micromeritics AutoChem II 2920 from the United States.3. Results and discussion
3.1 Structure, morphology, and composition
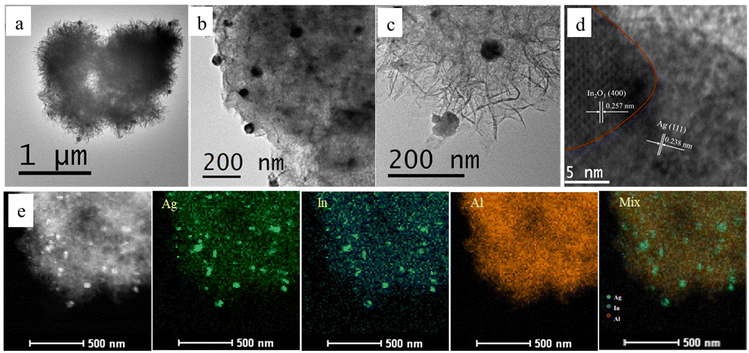
X-ray diffraction (XRD) is used to analyse the structure of the catalyst first. As shown in Fig. S2,† Al2O3 is an amorphous structure. The diffraction peak of In cannot be found on XRD because of the small content and good dispersion of In, but the diffraction peaks of Ag corresponding to the (111), (200), and (220) planes can be clearly observed. The Ag peaks of the catalyst doped with In are widened, which shows that the doping of In is beneficial to the dispersion of Ag.To further understand the microstructure of the catalyst, high resolution transmission electron microscopy (HRTEM) was performed, as shown in Fig. 1. From Fig. 1a–c, it is worth noting that mesoporous Al2O3 appears as a fluffy structure. Compared with the conventional spherical structure, this special fluffy structure has a larger surface area and pore volume, which can improve diffusion efficiency. The active components of the catalyst supported on mesoporous Al2O3 are uniformly dispersed, and the particle size is about 30 nm.
As shown in Fig. 1d, the lattice spacing of 0.238 nm corresponds to the Ag (111) plane, while 0.257 nm is the lattice spacing of the In2O3 (400) plane. It can be observed that Ag is in close contact with In2O3, which agrees with the formation conditions of the Schottky junction. Fig. 1e depicts a schematic diagram of the element distribution of the Ag––In2O3/f-m-Al2O3 catalyst. It can be observed that the active components Ag and In on the catalyst are uniformly dispersed and highly overlapped.
3.2 Electron transfer and Schottky junction
X-ray photoelectron spectroscopy (XPS) was used to examine the valence state of the elements on the catalyst surface (Fig. 2). As shown in Fig. 2a, the binding energy of Ag is reduced by 0.15 eV after combination with In2O3, reflecting an increase in the electron density. According to the In 3d spectra (Fig. 2b), In exists as an oxidation state, and the binding energy increases by 0.10 eV upon contact with Ag, indicating that In2O3 has lost some electrons. This shows that electron transfer occurs from In2O3 to Ag, forming a metal–n-type semiconductor Schottky junction. This is very advantageous for the hydrogenation of UAL to UOL because the electron-rich Ag repels the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond31 and promotes the heterolytic of H2.21,22,46 Meanwhile, the electron-deficient In2O3 serves as an electrophilic site promoting C
C bond31 and promotes the heterolytic of H2.21,22,46 Meanwhile, the electron-deficient In2O3 serves as an electrophilic site promoting C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O adsorption.31
O adsorption.31
The work function is a parameter that reflects the ability of a substance to donate or gain electrons. The larger the work function, the easier it is for the substance to gain electrons, while the smaller the work function, the easier it is for the substance to donate electrons. When a metal and semiconductor contact, electrons spontaneously flow from the side with a smaller work function to the side with a larger work function until their work functions are the same. UPS characterization is a common method for analysing the work function of substances. Knowing the change in the work function of the two materials can help us understand the direction of the electron transfer of catalysts more intuitively. As shown in Fig. S3,† the work functions of Ag/f-m-Al2O3, In2O3/f-m-Al2O3 and Ag–In2O3/f-m-Al2O3 are about 3.70 eV, 2.83 eV and 3.20 eV, respectively. It can be explained that electrons are spontaneously transferred from In2O3 with a smaller work function to Ag with a larger work function, which further supports the direction of electron transfer in the Ag–In2O3 Schottky junction.
3.3 Physical and chemical properties
Fig. 3a and b show the N2 isothermal adsorption and desorption curves and pore size distribution of Ag–In2O3/f-m-Al2O3. The type IV isotherm and type H1 hysteresis loop proved that the catalyst was a typical mesoporous material. The pore size is about 5.8 nm. Table S1† shows the pore volume and specific surface area of the material. As can be observed from the table, owing to the fluffy structure of mesoporous Al2O3, compared with commercial Al2O3, the specific surface area is increased more than 10 times, and the pore volume is also increased several times, which makes the catalyst expose more active sites. | ||
| Fig. 3 (a) N2 adsorption–desorption isotherm. (b) Pore size distribution diagram of the Ag–In2O3/f-m-Al2O3 catalyst. (c) NH3-TPD of different catalysts. (d) Raman spectra for different catalysts. | ||
Furthermore, the fluffy structure can expose more Al unsaturated coordination sites and increase the Lewis acid content. As shown in Fig. 2c, the content of oxygen vacancy (Ov) in Ag–In2O3/f-m-Al2O3 (40.1%) increased significantly compared to that in Ag–In2O3/γ-Al2O3 (31.5%). Meanwhile, the binding energy of Al 2p decreased (Fig. 2d), meaning that Al has a higher electron density and thus a lower valence state, which is in accordance with the deficiency of oxygen. With the deficiency of oxygen, the degree of coordination unsaturation of Al increases, resulting in more empty orbitals and, thus, more Lewis acidity.
To prove the increase in Lewis acidity, NH3-TPD was used to detect the acid amounts of the different catalysts (Fig. 3c). Compared with SiO2 and γ-Al2O3, self-made f-m-Al2O3 can provide more acidic sites for the catalyst, which once again shows the advantages of the fluffy structure. Lewis acid acts as an electrophilic site to adsorb a lone pair on O with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, so the selectivity of UOL can be further improved. Consistent with the change in acid amounts, the UOL selectivity of Ag–In2O3 supported on f-m-Al2O3 increased by 17% and 45% compared to those on γ-Al2O3 and SiO2, respectively.
O, so the selectivity of UOL can be further improved. Consistent with the change in acid amounts, the UOL selectivity of Ag–In2O3 supported on f-m-Al2O3 increased by 17% and 45% compared to those on γ-Al2O3 and SiO2, respectively.
To test the degree of coordination unsaturation of Al, the catalysts were analyzed by Raman, and the results are shown in Fig. 3d. The spectral peak of 705 cm−1 belongs to the symmetric stretching vibration caused by four-coordinated Al–O. Because the hexa-coordinated octahedral structure of Al cannot absorb electrons, the unsaturated coordination of Al is the main reason for Lewis acidity. Raman shows that f-m-Al2O3 contains a large number of unsaturated tetra-coordinated Al–O bonds, which can be used as Lewis acid sites, further proving that the unique fluffy structure of f-m-Al2O3 can increase the number of electrophilic sites.
3.4 Catalytic performance
To test the catalytic performance of Ag–In2O3/f-m-Al2O3, three typical UAL, 2-pentenal, cinnamaldehyde, and furfural (representing aliphatic, aromatic and heterocyclic groups, respectively) were tested (Table 1). Notably, 98% conversion of 2-pentenal and 92% selectivity of 2-pentenol were obtained for the hydrogenation of 2-pentenal using Ag–In2O3/f-m-Al2O3 catalyst at 160 °C and 2.5 MPa hydrogen pressure for 3 hours. The other two types of UAL also have good performance. The stability test is shown in Fig. S4.† The activity can be maintained after 5 cycles, which shows that the catalyst is very stable.| Entry | Substrate | Conversion (%) | Selectivity (%) |
|---|---|---|---|
| a Reaction conditions: substrate (1.8 mmol), catalyst (5 mol%), and isopropanol (20.0 mL) under a 2.5 MPa H2 atmosphere at 160 °C for 3 h. | |||
| 1 |

|
98 | 92 |
| 2 |

|
91 | 82 |
| 3 |

|
85 | 94 |
Fig. 4 shows the proposed mechanism of the reaction. The electron-rich Ag repels the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond while attracting the electron-deficient C in the C
C bond while attracting the electron-deficient C in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, and the electron-deficient indium oxide attracts the electron-rich oxygen in the C
O bond, and the electron-deficient indium oxide attracts the electron-rich oxygen in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, thus achieving diσCO adsorption mode. Oxygen vacancies in the support and Lewis acid sites simultaneously help attract O in the C
O bond, thus achieving diσCO adsorption mode. Oxygen vacancies in the support and Lewis acid sites simultaneously help attract O in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, further improving selectivity. H2 is broken into active H+ and H− at the heterojunction interface via hetero cleavage and is then selectively added to the polar C
O bond, further improving selectivity. H2 is broken into active H+ and H− at the heterojunction interface via hetero cleavage and is then selectively added to the polar C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond to form UOL. Then, the UOL desorbs, and the catalyst recovers and enters the next cycle.
O bond to form UOL. Then, the UOL desorbs, and the catalyst recovers and enters the next cycle.
4. Conclusions
In summary, Ag–In2O3/f-m-Al2O3 showed high selectivity for the hydrogenation of UAL to UOL. The analysis shows that the addition of In can regulate the electronic distribution of the catalyst. A Schottky junction can be formed between Ag and In2O3. Because of the larger work function of Ag, electrons flow from In2O3 to Ag. Electron-rich Ag reduces the probability of adsorption of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond owing to the “four-electron repulsion” effect. Electron-deficient In2O3 can increase the probability of C
C bond owing to the “four-electron repulsion” effect. Electron-deficient In2O3 can increase the probability of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond adsorption. Furthermore, the unsaturated coordination of Al–O in f-m-Al2O3 not only improves the selectivity of the C
O bond adsorption. Furthermore, the unsaturated coordination of Al–O in f-m-Al2O3 not only improves the selectivity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond by generating electrophilic sites, such as the oxygen vacancy and Lewis acid site, but also increases the specific surface area of the catalyst and reduces the mass transfer resistance. High selectivity of UOL can be obtained from UAL containing aliphatic, aromatic and heterocyclic groups. The design of this catalyst is expected to provide a new idea for achieving highly selective hydrogenation of UALs to UOLs.
O bond by generating electrophilic sites, such as the oxygen vacancy and Lewis acid site, but also increases the specific surface area of the catalyst and reduces the mass transfer resistance. High selectivity of UOL can be obtained from UAL containing aliphatic, aromatic and heterocyclic groups. The design of this catalyst is expected to provide a new idea for achieving highly selective hydrogenation of UALs to UOLs.
Author contributions
Jiasheng Wang: conceptualization, supervision, funding acquisition, and Writing–review and editing. Tianyu Zhang and Fengxin Zhang: investigation and Writing–original draft. Jiliang Song and Hong Liu: methodology. Wan-Hui Wang and Ming Bao: resources and Supervision.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support by the National Natural Science Foundation of China (22278063) and the Research and Innovation Team Project of Dalian University of Technology (DUT2022TB10).References
- Q. Liu, Q. Liu, Y. Chen, Y. Li, H. Su, Q. Liu and G. Li, Chin. Chem. Lett., 2022, 33, 374–377 CrossRef CAS.
- X. Wang, X. Liang, P. Geng and Q. Li, ACS Catal., 2020, 10, 2395–2412 CrossRef CAS.
- H. Yu, J. Zhao, C. Wu, B. Yan, S. Zhao, H. Yin and S. Zhou, Langmuir, 2021, 37, 1894–1901 CrossRef CAS PubMed.
- S. Zhou, Y. Yang, T. Shen, P. Yin, L. Wang, Z. Ren, L. Zheng, B. Wang, H. Yan and M. Wei, ACS Appl. Mater. Interfaces, 2024, 16, 13685–13696 CrossRef CAS PubMed.
- Q. Liu, J. Wu, J. Kang, Q. Liu, P. Liao and G. Li, Nanoscale, 2022, 14, 15462–15467 RSC.
- Y. Wang, P. Wang, M. Zhang, X. Yi, Y. Wei and J. Zhu, Fine Chem., 2024, 41, 1581–1589 CAS.
- X. Lan and T. Wang, ACS Catal., 2020, 10, 2764–2790 CrossRef CAS.
- N. Luo, J. Liao, L. Ouyang, H. Wen, J. Liu, W. Tang and R. Luo, Organometallics, 2019, 38, 3025–3031 CrossRef CAS.
- C. A. Barrales-Cortés, H. Pérez-Pastenes, J. C. Piña-Victoria and T. Viveros-García, Top. Catal., 2020, 63, 468–480 CrossRef.
- Z. Gao, L. Cai, C. Miao, T. Hui, Q. Wang, D. Li and J. Feng, ChemCatChem, 2022, 14, e202200634 CrossRef CAS.
- M. Luneau, J. S. Lim, D. A. Patel, E. C. H. Sykes, C. M. Friend and P. Sautet, Chem. Rev., 2020, 120, 12834–12872 CrossRef CAS PubMed.
- Y. Liang, J. He, Y. An, J. Zhang, G.-S. Park, L. Zhao, R. Oh, X. Huang, J. Dong and L. Liu, Chem. Eng. J., 2024, 484, 149670 CrossRef CAS.
- P. Adamski, H. Zhang, S. Kaur, X. Chen, C. Liang and M. Armbrüster, Chem. Mater., 2024, 36, 10383–10407 CrossRef CAS.
- F. Delbecq and P. Sautet, J. Catal., 1995, 152, 217–236 CrossRef CAS.
- F. Delbecq and P. Sautet, J. Catal., 2002, 211, 398–406 CrossRef CAS.
- L. Zhong, X. Liao, H. Cui, J. Huang, H. a. Luo, Y. Lv and P. Liu, ACS Catal., 2024, 14, 15799–15810 CrossRef CAS.
- Z. Tian, L. Wang, T. Shen, P. Yin, W. Da, Z. Qian, X. Zhao, G. Wang, Y. Yang and M. Wei, Chem. Eng. J., 2023, 472, 144876 CrossRef CAS.
- L. Zhong, X. Liao, H. Cui, H. a. Luo, Y. Lv and P. Liu, ACS Catal., 2024, 14, 857–873 CrossRef CAS.
- K. K. Ghuman, L. B. Hoch, P. Szymanski, J. Y. Y. Loh, N. P. Kherani, M. A. El-Sayed, G. A. Ozin and C. V. Singh, J. Am. Chem. Soc., 2016, 138, 1206–1214 CrossRef CAS PubMed.
- X. Deng, B. Qin, R. Liu, X. Qin, W. Dai, G. Wu, N. Guan, D. Ma and L. Li, J. Am. Chem. Soc., 2021, 143, 20898–20906 CrossRef CAS PubMed.
- J. Wang, H. Jin, W.-H. Wang, Y. Zhao, Y. Li and M. Bao, ACS Appl. Mater. Interfaces, 2020, 12, 19581–19586 CrossRef CAS PubMed.
- X. Xu, N. Luo, W.-H. Wang, M. Bao and J. Wang, ACS Appl. Mater. Interfaces, 2024, 16, 70489–70497 CrossRef CAS PubMed.
- Q. Liu, J. Xian, Y. Li, Q. Zhang, H. Kitagawa and G. Li, CCS Chem., 2022, 4, 3275–3284 CrossRef CAS.
- W. Yang, Q. Liu, J. Yang, J. Xian, Y. Li, G. Li and C.-Y. Su, CCS Chem., 2022, 4, 2276–2285 CrossRef CAS.
- Y. Chen, X. He, L. Hou, B. Liu, L. Dong and X. Ge, Chem. Eng. J., 2024, 500, 156934 CrossRef CAS.
- H. Wang, S. Bai, Y. Pi, Q. Shao, Y. Tan and X. Huang, ACS Catal., 2019, 9, 154–159 CrossRef CAS.
- Y. Zhong, P. Liao, J. Kang, Q. Liu, S. Wang, S. Li, X. Liu and G. Li, J. Am. Chem. Soc., 2023, 145, 4659–4666 CrossRef CAS PubMed.
- J. Xian, S. Li, H. Su, P. Liao, S. Wang, Y. Zhang, W. Yang, J. Yang, Y. Sun, Y. Jia, Q. Liu, Q. Liu and G. Li, Angew. Chem., Int. Ed., 2023, 62, e202304007 CrossRef CAS PubMed.
- X.-H. Li and M. Antonietti, Chem. Soc. Rev., 2013, 42, 6593–6604 RSC.
- J. Michel, D. Splith, J. Rombach, A. Papadogianni, T. Berthold, S. Krischok, M. Grundmann, O. Bierwagen, H. von Wenckstern and M. Himmerlich, ACS Appl. Mater. Interfaces, 2019, 11, 27073–27087 CrossRef CAS PubMed.
- M. Deng, D. Wang and Y. Li, Appl. Catal., A, 2023, 666, 119423 CrossRef CAS.
- Y. Ma, D. He, Z. Hu, H. Li and X. Wang, J. Phys. Chem. C, 2024, 128, 5064–5074 CrossRef CAS.
- C. Wang, Z. Zhao, Y. Peng, L. Ma, X. Liu, F. Fu and Y. Wu, Fuel, 2024, 372, 132231 CrossRef CAS.
- Y. Ren, H. Xu, B. Han and J. Xu, Molecules, 2023, 28, 4136 CrossRef CAS PubMed.
- Z. Yin, F. Yang, J. Chen, C. Sun and S. Cao, Appl. Catal., A, 2022, 644, 118807 CrossRef CAS.
- E. Plessers, D. E. De Vos and M. B. J. Roeffaers, J. Catal., 2016, 340, 136–143 CrossRef CAS.
- X. Yang, A. Wang, X. Wang, T. Zhang, K. Han and J. Li, J. Phys. Chem. C, 2009, 113, 20918–20926 CrossRef CAS.
- L. Tian, Q. Yang, Z. Jiang, Y. Zhu, Y. Pei, M. Qiao and K. Fan, Chem. Commun., 2011, 47, 6168–6170 RSC.
- Y. Hinuma, T. Toyao, N. Hamamoto, M. Takao, K.-i. Shimizu and T. Kamachi, J. Phys. Chem. C, 2020, 124, 27621–27630 CrossRef CAS.
- J. Jin, C. Liu, C. Dai, C. Zeng, Y. Jia and X. Liu, Environ. Res., 2024, 251, 118649 CrossRef CAS PubMed.
- Y. Zhang, S. Zhang, X. Pan, M. Bao, J. Huang and W. Shen, Catal. Lett., 2017, 147, 102–109 CrossRef CAS.
- Y. Wang, S. Wang, Q. Xu, X. Feng, W. Liu, Y. Yamamoto, Y. Shi and M. Bao, ACS Appl. Nano Mater., 2024, 7, 10739–10747 CrossRef CAS.
- J. Zhu, S. Yan, Y. Qian, X. Zhu and F. Yang, Microporous Mesoporous Mater., 2023, 351, 112465 CrossRef CAS.
- F. Ebert, P. Ingale, S. Vogl, S. Praetz, C. Schlesiger, N. Pfister, R. N. d’Alnoncourt, B. R. Cuenya, A. Thomas, E. Gioria and F. Rosowski, ACS Catal., 2024, 14, 9993–10008 CrossRef CAS.
- S. Arora, R. Khan and S. Sivakumar, ChemCatChem, 2025, 17, e202401673 Search PubMed.
- J. Wang, Y. Zhang, X. Xu and M. Bao, ACS Appl. Mater. Interfaces, 2023, 15, 8149–8156 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr05518g |
| This journal is © The Royal Society of Chemistry 2025 |