 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Atomically precise large-sized Aun(SR)m nanoclusters and scaling relationships among size, bandgap and excited-state lifetime†
Xiangsha Du‡
a,
Tatsuya Higaki‡ a,
Hedi Ma‡b,
Xinwen Zhang‡c,
Gangli Wang
a,
Hedi Ma‡b,
Xinwen Zhang‡c,
Gangli Wang *b,
He Wang
*b,
He Wang *c and
Rongchao Jin
*c and
Rongchao Jin *a
*a
aDepartment of Chemistry, Carnegie Mellon University, Pittsburgh, Pennsylvania 15213, USA. E-mail: rongchao@andrew.cmu.edu
bDepartment of Chemistry, Georgia State University, Atlanta, Georgia 30302, USA. E-mail: glwang@gsu.edu
cDepartment of Physics, University of Miami, Coral Gables, Florida 33146, USA. E-mail: hewang@miami.edu
First published on 14th May 2025
Abstract
The synthesis of atomically precise, large-sized Aun(SR)m nanoclusters (SR = thiolate) with n of 100 or more atoms has long been a challenge. In this work, we report the synthesis of Au100(Napt)42 and Au102(IPBT)44 nanoclusters (where Napt stands for 1-naphthalenethiolate and IPBT for 4-isopropylbenzenethiolate). Their optical absorption, single-electron charging, and femtosecond electron dynamics are investigated. Both Au100(Napt)42 and Au102(IPBT)44 exhibit multiple discrete absorption bands in their optical spectra owing to a quantized electronic structure in ultrasmall metal nanoclusters. Electrochemical analysis determines the precise HOMO–LUMO gaps of Au100(Napt)42 and Au102(IPBT)44 by single-electron charging. The electronic excited-state lifetimes of Au100(Napt)42 and Au102(IPBT)44 are determined by femtosecond transient absorption spectroscopy. The scaling relationship between the Eg and size (n) and the relationship of the excited state lifetime versus the Eg of large-sized Aun(SR)m are discussed. The findings provide a fundamental understanding of large-sized metal nanoclusters, which will benefit their future applications in optics, electronics, and other fields.
Introduction
When the size of metal nanoparticles is reduced to ∼2.2 nanometers, distinct quantum confinement effects come into play, manifested in the emergence of a sizable HOMO–LUMO gap (Eg) and the disappearance of plasmon resonance.1–3 Fundamental studies of the electronic and optical properties of such quantum-sized nanoclusters (NCs) require precise size control at the single-atom level.4–8 In recent years, breakthroughs in the synthesis have led to the establishment of a library of atomically precise Aun(SR)m NCs.9 These Au NCs can be protected by ligands such as thiolate,1 phosphine,2 alkynyl,6,10 N-heterocyclic carbene,7,11 and halide.12 The structural diversity of the Au kernel and Au–ligand interface, as well as the organic outer shell, provides great tunability of the functionality of NCs for diverse applications.13–18In the quantum-size regime (e.g., tens to hundreds of atoms per core), the material properties of gold NCs become very sensitive even to a small variation; for example, a single-atom variation can trigger structural transformation, and single-atom doping may induce significant changes to the optical absorption, photoluminescence, catalytic activity, and magnetic properties.19–21 The same adamantanethiolate-protected Au21(SR)15, Au22(SR)16, Au22Cd(SR)16, and Au24(SR)16 series offers a neat platform for atom-by-atom evolution of small-sized NCs.22 Structurally, a single cadmium addition to Au22(SR)16 induces a surface reconstruction in Au22Cd1(SR)16 as well as a kernel reconstruction from the Au10 bioctahedron in Au22(SR)16 to the Au13 cuboctahedron in Au22Cd1(SR)16.22 The addition of metal atoms to the NC in an atom-by-atom manner also causes dramatic changes to the optical absorption, reflected in the distinct color changes to the NCs.22 Such a high sensitivity of the structural and optical properties to single-atom variations is due to the strong quantum confinement effect in small NCs. In addition to the optical effects, recent work on various crystalline phases of Au NCs also found that the electronic excited-state relaxation can be drastically varied by the metal core's phase,23 with the hexagonal close-packed Au30(SR)18 and body-centered cubic Au38S2(SR)20 NCs protected by the same type of ligand showing a three-orders-of-magnitude variation of the photoexcited carrier lifetime. On the other hand, for larger sized Aun(SR)m NCs, the sensitivity of properties to the variation in size (n) remains less studied, as more efforts on the synthesis of large-sized NCs are still needed.
In the investigation of how the optical properties of gold NCs evolve with increasing size (n, the number of gold atoms), Negishi et al. previously reported a series of Aun(SC12)m NCs with n ranging from 38 to ∼520, in which the critical region—where a molecule-like electronic structure and a non-bulk geometric structure emerge—was determined to be between Au144 and Au187.24 Higaki et al. determined the transition from nonmetallic Au246 to metallic Au279.25,26 When taking the shape factor into consideration, Pei's group carried out theoretical work and reported that Au264(SH)96 is a critical size for the transition in cuboidal NCs.27 Zhou et al. drew a grand picture of the three-stage evolution from non-scalable to scalable optical properties of Aun(SR)m NCs.28 Nevertheless, the large-sized NCs with n ≥ 100 atoms still remain scarce and synthetically challenging. To further investigate the scaling relationship of the optical and electronic properties with the number of Au atoms in the NCs, more large-sized NCs should be synthesized and their electronic and optical properties should be investigated.
Herein, we report the synthesis, optical absorption and electronic properties of Au100(Napt)42 and Au102(IPBT)44 NCs. These NCs exhibit ultrasmall gaps (Eg < 0.5 eV), and precise determination of such gaps is not trivial.2 Their steady-state and transient optical absorption properties are studied. Furthermore, we summarize the scaling relationships of the gap Eg vs. size (n), as well as the carrier lifetime vs. Eg in the large-size regime. The findings of this work provide a deeper understanding of the size evolution behavior of the electronic properties of NCs in the large-size regime.
Results and discussion
Synthesis and characterization of Au100(Napt)42 and Au102(IPBT)44
Au100(Napt)42 (Napt = 1-naphthalenethiolate) was prepared by a ligand-exchange reaction from polydispersed Aux(2,4-DMBT)y nanoclusters (2,4-DMBT = 2,4-dimethylbenzenethiol) at 80 °C overnight; of note, the Aux(2,4-DMBT)y crude product was prepared by NaBH4 reduction of HAuCl4·3H2O in a two-phase solution (toluene/water). Separation of Au100(Napt)42 from side products was achieved by thin-layer chromatography (TLC); further synthetic details are provided in the ESI.† The high purity and molecular formula of Au100(Napt)42 are confirmed by matrix-assisted laser desorption ionization (MALDI) and electrospray ionization (ESI) mass spectrometry analyses. As can be seen in Fig. 1(a), the MALDI spectrum shows a very narrow peak at ∼25 kDa for Au100(Napt)42, indicating its high purity. Its precise mass was determined by ESI-MS (Fig. 1a, inset). A dominant peak of the molecular ion at m/z 8794.09 (M3+) was observed, along with a minor peak at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 192.75 (not shown) corresponding to the 2+ ion. These ions are formed under the ESI conditions, as the native state of the NC is charge neutral. Both peaks match well with the calculated mass of Au100(Napt)42 (FW = 26
192.75 (not shown) corresponding to the 2+ ion. These ions are formed under the ESI conditions, as the native state of the NC is charge neutral. Both peaks match well with the calculated mass of Au100(Napt)42 (FW = 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 384.20 Da). The UV–vis spectrum of Au100(Napt)42 shows multiple absorption bands at 430, 520, 610, and 730 nm in the visible range (Fig. 1b) due to ultrasmall size-induced quantization of the electronic structure, as opposed to the quasi-continuous conduction band in metallic-state nanoparticles. All these bands are highly congested, which is typical of large-sized NCs due to their much denser electronic states compared to small sized NCs such as Au25(SR)18, where the peaks are well separated.9 The highly structured, molecular-like optical features in gold nanoclusters often indicate high purity, as size-mixed nanoclusters readily wash out such features and give rise to a featureless, decay-like spectrum.
384.20 Da). The UV–vis spectrum of Au100(Napt)42 shows multiple absorption bands at 430, 520, 610, and 730 nm in the visible range (Fig. 1b) due to ultrasmall size-induced quantization of the electronic structure, as opposed to the quasi-continuous conduction band in metallic-state nanoparticles. All these bands are highly congested, which is typical of large-sized NCs due to their much denser electronic states compared to small sized NCs such as Au25(SR)18, where the peaks are well separated.9 The highly structured, molecular-like optical features in gold nanoclusters often indicate high purity, as size-mixed nanoclusters readily wash out such features and give rise to a featureless, decay-like spectrum.
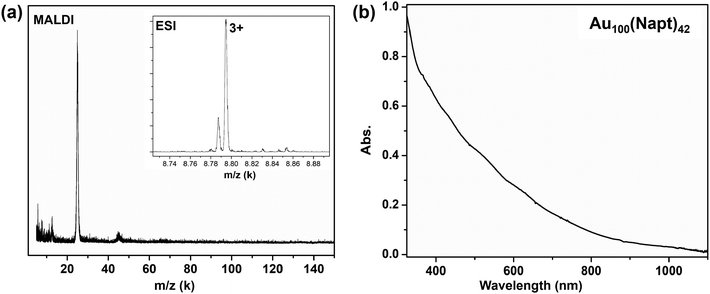 | ||
| Fig. 1 (a) MALDI and ESI (inset) mass spectra of Au100(Napt)42; (b) absorption spectrum of Au100(Napt)42. | ||
We next discuss the case of Au102(IPBT)44, which was synthesized by a two-step, size-focusing method. Briefly, a poly-dispersed Aux(IPBT)y mixture was first obtained by the reactions of HAuCl4·3H2O with 4-isopropylbenzenethiol (IPBT) and reduction by NaBH4. Further thermal etching of the poly-dispersed Aux(IPBT)y was conducted in toluene with 0.5 mL IPBT at 80 °C. After washing with methanol, pure Au102(IPBT)44 was collected. In MALDI-MS analysis, a single peak was observed at ∼25 kDa (Fig. 2a), demonstrating the high purity of the sample. The ESI spectrum gives two sets of peaks at m/z 8915.59 (3+) and 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 373.20 (2+), corresponding to the 3+ and 2+ ions, respectively. Both peaks are consistent with the expected formula weight of Au102(IPBT)44 (FW = 26
373.20 (2+), corresponding to the 3+ and 2+ ions, respectively. Both peaks are consistent with the expected formula weight of Au102(IPBT)44 (FW = 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 746.38 Da). Of note, the slight fragmentation within each set (Fig. 2a inset) is caused by the subtraction or addition of one IPBT ligand. The optical absorption spectrum of Au102(IPBT)44 shows peaks at 420, 505, 600, and 730 nm (Fig. 2b), which are comparable to the bands of Au100(Napt)42, indicating an intrinsic relationship (see below), in particular, given the fact that these two NCs differ by only two gold atoms and two ligands.
746.38 Da). Of note, the slight fragmentation within each set (Fig. 2a inset) is caused by the subtraction or addition of one IPBT ligand. The optical absorption spectrum of Au102(IPBT)44 shows peaks at 420, 505, 600, and 730 nm (Fig. 2b), which are comparable to the bands of Au100(Napt)42, indicating an intrinsic relationship (see below), in particular, given the fact that these two NCs differ by only two gold atoms and two ligands.
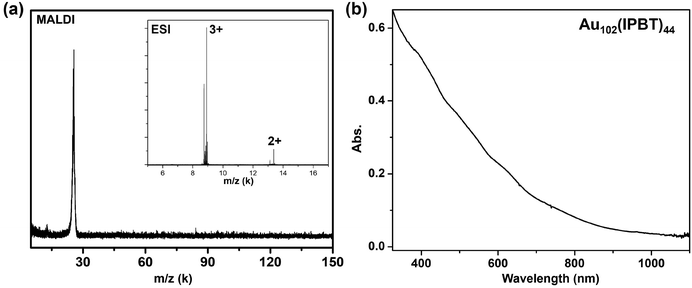 | ||
| Fig. 2 (a) MALDI and ESI (inset) mass spectra of Au102(IPBT)44; (b) absorption spectrum of Au102(IPBT)44. | ||
Structural insights
The highly structured optical absorption spectra of Aun(SR)m NCs reflect the metal core structures and can indeed serve as “fingerprints”.9 We find that the optical spectra of both Au100(Napt)42 and Au102(IPBT)44 are quite similar to those of previously reported Au102(p-MBA)44 and Au103S2(Napt)41 (Fig. S1†) having solved structures.29,30 This can be explained by the fact that all these four NCs (charge-neutral) possess the same number of nominal free-electron count, i.e., 58e, e.g., 100 (Au 6s1) − 42 (monovalent SR ligand) = 58e. It should be noted that the S species in the Au103S2(Napt)41 nanocluster localizes two electrons per S atom due to its divalent nature (S2−), so the free electron number = 103 − 2 × 2 − 41 = 58e. It is worth noting that the Au102(IPBT)44 nanocluster from the current work shares the same number of gold atoms and ligands (though hydrophobic) with the previously reported aqueous phase Au102(p-MBA)44 (where p-MBA = SPh-p-COOH), with the only difference being in the para-substitution on the benzene ring (i.e., isopropyl vs. –COOH). Based on the empirical rule that the kernel structure plays a decisive role in the optical absorption spectra,9 plus the fact that the four abovementioned NCs share the same 58e count, we deduce that Au102(IPBT)44 should have the same Au79 Marks decahedral kernel, i.e., a shell-by-shell Au7@Au32@Au40 arrangement, and the surface should be composed of 19 monomeric (SR–Au–SR) and two dimeric (SR–Au–SR–Au–SR) staple-like motifs as in the structure of its aqueous soluble Au102(p-MBA)44 counterpart.29 With respect to Au100(Napt)42, we also deduce that the same Au79 decahedron should be adopted, but the surface should comprise 21 monomeric (SR–Au–SR) staple motifs protecting the Au79 kernel, similar to the pair of Au102(p-MBA)44 and Au103S2(Napt)41 NCs, which share the same Au79 kernel but slightly different surface motifs.29,30Compared to the previously reported aqueous Au102(p-MBA)44,29 the attainment of organic soluble Au102(IPBT)44 in the current work allows for a precise determination of Eg and a detailed study on the single-electron charging energy, which was previously inaccessible with the aqueous Au102(p-MBA)44 (vide infra).
HOMO–LUMO gap determination and the scaling relationship with size
The 58e system with close sizes (n = 100, 102 and 103) and various ligands is a great platform for a comparative study of their electronic properties. Here, we carried out further electrochemical measurements. Differential pulse voltammetry (DPV) analysis indicates that both Au100(Napt)42 and Au102(IPBT)44 exhibit discrete electronic energy levels and rich charge states (Fig. 3). Following a meticulous method established by Wang and coworkers,31 the Eg of Au100(Napt)42 was deduced to be 0.40 eV from the electrochemical gap between the first oxidation and the first reduction potentials after subtracting the charging energy (i.e., 0.612–0.216 = 0.40 eV; the subscripts indicate single-meV accuracy31). Similarly, the Eg of Au102(IPBT)44 is 0.33 eV (i.e., 0.572–0.244 = 0.33 eV). The gaps of these two 58e NCs are similar to those of Au102(p-MBA)44 (0.45 eV)29 and Au103S2(Napt)41 (0.38 eV).30 The similar Eg values of these four 58e NCs support the abovementioned rationale for using the same Au79 kernel.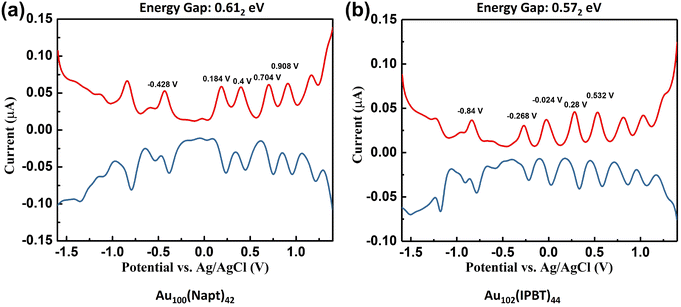 | ||
| Fig. 3 Differential pulse voltammograms of (a) Au100(Napt)42 and (b) Au102(IPBT)44. The gaps of 0.612 and 0.572 eV are without subtraction of charging energy. | ||
It is worth comparing the determined Eg of Au100 and Au102 NCs (quasi-spherical) with other NCs of slightly smaller or larger sizes, such as Au92(TBBT)4432 and Au99(SR)42,33,34 and two rod-shaped NCs of Au96(PET)68 and Au114(PET)80.35 Fig. 4 shows a plot of Eg versus the number of gold atoms (n) in the size range between 92 and 114, as well as those with larger sizes, including Au130(SR)50 (various types of ligands),36–39 Au133(SR)52,40,41 and Au144(SR)60.42–44 One can see that the spherical (or nearly spherical) NCs exhibit a trend (Fig. 4, the black solid line is a guide). Interestingly, their Eg is generally smaller than that of the rod-shaped Au96(PET)68 and Au114(PET)80 by ∼0.27 eV (i.e., the gap between the top and lower lines, Fig. 4) at comparable sizes. We attribute this to two factors: (i) the shape-induced quantum effect on the electronic structure, i.e., the lower symmetry of quantum rods35 leads to a stronger quantum confinement effect in the smaller dimension of the rods, and (ii) the higher ligand-to-gold ratio in rod-shaped NCs, e.g., the Au96(PET)68 rods possess many more ligands (∼43% higher) than Au100(Napt)42 and Au102(IPBT)44. The latter factor is expected to enlarge the Eg because more valence electrons (6s of gold atoms) are localized in the covalent bonds of Au–SR motifs.
 | ||
| Fig. 4 Scaling relationship between the Eg of large-sized Aun(SR)m NCs and the number of gold atoms (n). | ||
Ultrafast electron dynamics in Au100 and Au102 NCs
Gold NCs with 100 atoms or more have ultrasmall energy gaps (Eg < 0.5 eV), which are hard to reach for other molecular materials; thus, such NCs are quite unique and worthy of investigation due to their electron dynamics. Here, we probe the excited-state dynamics of Au100(Napt)42 and Au102(IPBT)44 by performing femtosecond transient absorption (fs-TA for short) measurements. After photoexcitation at 400 nm, the NCs exhibit significant fast and slow decays in the relaxation process (Fig. 5a and b). Au100(Napt)42 and Au102(IPBT)44 show very similar TA spectra and relaxation dynamics, which further indicates that they should share a similar atomic structure (Au79 kernel). Specifically, a distinct ground-state bleaching (GSB) signal is seen at ∼525 nm, which matches with the ground state absorption band in both NCs. Strong excited-state absorption (ESA) signals are observed at 580 and 675 nm. Overall, the similar GSB and ESA signals for both NCs also indicate their similar electronic structures.Global analysis on the fs-TA data gives three decay components for both NCs (Fig. 5c and d). The fast relaxation can be fitted by two processes: the <1 ps process is assigned to the relaxation from the higher excited state to the lower excited state, while the few-picosecond process should be considered as the energy rearrangement process around the lowest excited state (ESI Fig. S2†). The slow process takes 266 ps for Au100(Napt)42 and 466 ps for Au102(IPBT)44, which are the relaxation from the lowest excited state to the ground state (i.e. exciton recombination). Based on the above observations, we can deduce that upon photoexcitation, transitions from the ground state to the excited state occur predominantly through core-based orbitals, and the excited core then relaxes via core phonons and hence exhibits similar relaxation dynamics.
Among the 58e NCs (organic soluble ones, including Au100(Napt)42 (Eg = 0.40 eV), Au102(IPBT)44 (Eg = 0.33 eV), and Au103S2(Napt)41 (Eg = 0.4 eV)), a variation of ∼1.8 times (266 vs. 466 ps) can be seen in their excited-state lifetimes (ESI Fig. S3†). This is attributed to the surface effect because the excited state relaxation also involves the surface, while the Eg value and optical absorption peaks are primarily dictated by the kernel. When compared to the water phase Au102(p-MBA)44 (Eg = 0.45 eV), a 10-time increase in the excited lifetime is observed in Au102(p-MBA)44 (ESI Fig. S3†). Such a large effect was also found in the case of organic soluble Au130(SR)50 versus water soluble Au130(SR)50.37,38 This distinct difference may be caused by the –COOH perturbation to the surface and/or the hydrogen-bonding effect in aqueous NCs.
Scaling relationship of the excited state lifetime with the energy gap
We further discuss the scaling relationship of the excited-state lifetime (τ) with Eg for large-sized Aun(SR)m NCs. Here, we focus on (quasi)spherical NCs since more sizes have become available.2 The lifetimes of Au100(Napt)42 and Au102(IPBT)44 are much higher than that of the well-known Au144(SR)60 (a few ps)42–44 due to their Eg difference as well as the ligand effects. Our results suggest that when Eg decreases (i.e., approaching the metallic state), the ratio of the fast decay process (on the ps scale) increases in the whole decay process in large-sized NCs such as Au130, Au133, Au144 and Au246 NCs,28 which may be ascribed to the difficulty in separation between the fast decay (the relaxation from the higher excited state to the lowest excited state) and slow decay (the relaxation from the lowest excited state to the ground state) when the energy gap is so small. Thus, the excited-state relaxation cannot be treated as a sequential model since there is no population accumulation in the lower excited state for those Au NCs with very small Eg. By fitting the band gaps and excited-state lifetimes of Au100(Napt)42, Au102(IPBT)44 and other large-sized NCs,28 we observed a quantitative relationship in which the excited-state lifetimes of Au NCs decrease exponentially with the energy gaps (ESI Fig. S4a†). Here, we fit the data with the energy gap law,| knr = Ae−γEg/ħω | (1) |
 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) knr and Eg (Fig. S4b†), with the fitted intercept (ln
knr and Eg (Fig. S4b†), with the fitted intercept (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A) being 27.5 and the slope (−γ/ħω) being −15 eV−1.
A) being 27.5 and the slope (−γ/ħω) being −15 eV−1.
Conclusions
In summary, we devised synthetic routes for two large-sized nanoclusters Au100(Napt)42 and Au102(IPBT)44 and characterized their optical and electronic properties. Together with Au102(p-MBA)44 and Au103S2(Napt)41, a family of four 58e NCs is formed, which allows us to compare their atomic and electronic structures, as well as the electron relaxation dynamics. For the atomic structure, the two new NCs are rationalized to possess the same Au79 decahedral kernel as that observed in the crystallographically characterized Au102(p-MBA)44 and Au103S2(Napt)41 NCs, evidenced by the similarity in their steady-state absorption and transient absorption spectral features (e.g., common GSB signals at 525 nm and ESA signals at 580 and 675 nm), as well as comparable ultrafast dynamics. The determination of ultrasmall HOMO–LUMO gaps of Au100(Napt)42 and Au102(IPBT)44 is nontrivial but is successfully done by meticulous DPV analysis. The Eg values (∼0.4 eV) of Au100(Napt)42 and Au102(IPBT)44 are similar to those of their crystallographically characterized counterparts, indicating the decisive role of the kernel in the ground-state electronic properties, while the excited-state dynamics involves both the kernel and surface (i.e., the ligand dependence). Finally, extensive analyses done by summarizing the NCs from Au92(SR)44 to Au279(SR)84 give rise to the scaling relationships between the Eg and size (n), as well as the relationship between the excited-state lifetime and Eg, from which we identified a strong shape effect on Eg and also strong nonradiative decay. Such findings greatly deepen our fundamental understanding of the optical and electronic properties of Aun(SR)m NCs in the large-size regime, which will benefit the future development of applications of such atomically precise materials.Data availability
All the data are available and reported in the manuscript and its ESI.†Conflicts of interest
The authors have no conflicts of interest to declare.Acknowledgements
We thank Dr Meng Zhou for helpful discussions. This material is based upon the work supported as part of the Atomic-C2E project by the U.S. Department of Energy, Office of Science under award number DE-SC-0024716. H. W. acknowledges financial support by the Air Force Office of Scientific Research (AFOSR) under award number FA9550-21-1-0192.References
- H. Qian, M. Zhu, Z. Wu and R. Jin, Acc. Chem. Res., 2012, 45, 1470–1479 CrossRef CAS PubMed.
- T. Kawawaki and Y. Negishi, Dalton Trans., 2023, 52, 15152–15167 RSC.
- M. Zhou, C. Zeng, Y. Song, J. W. Padelford, G. Wang, M. Y. Sfeir, T. Higaki and R. Jin, Angew. Chem., Int. Ed., 2017, 56, 16257–16261 CrossRef CAS PubMed.
- Y. Li, M. Zhou, Y. Song, T. Higaki, H. Wang and R. Jin, Nature, 2021, 594, 380–384 CrossRef CAS PubMed.
- N. Yan, N. Xia, L. Liao, M. Zhu, F. Jin, R. Jin and Z. Wu, Sci. Adv., 2018, 4, eaat7259 CrossRef CAS PubMed.
- J.-Q. Wang, S. Shi, R.-L. He, S.-F. Yuan, G.-Y. Yang, G.-J. Liang and Q.-M. Wang, J. Am. Chem. Soc., 2020, 142, 18086–18092 CrossRef CAS PubMed.
- M. R. Narouz, K. M. Osten, P. J. Unsworth, R. W. Y. Man, K. Salorinne, S. Takano, R. Tomihara, S. Kaappa, S. Malola, C. T. Dinh, J. D. Padmos, K. Ayoo, P. J. N. M. Garrett, J. H. Horton, E. H. Sargent, H. Häkkinen, T. Tsukuda and C. M. Crudden, Nat. Chem., 2019, 11, 419–425 CrossRef CAS PubMed.
- H. Li, P. Wang, C. Zhu, W. Zhang, M. Zhou, S. Zhang, C. Zhang, Y. Yun, X. Kang, Y. Pei and M. Zhu, J. Am. Chem. Soc., 2022, 144, 23205–23213 CrossRef CAS PubMed.
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116, 10346–10413 CrossRef CAS PubMed.
- Z. Lei, X.-K. Wan, S.-F. Yuan, Z.-J. Guan and Q.-M. Wang, Acc. Chem. Res., 2018, 51, 2465–2474 CrossRef CAS PubMed.
- A. V. Zhukhovitskiy, M. J. MacLeod and J. A. Johnson, Chem. Rev., 2015, 115, 11503–11532 CrossRef CAS PubMed.
- R. P. Herrera and M. C. Gimeno, Chem. Rev., 2021, 121, 8311–8363 CrossRef CAS PubMed.
- Y. Li and R. Jin, J. Am. Chem. Soc., 2020, 142, 13627–13644 CrossRef CAS PubMed.
- W. Dong, F. Zhang, T. Li, Y. Zhong, L. Hong, Y. Shi, F. Jiang, H. Zhu, M. Lu, Q. Yao, W. Xu, Z. Wu, X. Bai and Y. Zhang, J. Am. Chem. Soc., 2024, 146, 22180–22192 CrossRef CAS PubMed.
- Y. Li, T. Higaki, X. Du and R. Jin, Adv. Mater., 2020, 32, e1905488 CrossRef PubMed.
- W. Xu, E. Wang and X. Zeng, Acc. Mater. Res., 2024, 5, 1134–1145 CrossRef CAS.
- S. Li, N.-N. Li, X.-Y. Dong, S.-Q. Zang and T. C. W. Mak, Chem. Rev., 2024, 124, 7262–7378 CrossRef CAS PubMed.
- H. Li, X. Kang and M. Zhu, Acc. Chem. Res., 2024, 57, 3194–3205 CrossRef CAS PubMed.
- C. M. Aikens, Acc. Chem. Res., 2018, 51, 3065–3073 CrossRef CAS PubMed.
- A. Ghosh, O. F. Mohammed and O. M. Bakr, Acc. Chem. Res., 2018, 51, 3094–3103 CrossRef CAS PubMed.
- K. Kwak and D. Lee, Acc. Chem. Res., 2019, 52, 12–22 CrossRef CAS PubMed.
- Y. Li, M. J. Cowan, M. Zhou, T.-Y. Luo, Y. Song, H. Wang, N. L. Rosi, G. Mpourmpakis and R. Jin, J. Am. Chem. Soc., 2020, 142, 20426–20433 CrossRef CAS PubMed.
- M. Zhou, T. Higaki, G. Hu, M. Y. Sfeir, Y. Chen, D.-E. Jiang and R. Jin, Science, 2019, 364, 279–282 CrossRef CAS PubMed.
- Y. Negishi, T. Nakazaki, S. Malola, S. Takano, Y. Niihori, W. Kurashige, S. Yamazoe, T. Tsukuda and H. Häkkinen, J. Am. Chem. Soc., 2015, 137, 1206–1212 CrossRef CAS PubMed.
- T. Higaki, M. Zhou, K. J. Lambright, K. Kirschbaum, M. Y. Sfeir and R. Jin, J. Am. Chem. Soc., 2018, 140, 5691–5695 CrossRef CAS PubMed.
- C. Zeng, Y. Chen, K. Kirschbaum, K. J. Lambright and R. Jin, Science, 2016, 354, 1580–1584 CrossRef CAS PubMed.
- L. Xiong and Y. Pei, J. Phys. Chem. C, 2021, 125, 20670–20675 CrossRef CAS.
- M. Zhou, T. Higaki, Y. Li, C. Zeng, Q. Li, M. Y. Sfeir and R. Jin, J. Am. Chem. Soc., 2019, 141, 19754–19764 CrossRef CAS PubMed.
- E. Hulkko, O. Lopez-Acevedo, J. Koivisto, Y. Levi-Kalisman, R. D. Kornberg, M. Pettersson and H. Häkkinen, J. Am. Chem. Soc., 2011, 133, 3752–3755 CrossRef CAS PubMed.
- T. Higaki, C. Liu, M. Zhou, T. Y. Luo, N. L. Rosi and R. Jin, J. Am. Chem. Soc., 2017, 139, 9994–10001 CrossRef CAS PubMed.
- S. Chen, T. Higaki, H. Ma, M. Zhu, R. Jin and G. Wang, ACS Nano, 2020, 14, 16781–16790 CrossRef CAS PubMed.
- C. Zeng, C. Liu, Y. Chen, N. L. Rosi and R. Jin, J. Am. Chem. Soc., 2016, 138, 8710–8713 CrossRef CAS PubMed.
- G. Li, C. Zeng and R. Jin, J. Am. Chem. Soc., 2014, 136, 3673–3679 CrossRef CAS PubMed.
- P. R. Nimmala and A. Dass, J. Am. Chem. Soc., 2014, 136, 17016–17023 CrossRef CAS PubMed.
- L. Luo, Z. Liu, J. Kong, C. G. Gianopoulos, I. Coburn, K. Kirschbaum, M. Zhou and R. Jin, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2318537121 CrossRef CAS PubMed.
- Y. Chen, C. Zeng, C. Liu, K. Kirschbaum, C. Gayathri, R. R. Gil, N. L. Rosi and R. Jin, J. Am. Chem. Soc., 2015, 137, 10076–10079 CrossRef CAS PubMed.
- X. Du, X. Zhang, H. Ma, M. Zhou, T. Higaki, G. Wang, H. Wang and R. Jin, ChemElectroChem, 2024, 11, e202300528 CrossRef CAS.
- S. Mustalahti, P. Myllyperkiö, T. Lahtinen, S. Malola, K. Salorinne, T.-R. Tero, J. Koivisto, H. Häkkinen and M. Pettersson, J. Phys. Chem. C, 2015, 119, 20224–20229 CrossRef CAS.
- Y. Chen, C. Zeng, D. R. Kauffman and R. Jin, Nano Lett., 2015, 15, 3603–3609 CrossRef CAS PubMed.
- C. Zeng, Y. Chen, K. Kirschbaum, K. Appavoo, M. Y. Sfeir and R. Jin, Sci. Adv., 2015, 1, e1500045 CrossRef PubMed.
- K. Iida, M. Noda and K. Nobusada, J. Phys. Chem. C, 2016, 120, 2753–2759 CrossRef CAS.
- M. Zhou, X. Du, H. Wang and R. Jin, ACS Nano, 2021, 15, 13980–13992 CrossRef CAS PubMed.
- W. Zhang, J. Kong, Y. Li, Z. Kuang, H. Wang and M. Zhou, Chem. Sci., 2022, 13, 8124–8130 RSC.
- W. R. Jeffries, S. Malola, M. A. Tofanelli, C. J. Ackerson, H. Häkkinen and K. L. Knappenberger Jr., J. Phys. Chem. Lett., 2023, 14, 6679–6685 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5nr01244a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |

