Open Access Article
Open Access ArticleCucurbit[7]uril encapsulation completely protects reactive imine in weak acid†
Kejia Shi and
Bradley D. Smith
and
Bradley D. Smith *
*
Department of Chemistry and Biochemistry, University of Notre, Dame, 251 Nieuwland Science Hall, Notre Dame, Indiana 46556, USA. E-mail: smith.115@nd.edu
First published on 6th February 2025
Abstract
An imine derived from 4-(N,N-dimethylamino)benzaldehyde and a primary amine undergoes very rapid hydrolysis in weak acid, however, it can be encapsulated within cucurbit[7]uril (CB7) to give a host/guest complex that completely protects the labile imine bond.
Imines (also known as Schiff bases) are formed when primary amines are condensed with aldehydes or ketones.1 Imines are ubiquitous structures in biochemistry and they are commonly employed as linkers or protecting groups in different molecular research fields, including pharmaceutical science and functional materials.2–5 The kinetics and thermodynamics of imine hydrolysis in water are structure and pH dependent. In general imines undergo relatively slow hydrolysis in neutral and basic pH but fast and nearly complete hydrolysis in weakly acidic pH.6 There is an increasing need for effective methods to stabilize imine bonds under specific aqueous conditions, and also innovative strategies that permit controlled hydrolytic cleavage.
One way to improve aqueous stability is to employ structurally modified imines with ortho-substituted hydroxyl or boronic acids that can form stabilizing interactions with the imine nitrogen.7,8 There has also been considerable research on supramolecular methods to capture and protect unmodified imines from hydrolytic attack. One supramolecular strategy is to generate a self-assembled, amphiphilic aggregate, such as a micelle or vesicle, that buries the reactive imine group in a hydrophobic, non-aqueous microenvironment.9,10 An alternative supramolecular strategy is to form a discrete and structurally defined host/guest complex that encapsulates and protects an imine-containing guest molecule within a protective macrocycle or cage. This approach has been used effectively to complex iminium cations derived from secondary amines which are relatively stable compounds with a permanent charge.11–14 In comparison, imine derivatives of primary amines are more easily hydrolysed and supramolecular stabilization is much more challenging. There is a report of high imine stabilization inside a water-soluble spherical polyaromatic cage at neutral pH,15 but capture and protection of an imine in acidic solution is very rare. Here, we describe a new method for supramolecular protection of a protonated imine using cucurbit[7]uril (CB7) a readily-available and non-toxic macrocyclic host molecule.16,17 CB7 is known to promote or inhibit the reactivity of captured guest molecules,18–20 including guests that have a labile C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond.21–23 Focusing on imines derived from primary amines,‡ the group of Nau and coworkers reported that CB7 promoted hydrolysis of an oxime derivative of benzaldehyde in weak acid by rate-enhancement factors of 50 to 285,22 and the group of Liu and coworkers found that the presence of CB7 slightly favoured hydrolysis of a benzaldehyde imine in basic pH.21 In striking contrast to these hydrolysis enhancement effects, we report the first example of an imine that can be captured and completely stabilized by CB7 in weak acid which is a common environmental condition in biomedicine.
N bond.21–23 Focusing on imines derived from primary amines,‡ the group of Nau and coworkers reported that CB7 promoted hydrolysis of an oxime derivative of benzaldehyde in weak acid by rate-enhancement factors of 50 to 285,22 and the group of Liu and coworkers found that the presence of CB7 slightly favoured hydrolysis of a benzaldehyde imine in basic pH.21 In striking contrast to these hydrolysis enhancement effects, we report the first example of an imine that can be captured and completely stabilized by CB7 in weak acid which is a common environmental condition in biomedicine.
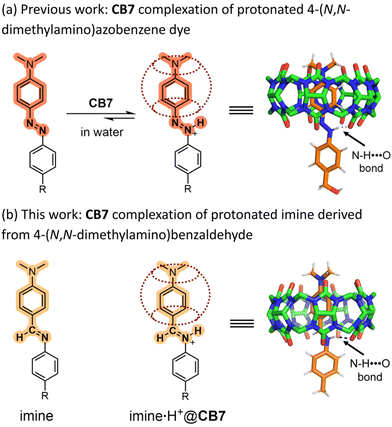
A well-known supramolecular feature of CB7 is its capacity to capture a nitrogen-containing guest molecule as its cationic protonated structure (complexation-induced pKa shift).24 This supramolecular property was apparent in our recent studies of azobenzene complexation by CB7, where we found that CB7 captures 4-(N,N-dimethylamino)azobenzene dyes as their protonated azonium structures with complex stabilization by a combination of attractive intermolecular N–H⋯O and C–H⋯O interactions (Fig. 1a).25,26 We reasoned that structurally similar imine derivatives of 4-(N,N-dimethylamino)benzaldehyde would have the same shape and polarity surface, and thus be complementary guests for CB7 (Fig. 1b). Moreover, complexation inside CB7 should protect the protonated imine from attack by water which is the initiating step for imine hydrolysis in acid.27,28 To test our hypothesis we prepared imine 1 (Scheme 1) and found that it exhibited sufficiently high solubility in weak aqueous acid for analysis by NMR and absorption spectroscopy.
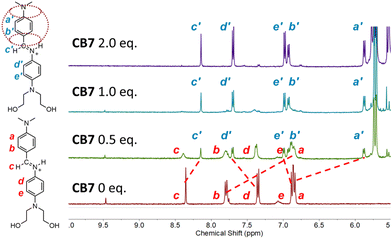
1H NMR spectroscopy was employed to characterize the complexation of imine 1·H+ by CB7 in D2O/acetic acid, pD = 4.70. A titration study mixed 1·H+ with 0–2.0 molar equivalents of CB7. As shown in Fig. 2 and Fig. S1, S2,† the presence of CB7 induced a new set of peaks in the 1H NMR spectra indicating 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex formation and slow host/guest exchange with NMR peak assignments verified by 2D COSY NMR (Fig. S3†). Notably, complexation induced large upfield changes in the chemical shifts of imine protons Ha (Δδmax = −0.97 ppm, where Δδmax = δcomplex − δfree), Hb (Δδmax = −0.85 ppm), and Hc (Δδmax = −0.22 ppm), along with a downfield change in chemical shift of Hd (Δδmax = +0.35 ppm). In contrast, He and peaks for the N,N-diethanolamino protons remained largely unchanged (Fig. S1†), suggesting that these structural regions were not encapsulated by CB7. A 2D NOESY specrum of the 1·H+@CB7 complex was not very informative (Fig. S4†). Therefore, we employed density functional theory (DFT) to calculate the low-energy structure of the 1·H+@CB7 complex in water and identify the important atomic features. For simplicity and clarity, the N,N-diethanolamino group was modelled as an N,N-dimethylamino group. The calculated structure in Fig. 1b and Fig. S15† is highly consistent with the observed complexation-induced changes in 1H NMR chemical shift (Fig. 2). Together, the NMR data and molecular modelling reveals that the upper aryl ring of the imine structure is a complementary match for the hydrophobic cavity of CB7, and that the 1·H+@CB7 complex is stabilized by specific directional bonds to oxygen atoms within each portal of the surrounding CB7, namely, (i) NH⋯O hydrogen bond between the iminium NH and an oxygen within the bottom portal; (ii) weak CH⋯O interactions between the six N,N-dimethylamino hydrogens and the oxygens within the top portal.29
1 complex formation and slow host/guest exchange with NMR peak assignments verified by 2D COSY NMR (Fig. S3†). Notably, complexation induced large upfield changes in the chemical shifts of imine protons Ha (Δδmax = −0.97 ppm, where Δδmax = δcomplex − δfree), Hb (Δδmax = −0.85 ppm), and Hc (Δδmax = −0.22 ppm), along with a downfield change in chemical shift of Hd (Δδmax = +0.35 ppm). In contrast, He and peaks for the N,N-diethanolamino protons remained largely unchanged (Fig. S1†), suggesting that these structural regions were not encapsulated by CB7. A 2D NOESY specrum of the 1·H+@CB7 complex was not very informative (Fig. S4†). Therefore, we employed density functional theory (DFT) to calculate the low-energy structure of the 1·H+@CB7 complex in water and identify the important atomic features. For simplicity and clarity, the N,N-diethanolamino group was modelled as an N,N-dimethylamino group. The calculated structure in Fig. 1b and Fig. S15† is highly consistent with the observed complexation-induced changes in 1H NMR chemical shift (Fig. 2). Together, the NMR data and molecular modelling reveals that the upper aryl ring of the imine structure is a complementary match for the hydrophobic cavity of CB7, and that the 1·H+@CB7 complex is stabilized by specific directional bonds to oxygen atoms within each portal of the surrounding CB7, namely, (i) NH⋯O hydrogen bond between the iminium NH and an oxygen within the bottom portal; (ii) weak CH⋯O interactions between the six N,N-dimethylamino hydrogens and the oxygens within the top portal.29
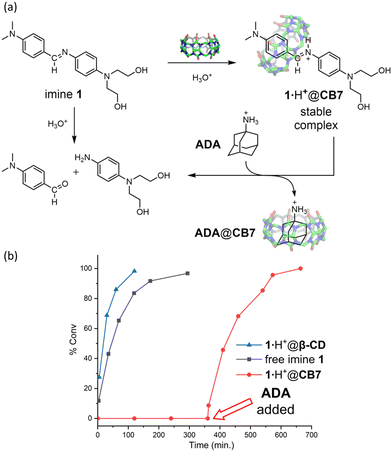
We next used 1H NMR spectroscopy to track the hydrolysis of imine 1·H+ in weak acid (Fig. 3a). A solution of 1·H+ (1.0 mM) in D2O/acetic acid, pD = 4.70, was monitored over time, and the extent of hydrolysis (% conversion) was determined by integrating the NMR peaks (Fig. S7†). Standard kinetic analysis of the plot in Fig. 3b produced a half-life t1/2 = 44.7 min (k = 1.55 × 10−2 M min−1) for the hydrolysis of free 1·H+. In contrast, 1H NMR spectra of a solution containing imine 1·H+ (1 mM) + CB7 (2.0 mM) exhibited no significant change over a period of 2 weeks, indicating a highly stable 1·H+@CB7 complex (Fig. S9†). In comparison, a second NMR experiment monitored a solution of imine 1·H+ (1 mM) plus β-cyclodextrin (β-CD, 2.0 mM), a widely used container macrocycle with similar cavity size as CB7, and, observed rapid hydrolysis of imine 1·H+ (Fig. 3b and Fig. S8†) with a half-life t1/2 = 17.3 min. These results show clearly that CB7 has a unique capability to capture imine 1·H+ and completely prevent its hydrolysis in weak acid.§
Additional NMR evidence that imine 1·H+ was encapsulated and protected by CB7 was gained by conducting a guest displacement experiment using 1-adamantylamine (ADA) which is well-known to have ultra-high affinity for the cavity of CB7 (Fig. 3a). As shown by the kinetic plot in Fig. 3b, multiple NMR spectra of a solution containing 1·H+ (1.0 mM) + CB7 (2.0 mM) in D2O/acetic acid, pD = 4.70, indicated no imine hydrolysis after 360 min. At that timepoint an aliquot of ADA (2.0 mM) was added, which displaced 1·H+ from the CB7 cavity and triggered imine hydrolysis at the same rapid rate as free imine (Fig. S10†).
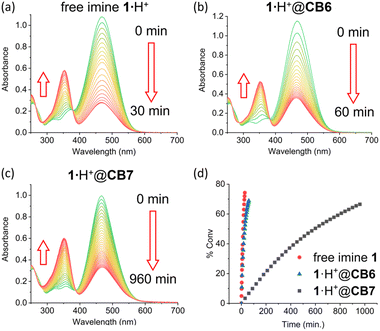
The supramolecular protection of imine 1·H+ by CB7 in weak acid was also characterized at much lower concentration using absorption spectroscopy (Fig. 4). An absorption spectrum of free 1·H+ (25 μM) in pH 6.0 citrate buffer exhibited an intense peak at 460 nm corresponding to the protonated iminium cation,30,31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ¶ and this peak disappeared upon imine hydrolysis with a half-life (t1/2 = 14.1 min (k = 4.93 × 10−2 M min−1)). In comparison, imine hydrolysis for a solution containing imine 1·H+ (25 μM) + CB7 (500 μM) was much slower with a half-life t1/2 = 602 min (k = 1.15 × 10−3 M min−1). While the protection of imine 1·H+ by CB7 was still quite substantial (forty-three times longer half-life), the effect was not as comprehensive as the NMR experiment because not all 1·H+ was complexed by the CB7 at the lower concentrations used for the absorption study. Another absorption-based experiment tested the capacity of cucurbit[6]uril (CB6) to inhibit hydrolysis of imine 1 in pH 6.0 citrate buffer and observed very little stabilization (Fig. 4d), with a half-life t1/2 = 29.5 min (k = 2.35 × 10−2 M min−1). Apparently, the CB6 cavity size is too small to fully encapsulate and stabilize 1·H+.
¶ and this peak disappeared upon imine hydrolysis with a half-life (t1/2 = 14.1 min (k = 4.93 × 10−2 M min−1)). In comparison, imine hydrolysis for a solution containing imine 1·H+ (25 μM) + CB7 (500 μM) was much slower with a half-life t1/2 = 602 min (k = 1.15 × 10−3 M min−1). While the protection of imine 1·H+ by CB7 was still quite substantial (forty-three times longer half-life), the effect was not as comprehensive as the NMR experiment because not all 1·H+ was complexed by the CB7 at the lower concentrations used for the absorption study. Another absorption-based experiment tested the capacity of cucurbit[6]uril (CB6) to inhibit hydrolysis of imine 1 in pH 6.0 citrate buffer and observed very little stabilization (Fig. 4d), with a half-life t1/2 = 29.5 min (k = 2.35 × 10−2 M min−1). Apparently, the CB6 cavity size is too small to fully encapsulate and stabilize 1·H+.
It is worth noting that the hydrolysis of imine 1 in the absence of CB7 is unusually slow when compared to other aryl aldehyde imines. For example, the homologous imine derivative of benzaldehyde (i.e., imine 2 in Scheme 1) undergoes instant hydrolysis in weak acid with little stabilization gained by the presence of CB7 (Fig. S11 and S12†). This reactivity difference reflects the capacity of the strongly electron donating 4-(N,N-dimethylamino) substituent to reduce the imine electrophilicity within 1·H+.32 This intramolecular imine stabilization effect is further enhanced by the structurally precise supramolecular complexation of 1·H+ by CB7 which sterically prevents attack of the protonated imine group by water. We speculate that the encapsulated 1·H+ is clamped within the surrounding CB7 by the simultaneous electrostatic interactions with both CB7 portals (Fig. 1b) which reduces transient exposure of the labile imine group to nucleophilic attack by surrounding water.
In conclusion, CB7 has a remarkable capacity to capture an imine derivative of 4-(N,N-dimethylamino)benzaldehyde (imine 1) and extend the half-life for hydrolysis in weak acid from minutes to many hours or even weeks depending on concentration.‡ The protective effect is maximal in weak acid where the imine is protonated and it can be instantly reversed by molecular displacement of the imine guest from the CB7 cavity. It should be possible to exploit this discovery for development of supramolecular formulations that stabilize valuable imine derivatives of primary amines under acidic conditions, with potential applications in pharmaceutical science and advanced functional materials.5,33–35
Data availability
All experimental procedures and associated data are provided in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the US National Science Foundation under grant CHE-2103598.References
- T. T. Tidwell, Angew. Chem., Int. Ed., 2008, 47, 1016–1020 CrossRef PubMed.
- C. Ding, C. Chen, X. Zeng, H. Chen and Y. Zhao, ACS Nano, 2022, 16, 13513–13553 CrossRef PubMed.
- J. Xu, Y. Liu and S. Hsu, Molecules, 2019, 24, 3005 CrossRef PubMed.
- X. Qu and Z. Yang, Chem. – Asian J., 2016, 11, 2633–2641 CrossRef PubMed.
- X. Hu, A. M. Jazani and J. K. Oh, Polymer, 2021, 230, 124024 CrossRef.
- I. A. Müller, F. Kratz, M. Jung and A. Warnecke, Tetrahedron Lett., 2010, 51, 4371–4374 CrossRef.
- S. Cambray and J. Gao, Acc. Chem. Res., 2018, 51, 2198–2206 CrossRef PubMed.
- J. Weaver, G. B. Craven, L. Tram, H. Chen and J. Taunton, J. Am. Chem. Soc., 2024, 146, 24233–24237 CrossRef PubMed.
- D. Sawada, A. Hirono, K. Asakura and T. Banno, RSC Adv., 2020, 10, 34247–34253 RSC.
- F. Liu, N. Anton, Y. Niko and A. S. Klymchenko, ACS Appl. Bio Mater., 2023, 6, 246–256 CrossRef CAS.
- T. He, X. Hu and S. Liu, Org. Lett., 2023, 25, 246–250 CrossRef CAS PubMed.
- M. S. Linares and M. R. Longhi, Pharmazie, 2003, 58, 32–37 CAS.
- Z. Miskolczy, M. Megyesi, G. Tárkányi, R. Mizsei and L. Biczók, Org. Biomol. Chem., 2011, 9, 1061–1070 RSC.
- V. M. Dong, D. Fiedler, B. Carl, R. G. Bergman and K. N. Raymond, J. Am. Chem. Soc., 2006, 128, 14464–14465 CrossRef PubMed.
- M. Yuasa, R. Sumida, Y. Tanaka and M. Yoshizawa, Chem. – Eur. J., 2022, 28, e202104101 CrossRef.
- K. I. Kuok, S. Li, I. W. Wyman and R. Wang, Ann. N. Y. Acad. Sci., 2017, 1398, 108–119 CrossRef.
- D. Das, K. I. Assaf and W. M. Nau, Front. Chem., 2019, 7, 619 CrossRef PubMed.
- K. Droguett, G. E. Quintero, J. G. Santos and M. E. Aliaga, J. Inclusion Phenom. Macrocyclic Chem., 2023, 103, 1–20 CrossRef.
- S. Moorthy, A. C. Bonillo, H. Lambert, E. Kalenius and T.-C. Lee, Chem. Commun., 2022, 58, 3617–3620 RSC.
- B. Tang, J. Zhao, J. Xu and X. Zhang, Chem. – Eur. J., 2020, 26, 15446–15460 CrossRef PubMed.
- W. Gong, J. Ma, Z. Zhao, F. Gao, F. Liang, H. Zhang and S. Liu, J. Org. Chem., 2017, 82, 3298–3301 CrossRef PubMed.
- C. Klöck, R. N. Dsouza and W. M. Nau, Org. Lett., 2009, 11, 2595–2598 CrossRef PubMed.
- J. J. Alcázar, N. Geue, V. Valladares, A. Cañete, E. G. Pérez, L. García-Río, J. G. Santos and M. E. Aliaga, ACS Omega, 2021, 6, 10333–10342 CrossRef.
- I. Ghosh and W. M. Nau, Adv. Drug Delivery Rev., 2012, 64, 764–783 CrossRef PubMed.
- S. S. R. Kommidi and B. D. Smith, Molecules, 2022, 27, 5440 CrossRef CAS PubMed.
- S. S. R. Kommidi and B. D. Smith, J. Org. Chem., 2023, 88, 8431–8440 CrossRef CAS.
- A. V. Willi and R. E. Robertson, Can. J. Chem., 1953, 31, 361–376 CrossRef.
- R. W. Layer, Chem. Rev., 1963, 63, 489–510 CrossRef.
- G. Desiraju and T. Steiner, The Weak Hydrogen Bond: In Structural Chemistry and Biology, Oxford University Press, 2001, pp. 1–28 Search PubMed.
- R. L. Reeves and W. F. Smith, J. Am. Chem. Soc., 1963, 85, 724–728 CrossRef.
- A. Saeed, Indian J. Chem., 1979, 17, 105–107 Search PubMed.
- H. Dehne and H.-H. Rüttinger, Z. Chem., 1973, 13, 138–139 CrossRef.
- A. K. Gilbert, Y. Zhao, C. E. Otteson and M. D. Pluth, J. Org. Chem., 2019, 84, 14469–14475 CrossRef PubMed.
- Z. Ding, J. Cen, Y. Wu, K. Zhong, G. Liu, J. Hu and S. Liu, Giant, 2020, 1, 100012 CrossRef.
- K. Bairagi, J. T. Liu, A. Thinphang-nga and J. K. Oh, Macromolecules, 2023, 56, 4307–4317 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedure, compound and complex characterization, hydrolysis and titration data, and computational modelling. See DOI: https://doi.org/10.1039/d4ob01879f |
| ‡ As shown in ref. 21, CB7 can capture and protect cationic iminium guests derived from a secondary amine, which is distinct but complementary to the focus here on a protonatable imine guest derived from a primary amine. |
| § The hydrolysis experiment was repeated for imine 1 in neutral pH D2O with 2% DMSO-d6. The results revealed a half-life of ∼50 min for the hydrolysis of free 1, and ∼120 min for 1 in the presence of CB7 (Fig. S13 and S14†). The corresponding NMR spectra suggest that the relatively weak protection provided by CB7 at neutral pH is due to weaker imine guest affinity and a faster rate of host/guest exchange. |
| ¶ Absorption spectroscopy was used to determine a pKa of 7.51 ± 0.01 for imine 1·H+ and 9.71 ± 0.03 for 1·H+@CB7 complex (Fig. S5†), thus confirming the expectation of a large complexation-induced pKa shift. The large complexation-induced change in absorption spectrum enabled determination of the association constant for formation of 1·H+@CB7 in pH 8.80, sodium bicarbonate buffer (10 mM) to be 1.0 × 106 M−1 (Fig. S6†). |
| This journal is © The Royal Society of Chemistry 2025 |