Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
In pursuit of larger lipophilicity enhancement: an investigation of sugar deoxychlorination†
Jonas
Van De Velde
 a,
Ariadna
Calderón Rodríguez
a,
Zhong
Wang
b,
David E.
Wheatley
a,
Ariadna
Calderón Rodríguez
a,
Zhong
Wang
b,
David E.
Wheatley
 b and
Bruno
Linclau
b and
Bruno
Linclau
 *ab
*ab
aDepartment of Organic and Macromolecular Chemistry, Ghent University, Ghent 9000, Belgium. E-mail: Bruno.linclau@ugent.be
bSchool of Chemistry, University of Southampton, Southampton SO17 1BJ, UK
First published on 5th February 2025
Abstract
The excessive hydrophilicity of carbohydrates hampers their application in drug discovery. Deoxyfluorination is one of the strategies to increase sugar lipophilicity. However, lipophilicities of dideoxy-difluorinated monosaccharides are still well below the desired range for oral drug candidates. Here we investigate the power of deoxychlorination to increase sugar lipophilicities. A series of dideoxygenated chloro-fluorosugars was synthesized and for these substrates it was shown that deoxychlorination increased the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P by an average of 1.37
P by an average of 1.37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P units, compared to 0.83
P units, compared to 0.83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P units for analogous deoxyfluorination. This shows the potential of deoxychlorination of carbohydrates to increase lipophilicity while limiting the number of potentially important hydrogen bond donating groups to be sacrificed, and will be of interest for glycomimetic development.
P units for analogous deoxyfluorination. This shows the potential of deoxychlorination of carbohydrates to increase lipophilicity while limiting the number of potentially important hydrogen bond donating groups to be sacrificed, and will be of interest for glycomimetic development.
Given the pivotal role of carbohydrates in human health,1 there is much interest in investigating and manipulating protein–carbohydrate interactions or activities of carbohydrate-processing enzymes.2 The sugar scaffold itself is a very challenging starting point for drug development, with its very high hydrophilicity/very low lipophilicity (log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) as one of the main reasons.3 One of the strategies in glycomimetic design thus rests on reducing the hydrophilic character, for example by the functionalization of sugar alcohols with apolar groups, alcohol deoxygenation or deoxyfluorination.4–7 Our group reported a straightforward method for lipophilicity determination of the non UV-active fluorinated carbohydrates, and it was established that each successive deoxyfluorination increased the log
P) as one of the main reasons.3 One of the strategies in glycomimetic design thus rests on reducing the hydrophilic character, for example by the functionalization of sugar alcohols with apolar groups, alcohol deoxygenation or deoxyfluorination.4–7 Our group reported a straightforward method for lipophilicity determination of the non UV-active fluorinated carbohydrates, and it was established that each successive deoxyfluorination increased the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P by an order of magnitude, with variations depending on fluorination position and stereochemistry.8 This latter aspect has been further investigated in detail by the Giguère group.9,10
P by an order of magnitude, with variations depending on fluorination position and stereochemistry.8 This latter aspect has been further investigated in detail by the Giguère group.9,10
While there are reports that chlorinated glycans bind to proteins, including examples with higher and lower affinity,11 it is remarkable that compared to sugar fluorination,12 sugar chlorination is much less investigated in glycomimetic design. This is surprising given that sucralose (Fig. 1), a trichlorinated sucrose derivative which is used as an artificial sweetener,13,14 is arguably the most synthesized halogenated sugar. It is resistant against enzymatic hydrolysis – hence its non-calorific properties – and generally possesses good chemical stability due to the strengthening of the C–Cl bonds by the combined effect of the many electronegative substituents.
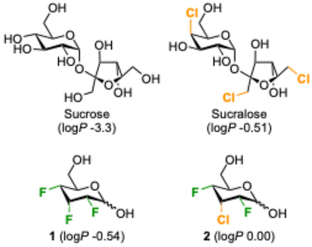 | ||
| Fig. 1 Examples of increase in lipophilicity upon deoxychlorination or fluorine-chlorine replacement of sugars.17–19 | ||
Chlorine introduction is also very well established in drug development, albeit mostly on aromatic rings, as a monovalent hydrophobic substituent. There is the possibility for beneficial halogen bonding effects, which in some cases contributes to marked affinity increases, and chlorination is typically associated with a lipophilicity increase on a par with methyl group introduction.15,16 Lipophilicity information for chlorinated sugars is scarce. The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P of sucralose (−0.51
P of sucralose (−0.51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P units)17 is three orders of magnitude higher than that of sucrose (−3.3 units).18 Recently the Giguère group reported the higher lipophilicity of the chlorodifluoroallose analogue 2 compared to its trifluorinated analogue 1.19
P units)17 is three orders of magnitude higher than that of sucrose (−3.3 units).18 Recently the Giguère group reported the higher lipophilicity of the chlorodifluoroallose analogue 2 compared to its trifluorinated analogue 1.19
The alcohol groups in sugars are often essential hydrogen bond donors and/or acceptors in a binding event, imposing limitations on the number of alcohol groups that can be sacrificed for increasing lipophilicity. Hence, methods to maximise the increase in lipophilicity without significant addition to the sugar conformation and steric footprint are of interest. In this context, we became interested in investigating sugar deoxychlorination and to quantify the effect of deoxychlorination on sugar lipophilicity. In this communication, we report on the effect of mono-deoxychlorination of sugars (Fig. 2).
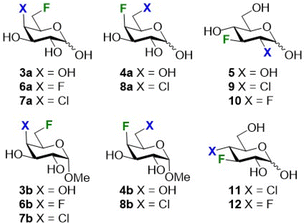
The selection of the substrates was in the first instance guided both by synthetic and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P determination considerations. As starting points we used 6- and 4-deoxyfluorogalactose (3a and 4a), as well as 3-fluoroglucose 5, with the fluorine atom serving as handle for 19F NMR based log
P determination considerations. As starting points we used 6- and 4-deoxyfluorogalactose (3a and 4a), as well as 3-fluoroglucose 5, with the fluorine atom serving as handle for 19F NMR based log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P determination.8 The corresponding methyl galactosides 3b and 4b were also investigated (Fig. 2).
P determination.8 The corresponding methyl galactosides 3b and 4b were also investigated (Fig. 2).
The monofluorinated galactoses at C6 (3a–b) and C4 (4a–b) were obtained as described in the literature,20–22 and the difluorinated analogues 6a–b were synthesized starting from methyl α-D-glucopyranoside as reported by our group (not shown).23 The novel galactose derivatives 7a and 8a (Scheme 1) were synthesised from known 7b and 8b, both also obtained from commercially available methyl α-D-glucopyranoside,23 by anomeric hydrolysis in good yields.
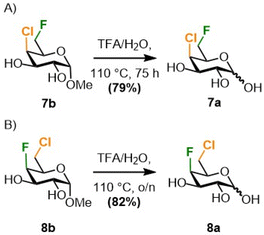 | ||
| Scheme 1 Anomeric hydrolysis of the methyl chloro-fluorogalctosides 7b23 and 8b23 towards the reducing sugars 7a and 8a. | ||
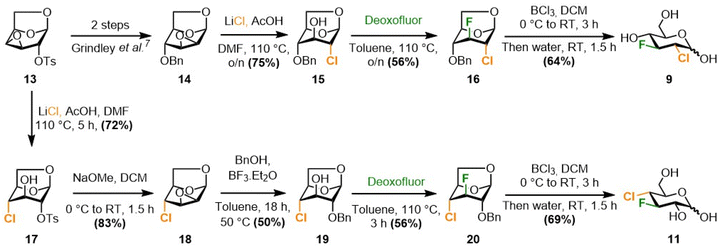
The synthesis of 9 and 11 (Scheme 2) was achieved from levoglucosan, using a synthetic route that mirrored the known8,10 syntheses of the corresponding difluorinated sugars. The two required epoxide intermediates, 13 and 14, are easily available from levoglucosan on multigram scale,24,25,26–28 and were chosen as handles for chlorine introduction. Procter et al. had reported that reaction of 14 with in situ generated allyl magnesium chloride in THF as solvent delivered the 2-deoxy-2-chloro derivative 15 in 76% yield instead of the aniticipated allylation product.24 However, in our hands, reaction of a commercially available 2 M solution of allyl magnesium chloride in THF with 14 led to the allylation product. A procedure by Paulsen et al., in which reaction of 13 with an ammonium fluoride and chloride mixture was reported to give 17,25 gave no conversion. In contrast, a method using lithium chloride, reported by Sofian and Lee on disaccharides,29 successfully afforded compound 15 from 14 in good yield. This reaction could easily be upscaled to a 3 g scale. The same method was then used to synthesize the 4-deoxy-4-chloro derivative 17 from 13. Treatment of the latter with base afforded the 2,3-anhydro group in 18,25 which allowed benzyloxy introduction at the 2-position. With 15 and 19 in hand, the stage was set for fluorine introduction at C3, which is typically effected by DAST or Deoxyfluor with retention of configuration.30,31 In both cases, this reaction was successful, delivering the 2,3-dideoxy-2-chloro-3-fluoro and 3,4-dideoxy-4-chloro-3-fluoro derivatives 16 and 20, both in 56% yield. A chlorine atom is a more powerful partner in neighboring group participation, potentially leading to a weaker bond between the chlorine and C2/C4, yet the epoxide opening remained fully regioselective, as dictated by the Fürst–Plattner effect32 (chairlike transition state). The regio- and stereoselective introduction of the C–F bond was easily established by 1H and 19F J-value analysis. Finally, preparation of the desired final compounds 9 and 11 could be established by hydrolytic cleavage of the 1,6-anhydro bridge with concomittent benzyl group removal in good yields.
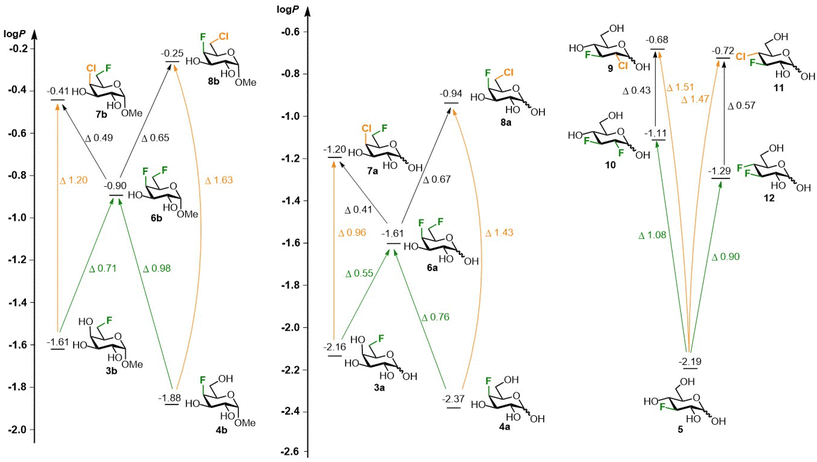
The lipophilicity data of the chloro-fluorosugars is shown in Fig. 3. The chlorinated sugar derivatives invariably have a higher lipophilicity compared to the corresponding fluorinated derivatives. The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values of the regioisomeric 4,6-dihalogenated galactoses 7b (log
P values of the regioisomeric 4,6-dihalogenated galactoses 7b (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P −0.41) and 8b (log
P −0.41) and 8b (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P −0.25), have an appreciable difference and are higher than the difluorinated analogue 6b (log
P −0.25), have an appreciable difference and are higher than the difluorinated analogue 6b (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P −0.90, average increase of 0.57
P −0.90, average increase of 0.57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P units). Compared to the corresponding monofluorinated saccharides 3b/4b (log
P units). Compared to the corresponding monofluorinated saccharides 3b/4b (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P −1.61/−1.88) there is a significant increase of 1.20/1.63
P −1.61/−1.88) there is a significant increase of 1.20/1.63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P units upon deoxychlorination at C4/C6. In contrast, analogous deoxyfluorination ‘only’ delivers a 0.71/0.98
P units upon deoxychlorination at C4/C6. In contrast, analogous deoxyfluorination ‘only’ delivers a 0.71/0.98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P increase. A similar picture is seen for the more polar reducing halogenated galactose equivalents 3a–8a, with similar differences between the difluorinated galactose 6a (log
P increase. A similar picture is seen for the more polar reducing halogenated galactose equivalents 3a–8a, with similar differences between the difluorinated galactose 6a (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P −1.61)33 and the chlorofluorogalactoses 7a/8a (log
P −1.61)33 and the chlorofluorogalactoses 7a/8a (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P −1.20/−0.94), but with slightly reduced differences compared to the monofluorinated galactoses 3a and 4a (log
P −1.20/−0.94), but with slightly reduced differences compared to the monofluorinated galactoses 3a and 4a (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P −2.16/−2.37). This is due to the lower increase in lipophilicity upon methyl glycosidation of the monofluorinated galactoses compared to the dihalogenated ones (the difference between 3a/4a with 3b/4b is ∼0.53
P −2.16/−2.37). This is due to the lower increase in lipophilicity upon methyl glycosidation of the monofluorinated galactoses compared to the dihalogenated ones (the difference between 3a/4a with 3b/4b is ∼0.53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P units, compared to ∼0.73 for the other derivatives). The reducing glucoses show larger lipophilicity differences. The log
P units, compared to ∼0.73 for the other derivatives). The reducing glucoses show larger lipophilicity differences. The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P-values of 9 (log
P-values of 9 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P −0.68) and 11 (log
P −0.68) and 11 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P −0.72) are very similar, with a 1.5
P −0.72) are very similar, with a 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P increase compared to 3-fluoroglucose 5 (log
P increase compared to 3-fluoroglucose 5 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P −2.19).10 The corresponding difluorinated glucoses 10 (log
P −2.19).10 The corresponding difluorinated glucoses 10 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P −1.11)8 and 12 (log
P −1.11)8 and 12 (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P −1.29)10 have a larger difference in lipophilicity but on average, the lipophilicity increase compared to 5 is ‘only’ 1.0
P −1.29)10 have a larger difference in lipophilicity but on average, the lipophilicity increase compared to 5 is ‘only’ 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P units.
P units.
In summary, the synthesis of a series of dideoxygenated chloro-fluoro galactoses and glucoses has been achieved and their lipophilicities were determined. These values were compared to those measured for analogous difluorinated and monofluorinated monosaccharides. It was established that deoxychlorination leads to an increase of the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P with an average of 1.37
P with an average of 1.37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P units, compared to 0.83
P units, compared to 0.83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P units for analogous deoxyfluorination. Substitution of fluorine for chlorine in carbohydrates thus results in a significant average increase in lipophilicity of 0.54
P units for analogous deoxyfluorination. Substitution of fluorine for chlorine in carbohydrates thus results in a significant average increase in lipophilicity of 0.54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P units. With these results, we show that deoxychlorination is a powerful tool to increase lipophilicity while limiting the number of potentially important hydrogen bond donating groups to be sacrificed, which will be of interest in glycomimetic design.
P units. With these results, we show that deoxychlorination is a powerful tool to increase lipophilicity while limiting the number of potentially important hydrogen bond donating groups to be sacrificed, which will be of interest in glycomimetic design.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the Research Foundation Flanders (FWO, Odysseus Type I G0F5621N), and by the Industrial Biotechnology Catalyst (Innovate UK, BBSRC, EPSRC, BB/M028941/1). We also thank the EPSRC (core capability EP/K039466/1) for funding, The FWO (G011015N and I006920N) and Ghent University (BOF.BAS.20200019.01) are thanked for equipment grants.References
- A. Varki, Glycobiology, 2017, 27, 3–49 CrossRef CAS PubMed.
- C. R. Bertozzi and L. L. Kiessling, Science, 2001, 291, 2357–2364 CrossRef CAS PubMed.
- B. Ernst and J. L. Magnani, Nat. Rev. Drug Discovery, 2009, 8, 661–677 CrossRef CAS PubMed.
- R. Hevey, Pharmaceuticals, 2019, 12, 55 CrossRef CAS PubMed.
- R. Hevey, Biomimetics, 2019, 4, 53 CrossRef CAS PubMed.
- A. Tamburrini, C. Colombo and A. Bernardi, Med. Res. Rev., 2020, 40, 495–531 CrossRef CAS.
- X. Wei, P. Wang, F. Liu, X. Ye and D. Xiong, Molecules, 2023, 28, 6641 CrossRef CAS PubMed.
- B. Linclau, Z. Wang, G. Compain, V. Paumelle, C. Q. Fontenelle, N. Wells and A. Weymouth-Wilson, Angew. Chem., Int. Ed., 2016, 55, 674–678 CrossRef CAS PubMed.
- J. St-Gelais, M. Bouchard, V. Denavit and D. Giguère, J. Org. Chem., 2019, 84, 8509–8522 CrossRef CAS PubMed.
- J. St-Gelais, É. Côté, D. Lainé, P. A. Johnson and D. Giguère, Chem. – Eur. J., 2020, 26, 13499–13506 CrossRef CAS.
- A. Maradufu and A. S. Perlin, Carbohydr. Res., 1974, 32, 93–99 CrossRef CAS.
- R. Hevey, Chem. – Eur. J., 2021, 27, 2240–2253 CrossRef CAS PubMed.
- L. Hough and S. P. Phadnis, Nature, 1976, 263, 800–800 CrossRef CAS.
- L. Hough and R. Khan, Trends Biochem. Sci., 1978, 3, 61–63 CrossRef CAS.
- D. Chiodi and Y. Ishihara, J. Med. Chem., 2023, 66, 5305–5331 CrossRef CAS PubMed.
- C. Hansch, A. Leo, S. H. Unger, K. H. Kim, D. Nikaitani and E. J. Lien, J. Med. Chem., 1973, 16, 1207–1216 CrossRef CAS PubMed.
- M. R. Jenner and A. Smithson, J. Food Sci., 1989, 54, 1646–1649 CrossRef CAS.
- M. F. Mazzobre, M. V. Roman, A. F. Mourelle and H. R. Corti, Carbohydr. Res., 2005, 340, 1207–1211 CrossRef CAS PubMed.
- O. Lessard, D. Lainé, C.-É. Fecteau, P. A. Johnson and D. Giguère, Org. Chem. Front., 2022, 9, 6566–6572 RSC.
- M.-C. Chapeau and P. A. Frey, J. Org. Chem., 1994, 59, 6994–6998 CrossRef CAS.
- C.-L. Schengrund and P. Kovàc, Carbohydr. Res., 1999, 319, 24–28 CrossRef CAS PubMed.
- P. Keitz, G. Bergnes, M. A. Gallop and M. Quarta, US Pat, 2023039292A2, 2023 Search PubMed.
- D. E. Wheatley, C. Q. Fontenelle, R. Kuppala, R. Szpera, E. L. Briggs, J. B. Vendeville, N. J. Wells, M. E. Light and B. Linclau, J. Org. Chem., 2021, 86, 7725–7756 CrossRef CAS PubMed.
- G. Procter and D. Genin, Carbohydr. Res., 1990, 202, 81–92 CrossRef CAS.
- H. Paulsen, B. Schröder, H. Böttcher and R. Hohlweg, Chem. Ber., 1981, 114, 322–332 CrossRef CAS.
- M. Cerny, I. Buben and J. Pacak, Collect. Czech. Chem. Commun., 1963, 28, 1569–1578 CrossRef.
- T. B. Grindley, G. J. Reimer, J. Kralovec, R. G. Brown and M. Anderson, Can. J. Chem., 1987, 65, 1065–1071 CrossRef CAS.
- M. Oikawa, T. Shintaku, H. Sekljic, K. Fukase and S. Kusumoto, Bull. Chem. Soc. Jpn., 1999, 72, 1857–1867 CrossRef CAS.
- A. S. Sofian and C. Kuan Lee, J. Carbohydr. Chem., 2003, 22, 185–206 CrossRef CAS.
- L. Mtashobya, L. Quiquempoix and B. Linclau, J. Fluorine Chem., 2015, 171, 92–96 CrossRef CAS.
- V. Denavit, D. Laine, C. Bouzriba, E. Shanina, E. Gillon, S. Fortin, C. Rademacher, A. Imberty and D. Giguère, Chem. – Eur. J., 2019, 25, 4478–4490 CrossRef CAS PubMed.
- A. Fürst and P. A. Plattner, Helv. Chim. Acta, 1949, 32, 275–283 CrossRef.
- Z. Wang, H. R. Felstead, R. I. Troup, B. Linclau and P. T. F. Williamson, Angew. Chem., Int. Ed., 2023, 62, e202301077 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ob00037h |
| This journal is © The Royal Society of Chemistry 2025 |