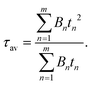Open Access Article
Open Access ArticleFlexible and rigid “chirally distorted” π-systems: binaphthyl conjugates as organic CPL-active chromophores†
Giovanni
Preda
a,
Elisa Maria
Ciccarello
a,
Alessio
Bianchi
a,
Francesco
Zinna
 b,
Chiara
Botta
c,
Lorenzo
Di Bari
b,
Chiara
Botta
c,
Lorenzo
Di Bari
 b and
Dario
Pasini
b and
Dario
Pasini
 *a
*a
aDepartment of Chemistry and INSTM, University of Pavia Via Taramelli 12, 27100 Pavia, Italy. E-mail: dario.pasini@unipv.it
bDipartimento di Chimica e Chimica Industriale, Università di Pisa, Via Giuseppe Moruzzi 13, 56124 Pisa, Italy
cSCITEC−CNR, Consiglio Nazionale delle Ricerche, Istituto di Scienze e Tecnologie Chimiche ‘G. Natta’, Via A. Corti 12, 20133 Milano, Italy
First published on 19th February 2025
Abstract
The inclusion of high-performance dyes into chiral π-conjugated systems is an effective strategy for activating significant chiroptical properties. We report the preparation and characterization of configurationally stable, axially-chiral π-conjugated systems in which acridone or 2,5-diarylamino-terephthalate has been fused into the chiral scaffold of a 1,1′-binaphthyl moiety. The high-yielding synthesis afforded π-conjugated systems with characteristics essentially matching those of the parent dyes while introducing detectable CPL activity in solution. In the acridone conjugate, good fluorescence is maintained in solution, but in the solid state, the distortion introduced by the binaphthyl system does not substantially help in restoring emissive properties; the flexibility and the emissive properties of the 2,5-diphenylamino-terephthalate chromophore are maintained in the conjugate. The new chiral chromophoric systems show absorption in the UV-vis domain, with good fluorescence properties in the visible range (quantum yields up to 23% and glum values up to 4 × 10−4).
Introduction
Chiral organic chromophores are a subject of increasing interest because they can absorb and emit polarized light, which can be measured through circular dichroism (CD) and circularly polarized luminescence (CPL) techniques, respectively. CPL can provide specific further information about the system, and CPL-active materials find potential applications in 3D displays, chiroptical materials, and optical sensors.1,2A variety of chiral organic emitters have been proposed as CPL materials. Lanthanide-based chiral metal complexes exhibit excellent chiroptical properties and unsurpassed glum values;3 however, problems associated with their strategic sourcing, use and disposal have significantly increased interest in the development of CPL-active soft organic materials. The main advantage in using organic molecules to build CPL-active systems arises from the possibility of regulating their emission in response to changes in the energy levels of their excited states. Unlike inorganic systems, organic molecules do not yet show sufficiently high gabs and glum values, so to find suitable solutions, approaches have been pursued towards unusual combinations of molecular architectures and/or their self-assembly into nanoaggregates, in order to boost CPL activity.4
Substituted 1,1′-binaphthyl derivatives are particularly interesting, in our view, for application in chiral nanomaterials, since the expression of chirality (the asymmetry induced by the stereogenic axis) is directly embedded in the two π-extended chromophoric regions. Their use in the field of chiroptical sensing has been recently explored, given that the conformational change in the dihedral angle defined by the two naphthyl rings occurs with an intense CD signal modulation.5
Acridone, quinacridone and their derivatives are industrially relevant dyes/pigments owing to their π-conjugation, internal charge transfer effects, and structural rigidity.6 In fact, they are not fluorescent in the solid state because of aggregation-induced quenching effects, driven by intermolecular π–π stacking and hydrogen bonding interactions.
We were thus interested in understanding whether the annulation of acridones into the π-extended skeleton of suitable 1,1′-binaphthyl units could impart peculiar chiroptical properties, and eventually, given the steric hindrance of the orthogonally positioned chromophores around the chiral axis, activate CPL emission. Furthermore, we envisaged to compare such properties with those of a recently emerged family of flexible, solid-state luminophores, 2,5-diarylamino-terephthalates.7 They are formally obtained from quinacridones when appropriate bonds are broken, and thus their characteristic structural rigidity is lost (Fig. 1).
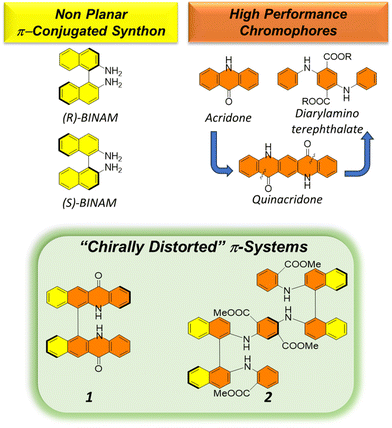 | ||
| Fig. 1 Outline of the synthons that are fused to obtain flexible and rigid “chirally distorted” π-systems. | ||
In this work, we efficiently fuse enantiomerically stable, substituted 1,1′-binaphthyl derivatives bearing amino functionalities at the 2,2′ positions with high-performance fully organic chromophores (acridones and 2,5-diarylamino terephthalates). We present the solution- and solid-state (chir)optical properties of the newly synthesized compounds 1 and 2 (Fig. 1), in which the π-systems, while maintaining a certain degree of symmetry, are locked in a chiral environment.
Results and discussion
Synthetic procedures
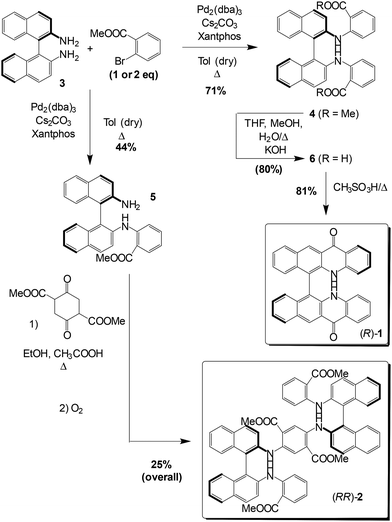
The synthetic approach for obtaining the target compounds 1 and 2 is reported in Scheme 1. In the case of 1, our retrosynthetic approach took advantage of the established synthesis of acridones and quinacridones, in which an arylamine substrate is functionalized with an o-chloro or o-bromo benzoate through a transition-metal mediated coupling.6 As the starting point, we considered having the arylamino moiety on the binaphthyl units and thus started from enantiopure and stable (R)- or (S)-3,3′-dimethyl-[1,1′-binaphthalene]-2,2′-diamine (BINAM) 3, since the synthesis of both enantiomers is reported in the literature. The coupling with methyl o-bromobenzoate was conducted through a Buchwald–Hartwig reaction. After the screening of reaction conditions reported in the literature, the best results were obtained using Pd(dba)3 as the catalyst, Xantphos as the phosphorus-based ligand and Cs2CO3 as the base. The monosubstituted and disubstituted products (R)-4 and (R)-5 could be isolated by column chromatography and obtained in 44% and 71% optimized yields, respectively.We observed (Scheme 1) that the final cyclization step to obtain compound 1 could be conducted with higher yields when using the hydrolysed compound 6; it was obtained in excellent yield (80%) and, since the product directly precipitates from the reaction mixture, it does not require further purification. The final cyclization to obtain (R)-1 proceeds via an intramolecular electrophilic aromatic substitution in the presence of methanesulfonic acid, which acts both as the catalyst and the reaction solvent.
The monosubstituted product (R)-5 (2 equiv.) was reacted with dimethyl 2,5-dioxocyclohexane-1,4-dicarboxylate, followed by molecular oxidative aromatization induced by atmospheric O2 to yield (RR)-2. We attempted the subsequent cyclization following the synthetic sequence described for compound 1 (saponification and cyclization in the presence of methanesulfonic acid) to obtain a mixed binaphthyl-fused quinacridone and acridone system, but the desired product was detected only in trace amounts. We believe that the large conjugation of the central core could cause the insurgence of complex secondary reactions in strongly acidic media, which prevented the obtainment of the desired cyclized compound. The synthetic sequences discussed above were repeated with the starting material (S)-BINAM, obtaining (S)-1 and (SS)-2. No racemization was observed for (S)-1 and even in the case of compound (SS)-2, bearing two binaphthyl units, as shown by chiral HPLC analysis (Fig. S1†).
Photophysical and chiroptical characterization
Acridone is a bright yellow solid with negligible solvatochromic behaviour in solution. In a CHCl3/MeOH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solution, the compound shows (Fig. S2†) an intense absorption peak at 254 nm (ca. ε ≈ 60
1 solution, the compound shows (Fig. S2†) an intense absorption peak at 254 nm (ca. ε ≈ 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) and two less intense peaks at 381 and 399 nm (ε ≈ 10
000 M−1 cm−1) and two less intense peaks at 381 and 399 nm (ε ≈ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) associated with its visible HOMO–LUMO excitation (the absorption of blue-violet wavelengths gives the complementary yellow tint).
000 M−1 cm−1) associated with its visible HOMO–LUMO excitation (the absorption of blue-violet wavelengths gives the complementary yellow tint).
The absorption and CD spectra of compounds (R)-1 and (S)-1 in the same solvent mixture are shown in Fig. 2. The UV/vis spectrum of 1 has remarkable similarity to that of acridone, but the main peaks are shifted towards longer wavelengths, demonstrating that the annulation of an additional benzene ring into the acridone conjugated system is indeed effective in extending the conjugation and lowering the HOMO–LUMO gap (Fig. 2 top).
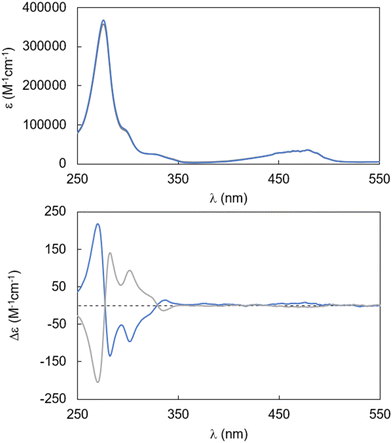 | ||
Fig. 2 UV/vis (top) and CD (bottom) spectra of compounds (R)-1 (blue trace) and (S)-1 (grey trace) in CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH 1 MeOH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (1.13 μM for UV/vis, 10 μM for CD). 1 (1.13 μM for UV/vis, 10 μM for CD). | ||
The λmax were observed at 276 nm and 477 nm, with molar absorptivities almost double with respect to that of acridone (ca. 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 15
000 and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 for the two bands, respectively), in line with a compound bearing two identical acridone chromophores. CD spectra of (R)-1 and (S)-1 revealed perfectly symmetric signatures (Fig. 2 bottom), with an exciton couplet corresponding to the main UV absorption band of the fused acridone chromophore: it is the classical CD signature of chromophores in a skewed orientation, as commonly found in atropisomeric biaryl compounds. While substantial CD activity is observed in relation to the shoulder in the UV/vis spectrum at ca. 300 nm, negligible CD activity could be observed corresponding to the red-shifted (477 nm) band. The maximum absorbance dissymmetry factor gabs is at 305 nm with a value of 1.27 × 10−3 (Fig. S3†), which is in line with the majority of non-aggregated/monomeric organic compounds.
000 M−1 cm−1 for the two bands, respectively), in line with a compound bearing two identical acridone chromophores. CD spectra of (R)-1 and (S)-1 revealed perfectly symmetric signatures (Fig. 2 bottom), with an exciton couplet corresponding to the main UV absorption band of the fused acridone chromophore: it is the classical CD signature of chromophores in a skewed orientation, as commonly found in atropisomeric biaryl compounds. While substantial CD activity is observed in relation to the shoulder in the UV/vis spectrum at ca. 300 nm, negligible CD activity could be observed corresponding to the red-shifted (477 nm) band. The maximum absorbance dissymmetry factor gabs is at 305 nm with a value of 1.27 × 10−3 (Fig. S3†), which is in line with the majority of non-aggregated/monomeric organic compounds.
Diphenylamino dimethylterephthalate has an absorption maximum at 465 nm in CHCl3 (molar absorptivity ca. 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1).8 The absorption and CD spectra of compounds (RR)-2 and (SS)-2 in the same solvent are shown in Fig. 3. The absorbance spectrum of 2 is not red shifted in terms of absorption maxima (λmax = 466 nm), indicating that the insertion of one more benzene unit into the diphenylamino dimethylterephthalate moiety does not perturb significantly the HOMO–LUMO levels; a similar molar absorptivity is detected for this band (ca. 13
000 M−1 cm−1).8 The absorption and CD spectra of compounds (RR)-2 and (SS)-2 in the same solvent are shown in Fig. 3. The absorbance spectrum of 2 is not red shifted in terms of absorption maxima (λmax = 466 nm), indicating that the insertion of one more benzene unit into the diphenylamino dimethylterephthalate moiety does not perturb significantly the HOMO–LUMO levels; a similar molar absorptivity is detected for this band (ca. 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1). The spectrum also contains other more intense bands (352 and 281 nm) related to the chromophoric units in the scaffold (including the binaphthyl units), suggesting localization rather than delocalization of the π-structure of the molecule. The ECD spectrum is characterized by a series of Cotton effects with alternating signs, which is strongly suggestive of a rich exciton coupling network between the manifold of electric dipole transition moments present in the complex chromophores. It is noteworthy that, similarly to that of the enantiomers of 1, the ECD activity related to the HOMO–LUMO transition is weak. The absorbance maximum dissymmetry factor gabs is found at 390 nm with a value of 3 × 10−3 (Fig. S4†).
000 M−1 cm−1). The spectrum also contains other more intense bands (352 and 281 nm) related to the chromophoric units in the scaffold (including the binaphthyl units), suggesting localization rather than delocalization of the π-structure of the molecule. The ECD spectrum is characterized by a series of Cotton effects with alternating signs, which is strongly suggestive of a rich exciton coupling network between the manifold of electric dipole transition moments present in the complex chromophores. It is noteworthy that, similarly to that of the enantiomers of 1, the ECD activity related to the HOMO–LUMO transition is weak. The absorbance maximum dissymmetry factor gabs is found at 390 nm with a value of 3 × 10−3 (Fig. S4†).
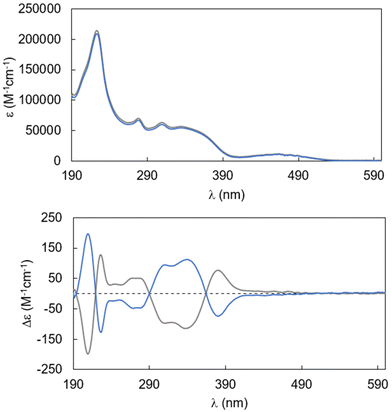 | ||
| Fig. 3 Absorption and CD spectra of compounds (RR)-2 (blue trace) and (SS)-2 (grey trace) in CHCl3 (27.1 μM for UV, 26.6 μM for CD). | ||
For compounds 1 and 2, fluorescence properties in solution and in the solid state were also analyzed. In the case of 1, a rather narrow emission band with a small Stokes shift (<50 nm) was recorded, as expected on account of its rigid structure (Fig. S5†); in compound 2, however, intense orange fluorescence was evident even when a solution of the molecule was evaporated from MeOH, EtOH or CHCl3 to form a thin film (Fig. S6†). Compound 2 in solution shows a broad emission band, compatible with a more flexible structure with substantial degrees of conformational freedom (Fig. S7†), and a large Stokes shift was observed (104 nm).
Fluorescence quantum yields in solution and in films were determined for both enantiomers of 1 and 2 (Table 1 and Fig. S8–S13†). In the case of 1, the good luminescence performance of the parent acridone dye in solution was confirmed (quantum yield of 23% in both cases). In the solid state the fluorescence is strongly quenched: the introduction of chirality and steric hindrance protecting the acridone moiety does not substantially reduce the aggregation-induced quenching, probably due to strong intermolecular hydrogen bonding. Indeed, the FTIR spectra of 1 (Fig. S14†) are compatible with hydrogen-bonded NH stretching resonances, and are very similar to those of acridone in the solid state.9 Lifetimes and monoexponential decays of the fluorescence in solution are, however, consistent with simple molecular decays.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and CHCl3, respectively) and as thin films from spin-coating or dropcasting from solutiona
1 and CHCl3, respectively) and as thin films from spin-coating or dropcasting from solutiona
| Solution | Film | |||||
|---|---|---|---|---|---|---|
| λ em (nm) (λexc = 488 nm) | ϕ (λexc = 488 nm) | τ (ns) (λexc = 407 nm) | λ em (nm) (λexc = 407 nm) | ϕ (λexc = 407 nm) | τ (ns) (λexc = 407 nm) | |
| a See the ESI for details.† b Average lifetime τav from 2-exponential fit. | ||||||
| (R)-1 | 510, 540 | 23 | 7.5 | 544, 645 | <0.1 | <0.1 (407) |
| (S)-1 | 510, 540 | 23 | 7.5 | 544, 645 | <0.1 | <0.1 (407) |
| (RR)-2 | 575 | 11 | 3.2b | 583 | 9 | 1.8b (407) |
| (SS)-2 | 575 | 11 | 3.1b | 585 | 12 | 1.8b (407) |
Interestingly, in the case of 2, good fluorescence quantum yields were obtained both in solution and in thin films, as previously reported for diphenylamino terephthalates as chromophores. The small difference in quantum yields in the case of the emission in the solid state between the two enantiomeric forms of 2 can be rationalized by differences in packing and orientation in the supramolecular structures obtained in the solid state.
CPL properties were also determined. The enantiomers of 1 displayed significant positive and negative signals in the visible domain (Fig. 4), with a luminescence dissymmetry factor (|glum|) of 4 × 10−4 in correspondence to the emission maximum. In the case of 2, luminescence dissymmetry factors (|glum|) of up to 2 × 10−4 were detected. In both cases, glum is of the same order of magnitude as the corresponding gabs for the HOMO–LUMO transitions; some of us have previously introduced a new quantity, named CPL brightness (BCPL), to have a complete picture of the efficiency of a CPL emitter, since dissymmetry factor (|glum|) alone is not enough. BCPL takes into account the absorption extinction coefficient and quantum yield along with (|glum|). In the case of both 1 and 2BCPL was estimated to be ≈0.5 M−1 cm−1.10 Such a value is close to the average value for the class of cationic helicenes, which are certainly more complex to achieve synthetically.10
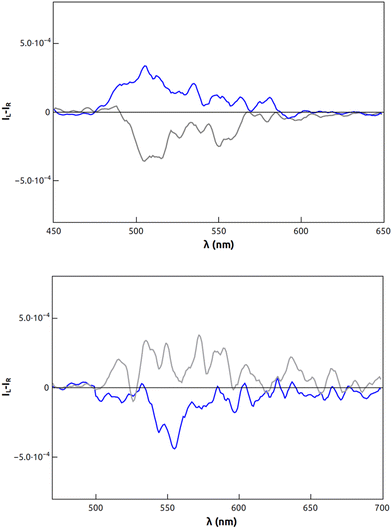 | ||
Fig. 4 CPL spectra of compounds (R)-1 (blue trace) and (S)-1 (grey trace) in CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH 1 MeOH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (top); (RR)-2 (blue trace) and (SS)-2 (grey trace) in CHCl3 (bottom). 1 (top); (RR)-2 (blue trace) and (SS)-2 (grey trace) in CHCl3 (bottom). | ||
Conclusions
We have reported the preparation and characterization of chiral π-conjugated systems embedding two types of high-performance dyes with differing “flexibility” characteristics. The synthetic approach is high yielding, flexible, and potentially allows for other substituents to be incorporated into the binaphthyl skeleton.In the acridone conjugate, good fluorescence is maintained in solution.
The flexibility and the emissive properties of the 2,5-diphenylamino-terephthalate chromophore are conserved in the conjugate, affording novel chiral dyes which are emissive both in solution and in the solid state. Compounds 2 are, to our knowledge, the first chiral versions of 2,5-diphenylamino-terephthalates utilizing the widely used and accessible binaphthyl synthons as the source of chirality. Their interesting Stokes shifts could be important in selected applications where self-absorption phenomena become an issue, such as in LSC devices.
In both cases, CPL activity in the visible region could be observed, with CPL brightness comparable to that of other, more complex, fully organic chromophores.
Experimental
General synthetic procedures
Commercial quinacridone was purchased from TCI (purity > 93%). (R)- and (S)-3,3′-dimethyl-[1,1′-binaphthalene]-2,2′-diamine (BINAM) 3 were prepared by Symo-Chem within the framework of a project funded by the European Consortium EUSMI. All other commercially available reagents and solvents were purchased from Sigma-Aldrich, Fluorochem, TCI and Alfa Aesar. They were all used as received. Anhydrous solvents such as THF and dichloromethane (DCM) were obtained by conventional methods through distillation with certain drying agents (Na for THF and CaH2 for dichloromethane). Flash chromatography was carried out using Merck silica gel 60 (pore size 60 Å, 270–400 Mesh). 1H and 13C NMR spectra were recorded from solutions in deuterated solvents on 300 or 400 MHz Bruker spectrometers with the residual solvent as the internal standard. FT-IR spectra were recorded using a ThermoFisher Nicolet FT-IR spectrometer. Mass spectra of pure compounds were recorded using an Electron Spray Ionization Agilent Technologies mass spectrometer, a Direct Exposure Probe mass spectrometer and a GC-MS ThermoScientific spectrometer.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether 3
petroleum ether 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 to 9
7 to 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain the disubstituted compound (R)-4 (1.1 g, 71%) and the monosubstituted product (R)-5 (300 mg, 25%). Characterization for (R)-4 (white solid): 1H NMR (400 MHz, CDCl3) δ 8.66 (s, 1H), 7.93 (dd, J = 8.8 Hz, 1H), 7.89 (dd, J = 8.2, 1.0 Hz, 1H), 7.78 (d, J = 8.9 Hz, 1H), 7.72 (dd, J = 8.0, 1.6 Hz, 1H), 7.37 (td, J = 8.1, 6.6, 1.5 Hz, 2H), 7.28 (dd, J = 1.2 Hz, 1H), 7.25–7.19 (m, 3H), 7.11 (td, J = 8.6, 7.1, 1.7 Hz, 1H), 6.62 (td, J = 8.1, 7.1, 1.1 Hz, 1H), 3.44 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 167.79, 146.18, 137.83, 134.08, 133.40, 131.44, 130.61, 128.91, 128.16, 126.85, 125.01, 124.44, 123.45, 121.29, 117.71, 115.17, 113.70, 51.31. HRMS m/z (%) = expected: 552.1681; found 591.1648 [M + K]+ (100), error −5.5 ppm.
1) to obtain the disubstituted compound (R)-4 (1.1 g, 71%) and the monosubstituted product (R)-5 (300 mg, 25%). Characterization for (R)-4 (white solid): 1H NMR (400 MHz, CDCl3) δ 8.66 (s, 1H), 7.93 (dd, J = 8.8 Hz, 1H), 7.89 (dd, J = 8.2, 1.0 Hz, 1H), 7.78 (d, J = 8.9 Hz, 1H), 7.72 (dd, J = 8.0, 1.6 Hz, 1H), 7.37 (td, J = 8.1, 6.6, 1.5 Hz, 2H), 7.28 (dd, J = 1.2 Hz, 1H), 7.25–7.19 (m, 3H), 7.11 (td, J = 8.6, 7.1, 1.7 Hz, 1H), 6.62 (td, J = 8.1, 7.1, 1.1 Hz, 1H), 3.44 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 167.79, 146.18, 137.83, 134.08, 133.40, 131.44, 130.61, 128.91, 128.16, 126.85, 125.01, 124.44, 123.45, 121.29, 117.71, 115.17, 113.70, 51.31. HRMS m/z (%) = expected: 552.1681; found 591.1648 [M + K]+ (100), error −5.5 ppm.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether 3
petroleum ether 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 to 9
7 to 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain the monosubstituted product (R)-5 (192.2 mg, 44%) and the disubstituted compound (R)-4 (164.4 mg, 29%). Characterization for (R)-5 (white solid): 1H NMR (400 MHz, CDCl3) δ 8.80–8.75 (m, 1H), 7.97 (d, J = 8.9 Hz, 1H), 7.92 (s, 0H), 7.90–7.78 (m, 4H), 7.47–7.38 (m, 2H), 7.36–7.15 (m, 6H), 7.09 (dd, J = 8.4, 1.3 Hz, 1H), 6.74 (td, J = 8.1, 7.0, 1.1 Hz, 1H), 3.52 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 167.81, 146.30, 142.51, 138.05, 133.95, 133.90, 133.67, 131.96, 130.80, 129.58, 128.93, 128.39, 128.16, 126.96, 126.76, 125.35, 124.61, 123.83, 122.99, 122.39, 121.43, 118.32, 117.96, 114.70, 113.99, 113.05, 51.50. UPLC-ESI m/z = 419.25 [M + H]+.
1) to obtain the monosubstituted product (R)-5 (192.2 mg, 44%) and the disubstituted compound (R)-4 (164.4 mg, 29%). Characterization for (R)-5 (white solid): 1H NMR (400 MHz, CDCl3) δ 8.80–8.75 (m, 1H), 7.97 (d, J = 8.9 Hz, 1H), 7.92 (s, 0H), 7.90–7.78 (m, 4H), 7.47–7.38 (m, 2H), 7.36–7.15 (m, 6H), 7.09 (dd, J = 8.4, 1.3 Hz, 1H), 6.74 (td, J = 8.1, 7.0, 1.1 Hz, 1H), 3.52 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 167.81, 146.30, 142.51, 138.05, 133.95, 133.90, 133.67, 131.96, 130.80, 129.58, 128.93, 128.39, 128.16, 126.96, 126.76, 125.35, 124.61, 123.83, 122.99, 122.39, 121.43, 118.32, 117.96, 114.70, 113.99, 113.05, 51.50. UPLC-ESI m/z = 419.25 [M + H]+.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of ice and water (100 mL). The precipitated solid was filtered to yield pure (R)-1 (75 mg, 81%) as a dark red solid. 1H NMR (400 MHz, DMSO) δ 9.81 (s, 1H), 9.29 (s, 1H), 8.37 (dd, J = 8.2, 0.9 Hz, 1H), 8.27 (dd, J = 8.2, 1.6 Hz, 1H), 7.58–7.39 (m, 3H), 7.31 (td, J = 8.8, 6.7, 1.3 Hz, 1H), 7.16 (td, J = 8.0, 6.7, 1.2 Hz, 1H), 6.81 (dd, J = 8.7, 1.0 Hz, 1H).13C NMR (101 MHz, DMSO) δ 179.13, 142.87, 138.28, 136.21, 134.36, 131.01, 129.77, 129.73, 128.82, 126.56, 124.56, 124.35, 122.52, 121.05, 119.62, 118.14, 116.16. HRMS m/z (%) = expected: 488.1598; found: 489.1588 [M + H]+ (100), error −2.0 ppm. FT IR (cm−1) = 773, 1146, 1477, 1498, 1515, 2855, 2922, 3050, 3185, 3253, 3317.
1 mixture of ice and water (100 mL). The precipitated solid was filtered to yield pure (R)-1 (75 mg, 81%) as a dark red solid. 1H NMR (400 MHz, DMSO) δ 9.81 (s, 1H), 9.29 (s, 1H), 8.37 (dd, J = 8.2, 0.9 Hz, 1H), 8.27 (dd, J = 8.2, 1.6 Hz, 1H), 7.58–7.39 (m, 3H), 7.31 (td, J = 8.8, 6.7, 1.3 Hz, 1H), 7.16 (td, J = 8.0, 6.7, 1.2 Hz, 1H), 6.81 (dd, J = 8.7, 1.0 Hz, 1H).13C NMR (101 MHz, DMSO) δ 179.13, 142.87, 138.28, 136.21, 134.36, 131.01, 129.77, 129.73, 128.82, 126.56, 124.56, 124.35, 122.52, 121.05, 119.62, 118.14, 116.16. HRMS m/z (%) = expected: 488.1598; found: 489.1588 [M + H]+ (100), error −2.0 ppm. FT IR (cm−1) = 773, 1146, 1477, 1498, 1515, 2855, 2922, 3050, 3185, 3253, 3317.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCM 6
DCM 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) to obtain the pure product (RR)-2 (60 mg, 25% overall) as a highly fluorescent orange solid. 1H NMR (400 MHz, CDCl3) δ 8.72 (s, 1H), 7.97–7.83 (m, 4H), 7.80 (d, J = 8.9 Hz, 1H), 7.71 (s, 1H), 7.66 (d, J = 8.9 Hz, 1H), 7.62 (dd, J = 8.0, 1.7 Hz, 1H), 7.39 (td, 1H), 7.33 (td, 1H), 7.29–7.18 (m, 5H), 7.17–7.13 (dd, 1H), 7.07 (td, J = 8.6, 7.1, 1.7 Hz, 1H), 6.48 (td, J = 8.1, 6.6, 1.4 Hz, 1H), 3.38 (s, 3H), 3.26 (s, 3H).13C NMR (101 MHz, CDCl3) δ 167.65, 166.76, 146.18, 138.59, 137.80, 136.87, 134.15, 134.09, 133.50, 131.41, 130.65, 130.07, 129.19, 128.86, 128.15, 126.93, 126.81, 125.17, 124.72, 124.52, 123.91, 123.44, 121.46, 121.22, 119.90, 119.74, 119.06, 117.66, 114.60, 113.43, 58.51, 51.50, 51.21. HRMS m/z (%) = expected: 1026.3701; found: 1027.3667 [M + H]+ (100), 1049.3505 [M + Na]+ (70), error 3.3 ppm.
4) to obtain the pure product (RR)-2 (60 mg, 25% overall) as a highly fluorescent orange solid. 1H NMR (400 MHz, CDCl3) δ 8.72 (s, 1H), 7.97–7.83 (m, 4H), 7.80 (d, J = 8.9 Hz, 1H), 7.71 (s, 1H), 7.66 (d, J = 8.9 Hz, 1H), 7.62 (dd, J = 8.0, 1.7 Hz, 1H), 7.39 (td, 1H), 7.33 (td, 1H), 7.29–7.18 (m, 5H), 7.17–7.13 (dd, 1H), 7.07 (td, J = 8.6, 7.1, 1.7 Hz, 1H), 6.48 (td, J = 8.1, 6.6, 1.4 Hz, 1H), 3.38 (s, 3H), 3.26 (s, 3H).13C NMR (101 MHz, CDCl3) δ 167.65, 166.76, 146.18, 138.59, 137.80, 136.87, 134.15, 134.09, 133.50, 131.41, 130.65, 130.07, 129.19, 128.86, 128.15, 126.93, 126.81, 125.17, 124.72, 124.52, 123.91, 123.44, 121.46, 121.22, 119.90, 119.74, 119.06, 117.66, 114.60, 113.43, 58.51, 51.50, 51.21. HRMS m/z (%) = expected: 1026.3701; found: 1027.3667 [M + H]+ (100), 1049.3505 [M + Na]+ (70), error 3.3 ppm.
UV-vis and emission studies
UV-vis spectra were collected using a Varian Cary 50 SCAN spectrophotometer, with quartz cuvettes of an appropriate path length at 25.0 ± 0.1 °C.The solid UV-vis diffuse reflectance spectra were recorded using a Shimadzu UV3600 spectrophotometer with BaSO4 as a reference. Steady-state emission and excitation spectra and photoluminescence lifetimes were obtained using both an FLS 980 (Edinburgh Instruments Ltd) and a Nanolog (Horiba Scientific) spectrofluorometer composed of an iH320 spectrograph equipped with a Synapse QExtra charge-coupled device. The spectra were corrected for the instrument response. PL quantum yields of solutions were obtained by using rhodamine 6G as the reference. PL QYs of solid-state samples were measured with a homemade integrating sphere according to the procedure reported elsewhere.11 Time-resolved TCSPC measurements were obtained with a PPD-850 single photon detector module and a DD-405L DeltaDiode Laser and analyzed with the instrument software DAS6. Decay fits were performed with multi-exponential functions and average lifetimes were obtained as follows:
CD and CPL spectroscopy
CD spectra were collected on a JASCO J1500 spectropolarimeter equipped with a Peltier temperature controller. A quartz cell of 1 cm optical path length was used for all measurements. The circularly polarized luminescence experiments for compounds S/R-1-and SS/RR-2 were carried out with a home-built CPL spectrofluoropolarimeter, in a <10−5 M solution (CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH 1
MeOH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for S/R-1 and CHCl3 for SS/RR-2). The following parameters were used: excitation: 365 nm, slit width: 5 nm, scan speed: 2 nm s−1, integration time: 2 s, PMT voltage: 450 V, accumulation: 12.9
1 for S/R-1 and CHCl3 for SS/RR-2). The following parameters were used: excitation: 365 nm, slit width: 5 nm, scan speed: 2 nm s−1, integration time: 2 s, PMT voltage: 450 V, accumulation: 12.9
Author contributions
Conceptualization: G. P. and D. P.; data curation: G. P., E. M. C., A. B., C. B. and F. Z.; formal analysis: C. B. and F. Z.; funding acquisition: L. D. B. and D. P.; supervision: L. D. B. and D. P.; writing – original draft: G. P., E. M. C., A. B. and D. P.; writing – review & editing: G. P., F. Z., L. D. B., C. B. and D. P.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge Regione Lombardia (POR FESR 2014-2020-Call HUB Ricerca e Innovazione, Progetto 1139857 CE4WE: Circular Economy for Water and Energy) for financial support. G. P. and D. P. acknowledge support from the Ministero dell'Università e della Ricerca (MUR), the University of Pavia through the program “Dipartimenti di Eccellenza 2023–2027”, and financial support from the European Commission under the Horizon 2020 Programme by means of the grant agreement No. 200700429 EUSMI. We thank Dr Roberto Cirilli (ISS Rome) for the confirmation of the enantiomeric purity via chiral HPLC analysis.References
- (a) C. Zhang, S. Li, X. X.-Y. Dong and S.-Q. Zang, Aggregate, 2021, 2, e48 CrossRef CAS; (b) D.-Y. Kim, J. Korean Phys. Soc., 2006, 49, 505 Search PubMed.
- (a) K. Yu, T. Fan, S. Lou and D. Zhang, Prog. Mater. Sci., 2013, 58, 825–873 CrossRef; (b) Y. Kim, B. Yeom, O. Arteaga, S. J. Yoo, S.-G. Lee, J.-G. Kim and N. A. Kotov, Nat. Mater., 2016, 15, 461–468 CrossRef CAS PubMed; (c) Y. Sang, J. Han, T. Zhao, P. Duan and M. Liu, Adv. Mater., 2020, 32, 1900110 CrossRef CAS PubMed; (d) L. E. MacKenzie and R. Pal, Nat. Rev. Chem., 2021, 5, 109–124 CrossRef CAS PubMed; (e) R. Brandt, F. Salerno and M. J. Fuchter, Nat. Rev. Chem., 2017, 1, 0045 CrossRef; (f) F. Zinna, U. Giovanella and L. Di Bari, Adv. Mater., 2015, 27, 1791 CrossRef CAS PubMed; (g) Y. Yang, R. C. da Costa, D.-M. Smilgies, A. J. Campbell and M. J. Fuchter, Adv. Mater., 2013, 25, 2624 CrossRef CAS PubMed.
- (a) R. Carr, N. Evans and D. Parker, Chem. Soc. Rev., 2012, 41, 7673–7686 RSC; (b) Z.-L. Gong, Z.-Q. Li and Y.-W. Zhong, Aggregate, 2022, 3, e177 CrossRef CAS; (c) Y. Zhou, H. Li, T. Zhu, T. Gao and P. Yan, J. Am. Chem. Soc., 2019, 141, 19634 CrossRef CAS PubMed.
- (a) W.-L. Zhao, M. Li, H.-Y. Lu and C.-F. Chen, Chem. Commun., 2019, 55, 13793–13803 RSC; (b) H. Oyama, M. Akiyama, K. Nakano, M. Naito, K. Nobusawa and K. Nozaki, Org. Lett., 2016, 18, 3654–3657 CrossRef CAS PubMed; (c) J. Kumar, T. Nakashima and T. Kawai, J. Phys. Chem. Lett., 2015, 6, 3445–3452 CrossRef CAS PubMed; (d) A. Macé, K. Hamrouni, E. S. Gauthier, M. Jean, N. Vanthuyne, L. Frédéric, G. Pieters, E. Caytan, T. Roisnel, F. Aloui, M. Srebro-Hooper, B. Carboni, F. Berrée and J. Crassous, Chem. – Eur. J., 2021, 27, 7959–7967 CrossRef PubMed; (e) P. M. Lorente, A. Wallabregue, F. Zinna, C. Besnard, L. Di Bari and J. Lacour, Org. Biomol. Chem., 2020, 18, 7677–7684 RSC.
- (a) P. K. Baruah, R. Gonnade, P. R. Rajamohanan, H.-J. Hofmann and G. J. Sanjayan, J. Org. Chem., 2007, 72, 5077–5084 CrossRef CAS PubMed; (b) L. Di Bari, G. Pescitelli and P. Salvadori, J. Am. Chem. Soc., 1999, 121, 7998–8004 CrossRef CAS; (c) M. Caricato, C. Coluccini, D. Dondi, D. A. Vander Griend and D. Pasini, Org. Biomol. Chem., 2010, 8, 3272–3280 RSC; (d) M. Caricato, N. J. Leza, K. Roy, D. Dondi, G. Gattuso, L. S. Shimizu, D. A. Vander Griend and D. Pasini, Eur. J. Org. Chem., 2013, 6078–6083 CrossRef CAS; (e) M. Caricato, A. Olmo, C. Gargiulli, G. Gattuso and D. Pasini, Tetrahedron, 2012, 68, 7861–7866 CrossRef CAS; (f) M. Agnes, A. Nitti, D. A. Vander Griend, D. Dondi, D. Merli and D. Pasini, Chem. Commun., 2016, 52, 11492–11495 RSC; (g) R. Mobili, G. Preda, S. La Cognata, L. Toma, D. Pasini and V. Amendola, Chem. Commun., 2022, 58, 3897–3900 RSC; (h) D. Pasini and A. Nitti, Chirality, 2016, 28, 116–123 CrossRef CAS PubMed.
- (a) C. F. H. Allen and G. H. W. McKee, Org. Synth., 1939, 19, 6 CrossRef CAS; (b) W. T. Wei, J.-F. Sheng, H. Miao, X. Luo, X.-H. Song, M. Yan and Y. Zou, Adv. Synth. Catal., 2018, 360, 2101–2106 CrossRef CAS; (c) P. Belmont and I. Dorange, Expert Opin. Ther. Pat., 2008, 18, 1211–1224 CrossRef CAS; (d) G. Preda, A. Aricò, C. Botta, D. Ravelli, D. Merli, S. Mattiello, L. Beverina and D. Pasini, Org. Lett., 2023, 25, 6490–6494 CrossRef CAS PubMed.
- (a) Z. Liu, Y. Liu, F. Qi, H. Yan, Z. Jiang and Y. Chen, Chem. – Eur. J., 2020, 26, 14963–14968 CrossRef CAS PubMed; (b) B. Tang, Z. Zhang, H. Liu and H. Zhang, Chin. Chem. Lett., 2017, 28, 2129–2132 CrossRef CAS; (c) K. Nowak, O. Morawski, F. Zinna, G. Pescitelli, L. Di Bari, M. Górecki and M. Grzybowski, Chem. – Eur. J., 2023, 29, e202300932 CrossRef CAS PubMed.
- J. H. Jin, J. M. An, D. Kim, S. Lee, M. Kim and D. Kim, Dyes Pigm., 2024, 221, 111811 CrossRef CAS.
- K. V. Berezin, T. V. Krivokhizhina and V. V. Nechaev, Opt. Spectrosc., 2006, 100, 15–22 CrossRef CAS.
- L. Arrico, L. Di Bari and F. Zinna, Chem. – Eur. J., 2021, 27, 2920–2934 CrossRef CAS PubMed.
- J. Moreau, U. Giovanella, J.-P. Bombenger, W. Porzio, V. Vohra, L. Spadacini, G. Di Silvestro, L. Barba, G. Arrighetti, S. Destri, M. Pasini, M. Saba, F. Quochi, A. Mura, G. Bongiovanni, M. Fiorini, M. Uslenghi and C. Botta, ChemPhysChem, 2009, 10, 647–653 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ob00086f |
| This journal is © The Royal Society of Chemistry 2025 |