Reusable cobalt–copper-catalyzed cross-coupling of (hetero)aryl halides with primary amides under air: investigating a new Co0/CoII-based catalytic cycle†
Anay
Saha
a,
Keya
Roy
a,
Sampa
Mondal
a,
Bijay
Saha
 b,
Jayabrata
Sumar
a,
Sumanta Kumar
Sahu
b,
Jayabrata
Sumar
a,
Sumanta Kumar
Sahu
 b and
Laksmikanta
Adak
b and
Laksmikanta
Adak
 *a
*a
aDepartment of Chemistry, Indian Institute of Engineering Science and Technology, Shibpur, Botanic Garden, Howrah 711103, India. E-mail: ladak@chem.iiests.ac.in; ladak.chem@faculty.iiests.ac.in
bDepartment of Chemistry and Chemical Biology, Indian Institute of Technology (ISM), Dhanbad, Jharkhand 826004, India
First published on 17th February 2025
Abstract
We report an efficient ligand-free cobalt–copper-catalyzed cross-coupling reaction of aryl halides with primary amides and also investigate a new Co0/CoII-based catalytic cycle for this transformation. This reaction successfully couples a wide range of aryl and heteroaryl halides (including chlorides, bromides, and iodides) with various aryl, heteroaryl, and aliphatic primary amides in air under solvent-minimized conditions. This cost-effective method efficiently produces the desired cross-coupling products (N-arylamides) in good to excellent yields, showcasing a broad substrate scope (51 examples) and tolerance to many sensitive functional groups (including heterocycles). This protocol requires no conventional work-up and can be performed without the need for strict inert conditions. The established method is also suitable for gram-scale synthesis. Importantly, the catalyst is environmentally benign, inexpensive, and can be reused several times with insignificant loss of catalytic activity. A series of experiments, including UV spectroscopy, transmission electron microscopy (TEM), powder X-ray diffraction (PXRD), and cyclic voltammetry (CV), were carried out to determine the oxidation state of the active catalytic species, and a radical clock experiment using a radical probe was conducted to examine the reaction mechanism. The experiments support the proposed novel mechanism and eliminate the possibility of a radical-based pathway.
Introduction
Synthesis of N-arylamides has drawn significant interest due to their importance in pharmaceutical, biological, and materials science.1a–g In fact, N-arylamides are the fundamental building blocks for the synthesis of drugs and highly biologically active compounds (Fig. 1).1h–j Consequently, the synthesis of N-arylamides has become one of the most thought-provoking tasks for organic chemists. In this regard, among the techniques that have been explored to date, using transition metal-catalyzed cross-coupling of amides has garnered more interest in recent years. When considering amides in comparison with simple amines, their nucleophilicity is greatly diminished due to the electron-withdrawing properties of the carbonyl group. In metal-catalyzed cross-coupling reactions, this feature presents challenges since the success of these reactions frequently depends on effective nucleophilicity. Even though Pd catalysis was used to perform the C(sp2)–N coupling of amides with aryl halides,2 this reaction has drawbacks such as a high catalyst cost, the presence of ligands, and laborious Pd catalyst extraction from polar reaction media, particularly in the latter phases of the reaction. Other less expensive metals, such as nickel in the presence of phosphine ligands or redox-active diamine-based ligands, have also been employed for similar reactions (eqn (1)).3 Nevertheless, these techniques also have disadvantages, including the use of costly and hazardous ligands, metal catalysts that are sensitive to air, alkali metal alkoxides or inorganic bases, and a limited selection of substrates.4 During the past few decades, Goldberg's5 Cu-catalyzed N-arylation of amides has also been extensively utilized. However, these methods often need high temperature (nearly 200 °C) and stoichiometric amounts of copper, which create a waste disposal issue on scale-up.6 In an effort to overcome the limitations of traditional Goldberg reactions for N-arylation of amides with aryl halides, scientists have explored the use of electron-rich bi- or tridentate chelating ligands with copper-based catalysts (eqn (1)).7 The ligands that are chelated with the metal core are crucial for the catalytic activity of these homogeneous reactions. However, these procedures pose significant drawbacks due to the costly and time-consuming ligand production. A recent research study describes the use of a ligand-free copper-catalyzed system to promote the amidation reaction of aryl iodides (eqn (2)).8 Nevertheless, the substrate scope remains limited. Indeed, one of the problems with many ligand-free catalytic systems that have been explored so far is their restricted applicability to aryl bromides, which naturally provide lower yields compared to aryl iodides.9Bolm and his group made substantial contributions to ligand-promoted iron-catalyzed N-arylation reactions between primary amides and aryl iodides (eqn (3)).10a,b The catalyst system used in this protocol was a mixture of FeCl3 and N,N-dimethylethylenediamine (DMEDA). Subsequent studies by Buchwald and Bolm revealed that the iron-catalyzed N-arylation reaction of primary amides with aryl iodides might be influenced by trace amounts of copper impurities.10c Teo developed a procedure for iron-catalyzed N-arylation of benzamides with aryl iodides in the presence of an N,N-dimethylethylenediamine (DMEDA) ligand that furnished the corresponding N-arylamide product in moderate yield (46–55%).10d To achieve the ligand-free iron-catalyzed cross-coupling reaction of benzamide with iodobenzene, Rao built a heterogeneous iron catalytic system (Fe/Cg), which produced the N-phenylbenzamide product in moderate yield (51%).10e Although a few ligand-assisted iron-catalyzed systems were established for the N-arylation of primary amides, the substrate scope was limited to only aryl iodides, and the products were obtained after a lengthy reaction time (24–40 h).
A few groups have previously investigated the use of Mn/Cu catalytic systems in the presence of bidentate nitrogenous ligands in cross-coupling reactions of primary amides with aryl iodides.11 However, the application of these techniques to aryl bromides as coupling partners was not extended smoothly. In addition to this, silver (Ag) and lanthanum oxide (La2O3) in combination with the N,N-dimethylethylenediamine (DMEDA) ligand as a catalytic system for the N-arylation of benzamides are also reported in the literature. However, this approach typically includes elevated temperatures and polar solvents to promote the coupling reaction of benzamides with aryl iodides.12
Despite the significant advancement made with palladium-, nickel-, copper-, and iron-catalyzed reactions of this type, the development of any sustainable and new protocols and ligand-free approaches for the synthesis of these target molecules can be of great synthetic importance and industrial significance. Cobalt salts have emerged as a practical alternative in the field of catalysis owing to their ready availability, low cost, and environmentally benign nature.13
Teo and Chua established a protocol for the cobalt-catalyzed N-arylation of nitrogen nucleophiles with aryl iodides in the presence of a chelating diamine ligand, N,N′-dimethylethylenediamine (DMEDA).14a This protocol was applied only to two aryl iodides for coupling with benzamide and provided the coupling product in low yield (24–28%). Moreover, no reaction mechanism for this transformation was provided or discussed. Tan et al. reported a cross-coupling reaction of benzamides with aryl iodides to assemble N-aryl benzamides (eqn (4))14b using a mixed Co(C2O4)·2H2O/DMEDA catalytic system. This protocol cannot be applied to aryl bromides, chlorides, and heteroaryl halides and they did not conduct any mechanistic studies for their proposed Co(II)/Co(IV)-based mechanism. Additionally, no reference was cited to support their anticipated mechanistic cycle. Furthermore, Tan and Teo reported a protocol for the C–N cross-coupling of aliphatic amides and iodobenzene using a combination of cobalt catalysts and the N,N′-dimethylethylenediamine (DMEDA) ligand.14c Again this protocol cannot be applicable to aryl bromides and chlorides. Also, the reaction mechanism for this transformation was not presented or discussed. Ranu et al. subsequently reported a cobalt–copper-catalyzed C(sp2)–N cross-coupling of cyclic secondary amides and aliphatic primary amides with styrenyl bromides and aryl iodides.13e The coupling reaction was extended to include the coupling of nitrogen-containing heterocycles with styrenyl bromides and aryl halides. Two examples were identified where aromatic primary amides react solely with styrenyl bromides, but there is no report of coupling between aromatic primary amides and aryl halides. Moreover, this method required a strict argon atmosphere. It is notable that a Co(I)/Co(III)-based catalytic pathway was proposed for their transformation. However, they did not perform any experimental studies to determine the oxidation state of the active catalytic species as Co(I), nor did they provide literature support or investigate their Co(I)/Co(III)-based mechanism. In addition, there is no convincing experimental evidence or literature support regarding the exact role of the acetylacetone moiety in the reduction process of the Co(acac)2 pre-catalyst to Co(I), as proposed in their mechanistic cycle. Using aryl bromides and chlorides as substrates for cross-coupling with primary amides is still a challenge for cobalt catalysis.14d Aryl bromides and chlorides, known for their lower reactivity with respect to aryl iodides, required innovative catalytic approaches for undergoing efficient amidation. Furthermore, experimental studies to investigate the reaction mechanism involved in the cobalt-catalyzed cross-coupling of primary amides with aryl halides still remain unexplored.
Results and discussion
Optimization of the reaction conditions
To achieve the optimized reaction conditions, a series of experiments were performed with variations of the reaction parameters, such as catalyst, base, solvent, temperature, etc., for a representative reaction of benzamide with iodobenzene.15a The results are summarized in Table 1. The best results were obtained using 10 mol% of both Co(acac)2 and CuI and 1.5 equiv. of K2CO3 in NMP (100 μL for 1 mmol scale of reaction) at 110 °C in open air (entry 20, Table 1).| Entry | Catalyst | Base | Solvent | Temperature (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: amide (0.5 mmol), aryl halide (0.5 mmol), co-catalyst (10 mol%), CuI (10 mol%), base (0.75 mmol), and solvent (1 ml) under an argon atmosphere. b Isolated yields. c 100 μL of NMP was used for 1.0 mmol scale of reaction. d Reaction was carried out under a nitrogen atmosphere. e Reaction was carried out in open air. | ||||||
| 1 | Co(acac)2/CuI | Cs2CO3 | NMP | 110 | 24 | 86 |
| 2 | Co(acac)2/CuI | Cs2CO3 | DMF | 110 | 24 | 45 |
| 3 | Co(acac)2/CuI | Cs2CO3 | DMSO | 110 | 24 | 76 |
| 4 | Co(acac)2/CuI | Cs2CO3 | Toluene | 110 | 24 | 31 |
| 5 | Co(acac)2/CuI | Cs2CO3 | PhCI | 110 | 24 | 55 |
| 6 | Co(acac)2/CuI | Cs2CO3 | H20 | 110 | 24 | 40 |
| 7 | Co(acac)2/CuI | Cs2CO3 | — | 110 | 24 | 80 |
| 8c | Co(acac)2/CuI | Cs2CO3 | NMP | 110 | 24 | 88 |
| 9c | Co(OAc)2/CuI | Cs2CO3 | NMP | 110 | 24 | 67 |
| 10c | CoBr2/CuI | Cs2CO3 | NMP | 110 | 24 | 43 |
| 11c | Col2/CuI | Cs2CO3 | NMP | 110 | 24 | 51 |
| 12c | Co(acac)2/CuI | K2CO3 | NMP | 110 | 24 | 90 |
| 13c | Co(acac)2/CuI | Na2CO3 | NMP | 110 | 24 | 70 |
| 14c | Co(acac)2/CuI | K2CO3 | NMP | 90 | 24 | 58 |
| 15c | Co(acac)2/— | K2CO3 | NMP | 110 | 24 | 0 |
| 16c | —/CuI | K2CO3 | NMP | 110 | 24 | 11 |
| 17c | Co(acac)2/CuI | K2CO3 | NMP | 110 | 15 | 91 |
| 18c | Co(acac)2/CuI | K2CO3 | NMP | 110 | 12 | 81 |
| 19c,d | Co(acac)2/CuI | K2CO3 | NMP | 110 | 15 | 90 |
| 20 , | Co(acac) 2 /CuI | K 2 CO 3 | NMP | 110 | 15 | 93 |
Substrate scope and a plausible reaction mechanism
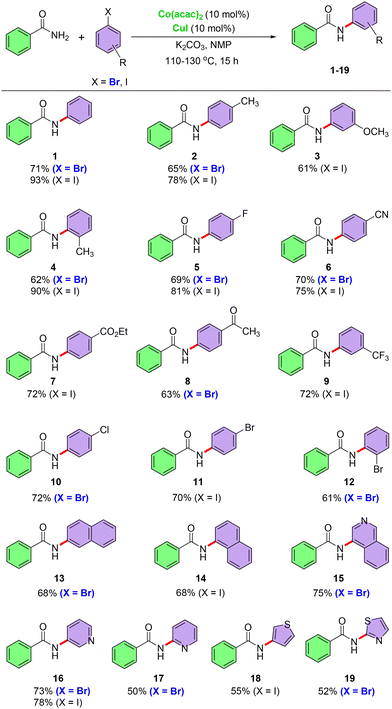
After standardizing the reaction conditions (entry 20, Table 1), we explored the reaction scope. A series of diversely substituted aryl halides (Br and I) were first examined, and the outcomes are summarized in Scheme 1. Aryl halides with electron-neutral, electron-donating, and electron-withdrawing substituents reacted with benzamide to afford the corresponding cross-coupled products (1, 2–4, and 5–9) in good to excellent yields (61–93%). Fluorinated aryl bromides and iodides reacted with benzamide to give products 5 and 9 with good yields. The standardized reaction conditions were tested with a variety of aryl halides containing sensitive functional groups (–CN, –CO2Et, and –COCH3) to assess their compatibility and reactivity. The results of these experiments indicate that such sensitive groups can also be accommodated without adversely affecting the yield of the reaction, giving products 6–8 in very good yields. 1-Bromo-4-chlorobenzene, 1-bromo-4-iodobenzene and 1,2-dibromobenzene selectively cross-coupled with benzamide to provide products 10–12 in 61–72% yields. The existence of halogen functionalities (Br and Cl) in the obtained products suggests their potential utility in further cross-coupling reactions, expanding the scope for potential chemical transformations. The established cross-coupling reaction effectively proceeded with sterically hindered aryl halides, providing products 4 and 12 in good to excellent yields. 2-Bromonaphthalene and 1-iodonaphthalene also reacted smoothly to give 13 and 14 with 68% yields in both cases. Heteroaryl bromides and iodides were successfully reacted with benzamide under the optimal reaction conditions, yielding products 15–19 in good yields. The outcomes show how widely the reaction conditions can be applied to various halogenated substrates, including those with heteroaromatic structures.The established cross-coupling reaction was explored using a series of substituted benzamides and aryl halides to investigate the scope and versatility of the protocol (Scheme 2). Aryl amides with electron-donating substituents were successfully coupled with electron-neutral and electron-deficient aryl halides and products 20–24 were obtained in good to excellent yields. Aryl amides with electron-withdrawing substituents react efficiently with a variety of aryl halides (Br and I), including electron-neutral and electron-rich types. The cross-coupled products (25–27) were obtained in excellent yields, highlighting the wide applicability and robustness of the protocol. Sterically hindered aryl bromide also furnishes the desired product 28 in 54% yield after reacting with 4-(tert-butyl)benzamide under the optimal reaction conditions. Heteroaryl amide reacts with both aryl and heteroaryl bromides, leading to the formation of products 29 and 30 with moderate to good yields.
This coupling reaction is not limited to aromatic substrates but effectively proceeded with aliphatic amides, yielding the corresponding cross-coupled products. This is illustrated in Scheme 3. Aliphatic amides with primary, secondary, and tertiary side chains successfully reacted with aryl and polyaromatic bromides and iodides under the cross-coupling reaction conditions, yielding products 31–34 in good yields. Pivalamide also reacted with heteroaryl halides (Br and I) under the standard reaction conditions to produce coupling products 35 and 36 in high yields. This extension highlights the broad applicability of this cross-coupling reaction and opens avenues for the further exploration of different types of substrates.
The optimized conditions are also effective for the coupling of benzamide with more challenging substrates such as aryl chlorides (Scheme 4). Although the substrate scope was found to be limited.
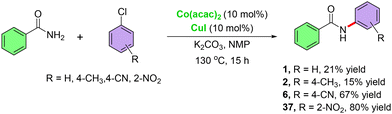 | ||
| Scheme 4 Cobalt–copper-catalyzed cross-coupling of benzamide with aryl chlorides.15a | ||
To our delight,15b as an extension of our method, we have applied our established protocol to the coupling of aryl sulfonamides with bromobenzene (Scheme 5). The performed reactions afforded the desired coupling products (38–40) in good to moderate yields.
 | ||
| Scheme 5 Cobalt–copper-catalyzed cross-coupling of aryl sulfonamides with bromobenzene.15a | ||
A gram-scale synthesis was carried out using benzamide and 3-bromopyridine as model substrates under the standard reaction conditions to illustrate the applicability of this cross-coupling reaction. The reaction proceeded smoothly, furnishing the desired bioactive product 16 in a similar yield to the lower millimolar scale in the same time frame (eqn (6)).
 | (6) |
The cobalt–copper catalyst was recycled for up to four runs without significant loss of catalytic efficiency for the representative reaction of benzamide with bromobenzene (Fig. 2).15c
To explore the mechanism behind this cross-coupling reaction, initial mechanistic investigations were conducted. At first, a radical clock experiment was carried out15d,e using a radical probe, 1-(allyloxy)-2-bromobenzene, and benzamide under the optimal reaction conditions (eqn (7)). This experiment provided the direct cross-coupling product (41) without forming the ring-closing cross-coupled product, suggesting that radical intermediates are not involved. Subsequently, the reaction was carried out in the presence of a radical scavenger, TEMPO, and a significant amount of product 1 was isolated (eqn (8)), which further supports the absence of radical species in the reaction mechanism. These results collectively suggest that the established reaction proceeds via a non-radical pathway.
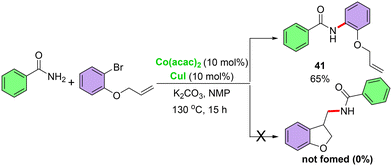 | (7) |
 | (8) |
Furthermore, a series of experimental studies were conducted to determine the oxidation state of active catalytic species and investigate the reaction mechanism. The reaction of benzamide and bromobenzene was studied by UV (NMP) spectroscopy. For comparison, the spectra of a solution of Co(acac)2 in NMP was recorded as a reference. The peak at 277 nm for Co(acac)2 was absent in the reaction mixture, which suggests that Co(acac)2, that is Co(II), was converted to different species with different oxidation states in the reaction mixture (Fig. 3a). Moreover, the absence of any peak may indicate the presence of Co(0) as the active catalytic species within the system. To explore further, transmission electron microscopy (TEM) was performed using the same reaction mixture. The TEM image (Fig. 3b) and EDS (energy dispersive X-ray spectroscopy)15f confirmed the presence of Co nanoparticles (with a size of about 5 nm). It is suggested that Co(II) was reduced to Co(0) during the reaction.
 | ||
| Fig. 3 (a) UV spectra of the reaction mixture and (b) the TEM image of the Co-nanoparticles formed in the reaction mixture. | ||
The XRD (X-ray diffraction) pattern of the residual powder that was taken by extraction of organic compounds after completion of the reaction showed the presence of Co(0) (Fig. 4). For the next four runs, this solid was equally effective with no appreciable drop in efficiency (Fig. 2).
 | ||
| Fig. 4 XRD pattern of Co(0) in the residual powder that was obtained by extraction of organic compounds. | ||
Cyclic voltammetry of Co(acac)2 in water/0.1 M K2CO3 exhibited a two-electron reduction response in the potential range of 1.25 to −1.0 V (see the ESI, Fig. S2†). The corresponding voltammogram exhibited a cathodic reduction process of the Co(II) to Co(I) response at −0.15 V and the Co(I) to Co(0) response at 0.42 V.15g This finding is in agreement with a reaction mechanism involving the reduction of Co(II) to Co(0) facilitated by K2CO3 in the preliminary step of the catalytic cycle.
Based on our mechanistic investigations and previous literature reports,13g,h we propose a new Co(0)/Co(II)-based mechanism for the cross-coupling reaction of aryl halides with primary amides (Scheme 6). The reaction is initiated by in situ reduction of Co(II) facilitated by potassium carbonate to form Co(0), which then undergoes oxidative addition with aryl- or heteroaryl halides, leading to the formation of intermediate A. In a different cycle, amide and Cu(I) interact in the presence of a base, forming amide–Cu(I) intermediate Bvia nitrogen chelation. Subsequently, transmetallation between the Co(II) center of A and the Cu(I) center of B results in the generation of intermediate C. Finally, this intermediate C undergoes reductive elimination, furnishing the coupling product and regenerating the active Co(0) species which participates in the subsequent cycle.
Conclusions
In conclusion, we have established an efficient ligand-free cobalt–copper-catalyzed cross-coupling reaction that successfully couples a range of aryl and heteroaryl halides (including chlorides, bromides, and iodides) with various primary aliphatic, aromatic, and heteroaromatic amides. A new catalytic cycle based on Co(0)/Co(II) for this method was investigated through a series of experimental studies and control reactions. This reaction was carried out under solvent-minimized conditions and does not require an inert atmosphere, thereby improving its sustainability and efficiency. This protocol efficiently yields the desired N-arylamide cross-coupling products in good to excellent yields, demonstrating a broad substrate scope with 51 examples and high tolerance to various sensitive functionalities, including heterocycles and aryl sulfonamides. The developed methodology is applicable to gram-scale synthesis. Additionally, this protocol eliminates the need for traditional work-up, making it more efficient and straightforward. Notably, the catalyst is cost-effective, environmentally benign, and can be reused at least four times with nominal loss of catalytic activity. A series of experiments, including TEM, UV spectroscopy, PXRD, and CV, were conducted to detect the oxidation state of the active catalytic species involved in the mechanistic cycle. In addition, a radical clock experiment with a radical probe was conducted to explore the reaction mechanism.Author contributions
A. S. took part in conceptualization, methodology, investigation, and data curation. K. R. wrote the original draft and took part in methodology and data curation. B. S. and S. K. S. helped in mechanistic investigations. J. S. contributed to the experimental work. S. M. contributed to the final version of the manuscript. L. A. wrote the original draft, supervised the work, and was involved in funding acquisition, conceptualization, review, and the editing process.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
L. Adak gratefully acknowledges the support of the funding agency SERB, DST, Govt. of India (Project: SRG/2020/001350) and the Department of Science & Technology and Biotechnology, Govt. of West Bengal [Project: 1854 (Sanc.)/ST/P/S&T/15G-7/2019]. A. S. is grateful to IIESTS for fellowship support. K. R. is grateful to DST, Govt. of India, New Delhi, for providing her with a Senior Research Fellowship. We thank DST for sponsoring SAIF IIESTS, which is acknowledged for providing NMR and HRMS facilities.References
- (a) J. M. Humphrey and A. R. Chamberlin, Chem. Rev., 1997, 97, 2243–2266 CrossRef CAS PubMed; (b) J. S. Carey, D. Laffan, C. Thomson and M. T. Williams, Org. Biomol. Chem., 2006, 4, 2337–2347 RSC; (c) T. Cupido, J. Tulla-Puche, J. Spengler and F. Albericio, Curr. Opin. Drug Discovery Dev., 2007, 10, 768–783 CAS; (d) M. A. Mintzer and E. E. Simanek, Chem. Rev., 2009, 109, 259–302 CrossRef CAS PubMed; (e) P. Anastas and N. Eghbali, Chem. Rev., 2009, 109, 259–302 CrossRef PubMed; (f) E. Valeur and M. Bradley, Chem. Soc. Rev., 2010, 39, 301–312 RSC; (g) C. L. Allen and J. M. J. Williams, Chem. Soc. Rev., 2011, 40, 3405–3415 RSC; (h) M. Halder, M. M. Islam, Z. Ansari, S. Ahammed, K. Sen and S. M. Islam, ACS Sustainable Chem. Eng., 2017, 5, 648–657 CrossRef CAS; (i) B. Clarke, E. Demont, C. Dingwall, R. Dunsdon, A. Faller, J. Hawkins, I. Hussain, D. MacPherson, G. Maile and R. Matico, Bioorg. Med. Chem. Lett., 2008, 18, 1011 CrossRef CAS PubMed; (j) A. Saha, K. Roy, S. Banerjee, S. Panja, M. Khatua, C. D. Mukhopadhyay, S. Samanta and L. Adak, J. Mol. Struct., 2025, 1322, 140177 CrossRef CAS.
- (a) W. C. Shakespere, Tetrahedron Lett., 1999, 40, 2035–2038 CrossRef; (b) J. Yin and S. L. Buchwald, Org. Lett., 2000, 2, 1101–1104 CrossRef CAS PubMed; (c) G. A. Artamkina, A. G. Sergeev and I. P. Beletskaya, Tetrahedron Lett., 2001, 42, 4381–4384 CrossRef CAS; (d) S. Cacchi, G. Fabrizi, A. Goggiamani and G. Zappia, Org. Lett., 2001, 3, 2539–2541 CrossRef CAS PubMed; (e) J. Yin and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 6043–6048 CrossRef CAS PubMed; (f) D. J. Wallace, D. J. Klauber, C. Chen and R. P. Volante, Org. Lett., 2003, 5, 4749–4752 CrossRef CAS PubMed; (g) M. A. Soussi, D. Audisio, S. Messaoudi, O. Provot, J. D. Brion and M. Alami, Eur. J. Org. Chem., 2011, 5077–5088 CrossRef CAS; (h) J. E. Rixon, T. Chaloner, C. H. Heath, L. F. Tietze and S. G. Stewart, Eur. J. Org. Chem., 2012, 544–558 CrossRef.
- (a) M. J. Cotnam, N. Martinek and M. Stradiotto, Eur. J. Org. Chem., 2023, e202301004 CrossRef CAS; (b) T. Lundrigan, J. P. Tassone and M. Stradiotto, Synlett, 2021, 1665–1669 CAS; (c) R. Sikari, S. Sinha, G. Chakraborty, S. Das, N. P. V. Leest and N. D. Paul, Adv. Synth. Catal., 2019, 361, 4342–4353 CrossRef CAS; (d) F. M. Moghaddam, G. Tavakoli, A. Moafi, V. Saberi and H. R. Rezvani, ChemCatChem, 2014, 6, 3474–3481 CrossRef CAS.
- R. Y. Liu, J. M. Dennis and S. L. Buchwald, J. Am. Chem. Soc., 2020, 142, 4500–4507 CrossRef CAS PubMed.
- (a) F. Ullmann and B. Dtsch, Chem. Ges., 1903, 36, 2382–2384 CrossRef; (b) J. Hassan, M. Sevignon, C. Gozzi, E. Schulz and M. Lemaire, Chem. Rev., 2002, 102, 1359–1470 CrossRef CAS PubMed; (c) F. Y. Kwong and S. L. Buchwald, Org. Lett., 2002, 4, 3517–3520 CrossRef CAS PubMed; (d) T. Itoh and T. A. Mase, Org. Lett., 2004, 6, 4587–4590 CrossRef CAS PubMed; (e) M. Schnürch, R. Flasik, A. F. Khan, M. Spina, M. D. Mihovilovic and P. Stanetty, Eur. J. Org. Chem., 2006, 3283–3307 CrossRef; (f) M. Taillefer, N. Xia and A. Ouali, Angew. Chem., Int. Ed., 2007, 46, 934–936 CrossRef CAS PubMed; (g) M. G. Wang, H. Yu, X. W. You, J. Wu and Z. C. Shang, Chin. J. Chem., 2012, 30, 2356–2362 CrossRef CAS; (h) Y.-C. Teo, F.-F. Yong, I. K. Ithnin, S.-H. T. Yio and Z. Lin, Eur. J. Org. Chem., 2013, 515–524 CrossRef CAS.
- (a) J. Lindley, Tetrahedron, 1984, 40, 1433–1456 CrossRef CAS; (b) M. A. Khan, Rec. Chem. Prog., 1970, 31, 43–50 CAS; (c) R. G. R. Bacon and H. A. O. Hill, Q. Rev., Chem. Soc., 1965, 19, 95–125 RSC; (d) A. Ouali, J.-F. Spindler, A. Jutand and M. Taillefer, Organometallics, 2007, 26, 65–74 CrossRef CAS.
- (a) P.-F. Larsson, A. Correa, M. Carril, P.-O. Norrby and C. Bolm, Angew. Chem., Int. Ed., 2009, 48, 5691–5693 CrossRef CAS PubMed; (b) J. Mao, Q. Hua, J. Guo and D. Shi, Catal. Commun., 2008, 10, 341–346 CrossRef CAS; (c) R. Hosseinzadeh, N. Aghili, M. Tajbakhsh and J. Mao, Catal. Lett., 2016, 146, 193–203 CrossRef CAS; (d) H.-J. Cristau, P. P. Cellier, J.-F. Spindler and M. Taillefer, Chem. – Eur. J., 2004, 10, 5607–5622 CrossRef CAS PubMed; (e) Z. Yao and X. Wei, Chin. J. Chem., 2010, 28, 2260–2268 CrossRef CAS; (f) K. Moriwaki, K. Satoh, M. Takada, Y. Ishino and T. Ohno, Tetrahedron Lett., 2005, 46, 7559–7562 CrossRef CAS; (g) R. Hosseinzadeh, M. Tajbakhsh, M. Mohadjerani and E. Ghorbani, Chin. J. Chem., 2008, 26, 2120–2124 CrossRef CAS; (h) C. Wang, L. Liu, W. Wang, D.-S. Ma and H. Zhang, Molecules, 2010, 15, 1154–1160 CrossRef CAS PubMed; (i) R. Hosseinzadeh, M. Tajbakhsh, M. Mohadjerani and H. Mehdinejad, Synlett, 2004, 1517–1520 CrossRef CAS; (j) M. Halder, M. M. Islam, Z. Ansari, S. Ahammed, K. Sen and S. M. Islam, ACS Sustainable Chem. Eng., 2017, 5, 648–657 CrossRef CAS; (k) Z.-J. Quana, H.-D. Xiaa, Z. Zhanga, Y.-X. Daa and X.-C. Wanga, Appl. Organomet. Chem., 2014, 28, 81–85 CrossRef; (l) K. Swapna, S. N. Murthy and V. D. Nageswar, Eur. J. Org. Chem., 2010, 6678–6684 CrossRef CAS; (m) C. Sambiagio, R. H. Munday, A. J. Blacker, S. P. Marsdena and P. C. McGowana, RSC Adv., 2016, 6, 70025–70032 RSC; (n) W. Deng, Y.-F. Wang, Y. Zou, L. Liu and Q.-X. Guo, Tetrahedron Lett., 2004, 45, 2311–2315 CrossRef CAS; (o) Y.-C. Teo, F.-F. Yong, I. K. Ithnin, S.-H. T. Yio and Z. Lin, Eur. J. Org. Chem., 2013, 515–524 CrossRef CAS; (p) M. L. P. R. Alapati, S. R. Abburu, K. R. Mutyala and S. B. Mukkamala, Synth. Commun., 2016, 46, 1242–1248 CrossRef CAS; (q) I. Güell and X. Ribas, Eur. J. Org. Chem., 2014, 3188–3195 CrossRef; (r) A. Klapars, J. C. Antilla, X. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2001, 123, 7727–7729 CrossRef CAS PubMed; (s) D. Nandi, S. Siwal and K. Mallick, New J. Chem., 2017, 41, 3082–3088 RSC; (t) S. De, J. Yin and D. Ma, Org. Lett., 2017, 19, 4864–4867 CrossRef CAS PubMed; (u) C. Cheng, G. Sun, J. Wan and C. Sun, Synlett, 2009, 2663–2668 Search PubMed; (v) L. Mohammadi, M. A. Zolfigol, A. Khazaei, M. Yarie, S. Ansari, S. Azizian and M. Khosravi, Appl. Organomet. Chem., 2017, 32, e3933 CrossRef; (w) M. Abaszadeh, R. Hosseinzadeh, M. Tajbakhsh and S. Ghasemi, J. Mol. Struct., 2022, 1261, 132832 CrossRef CAS; (x) A. Klapars, J. C. Antilla, X. Huang and S. L. A. Buchwald, J. Am. Chem. Soc., 2001, 123, 7727–7729 CrossRef CAS PubMed; (y) A. Klapars, X. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 7421–7428 CrossRef CAS PubMed; (z) Z. Yao and X. C. Wei, J. Org. Chem., 2010, 28, 2260–2268 CAS.
- (a) I. Güell and X. Ribas, Eur. J. Org. Chem., 2014, 3188–3195 CrossRef; (b) S. Jammi, S. Sakthivel, L. Rout, T. Mukherjee, S. Mandal, R. Mitra, P. Saha and T. Punniyamurthy, J. Org. Chem., 2009, 74, 1971–1976 CrossRef CAS PubMed; (c) S. Jammi, S. Krishnamoorthy, P. Saha, D. S. Kundu, S. Sakthivel, M. A. Ali, R. Paul and T. Punniyamurthy, Synlett, 2009, 3323–3327 CAS; (d) F.-F. Yong, Y.-C. Teo, G.-L. Chua, G. S. Lim and Y. Lin, Tetrahedron Lett., 2011, 52, 1169–1172 CrossRef CAS; (e) C. R. Tubío, J. Azuaje, L. Escalante, A. Coelho, F. Guitián, E. Sotelo and A. Gil, J. Catal., 2016, 334, 110–115 CrossRef; (f) M. A. Ali, P. Saha and T. Punniyamurthy, Synthesis, 2010, 908–910 CAS; (g) D.-X. Bai, R. S.-E. Lim, H.-F. Ng and Y.-C. Teo, Synth. Commun., 2021, 51, 1398–1405 CrossRef CAS; (h) P. Maity, D. Kundu and B. C. Ranu, Adv. Synth. Catal., 2015, 357, 3617–3626 CrossRef CAS.
- (a) H. Xu and C. Wolf, Chem. Commun., 2009, 13, 1715–1717 RSC; (b) D. Nandi, S. Siwal and K. A. Mallick, New J. Chem., 2017, 41, 3082–3088 RSC; (c) A. Kumar and A. K. Bishnoi, RSC Adv., 2014, 4, 41631–41635 RSC.
- (a) A. Correa and C. Bolm, Angew. Chem., Int. Ed., 2007, 46, 8862–8865 CrossRef CAS PubMed; (b) A. Correa, S. Elmore and C. Bolm, Chem. – Eur. J., 2008, 14, 3527–3529 CrossRef CAS PubMed; (c) S. L. Buchwald and C. Bolm, Angew. Chem., Int. Ed., 2009, 48, 5586–5587 CrossRef CAS PubMed; (d) Y.-C. Teoa, Adv. Synth. Catal., 2009, 351, 720–724 CrossRef; (e) K. Swapna, A. V. Kumar, V. P. Reddy and K. R. Rao, J. Org. Chem., 2009, 74, 7514–7517 CrossRef CAS PubMed.
- (a) Y.-C. Teo, F.-F. Yong, I. K. Ithnin, S.-H. T. Yio and Z. Lin, Eur. J. Org. Chem., 2013, 515–524 CrossRef CAS; (b) M. L. P. R. Alapati, S. R. Abburu, K. R. Mutyala and S. B. Mukkamala, Synth. Commun., 2016, 46, 1242–1248 CrossRef CAS.
- (a) R. Das, M. Mandal and D. Chakraborty, Asian J. Org. Chem., 2013, 2, 579–585 CrossRef CAS; (b) S. N. Murthy, B. Madhav, V. P. Reddy and Y. V. D. Nageswara, Adv. Synth. Catal., 2010, 352, 3241–3245 CrossRef CAS.
- (a) C. Gosmini, J.-M. Bégouin and A. Moncomble, Chem. Commun., 2008, 2008, 3221–3233 RSC; (b) G. Cahiez and A. Moyeux, Chem. Rev., 2010, 110, 1435–1462 CrossRef CAS PubMed; (c) B. Desai, A. Uppuluru, A. Dey, N. Deshpande, B. Z. Dholakiya, A. Sivaramakrishna, T. Naveen and K. Padala, Org. Biomol. Chem., 2023, 21, 673–699 RSC; (d) S. Gupta, R. Fernandes, R. Patel, M. Spreitzer and N. Patel, Appl. Catal., A, 2023, 661, 119254 CrossRef CAS; (e) T. Ghosh, P. Maity and B. C. Ranu, ChemistrySelect, 2018, 3, 4406–4412 CrossRef CAS; (f) M. M. Tohidi, B. Paymard, S. R. Vasquez-García and D. Fernández-Quiroz, Tetrahedron, 2023, 136, 133352 CrossRef CAS; (g) L. Feng, F. Liu, P. Sun and J. Bao, Synlett, 2008, 1415–1417 CAS; (h) S. Asghar, S. B. Tailor, D. Elorriaga and R. B. Bedford, Angew. Chem., Int. Ed., 2017, 56, 16367–16370 CrossRef CAS PubMed.
- (a) Y.-C. Teo and G.-L. Chua, Chem. – Eur. J., 2009, 15, 3072–3075 CrossRef CAS PubMed; (b) B. Y.-H. Tan and Y.-C. Teo, Org. Biomol. Chem., 2014, 12, 7478–7481 RSC; (c) B. Y.-H. Tan and Y.-C. Teo, Synlett, 2015, 1697–1701 CAS; (d) See Table S2a in the ESI† for a comparison of the results of the present work with those of reported cobalt-catalyzed methodologies.
- (a) See the ESI for details;†; (b) S. Ghosh, P. P. Pal and A. Hajra, Adv. Synth. Catal., 2023, 365, 3020–3043 CrossRef CAS; (c) See Scheme S1 in the ESI for a detailed procedure of catalyst recovery;†; (d) L. Adak, S. Kawamura, G. Toma, T. Takenaka, K. Isozaki, H. Takaya, A. Orita, H. C. Li, T. K. M. Shing and M. Nakamura, J. Am. Chem. Soc., 2017, 139, 10693–10701 CrossRef CAS PubMed; (e) J. Breitenfeld, M. D. Wodrich and X. Hu, Organometallics, 2014, 33, 5708–5715 CrossRef CAS; (f) See Fig. S1 in the ESI for the EDS image of Co-nanoparticles formed in the reaction mixture;†; (g) See Fig. S2 in the ESI for the cathodic reduction process of Co(II) to Co(0).†.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ob00178a |
| This journal is © The Royal Society of Chemistry 2025 |