Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Systematic investigation of the structure–property relationship of substituted p-alkoxy-azothiophenes†
Conrad
Averdunk
ab and
Hermann A.
Wegner
 *ab
*ab
aInstitute of Organic Chemistry, Justus Liebig University Giessen, Heinrich-Buff-Ring 17, 35392 Giessen, Germany. E-mail: Hermann.A.Wegner@org.chemie.uni-giessen.de
bCenter of Materials Research (ZfM/LaMa), Justus Liebig University Giessen, Heinrich-Buff-Ring 16, 35392 Giessen, Germany
First published on 13th May 2025
Abstract
Differently substituted p-alkoxy azothiophenes with increasing alkoxy chains were systematically investigated in terms of their structure–property relationships. In particular, it was observed that increasing the length of the alkoxy chain had an unusual effect on the melting point, which did not follow the expected odd–even effect. It was also shown that changing the length of the alkoxy chain did not significantly affect the thermal half-life, a finding that disagrees with results reported in other studies. These observations provide valuable insights into structure–property relationships with important implications for the design and development of azobenzenes as molecular materials for various applications. Furthermore, each p-alkoxy azothiophene was investigated in terms of neat solid-state photoisomerisation or photoinduced liquefaction, which is a critical parameter for application as a molecular solar thermal phase-change (MOST-PCM) energy storage system.
Introduction
Azobenzenes (ABs) are well-known photoswitches that can reversibly be switched with light irradiation between their thermodynamically stable (E)-isomer and the energetically excited (Z)-isomer.1 Due to their photochromism, ABs have a wide range of potential applications, including as a dye,2 in photopharmacology,3 as a molecular wind-up meter4 or energy storage systems.5 In recent years, there has been growing interest in heteroaryl-substituted ABs due to their unique properties.6 Thiophenylazobenzenes (TphABs) represent particularly interesting examples of heteroaryl-substituted ABs (Fig. 1).7 On the one hand, the (E)-isomers of TphABs exhibit a significantly red-shifted absorption band of the π–π* transition, which can range from 365 nm to 405 nm depending on the substitution. On the other hand, TphABs show an almost quantitative (E) → (Z) photoisomerization, which is promoted by a strong separation of the π–π* transitions of the (E)- and (Z)-isomers. Furthermore, due to this band separation and the comparable high quantum yields of up to 44%, the photochemical back-isomerization of the (Z)-isomer is remarkably efficient. Additionally, the (Z)-isomers of TphABs show a T-shaped structure, in which the phenyl ring is orthogonal to the thiophene ring and the azo bond realizing a stabilizing intramolecular interaction between the lone pair of the sulphur atom and the π-system of the phenyl ring. This lone pair⋯π interaction only occurs in the (Z)-isomer and allows tuning of the thermal stability. Electron-withdrawing substituents on the phenyl ring can enhance the electron-donating interaction of the sulphur lone pair with the π-system, increasing the half-life of the (Z)-isomer.8 Due to these different properties compared to pristine azobenzenes, the investigation of the structure–property relationship is essential for the development and optimization of heterocyclic azobenzenes as molecular materials. One potential application is the usage of azobenzenes as molecular solar thermal energy storage (MOST) systems, as alternative renewable and low-emission energy harvesting and storage systems. Such MOST systems utilize the energy difference between a higher energy meta-stable state and the ground state.9 The class of TphABs represents a promising candidate for this application.10 Although ABs have in general lower energy densities than the norbornadiene-quadricyclane (NBD-QC) system,11 they excel due to their good synthetic accessibility and chemical stability compared to other photoswitches. These properties make them more suitable for commercial utilisation.12 In recent years, a number of attempts have been made to increase the energy density.13One promising approach is the combination of photoswitches with the concept of phase change materials (PCM). ABs are particularly well suited for this application because their photoisomers exhibit a significant change in geometry during photoisomerization compared to other photoswitches, leading to substantial differences in physical properties such as melting point.14 However, the photoisomerization of crystalline ABs is challenging and has long been considered unfeasible, since crystalline structures usually provide insufficient free pore volume for molecular movement, necessary for photoisomerization.15 In recent years, however, several AB-based MOST-PCM systems have been developed in which photoisomerization and liquefaction of crystalline ABs upon irradiation were achieved.16 In these systems, the ABs are typically equipped with at least one alkyl chain. Systems with additional substituents are also known.17–19 Several azopyrazole- and azoisoxazole-based systems have also been reported that can be used as MOST-PCM systems.20,21 The combination of heterocyclic ABs with MOST-PCM systems is particularly interesting for tuning the properties of such systems.
For this reason, TphABs with different alkoxy chains at the para-position of the phenyl moiety were synthesised in this study. The para-substitution was chosen because almost all literature known MOST-PCM systems are para-substituted although we have shown that meta-substitution would significantly prolong the thermal half-life.8 Furthermore, substitution in the para-position enables much better synthetic accessibility. In addition, the substituent on the thiophene ring was varied. A methyl and a methoxy group were used to investigate the influence of a second substituent on the physicochemical properties. The methoxy group was chosen to study whether introducing a second, free rotating substituent could have a positive effect on molecular mobility within the crystal structure and enhance photoliquefaction. The shorter, more rigid methyl group was used as a reference. In this study melting points, kinetic half-lives, crystal structures, absorption spectra and solid-state photoisomerization were investigated to establish the valuable structure–property relationship which will contribute to the development of new MOST-PCM systems.
Results and discussion
Synthesis
First, 4-alkoxyphenyl-5-methoxythiophenyldiazenes (4a–i) were successfully synthesised by a two-step synthesis involving an azo-coupling reaction followed by Williamson etherification (Fig. 2a). Therefore, 4-aminophenol (2) was converted to a diazonium salt and treated with the electron-rich 2-methoxythiophene under basic conditions to obtain the hydroxy substituted methoxy TphAB 3via an azo-coupling reaction.22 The electron-donating effect of the methoxy group at the C5 position of the thiophene increases the acidity of the proton providing good nucleophilicity. The resulting TphAB 3 was then etherified to obtain the desired TphABs 4a–i. Due to the insufficient electron density of the methyl substituted thiophene, a slightly different method was applied for the synthesis of TphABs 9a–e (Fig. 2b). In these cases, the corresponding bromides were used and first converted to the corresponding Grignard reagents.23 These intermediates were then treated with zinc bromide to obtain the corresponding organozinc compounds to obtain a suitable nucleophile, which undergoes efficient azo-coupling reactions under mild conditions with the corresponding diazonium salts 8a–e to provide the TphABs 9a–e.24Melting points
A deep understanding of the structure–property relationship is essential for the development and optimisation of new molecular materials. One of the most important parameters in this context is the melting point of the (E)- and (Z)-isomers, as it determines the temperature range in which the system can be operated. The melting point of the isomers is also a critical factor in the success and efficiency of photoliquefaction, as excessively high melting points, particularly of the (E)-isomer, can make the irradiation induced liquefaction process less advantageous. In such cases, a larger temperature difference may need to be compensated by a higher proportion of the (Z)-isomer. In this study, the melting points of the respective (E)-isomers of different TphABs were determined in order to gain insights into the relationship between melting points, chain length and substituents (Fig. 3). The (E)-isomers of the methoxy-substituted TphABs 4a–i revealed an increase in melting point with increasing chain length, which can be attributed to the increasing intermolecular interactions between the alkoxy chains (Fig. 3; red dots). A commonly observed phenomenon in compounds with long alkyl chains is the so-called odd–even effect, where stronger interactions between the chains occur in compounds with an even number chain length compared to those with an odd number chain length.25 In general, it can be assumed that compounds with an even number of carbon atoms in their chains tend to have slightly higher melting points than those with an odd number of carbon atoms.26 Here, an unusual trend in the melting points as a function of chain length was observed. Specifically, a significant decrease in melting point occurred for every third additional carbon of the alkoxy chain, while the remaining chain lengths followed a relatively linear behaviour. | ||
| Fig. 3 Melting points of the (E)-isomers of methyl and methoxy substituted TphABs 4a–i and TphABs 9a–e with different chain lengths. | ||
It seemed that the melting points of TphABs 4a–i follow two distinct trends. In particular, TphABs 4a–i with chain lengths of C4, C7 and C10 showed significantly lower melting points. In contrast, the methyl-substituted TphABs neither showed a comparable trend nor any linear or increasing behaviour of the melting points with increasing chain length. With the more rigid molecular geometry, a rather linear behaviour of the melting point could be expected. As only alkoxy groups with an even chain length were investigated for the methyl-substituted TphABs, no conclusions can be made concerning the influence of the odd–even effect. This shows that in general there is no trivial linear relationship between chain length and melting point. This is in accordance with literature known MOST-PCM systems, where also no correlation of the melting point with increasing chain length has been observed so far.17,19,21
Spectroscopic properties
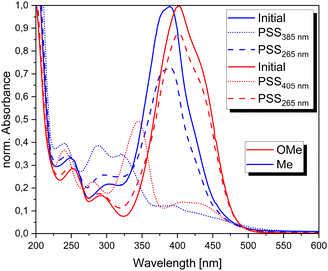
The methyl and methoxy substituted TphABs 4a–i and TphABs 9a–e were dissolved in acetonitrile and irradiated at different wavelengths to determine the wavelength for the most effective photoisomerization (Fig. 4). The absorption spectrum of the initial state of the TphABs 4 shows a strong absorption band around 400 nm corresponding to the π–π* transition and a shoulder around 450 nm corresponding to the n–π* transition (Fig. 4, red). Irradiation with a wavelength of 405 nm leads to a significant decrease in the intensity of the π–π* band and the appearance of a new band around 330 nm. Different wavelengths were tested to determine the most efficient (Z) → (E) isomerization. It was found that a wavelength of 265 nm resulted in almost quantitative back-isomerization. Compared to the methoxy substituted TphABs 4a–i, the methyl substituted TphABs 9a–e show a less red-shifted absorption maximum of the π–π* transition band at approx. 385 nm. Back switching was also carried out with a wavelength of 265 nm. This process was not as effective as for the methoxy substituted TphABs 4a–i. However, it was not possible to accurately determine the composition of the (E)- and (Z)-isomers in the photostationary state at the different wavelengths, as the thermal half-lives of the TphABs 4a–i and 9a–e in solution are too short to determine their composition by NMR spectroscopy or HPLC (Fig. 5).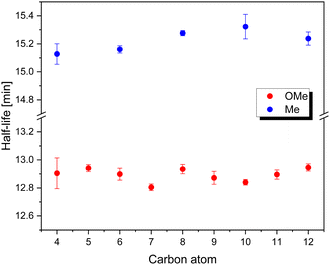 | ||
| Fig. 5 Thermal half-lives of methoxy and methyl substituted TphABs 4a–i and TphABs 9a–e at 20 °C in acetonitrile. | ||
Thermal isomerization kinetics
The thermal half-lives of methoxy and methyl substituted TphABs 4a–i and TphABs 9a–e have been determined using UV-Vis spectroscopy. The methoxy-substituted TphABs 4a–i showed an average thermal half-life of just under 13 min. In comparison, the methyl-substituted TphABs 9a–e showed a slightly longer thermal half-life of just over 15 min on average. This drastically reduced half-life can be explained by electronic effects. Due to the electron-donating effect of the alkoxy group on the phenyl ring, the electron density is increased, which reduces the electron-donating intramolecular Lp⋯π interaction between the lone pair of the sulphur atom and the π system of the phenyl ring. This effect leads to less stabilization of the (Z)-isomer, resulting in a significant decrease in its thermal half-life. Similar observations have been made in comparable TphAB systems.8 Furthermore, the thermal half-life is not affected by chain length, which is in contrast to analogous literature-known systems.27Photoliquefaction properties
All synthesised TphABs were irradiated in the solid state at different wavelengths and temperatures to investigate their potential as candidates for MOST-PCM materials. It was found that the methoxy-substituted TphABs 4a–i do not show any photoliquefaction upon irradiation with 405 nm, which corresponds to the absorption maximum of the π–π* transition of the (E)-isomer in solution. The same observation was made for the methyl-substituted TphABs 9a–e upon irradiation with 385 nm, which could be caused by a significant shift of the π–π* transition band in the solid state compared to that in solution, preventing effective excitation with 405 nm or 385 nm. In other MOST-PCM systems, a strong redshift of the π–π* transition has been observed in the solid state, with a shift in excitation in solution from around 340 nm to around 365 nm in the solid state.17 During irradiation a colour change was observed indicating possible photoisomerization in the solid state. Therefore, a potential reason for unsuccessful photoliquefaction could be the high melting points of the (E)- and (Z)-isomers, which may prevent effective liquefaction of the solid phase upon irradiation. To promote photoliquefaction, the respective compounds were heated during irradiation to temperatures just below the melting point of the (E)-isomer. However, these conditions also did not lead to a successful photoliquefaction. In this case it is also important to consider the short half-lives, which are further reduced by the additional heating, potentially making effective photoisomerization more challenging. The most likely cause of unsuccessful photoliquefaction is an insufficient mobility of the molecules within the crystal structure, which suppresses and prevents photoisomerization in the solid phase. In addition, NMR-spectroscopy revealed decomposition after prolonged irradiation, which may also cause colour change.Solid state structures
The solid-state structures of methoxy-substituted TphABs 4f–h with chain lengths of C9, C10 and C11 and that of methyl substituted TphAB 9d were obtained (Fig. 6). A comparison of the crystal structures of the C9 (Fig. 6a) and C10 (Fig. 6b) derivatives showed that they have similar crystal packing. The only difference, apart from the chain length, is the orientation of the methoxy group. For the TphAB 4f with a C9 chain, the methoxy group is in the plane of the thiophene ring and faces the sulphur atom, whereas for the TphAB 4f with a C10 chain it is oriented in the opposite direction. Thus, the orientation of the methoxy group could potentially explain the unusual decrease of melting point. Compound 4h adopts a completely different packing, which makes it only partially comparable with the crystal structures of TphABs 4f and g. In the solid-state structure of 4h we also find that here the methoxy group points in the opposite direction to the sulphur atom. | ||
| Fig. 6 Crystal structures of (E)-4-alkoxyphenyl-5-methoxythiophenyldiazenes with (a) C9, (b) C10 and (c) C11 chain lengths and (d) (E)-4-alkoxyphenyl-5-methylthiophenyldiazene with C10 chain length. | ||
The methyl substituted TphAB 9d shows a similar packing to the methoxy substituted TphABs 4f and g indicating that the alkoxy group mainly determines the crystal packing. Since the methyl substituent in TphABs 9a–e is fixed and cannot change its orientation it could be assumed that the melting points should show a more linear behaviour with increasing chain length. In summary, no clear correlation between melting points and any structural properties could be assigned.
Conclusions
Various substituted TphABs with different substituents and chain lengths were successfully synthesised and their physical properties were investigated. It was shown that methoxy and methyl substituted TphABs 4a–i and TphABs 9a–e show promising properties for lower energy absorption, as they exhibit a significant red shift of the π–π* transition reaching up to 405 nm. It was shown that there is no correlation between chain length and thermal half-life in solution for the synthesised TphABs. An unusual trend in the melting points as a function of chain length was also observed for the methoxy substituted TphABs 9a–e, with a significant decrease in melting point for every third additional carbon in the alkoxy chain. This behaviour is contrary to other MOST-PCM systems and the textbook-known odd–even effect.28 In addition, single crystals were obtained which allowed a more detailed analysis of the structural effects in the solid state via X-ray analysis. For the methyl substituted TphABs 9a–d no correlation of the melting point or the chain length was observed, though. Photoliquefaction could not be achieved for any of the TphABs by irradiation with different wavelengths and temperatures. This behaviour might be due to reduced molecular mobility in the solid state, the high melting points of the (E)-isomers, short thermal half-lives as well as decomposition. To realize the concept of MOST-PCM systems with azothiophenes, it is necessary to increase the thermal half-life to achieve sufficient photoisomerization in the condensed phase. Furthermore, it is essential to prevent irradiation-induced decomposition by selective substitution of the thiophene moiety. Additionally, an increase of the free pore volume and a reduction of the melting points of the (E)-isomers could enhance photoliquefaction. For example, the substitution of the alkoxy chain in the meta-position could not only offer an increased thermal half-life but could also adapt the melting point and the free pore volume due to the lower symmetry. Nevertheless, valuable information has been gained, which supports the design of novel molecular materials with application potential as MOST-PCMs in the future.Author contributions
H. A. W. and C. A. conceptualized the project and prepared the manuscript. C. A. synthesized all compounds and collected all experimental data. Both authors were involved in discussing the data and have given approval to the final version of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support from the Deutsche Forschungsgemeinschaft (DFG) within the Research Unit FOR 5499 “Molecular Solar Energy Management – Chemistry of MOST Systems” (project 496207555). We thank Dominic Schatz, Institute of Organic Chemistry of the Justus Liebig University, for X-ray analysis.References
- A. A. Beharry and G. A. Woolley, Chem. Soc. Rev., 2011, 40, 4422–4437 RSC.
- A. Bafana, S. S. Devi and T. Chakrabarti, Environ. Rev., 2011, 19, 350–371 CrossRef CAS.
- V. A. Gutzeit, A. Acosta-Ruiz, H. Munguba, S. Häfner, A. Landra-Willm, B. Mathes, J. Mony, D. Yarotski, K. Börjesson, C. Liston, G. Sandoz, J. Levitz and J. Broichhagen, Cell Chem. Biol., 2021, 28, 1648–1663 CrossRef CAS PubMed.
- C. Averdunk, K. Hanke, D. Schatz and H. A. Wegner, Acc. Chem. Res., 2024, 57, 257–266 CrossRef CAS PubMed.
- (a) E. N. Cho, D. Zhitomirsky, G. G. D. Han, Y. Liu and J. C. Grossman, ACS Appl. Mater. Interfaces, 2017, 9, 8679–8687 CrossRef CAS PubMed; (b) J. Huang, Y. Jiang, J. Wang, C. Li and W. Luo, Thermochim. Acta, 2017, 657, 163–169 CrossRef CAS; (c) A. M. Kolpak and J. C. Grossman, Nano Lett., 2011, 11, 3156–3162 CrossRef CAS PubMed; (d) A. M. Kolpak and J. C. Grossman, J. Chem. Phys., 2013, 138, 34303 CrossRef PubMed; (e) W. Pang, J. Xue and H. Pang, Sci. Rep., 2019, 9, 5224 CrossRef PubMed; (f) B. Zhang, Y. Feng and W. Feng, Nano Lett., 2022, 14, 138 CrossRef CAS PubMed.
- S. Crespi, N. A. Simeth and B. König, Nat. Rev. Chem., 2019, 3, 133–146 CrossRef CAS.
- C. Slavov, C. Yang, A. H. Heindl, H. A. Wegner, A. Dreuw and J. Wachtveitl, Angew. Chem., Int. Ed., 2020, 59, 380–387 CrossRef CAS PubMed.
- A. H. Heindl and H. A. Wegner, Chemistry, 2020, 26, 13730–13737 CrossRef CAS PubMed.
- Z. Wang, P. Erhart, T. Li, Z.-Y. Zhang, D. Sampedro, Z. Hu, H. A. Wegner, O. Brummel, J. Libuda, M. B. Nielsen and K. Moth-Poulsen, Joule, 2021, 5, 3116–3136 CrossRef CAS.
- E. Franz, A. Kunz, N. Oberhof, A. H. Heindl, M. Bertram, L. Fusek, N. Taccardi, P. Wasserscheid, A. Dreuw, H. A. Wegner, O. Brummel and J. Libuda, ChemSusChem, 2022, 15, e202200958 CrossRef CAS PubMed.
- (a) F. Hemauer, H.-P. Steinrück and C. Papp, ChemPhysChem, 2024, 25, e202300806 CrossRef CAS PubMed; (b) M. J. Kuisma, A. M. Lundin, K. Moth-Poulsen, P. Hyldgaard and P. Erhart, J. Phys. Chem. C, 2016, 120, 3635–3645 CrossRef CAS PubMed; (c) J. Orrego-Hernández, A. Dreos and K. Moth-Poulsen, Acc. Chem. Res., 2020, 53, 1478–1487 CrossRef PubMed; (d) A. U. Petersen, A. I. Hofmann, M. Fillols, M. Mansø, M. Jevric, Z. Wang, C. J. Sumby, C. Müller and K. Moth-Poulsen, Adv. Sci., 2019, 6, 1900367 CrossRef PubMed; (e) M. Quant, A. Lennartson, A. Dreos, M. Kuisma, P. Erhart, K. Börjesson and K. Moth-Poulsen, Chemistry, 2016, 22, 13265–13274 CrossRef CAS PubMed; (f) F. Waidhas, M. Jevric, L. Fromm, M. Bertram, A. Görling, K. Moth-Poulsen, O. Brummel and J. Libuda, Nano Energy, 2019, 63, 103872 CrossRef CAS; (g) R. Schulte, S. Afflerbach, T. Paululat and H. Ihmels, Angew. Chem., Int. Ed., 2023, 62, e202309544 CrossRef CAS PubMed.
- (a) E. Merino, Chem. Soc. Rev., 2011, 40, 3835–3853 RSC; (b) C.-L. Sun, C. Wang and R. Boulatov, ChemPhotoChem, 2019, 3, 268–283 CrossRef CAS.
- (a) X. Xu, J. Feng, W.-Y. Li, G. Wang, W. Feng and H. Yu, Prog. Polym. Sci., 2024, 149, 101782 CrossRef CAS; (b) A. Kunz, A. H. Heindl, A. Dreos, Z. Wang, K. Moth-Poulsen, J. Becker and H. A. Wegner, ChemPlusChem, 2019, 84, 1145–1148 CrossRef CAS PubMed; (c) A. R. Ibrahim, M. F. Khyasudeen, J. Husband, S. M. Alauddin, N. F. K. Aripin, T. S. Velayutham, A. Martinez-Felipe and O. K. Abou-Zied, J. Phys. Chem. C, 2021, 125, 22472–22482 CrossRef CAS; (d) Z. Wang, R. Losantos, D. Sampedro, M.-A. Morikawa, K. Börjesson, N. Kimizuka and K. Moth-Poulsen, J. Mater. Chem. A, 2019, 7, 15042–15047 RSC.
- (a) G. S. Kumar and D. C. Neckers, Chem. Rev., 1989, 89, 1915–1925 CrossRef CAS; (b) W.-C. Xu, S. Sun and S. Wu, Angew. Chem., Int. Ed., 2019, 58, 9712–9740 CrossRef CAS PubMed.
- A. Gonzalez, E. S. Kengmana, M. V. Fonseca and G. G. D. Han, Mater. Today Adv., 2020, 6, 100058 CrossRef.
- (a) J. Ge, M. Qin, X. Zhang, X. Yang, P. Yang, H. Wang, G. Liu, X. Zhou, B. Zhang, Z. Qu, Y. Feng and W. Feng, SmartMat, 2024, 5, e1300 CrossRef CAS; (b) J. Hu, S. Huang, M. Yu and H. Yu, Adv. Energy Mater., 2019, 9, 1901363 CrossRef CAS; (c) H. Liu, Y. Feng and W. Feng, Compos. Commun., 2020, 21, 100402 CrossRef; (d) J. Tang, Y. Feng and W. Feng, Compos. Commun., 2021, 23, 100575 CrossRef.
- J. Gao, Y. Feng, W. Fang, H. Wang, J. Ge, X. Yang, H. Yu, M. Qin and W. Feng, Energy Environ. Mater., 2024, 7, e12607 CrossRef CAS.
- (a) Y. Shi, M. A. Gerkman, Q. Qiu, S. Zhang and G. G. D. Han, J. Mater. Chem. A, 2021, 9, 9798–9808 RSC; (b) J. Hu, M. Yu and H. Yu, J. Mater. Chem. C, 2025, 35, 4621 CrossRef.
- Y. Yang, S. Huang, Y. Ma, J. Yi, Y. Jiang, X. Chang and Q. Li, ACS Appl. Mater. Interfaces, 2022, 14, 35623–35634 CrossRef CAS PubMed.
- (a) A. Gonzalez, M. Odaybat, M. Le, J. L. Greenfield, A. J. P. White, X. Li, M. J. Fuchter and G. G. D. Han, J. Am. Chem. Soc., 2022, 144, 19430–19436 CrossRef CAS PubMed; (b) X. Huang, Z. Shangguan, Z.-Y. Zhang, C. Yu, Y. He, D. Fang, W. Sun, Y.-C. Li, C. Yuan, S. Wu and T. Li, Chem. Mater., 2022, 34, 2636–2644 CrossRef CAS; (c) Z. Shangguan, W. Sun, Z.-Y. Zhang, D. Fang, Z. Wang, S. Wu, C. Deng, X. Huang, Y. He, R. Wang, T. Li, K. Moth-Poulsen and T. Li, Chem. Sci., 2022, 13, 6950–6958 RSC; (d) L. Burg, L. Kortekaas, A. Gibalova, C. Daniliuc, J. Heßling, M. Schönhoff and B. J. Ravoo, RSC Appl. Interfaces, 2025, 2, 373–380 RSC; (e) L. Kortekaas, J. Simke, D. W. Kurka and B. J. Ravoo, ACS Appl. Mater. Interfaces, 2020, 12, 32054–32060 CrossRef CAS PubMed.
- Z.-Y. Zhang, Y. He, Z. Wang, J. Xu, M. Xie, P. Tao, D. Ji, K. Moth-Poulsen and T. Li, J. Am. Chem. Soc., 2020, 142, 12256–12264 CrossRef CAS PubMed.
- A. Matharu, P. Huddleston, S. Jeeva, M. Wood and D. Chambers-Asman, Dyes Pigm., 2008, 78, 89–92 CrossRef CAS.
- (a) A. Krasovskiy and P. Knochel, Angew. Chem., Int. Ed., 2004, 43, 3333–3336 CrossRef CAS PubMed; (b) A. Krasovskiy and P. Knochel, Angew. Chem., 2004, 116, 3396–3399 CrossRef.
- (a) B. Haag, Z. Peng and P. Knochel, Org. Lett., 2009, 11, 4270–4273 CrossRef CAS PubMed; (b) D. Y. Curtin and J. A. Ursprung, J. Org. Chem., 1956, 21, 1221–1225 CrossRef CAS; (c) D. Y. Curtin and J. L. Tveten, J. Org. Chem., 1961, 26, 1764–1768 CrossRef CAS.
- W. H. de Jeu, J. van der Veen and W. J. A. Goossens, Solid State Commun., 1973, 12, 405–407 CrossRef CAS.
- K. Yang, Z. Cai, A. Jaiswal, M. Tyagi, J. S. Moore and Y. Zhang, Angew. Chem., Int. Ed., 2016, 55, 14090–14095 CrossRef CAS PubMed.
- (a) Q. Qiu, M. A. Gerkman, Y. Shi and G. G. D. Han, Chem. Commun., 2021, 57, 9458–9461 RSC; (b) Z. Abdin, M. A. Alim, R. Saidur, M. R. Islam, W. Rashmi, S. Mekhilef and A. Wadi, Renewable Sustainable Energy Rev., 2013, 26, 837–852 CrossRef CAS; (c) L. Jiang, Z. Wu, H. Liu, L. Zhang and X. Luo, Appl. Mater. Today, 2025, 42, 102531 CrossRef; (d) Q. Yang, J. Ge, M. Qin, H. Wang, X. Yang, X. Zhou, B. Zhang, Y. Feng and W. Feng, Sci. China Mater., 2023, 66, 3609–3620 CrossRef CAS; (e) L. Fei, Y. Yin, J. Zhang and C. Wang, Sol. RRL, 2020, 4, 105 Search PubMed; (f) L. Fei, W. Yu, J. Tan, Y. Yin and C. Wang, Adv. Fiber Mater., 2023, 5, 955–967 CrossRef.
- J. C. S. Costa and L. M. N. B. F. Santos, J. Chem. Eng. Data, 2019, 64, 2229–2246 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2425327, 2425326, 2425324 and 2425325. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ob00506j |
| This journal is © The Royal Society of Chemistry 2025 |