Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Towards the synthesis of polythiazolines: a post-polymerization approach†
Aikaterini
Mathianaki
a,
Aysha Kinjo
Demeler
a,
Adrian
Dömling
b,
Federico
Ferrari
c,
Frieda Clara M.
Scheelje
c,
Hilke
Bahmann
 bd and
Guillaume
Delaittre
bd and
Guillaume
Delaittre
 *ad
*ad
aOrganic Functional Molecules, Organic Chemistry, University of Wuppertal, Gaußstraße 20, 42119 Wuppertal, Germany. E-mail: delaittre@uni-wuppertal.de
bPhysical and Theoretical Chemistry, University of Wuppertal, Gaußstraße 20, 42119 Wuppertal, Germany
cLaboratory of Applied Chemistry, Institute of Organic Chemistry (IOC), Karlsruhe Institute of Technology (KIT), Straße am Forum 7, 76131 Karlsruhe, Germany
dWuppertal Center for Smart Materials & Systems (CM@S), University of Wuppertal, Gaußstraße 20, 42119 Wuppertal, Germany
First published on 5th December 2024
Abstract
The synthesis of novel tertiary polythioamide copolymers, analogues of poly(2-ethyl-2-oxazoline) (PEtOx), is reported. Firstly, the direct synthesis of poly(2-methyl-2-thiazoline) was attempted via the cationic ring-opening polymerization of 2-methyl-2-thiazoline, in analogy to the well-known 2-alkyl-2-oxazoline monomers. Since no conversion was monitored under several conditions – which was investigated in parallel by density functional theory calculations – the post-polymerization modification of PEtOx using Lawesson's reagent was successfully achieved, yielding poly(2-ethyl-2-thiazoline)-co-(2-ethyl-2-oxazoline) copolymers with up to 95 mol% of the thioamide unit. The newly synthesized copolymers exhibited significantly lower water solubility and thermal stability than the pristine PEtOx, as demonstrated during cloud point temperature determination and thermal gravimetric analysis, respectively. Moreover, the glass transition temperature of the copolymers increases linearly with increasing oxygen–sulfur exchange.
Introduction
Poly(2-alkyl/aryl-2-oxazoline)s (PAOx) constitute one of the most promising classes of polyamides and have been extensively investigated.1–4 PAOx are obtained by the cationic ring-opening polymerization (cROP) of 2-alkyl/aryl-2-oxazolines (Scheme 1). One of their great advantages is the facile access to different solution properties by simple structural variation of the pendant alkyl chain (length, branching, or the presence of heteroatoms).5 However, substitution of the carbonyl oxygen atom to yield the polythioamide analogues has never been reported. Recent advances have proved that the properties of thioamides differ from those of the corresponding amides. For example, thioamides can act as ligands in coordination chemistry due to their high affinity for certain metals such as copper.6–8 The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond is weaker (BDE ∼130 kcal mol−1) than the C
S bond is weaker (BDE ∼130 kcal mol−1) than the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond (∼170 kcal mol−1).9,10 Thioamides are less flexible due to their higher rotational barrier9,10 and also appear to be less hydrophilic since sulfur is a poorer hydrogen bond acceptor than oxygen.9 Furthermore, there is an increased interest in sulfur-containing polymers in general, since they possess significant properties for a variety of applications such as in biomedicine and energy storage, or as flame retardants.11 Interestingly, the synthesis of poly(2-alkyl-2-thiazoline) (PAThz) homopolymers has not been reported yet, despite the commercial availability of at least one sulfur analogue of AOx, namely 2-methyl-2-thiazoline (MeThz), and the reported efficient alkylation of a variety of AThz using alkyl (pseudo)halogenides, which would represent the first mechanistic step in cROP. The only polymer-related AThz study reported the spontaneous copolymerization of MeThz with acrylic acid in the absence of an initiator, following a zwitterionic mechanism,12 similar to the behavior of 2-alkyl-2-oxaz(ol)ines.13 However, the final polymer structures resulted from the nucleophilic attack of the sulfur atom yielding iminothioether-based repeating units rather than thioamides. We also note that the term polythiazolines has already been introduced in 1969, when polyureas were synthesized by polycondensation and subsequently treated with poly(phosphoric acid) to yield in-chain five-membered thiazoline rings.14
O bond (∼170 kcal mol−1).9,10 Thioamides are less flexible due to their higher rotational barrier9,10 and also appear to be less hydrophilic since sulfur is a poorer hydrogen bond acceptor than oxygen.9 Furthermore, there is an increased interest in sulfur-containing polymers in general, since they possess significant properties for a variety of applications such as in biomedicine and energy storage, or as flame retardants.11 Interestingly, the synthesis of poly(2-alkyl-2-thiazoline) (PAThz) homopolymers has not been reported yet, despite the commercial availability of at least one sulfur analogue of AOx, namely 2-methyl-2-thiazoline (MeThz), and the reported efficient alkylation of a variety of AThz using alkyl (pseudo)halogenides, which would represent the first mechanistic step in cROP. The only polymer-related AThz study reported the spontaneous copolymerization of MeThz with acrylic acid in the absence of an initiator, following a zwitterionic mechanism,12 similar to the behavior of 2-alkyl-2-oxaz(ol)ines.13 However, the final polymer structures resulted from the nucleophilic attack of the sulfur atom yielding iminothioether-based repeating units rather than thioamides. We also note that the term polythiazolines has already been introduced in 1969, when polyureas were synthesized by polycondensation and subsequently treated with poly(phosphoric acid) to yield in-chain five-membered thiazoline rings.14
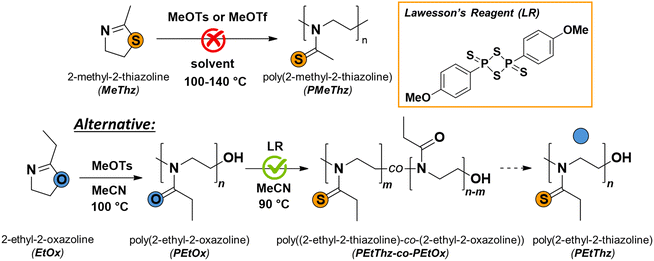 | ||
| Scheme 1 Schematic representation of the unsuccessful cROP of MeThz (top) and the suggested alternative by thionation of PEtOx with Lawesson's reagent (bottom). | ||
As a consequence of the above, we first considered the direct cROP of MeThz (Scheme 1, top). However, since no conversion could be achieved under a range of experimental conditions suitable for the cROP of AOx, we turned to a post-polymerization approach in which poly(2-ethyl-2-oxazoline) (PEtOx) was reacted with Lawesson's reagent (LR)15–20 as a known oxygen-to-sulfur exchange agent (Scheme 1, bottom).
Results and discussion
The cROP of cyclic iminoethers such as AOx is typically initiated by the nucleophilic attack of an alkylating agent such as methyl tosylate or benzyl bromide by the nitrogen free electron pair of an AOx monomer. A careful literature search reveals that the alkylation of methyl, ethyl, n-propyl, i-butyl, and benzyl thiazolines could efficiently be carried out with methyl iodide or ethyl ethanesulfonate.21–24 MeThz was used for the initial studies of the polymerization (Scheme 1) since it is a cheap and commercially available potential monomer. Methyl tosylate (MeOTs) and methyl triflate (MeOTf) were used as initiators under various stoichiometries and experimental conditions (Table S1†). However, in all cases, zero conversion was monitored (Fig. S1–S3†). In order to gain insight into the non-reactivity of MeThz under classic cROP conditions, we turned to quantum chemical calculations based on density functional theory. Fig. S12† depicts the reaction enthalpies and Gibbs free energies for the dimerization and trimerization reactions of both 2-methyl-2-oxazoline (MeOx) and MeThz at various temperatures in acetonitrile (MeCN), assuming that initiation had already taken place, that is, oxazolinium and thiazolinium cations would react with the corresponding monomers. While all reactions are exothermic with enthalpy values essentially temperature-independent, the average reaction enthalpy for MeOx dimerization and trimerization (approx. −80 kJ mol−1) is about four times more negative than that for MeThz (Fig. S12,† left). The Gibbs free energies are even more distinct, revealing exergonic dimerization and trimerization for MeOx up to 160 °C, while these reactions for MeThz are largely endergonic within the 0–180 °C range (Fig. S12,† right), thus highly unfavorable. These findings are also independent of the solvent (Fig. S13†). More details on DFT calculations can be found in the ESI (ESI, pages S15–S19†).LR is possibly the most successful thionation agent, typically used to convert carbonyl functional groups into thiocarbonyl moieties.15–20 In polymer chemistry, it is mostly known for the synthesis of some chain transfer agents.25 Interestingly, at least one example of polythioamide synthesis by thionation of classic in-chain polyamides can also be found.26 We therefore set out to obtain PAThz by reaction of PAOx with LR, here particularly focusing on PEtOx for a proof-of-principle.
To this aim, PEtOx with a targeted molecular weight of about 6 kg mol−1 was first synthesized (see the ESI†). Briefly, EtOx was polymerized at 100 °C in MeCN at 4 M under inert atmosphere and dry conditions using MeOTs as the initiator and with [EtOx]/[MeOTs] = 60. The polymerizations were terminated with either 5 wt% aqueous Na2CO3 or 25 wt% methanolic tetramethylammonium hydroxide (TMAH),27 yielding PEtOx A and PEtOx B, respectively, with low dispersity (Table 1). Subsequently, thionation was performed using LR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEtOx molar ratios related to the EtOx units and varying them from 0.025 to 0.5. The latter ratio is designed to fully convert the EtOx units to EtThz units since one equivalent of LR can theoretically convert two equivalents of carbonyl moieties. Obviously, lower ratios should lead to copolymers of EtOx and MeThz.
PEtOx molar ratios related to the EtOx units and varying them from 0.025 to 0.5. The latter ratio is designed to fully convert the EtOx units to EtThz units since one equivalent of LR can theoretically convert two equivalents of carbonyl moieties. Obviously, lower ratios should lead to copolymers of EtOx and MeThz.
| Sample code | [LR]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PEtOx] [PEtOx] |
Theoretical thionation degree (%) | Experimental thionation degree (%) | M n | M w | Đ |
|---|---|---|---|---|---|---|
| a Calculated from DMAc SEC using linear PMMA standards. b Calculated from HFIP SEC using linear PMMA standards. c Bimodal distribution due to end-to-end disulfide coupling. | ||||||
| PEtOx A | — | — | — | 6800a | 7400a | 1.10 |
| 5% | 0.025 | 5 | 5 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900a 900a |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600a 600a |
1.29c |
| 11% | 0.050 | 10 | 11 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500a 500a |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700a 700a |
1.22c |
| 23% | 0.100 | 20 | 23 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500a 500a |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900a 900a |
1.24c |
| 28% | 0.125 | 25 | 28 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800a 800a |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600a 600a |
1.26c |
| 33% | 0.150 | 30 | 33 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300a 300a |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400a 400a |
1.28c |
| PEtOx B | — | — | — | 9800b | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300b 300b |
1.05 |
| 40% | 0.165 | 33 | 40 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000b 000b |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600b 600b |
1.06 |
| 55% | 0.250 | 50 | 55 | 9200b | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400b 400b |
1.24 |
| 91% | 0.400 | 80 | 91 | 9300b | 9700b | 1.04 |
| 95% | 0.500 | 100 | 95 | 9900b | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100b 100b |
1.02 |
Due to the unprecedented character of the thionation performed on PAOx, several initial trials were conducted to understand the critical aspects of the reaction and optimize the conditions. The present investigation employed a methodology inspired by a prior publication dealing with the thionation of low-molar mass compounds.28 Importantly, incomplete drying of PEtOx led to a complete absence of reaction. Therefore, before reacting with LR, PEtOx was thoroughly dried under vacuum (P = 1.1 × 10−2 mbar). Furthermore, for LR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEtOx above 0.4, LR was added in portions because a batch protocol led to early precipitation of the polymer and reduced the thionation degree. The reactions were run at 90 °C for 2 to 2.5 hours. After cooling down to room temperature and continuous stirring overnight, MeCN was removed under reduced pressure and the residues were redissolved in dichloromethane before precipitation in diethyl ether, followed by elution through a basic alumina column using chloroform as the eluent for samples obtained with LR
PEtOx above 0.4, LR was added in portions because a batch protocol led to early precipitation of the polymer and reduced the thionation degree. The reactions were run at 90 °C for 2 to 2.5 hours. After cooling down to room temperature and continuous stirring overnight, MeCN was removed under reduced pressure and the residues were redissolved in dichloromethane before precipitation in diethyl ether, followed by elution through a basic alumina column using chloroform as the eluent for samples obtained with LR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEtOx up to 0.15, and final precipitation in Et2O or n-heptane for all. It is worth mentioning that since LR and its byproduct, namely (4-methoxyphenyl)(thioxo)phosphine oxide (denoted as LR′ in the NMR spectra – possibly in the form of a trimer), have similar solubility behavior to the final polymers, several different purification techniques were attempted for their complete removal from the final polymers. This includes multiple reprecipitations, manual size-exclusion chromatography (crosslinked polystyrene, exclusion range 0.6–14 kDa), extraction, methanol filtration, and elution through a basic alumina column, with the last one yielding the best results. In addition, it has been observed that the solubility of the copolymers evolves significantly with increasing degrees of thionation. Interestingly, for LR
PEtOx up to 0.15, and final precipitation in Et2O or n-heptane for all. It is worth mentioning that since LR and its byproduct, namely (4-methoxyphenyl)(thioxo)phosphine oxide (denoted as LR′ in the NMR spectra – possibly in the form of a trimer), have similar solubility behavior to the final polymers, several different purification techniques were attempted for their complete removal from the final polymers. This includes multiple reprecipitations, manual size-exclusion chromatography (crosslinked polystyrene, exclusion range 0.6–14 kDa), extraction, methanol filtration, and elution through a basic alumina column, with the last one yielding the best results. In addition, it has been observed that the solubility of the copolymers evolves significantly with increasing degrees of thionation. Interestingly, for LR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEtOx larger than 0.1, the polymers were not soluble in water anymore and above 0.25, solubility decreased significantly in common organic solvents too. That is why the complete removal of LR by-products was conducted only up to LR
PEtOx larger than 0.1, the polymers were not soluble in water anymore and above 0.25, solubility decreased significantly in common organic solvents too. That is why the complete removal of LR by-products was conducted only up to LR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEtOx = 0.15. The isolated yields span a broad range, i.e., 11 to 88%, depending on thionation degrees and required purification protocols. Since the focus of this article was placed on the generation and physical investigation of these new thioazoline copolymers, no optimization was carried out.
PEtOx = 0.15. The isolated yields span a broad range, i.e., 11 to 88%, depending on thionation degrees and required purification protocols. Since the focus of this article was placed on the generation and physical investigation of these new thioazoline copolymers, no optimization was carried out.
The occurrence of a reaction could first be assessed using 1H NMR spectroscopy. For LR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
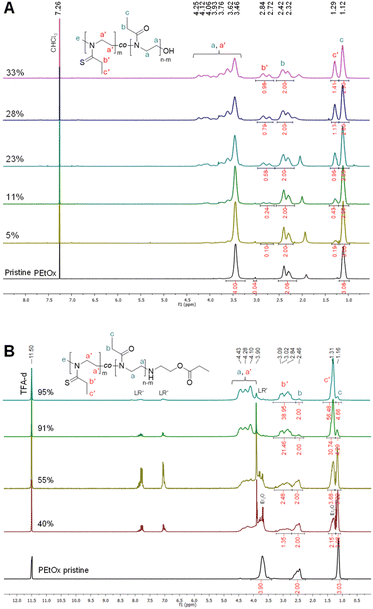
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEtOx up to 0.25, that is, a targeted fraction of 0.5 in sulfur-containing monomeric units, the product could be analyzed in CDCl3 (Fig. 1A). However, for higher targeted modification degrees, deuterated trifluoroacetic acid (TFA-d) was required to fully dissolve the polymers (Fig. 1B). In each spectrum of reacted PEtOx, a series of new peaks appears slightly shifted downfield in comparison with those of the oxazoline units. More specifically, in CDCl3, the methylene protons b′ of the thiazoline unit side chain appear at 2.84–2.72 ppm in comparison with those of the oxazoline unit at 2.42–2.32 ppm. The side-chain methyl protons c′ logically experience a weaker but distinct shift from 1.12 to 1.29 ppm. Finally, the backbone protons a and a′ appear within an extended range of 4.25 to 3.46 ppm (maxima) in comparison with the single broad peak at 3.46 ppm for pristine PEtOx. The assignment of these protons is more complex since they potentially correspond to various sequences of both EtOx and EtThz repeating units in the copolymers. Similar observations can be made in TFA-d, with different chemical shifts and the progressive disappearance of the EtOx repeating unit signals.
PEtOx up to 0.25, that is, a targeted fraction of 0.5 in sulfur-containing monomeric units, the product could be analyzed in CDCl3 (Fig. 1A). However, for higher targeted modification degrees, deuterated trifluoroacetic acid (TFA-d) was required to fully dissolve the polymers (Fig. 1B). In each spectrum of reacted PEtOx, a series of new peaks appears slightly shifted downfield in comparison with those of the oxazoline units. More specifically, in CDCl3, the methylene protons b′ of the thiazoline unit side chain appear at 2.84–2.72 ppm in comparison with those of the oxazoline unit at 2.42–2.32 ppm. The side-chain methyl protons c′ logically experience a weaker but distinct shift from 1.12 to 1.29 ppm. Finally, the backbone protons a and a′ appear within an extended range of 4.25 to 3.46 ppm (maxima) in comparison with the single broad peak at 3.46 ppm for pristine PEtOx. The assignment of these protons is more complex since they potentially correspond to various sequences of both EtOx and EtThz repeating units in the copolymers. Similar observations can be made in TFA-d, with different chemical shifts and the progressive disappearance of the EtOx repeating unit signals.
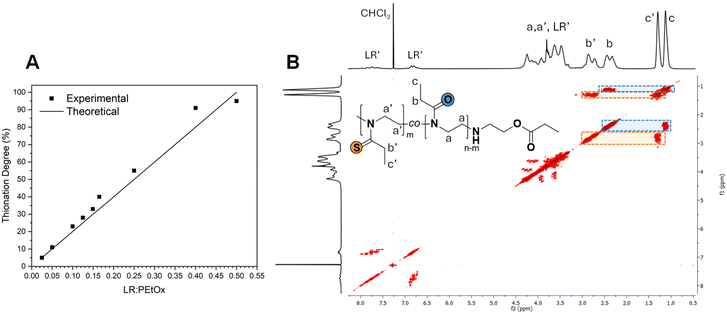
Integration of the b and b′ signals (i.e., the most separated signals) allowed for the calculation of the experimental thionation degree, which could be compared with the theoretical one assuming a quantitative reaction of LR (Fig. 2A). It can clearly be seen that, within the NMR integration error range, the thionation could be quantitatively performed at all stoichiometric ratios, except for the targeted full modification, which nevertheless led to 95% of EtThz units. Furthermore, the COSY 2D spectrum displayed in Fig. 2B clearly evidences cross-correlation peaks within each of the proposed monomer unit side chains, here highlighted in blue for EtOx and in orange for EtThz. Fourier-transform infrared spectroscopy (Fig. S5†) also confirmed the reaction through the appearance of the characteristic vibration of the symmetrical stretching of the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N– thioamide bond at 1120 cm−1 (peak d)19,29 and the progressive depletion of the characteristic vibration of the O
C–N– thioamide bond at 1120 cm−1 (peak d)19,29 and the progressive depletion of the characteristic vibration of the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N– amide bond at 1630 cm−1 (peak a), alongside additional vibration variations. Elemental analysis carried out on the pristine PEtOx A, as well as on samples 5%, 28%, and 95%, confirmed the incorporation of sulfur, with experimental values close to theoretical values based on NMR-determined thionation degrees (Fig. S9A†). In particular, experimental S/N mass ratios match very well with the expected values (Fig. S9B†).
C–N– amide bond at 1630 cm−1 (peak a), alongside additional vibration variations. Elemental analysis carried out on the pristine PEtOx A, as well as on samples 5%, 28%, and 95%, confirmed the incorporation of sulfur, with experimental values close to theoretical values based on NMR-determined thionation degrees (Fig. S9A†). In particular, experimental S/N mass ratios match very well with the expected values (Fig. S9B†).
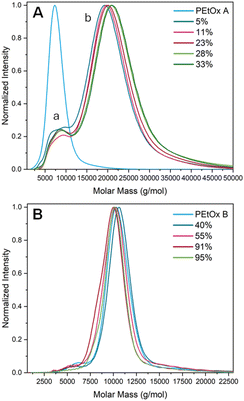
The next step was to confirm that the proposed post-polymerization modification does not lead to a degradation of the polymer backbone and hence preserves the well-defined character of the PEtOx starting polymer. Size-exclusion chromatography (SEC) was therefore performed. While the classic N,N-dimethylacetamide (DMAc) was a suitable eluent for low thionation degrees (up to 33%), hexafluoroisopropanol (HFIP), a significantly less common yet known SEC eluent for polyamides and other polar polymers, was required for a proper dissolution of the high-thionation-degree products. Fig. 3 depicts the chromatograms of the PEtThz-co-PEtOx copolymers with different thionation degrees ranging from 5% to 95% in comparison with the pristine PEtOx.
To our surprise, all copolymers with a low degree of thionation (5–33%) exhibited bimodal distributions, with the low-molar mass maximum (indicated by a) matching the elution of the pristine PEtOx and the high-molar mass maximum (b) falling roughly at double the molar mass (Fig. 3A). In contrast, all polymers with thionation degrees of at least 40%, hence analyzed by HFIP SEC, showed the conservation of the original molar mass distribution with rather high fidelity. It is crucial to note that the polymers with 5–33% thionation were also subjected to HFIP SEC (Fig. S6†) and still showed bimodal distributions, discarding the potential unsuitability of the DMAc SEC system. Importantly, the common feature of low-thionation-degree polymers is the method employed for termination during the synthesis of the original PEtOx A, namely with aqueous Na2CO3, as opposed to methanolic TMAH for PEtOx B. While TMAH was claimed to provide clean OH chain ends,27 in our hands, it was found to be less potent than aqueous Na2CO3 and led to an ω-propionate ester group due to water termination, as observed by 1H NMR spectroscopy (see Fig. S7†).27,30 Therefore, the disparate SEC results between low and high thionation degrees lie in the nature of the end group. While LR is usually employed for the thionation of carbonyl groups, it has been reported that hydroxyl groups are more reactive, notably faster than amide groups which come second.15 Thus, during the thionation reaction, the ω-hydroxyl groups of low-thionation-degree copolymers were converted into thiols, which are prone to forming disulfide bridges, here yielding end-to-end chain coupling into dimers. In order to further support this assumption, an additional sample at a lower thionation degree was produced using PEtOx B rather than PEtOx A. In that case, the monomodality was conserved (see Fig. S8A†), implying that the thionation degree itself is not the cause for bimodality. All in all, it can safely be concluded that the backbone remained intact during the post-polymerization modification and, thanks to the unintentional end-group discrepancy, it is highlighted that the presence of other oxygen-based functional groups in the PEtOx structure needs to be considered.
Subsequently, in order to investigate the properties of the newly synthesized copolymers, we first turned to differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) to investigate their thermal properties.
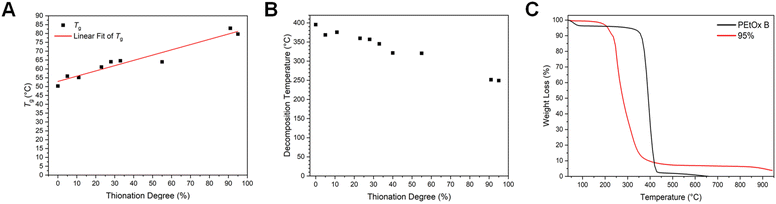
For the DSC measurements and the determination of glass transition temperature (Tg), four heating–cooling cycles were performed at a heating rate of 10 K min−1, the first one to remove the thermal history of the sample. The third heating curves are presented in Fig. S10,† while Tg values are compiled in Fig. 4A. A linear increase in Tg with rising thionation degrees was observed, evidencing no phase separation in the solid state. Since the solubility of Thz-rich polymers is strongly diminished and no partitioning of polymers or self-assembly was observed under any tested solvent conditions for low thionation degrees, chain-wise or block-wise thionation in a proximity-based self-catalyzed manner is excluded. This therefore suggests the formation of random copolymers. The increase in Tg can be rationalized by consideration of the literature-known higher rotational barrier of the C–N bond in thioamides in comparison with that in amides.9,31 This leads to lower flexibility in thiazoline monomeric units compared to the oxazoline ones. Hence, more energy is required to reach the glass transition for higher thionation degrees. Furthermore, no crystallization or melting transitions were detected. Extrapolation of the linear fit would suggest that pure PEtThz would undergo glass transition at approx. 83 °C. All individual TGA measurements are included in the ESI (Fig. S11†) and also reveal a clear trend: an increasing thionation degree leads to a lower decomposition temperature, as illustrated in Fig. 4B. Notably, a difference of approx. 150 °C between the decomposition temperature of pure PEtOx and that of the PEtThz-co-PEtOx copolymer with 95% EtThz units was observed, evidencing a significantly higher thermal instability of polythioamides in comparison with polyamides. We note that the samples with intermediate thionation degrees exhibit a complex degradation behavior with multiple steps, which would require analysis of the degradation products, for instance, by TGA coupled to mass spectrometry.
Lastly, we investigated whether the sulfur-containing monomeric units influence one of the most interesting properties of PAOx, namely the temperature-dependent solution behavior in aqueous solution. While poly(2-methyl-2-oxazoline) is very hydrophilic and dissolves in water at any temperature under atmospheric conditions, poly(2-butyl-2-oxazoline) is water-insoluble. However, PEtOx and all variants of poly(2-propyl-2-oxazoline)s (n-, iso-, and cyclopropyl-) possess lower critical solution temperatures (LCST), representing a transition from fully solvated polymer chains at low temperatures to collapsed chains at high temperatures, generally manifested via the appearance of turbidity. This phase transition depends on the concentration of the sample and the LCST represents the absolute minimal temperature at which it occurs. Generally, cloud point temperatures (Tcp) are determined for a set of specific conditions. Importantly, the degree of polymerization plays a role,5 which is a very relevant aspect for PEtOx: According to the literature,32 a minimum of 100 EtOx units is needed to observe the appearance of turbidity in pure water below 100 °C. In the current study, a concentration of 5 mg mL−1 was used in double deionized water. Measurements were carried out using a dynamic light scattering (DLS) device, able to detect the appearance of aggregates. Therefore, the intensity of the scattered light was monitored between 10 and 70 °C (the range allowed by our DLS device), using a heating rate of 1 °C min−1 (Fig. 5). Only the copolymers with up to 23% thionation degree could be examined in aqueous solutions because higher EtThz contents led to temperature-independent water-insolubility. Since the existence of disulfide bridges was established for these samples, and as mentioned above, the solution behavior is molar mass-dependent, not only pristine PEtOx A but also a well-defined PEtOx with double the molecular weight (i.e., 13 kg mol−1) were included as references.
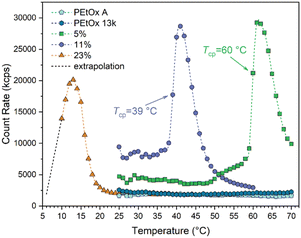 | ||
| Fig. 5 Evolution of the scattered light intensity for 5 mg mL−1 aqueous solutions of PEtOx and PEtThz-co-PEtOx during heating between 10 and 70 °C at a rate of 1 °C min−1. | ||
No cloud point was observed for both PEtOx under the current conditions, as expected. However, even for a thionation degree as low as 5 mol%, a cloud point was detected at 60 °C. At 11 mol% thionation, Tcp decreased to 39 °C. At 23 mol%, the experimental limits were already reached. While the sample was fully soluble in water at 4 °C (kept in the refrigerator), it became cloudy after a few seconds at room temperature. Yet, with special care, a measurement could be conducted and it was clearly evidenced that at 10 °C, the phase transition had already started. Using a rough extrapolation, it could be assumed that Tcp in this case lies around 8–9 °C. It is obvious that the higher the thionation degree, the lower the Tcp and this can be explained considering that the sulfur lone pairs of thioamides are weaker hydrogen bond acceptors compared to the oxygen lone pairs in amides.9,33
Conclusions
In this work, the synthesis of polythiazolines was attempted and led to the successful synthesis of novel PEtThz-co-PEtOx copolymers. Various experimental trials showed that the direct polymerization of 2-methyl-2-thiazoline using cROP was not accessible, at least under conditions similar to those used for the cROP of AOx. An alternative approach towards polythiazolines, namely the post-polymerization modification of PEtOx with the most well-known thionating agent, Lawesson's reagent (LR), was therefore investigated to convert amides into thioamides. Different thionation degrees, ranging from 5% to 95%, were achieved via a one-pot addition of LR (for lower than 80%) or stepwise addition (for higher than 80% conversion) in acetonitrile. In the presence of hydroxyl end groups, the formation of terminal thiols occurred, which led to end-to-end chain coupling, as observed in size-exclusion chromatograms. The newly synthesized copolymers exhibited a higher Tg than PEtOx, which linearly increases as the number of sulfur-containing monomeric units increases. In addition, it was observed that the thermal stability of the copolymers decreased with increasing fractions of thiazoline units. Finally, the thiazoline-containing copolymers exhibited decreased solubility, notably in water, in which thionation of at least 28% led to full water insolubility. For lower thionation degrees, the copolymers possessed LCST-like behavior. Here, a related contribution of Luxenhofer and coworkers, which appeared during the revision of the present manuscript and tackles the introduction of thiazoline units through a different angle and with high-dispersity polymers, yet with a similar protocol, should be acknowledged.34 Future research should be dedicated to attaining PEtThz homopolymers, improving purification, and further expanding the methodology to oxazoline and oxazine derivatives. These (co)polymers could potentially find applications as flame retardants or well-defined polyligands in coordination chemistry.Author contributions
A. M. and A. K. D. carried out all syntheses and performed NMR, FTIR, and DMAc-SEC measurements and analyses. A. M. performed DSC and DLS measurements and analyses. A. D. and H. B. performed DFT calculations. F. C. M. S. and F. F. acquired and analyzed HFIP-SEC data. G. D. conceptualized the initial idea and supervised the entire study. A. M. and G. D. wrote the manuscript, with contributions from all coauthors.Data availability
The data supporting this article have been included as part of the ESI† and all raw data are available through the corresponding author.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Ms Sylwia Adamczyk and Mr Ferdinand M. Wachter (both BUW) are gratefully acknowledged for TGA measurements and initial calculations, respectively. A. M. would like to thank the BUW scholarship committee for a finishing doctorate scholarship (Abschlussstipendium).References
- F. Wiesbrock, R. Hoogenboom, C. H. Abeln and U. S. Schubert, Macromol. Rapid Commun., 2004, 25, 1895–1899 CrossRef CAS.
- R. Hoogenboom, M. W. M. Fijten, H. M. L. Thijs, B. M. Van Lankvelt and U. S. Schubert, Des. Monomers Polym., 2005, 8, 659–671 CAS.
- M. Bauer, C. Lautenschlaeger, K. Kempe, L. Tauhardt, U. S. Schubert and D. Fischer, Macromol. Biosci., 2012, 12, 986–998 CAS.
- O. Sedlacek, K. Lava, B. Verbraeken, S. Kasmi, B. G. De Geest and R. Hoogenboom, J. Am. Chem. Soc., 2019, 141, 9617–9622 CAS.
- R. Hoogenboom and H. Schlaad, Polym. Chem., 2017, 8, 24–40 CAS.
- R. R. Kumar, R. Ramesh and J. G. Małecki, New J. Chem., 2017, 41, 9130–9141 RSC.
- A. A. Eroy-Reveles and P. K. Mascharak, Chemtracts, 2005, 18, 87–92 CAS.
- T. S. Jagodziński, Chem. Rev., 2003, 103, 197–227 CrossRef PubMed.
- N. Mahanta, D. M. Szantai-Kis, E. J. Petersson and D. A. Mitchell, ACS Chem. Biol., 2019, 14, 142–163 CAS.
- K. B. Wiberg and Y. Wang, ARKIVOC, 2011, 2011, 45–56 Search PubMed.
- H. Mutlu, E. B. Ceper, X. Li, J. Yang, W. Dong, M. M. Ozmen and P. Theato, Macromol. Rapid Commun., 2019, 40, 1–51 Search PubMed.
- B. L. Rivas, G. S. Canessa and S. A. Pooley, Eur. Polym. J., 1993, 29, 1089–1093 CAS.
- J. Steinkoenig, P. A. J. M. De Jongh, D. M. Haddleton, A. S. Goldmann, C. Barner-Kowollik and K. Kempe, Macromolecules, 2018, 51, 318–327 CAS.
- Y. Iwakura, K. Kurita and F. Hayako, J. Polym. Sci., Part A-1, 1969, 7, 3075–3087 CAS.
- T. Ozturk, E. Ertas and O. Mert, Chem. Rev., 2007, 107, 5210–5278 CAS.
- H. Khatoon and E. Abdulmalek, Molecules, 2021, 26, 6937–6979 CrossRef CAS.
- M. Jesberger, T. P. Davis and L. Barner, Synthesis, 2003, 1929–1958 CrossRef CAS.
- S. Scheibye, B. S. Pedersen and S.-O. Lawesson, Bull. Soc. Chim. Belg., 1978, 87, 229–238 CrossRef CAS.
- S. Scheibye, B. S. Pedersen and S.-O. Lawesson, Bull. Soc. Chim. Belg., 1978, 87, 299–306 CrossRef CAS.
- B. S. Pedersen, S. Scheibye, N. H. Nilsson and S.-O. Lawesson, Bull. Soc. Chim. Belg., 1978, 87, 223–228 CAS.
- A. C. Gaumont, M. Gulea and J. Levillain, Chem. Rev., 2009, 109, 1371–1401 CAS.
- A. F. Ferris, L. Salerni and B. A. Schütz, J. Med. Chem., 1966, 9, 391–394 CrossRef CAS PubMed.
- A. D. Clark and P. Sykes, J. Chem. Soc. C, 1971, 103–110 Search PubMed.
- H. Singh and S. Rakesh, Tetrahedron, 1986, 42, 1449–1460 CAS.
- D. J. Keddie, G. Moad, E. Rizzardo and S. H. Thang, Macromolecules, 2012, 45, 5321–5342 CrossRef CAS.
- M. Delêtre and G. Levesque, Macromolecules, 1990, 23, 4876–4878 CrossRef.
- V. R. De La Rosa, S. Tempelaar, P. Dubois, R. Hoogenboom and L. Mespouille, Polym. Chem., 2016, 7, 1559–1568 RSC.
- S. Scheibye, J. Kristensen and S. O. Lawesson, Tetrahedron, 1979, 35, 1339–1343 CrossRef CAS.
- K. A. Jensen and P. H. Nielsen, Acta Chem. Scand., 1966, 20, 597–629 CAS.
- B. Verbraeken, B. D. Monnery, K. Lava and R. Hoogenboom, Eur. Polym. J., 2017, 88, 451–469 CAS.
- K. B. Wiberg and P. R. Rablen, J. Am. Chem. Soc., 1995, 117, 2201–2209 CAS.
- R. Hoogenboom, H. M. L. Thijs, M. J. H. C. Jochems, B. M. Van Lankvelt, M. W. M. Fijten and U. S. Schubert, Chem. Commun., 2008, 5758–5760 CAS.
- M. Hollósi, M. Zewdu, E. Kollát, Z. Majer, M. Kajtár, G. Batta, K. Kövér and P. Sándor, Int. J. Pept. Protein Res., 1990, 36, 173–181 CrossRef.
- A. Hacioglu, V. Baddam, A. Kerr, J. Kehrein, A. Bunker and R. Luxenhofer, Macromolecules, 2024, 57, 10368–10378 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: All experimental details, additional characterization data, and DFT calculation results. See DOI: https://doi.org/10.1039/d4py00930d |
| This journal is © The Royal Society of Chemistry 2025 |