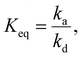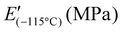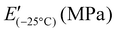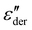Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Spiropyran-based supramolecular elastomers with tuneable mechanical properties and switchable dielectric permittivity†
Malte Sebastian
Beccard
ab,
Frank A.
Nüesch
 ac,
Thulasinath
Raman Venkatesan
ac,
Thulasinath
Raman Venkatesan
 *a and
Dorina M.
Opris
*a and
Dorina M.
Opris
 *ab
*ab
aFunctional Polymers, Empa, Swiss Federal Laboratories for Materials Science and Technology (EMPA), 8600 Dübendorf, Switzerland. E-mail: dorina.opris@empa.ch; Thulasinath.RamanVenkatesan@empa.ch
bEidgenössische Technische Hochschule Zürich (ETHZ), 8092 Zurich, Switzerland
cInstitute of Chemical Sciences and Engineering, Ecole Polytechnique Federale de Lausanne, EPFL, Station 6, CH-1015 Lausanne, Switzerland
First published on 27th November 2024
Abstract
Silicone elastomers are widely used in various applications, each demanding different properties and functionalities. To be used in such a broad spectrum, silicones with easily tunable or switchable properties are needed. We showed this is achievable with novel metallo-supramolecular polysiloxanes. Poly(dimethylsiloxane-co-3-aminopropylmethylsiloxane) was reacted with an epoxy-modified spiropyran (SP) in the presence of ZnCl2 as a catalyst. We have found that the ZnCl2 allows the formation of metallo-supramolecular polymers with tuneable mechanical propertiers. The influence of the amount of ZnCl2 used on the thermal and mechanical properties of the synthesized materials was investigated by DSC, tensile test, and DMA. The ability of SP to act not only as a physical cross-linker, but also as a molecular switch was investigated by UV-Vis spectroscopy and dielectric permittivity measurements. It was found that depending on the amount of ZnCl2 used, the dielectric permittivity can either increase or decrease after exposure to UV or visible light, respectively. Additionally, the developed materials can be reprocessed similarly to thermoplastic elastomers. Furthermore, their solubility can be manipulated from insoluble in practically any solvent to highly soluble by simply adding ZnCl2.
Introduction
Silicones are inorganic polymers with a backbone consisting of alternating Si–O bonds, contributing to outstanding properties such as flexibility, low glass transition temperature (Tg), good dielectric properties, chemical resistance, and physiological inertness.1–3 It is not surprising that silicones can be found in many applications, including bake and cookware,4 medicine,5 cosmetics, and electronics.6 However, as research progresses, new applications for silicones are being explored, such as active components in dielectric elastomer actuators (DEAs), sensors, generators, and stretchable electronics, to name a few. In DEAs, silicones are particularly popular due to their easy processability in thin films and low Young's modulus,7 as well as the possibility of attaching different side groups to the backbone, resulting in even better properties for actuation.8 However, to achieve the desired properties tailored for specific applications, fine-tuning must be done by different fillers,9 post-polymerization modification,2 or cross-linking density.10 Since most silicones have a low Tg, cross-linking is needed to achieve robust materials. This is normally done by chemical cross-linking, which has the major disadvantage of leading to unprocessable and non-recyclable materials. While thermoplastic silicones are known, their synthesis is tedious, and their properties cannot be easily tuned. It would be advantageous if silicones were available and a stimulus could easily tune their properties.To meet the requirements set, we aimed to produce a supramolecular polymer with a physical network instead of a chemical network to obtain an elastomer that can be reprocessed. Chemical networks consisting of permanent covalent cross-links afford tough and temperature-stable materials, but their permanent cross-linking has the disadvantage that it does not allow for materials to be recycled or reprocessed.11 A physical network, on the other hand, consists of complementary binding motifs that can associate due to non-covalent interactions but also dissociate in a given time.12 The resulting equilibrium can be described by the equilibrium constant:
For the design of our metallo-supramolecular polymer (MSP), spiropyran (SP) was chosen as the binding motif. SP is known for dipole–dipole interactions23 and for forming complexes with various metal-ions,24 which makes it attractive for forming MSPs. Moreover, SP is a molecular switch that reverses isomerization from a ring-closed SP form to an opened merocyanine (MC) form. Because of its switching behavior, SP is being considered for a wide range of applications, such as strain sensors,25 drug delivery systems,26 sensors for cations,27 and optics for photoswitchable holograms.28,29 The isomerization can be triggered by heat, pressure, pH change, and light.30 Upon irradiation with UV light, a C–O bond in the SP structure dissociates, resulting in the open zwitterionic MC form.31 The zwitterionic MC form has a much higher dipole moment (14–18 D) than the SP-form (4–6 D).32 The MC form has an extended conjugated system of π-electrons and absorbs light in the visible range. Irradiation with visible light or treatment with heat leads to further isomerization back to the SP form.33 SP switching ability may allow designing materials whose properties can easily be changed by an external stimulus.
Here, a poly(dimethylsiloxane-co-3-aminopropylmethylsiloxane) (PDMS-NH2) was functionalized with SP groups by reacting the primary amine with an oxirane attached to the SP. The amount of ZnCl2, acting as both catalyst and complexing agent, was varied and allowed us to tune the mechanical properties of the SP functionalized PDMS in a wide range. In addition, the photoswitching abilities and their impact on the dielectric properties of the synthesized materials were investigated.
Experimental
The following reagents were used without further purification: 2,3,3-trimethyl-3H-indole, and 2-hydroxy-5-nitrobenzaldehyde from Apollo Scientific; 2-bromoethanol from Fisher Scientific; 2-(bromomethyl)oxirane, acetonitrile and K2CO3 from Sigma Aldrich; ZnCl2 from Alfa Aesar; KOH from Fluka; and tetrahydrofuran, ethanol, ethylacetate, n-pentane from VWR. PDMS-NH2 (6–7 mol% amino groups), trimethylsilyl terminated, Mn = 7200 g mol−1, 80–120 cst. from ABCR. The content of aminopropyl in PDMS-NH2 was calculated from 1H NMR spectrum and found to be only 5%.1H and 13C NMR spectra were recorded on a Bruker Avance 400 NMR spectrometer at 298 K using a 5 mm broadband probe at 400.18 and 100.63 Hz. Chemical shifts are given relative to the solvents (CHCl3: δ = 7.26 ppm and 77.16 ppm; DMSO: δ = 2.50 ppm and 39.52 ppm). IR spectra were recorded on a Brucker Tensor 27 FT-IR spectrometer in a range of 4000–600 cm−1.
Thermogravimetric analysis was performed on a PerkinElmer TGA8000, heating the samples from 30 to 600 °C at 20 °C min−1 under air.
Differential scanning calorimetry was performed on a PerkinElmer DSC8000 in a temperature range of −80 to 80 °C with a heating/cooling rate of 20 °C min−1 under N2.
Tensile tests of dog-bone-shaped samples with a width of 2 mm and a length of 18 mm were performed on a Zwick Z010 machine. The applied preload force was 0.005 N, and the sample was stretched at a speed of 50 mm min−1. At least five samples were measured and averaged. Young's modulus was determined by a linear fit to the data points from 0 to 10% strain.
Dynamic mechanical analysis was performed on a RSA 3 from TA Instruments. Stripes with a width of 10 mm and a length of 12 mm were measured. The sample was measured at least three times at room temperature for each method to determine average values. For frequency-dependent measurements, a strain of 2% and a preload force of 2 g were applied, and the frequency increased from 0.05 to 10 Hz. A preload force of 2 g and a frequency of 0.1 Hz were used for the strain-dependent measurements. The measurements occurred at a strain rate of 50 mm min−1 from 0.05 to 10%. For the temperature-dependent measurements, a frequency of 0.1 Hz and a strain of 0.5% were used. The measurements were conducted in a temperature range from −120 to 110 °C.
A Novocontrol Alpha-A frequency analyzer was used to supply a voltage of 1 V for the dielectric relaxation spectroscopy measurements carried out between 1 and 106 Hz. A Novocontrol Quatro cryosystem was used to control the sample temperature with a 2.5 K temperature step under a dry nitrogen atmosphere. For obtaining the derivative curves and fitting the dielectric data DCALC program developed by Wübbenhorst was used.34,35
UV-Vis measurements were conducted with a Varian Cary 50 UV-Vis spectrophotometer ranging from 300 to 800 nm. As a UV source for the irradiation experiments, 6 LEDs from Distrelec with a wavelength of 365 nm and a radiant power of 1 W per LED were mounted on a PCB plate on a surface of 2 cm2. For the green light, 6 LEDs from Distrelec, with a wavelength of 505 nm and a radiant power of 1.1 W, were mounted on a PCB plate on a surface of 2 cm2.
The synthesis of compounds 1, 2, SP-OH, and SP-Ep was carried out following established literature36,37 and a comprehensive description is available in the ESI section.†
Synthesis of PDMS-SP-Ep-(Zn)
3′,3′-Dimethyl-6-nitro-1′-(2-(oxiran-2-ylmethoxy)ethyl)spiro[chromene-2,2′-indoline] (0.26 g, 0.63 mmol), varying amounts of ZnCl2, and PDMS-NH2 (1 mL) were dissolved in THF (20 mL) to give PDMS-SP-Ep-(Zn) with different molar ratios of SP to ZnCl2 (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y). After refluxing the solution overnight, the solvent was evaporated, and the polymer was dried in the vacuum oven at 65 °C. The product was used without further purification steps and was obtained as an orange-brownish solid. Sample 1
y). After refluxing the solution overnight, the solvent was evaporated, and the polymer was dried in the vacuum oven at 65 °C. The product was used without further purification steps and was obtained as an orange-brownish solid. Sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 was refluxed for 2 days. Sample 1
0.01 was refluxed for 2 days. Sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.12 was obtained by mixing a 1
1.12 was obtained by mixing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of two solutions of samples 1
1 ratio of two solutions of samples 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25. The complete functionalization was confirmed by the absence of the oxirane band at 908 cm−1 (Fig. S1†). 1H NMR spectroscopy was conducted on the soluble sample 1
1.25. The complete functionalization was confirmed by the absence of the oxirane band at 908 cm−1 (Fig. S1†). 1H NMR spectroscopy was conducted on the soluble sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S2†), but the resolution was poor. Nevertheless, the signals from the SP-Ep showed peak broadening, and the NH2 peak at 2.2 ppm disappeared, indicating complete functionalization.
1 (Fig. S2†), but the resolution was poor. Nevertheless, the signals from the SP-Ep showed peak broadening, and the NH2 peak at 2.2 ppm disappeared, indicating complete functionalization.
Preparation of the thin films
PDMS-SP-Ep-(Zn) was placed between two Teflon films attached to metal plates and separated by spacers with a thickness of 40 μm or 200 μm, respectively. The films were pressed with 3 t at 100 °C for three minutes. For 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01, the samples were pressed for 2 hours.
0.01, the samples were pressed for 2 hours.
UV-Vis spectroscopy
The samples 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1
1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 1
0.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 were pressed into a film, but without spacers. After each measurement, the samples were exposed to the light sources at a distance of 1 cm and in steps of 2 min for green light and 10 s for UV light.
0.01 were pressed into a film, but without spacers. After each measurement, the samples were exposed to the light sources at a distance of 1 cm and in steps of 2 min for green light and 10 s for UV light.
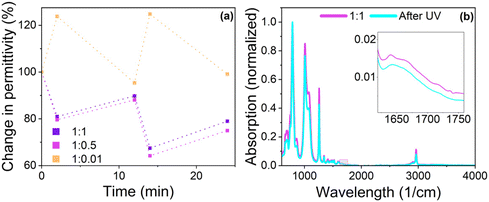
Switch in permittivity
The samples 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, and 1
0.5, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 were pressed into films with thickness >40 μm. Dielectric permittivity measurements were conducted three times for each sample. Exposure to UV light lasted 2 min, while treatment with green light took 10 min. The samples were placed at a distance of 1 cm from the light source.
0.01 were pressed into films with thickness >40 μm. Dielectric permittivity measurements were conducted three times for each sample. Exposure to UV light lasted 2 min, while treatment with green light took 10 min. The samples were placed at a distance of 1 cm from the light source.
Results and discussion
Scheme 1a outlines the synthetic strategy employed for the preparation of SP-Ep. In the first step, 2,3,3-trimethyl-3H-indole was reacted with 2-bromoethanol, yielding product 1. Treatment of 1 with KOH afforded product 2, which was subsequently reacted with 2-hydroxy-5-nitrobenzaldehyde to afford SP-OH. Finally, SP-Ep was synthesized by reacting SP-OH with 2-(bromomethyl)oxirane. The composition of commercial PDMS-NH2 with 5 mol% of amino functional groups was determined by 1H NMR spectroscopy. Based on this information, the amount of SP-Ep required for complete functionalization of the polymer was calculated using the molecular weight of a theoretical average repeating unit (RU):| [0.95 × M(RUPDMS) + 0.05 × M(RUNH2 functionalized PDMS)]. |
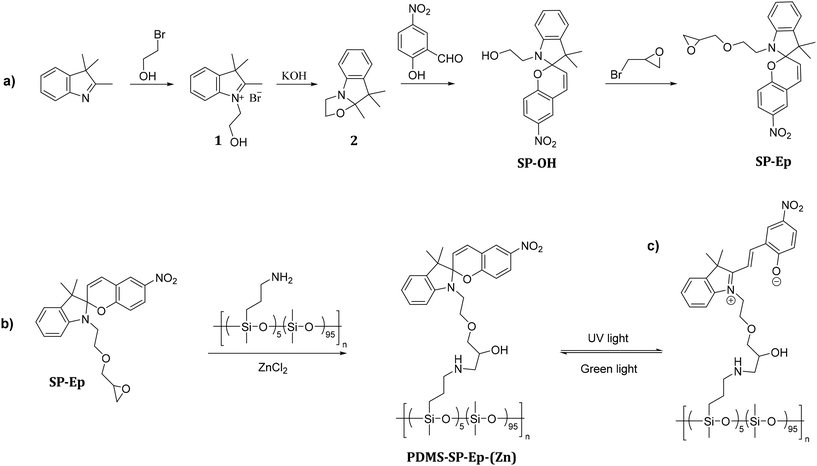 | ||
| Scheme 1 Synthetic pathway for the photoswitch SP-Ep (a) and post-polymerization modification of PDMS-NH2 with SP-Ep catalyzed by ZnCl2 (b), and conversion of SP to MC (c). | ||
Functionalization of PDMS-NH2 with the chromophore SP-Ep was achieved using ZnCl2 as a catalyst (Scheme 1b). Immediately after the synthesis, PDMS-SP-Ep was well soluble in THF and chloroform. However, after washing it with water, and thus ZnCl2 removal, the polymer turned insoluble. The addition of a small amount of ZnCl2 rendered the polymer soluble again. Indeed, ZnCl2 was crucial for both the reaction between PDMS-NH2 and SP-Ep and for forming solid supramolecular polymers (SMPs). While the unreacted PDMS-NH2 and SP-Ep mixture 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 and the PDMS-NH2 and ZnCl2 mixture remained liquid, PDMS-SP-Ep and ZnCl2 mixture solidified. This suggests that functionalization with the chromophore leads to non-covalent interactions responsible for the SMPs’ solidification. Therefore, we synthesized materials with varying SP-EP to ZnCl2 ratios of 1
0 and the PDMS-NH2 and ZnCl2 mixture remained liquid, PDMS-SP-Ep and ZnCl2 mixture solidified. This suggests that functionalization with the chromophore leads to non-covalent interactions responsible for the SMPs’ solidification. Therefore, we synthesized materials with varying SP-EP to ZnCl2 ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.12, 1
1.12, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 1
0.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, and 1
0.1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01, named the resulting materials PDMS-SP-Ep (x
0.01, named the resulting materials PDMS-SP-Ep (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y), and investigated the impact of ZnCl2 content on the solubility and appearance of different materials (Table 1). Thus, all prepared materials have the same polysiloxane backbone and content of SP-Ep, but differ in their relative molar ratio of SP-Ep to ZnCl2. This ratio significantly impacts the solubility of the SMPs in THF and CHCl3. Specifically, a SP-Ep to ZnCl2 ratio of 1
y), and investigated the impact of ZnCl2 content on the solubility and appearance of different materials (Table 1). Thus, all prepared materials have the same polysiloxane backbone and content of SP-Ep, but differ in their relative molar ratio of SP-Ep to ZnCl2. This ratio significantly impacts the solubility of the SMPs in THF and CHCl3. Specifically, a SP-Ep to ZnCl2 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05 or even higher amounts of ZnCl2 result in soluble SMPs, while a decrease of ZnCl2 leads to insoluble SMPs. Experiments showed that the material 1
05 or even higher amounts of ZnCl2 result in soluble SMPs, while a decrease of ZnCl2 leads to insoluble SMPs. Experiments showed that the material 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01, initially insoluble in THF and CHCl3, could be dissolved by adding small amounts of ZnCl2 to the solution (Fig. S3†). However, an excess of ZnCl2, as in sample 1
0.01, initially insoluble in THF and CHCl3, could be dissolved by adding small amounts of ZnCl2 to the solution (Fig. S3†). However, an excess of ZnCl2, as in sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, leads to a viscous liquid.
2, leads to a viscous liquid.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2 (x
ZnCl2 (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y) ratios, composition, solubility in THF or chloroform, and appearance
y) ratios, composition, solubility in THF or chloroform, and appearance
| Sample | Eq. SP-Ep per eq. NH2-group | Eq. ZnCl2 per eq. NH2-group | Soluble in THF/CHCl3 | Appearance |
|---|---|---|---|---|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25 1.25 |
1 | 1.25 | Yes | Highly viscous |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.12 1.12 |
1 | 1.12 | Yes | Solid |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 1 | Yes | Solid |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
1 | 0.5 | Yes | Solid |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
1 | 0.1 | No | Solid |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 0.01 |
1 | 0.01 | No | Solid |
To confirm that SP-Ep was indeed chemically bonded to the polysiloxane chain, both 1H NMR spectroscopy and IR spectroscopy were used. 1H NMR spectroscopy was used for the samples soluble in CHCl3. Complex spectra were observed due to comparable low amounts of SP-Ep and complex formation with the Zn2+ ions. For the sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
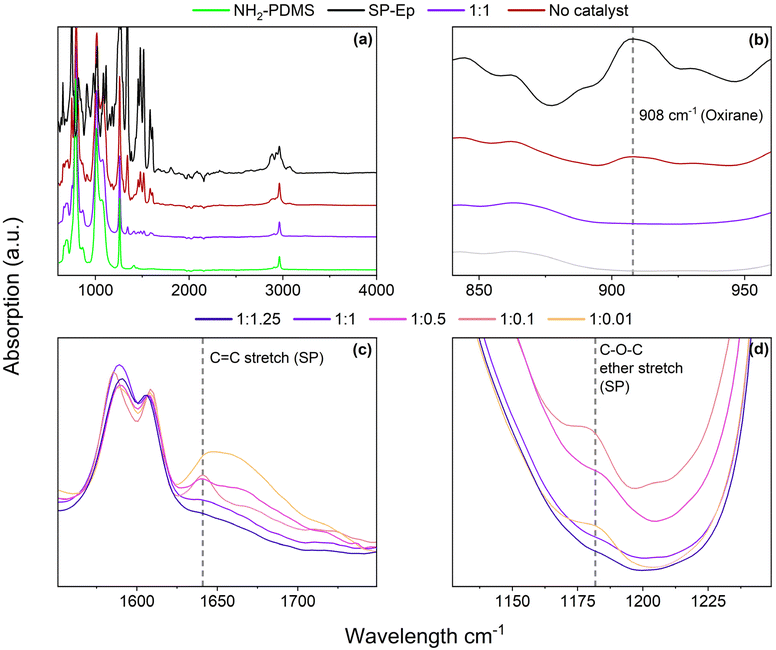
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, it was observed that the NH2 peak at 2.2 ppm disappeared (Fig. S2†), while the signals for the propyl chain remained at the same chemical shift, indicating successful attachment of SP-Ep to the main chain. IR spectroscopy (Fig. 1a) allowed the complete reaction of SP-Ep to be monitored by analyzing the absorbance peak of the oxirane at 908 cm−1 (Fig. 1b and S9†). The peak disappeared in the samples where ZnCl2 was used as a catalyst, while the peak persisted in the mixture of PDMS-NH2 and SP-Ep without the catalyst. The IR absorption bands from SP-Ep provide a better understanding of the influence of ZnCl2 on the SMPs. Fries et al. found significant changes in the IR spectra between the closed SP and open MC forms. For instance, the stretching band of C–N or O–C–N disappears after opening SP up to the MC form, while other bands like the stretch of C–O− and C
1, it was observed that the NH2 peak at 2.2 ppm disappeared (Fig. S2†), while the signals for the propyl chain remained at the same chemical shift, indicating successful attachment of SP-Ep to the main chain. IR spectroscopy (Fig. 1a) allowed the complete reaction of SP-Ep to be monitored by analyzing the absorbance peak of the oxirane at 908 cm−1 (Fig. 1b and S9†). The peak disappeared in the samples where ZnCl2 was used as a catalyst, while the peak persisted in the mixture of PDMS-NH2 and SP-Ep without the catalyst. The IR absorption bands from SP-Ep provide a better understanding of the influence of ZnCl2 on the SMPs. Fries et al. found significant changes in the IR spectra between the closed SP and open MC forms. For instance, the stretching band of C–N or O–C–N disappears after opening SP up to the MC form, while other bands like the stretch of C–O− and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+ are only present in the MC form.38 Sample 1
N+ are only present in the MC form.38 Sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 exhibited a band at 1641 cm−1. The band was weaker in samples 1
0.01 exhibited a band at 1641 cm−1. The band was weaker in samples 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 and 1
0.1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 (Fig. 1c). As the amount of ZnCl2 in the sample increased, the band weakened and ultimately disappeared entirely in sample 1
0.5 (Fig. 1c). As the amount of ZnCl2 in the sample increased, the band weakened and ultimately disappeared entirely in sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25. The band was assigned to the C
1.25. The band was assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch of the SP. Samples 1
C stretch of the SP. Samples 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 and 1
0.01 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, with low amounts of ZnCl2, exhibit an absorption band at 1181 cm−1 (Fig. 1d). This band becomes less pronounced in sample 1
0.1, with low amounts of ZnCl2, exhibit an absorption band at 1181 cm−1 (Fig. 1d). This band becomes less pronounced in sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and disappears in samples 1
0.5 and disappears in samples 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25 with higher amounts of ZnCl2. The observed band corresponds to the asymmetrical C–O–C ether stretch, which is present only in the SP-isomer and not in the MC-isomer. The results suggest that the presence of ZnCl2 induces SP to open up, presumably to form complexes. In the absence of ZnCl2, the closed SP form is favored.
1.25 with higher amounts of ZnCl2. The observed band corresponds to the asymmetrical C–O–C ether stretch, which is present only in the SP-isomer and not in the MC-isomer. The results suggest that the presence of ZnCl2 induces SP to open up, presumably to form complexes. In the absence of ZnCl2, the closed SP form is favored.
Tensile tests were performed to investigate the mechanical properties of samples with different SP-Ep to ZnCl2 ratios. Fig. 2 and Table 2 illustrate the relationship between the mechanical properties and the ratios of ZnCl2 to SP-Ep for different samples. Samples of SMPs 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 and 1
0.01 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 with a low content of ZnCl2 exhibit a low strain at break, low elastic modulus, and low tensile strength. Despite the low salt content and the absence of any chemical cross-links, the materials are elastic. These results suggest that SP-Ep functions as a physical cross-linker. As the amount of ZnCl2 was increased, an improvement in mechanical properties was observed. Increasing the amount of ZnCl2 in the materials results in an increase in stress at break from 0.52 to 0.45, 1.09, and 1.55 MPa, an increase in Young's modulus from 0.87, 0.84, 2.51, and 3.36 MPa and an increase in toughness from 0.20, 0.17, 0.98 to 1.44 MPa for SMPs 1
0.1 with a low content of ZnCl2 exhibit a low strain at break, low elastic modulus, and low tensile strength. Despite the low salt content and the absence of any chemical cross-links, the materials are elastic. These results suggest that SP-Ep functions as a physical cross-linker. As the amount of ZnCl2 was increased, an improvement in mechanical properties was observed. Increasing the amount of ZnCl2 in the materials results in an increase in stress at break from 0.52 to 0.45, 1.09, and 1.55 MPa, an increase in Young's modulus from 0.87, 0.84, 2.51, and 3.36 MPa and an increase in toughness from 0.20, 0.17, 0.98 to 1.44 MPa for SMPs 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01, 1
0.01, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.10, 1
0.10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 1
0.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. These results indicate that the mechanical properties of the SMPs are weakened for samples with reduced amounts of complexes acting as physical cross-links. The average elongation at break remains constant at 142 and 148% for samples 1
1, respectively. These results indicate that the mechanical properties of the SMPs are weakened for samples with reduced amounts of complexes acting as physical cross-links. The average elongation at break remains constant at 142 and 148% for samples 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5. However, a further reduction in ZnCl2 for the 1
0.5. However, a further reduction in ZnCl2 for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 and 1
0.1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 samples reduces elongation at break to 70 and 75%, respectively. These results suggest that a certain concentration of ZnCl2 is required for a good network of physical cross-links (1
0.01 samples reduces elongation at break to 70 and 75%, respectively. These results suggest that a certain concentration of ZnCl2 is required for a good network of physical cross-links (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) leading to good mechanical properties. However, after a critical concentration of 1
0.5) leading to good mechanical properties. However, after a critical concentration of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a further increase in ZnCl2 leads to a drastic increase in elongation and a decrease in Young's modulus. Even a small excess of ZnCl2 (1
1, a further increase in ZnCl2 leads to a drastic increase in elongation and a decrease in Young's modulus. Even a small excess of ZnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.12) results in more than twice the strain at break (337%) and a large reduction in the stress at break (0.56 MPa) and Young's modulus (1.64 MPa). Interestingly, the toughness remains the same (1.47 MPa) when compared to the 1
1.12) results in more than twice the strain at break (337%) and a large reduction in the stress at break (0.56 MPa) and Young's modulus (1.64 MPa). Interestingly, the toughness remains the same (1.47 MPa) when compared to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 sample. Adding ZnCl2 has a plasticizing effect, allowing deformation at lower stresses.
1 sample. Adding ZnCl2 has a plasticizing effect, allowing deformation at lower stresses.
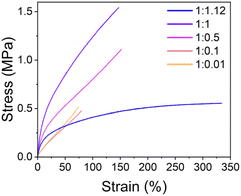 | ||
Fig. 2 Representative curves for the tensile test of samples with different SP-Ep![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2 (x ZnCl2 (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y) ratios. y) ratios. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2 (x
ZnCl2 (x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y) ratios
y) ratios
| Sample | ε (Break) (%) | δ (Break) (MPa) | Y 10% , (MPa) | Toughnessa (MPa) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a At least six values were taken. b Calculated by linear fitting the data points from 0 to 10% strain. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.12 1.12 |
337 ± 32 | 0.56 ± 0.06 | 1.64 ± 0.11 | 1.47 ± 0.24 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
142 ± 8 | 1.55 ± 0.06 | 3.36 ± 0.15 | 1.44 ± 0.15 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
148 ± 9 | 1.09 ± 0.06 | 2.51 ± 0.10 | 0.98 ± 0.09 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
70 ± 10 | 0.45 ± 0.05 | 0.84 ± 0.05 | 0.17 ± 0.04 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 0.01 |
75 ± 3 | 0.52 ± 0.03 | 0.87 ± 0.02 | 0.20 ± 0.01 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
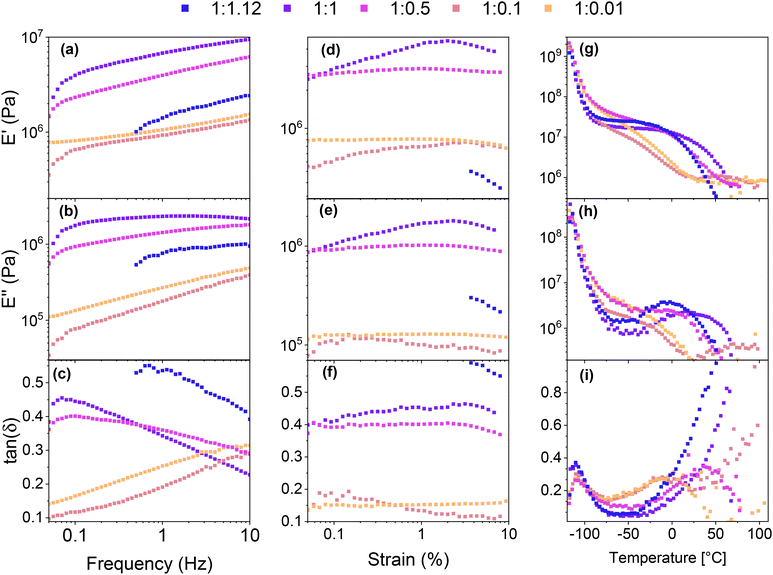
Frequency-dependent viscoelastic measurements were carried out at room temperature to investigate the relationship between the mechanical properties and the SP-Ep![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2 ratio. Sample 1
ZnCl2 ratio. Sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.12 could not be measured at low frequencies due to unstable values. All samples exhibited a storage modulus E′ higher than the loss modulus E′′ over the frequency range investigated. With a decreasing amount of ZnCl2, E′ and E′′ decreased, showing a similar behavior to the tensile test. For the polymer with a high amount of ZnCl2 (1
1.12 could not be measured at low frequencies due to unstable values. All samples exhibited a storage modulus E′ higher than the loss modulus E′′ over the frequency range investigated. With a decreasing amount of ZnCl2, E′ and E′′ decreased, showing a similar behavior to the tensile test. For the polymer with a high amount of ZnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.12, 1
1.12, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5), tan(δ) decreased with increasing frequency (Fig. 3a–c), indicating a better elastomeric behavior at high frequencies. The opposite trend was observed in the samples with lower amounts of ZnCl2 (1
0.5), tan(δ) decreased with increasing frequency (Fig. 3a–c), indicating a better elastomeric behavior at high frequencies. The opposite trend was observed in the samples with lower amounts of ZnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, 1
0.1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01), indicating that the viscous response becomes more pronounced at higher frequencies. These observations suggest that at room temperature, relatively low amounts of the complex are sufficient to yield solid-like behavior at low frequencies. However, this response becomes less pronounced as the frequency increases due to the inability of the physical cross-links between the SPs to store and release energy rapidly. On the other hand, as the ZnCl2 content increases, more complexes are formed and the materials can store more energy at higher frequencies. This is evidenced by the decreased tan(δ) for the 1
0.01), indicating that the viscous response becomes more pronounced at higher frequencies. These observations suggest that at room temperature, relatively low amounts of the complex are sufficient to yield solid-like behavior at low frequencies. However, this response becomes less pronounced as the frequency increases due to the inability of the physical cross-links between the SPs to store and release energy rapidly. On the other hand, as the ZnCl2 content increases, more complexes are formed and the materials can store more energy at higher frequencies. This is evidenced by the decreased tan(δ) for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.12, 1
1.12, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 1
1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 samples. One potential explanation for this behavior is that at low ZnCl2 amounts, intermolecular complexes between two distinct chains are the predominant species, thereby leading to higher losses at higher frequencies, whereas, at higher concentrations, intramolecular complexes within the same chain are formed, which lowers the elastic modulus at increased frequencies.
0.5 samples. One potential explanation for this behavior is that at low ZnCl2 amounts, intermolecular complexes between two distinct chains are the predominant species, thereby leading to higher losses at higher frequencies, whereas, at higher concentrations, intramolecular complexes within the same chain are formed, which lowers the elastic modulus at increased frequencies.
Strain-dependent DMA measurements were performed to investigate the elastic properties of the different samples (Fig. 3d–f). Sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.12 could only be measured at higher strains as the losses were too high for the instrument to measure at lower strains. The results show that similar to the frequency-dependent measurements, the storage modulus increased with increasing ZnCl2 content (from 1
1.12 could only be measured at higher strains as the losses were too high for the instrument to measure at lower strains. The results show that similar to the frequency-dependent measurements, the storage modulus increased with increasing ZnCl2 content (from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 and 1
0.01 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 to 1
0.1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). However, for the 1
1). However, for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.12 sample with a small excess of ZnCl2 to SP, the modulus decreased. Interestingly, for all samples tested, the influence of the strain on E′ and E′′ was limited. Notably, for samples with low amounts of ZnCl2, both the modulus and tan(δ) exhibited a notable reduction compared to samples with a higher amount of the complex. This finding is consistent with the results of the frequency sweep, which demonstrated that samples with low amounts of complex exhibited a lower tan(δ) at low frequencies. The results provide further evidence supporting the hypothesis that two distinct types of complex cross-links are present. In samples with a low complex concentration (1
1.12 sample with a small excess of ZnCl2 to SP, the modulus decreased. Interestingly, for all samples tested, the influence of the strain on E′ and E′′ was limited. Notably, for samples with low amounts of ZnCl2, both the modulus and tan(δ) exhibited a notable reduction compared to samples with a higher amount of the complex. This finding is consistent with the results of the frequency sweep, which demonstrated that samples with low amounts of complex exhibited a lower tan(δ) at low frequencies. The results provide further evidence supporting the hypothesis that two distinct types of complex cross-links are present. In samples with a low complex concentration (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 and 1
0.01 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1), intermolecular cross-links are formed, resulting in low losses and a lower modulus. Conversely, higher complex amounts lead to intramolecular cross-links, which exhibit a higher modulus and losses due to their susceptibility to rupture under high strain.
0.1), intermolecular cross-links are formed, resulting in low losses and a lower modulus. Conversely, higher complex amounts lead to intramolecular cross-links, which exhibit a higher modulus and losses due to their susceptibility to rupture under high strain.
The thermal stability of the samples was investigated using TGA (Fig. S4a†). All samples exhibit great stability up to 150 °C and show a mass loss of 2% above 170 °C.
Differential scanning calorimetry (DSC) measurements were performed to investigate the thermal transitions of SP-Ep and PDMS-SP-Ep. Upon cooling, pure SP-Ep shows a broad exothermal peak at 36 °C, attributed to a crystallization process (Fig. S4b†). Upon heating, an endothermal peak is observed at 45 °C, corresponding to the melting of the crystalline phase (Fig. S4c†). In fact, no thermal transitions were detected between −80 and 80 °C for any of the samples, neither on cooling nor on heating, suggesting that the grafting of SP-Ep onto the PDMS chain prevents SP-Ep crystallization (Fig. S4d and e†). Even sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01, with the lowest amount of ZnCl2, does not crystallize. All materials exhibited an elastic behavior at room temperature, suggesting that all should have a Tg below −80 °C and another transition temperature above 80 °C, responsible for the physical cross-links. Unfortunately, our DSC setup did not allow measurements to be conducted at even lower temperatures. Therefore, to investigate the temperature-related transitions in the polymer structure, temperature-dependent DMA measurements were conducted for samples 1
0.01, with the lowest amount of ZnCl2, does not crystallize. All materials exhibited an elastic behavior at room temperature, suggesting that all should have a Tg below −80 °C and another transition temperature above 80 °C, responsible for the physical cross-links. Unfortunately, our DSC setup did not allow measurements to be conducted at even lower temperatures. Therefore, to investigate the temperature-related transitions in the polymer structure, temperature-dependent DMA measurements were conducted for samples 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.12, 1
1.12, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 1
0.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, and 1
0.1, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 (Fig. 3g–i). All samples exhibited a decrease in modulus as temperatures increased from −115 °C. The maximum in tan(δ) was observed at temperatures around −110 °C (for 1
0.01 (Fig. 3g–i). All samples exhibited a decrease in modulus as temperatures increased from −115 °C. The maximum in tan(δ) was observed at temperatures around −110 °C (for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.12 at −111 °C, 1
1.12 at −111 °C, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at −110 °C, 1
1 at −110 °C, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 at −110 °C, 1
0.5 at −110 °C, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 at −109 °C, and 1
0.1 at −109 °C, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 at −110 °C) (Table 3). This maximum in tan(δ) corresponds to the Tg of the PDMS phase and is independent of the amount of ZnCl2. As the temperature increased, E′ decreased for all samples. A plateau in E′ was reached for samples with a high amount of ZnCl2, however, samples 1
0.01 at −110 °C) (Table 3). This maximum in tan(δ) corresponds to the Tg of the PDMS phase and is independent of the amount of ZnCl2. As the temperature increased, E′ decreased for all samples. A plateau in E′ was reached for samples with a high amount of ZnCl2, however, samples 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 and 1
0.1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 exhibited a steady decrease in E′ up to about 25 °C where E′ reached a plateau. This suggests that a complex is responsible for the plateau in the low-temperature range. DMA measurements suggest the presence of a second Tg, which was strongly dependent on the content of ZnCl2 in the SMPs. With an increasing amount of ZnCl2, the peak of tan(δ) is shifted to higher temperatures, from around −10 °C for 1
0.01 exhibited a steady decrease in E′ up to about 25 °C where E′ reached a plateau. This suggests that a complex is responsible for the plateau in the low-temperature range. DMA measurements suggest the presence of a second Tg, which was strongly dependent on the content of ZnCl2 in the SMPs. With an increasing amount of ZnCl2, the peak of tan(δ) is shifted to higher temperatures, from around −10 °C for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 and 1
0.01 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 to about 37 °C for 1
0.1 to about 37 °C for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, and goes up to higher temperatures for the 1
0.5, and goes up to higher temperatures for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Thus, tan(δ) increased strongly for samples with high amounts of ZnCl2 to elevated temperatures. For sample 1
1. Thus, tan(δ) increased strongly for samples with high amounts of ZnCl2 to elevated temperatures. For sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.12, tan(δ) reached a value of 1 at temperatures of 44 °C. Higher amounts of the complex led to a higher storage modulus at 25 °C, while an excess of ZnCl2 resulted in a lower modulus. These findings are consistent with the results from tensile testing and frequency sweep-dependent DMA measurements. Thus, at room temperatures, sample 1
1.12, tan(δ) reached a value of 1 at temperatures of 44 °C. Higher amounts of the complex led to a higher storage modulus at 25 °C, while an excess of ZnCl2 resulted in a lower modulus. These findings are consistent with the results from tensile testing and frequency sweep-dependent DMA measurements. Thus, at room temperatures, sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 showed the highest storage modulus of 7.3 MPa, followed by 1
1 showed the highest storage modulus of 7.3 MPa, followed by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 with 3.71 MPa, 1
0.5 with 3.71 MPa, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.12 with 2.97 MPa, 1
1.12 with 2.97 MPa, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 with 0.95 MPa, and 1
0.1 with 0.95 MPa, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 with 0.92 MPa, respectively (Table 3). The aforementioned observations also explain why the polymers exhibit elastomeric properties but can be processed like a thermoplastic polymer at elevated temperatures. The relatively low tan(δ) at room temperature provides stability and an elastomeric behavior, but as temperature increases, the material softens and can be melt pressed, similarly to a thermoplastic elastomer.
0.01 with 0.92 MPa, respectively (Table 3). The aforementioned observations also explain why the polymers exhibit elastomeric properties but can be processed like a thermoplastic polymer at elevated temperatures. The relatively low tan(δ) at room temperature provides stability and an elastomeric behavior, but as temperature increases, the material softens and can be melt pressed, similarly to a thermoplastic elastomer.
| Sample | T g (°C) | T (tan(δ)=1) (MPa) | E′; E′′ at 0.1 Hz (MPa) | E′; E′′ at 1 Hz (MPa) | E′; E′′ at 10 Hz (MPa) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All samples were measured at least 3 times and the average was taken.a Not for every sample tan(δ) = 1 could be determined until T = 110 °C.b No sample showed a tan(δ) = 1 up to T = 110 °C. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.12 1.12 |
−111 ± 1 | 1360 ± 70 | 2.97 ± 0.23 | 44 ± 6 | Not determined | 1.4 ± 0.2; 0.76 ± 0.14 | 2.4 ± 0.2; 0.95 ± 0.06 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
−110 ± 1 | 1190 ± 70 | 7.3 ± 0.8 | 66 ± 6 | 4.0 ± 0.2; 1.8 ± 0.1 | 6.8 ± 0.3; 2.3 ± 0.1 | 9.5 ± 0.4; 2.2 ± 0.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
−110 ± 1 | 1690 ± 170 | 3.71 ± 0.08 | Above 75a | 2.3 ± 0.1; 0.93 ± 0.03 | 4.0 ± 0.1; 1.4 ± 0.1 | 6.2 ± 0.3; 1.8 ± 0.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
−109 ± 1 | 1300 ± 110 | 0.95 ± 0.05 | Above 97a | 0.66 ± 0.04; 0.077 ± 0.001 | 0.93 ± 0.03; 0.18 ± 0.01 | 1.35 ± 0.06; 0.40 ± 0.02 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 0.01 |
−110 ± 1 | 1590 ± 110 | 0.92 ± 0.06 | Not determinedb | 0.82 ± 0.02; 0.13 ± 0.01 | 1.08 ± 0.03; 0.28 ± 0.01 | 1.56 ± 0.05; 0.49 ± 0.02 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
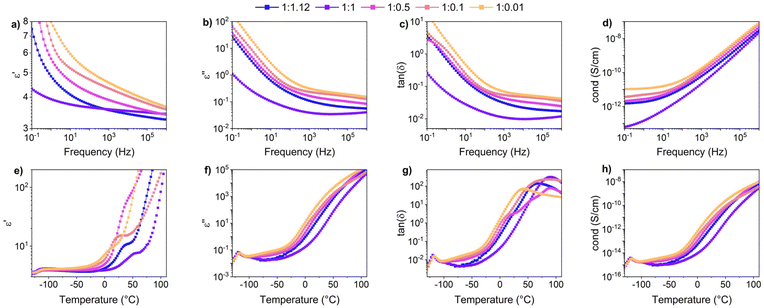
Dielectric properties were measured for samples with different amounts of ZnCl2. As shown in Fig. 4a–c, for all samples, permittivity (ε′) and dielectric losses (ε′′) increased with decreasing frequency, while in contrast, conductivity (Fig. 4d) increased with increasing frequency. Fig. 4a shows that at 1 kHz the dielectric permittivity of all materials is higher than pure PDMS (ε′ = 3), even if the polysiloxane backbone was modified with only 5 mol% SP-Ep side chains.39 The permittivity at 1 kHz decreases with an increasing amount of ZnCl2 in the sample from 4.7 for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01, to 4.4 for 1
0.01, to 4.4 for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, to 3.9 for 1
0.1, to 3.9 for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, to 3.6 for 1
0.5, to 3.6 for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.12. Similarly, a decrease in ε′′ and conductivity is observed with a decreasing amount of ZnCl2. It can be concluded that the dipoles of SP-Ep interact with the Zn-ion and the formed SP-Ep-Zn complex is less susceptible to polarization.
1.12. Similarly, a decrease in ε′′ and conductivity is observed with a decreasing amount of ZnCl2. It can be concluded that the dipoles of SP-Ep interact with the Zn-ion and the formed SP-Ep-Zn complex is less susceptible to polarization.
Dielectric spectroscopy measurements as a function of temperature can help study the transitions occurring in the sample and complement the DMA measurements. Fig. 4e–h lists the real and imaginary components of the complex dielectric permittivity, including the dissipation factor tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ and the conductivity of all the samples with varying amounts of ZnCl2, measured from −130 °C to 110 °C at a constant frequency of 0.1 Hz. From the ε′ plot, all samples show a step increase in permittivity below −100 °C. This is manifested as a peak in both dielectric loss and tan
δ and the conductivity of all the samples with varying amounts of ZnCl2, measured from −130 °C to 110 °C at a constant frequency of 0.1 Hz. From the ε′ plot, all samples show a step increase in permittivity below −100 °C. This is manifested as a peak in both dielectric loss and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ. Comparing with the data from DMA, this should correspond to the glass-transition relaxation process of PDMS and, as expected, is independent of the amount of ZnCl2. Above the first Tg loss peaks, we see an additional shoulder for high concentration of ZnCl2 (1
δ. Comparing with the data from DMA, this should correspond to the glass-transition relaxation process of PDMS and, as expected, is independent of the amount of ZnCl2. Above the first Tg loss peaks, we see an additional shoulder for high concentration of ZnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.12). The shoulder becomes broader for lower concentrations of ZnCl2.
1.12). The shoulder becomes broader for lower concentrations of ZnCl2.
From DMA measurements, we expect to see a relaxation around −25 °C for the samples with low ZnCl2 content, moving progressively to higher temperatures with increasing ZnCl2. For 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 sample, we observed a relaxation process reflected by the shoulder in the permittivity spectrum at 10 °C. This relaxation process is more clearly seen in the 1
0.01 sample, we observed a relaxation process reflected by the shoulder in the permittivity spectrum at 10 °C. This relaxation process is more clearly seen in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 sample at 25 °C. A further increase in the ZnCl2 concentration shifts the relaxation to higher temperatures, albeit a lower change in permittivity during this process. While, this process is clearly visible in permittivity, it cannot be easily identified in their corresponding loss plots. This is due to the enhanced conductivity of all samples above −25 °C. Further analysis is therefore required to explain the various transitions observed in dielectric spectroscopy.
0.1 sample at 25 °C. A further increase in the ZnCl2 concentration shifts the relaxation to higher temperatures, albeit a lower change in permittivity during this process. While, this process is clearly visible in permittivity, it cannot be easily identified in their corresponding loss plots. This is due to the enhanced conductivity of all samples above −25 °C. Further analysis is therefore required to explain the various transitions observed in dielectric spectroscopy.
Sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 was chosen for detailed analysis. As a first step, contributions from d.c. conductivity (ohmic conduction) was removed by performing a frequency derivative of permittivity, as stated in eqn (1).
0.1 was chosen for detailed analysis. As a first step, contributions from d.c. conductivity (ohmic conduction) was removed by performing a frequency derivative of permittivity, as stated in eqn (1).
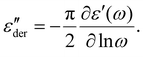 | (1) |
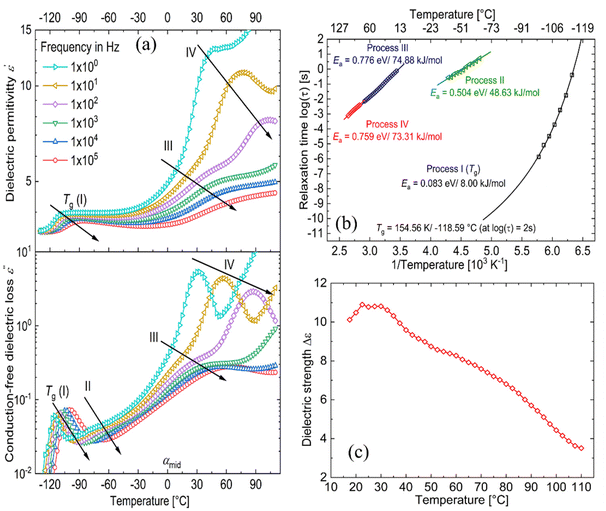
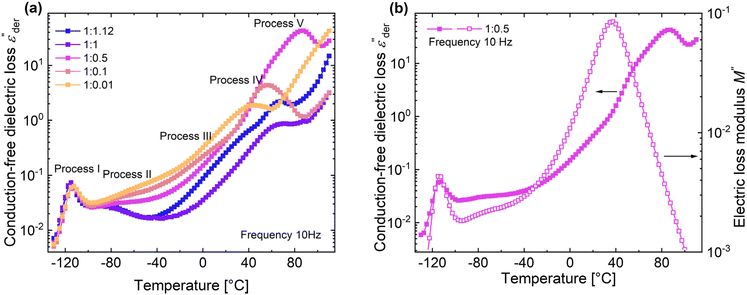
Fig. 5a shows the d.c. conduction-free dielectric loss (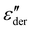 ) and permittivity (ε′) plotted as a function of temperature at selected frequencies. Comparing
) and permittivity (ε′) plotted as a function of temperature at selected frequencies. Comparing 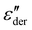 curves to Fig. 4f, in addition to the two transitions (process I and II) found at lower temperatures, we observe two additional processes above 0 °C named as process III and IV. As stated earlier, process I is assigned to a glass-transition process. The peaks shift to higher temperatures with increased frequency, as expected in a relaxation process.40 The Havriliak–Negami (HN) function41 was used to fit the loss peaks of the process I. The relaxation times obtained from peak fitting are plotted in Fig. 5b. The fit is non-linear and obeys Vogel–Fulcher–Tammann (VFT) law affirming a glass-transition relaxation.41 The dynamic Tg can be calculated by extrapolating the curve to a relaxation time of 100 s (log
curves to Fig. 4f, in addition to the two transitions (process I and II) found at lower temperatures, we observe two additional processes above 0 °C named as process III and IV. As stated earlier, process I is assigned to a glass-transition process. The peaks shift to higher temperatures with increased frequency, as expected in a relaxation process.40 The Havriliak–Negami (HN) function41 was used to fit the loss peaks of the process I. The relaxation times obtained from peak fitting are plotted in Fig. 5b. The fit is non-linear and obeys Vogel–Fulcher–Tammann (VFT) law affirming a glass-transition relaxation.41 The dynamic Tg can be calculated by extrapolating the curve to a relaxation time of 100 s (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) τ = 2 s),42 which yields a temperature of −118.6 °C, agreeing well with the value obtained from DMA. Similar to process I, the other processes were also subjected to a HN-fit, and the corresponding relaxation map is plotted in Fig. 5b. In contrast to process I, all the other processes result in a linear fit obeying an Arrhenius-type equation.41 Since all these processes also shift with frequency and the polymer samples are amorphous, structural transition processes such as melting and crystalline phase change can be excluded.40 To obtain an overview of these different processes across the samples with different amounts of ZnCl2,
τ = 2 s),42 which yields a temperature of −118.6 °C, agreeing well with the value obtained from DMA. Similar to process I, the other processes were also subjected to a HN-fit, and the corresponding relaxation map is plotted in Fig. 5b. In contrast to process I, all the other processes result in a linear fit obeying an Arrhenius-type equation.41 Since all these processes also shift with frequency and the polymer samples are amorphous, structural transition processes such as melting and crystalline phase change can be excluded.40 To obtain an overview of these different processes across the samples with different amounts of ZnCl2, 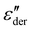 curves of all the samples as a function of temperature at a fixed frequency of 10 Hz are shown in Fig. 6a. Looking into process II, we find that the relaxation is stronger for samples with a higher amount of ZnCl2 and progressively becomes weaker with decreasing amounts of ZnCl2. Correlating this with the change in permittivity during this temperature range for the different samples (Fig. 4e), we find that samples with higher amount of ZnCl2, i.e., higher amount of complex formation (1
curves of all the samples as a function of temperature at a fixed frequency of 10 Hz are shown in Fig. 6a. Looking into process II, we find that the relaxation is stronger for samples with a higher amount of ZnCl2 and progressively becomes weaker with decreasing amounts of ZnCl2. Correlating this with the change in permittivity during this temperature range for the different samples (Fig. 4e), we find that samples with higher amount of ZnCl2, i.e., higher amount of complex formation (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.12 and 1
1.12 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), show a dip in permittivity, while the SP-Ep with lower fraction of ZnCl2 (1
1), show a dip in permittivity, while the SP-Ep with lower fraction of ZnCl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 and 1
0.1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01) show a small increase. As previously stated, the complexation of SP-Ep by ZnCl2 reduces the permittivity of the samples, and hence, process II can be assigned to the relaxation of these complex units.
0.01) show a small increase. As previously stated, the complexation of SP-Ep by ZnCl2 reduces the permittivity of the samples, and hence, process II can be assigned to the relaxation of these complex units.
Moving on to process III, looking at the loss curves in Fig. 5a, it appears as a shoulder at a frequency of 10 Hz and transforms into a peak at higher frequencies. From Fig. 6a, we also notice that the process is stronger and occurs at a higher temperature for samples with higher ZnCl2. This relaxation can be directly correlated with the loss modulus peaks occurring between −25 °C and +50 °C across different samples in DMA measurements (Fig. 3h). As mentioned earlier, the mechanical failure of samples above this transition hints at the disassociation of the complex in the samples. This also explains the higher temperature at which the shoulder is observed for samples with higher ZnCl2. A similar trend is observed with the process IV peaks, and by looking at the Arrhenius plots for both process III and IV in Fig. 5b, we observe similar activation energies and distribution of relaxation times. All these suggest that both these processes are related and can be assigned to the dissociation of the two types of complexes hypothesized to exist in the samples. Plotting the dielectric strengths (Δε) of both these processes (Fig. 5c) obtained from the HN fitting of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 sample reveals a continuous curve with a peak at 27.5 °C and a shoulder at 77.5 °C corresponding to processes III and IV, respectively. Process III can be assigned to the intermolecular complexes, which are thermally less stable than intramolecular complexes, which dissociate around 80 °C (process IV).
0.1 sample reveals a continuous curve with a peak at 27.5 °C and a shoulder at 77.5 °C corresponding to processes III and IV, respectively. Process III can be assigned to the intermolecular complexes, which are thermally less stable than intramolecular complexes, which dissociate around 80 °C (process IV).
For a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 sample, above process IV, we can observe yet another peak at 87.5 °C in Fig. 6a. Since, the complexes already dissociate at this temperature, the ions can move to their respective counter electrodes, resulting in electrode polarization.43 To check this, the imaginary part of complex electric modulus M′′ = ε′′/ε′2 + ε′′2 is plotted in Fig. 6b along with
0.5 sample, above process IV, we can observe yet another peak at 87.5 °C in Fig. 6a. Since, the complexes already dissociate at this temperature, the ions can move to their respective counter electrodes, resulting in electrode polarization.43 To check this, the imaginary part of complex electric modulus M′′ = ε′′/ε′2 + ε′′2 is plotted in Fig. 6b along with 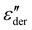 for a 1
for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 sample. The advantage of using a M′′ loss plot is suppressing electrode polarization.44,45 From the figure, we clearly observe the absence of process V in the M′′ loss curve, confirming it to be electrode polarization. Such a process can be expected to be observed at higher temperatures for the rest of the samples, indicated by the increase in losses in Fig. 6a. On the other hand, the peak observed at 37.5 °C in the M′′ loss curve can be delegated to the shoulder of process IV relaxation observed in
0.5 sample. The advantage of using a M′′ loss plot is suppressing electrode polarization.44,45 From the figure, we clearly observe the absence of process V in the M′′ loss curve, confirming it to be electrode polarization. Such a process can be expected to be observed at higher temperatures for the rest of the samples, indicated by the increase in losses in Fig. 6a. On the other hand, the peak observed at 37.5 °C in the M′′ loss curve can be delegated to the shoulder of process IV relaxation observed in 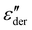 .
.
Using dielectric spectroscopy and detailed analysis, it is possible to identify the different relaxations taking place in the samples under investigation. On heating above −110 °C, we first observe the glass transition at low temperatures, immediately followed by the relaxation of complexes. Above −30 °C, we observe the disassociation of complexes at two different temperatures. While process III is observed as a shoulder, process IV is observed as a peak. On further heating the samples to higher temperatures above 80 °C, the disassociated ions lead to the observation of electrode polarization. Except for glass transition, all other processes depend on the concentration of ZnCl2 in the sample. In general, a higher fraction of ZnCl2 increases the strength of these processes. While the relaxation of the complexes (process II) in samples with a higher presence of ZnCl2 is observed at a lower temperature, the opposite holds for processes III and IV.
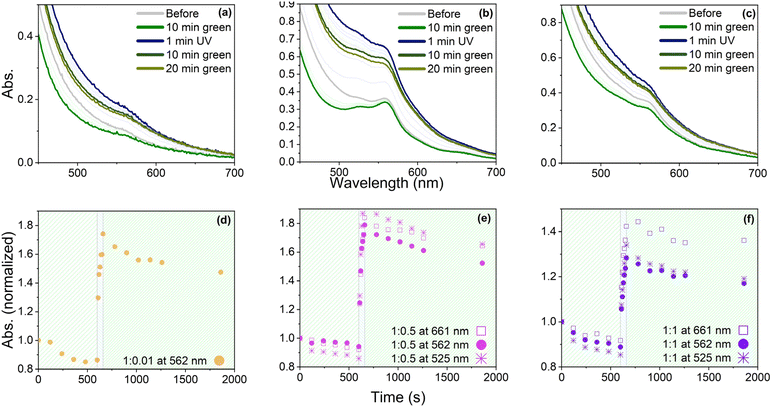
To investigate the light-triggered switching capabilities of the synthesized materials, UV-Vis spectra of three selected samples 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, and 1
0.5, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 were recorded before and after exposure to green and UV light (Fig. 7a–c). Sample 1
0.01 were recorded before and after exposure to green and UV light (Fig. 7a–c). Sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 (Fig. 7a) showed one absorption band at 562 nm, whereas samples 1
0.01 (Fig. 7a) showed one absorption band at 562 nm, whereas samples 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 1
0.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 7b and c) exhibited three absorption bands, two weak ones at 661 and 525 nm, and a stronger one at 525 nm, with 1
1 (Fig. 7b and c) exhibited three absorption bands, two weak ones at 661 and 525 nm, and a stronger one at 525 nm, with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 showing a more pronounced band at 525 and 661 nm. These results suggest that the 661 and 525 nm bands result from complex formation. To ensure that SP-Ep did not contain open MC form, all samples were first exposed to green light at a wavelength of 505 nm for 10 min. The resulting decrease in absorption suggests that some SP-Ep had already undergone isomerization to the open MC form and subsequently switched back to the closed form upon irradiation with green light. Nevertheless, a small peak at 562 nm was observed in all samples, which was attributed to the MC form. This phenomenon was more pronounced in samples 1
0.5 showing a more pronounced band at 525 and 661 nm. These results suggest that the 661 and 525 nm bands result from complex formation. To ensure that SP-Ep did not contain open MC form, all samples were first exposed to green light at a wavelength of 505 nm for 10 min. The resulting decrease in absorption suggests that some SP-Ep had already undergone isomerization to the open MC form and subsequently switched back to the closed form upon irradiation with green light. Nevertheless, a small peak at 562 nm was observed in all samples, which was attributed to the MC form. This phenomenon was more pronounced in samples 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, which had a higher ZnCl2 content. This suggests that ZnCl2 stabilizes the MC form and deactivates the switch back to the SP form. The absorbance significantly increased after applying UV light with a wavelength of 365 nm for 1 min. The highest increase was observed for 1
0.5, which had a higher ZnCl2 content. This suggests that ZnCl2 stabilizes the MC form and deactivates the switch back to the SP form. The absorbance significantly increased after applying UV light with a wavelength of 365 nm for 1 min. The highest increase was observed for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 (Fig. 7e), followed by 1
0.5 (Fig. 7e), followed by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 (Fig. 7d), and the smallest change in absorbance was observed for 1
0.01 (Fig. 7d), and the smallest change in absorbance was observed for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 7f). Upon exposure to green light for 20 min, the absorbance decreased, but it was impossible to re-obtain the original spectra, which would have indicated a complete switch back. The results suggest that despite being chemically bonded to the polymer and incorporated into a complex, SP-Ep still exhibits photo-switching behavior. However, photoisomerization towards the open MC form is faster and more efficient than the switch back with a green light, which was incomplete. Several possible explanations include photo-induced oxidation processes resulting from highly energetic UV light exposure. Additionally, metal ions and stacking phenomena in the physical cross-links may stabilize the opened form. It is known from the literature23 that the open MC form can be stacked. Eckhardt et al. also reported that the ability to shift back into the closed-ring form depends on the degree of MC association and sometimes cannot be changed back without degradation.46
1 (Fig. 7f). Upon exposure to green light for 20 min, the absorbance decreased, but it was impossible to re-obtain the original spectra, which would have indicated a complete switch back. The results suggest that despite being chemically bonded to the polymer and incorporated into a complex, SP-Ep still exhibits photo-switching behavior. However, photoisomerization towards the open MC form is faster and more efficient than the switch back with a green light, which was incomplete. Several possible explanations include photo-induced oxidation processes resulting from highly energetic UV light exposure. Additionally, metal ions and stacking phenomena in the physical cross-links may stabilize the opened form. It is known from the literature23 that the open MC form can be stacked. Eckhardt et al. also reported that the ability to shift back into the closed-ring form depends on the degree of MC association and sometimes cannot be changed back without degradation.46
To further investigate changes in the properties of the samples, permittivity was measured before and after exposure to UV and green light (Fig. 8a). The initial value before light exposure was set as 100%. After exposure for 2 min with UV light, the values decreased to 81 and 80% for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, respectively. Exposure to green light for 10 min led to a permittivity value of up to 90 and 88% of the original value for 1
0.5, respectively. Exposure to green light for 10 min led to a permittivity value of up to 90 and 88% of the original value for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 samples, respectively. Repeating the procedure resulted in a decrease to 67 and 64%, followed by an increase after exposure to UV light to 79 and 75%, respectively. Even though the switching behavior can be repeated, the original permittivity values cannot be restored. On the contrary, exposure of the 1
0.5 samples, respectively. Repeating the procedure resulted in a decrease to 67 and 64%, followed by an increase after exposure to UV light to 79 and 75%, respectively. Even though the switching behavior can be repeated, the original permittivity values cannot be restored. On the contrary, exposure of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01 sample to UV light for 2 min resulted in an increase in permittivity of +24%, followed by a decrease to 95% after exposure to green light for 10 min. This switching behavior could be repeated, and in the second run, exposure to UV light led to an increase of up to +25%, followed by a decrease to 99%. Hence, for sample 1
0.01 sample to UV light for 2 min resulted in an increase in permittivity of +24%, followed by a decrease to 95% after exposure to green light for 10 min. This switching behavior could be repeated, and in the second run, exposure to UV light led to an increase of up to +25%, followed by a decrease to 99%. Hence, for sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01, exposure to UV light is accompanied by an increase in permittivity, while exposure to green light can restore values close to the original ones. We also observed that sample handling became more difficult for all materials after exposure to UV light, possibly due to changes in the internal structure caused by stacking phenomena, as discussed earlier. Comparing the results for 1
0.01, exposure to UV light is accompanied by an increase in permittivity, while exposure to green light can restore values close to the original ones. We also observed that sample handling became more difficult for all materials after exposure to UV light, possibly due to changes in the internal structure caused by stacking phenomena, as discussed earlier. Comparing the results for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 with those of 1
0.5 with those of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01, it can be seen that the presence of a complex in the sample significantly influences the photo-switching behavior. For sample 1
0.01, it can be seen that the presence of a complex in the sample significantly influences the photo-switching behavior. For sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01, UV light-induced ring opening of the chromophore increases the dielectric permittivity. However, the dielectric permittivity for the ring-opened MC form in 1
0.01, UV light-induced ring opening of the chromophore increases the dielectric permittivity. However, the dielectric permittivity for the ring-opened MC form in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 1
0.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 decreased. The open MC form has a higher dipole moment than the closed SP form,47,48 however, the results suggest that the Zn complex of MC form has a lower dipole moment than its closed SP counterpart. This would also explain the contrary switching behavior in permittivity after UV and green light treatment.
1 decreased. The open MC form has a higher dipole moment than the closed SP form,47,48 however, the results suggest that the Zn complex of MC form has a lower dipole moment than its closed SP counterpart. This would also explain the contrary switching behavior in permittivity after UV and green light treatment.
Based on the UV measurements, it has been shown that exposure to UV light leads to irreversible changes in the absorbance spectra of the samples. To investigate these changes further, IR-spectroscopy was performed on sample 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 before and after subsequent exposure to UV light for 20 min (Fig. 8b). The spectra before and after irradiation do not show any new bands, indicating that no degradation by oxidation occurred. Notably, the band assigned to the C
0.5 before and after subsequent exposure to UV light for 20 min (Fig. 8b). The spectra before and after irradiation do not show any new bands, indicating that no degradation by oxidation occurred. Notably, the band assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch showed two absorption maxima for the untreated 1
C stretch showed two absorption maxima for the untreated 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 (1641 and 1666 cm−1), but only one for the irradiated sample (1643 cm−1). As previously discussed, the band at higher wavenumbers can be assigned to the C
0.5 (1641 and 1666 cm−1), but only one for the irradiated sample (1643 cm−1). As previously discussed, the band at higher wavenumbers can be assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch of the SP, which shifts towards lower wavenumbers when SP is converted to MC. The results indicate that the changes occurring in the sample are not chemical but related to the arrangement of SP in the polymer matrix. This could be due to the aggregation of the MC stacks, which, according to Eckhardt et al. can be very stable and irreversible.46 The aggregation can lead to shrinking phenomena,49 which was also observed in the sample. Another explanation for the observed fatigue is the presence of ZnCl2 in the sample. Previous research has demonstrated that ions can stabilize the open MC form, which may impede the molecule's ability to revert to the SP form.50,51 These irreversible changes display a significant drawback for the presented system as they would only allow the construction of devices with a functional of a limited amount of cycles and decreasing performance. Further investigations into these stacking phenomena and the means of preventing them would be of interest. Potential methods for this investigation could include X-ray spectroscopy,52–54 fluorescence spectroscopy,55 or electron microscopy.46
C stretch of the SP, which shifts towards lower wavenumbers when SP is converted to MC. The results indicate that the changes occurring in the sample are not chemical but related to the arrangement of SP in the polymer matrix. This could be due to the aggregation of the MC stacks, which, according to Eckhardt et al. can be very stable and irreversible.46 The aggregation can lead to shrinking phenomena,49 which was also observed in the sample. Another explanation for the observed fatigue is the presence of ZnCl2 in the sample. Previous research has demonstrated that ions can stabilize the open MC form, which may impede the molecule's ability to revert to the SP form.50,51 These irreversible changes display a significant drawback for the presented system as they would only allow the construction of devices with a functional of a limited amount of cycles and decreasing performance. Further investigations into these stacking phenomena and the means of preventing them would be of interest. Potential methods for this investigation could include X-ray spectroscopy,52–54 fluorescence spectroscopy,55 or electron microscopy.46
Conclusions
The present study reports on a novel and straightforward synthesis strategy for metallo-supramolecular polymers based on PDMS modified with aminopropyl groups functionalized with spiropyran in a ZnCl2-catalyzed reaction. The ZnCl2 acts not only as a catalyst but also forms a complex with spiropyran. The spiropyran moieties on the PDMS can form physical cross-links by either spyran-spyran interactions or spiropyran Zn-complex. The metallo-supramolecular polymers behave as thermoplastic elastomers, which can be easily reprocessed. The amount of ZnCl2 allows for mechanical properties of the materials to be tuned. Additionally, spiropyran exhibits photo-switching behavior, where exposure to UV light induces ring opening to the merocyanine form, which can be partially reversed by exposure to green light. Notably, the uncomplexed spiropyran shows an increase in the dielectric permittivity upon exposure to UV light, whereas the complexed form shows lower dielectric permittivity values. Although the switching behavior is reversible, the open merocyanine form can stack irreversibly, leading to changes in polymer structure and properties.Author contributions
M. B. performed the synthesis, characterization of all materials, and wrote the first draft. T. R. V. conducted the impedance spectroscopy investigations and analysis. D. M. O. initiated the activity, designed the materials, received funding acquisition, and coordinated and supervised this research. All authors contributed with discussions, reviewing, and editing and have approved the final version of the manuscript.Data availability
The data, software or code supporting the results reported in a published article can be found in the ESI† and on https://dx.doi.org/10.5281/zenodo.13740447.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement no 101001182) for financial support. We also acknowledge J. Tschudin (Empa) for DSC and TGA measurements.References
- S. C. Shit and P. Shah, Natl. Acad. Sci. Lett., 2013, 36, 355–365 CrossRef CAS.
- E. Yilgör and I. Yilgör, Prog. Polym. Sci., 2014, 39, 1165–1195 CrossRef.
- M. A. Brook, Chem. Commun., 2023, 59, 12813–12829 RSC.
- N. Barber, J. Scarcelli, B. A. Almanza, J. R. Daniel and D. Nelson, J. Foodserv., 2007, 18, 43–51 Search PubMed.
- M. Zare, E. R. Ghomi, P. D. Venkatraman and S. Ramakrishna, J. Appl. Polym. Sci., 2021, 138, e50969 Search PubMed.
- J. P. Reynders, I. R. Jandrell and S. M. Reynders, IEEE Trans. Dielectr. Electr. Insul., 1999, 6, 620–631 CAS.
- D. M. Opris, Adv. Mater., 2018, 30, 1703678 Search PubMed.
- Y. Sheima, Y. Yuts, H. Frauenrath and D. M. Opris, Macromolecules, 2021, 54, 5737–5749 CAS.
- N. S. Gupta, K. S. Lee and A. Labouriau, Polymers, 2021, 13, 1141 CrossRef CAS.
- A. R. Babu and N. Gundiah, Exp. Mech., 2014, 54, 1177–1187 CAS.
- S. Seiffert and J. Sprakel, Chem. Soc. Rev., 2012, 41, 909–930 CAS.
- E. A. Appel, J. del Barrio, X. J. Loh and O. A. Scherman, Chem. Soc. Rev., 2012, 41, 6195–6214 CAS.
- J. L. Mann, A. C. Yu, G. Agmon and E. A. Appel, Biomater. Sci., 2018, 6, 10–37 CAS.
- M. Fujita, Molecular Self-Assembly, Organic versus Inorganic Approaches, Springer-Verlag, Berlin, 2000 Search PubMed.
- M. Guo, L. M. Pitet, H. M. Wyss, M. Vos, P. Y. W. Dankers and E. W. Meijer, J. Am. Chem. Soc., 2014, 136, 6969–6977 CAS.
- B. Yang, H. Zhang, H. Peng, Y. Xu, B. Wu, W. Weng and L. Li, Polym. Chem., 2014, 5, 1945–1953 CAS.
- D. Hrabowyj, L. A. Wittenberg, E. M. Donahue-Boyle, Y. Chen and M. A. Brook, Can. J. Chem., 2024, 102, 91–98 CrossRef CAS.
- N. Holten-Andersen, M. J. Harrington, H. Birkedal, B. P. Lee, P. B. Messersmith, K. Yee, C. Lee and J. H. Waite, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 2651–2655 CrossRef CAS.
- T. F. A. De Greef, M. M. J. Smulders, M. Wolffs, A. P. H. J. Schenning, R. P. Sijbesma and E. W. Meijer, Chem. Rev., 2009, 109, 5687–5754 CrossRef CAS.
- A. S. Fawcett and M. A. Brook, Macromolecules, 2014, 47, 1656–1663 CrossRef CAS.
- N. M. Gallagher, A. V. Zhukhovitskiy, H. V. T. Nguyen and J. A. Johnson, Macromolecules, 2018, 51, 3006–3016 CrossRef CAS.
- G. Gréger, W. Meyer-Zaika, C. Böttcher, F. Grähn, C. Ruthard and C. Schmuck, J. Am. Chem. Soc., 2011, 133, 8961–8971 Search PubMed.
- C. P. McCoy, L. Donnelly, D. S. Jones and S. P. Gorman, Tetrahedron Lett., 2007, 48, 657–661 CAS.
- H. Görner and A. K. Chibisov, J. Chem. Soc., Faraday Trans., 1998, 94, 2557–2564 Search PubMed.
- M. Li, Q. Zhang, Y. N. Zhou and S. Zhu, Prog. Polym. Sci., 2018, 79, 26–39 CAS.
- A. Fagan, M. Bartkowski and S. Giordani, Front. Chem., 2021, 9, 720087 CAS.
- M. Natali and S. Giordani, Chem. Soc. Rev., 2012, 41, 4010–4029 RSC.
- S. S. Xue, G. Manivannan and R. A. Lessard, Thin Solid Films, 1994, 253, 228–232 CrossRef CAS.
- R. Ramos-Garcia, R. Delgado-Macuil, D. Iturbe-Castillo, E. G. de Los Santos and F. S. Corral, Opt. Quantum Electron., 2003, 35, 641–650 CAS.
- J. Garcia, J. B. Addison, S. Z. Liu, S. Lu, A. L. Faulkner, B. M. Hodur, E. I. Balmond, V. W. Or, J. H. Yun, K. Trevino, B. Shen, J. T. Shaw, N. L. Frank and A. Y. Louie, J. Phys. Chem. B, 2019, 123, 6799–6809 CAS.
- L. Kortekaas and W. R. Browne, Chem. Soc. Rev., 2019, 48, 3406–3424 CAS.
- J. Keyvan Rad, Z. Balzade and A. R. Mahdavian, J. Photochem. Photobiol., C, 2022, 51, 100487 CAS.
- G. Such, R. A. Evans, L. H. Yee and T. P. Davis, J. Macromol. Sci., Polym. Rev., 2003, 43, 547–579 Search PubMed.
- M. Wübbenhorst and J. van Turnhout, J. Non-Cryst. Solids, 2002, 305, 40–49 Search PubMed.
- J. van Turnhout and M. Wübbenhorst, J. Non-Cryst. Solids, 2002, 305, 50–58 CAS.
- Y. Xue, J. Tian, W. Tian, K. Zhang, J. Xuan and X. Zhang, Spectrochim. Acta, Part A, 2021, 250, 119385 CrossRef CAS.
- W. Cao, C. Wang, S. Wang, Y. Zhang and R. Zhao, Chem. Res. Chin. Univ., 2021, 37, 512–521 CrossRef CAS.
- K. H. Fries, J. D. Driskell, S. Samanta and J. Locklin, Anal. Chem., 2010, 82, 3306–3314 CrossRef CAS PubMed.
- C. Racles, V. Cozan, A. Bele and M. Dascalu, Des. Monomers Polym., 2016, 19, 496–507 CrossRef CAS.
- T. R. Venkatesan, A. A. Gulyakova, P. Frubing and R. Gerhard, IEEE Trans. Dielectr. Electr. Insul., 2018, 25, 2229–2235 CAS.
- F. Kremer and A. Schönhals, Broadband Dielectric Spectroscopy, Springer-Verlag, Berlin, 2003 Search PubMed.
- R. Richert and M. Yang, J. Phys.: Condens. Matter, 2003, 15, S1041 CAS.
- T. R. Venkatesan, D. Smykalla, B. Ploss, M. Wübbenhorst and R. Gerhard, Appl. Phys. A, 2021, 127, 756 Search PubMed.
- F. Tian and Y. Ohki, J. Phys. D: Appl. Phys., 2014, 47, 045311 CAS.
- H. Hammami, M. Arous, M. Lagache and A. Kallel, J. Alloys Compd., 2007, 430, 1–8 CrossRef CAS.
- H. Eckhardt, A. Bose and V. A. Krongauz, Polymer, 1987, 28, 1959–1964 CrossRef CAS.
- M. Bletz, U. Pfeifer-Fukumura, U. Kolb and W. Baumann, J. Phys. Chem. A, 2002, 106, 2232–2236 CrossRef CAS.
- A. B. Kanj, A. Chandresh, A. Gerwien, S. Grosjean, S. Bräse, Y. Wang, H. Dube and L. Heinke, Chem. Sci., 2020, 11, 1404–1410 RSC.
- T. Wismontski-Knittel and V. Krongauz, Macromolecules, 1985, 18, 2124–2126 CrossRef CAS.
- T. J. Feuerstein, R. Müller, C. Barner-kowollik and P. W. Roesky, Inorg. Chem., 2019, 58, 15479–15486 CrossRef CAS.
- J. Ren and H. Tian, Sensors, 2007, 7, 3166–3178 CAS.
- D. Zhang, P. K. Shah, H. R. Culver, S. N. David, J. W. Stansbury and C. N. Bowman, Soft Matter, 2019, 15, 3740–3750 CAS.
- B. Fang, M. Fan, H. Gao, Y. Wang, D. Gao, J. Yu, M. Yin and Y. Dai, J. Mater. Chem., 2024, 12, 14361–14367 CAS.
- D. P. Lydon, P. Li, A. C. Benniston, W. Mcfarlane, R. W. Harrington and W. Clegg, Eur. J. Org. Chem., 2007, 1653–1658 CAS.
- P. Uznanski, Synth. Met., 2000, 109, 281–285 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra, IR soectra, UV vis, TGA, DSC measurements. See DOI: https://doi.org/10.1039/d4py00964a |
| This journal is © The Royal Society of Chemistry 2025 |