Open Access Article
Open Access ArticleInsights into the bulk kinetics of a 2K radical polymerization system based on the copper catalyzed cleavage of diboranes and its perspectives†
F.
Pieringer
 a,
Y.
Catel
b,
R.
Liska
a,
Y.
Catel
b,
R.
Liska
 a and
P.
Knaack
a and
P.
Knaack
 *a
*a
aInstitute of Applied Synthetic Chemistry, TU Wien, 1060 Vienna, Austria. E-mail: patrick.knaack@tuwien.ac.at
bIvoclar AG, Bendererstrasse 2, Schaan 9494, Principality of Liechtenstein
First published on 13th December 2024
Abstract
Many applications of polymer materials, such as adhesives, require a polymerization process at room temperature and ambient atmosphere. In those cases, two-component (2K) systems based on redox initiation truly stand out due to their reliable performance. Herein, we present a deep insight into the polymerization kinetics of a newly developed initiation system based on the copper catalyzed cleavage of diborane compounds, followed by rheology coupled with NIR. The analysis of different diboranes led us to further investigate the diborane concentration dependency and the effects on gel time that can be observed. Furthermore, it is shown that the diborane/Cu system yields polymers with high molecular weight at high double bond conversions. In addition, the perspectives of diborane/Cu initiation for radical polymerization are presented, as various different monomer classes showed excellent reactivity towards polymerization, enabling the great potential of this initiation system for various applications in polymer chemistry.
Introduction
Polymers have transformed numerous industries such as packaging, dental restoration, and coatings, through their versatile applications.1–3 However, the curing of polymers is often an energy-intensive process when using e.g., thermal methods. This has led to significant advancements in developing more energy-efficient polymerization processes such as photopolymerization and redox polymerization.1 Recently, photopolymerization methods have utilized less hazardous monomers, such as methacrylates, offering new possibilities for curing speed and safety.4 Despite these benefits, one limitation of photopolymerization is its inability to deeply cure bulk materials.5,6 To address this issue, the adoption of two-component (2K) systems for curing at room temperature under ambient conditions has gained traction for bulk curing applications.1,7,8 These 2K systems require minimal energy to initiate polymerization compared to conventional thermal curing methods.1 Typically, they consist of a redox pair that starts the polymerization via a redox reaction when the two distinct formulations are mixed. In these systems, one formulation contains an oxidizing agent while the other contains a reducing agent.1 Commonly, tertiary amines are used as reducing agents and (hydro)peroxides used as oxidizing agents. Those initiation systems bear several disadvantages such as high toxicity and thermal lability.9–12 Therefore, alternatives are looked for and are found in initiation systems containing e.g. silanes and metal salts,9 thiourea derivatives,13 ascorbic acid redox systems,14 or recently developed diboranes and copper salts.15 These systems, however, also show disadvantages, such as discoloration, low stability of formulations and often are prone to oxygen inhibition. The reactivity towards polymerization of the bulk monomer using such initiation systems is often characterized using polymerization temperature measurements or pyrometric measurements.1,16 However, those methods only offer limited perspective on the polymerization, leaving out rheological data during the polymerization which is a highly important metric for the investigation of e.g. gel time.17Herein, we present the use of a rheology/IR method to determine rheological as well as chemical (double bond conversion (DBC)) data during the bulk polymerization of radically polymerizable monomers, applying a new and promising amine and peroxide free 2K initiation system.
Results and discussion
Rheology/IR characterization of 2K radical polymerization
Rheological evaluation of polymerizing substrates is an often-used tool in polymer chemistry to understand the kinetics and the progression of the respective polymerization reaction.18 The data acquired from rheology measurements is of immense value to analyze the gel time, which is described in literature as the cross section of the storage modulus G′ and the loss modulus G′′. In very fast reacting systems (e.g. photopolymerization17) this might seem convenient, however, in slower polymerizations methods (e.g. 2K systems) this evaluation causes errors, which is why in this work the steepest increase of G′ was defined as the gel time (tgel).15The combination of rheology/IR is a method to measure NIR of polymerizing resins, while simultaneously acquiring rheological data. The NIR region from 4000–7000 cm−1 is a convenient range for the CH2 band of double bond of radically polymerizable double bonds including methacrylates, acrylates, acrylamides, vinyl esters and styrene.19 The decrease of the area of the respective NIR band (e.g. acrylates ∼6140 cm−1) in relation to the band of the unreacted monomer resin can be evaluated and the double bond conversion can be calculated as a function of time. This simultaneous rheology/IR coupling for polymerization monitoring of 2K systems is not reported in literature to the best of our knowledge.
In this work, a 2K radical polymerization system which was recently developed by our group15 was investigated, applying the rheology/IR method to investigate the perspective that this method can offer for the investigation of linear polymers.
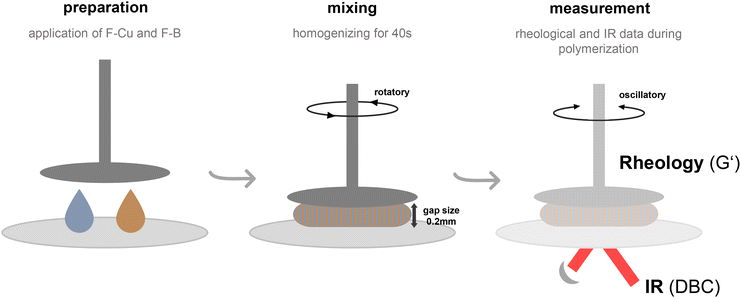
The work-flow for rheology/IR measurements presented in this study would include the preparation of the respective formulations (F–Cu = monomer + copper compound; F–B = monomer + diborane compound) and the application of those to the rheometer. A subsequent mixing phase of 40 s in rotary mode ensures the homogeneous mixing of the 2K systems, which is extremely important for acquiring reproducible results. Thereafter, the rheological measurement starts in oscillatory mode recording G′ and G′′, while the NIR records spectra leading to the evaluation of the DBC (Fig. 1).
Polymerization of BzMA using different diboranes
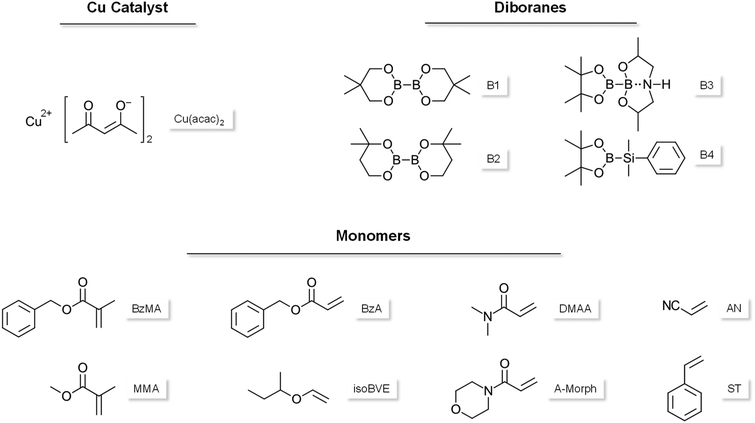
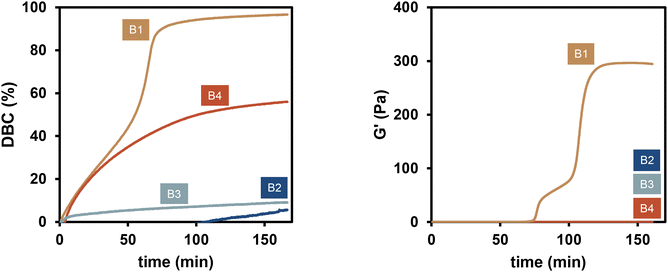
The polymerization of benzylmethacrylate (BzMA) (Scheme 1) was investigated using a 2K system based on the copper catalyzed cleavage of diborane compounds that initiate radical polymerization without the use of peroxides or amines.15 To this end, four diborane compounds depicted in Scheme 1 were evaluated. Formulations containing 3.5 mol% of each diborane were prepared and mixed with a BzMA mixture containing 0.2 mol% Cu(acac)2. The polymerization was monitored via rheology/IR following the general procedure described in Fig. 1.The DBC results clearly showed that the choice of diborane initiator strongly influences the reactivity of the polymerization reaction (Fig. 2). B1 and B2 seem structurally very similar. However, B2, bearing the methyl groups in close proximity to the boron-boron bond, is less reactive due to a higher steric demand. Diborane B3, which proved to be a highly reactive initiator in a previous work,15 showed almost no conversion due to very poor solubility in the monomer. However, silylborane B4 led to more than 50% DBC, owing this to the higher reactive silyl radical.20,21 The highest DBC (96%) was achieved using B1 as the diborane initiator, observing also a very prominent increase of the slope of the DBC due to the Trommsdorff effect.18
The rheology/IR method allows for further investigation of the progression of G′ during the polymerization. Based on the progression of the DBC one might have thought that G′ would follow a similar trend. However, monitoring both characteristic values simultaneously shows that only the polymer derived from B1 shows a significant increase in G′ and a gel time (tgel = 108 min). This is explained by an overall lesser conversion of the monomer using B2, B3 and B4. The molecular weight of the obtained polymers is in the same molecular weight region (Mn ≈ 650 kDa; ESI-Table 1†) for all diboranes, however, due to the less DBC of B2, B3 and B4, no entangling of these few high molecular weight chains can be observed, which would result in a gel point and an increase in G′. The logarithmic plot of the progression of G′ reveals a minor increase of G′ during the polymerization with B3 and B4 (ESI-Fig. 2†).
Due to the high reactivity of B1 towards polymerization of BzMA, this diborane initiator was used in further studies in this work.
Understanding the bulk kinetics of diborane/Cu in BzMA
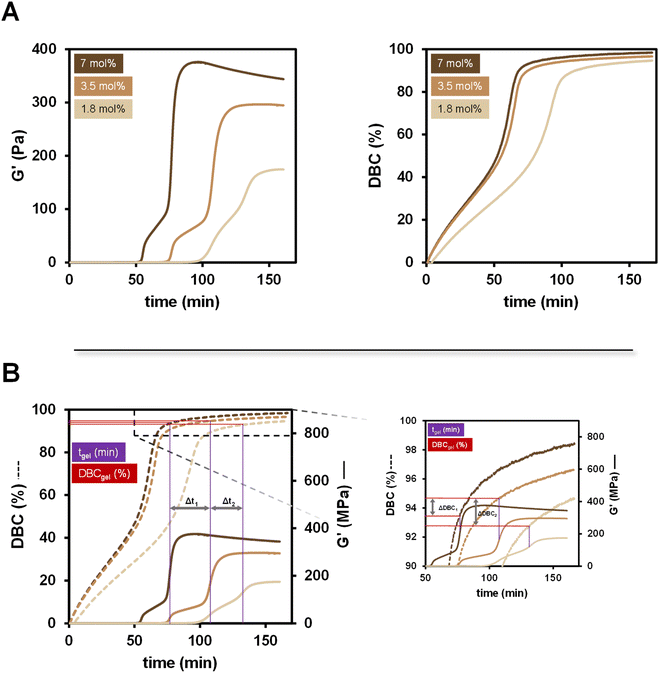
The bulk polymerization of benzylmethacrylate (BzMA) is investigated in detail via rheology/IR in this chapter. The radical polymerization system (RPS) consisting of the diborane B1 and the copper catalyst Cu(acac)2 is used (0.2 mol%) (see Scheme 1) and the concentration of B1 is varied between 1.8 mol%, 3.5 mol% and 7 mol% with respect to the methacrylic double bond. During the polymerization, the rheological data including G′ is monitored and the DBC is simultaneously calculated from the measured NIR spectra (Fig. 3A)Looking at the progression of G′ (Fig. 3A left), the reactivity towards polymerization is strongly depending on the concentration of the diborane initiator. It is shown that 7 mol% diborane initiator leads to an earlier tgel than 3.5 mol% and 1.8 mol%. Interestingly, also the final storage modulus at the end of the polymerization, after the formation of a plateau-like state increases with the initiator concentration. The formulation containing 7 mol% B1 exhibits a decrease of G′ after reaching a maximum, which may be due to an arrangement of the polymer chains caused by the oscillatory movement of the rheometer.22
In all measurements the G′ progression follows the same pattern, consisting of an initial increase, followed by a relaxation, followed by the steepest increase (including tgel) and the formation of a final G′ plateau. A logarithmic plot gives even more insights, as the polymerization process includes even more of these “increase-relaxation” steps (ESI-Fig. 2†). These losses in G′ are explained by arrangements of the polymer chains in the formulation, caused by the oscillation of the rheometer.
Looking at the double bond conversion (Fig. 3A right), it is shown that it is in perfect alignment with existing literature investigating similar bulk polymerizations.18 A higher B1 diborane concentration facilitates the start of the polymerization reaction and therefore leads to higher conversions in a shorter amount of time. It is remarkable that the Trommsdorff effect is visible very clearly resulting in a steeper increase of the DBC curve of the respective measurement.
However, a separate analysis of the rheological (G′) and kinetic (DBC) data is limited in its possibilities. The method of rheology/IR overcomes exactly these limitations by measuring both simultaneously and therefore leading to a direct comparison of rheology and kinetic, allowing for a plotting in one diagram (Fig. 3B). The left diagram shows the progression of the DBC with a direct comparison to the respective G′ progression. The delayed gel times using less initiator are marked (Δt1, Δt2; Fig. 3B left) and the DBC at the respective times is analyzed (DBCgel). Even though different concentrations of initiator are used and the gel times are delayed (Δt1, Δt2), the DBC at the gel point is almost the same for each measurement (ΔDBC1, ΔDBC2; Fig. 3B right). This leads to the conclusion that even though the gel point is delayed using less initiator, a certain threshold in DBC is necessary to lead to a gelling behavior. This was also observed in the investigation of different diboranes previously, as B4 did not cause the formulation to gel even though a DBC of ∼50% was reached (Table 1).
| t gel (min) | DBCgel (%) | DBCend (%) | |
|---|---|---|---|
| 7 mol% | 77 | 94 | 99 |
| 3.5 mol% | 108 | 93 | 96 |
| 1.8 mol% | 133 | 93 | 95 |
Investigating bulk influences on polymerization via rheology/IR
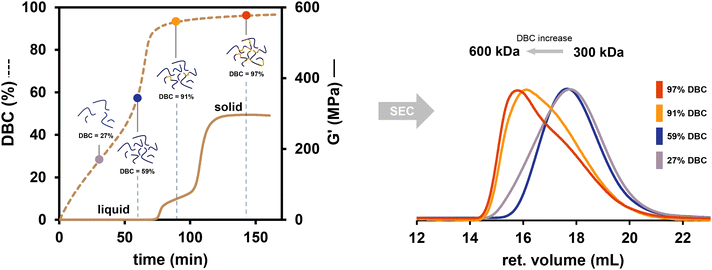
A deep insight into the polymerization of BzMA with B1/Cu(acac)2 is presented using the coupling of rheology/IR with size exclusion chromatography (SEC). The polymerization reaction during rheology/IR measurements was interrupted and was quenched at four different DBCs (27%, 59%, 91% and 97%). The isolated polymers were subsequently analyzed by SEC. This setup led to the diagram depicted in Fig. 4 showing a direct comparison of DBC, G′ and molecular weight. Furthermore, the reproducibility of the method was emphasized as the progression of the DBC for all measurements proved to be congruent (ESI-Fig. 3†).The results show an interconnection between the three parameters DBC, G′ and molecular weight (Fig. 4). Going from low conversions to high conversions in Fig. 4 (left), at 27% DBC the bulk formulation is still a liquid; however, the SEC shows the formation of polymer (Mn = 300 kDa). High molecular weight is reached very fast and at already low conversions, leading to few polymer chains dissolved in a lot of bulk monomer.
When looking at the data point at 59% DBC, the DBC starts to increase steeper due to the Trommsdorff effect, however, the formulation is still liquid (low G′) and the molecular weight of the chains remained the same as with 27% DBC (Fig. 4 right). This is interpreted as a higher quantity of polymer chains dissolved in less monomer (due to higher conversion).
At 91% DBC the measurement already surpassed the Trommsdorff effect phase and has reached a plateau-like progression of the DBC. Additionally, the first increase in G′, which is a result of the Trommsdorff effect due to a high polymerization rate is already surpassed. This leads to very high molecular weight polymer chains with around 600 kDa, which caused the assumption that many of the chains that are present link together forming very high molecular weight chains. This is supported by the SEC trace (Fig. 4 right; yellow) showing a remarkable shoulder leaning to lower molecular weights, which indicates a formation of 600 kDa polymer chains from 300 kDa chains.
At the last data point at 97% DBC the formulation is solid, having already surpassed its gel point (tgel = 108 min). The SEC trace shows a less distinct shoulder, so it can be assumed that higher quantities of the very high molecular weight (Mn = 600 kDa) polymer chains are present in the gelled formulation.
It is remarkable, that using the rheology/IR and SEC coupling allowed us to show that 6% difference in DBC from 91% to 97% causes the formulation to gel. This is explained as the high molecular weight chains completely entangle and very little monomer is left in between them.
Polymerization of various monomer classes using diborane/Cu initiation
Herein, the versatility of the presented initiation method using B1/Cu(acac)2 as 2K initiation system shall be highlighted (3.5 mol% B1; 0.2 mol% Cu(acac)2). Rheology/IR was used to investigate the initiation reactivity towards radical polymerization of different monomer classes, including methacrylates, acrylates, acrylamides, acrylonitrile, styrene and vinyl ethers.The results show expectedly significant differences depending on the type of monomer used for bulk polymerization (Fig. 5A). Interestingly, all the investigated monomers showed polymerization in the rheology/IR measurements. Especially high reactivity was observed for BzA, isoBVE and AN. The progression of the DBC does not develop any increase due to the Trommsdorff effect, as the formulations reach conversions over >99% in a comparatively short time. In the case of BzA, the rate of polymerization Rp is an order of magnitude higher for the acrylate monomer, compared to the respective methacrylate (ESI-Table 3†).
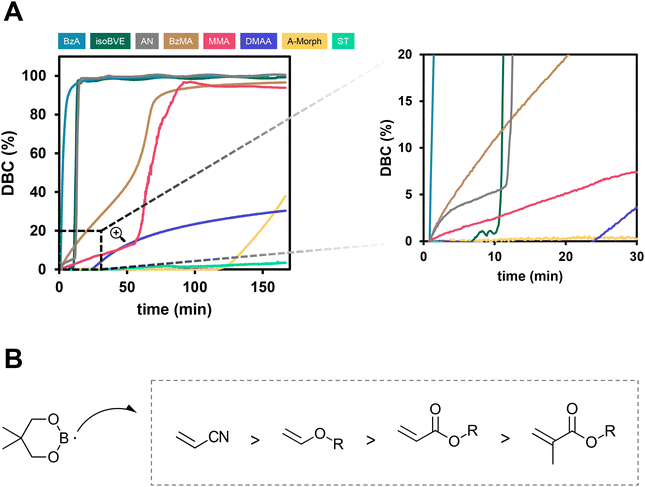 | ||
| Fig. 5 (A) Rheology/IR measurement of the polymerization of BzA, isoBVE, AN, BzMA, MMA, DMAA, A-Morph and ST using 3.5 mol% B1 and 0.2 mol% Cu(acac)2 as initiators. A close-up view gives a more detailed insight into the kinetics at the start of the polymerization reaction. (B) Reactivity of B1 radical towards polymerization of different monomer types based on the evaluation of the polymerization rate Rp (ESI-Table 3†). | ||
Interestingly, the acrylamides, which would be expected to be highly reactive, showed poor DBC with the B1/Cu initiation system. In this regard, it was shown in previous work that amides might interact with the diborane compound, leading to side reactions, which could explain such a loss in reactivity.15 Also, styrene would be expected to show high conversions, however, only up to 4% DBC were reached (Table 2).
| t gel (min) | DBCgel (%) | DBCend (%) | |
|---|---|---|---|
| BzMA | 107 | 92.6 | 96.1 |
| MMA | 66 | 49.1 | 96.9 |
| BzA | 4 | 83.7 | 99.9 |
| DMAA | 69 | 18.2 | 30.4 |
| A-Morph | 127 | 3.5 | 38 |
| AN | 34 | 99.8 | 99.6 |
| ST | >12 h | — | 4 |
| isoBVE | — | — | 99.7 |
When looking at the start of the polymerization reaction in a close-up view (Fig. 5A, right), the different kinetics of polymerization of the monomers can be understood very clearly. The acrylate monomer showed very high conversion rates immediately after the mixing phase, while isoBVE and AN have a delay of about 10 min, after which high conversion rates are reached. The methacrylic monomers BzMA and MMA show low conversion rates right after mixing, however, increasing throughout the polymerization reaction. Overall, the reactivity increases from methacrylates to acrylates, vinyl ethers and acrylonitrile (Fig. 5B)
It was shown that the initiation with B1 and Cu(acac)2 is a very feasible system for various different monomer classes. However, it is strongly limited when applied in styrene and DMAA monomers. Especially the very high reactivity towards the polymerization of acrylates can enable many applications in adhesives industry.
Conclusion
A recently developed 2K system for radical polymerization of methacrylates based on the copper catalyzed cleaving of diborane compounds was investigated in monofunctional bulk formulations using the specialized method of rheology/IR. It was shown that the use of B1 as the diborane compound, in combination with Cu(acac)2 led to very high conversions (96%) and surprisingly high molecular weights (>600 kDa).The kinetics of the polymerization using this initiation system in bulk benzylmethacrylate formulations were investigated applying different concentrations of the diborane initiator B1. A threshold double bond conversion (DBC) was determined at around 90% that causes a very steep increase in storage modulus due to a shift in molecular weight of the polymer chains. This shift is likely interpreted as an addition of the polymer chains to one another, as an increase was observed from 300 kDa to 600 kDa.
Furthermore, a variety of radically polymerizable monomers proved to be highly reactive towards polymerization using the diborane/Cu 2K system. This includes acrylonitrile, vinyl ethers, acrylates and methacrylates. Especially acrylates showed a very fast polymerization right after mixing, potentially enabling applications in adhesion industries.
To conclude, diborane/Cu 2K initiation was investigated in detail regarding its kinetics and DBC as well as G′ progression during the polymerization reaction. Rheology/IR proved to be the ideal tool for the determination of crucial correlations that require such a specialized method in this context. In addition, the spectrum of polymerizable monomers showcased the potential of this initiation system and displays its potential also in industrial applications.
Experimental part
Synthesis of bis(3-methylbutane-1,3-diol)diborane (B2)
A solution of B2(NMe2)4 (1 eq., 4.64 mmol) in dry Et2O (20 mL) was prepared inside a glovebox. 3-Methylbutane-1,3-diol (2.22 eq., 10.3 mmol) in 20 mL dry Et2O were added under argon atmosphere. The reaction mixture was stirred at room temperature for 12 h. Thereafter, a 1 M solution of HCl in Et2O (4 eq.) was added and the mixture was stirred for another 6 h at room temperature. The reaction mixture was filtered and all volatiles were removed in vacuo. The product was recrystallized from n-hexane to yield white solid needles (m.p. 62.3 °C).2311B NMR (128 MHz, chloroform-d) δ 27.87 ppm. 1H NMR (200 MHz, chloroform-d) δ 4.06–3.93 ppm (m, 1H), 1.85–1.72 ppm (m, 1H), 1.31 ppm (s, 3H) (ESI-Fig. 5 and 6†).Rheology/IR measurements
Rheology measurements were performed with a real time-near infrared rheometer consisting of an Anton Paar MCR 302 WESP with a P-PTD 200/GL Peltier glass plate and a PP25 measuring system coupled with a Bruker Vertex 80 FTIR spectrometer with an MCT detector. 100 mg of each respective 2K-formulation was placed at the center of the glass plate, which was covered with a PE tape and the measurements were conducted at 25 °C with an Anton Paar H-PTD 200 furnace and a gap size of 200 μm. The rheology stamp was rotated for 40 s to ensure homogeneous mixture of the 2K formulations. The polymerizing sample was sheered with a strain of 1% and a frequency of 1 Hz. The NIR-spectra were evaluated using OPUS 7.0 software, integrating the signals at a wavelength of ∼6140 cm−1. DBC was determined as the ratio of peak areas at the start and each respective time during the measurement.SEC
SEC measurements were carried out in THF on a Waters GPC using 3 columns (Styragel HR 0.5, Styragel HR 3 and Styragel HR 4) and a Waters 2410 RI detector, a UV Detector Module 2550 for TDA 305 and a VISCOTEK SEC-MALS 9 light scattering detector. A calibration with polystyrene standards (375–177000 Da) was used to determine the molecular weight of the polymers.Author contributions
Florian Pieringer: data curation, formal analysis, investigation, methodology, visualization, writing – original draft, writing – review & editing; Yohann Catel: conceptualization, supervision, project administration; Robert Liska: conceptualization, supervision, project administration; Patrick Knaack: conceptualization, supervision, project administration.Data availability
The data that support the findings of this study are available from the corresponding author, Patrick Knaack, upon reasonable request.Conflicts of interest
The authors declare no competing financial interest.References
- P. Garra, C. Dietlin, F. Morlet-Savary, F. Dumur, D. Gigmes, J.-P. Fouassier and J. Lalevée, Redox two-component initiated free radical and cationic polymerizations: Concepts, reactions and applications, Prog. Polym. Sci., 2019, 94, 33–56, DOI:10.1016/j.progpolymsci.2019.04.003.
- R. A. Guggenberger, R. Hecht, M. Ludsteck and G. Raia, Stippschild-Boxler Twocomponent self-adhesive dental composition, process of production and use thereof, 10932994B2, 2015.
- Z. Y. Duymus, N. D. Yanikoğlu and M. Alkurt, Evaluation of the flexural strength of dual-cure composite resin cements, J. Biomed. Mater. Res., Part B, 2013, 101B(5), 878–881, DOI:10.1002/jbm.b.32892.
- G. Peer, A. Eibel, C. Gorsche, Y. Catel, G. Gescheidt, N. Moszner and R. Liska, Ester-Activated Vinyl Ethers as Chain Transfer Agents in Radical Photopolymerization of Methacrylates, Macromolecules, 2019, 52(7), 2691–2700, DOI:10.1021/acs.macromol.9b00085.
- S. C. Ligon, B. Husár, H. Wutzel, R. Holman and R. Liska, Strategies to reduce oxygen inhibition in photoinduced polymerization, Chem. Rev., 2014, 114(1), 557–589, DOI:10.1021/cr3005197 From NLM.
- C. Haslinger, L. P. Leutgeb, M. Haas, S. Baudis and R. Liska, Synthesis and Photochemical Investigation of Tetraacylgermanes, ChemPhotoChem, 2022, 6(10), e202200108, DOI:10.1002/cptc.202200108.
- A. Arar, H. Mokbel, F. Dumur and J. Lalevée, High Performance Redox Initiating Systems Based on the Interaction of Silane with Metal Complexes: A Unique Platform for the Preparation of Composites, Molecules, 2020, 25(7), 1602 CrossRef PubMed.
- P. Garra, F. Morlet-Savary, B. Graff, F. Dumur, V. Monnier, C. Dietlin, D. Gigmes, J. P. Fouassier and J. Lalevée, Metal Acetylacetonate–Bidentate Ligand Interaction (MABLI) as highly efficient free radical generating systems for polymer synthesis, Polym. Chem., 2018, 9(12), 1371–1378, 10.1039/C8PY00238J.
- A. Arar, A. A. Mousawi, C. Boyadjian, P. Garra, J. P. Fouassier and J. Lalevee, Diphenylsilane-Manganese Acetylacetonate Redox Initiating Systems: Toward Amine-Free and Peroxide-Free Systems, Macromol. Chem. Phys., 2020, 221(11), 2000058, DOI:10.1002/macp.202000058.
- D. S. Achilias and I. Sideridou, Study of the effect of two BPO/amine initiation systems on the free-radical polymerization of MMA used in dental resins and bone cements, J. Macromol. Sci., Part A:Pure Appl. Chem., 2002, 39 (12), 1435–1450, DOI:10.1081/MA-120016045.
- D. S. Achilias and I. D. Sideridou, Kinetics of the benzoyl peroxide/amine initiated free-radical polymerization of dental dimethacrylate monomers: Experimental studies and mathematical modeling for TEGDMA and Bis-EMA, Macromolecules, 2004, 37(11), 4254–4265, DOI:10.1021/ma049803n Scopus.
- I. D. Sideridou, D. S. Achilias and O. Karava, Reactivity of Benzoyl Peroxide/Amine System as an Initiator for the Free Radical Polymerization of Dental and Orthopaedic Dimethacrylate Monomers: Effect of the Amine and Monomer Chemical Structure, Macromolecules, 2006, 39(6), 2072–2080, DOI:10.1021/ma0521351.
- Y. Catel, J. Angermann, B. Grob, P. Fässler, I. Lamparth and T. Schnur, Acylthiourea oligomers as promising reducing agents for dimethacrylate-based two-component dental materials, Dent. Mater., 2023, 39(10), 886–893, DOI:10.1016/j.dental.2023.07.007.
- P. Garra, A. Kermagoret, A. Al Mousawi, F. Dumur, D. Gigmes, F. Morlet-Savary, C. Dietlin, J. P. Fouassier and J. Lalevée, New copper(I) complex based initiating systems in redox polymerization and comparison with the amine/benzoyl peroxide reference, Polym. Chem., 2017, 8(28), 4088–4097, 10.1039/C7PY00726D.
- F. Pieringer, K. Knaipp, R. Liska, N. Moszner, Y. Catel, G. Gescheidt and P. Knaack, Boron–boron bonds: boldly breaking boundaries towards amine- and peroxide-free 2K radical polymerization, Polym. Chem., 2024, 15, 3127–3138, 10.1039/D4PY00445K.
- P. Garra, F. Dumur, M. Nechab, F. Morlet-Savary, C. Dietlin, B. Graff, E. P. Doronina, V. F. Sidorkin, D. Gigmes, J.-P. Fouassier and J. Lalevée, Peroxide-Free and Amine-Free Redox Free Radical Polymerization: Metal Acetylacetonates/Stable Carbonyl Compounds for Highly Efficient Synthesis of Composites, Macromolecules, 2018, 51(16), 6395–6404, DOI:10.1021/acs.macromol.8b01360.
- C. Gorsche, R. Harikrishna, S. Baudis, P. Knaack, B. Husar, J. Laeuger, H. Hoffmann and R. Liska, Real Time-NIR/MIR-Photorheology: A Versatile Tool for the in Situ Characterization of Photopolymerization Reactions, Anal. Chem., 2017, 89(9), 4958–4968, DOI:10.1021/acs.analchem.7b00272.
- Y. Suzuki, R. Mishima, E. Kato and A. Matsumoto, Analysis of the glass effect and Trommsdorff effect during bulk polymerization of methyl methacrylate, ethyl methacrylate, and butyl methacrylate, Polym. J., 2023, 55(3), 229–238, DOI:10.1038/s41428-022-00746-5.
- J. W. Stansbury and S. H. Dickens, Determination of double bond conversion in dental resins by near infrared spectroscopy, Dent. Mater., 2001, 17(1), 71–79, DOI:10.1016/S0109-5641(00)00062-2.
- M. Suginome and Y. Ito, Transition-Metal-Catalyzed Additions of Silicon–Silicon and Silicon–Heteroatom Bonds to Unsaturated Organic Molecules, Chem. Rev., 2000, 100(8), 3221–3256, DOI:10.1021/cr9902805.
- C. Kleeberg and C. Borner, On the Reactivity of Silylboranes toward Lewis Bases: Heterolytic B–Si Cleavage vs. Adduct Formation, Eur. J. Inorg. Chem., 2013, 2013(15), 2799–2806, DOI:10.1002/ejic.201300119.
- L. Sangroniz, M. Fernández and A. Santamaria, Polymers and rheology: A tale of give and take, Polymer, 2023, 271, 125811, DOI:10.1016/j.polymer.2023.125811.
- W. Clegg, T. R. F. Johann, T. B. Marder, N. C. Norman, A. Guy Orpen, T. M. Peakman, M. J. Quayle, C. R. Rice and A. J. Scott, Platinum-catalysed 1,4-diboration of 1,3-dienes, J. Chem. Soc., Dalton Trans., 1998,(9), 1431–1438, 10.1039/A800108A.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4py01102c |
| This journal is © The Royal Society of Chemistry 2025 |