Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Novel hyperbranched polymers from transfer-dominated branching radical telomerisation (TBRT) of diacrylate taxogens†
Samuel
Mckeating
ab,
Corinna
Smith
ab,
Oliver
Penrhyn-Lowe
ab,
Sean
Flynn
ab,
Stephen
Wright
ab,
Pierre
Chambon
ab,
Andrew
Dwyer
ab and
Steve
Rannard
 *ab
*ab
aDepartment of Chemistry, University of Liverpool, Crown Street, L69 7ZD, UK. E-mail: srannard@liv.ac.uk
bMaterials Innovation Factory, University of Liverpool, Crown Street, L69 7ZD, UK
First published on 21st February 2025
Abstract
Transfer-dominated Branching Radical Telomerisation (TBRT) allows the free radical homopolymerisation of multi-vinyl monomers whilst avoiding gelation. When using dimethacrylates, high molecular weight branched polyesters are formed with complete reaction of all vinyl functional groups. Here, we present the first report of TBRT using diacrylates and compare their homopolymerisation with that of analogous dimethacrylates. To establish very high molecular weight polyesters, diacrylate TBRT reactions required elevated temperatures. The reasons for this are investigated, including the use of model linear telomerisations of analogous mono-vinyl methacrylate and acrylate monomers.
Introduction
Transfer-dominated branching radical telomerisation (TBRT) has recently been shown to be an effective strategy for the homopolymerisation of multi-vinyl methacrylates.1 As a chain-growth process employing conventional free radical chemistries, TBRT provides ready and scalable access to a considerable array of new polymeric structures containing backbone chemistries typically associated with step-growth polymers (for example, polyesters from dimethacrylates).2Since its initial report in 2020, TBRT has been employed to synthesise soluble, high molecular weight, highly branched polymers and new copolymers with a range of physical properties and potential applications.3 For example, the production of functional materials with polyester backbones, with weight-average molecular weight (Mw) values greater than 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, has been consistently achieved by TBRT.4
000 g mol−1, has been consistently achieved by TBRT.4
TBRT differs from other approaches that have aimed to homopolymerise multi-vinyl monomers which require quenching at vinyl conversions <80% to avoid gelation.5,6 As a telomerisation reaction,7 monomers are more appropriately referred to as taxogens,8 and multi-vinyl taxogens (MVTs) employed in TBRT have included bisphenol-A dimethacrylate, N,N-bis(methacryloxyethyl) methylamine, 1,6-hexanediol dimethacrylate, 1,12-dodecanediol dimethacrylate, and ethylene glycol dimethacrylate.2,3,9,10 Thiols are used in high concentrations and are known as telogens rather than the conventional term ‘chain transfer agent’ when used at relatively low levels.8
Whilst methacrylic MVTs have been instrumental in the determination of many of the mechanistic features of the polymerisation, and characterisation of the resulting TBRT polymer architectures,11 MVTs containing acrylate functionality are also of interest. Acrylic and methacrylic monomers have remarkably different reactivity given their very small structural differences.12,13 During radical polymerisation, acrylic monomers form secondary radicals as a propagating end group, as opposed to methacrylic monomers which form more stable tertiary radicals.14 As a result, the free radical homopolymerisations of acrylic monomers proceed at a greater rate compared to their methacrylic counterparts.15
Under ideal TBRT conditions the molar ratio of MVT to telogen in the final polymer is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
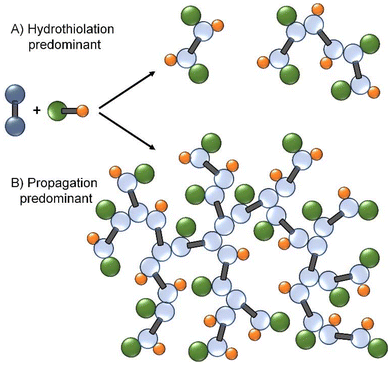
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and thiols may be considered as side-chains within the nominal polymer repeat structure.3 Thiols are excellent Michael donors, due to the polarisable nature of the sulfur atom, and are known to undergo radical-mediated thiol–ene addition reactions; examples have been reported under photo- and thermal initiation.16,17 Within a TBRT diacrylate homopolymerisation, one would therefore expect the observed vinyl group consumption to result from a significant competition between radical-mediated thiol-acrylate Micheal addition and propagation to form telomer chains. If the balance of this competition favours the hydrothiolation reaction, propagation will be highly restricted, possibly leading to limited growth in molecular weight, Fig. 1A. If propagation is favoured, the TBRT of diacrylate MVTs may allow the formation of highly branched polyesters in a manner similar to those already reported for dimethacrylates, Fig. 1B.
1, and thiols may be considered as side-chains within the nominal polymer repeat structure.3 Thiols are excellent Michael donors, due to the polarisable nature of the sulfur atom, and are known to undergo radical-mediated thiol–ene addition reactions; examples have been reported under photo- and thermal initiation.16,17 Within a TBRT diacrylate homopolymerisation, one would therefore expect the observed vinyl group consumption to result from a significant competition between radical-mediated thiol-acrylate Micheal addition and propagation to form telomer chains. If the balance of this competition favours the hydrothiolation reaction, propagation will be highly restricted, possibly leading to limited growth in molecular weight, Fig. 1A. If propagation is favoured, the TBRT of diacrylate MVTs may allow the formation of highly branched polyesters in a manner similar to those already reported for dimethacrylates, Fig. 1B.
Here, we explore the potential for forming new polymers from the homopolymerisation of diacrylates using TBRT approaches. Comparative reactions with analogous dimethacrylate MVTs have been conducted, and we present studies aimed at enabling very high molecular weight branched polymer synthesis that form the basis for future studies.
Results and discussion
TBRT of acrylic and methacrylic multi-vinyl taxogens
Numerous TBRT polymerisations have demonstrated the effectiveness of 1-dodecanethiol (DDT) under TBRT conditions, therefore DDT was selected for this initial study of diacrylate homopolymerisation.1–4 Four MVTs, two diacrylates and two dimethacrylates, were selected for comparative TBRT reactions with DDT; namely, 1,4-butanediol diacrylate (BDA), 1,6-hexanediol diacrylate (HDA), 1,4-butanediol dimethacrylate (BDMA), and 1,6-hexanediol dimethacrylate (HDMA), Scheme 1. The acrylate/methacrylate comparative pairs chosen here enable the impact of the different reactivities to be studied directly.The TBRT conditions were standardised across all reactions of BDA, BDMA, HDA, and HDMA for consistency. In summary, the homopolymerisations were conducted in toluene (50 wt% solids content), using α,α′-azoisobutyronitrile as the radical source (AIBN, 1.5 mol% relative to total vinyl groups), and at a temperature of 70 °C. The concentration of telogen required to avoid gelation and create a soluble high molecular weight polymer is not immediately clear for unstudied MVTs, therefore the ratio of MVT to telogen ([MVT]0/[DDT]0) was varied in each reaction. In this manner, the limiting [MVT]0/[DDT]0 gel point ratios may also be determined. To accurately report the reagent ratios, 1H nuclear magnetic resonance spectroscopy (NMR) was performed on all reactions prior to initiation, ESI Fig. S1–7.†
Under TBRT conditions, the expected differences in reactivity of the diacrylate and dimethacrylate MVTs were immediately apparent at 70 °C, Table 1. Triple-detection size exclusion chromatography (TD-SEC, ESI Fig. S8†) showed systematic increases in [MVT]0/[DDT]0 during the TBRT of BDMA led to a steadily increasing weight-average molecular weight (Mw) as reported previously for dimethacrylate MVTs, ESI Fig. S9.† When BDA was subjected to the same study, it was clear that the purified recovered polymers showed far lower Mw values than the analogous BDMA reactions. For example, reactions conducted at [MVT]0/[DDT]0 = 0.60 showed Mw values of 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g mol−1 and 8700 g mol−1 for BDMA and BDA respectively. As the ratio increased, the resulting polymers followed this trend ([MVT]0/[DDT]0 = 0.70, BDMA: Mw = 468
200 g mol−1 and 8700 g mol−1 for BDMA and BDA respectively. As the ratio increased, the resulting polymers followed this trend ([MVT]0/[DDT]0 = 0.70, BDMA: Mw = 468![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 g mol−1; BDA: Mw = 27
900 g mol−1; BDA: Mw = 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 g mol−1). The limiting [MVT]0/[DDT]0 gel point ratios were also substantially different with BDMA reaching an observable gel point at [MVT]0/[DDT]0 > 0.72 (and <0.75) and BDA progressing through to [MVT]0/[DDT]0 ratios up to 0.82 (and <0.85) whilst avoiding gelation.
300 g mol−1). The limiting [MVT]0/[DDT]0 gel point ratios were also substantially different with BDMA reaching an observable gel point at [MVT]0/[DDT]0 > 0.72 (and <0.75) and BDA progressing through to [MVT]0/[DDT]0 ratios up to 0.82 (and <0.85) whilst avoiding gelation.
| MVT | Temperature (°C) | 1H NMR | TD-SECd | |||||
|---|---|---|---|---|---|---|---|---|
| [MVT]0/[DDT]0a | [MVT]F/[DDT]F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
M w (g mol−1) | M n (g mol−1) | Đ | α | dn/dc | ||
| a Calculated from 1H NMR analysis of the reaction mixture at t = 0 (examples ESI Fig. S1 and S5†). b Determined by 1H NMR analysis of purified polymers (examples ESI Fig. S3, S4 and S7†). c Formation of a microgel indicated by observed pressure during sample filtration prior to TD-SEC analysis, values shown are from the filtered sample; gel formation confirmed by visual analysis of the crude reaction. d Determined by triple-detection size exclusion chromatography using a 0.5% v/v TEA/THF eluent system (examples ESI Fig. S14 and S15†). e All polymerisations achieved >99% vinyl group consumption as determined by 1H NMR (CDCl3) analysis of crude samples of the reaction mixtures after 24 h (ESI Fig. S2 and S6†). | ||||||||
| BDMA | 70 | 0.50 | 0.77 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
3.45 | 0.44 | 0.067 |
| 0.60 | 0.82 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
3.24 | 0.43 | 0.068 | ||
| 0.65 | 0.93 | 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
4.98 | 0.36 | 0.071 | ||
| 0.68 | 0.90 | 210![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
12.29 | 0.43 | 0.069 | ||
| 0.70 | 1.01 | 468![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
35.26 | 0.39 | 0.070 | ||
| 0.72 | 0.95 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
50.47 | 0.40 | 0.071 | ||
| 0.75 | Gel formedc | |||||||
| BDA | 70 | 0.60 | 0.94 | 8700 | 3700 | 2.35 | 0.21 | 0.083 |
| 0.65 | 0.90 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
5100 | 2.43 | 0.29 | 0.084 | ||
| 0.70 | 0.98 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
5000 | 5.46 | 0.29 | 0.084 | ||
| 0.75 | 0.97 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
6100 | 5.20 | 0.36 | 0.087 | ||
| 0.80 | 0.96 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
5700 | 7.18 | 0.33 | 0.088 | ||
| 0.82 | 0.99 | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
7200 | 8.93 | 0.35 | 0.088 | ||
| 0.85 | Gel formedc | |||||||
| BDA | 100 | 0.80 | 0.93 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
2020 | 9.46 | 0.35 | 0.078 |
| 0.83 | 0.92 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
2150 | 9.49 | 0.27 | 0.085 | ||
| 0.89 | 0.99 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
2510 | 12.67 | 0.28 | 0.086 | ||
| 0.95 | 1.03 | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
4950 | 14.51 | 0.30 | 0.084 | ||
| 1.01 | 1.00 | 194![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
6520 | 29.86 | 0.37 | 0.083 | ||
| 1.05 | 0.99 | 568![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
9490 | 59.86 | 0.39 | 0.083 | ||
| 1.09 | Microgel fractionc | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 147 147![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 430 430 |
41.75 | 0.47 | |||
| 1.15 | Gel formedc | |||||||
| HDMA | 70 | 0.50 | 0.85 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.94 | 0.40 | 0.083 |
| 0.55 | 0.93 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
2.29 | 0.41 | 0.083 | ||
| 0.60 | 0.89 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
9500 | 3.76 | 0.39 | 0.085 | ||
| 0.65 | 0.91 | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
5.66 | 0.42 | 0.084 | ||
| 0.67 | 0.94 | 104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410 410 |
10.08 | 0.40 | 0.087 | ||
| 0.71 | 1.00 | 493![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
24.19 | 0.41 | 0.086 | ||
| 0.73 | 0.98 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 202 202![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
40.21 | 0.39 | 0.087 | ||
| 0.76 | Gel formedc | |||||||
| HDA | 70 | 0.60 | 0.82 | 5800 | 3000 | 1.93 | 0.30 | 0.081 |
| 0.65 | 0.90 | 8400 | 3400 | 2.47 | 0.28 | 0.083 | ||
| 0.70 | 0.87 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
5300 | 3.25 | 0.35 | 0.082 | ||
| 0.75 | 0.95 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
6000 | 2.82 | 0.35 | 0.076 | ||
| 0.77 | 0.95 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
7000 | 6.07 | 0.36 | 0.076 | ||
| 0.80 | 0.98 | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
6300 | 8.70 | 0.35 | 0.077 | ||
| 0.83 | Gel formedc | |||||||
| HDA | 100 | 0.79 | 0.91 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
2370 | 6.20 | 0.43 | 0.080 |
| 0.85 | 0.95 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4490 | 4.23 | 0.30 | 0.082 | ||
| 0.90 | 0.97 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
4030 | 5.73 | 0.34 | 0.078 | ||
| 0.93 | 0.99 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1990 | 22.36 | 0.31 | 0.080 | ||
| 0.99 | 0.97 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
7020 | 12.85 | 0.33 | 0.079 | ||
| 1.03 | 1.00 | 523![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 020 020 |
47.47 | 0.36 | 0.079 | ||
| 1.08 | Microgel fractionc | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 786 786![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
271![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
13.96 | 0.39 | — | ||
| 1.11 | Gel formedc | |||||||
TBRT reactions utilising HDMA and HDA showed the same trend, with HDMA achieving Mw > 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 at [MVT]0/[DDT]0 as low as 0.65 and HDA failing to match these values at [MVT]0/[DDT]0 ≤ 0.80. Very high molecular weight polymer (Mw > 2
000 g mol−1 at [MVT]0/[DDT]0 as low as 0.65 and HDA failing to match these values at [MVT]0/[DDT]0 ≤ 0.80. Very high molecular weight polymer (Mw > 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) was able to be formed using HDMA prior to reaching the limiting [MVT]0/[DDT]0 gel point ratio but purified polymers with Mw < 60
000 g mol−1) was able to be formed using HDMA prior to reaching the limiting [MVT]0/[DDT]0 gel point ratio but purified polymers with Mw < 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 were only achievable with HDA before further reductions of DDT led to network formation. Under these reaction conditions, p(DDT-BDMA), p(DDT-BDA), p(DDT-HDMA), and p(DDT-HDA) all exhibited [MVT]F/[DDT]F ratios close to 1, when appreciable molecular weight material was formed, strongly suggesting near ideal structures and the absence of significant cyclisation.11
000 g mol−1 were only achievable with HDA before further reductions of DDT led to network formation. Under these reaction conditions, p(DDT-BDMA), p(DDT-BDA), p(DDT-HDMA), and p(DDT-HDA) all exhibited [MVT]F/[DDT]F ratios close to 1, when appreciable molecular weight material was formed, strongly suggesting near ideal structures and the absence of significant cyclisation.11
Kinetic analysis of TBRTs utilising structurally similar acrylic and methacrylic taxogens
As previously reported, the disappearance of 1H NMR resonances relating to vinyl functionality is conventionally considered as being indicative of monomer being ‘converted’ to polymer within mono-vinyl polymerisations; however, within the homopolymerisation of multi-vinyl monomers a decreasing vinyl group concentration is not necessarily related to polymer formation or molecular weight growth.4 With two vinyl functionalities within a divinyl monomer and the presence of pendant unsaturation within the growing polymer, it is possible that vinyl groups undergo reactions that are not related to propagation. This is especially true under TBRT conditions where the formation of structures with a degree of polymerisation of just one vinyl group (DP1) is critical to avoid gelation, Fig. 1. The term ‘vinyl group consumption’ is therefore used here.To compare the TBRT polymerisation of diacrylates and dimethacrylates in finer detail, kinetic studies were undertaken for each of the MVTs using the conditions described above, Table 1.
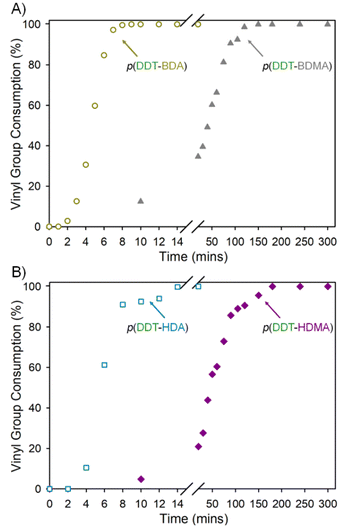
As mentioned previously, acrylates are known to react faster than methacrylates,17 and this was also seen in the consumption of the different vinyl functionalities under TBRT conditions, Fig. 2. When monitored by 1H NMR, all TBRT polymerisations reached >99% vinyl group consumption with BDA achieving this faster than HDA and BDMA reacting faster than HDMA. Full disappearance of vinyl resonances within 3.5 hours of initiation is entirely consistent with previous reports; for example, EGDMA exhibits >99% vinyl group consumption within 3 hours when using DDT as the telogen.1
In stark contrast, the TBRT reactions utilising the diacrylate taxogens showed no detectable vinyl resonances within the 1H NMR spectra of samples from BDA and HDA polymerisations after 8 minutes and 14 minutes, respectively, Fig. 2. As mentioned above, consumption of acrylic vinyl functionality may progress via two routes and it is clear from the molecular weight data, Table 1, that reactions between vinyl groups (i.e. propagation) may be playing a lesser role compared to hydrothiolation. The rapid formation of DP1 structures within TBRT polymerisations of diacrylates would, however, have two effects. Firstly, and even in the absence of propagation, each DP1 formed removes a vinyl group, even in the early stages of the reaction, thereby decreasing the concentration of vinyl groups overall, and secondly, rapid DP1 structure formation minimises the potential for intermolecular branching reactions and high molecular weight polymers would only be formed if the rate of hydrothiolation can be moderated. Under these conditions, this is readily achieved by reducing the DDT telogen concentration relative to MVT.
The impact of reaction temperature on the TBRT of diacrylate taxogens
Studies of the effect of temperature on the TBRT of dimethacrylates showed that a thermally driven increase in chain transfer coefficient (CT) allowed a reduction in the concentration of thiol telogen to be used, with a subsequent increase in [MVT]0/[DDT]0 ratios that would successfully avoid gelation.18 Essentially, the rate of chain transfer increased relative to the rate of propagation, therefore shorter telomer formation was preferred and intermolecular reactions were reduced; at any given [MVT]0/[DDT]0 ratio, lower molecular weight TBRT polymers were formed at higher temperatures.18To establish the impact of temperature on diacrylate TBRT, the reactions of BDA and HDA with DDT were repeated at 100 °C. The competing reactions are potentially more complex in this scenario as hydrothiolation, chain transfer, and propagation all need to be considered.
As seen with the TBRT of dimethacrylates at elevated temperature, the limiting [MVT]0/[DDT]0 gel point ratios were again considerably increased when conducting the diacrylate TBRT reactions at 100 °C, Table 1. Homopolymerisations of BDA were successful at [MVT]0/[DDT]0 values <1.09 (previously <0.85 at 70 °C) and the TBRT of HDA avoided gelation at [MVT]0/[DDT]0 < 1.08 (previously <0.83 at 70 °C).
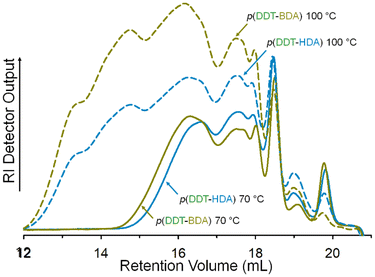
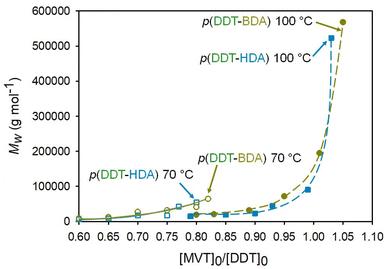
By conducting the diacrylate TBRTs at 100 °C and accessing the higher [MVT]0/[DDT]0 ratios, much higher molecular weight branched polyesters were achievable; for the homopolymerisations of BDA and HDA it was possible to achieve Mw > 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 at comparable [MVT]0/[DDT]0 ratios of 1.05 and 1.03 respectively, representing values approximately an order of magnitude higher than those achievable at 70 °C, Table 1 & Fig. 3.
000 g mol−1 at comparable [MVT]0/[DDT]0 ratios of 1.05 and 1.03 respectively, representing values approximately an order of magnitude higher than those achievable at 70 °C, Table 1 & Fig. 3.
As molecular weight evolution within TBRT is governed by the intermolecular reaction of pendant vinyl groups,1 it is clear that the elevated reaction temperature enables a higher degree of propagation. In addition, this must be accompanied with a concomitant higher rate of chain transfer to avoid gelation at lower concentrations of DDT. The behaviour of both BDA and HDA under TBRT conditions is remarkably similar in this respect, Fig. 4.
Investigating the temperature dependence of linear telomerisations using mono-vinyl acrylate taxogens
In our previous report of the impact of temperature on the TBRT of dimethacrylate taxogens, branched polyesters with comparable Mw values were ultimately achievable even though higher [MVT]0/[DDT]0 ratios (reduced telogen) were able to be employed.18 For the formation of branched polyesters from diacrylate taxogens it is evident that far higher Mw polymers are available when conducting the polymerisations at elevated temperature and reduced DDT concentration. It is not clear, however, whether this is due to a considerable increase in CT relative to hydrothiolation, therefore linear telomerisation studies of mono-vinyl taxogens were conducted over a range of reaction temperatures (70 °C–100 °C).Both n-butyl acrylate (BuA) and n-butyl methacrylate (BuMA) mono-vinyl taxogens were employed in the linear telomerisation study as contrasting, or similar, behaviour was expected to be readily observed.2,19 Each reaction was carried out at 50 wt% solids in toluene and a [Taxogen]0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [DDT]0 molar ratio of 2
[DDT]0 molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
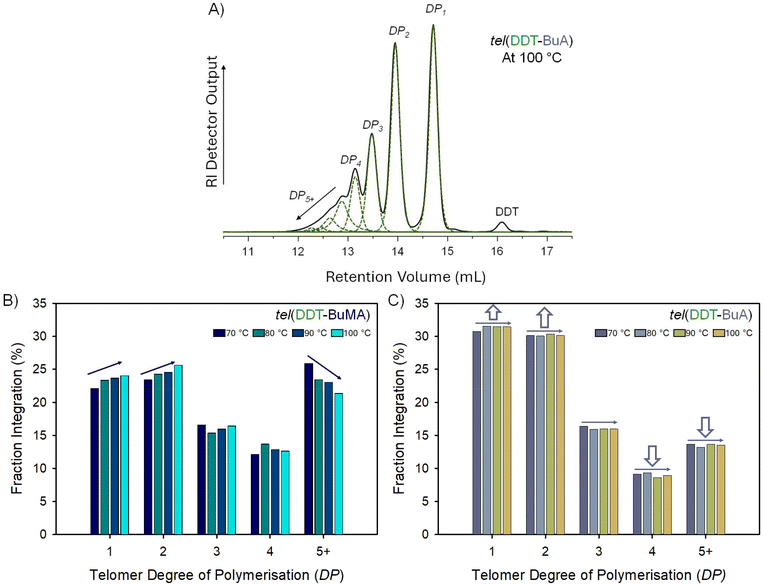
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with AIBN (1.5 mol% relative to vinyl group), ESI Fig. S10–12, Table S1 and Scheme S1.† The resulting telomer products were characterised by oligomer-SEC with each multimodal distribution subjected to deconvolution analysis using a Gaussian-Loren Cross function to establish the proportions of each telomeric species relative to the total chain length distribution, ESI Fig. S13 and 14.† To compensate for the reduced resolution of peaks at lower retention volume, the data was grouped into one peak representative of telomer chains with DP5 or higher and the corresponding total area was integrated, Fig. 5A.
1 with AIBN (1.5 mol% relative to vinyl group), ESI Fig. S10–12, Table S1 and Scheme S1.† The resulting telomer products were characterised by oligomer-SEC with each multimodal distribution subjected to deconvolution analysis using a Gaussian-Loren Cross function to establish the proportions of each telomeric species relative to the total chain length distribution, ESI Fig. S13 and 14.† To compensate for the reduced resolution of peaks at lower retention volume, the data was grouped into one peak representative of telomer chains with DP5 or higher and the corresponding total area was integrated, Fig. 5A.
Comparison of the telomeric products tel(DDT-BuMA) and tel(DDT-BuA) from the temperature study showed a number of trends, Fig. 5B & C, ESI Table S2 and Fig. S15.† Firstly, as the temperature was increased within the tel(DDT-BuMA) synthesis, a general increase in the proportion of DP1 and DP2 structures was observed with a subsequent decrease in DP5+ telomers. This is consistent with an increasing CT, the enhancement in chain transfer relative to propagation, and the formation of shorter chain species. This is entirely consistent with our previous reports.2,4,18
When considering the formation of tel(DDT-BuA)at different temperatures, it is clear that DP1 and DP2 structures contribute a far higher percentage of the overall distribution than their equivalents within the tel(DDT-BuMA), and structures >DP4 are dramatically reduced in number. Far more striking is the lack of any noticeable impact across a 30 °C increase in reaction temperature on the overall ratio of telomers formed. Not only do the relative proportions of each telomer chain species stay essentially unchanged in samples generated at each temperature, there is also negligible variation in absolute percentage of these species between samples, Fig. 5C. The rationale for this lack of variation is not entirely clear but it would suggest that the rates of chain transfer, propagation, and, potentially, hydrothiolation have increased but remain in proportion, or hydrothiolation has potentially become unimportant at higher temperature and the rate of chain transfer has increased far faster than propagation. This latter possibility is unlikely given how consistent the data is across the four temperatures studied.
Mechanistic considerations for TBRT of diacrylate MVTs
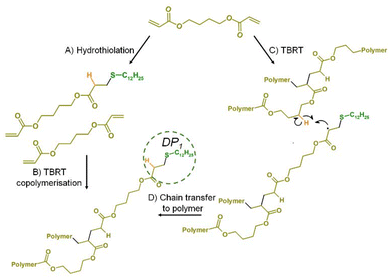
The lack of any temperature dependence on the linear telomer distributions within tel(DDT-BuA) samples is contrary to the results seen when p(DDT-BDA) as synthesised under TBRT conditions at high temperatures. If the rates of chain transfer, propagation, and, presumably, hydrothiolation are not affected relative to each other it would be unlikely that higher [MVT]0/[DDT]0 ratios would be accessible or higher Mw polymers would be achievable at 100 °C.One plausible mechanism involves the formation of DP1 structures early in the TBRT of diacrylates by hydrothiolation, Scheme 2A. As mentioned previously, it is generally believed that DP1 structures are formed predominantly in the latter stages of dimethacrylate TBRT reactions, however, if one of the acrylate functional groups is modified to form a DP1 structure, leaving the second acrylate to still react into the growing branched polymer, this would be analogous to a copolymerisation using a small concentration of mono-vinyl taxogen, Scheme 2B. This has been explored within previous reports and even very low incorporation of mono-vinyl taxogen decreases the molecular weight of the polymers formed at any given [MVT]0/[DDT]0 ratio. This is readily understood as incorporation of mono-vinyl species decreases the average vinyl functionality of any telomeric substructure and reduces the probability of intermolecular branching.3 This allows reduced telogen to be used in further reactions and higher molecular weights to be accessed.
From a different perspective, the primary difference between the linear telomerisations studied here and TBRT reactions is the presence of divinyl functionality. Flory–Stockmayer theory assumes that the reactivity of vinyl species is unchanged during polymerisation, therefore pendant vinyl groups have the same reactivity as unreacted monomers.20–23 This assumption has been widely debated and is too simplistic when considering TBRT.
During linear telomerisations, all vinyl functional groups are monomeric and the impact of steric hinderance on the reaction of the active radical species is presumably relatively small when generating such short chains (DPn < 5 monomer units). TBRT utilises the intermolecular branching that occurs when pendant vinyl groups on growing polymeric structures react together. The steric constraints increase with vinyl consumption and DP1 structures are assumed to predominantly form towards the end of the reaction and hinder gelation. By definition, DP1 structures do not require propagation and are formed by either chain transfer to a small molecule telogen (DDT used here) or hydrothiolation in the case of diacrylates. The linear telomerisation model, therefore, potentially holds limited relevance to the mechanistic understanding of diacrylate TBRT reactions.
The question of what is allowing additional control of diacrylate TBRT at high temperatures remains. Acrylate polymerisations under free radical conditions are known to undergo backbiting reactions resulting from intramolecular chain-transfer. Intermolecular chain-transfer to polymer is also well understood.24 TBRT does not generate extended C–C polymer backbones, hence the formation of polyesters from di(meth)acrylate MVTs, however, backbiting reactions do not require very long chains to exist, and are understood to proceed through the formation of a six-membered transition state.13 Along with backbiting, intermolecular chain transfer to polymer provides additional mechanisms for DP1 formation that are not available in the TBRT of dimethacrylates, and are not reliant on the action of a telogen, Scheme 2C & D.
This would allow successful TBRT at reduced telogen concentrations (higher [MVT]0/[DDT]0 ratios). The formation of new backbone-situated secondary and tertiary radical species via these routes may become more important for diacrylate TBRT at higher temperatures, and provide additional routes for propagation, resulting in the observed higher molecular weight polymers.
As discussed earlier, TBRT has been reported to form intramolecular cycles which also aid the avoidance of gelation.11 As with the reactions carried out at 70 °C, the [MVT]F/[DDT]F ratios of purified branched p(BDA-DDT) and p(HDA-DDT) polyesters formed at 100 °C are also very close to 1, Table 1, indicating a lack of any significant cyclisation.
Conclusions
As a novel polymerisation technology, the scope of TBRT for producing functional materials still requires detailed exploration. Numerous descriptions of dimethacrylate homopolymerisation have shown the formation of a range of new branched polymer structures, however, diacrylate homopolymerisation has, until now, been unreported. TBRT has again been shown to be a versatile synthetic platform for producing high molecular weight branched macromolecules, but to successfully achieve very high molecular weight samples, elevated temperatures were required in this case. Attempting TBRT with varying vinyl functionalities may require additional considerations, but branched polyesters derived from diacrylates may offer new opportunities and novel copolymers. This will require future study, especially when assessing the use of different telogens, the impact of polymerisation solvent and the structure of the diacrylate taxogens themselves.Author contributions
SMck, OPL and CS were responsible for conceptualisation, methodology, experimentation, investigation, data curation, formal analysis, visualisation and writing of the original draft. SF and SW contributed to validation and scrutiny of data. AD contributed to supervision, project administration, analytical services and validation. SPR was responsible for funding acquisition, conceptualisation of the research programme, methodology, validation, visualisation, supervision, project administration and manuscript review and editing.Data availability
All data generated during this study supporting its findings are available within the manuscript and the ESI.† All data is available from the corresponding author upon reasonable request.Conflicts of interest
SPR and SW are co-inventors on patents that protect TBRT chemistry; several of these patents have been licensed to Scott Bader and form the basis of Polymer Mimetics Ltd (Company number 12598928).Acknowledgements
The Engineering & Physical Sciences Research Council (EPSRC) are gratefully acknowledged for funding through grant EP/X010864/1. CS is grateful to the Centre of Excellence for Long-acting Therapeutics (CELT) for PhD funding. The authors would like to thank the Materials Innovation Factory (University of Liverpool) for analytical support.References
- S. R. Cassin, P. Chambon and S. P. Rannard, Polym. Chem., 2020, 11, 7637–7649 RSC.
- O. B. Penrhyn-Lowe, S. Flynn, S. R. Cassin, S. Mckeating, S. Lomas, S. Wright, P. Chambon and S. P. Rannard, Polym. Chem., 2021, 12, 6472–6483 Search PubMed.
- S. R. Cassin, S. Flynn, P. Chambon and S. P. Rannard, Polym. Chem., 2022, 13, 2295–2306 RSC.
- S. Flynn, B. Linthwaite, O. B. Penrhyn-Lowe, S. Mckeating, S. Wright, S. R. Cassin, P. Chambon and S. P. Rannard, Polym. Chem., 2023, 14, 5102–5114 RSC.
- T. Zhao, Y. Zheng, J. Poly and W. Wang, Nat. Commun., 2013, 4, 1873 CrossRef PubMed.
- M. L. Koh, D. Konkolewicz and S. Perrier, Macromolecules, 2011, 44, 2715–2724 Search PubMed.
- C. Boyer, G. Boutevin, J. J. Robin and B. Boutevin, Polymer, 2004, 45, 7863–7876 CrossRef CAS.
- B. Boutevin, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 3235–3243 Search PubMed.
- O. B. Penrhyn-Lowe, S. R. Cassin, P. Chambon and S. Rannard, Nanoscale Adv., 2022, 4, 4051–4058 RSC.
- S. Cassin, S. Flynn, P. Chambon and S. P. Rannard, RSC Adv., 2021, 11, 24374–24380 Search PubMed.
- S. R. Cassin, S. Wright, S. Mckeating, O. B. Penrhyn-Lowe, S. Flynn, S. Lomas, P. Chambon and S. P. Rannard, Polym. Chem., 2023, 14, 1905–1914 RSC.
- E. Takács and L. Wojnárovits, Radiat. Phys. Chem., 1995, 46, 1007–1010 Search PubMed.
- T. Pirman, M. Ocepek and B. Likozar, Ind. Eng. Chem. Res., 2021, 60, 9347–9367 CrossRef CAS.
- N. Ballard and J. M. Asua, Prog. Polym. Sci., 2018, 79, 40–60 CrossRef CAS.
- C. Corsaro, G. Neri, A. Santoro and E. Fazio, Materials, 2022, 15, 282 CrossRef CAS PubMed.
- D. P. Nair, M. Podgórski, S. Chatani, T. Gong, W. Xi, C. R. Fenoli and C. N. Bowman, Chem. Mater., 2014, 26, 724–744 CrossRef CAS.
- A. B. Lowe, Polym. Chem., 2010, 1, 17–36 RSC.
- S. Flynn, O. B. Penrhyn-Lowe, S. McKeating, S. Wright, S. Lomas, S. R. Cassin, P. Chambon and S. P. Rannard, RSC Adv., 2022, 12, 31424–31431 RSC.
- M. Destarac, B. Pees and B. Boutevin, Macromol. Chem. Phys., 2000, 201, 1189–1199 CrossRef CAS.
- P. J. Flory, J. Am. Chem. Soc., 1941, 63, 3083–3090 CrossRef CAS.
- W. H. Stockmayer, J. Chem. Phys., 1944, 12, 125–131 CrossRef CAS.
- C. Walling, J. Am. Chem. Soc., 1945, 67, 441–447 CrossRef.
- J. Lyu, Y. Gao, Z. Zhang, U. Greiser, H. Tai and W. Wang, Sci. China: Chem., 2018, 61, 319–327 CrossRef.
- T. Junkers and C. Barner-Kowollik, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 7585–7605 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, full experimental details and characterisation. See DOI: https://doi.org/10.1039/d5py00062a |
| This journal is © The Royal Society of Chemistry 2025 |