Open Access Article
Open Access ArticleRing-opening polymerization of six-membered 1,3-dioxa-2-silacycloalkanes by an organobase catalyst: precision polymerization and monomer regeneration by polymer degradation†
Ikuto
Tanaka
,
Sadahito
Aoshima
 and
Arihiro
Kanazawa
and
Arihiro
Kanazawa
 *
*
Department of Macromolecular Science, Graduate School of Science, The University of Osaka, Toyonaka, Osaka 560-0043, Japan. E-mail: kanazawaa11@chem.sci.osaka-u.ac.jp
First published on 21st April 2025
Abstract
Exploring monomers that undergo polymerization–depolymerization processes is of great importance for constructing closed-loop chemical recycling systems. In this study, six-membered 1,3-dioxa-2-silacycloalkanes were demonstrated to undergo ring-opening polymerization with 1,5,7-triazabicyclo[4.4.0]dec-5-ene as a catalyst. Moreover, monomer regeneration via polymer degradation was achieved by a distillation-combined reaction with an acid catalyst.
Ring-opening polymerization (ROP) of various cyclic monomers proceeds via the propagation reactions consisting of bond cleavage in a monomer and subsequent formation of the same bond as that existing in a monomer. Such propagation reactions can be catalyzed by different mechanisms in many cases. For example, oxiranes undergo cationic and anionic ROP by acid and base catalysts, respectively, via the cleavage and formation of C–O bonds.1,2 The difference of the mechanisms is also related to side reactions that occur during ROP, such as proton abstraction by an oxyanionic species in anionic ROP of oxiranes and cyclic oligomer formation in cationic ROP.
Cyclosiloxanes are valuable cyclic monomers that are used for the synthesis of polysiloxanes (silicones) by ROP.3–5 Polysiloxanes are widely synthesized and used in industry due to the characteristics associated with Si–O bonds in the main chain. Both cationic and anionic ROP are applicable for cyclosiloxanes, while side reactions such as backbiting reactions and intermolecular exchange reactions often occur, which makes the control of polymer molecular weights (MWs) difficult. Fuchise and coworkers reported that organobase catalysts, such as guanidine bases and phosphazene bases, are effective for controlled/living ROP of cyclosiloxanes.6 Polymerization accompanying negligible side reactions was achieved in their system, resulting in polysiloxanes with controlled MWs and narrow molecular weight distributions (MWDs).
Recently, we focused on 1,3-dioxa-2-silacycloalkanes as promising silicon-containing cyclic monomers for ROP.7–9 These silyl compounds are synthesized from dialkyldichlorosilanes or dialkoxydialkylsilanes with a diol. 1,3-Dioxa-2-silacycloalkanes were reported to undergo unintended polymerization during storage after synthesis.9 1,3-Dioxa-2-silacycloalkanes correspond to silicon counterparts of cyclic acetals. Cyclic acetals undergo cationic ROP;10 hence, we recently examined cationic ROP of 1,3-dioxa-2-silacycloalkanes. In our previous study, we demonstrated that cationic terpolymerization of six-membered 1,3-dioxa-2-silacycloalkanes with vinyl ethers and aliphatic aldehydes successfully proceeded to yield polymers with high MWs and controlled monomer sequences.11 Moreover, the obtained terpolymers exhibit acid-, alkaline-, or fluoride anion-triggered degradability due to the O–Si–O bonds in the main chain. Unlike successful terpolymerization, however, cationic homopolymerization did not proceed, resulting in very low monomer conversion and/or low-MW products.
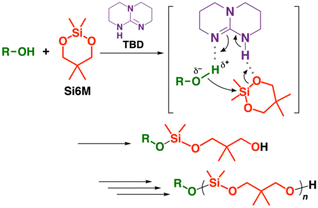
In this study, we aimed to examine homopolymerization of 1,3-dioxa-2-silacycloalkanes. In particular, we focused on various organocatalysts that are suitable for the ROP of cyclosiloxanes.6,12 Both 1,3-dioxa-2-silacycloalkanes and cyclosiloxanes have O–Si–O bonds; hence, organocatalysts potentially catalyze ROP of 1,3-dioxa-2-silacycloalkanes (Scheme 1). When we were preparing this manuscript,13 very recently, Coates, Balsara, and coworkers reported that the anionic ROP of eight-membered 1,3-dioxa-2-silacycloalkanes successfully proceeds in a controlled manner with a suitable anionic initiator.14 Unlike their study, an organobase catalyst was demonstrated to catalyze the ROP of a six-membered 1,3-dioxa-2-silacycloalkane in this study. Moreover, the product polymer was successfully transformed into the original monomer by an acid-catalyzed reaction and isolated with a distillation apparatus.
First, we examined cationic ROP of 2,2,5,5-tetramethyl-1,3-dioxa-2-silacyclohexane (Si6M). However, only oligomers were obtained in the reactions with various Lewis acids or cationizing agents at different temperatures (Table S1†). Analyses of the products by ESI-MS and 1H NMR suggested that cyclic oligomers were mainly produced by the cationic reactions (Fig. S1 and S2†).
Considering the effective organobase-catalyzed ROP of cyclosiloxanes,6,12 we conducted polymerization of Si6M with 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD), 1,8-diazabicyclo[5.4.0]-7-undecene (DBU), or phosphazene base (tert-butylimino-tri(pyrrolidino)phosphorane; BTTP) as an organocatalyst (Table S2† and Scheme 1). As a result, TBD exhibited excellent catalytic activity. A polymer with a number-average molecular weight (Mn) of over 20 × 103 was obtained when combined with ethanol as an initiator at 25 °C. DBU and BTTP were not effective for the Si6M polymerization under the examined conditions.
The polymerization of Si6M with TBD as a catalyst was next examined in more detail. Triethylene glycol (TEG) was used as a bifunctional initiator.‡ The polymerization smoothly proceeded to reach conversion of 69% in 60 h (Fig. 1A), resulting in polymers with very narrow MWDs (entry 1 in Table 1). The Mn values increased linearly with an increase in monomer conversion (Fig. 1B), suggesting that living propagating species were generated. When the initial concentrations of the initiator (TEG) were varied, the Mn values of the products changed depending on the concentrations (Fig. S4†), which indicates that TEG functioned as an initiator for polymerization.
1H NMR analysis of the product polymer exhibited peaks assigned to the TEG moiety (Fig. 1C; see Fig. S5† for the 13C NMR spectrum). The Mn values determined by gel permeation chromatography (GPC) with polystyrene calibration were lower than the theoretical values based on the initial monomer and TEG concentrations (Fig. 1B). Unintended initiation reactions from adventitious water and/or the residual alcohol (2,2-dimethyl-1,3-propanediol) used in monomer synthesis are most likely responsible for the lower values. Indeed, peaks whose m/z are consistent with those of polymer chains initiated from TEG, water, or the residual alcohol were detected by the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) analysis of the polymer (Fig. S6†).
 | ||
| Fig. 1 (A) Time–conversion and first-order plots for the polymerization of Si6M, (B) the Mn (open) and Mw/Mn (filled) values of poly(Si6M)s, and (C) 1H NMR spectrum of poly(Si6M) (entry 1 in Table 1; in CDCl3 at 30 °C; * Me4Si, water, or CHCl3; # 13C satellite; † very minor peaks observed near peaks b and c are not assigned yet). The data correspond to entry 1 in Table 1 (see Table 1 for the polymerization conditions). | ||
| Entry | Monomer | Time (h) | Conv. (%) |
M
n × 10−3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
M
w/Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
|---|---|---|---|---|---|
| a [Monomer]0 = 2.4 (except for entry 4) or 2.0 (entry 4) M, [TEG]0 = 5.0 mM, [TBD]0 = 5.0 mM, in toluene at −30 °C. b By GPC (polystyrene calibration). c For a main peak. d A polymer was not obtained. | |||||
| 1 | Si6M | 60 | 69 | 24.9 | 1.06 |
| 2 | Si6mM | 8 | 86 | 27.8 | 1.12 |
| 3 | Si6 | 2 | 77 | 2.2c | 1.71c |
| 4 | Si5M | 24 | 40d | — | — |
| 5 | Si7 | 72 | 6d | — | — |
| 6 | Si8O | 24 | <1 | — | — |
TBD is considered to catalyze the ROP of Si6M by the dual activation of both the hydroxy group at the propagating end and a Si6M monomer (Scheme 2). The dual activation mechanism was suggested for the ROP of cyclic esters by TBD in several studies.15–17 This mechanism was also applied to ROP of cyclosiloxanes.6 The interaction between an alcohol and TBD was indicated by 1H NMR analysis in a past study.18 DBU and BTTP were ineffective for polymerization; hence, the dual activation mechanism concerning TBD, hydroxy group, and monomer, which is characteristic to TBD, is likely of importance for smooth propagation reactions. A detailed study is required to elucidate the polymerization mechanism.
To confirm livingness of the polymerization, a monomer addition experiment was conducted. After a fresh feed of Si6M was added at monomer conversion of 75%, the polymerization smoothly proceeded with a consumption of the added monomer (Fig. 2A). Moreover, the Mn values of the polymers linearly increased even after the monomer addition (Fig. 2B). The MWD curve also shifted to the higher-MW region without tailing peaks (Fig. 2C). The results indicate that the polymerization of Si6M with TBD proceeded in a living manner.
 | ||
| Fig. 2 Monomer addition experiments: (A) time–conversion plots for the polymerization of Si6M, (B) Mn and Mw/Mn values (black: before monomer addition, blue: after monomer addition), and (C) MWD curves of poly(Si6M)s ([Si6M]0 = 2.4 M, [Si6M]added = 2.4 M, [TEG]0 = 5.0 mM, [TBD]0 = 5.0 mM, in toluene at −30 °C). (D) Polymerization of Si6M at −30 (black), 0 (green), 25 (brown), or 40 (red) °C ([Si6M]0 = 2.4 M, [TEG]0 = 5.0 mM, [TBD]0 = 5.0 mM, in toluene; the first four data at −30 °C are the same as those in Fig. 1A). | ||
When the polymerization time was prolonged to 140 h, the monomer conversion ceased at approximately 80% (red symbols in Fig. 2D). We hypothesized that the ceiling temperature (Tc) of the Si6M polymerization is not high. To examine the effect of the ceiling temperature, which is also related to the equilibrium concentration, we conducted polymerization at different monomer concentrations or at higher temperatures. The concentrations of remaining monomers were found to be almost constant (approximately 0.4 M; the first-order plot shown in Fig. 1A is based on this concentration) when polymerization was conducted at a monomer concentration of 2.4, 1.2, or 0.60 M at −30 °C (Fig. S7†). Moreover, the polymerization ceased at lower monomer conversion at 40, 20, or 0 °C than that at −30 °C (Fig. 2D). From the polymerization results at different temperatures, the Tc was determined to be 46 °C (Fig. S8†). In addition, the heat of polymerization (ΔH) and the entropy change of polymerization (ΔS°) were calculated to be −5.5 kJ mol−1 and −17 J mol−1 K−1, respectively.
The Si6M monomer was synthesized from dimethoxydimethylsilane and 2,2-dimethyl-1,3-propanediol with indium trifluoromethanesulfonate [In(OTf)3] as a catalyst by a distillation method. In the monomer synthesis, reactions among various compounds, such as the silane substrate, diol, methanol, Si6M, and oligomer/polymer, are likely in an equilibrium. The removal of methanol by distillation promotes the generation of Si6M and oligomers/polymers. After almost complete removal of methanol, Si6M is distilled, which also facilitates the conversion of oligomers/polymers to a Si6M monomer. Therefore, a Si6M polymer is potentially converted to a Si6M monomer by an acid catalyst. This kind of monomer regeneration from a polymer is attracting much attention as exemplified by the polymerization–depolymerization cycles of five-membered lactones and cyclic acetals.19,20 Depolymerization of polysiloxanes has also been extensively studied.21
We conducted polymer degradation into a monomer with a distillation apparatus shown in Fig. 3A. When a solution containing poly(Si6M) (5.0 g, Mn = 37.0 × 103), In(OTf)3, and dichloromethane was heated at 80 °C under reduced pressure (30 kPa), dichloromethane was first distilled off. Subsequently, after temperature was raised (130 °C) and pressure was lowered (10 kPa), colorless, transparent liquid (4.0 g; yield = 80%) was distilled. This liquid was a Si6M monomer, as confirmed by 1H NMR analysis (Fig. 3B, lower). The collected monomer was successfully used for repolymerization, resulting in a high-MW polymer (Fig. S9†). This experiment demonstrated that the polymerization–depolymerization cycle was constructed for this silyl monomer (Scheme 1).
We also examined polymerization of six-membered 1,3-dioxa-2-silacycloalkanes with the smaller number of methyl groups (Scheme 1). A six-membered monomer with one methyl group in the diol-derived unit (Si6mM) underwent smooth polymerization, resulting in polymers with high MWs and narrow MWDs (entry 2 in Table 1; Fig. S10, S11†). The polymerization was faster than that of Si6M. Unlike Si6M and Si6mM, polymers with high MWs were not obtained from a six-membered monomer that does not have methyl groups in the diol-derived unit (Si6) (entry 3 in Table 1; Fig. S12, S13†). The products had bimodal MWDs consisting of a broad polymer peak and a sharp oligomer peak. Side reactions, such as backbiting cyclization, likely occurred during the polymerization of Si6.
Other 1,3-dioxa-2-silacycloalkane monomers with different ring members (Scheme 1) were subjected to polymerization under the conditions suitable for the Si6M polymerization. However, polymers were not obtained from five- (Si5M), seven- (Si7), and eight-membered (Si8O) monomers (entries 4–6 in Table 1). The use of BTTP instead of TBD also resulted in ineffective reactions (Table S3†). Si8O was reported to yield a polymer with an anionic initiator by Coates, Balsara, and coworkers,14 while Si8O did not undergo polymerization with the organocatalysts.
We examined the thermal properties of the Si6M polymer by differential scanning calorimetry (DSC) analysis. The glass-transition temperature (Tg) and melting point (Tm) were detected at −69 °C and 33 °C, respectively (Fig. S14†). The Tg and Tm are higher than those of polydimethylsiloxane22 (Tg = −120 °C, Tm = −50 °C). Thermogravimetric analysis (TGA) indicated that poly(Si6M) started to degrade at approximately 200 °C on heating (Fig. S15†).
In conclusion, six-membered 1,3-dioxa-2-silacycloalkanes were demonstrated to undergo ROP with TBD as an organobase catalyst. Si6M exhibited equilibrium polymerization behavior; hence, the polymerization at a low temperature at a high monomer concentration was suitable for effective polymerization. Moreover, the Si6M polymer was successfully converted to the original monomer by an acid catalyst. The polymerization–depolymerization cycle is very promising for a closed-loop chemical recycling system of silicon-containing cyclic monomers. The results obtained in this study contribute to the development of silicon-containing polymers, Si–O group related-organocatalyzed ROP, and closed-loop chemical recycling systems.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by JSPS KAKENHI Grant 23K26703.References
- J. Herzberger, K. Niederer, H. Pohlit, J. Seiwert, M. Worm, F. Wurm and H. Frey, Chem. Rev., 2016, 116, 2170–2243 CrossRef CAS PubMed.
- S. Hu, J. Zhao, G. Zhang and H. Schlaad, Prog. Polym. Sci., 2017, 74, 34–77 CrossRef CAS.
- J. E. McGrath, J. S. Riffle, A. K. Banthia, I. Yilgor and G. L. Wilkes, ACS Symp. Ser., 1983, 212, 145–172 CrossRef CAS.
- P. Sigwalt, Polym. J., 1987, 19, 567–580 CrossRef CAS.
- T. Köhler, A. Gutacker and E. Mejía, Org. Chem. Front., 2020, 7, 4108–4120 RSC.
- K. Fuchise, M. Igarashi, K. Sato and S. Shimada, Chem. Sci., 2018, 9, 2879–2891 RSC.
- R. H. Krieble and C. A. Burkhard, J. Am. Chem. Soc., 1947, 69, 2689–2692 CrossRef CAS.
- R. H. Cragg and R. D. Lane, J. Organomet. Chem., 1984, 267, 1–71 CrossRef CAS.
- R. H. Cragg and R. D. Lane, J. Organomet. Chem., 1984, 268, 1–3 CrossRef CAS.
- P. Kubisa and J. P. Vairon, in Polymer Science: A Comprehensive Reference, K. Matyjaszewski and M. Möller, Elsevier B.V., Amsterdam, 2012; Vol. 4.10 Search PubMed.
- R. Hada, A. Kanazawa and S. Aoshima, Macromolecules, 2022, 55, 5474–5484 CrossRef CAS.
- J. Shi, N. Zhao, S. Xia, S. Liu and Z. Li, Polym. Chem., 2019, 10, 2126–2133 RSC.
- I. Tanaka, A. Kanazawa and S. Aoshima, Polym. Prepr., Jpn., 2024, 73(1), 1C11 Search PubMed.
- H. J. Rugh, J. Lee, C. Sun, E. E. Abdo, J. N. Bem, N. P. Balsara and G. W. Coates, Angew. Chem., Int. Ed., 2024, e202415069 Search PubMed.
- A. Chuma, H. W. Hom, W. C. Swope, R. C. Pratt, L. Zhang, B. G. G. Lohmeijer, C. G. Wade, R. M. Waymouth, J. L. Hedrick and J. E. Rice, J. Am. Chem. Soc., 2008, 130, 6749–6754 CrossRef CAS PubMed.
- L. Simón and J. M. Goodman, J. Org. Chem., 2007, 72, 9656–9662 CrossRef PubMed.
- A. Pascual, H. Sardón, F. Ruipérez, R. Gracia, P. Sudam, A. Velose and D. Mecerreyes, J. Polym. Sci., Part A: Polym. Chem., 2015, 53, 552–561 CrossRef CAS.
- X. Wang and N. Hadjichristidis, ACS Macro Lett., 2020, 9, 464–470 CrossRef CAS PubMed.
- M. Hong and E. Y.-X. Chen, Nat. Chem., 2016, 8, 42–49 CrossRef CAS PubMed.
- B. A. Abel, R. L. Snyder and G. W. Coates, Science, 2021, 373, 783–789 CrossRef CAS PubMed.
- S. Salahudeen, T. A. Thiel and E. Mejía, RSC Sustainability, 2024, 2, 1904–1929 RSC.
- E. Yilgör and I. Yilgör, Prog. Polym. Sci., 2014, 39, 1165–1195 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental section, polymerization data, NMR and MALDI-TOF-MS spectra of polymers, and thermal analysis data. See DOI: https://doi.org/10.1039/d5py00204d |
| ‡ We first used ethanol as an initiator; however, polymers with bimodal MWDs were obtained (Fig. S3†) likely due to simultaneous initiation from adventitious water and/or 2,2-dimethyl-1,3-propanediol, which remained in a monomer. Water and the diol function as bifunctional initiators. |
| This journal is © The Royal Society of Chemistry 2025 |