Open Access Article
Open Access ArticleReinvestigation of the ionic conductivity of a layered Li(BH3NH2BH2NH2BH3) salt†
A.
Prus
 a,
R.
Owarzany
a,
R.
Owarzany
 a,
D.
Jezierski
a,
D.
Jezierski
 a,
M.
Rzepecka
a,
M.
Rzepecka
 a,
W.
Grochala
a,
W.
Grochala
 a,
P.
Połczyński
a,
P.
Połczyński
 *b and
K. J.
Fijalkowski
*b and
K. J.
Fijalkowski
 *a
*a
aUniversity of Warsaw, Centre of New Technologies, ul. Banacha 2c, 02-097 Warsaw, Poland. E-mail: karol.fijalkowski@cent.uw.edu.pl
bUniversity of Warsaw, Faculty of Chemistry, ul. Pasteura 1, 02-089 Warsaw, Poland. E-mail: piotrp@chem.uw.edu.pl
First published on 10th October 2024
Abstract
We reinvestigated the ionic conductivity of lithium ions for Li(BH3NH2BH2NH2BH3), an ammonia borane derivative. The observed conductivity (4.0 × 10−6 S cm−1 at 65 °C) was found to be over four orders of magnitude higher than the value reported previously at 70 °C for this compound. Since very slow thermal decomposition of Li(BH3NH2BH2NH2BH3) progresses already below 100 °C, the previous results reported for 70–130 °C most likely correspond to decomposed samples. The activation energy for the lithium conductivity of polycrystalline layered Li(BH3NH2BH2NH2BH3) (57 kJ mol−1) resembles that of powdered Li3N (59 kJ mol−1), suggesting a similar mechanism of lithium diffusion in both materials.
Introduction
The rapid development of portable electronics and mobile applications has put immense pressure on the development of highly efficient energy storage units. Currently, lithium-ion batteries are preferred owing to their very beneficial large electrical charge-to-mass ratio. However, despite the 40 years development of lithium batteries,1 safety issues still arise due to flammability and the relatively large mass of the components used.All types of commercially available lithium-ion batteries use liquid electrolytes to some extent.2 Organic-solvent-based liquid electrolytes used in Li-ion batteries have numerous advantages (e.g. very high lithium solubility and conductivity, excellent wetting properties, and sufficient electric contact with the electrode), but they might be problematic due to flammability when exposed to air, low thermal and electrochemical stability, and poor ionic selectivity.3 Moreover, organic solvents decrease the effective energy density of the system and promote the formation of dendrites at the surface of lithium electrodes.
The minimisation of the amount of liquid electrolytes used in Li-ion batteries has been one of the hottest research targets in recent years. Systems with an ultra-small volume of electrolytes have been successfully constructed (e.g. 10 μL cm−2 (ref. 4)); however, the ultimate goal is the construction of an all-solid-state Li-ion battery with no liquid electrolyte, which was first considered as early as the 1950s but was attempted for the first time in the 1990s, followed by numerous successful studies in the 21st century.5–8 All-solid-state lithium batteries are believed to be lighter, safer, and reach higher capacities than currently used standard batteries; however, issues concerning insufficient cell conductivity at low temperatures and sensitivity to mechanical stress can be expected. None of the studied and constructed all-solid-state lithium-ion batteries are commercially available considering their production problems and relatively high cost. The introduction of such batteries is limited mostly by the inaccessibility of suitable solid electrolyte materials.
Various promising lithium-ion conducting materials were already known in the early 2000s.9–11 Li-β-alumina (Li20·11Al203) with a layered crystal structure facilitating the migration of lithium ions exhibits a conductivity of 1.3 × 10−4 S cm−1 at room temperature (RT) but cannot be used as an electrolyte because of its high hygroscopicity.12 Layered Li3N shows a very high conductivity of 1.2 × 10−3 S cm−1 at RT in the single-crystal form along structural layers but exhibits a low decomposition voltage of 0.44 V.13–16 LIPON (Li2.88PO3.86N) shows good overall properties but suffers from moderate conductivity of only 2 × 10−6 S cm−1, which is insufficient for practical use.17 In recent years, significant progress has been observed in the field of lithium-ion conducting materials. Currently, Li10GeP2S12 is believed to be the best solid-state candidate for solid electrolyte application, having a conductivity of 1.2 × 10−2 S cm−1 but a slightly too narrow electrochemical stability window of 5 V.18
Hydride-based materials have also been reported as lithium-ion conductors. LiBH4 exhibits a conductivity of 1 × 10−3 S cm−1 after transition to the high-temperature (HT) form above 115 °C, while the low-temperature (LT) form shows a conductivity three orders of magnitude lower.19 It was subsequently discovered that some doped lithium borohydride materials (e.g. 7LiBH4·LiI;20 LiCe(BH4)3Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21) retain the HT-LiBH4 crystal structure below 115 °C, showing high conductivity at RT (4.5 × 10−5 S cm−1;20 1.0 × 10−4 S cm−1 (ref. 21)).
21) retain the HT-LiBH4 crystal structure below 115 °C, showing high conductivity at RT (4.5 × 10−5 S cm−1;20 1.0 × 10−4 S cm−1 (ref. 21)).
Protonic-hydridic materials are also known to conduct lithium cations in the solid state. Lithium amidoborane Li(NH2BH3) has a relatively poor ionic conductivity of 5.5 × 10−9 S cm−1 at RT.22 However, the adducts of LiBH4 with molecular ammonia borane were reported to show very high conductivity at 40 °C: (LiBH4)(NH3BH3) 4.9 × 10−3 S cm−1 and (LiBH4)2(NH3BH3) 1.2 × 10−4 S cm−1.23 Recently, Cascallana-Matías et al. reported ammonia borane adduct-related materials exhibiting high lithium conductivity at 70 °C: (LiBH4)2(NH3BH3) 2.83 × 10−5 S cm−1, (LiI)(NH3BH3) 1.80 × 10−6 S cm−1 and (LiI)(NH3BH3)2 1.79 × 10−5 S cm−1, while Li(BH3NH2BH2NH2BH3) had a much poorer conductivity of 4.11 × 10−9 S cm−1 at 70 °C.24 Surprisingly, the figure for Li(BH3NH2BH2NH2BH3) was as low as the conductivity of Li(NH2BH3) (5.5 × 10−9 S cm−1 at RT22), having an unfavourable crystal structure with neither the 1D nor 2D lithium cation substructure.
Herein, we present a detailed reinvestigation of the ionic conductivity of Li(BH3NH2BH2NH2BH3) performed using an in-house designed IMPED CELL.25 We present the results of electrochemical impedance spectroscopy (EIS) supported by spectroscopic (FTIR, NMR, Raman, 7Li NMR) and structural (PXRD) studies of the title compound with respect to earlier reports.24,26,27 We discuss the influences of the chosen synthesis method, the purity of the samples, and the methodology of the solid-state EIS investigation on the material's conductivity.
Results and discussion
Li(BH3NH2BH2NH2BH3) (in short: Li[B3N2]) was previously reported by us as a promising hydrogen storage material with a total hydrogen content of 15.2%.26,27 Its layered crystal structure, with flat sheets of lithium cations separated by thick layers of perpendicularly oriented (B3N2)− anions,26 resembles that of Li3N,13 a well-known super-ionic lithium conductor.14–16For this study, we have designed a novel approach to the synthesis of Li[B3N2], which is an improvement of the previously reported mechanochemical method.26 Instead of milling the solid mixture of LiH and NH3BH3 in a high-energy mill, we ground the reactants using an agate mortar and pestle to receive an unreacted quasi-homogeneous mixture of the starting compounds, which was further subjected to a 2 hour thermal treatment at 80 °C. The novel method is much easier and more efficient than the classical one26 and it also avoids the use of metal tools and vessels, ensuring the highest purity of the product received with no electron-conducting metal impurities added upon treatment, which could easily interfere with the results of the EIS investigation. The high purity of Li[B3N2] samples obtained via the novel method was confirmed by Rietveld analysis‡ of its X-ray powder pattern and spectroscopic analyses showing the sole presence of the Li[B3N2] crystalline phase in the product and no contamination with moisture absorbed, which is crucial for the stability of the compound known to be highly hygroscopic.
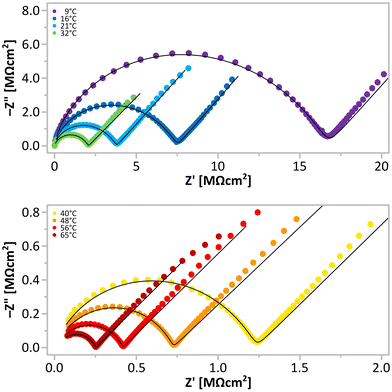
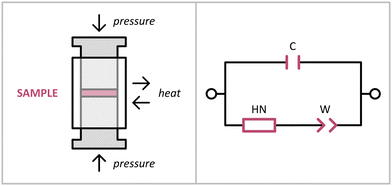
The EIS measurements were conducted using an in-house developed setup, IMPED CELL.25 We have acquired several series of temperature-resolved EIS spectra (temperature range of 9–65 °C) for three different batches of Li[B3N2], obtaining consistent results (Fig. 2 and 3). We observed Nyquist plots having shapes of a semicircle followed by spurs at lower frequencies (Fig. 2), typical of materials exhibiting ionic conductivity. The observed semicircles were slightly flattened, similar to the shape of the spectra of related ammonia borane derivatives characterised before.22 For fitting the spectra, various forms of the dielectric function were tested: Cole–Cole, Davidson–Cole, Jonscher, and Havrilak–Negami. The best quality of the fit was obtained using an equivalent circuit consisting of two branches: one with a single capacitor, and the second with a series connection of Havriliak–Negami and Warburg elements (Fig. 6).28 The Havriliak–Negami element models ionic relaxation behaviour for the broad and asymmetric distribution of relaxation times characteristic of amorphous materials. The high-frequency capacitance observed at low temperatures takes into account the influence of polymer/anion relaxations at high frequencies.29 The Warburg element describes diffusive transport mechanisms at low frequencies with charge accumulation at blocking electrodes. The errors of all fitted parameters were below 3%.
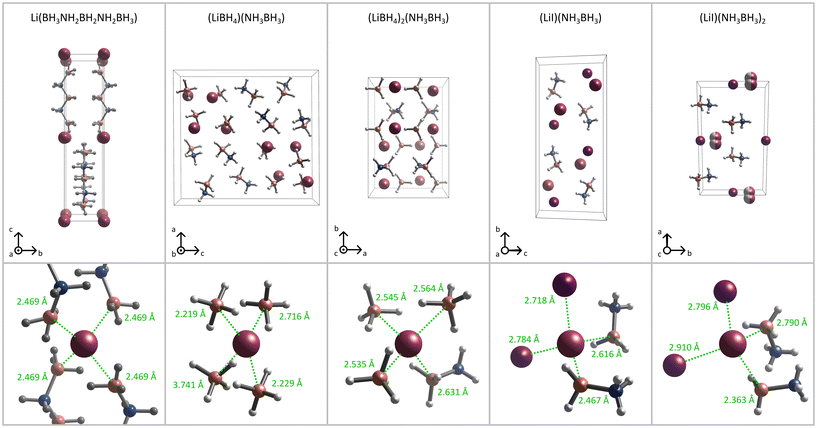 | ||
| Fig. 1 Top: A comparison of the crystal structures of Li[B3N2],26 (LiBH4)(NH3BH3),23 (LiBH4)2(NH3BH3),23 (LiI)(NH3BH3),24 and (LiI)(NH3BH3)2.24 Bottom: coordination spheres of lithium cations with Li–B and Li–I distances. For clarity of visualisation, Li coordination spheres are shown with no distortion of (B3N2)− anions in Li[B3N2]26 and Li+ cations in (LiI)(NH3BH3)2.24 | ||
We observed the moderate conductivity of Li[B3N2] of 2.6 × 10−7 S cm−1 at RT with an activation energy of 57 kJ mol−1, and the expected growth of the material's conductivity at elevated temperatures reached 4.0 × 10−6 S cm−1 at 65 °C (Fig. 4 and Table 1). The values at roughly this level of conductivity are characteristic of doped LiBH4 materials exhibiting the HT-LiBH4 crystal structure at lower temperatures.20
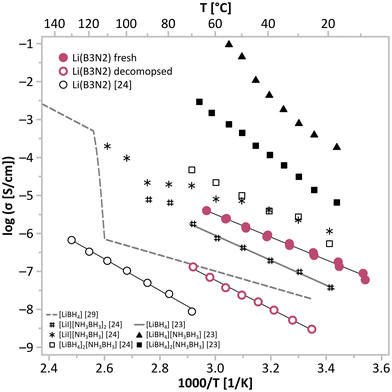 | ||
| Fig. 4 Comparison of the values of conductivity and activation energy of Li[B3N2] measured in this study (fresh and decomposed at 200 °C) and reported by Cascallana-Matías et al.,24 together with the values characteristic of other ammonia borane-based materials23,24 and reference values for LiBH4.23,31 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 and Li3N
22 and Li3N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14–16
14–16
| Compound | E a [kJ mol−1] | σ [S cm−1] | T [°C] | Ref. |
|---|---|---|---|---|
| Li(BH3NH2BH2NH2BH3) – fresh | 57 | 6.0 × 10−8 | 9 | [Here] |
| Li(BH3NH2BH2NH2BH3) – fresh | 57 | 2.6 × 10−7 | 21 | [Here] |
| Li(BH3NH2BH2NH2BH3) – fresh | 57 | 4.0 × 10−6 | 65 | [Here] |
| Li(BH3NH2BH2NH2BH3) – dec. | 71 | 3.0 × 10−9 | 25 | [Here] |
| Li(BH3NH2BH2NH2BH3) – dec. | 71 | 1.3 × 10−7 | 69 | [Here] |
| Li(BH3NH2BH2NH2BH3) | 83 | 8.7 × 10−9 | 70 | 24 |
| (LiBH4)(NH3BH3) | 12 | 4.9 × 10−3 | 40 | 23 |
| (LiBH4)2(NH3BH3) | 24 | 1.2 × 10−4 | 40 | 23 |
| (LiBH4)2(NH3BH3) | 69 | 2.8 × 10−5 | 70 | 24 |
| (LiI)(NH3BH3) | 67 | 1.8 × 10−6 | 70 | 24 |
| (LiI)(NH3BH3)2 | 46 | 1.8 × 10−5 | 70 | 24 |
| LT-LiBH4 | 67 | 2.0 × 10−8 | 25 | 19 |
| HT-LiBH4 | 51 | 1.0 × 10−3 | 117 | 19 |
| Li(NH2BH3) | 140 | 5.5 × 10−9 | 25 | 22 |
| NH3BH3 | 117 | 5.2 × 10−11 | 60 | 22 |
| Li3N (powder) | 59 | 3.7 × 10−8 | 25 | 14 |
| Li3N (sintered powder) | 24 | 6.6 × 10−4 | 25 | 15 |
| sc-Li3N (out-of-plane) | 47 | 1.0 × 10−5 | 27 | 16 |
| sc-Li3N (in-plane) | 27 | 1.2 × 10−3 | 27 | 16 |
Surprisingly, the conductivity observed here for polycrystalline Li[B3N2] (2.6 × 10−7 S cm−1 at 21 °C, 57 kJ mol−1) is ca. fifteen times higher than the conductivity shown by powdered Li3N (3.7 × 10−8 S cm−1 at RT; 59 kJ mol−1)14 having a comparable activation energy, similar layered crystal structure (Table 1) and comparable lithium-lithium distances within the layers (4.020 Å for Li(B3N2),26 3.648 Å for Li3N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13). This may stem from differences in electrostatic anion–cation interactions. In Li(B3N2), a single negative charge is diluted along the whole (B3N2)− anion, with partial negative charges localised on nitrogen atoms and hydridic atoms bonded to boron atoms, while in Li3N a triple-negative charge is localised on nitrogen atoms in Li2N− layers, which might inhibit the mobility of Li+ in lithium. On the other hand, comparable values of conductivity, together with structural similarities of both systems, suggest that lithium diffusion in the crystals of Li[B3N2] may resemble the mechanism present in Li3N,16 which involves the out-of-plane hopping of Li+ cations when migrating along the [Li2N]− layers in the crystal structure. A closer look at the layered structure of Li[B3N2] revealed that for Li+ cations to move between the occupied and vacant tetrahedral sites (T) in the lithium layers, they need to pass through out-of-plane octahedral sites (O) (Fig. 5). Such a T–O–T lithium migration pathway is observed in most solid-state lithium conductors that, unfortunately, do not show ultra-high values of conductivity due to the significant energy barrier of crossing the unstable octahedral sites.30 Numerous similarities between Li[B3N2] and Li3N also suggest that the conductivity of Li[B3N2] might be several orders of magnitude higher and the activation energy much lower if measured in-plane in single crystals, just as observed for Li3N (1.2 × 10−3 S cm−1 at 27 °C, 27 kJ mol−1 (ref. 16)).
13). This may stem from differences in electrostatic anion–cation interactions. In Li(B3N2), a single negative charge is diluted along the whole (B3N2)− anion, with partial negative charges localised on nitrogen atoms and hydridic atoms bonded to boron atoms, while in Li3N a triple-negative charge is localised on nitrogen atoms in Li2N− layers, which might inhibit the mobility of Li+ in lithium. On the other hand, comparable values of conductivity, together with structural similarities of both systems, suggest that lithium diffusion in the crystals of Li[B3N2] may resemble the mechanism present in Li3N,16 which involves the out-of-plane hopping of Li+ cations when migrating along the [Li2N]− layers in the crystal structure. A closer look at the layered structure of Li[B3N2] revealed that for Li+ cations to move between the occupied and vacant tetrahedral sites (T) in the lithium layers, they need to pass through out-of-plane octahedral sites (O) (Fig. 5). Such a T–O–T lithium migration pathway is observed in most solid-state lithium conductors that, unfortunately, do not show ultra-high values of conductivity due to the significant energy barrier of crossing the unstable octahedral sites.30 Numerous similarities between Li[B3N2] and Li3N also suggest that the conductivity of Li[B3N2] might be several orders of magnitude higher and the activation energy much lower if measured in-plane in single crystals, just as observed for Li3N (1.2 × 10−3 S cm−1 at 27 °C, 27 kJ mol−1 (ref. 16)).
 | ||
| Fig. 5 Visualisations of the crystal structure of Li[B3N2] in the side and top views26 (left), and the alternating occupied (purple) and vacant (grey) tetragonal sites (T) present within the cationic lithium layers shown in the side and top views (right). Vacant octahedral sites (O) (facing tetragonal sites) are formed on both sides of the cationic layers. | ||
The conductivity and activation energy of Li[B3N2] obtained here (4.0 × 10−6 S cm−1 at 65 °C, 57 kJ mol−1) significantly differ from the data reported earlier by Cascallana-Matías et al.24 (8.7 × 10−9 S cm−1 at 70 °C; 83 kJ mol−1) (Table 1 and Fig. 5). The differences in both conductivity and activation energy are so large that they cannot be simply explained by differences in the measurement setup used or sample quality. Analysis of the results reported by Cascallana-Matías et al.24 led us to conclude that their samples were at least partially thermally decomposed during EIS measurements. Cascallana-Matías et al.24 presented the results of temperature-resolved EIS measurements conducted in the temperature range of ca. 70–130 °C (343–403 K), i.e., nominally below the temperature of the thermal decomposition and hydrogen evolution of Li[B3N2] observed at ca. 135 °C.26,27 However, this value was obtained in non-stationary conditions upon dynamic heating,26,27 while the onset of this process was observed below 100 °C, especially when heated at a low rate.27 Performing this study, trying to collect data in the temperature range reported in the previous study,24 we could not obtain stable or reproducible EIS spectra at ca. 95 °C, which we believe was a result of the slow decomposition of Li[B3N2]. Thus, Cascallana-Matías et al.,24 while performing their tests at temperatures reaching 130 °C with ≥1 h temperature equilibration, and willing to get reproducible values, must have recorded the data several times in the presented temperature range, effectively decomposing the material. In other words, their conductivity data correspond to at least partially decomposed Li[B3N2].
To check this hypothesis, we subjected a fully thermally decomposed Li[B3N2] sample (1 h at 200 °C under argon) to an EIS study in the temperature range of 13–69 °C. We repeatably obtained the conductivity of 1.3 × 10−7 S cm−1 at 69 °C, with an activation energy of 71 kJ mol−1, comparable to the conductivity of LT-LiBH4. These values are still one order of magnitude higher than the figures reported by Cascallana-Matías et al.,24 however, our simple experiment proves that at elevated temperatures Li[B3N2] undergoes substantial chemical changes that adversely affect its electronic conductivity. It should be added here that sample operations reported by Cascallana-Matías et al.24 (the exposure of the sample to air and moisture, presence of platinum paste, heating of a compressed pallet, etc.) added so many uncontrollable factors that it would be surprising if their and our samples (thermally decomposed and measured in inert gas conditions) were not different.
We have also compared the figures obtained for Li[B3N2] with the conductivity reported earlier for ammonia borane-based adducts with LiI and LiBH4.23,24 We noticed that Li[B3N2] showed similar conductivity to LiI-NH3BH3 adducts (Fig. 4) whose crystal structures comprise lithium chains, pseudo-layers, and columns (Fig. 1).24 Li–Li in-chain distances in LiI(NH3BH3) (4.291 Å (ref. 24)) and LiI(NH3BH3)2 (4.442 Å (ref. 24)) are only slightly larger than the nearest in-plane distances in Li[B3N2] (4.020 Å (ref. 26)), which is in good agreement with the observed similar conductivities (Tables 1 and 2). Interestingly, lithium chains in LiI(NH3BH3)2 lie close to each other, with a Li–Li distance between the chains of just 4.391 Å, forming non-flat pseudo-layers (rendering lithium diffusion easier), while lithium chains in LiI(NH3BH3) are well separated from each other, resulting in inferior conductivity.
Li[B3N2] exhibits lower conductivity than LiBH4-NH3BH3 adducts23,24 (Fig. 4), and its crystal structures seem to be more favourable for lithium conductivity. (LiBH4)2(NH3BH3) is comprised of alternating lithium borohydride ionic layers separated by ammonia borane molecular layers. Lithium cations do not form clear chains or layers, but the Li–Li distances are very short within the lithium borohydride layers (3.417 Å (ref. 30)), and only slightly longer (4.295 Å (ref. 30)) between these layers, which may explain the high conductivity of this material. On the other hand, the crystal structure of (LiBH4)(NH3BH3) consists of borohydride columns separated by regions built exclusively of ammonia borane molecules. Lithium cations do not form straight chains within these columns but rather a network of cations with short interaction distances (3.158–4.363 Å (ref. 33)), while the closest Li–Li distances between the borohydride columns are noticeably longer (5.380 Å (ref. 33)). The high conductivity of (LiBH4)(NH3BH3) can also be explained by the remarkably large volume of the unit cell caused by a substantial share of ammonia borane in the structure. The volume of the unit cell of (LiBH4)(NH3BH3) is 1.4 times larger than the volume of the unit cell of (LiBH4)2(NH3BH3) (both with Z = 8) resulting in an effective decrease in the volume density of lithium cations and this in turn favours high Li-ion conduction.
Conclusions
We have presented a detailed EIS study on the lithium ionic conductivity of Li[B3N2]. The observed conductivity (σ = 4.0 × 10−6 S cm−1 at 65 °C; Ea = 57 kJ mol−1) was found to be significantly higher than the value reported previously for Li[B3N2] (8.7 × 10−9 S cm−1 at 70 °C; 83 kJ mol−1 (ref. 24)) which is now believed to have shown properties of a thermally decomposed sample. We found that Li[B3N2] undergoes very slow thermal decomposition at ca. 95 °C, consistent with earlier thermogravimetric observations.26,27 This explains why samples of Li[B3N2] studied earlier24 were largely thermally decomposed, and thus incorrect lithium-ion conductivities were measured for their specimen. Moreover, the conductivity of Li[B3N2] was found to be comparable with that of ammonia borane hybrid adducts with LiI.24 Surprisingly, polycrystalline Li[B3N2] was found to have a higher conductivity and similar energy barrier for conductivity compared to powdered Li3N (3.7 × 10−8 at RT; 59 kJ mol−1 (ref. 14)). The measurement setup used here (IMPED CELL), allows for the easy and highly precise EIS investigation of air/moisture-sensitive samples of powdered solids.Experimental
All manipulations were performed under an inert atmosphere of argon (5.0) in an MBRAUN Labmaster DP or Vigor SG1200 glovebox (O2 < 1.0 ppm; H2O < 1.0 ppm), avoiding the use of metal tools and vessels. High-purity reactants were used: NH3BH3 (98%, JSC Aviabor), and LiH (95%, Sigma Aldrich).Synthesis
Li[B3N2] was obtained using a novel method derived from a dry mechanochemical approach reported earlier by us.26 The entire procedure was carried out without the use of metal tools and vessels to ensure no sample contamination with electronically conducting fractions. LiH and NH3BH3 in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 were ground using an agate mortar and pestle for ca. 60 seconds until a fine powder was obtained. The as-obtained mixture of starting reactants was heated for 2 h at 75 °C. The process can be described by eqn (1):
3 were ground using an agate mortar and pestle for ca. 60 seconds until a fine powder was obtained. The as-obtained mixture of starting reactants was heated for 2 h at 75 °C. The process can be described by eqn (1):| LiH + 3NH3BH3 → Li(BH3NH2BH2NH2BH3) + NH3↑ + 2H2↑ | (1) |
The quality of the product was comparable with the purity of the samples obtained with other known methods26,27 as judged from PXD, FTIR, and Raman measurements.
Powder X-ray diffraction
PXRD measurements were obtained using a Panalytical X'Pert Pro with linear PIXcel Medipix2 detector (parallel beam; the CoKα1 and CoKα2 radiation intensity ratio of ca. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, λ ∼1.7890 Å). Samples were sealed in 0.5 mm thick quartz capillary tubes under an inert argon atmosphere.
1, λ ∼1.7890 Å). Samples were sealed in 0.5 mm thick quartz capillary tubes under an inert argon atmosphere.
Nuclear magnetic resonance
7Li NMR spectra were obtained using a Bruker AVANCE II 500 MHz spectrometer. Pb(NO3)2 was used as an intrinsic temperature reference. Due to the risk of explosion (lead nitrate is an oxidant while Li[B3N2] is a reductant), only non-metal tools were used during the sample preparation to avoid sparks. For safety reasons, the temperature range of the measurements was narrowed down to 272–348 K.Electric impedance spectroscopy
EIS measurements were performed in the frequency range of 10−2–106 Hz using a Solartron 1260A Frequency Response Analyzer with a 1296A Dielectric Interface using the approach reported earlier.22 All measurements were performed using an in-house designed IMPED CELL25 available from KEMI (Warsaw, Poland). The IMPED CELL housed the sample under an inert atmosphere (if needed) in an air-tight measurement module that can be loaded inside a glovebox under an inert atmosphere (Fig. 6) with no need for binders or electric contact deposition, enabling the reliable investigation of reactive solids in pure form, which is crucial for moisture sensitive Li(B3N2). The IMPED CELL stabilized the temperature (T ± 0.01 °C) and pressure (p ± 0.1 MPa) with the continuous real-time monitoring of these parameters, together with monitoring the sample's thickness (d ± 1 μm) throughout the entire test, allowing the reliable correction of the results. Each sample (ca. 100 mg) was placed inside a sealed stainless-steel module directly between two electrodes (working surface of 0.785 cm2) and compressed under a pressure of ca. 200 MPa during the entire measurement. The temperature was stabilised with a dedicated water jacket coupled with a Lauda RE 1050 thermostat and controlled via SMART software from Solartron. The pressure was stabilised using a SPECAC ATLAS 15T laboratory press. The sample thickness was monitored using a SICK OD Mini LIDAR distance sensor. All the auxiliary data (T, p, d) were collected and synchronised using a dedicated data acquisition unit. The IMPED CELL was loaded under argon gas inside the glovebox. Each experimental spectrum was measured until the value was reproduced. Fitting was carried out using the ZPlot software from Scribner Associates, Inc.The IMPED CELL was designed to provide a versatile, user-friendly tool for EIS research. The development was performed under the Stanford Design Thinking Model approach with a functional usability study based on qualitative analysis employing in-depth interviews and mini-focus group workshops with participants representing the International Electrochemical Society.
Graphical presentation
Visualisations of crystal structures have been performed with Vesta.35 Illustrations have been prepared using Inkscape 0.92.1.36Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by Polish National Science Centre within the projects Sonata Bis 8 (UMO/2018/30/E/ST5/00854) and Preludium 13 (UMO/2017/25/N/ST5/01977). Development and prototyping of IMPED CELL was funded by Polish National Centre for Research and Development within the project Tango 2 (TANGO2/340584/NCBR/2017). The authors thank all the participants of the functional usability study from Cambridge in the UK, Belgrade in Serbia, and Warsaw in Poland. The authors thank Mr Krzysztof Dawid (KEMI, Warsaw, Poland) for his support in the development of IMPED CELL. The research was carried out with the use of CePT infrastructure financed by the European Union – the European Regional Development Fund within the Operational Programme “Innovative economy” for 2007–2013 (POIG.02.02.00-14-024/08-00). This work is dedicated to Prof. Zbigniew Galus at his 90th birthday.References
- M. S. Whittingham, Electrical energy storage and intercalation chemistry, Science, 1976, 192, 1126–1127 CrossRef CAS.
- J. M. Tarascon and M. Armand, Issues and challenges facing rechargeable lithium batteries, Nature, 2001, 414, 359–367 CrossRef CAS.
- E. Quartarone and P. Mustarelli, Electrolytes for solid-state lithium rechargeable batteries: recent advances and perspectives, Chem. Soc. Rev., 2011, 40, 2525–2540 RSC.
- B. Hamankiewicz, A. Czerwiński, M. Krajewski, M. Michalska, L. Lipińska, J. Kozakiewicz, J. Przybylski, K. Sylwestrzak and W. Sarna, Lithium-ion cel, Polish patent application noP.416704, 2016 Search PubMed.
- K. Iwamoto, N. Aotani, K. Takada and S. Kondo, Application of Li3PO4-L2S-SiS2 glass to the solid state secondary batteries, Solid State Ionics, 1995, 79, 288–291 CrossRef CAS.
- Y. Kato, S. Hori, T. Saito, K. Suzuki, M. Hirayama, A. Mitsu, M. Yonemura, H. Iba and R. Kanno, High-power all-solid-state batteries using sulphide superionic conductors, Nat. Energy, 2016, 1, 16030 CrossRef CAS.
- T. Takeuchi, H. Kageyama, K. Nakanish, M. Tabuchi, H. Sakaebe, T. Ohta, H. Senoh, T. Sakai and K. Tatsumi, All-Solid-State Lithium Secondary Battery with Li2S-C Composite Positive Electrode Prepared by Spark-Plasma-Sintering Process, J. Electrochem. Soc., 2010, 157, A1196–A1201 CrossRef CAS.
- K. Yoon, J. J. Kim, W. M. Seong, M. H. Lee and K. Kang, Investigation on the interface between Li10GeP2S12 electrolyte and carbon conductive agents in allsolid-state lithium battery, Sci. Rep., 2018, 8, 8066 CrossRef PubMed.
- V. Thangadurai and W. Weppner, Recent progress in solid oxide and lithium ion conducting electrolytes research, Ionics, 2016, 12, 81–92 CrossRef.
- S. Stramare, V. Thangadurai and W. Weppner, Lithium Lanthanum Titanates: A Review, Chem. Mater., 2003, 15, 3974–3990 CAS.
- V. Thangadurai, H. Kaack and W. Weepner, Novel Fast Lithium Ion Conduction in Garnet-Type Li5La3M2O12 (M = Nb, Ta), J. Am. Ceram. Soc., 2003, 86, 437 CAS.
- J. L. Briant and G. C. Farrington, Ionic Conductivity in Lithium and Lithium-Sodium Beta Alumina, J. Electrochem. Soc., 1981, 128, 1830–1934 CrossRef CAS.
- A. Rabenau and H. Schulz, Re-evaluation of the lithium nitride structure, J. Less-Common Met., 1976, 50, 155–159 CAS.
- B. A. Boukamp and R. A. Huggins, Lithium ion conductivity in lithium nitride, Phys. Lett. A, 1976, 58, 231–233 CrossRef.
- B. A. Boukamp and R. A. Huggins, Fast ionic conductivity in lithium nitride, Mater. Res. Bull., 1978, 13, 23–32 CrossRef CAS.
- U. v. Alpen, A. Rabenau and G. H. Talat, Ionic conductivity in Li3N single crystals, Appl. Phys. Lett., 1977, 30, 621–623 CrossRef.
- J. B. Bates, N. J. Dudney, G. R. Gruzalski, R. A. Zuhr, A. Choudhury, C. F. Luck and J. D. Robertson, Electrical properties of amorphous lithium electrolyte thin films, Solid State Ionics, 1992, 53–56, 647–654 CrossRef CAS.
- N. Kamaya, K. Homma, Y. Yamakawa, M. Hirayama, R. Kanno, M. Yonemura, T. Kamiyama, Y. Kato, S. Hama, K. Kawamoto and A. Mitsui, A lithium superionic conductor, Nat. Mater., 2011, 10, 682–686 CrossRef CAS PubMed.
- M. Matsuo, Y. Nakamori, S. Orimo, H. Maekawa and H. Takamura, Lithium superionic conduction in lithium borohydride accompanied by structural transition, Appl. Phys. Lett., 2007, 91, 224103 Search PubMed.
- H. Maekawa, M. Matsuo, H. Takamura, M. Ando, Y. Noda, T. Karahashi and S. I. Orimo, Halide-Stabilized LiBH4, a Room-Temperature Lithium Fast-Ion Conductor, J. Am. Chem. Soc., 2009, 131, 894–895 Search PubMed.
- M. B. Ley, D. B. Ravnsbek, Y. Filinchuk, Y. S. Lee, R. Janot, Y. W. Cho, J. Skibsted and T. R. Jensen, LiCe(BH4)3Cl, a New Lithium-Ion Conductor and Hydrogen Storage Material with Isolated Tetranuclear Anionic Clusters, Chem. Mater., 2012, 24, 1654–1663 Search PubMed.
- K. J. Fijalkowski, R. Jurczakowski, W. Koźmiński and W. Grochala, Insights from impedance spectroscopy into the mechanism of thermal decomposition of M(NH2BH3), M = H, Li, Na, Li0.5Na0.5, hydrogen stores, Phys. Chem. Chem. Phys., 2012, 14, 5778–5784 CAS.
- H. Liu, Z. Ren, X. Zhang, J. Hu, M. Gao, H. Pan and Y. Liu, Incorporation of Ammonia Borane Groups in the Lithium Borohydride Structure Enables Ultrafast Lithium Ion Conductivity at Room Temperature for Solid-State Batteries, Chem. Mater., 2020, 32, 671–678 CAS.
- I. Cascallana-Matías, J. Breternitz, A. Baker, H. Davis, E. J. Cussen and D. H. Gregory, Molecular-salt hybrids; integration of ammonia borane into lithium halides, Inorg. Chem. Front., 2019, 6, 808–812 RSC.
- K. J. Fijalkowski and R. Jurczakowski, Cell and method for electrical measurements of highly reactive powder and liquid samples, Patents no. PL221643 (2016), EP2788745 (2016), US9651595 (2017), JP6219831 (2017).
- K. J. Fijalkowski, T. Jaroń, P. J. Leszczyński, E. Magoś-Palasyuk, T. Palasyuk, M. K. Cyrański and W. Grochala, M(BH3NH2BH2NH2BH3) – the missing link in the mechanism of the thermal decomposition of light alkali metal amidoboranes, Phys. Chem. Chem. Phys., 2014, 16, 23340–23346 RSC.
- R. Owarzany, K. J. Fijałkowski, T. Jaroń, P. J. Leszczyński, Ł. Dobrzycki, M. K. Cyrański and W. Grochala, Complete Series of Alkali-Metal M(BH3NH2BH2NH2BH3) Hydrogen-Storage Salts Accessed via Metathesis in Organic Solvents, Inorg. Chem., 2016, 55, 37–45 CrossRef CAS.
- J. R. Dygas, Dielectric function of ionic conductors studied by impedance spectroscopy, Solid State Ionics, 2005, 176, 2065–2078 CrossRef CAS.
- I. B. Pehlivan, R. Marsal, P. Georén, C. G. Granqvist and G. A. Niklasson, Ionic relaxation in polyethyleneimine-lithium bis (trifluoromethylsulfonyl) imide polymer electrolytes, J. Appl. Phys., 2010, 108, 074102 CrossRef.
- Y. Wang, W. D. Richards, S. P. Ong, L. J. Miara, J. C. Kim, Y. Mo and G. Ceder, Design principles for solid-state lithium superionic conductors, Nat. Mater., 2015, 14, 1026–1032 CrossRef CAS PubMed.
- D. Sveinbjornsson, J. S. G. Myrdal, D. Blanchard, J. J. Bentzen, T. Hirata, M. B. Mogensen, P. Norby, S. I. Orimo and T. Vegge, Effect of Heat Treatment on the Lithium Ion Conduction of the LiBH4−LiI Solid Solution, J. Phys. Chem. C, 2013, 117, 3249–3257 Search PubMed.
- H. Wu, W. Zhou, F. E. Pinkerton, M. S. Meyer, G. Srinivas, T. Yildirim, T. J. Udovic and J. J. Rush, A new family of metal borohydride ammonia borane complexes: Synthesis, structures, and hydrogen storage properties, J. Mater. Chem., 2010, 20, 6550–6556 RSC.
- J. Luo, H. Wub, W. Zhou, X. Kang, Z. Fang and P. Wang, LiBH4·NH3BH3: A new lithium borohydride ammonia borane compound with a novel structure and favorable hydrogen storage properties, Int. J. Hydrogen Energy, 2012, 37, 10750–10757 Search PubMed.
- Y. Filinchuk, D. Chernyshov and R. Cerny, Lightest Borohydride Probed by Synchrotron X-ray Diffraction: Experiment Calls for a New Theoretical Revision, J. Phys. Chem. C, 2008, 112, 10579–10584 CAS.
- K. Momma and F. Izumi, VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data, J. Appl. Crystallogr., 2011, 44, 1272–1276 CAS.
- Inkscape project, 2017.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis, 7Li MAS NMR, Rietveld analysis of Li(B3N2) PXRD, EIS of pristine Li(B3N2). See DOI: https://doi.org/10.1039/d4qi01595a |
‡ Obtained Li[B3N2] lattice parameters: a = 4.014 Å, c = 16.924 Å in P![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 2c, purity = 99%, GOF = 1.21, Rp = 0.97, wRp = 1.38. 2c, purity = 99%, GOF = 1.21, Rp = 0.97, wRp = 1.38. |
| This journal is © the Partner Organisations 2025 |