Stimuli-responsive gold–polyoxometalate multielectron transformers
Kirill
Monakhov

Leibniz Institute of Surface Engineering (IOM), Permoserstraße 15, Leipzig 04318, Germany. E-mail: kirill.monakhov@iom-leipzig.de
First published on 18th December 2024
Abstract
Gold and atomically precise polyoxometalates (POMs) are widely used materials with electron reservoir redox properties. The combination of optically excited gold nanoparticles, gold surface/tip electrodes, or molecular gold(I) complexes with {VV6} Lindqvist-, {MnMoVI6} Anderson-Evans-, and {PWVI12} Keggin-type POM structures leads to interfacial multielectron transformations with far-reaching implications for electronics, photonics, biomedical diagnostics, and catalysis.
Gold and its features
Although gold (Au) is a chemically “inert” noble metal, it is often the first choice when testing different ideas and concepts for preparing responsive materials and interfaces for application-oriented basic research. Gold is a conductor and is resistant to air and water. Gold surfaces can be easily modified by a variety of structurally exposed functionalities through, e.g., covalent (e.g., Au–SR) and coordinative (e.g., Au⋯NH2R) interactions.1 These characteristics allow materials scientists to efficiently process and use gold as a bottom electrode and/or as a sharp metal tip in scanning tunneling microscopy experiments, as nanoparticles with one-step chemically modified surfaces, or as metalorganic synthons in the synthesis of heterometallic ensembles under a range of conditions.Gold in compounds has two main oxidation states (+I and +III), can be easily converted into gold(0) nanoparticles (AuNPs) of different sizes and morphologies (e.g., nanorods and nanoclusters) and, thus, exhibit different optical and electronic properties. AuNPs can be obtained by a conventional citrate reduction method or solid-state dewetting with Ar+ bombardment to control the oxidation state of gold.2 When AuNPs are capped with electron acceptor agents (or so-called electron sponges), these engineered gold–sample interfaces can lead to plasmon-induced charge transfer triggered by an external stimulus such as light irradiation.3
Gold–polyoxometalate interfaces
Polyoxometalates (POMs) are atomically precise metal–oxygen clusters and are outstanding examples of electron sponges that exhibit element-selective redox activity in solution.4 Their assembly and use as anionic protecting ligands on AuNPs have been described in several studies, with particular emphasis on catalytic applications.5–7The latest study showed8 that attachment of H3PW12O40 to the surface of AuNPs under UV light leads to the transfer and storage of hot electrons in these Kegging-type POM structures (Fig. 1A). Such hybrid ensembles with donor–acceptor interactions hold great potential for the development of experimental prototype devices with the underlying charge separation, recombination dynamics, and redox switching mechanisms. Their wavelength-selective photon-electron control could be used for future practical applications in osmotic energy conversion, photocatalysis, optical multiplexed bioassays, and opto-electronic transducers.
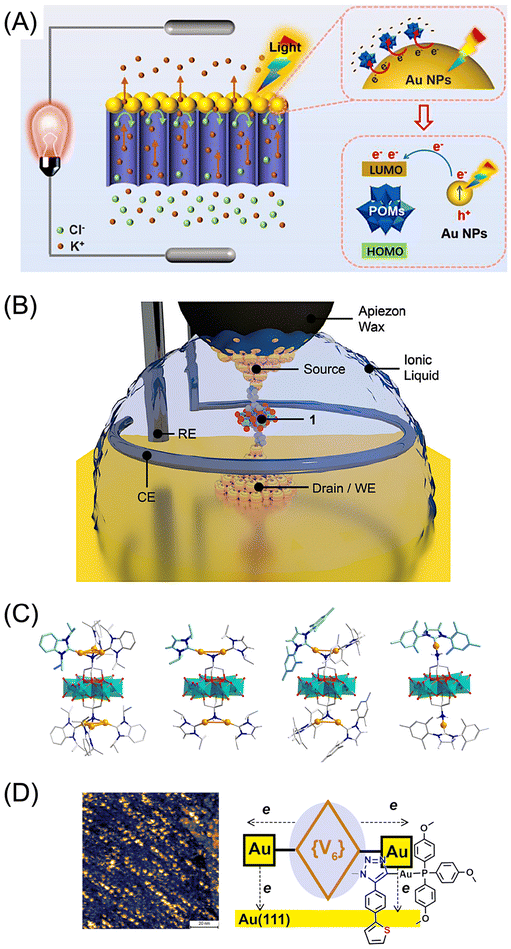 | ||
| Fig. 1 (A) Optically activated AuNPs (35 nm) act as electron donors for POM acids H3PW12O40 ({PW12}), which behave as electron acceptors in POM-based plasmonic electron sponge membranes. (B) A single-POM junction [MnMo6O18{(OCH2)3CC5H4N}2]3− in the four-electrode configuration immersed in the ionic liquid BMIM-OTf. 1 is the POM junction. WE is a working electrode (Au substrate), CE is a counter electrode (Pt), and RE is a reference electrode (Pt). (C) Anderson-Evans-type POMs [MnMo6O18{(OCH2)3CNH2}2]3− ({MnMo6}) bis-functionalized with monovalent Au3(carbene)3, Au2(carbene)2, and Au(carbene) moieties. Color code: Au = yellow; Mo = green; Mn = brown; O = red; N = blue; C = gray and light blue. (D) STM image of a sample obtained by drop-casting of CH2Cl2 solution of a Lindqvist-type hexavanadate ({V6}) bis-functionalized with monovalent Au(phosphine) moieties and coordinative S-termini on the Au(111) surface. The heterometallic dianion was synthesized from a [V6O13{(OCH2)3CCH2N3}2]2− precursor ({V6}) via Cu(I)-catalyzed alkyne–azide cycloaddition. For (A)–(D) H atoms and counterions are omitted for clarity. Reproduced/adapted from ref. 8 (A, copyright Springer Nature), ref. 9 (B, copyright Wiley), ref. 10 (C, copyright Wiley), and ref. 14 (D, copyright RSC). | ||
Gold–POM interfaces with multielectron transformation processes were also observed9 under electrochemical control by incorporating a tris(tetrabutylammonium) salt of the pyridyl-terminated Anderson-Evans-type POM [MnMo6O18{(OCH2)3CC5H4N}2]3− as a single-molecule junction in a four-electrode cell configuration (Fig. 1B). Electrochemical scanning tunneling microscopy (EC-STM) measurements were performed in the ionic liquid 1-butyl-3-methylimidazolium trifluoromethanesulfonate (BMIM-OTf). The results showed that this redox-active POM exhibits the behavior of a three-state electrochemical transistor.
Chemical post-functionalization of the related amine-terminated POM [MnMo6O18{(OCH2)3CNH2}2]3− with N-heterocyclic carbene (NHC) gold(I) complexes resulted in the formation of molecular triads,10 in which the POM acceptor is covalently sandwiched by low-valent Au3(NHC)3, Au2(NHC)2, or Au(NHC) donor moieties (Fig. 1C). The synergistic effect between gold and POM also plays a key role here. The triads showed excellent performance in the catalytic conversion of H2O2. Moreover, these can be viewed as molecular models of the POM-based EC-STM break junction, albeit direct comparison is indeed difficult due to the different type of gold and the different binding situations in these gold–POM heterostructures (Au⋯NH4C5-POM in the single-molecule junctions vs. (NHC)3Au3–N-POM, (NHC)2Au2–NH-POM, and (NHC)Au–NH2-POM in the individual molecules).
It is known from the chemistry of low-valent metalates that their highly reduced nature can be of great importance for the activation of inert bonds and for catalytically relevant multielectron transformations.11 However, data on the covalent derivatization of POM-based inorganic–organic hybrids with organogold(I) moieties and their application on metal surfaces are still scarce. This contrasts with the extensive data on intermetallic compounds consisting of negatively charged POM structures counter-balanced with mono- or polynuclear gold(I) complexes acting as cations.12 Discrete POMs such as, e.g., [NaAu4Pd8O8(AsO4)8]11− with incorporated gold(III) centers were also reported.13 The combination of POM electron sponges and highly reduced organometallic compounds or even AuNPs may be critical for the development of memory devices with extremely high data capacity.
It has recently been demonstrated that organogold(I) substituents introduced at the periphery of the trisalkoxo ligands of the Lindqvist-type hexavanadate precursors such as, e.g., [V6O13{(OCH2)3CCH2N3}2]2− affect the electrical properties of these four-state {V6}POM switches in new {Au(I)–V6–Au(I)} POMs on the Au(111) surface (Fig. 1D).14 The enhanced multielectron transformations observed at the “gold//{Au(I)–V6–Au(I)}-POM//vacuum–Pt-tip” interface are due to the efficient Au(I)-mediated charge redistribution across molecular linkages. This resulted in more than four distinct charge states of the POM under the influence of applied bias voltages and electron tunneling through vacuum. The described stimulus-responsive behavior is significantly different from the situation when Lindqvist-type hexavanadate structures bear non-metallic ligand substituents. In that case, no effect on the resistance steps in the current–voltage profiles was observed.15
Concluding remarks
The solution-processable POMs at gold interfaces prove to be promising redox-active hybrid materials with multielectron transforming character, which should be further explored using correlative microscopies at the nanoscale and electrical contact engineering at the micron-scale. For example, electrochemical STM and scanning electrochemical cell microscopy could be used to study their electrochemical activity under industry-relevant conditions. These methods should accelerate progress in the use of responsive gold–POM heterostructures,16 particularly in (liquid electrolyte) battery and computer data storage technologies.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The author declares no competing interests.Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) through the SPP 2262 program MemrisTec (Memristive Devices Toward Smart Technical Systems). Project number 536022773 (2DPOMristor).References
- T. Li, V. K. Bandari and O. G. Schmidt, Molecular electronics: creating and bridging molecular junctions and promoting its commercialization, Adv. Mater., 2023, 35, 2209088 CrossRef CAS.
- G. Lanza, M. J. Martinez Jimenez, F. Alvarez, J. A. Perez-Taborda and A. Avila, Valence state tuning of gold nanoparticles in the dewetting process: an X-ray photoelectron spectroscopy study, ACS Omega, 2022, 7, 34521 CrossRef CAS PubMed.
- Y. Hattori, S. Gutiérrez Álvarez, J. Meng, K. Zheng and J. Sá, Role of the metal oxide electron acceptor on gold–plasmon hot-carrier dynamics and its implication to photocatalysis and photovoltaics, ACS Appl. Nano Mater., 2021, 4, 2052 CrossRef CAS.
- W. Ahmad, N. Ahmad, K. Wang, S. Aftab, Y. Hou, Z. Wan, B.-B. Yan, Z. Pan, H.-L. Gao, C. Peung, Y. Junke, C. Liang, Z. Lu, W. Yan and M. Ling, Electron-sponge nature of polyoxometalates for next-generation electrocatalytic water splitting and nonvolatile neuromorphic devices, Adv. Sci., 2024, 11, 2304120 CrossRef CAS.
- Y. Wang, A. Neyman, E. Arkhangelsky, V. Gitis, L. Meshi and I. A. Weinstock, Self-assembly and structure of directly imaged inorganic-anion monolayers on a gold nanoparticle, J. Am. Chem. Soc., 2009, 131, 17412 CrossRef CAS PubMed.
- Y. Wang, O. Zeiri, M. Raula, B. Le Ouay, F. Stellacci and I. A. Weinstock, Host–guest chemistry with water-soluble gold nanoparticle supraspheres, Nat. Nanotechnol., 2017, 12, 170 CrossRef CAS.
- K. Xia, T. Yatabe, K. Yonesato, S. Kikkawa, S. Yamazoe, A. Nakata, R. Ishikawa, N. Shibata, Y. Ikuhara, K. Yamaguchi and K. Suzuki, Ultra-stable and highly reactive colloidal gold nanoparticle catalysts protected using multi-dentate metal oxide nanoclusters, Nat. Commun., 2024, 15, 851 CrossRef CAS PubMed.
- C. Zhu, L. Xu, Y. Liu, J. Liu, J. Wang, H. Sun, Y.-Q. Lan and C. Wang, Polyoxometalate-based plasmonic electron sponge membrane for nanofluidic osmotic energy conversion, Nat. Commun., 2024, 15, 4213 CrossRef CAS.
- C. Wu, X. Qiao, C. M. Robertson, S. J. Higgins, C. Cai, R. J. Nichols and A. Vezzoli, A chemically soldered polyoxometalate single-molecule transistor, Angew. Chem., Int. Ed., 2020, 59, 12029 CrossRef CAS.
- R. Chen, X.-H. Ma, P. Luo, C.-H. Gong, J.-J. Sun, Y.-B. Si, X.-Y. Dong, F. Pan and S.-Q. Zang, Atomically precise ternary cluster: polyoxometalate cluster sandwiched by gold clusters protected by N-heterocyclic carbenes, Angew. Chem., Int. Ed., 2024, 63, e202408310 CrossRef CAS PubMed.
- V. R. Landaeta, T. M. Horsley Downie and R. Wolf, Low-valent transition metalate anions in synthesis, small molecule activation, and catalysis, Chem. Rev., 2024, 124, 1323 CrossRef CAS.
- A. Blanc and P. de Frémont, When gold cations meet polyoxometalates, Chem. – Eur. J., 2019, 25, 9553 CrossRef CAS PubMed.
- N. V. Izarova, A. Kondinski, N. Vankova, T. Heine, P. Jäger, F. Schinle, O. Hampe and U. Kortz, The mixed gold–palladium polyoxo-noble-metalate [NaAuIII4PdII8O8(AsO4)8]11−, Chem. – Eur. J., 2014, 20, 8556 CrossRef CAS PubMed.
- S. K. Petrovskii, M. Moors, S. Schmitz, E. V. Grachova and K. Y. Monakhov, Increasing the redox switching capacity of Lindqvist-type hexavanadates by organogold post-functionalization, Chem. Commun., 2023, 59, 9517 RSC.
- F. Yang, G. Kalandia, M. Moors, J. Lorenz, M. Rohdenburg, X.-B. Wang, W. Cao, M. A. Moussawi, D. Volke, R. Hoffmann, J. Warneke, T. N. Parac-Vogt and K. Y. Monakhov, Ligand substituent effects on the electronic properties of Lindqvist-type polyoxometalate multi-level-switches in the gas phase, solution and on surfaces, Adv. Mater. Interfaces, 2024, 11, 2400411 CrossRef CAS.
- C. Huez, D. Guérin, F. Volatron, A. Proust and D. Vuillaume, Low-frequency noise in nanoparticle–molecule networks and implications for in materio reservoir computing, Nanoscale, 2024, 16, 21571 RSC.
| This journal is © the Partner Organisations 2025 |
